Abstract
Background
Given the potential importance of epithelial plasticity (EP) to cancer metastasis, we sought to investigate biomarkers related to EP in men with localized prostate cancer (PC) for the association with time to PSA recurrence and other clinical outcomes after surgery.
Methods
Men with localized PC treated with radical prostatectomy at the Durham VA medical center and whose prostatectomy tissues were included in a tissue microarray (TMA) linked to long-term outcomes. We performed immunohistochemical studies using validated antibodies against E-cadherin and Ki-67 and mesenchymal biomarkers including N-cadherin, vimentin, SNAIL, ZEB1, and TWIST. Association studies were conducted for each biomarker with baseline clinical/pathologic characteristics and risk of PSA recurrence over time.
Results
Two hundred and five men contributed TMA tissue and had long-term follow-up (median 11 years). Forty-three percent had PSA recurrence; 3 died of PC. The majority had high E-cadherin expression (86%); 14% had low/absent E-cadherin expression. N-cadherin was rarely expressed (<4%) and we were unable to identify an E-to-N cadherin switch as independently prognostic. No associations with clinical risk group, PSA recurrence, or Gleason sum were noted for SNAIL, ZEB1, vimentin, or TWIST, despite heterogeneous expression between patients. We observed an association of higher Ki-67 expression with Gleason sum (p=0.043), NCCN risk (p=0.013), and PSA recurrence (HR 1.08, p=0.0095).
Conclusions
The expression of EP biomarkers in this cohort of men with a low risk of PC-specific mortality was not associated with aggressive features or PSA relapse after surgery.
Keywords: epithelial to mesenchymal transition, prostate cancer, tumor biomarkers, prognosis, PSA recurrence
Background
Localized prostate cancer (PC) is a heterogeneous disease, in which men have widely disparate outcomes based on key clinical and pathologic factors including Gleason sum, PSA levels, tumor stage, and extent of invasion(1, 2). Current models of risk of recurrence or PC mortality after surgery are reasonably accurate at assessing long-term outcomes(1). However, some low and intermediate risk tumors still relapse while some high-risk tumors may be cured with surgery alone and our ability to predict these discordant results is imperfect, illustrating the biologic heterogeneity even within well-defined risk categories(3). Thus, additional biomarkers of biologic aggressiveness in localized PC are needed.
Epithelial plasticity (EP), defined as the ability of cells to reversibly undergo phenotypic changes, may underlie the ability of many solid tumors, including PC, to disseminate and resist commonly used therapies, including surgery, radiation, hormonal therapies, and chemotherapy(4, 5). During the loss of the more differentiated epithelial phenotype, cancer cells may up-regulate stemness biomarkers(6) or biomarkers of a mesenchymal or invasive phenotype(7), associated with an epithelial-to-mesenchymal transition (EMT). An EMT has been associated with metastatic risk in multiple tumor types, and PC cell lines and human metastases expressing EMT biomarkers appear to be more androgen receptor independent and aggressive(8). We have shown that circulating tumor cells (CTCs) from men with metastatic castration resistant PC commonly express these plasticity biomarkers, indicating their potential importance in lethal disease(9), and others have shown that loss of epithelial biomarkers and/or an increase in mesenchymal or stemness biomarkers in localized PC may be associated with recurrent disease and PC mortality(6, 7, 10).
Several studies have specifically analyzed mesenchymal biomarker expression in radical prostatectomy specimens, identifying an E- to N-cadherin switch(7), loss of cytokeratin or PSA expression(6, 11), gain of hedgehog or NOTCH signaling(6), or gain of expression of the EMT transcriptional regulators TWIST and SNAIL(10), as adversely prognostic and independently associated with recurrent disease. However, others have not found associations between SNAIL or vimentin expression and clinical outcomes(12, 13), and currently EP biomarkers are not routinely assessed during the pathologic examination of the prostate. We thus sought to evaluate the association of EP biomarker expression in a contemporary series of men with localized PC treated with radical prostatectomy and who had long-term follow-up for recurrence.
Materials and Methods
Patient population
The current cohort includes men with localized PC treated with radical prostatectomy performed between 1993–2004 at the Durham Veteran’s Affairs Medical Center (VAMC) in Durham NC. Clinical data was extracted and included in the Shared Equal Access Research Center Hospital (SEARCH) database, under Duke University and Durham VAMC IRB approval. Data recorded included age, demographics, PSA levels at diagnosis and recurrence, prostatectomy pathologic characteristics, stage and NCCN risk score, prior and subsequent therapies, biopsy information, and long term recurrence, metastasis, and survival outcomes. PSA recurrence was defined as a single PSA >0.2 ng/ml, two values at 0.2 ng/ml, or secondary treatment for a rising PSA prior to reaching 0.2 ng/ml and were typically followed every 6–12 months with serial PSA monitoring after surgery. Men who received adjuvant therapy with an undetectable PSA were censored for PSA recurrence at that time. A tissue microarray on a random subset of patients in the SEARCH database treated at the Durham VA was developed after institutional review board approval in which prostatectomy histologic sections were arrayed on slides for biomarker evaluation with four cores of cancer per patient on each microarray, along with benign negative control tissues. We focused on the dominant highest grade lesion in a given patient for the TMA creation for biomarker development.
Antibodies and validation
We performed antibody optimization and analytic validation for all antibodies tested, determining the optimal concentration using both negative and positive control tissues prior to application to the TMA. Antibodies against E-cadherin, Ki-67, N-cadherin, vimentin, SNAIL1/2, TWIST, and ZEB1 were evaluated in parallel with hematoxylin and eosin (H&E) by an expert PC pathologist blinded to outcomes and other biomarker results (RV). Scoring of each biomarker followed an ordinal scale ranging from 0–2 (E-cadherin, ZEB1, vimentin) or 0–3 (SNAIL, TWIST) based on intensity and frequency of expression in each TMA section. The scoring range for each biomarker was selected by the pathologist based on the heterogeneity and range of expression between patients. Ki-67 was scored on a 0–100% scale based on frequency of expression in tumor cells. In order to account for tumor heterogeneity for each biomarker, four tumor containing TMA sections were obtained from radical prostatectomy tissue per patient which was then linked back to the subject ID by a master code for clinical database association studies. For each biomarker, minimum and maximum expression levels per subject as well as average expression was associated with outcomes and pathologic/clinical features. Scoring of epithelial tumor cells rather than benign stroma was performed for all EP biomarkers. Table 1 provides a listing of each antibody used, the source and clone, isotype, positive and negative controls, and concentrations used.
Table 1.
Antibodies used for immunohistochemical studies performed in the present study.
| Antigen | Antibody Source | Catalog/clone ID | Host | Isotype | Dilution | Retrieval | Positive Control Tissue | Negative Control Tissue |
|---|---|---|---|---|---|---|---|---|
| Vimentin | Dako | M7020 | M/m/aH | IgG2a | 1:150 | (0.5% in 5mM HCl) Pepsin, 40C°/15mints | Tonsil, Kidney | Internal stroma control |
| E Cadherin | Dako | M3612 | M/m/aH | IgG1(k) | 1:100 | 10mMTris Base, 1mM EDTA, 0.05% Tween20, pH 9.0 | Breast Cancer | Internal epithelial control |
| N Cadherin | Dako | M3613 | M/m/aH | IgG1(k) | 1:50 | 10mM Na Citrate / pH6.1 95C°/20mints | Heart | Internal stroma control |
| Ki67 | Dako | M7240 | M/m/aH | IgG1(k) | 1–50 | 10mM NaCitrate/0.05%Tween 20 pH6.1, 95C°/20mints | Tonsil | Benign tissue |
| Zeb1 | NovusBio | NBP1-88854 | R/p/aH | IgG | 1:250 | 1mM EDTA pH 8.0, 95C°/20mints | Breast Cancer | Normal breast Tissue |
| Snail/Slug | Abcam | Ab85936 | R/p/aH | IgG | 1:500 | 1mM EDTA pH 8.0, 95C°/20mints | Breast Cancer | Normal breast tissue |
| Twist | Abcam | Ab49254 | R/p/aH | IgG | 1:100 | 10mM NaCitrate / pH6.1 95C°/20mints | Testis | Normal Prostate |
Statistical methods and analysis plan
The primary objective was to assess the association of each EP biomarker with PSA recurrence over time. PSA recurrence was defined as the time from the date of RP to PSA recurrence, with an increase in recurrence hypothesized for higher levels of Ki-67 and mesenchymal biomarkers (SNAIL, TWIST, N-cadherin, vimentin), and lower levels of epithelial biomarkers (E-cadherin). Secondary objectives included the association of each EP biomarker with adverse clinical/pathologic characteristics (PSA, Gleason sum, NCCN risk, stage, survival and risk of metastasis). Descriptive statistics were generated for each marker. Patients who had not failed biochemically as of last follow up were censored at time of last follow up or death, whichever occurred first. Survival and recurrence was documented through annual updates to the SEARCH database and chart review. The Kaplan-Meier approach was used to estimate the PSA recurrence and overall survival distributions. In addition, the proportional hazards model was used to determine the association between the markers and PSA recurrence. Univariate hazard ratios and 95% confidence intervals were estimated.
Results
Two-hundred five men with localized PC were identified and included in this analysis who contributed tissue to the tissue microarray. The median age was 63 years (range 47–73). Fifty percent of men were White and 48% of men were Black. By D’Amico/NCCN risk classification, 47% were low risk, 33% intermediate risk, and 20% high risk (Table 2). At surgery, 12 had Gleason 8–10 tumors, 67% were Gleason 7, and 21% were Gleason 6 or under. Fifteen percent had seminal vesicle invasion, 27% had extracapsular extension, and PSA at the time of surgery was a median of 7.4 (range 0.6–75.4). Over a median follow-up period of 11.3 years, 71 (35%) men died, with 3 men (1.4%) dying of metastatic prostate cancer out of 4 men (2%) who developed metastatic disease. Eighty-nine (43.4%) had biochemical (PSA) recurrence and 77 (38%) of men were treated with adjuvant or salvage radiation to the prostate bed. Fifteen percent of men (n=32) required androgen deprivation therapy (ADT) at any time; however, no patients received ADT prior to surgery. Table 2 provides demographic and clinical characteristics, and Supplementary Figure 1 shows the REMARK diagram for patient and specimen/biomarker analysis.
Table 2. Baseline characteristics of the patients included in the present tissue microarray study.
ADT=androgen deprivation therapy. PSA=prostate specific antigen. NCCN=National Comprehensive Cancer Network.
| Clinical and Pathologic Characteristics of the Patients | N (%), range |
|---|---|
|
| |
| Age, years | 63 (47–73) |
| Race/Ethnicity | |
| White (%) | 104 (51) |
| Black (%) | 99 (48) |
| Hispanic (%) | 0 (0) |
| PSA at the time of surgery, ng/dl | 7 (1–75) |
| Pathologic Gleason sum | |
| ≤6 | 42 (21) |
| 7 | 138 (67) |
| 8–10 | 25 (12) |
| NCCN Risk Group | |
| Low | 96 (47) |
| Intermediate | 68 (33) |
| High | 40 (20) |
| Extracapsular extension (%) | 56 (27) |
| Seminal Vesicle Invasion (%) | 31 (15) |
| Positive Surgical Margins (%) | 125 (61) |
| Prior ADT, % | 0 (0) |
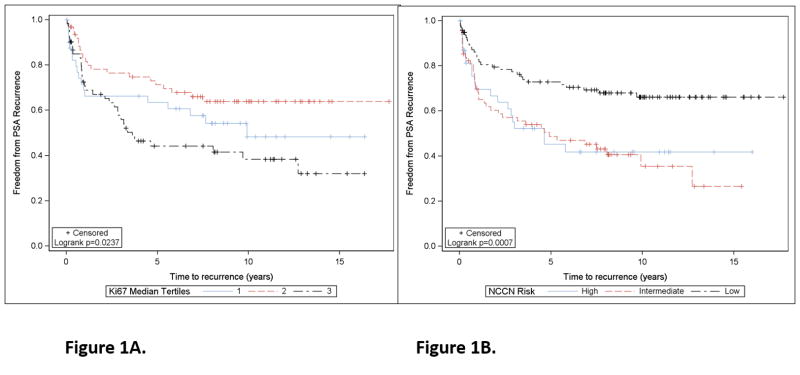
We initially examined Ki-67 as a biomarker of tumor proliferation rate, given the association of higher Ki-67 expression with recurrence and adverse pathology in multiple prior studies (14–18). Median Ki-67 expression was 2.5% (range 0–18.7%), while the median maximum Ki-67 expression was 4.7% (range 0–21.7%), and was evaluable in 178/205 men. Of these 178, we identified 14 men who had Ki-67 scores of 0, in which benign prostate tissue was likely scored in the present study, leaving 164 men evaluable for Ki-67 analysis (see Supplementary Figure 1 for REMARK diagram). Supplemental Figure 2 presents a spaghetti plot demonstrating the variability of Ki-67 expression levels by site of the core on a per patient basis. Using the median maximum Ki-67 score (0–100% range) as a continuous variable, Ki-67 percentage was associated with PSA recurrence (HR 1.07 for each unit increase in Ki-67, p=0.016, 95% CI 1.01–1.14). In multivariable analysis adjusting for NCCN risk, Ki-67 remained associated with PSA recurrence (HR 1.07 per unit increase, 95% CI 1.01–1.14), although risk was not uniform across Ki-67 tertiles (Figure 1a). NCCN risk was also associated with PSA recurrence (HR 1.91 and 2.20 for high and intermediate vs. low risk, respectively, Figure 1b). The time to PSA recurrence was similar in the two lowest tertiles as compared to the highest tertile (median 9.9 years, not reached, vs. 3.6 years, respectively) and 10-year recurrence-free proportion was 46, 64, and 38%, respectively. These data suggest that men in the highest tertile of Ki-67 have a higher risk of PSA recurrence over time. While patients with low risk prostate cancer had favorable outcomes (73% of men were free of PSA progression at 5 years, median time to recurrence not yet reached), men with intermediate or high prostate cancer had a higher risk of recurrence (49 and 45% of men free of PSA progression at 5 years, respectively, and median time to recurrence of 4.9 and 4.6 years). The differences in PSA recurrence across NCCN risk groups was statistically significant (log rank p=0.0057 and 0.0005 for low vs. high and low vs. intermediate risk, respectively).
Figure 1. Association of Ki-67 biomarker expression (average score per subject across TMA) and NCCN clinical risk group with PSA relapse.
A. Association of Ki-67 expression by tertiles with recurrence-free survival (PSA relapse), shown in a Kaplan-Meier survival plot. B. Association of NCCN risk groups with PSA relapse, shown in a Kaplan-Meier survival plot. Low risk includes PSA<10, Gleason 6 or less, and pT2a or less pathologic stage. Intermediate risk includes PSA 10–20, Gleason 7, or pT2b. High risk includes PSA>20, Gleason 8–10, or stage T2c or higher.
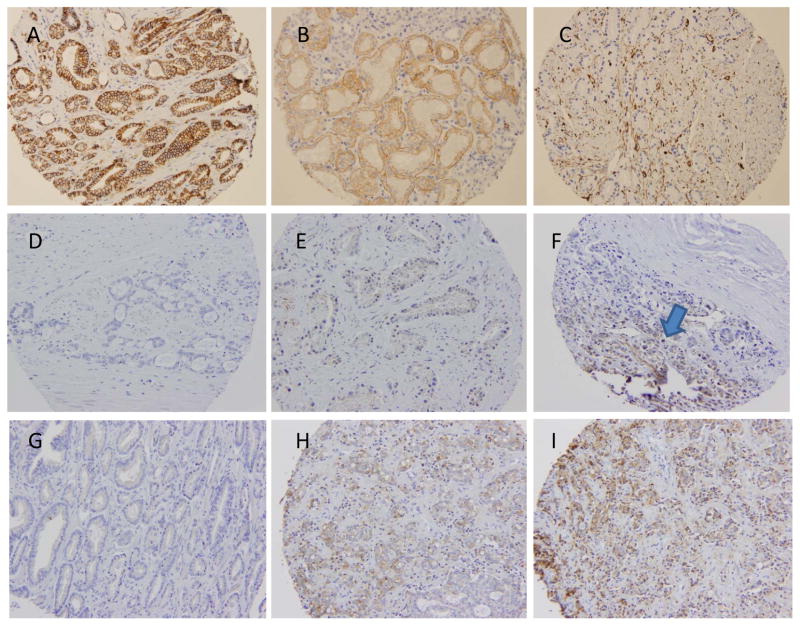
Having validated Ki-67 and NCCN risk in our clinical dataset, we next evaluated a series of EP biomarkers in primary PC tissues. Examples of representative IHC images of each biomarker are shown in Figure 2 and Supplementary Figure 3. We found that only 1.5% and 12.7% of patients had absent or low E-cadherin expression, respectively; the majority (85.6%) had high E-cadherin expression. For all mesenchymal biomarkers, we used the maximal expression between cores, given the heterogeneity of expression between cores. For all epithelial biomarkers, we used the minimum expression between cores. Of evaluable tissue, N-cadherin was rarely expressed, and was present in only 3.9% of PCs, while 96.0% had absent N-cadherin expression. We observed that vimentin expression to be largely stromal in distribution and only tumor cell vimentin was scored. We found that 22.4% and 5.9% of patients expressed intermediate and high vimentin, respectively, and most cancers had absent vimentin expression (70.7%). Zeb1 was expressed in 15.3% of cases, with only 3 cases demonstrating intense staining. SNAIL was expressed more heterogeneously among 188 evaluable men for this biomarker, with 31%, 39%, 24%, and 5% of PCs having 0, 1, 2, and 3+ SNAIL expression, respectively. Finally, TWIST expression was also heterogeneously expressed in the 148 evaluable men, with 1%, 45%, 29%, and 64% of PCs having 0, 1, 2, and 3+ TWIST expression.
Figure 2.
Representative prostate cancer tissue microarray (TMA) immunohistochemical (IHC) staining examples from the Durham VA SEARCH database, stained for A) E-cadherin (2+), B) N-cadherin (1+), and C) vimentin (2+). D-F shows ZEB1 expression (0, 1, and focal 2 (arrow), respectively). G-I shows SNAIL expression (0, 1, 2, respectively). Images of TWIST expression are available in supplementary figure 1.
In univariate analysis, we found no association with any EP biomarker with PSA recurrence (Table 3). When examined using mean expression, minimum expression, or maximum expression, we likewise found no associations with outcome for low E-cadherin, high N-cadherin, high SNAIL, high vimentin, high ZEB1, or high TWIST expression. In several cases, higher mesenchymal protein expression was numerically associated with improved outcome, although this was not statistically significant. For example, men with high levels of SNAIL expression had a 5-year PSA progression-free probability of 69% as compared to 53% for men with low SNAIL expression. Similar trends were seen for vimentin (5 year PSA recurrence-free probability of 68% for high vimentin, vs 57% for absent vimentin). High E-cadherin was associated with a greater probability of PSA relapse at 5 years (43%) as compared to men with low to absent E-cadherin in their prostate cancer (24% risk of PSA relapse). N-cadherin was not evaluated for associations with PSA recurrence given the low number of men who had high N-cadherin expression (n=8). TWIST expression was also not associated with PSA relapse (Table 3). In addition, we found no statistically significant prognostic associations for composites of EP biomarkers (0, 1, vs. 2 or more EP biomarkers) for PSA recurrence. Finally, there was little concordance between individual EP biomarkers (kappa <0.05). Given the low number of PC-specific deaths or metastatic events over time, we were unable to identify an association of EP biomarker expression with these endpoints.
Table 3.
Association of epithelial plasticity biomarker expression with PSA recurrence.
| Biomarker (number evaluable) | Univariate Hazard Ratio for PSA recurrence, 95% CI | 5-year PSA recurrence rate(%) and 95% CI | 10-year PSA recurrence rate (%)and 95% CI |
|---|---|---|---|
|
| |||
| Median Ki-67 (n=164) | |||
| Ki67 tertile 1 (n=57) | 0.84 (0.50, 1.40) | 60% (45%, 72%) | 46% (30%, 60%) |
| Ki-67 tertile 2 (n=52) | 0.48 (0.28, 0.83) | 71% (58%, 81%) | 64% (50%, 75%) |
| Ki-67 tertile 3 (n=55) | REF | 44% (31%, 57%) | 38% (25%, 52%) |
| SNAIL (maximum), n=188 | 47% (35%, 61%) | ||
| Absent SNAIL (n=58) | 1.47 (0.84, 2.58) | 45% (34%, 58%) | 54% (41%, 69%) |
| SNAIL 1+ (n=74) | 1.39 (0.82, 2.37) | 31% (21%, 45%) | 52% (40%, 64%) |
| SNAIL 2–3+ (n=56) | REF | 42% (29%, 59%) | |
| ZEB1 (maximum), n=189 | |||
| Absent ZEB1 (n=160) | 0.91 (0.51, 1.65) | 41% (34%, 49%) | 49% (41%, 57%) |
| ZEB1 1–2+ (n=29) | REF | 48% (30%, 69%) | 61% (37%, 86%) |
| Vimentin (maximum), n=203 | |||
| Absent vimentin (n=145) | 1.40 (0.86, 1.29) | 43% (35%, 52%) | 51% (43%, 61%) |
| Vimentin 1–2+ (n=58) | REF | 32% (22%, 47%) | 38% (27%, 53%) |
| E-cadherin (minimum), n=205 | |||
| Absent-low E-cadherin (n=29) | 0.62 (0.32, 1.19) | 24% (12%, 44%) | 36% (21%, 57%) |
| E-cadherin 2+ (n=176) | REF | 43% (36%, 51%) | 50% (42%, 58%) |
| TWIST (maximum), n=148 | |||
| Absent-low TWIST (n=10) | 1.56 (0.67, 3.61) | 50% (25%, 82%) | 60% (33%, 88%) |
| TWIST 2–3+ (n=138) | REF | 40% (32%, 49%) | 47% (38%, 56%) |
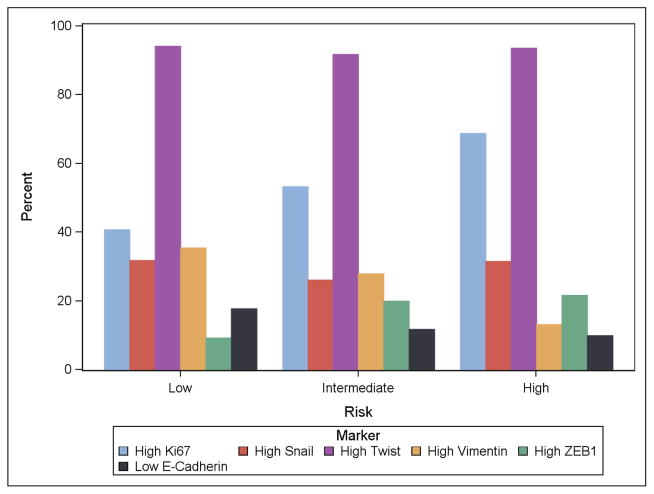
We next examined whether EP biomarkers were associated with known prognostic clinical/pathologic risk factors. Ki-67 was directly linked to NCCN risk groups, with 40%, 53%, and 69% of low, intermediate, and high risk cases having Ki-67 expression levels over the median value. The median Ki-67 expression (using median score across the TMA for each subject) was 2.0, 2.9, and 4.7 percent for low, intermediate, and high NCCN risk (p=0.013 by Kruskal-Wallis test, Figure 3). Ki-67 was also associated with Gleason sum (p=0.043 by Kruskal-Wallis testing). Plots of the proportion of men with high Ki-67 (>median), TWIST (2–3+), SNAIL (2–3+), vimentin (1–2+), and ZEB1 (1–2+) and low E-cadherin (0–1+) expression according to NCCN/classic D’Amico risk groups are shown in Figure 3. While Ki67 increased with NCCN risk, we did not observe any associations of EP biomarkers with increasing NCCN risk. Other mesenchymal biomarkers decreased with NCCN risk (vimentin) or had no association with risk group (SNAIL, loss of E-cadherin, TWIST, N-cadherin).
Figure 3. Bar graph demonstrating the association of epithelial plasticity biomarkers with NCCN risk group.
Low risk includes PSA<10, Gleason 6 or less, and pT2a or less pathologic stage. Intermediate risk includes PSA 10–20, Gleason 7, or pT2b. High risk includes PSA>20, Gleason 8–10, or stage T2c or higher. Biomarkers are classified by the proportion high: average Ki67 above median, maximum SNAIL 2–3+, maximum TWIST 2–3+, maximum vimentin 1–2+, maximum ZEB1 2–3+. For E-cadherin, classification is by the proportion low (0–1+) using the minimum value per subject across the TMA.
Discussion
We examined the association of a range of EP biomarkers for their prognostic association with PSA recurrence in a contemporary series of men with localized PC treated with curative intent radical prostatectomy. The outcomes in this cohort of VA men were excellent, with only three deaths from PC and four patients developing metastatic disease with over 11 year median follow-up despite a relatively high 43% PSA recurrence rate. While a biomarker of proliferation, Ki-67, was validated as being associated with NCCN risk and risk of PSA relapse after surgery, we found no associations of EP biomarkers with clinical risk groups or PSA recurrence in our study.
There are several possible explanations for the lack of association of mesenchymal biomarker expression with outcomes after surgery (7, 10, 19). The first is the overall excellent long-term outcomes in our cohort, limiting the ability to demonstrate associations of biomarkers with PC metastasis or death due to low event rates. This reflects the improving prognosis of men treated for localized PC over time, and the limitations of any biomarker for improving upon clinical risk stratification. In addition, we could not validate the prognostic relevance of E/N-cadherin switch given the low level N-cadherin expression observed. While the E- to N-cadherin switch data(7) has not yet been validated externally, a larger cohort of high-risk men followed long-term through metastatic relapse and death would be needed.
Second, we found several mesenchymal biomarkers to be quite commonly expressed in low-grade tumors. For example, loss of E-cadherin or low E-cadherin expression was more commonly seen in low grade Gleason 6 disease, while most high-grade tumors had abundant and intense E-cadherin staining. Similarly, TWIST expression was ubiquitous across NCCN risk groups, and vimentin expression in PC cells actually decreased with increasing grade. SNAIL and ZEB1 expression was not associated with grade or clinical risk in our series. These data suggest that in localized PC, broad mesenchymal biomarker expression in high grade disease is not common or associated with recurrence.
There are several limitations present in our study. The first includes the lack of rare cell isolation within tumors that lack epithelial biomarker expression. These alternative and more complex histologic methods, using quantitative imaging and dual-color immunofluorescence, have associated a loss of PSA expression or cytokeratin staining with high grade, poor risk disease and adverse outcomes after surgery(6, 11). Second, a TMA is unable to assess regional or geographic variability of biomarkers, for example mesenchymal biomarker expression only at the invasive tumor front. Our TMA did not include this geographic distribution information, and future studies should consider annotation of biomarker expression according to geographic distribution (central, peripheral, invasive strands, capsular invasion regions) and heterogeneity/multifocality of individual tumors in order to better ascertain the relationship of a given biomarker distribution with outcome, similar to that described in other solid tumors (20, 21, 22).
Finally, our cohort of men was relatively small and despite the long-term follow-up, the number of metastatic and PC-specific mortality events was low, limiting our power to observe associations of EP biomarkers with more clinically relevant outcomes. Our prior work identified the common presence of these EP biomarkers in circulating tumor cells (CTCs) from men with castration-resistant PC, suggesting the importance of EP with lethal forms of the disease(4, 9, 23). Others have confirmed these findings, but suggested that these EP biomarkers may be less prevalent in hormone-sensitive disease(24). Castration itself may promote an EMT, possibly linked to the development of androgen receptor alterations such as splice variants(25–27). Our cohort of men was unexposed to ADT and had a low metastatic rate, limiting our ability to detect an EMT if EP is more relevant to castration-resistant progression and/or metastasis. Only 12% of our patients had high grade Gleason 8–10 disease, which limits our ability to determine associations within this high risk subpopulation. Nevertheless, we observed that the majority of high grade tumors had an epithelial phenotype, and that mesenchymal biomarker expression was often more commonly expressed in lower risk cancers. These data suggest the importance of a mesenchymal to epithelial transition (MET) in PC, or that the majority of primary PCs have largely not undergone an EMT, at least in the majority of their cells. We cannot rule out rare cellular events relevant to EMT and cancer dissemination in this study, however.
In conclusion, our data suggest that EP biomarker expression in men with localized PC does not add clinical utility or prognostic information. Further study of rare de-differentiated cellular populations and geographic distribution of EP biomarkers in PC and the association with PC specific mortality or metastasis is warranted however.
Supplementary Material
Acknowledgments
This work was supported by the Robert B. Goergen Prostate Cancer Foundation Young Investigator Award, and the Department of Defense Physician Research Training Award (W81XWH-10-1-0483).
Footnotes
Conflict of interest: none
Reference List
- 1.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Amico AV, Whittington R, Malkowicz SB, et al. A multivariate analysis of clinical and pathological factors that predict for prostate specific antigen failure after radical prostatectomy for prostate cancer. J Urol. 1995;154:131–8. [PubMed] [Google Scholar]
- 3.Irshad S, Bansal M, Castillo-Martin M, et al. A molecular signature predictive of indolent prostate cancer. Sci Transl Med. 2013;5:202ra122. doi: 10.1126/scitranslmed.3006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitting RL, Schaeffer D, Somarelli JA, Garcia-Blanco MA, Armstrong AJ. The role of epithelial plasticity in prostate cancer dissemination and treatment resistance. Cancer Metastasis Rev. 2014 doi: 10.1007/s10555-013-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–88. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Kono E, Tran CP, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–20. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong AJ, Marengo MS, Oltean S, et al. Circulating Tumor Cells from Patients with Advanced Prostate and Breast Cancer Display Both Epithelial and Mesenchymal Markers. Mol Cancer Res. 2011 doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behnsawy HM, Miyake H, Harada K, Fujisawa M. Expression patterns of epithelial-mesenchymal transition markers in localized prostate cancer: significance in clinicopathological outcomes following radical prostatectomy. BJU Int. 2013;111:30–7. doi: 10.1111/j.1464-410X.2012.11551.x. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Liu X, Laffin B, et al. The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell. 2012;10:556–69. doi: 10.1016/j.stem.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeboll S, Borre M, Ottosen PD, Dyrskjot L, Orntoft TF, Torring N. Snail1 is over-expressed in prostate cancer. APMIS. 2009;117:196–204. doi: 10.1111/j.1600-0463.2008.00007.x. [DOI] [PubMed] [Google Scholar]
- 13.Ayala G, Tuxhorn JA, Wheeler TM, et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–801. [PubMed] [Google Scholar]
- 14.Khor LY, Bae K, Paulus R, et al. MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92-02. J Clin Oncol. 2009;27:3177–84. doi: 10.1200/JCO.2008.19.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker AS, Heckman MG, Wu KJ, et al. Evaluation of ki-67 staining levels as an independent biomarker of biochemical recurrence after salvage radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:1364–70. doi: 10.1016/j.ijrobp.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 16.Pollack A, DeSilvio M, Khor LY, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133–40. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 17.Verhoven B, Yan Y, Ritter M, et al. Ki-67 is an independent predictor of metastasis and cause-specific mortality for prostate cancer patients treated on Radiation Therapy Oncology Group (RTOG) 94-08. Int J Radiat Oncol Biol Phys. 2013;86:317–23. doi: 10.1016/j.ijrobp.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berney DM, Gopalan A, Kudahetti S, et al. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer. 2009;100:888–93. doi: 10.1038/sj.bjc.6604951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak P, Leav I, Pursell B, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–22. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haffner MC, De Marzo AM, Yegnasubramanian S, Epstein JI, Carter HB. Diagnostic Challenges of Clonal Heterogeneity in Prostate Cancer. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.50.3540. [DOI] [PubMed] [Google Scholar]
- 23.Bitting RL, Boominathan R, Rao C, et al. Development of a method to isolate circulating tumor cells using mesenchymal-based capture. Methods. 2013;64:129–36. doi: 10.1016/j.ymeth.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CL, Mahalingam D, Osmulski P, et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate. 2013;73:813–26. doi: 10.1002/pros.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottard F, Asmane I, Erdmann E, Bergerat JP, Kurtz JE, Ceraline J. Constitutively active androgen receptor variants upregulate expression of mesenchymal markers in prostate cancer cells. PLoS ONE. 2013;8:e63466. doi: 10.1371/journal.pone.0063466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun F, Chen HG, Li W, et al. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. 2014;289:1529–39. doi: 10.1074/jbc.M113.492140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Wang BE, Leong KG, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.