Abstract
A critical step in mosquito reproduction is the ingestion of a blood meal from a vertebrate host. In mosquitoes like Aedes aegypti, blood feeding stimulates the release of ovary ecdysteroidogenic hormone (OEH) and insulin-like peptide 3 (ILP3). This induces the ovaries to produce ecdysteroid hormone (ECD), which then drives egg maturation. In many immature insects, prothoracicotropic hormone (PTTH) stimulates the prothoracic glands to produce ECD that directs molting and metamorphosis. The receptors for OEH, ILP3 and PTTH are different receptor tyrosine kinases with OEH and ILP3 signaling converging downstream in the insulin pathway and PTTH activating the mitogen-activated protein kinase pathway. Calcium (Ca2+) flux and cAMP have also been implicated in PTTH signaling, but the role of Ca2+ in OEH, ILP3, and cAMP signaling in ovaries is unknown. Here, we assessed whether Ca2+ flux affects OEH, ILP3, and cAMP activity in A. aegypti ovaries and also asked whether PTTH stimulated ovaries to produce ECD. Results indicated that Ca2+ flux enhanced but was not essential for OEH or ILP3 activity, whereas cAMP signaling was dependent on Ca2+ flux. Recombinant PTTH from Bombyx mori fully activated ECD production by B. mori PTGs, but exhibited no activity toward A. aegypti ovaries. Recombinant PTTH from A. aegypti also failed to stimulate either B. mori PTGs or A. aegypti ovaries to produce ECD. We discuss the implications of these results in the context of mosquito reproduction and ECD biosynthesis by insects generally.
Keywords: ovary ecdysteroidogenic hormone, insulin-like peptide, prothoracicotropic hormone, prothoracic gland, Bombyx mori, Aedes aegypti
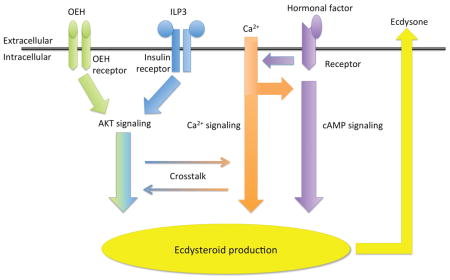
Graphical Abstract
1. Introduction
Female mosquitoes must usually consume blood from a human or other vertebrate host to mature a clutch of eggs. This nutritional requirement can also result in the acquisition and transmission of disease-causing pathogens to other hosts during subsequent bouts of blood feeding and oviposition. Blood digestion provides amino acids to the fat body for the production of yolk proteins, which are then packaged into primary oocytes that develop into mature eggs. Studies conducted primarily with Aedes aegypti indicate that ecdysteroid hormone (ECD) produced by the ovaries is the primary factor that activates the fat body to synthesize yolk proteins (Attardo et al., 2005; Baldridge and Feyereisen, 1986; Gulia-Nuss et al., 2012; Gulia-Nuss et al., 2015; Pondeville et al., 2008; 2013; Roy et al., 2015). Prior studies further show that ovaries produce ECD in response to insulin-like peptides (ILPs) and ovary ecdysteroidogenic hormone (OEH), which are released from neurosecretory cells in the brain within a few hours after females consume a blood meal (Riehle and Brown, 1999; Riehle and Brown, 2002; Gulia-Nuss et al., 2011; Dhara et al., 2013).
A. aegypti encodes eight ILPs (Brown et al., 2008). ILP3 directly stimulates the ovaries to produce ECD by binding to the insulin receptor (IR), which is a receptor tyrosine kinase (RTK) that activates the insulin signaling pathway (Riehle and Brown, 1999; Riehle and Brown, 2002; Brown et al., 2008; Wen et al., 2010; Gulia-Nuss et al., 2011; Dhara et al., 2013). OEH also directly stimulates ovaries to produce ECD but does so by binding to the OEH receptor (OEHR), which is an RTK that is closely related to the IR (Vogel et al. 2013; 2015). Recent studies further show that OEH binding to the OEHR activates components of the insulin signaling pathway such as Akt. These data collectively support that OEH and ILP3 stimulate ECD production by activating different receptors whose signaling activities converge downstream in the insulin signaling pathway (Dhara et al., 2013; Vogel et al., 2015).
ECD is also well known to direct molting and metamorphosis in immature insects with studies identifying the prothoracic glands (PTGs) as the primary source of ECD biosynthesis (Smith and Rybczynski, 2012). Studies conducted primarily in Lepidoptera identify the neuropeptide prothoracicotropic hormone (PTTH) as the key factor that stimulates PTGs to produce ECD (De Loof et al., 2015; Marchal et al., 2010; Yamanaka et al., 2013). Genetic studies in Drosophila melanogaster indicate that PTTH interacts with Torso, which similar to the IR and OEHR is also an RTK (McBrayer et al., 2007; Rewitz et al., 2009). Phylogenetic studies, however, indicate that Torso is structurally distinct from the IR and OEHR (Vogel et al., 2013), while functional assays indicate that Torso signals through the mitogen-activated protein kinase (MAPK) pathway (Rewitz et al., 2009). The mosquito literature suggests ECD stimulates molting and metamorphosis, but notably identifies the body wall of larvae rather than PTGs as the source of ECD (Jenkins et al., 1992; Telang et al., 2007). While mosquitoes encode orthologs for ptth and torso (Predel et al., 2010; Akbari et al., 2013), their functional roles in ECD biosynthesis are unknown.
A final interesting feature of the comparative literature are data indicating that other factors besides neurohormones like PTTH, OEH and ILP3 affect ECD production by PTGs and/or the mosquito ovary. Studies from Lepidoptera, for example, show that PTTH elevates calcium (Ca2+) flux and cAMP levels in PTG cells (Smith et al., 1985; Gu et al. 2000; Fellner et al., 2005), while demonstrating that cAMP analogs stimulate PTGs to produce ECD in the absence of PTTH (Smith et al., 1984). Experiments from D. melanogaster and Bombyx mori also implicate insulin and target of rapamycin (TOR) pathway signaling in regulating PTG function (Ishizaki and Suzuki, 1994; Mizoguchi and Okamoto, 2013; Columbani et al., 2005; Walkiewicz and Stern, 2009; Ohhara et al., 2015; Gu et al., 2011; 2012; 2015). An early study by Shapiro (1983) before ILPs and OEH were known showed that a cAMP analog and head extract both stimulate A. aegypti ovaries to produce ECD. More recent studies also found that TOR signaling enhances ECD production by A. aegypti ovaries after activating by ILP3 or OEH (Gulia-Nuss et al., 2011; Dhara et al., 2013), while transcriptome data show that blood feeding upregulates expression of the ptth gene in mosquitoes (Marinotti et al., 2005; Zhang and Denlinger, 2011) and a torso ortholog in A. aegypti ovaries (Akbari et al., 2013).
While the mosquito ovary and PTG literature have largely accrued independently, the above summary suggests ECD production by these tissues may share more features than is generally recognized. On the other hand it is also difficult to draw clear conclusions about potential similarities because studies conducted with PTGs and mosquito ovaries have applied different approaches or reagents. Given our primary interest is mosquito endocrinology, one key deficiency we clearly recognize is the lack of any studies examining the role of Ca2+ flux relative to the known ability of OEH, ILPs, and cAMP to stimulate ECD production by ovaries. Another deficiency, remarkably, is that no functional studies of mosquito PTTH have been conducted in any context, including whether this neurohormone has any effect on ovaries. Here, we address these issues by conducting experiments with A. aegypti ovaries using similar methodology to the lepidopteran PTG literature. Our results show that ECD production by ovaries treated with ILP3 and OEH is enhanced by but does not depend on Ca2+ flux, while stimulation by a cAMP analog was Ca2+ dependent. Our results also indicated that recombinant PTTH from A. aegypti and B. mori did not stimulate ovaries to produce ECD.
2. Materials and Methods
2.1. Mosquitoes
The UGAL strain of A. aegypti was reared as described (Dhara et al., 2013). Adults were provided water continuously but fed a 5% sucrose solution (weight/volume) on the second day after eclosion. The p20 strain of the silkmoth, Bombyx mori, was reared on artificial diet (Coastal Exotics) at 27° C and a 16 h light: 8 h dark photoperiod (Clark and Strand, 2013). Larvae used in experiments were monitored so that timing of the molt to the fifth instar was known. Larvae were then collected on day 6 for use in experiments.
2.2. Neuropeptides
A. aegypti ILP3 was synthesized by CPC Scientific Inc. (90% purity, Sunnyvale, CA) and exhibited bioactivity identical to previous reports (Brown et al., 2008; Gulia-Nuss et al., 2015). Recombinant long OEH from A. aegypti was produced in Escherichia coli and purified as described (Gulia-Nuss et al., 2012). Recombinant PTTHs from A. aegypti (AaPTTH) and B. mori (BmPTTH) were also produced in E. coli. Total RNA was isolated from A. aegypti and B. mori larvae using TRIzol (Life Technologies, U.S.A.) followed by first-strand cDNA synthesis using the Transcriptor High Fidelity cDNA synthesis Kit (Roche). PTTH is expressed as a preproprotein, which is processed at a dibasic (Arg-Lys) cut site to its mature form (Kawakami et al., 1990). Amplicons corresponding to mature B. mori and A. aegypti PTTH were PCR amplified using the above cDNA pools as templates and specific primers designed for mature B. mori PTTH (5′-ATCGTTCAGTTGAGTTATCCAGCATTC-3′ and 5′-AATTCGATTCGGAAC AAATCATCA G-3′) (Kawakami et al., 1990) and mature A. aegypti PTTH derived from an expressed sequence tag (EST) (reverse complement of GenBank: DV370510.1) (5′-ATGAAGTTAGTGTTCATATTAATATGTGCCATC-3′ and 5′-CTATATCGAACA TTGGCAAGCGGC-3′).
The above products were cloned into pCR2.1 TA (Invitrogen) followed by subcloning into pET30 Ek/LIC (Novagen) using primer sets with Ek/LIC ovarhangs (B. mori, 5′-GACGACGACAAGATGGGAAACATTCAAGTT-3′ and 5′-GAGGAGAAGCCCGGT TTATTATTATTATTATATCGTAGTTGGTAGTC-3′: A. aegypti, 5′-GACGACGACAAGA TGAACGACAAGCATGGCGATCT-5′ and 5′-GAGGAGAAGCCCGGTATCGAACAT TGGCAAGCGG-3′). After sequencing to confirm both constructs were correct, plasmids were transformed into E. coli BL21 (DE3) cells, followed by culture in SOC medium (0.5% yeast extract, 2% tryptone, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, 20 mM glucose), supplemented with 10 μg/ml of kanamycin. Bacteria were grown to an optical density of 1.0 at 37 °C, induced with isopropyl-β-d-thiogalactopyranoside (IPTG) up to a final concentration of 0.1 mM, and grown as 800 ml cultures for 16 h at 16° C. Bacterial cells were then harvested by centrifugation at 5000 x g for 10 min followed by storage at −20 °C. Bacterial pellets were resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole) on ice for 1 h followed by addition of lysozyme (1 mg/ml) in 50 mM Tris buffer (pH 8.0) and sonication. After centrifugation of the lysate at 10,000 x g for 25 min, supernatants were bound to a Ni-NTA matrix (5 Prime) pre-equilibrated with lysis buffer. Bound proteins were washed 5x with wash buffer (8 M urea, 100 mM NaH2PO4, 10 mM imidazole, 10 mM Tris-Cl pH 5.9) followed by elution with three column volumes of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 40–300 mM imidazole). The eluted proteins from wash fractions were loaded onto 10% SDS-PAGE gels and visualized by coomassie blue staining. Proteins eluted using 300 mM imadizole were desalted and concentrated by Centricon 10 (Millipore, USA). Approximately 125 μg of soluble rAaPTTH and rBmPTTH were produced, which had N-terminal cleavable 6xHis- and S-tags, and a C-terminal His-tag.
2.3. Reverse transcriptase PCR
Total RNA was isolated from A. aegypti 48 h old pupae, newly emerged adult females (6 h post eclosion), or 3 day old females at 24 h post blood ingestion using TRIzol reagent (Thermo Fisher). Samples were then quantified using a Nanodrop spectrometer (Thermo Fisher). First-strand cDNA synthesis reactions were performed using 100 ng of total RNA, oligo dT primer, and Superscript III (Invitrogen). Reverse transcription (RT) PCR reactions were then run using a BioRad thermocycler and 25 μl volumes containing 1 μl of cDNA and 2.5 μM of primers specific for A. aegypti ptth (5′-ATGT CTGCCGGTCCAGTGCT-3′ and 5′-CTAAGCTGACTCGCTACTGT AGTAA-3′), torso (AAEL002404) (5′-TGAGCACTTTGTACCTTCT-3′ and 5′-TCTGT TCCAGTTCCTTGAAT-3′), or ribosomal S7 (5′-ACCGCCGTCTACGAT-′3 and 5′-ATGGTGGTCTGCTGGTTCTT-3′), which served as a loading control. Reactions were carried out using AccuPower RT premix and AccuPower HotStart PCR premix (Bioneer) with the following conditions: RT at 50° C for 60 min and denaturation at 95° C for 5 min; HotStart activation cycle for 6 m at 94° C; and 35 cycles of 30 s at 94 °C, 30 s at 55 °C, and 60 s at 68 °C. Following a final extension step for 5 min at 68 °C, products were run on 1% agarose gels and visualized using ethidium bromide.
2.4. Salines
Ovary ECD assays were conducted in four saline solutions referred to as standard saline, nominally Ca2+ free saline, Ca2+ free saline, and high Ca2+ saline. Previous studies established that ovaries dissected from A. aegypti females sustain ECD production in vitro over multiple hours when cultured in standard saline (1.7 mM CaCl2, 1 mM MgCl2, 1.8 mM NaHCO3, 3.4 mM KCl, 5 mM trehalose, 25 mM HEPES, 150 mM NaCl) (Riehle and Brown, 1999). Nominally Ca2+ free saline was standard saline without CaCl2. Ca2+ free saline lacked CaCl2 and contained EGTA (2 mM) while high Ca2+ saline contained 8.5 mM Ca2+. Other modifications to standard saline included the addition of 10 or 100 nM gadolinium chloride (GdCl3) (Sigma G7532), 2 or 20 μM ionomycin (Sigma 10634), or 10 μM thapsigargin (Calbiochem 586005). Stocks of thapsigargin and ionomycin (2 mM) were prepared in ethanol while GdCl3 (1 mM) was prepared in water. NaHCO3 was replaced in standard saline with additional NaCl to 151.8 mM to avoid formation of insoluble Gd2(CO3)3 when GdCl3 was used at a working concentration of 100 nM. Preliminary experiments showed this modification in the absence of GdCl3 had no effect on ovary ECD production. 8-pCPT-cAMP (Sigma C3912) was made as a 10 mM stock in water and added to saline solutions at 100 uM.
2.5. Labeled calcium assays
A stock of Fluo-3, AM (F-23915, ThermoFisher Scientific) was prepared fresh in 1:1 DMSO (D-12345) and Pluronic F-127 (P-3000MP), according to instructions. Ovaries from 3–5 day-old A. aegypti were dissected into Ca2+-free saline (2 mM EGTA) with Fluo-3, AM (15 uM final) and Hoechst 33342 (1:3000 of the 10 mg/ml stock) for 1 h incubation at 27° C. Other ovaries were treated with OEH (330 nM), ILP3 (400 nM), or ionomycin (2.5 uM). Thereafter, ovaries were transferred to slides with a pool of Ca2+-free saline (2 mM EGTA), mechanically dispersed, and then an equal volume of saline with 10 mM CaCl was added. After applying cover slips, digital images of ovaries before and within 3 min after the addition of the Ca2+ saline were captured using a Leica SP5 microscope (Georgia Electron Microscopy core facility) in confocal mode as illuminated with a 405 nm UV laser and an Argon laser set to 488 nm. Three or more ovaries treated as above with each reagent were processed, and images were captured for 6 or more ovarioles. Capture settings were the same for all images taken simultaneously in two channels: 415–480 nm for the Hoechst stain and 505–550 nm for the Fluo-3. Images were uniformly adjusted for brightness and contrast followed by final figure assembly using Adobe Photoshop Cs5 (v12.1).
2.6. cAMP assay
cAMP levels were quantified in OEH and ILP3 treated ovaries with a commercial enzyme immunoassay (EIA, Arbor Assays K019-C1). For each treatment, 10 ovary pairs were dissected in saline with 1x protease inhibitor from 3–5 day old females and transferred to a microtube cap containing 120 μl (total) of Sf-900 II SFM medium (ThermoFisher Scientific). Triplicate ovary samples were incubated (30 min, 27°C) alone (negative control) or with OEH (330 nM), ILP3 (330 nM), or NKH 477 (8.3 mM, Sigma-Aldrich, N3290). NKH 477 (colforsin dapropate hydrochloride) is a water soluble derivative of forskolin, an adenyl cyclase activator, which is often used as positive control for cAMP production (Toya et al., 1998). The cAMP phosphodiesterase inhibitor, isobutyl methylxanthine (IBMX, Sigma-Aldrich I5879, final concentration 0.83 mM), was added to all samples to inhibit cAMP degradation. After incubation, caps with ovaries were attached to tubes and centrifuged to pellet the ovaries for removal of the incubation medium. EIA sample diluent (100 μl) was added to each sample followed by incubation (10 min, room temperature (RT)), sonication, and freezing at −80°C. Samples were thawed on ice, mixed, and centrifuged (1000 x g, 4°C, 15 min) to collect supernatants (50 μl per well) for the EIA. Reagents, DetecX® cAMP antibody, and cAMP standards were prepared and added to the plate following kit instructions. The covered plate was held on a shaker for 2 h at RT. After incubation, the plate was washed extensively prior to the addition of chemiluminescent substrate. Immediately afterwards, the plate was loaded for chemiluminescent absorption at 0.1 sec per well (BioTek uQuant). A regression line equation was generated from the cAMP standards and used to calculate cAMP content of the ovary samples.
2.7. Ecdysteroid production assays
In vitro ECD production assays with A. aegypti ovaries were conducted by dissecting non-blood fed females (3–5 days post-eclosion) in standard saline and removing the paired ovaries attached to the last two abdominal segments. Ovaries from two females were transferred to a small polypropylene cap containing 60 μl of one of the salines described above and incubated in a humidified chamber for 6 h at 27° C. OEH (330 nM) or ILP3 (400 nM) was added at the start of an incubation. For the PTTH assays, ovaries were placed in 60 μl standard saline, Sf-900 II SFM medium, or TC-100 medium (Sigma) followed by addition of AaPTTH or BmPTTH. At the end of the incubation period, medium from each sample was collected and stored at −80°C. In vitro ECD assays with PTGs were conducted by dissecting day 6 fifth instar B. mori in phosphate buffered saline, followed by incubation of single glands in 50 μl of TC-100 medium alone or medium plus 100 nM of BmPTTH or AaPTTH for 3 h at 25° C (Yokoyama et al., 1996; Pruijssers et al., 2009). All treatments were setup as triplicate samples of ovaries or PTGs and performed a minimum of three times with different cohorts of insects.
Secreted ECD was measured in some assays using a well-established radioimmunoassay (RIA) (Sieglaff et al., 2005). Briefly, sample medium or ecdysone standards (50 μl; 2 to 2,000 pg) were incubated overnight at 4° C with ECD antiserum (50 μl; 1:21,000 final dilution; L2 rabbit serum provided by J.-Paul Delbecque, Université Bordeaux 1, Talence, France) and [23, 24-3H(N)]-ecdysone (3H-ECD; 50 μl; ~12,000 cpm; PerkinElmer). Antibody bound and free 3H-ECD in the sample and standard solutions were separated by the ammonium sulfate method. Pelleted bound 3H-ECD was dispersed in scintillation fluid (10% water) and counted using a scintillation counter (Beckman).
To complete the study, however, we had to develop an alternative approach for measuring ECD because commercial production of 3H-ECD was discontinued. We therefore established an enzyme immunoassay (EIA) based on methods originally outlined by Kingan (1989). Plate wells (Costar 3590 96 well plates) were coated with an ecdysone-lactoglobulin conjugate. Sample medium (50 μl) or ecdysone standards (4 – 2000 pg/50 μl) were added to the wells, followed by ECD antiserum (50 μl, 1:30,000 final dilution). After washing, horseradish peroxidase-labeled secondary antibody (Sigma-Aldrich SAB3700949) was added to each well followed by the substrate 3,3′,5,5′-tetramethylbenzidine using the Microwell Peroxidase Substrate System (KPL). Absorbance values were used to calculate percentage absorbance, and ECD content in each sample was calculated from a standard regression line (typically 4 – 250 pg/well). Medium from the B. mori PTG samples was diluted 1:10 and up to 1:1000 for those incubated with B. mori PTTH to fall in the linear range of the immunoassays. Sample values were reported as pg of ECD because the L2 antiserum detects ecdysone and 20-hydroxyecdsone equally over the same range (Sieglaff et al., 2005). While lepidopteran PTGs are known to secrete 3-dehydroecdysone, it is rapidly converted to ecdysone, which is the main form of ECD produced by mosquito ovaries (Hagedorn et al., 1975; Lafont et al., 2012). Ecdysone is also known to be converted to 20-hydroxyecdysone by enzymes from tissues or cells associated with lepidopteran PTGs or mosquito ovaries in primary cultures. ECD per sample was determined by RIA or EIA with each sample internally replicated in triplicate. Sample replicates were then averaged to generate ECD values for the data points. Treatment effects were analyzed by ANOVA followed by a post-hoc Tukey-Kramer honest significant difference (HSD) test using Graphpad Prism (5.0). Graphs were also generated using Graphpad Prism followed by labeling in Adobe Illustrator.
3. Results
3.1. Extracellular Ca2+ enhances OEH and ILP3 activity
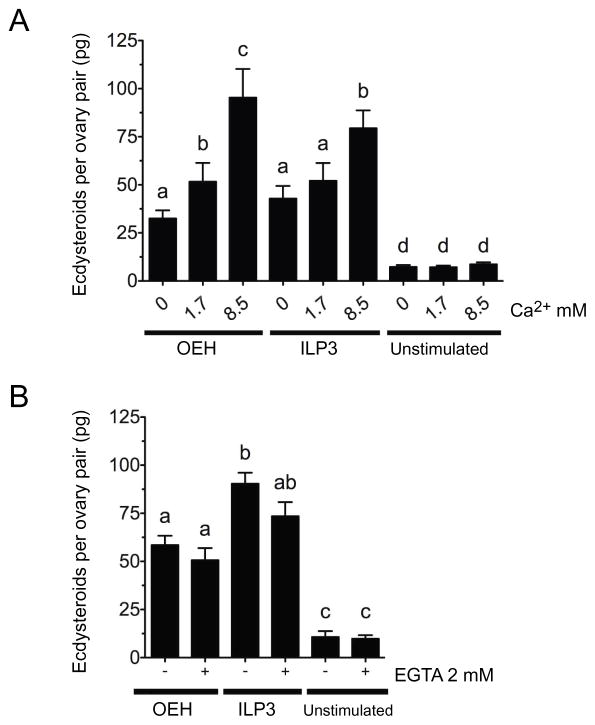
Each A. aegypti ovary consists of 60–70 ovarioles, and each ovariole contains a primary follicle comprised of an oocyte and nurse cells plus enveloping follicle cells (Fig. 3A). Previous studies established that follicle cells are the site of ECD biosynthesis (Riehle and Brown, 2002), which requires cholesterol and shuttling of sterol metabolites between the endoplasmic reticulum and biosynthetic enzymes in the mitochondria, as in PTG cells (Brown et al., 2009). Prior studies also indicate that ovaries produce ECD in standard saline up to 8 h in vitro when stimulated by ILP3 or OEH (Riehle and Brown, 1999; Brown et al. 1998; Brown et al. 2008; Dhara et al., 2013). We therefore began this study by testing whether extracellular Ca2+ stimulated ovaries to produce ECD as previously reported for PTGs from larval stage B. mori and Manduca sexta (Smith et al., 1985; Gu et al., 1998). Assays conducted in nominally Ca2+ free (0 mM), standard (1.7 mM Ca2+), or high Ca2+ (8.5 mM) saline indicated that Ca2+ alone had no effect on ECD production, whereas adding ILP3 or OEH to each treatment showed that ECD production dose-dependently increased with Ca2+ concentration (Fig. 1A). Comparing between these treatments indicated that ovaries produced more ECD when OEH or ILP3 was present in nominally Ca2+ free saline than in high Ca2+ saline without any hormone (Fig. 1A). Follow-up experiments indicated that OEH and ILP3 also stimulated ECD production in Ca2+ free saline that contained the chelator EGTA (Fig. 1B).
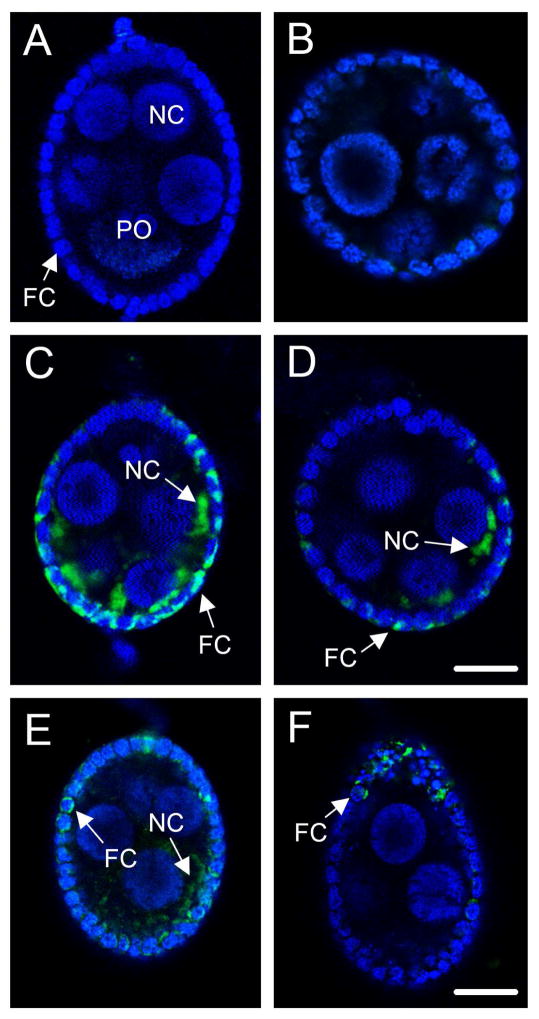
Fig. 3.
The indicator Fluo-3, AM visualizes Ca+2 influx into primary follicles following exposure to ionomycin (2.5 μM), OEH (330 nM), or ILP3 (400 nM). (A) A primary follicle preloaded with Fluo-3, AM and counterstained with nuclear dye Hoechst 33342 from an ovary cultured Ca2+-free saline (2 mM EGTA). All nuclei are labeled blue by Hoechst 33342. The nucleus of the primary oocyte (PO) is indicated as is the nucleus of a representative nurse (NC) and follicle cell (FC). No fluorescence of Fluo-3, AM (green) indicative of Ca2+ binding is visible. (B) A primary follicle from an ovary treated with 10 mM CaCl2. Hoechst labeling of cell nuclei and the absence of Fluo-3, AM fluorescence is identical to (A). (C and D) Two representative primary follicles from ovaries treated with 10 mM CaCl2 plus ionomycin. Arrows identify increased fluorescence of Fluo-3, AM (green) in the cytoplasm of a follicle (FC) and nurse cell (NC). Numerous follicle cells with increased Fluo-3, AM fluorescence are also visible. Magnification of A–D is identical with the scale bar in D equaling 48 μm. (E) A primary follicle from an ovary treated with 10 mM CaCl2 plus OEH. (F) A primary follicle from an ovary treated with 10 mM CaCl2 plus ILP3. Arrows identify increased fluorescence of Fluo-3, AM (green) in a follicle (FC) and nurse cell (NC) but also note that fewer cells exhibit a signal than in C and D. Magnification of E and F is identical with the scale bar in F equaling 96 μm.
Fig. 1.
Extracellular calcium (Ca+2) enhances ecdysteroid hormone (ECD) production by ovaries after treatment with OEH (330 nM) or ILP3 (400 nM). (A) Ovaries were placed into saline containing 0, 1.7 or 8.5 mM Ca+2 plus OEH, ILP3, or no hormone (unstimulated) followed by measurement of ECD content in the medium after a 6 h incubation. A one-way ANOVA indicated that treatments overall differed significantly from one another (F8, 230 = 30.95, P < 0.0001). Bars with different letters indicate treatments significantly differed as determined by a post-hoc Tukey-Kramer HSD test (p≤0.05). (B) Ovaries were placed into standard saline containing 1.7 mM Ca+2 with or without 2 mM EGTA followed by addition of OEH, ILP3 or no hormone. ECD in the medium was then determined and statistically analyzed as in (A) (F5, 114 = 40.06, P < 0.0001). Bars in each graph show the mean ± standard error (SE) for each treatment.
3.2. Increased Ca2+ flux also enhances OEH and ILP3 activity
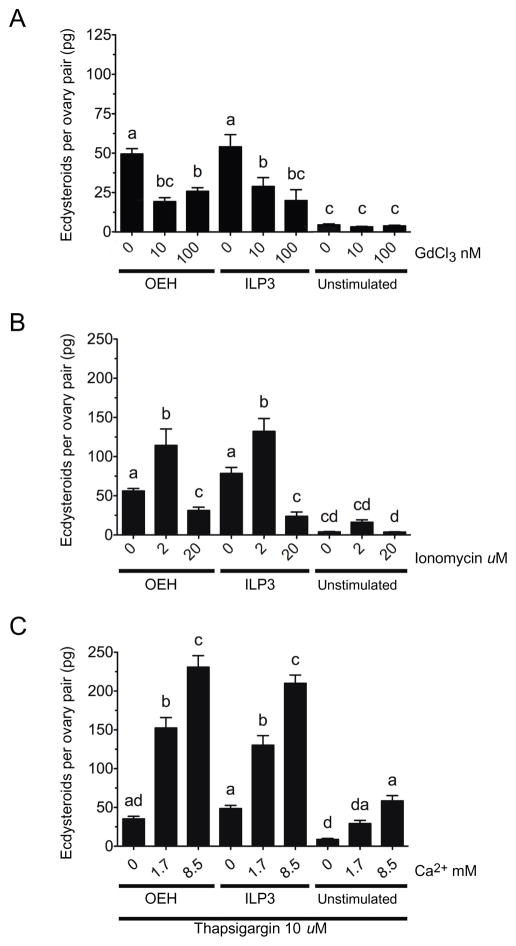
Pharmacological agents have long been used to study the effects of Ca2+ flux on cellular functions including the response of PTGs to PTTH (Birkenbeil and Dedos, 2002; Fellner et al., 2005). The literature also indicates that resting cells normally maintain low cytoplasmic Ca2+ levels (nM range) in the presence of excess of extracellular Ca2+ (mM range), with the endoplasmic reticulum (ER) serving as an intracellular store of Ca2+ (μM range) (Berridge et al., 2000). In contrast, activation of cell surface receptors by neurohormones can result in second messenger signaling that initially promotes Ca2+ release from the ER followed by activation signals that promote the influx of extracellular Ca2+ via the plasma membrane in a process known as store-operated Ca2+ entry (SOCE) (Putney, 2010). We therefore compared the effects of gadolinium chloride (GdCl3), a lanthanide that blocks influx of extracellular Ca2+, to the ionophore ionomycin, which promotes extracellular Ca2+ movement through the plasma membrane (Adding et al., 2001; Putney, 2010). Each was added to standard saline containing OEH or ILP3. Ten and 100 nM GdCl3 reduced ECD production to levels that were intermediate between treatments containing OEH or ILP3 but no GdCl3 (positive control) and treatments containing no OEH or ILP3 (negative control) (Fig. 2A). Reciprocally, 2 μM ionomycin increased ECD production but 20 μM ionomycin reduced ECD production to levels below our positive control (Fig. 2B).
Fig. 2.
The extracellular Ca+2 channel blocker, gadolinium chloride (GdCl3) (A), ionophore ionomycin (B), and SERCA inhibitor thapsigargin (C) differentially affect ECD production by ovaries following treatment with OEH or ILP3. In (A), ovaries were placed into standard saline containing 1.7 mM Ca+2 without or with GdCl3 (100 nM) followed by addition of OEH (330 nM), ILP3 (400 nM) or no hormone. (F8, 146 = 21.57, P < 0.0001). In (B), ovaries were placed in standard saline without or with ionomycin (2 and 20 μM) followed by addition of OEH, ILP3 or no hormone (F8, 210 = 41.80, P < 0.0001). In (C), ovaries were placed in saline containing 0–8.5 mM Ca+2 plus thapsigargin (10 μM) by addition of OEH, ILP3 or no hormone (F8, 102 = 93.97, P < 0.0001). Determination of ECD in the medium, data presentation, and statistical analyses for each experiment were performed as in Fig. 1.
We then examined the effects of thapsigargin, which increases intracellular Ca2+ by inhibiting sarco/endoplasmic reticulum Ca2+-ATPases (SERCA) that deplete Ca2+ while secondarily activating a SOCE response via Orai plasma membrane Ca2+ channels (Stathopulos et al., 2013). For these experiments, we used the same concentration of thapsigargin (10 μM) previously used to enhance ECD production by lepidopteran PTGs in response to PTTH (Gu et al., 1998) in saline with increasing Ca2+ concentration. In the absence of OEH and ILP3, thapsigargin in high Ca2+ saline increased ECD production to similar levels as OEH or ILP3 in nominally Ca2+ free saline (Fig. 2C; see Fig. 1). In contrast, thapsigargin substantially increased ECD production when OEH or ILP3 was added to standard or high Ca2+ saline (Fig. 2C).
We assessed whether Ca2+ flux into primary follicles could be directly visualized by preincubating ovaries in Ca2+-free saline (2 mM EGTA) plus the indicator Fluo-3, AM, which exhibits a large increase in fluorescence intensity upon Ca2+ binding. Ovaries in Ca2+-free saline served as the negative control while other treatments consisted of adding 10 mM CaCl2 only or 10 mM CaCl2 plus ionomycin, OEH or ILP3. Counter labeling with Hoechst 33342 and confocal microscopy showed across all treatments including the negative control that each follicle consisted of a primary oocyte plus nurse cells with large nuclei that were surrounded by a monolayer of follicle cells with small nuclei (Fig. 3A). Follicles to which 10 mM CaCl2 was added were indistinguishable from the negative control (Fig. 3B), whereas ionomycin treatment followed by10 mM CaCl2 induced a prominent Fluo-3, AM signal in the cytoplasm of many follicle cells plus a weaker signal in some nurse cells and primary oocytes (Fig. 3C, D). This Fluo-3, AM signal intensity was visible within 1 min but subsequently diminished and was fully lost by 15 min. OEH or ILP3 treatment followed by CaCl2 also induced a Fluo-3, AM signal in the cytoplasm of some follicle cells, nurse cell and primary oocytes (Fig. 3E, F). Detection of a Fluo-3 signal in these follicle cells occurred within 1 min of exposure and persisted for approximately 10 min before it was no longer detected. Yet, comparison between treatments also clearly showed a greater number and intensity of Fluo-3, AM stained follicle cells in the ionomycin treated follicles relative to the OEH and ILP3 treated follicles (Fig. 3C–F). The persistence of a Fluo-3, AM signal for ~ 10 min in ionomycin, OEH, and ILP3 treated follicle cells was similar to that of mammalian cells in which prolonged Ca2+ flux is associated with inducible gene expression (Uhlén and Fritz, 2010). In contrast, we observed no rapid oscillations (< 1 min) in fluorescence, which is commonly observed in muscle cells (Uhlén and Fritz, 2010) but has also been reported in PTG cells from M. sexta (Fellner et al., 2005).
3.3. cAMP signaling plays a Ca+2 dependent role in ovary ECD production but is not elevated by OEH or ILP3
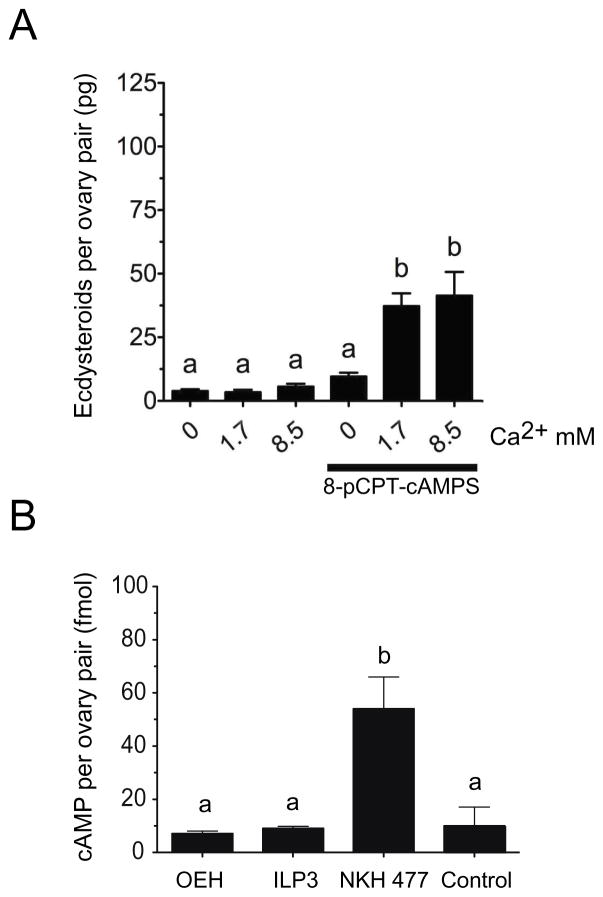
cAMP is the second intracellular signaling component implicated in the activation of ECD production by lepidopteran PTGs (Smith and Rybczynski, 2012) and mosquito ovaries (Shapiro, 1983). The membrane permeable analogue 8-pCPT-cAMP had no effect on ECD production by A. aegypti ovaries in nominally Ca2+ free saline, but it did increase ECD production in standard or high Ca2+ saline (Fig. 4A). However, this stimulatory effect was approximately half the level induced by OEH or ILP3 in high Ca2+ saline (see Fig. 1). The requirement of Ca2+ for cAMP activity also differed from our results showing that OEH and ILP signaling did not require Ca2+, which in turn also suggested that OEH and ILP signaling did not depend on cAMP.
Fig. 4.
cAMP signaling requires extracellular Ca+2 to activate ovary ECD production but is not elevated by OEH or ILP3. (A) The cAMP analog 8-pCPT-cAMP stimulated ECD production by ovaries only in the presence of extracellular Ca+2. Ovaries were placed into saline containing 0, 1.7, or 8.5 mM Ca+2 without or with 8-pCPT-cAMP (100 uM) followed by determination of ECD in the medium and statistical analysis as described in Fig. 1 (F6, 54= 17.6, P < 0.0001). (B) OEH and ILP3 treatment did not elevate intracellular cAMP in ovaries above the medium only negative control, whereas NKH 477 increased intracellular cAMP levels 5 fold. Triplicate sets of ovaries were incubated for 30 min in Sf-900 II SFM medium only (control) or medium containing OEH (330 nM), ILP3 (400 nM), or NKH 477 (8.3 mM) plus IBMX (0.83 mM). cAMP per ovary pair was then determined by EIA and analyzed as described in Fig. 1 (F3, 11 = 6.796, P = 0.0074 and Tukey’s multiple comparison test, α = 0.05).
To directly assess this, we determined whether OEH and ILP treatment elevated cAMP levels by incubating ovaries in OEH, ILP3, NHK 477 (positive control) or medium only (negative control) together with IBMX, which is an inhibitor of cAMP degradation. We then, quantified cAMP using a commercially available EIA. As shown in Fig. 4B, OEH and ILP3 had no significant effect on cAMP levels in ovaries relative to our negative control, whereas NHK 477 increased cAMP levels five-fold.
3.4. A. aegypti and B. mori PTTHs do not stimulate ECD production by ovaries
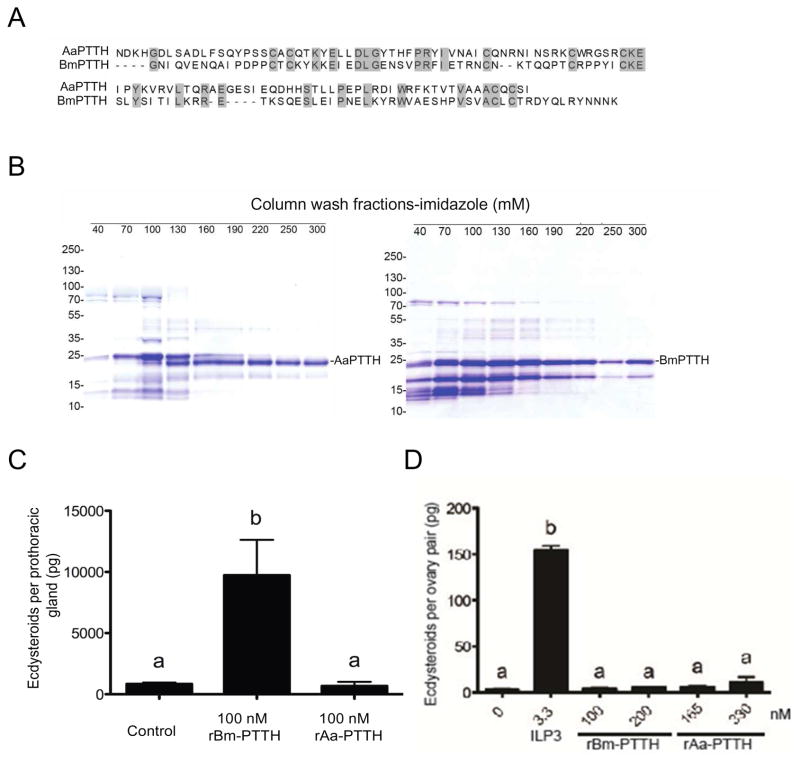
Comparative genomic and transcriptome data indicate that mosquitoes encode a single ptth gene (Predel et al., 2010), which in A. aegypti would yield a predicted 217 amino acid preproprotein. This preproprotein contains a tribasic cleavage site as seen for prepro-PTTHs of Lepidoptera like B. mori to yield a 111 amino acid mature peptide from the C-terminus (Fig. S1). Alignment of predicted mature PTTHs for A. aegypti (AaPTTH) and B. mori (BmPTTH) (Kawakami et al., 1990) showed a similar spacing pattern for the seven cysteines that form predicted intramonomeric bonds (Rybczynski, 2005), but overall identity was only 28% (Fig. 5A). RT-PCR assays detected expression of A. aegypti ptth in pupae, newly emerged females, and older females 24 h after ingesting a blood meal (Fig. S2A). RT-PCR assays also detected expression of A. aegypti torso, which consistent with transciptome data for ovaries following blood feeding (Akbari et al., 2013), was seemingly stronger in females at 24 h after a blood meal than in pupae or newly emeged adult females (Fig. S2A).
Fig. 5.
PTTH has no stimulatory effect on ovaries. (A) Alignment of mature PTTH from A. aegypti (AaPTTH) and B. mori (BmPTTH). Identical amino acids including 7 conserved cysteines are shaded in grey. (B) For each PTTH expression construct, the soluble exclusion body of E. coli was collected after induction with IPTG and the corresponding recombinant protein purified by Ni-NTA chromatography. The soluble lysate and corresponding eluates after Ni-NTA chromatography and elution using increasing concentrations of imidazole (mM) were subjected to SDS-PAGE under reducing conditions followed by staining with Coomassie Brilliant blue. Each lane shows the elution products at a given imidazole concentration with >220 mM imidazole primarily eluting proteins corresponding to the predicted masses of AaPTTH (left gel) and BmPTTH (right gel). Molecular mass markers are indicated to the left of each gel. (C) ECD production by one B. mori PTG after a 3 h incubation in TC100 medium alone (Control) or with 100 nM of rBmPTTH or rAaPTTH. rBmPTTH stimulated a significance increase in ECD in the medium relative to the control (F2,26=14.4; P<0.0001). (D) ECD production by one A. aegypti ovary pair after a 6 h incubation in Sf-900 SFM II medium alone, medium plus 330 nM ILP3 or two concentrations of rBmPTTH (100 or 200 nM) or rAaPTTH (165 and 330 nM) (F7, 59= 69.2, P < 0.0001). Determination of ECD in the medium, data presentation and statistical analyses in (C) and (D) were performed as in Fig. 1.
We therefore asked whether PTTH stimulated ovaries to produce ECD. The approach we took was to produce rAaPTTH in E. coli because prior results with B. mori and Manduca sexta indicated that rPTTHs produced in E. coli had similar activity to purified PTTH or enriched brain extracts containing PTTH (Kataoka et al., 1991; Shionoya et al., 2003). Since no studies of a mosquito PTTH had previously been conducted, we also produced rBmPTTH by identical methods. rAaPTTH and rBmPTTH were present in the soluble fraction from lysed E. coli. Ni-NTA chromatography followed by elution with imidazole buffer, and SDS-PAGE showed that high imidazole eluted proteins corresponding to the predicted molecular masses of rAaPTTH and rBmPTTH with N- and C-terminal epitope tags (Fig. 5B; Fig. S2B). Fractions from high imidazole buffer were then transferred to Centricon filters for buffer exchange into distilled water and concentration. We first conducted in vitro ECD assays with B. mori PTGs using a 100 nM dose of each rPTTH. These experiments showed that rBmPTTH strongly stimulated PTGs to produce ECD, whereas rAaPTTH did not (Fig. 5C). We then conducted in vitro ECD assays with A. aegypti ovaries using a single concentration of ILP3 as a positive control and two concentrations of rAaPTTH and rBmPTTH. ILP3 stimulated ovaries to produce ECD but neither rPTTH did (Fig. 5D).
4. Discussion
ILP3 and OEH stimulate A. aegypti ovaries to produce ECD by binding to different receptors that activate the insulin signaling pathway (Brown et al., 2008; Wen et al., 2010; Dhara et al., 2013; Vogel et al., 2015). In the first part of this study, we examined the effects of Ca2+ flux on ILP3 and OEH activity as previously examined for PTTH and PTGs from lepidopteran larvae (Fellner et al., 2005; Rybczynski and Gilbert, 2006; Gu et al., 2010; Smith and Rybczynski, 2012). Our results overall indicate the influx of extracellular Ca2+ into follicle cells enhance OEH and ILP activity as measured by increased production of ECD. However, they also show that ILP3 and OEH stimulate ovaries to produce ECD in saline without Ca2+, which indicates that neither hormone fully depends on extracellular Ca2+ for function.
Additional experiments with reagents that selectively alter the flux of extracellular or intracellular Ca2+ supported the positive interaction with ILP3 and OEH signaling. That GdCl3 reduces activity but thapsigargin increases activity suggests a role for SOCE in amplifying hormonal signaling, ECD biosynthesis, or both. Our finding that 2 μM ionomycin enhances ILP3 and OEH activity supports a role for the influx of extracellular Ca2+ in ECD production by ovaries, while the strong Fluo-3, AM signal induced following ionomycin exposure confirmed this ionophore induces Ca2+ entry into follicle cells, which are the site of ECD biosynthesis in ovaries (Riehle and Brown, 2002). However, the decreased activity of ILP3 and OEH in the presence of 20 μM ionomycin suggests excess free cytoplasmic Ca2+ either disrupts signaling or ECD biosynthesis. Lastly, the outcomes of our Fluo-3, AM assays indicate that OEH and ILP3 only weakly stimulate Ca2+ entry into follicle cells relative to ionomycin, which is consistent with both of these hormones binding RTKs, which are not known to function through Ca2+/cAMP signaling pathways.
In the absence of a direct role for Ca2+ in OEH or ILP3 signaling, several factors could account for why Ca2+ flux enhances OEH or ILP3-mediated stimulation of ECD production. The positive effects of Ca2+ flux through SOCE channels on ILP3 and OEH activity, for example, are interesting in light of studies from the insect and mammalian literature, which supports a connection between Ca2+ flux, inositol triphosphate signaling, and potentiation of insulin signaling (Worrall and Olefsky, 2002; Fellner et al., 2005; Lanner et al., 2008; Krüger et al., 2008). Ca2+ flux may also play a role in regulating the rate of ECD production rather than its activation, which is consistent with data showing that steroid biosynthesis and release in mammalian adrenal cortical cells is Ca2+ dependent (Rossier, 2006). In particular, shuttling of steroid intermediates may be affected by SOCE given that ovaries produced more ECD in the presence of thapsigargin and increased Ca2+ concentrations even in the absence of ILP3 or OEH. This could be through Ca2+ flux into the mitochondria, which play a primary role in steroid hormone synthesis in both mammalian and insect cells (Cherradi et al., 1998). Another possibility long suspected in the literature is that ECD release from PTG cells in Lepidoptera is a Ca2+-dependent event (Birkenbeil, 1983; Hanton et al., 1993), which was also recently demonstrated in D. melanogaster (Yamanaka et al., 2015). If Ca2+-dependent release of ECD is a conserved feature of steroidogenic cells in insects, it may explain why Ca2+enhances OEH and ILP3 activity in ovaries. On the other hand this explanation fails to account for the increase in ECD production we observed following treatment of ovaries with ionomycin and thapsigargin or that OEH and ILP3 stimulated ovaries to produce and release ECD in the absence of extracellular Ca2+.
As previously noted, Shapiro (1983) reported that cAMP levels increase in A. aegypti ovaries after a blood meal or treatment with head extract, and a cell permeable cAMP analog stimulated ovaries to produce ECD. This work preceded identification of ILP3 and OEH as the neurohormones that stimulate A. aegypti ovaries to produce ECD. Later work also showed that PTTH elevates cAMP levels in lepidopteran PTG cells in the presence of extracellular Ca2+, but in the absence of Ca2+ PTTH has no effect on cAMP levels, phosphorylation of MAPK (also called extracellular signal-regulated kinases (ERK)), or ECD biosynthesis (Smith and Rybczynski, 2012). This led to the suggestion PTTH increases Ca2+ flux via activation of phospholipase C and phosphokinase C, and may elevate cAMP levels through a Ca2+/calmodulin dependent adenylyl cyclase (summarized in Smith and Rybczynski 2012). Yet studies also report that a cAMP analog activates ECD production by lepidopteran PTGs in the absence of a Ca2+, which indicates a role for cAMP that is independent of PTTH mediated alterations in Ca2+ flux (Smith et al., 1984; Smith and Rybczynski, 2012).
Our experiments corroborate the findings of Shapiro (1983) by showing that 8-pCPT-cAMP stimulates ovaries to produce ECD in the absence of ILP3 and OEH, but differ from studies of PTGs because this effect only occurred in the presence of extracellular Ca2+. The highest amount of ECD produced by A. aegypti ovaries in response to 8-pCPT-cAMP was also similar to the response elicited ILP3 and OEH in the absence of Ca2+. Thus, our data support a role for cAMP in ECD production by A. aegypti ovaries, but the ability of ILP3 and OEH to stimulate ovaries without extracellular Ca2+ while also not increasing intracellular cAMP levels strongly suggests signaling by these hormones is cAMP independent. We also note parallels between the findings of this study and work conducted in the blowfly, Phormia regina, whose ovaries also produce ECD required for egg maturation following consumption of a protein meal (Manière et al., 2000). Like A. aegypti, P. regina ovaries exhibit an increase in intracellular cAMP after feeding and produce ECD when incubated in vitro with bovine insulin, but this response is not cAMP dependent (Manière et al., 2004). These data together with the findings of Shapiro (1983) thus suggest cAMP may function as a second messenger for another, currently unknown, peptide hormone that stimulates ovaries to produce ECD in dipterans requiring a protein meal for egg maturation.
As noted in our introduction, prior studies indicate that ECD regulates molting and metamorphosis of A. aegypti larvae, yet experiments also indicate that PTGs are not the source of the ECD that is released into the hemolymph of larvae to stimulate molting (Jenkins et al., 1992; Telang et al., 2007). Moreover, no study has used a purified or recombinant PTTH from mosquitoes for any functional assay. Nonetheless, we were interested in assessing whether A. aegypti PTTH could play a role in stimulating ECD production by ovaries because of: 1) previous data suggesting lepidopteran PTTH activity depends on Ca2+ and cAMP (Smith and Rybczynski 2012), 2) evidence that the ptth gene is transcribed in A. aegypti (Telang et al., 2010), and 3) data reporting elevated ptth expression in Anopheles gambiae and Culex pipiens following blood feeding (Marinotti et al., 2005; Zhang and Denlinger, 2011) and torso expression in ovaries following blood feeding by A. aegypti (Akbari et al., 2013).
Our RT-PCR assays support earlier findings by qualitatively showing that transcript abundance of ptth and torso increase in adult female A. aegypti following consumption of a blood meal. However, our bioassays detect no increase in ECD production by ovaries in response to rAaPTTH or rBmPTTH. Given the rBmPTTH we produced strongly stimulated B. mori PTGs to produce ECD, we conclude it was biologically active in B. mori and that the lack of activity against A. aegypti ovaries was not due to improper folding or dimerization. On the other hand, we cannot conclude with certainty the basis for the lack of activity of rAaPTTH due to there is no known function for PTTH in mosquitoes and thus no established assay for measuring whether a mosquito PTTH has biological activity. This is why we included rBmPTTH in our experimental design and conducted functional assays using B. mori PTGs before conducting experiments on A. aegypti ovaries. While produced identically to B. mori rPTTH, it is possible improper folding underlies the lack of rAaPTTH activity in our ovary assays. Alternatively, the lack of activity of rAaPTTH against B. mori PTGs and rBmPTTH against A. aegypti ovaries could reflect cross-species barriers given amino acid identity between these PTTHs is only 28%, and studies showing that PTTHs or brain extracts often exhibit greatly reduced or no activity in cross-species experiments (summarized by Smith and Rybczynski, 2012). An earlier study using brain extracts from M. sexta larvae also showed no activity in stimulating mosquito ovaries to produce ECD (Kelly et al., 1986). The third option is that PTTH does not stimulate A. aegypti ovaries to produce ECD.
We well recognize the need for caution in interpreting these findings but concluded that reporting these negative data was a useful contribution because they are first experimental outcomes using rPTTHs against a known ecdysteroidogenic tissue in mosquitoes. In light of our results, future studies include the need to identify the source of ECD in A. aegypti larvae and whether A. aegypti PTTH regulates ECD production in these cells. Another need is to determine whether A. aegypti PTTH activates A. aegypti Torso. However, these objectives differ from the primary purpose of this study, which was to examine the role of other signaling factors besides OEH and ILP3 in ECD production by ovaries. Other factors that could potentially play a role in stimulating mosquito ovaries to produce ECD via Ca2+/cAMP signaling are unclear. The most likely candidates, however, would be neuropeptides that activate G protein coupled receptors, which often function through Ca2+/cAMP signaling pathways (Vogel et al., 2013). Many such neuropeptides exist in mosquitoes (Predel et al., 2010; Vogel et al., 2013) including orthologs of pigment dispersing factor, which was recently reported to stimulate PTGs from B. mori to produce ECD (Iga et al., 2014). Several other peptide hormones in B. mori have also been suggested to play roles in PTG activation (Marchal et al., 2010).
Supplementary Material
Fig. S1. Nucleotide and amino acid sequences for predicted prepro-PTTH from A. aegypti (A) and the known sequence for prepro-PTTH for B. mori (B). Grey highlights the predicted sequence for mature AaPTTH and BmPTTH.
Fig. S2. (A) RT-PCR of A. aegypti ptth and torso (right) in whole body samples of pupae (P), newly emerged adult females (NA) and 4 day old females blood fed 24 h earlier (BFA). Amplification of A. aegypti S7 ribosomal protein (S7) served as a loading control. The image shown is from one replicate but this assay was repeated three times using independently collected samples, which each time yielded the same qualitative patterns shown. (B) Nucleotide and amino acid sequences for rAaPTTH and rBmPTTH produced in E. coli. Note that each recombinant PTTH used in bioassays consisted of the mature peptide plus N- and C-terminal His tags.
Highlights.
Ca2+ can enhance OEH and ILP3 activated ecdysteroid production by mosquito ovaries.
cAMP analog activation of this process is dependent on Ca2+.
Prothoracicotropic hormones do not stimulate this process in ovaries.
Acknowledgments
We thank Anne Elliot and Sarah Robertson for their assistance in maintaining the mosquito colony. This work was a grant from the National Institutes of Health (RO1AI033108) to MRB and MRS, and the Georgia Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adding LC, Bannenberg GL, Gustafsson LE. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardio Drug Rev. 2001;19:41–56. doi: 10.1111/j.1527-3466.2001.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Akbari OS, Antoshechkin I, Amrhein H, Williams B, Diloreto R, Sandler J, Hay BA. The developmental transcriptome of the mosquito Aedes aegypti, an invasive species and major arbovirus vector. G3. 2013;3:1493–1509. doi: 10.1534/g3.113.006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Baldridge G, Feyereisen R. Ecdysteroid titer and oocyte growth in the northern house mosquito, Culex pipiens L. Comp. Biochem Physiol A. 1986;83:325–329. doi: 10.1016/0300-9629(86)90583-9. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Birkenbeil H. Ultrastructural and immunocytochemical investigation of ecdysteroid secretion by the prothoracic gland of the waxmoth Galleria mellonella. Cell Tissue Res. 1983;229:433–441. doi: 10.1007/BF00214984. [DOI] [PubMed] [Google Scholar]
- Birkenbeil H, Dedos SG. Ca2+ as second messenger in PTTH-stimulated prothoracic glands of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2002;32:1625–1634. doi: 10.1016/s0965-1748(02)00101-7. [DOI] [PubMed] [Google Scholar]
- Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO. Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem. 1998;273:3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Sieglaff DH, Rees HH. Gonadal ecdysteroidogenesis in arthropoda: occurrence and regulation. Ann Rev Entomol. 2009;54:105–125. doi: 10.1146/annurev.ento.53.103106.093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherradi N, Brandenburger Y, Capponi AM. Mitochondrial regulation of mineralocorticoid biosynthesis by calcium and the StAR protein. Euro J Endocrin. 1998;139:249–256. doi: 10.1530/eje.0.1390249. [DOI] [PubMed] [Google Scholar]
- Clark KD, Strand MR. Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J Biol Chem. 2013;288:14476–14487. doi: 10.1074/jbc.M113.459222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carré C, Noselli S, Léopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- De Loof A, Vandersmissen T, Marchal E, Schoofs L. Initiation of metamorphosis and control of ecdysteroid biosynthesis in insects: The interplay of absence of juvenile hormone, PTTH, and Ca2+-homeostasis. Peptides. 2015;68:120–129. doi: 10.1016/j.peptides.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Dhara A, Eum JH, Robertson A, Gulia-Nuss M, Vogel KJ, Clark KD, Graf R, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2013;43:1100–1108. doi: 10.1016/j.ibmb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner SK, Rybczynski R, Gilbert LI. Ca2+ signaling in prothoracicotropic hormone-stimulated prothoracic gland cells of Manduca sexta: evidence for mobilization and entry mechanisms. Insect Biochem Mol Biol. 2005;35:263–275. doi: 10.1016/j.ibmb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Gu SH, Chow YS, O’Reilly DR. Role of calcium in the stimulation of ecdysteroidogenesis by recombinant prothoracicotropic hormone in the prothoracic glands of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 1998;28:861–867. [Google Scholar]
- Gu SH, Tsia WH, Chow YS. Temporal analysis of ecdysteroidogenic activity of the prothoracic glands during the fourth larval instar of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2000;30:499–505. doi: 10.1016/s0965-1748(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Gu SH, Lin JL, Lin PL. PTTH-stimulated ERK phosphorylation in prothoracic glands of the silkworm, Bombyx mori: role of Ca2+/calmodulin and receptor tyrosine kinase. J Insect Physiol. 2010;56:93–101. doi: 10.1016/j.jinsphys.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Gu SH, Young SC, Lin JL, Lin PL. Involvement of PI3K/Akt signaling in PTTH-stimulated ecdysteroidogenesis by prothoracic glands of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2011;41:197–202. doi: 10.1016/j.ibmb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Gu SH, Yeh WL, Young SC, Lin PL, Li S. TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2012;42:296–303. doi: 10.1016/j.ibmb.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Gu SH, Chen CH, Hsieh YC, Lin PL, Young SC. Modulatory effects of bombyxin on ecdysteroidogenesis in Bombyx mori prothoracic glands. J Insect Physiol. 2015;72:61–69. doi: 10.1016/j.jinsphys.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PloS One. 2011;6:e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Eum JH, Strand MR, Brown MR. Ovary ecdysteroidogenic hormone activates egg maturation in the mosquito Georgecraigius atropalpus after adult eclosion or a blood meal. J Exp Biol. 2012;215:3758–3767. doi: 10.1242/jeb.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Elliot A, Brown MR, Strand MR. Multiple factors contribute to anautogenous reproduction by the mosquito Aedes aegypti. J Insect Physiol. 2015;82:8–16. doi: 10.1016/j.jinsphys.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn H, O’connor J, Fuchs MS, Sage B, Schlaeger DA, Bohm M. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc Natl Acad Sci USA. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton WK, Watson RD, Bollenbacher WE. Ultrastructure of prothoracic glands during larval-pupal development of the tobacco hornworm, Manduca sexta: A reappraisal. J Morphol. 1993;216:95–112. doi: 10.1002/jmor.1052160110. [DOI] [PubMed] [Google Scholar]
- Iga M, Nakaoka T, Suzuki Y, Kataoka H. Pigment dispersing factor regulates ecdysone biosynthesis via bombyx neuropeptide G protein coupled receptor-B2 in the prothoracic glands of Bombyx mori. PLoS One. 2014;9:e103239. doi: 10.1371/journal.pone.0103239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki H, Suzuki A. The brain secretory peptides that control moulting and metamorphosis of the silkmoth, Bombyx mori. Int J Dev Biol. 1994;38:301–310. [PubMed] [Google Scholar]
- Jenkins SP, Brown MR, Lea AO. Inactive prothoracic glands in larvae and pupae of Aedes aegypti: ecdysteroid release by tissues in the thorax and abdomen. Insect Biochem Mol Biol. 1992;22:553–559. [Google Scholar]
- Kataoka NH, Isogai A, Ishizaki H, Suzuki A. Prothoracicotropic hormone of the silkworm, Bombyx mori: amino acid sequence and dimeric structure. Agric Biol Chem. 1991;55:73–86. [PubMed] [Google Scholar]
- Kawakami A, Kataoka H, Oka T, Mizoguchi A, Kimura-Kawakami M, Adachi T, Iwami M, Nagasawa H, Suzuki A, Ishizaki H. Molecular cloning of the Bombyx mori prothoracicotropic hormone. Science. 1990;247:1333–1335. doi: 10.1126/science.2315701. [DOI] [PubMed] [Google Scholar]
- Kelly TJ, Whisenton LR, Katahira EJ, Fuchs MS, Bo3kovec AB, Bollenbacher WE. Inter-species cross-reactivity of the prothoracicotropic hormone of Manduca sexta and egg-development neurosecretory hormone of Aedes aegypti. J Insect Physiol. 1986;32:757–762. [Google Scholar]
- Kingan TG. A competitive enzyme-linked immunosorbent assay: applications in the assay of peptides, steroids, and cyclic nucleotides. Anal Biochem. 1989;183:283–289. doi: 10.1016/0003-2697(89)90481-8. [DOI] [PubMed] [Google Scholar]
- Krüger M, Kratchmarova I, Blagoev B, Tseng YH, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci USA. 2008;105:2451–2456. doi: 10.1073/pnas.0711713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont R, Warren JT, Rees H. Ecdysteroid chemistry and biochemistry. In: Gilbert LI, editor. Insect Endocrinology. Elsevier/Academic; New York: 2012. pp. 106–176. [Google Scholar]
- Lanner JT, Bruton JD, Katz A, Westerblad H. Ca2+ and insulin-mediated glucose uptake. Curr Opin Pharmacol. 2008;8:339–45. doi: 10.1016/j.coph.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Manière G, Vanhems E, Delbecque J. Cyclic AMP-dependent and independent stimulations of ovarian steroidogenesis by brain factors in the blowfly, Phormia regina. Mol Cell Endocrinol. 2000;168:31–40. doi: 10.1016/s0303-7207(00)00312-9. [DOI] [PubMed] [Google Scholar]
- Manière G, Rondot I, Büllesbach EE, Gautron F, Vanhems E, Delbecque JP. Control of ovarian steroidogenesis by insulin-like peptides in the blowfly (Phormia regina) J Endocrinol. 2004;181:147–156. doi: 10.1677/joe.0.1810147. [DOI] [PubMed] [Google Scholar]
- Marchal E, Vandersmissen HP, Badisco L, Van de Velde S, Verlinden H, Iga M, Van Wielendaele P, Huybrechts R, Simonet G, Smagghe G, Vanden Broeck J. Control of ecdysteroidogenesis in prothoracic glands of insects: a review. Peptides. 2010;31:506–519. doi: 10.1016/j.peptides.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Marinotti O, Nguyen QK, Calvo E, James AA, Ribeiro J. Microarray analysis of genes showing variable expression following a blood meal in Anopheles gambiae. Insect Mol Biol. 2005;14:365–373. doi: 10.1111/j.1365-2583.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, Warren JT, Thummel CS, Dauphin-Villemant C, Gilbert LI, O’Connor MB. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Okamoto N. Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: discovery, structure, secretion, and function. Front Physiol. 2013;4:217. doi: 10.3389/fphys.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohhara Y, Shimada-Niwa Y, Niwa R, Kayashima Y, Hayashi Y, Akagi K, Ueda H, Yamakawa-Kobayashi K, Kobayashi S. Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc Natl Acad Sci USA. 2015;112:1452–1457. doi: 10.1073/pnas.1414966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci USA. 2008;105:19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondeville E, David JP, Guittard E, Maria A, Jacques JC, Ranson H, Bourgouin C, Dauphin-Villemant C. Microarray and RNAi analysis of P450s in Anopheles gambiae male and female steroidogenic tissues: CYP307A1 is required for ecdysteroid synthesis. PloS One. 2013;8:e79861. doi: 10.1371/journal.pone.0079861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predel R, Neupert S, Garczynski SF, Crim JW, Brown MR, Russell WK, Kahnt JR, Russell DH, Nachman RJ. Neuropeptidomics of the mosquito Aedes aegypti. J Proteome Res. 2010;9:2006–2015. doi: 10.1021/pr901187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijssers AJ, Falabella P, Eum JH, Pennacchio F, Brown MR, Strand MR. Infection by a symbiotic polydnavirus induces wasting and inhibits metamorphosis of the moth Pseudoplusia includens. J Exp Biol. 2009;212:2998–3006. doi: 10.1242/jeb.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. Pharmacology of store-operated calcium channels. Mol Interv. 2010;10:209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz KF, Yamanaka N, Gilbert LI, O’connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase Torso to initiate metamorphosis. Science. 2009;326:1403–1405. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 1999;29:855–860. doi: 10.1016/s0965-1748(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tissue Res. 2002;308:409–420. doi: 10.1007/s00441-002-0561-8. [DOI] [PubMed] [Google Scholar]
- Rossier MF. T channels and steroid biosynthesis: in search of a link with mitochondria. Cell Calcium. 2006;40:155–164. doi: 10.1016/j.ceca.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Roy S, Saha TT, Johnson L, Zhao B, Ha J, White KP, Girke T, Zou Z, Raikhel AS. Regulation of gene expression patterns in mosquito reproduction. PLoS Genet. 2015;11:e1005450. doi: 10.1371/journal.pgen.1005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybczynski R. Prothoracicotropic hormone. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. Vol. 3. Elsevier; Amsterdam: 2005. pp. 61–123. [Google Scholar]
- Rybczynski R, Gilbert LI. Protein kinase C modulates ecdysteroidogenesis in the prothoracic gland of the tobacco hornworm, Manduca sexta. Mol Cell Endocrinol. 2006;251:78–87. doi: 10.1016/j.mce.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Shapiro JP. Ovarian cyclic AMP and response to a brain hormone from the mosquito Aedes aegypti. Insect Biochem. 1983;13:273–279. [Google Scholar]
- Sieglaff DH, Duncan KA, Brown MR. Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:471–490. doi: 10.1016/j.ibmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Shionoya M, Matsubayashi H, Asahina M, Kuniyoshi H, Nagata S, Riddiford LM, Kataoka H. Molecular cloning of the prothoracicotropic hormone from the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2003;33:795–801. doi: 10.1016/s0965-1748(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Smith WA, Gilbert LI, Bollenbacher WE. The role of cyclic AMP in the regulation of ecdysone synthesis. Mol Cell Endocrinol. 1984;37:285–294. doi: 10.1016/0303-7207(84)90098-4. [DOI] [PubMed] [Google Scholar]
- Smith WA, Gilbert LI, Bollenbacher WE. Calcium-cyclic AMP interactions in prothoracicotropic hormone stimulation of ecdysone synthesis. Mol Cell Endocrinol. 1985;39:71–78. doi: 10.1016/0303-7207(85)90093-0. [DOI] [PubMed] [Google Scholar]
- Smith W, Rybczynski R. Prothoracicotropic Hormone. In: Gilbert LI, editor. Insect Endocrinology. Elsevier/Academic; New York: 2012. pp. 1–62. [Google Scholar]
- Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun. 2013;4:2963. doi: 10.1038/ncomms3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang A, Frame L, Brown MR. Larval feeding duration affects ecdysteroid levels and nutritional reserves regulating pupal commitment in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae) J Exp Biol. 2007;210:854–864. doi: 10.1242/jeb.02715. [DOI] [PubMed] [Google Scholar]
- Telang A, Peterson B, Frame L, Baker E, Brown M. Analysis of molecular markers for metamorphic competency and their response to starvation or feeding in the mosquito, Aedes aegypti (Diptera: Culicidae) J Insect Physiol. 2010;56:1925–1934. doi: 10.1016/j.jinsphys.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya Y, Schwencke C, Ishikawa Y. Forskolin derivatives with increased selectivity for cardiac adenylyl cyclase. J Mol Cell Cardiol. 1998;30:97–108. doi: 10.1006/jmcc.1997.0575. [DOI] [PubMed] [Google Scholar]
- Uhlén P1, Fritz N. Biochemistry of calcium oscillations. Biochem Biophys Res Commun. 2010;396:28–32. doi: 10.1016/j.bbrc.2010.02.117. [DOI] [PubMed] [Google Scholar]
- Vogel KJ, Brown MR, Strand MR. Phylogenetic investigation of peptide hormone and growth factor receptors in five dipteran genomes. Front Endocrinol. 2013;4:193. doi: 10.3389/fendo.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a novel receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2015;112:5057–5062. doi: 10.1073/pnas.1501814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Marqués G, O’Connor MB. Vesicle-mediated steroid hormone secretion in Drosophila melanogaster. Cell. 2015;163:907–919. doi: 10.1016/j.cell.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama I, Endo K, Yamanaka A, Kumagai K. Species-specificity in the action of big and small prothoracicotropic hormones (PTTHs) of the swallowtail butterflies, Papilio xuthus, P. machaon, P. bianor and P. helenus. Zool Sci. 1996;13:449–454. [Google Scholar]
- Walkiewicz MA, Stern M. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS One. 2009;4:e5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR, Brown MR. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol. 2010;328:47–55. doi: 10.1016/j.mce.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall DS, Olefsky JM. The effects of intracellular calcium depletion on insulin signaling in 3T3-L1 adipocytes. Mol Endocrinol. 2002;16:378–389. doi: 10.1210/mend.16.2.0776. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Denlinger DL. Molecular structure of the prothoracicotropic hormone gene in the northern house mosquito, Culex pipiens, and its expression analysis in association with diapause and blood feeding. Insect Mol Biol. 2011;20:201–213. doi: 10.1111/j.1365-2583.2010.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Nucleotide and amino acid sequences for predicted prepro-PTTH from A. aegypti (A) and the known sequence for prepro-PTTH for B. mori (B). Grey highlights the predicted sequence for mature AaPTTH and BmPTTH.
Fig. S2. (A) RT-PCR of A. aegypti ptth and torso (right) in whole body samples of pupae (P), newly emerged adult females (NA) and 4 day old females blood fed 24 h earlier (BFA). Amplification of A. aegypti S7 ribosomal protein (S7) served as a loading control. The image shown is from one replicate but this assay was repeated three times using independently collected samples, which each time yielded the same qualitative patterns shown. (B) Nucleotide and amino acid sequences for rAaPTTH and rBmPTTH produced in E. coli. Note that each recombinant PTTH used in bioassays consisted of the mature peptide plus N- and C-terminal His tags.