Abstract
Objectives:
To assess whether black race and other cerebrovascular risk factors have a differential effect on first vs recurrent stroke events.
Methods:
Estimate the differences in the magnitude of the association of demographic (age, back race, sex) or stroke risk factors (hypertension, diabetes, cigarette smoking, atrial fibrillation, left ventricular hypertrophy, or heart disease) for first vs recurrent stroke from a longitudinal cohort study of 29,682 black or white participants aged 45 years and older.
Results:
Over an average 6.8 years follow-up, 301 of 2,993 participants with a previous stroke at baseline had a recurrent stroke, while 818 of 26,689 participants who were stroke-free at baseline had a first stroke. Among those stroke-free at baseline, there was an age-by-race interaction (p = 0.0002), with a first stroke risk 2.70 (95% confidence interval: 1.86–3.91) times greater for black than white participants at age 45, but no racial disparity at age 85 (hazard ratio = 0.91; 95% confidence interval: 0.70–1.18). In contrast, there was no evidence of a higher risk of recurrent stroke at any age for black participants (p > 0.05). The association of traditional stroke risk factors was generally similar for first and recurrent stroke.
Conclusion:
The association of age and black race differs substantially on first vs recurrent stroke risk, with risk factors playing a similar role.
Risk factors for first stroke have been well studied,1 providing a framework for primary stroke prevention. Multivariable risk functions developed in the Framingham2 and Cardiovascular Health Study3 cohorts have identified age, sex, hypertension, diabetes, cigarette smoking, left ventricular hypertrophy, atrial fibrillation, and heart disease as predictors of incident stroke in a stroke-free population. A limitation of those studies was their inability to address the role of race (black race specifically) as a risk factor for stroke; however, there are substantial differences in stroke incidence between black and white persons, with incident stroke in black persons approximately 3 times higher between the ages of 45 and 65 years, but with disparities attenuating by age 85.4–8
While substantial data document the risk factors for first stroke, there are fewer data establishing the risk factors for recurrent stroke (that is, risk factors for a subsequent stroke in a population with a previous stroke). The 2011 American Heart/American Stroke Association Guidelines for Stroke Prevention in Patients with Stroke or Transient Ischemic Attack noted “few trials directly address the role of BP treatment in secondary prevention,” and “the data supporting diabetes for recurrent stroke are more sparse.”9 Data on the role of black race as a risk factor for recurrent stroke are rare.
The goal of this report was to assess a potential differential effect of risk factors for first vs recurrent stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort.
METHODS
REGARDS is a population-based study of 30,239 white and black participants aged 45 years or older at baseline recruited from the 48 contiguous US states between 2003 and 2007. The study sampled the general population, including both those stroke-free and those with prevalent stroke or TIA at baseline. Potential participants were randomly selected from a commercially available list (Genesys) and contacted by a combination of mail and telephone. Those willing to participate completed a telephone interview to assess cardiovascular risk factors and other measures. This was followed by an in-home assessment conducted approximately 2 to 3 weeks later, where blood and urine specimens were collected, an ECG performed, and anthropometric measures taken. The cooperation rate among eligible participants contacted was 49%. Further details of the study design are provided elsewhere.10
The focus of this report is the difference in risk factors between first and recurrent stroke. REGARDS is a study of non-Hispanic black and white participants. Ethnicity was established by self-report to the question, “Are you Hispanic or Latino?” with those indicating Hispanic ethnicity excluded. Race was also defined by self-report in response to the question, “What is your race? Would you say White, Black or African American, Asian, Native Hawaiian or Other Pacific Islander, American Indian, Alaska Native or some other race?” with only white and black respondents included. History of stroke or TIA was determined at baseline on the basis of the following questions: (1) “Were you ever told by a physician that you had a stroke?” and (2) “Were you ever told by a physician that you had a mini-stroke or TIA, also known as a transient ischemic attack?” The effects of risk factors for first stroke during follow-up were estimated among those who were stroke/TIA-free at baseline, while the risk factors for recurrent stroke during follow-up were estimated among those who reported having a stroke or TIA before baseline. Participants were followed up by telephone at 6-month intervals. While medical records for strokes occurring during the follow-up were retrieved and physician-reviewed,5 challenges of retrieving medical records describing events that occurred before participants were in the study required the prevalent strokes at baseline to be defined by participant self-report of a physician diagnosis.
Hypertension was defined as systolic blood pressure at the time of the initial in-home visit ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. Blood pressure was assessed as the average of 2 blood pressures after the participant had been seated for at least 5 minutes. Diabetes was defined as a fasting baseline glucose ≥126 mg/dL (or ≥200 mg/dL for those who failed to fast) or current use of medications to treat diabetes. Current smoking was defined by self-report. Atrial fibrillation was defined by ECG evidence of atrial fibrillation or a self-report of a physician diagnosis of atrial fibrillation. Left ventricular hypertrophy was defined on the basis of ECG evidence using the Sokolow criteria.11 History of heart disease was defined by ECG evidence of myocardial infarction or self-reported myocardial infarction, coronary artery bypass, angioplasty, or stent.
The association of stroke risk during follow-up with risk factors was assessed using proportional hazards analysis. Age was assessed as a continuous factor, with an interaction term for age-by-race interaction reflecting the association previously reported among those stroke-free at baseline.5,6 Analysis was performed “individually” for each risk factor in a model with variables that also included age, race, and age-by-race interaction, sex, previous stroke/TIA at baseline, and the interaction of a previous stroke/TIA with the risk factor. Analogous multivariable models estimated the joint effect of risk factors in models that simultaneously included all risk factors. Multiple imputation for suspected stroke events that could not be adjudicated because of inability to retrieve records and for records currently in the adjudication process (approximately 10% of suspected stroke events for each) was used to reduce potential bias; details of this approach are described elsewhere.12
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review boards of all participating universities and all participants provided written informed consent.
RESULTS
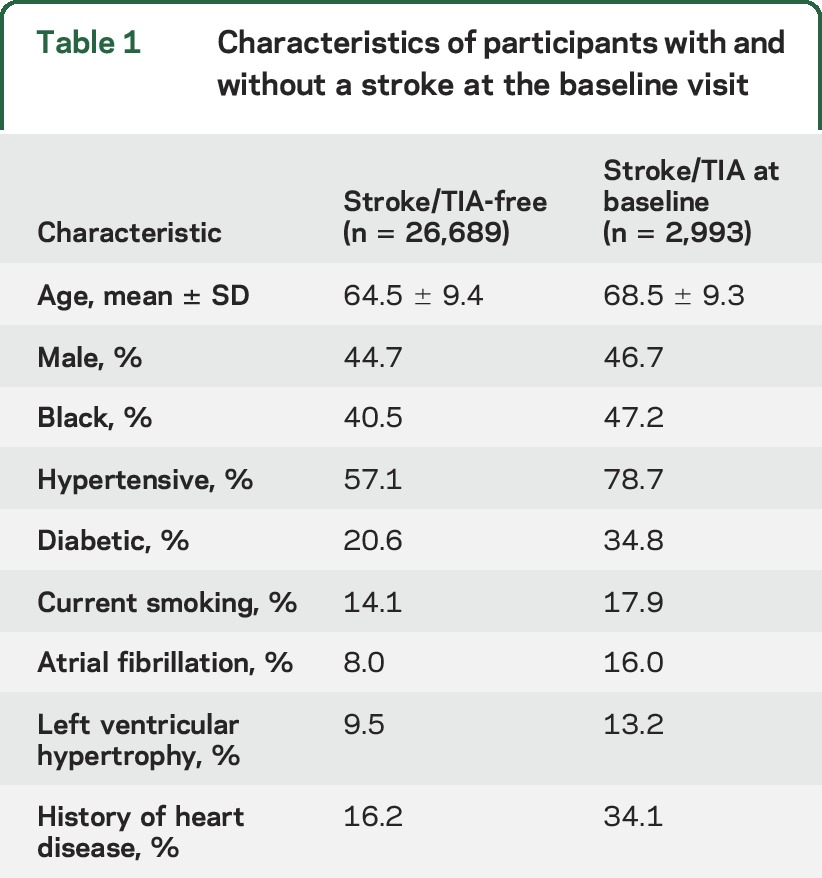
Of the 30,239 REGARDS participants, 29,682 (98%) had follow-up and were included in this analysis. Of these, 2,993 (10%) reported having a physician diagnosis of stroke/TIA at baseline, while 26,689 (90%) reported no such history. Characteristics are described in table 1. As expected, those with a history of prevalent stroke/TIA were older, more likely male and black, and more likely to have stroke risk factors.
Table 1.
Characteristics of participants with and without a stroke at the baseline visit
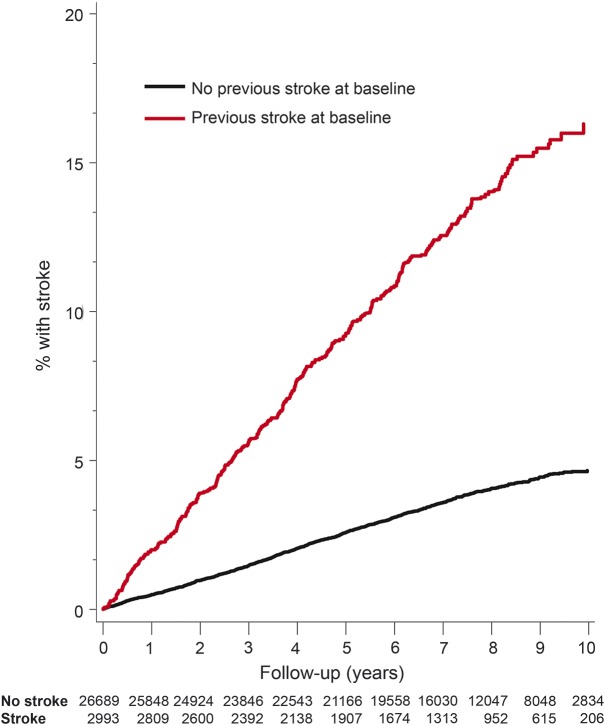
During an average follow-up of 6.8 years, there were 301 recurrent stroke events among those with stroke/TIA at baseline (10.1%), and 818 first stroke events among those stroke/TIA free at baseline (3.1%). The Kaplan-Meier estimated 10-year risk of a stroke during follow-up for first or recurrent stroke is shown in figure 1, with a univariate hazard ratio of 3.72 (95% confidence interval [CI]: 3.28–4.22) associated with having a prior stroke/TIA.
Figure 1. Estimated stroke rates for those without (black) and with (red) prevalent stroke/TIA at baseline.
Kaplan-Meier estimates of stroke rates for those without (black) and with (red) prevalent stroke/TIA at baseline. The number at risk at the beginning of each year of follow-up is at the top of the figure.
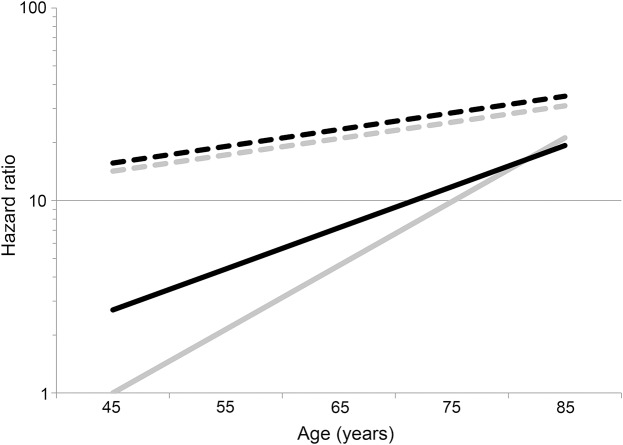
Both in the individual and multivariable analysis, the interplay of age and black race on stroke risk was remarkably different for first stroke compared to recurrent stroke (table 2). In the individual analysis for first stroke, there was a highly significant interaction between age and race (pinteraction = 0.0002), with black participants having a risk 2.70 (95% CI: 1.86–3.91) times higher than white participants at age 45. However, risk of first stroke increased 2.15 times (95% CI: 1.95–2.36) per 10 years for white participants, but only 1.63 times (95% CI: 1.47–1.82) per 10 years in age in black participants, completely attenuating the black-to-white stroke risk ratio by age 85 to 0.91 (95% CI: 0.70–1.18). By contrast, there was no evidence of race-by-age interaction for recurrent stroke (pinteraction = 0.98), with recurrent stroke risk being approximately 11% higher in black participants than in white participants (p > 0.05) across the age spectrum. Results were similar in the multivariable analysis, with the black–white differences showing a nonsignificantly lower risk of recurrent stroke in black participants. Figure 2 provides a graphical representation of these hazard ratios for first and recurrent stroke (relative to a 45-year-old white participant who was stroke-free at baseline).
Table 2.
Hazard ratio (with 95% CI) on the individual and multivariable association of “Framingham” risk factors with first (incident) and recurrent stroke, with a test of whether the magnitude of the association of the risk factor differs for incident vs recurrent (interaction test)

Figure 2. Hazard ratio for first vs recurrent stroke and black race.
Hazard ratio for stroke relative to a 45-year-old participant stroke/TIA-free at baseline. White participants shown in gray, black participants shown in black; participants stroke/TIA-free at baseline shown as solid lines, participants self-reporting a physician diagnosis of stroke or TIA as a dashed line. The 95% confidence bounds were omitted for clarity.
The hazard for stroke in black compared to white individuals can be calculated both for first and recurrent stroke from data in figure 2 as shown in figure e-1 on the Neurology® Web site at Neurology.org. For those who were stroke/TIA-free at baseline, there is a clear decreasing magnitude of the black–white disparity in first stroke risk with increasing age, where at age 45, black participants are at nearly 3 times the risk of white participants, while at age 85, black participants are at marginally lower risk than white participants. This change reflects the pattern in figure 2, where young black participants have notably higher risk at age 45, but risk increases more rapidly in white participants with differences diminishing at older ages. In contrast, the black–white differences in the risk of recurrent stroke are consistent over age, with risk nonsignificantly elevated (hazard ratio [HR] ≈ 1.1) in the individual analysis and nonsignificantly lower (HR ≈ 0.85) in the multivariable models.
Also using the data presented in figure 2, the hazard associated with recurrent stroke (relative to first stroke) can be calculated (see figure e-2). In figure 2, for white participants, the difference between first and recurrent stroke risk is large at young ages but decreases at older ages. This difference is reflected in figure e-2, where for white participants there is a 14.2-times increased risk if they had a previous stroke/TIA, but this risk was attenuated to 1.81-times increased risk by age 85. In contrast, for black participants, the higher risk of first stroke among young black participants implies that the difference between risk of first and recurrent stroke is smaller. This smaller difference is reflected in figure e-2, where there is “only” a 5.8-times increased risk at age 45, but a similar attenuation of risk to 1.48 times by age 85.
Table 2 also provides the associations of male sex and risk factors with the risk of first and recurrent stroke, both individually and multivariably. In the individual analysis, the magnitude of association of all factors was larger for first vs recurrent stroke, was significantly larger for heart disease (HR = 2.11 for first stroke, HR = 1.37 for recurrent stroke; p = 0.0031), and was marginally significantly smaller for atrial fibrillation (HR = 1.95 for first stroke, HR = 1.39 for recurrent stroke; p = 0.060). Univariately, all of these traditional risk factors were significantly related to incident stroke, and with the exceptions of current smoking and male sex, the other risk factors were significantly (p < 0.05) associated with recurrent stroke. Multivariable analysis generally attenuated the magnitude of the association of risk factors for both first and recurrent stroke, and the individual difference in the magnitude of the association of heart disease for first vs recurrent stroke was no longer significant (p = 0.092), while the association with current smoking and recurrent stroke became significant (p < 0.05).
DISCUSSION
The interplay of black race and age appears remarkably different for risk of first vs recurrent stroke. Among those stroke/TIA-free at baseline, we had previously reported a strong age-by-race interaction, with approximately a 3-times-higher risk of a first stroke at age 45, but a diminishing racial disparity at older ages so that at age 85 the stroke risk was similar for black and white participants.5,6 This pattern was confirmed with longer follow-up. In contrast, while a previous stroke/TIA proved to be a powerful risk factor for a subsequent stroke, the black–white difference in the risk of recurrent stroke was consistent and small across the age spectrum.
These differences in the magnitude of the association of race and age on the risk of a first vs recurrent stroke imply that the risk associated with having had a prior stroke (1) substantially differs for white and black individuals and (2) will differ with age (for both races). At young ages (45 years), among those stroke/TIA-free at baseline, black individuals were at a substantially higher risk than those who were white; however, for those with a stroke/TIA at baseline there was little racial difference in risk of a subsequent stroke. This different role of race for first vs recurrent stroke at age 45 implies the stroke risk associated with having a previous stroke is much larger for white (HR = 14.2) than black individuals (HR = 5.7). However, at younger ages, the apparent larger effect of a previous stroke on the risk of recurrent stroke in white than in black individuals is not because white individuals with a previous stroke have a much greater stroke risk than black individuals with a previous stroke, but rather that the risk of first stroke is substantially lower than the risk of first stroke among black individuals. In addition, the risk of a first stroke increases more rapidly with age than the risk of a recurrent stroke (i.e., for both black and white individuals) implying that at older age the stroke risk associated with having had a previous stroke is smaller than at younger ages. Hence at older ages, the stroke risk associated with having had a previous stroke is relatively small and similar for black (HR = 1.81) and white (HR = 1.48) individuals.
The “Framingham” traditional stroke risk factors were confirmed in this analysis as risk factors for a first stroke. In the analysis of the individual risk factors, with the exception of heart disease (and perhaps atrial fibrillation), the contributions of these factors were generally similar for first vs recurrent stroke. The apparent larger magnitude of association for heart disease (and perhaps atrial fibrillation) for first compared with recurrent stroke may reflect that they are risk factors for which treatment is more effective, and the stroke event itself may serve to ensure the identification and treatment of these risk factors. The significance of these risk factors generally persisted in multivariable analysis, with similar effects for first vs recurrent stroke. This suggests that equal vigilance for risk-factor prevention and control is appropriate for primary and secondary stroke prevention.
There have been many studies of blood pressure lowering in stroke patients that have been summarized in several meta-analyses13–16; however, there are few studies in stroke/TIA populations examining the association between systolic blood pressure levels and recurrent stroke risk. In addition, recent efforts to show reduced risk with aggressive blood pressure treatment (target of 130 mm Hg or less compared to a target of 130–149 mm Hg) for stroke prevention following a lacunar infarction were at most only suggestive of a benefit of stroke reduction.17 A large cohort like REGARDS is needed to have a sufficient number of individuals with a prevalent stroke that can be followed to provide a sufficient number of recurrent strokes for analysis. However, the substantial sample size of REGARDS (29,682 in this report) provided 2,993 individuals with a prevalent stroke/TIA at baseline, of whom 301 had subsequent strokes during follow-up. This number of recurrent stroke events is sufficient to detect an HR for a dichotomous variable with 50% prevalence of 1.38 with 80% power (or 1.45 with 90% power).
This work has several limitations. Most notably, while we reviewed medical records on suspected stroke events occurring during follow-up, we had to rely on self-reported stroke events occurring before participants were enrolled in study. However, these self-reported strokes at baseline have previously been found to be a reliable predictor of recurrent events,18 a relationship confirmed herein. Perhaps more important, the specificity of self-reported stroke has been generally reported to be above 95% (and the sensitivity is generally above 80%),19–23 suggesting that few individuals falsely self-reported stroke. With the relatively low prevalence of stroke in the general population, there will also be few individuals among those self-reporting the absence of stroke that truly have had an event. There are also substantial strengths to the work. Most important, REGARDS undertook a risk-factor assessment of a sufficient number of participants with and without stroke/TIA at baseline to reliably describe the differences in risk factors in these 2 populations. In addition, the stroke events during follow-up were adjudicated by physician review of medical records, ensuring a high likelihood that true strokes were detected.
This work documents a remarkably different association for first vs recurrent stroke for black race and age. The substantial excess risk of incident stroke for “young” (age 45–65) black individuals was not present for recurrent stroke; rather, the black–white differences in recurrent stroke risk were minimal. Almost all of the “traditional” stroke risk factors for incident stroke proved to also be a risk factor for recurrent stroke, suggesting that equal vigilance for risk-factor prevention and control is appropriate for primary and secondary stroke prevention.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the investigators, staff, and participants of the REGARDS Study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
GLOSSARY
- CI
confidence interval
- HR
hazard ratio
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. G. Howard, Kissela, Kleindorfer, McClure, Soliman, Judd, Cushman, and V. Howard designed the research, were responsible for obtaining funding, and actively participated in the acquisition of the data. Dr. G. Howard drafted the manuscript. Mr. Rhodes participated in the design of the research and acquisition of the data. Drs. G. Howard and Judd performed the statistical analysis and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors made critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This research project was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–584. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 3.Manolio TA, Kronmal RA, Burke GL, O'Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke 1996;27:1479–1486. [DOI] [PubMed] [Google Scholar]
- 4.Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995;155:1319–1324. [PubMed] [Google Scholar]
- 5.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke 2011;42:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004;35:426–431. [DOI] [PubMed] [Google Scholar]
- 8.Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke 2006;37:2473–2478. [DOI] [PubMed] [Google Scholar]
- 9.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 11.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Ann Noninvasive Electrocardiol 2001;6:343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard G, McClure LA, Moy CS, et al. Imputation of incident events in longitudinal cohort studies. Am J Epidemiol 2011;174:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741–2748. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AI, Sapkota BL. Blood pressure reduction in secondary stroke prevention. Continuum 2011;17:1233–1241. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Wang Z, Gong L, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res 2009;32:1032–1040. [DOI] [PubMed] [Google Scholar]
- 16.Grassi G, Arenare F, Trevano FQ, Dell'Oro R, Mancia AG. Primary and secondary prevention of stroke by antihypertensive treatment in clinical trials. Curr Hypertens Rep 2007;9:299–304. [DOI] [PubMed] [Google Scholar]
- 17.SPS3 Study Group, Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judd SE, Kleindorfer DO, McClure LA, et al. Self-report of stroke, transient ischemic attack, or stroke symptoms and risk of future stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke 2013;44:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machon M, Arriola L, Larranaga N, et al. Validity of self-reported prevalent cases of stroke and acute myocardial infarction in the Spanish cohort of the EPIC Study. J Epidemiol Community Health 2013;67:71–75. [DOI] [PubMed] [Google Scholar]
- 20.Carter K, Barber PA, Shaw C. How does self-reported history of stroke compare to hospitalization data in a population-based survey in New Zealand? Stroke 2010;41:2678–2680. [DOI] [PubMed] [Google Scholar]
- 21.Horner RD, Cohen HJ, Blazer DG. Accuracy of self-reported stroke among elderly veterans. Aging Ment Health 2001;5:275–281. [DOI] [PubMed] [Google Scholar]
- 22.Engstad T, Bonaa KH, Viitanen M. Validity of self-reported stroke: the Tromso Study. Stroke 2000;31:1602–1607. [DOI] [PubMed] [Google Scholar]
- 23.O'Mahony PG, Dobson R, Rodgers H, James OF, Thomson RG. Validation of a population screening questionnaire to assess prevalence of stroke. Stroke 1995;26:1334–1337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.