Abstract
Although potentially modifiable risk factors for interferon-alpha (IFN-α)-associated depression (IFN-MDD) have been identified, it is not currently known how they interact to confer risk. In the present study we prospectively investigated interactions among poor sleep quality, high stress, pre-existing depressive symptoms, and polyunsaturated fatty acid status. Non-depressed hepatitis C patients (n=104) were followed prospectively during IFN-α therapy. IFN-MDD occurs in 20-40% of patients and was diagnosed using the Structured Clinical Interview of DSM-IV (SCID-IV), with incidence examined using Cox regression. Baseline Pittsburgh Sleep Quality Inventory (PSQI), Perceived Stress Scale (PSS), Beck Depression Inventory (BDI), and a range of plasma long-chain fatty acid levels were measured (gas chromatography) -- focusing on the ratio of arachidonic acid (AA) to docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (AA/EPA+DHA). The AA/EPA+DHA ratio (B=0.40±0.16; p=0.006), PSQI (B=0.12±0.04; p=0.001), PSS (B=0.07±0.02; p<0.001), and baseline BDI (B=0.05±0.02; p<0.001) each individually predicted IFN-MDD incidence. In step-wise Cox regression eliminating non-significant variables, two interactions remained significantly predictive: PSQI*AA/EPA+DHA (p=0.008) and PSS*AA/EPA+DHA (p=0.01). Receiver Operator Curves (ROC) were used to examine the specificity and sensitivity of IFN-MDD prediction. When sleep was normal (PSQI<5), AA/EPA+DHA was strongly predictive of IFN-MDD (AUC = 91 +/− 6; p=0.002). For example, among those with AA/EPA+DHA less than the median (4.15), none with PSQI<5 developed depression. Conversely, neither PSS nor PSQI was statistically associated with depression risk in those with an elevated AA/EPA+DHA ratio. These data demonstrate that the AA/EPA+DHA ratio moderates the effect of poor sleep on risk for developing IFN-MDD and may have broader implications for predicting and preventing MDD associated with inflammation.
Keywords: Polyunsaturated fatty acids, Docosahexaenoic acid, Eicosapentaenoic acid, Arachidonic acid, Interferon-alpha, Sleep quality, Stress, Major depressive disorder
1. INTRODUCTION
Emerging evidence suggests that the pathophysiology of major depressive disorder (MDD) is frequently associated with chronic low-grade inflammation [1]. This is supported in part by findings that some MDD patients exhibit elevated levels of prostaglandin E2 [2] and pro-inflammatory cytokines such as interleukin-6 [3]. Moreover, many initially non-depressed subjects develop a depressive episode during chronic administration of the pro-inflammatory cytokine interferon-alpha (IFN-α) [4-6]. Because 25-40% of patients develop interferon-associated depression (IFN-MDD) with 2-4 months of treatment [7], prospective examination of patients treated with IFN-α represents a valuable opportunity to identify risk and resilience factors. To date several putative modifiable risk factors for IFN-MDD have been identified [8], and developing a better understanding of how these risk factors interact may inform novel preventative strategies for IFN-MDD specifically and inflammation-associated MDD more generally.
1.1 Sleep is a risk factor for MDD
Poor sleep quality is commonly associated with subsequent depression incidence [9-11], and also predicts IFN-MDD [12, 13]. In particular, a physiological predictor of IFN-MDD is lower early night delta power [14], which also predicts MDD recurrence [15-17]. Whether improving sleep quality – specifically improving delta power during sleep – can prevent depression remains to be determined.
1.2 Long Chain fatty acid status is a risk factor for MDD
Deficits in the long-chain omega-3 (LCn-3) fatty acids eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) are associated with MDD [18]. In particular, the ratio of the omega-6 arachidonic acid (AA, 20:n-6) to LCn-3 fatty acids (the AA/EPA+DHA ratio) is elevated in MDD patients [19-24]. Likewise, IFN-MDD is also associated with a high AA/EPA+DHA ratio [25]. In fact, LCn-3 supplements have recently been demonstrated to prevent IFN-MDD in some people [26]. On proposed mechanism is via decreased inflammation [27], though direct brain effects may be plausible [28]. Although LCn-3 fatty acid levels may also interact with poor sleep quality to moderate adverse effects cognition [29], how or whether they interact with sleep quality to effect depression risk is not known.
1.3 Pre-existing sub-syndromal depression symptoms are a risk factor for MDD
Third, a history of prior MDD episodes and/or current sub-syndromal symptoms may predispose to IFN-MDD [30]. Prophylactic selective serotonin reuptake inhibitors (SSRIs) can halve the incidence of IFN-MDD [31-34]. Unfortunately, SSRIs don't eliminate the IFN-MDD incidence, and may have side effects such as retinopathy [33] and worsened irritable anger [35, 36]. Although LCn-3 fatty acids have antidepressant properties [37] and may augment SSRI antidepressant effects [38, 39] it is not known how or whether LCn-3 fatty acid biostatus and MDD history interact to modify IFN-MDD risk.
1.4 Stress is a risk factor for MDD
Fourth, psychosocial stressors are a significant predictor of depressive episodes [40]. Consistent with this, altered hypothalamic-pituitary-adrenal axis activity may predict IFN-MDD [41, 42]. Interestingly, a fatty acid synthase polymorphism moderated the effect of perceived stress on depression [43] suggesting a plausible interaction between AA/EPA+DHA and stress.
The present study thus sought to prospectively investigate the interactions between the AA/EPA+DHA ratio and other risk factors such as psychosocial stress and sleep quality on depression vulnerability. Our primary hypothesis was that these risk factors would interact in a synergistic manner to increase risk for developing subsequent IFN-MDD. Based on prior evidence linking pro-inflammatory processes with poor sleep quality [44, 45], stressful life events [46-48], and an elevated AA/EPA+DHA ratio [24, 49-51], these findings may have relevance for predicting and preventing inflammation-associated MDD more broadly.
2. Materials and Methods
2.1. Subjects
Non-depressed adult subjects (between ages 18-80) were examined for plasma fatty acid levels and completed a set of questionnaires prior to subsequent IFN-α therapy for HCV (n=104). Our primary interest is IFN-MDD incidence. These subjects partially overlapped with those in a prior study [25]. Exclusion criteria were active mood, anxiety, psychotic, or drug/alcohol use disorders within 6 months prior to starting IFN-α treatment – using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV); known neurologic disease; or taking corticosteroids, antidepressants, anticonvulsants, and/or antipsychotics (although they could be taking as-needed sleeping medications). All subjects started weekly injections of pegylated (PEG) IFN-α2 (PEG-IFN-α2a: 135 μg/week or PEG-IFN-α2b: 120 or 150 μg/week) augmented with oral ribavirin. This study was approved by the University of Pittsburgh Institutional Review Board.
2.2. Assessments
Prior to initiating IFN-α therapy, and monthly for four months after therapy was initiated, depression was assessed using the Beck Depression Inventory-II (BDI) and the Montgomery-Asberg Depression Rating Scale (MADRS) as previously described (Lotrich et al., 2009). IFN-MDD was diagnosed using either an abbreviated SCID-IV interview focused on depression and/or in any subject started on an antidepressant by their physician -- as previously described in more detail [25]. The Perceived Stress Scale (PSS) [52], the life events scale (LES) [53, 54], and the Pittsburgh Sleep Quality Inventory (PSQI)(Buysse et al., 1989) were administered at baseline. For the LES, we examined both total number of stressful life events in the past year, as well the total score after adjusting each event for impact (self-rated on a scale from −3 to +3).
2.3. Gas chromatography
Plasma from whole blood was obtained from all subjects between 10AM and 4PM prior to initiating treatment for hepatitis C (HCV), and stored at −80°C until analysis. Folch reagent (2 mL Chloroform/Methanol 2:1) was added to 0.3ml of plasma to extract the lipid layer, dried under nitrogen, and reconstituted with chloroform (100 uL). The lipid extract was then transferred to a reversed-phase packed SPE column (Alltech, Nicholasville, KY) and washed with chloroform (10 mL), to remove triglycerides, and then acetone (10mL) to remove the cholesteryl esters. Phospholipids were then eluted with methanol (20mL), and the combined methanol fractions evaporated. The sample was methylated using NaOH/MeOH (0.5 mL) and the derivatization was completed with BF3/MeOH followed by heating for 15 minutes at 85°C. To ensure total fatty acid methyl ester (FAME) extraction, NaCl (0.3 mL) was used before extraction with hexane. Sodium sulfate was added to the hexane layer to remove water, and the organic phase decanted and evaporated using nitrogen. Samples were then reconstituted with hexane (0.5 mL) and analyzed.
FAME's were analyzed using an HP 6890/5973 gas chromatograph/mass selective detector (Agilent Technologies, Santa Clara, CA). The column used to separate FAME's was an Agilent DB-FFAP 15m × 0.1 mm with 0.1 um of film thickness. Helium was used as carrier gas at a flow rate of 17.6 ml/min and a constant pressure of 53.8 psi. The initial temperature was set at 160°C and increased after injection of 1 ul of sample to 240°C at a rate of 15°C per minute. Once the temperature of 240°C was reached, it was maintained for 6 minutes for a total run time of 14.33 minutes. The transfer line was maintained at 280°C and the filament at 70Ev for EI. The data were evaluated using a TIC for compound identification and SCAN mode to measure relative percent of each fatty acid. Fatty acid identification was based on retention times of authenticated FAME standards (GLC 473B) and controls (GLC 462 and GLC 463) to ensure reproducibility (NuCheck Prep, Elysian, MN). Data are expressed as weight percent of total fatty acid pool (mg fatty acid/100 mg fatty acids). We assessed the omega-6 fatty acids linoleic acid (LA; C18:2n-6), gamma-linolenic acid (GLA; C18:3n-6), dihomo-gamma-linolenic acid (DGLA; C20:3n-6), and arachidonic acid (AA; C20:4n-6), and the omega-3 fatty acids alpha-linolenic acid (ALA; C18:3n-3), eicosapentaenoic acid (EPA; C20:5n-3), and docosahexaenoic acid (DHA; C22:6n-3).
2.5. Statistical analyses
All statistics employed SPSS 22.0. We first examined IFN-MDD incidence (i.e. days after starting IFN-α therapy until MDD developed), following patients for 120 days after starting IFN-α therapy. Potential reasons for censored data were that patients quit IFN-α therapy or IFN-α therapy was stopped prematurely because of medical side effects. Cox regression analyses were employed in four stages. First, we tested the assumption of proportional hazards using two techniques: i. Graphical inspection of log-hazard vs. time, and the log (−log) survival plot. ii. testing the interaction of time by study group.
Next, we examined individual candidate variables that are likely influences on risk for IFN-MDD, both without covariates and then including co-variates such as age and weight. Third, we examined hypothesized interactions, both with and without covariates. Finally, we then included each variable (including interactions and covariates) into a step-wise Cox regression model, using conditional modeling to eliminate non-contributing variables.
All variables met the proportional hazards assumption except for AA/DHA+EPA, thus any interactions wit fatty acids found using Cox-regression were suspect. We therefore examined interactions with AA/DHA+EPA using several additional statistical approaches. First, we divided variables (AA/DHA+EPA, PSQI, and PSS) into quartiles. We utilized Kaplan-Meier with Mantel-Cox log rank comparisons to examine the AA/DHA+EPA quartiles, stratified by either PSQI or PSS. Secondly, we also used Receiver Operating Curve (ROC) analyses to examine sensitivity and specificity. ROC analyses examine predictive tests by plotting the false positive rate (1-specificity) against the true positive rate (sensitivity). The area under the curve (AUC) for this plot ranges from 100 (perfect performance) to 50 (uninformative) [55]. We examined the ability of baseline AA/DHA+EPA to predict subsequent IFN-MDD, when stratified by PSQI.
Finally, the categorical diagnosis of “MDD” is only one way of defining depression. To examine interactions using a quantitative measure and confirm any findings from the survival analyses, we analyzed MADRS scores over time (where day one was the first day of IFN-α therapy) using a mixed-effect repeated-measure analysis. We dichotomized the variables (AA/DHA+EPA, PSQI, and PSS) using a median split and test for interactions: AA/EPA+DHA*PSQI*time and AA/EPA+DHA*PSS*time.
3. Results
3.1 Subject characteristics
Subjects were 2/3 male, mostly Caucasian, often overweight, and 40% had a history of any mood disorder in remission (Table 1). Both age and female gender were associated with lower ratios of AA/EPA+DHA (Table 2). None of the long-chain fatty acids were associated with weight; but self-reported African-American heritage was associated with evidence of greater delta-5 desaturase activity (as manifest in an elevated AA/DGLA ratio) and higher levels of DHA+EPA, as consistent with prior reports [56]. Age was associated with higher DHA levels; and females had lower AA/DGLA ratios and higher ALA levels (Table 2). Because of these various relationships, in subsequent analyses we controlled for age, weight, gender, and race. Consistent with prior studies, the only fatty acid variable predictive of IFN-MDD development was AA/EPA+DHA (Table 2), whether controlling for covariates or not.
Table 1.
Demographic characteristics (mean +/− standard deviation) of the study population, and correlations with perceived stress (PSS), sleep quality (PSQI), and ratio of omega-6/omega3 fatty acids (AA/EPA+DHA). Significant (p<0.05) results for correlations (R) are indicated in bold and with an asterisk.
| Mean +/− SD | R with PSS | R with PSQI | R with AA/EPA+DHA | |
|---|---|---|---|---|
| Female | 33% | 0.073 | 0.04 | 0.27* |
| African-American | 15% | 0.10 | 0.03 | 0.07 |
| Age | 47.9 +/− 11 | 0.20* | 0.09 | 0.27* |
| Weight (Kg) | 86.6 +/− 17.6 | 0.09 | 0.05 | 0.01 |
| CRP | 2.1 +/− 2.6 | 0.05 | 0.02 | 0.01 |
| MADRS | 3.6 +/− 4.4 | 0.38* | 0.36* | 0.19 |
| BDI | 9./1 +/− 7.9 | 0.70* | 0.52* | 0.26* |
| History of depression | 40.0% | 0.37* | 0.25* | 0.17 |
| LES Total number | 6.1 +/− 4.3 | 0.31* | 0.28* | 0.04 |
| LES Total score | −2.5 +/− 8.7 | 0.28* | 0.23* | 0.20 (p=0.07) |
| PSS | 19.6 +/− 8.6 | 0.38* | 0.12 | |
| PSQI | 6.6 +/− 4.1 | 0.31* | ||
| AA/EPA+DHA | 4.2 +/− 1.1 |
Table 2.
Mean percent of total fatty acids (+/− standard deviations) for seven long chain fatty acids, and correlations (R values) with demographics are presented. Statistical significance (p<0.05), not correcting for multiple testing, is labeled in bold and with an asterisk. Only one of these (AA/EPA+DHA) predicted depression during interferon treatment (IFN-MDD).
| Fatty Acids | Percent of total | Correlations | Cox-Regression with IFN-MDD | ||||
|---|---|---|---|---|---|---|---|
| Gender | Race | Age | Weight | ||||
| LA | C18:2n-6 | 23.6 +/− 2.8% | .02 | .16 | .06 | .1 | B = −0.08 +/− 0.06; N.S. |
| GLA | C18:3n-6 | 0.08 +/− 0.05% | .01 | .17 | .08 | .1 | B = −0.73 +/− 3.0; N.S. |
| DGLA | C20:3n-6 | 3.5 +/− 0.8% | .14 | .29* | .08 | .09 | B = 0.09 +/− 0.18; N.S. |
| AA | C20:4n-6 | 10.9 +/− 2.1% | .12 | .17 | .05 | .11 | B = 0.05 +/− 0.07; N.S. |
| ALA | C18:3n-3 | 0.19 +/− 0.08% | .25* | .04 | .04 | .19 | B = −0.13 +/− 1.9; N.S. |
| EPA | C20:5n-3 | 0.46 +/− 0.19% | .12 | .03 | .07 | .08 | B = 1.1 +/− 0.8; N.S. |
| DHA | C22:6n-3 | 2.3 +/− 0.8% | .14 | .24* | .21* | .08 | B = −0.3 +/− 0.2; N.S. |
| GLA/LA | Delta-6 desaturase index | .01 | .15 | .1 | .09 | B = 4.7 +/− 6.2; N.S. | |
| DGLA/LA | Delta-6 desaturase index | .11 | .18 | .08 | .12 | B = 3.4 +/− 3.6; N.S. | |
| AA/DGLA | Delta-5 desaturase index | .22* | .35* | .12 | .03 | B = −0.03 +/− 0.145; N.S. | |
| EPA+DHA | Combined LCn-3 | .15 | .22* | .21* | .06 | B = −0.23 +/− 0.18; N.S. | |
| AA/EPA+DHA | Ratio of LCn-6/3. | .27* | .07 | .27* | .01 | B = 0.4 +/− 0.15; p=0.008 | |
3.21 Depression incidence with Cox regression
In Cox-regression analyses of depression development, the AA/EPA+DHA ratio, PSQI, PSS, and BDI all predicted IFN-MDD incidence, when examined individually or when co-varying for race, weight, age, and gender (Table 3). When also controlling for baseline BDI, AA/EPA+DHA and PSQI both continued to predict IFN-MDD (p=0.002 for each). Actual stressful life events did not influence risk for IFN-MDD, but rather the subjective perception of stress (PSS) did (Table 3). However, when controlling for baseline BDI, PSS no longer was significant (p=0.13).
Table 3.
The fatty acid ratio (AA/EPA+DHA), perceived stress (PSS), sleep quality (PSQI), and baseline depression symptoms (BDI) all predicted IFN-MDD in Cox-regression survival analyses. Two measures of stressful life events did not. With covariates included, two interactions also predicted depression. The interaction between depression and fatty acids lost significance when covariates were included.
| Without covariates | With covariates (age, weight, gender, race) | |
|---|---|---|
| AA/EPA+DHA | B=0.40±0.15; p=0.008 | B=0.56+/−0.17; p=0.001 |
| PSS | B=0.07±0.02; p<0.001 | B=0.036 +/−0.014; p=0.01 |
| PSQI | B=0.12±0.04; p<0.002 | B=0.13+/−0.04; p<0.001 |
| BDI | B=0.05±0.02; p<0.001 | B=0.06+/−0.02; p<0.001 |
| LES (total #) | B=0.06 +/−0.03; p=0.07 | B=−0.02 +/−0.05; p=0.6 |
| LES (total score) | B=0.0+/−0.02; p=0.9 | B=0.03+/−0.02; p=0.12 |
| PSS*AA/EPA+DHA | B=0.01+/−0.004; p=0.006 | B=0.009+/−0.004; p=0.035 |
| PSQI*AA/EPA+DHA | B=0.03+/−0.007; p<0.001 | B=0.023+/−0.009; p=0.012 |
| BDI*AA/EPA+DHA | B=0.019+/−0.005; p<0.001 | B=0.011+/−0.007; p=0.13 |
AA/EPA+DHA interacted with both PSQI and PSS to predict IFN-MDD when other covariates were included (Table 3). When additionally co-varying for baseline BDI, both of these interactions remained significant (P<0.001 and p=0.011, respectively). We next added each of these variables stepwise into a single multivariate Cox regression model – sequentially including both interactions (PSQI*AA/EPA+DHA and PSS*AA/EPA+DHA), as well as AA/EPA+DHA, BDI, PSQI, PSS, race, age, gender, and weight. Because most of these variables are correlated with the others (Table 1), conditional modeling was employed to eliminate non-contributing variables (both the Wald or Likelihood Ratio produced similar results). The only remaining three variables that predicted IFN-MDD incidence were PSQI*AA/EPA+DHA (B=0.029+/−0.008; p=0.008), PSS*AA/EPA+DHA (B=0.01+/0.004; p=0.01) and race (B=1.038 +/− 0.008; p=0.04).
Because AA/EPA+DHA and PSQI are correlated at baseline (Table 1), we also examined if one might mediate the other's effects. When co-entered into Cox-regression analyses of IFN-MDD incidence (along with weight, age, gender, race), both continued to be significant (B=0.57+/−0.18; p=0.002 and B=0.12+/−0.04; p=0.007 respectively). Thus the influence of AA/EPA+DHA on depression risk is not because it is correlated with PSQI.
3.22 Depression incidence with Kaplan-Meier
Although the interactions between AA/EPA+DHA were robust, the assumption of proportional hazards was not met by AA/DHA+EPA. Therefore, we divided AA/EPA+DHA (as well as PSQI and PSS) into quartiles for analysis using log-rank comparisons. When PSQI was in the lowest quartile (PSQI<5), AA/EPA+DHA was strongly predictive of IFN-MDD (X2 = 17.7; p=0.001). In fact, 100% of subjects with the highest quartile of AA/EPA+DHA (AA/EPA+DHA>4.95%) developed IFN-MDD while 0% of the subjects in the lower fatty acid quartile (AA/EPA+DHA<3.5%) did. Conversely, for the other three quartiles of PSQI, AA/EPA+DHA was no longer predictive at all (p>0.3). This supports the interaction between AA/EPA+DHA and PSQI noted above – where fatty acids are only predictive when PSQI is low. The interaction with stress was less evident. AA/EPA+DHA was no longer predictive for any quartile of PSS (p >0.13 for all four quartiles).
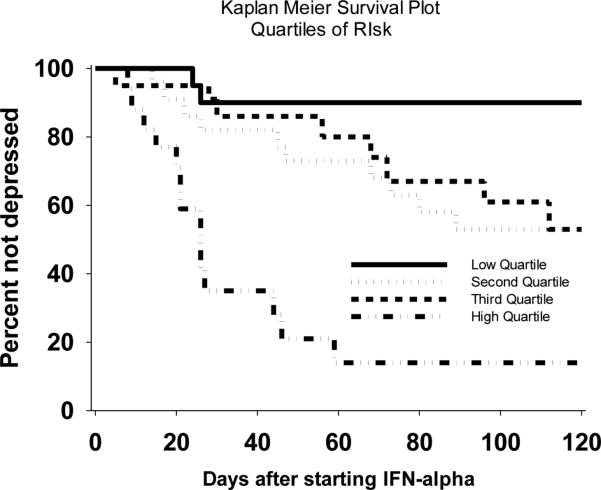
To explore potential clinical utility, we also combined z-scores for PSS, PSQI, and AA/DHA+EPA into a single measure for log-rank analysis. In Kaplan-Meier analyses, the lowest quartile of this combined z-score were almost all resilient whereas the highest quartile were almost all vulnerable to depression during IFN-α treatment (X2 = 18.2; p<0.001) (Fig. 1).
Figure 1.
Kaplan-Meier survival plot of the quartiles for combined Z-score for fatty acids, PSS, and PSQI. Few patients in the lower quartile developed depression whereas the majority of patients in the upper quartile developed depression.
3.3 Receiver Operating Curve (ROC) analyses of depression prediction
To better define the nature of the significant interactions, we split AA/EPA+DHA ratios into greater or less than the median (median=4.15, range 1.47 to 7.06); and then examined the predictive utility of baseline BDI, PSS, and PSQI using ROC analyses. When AA/EPA+DHA was <4.15, the ROC AUC for PSQI was 76 +/− 9 (p=0.02). In fact for all subjects with PSQI<5 and AA/EPA+DHA<4.15, sensitivity at predicting total resilience to IFN-MDD was 100%. However, PSQI was not predictive of IFN-MDD when AA/EPA+DHA>4.5 (p=0.21).
Baseline BDI was similarly predictive of IFN-MDD regardless of having high or low AA/EPA+DHA (AUC = 68 +/− 9; p=0.045 and 67 +/− 8; p=0.045). PSS lost power to predict subsequent IFN-MDD, whether AA/EPA+DHA was less than or greater than the median, (p=0.49 and 0=0.72, respectively). Thus the moderation of sleep quality's effects by AA/EPA+DHA was the only consistent interaction associated with IFN-MDD development.
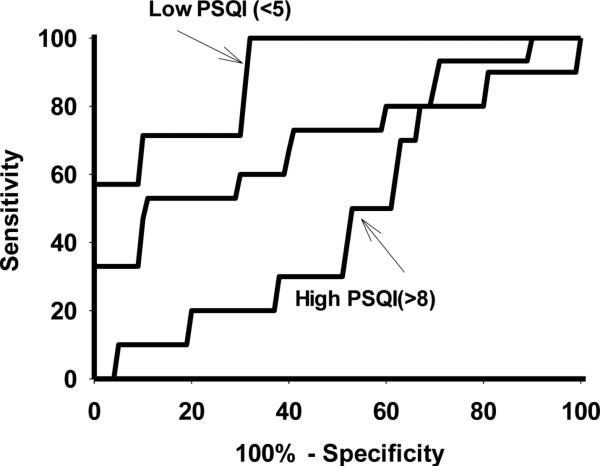
We also examined the ability of AA/EPA+DHA to predict IFN-MDD, as moderated by PSQI. PSQI was split into tertiles (<5, 5-8, >8), and ROC curves were assessed for AA/EPA+DHA (Figure 2). When PSQI was low (<5), AA/EPA+DHA was strongly predictive of IFN-MDD (AUC = 91 +/− 6; p=0.002). However when PSQI was medium (5-8) or high (>8), AA/EPA+DHA was no longer predictive (AUC = 71 +/− 10 p=0.08 and AUC = 46+/− 11; p=0.8).
Figure2.
Receiver Operating Curves (ROC) for fatty acid ratio (AA/EPA+DHA). Three ROC curves were obtained: when Pittsburgh Sleep Quality Inventory tertiles of <5 (good sleep), 5-8 (mild sleep problems), and >8 (significant sleep problems).
3.4 Repeated-measure mixed-effect analysis of symptom development
This interaction between sleep and fatty acids was confirmed in repeated-measure mixed-effect analyses of MADRS scores over time. For this, we examined median splits for PSS, PSQI, and AA/EPA+DHA to dichotomize each into ‘high’ and ‘low’ groups. We replicated the interaction between AA/EPA+DHA and time - those with greater AA/EPA+DHA have increasing MADRS scores after IFN-α is started (F=3.9(9,42.9); p=0.001). Consistent with the findings above, there was an interaction between PSQI*AA/EPA+DHA*time (F=2.2(10,39.3); p=0.042). However, there was no similar PSS*AA/EPA+DHA*time interaction (p>0.4).
4. Discussion
Self-reported sleep quality and LCn-3 status interact to affect resilience to inflammatory-cytokine-associated depression. This interaction indicates that LCn-3 status (i.e. AA/EPA+DHA) moderates the influence of sleep quality on depression risk. We have previously reported on the beneficial effect of good sleep [13], which is often reflected by PSQI <5 [57]. However, a number of patients still develop IFN-MDD despite sleeping well. We now report that in these good sleeping subjects, the AA/EPA+DHA ratio was highly influential on risk for IFN-MDD. The AUC for the receiver operating curve was >0.9 for AA/EPA+DHA, indicative of a test with excellent predictive performance [58]. However, in those sleeping poorly, the AA/EPA+DHA ratio lost much of its predictive power.
Our findings suggest that AA/EPA+DHA levels are less influential in those with poor sleep. In a recent placebo-controlled prevention trial, supplementation with EPA improved resilience, but 10% still developed IFN-MDD [26], indicating a significant amount of IFN-MDD risk remains even following supplementation. Our results predict that (i) optimal benefits of LCn-3 supplementation will be seen in those that are sleeping well, and (ii) optimal benefits of sleep interventions will occur in those with low AA/EPA+DHA ratios.
We also observed that PSQI scores correlated with AA/EPA+DHA at baseline, prior to any IFN-α injection. In fact, this correlation continued to be significant even when co-varying for both BDI and PSS. LCn-3 fatty acids may influence sleep quality [59-61]; and consistent with our observation, poor sleep correlated with the AA/DHA ratio in children [62] and with lower EPA and DHA levels in depressed inpatients [63]. It is thus possible that fatty acid supplementation can also improve sleep [60, 64]. However, any causal link between fatty acids and sleep is speculative at this point. Of note, we did not find any evidence that sleep mediated the effects of AA/EPA+DHA on depression risk.
Unlike the interaction with sleep, our findings did not support any influence of AA/EPA+DHA on the risk related to baseline BDI. Treating pre-existing sub-syndromal depression symptoms may be important in preventing IFN-MDD [32, 65], but our results predict that these benefits of SSRIs do not depend on LCn-3 levels. Also, stress had limited predictive power. PSS scores were strongly correlated with BDI scores (R=0.7), and only somewhat correlated with stressful life events. This is consistent with observations that the subjective perception of stress is likely related to mood state and can improve with antidepressant treatment [66]. PSS lost the power to predict IFN-MDD when AA/EPA+DHA was used to split the population into subgroups; and LES had no predictive power. PSS also did not interact with AA/EPA+DHA in the repeated measures analysis of MADRS depression symptoms during IFN–α therapy. It is possible that we lacked statistical power to detect smaller effect sizes. Thus potential findings are only suggestive, and the role of stress in increasing vulnerability to inflammation requires further investigation. Nonetheless, patients with combined poor sleep quality, high perceived stress, and elevated AA/(EPA+DHA) are at highest risk of developing depression in response to IFN-α treatment.
Importantly in these analyses, we controlled for race, gender, weight, and age. Delta-5-desaturase and delta-6 desaturase are the rate-limiting enzymes for polyunsaturated fatty acid conversion, and are recognized as main determinants (other than diet) of PUFA levels. Genetic polymorphism studies [67] support our findings of higher delta-5-desaturase activity in African-Americans [68, 69]. We also observed evidence that females have higher delta-5-desaturase activity [70]. Interestingly African-Americans had higher EPA+DHA levels. This is despite the observation that African-Americans were about 4% more likely to develop IFN-MDD. There are likely other influences on risk for IFN-MDD that co-vary with racial background and gender [71, 72]. Thus, controlling for these variables is important in clinical studies of LCn-3 and depression.
One limitation of our study is that neither diet nor exercise was measured. Exercise can influence sleep quality [73], and diet is an obvious influence on fatty acid levels. A strength of this study is the prospective design and longitudinal assessment of IFN-MDD incidence. Another strength is the measurement of the ratio of AA/EPA+DHA, which may be more physiologically meaningful than straightforward LCn-3 levels [74-76]. This study did not address the physiological mechanism that might underlie the interactions. LCn-3 levels correlate with brain serotonin [77, 78] [79]; and with glutamate receptor subunit expression [80] [81, 82] [83]. Brain-derived neurotrophic factor (BDNF) genotype is associated with increased vulnerability to IFN-MDD [84], and LCn-3 fatty acids increase BDNF production [28, 85]. The effects of sleep on depression risk may also involve serotonin [86], glutamate [87], and growth factors [88, 89]. LCn-3 fatty acids may also blunt stress-induced elevations in inflammatory cytokines [27]. Thus, there are several mechanisms by which interactions are plausibly feasible.
Regardless of mechanism, the interaction between sleep and fatty acids on depression risk has important clinical implications. MDD is a leading cause of disability and suicide [90-92] and has adverse effects on the prognosis for those with co-morbid medical illness [93]. Targeted attention to modifiable, interacting risk factors like sleep and fatty acid levels may be critical for depression prevention. In brief, efforts to improve resiliency will be optimal if both sleep quality and AA/EPA+DHA are concomitantly addressed.
Highlights.
Sleep, the AA/EPA+DHA ratio, and stress each influence risk for depression
Sleep and AA/EPA+DHA interact - the AA/EPA+DHA effect is strongest in good sleepers
Conversely, the role of sleep is strongest in those with low AA/EPA+DHA ratios
The role of baseline depression remained independent of AA/EPA+DHA ratios
The role of stress was less robust, but may interact with AA/EPA+DHA
Acknowledgements
Funding for this study was provided in part by NIH R01 grants MH090250 (FEL) and DK097599 (RKM). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors thank Mary Dinehart-Perry and Fermin Castro for their technical assistance.
Abbreviations
- ALA
Alpha-linolenic acid
- AA
Arachidonic acid
- BDI
Beck Depression Inventory
- DGLA
Dihomo-gamma-linolenic acid
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FAME
Fatty acid methyl ester
- GLA
Gamma-linolenic acid
- IFN-α
Interferon-alpha
- IFN-MDD
Interferon-alpha-associated depression
- LES
Life events scale
- LA
Linoleic acid
- LCn-3
Long chain omega-3
- MDD
Major depressive disorder
- MADRS
Montgomery-Asberg Depression Rating Scale
- PSS
Perceived Stress Scale
- PSQI
Pittsburgh Sleep Quality Inventory
- PUFA
Polyunsaturated fatty acid
- ROC
Receiver operator curve
- SSRI
Selective serotonin reuptake inhibitor
- SCID-IV
Structured Clinical Interview of DSM-IV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McNamara R, Lotrich FE. Elevated Immune-Inflammatory Signaling in Mood Disorders: A New Therapeutic Target? Expert Review of Neurotherapeutics. 2012;12:1143–1161. doi: 10.1586/ern.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins, Leukotrienes and Medicine. 1983;10 doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- 3.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-a in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 5.Lotrich FE, Rabinovitz M, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. Journal of Psychosomatic Research. 2007;63:131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric Adverse Effects of Interferon-a: Recognition and Management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotrich FE. Inflammatory cytokine associated depression. Brain Research. 2015 doi: 10.1016/j.brainres.2014.06.032. in press 10.1016/j.brainres.2014.1006.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuno N, Besset A, Ritchie K. Sleep and depression. Journal of Clinical Psychiatry. 2005;66:12541269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 10.Perlis ML, Giles DE, Buysse DJ, Thase ME, Tu X, Kupfer DJ. Which depressive symptoms are related to which sleep electroencephalographic variables? Biological Psychiatry. 1997;42:904–913. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- 11.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 12.Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-α treatment: the role of IL-6 and sleep quality. Brain, Behavior, & Immunity. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzen PL, Buysse DJ, Rabinovitz M, Pollock BG, Lotrich FE. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irritability during interferon-alpha treatment. Journal of Psychiatric Research. 2009;177:240–245. doi: 10.1016/j.psychres.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotrich FE, Germain M. Decreased delta sleep ratio and elevated alpha power predict vulnerability to depression during interferon-alpha treatment. Acta Neuropsychiatrica. 2015;27:14–24. doi: 10.1017/neu.2014.30. [DOI] [PubMed] [Google Scholar]
- 15.Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Archives of General Psychiatry. 1990;47:1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- 16.Spanier C, Frank E, McEachran AB, Grochocinski VJ, Kupfer DJ. The prophylaxis of depressive episodes in recurrent depression following discontinuation of drug therapy: integrating psychological and biological factors. Psychol Med. 1996;26:461–475. doi: 10.1017/s0033291700035546. [DOI] [PubMed] [Google Scholar]
- 17.Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ, Ehlers CL. EEG sleep correlates of recurrence of depression on active medication. Depression. 1993;1:300–308. [Google Scholar]
- 18.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biological Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosomatic Medicine. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 20.Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam study. American Journal of Clinical Nutrition. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 21.Frasur-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biological Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. Journal of Affective Disorders. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 23.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 24.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: Relationshipwith central serotonin turnover. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;83:185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotrich FE, Sears B, McNamara R. Elevated Ratio of Arachidonic acid to Long-Chain Omega-3 Fatty Acids Predicts Depression Development Following Interferon-alpha Treatment: Relationship with Interleukin-6. Brain Behavior and Immunity. 2012 doi: 10.1016/j.bbi.2012.08.007. (in press) doi 10.1016/j.bbi.2012.1008.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su K-P, Lai H-C, Yang H-T, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biological Psychiatry. 2014;76:559–566. doi: 10.1016/j.biopsych.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Kiecolt-Glaser J, Belury MA, Andridge R, Malarkey WB, Glase r.R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behavior and Immunology. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao JS, Ertley RN, Lee HJ, et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Molecular Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 29.Johnston DT, Deuster PA, Harris WS, Macrae H, Dretsch MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed U.S. Servicemembers. Nutritional Neuroscience. 2013;16:30–38. doi: 10.1179/1476830512Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 30.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patients initial affective state. New England Journal of Medicine. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 31.Lotrich FE. Risk factors and prevention of interferon-induced depression. Dialogues in Clinical Neuroscience. 2009;11:417–426. doi: 10.31887/DCNS.2009.11.4/felotrich. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Alimentary Pharmacology & Therapeutics. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 33.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. New England Journal of Medicine. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-alpha-2a-associated depression in hepatitis C virum - infected patients without previous psychiatric disease. Annals of Internal Medicine. 2012;157:94–103. doi: 10.7326/0003-4819-157-2-201207170-00006. [DOI] [PubMed] [Google Scholar]
- 35.Lotrich FE, Sears B, McNamara R. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. J Psychosom Res. 2013 doi: 10.1016/j.jpsychores.2013.07.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strawn JR, Adler CM, McNamara RK, et al. Antidepressant tolerability in anxious and depressed youth at high-risk for bipolar disorder: A prospective naturalistic treatment study. 2013 doi: 10.1111/bdi.12113. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. Journal of Clinical Psychopharmacology. 2012;32:61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. American Journal of Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 41.Capuron L, Dantzer R, Miller AH. Neuro-immune interactions in psychopathology with the example of interferon-alpha-induced depression. Journal de la Societe de Biologie. 2003;197:151–156. [PubMed] [Google Scholar]
- 42.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt GJ, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Molecular Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuboi H, Sakakibara H, Yamakawa-Kobayashi K, et al. Val1483Ile polymorphism in the fatty acid synthase gene was associated with depressive symptoms under the influence of psychological stress. Journal of Affective Disorders. 2011;134:448–452. doi: 10.1016/j.jad.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Mullington JM, Hinze-Selch D, Pollmacher T. Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Annals of the New York Academy of Sciences. 2001;933:201–210. doi: 10.1111/j.1749-6632.2001.tb05825.x. [DOI] [PubMed] [Google Scholar]
- 45.Ali T, Choe J, Awab A, Wagener TL, Orr WC. Sleep, immunity and inflammation in gastrointestinal disorders. World Journal of Gastroenterology. 2013;19:9231–9239. doi: 10.3748/wjg.v19.i48.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain Behavior and Immunology. 2002;16:513–524. doi: 10.1016/s0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 47.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 48.Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behavior and Immunology. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? British Journal of Clinical Pharmacology. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukotrienes and Essential Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Mingam R, Moranis A, Bluthe R-M, et al. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. European Journal of Neuroscience. 2008;28:1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 53.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. Journal of Consulting & Clinical Psychology. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 54.Reilly-Harrington NA, Alloy LB, Fresco DM, Whitehouse WG. Cognitive styles and life events interact to predict bipolar and unipolar symptomatology. Journal of Abnormal Psychology. 1999;108:567–578. doi: 10.1037//0021-843x.108.4.567. [DOI] [PubMed] [Google Scholar]
- 55.Beck AT, Brown G, Berchick RJ, Stewart BL, Steer RA. Relationship between hopelessness and ultimate suicide: A replication with psychaitric outpatients. American Journal of Psychiatry. 1990;147:190–195. doi: 10.1176/ajp.147.2.190. [DOI] [PubMed] [Google Scholar]
- 56.Mathias RA, Sergeant S, Ruczinski I, et al. The impact of FADS genetic variants on omega6 polyunsaturated fatty acid metabolism in African Americans. BMC Genetics. 2011;12:50. doi: 10.1186/1471-2156-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatric Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 59.Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. American Journal of Clinical Nutrition. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- 60.Hansen AL, Dahl L, Olson G, et al. Fish consumption, sleep, daily functioning, and heart rate variability. Journal of Clinical Sleep Medicine. 2014;10:567–575. doi: 10.5664/jcsm.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Judge MP, Cong X, Harel O, Courville AB, Lammi-Keefe CJ. Maternal consumption of a DHA-containing functional food benefits infant sleep patterning: an early neurodevelopmental measure. Early Human Development. 2012;88:531–537. doi: 10.1016/j.earlhumdev.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Fatty acids and sleep in UK children: subjective and pilot objective sleep results from the DOLAB study--a randomized controlled trial. Journal of Sleep Research. 2014;23:364–388. doi: 10.1111/jsr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irmisch G, Schlafke D, Gierow W, Herpertz S, Richter J. Fatty acids and sleep in depressed inpatients. Prostaglandins Leukotrienes & Essential Fatty Acids. 2007;76:1–2. doi: 10.1016/j.plefa.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Huss M, Volp A, Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems - an observational cohort study. Lipids in Health & Disease. 2010;9:105. doi: 10.1186/1476-511X-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Candrian M, Schwartz F, Farabaugh A, Perlis RH, Ehlert U, Fava M. Personality disorders and perceived stress in major depressive disorder. Psychiatry Research. 2008;160:184–191. doi: 10.1016/j.psychres.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemaitre RN, Tanaka T, Tang W, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genetics. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sergeant S, Hugenschmidt CE, Rudock ME, et al. Difference in arachidonic acid levels and fatty acid desaturase (FAD5) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. British Journal of Nutrition. 2012;107:547–555. doi: 10.1017/S0007114511003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathias RA, Fu W, Akey JM, et al. Adaptive Evolution of the FADS Gene Cluster within Africa. PLoS One. 2012;7:e44926. doi: 10.1371/journal.pone.0044926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. American Journal of Clinical Nutrition. 2011;94:1914S–1919S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- 71.Blackmore ER, Groth SW, Chen D-GD, Gilchrist MA, O'Connor TG, Moynihan JA. Depressive symptoms and proinflammatory cytokines across the perinatal period in African American women. Journal of Psychosomatic Obstetrics & Gynecology. 2014;35:8–15. doi: 10.3109/0167482X.2013.868879. [DOI] [PubMed] [Google Scholar]
- 72.Morris AA, Zhao L, Ahmed Y, et al. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosomatic Medicine. 2011;73:462–468. doi: 10.1097/PSY.0b013e318222379c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kline CE, Irish LA, Krafty RT, et al. Consistently High Sports/Exercise Activity Is Associated with Better Sleep Quality, Continuity and Depth in Midlife Women: The SWAN Sleep Study. Sleep. 2013;36:1279–1288. doi: 10.5665/sleep.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doug, B B. Lands Balancing proportions of competing omega-3 and omega-6 highly unsaturated fatty acids (HUFA) in tissue lipids. Prostaglandins Leukotrienes & Essential Fatty Acids. 2015 doi: 10.1016/j.plefa.2015.04.005. in press (doi:10.1016/j.plefa.2015.1004.1005) [DOI] [PubMed] [Google Scholar]
- 75.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid raion in cardiovascular disease and other chronic diseases. Experimental Biological Medicine. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 76.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Molecular Neurobiology. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 77.Olsson NU, Shoaf SE, Salem JN. The effect of dietary polyunsatured fatty acids and alcohol on neurotransmitter levels in rat brain. Nutritional Neuroscience. 1998;1:133–140. doi: 10.1080/1028415X.1998.11747222. [DOI] [PubMed] [Google Scholar]
- 78.Park Y, Moon H-J, Kim S-H. N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. Journal of Nutritional Biochemistry. 2012;23:924–928. doi: 10.1016/j.jnutbio.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 79.Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem JN. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biological Psychiatry. 1998;44:235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 80.Harbeby E, Jouin M, Alessandri J-M, et al. n-3 PUFA status affects expression of genes involved in neuroenergetics differently in the fronto-parietal cortex compared to the CA1 area of the hippocampus: effect of rest and neuronal activation in the rat. Prostaglandins Leukotrienes & Essential Fatty Acids. 2012;86:211–220. doi: 10.1016/j.plefa.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiology of Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Kariv-Inbal Z, Yacobson S, Berkecz R, et al. The isoform-specific pathological effects of apoE4 in vivo are prevented by a fish oil (DHA) diet and are modified by cholesterol. Journal of Alzheimer's Disease. 2012;28:667–683. doi: 10.3233/JAD-2011-111265. [DOI] [PubMed] [Google Scholar]
- 83.Kim H-Y, Spector AA, Xiong Z-M. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins & Other Lipid Mediators. 2011;96:114–120. doi: 10.1016/j.prostaglandins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lotrich FE, Albusaysi S, Ferrell RE. Brain-derived neurotrophic factor serum levels and genotype: association with depression during interferon-alpha treatment. Neuropsychopharmacology. 2013;38:989–995. doi: 10.1038/npp.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rao JS, Ertley RN, DeMar JC, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprovation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Molecular Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 86.Thase ME. Depression and sleep: pathophysiology and treatment. Dialogues in Clinical Neuroscience. 2006;8:217–226. doi: 10.31887/DCNS.2006.8.2/mthase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamashita A, Hamada A, Suhara Y, et al. Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse. 2014;68:235–247. doi: 10.1002/syn.21733. [DOI] [PubMed] [Google Scholar]
- 88.Guindalini C, Mazzotti DR, Castro LS, et al. Brain-derived neurotrophic factor gene polymorphism predicts interindividual variation in the sleep electroencephalogram. Journal of Neuroscience Research. 2014;92:1018–1023. doi: 10.1002/jnr.23380. [DOI] [PubMed] [Google Scholar]
- 89.Zhang L, Zhang H-Q, Liang X-Y, Zhang H-F, Zhang T, Liu F-E. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behavioural Brain Research. 2013;256:72–81. doi: 10.1016/j.bbr.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 90.Insel TR, Charney DS. Research on Major Depression Strategies and Priorities. JAMA. 2003;289:3167–3168. doi: 10.1001/jama.289.23.3167. [DOI] [PubMed] [Google Scholar]
- 91.Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nature Medicine. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 92.Panzarino PJJ. The costs of depression: direct and indirect; treatment versus nontreatment. Journal of Clinical Psychiatry. 1998;59:1–141. [PubMed] [Google Scholar]
- 93.Coyne JC, Fechner-Bates S, Schwenk TL. Prevalence, nature, and comorbidity of depressive disorders in primary care. General Hospital Psychiatry. 1994;16:267–276. doi: 10.1016/0163-8343(94)90006-x. [DOI] [PubMed] [Google Scholar]