Highlight
Attack by different below- and aboveground organisms alters the condition of the host plant through systemic changes in phytohormone levels, which modulate defence responses and affect subsequent attackers.
Key words: Aboveground–belowground interactions, Frankliniella occidentalis, herbivores, Heterodera schachtii, phytohormones, plant-parasitic nematodes, systemic responses, Tetranychus urticae.
Abstract
Above- and belowground plant parts are simultaneously attacked by different pests and pathogens. The host mediates these interactions and physiologically reacts, e.g. with local and systemic alterations of endogenous hormone levels coupled with coordinated transcriptional changes. This in turn affects attractiveness and susceptibility of the plant to subsequent attackers. Here, the model plant Arabidopsis thaliana is used to study stress hormone-based systemic responses triggered by simultaneous root parasitism by the cyst nematode Heterodera schachtii and shoot herbivory by the thrips Frankliniella occidentalis and the spider mite Tetranychus urticae. First, HPLC/MS and quantitative reverse transcriptase PCR are used to show that nematode parasitism strongly affects stress hormone levels and expression of hormone marker genes in shoots. Previous nematode infection is then demonstrated to affect the behavioural and life history performance of both arthropods. While thrips explicitly avoid nematode-infected plants, spider mites prefer them. In addition, the life history performance of T. urticae is significantly enhanced by nematode infection. Finally, systemic changes triggered by shoot-feeding F. occidentalis but not T. urticae are shown to make the roots more attractive for H. schachtii. This work emphasises the importance of above- and belowground signalling and contributes to a better understanding of plant systemic defence mechanisms against plant-parasitic nematodes.
Introduction
Plants have evolved sophisticated defence strategies to effectively combat attack by different pathogens and herbivorous arthropods. Thus, they perceive an attack and translate this ‘perception’ into an appropriate defensive response. For many years, locally occurring reactions were the focus of various studies (Dangl and Jones, 2001; Dicke and Hilker, 2003; Pieterse and van Loon, 2004). However, in both natural and agricultural ecosystems, attacks by above- or belowground pathogens and herbivores may induce systemic effects in addition to local responses. These can either occur through induction of defence pathways (Ithal et al., 2007; Hamamouch et al., 2011) or through their active suppression by the invader (Glas et al., 2014; Goverse and Smant, 2014; Alba et al., 2015). These multilayered interactions are very likely more important ecologically than the local responses (Groen et al., 2013). Via pathogen-associated molecular patterns (PAMP) or herbivore-associated molecular patterns (HAMP) simultaneous attacks may activate PAMP- or HAMP-triggered immunity (Jones and Dangl, 2006; Acevedo et al., 2015). Both can trigger stress-signalling and systemic resistance phenomena in the whole plant that lead to a number of different defence responses, such as changes in gene expression and in endogenous hormone levels (Choudhary et al., 2007; Fu and Dong, 2013). Indeed, this interplay is fine-tuned by a set of phytohormones, including jasmonic acid (JA), salicylic acid (SA), and ethylene (ET), and is highly species-specific (Pieterse et al., 2009; Kutyniok and Müller, 2012; Groen et al., 2013). In general, it is widely accepted that ET and JA are responsible for defence against herbivores and necrotrophic pathogens (Kessler and Baldwin, 2002; Thaler et al., 2004; Pieterse et al., 2009; Wei et al., 2011; Wei et al., 2014), whereas SA mainly acts against biotrophic pathogens and some phloem-sucking insects, such as whiteflies (Thaler et al., 2004; Spoel et al., 2007; Pieterse et al., 2009; Zhang et al., 2009; Wei et al., 2014). However, there are exceptions to these principles. For instance, the JA pathway is also effective against some biotrophic pathogens (Ellis et al., 2002; Pozo et al., 2004; Thaler et al., 2004). Moreover, recent studies suggest that microorganisms in the saliva of herbivores may suppress the herbivore-typical JA pathway and, instead, elicit the SA pathway that is typical for biotrophic pathogens (Chung et al., 2013).
In case of herbivorous arthropods, the elicitation of plant defence also strongly depends on the type of damage to the plant tissue, which varies widely among species. Chewing insects, such as caterpillars, consume significant portions of plant tissue, whereas phloem-feeding insects like aphids provoke minimal direct damage. Chelicerates, such as the two-spotted spider mite Tetranychus urticae, a major pest on many different crop species, pierce parenchyma cells to feed on cell contents (Zhurov et al., 2014). Frankliniella occidentalis, the Western flower thrips, another agricultural pest of worldwide relevance, feeds on epidermal and sub-epidermal cells by rasping and sucking (Kirk and Terry, 2003). In general, plant responses to herbivore damage suggest that plants perceive this type of attack by recognizing specific elicitors, which mainly originate from the arthropods’ oral secretions (Bonaventure, 2012) and/or by damage-associated molecular patterns (DAMPs) that result from wounding or enzymatic damage caused by the herbivore (Erb et al., 2012). This includes basal defence responses, in which, besides salicylic acid (SA) and ethylene (ET), jasmonic acid (JA) plays a major role (Zhurov et al., 2014). Accordingly, host preference and herbivore oviposition can be affected either negatively or positively by exogenous JA or methyl jasmonate (mJA) application. JA-treatment of Brassica oleracea plants, for instance, resulted in avoidance by Pieris rapae and P. brassicae but preference by Plutella xylostella (Bruinsma et al., 2007). For F. occidentalis, some previous findings indicate that both adults and larvae respond negatively to jasmonates (Thaler et al., 2001; Egger and Kochier, 2014). First choice experiments with the spider mite T. urticae (Wei et al., 2014) showed that JA application makes plants less attractive for females at earlier time points but more attractive at later time points after hormone treatment. Similarly, SA alone and joint application of SA and JA enhanced the attraction of plants for female mites (Wei et al., 2014).
Several experiments have been conducted to identify systemic effects triggered by simultaneous attack by different pathogens. For instance, an early Pseudomonas syringae infection can affect subsequent plant–pathogen and plant–herbivore interactions, either compromising or aiding the response to a second attacker of similar or different identity (Cui et al., 2005; Groen et al., 2013; Fu and Dong, 2013). Cui et al. (2002) described P. syringae infection of lower rosette leaves triggering a systemic induced susceptibility to herbivorous Trichoplusia ni larvae in the upper leaves. Further, the beet armyworm Spodoptera exigua showed reduced growth rates when fed on cotton leaves of plants with prior root damage caused by the click beetle Agriotes lineatus (Bezemer et al., 2003).
Plant-parasitic nematodes (PPN), such as cyst nematodes (CN) and root-knot nematodes (RKN), are biotrophic pathogens that trigger a wide range of transcriptional and metabolic changes as well as altered levels in endogenous hormones (Alkharouf et al., 2006; Ithal et al., 2007; Puthoff et al., 2007; Szakasits et al., 2009; Hofmann et al., 2010; Mazarei et al., 2011; Cabello et al., 2014; Kammerhofer et al., 2015). Phytohormones in particular play important roles in host responses to the nematode infection (Wubben et al., 2001; Lohar et al., 2004; Wubben et al., 2008; Hamamouch et al., 2011). For instance, auxin (indole-3-acetic acid, IAA) was found to be indispensable for successful syncytium formation (Karczmarek et al., 2004; Wang et al., 2007; Grunewald et al., 2008; Gutjahr and Paszkowski, 2009; Swiecicka et al., 2009). In addition, Wubben et al. (2001, 2004) showed ET-overproducing mutants to be hyper-susceptible to Heterodera schachtii, whereas ET-insensitive mutants exhibited a lower susceptibility. Active suppression of JA biosynthesis and signalling also plays an important role during syncytium formation (reviewed in Gutjahr and Paszkowski, 2009). Wubben et al. (2008) showed that SA adversely affects development of H. schachtii females. However, in contrast to these well-described local changes, the systemic effects triggered by nematodes are poorly understood. For instance, Hamamouch et al. (2011) found up-regulation of SA marker genes PR-1 and PR-5 at 9 days after infection (dai), whereas the JA marker PR-4 (HEL) was down-regulated at 15 dai in shoots. Similarly, Wubben et al. (2008) found enhanced PR-1 transcription at 3 dai and a slight elevation of endogenous SA levels in shoots of infected plants at 4 dai. Therefore, it is assumed that systemic changes in hormone levels triggered by concurrent nematode attack and infestation by shoot attackers have significant impacts on all organisms involved in the above- and belowground sphere of the host. Accordingly, van Dam et al. (2005) found that the leaf-feeding larvae of P. rapae grow more slowly and are less likely to pupate on black mustard plants (Brassica nigra) infested with the root lesion nematode Pratylenchus penetrans.
Because insects and PPNs are frequently occurring and very diverse plant-attackers, their interaction mediated by plants is particularly interesting (reviewed in van Dam and Heil, 2011; Johnson et al., 2012; Soler et al., 2013; Wondafrash et al., 2013). Therefore, the aim of this study was to shed more light on induced resistance-related effects triggered by simultaneous root-feeding of the CN H. schachtii and shoot-feeding by the herbivores F. occidentalis and T. urticae. To this end, the effects triggered by nematodes were investigated to determine if they are strong enough to change host attractiveness to shoot herbivores or to modify their behavioural and life history performance (from below- to aboveground) on Arabidopsis thaliana. Second, it was determined whether shoot herbivores are triggering systemic effects in roots that change the host attractiveness to nematodes (from above- to belowground). Overall, the findings of this study should significantly contribute to a better understanding of the complex systemic defence mechanisms employed by plants that are simultaneously attacked by different organisms.
Material and methods
A. thaliana culture
Seeds of A. thaliana (Col-0) were surface-sterilized (0.7% NaClO, 40% EtOH) for 8min, washed in 70% EtOH, and immediately rinsed three times in distilled water. Ten seeds per dish (94mm in diameter) were placed on 0.2 Knop medium. Subsequently they were grown in a 16h light/8h dark (L16D8) photoperiod at 23°C for 12 days.
Nematode culture, inoculation, and attraction assay
H. schachtii was obtained from sterile stock culture on Sinapis alba cv. Albatros. Second-stage juveniles (J2) were surface sterilized in 0.05% HgCl2 for 3min and immediately washed three times in double-distilled H2O. Next, 12-day-old A. thaliana seedlings were inoculated with 50 J2s directly on the roots (Sijmons et al., 1991). Inoculated plates were kept in the dark for 24h and subsequently transferred into a growing chamber in a L16D8 photoperiod.
The nematode attraction assay was performed according to Dalzell et al. (2011). Briefly, 2% water agar plates with cylindrical counting wells (8mm in diameter) connected via a cylindrical channel (20mm × 2.5mm) were prepared. Agar discs containing root exudates, obtained from treated and non-treated A. thaliana plants grown on 0.2 Knop media as described above, were placed into the counting wells. One hundred J2s were placed in the middle of the connecting channel. Six plates for each treatment and each replicate were prepared and stored in the dark at room temperature. After 3.5h, the number of J2s that reached either one or the other well was counted and classified as attracted by the root exudate of the respective agar disc. Experiments were performed in three independent replicates with six plates each. Results were calculated as attraction rate (%) of the total number of used nematodes.
Thrips and mite culture, inoculation, and bioassays
F. occidentalis (class Insecta) was reared on detached Phaseolus vulgaris leaves on 1% water agar (Agar, Sigma-Aldrich, Vienna, Austria) in Petri dishes in a climate chamber at 24°C in a L16D8 photoperiod (Egger et al., 2014). T. urticae (class Arachnida) was reared on whole bean plants at room temperature in a L16D8 photoperiod (Hoffmann et al., 2011). For quantitative reverse transcriptase (qRT)-PCR and HPLC/MS analysis, two adult spider mite females or one adult thrips female was placed on each plant to feed for 24h. Whole shoot and root tissue was sampled and immediately put in liquid nitrogen for subsequent analysis.
F. occidentalis and T. urticae were further used for choice and no-choice performance assays comparing plants previously infected with H. schachtii and uninfected control plants. Plants used for these bioassays were singly grown in 12-well plates on Knop media containing 1% Daichin agar. Inoculation of 12-day-old seedlings was performed with 50 H. schachtii J2s. Two agar cylinders were obtained 24h after inoculation (hai) from the 12-well plates, containing either an infected or control plant, and placed next to each other in one petri dish (50mm ø). Subsequently, 10 second-stage thrips larvae (L2) were placed on a piece of filter paper between these two plants and their position was monitored immediately after release—immediate choice indicates the plant first chosen by the L2. The position of the larvae was monitored after 30min and then every hour for 4h in total. For the mite assay, one adult female was placed between the plants and its position on the infected or uninfected plant was monitored as described above.
For the spider mites, an additional no-choice performance assay was performed on single plants grown in agar plates (50mm ø). Here, a single adult female was placed on each plant, either previously infected with H. schachtii (24 hai) or uninfected. After 24 and 48h, the following parameters were monitored: presence (yes/no), location (adaxial or abaxial leaf surface), oviposition (number of eggs), and damage (categorized based on discoloration as no, light, or severe damage according to the leaf area visibly sucked by the mites). In case of female disappearance, after 24h a new mite was placed on the plant.
qRT-PCR analysis
Whole roots or shoots of approximately 20–30 A. thaliana seedlings were sampled in three independent biological replicates. RNA was extracted using Qiagen RNA Plant Mini Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). RNA was analysed using a Nanodrop 2000c Spectrophotometer (Thermo Scientific, Peqlab, Germany); cDNA synthesis was performed using SuperScriptIII reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Reference genes UBP22 and 18S RNA (Hofmann and Grundler, 2007) were used for analysis (see Supplementary Table S1 for all primer sequences and Supplementary Table S2 showing the validation of 18S rRNA and UBP22 as reference genes). qPCR was performed using ABI PRISM 7300 (Applied BioSystems, Waltham, MA, USA). The final reaction volume was 25 µl containing 12.5 µl SYBR Green reaction kit (Invitrogen), 0.5 µl 10μM primers, 9.5 µl double-distilled H2O, and 2 µl of cDNA template. Three independent biological replicates each in technical triplicates (averaged before statistical analysis) were tested. The PCR reaction was conducted as follows: 95°C for 10min, then 40 cycles of 95°C for 15 s and 60°C for 60 s. Changes in transcript levels were calculated using the 2-∆∆ct method (Schmittgen and Livak, 2008).
Hormone quantification
For hormone quantification, 12-day-old A. thaliana plants were infected with nematodes, thrips, or mites as described above. Shoots and roots of approximately 20–40 individual seedlings were pooled and collected 24 hai in four biological replicates, weighed, frozen in liquid nitrogen, and stored at −80°C. Hormones were purified and analysed according to Dobrev and Kaminek (2002) and Dobrev and Vanková (2012). Samples of ~200mg were homogenized and extracted with methanol/water/formic acid (15/4/1, v/v/v). The following labelled internal standards (10 pmol per sample) were added: 13C6-IAA (Cambridge Isotope Laboratories, Tewksbury, MA, USA), 2H4-SA (Sigma-Aldrich), 2H2-GA4, 2H2-GA19, 2H6-ABA, 2H5-transZ, 2H5-transZR, 2H5-transZ7G, 2H5-transZ9G, 2H5-transZOG, 2H5-transZROG, 2H5-transZRMP, 2H3-DHZ, 2H3-DHZR, 2H3-DHZ9G, 2H6-iP, 2H6-iPR, 2H6-iP7G, 2H6-iP9G, and 2H6-iPRMP (Olchemim, Olomouc, Czech Republic). Extracts were purified using an SPE-C18 column (SepPak-C18, Waters, Milford, MA, USA) and separated on a reverse phase-cation exchange SPE column (Oasis-MCX, Waters, Milford, MA, USA). The first hormone fraction, eluted with methanol, contained abscisic acid (ABA) and other acidic hormones; the second fraction, eluted with 0.35M NH4OH in 70% methanol, contained cytokinin metabolites and 1-aminocyclopropane-1-carboxylic acid (ACC). Both fractions were separated by HPLC (Ultimate 3000, Dionex, Sunnyvale, CA, USA) and the hormones were quantified using a hybrid triple quadrupole/linear ion trap mass spectrometer (3200 Q TRAP, Applied Biosystems) operated in selected reaction monitoring mode.
Statistical analyses
Stat Graphics plus 4.0 software (Statpoint Technologies Inc., Warrenton, VA, USA) was used to analyse the data obtained from qRT-PCR analysis and hormone quantification. A one-way ANOVA (post-hoc Tukey) was performed to determine statistical differences between the variants. Differences in nematode attraction to herbivore-infested plants were analysed using paired t-tests. SPSS 21 was used for the thrips and mite bioassays. For the thrips choice assay, generalized estimating equations (GEE) were used to analyse the preference of the larvae (counts of events; binomial distribution with logit link) for nematode-infected and uninfected plants over time (used as inner-subject variable, autocorrelation structure between observations). Similarly, GEE was used for the mite choice assay to analyse the preference of the mites (binomial distribution with logit link) for nematode-infected and uninfected plants over time (used as inner-subject variable, autocorrelation structure between observations). For the mite no-choice assay, GEEs were used to compare female presence (yes/no; binomial distribution with logit link), position (abaxial/adaxial; binomial distribution with logit link), activity (moving/stationary; binomial distribution with logit link), damage caused (Poisson distribution, log link), and the number of eggs laid (Poisson distribution, log link) over time (autocorrelation structure between observations) on nematode-infected and uninfected plants. The number of eggs laid at 24h and 48h was additionally analysed by separate generalized linear models (GLM; Poisson distribution with log link).
Results
H. schachtii infection modulates endogenous stress hormone levels and transcription of hormone-related genes in shoot
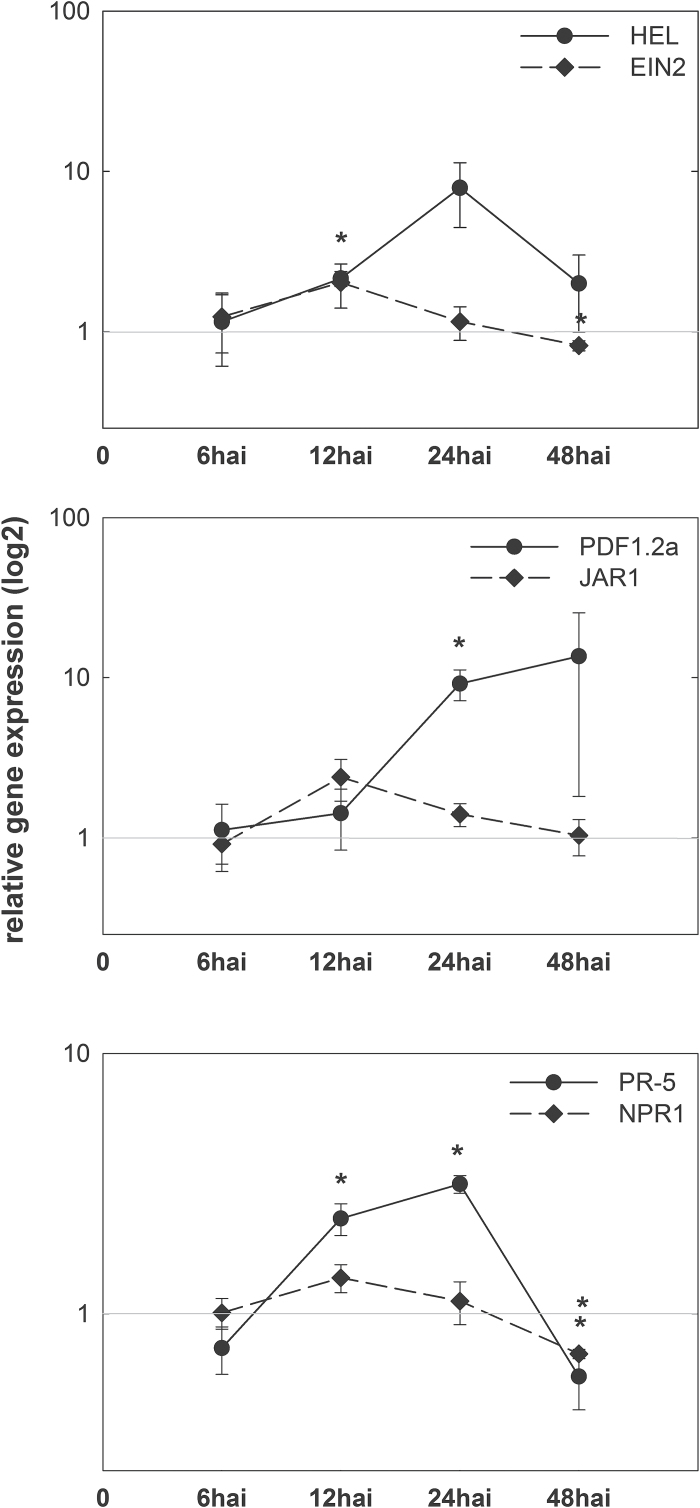
To monitor possible systemic effects triggered by root parasitism of H. schachtii in A. thaliana, a qRT-PCR was performed analysing recently used marker genes for JA, ET, and SA. Previously, UBP22 and 18S rRNA transcript levels have been shown to be stable in nematode-infected root tissue (Hofmann and Grundler, 2007). Here, first their stability in shoot tissue was tested by qRT-PCR. This analysis showed that transcript levels of both house-keeping genes in shoots of plants infected with H. schachtii were stable and could therefore be used as internal reference genes in the following experiments (Supplementary Table S2). Subsequent qRT-PCR showed a significant up-regulation of the JA/ET-responsive genes HEL at 12 hai (P = 0.0073) and PDF1.2a at 24 hai (P = 0.0151). The level of the ET-signalling gene EIN2 also changed in shoot, exhibiting a slight up-regulation at 12 hai and slight, but significant, down-regulation at 48 hai (P = 0.0384). The expression of the JA-signalling gene JAR1 was not altered at either tested time point. The SA-dependent PR-5 gene was significantly up-regulated at both 12 hai (P = 0.0150) and 24 hai (P = 0.0009), whereas at 48 hai it was down-regulated (P = 0.0437). NPR1 did not show any differential regulation until 48 hai, when it was slightly down-regulated (P = 0.0005) (Fig. 1).
Fig. 1.
Fold changes (log2) of hormone-related marker genes in shoots of H. schachtii-infected A. thaliana plants at 6, 12, 24, and 48 hai compared to uninfected control plants. Values are means ±SE, n = 3, asterisks indicate significant differences (P < 0.05).
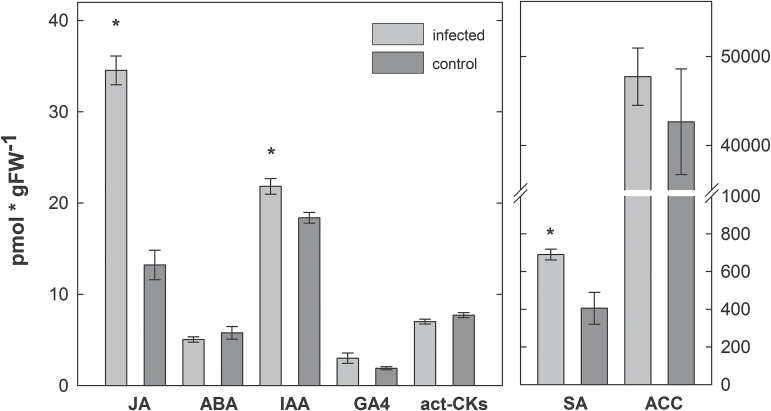
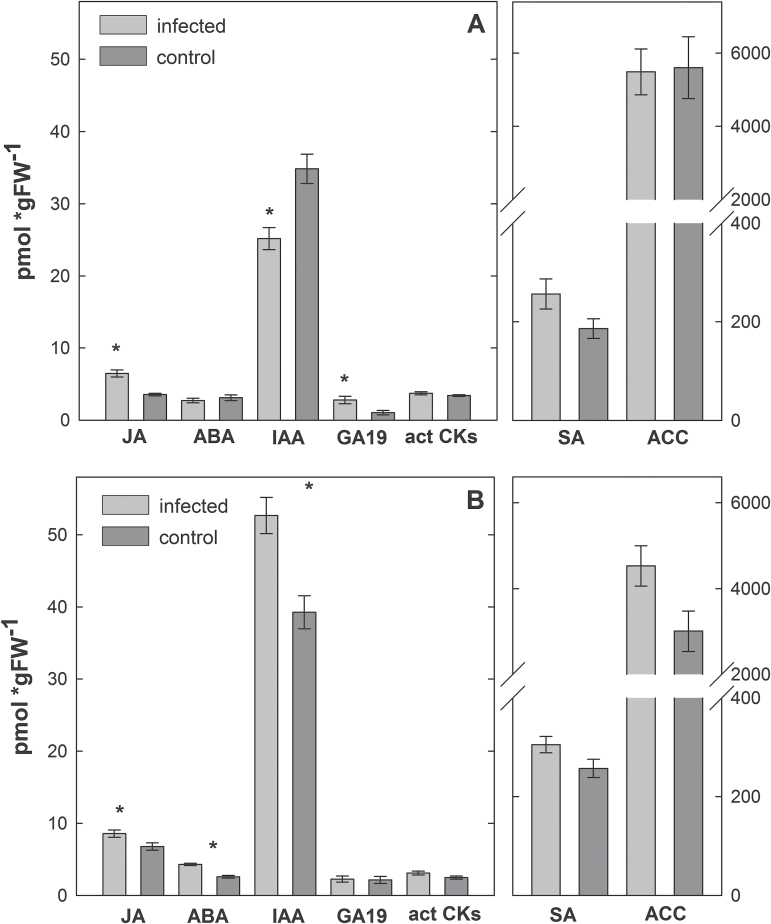
To verify these results, HPLC/MS analyses were conducted. Because the most severe effects on the expression levels of hormone-related genes triggered by nematode infection were found at 24 hai, this time point was used for subsequent hormone quantification. This analysis revealed elevated levels of JA and SA in shoots of nematode-infected plants. The level of ET-precursor ACC was not changed, whereas IAA was present at higher levels in shoots. Similarly, the level of ABA catabolite dihydrophaseic acid (DPA) was elevated (Supplementary Table S3), whereas the level of ABA itself did not change because of nematode root infection (Fig. 2).
Fig. 2.
Hormone quantification in shoots of H. schachtii-infected A. thaliana plants compared to uninfected control plants. Samples were collected at 24 hai. ACC, 1-aminocyclopropane-1-carboxylic acid; ABA, abscisic acid; act-CKs, active cytokinins (trans-zeatin, dihydrozeatin, isopentenyladenine, cis-zeatin, and the corresponding ribosides); GA4, gibberellin 4;IAA, indole-3-acetic acid; JA, jasmonic acid; SA, salicylic acid. Values are means ±SE, n = 4, asterisks indicate significant differences (P < 0.05).
Systemic effects triggered by H. schachtii change behaviour and performance of shoot herbivores
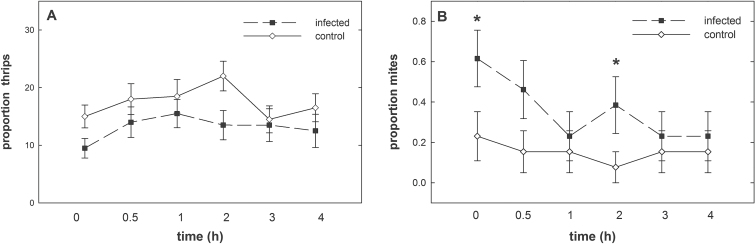
Choice assays performed with thrips L2 revealed a significant preference for the uninfected control over the plants infected with H. schachtii (Fig. 3A; GEE: Wald ӽ1 2 = 4.149, P = 0.042). This preference did not statistically change over time, although a slight reduction was observed (Wald ӽ10 2 = 15.766, P = 0.107). In contrast to thrips larvae, the spider mite females preferred plants infected with H. schachtii over the uninfected control plants (Fig. 3B; GEE: Wald ӽ1 2 = 3.725, P = 0.05). This preference was the strongest immediately after release and weakened over time (Wald ӽ5 2 = 230.117, P < 0.001).
Fig. 3.
Proportions of (A) F. occidentalis (GEE P = 0.042) and (B) T. urticae (GEE P = 0.05) choosing between an A. thaliana plant infected by H. schachtii (24 hai) and an uninfected control. Thrips L2s were released in groups of 10 (n = 20); adult spider mite females were released singly (n = 13). Values are means ±SE, asterisks indicate significant differences (P < 0.05).
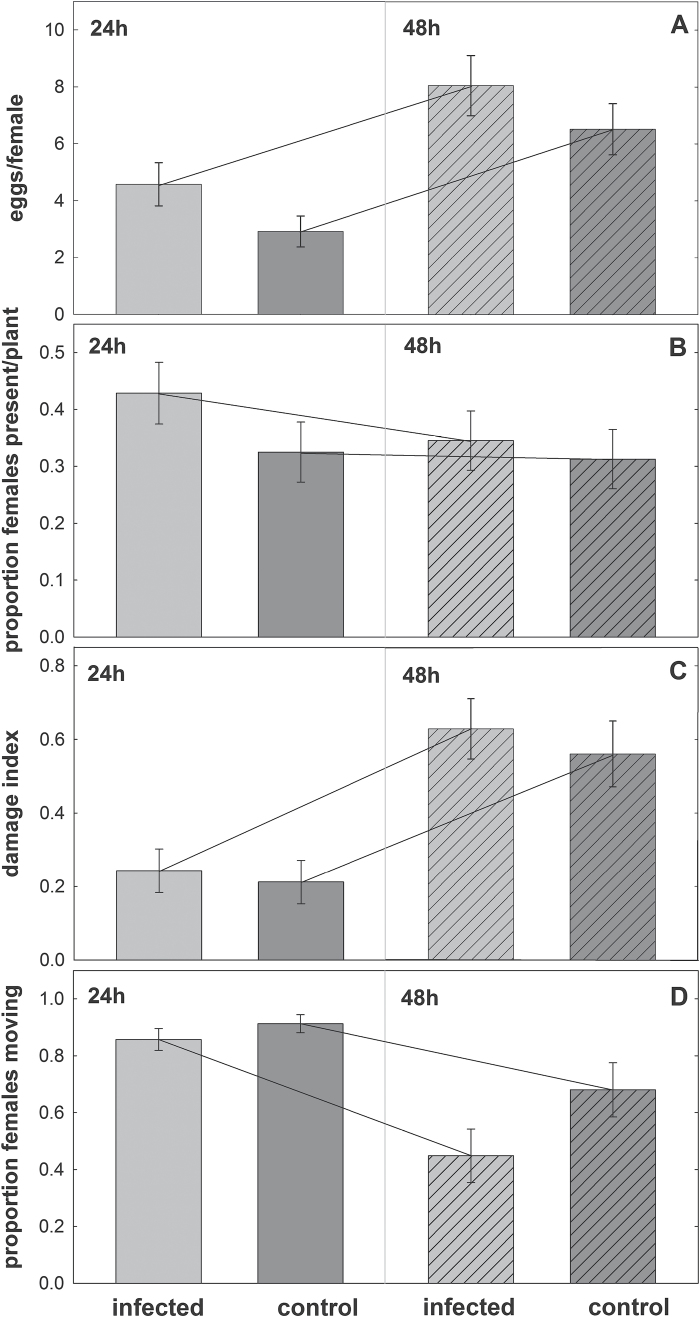
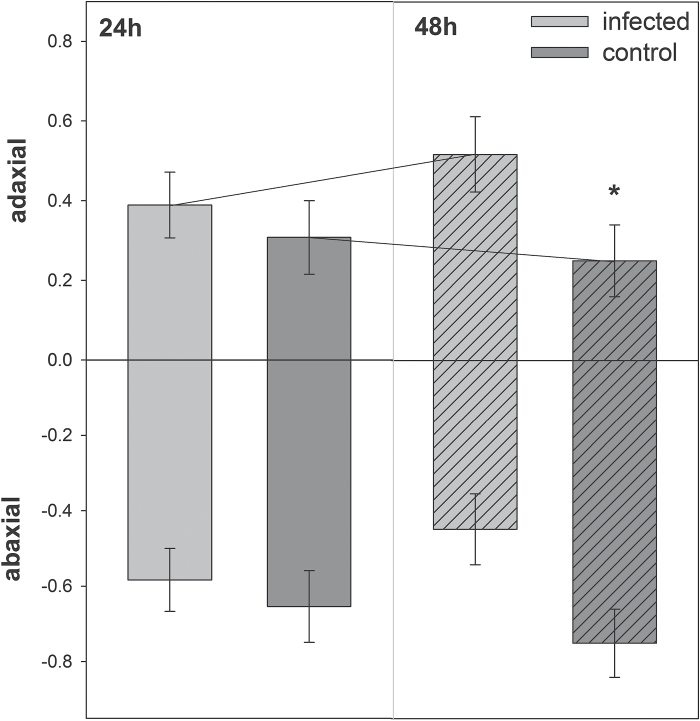
In the no-choice performance assay, the T. urticae females performed better on nematode-infected than uninfected plants. They were less active, caused more damage, tended to deposit more eggs, and had a stronger preference for the abaxial leaf surface on nematode-infected plants as compared to the uninfected control (Figs. 4 and 5, Table 1). Although oviposition across time was insignificant, time-dependent oviposition differed (Table 1), with females depositing more eggs on infected than uninfected plants at 24h (GLM: Wald ӽ1 2 = 3.725, P = 0.077) but not at 48h (Wald ӽ1 2 = 1.217, P = 0.270).
Fig. 4.
Spider mite no-choice performance assay. Single adult females were released on A. thaliana plant infected with H. schachtii (24 hai; n = 84) or uninfected control plants (n = 80) and monitored after 24 and 48h. Values are means ±SE.
Fig. 5.
Proportion of spider mite females on the abaxial and adaxial leaf surfaces of A. thaliana plants infected with H. schachtii. Single adult females were released on nematode-infected (24 hai; n = 84) or uninfected control (n = 80) plants and their position on the leaves was monitored after 24h and 48h. Values are means ±SE.
Table 1.
Results of generalized estimating equations for the performance of the spider mite T. urticae on nematode-infected and uninfected A. thaliana plants over time
| Infected/uninfected | Infected/uninfected (time) | |||
|---|---|---|---|---|
| Wald ӽ2 | P-value | Wald ӽ2 | P-value | |
| Eggs | 2.380 | 0.123 | 77.947 | <0.001 |
| Presence | 1.056 | 0.304 | 2.678 | 0.263 |
| Damage | 4.793 | 0.632 | 1.469 | <0.001 |
| Position | 0.230 | 0.029 | 54.748 | 0.480 |
| Activity | 5.563 | 0.018 | 24.419 | <0.001 |
Shoot herbivory triggers effects in systemic root
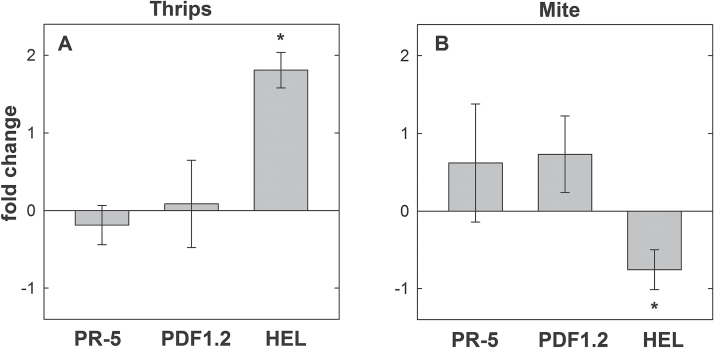
The expression of hormone-related marker genes differed between plants invaded by the two different arthropods. Feeding of F. occidentalis triggered a significant up-regulation of ET/JA-marker gene HEL in systemic roots. The SA-marker PR-5 and ET/JA-marker PDF1.2 did not show any changes. In contrast, feeding by T. urticae resulted in a significant down-regulation of HEL but did not cause any differential regulation of PR-5 or PDF1.2 (Fig. 6).
Fig. 6.
Fold changes (∆∆ct) of hormone-related marker genes in roots of A. thaliana plants infested by (A) F. occidentalis or (B) T. urticae compared to non-infested control plants (n = 3 for each herbivore). Values are means ±SE, asterisks indicate significant differences (P < 0.05).
Hormone quantification revealed a significant elevation of endogenous ABA and IAA levels in systemic roots of plants infested with spider mites; only a mild increase was observed in the case of active cytokinins (Fig. 7). Spider mite feeding did not significantly affect the concentration of the precursor of active gibberellins (GA19). In contrast, in roots of plants infested with thrips, IAA concentration was decreased, GA19 was elevated, and active cytokinin and ABA levels did not change. However, the concentration of the ABA catabolite DPA was elevated (Supplementary Table S4). Both herbivores significantly elevated JA levels in systemic root and did not change levels of SA or the ET precursor ACC.
Fig. 7.
Hormone quantification in roots of (A) thrips-infested and (B) spider mite-infested compared to non-infested A. thaliana plants. Samples were collected at 24 hai. ACC, 1-aminocyclopropane-1-carboxylic acid; ABA, abscisic acid; act-CKs, active cytokinins (trans-zeatin, dihydrozeatin, isopentenyladenine, cis-zeatin, and the corresponding ribosides); GA19, gibberellin 19;IAA, indole-3-acetic acid; JA, jasmonic acid; SA, salicylic acid. Values are means ±SE, n = 4, asterisks indicate significant differences (P < 0.05).
Systemic effects triggered by shoot herbivory change root attractiveness towards H. schachtii
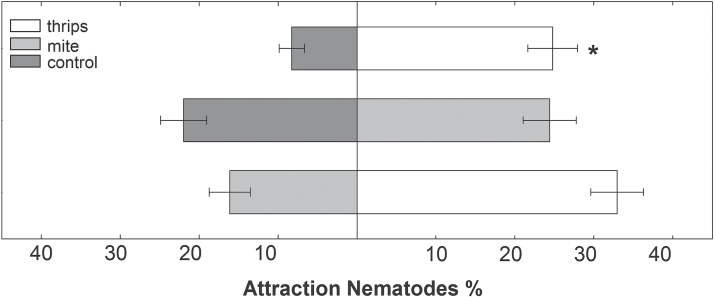
Possible effects triggered by shoot herbivory were tested in attraction assays analysing the attractiveness of plants previously infested with herbivores to J2s of H. schachtii. Agar discs containing root exudates from plants infested with either F. occidentalis or T. urticae were used for this analysis. Results show that nematodes were more attracted to root exudates from plants previously infested by thrips (P ≤ 0.001). In contrast, plants infested with spider mites did not show any difference in their attractiveness to nematodes in comparison to control (P = 0.6627). Further, when directly comparing thrips- and mite-infested plants, the exudates from plants fed on by thrips were slightly more attractive for H. schachtii juveniles (P = 0.0997), although this was not statistically significant (Fig. 8).
Fig. 8.
Nematode attraction assays illustrating movement of 100 H. schachtii J2s towards agar discs containing root exudates of previously thrips- or spider mite-infested A. thaliana plants (24 hai) and non-infested control plants. Values are means ±SE, n = 18, asterisks indicate significant differences (paired, P < 0.05).
Discussion
Most studies available to date have focused on the plant defence above- or belowground activated as a response to pathogen or herbivore attack. However, substantially less knowledge is available on systemic effects triggered by simultaneous attack by different pests above- and belowground. Cross talk between these two different spheres of the host plant is ecologically important and has profound effects on natural and agricultural food webs (Erb et al., 2008; Groen et al., 2013). The interactions between belowground PPN and aboveground herbivorous arthropods are especially crucial because of their ubiquitous occurrence (reviewed in van Dam and Heil, 2011; Johnson et al., 2012; Soler et al., 2013; Wondafrash et al., 2013). The results presented here provide novel insights into systemic responses triggered in the model plant A. thaliana by concomitant root infection by the CN H. schachtii and shoot infestation by two different herbivorous arthropods, two-spotted spider mites T. urticae and Wester flower thrips F. occidentalis. The way in which these different attackers induce hormone-based systemic responses are examined, as well as host susceptibility, and how they influence each other’s behavioural and life history performance.
H. schachtii root infection triggers hormone-related systemic responses in shoots
Nematodes induce severe alterations in affected root tissue during the infection process and further development, including changes in expression of genes involved in defence responses, metabolism, nutrient allocation, as well as phytohormone biosynthesis and signalling (Hofmann et al., 2010; Ithal et al., 2007; Alkharouf et al., 2006; Mazarei et al., 2011; Kammerhofer et al., 2015). These modifications have been intensively studied locally within the host root, but some studies have shown that systemic effects in aboveground tissue can mostly be attributed to changed levels of several phytohormones, such as JA, SA, and IAA, and the expression of hormone-related genes (Wubben et al., 2008; Hamamouch et al., 2011; Kyndt et al., 2012).
JA is mostly referred to as being active against necrotrophic pathogens and herbivores (Pieterse et al., 2009) and is often considered as a key component of the central platform triggering systemic plant responses (Farmer and Ryan, 1992; Erb et al., 2008). Moreover, several studies have also indicated that JA plays a crucial role during attack by biotrophic pathogens (Baldwin et al., 1996; van Dam et al., 2001; van Dam et al., 2005; Hamamouch et al., 2011; Kammerhofer et al., 2015). For instance, Hamamouch et al. (2011) analysed the transcript levels of several hormone- and defence-related genes in systemic A. thaliana shoots at later time points of H. schachtii infection. The authors detected a significant up-regulation of a JA-dependent gene, PR-3, at 5 and 9 dai, and a down-regulation of PR-4 (HEL) at 14 dai in shoots, although neither gene was regulated locally in roots at tested stages of infection. Similarly, the presented data show a clear up-regulation of PR-4 (HEL), starting at 12 hai, as well as PDF1.2 at 24 hai, followed by reduced expression at 48 hai. These results are in line with the strongly elevated endogenous JA levels measured in shoots, and are similar to root tissue data recently obtained for similar early stages of nematode infection (Kammerhofer et al., 2015). Interestingly, contrasting results were recently published for RKN infection. Kyndt et al. (2012) reported systemic changes in expression of defence-related genes in rice (Oryza sativa) infected by Meloidogyne graminicola. The authors found decreased expression of genes related to JA biosynthesis at 3 dai and suggested that RKN might suppress the systemic defence in shoots already at early stages of infection. This also indicates that the JA-related systemic responses vary considerably, being highly nematode species- and host-specific. Here, for CN, the JA-dependent defence pathways were induced during early stages of infection not only in nematode-infected root tissue, as previously shown (Kammerhofer et al., 2015), but also exerted systemic effects on shoot tissue, where these responses might be subsequently suppressed as soon as the infection is successfully completed and stable.
In contrast to JA, SA is known to be active against mainly biotrophic pathogens (Pieterse et al., 2009) but can also be induced by some herbivores, e.g., Bemisia tabaci (Zarate et al., 2007). For CN, recent research found no changes in endogenous SA levels or expression of SA-marker PR-5 during early H. schachtii root infection (Kammerhofer et al., 2015). However, significantly elevated endogenous levels of SA (24 hai) as well as increased PR-5 expression at 12 hai and 24 hai were found in this study in shoots. These results demonstrate that SA-dependent defence is already systemic in shoot tissue during early phases of CN parasitism. At later time points, Wubben et al. (2008) found a slight but not significant systemic elevation of endogenous SA levels in shoots and enhanced transcript levels for PR-1. Similarly, at 9 dai, Hamamouch et al. (2011) detected up-regulation of PR-1, PR-2, and PR-5 in shoots. These studies, together with results presented here, show that root parasitism by CN triggers durable induction of SA-dependent resistance in shoots. For RKN, a constant down-regulation of SA-responsive genes has been shown in shoots of A. thaliana (Hamamouch et al., 2011) and rice (Kyndt et al., 2012). This indicates that the role of SA in shoots fundamentally differs between CN and RKN parasitism.
In contrast to JA and SA, which function mainly as coordinators of plant defence responses, auxins are mainly attributed to regulation of plant growth and development. However, they are now also considered by some authors to be essential factors in defence responses to biotic stress (Mauch-Mani and Mauch, 2005; Fu and Wang, 2011) and in modulation of the feedback between above- and belowground plant parts (Erb et al., 2008). The elevated auxin levels in shoot tissue of nematode-infected plants observed here are in accordance with compensatory shoot growth after root attack by two herbivorous insects (Steinger and Müller-Schärer, 1992) and the up-regulation of an auxin marker gene in Zea mays shoots after root damage by Diabrotica virgifera (Erb et al., 2008).
Data obtained here for JA, SA, and IAA demonstrate that H. schachtii infection causes explicit systemic changes in stress hormone-based defence in shoot tissue, which significantly differ from those locally triggered in the infected roots. These effects essentially change plant responses to following shoot attackers, either in a positive or negative way.
Systemic responses triggered by H. schachtii modulate life history and behavioural performance of herbivores
Recently, growing interest has been dedicated to systemic defence induced by attackers simultaneously infesting different parts of the host plant. A negative influence on performance of shoot herbivores triggered by root-feeding insects or nematodes has previously been reported. For example, Bezemer et al. (2003) provided evidence that Agriotes lineatus, a root feeder, reduces the performance of the foliar-feeding insect Spodoptera exigua. For PPN, van Dam et al. (2005) found a decrease in performance of Pieris rapae larvae, which grew more slowly and pupated less on plants infected with the endoparasitic nematode Pratylenchus penetrans. For RKN, infection of certain chickpea cultivars can lead to breakdown of resistance against specific strains of Fusarium oxysporum (Castillo et al., 2003; Palomares-Rius et al., 2011). Other studies found that nematode root infection may negatively affect phloem-sucking aphids (Bezemer and van Dam, 2005; Wurst and van der Putten 2007; Kaplan et al., 2009; Hol et al., 2010). For example, ectoparasitic and migratory endoparasitic nematodes caused fecundity reduction in the aphid Rhopalosiphum padi on Agrostis capillaris and Anthoxanthum odoratum (Bezemer et al., 2005). Kaplan et al. (2011) demonstrated that Meloidogyne incognita parasitism in roots of Nicotiana tabacum causes severe declines in growth and fecundity of aphids. A study conducted with the CN H. glycines concluded that aphids prefer uninfected over infected soybean plants, whereupon the aphid behaviour rather than their life history is influenced (Hong et al., 2010). However, the influence of soil nematodes on shoot-feeding insects is rather variable (reviewed in Wondafrash et al., 2013). A number of studies reported positive (Kaplan et al., 2008a ) and neutral effects of nematode parasitism on the performance of shoot-feeders (Kabouw et al., 2011; Kutyniok and Müller, 2012; Wurst and van der Putten, 2007), indicating that the triggered responses are highly species-specific and also depend, among other factors, on the mode of feeding.
This study examined the way two shoot herbivores, an insect and a mite, with different feeding modes were affected by previous nematode root parasitism. The elevated levels of endogenous JA, SA, and IAA as well as the up-regulation of PDF1.2, HEL, and PR-5 in shoots of nematode-infected plants strongly suggests altered susceptibility and/or attraction of these plants to shoot invaders. Indeed, early H. schachtii infection explicitly changed the behavioural and life history performance of following shoot herbivores. Attractiveness of the shoots of nematode-infected plants to L2 of F. occidentalis was significantly reduced. Similarly, Abe et al. (2008, 2009) showed that thrips feeding induces JA-regulated plant defence, which negatively affects thrips oviposition and population density. These authors also showed that exogenous application of JA and mJA onto the leaf surface significantly reduces attraction and settling of F. occidentalis. Significantly lower numbers of thrips were found on JA-sprayed plants in the field (Thaler et al., 2001) and jasmonate-baited traps did not attract F. occidentalis (James, 2005). Most of these studies focused on the adult stage, but recently Egger and Koschier (2014) found that the larval stage may be similarly deterred by exogenous JA application. De Puysseleyr et al. (2011) detected higher susceptibility of the tomato mutant def1, which exhibits reduced JA-related defence responses, to F. occidentalis, and thus suggested that JA-regulated defence mechanisms restrict thrips damage. Accordingly, it is proposed that the deterrent effect of shoots of nematode-infected plants against thrips might be due, at least in part, to the elevated JA levels and enhanced JA-related defence in aboveground tissue, both of which are triggered belowground by H. schachtii root infection.
With regard to the spider mites, Bonte et al. (2010) reported reduced fecundity on Phaseolus vulgaris plants infected with the ectoparasitic nematodes Pratylenchus penetrans. However, CN infection in the presented study had an entirely opposite effect, which is also contrary to the results obtained for thrips. In the choice assays, adult females of T. urticae immediately chose the nematode-infected over the control plants. Additionally, subsequent behaviours such as their position on the abaxial and adaxial leaf surface as well as oviposition, damage inflicted, and activity were significantly altered. These results indicate that CN triggered substantial changes within the plant at the onset of parasitism, altering the performance of the mites. These effects declined over time. The most profound differences in mite performance were observed between infected and uninfected plants at the beginning of the bioassay. Wei et al. (2014) reported on a similar time-dependent response to JA application. The authors first observed that T. urticae showed avoidance behaviour, and then, at later time points, demonstrated an attraction towards treated plants. For SA treatments, they did not find avoidance effects, but observed an attraction later on. Concomitant application of JA and SA resulted in attraction of the mites 72h after hormone application.
The observations from the presented study are in line with several reports showing that root herbivory may enhance the performance of shoot herbivores (Kaplan et al., 2008a : Gange and Brown, 1989; Masters and Brown, 1992). Previous studies suggested that this effect might be due to stress-induced allocation and accumulation of amino acids and carbohydrates in leaves (Kaplan et al., 2008b ; White, 1984; Chapin, 1991). Therefore, it is suggested that the concomitant elevation of JA and SA in A. thaliana plants infected with H. schachtii as well as possible nutrient allocation shifts resulted in greater attractiveness, as well as improved quality as a host, to T. urticae females, whereas thrips were repelled by enhanced JA-regulated defence after CN infection. These findings underscore the complexity of the plant hormone-based mechanisms in the interaction between plant, nematodes, and different herbivores. Which substances or specific signalling pathways are responsible for these opposite results, and whether microorganisms in the saliva of the studied herbivores play a role in defence induction (Chung et al., 2013), needs further scrutiny.
Shoot herbivory triggers hormone-based systemic responses in roots and changes their attractiveness to H. schachtii
To date, far less information is available on the effects induced by aboveground herbivory on belowground pests, especially on PPN (reviewed in Wondafrash et al., 2013). This is presumably because the effects on belowground organisms are difficult to measure owing to their inaccessibility (Bruce and Pickett, 2007). However, there are strong indications that such interactions occur and have substantial effects on subsequent root attackers. The presence of shoot-feeders may either reduce (Moran and Whitham, 1990; Salt et al., 1996; Kaplan et al., 2009) or increase (Russin et al., 1989; Alston et al., 1993; Russin et al., 1993; Kaplan et al., 2009) or have no effects on the performance of belowground herbivores (Masters et al., 1993; Kaplan et al., 2011). This influence varies between nematode species and is at least partly determined by the feeding habits of the insects. For instance, Kaplan et al. (2011) found no reciprocal effects of aphid herbivory on performance of the RKN Meloidogyne incognita. In contrast, Kaplan et al. (2009) showed that mutually positive interactions take place between nematodes and chewing insects (e.g., caterpillars), whereas negative interactions occur with phloem sap-feeders (e.g., aphids). Kutyniok and Müller (2013) found deviating effects of aphid shoot-feeding on H. schachtii. At low nitrate supply, aphids had a promoting effect on the nematodes, whereas at high nitrate fertilization they reduced the nematode infestation compared to control plants. For chewing insects, increased numbers of H. glycines and M. incognita were found on Glycine max infested with two different caterpillars (Russin et al., 1989; Alston et al., 1993; Russin et al., 1993). The effects triggered by shoot-feeding insects on performance of nematodes might be due to sink competition (Larson and Whitham, 1997); re-allocation of primary metabolites, such as nitrogen and carbohydrates (Masters et al., 1993); or elevated concentrations of secondary metabolites, such as nicotine or proteinase inhibitors in Nicotiana attenuata (Baldwin et al., 1994), reducing survival or reproduction of herbivores.
In this study, shoot-feeding by two different herbivores changed the phytohormone homeostasis of the host, affecting the performance of H. schachtii. Feeding by thrips F. occidentalis, which damage the epidermal layer with their rasping-sucking mouthparts, resulted in significant up-regulation of both ET- and JA-dependent marker genes HEL and PDF1.2 in damaged shoot tissue. In comparison, the systemic effects in roots manifested only in up-regulation of HEL. These changes in marker gene expression corresponded to systemic changes in hormone levels in the root tissue. Thus, concentration of JA and its active conjugate JA-Ileu was elevated, whereas ACC and SA levels did not change significantly. Additionally, the ABA catabolite DPA and gibberellin GA19 were elevated. IAA and its precursor IAN were reduced compared to non-infested control roots. In contrast, feeding by the spider mite T. urticae did not change the hormone marker genes in damaged shoots, whereas in roots HEL was down- and PDF1.2 slightly up-regulated (Kammerhofer et al., 2015). It triggered, however, an elevation of JA and the receptor interacting conjugate as well as ABA and auxin in roots.
This study demonstrated that shoot-herbivory by thrips and spider mites causes different hormone-based systemic effects in root tissue. To check whether these changes manipulate root susceptibility towards nematodes, an attraction assay was performed to test the attractiveness of root exudates towards H. schachtii collected from plants infested with both herbivores. Only previous shoot-feeding by F. occidentalis but not T. urticae strongly increased the attractiveness of these plants to the nematodes. These herbivore-specific differences might be, at least partially, due to highly variable changes in hormone levels and defence pathways. It can only be speculated that, for instance, the elevated IAA levels in roots of plants infested with spider mites could have a negative effect, whereas the reduced IAA levels in roots of plants infested with thrips could have a positive effect on nematode attraction. The elevated gibberellin levels in roots of thrips-infested plants could be an indicator for a suitable host for the nematodes.
A number of different stress hormone-based systemic effects triggered in A. thaliana shoots by H. schachtii root infection have been demonstrated here. These responses significantly differ from those occurring locally in root tissue, which have recently been reported. Nematode infection significantly changed the host plant susceptibility to shoot herbivores, such as thrips and spider mites, modulating their behavioural and life history performance. Additionally, similar hormone-based systemic effects occurred in the opposite direction, from above- to belowground parts of the plant, significantly changing the host susceptibility towards H. schachtii. The observed effects and hormone-dependent pathways are highly complex and species-specific. Thus, additional and more comprehensive studies are needed to elucidate these plant-mediated interactions, especially with the aid of specific hormone-related A. thaliana mutants. Nonetheless, these results greatly contribute to a better understanding of how plants integrate the induced responses triggered by different plant attackers above- and belowground.
Supplementary data
Supplementary materials are available at JXB online.
Supplementary Table S1. Primers for hormone- and defence marker genes used in qRT-PCR analysis.
Supplementary Table 2. Validation of 18S rRNA and UBP22 for their suitability as reference genes for qRT-PCR analysis.
Supplementary Table S3. Hormone quantification of shoots of A. thaliana plants infected with H. schachtii.
Supplementary Table S4. Hormone quantification of roots of A. thaliana plants infested with F. occidentalis and T. urticae in comparison to roots of non-infested control plants.
Acknowledgements
The authors thank Elisabeth Koschier for providing Frankliniella occidentalis. NK was a recipient of a DOC fFORTE fellowship of the Austrian Academy of Sciences. JH and KW were supported by Austrian Science Fund grants L00687 and P21067-B12, respectively. RV was supported by MEYS CR project no. LD14120.
References
- Abe H, Ohnishi J, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M. 2008. Function of jasmonate in response and tolerance of Arabidopsis to thrips feeding. Plant Cell Physiology 49, 68–80. [DOI] [PubMed] [Google Scholar]
- Abe H, Shimoda T, Ohnishi J, Kugimiya S, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M. 2009. Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biology 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo FW, Rivera-Vega LJ, Chung SH, Ray S, Felton GW. 2015. Cues form chewing insects – the intersections of DAMPs, HAMPs, MAPMs and effectors. Current Opinion in Plant Biology 26, 80–86. [DOI] [PubMed] [Google Scholar]
- Alba JM, Schimmel BC, Glas JJ, Ataide LMS, Pappas ML, Villarroel CA, Schuurnik RC, Sabelis MW, Kant MR. 2015. Spider mites suppress tomato defences downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytologist 205, 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharouf NW, Klink VP, Chouikha IB, Beard HS, MacDonald MH, Meyer S, Knap HT, Khan R, Matthews BF. 2006. Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 224, 838–852. [DOI] [PubMed] [Google Scholar]
- Alston DG, Schmitt DP, Bradley JR, Coble HD. 1993. Multiple pest interactions in soybean – effects on Heterodera glycines egg populations and crop yield. Journal of Nematology 25, 42–49. [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE. 1994. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. Journal of Chemical Ecology 20, 2139–2157. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA, Zhang ZP. 1996. Effects of octadecanoid metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris . Journal of Chemical Ecology 22, 61–74. [DOI] [PubMed] [Google Scholar]
- Bezemer TM, De Deyn GB, Bossinga TM, Van Dam NM., Harvey JA, Van Der Putten WH. 2005. Soil community composition drives aboveground plant–herbivore–parasitoid interactions. Ecology Letters 8, 652–661. [Google Scholar]
- Bezemer TM, Van Dam NM. 2005. Linking aboveground and belowground interactions via induced plant defenses. Trends in Ecology & Evolution 20, 617–624. [DOI] [PubMed] [Google Scholar]
- Bezemer TM, Wagenaar R, Van Dam NM, Wäckers FL. 2003. Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562. [Google Scholar]
- Bonaventure G. 2012. Perception of insect feeding by plants. Plant Biology 14, 872–880. [DOI] [PubMed] [Google Scholar]
- Bonte D, De Roissart A, Vandegehuchte ML, Ballhorn DJ, Van Leeuwen T, De La Pena E. 2010. Local adaptation of aboveground herbivores towards plant phenotypes induced by soil biota. PLoS ONE 5, e11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce TJ, Pickett J.A. 2007. Plant defence signalling induced by biotic attacks. Current Opinion in Plant Biology 10, 387–392. [DOI] [PubMed] [Google Scholar]
- Bruinsma M, Van Dam NM, Van Loon JJ, Dicke M. 2007. Jasmonic acid-induced changes in Brassica oleracea affect oviposition preference of two specialist herbivores. Journal of Chemical Ecology 33, 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello S, Lorenz C, Crespo S, Cabrera J, Ludwig R, Escobar C, Hofmann J. 2014. Altered sucrose synthase and invertase expression affects the local and systemic sugar metabolism of nematode-infected Arabidopsis thaliana plants. Journal of Experimental Botany 65, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P, Navas-Cortes JA, Gomar-Tinoco D, Di Vito M, Jimenez-Diaz RM. 2003. Interactions Between Meloidogyne artiellia, the cereal and legume root-knot nematode, and Fusarium oxysporum f. sp. ciceris race 5 in chickpea. Phytopathology 93, 1513–1523. [DOI] [PubMed] [Google Scholar]
- Chapin FS. 1991. Integrated responses of plants to stress. Bioscience 41, 29–36. 1991. [Google Scholar]
- Choudhary DK, Prakash A, Johri BN. 2007. Induced systemic resistance (ISR) in plants: mechanism of action. Indian Journal of Microbiology 47, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Rosa C, Scully ED, Peiffer M, Tooker JF, Hoover K, Luthe DS, Felton GW. 2013. Herbivore exploits orally secreted bacteria to suppress plant defenses. PNAS 110, 15728–15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez–Guzman G, Bender CL, Pierce NE, Ausubel FM. 2005. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proceedings of the National Academy of Sciences U S A 102, 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Jander G, Racki LR, Kim PD, Pierce NE, Ausubel FM. 2002. Signals involved in Arabidopsis resistance to Trichoplusia ni caterpillars induced by virulent and avirulent strains of the phytopathogen Pseudomonas syringae . Plant Physiology 129, 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalzell JJ, Kerr R, Corbett MD, Fleming CC, Maule AG. 2011. Novel bioassays to examine the host-finding ability of plant-parasitic nematodes. Nematology 13, 211–220. [Google Scholar]
- Dangl JL, Jones JDG. 2001. Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. [DOI] [PubMed] [Google Scholar]
- De Puysseleyr V, Höfte M, De Clercq P. 2011. Ovipositing Orius laevigatus increase tomato resistance against Frankliniella occidentalis feeding by inducing the wound response. Arthropod–Plant Interactions 5, 71–80. [Google Scholar]
- Dicke M, Hilker M. 2003. Induced plant defences: from molecular biology to evolutionary ecology. Basic and Applied Ecology 4, 3–14. [Google Scholar]
- Dobrev PI, Kaminek M. 2002. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. Journal of Chromatography A 950, 21–29. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Vankova R. 2012. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. Methods in Molecular Biology 913, 251–261. [DOI] [PubMed] [Google Scholar]
- Egger B, Koschier EH. 2014. Behavioural responses of Frankliniella occidentalis Pergande larvae to methyl jasmonate and cis-jasmone. Journal of Pest Science 87, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Turner JG. 2002. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae . Molecular Plant Microbe Interactions 15, 1025–1030. [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. 2012. Role of phytohormones in insect-specific plant reactions. Trends in Plant Science 17, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Ton J, Degenhardt J, Turlings TC. 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiology 146, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. 1992. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wang S. 2011. Insights into auxin signaling in plant–pathogen interactions. Frontiers in Plant Science 2, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZQ, Dong X. 2013. Systemic acquired resistance: turning local infection into global defense. Annual Review of Plant Biology 64, 839–863. [DOI] [PubMed] [Google Scholar]
- Gange AC, Brown VK. 1989. Effects of root herbivory by an insect on a foliar-feeding species, mediated through changes in the host plant. Oecologia 81, 38–42. [DOI] [PubMed] [Google Scholar]
- Glas JJ, Alba JM, Simoni S, Villarroel CA, Stoops M, Schimmel BC, Schuurink RC, Sabelis MW, Kant MR. 2014. Defense suppression benefits herbivores that have a monopoly on their feeding site but can backfire within natural communities. BMC Biology 12, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverse A, Smant G. 2014. The activation and suppression of plant innate immunity by parasitic nematodes. Annual Review of Phytopathology 52, 243–265. [DOI] [PubMed] [Google Scholar]
- Groen SC, Whiteman NK, Bahrami AK, Wilkcek AM, Cui J, Russel JA, Cibrain-Jaramillo A, Butler IA, Rana JD, Huang GH, Bush J, Ausubel FM, Pierce NE. 2013. Pathogen-triggered ethylene signaling mediates systemic-induced susceptibility to herbivory in Arabidopsis . Plant Cell 25, 4755–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, Karimi M, Wieczorek K, Van de Cappelle E, Wischnitzki E, Grundler FMW, Inze D, Beeckman T, Gheysen G. 2008. A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiology 148, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Paszkowski U. 2009. Weights in the balance: jasmonic acid and salicylic acid signaling in root–biotroph interactions. Molecular Plant Microbe Interactions 22, 763–772. [DOI] [PubMed] [Google Scholar]
- Hamamouch N, Li C, Seo PJ, Park CM, Davis EL. 2011. Expression of Arabidopsis pathogenesis-related genes during nematode infection. Molecular Plant Pathology 12, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Vierheilig H, Schausberger P. 2011. Mycorrhiza-induced trophic cascade enhances fitness and population growth of an acarine predator. Oecologia 166, 141–149. [DOI] [PubMed] [Google Scholar]
- Hofmann J, El Ashry Ael N, Anwar S, Erban A, Kopka J, Grundler FMW. 2010. Metabolic profiling reveals local and systemic responses of host plants to nematode parasitism. Plant Journal 62, 1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Grundler FMW. 2007. Identification of reference genes for qRT-PCR studies of gene expression in giant cells and syncytia induced in Arabidopsis thaliana by Meloidogyne incognita and Heterodera schachtii . Nematology 9, 317–323. [Google Scholar]
- Hol WH, de Boer W, Termorshuizen AJ, Meyer KM, Schneider JH, Van Dam NM, Van Veen JA, Van der Putten WH. 2010. Reduction of rare soil microbes modifies plant–herbivore interactions. Ecology Letters 13, 292–301. [DOI] [PubMed] [Google Scholar]
- Hong SC, Donaldson J, Gratton C. 2010. Soybean cyst nematode effects on soybean aphid preference and performance in the laboratory. Environmental Entomology 39, 1561–1569. [DOI] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG. 2007. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Molecular Plant Microbe Interaction 20, 510–525. [DOI] [PubMed] [Google Scholar]
- James D. 2005. Further field evaluation of synthetic herbivor-induced plan volatiles as attractants for beneficial insects. Journal of Chemical Ecology 31, 481–495. [DOI] [PubMed] [Google Scholar]
- Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW, Koricheva J. 2012. Aboveground–belowground herbivore interactions: a meta-analysis. Ecology 93(10), 2208–2215. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kabouw P, Kos M, Kleine S, Vockenhuber EA, Van Loon JJA, Van der Putten WH, Van Dam NM, Biere A. 2011. Effects of soil organisms on aboveground multitrophic interactions are consistent between plant genotypes mediating the intraction. Entomologia Experimentalis et Applicata 139, 197–206. [Google Scholar]
- Kammerhofer N, Radakovic Z, Regis MAJ, Dobrev P, Vankova R, Grundler FMW, Siddique S, Hofmann J, Wieczorek K. 2015. Role of stress-related hormones in plant defence during early infection of the cyst nematode Heterodera schachtii in Arabidopsis . New Phytologist 207, 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan I, Halitschke R, Kessler A, Rehill BJ, Sardanelli S, Denno RF. 2008. a Physiological integration of roots and shoots in plant defense strategies links above- and belowground herbivory. Ecology Letters 11, 841–851. [DOI] [PubMed] [Google Scholar]
- Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF. 2008. b Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89, 392–406. [DOI] [PubMed] [Google Scholar]
- Kaplan I, Sardanelli S, Denno RF. 2009. Field evidence for indirect interactions between foliar-feeding insect and root-feeding nematode communities on Nicotiana tabacum . Ecological Entomology 34, 262–270. [Google Scholar]
- Kaplan I, Sardanelli S, Rehill BJ, Denno RF. 2011. Toward a mechanistic understanding of competition in vascular-feeding herbivores: an empirical test of the sink competition hypothesis. Oecologia 166, 627–636. [DOI] [PubMed] [Google Scholar]
- Karczmarek A, Overmars H, Helder J, Goverse A. 2004. Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Molecular Plant Pathology 5, 343–346. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. 2002. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review of Plant Biology 53, 299–328. [DOI] [PubMed] [Google Scholar]
- Kirk WDJ, Terry LI. 2003. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology 5, 301–310. [Google Scholar]
- Kutyniok M, Müller C. 2012. Crosstalk between above- and belowground herbivores is mediated by minute metabolic responses of the host Arabidopsis thaliana . Journal of Experimental Botany 63, 6199–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutyniok M, Müller C. 2013. Plant-mediated interactions between shoot-feeding aphids and root-feeding nematodes depend on nitrate fertilization. Oecologia 173, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Kyndt T, Nahar K, Haegeman A, De Vleesschauwer D, Hofte M, Gheysen G. 2012. Comparing systemic defence-related gene expression changes upon migratory and sedentary nematode attack in rice. Plant Biology 1, 73–82. [DOI] [PubMed] [Google Scholar]
- Larson KC, Whitham TG. 1997. Competition between gall aphids and natural plant sinks: plant architecture affects resistance to galling. Oecologia 109, 575–582. [DOI] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM. 2004. Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant Journal 38, 203–214. [DOI] [PubMed] [Google Scholar]
- Masters GJ, Brown VK, Gange AC. 1993. Plant mediated interactions between above- and belowground insect herbivores. Oikos 66, 148–151. [Google Scholar]
- Masters GJ, Brown VK. 1992. Plant-mediated interactions between two spatially separated insects. Functional Ecology 6, 175–179. [Google Scholar]
- Mauch-Mani B, Mauch F. 2005. The role of abscisic acid in plant-pathogen interactions. Current Opinion in Plant Biology 8, 409–414. [DOI] [PubMed] [Google Scholar]
- Mazarei M, Liu W, Al-Ahmad H, Arelli PR, Pantalone VR, Stewart CN. 2011. Gene expression profiling of resistant and susceptible soybean lines infected with soybean cyst nematode. Theoretical Applied Genetics 123, 1193–1206. [DOI] [PubMed] [Google Scholar]
- Moran NA, Whitham TG. 1990. Interspecific competition between root-feeding and leaf-galling aphids mediated by host-plant resistance. Ecology 71, 1050–1058. [Google Scholar]
- Palomares-Rius JE, Castillo P, Navas-Cortes JA, Jimenez-Diaz RM, Tena M. 2011. A proteomic study of in-root interactions between chickpea pathogens: the root-knot nematode Meloidogyne artiellia and the soil-borne fungus Fusarium oxysporum f. sp. ciceris race 5. Journal of Proteomics 74, 2034–2051. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. 2009. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Van Loon LC. 2004. NPR1: the spider in the web of induced resistance signaling pathways. Current Opinion in Plant Biology 7, 456–464. [DOI] [PubMed] [Google Scholar]
- Pozo M, Van Loon LC, Pieterse CJ. 2004. Jasmonates – signals in plant-microbe interactions. Journal of Plant Growth Regulation 23, 211–222. [Google Scholar]
- Puthoff DP, Ehrenfried ML, Vinyard BT, Tucker ML. 2007. GeneChip profiling of transcriptional responses to soybean cyst nematode, Heterodera glycines, colonization of soybean roots. Journal of Experimental Botany 58, 3407–3418. [DOI] [PubMed] [Google Scholar]
- Russin JS, Layton MB, Boethel DJ, Mcgawley EC, Snow JP, Berggren GT. 1989. Development of Heterodera glycines on soybean damaged by soybean looper and stem canker. Nematology 21, 108–114. [PMC free article] [PubMed] [Google Scholar]
- Russin JS, Mcgawley EC, Boethel DJ. 1993. Population development of Meloidogyne incognita on soybean defoliated by Pseudoplusia includens . Nematology 25, 50–54. [PMC free article] [PubMed] [Google Scholar]
- Salt DT, Fenwick P, Whittaker JB. 1996. Interspecific herbivore interactions in a high CO2 environment: root and shoot aphids feeding on Cardamine. Oikos 77, 326–330. [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, Von Mende N, Burrows PR, Wyss U. 1991. Arabidopsis thaliana as a new model host for plant–parasitic nematodes. The Plant Journal 1, 245–254. [Google Scholar]
- Soler R, Erb M, Kaplan I. 2013. Long distance root–shoot signalling in plant–insect community interactions. Trends in Plant Science 18(3), 149–156. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X. 2007. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proceedings of the National Academy of Science U S A 104, 18842–18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinger T, Müller-Schärer H. 1992. Physiological and growth responses of Centaurea maculosa (Asteraceae) to root herbivory under varying levels of interspecific plant competition and soil nitrogen availability. Oecologia 91, 141–149. [DOI] [PubMed] [Google Scholar]
- Swiecicka M, Filipecki M, Lont D, Van Vliet J, Qin L, Goverse A, Bakker J, Helder J. 2009. Dynamics in the tomato root transcriptome on infection with the potato cyst nematode Globodera rostochiensis . Molecular Plant Pathology 10, 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil DP, Sykacek P, Grundler FMW, Bohlmann H. 2009. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant Journal 57, 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Owen B, Higgins VJ. 2004. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiology 235, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Stout MJ, Karban R, Duffey SS. 2001. Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecological Entomology 26, 312–324. [Google Scholar]
- Van Dam N, Horn M, Mareš M, Baldwin I. 2001. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata . Journal of Chemical Ecology 27, 547–568. [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Heil M. 2011. Multitrophic interactions below and above ground: en route to the next level. Journal of Ecology 99(1), 77–88. [Google Scholar]
- Van Dam NM, Raaijmakers CE, Van Der Putten WH. 2005. Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra . Entomologia Experimentalis et Applicata 115, 161–170. [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. 2007. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Current Biology 17, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Wei J, van Loon JJ, Gols R, Menzel TR, Li N, Kang L, Dicke M. 2014. Reciprocal crosstalk between jasmonate and salicylate defence-signalling pathways modulates plant volatile emission and herbivore host-selection behaviour. Journal of Experimental Botany 65, 3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wang L, Zhao J, Li C, Ge F, Kang L. 2011. Ecological trade-offs between jasmonic acid-dependent direct and indirect plant defences in tritrophic interactions. New Phytologist 189, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TCR. 1984. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63, 90–105. [DOI] [PubMed] [Google Scholar]
- Wondafrash M, Van Dam NM, Tytgat TOG. 2013. Plant systemic induced responses mediate interactions between root parasitic nematodes and aboveground herbivorous insects. Frontiers in Plant Science 4, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubben MJ, Jin J, Baum TJ. 2008. Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Molecular Plant Microbe Interactions 21, 424–432. [DOI] [PubMed] [Google Scholar]
- Wubben MJ, Rodermel SR, Baum TJ. 2004. Mutation of a UDP-glucose-4-epimerase alters nematode susceptibility and ethylene responses in Arabidopsis roots. Plant Journal 40, 712–724. [DOI] [PubMed] [Google Scholar]
- Wubben MJ, Su H, Rodermel SR, Baum TJ. 2001. Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana . Molecular Plant Microbe Interactions 14, 1206–1212. [DOI] [PubMed] [Google Scholar]
- Wurst S, Van der Putten WH. 2007. Root herbivore identity matters in plant-mediated interactions between root and shoot herbivores. Basic and Applied Ecology 8, 491–499. [Google Scholar]
- Zarate SI, Kempema LA, Walling LL. 2007. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiology 143, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PJ, Zheng SJ, van Loon JJ, Boland W, David A, Mumm R, Dicke M. 2009. Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proceedings of the Natlional Academy of Sciences U S A 106, 21202–21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov V, Navarro M, Bruinsma KA, Arbona V, Santamaria ME, Cazaux M, Wybouw N, Osborne EJ, Ens C, Rioja C, Vermeirssen V, Rubio–Somoza I, Krishna P, Diaz I, Schmid M, Gomez-Cadenaz A, Van de Peer Y, Grbic M, Clark RM, Van Leuween T, Grbic V. 2014. Reciprocal responses in the interaction between Arabidopsis and the cell-content-feeding chelicerate herbivore spider mite. Plant Physiology 164, 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.