Abstract
The gut microbiome comprises the collective genome of the trillions of microorganisms residing in our gastrointestinal ecosystem. The interaction between the host and its gut microbiome is a complex relationship whose manipulation could prove critical to preventing or treating not only various gut disorders, like irritable bowel syndrome (IBS) and ulcerative colitis (UC), but also central nervous system (CNS) disorders, such as Alzheimer’s and Parkinson’s diseases. The purpose of this review is to summarize what is known about the gut microbiome, how it is connected to the development of disease and to identify the bacterial and biochemical targets that should be the focus of future research. Understanding the mechanisms behind the activity and proliferation of the gut microbiome will provide us new insights that may pave the way for novel therapeutic strategies.
Keywords: microbiome-gut-brain axis, Parkinson’s disease, gut microbiota, neurological diseases, Autism, manganese, metals, environmental factors, neurotoxicity
2. Introduction
Among the many microbial communities colonizing the human body, the gut microbiome is emerging as a major player influencing the health status of the host. The composition of the gut microbiome is established early during the host’s development and can undergo a myriad of changes throughout a lifetime. The complex interaction between host physiology and the gut microbiome is a topic of research that is still in its infancy. Yet broadening our understanding of this interaction could lead to beneficial therapeutic strategies for improving human health. Although we are only in the beginning stages of defining the biomarkers, bacterial species, and diets necessary to manipulate and study the human microbiome, we can appreciate the potential implications this research has on the future of medicine. The objective of this review is to summarize current findings linking host gut microbiome and health and to identify future directions necessary for unraveling the gut microbiome’s role in the pathogenesis of chronic metabolic and central nervous system diseases.
3. The Gut Microbiome
The gut microbiome comprises the collective genome of roughly 100 trillion microorganisms residing in the gastrointestinal tract (Tsai & Coyle, 2009). The gene repertoire of our gut bacteria contains 150 times more unique genes than the human genome (Qin, et al., 2010).
The fetal gastrointestinal tract (GIT) is sterile prior to birth with microbial colonization first occurring at delivery (Morelli, 2008). In vaginally delivered infants, the GIT is primarily colonized by bifidobacteria as well as lactobacilli, Bacteroides, Proteobacteria and Actinobacteria. In contrast, infants delivered via cesarean section have more Escherichia coli (E. coli) as well as Clostridia, especially C. difficile, and fewer Bacteroides and bifidobacteria (Penders, et al., 2006). Similarly, breast-fed infants showed a higher abundance of bifidobacteria, whereas in formula-fed infants, bifidobacteria and Bacteroides as well as Clostridia and Staphylococci were found in equal numbers (Harmsen, et al., 2000). Conversely, other groups have shown an abundance of Bifidobacterium, Actinomyces, and Haemophilus in breast-fed babies while formula-fed babies had an abundance of Firmicutes and Bacteroidetes in their gut (Yatsunenko, et al., 2012). This discrepancy in estimating the gut microbiome population is possibly due to differences in the techniques used to sample and analyze the data. However, despite these discrepancies, most studies emphasize that both the type of diet and mode of delivery can preferentially promote certain bacterial communities over others. While the mechanism remains unclear, it is known that the earliest gut microbiota primarily consists of bacteria that can metabolize the lactose absorbed from breast milk or infant formulas made from cow’s milk. However, with the introduction of solid food, the gut becomes dominated by bacterial species associated with carbohydrate, protein and fat utilization as well as vitamin synthesis (Koenig, et al., 2011). The host also selectively favors particular bacterial species in different regions of the gut. For example, butyrate-synthesizing bacteria such as the Firmicutes are present in higher proportions in the colon and are less well represented in the upper small intestine. This is beneficial and quite necessary since short chain fatty acids like butyrate are the main source of energy for colonic epithelial cells (Hooper, Midtvedt, & Gordon, 2002; Roediger, 1980; Wong, de Souza, Kendall, Emam, & Jenkins, 2006).
Not surprisingly, the interaction between an organism and its gut microbes is not unidirectional but involves feedback with the host environment affecting the gut microbiotic composition and the colonized bacteria, influencing host development. Following bacterial colonization, certain physiological changes have been observed in the gut, including increased production of neurotransmitters such as serotonin (5-HT) and γ-aminobutyric acid (GABA) as well as the expression of various cytokines. These changes are integral to gut homeostasis and to programming of the hypothalamic-pituitary-adrenal (HPA) axis, which plays an important role in stress responses (Diaz Heijtz, et al., 2011; Sudo, et al., 2004). As shown in Figure 1, early colonization of certain enterotypes can have a long-lasting influence on the health status of the host. For example, infants with a higher prevalence of Bifidobacterium and Collinsella at age 6 months showed lower adiposity at 18 months (Dogra, et al., 2015). In another study, overweight subjects exhibited decreased numbers of Bifidobacteria and the Bacteroides (Santacruz, et al., 2010). The gut microbiome composition throughout a host’s lifespan is not static, but measuring these changes proves difficult due to confounding changes in diet, environment, and disease state throughout life. As previously stated, numerous studies have studied gut microbiome composition early in life, yet much fewer studies have reported gut microbiome changes in middle-aged and elderly subjects. Shifts from Bifidobacterium to Clostridia and Bacteriodetes occur as the host develops from a newborn into an adult (Yatsunenko, et al., 2012). Decreases in Faecalibacterium prauznitzii and its anti-inflammatory relatives occur as young adults mature to become elderly or even centenarians (Biagi, et al., 2010). However, determining to what extent such changes reflect normal development and maturation versus dietary/environmental changes or pathological deficiencies will require further study.
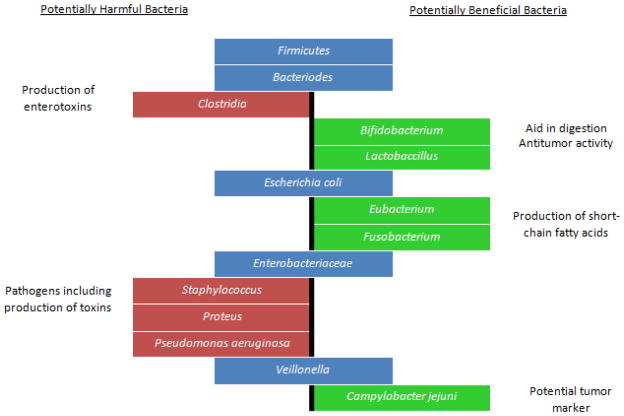
Figure 1.
Schematic representation of potentially harmful and potentially beneficial bacteria present in the gut microbiome. Pro-biotic bacteria such as Lactobacillus and Bifidobacteria modulate the gut environment by releasing bioactive compounds that enhance enteric epithelial barrier function as well as by competitively binding to the epithelium thus outcompeting pathogenic bacteria. Eubacterium rectale and Fusobacterium produce fatty acids such as acetic acid, propionate and butyrate that are important as an energy source for intestinal epithelial cells as well as for modulating mucosal immune responses. In contrast, higher counts of bacteria such as Staphylococcus and Pseudomonas are seen in various metabolic disorders such as diabetes and obesity. Clostridum tetani spores are resistant to the acidic environment of the stomach and to regular antibiotic treatments. Production of the tetanus toxin by these bacteria is thought to contribute to the “leaky gut syndrome” prevalent in autistic children. E.coli is a common commensal of the gut microbiome. However, certain serotypes are pathogenic and are known to cause gastroenteritis and urinary tract infections.
3.1 The Gut Microbiome and Health
It was initially thought that gut microbes were mainly commensals whose only benefit was controlling the population of pathogenic bacteria. We now know that these symbionts play important roles in aiding digestion, in the production of essential metabolites and in the development of the immune system (Walker & Lawley, 2013). Gut bacteria play a pivotal role in immune modulation and development of the nervous system and are the main source of vitamin K, and to a lesser extent the vitamin B complex (Kelly, King, & Aminov, 2007; Littman & Pamer, 2011; Resta, 2009). Indeed, germ-free rats require a higher intake of exogenous sources of vitamins K, B12 and B6 compared to their conventional counterparts (Gustafsson, 1959; Sumi, Miyakawa, Kanzaki, & Kotake, 1977; Wostmann, 1981). Recognition of the importance of the gut microbiota in infant development has been greatly aided by the development of gnotobiotic animal models. Germ-free rodents fed sterile chow, identical in all other respects to that consumed by their conventional mates, showed decreased basal metabolic rate, increased cholesterol in liver and blood as well as structural and functional differences in the enteric nervous system (ENS) (Danielsson & Gustafsson, 1959; Dupont, Jervis, & Sprinz, 1965; Wostmann, Wiech, & Kung, 1966). Furthermore, systemic T- and B-cell deficiencies in these animals are thought to result from the lack of exposure of the naïve immune cells to microbial products generally found in animals with normal intestinal microbiota (Falk, Hooper, Midtvedt, & Gordon, 1998; Mazmanian, Liu, Tzianabos, & Kasper, 2005; Szeri, Anderlik, Banos, & Radnai, 1976).
Other studies have shown that seemingly abrupt and chaotic shifts in the gut microbiota correspond to changes in the host’s environment, diet and genetic predisposition. Dysbiosis of the gut microbiome has been implicated in numerous disorders, ranging from intestinal diseases, such as colorectal cancer and inflammatory bowel disease (IBD), to more systemic diseases such as diabetes, metabolic syndrome and atopy (Walker & Lawley, 2013). The gut microbiome also influences various Type-2 Diabetes (T2D)-related complications (Fig. 2), including diabetic retinopathy, kidney toxicity, atherosclerosis, hypertension, diabetic foot ulcers, and cystic fibrosis (Zhang & Zhang, 2013). Recent research has also linked microbial dysbiosis to neurological disorders, such as Parkinson’s and Alzheimer’s diseases, multiple sclerosis and autism. The CNS connects with the gut via sympathetic and parasympathetic nerves. However, the importance of these connections had not been studied in depth until the past decade. Villaran and co-workers reported that peripheral inflammation in the form of dextran sodium sulfate (DSS)-induced colitis can aggravate LPS-induced neuroinflammation and neurodegeneration (Villaran, et al., 2010) as shown by increased mRNA transcripts of TNFα, iNOS and IL-6 in the midbrain. Interestingly, even rats with colitis alone (no midbrain injection of LPS) showed increased mRNA transcripts of TNFα, iNOS and IL-6 in the midbrain. Other studies have also shown that recurring systemic infections can increase the probability of developing multiple sclerosis, Alzheimer’s or Parkinson’s disease (Serres, et al., 2009; Tilvis, et al., 2004). A concise summary of the changes seen in the gut and CNS in various diseases is shown in Figure 3.
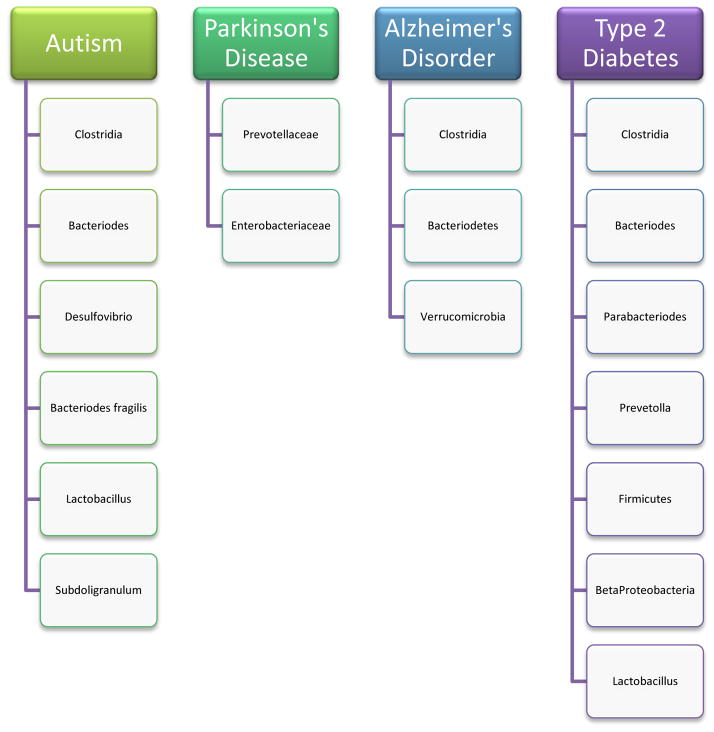
Figure 2.
Bacterial species relevant to research on Autism, Parkinson’s disease, Alzheimer’s disorder, and Type-2 Diabetes. Clostridia are prevalent in many diseases and notably, presence of C. tetani in the gut microbiome of autistic children may contribute the “leaky gut” syndrome seen in these children. Lower counts of probiotic bacteria like Lactobacillus are also seen in many disorders. Understanding the differences in bacterial species seen in healthy and diseased states is crucial to understanding their importance to host health.
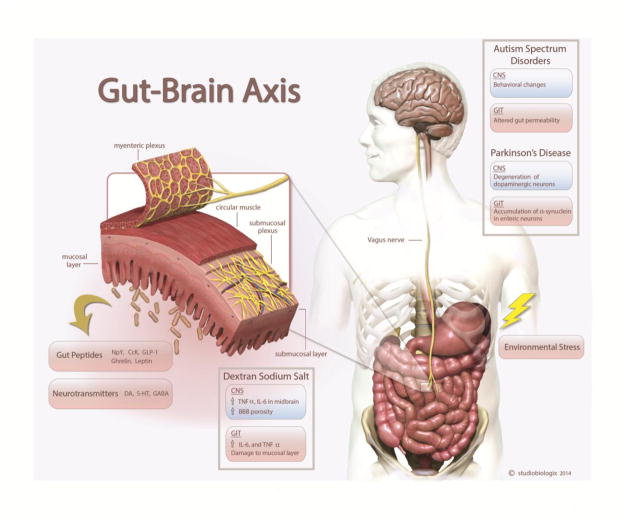
Figure 3.
The Gut-Brain axis: Bidirectional signaling between the gastrointestinal tract (GIT) and central nervous system (CNS) occurs through spinal afferents and the vagus nerve. This mode of communication is thought to occur through peptides such as Neuropeptide Y (NpY), Cholecystokinin (CcK), ghrelin, leptin as well as by neurotransmitters like dopamine (DA), serotonin (5-HT), GABA, acetylcholine (Ach) and glutamate. Human and animal studies of various diseases demonstrate that these two systems are not exclusive of one another but do in fact show some parallels in terms of expression of pro-inflammatory cytokines and altered physiological functions.
3.2 Systemic Diseases and Gut Microbiome Dysbiosis
Multi-disciplinary studies incorporating advances in next generation sequencing (NGS), have revealed that various systemic diseases, such as arthritis, atherosclerosis, IBD, as well as diabetes and obesity, show significant changes not only in the composition of the resident gut microbiota but also in the host’s gut homeostasis and metabolic processes (Musso, Gambino, & Cassader, 2011; Scher, et al., 2015; Vieira, et al., 2015; Yamashita, et al., 2015). In essence, the gut microbiota influence and even facilitate various metabolic processes such as regulating xenobiotic metabolism and energy production. As discussed later in this review, diet and environment play an important part in maintaining gut bacterial populations. Perturbations in this ecosystem can have negative and prolonged effects on host physiology. While there are many such systemic disorders that show an inherent gut microbiota dysbiosis, we summarize two common and well-studied diseases-ulcerative colitis and type-2 diabetes.
3.2.1 Ulcerative colitis
Ulcerative colitis (UC) is a type of IBD characterized by chronic inflammation and sores (ulcers) along the lining of the large intestine and rectum. In addition, neuroplastic changes in the ENS are observed in UC, including degeneration of ganglion cells and nerve hyperplasia (Vasina, et al., 2006). UC results from a combination of host genetic risk factors, environment, and alterations in gut microbiome composition. Dysbiosis in the gut microbiome of UC patients is characterized by a lower proportion of Firmicutes and a higher percentage of Gammaproteobacteria, sulfate-reducing Deltaproteobacteria, Actinobacteria and Proteobacteria compared to that of healthy hosts (Lepage, et al., 2011; Sokol, Lepage, Seksik, Dore, & Marteau, 2007; Sokol, et al., 2009). In general, Gram-negative bacteria populations tend to increase, while those of Gram-positive bacteria tend to decrease. Increases in Gram-negative bacteria like E.coli may lead to increased Lipopolysaccharide (LPS) translocation in the gut and consequently to a chronic state of low-grade inflammation as seen in UC. In addition to changes in the composition of the gut microbiome in IBD patients, gene expression for amino acid metabolism and biosynthesis are greatly downregulated while the expression of lysine, histidine, and arginine transport genes is upregulated. Expression in UC patients of transport and metabolism genes for the antioxidant glutathione (a tripeptide synthesized by bacterial species) also increases, thus possibly revealing the mechanism by which the gut microbiome responds to inflammatory oxidative stress (Gardiner, et al., 1993; Morgan, et al., 2012). Besides UC, gut microbiome dysbiosis also occurs in other forms of IBD where it causes vast changes in gut microbiome metabolic function and microbiotic reaction to the inflammatory response. Neither the extent to which dysbiosis alters GI microbiome function nor the specific consequences of such changes in gut microbiome composition are fully understood, and both require further metabolic and genetic study.
3.2.2 Diabetes
T2D is a chronic metabolic disorder wherein the body either does not produce enough insulin or cannot effectively metabolize glucose despite insulin production. Assessing the gut microbiome of healthy and T2D-diagnosed individuals showed that Betaproteobacteria were present in higher proportions in T2D individuals (Larsen, et al., 2010). This difference correlated more with increased glucose plasma levels than with body mass indices (BMI), suggesting that this species might be involved in glucose metabolism (Larsen, et al., 2010).
Although research linking PD to diabetes is still in its infancy, preliminary studies suggest that individuals with T2D have a higher incidence of PD than do non-T2D individuals (Hu, Jousilahti, Bidel, Antikainen, & Tuomilehto, 2007; Schernhammer, Hansen, Rugbjerg, Wermuth, & Ritz, 2011; Sun, et al., 2012). During a 9-year study, diabetic patients in Taiwan had a significantly increased risk of developing PD in both sexes and most age groups. Although the mechanistic link between T2D and PD is not known, chronic inflammation and oxidative stress are also linked in the risk of PD. Furthermore, insulin plays a role in regulating central dopaminergic signaling, and thus insulin dysregulation in T2D patients may contribute to nigrostriatal dopaminergic dysfunction. Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is an important gene involved in mitochondrial biogenesis, regulating energy homeostasis relative to environmental stimuli (Knusel, Michel, Schwaber, & Hefti, 1990; Puigserver & Spiegelman, 2003; Z. Wu, et al., 1999). PGC-1α and its downstream targets are downregulated in both PD and T2D patients (Mootha, et al., 2003; Zheng, et al., 2010). Recently, it has been found that the zinc finger protein Parkin Interacting Substrate (PARIS) binds to the insulin response sequences in the PGC-1α promoter. This interaction represses the expression of PGC-1α and Nuclear Respiratory Factor-1 (NRF-1) genes in dopaminergic neurons leading to their loss (J. H. Shin, et al., 2011). Thus, both T2D and PD share common pathophysiological mechanisms including oxidative stress, inflammation and mitochondrial dysfunction.
Since individuals with T2D run an increased risk of developing PD, we need to better understand the effect of anti-diabetic drugs on PD. A patient’s risk of developing PD increases 2.2 fold when diagnosed with T2D, but that risk is lowered to only 1.3 fold with oral anti-hyperglycemic agent (OAA) therapy, suggesting that OAAs could also protect against neurodegenerative diseases like Parkinson’s (Wahlqvist, et al., 2012). Metformin is an oral anti-hyperglycemic agent prescribed to treat T2D and metabolic syndrome (Knowler, et al., 2002). It is a well-tolerated drug that has been extensively used for decades, and thus, testing the drug’s efficacy against neurodegenerative diseases seems promising. Metformin’s mechanism of action was originally reported to involve activating AMPK through inhibition of mitochondrial respiration (El-Mir, et al., 2000; Owen, Doran, & Halestrap, 2000). However, recent evidence indicates that the metformin induced AMPK activation occurs independent of mitochondrial respiration (Bergheim, et al., 2006). We found that metformin does not inhibit mitochondrial respiration in neuronal cells at therapeutic concentrations. Recent evidence suggests that altering the gut microbiome to favor Akkermansia muciniphila, a newly discovered mucin-degrading bacterium, in mice fed a high-fat diet (HFD) may contribute to the anti-diabetic effects of metformin (N. R. Shin, et al., 2014). Treatment with A. muciniphila can reverse HFD-induced metabolic disorders such as adipose tissue inflammation, weight gain, and insulin resistance (Everard, et al., 2013). Metformin also affects host levels of methionine and folate, two nutrients essential to human health yet not produced de novo, but instead are synthesized by certain gut microbes. Folates are B-vitamins involved in the biosynthesis of purines and pyrimidines as well as the biotransformation of amino acids within the host. Interestingly, deficiencies in B-12 vitamins, and folate specifically, have been implicated in Alzheimer’s disease patients (Lahiri & Maloney, 2010). Methionine is a sulfur-containing amino acid that occurs in proteins with proposed antioxidant activity (Levine, Mosoni, Berlett, & Stadtman, 1996). Folate and methionine are just two examples of possible gut byproducts that influence bacterial and host metabolism and the development of CNS disease.
3.3 Central Nervous System Disorders and Gut Microbes
Although not well understood, the gut microbiome could have a significant influence on behavior and psychosis. Adult mice that had been separated as neonates from their mother for a protracted time showed elevated levels of basal and stress-evoked adrenocorticotropic hormone (ACTH) and corticotropic releasing hormone (CRH). This response was inversely related to the age of the mice at the time of maternal separation (Schmidt, Oitzl, Levine, & de Kloet, 2002), thus development of the HPA axis may have a maturation window influenced and regulated to some extent by the gut microbial composition. Sudo and colleagues showed that germ-free (GF) BALB/c mice had significantly higher levels of plasma corticosterone and ACTH compared to specific pathogen-free (SPF) mice in response to restraint stress (Sudo, et al., 2004). This response was tempered by the presence of Bifidobacterium infantis, which is known to be present in the neonate gut. In a highly intuitive experiment, they showed that gnotobiotic BALB/c mice colonized with enteropathogenic Escherichia coli (EPEC) developed an exaggerated response to stress, much higher than what the GF mice had shown. However, another set of mice colonized with the mutant EPEC strain ΔTir, did not display a heightened HPA response to stress. ΔTir bacteria lack the intimin receptor, thus preventing microbial adherence and internalization in the intestinal tissue. This study showed that early colonization by intestinal bacteria regulates the development of the HPA axis. Colonization with commensal bacteria that are known to produce pro-health micronutrients is essential because neurodevelopment is impaired if pathogenic bacteria colonize the gastrointestinal tract.
3.3.1 Neurodevelopmental disorders
Autism spectrum disorder (ASD)
Human studies have shown that gut problems in early childhood might contribute to autism development. Various disorders, such as Attention Deficit Hyperactive Disorder (ADHD) and Autism Spectrum Disorder (ASD) share behavioral abnormalities in sociability, communication, and/or compulsive activity (Hsiao, et al., 2013). An abnormal HPA response and altered microbial and metabolic profiles have been implicated in these disorders (Kaneko, Hoshino, Hashimoto, Okano, & Kumashiro, 1993; King, Barkley, & Barrett, 1998; Ming, Stein, Barnes, Rhodes, & Guo, 2012). Ming and colleagues showed altered amino acid metabolism and increased oxidative stress in ASD children relative to age-matched controls (Ming, Stein et al. 2012). A sub-group of ASD children with a history of gastrointestinal perturbations had an altered microbial profile compared to controls. The ASD group showed decreased levels of both 5-amino-valerate, which is thought to act as a weak GABA agonist, and 3-(3-hydroxyphenyl) propionic acid, an antioxidant.
ASD children usually have a higher abundance of Proteobacteria and Bacteroidetes and a lower abundance of the Firmicutes and bifidobacteria when compared to healthy controls (Finegold, et al., 2010; Finegold, Downes, & Summanen, 2012; Mezzelani, et al., 2014). Interestingly, of the many classes of bacteria that constitute the Firmicutes, one class in particular, the Clostridia, is shown to be present in higher numbers in autistic children with a history of GI problems (Song, Liu, & Finegold, 2004). Similarly, despite the overall abundance of Bacteroidetes in autistic subjects, lower counts of Prevotella are seen. Thus along with quantifying the relative increase or decrease in the populations of certain phyla, it is necessary to determine the populations of specific gut symbionts in order to understand the significance of certain physiological changes in the gut and/or the brain. One of the reasons why Clostridia are implicated in ASD is because autistic children subjected to oral Vancomycin treatment showed a regression of the characteristic symptoms of this disorder. Vancomycin is not absorbed in the intestine but can readily kill Gram-positive bacteria such as Clostridia. However, when the treatment was discontinued, the patients reverted back to their autistic behavior (Finegold, 2008). It is thought that clostridial spores, which are temperature-, pH- and antibiotic-resistant, also sporulate, further multiplying the mucosal populations of endotoxin-producing bacteria. Indeed, according to one current theory, the “leaky gut” syndrome, high counts of pathogenic bacteria in the gut impair the intestinal barrier by producing neuro- and endotoxins, which then expose the mucosa and sub-mucosa to bacteria. The bacterial invasion of this previously aseptic environment causes immune cell activation and infiltration as well as up-regulation of pro-inflammatory cytokines such as TNFα and Il-1β. This inflammatory response further increases barrier permeability thereby perpetuating an inflammatory cycle. Sandler et al. (2000) hypothesized that in a subgroup of children, disruption of indigenous gut microbiota might promote colonization by one or more neurotoxin-producing bacteria, contributing, at least in part, to eliciting autistic signs. These same investigators found that with broad-spectrum antimicrobial exposure followed by chronic persistent diarrhea, previously acquired skills deteriorated and autistic features emerged (Sandler, et al., 2000). In a recent study of offspring exposed to maternal immune activation (MIA), Hsaio et al. revealed that oral treatment with Bacteroides fragillis corrects gut permeability, alters microbial composition, and ameliorates defects in communicative, stereotypic, anxiety-like and sensorimotor behaviors mainly by altering the HPA axis response to stress. This experiment supports a microbiome-gut-brain connection in a mouse model of ASD and identifies a potential probiotic therapy for GI disorders and particular behavioral symptoms in human neurodevelopmental disorders (Hsiao, et al., 2013).
Besides ASD, there has been considerable interest in understanding the role the gut microbiota plays in the development of ADHD and schizophrenia. In one study, infants were given either Lactobacillus rhamnosus GG or placebo during the first 6 months of life and gut microbiota was assessed over a period of 13 years. The study found a correlation between lower counts of Bifidobacterium species and development of ADHD or Asperger’s Syndrome (Partty, Kalliomaki, Wacklin, Salminen, & Isolauri, 2015).
3.3.2 Neurodegenerative Disorders
Parkinson’s disease
The role of the gut microbiome in the pathogenesis of chronic neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases is beginning to emerge. In PD, GI dysregulation is often seen several years before the disease is even detected. Braak and colleagues hypothesized that the disease begins in the gut and spreads from gut to brain via the gut-brain axis, i.e., vagus nerve and spinal cord. Indeed, the parasympathetic fibers of the vagus nerve that innervate the intestine among other regions arise from the dorsal motor nucleus (DMVX). Lewy bodies (aggregated proteins, mainly alpha-synuclein and ubiquitin), which are the hallmark of PD, were found in the ENS in post-mortem cases of early PD (Braak, de Vos, Bohl, & Del Tredici, 2006). The presence of these protein aggregates correlated with the increasing stages of PD and was subsequently found in the spinal cord, DMVX, prefrontal cortex and finally the mid-brain region of postmortem PD subjects. A recent study demonstrated that alpha-synuclein injected in the gut wall of rats migrated to the brain stem via the vagus nerve at a rate estimated to be 5–10 mm/day (Holmqvist, et al., 2014). The idea that PD patients have a low-grade inflammation of the gut has been around for some time. Indeed, increased mRNA expression of pro-inflammatory cytokines has been observed in colonic biopsies of PD patients compared to control subjects (Devos, et al., 2013). This chronic low-grade inflammation might be the trigger that leads to blood brain barrier leakiness, immune cell activation and infiltration and ultimately neuro-inflammation in the CNS.
Investigators are developing considerable interest in understanding the role of the gut microbiota in the context of PD. In one study, fecal microbiota collected from 72 PD subjects and age-matched controls showed higher counts of Enterobacteriaceae and reduced Prevotellaceae in PD patients. Prevotella is known to break down complex carbohydrates, providing short chain fatty acids (SCFA’s) as well as thiamine and folate as byproducts that promote a healthy intestinal environment. Decreased Prevotella numbers are likely to result in reduced production of these important micronutrients. Unless compensated by dietary changes to supply these nutrients exogenously, decreased thiamine and folate might result in reduced production of essential vitamins and impaired secretions of gut hormones (Scheperjans, et al., 2015). However, the study did not evaluate whether or not the patients had a history of GI disturbances or significant inflammation. Nevertheless, taken together, these results suggest that changes in the gut microbiome could have a direct effect on the CNS via the gut-brain axis with chronic mild systemic inflammation possibly driving the pathogenesis. Cyanobacteria present in small numbers in the GI tract are thought to produce β-N-methylamino-L-alanine (BMAA), which is elevated in the brains of AD, PD and amyotrophic lateral sclerosis (ALS) patients. BMAA is an excitotoxin that activates metabotropic glutamate receptor 5, thereby inducing depletion of the major antioxidant glutathione. As a result, neurons and glial cells are unable to effectively control ROS and RNS production in the brain. The BMAA protein is also implicated in aiding protein misfolding and aggregation typically seen in AD, PD and ALS (Brenner, 2013). However, whether patients showing elevated levels of BMAA in their brain also show increased cyanobacteria populations in the gut is not clear and needs further study.
A majority of the clinically diagnosed PD cases are of the sporadic form, which is likely caused by a complex interplay of genetic and environmental factors. During the last few years, a number of epidemiological and clinical studies have suggested potential environmental risk factors for PD, including environmental exposure to certain pesticides and fungicides such as paraquat, rotenone and maneb, and some surrogate factors such as living in rural areas, drinking well water and farming. In addition, exposure to heavy metals such as iron, lead, mercury, cadmium, arsenic and manganese, as well as to metal-based nanoparticles, has also been shown to increase the risk of PD through the accumulation of metals in the mid brain and increased oxidative stress-mediated apoptosis (Aboud, et al., 2014; Afeseh Ngwa, et al., 2009; Afeseh Ngwa, et al., 2011; Harischandra, Jin, Anantharam, Kanthasamy, & Kanthasamy, 2015; Kanthasamy, et al., 2012; Milatovic, Zaja-Milatovic, Gupta, Yu, & Aschner, 2009). Currently, the effect of environmental factors on the host gut microbiome is completely unknown.
Manganese is an essential trace element vital for normal bone development, fat and carbohydrate metabolism, blood sugar regulation, and the biological functions of a number of enzymes (Aschner & Aschner, 2005; Bowman, Kwakye, Herrero Hernandez, & Aschner, 2011). However, excessive manganese is considered a neurotoxic pollutant in the environment and recently gained importance as a putative risk factor for environmentally-linked PD. Chronic exposure to occupational or environmental sources of manganese can cause a neurodegenerative disorder known as Manganism, characterized by severe neurological deficits that often resemble the involuntary extrapyramidal symptoms associated with PD. Manganism most frequently occurs from occupational manganese exposure during mining, welding metals, and dry battery manufacturing. Other manganese-containing products presenting a public exposure risk include fertilizers, varnish, fungicides, gasoline additives and livestock feeding supplements. The growing evidence indicates that manganese primarily causes damage to the basal ganglia in humans, where it affects dopamine release and causes GABA dysregulation (Brouillet, Shinobu, McGarvey, Hochberg, & Beal, 1993; Erikson & Aschner, 2003; Fitsanakis, Au, Erikson, & Aschner, 2006; Roth, Li, Sridhar, & Khoshbouei, 2013). Yet, despite its prevalence and potential risk to human health, the mechanisms by which manganese exerts its toxic effects on the gut and gut microbiome have yet to be elucidated.
Preliminary experiments were conducted in our laboratory to assess the impact of manganese in altering gut physiology by exposing mice to 15 mg/kg/day MnCl2 for 30 days via oral gavage. Manganese-exposed mice displayed increased whole gut transit time and an altered fecal metabolic profile. GC-MS studies carried out in stool samples showed that manganese exposure decreases butyrate production as well as α-tocopherol, which is involved in Vitamin-E synthesis, whereas it increases compounds such as cholic acid, which is known to saturate bile salts leading to the development of gall stones (Table 1). Thus, our results provide initial evidence that metal toxicity can influence gut motility and key gut microbial metabolites. Thus, it is likely that exposure to environmental toxins can influence the gut microbiome profile with potentially unfavorable physiological effects.
Table 1.
The fecal metabolic profile was altered by manganese treatment from day 10 until the completion of the study. The table above shows some key compounds that were different between treatment and control groups. Notably, decreased vitamin E and increased production of cholic acid were seen in manganese-exposed mice compared to healthy age-matched controls.
| Compound | Manganese-treated mice | Physiological relevance |
|---|---|---|
| α-tocopherol | Decreased levels | Antioxidant, requires proper nerve conduction |
| Cholesterol | Decreased levels | Structural component of cell membranes and an important pre-cursor for production of steroids, bile acids, etc. |
| Cholestane | Decreased levels | Reduces number of synaptic vesicles, helps regulate neurotransmitter release |
| Palmitic acid | Higher levels | Induces anxiety-like behavior in mice. Can be a mild antioxidant. |
| Cholic acid | Higher levels | Downregulates bile acid synthesis increasing the tendency to develop gall stones. |
4. Factors Influencing Microbiome-Gut-Brain regulation
4.1 Environment
A recently described epigenetic model of disease development, called the LEARn model (Latent Early-Life Associated Regulation), predicts that early-life exposures to nutritional imbalances, metals, maternal care variations, or other environmental stressors can lead to modified expression of disorder-associated genes over the course of an individual’s lifespan (Lahiri & Maloney, 2010). An early environmental influence is the maternal environment. The gut microbiota of mouse littermates are more similar than are those of pups born to different mothers despite being reared in adjacent boxes in the same animal room (Benson, et al., 2010). Bacteria present in the mother’s vaginal tract and on her skin serve as the first bacteria to colonize the neonate gut. Later, microbes present in the colostrum form a part of the gut microbiome, thus increasing the complexity of this environment. Our locality also plays an important role in determining individual gut microbiota profiles One study noted that ASD patients showed a gut microbiota profile similar to their healthy siblings, while unrelated healthy controls had significantly different profiles (Parracho, Bingham, Gibson, & McCartney, 2005).. Besides locality, additional environmental factors affecting the gut microbiome include ethnicity and culture (Annalisa, et al., 2014; De Filippo, et al., 2010; Holmes, Wilson, & Nicholson, 2008).
4.2 Diet, Microbes and Health
The relative abundance of gut microbiome is dependent upon the energy source available to them and their ability to utilize this source. In one study, switching from a low-fat, plant polysaccharide-rich diet to a high-fat, high-sugar diet shifted the community structure of the microbiome within a single day, changed the representation of metabolic pathways in the microbiome, and altered microbiome gene expression (Turnbaugh, et al., 2009). Firmicutes, especially Lactobacillaceae, Veillonellaceae, and Lachnospiraceae, were seen in greater proportions in obese individuals. Conversely, a high-fiber, low-fat diet decreased the population of Firmicutes (Parnell & Reimer, 2012).
While individual variations are seen, in general, diets high in animal fat and protein are associated with an abundance of Bacteroides, high-fiber diets with Prevotella, while plant-based diets are predominated by both Bacteroides and the Firmicutes (David, et al., 2014; G. D. Wu, et al., 2011). Furthermore, the gut microbiome composition is more complex in a host whose diet is primarily plant-based as opposed to animal-based (G. D. Wu, et al., 2011). Also, bacteria that can degrade complex carbohydrates are present in high-fiber diets in higher numbers, while bacteria that primarily break down proteins predominate in animal-based diets. However, the fact must be reiterated that while different studies state the overall change in bacterial phyla, it is important to understand which species show population changes. This is also necessary to know since commensal as well as pathogenic bacteria are present in each phylum. Summaries of the factors that potentially affect the host-gut microbiome relationship are shown in figure 4.
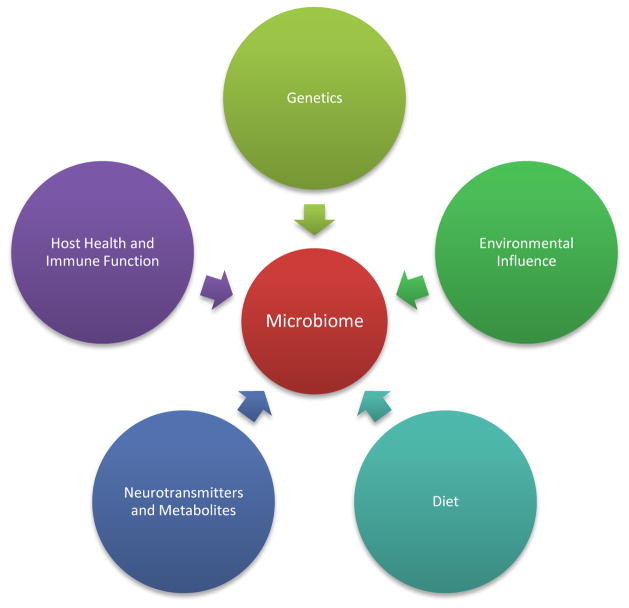
Figure 4.
Factors affecting gut microbiota composition: The host-microbiota interaction is a complex and dynamic symbiosis. While diet and environment are well known factors affecting the populations of different phyla in the gut, some reports suggest that the host’s genetic composition might pre-dispose the continued growth of certain microbiota. The microbiome population can also be modulated by the presence of neurotransmitters and metabolites secreted by the host.
Changing one’s diet towards complex carbohydrates such as less processed whole grain foods, fermented vegetables, and prebiotic-containing foods has been shown to induce weight loss, to reduce blood pressure, cholesterol and heart rate, and to decrease toxin-producing bacteria while increasing beneficial bacteria in the gut (Hvistendahl, 2012). Prebiotics are not digested by the host, but metabolized by the gut bacteria, thereby favoring specific changes in the activity and composition of the gut microbiome that benefit host health and wellness (Gibson, Probert, Loo, Rastall, & Roberfroid, 2004). Preliminary research suggests that the risk of developing cardiovascular disease, T2D, obesity, and osteoporosis is reduced with the addition of a prebiotic to the host’s diet (Roberfroid, 2000). One of the first prebiotics an infant receives is oligosaccharides present in the mother’s milk (Partty, et al., 2015). Unlike the sugars found in infant formula, the oligosaccharides present in breast milk are only partially digested in the small intestine and so reach the colon where they provide an energy source for the development of bifidobacteria. A probiotic, on the other hand, is a microbe (bacterial or yeast) that is administered to the host to confer health benefits (Maslowski & Mackay, 2011). They are found in our food (yogurt, fermented vegetables) and have been implicated in alleviating lactose intolerance and boosting immune function. Common probiotics include Propionibacterium, lactic acid bacteria (LAB), bifidobacteria, and certain yeasts to name a few. Numerous studies have investigated the effects of probiotics on behavior and intestinal health (Bravo, et al., 2011; D’Mello, et al., 2015; Ostan, et al., 2015; Savignac, Kiely, Dinan, & Cryan, 2014).
5. Biomarkers of interest
As mentioned before, gut microbiota produce various metabolites, some of which are involved in the host’s metabolic processes while others help maintain a healthy gut environment. Although the gut residence times of these metabolites differ, they can be detected in the host’s blood, urine, feces, or breath. Thus a useful and non-invasive way of diagnosing bacterial dysbiosis is by profiling biofluids for such compounds using metagenomics technology. For example, using 1H NMR spectroscopy and targeted analysis, Schicho and colleagues were able to identify a number of urine, serum and plasma metabolites found in higher numbers in colitis patients verses healthy controls (Schicho, et al., 2012). Another method employed to detect gut bacterial dysbiosis is by using selected ion flow tube mass spectrometry [SIFT-MS] to measure volatile compounds in the breath, stool or urine of patients. Volatile organic compounds (VOC), such as ethane, methane, pentane, dimethyl sulfide, ammonia, dimethylamine, trimethylamine, are produced in part by bacteria. These can be measured in biofluids like urine, and saliva or breath and stool. Changes in the levels of these compounds could serve as a potential diagnostic tool. For example, elevated levels of pentane and ethane are found in individuals diagnosed with asthma and chronic obstructive pulmonary disorder (Van Berkel, et al., 2010).
SCFAs are one of the most important gut microbial products. They affect a range of host processes, including energy utilization, host-microbe signaling, and colonic pH, with consequent effects on gut microbiome composition, gut motility, and epithelial cell proliferation (Musso, et al., 2011). Butyrate, a common gut microbiome byproduct, and propionate can inhibit histone deacetylase (HDAC) enzymes and alter the expression of specific genes via conformational changes in the active site of HDAC leading to its inactivation (Aoyama, Kotani, & Usami, 2010; Dashwood, Myzak, & Ho, 2006). Microbe-derived butyrate can trigger cell cycle arrest and apoptosis in rapidly dividing colonocytes, and thus has proven effective at preventing colon cancer (Rooks & Garrett, 2011; Shenderov, 2012; Waby, et al., 2010). In vitro studies have shown that butyrate amplifies the antioxidant properties of glutathione-S-transferase (Ebert, et al., 2003). Fecalibacterium prausnitzii, Roseburia intestinalis, Eubacteria hallii, E.coli and Butyricicoccus pullicaecorum are some of the bacteria that produce butyrate in the gut. During ulcerative colitis, the populations of these bacteria decline (Kumari, Ahuja, & Paul, 2013). Although gut microbial dysbiosis in T2D patients is moderate compared to the dramatic dysbiosis reported in obese patients, a study done by Qin et al. revealed that T2D patients exhibit fewer butyrate-producing bacteria and more opportunistic pathogens. They also reported an enrichment of microbial functions conferring sulphate reduction and increased oxidative stress, which are indicative of pathogenicity (Qin, et al., 2012). This promotes the idea that butyrate-producing bacteria protect the host against various diseases. Given that other intestinal diseases show a loss of butyrate-producing bacteria with a commensurate increase in opportunistic pathogens, it is worth considering butyrate as a potential biomarker for intestinal health.
Regarding the gut microbiome’s influence on neurotransmitters, a recent study has shown that patients diagnosed with depression have increased volatile fatty acids such as isovaleric acid in their stool (Szczesniak, Hestad, Hanssen, & Rudi, 2015). Isovaleric acid can cross the blood-brain barrier (BBB) and affect neurotransmitter release in the CNS, thus possibly worsening this disorder. This has diagnostic importance since a patient’s stool can be screened for increased volatile fatty acids. Factor-S, a sleep promoting substance, accumulates in the brain and fluids of sleep-deprived individuals. It is in fact derived from the bacterial cell wall and thus released by gut bacteria (Collins & Bercik, 2009) and it provides yet another example of how bacterial products can influence brain activity and function. Rats given a strict antibiotic regimen exhibited greatly reduced slow-wave sleep and increased sleep onset latency (Brown, Price, King, & Husband, 1990).
6. Current model systems
Currently, 16s rRNA gene sequence analysis or shotgun sequencing for identifying bacteria is widely used. By applying these techniques, changes in the gut microbiome composition, from phyla to species level, can be ascertained. To analyze the gut microbial content in T2D patients, Qin et al. developed a novel metagenome-wide association study (MGWAS) (Qin, et al., 2012). Using deep shotgun sequencing of the gut microbiome of 345 Chinese individuals, they identified roughly 60,000 T2D markers, thus allowing them to establish a linkage group for bacterial species-level analysis. Larsen et al. examined fecal content to characterize the composition of the colonic microbiome in adults with T2D using qPCR and tag-encoded amplicon pyrosequencing of the V4 region of the 16s rRNA gene (Larsen, et al., 2010). This hyper-variable V4 region is a bacterial gene segment used for distinguishing different bacteria, in this case phyla: Bacteroidetes, Firmicutes, Actinobacteria, Verrucomicrobia, and Proteobacteria. This study involved 36 males of varying BMIs, half with T2D and half serving as non-diabetic controls. Compared to controls, the diabetic group had elevated concentrations of plasma glucose and significantly reduced proportions of the phylum Firmicutes and class Clostridia.
One of the main obstacles in this area is the lack of an animal model system that successfully replicates a healthy or a diseased individual’s gut microbiome. Since rodent diets differ substantially from the diet of humans, making comparisons between human and mouse gut microbiota studies inherently problematic (Flint, 2011; Ravussin, et al., 2012). While studies have been done using conventional mice as the model organism, in some cases, the alterations seen in the intestinal bacterial population in mice are not validated by human data. For example, bacteria like Prevotella and Ruminococcus are dominant in the human gut but are under-represented in the mouse gut. Also, due to limited sequencing depth, many smaller microbial populations in both humans and mice may not be detected (Nguyen, Vieira-Silva, Liston, & Raes, 2015). However, while species differences exist between humans and rodents, the overall dominant families are more or less represented similarly between the two. Hence studies involving mouse models of different diseases/disorders can be used to understand certain changes in human gut microbiome structure. However, these findings must be validated with human gut microbiome sequencing studies. An integrative approach, involving detailed epidemiological surveys (e.g., lifestyle, diet, health history), high-throughput sequencing as well as the use of advanced analytical tools, is needed to better understand the gut microbiome and its role in various biological systems in the body. Furthermore, another approach to bridging the translational gap posed by rodent model systems is by making “humanized mice” by adding specific bacterial strains common to the human GIT into germ-free mice (Martin, et al., 2008; Turnbaugh, et al., 2006; Turnbaugh, et al., 2009).
7. Conclusions and Future directions
Understanding the complex transgenomic metabolic interactions within the gut microbiome perhaps poses the ultimate challenge in deciphering and learning how to optimize our gut microbiome to promote health and longevity. Understanding the gut microbiome has the potential to revolutionize the way human and animal diseases are treated. Novel antibiotics, prebiotics, probiotics, and even microbiome transplants could replace expensive surgeries or drug treatments. Actively managing the gut microbiome holds considerable potential in the realm of preventive medicine as well as the treatment of acquired diseases. The most difficult undertaking will be dissecting the complex metabolic, environmental, and genomic interactions between the host and its gut microbiome. Some of the pertinent areas to be studied are as follows:
The dynamics and impact of maternal gut microbiome transfer and the influence of infant nutrition on development of the gut microbiome in early childhood.
The influence of host genome variations and the fetal environment on the future gut microbiome and the influence of fetal development on the HMMA.
The effects of antibiotic use on the gut microbiome in utero, during childhood, and in the elderly population.
The influence of the gut microbiome on metabolism, pharmacokinetics, and the biotransformation of drugs and toxicants.
The effect of drugs and toxicants on gut microbiome population and gut metabolites.
The influence of the gut microbiome and the gut-brain axis on repair and remodeling in the CNS as well as on the development and progression of neurodegenerative diseases.
Developing a cost-effective technique to map an individual’s complete gut microbiome at any point in time to better help physicians create a therapeutic regimen.
Along with advances in metabolomics and metagenomics, a greater understanding of the potential health benefits of our gut microbiome is within reach. Considering the complex and dynamic nature of the microbial population and its effect on the individual, collaboration in the fields of microbiology, neurobiology, biochemistry, immunology, gastroenterology, genetics, epidemiology, pharmacology, and toxicology is warranted to gain a better understanding of this important host-bacteria relationship.
Acknowledgments
The authors acknowledge Gary Zenitsky for his assistance in the preparation of this manuscript. This work was in part supported by National Institutes of Health (NIH) [Grants ES19267, NS 074443, and ES10586]. The W. Eugene and Linda Lloyd Endowed Chair to A.G.K. is also acknowledged.
Footnotes
Conflict of Interest: A.G.K. is a shareholder of PK Biosciences Corporation (Ames, IA), which is interested in translating mechanistic studies into therapies targeting kinase signaling. The other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, Bowman AB. PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper. Neurobiol Dis. 2014;73C:204–212. doi: 10.1016/j.nbd.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol. 2009;240:273–285. doi: 10.1016/j.taap.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol. 2011;256:227–240. doi: 10.1016/j.taap.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annalisa N, Alessio T, Claudette TD, Erald V, Antonino de L, Nicola DD. Gut microbioma population: an indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediators Inflamm. 2014;2014:901308. doi: 10.1155/2014/901308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition. 2010;26:653–661. doi: 10.1016/j.nut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Herrero Hernandez E, Aschner M. Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner SR. Blue-green algae or cyanobacteria in the intestinal micro-flora may produce neurotoxins such as Beta-N-Methylamino-L-Alanine (BMAA) which may be related to development of amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson-Dementia-Complex in humans and Equine Motor Neuron Disease in horses. Med Hypotheses. 2013;80:103. doi: 10.1016/j.mehy.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Brown R, Price RJ, King MG, Husband AJ. Are antibiotic effects on sleep behavior in the rat due to modulation of gut bacteria? Physiol Behav. 1990;48:561–565. doi: 10.1016/0031-9384(90)90300-s. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, Surrette MG, Swain MG. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. J Neurosci. 2015;35:10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson H, Gustafsson B. On serum-cholesterol levels and neutral fecal sterols in germ-free rats; bile acids and steroids 59. Arch Biochem Biophys. 1959;83:482–485. doi: 10.1016/0003-9861(59)90056-6. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Myzak MC, Ho E. Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27:344–349. doi: 10.1093/carcin/bgi253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, Naveilhan P, Nguyen JM, Neunlist M, Derkinderen P. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Bruck WM, Berger B, Brussow H, Lee YS, Yap F, Chong YS, Godfrey KM, Holbrook JD, Group GS. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio. 2015;6 doi: 10.1128/mBio.02419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont JR, Jervis HR, Sprinz H. Auerbach’s plexus of the rat cecum in relation to the germfree state. J Comp Neurol. 1965;125:11–18. doi: 10.1002/cne.901250103. [DOI] [PubMed] [Google Scholar]
- Ebert MN, Klinder A, Peters WH, Schaferhenrich A, Sendt W, Scheele J, Pool-Zobel BL. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis. 2003;24:1637–1644. doi: 10.1093/carcin/bgg122. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–480. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM. Therapy and epidemiology of autism--clostridial spores as key elements. Med Hypotheses. 2008;70:508–511. doi: 10.1016/j.mehy.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA., 3rd Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;18:260–262. doi: 10.1016/j.anaerobe.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Au C, Erikson KM, Aschner M. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int. 2006;48:426–433. doi: 10.1016/j.neuint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Flint HJ. Obesity and the gut microbiota. J Clin Gastroenterol. 2011;45(Suppl):S128–132. doi: 10.1097/MCG.0b013e31821f44c4. [DOI] [PubMed] [Google Scholar]
- Gardiner KR, Erwin PJ, Anderson NH, Barr JG, Halliday MI, Rowlands BJ. Colonic bacteria and bacterial translocation in experimental colitis. Br J Surg. 1993;80:512–516. doi: 10.1002/bjs.1800800436. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gustafsson BE. Vitamin K deficiency in germfree rats. Ann N Y Acad Sci. 1959;78:166–174. doi: 10.1111/j.1749-6632.1959.tb53101.x. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. alpha-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson’s disease. Toxicol Sci. 2015;143:454–468. doi: 10.1093/toxsci/kfu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30:842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- Hvistendahl M. My microbiome and me. Science. 2012;336:1248–1250. doi: 10.1126/science.336.6086.1248. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hoshino Y, Hashimoto S, Okano T, Kumashiro H. Hypothalamic-pituitary-adrenal axis function in children with attention-deficit hyperactivity disorder. J Autism Dev Disord. 1993;23:59–65. doi: 10.1007/BF01066418. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Choi C, Jin H, Harischandra DS, Anantharam V, Kanthasamy A. Effect of divalent metals on the neuronal proteasomal system, prion protein ubiquitination and aggregation. Toxicol Lett. 2012;214:288–295. doi: 10.1016/j.toxlet.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D, King T, Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res. 2007;622:58–69. doi: 10.1016/j.mrfmmm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- King JA, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biol Psychiatry. 1998;44:72–74. doi: 10.1016/s0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knusel B, Michel PP, Schwaber JS, Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990;10:558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Ahuja V, Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J Gastroenterol. 2013;19:3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol. 2010;45:291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sorensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage P, Hasler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Dore J, Raedler A, Schreiber S. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227–236. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, Rezzi S, Ramadan Z, van Bladeren P, Fay LB, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mezzelani A, Landini M, Facchiano F, Raggi ME, Villa L, Molteni M, De Santis B, Brera C, Caroli AM, Milanesi L, Marabotti A. Environment, dysbiosis, immunity and sex-specific susceptibility: A translational hypothesis for regressive autism pathogenesis. Nutr Neurosci. 2014 doi: 10.1179/1476830513Y.0000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Stein TP, Barnes V, Rhodes N, Guo L. Metabolic perturbance in autism spectrum disorders: a metabolomics study. J Proteome Res. 2012;11:5856–5862. doi: 10.1021/pr300910n. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138:1791S–1795S. doi: 10.1093/jn/138.9.1791S. [DOI] [PubMed] [Google Scholar]
- Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–380. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostan R, Bene MC, Spazzafumo L, Pinto A, Donini LM, Pryen F, Charrouf Z, Valentini L, Lochs H, Bourdel-Marchasson I, Blanc-Bisson C, Buccolini F, Brigidi P, Franceschi C, d’Alessio PA. Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED study, an open-label randomized control trial. Clin Nutr. 2015 doi: 10.1016/j.clnu.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012;107:601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Partty A, Kalliomaki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015;77:823–828. doi: 10.1038/pr.2015.51. [DOI] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Meta HITC, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012;20:738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol. 2009;587:4169–4174. doi: 10.1113/jphysiol.2009.176370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71:1682S–1687S. doi: 10.1093/ajcn/71.6.1682S. discussion 1688S–1690S. [DOI] [PubMed] [Google Scholar]
- Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Garrett WS. Bacteria, food, and cancer. F1000 Biol Rep. 2011;3:12. doi: 10.3410/B3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA, Li Z, Sridhar S, Khoshbouei H. The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology. 2013;35:121–128. doi: 10.1016/j.neuro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, Nelson MN, Wexler HM. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, Marti-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, Manasson J, Pamer EG, Littman DR, Abramson SB. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B. Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care. 2011;34:1102–1108. doi: 10.2337/dc10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicho R, Shaykhutdinov R, Ngo J, Nazyrova A, Schneider C, Panaccione R, Kaplan GG, Vogel HJ, Storr M. Quantitative metabolomic profiling of serum, plasma, and urine by (1)H NMR spectroscopy discriminates between patients with inflammatory bowel disease and healthy individuals. J Proteome Res. 2012;11:3344–3357. doi: 10.1021/pr300139q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Oitzl MS, Levine S, de Kloet ER. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- Serres S, Anthony DC, Jiang Y, Broom KA, Campbell SJ, Tyler DJ, van Kasteren SI, Davis BG, Sibson NR. Systemic inflammatory response reactivates immune-mediated lesions in rat brain. J Neurosci. 2009;29:4820–4828. doi: 10.1523/JNEUROSCI.0406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenderov BA. Gut indigenous microbiota and epigenetics. Microb Ecol Health Dis. 2012:23. doi: 10.3402/mehd.v23i0.17195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- Sokol H, Lepage P, Seksik P, Dore J, Marteau P. Molecular comparison of dominant microbiota associated with injured versus healthy mucosa in ulcerative colitis. Gut. 2007;56:152–154. doi: 10.1136/gut.2006.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi Y, Miyakawa M, Kanzaki M, Kotake Y. Vitamin B-6 deficiency in germfree rats. J Nutr. 1977;107:1707–1714. doi: 10.1093/jn/107.9.1707. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care. 2012;35:1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesniak O, Hestad K, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. 2015 doi: 10.1179/1476830515Y.0000000007. [DOI] [PubMed] [Google Scholar]
- Szeri I, Anderlik P, Banos Z, Radnai B. Decreased cellular immune response of germ-free mice. Acta Microbiol Acad Sci Hung. 1976;23:231–234. [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009;11:307–313. doi: 10.1007/s11894-009-0045-z. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel JJ, Dallinga JW, Moller GM, Godschalk RW, Moonen EJ, Wouters EF, Van Schooten FJ. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir Med. 2010;104:557–563. doi: 10.1016/j.rmed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006;126–127:264–272. doi: 10.1016/j.autneu.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Vieira AT, Macia L, Galvao I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, Nicoli JR, Harper JL, Teixeira MM, Mackay CR. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015;67:1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- Villaran RF, Espinosa-Oliva AM, Sarmiento M, De Pablos RM, Arguelles S, Delgado-Cortes MJ, Sobrino V, Van Rooijen N, Venero JL, Herrera AJ, Cano J, Machado A. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson’s disease. J Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- Waby JS, Chirakkal H, Yu C, Griffiths GJ, Benson RS, Bingle CD, Corfe BM. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol Cancer. 2010;9:275. doi: 10.1186/1476-4598-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlqvist ML, Lee MS, Hsu CC, Chuang SY, Lee JT, Tsai HN. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord. 2012;18:753–758. doi: 10.1016/j.parkreldis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Walker AW, Lawley TD. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res. 2013;69:75–86. doi: 10.1016/j.phrs.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Wostmann BS. The germfree animal in nutritional studies. Annu Rev Nutr. 1981;1:257–279. doi: 10.1146/annurev.nu.01.070181.001353. [DOI] [PubMed] [Google Scholar]
- Wostmann BS, Wiech NL, Kung E. Catabolism and elimination of cholesterol in germfree rats. J Lipid Res. 1966;7:77–82. [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kasahara K, Emoto T, Matsumoto T, Mizoguchi T, Kitano N, Sasaki N, Hirata K. Intestinal Immunity and Gut Microbiota as Therapeutic Targets for Preventing Atherosclerotic Cardiovascular Diseases. Circ J. 2015;79:1882–1890. doi: 10.1253/circj.CJ-15-0526. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H. Microbiota associated with type 2 diabetes and its related complications. Food Science and Human Wellness. 2013;2:167–172. [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wullner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR, Global PDGEC. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]