Abstract
Purpose
Oncolytic herpes simplex viruses [oHSV] represent a promising therapy for glioblastoma [GB], but their clinical success has been limited. Early innate immune responses to viral infection reduce oHSV replication, tumor destruction, and efficacy. Here, we characterized the antiviral effects of macrophages and microglia on viral therapy for GB.
Experimental Design
Quantitative flow cytometry of mice with intracranial gliomas [± oHSV] was utilized to examine macrophage/microglia infiltration and activation. In vitro co-culture assays of infected glioma cells with microglia/macrophages were utilized to test their impact on oHSV replication. Macrophages from TNFα knockout mice and blocking antibodies were used to evaluate the biological effects of TNFα on virus replication. TNFα blocking antibodies were utilized to evaluate the impact of TNFα on oHSV therapy in vivo.
Results
Flow cytometry analysis revealed a 7.9 fold increase in macrophage infiltration after virus treatment. Tumor infiltrating macrophages/microglia were polarized towards a M1, pro-inflammatory phenotype and they expressed high levels of CD86, MHCII, and Ly6C. Macrophages/microglia produced significant amounts of TNFα in response to infected glioma cells in vitro and in vivo. Utilizing TNFα blocking antibodies and macrophages derived from TNFα knockout mice we discovered TNFα induced apoptosis in infected tumor cells and inhibited virus replication. Finally, we demonstrated the transient blockade of TNFα from the tumor microenvironment with TNFα blocking antibodies significantly enhanced virus replication and survival in GB intracranial tumors.
Conclusions
The results of these studies suggest FDA approved TNFα inhibitors may significantly improve the efficacy of oncolytic virus therapy.
Keywords: TNFα, macrophage, microglia, innate immune responses, oncolytic virus, apoptosis
Introduction
Glioblastoma [GB] is one of the most common and deadly types of primary brain tumors. These tumors are characterized by widespread invasion, extensive angiogenesis, and resistance to cell death (1). These features along with a restrictive blood brain barrier severely limit treatment options and result in a median patient survival of 15 months (2).
Oncolytic Herpes Simplex Viruses [oHSVs] are viruses genetically modified to specifically infect, replicate in, and target cancer cells for destruction. oHSVs represent a promising treatment modality for patients with GB, and in clinical trials these viruses are safe and well tolerated (3). Early phase clinical trials have produced promising results and there is currently a phase III clinical trial for patients with advanced melanoma [NCT00769704] (4–6).
The success of oHSV derived therapeutics is thought to depend on the oncolytic destruction of tumor cells and the activation of anti-tumor immune responses which can potentially lead to long term cancer remission. However, the pro-inflammatory immune responses generated by viral infection can also antagonize oHSV replication and spread. Innate immune responses destroy replicating virus and reduce tumor cell killing, and several studies have demonstrated the negative effects of innate immune responses to oHSV treatment (7–9).
Microglia and infiltrating macrophages are thought to be significant mediators of the innate immune response to viral infection in the CNS (10–14). Depletion of these cells with clodronate liposomes or cyclophosphamide [CPA] reduces antiviral responses and improves oHSV efficacy (15–20). As a result of these preclinical studies the combination of oncolytic measles virus with CPA is currently being evaluated in a phase I clinical trial for multiple myeloma [ClinicalTrials.gov Identifier: NCT00450814]. While these studies highlight the importance of modulating early immune responses to oHSV infection, the depletion of all phagocytic cells with clodronate liposomes or total immune suppression with high doses of CPA does not specifically address the mechanism by which macrophages and microglia limit oHSV replication, spread, and efficacy.
In this study, we investigated the impact of microglia and macrophages in oHSV therapy for GB. Quantitative flow cytometry analysis of mice with intracranial gliomas treated with oHSV revealed significant changes in the activation and infiltration of macrophages and microglia in oHSV treated animals relative to untreated mice. To evaluate the impact of these cells on oHSV propagation, we developed an in vitro co-culture system with infected glioma cells and microglia/macrophages. In these studies, macrophages and microglia significantly reduced virus replication. Furthermore, we identified microglia/macrophage secreted tumor necrosis factor α [TNFα] as a major factor which reduces viral replication through the induction of apoptosis in infected cells. In co-culture assays, we were able to rescue changes in virus replication with TNFα knockout macrophages or TNFα function blocking antibodies. Finally, we demonstrated the specific inhibition of TNFα produced by the tumor microenvironment could significantly enhance virus replication and efficacy in vivo. The results of these studies suggest FDA approved TNFα inhibitors may significantly enhance patient responses in oHSV clinical trials.
Materials and Methods
Cell Lines
Vero, LN229, U87ΔEGFR, U251-T2, and U251-T3-mCherry cells were maintained in DMEM supplemented with 10% fetal bovine serum [FBS]. U251-T2 and U251-T3-mCherry cells were created in our lab [May 2009] as tumorigenic clones of U251 cells by serially passaging these cells two and three times in mice, respectively. Monkey kidney epithelial derived Vero cells and U87ΔEGFR cells were obtained in April 2005 from Dr. E Antonio Chiocca [Ohio State University, Columbus, Ohio]. LN229 cells were obtained in January 2005 from Erwin Van Meir [Emory University, Atlanta, Georgia]. GB30 neurospheres were originally received in 2012 from Dr. EA Chiocca [Ohio State University, Columbus, OH]. GB30 neurospheres were maintained as tumor spheres in Neurobasal Medium supplemented with 2% B27, human EGF [50 ng/ml], and bFGF [50 ng/ml] in low-attachment cell culture flasks as previously described (21). Vero cells have not been authenticated since receipt. U87ΔEGFR [January 2015], LN229 [July 2013], GB30 [January 2015], and U251 [January 2015] cells were authenticated by the University of Arizona Genetics Core via STR profiling. Murine BV2 microglia were maintained in DMEM supplemented with 2% FBS. BV2 cells were obtained in January 2009 from J. Godbout [Ohio State University, Columbus, Ohio]. Murine RAW264.7 macrophages were obtained in RPMI supplemented with 10% FBS. RAW264.7 macrophages were received in June 2010 from S. Tridandapani [Ohio State University, Columbus, Ohio]. Murine BV2 and RAW264.7 cells have not been authenticated since receipt. All cells were incubated at 37°C in an atmosphere with 5% carbon dioxide and maintained with 100 units of penicillin/mL, and 0.1 mg of streptomycin/mL [Penn/Strep]. All cells are routinely monitored for changes in morphology and growth rate. All cells are negative for mycoplasma.
Viruses and virus replication assay
rHSVQ1, rHSVQ1-Luciferase, 1716, hrR3 and rQNestin34.5 were prepared and titered on Vero cells via a standard plaque forming unit assay as previously described.(22)
Co-culture Assays
550,000 glioma cells were plated in 6 well Falcon tissue culture plates and infected with virus at a multiplicity of infection [MOI] of 1 or 2 in DMEM supplemented with 0.05% FBS. Cells were washed 3 times over the course of an hour to remove unbound virus. Infected cells were then overlaid with 1,000,000 microglia or macrophages [2:1 ratio of macrophages/microglia to glioma cells] for 12 hours [pre-virus burst]. For TNFα blocking antibody assays, 1800 ng/ml of mouse TNFα neutralizing antibody [D2H4; Cell Signaling] or an isotype control was utilized. Concentrations of antibody were determined experimentally based on manufacturer’s specifications.
Western Blots
Cells were cultured with virus, TNFα, and/or microglia/macrophages as described above. BCA analysis [Pierce Biotechnology, Rockford, IL] was used to determine protein concentration. Equal amounts of protein were separated on a 4–20% Tris-HCL gel and transferred to a PVDF membrane. Caspase 8, cleaved Caspase 3, Cleaved PARP, and GAPDH [Cell signaling] were used at 1:1000 except GAPDH [1:5000] which was used as a loading control.
Microglia and macrophage antibody staining
Staining of surface antigens were performed as previously described.(23, 24) Briefly, Fc receptors were blocked with anti-CD16/CD32 antibody [eBioscience, San Diego, CA]. Cells were then incubated with the appropriate antibodies: CD45, CD11b, MHCII, CD86, LY6C, LY6G, and CD160 [eBioscience, San Diego, CA] for 45 minutes. Cells were re-suspended in FACS buffer [2% FBS in HBSS with 1 mg/ml sodium azide] for analysis. Non-specific binding was assessed via isotype-matched antibodies. Antigen expression was determined using a Becton-Dickinson FACS Caliber four color cytometer. Ten thousand events were recorded for each sample and isotype matched-conjugate. Data was analyzed using FlowJo software [Tree Star, CA].
Image Acquisition
Fluorescent and bright field images were acquired using an Olympus IX81 epi-fluorescence microscope equipped with a QImaging Retiga 2000R FAST camera and Olympus objectives. Image-Pro software [Version 6.2] was used for image acquisition. For luciferase imaging, mice received an intraperitoneal [IP] injection of luciferin [Caliper Life Sciences] and the luciferase signal was visualized/quantified utilizing a IVIS Lumina II imaging system.
Animal surgery
All animal experiments were performed in accordance with the Subcommittee on Research Animal Care of The Ohio State University guidelines and were approved by the institutional review board. 6–8-week-old, female athymic nude mice [NCI], were used for in vivo tumor studies. Intracranial surgeries were performed as previously described with stereotactic implantation of 100,000 U87ΔEGFR (22). Tumors were treated with HBSS, rQNestin34.5, or rHSVQ1-Luciferase virus at the location of tumor implantation. For antibody studies, mice were treated via IP injection at the days indicated with 400ug of anti-murine TNFα antibody [XT3.11] or isotype control antibody [BE0094; BE0088] from BioXCell. Animals were euthanized when they showed signs of morbidity.
Statistical Analysis
Student’s t-test or one-way ANOVA with Bonferroni multiple comparision post hoc tests were used to analyze changes in cell killing, viral plaque forming assays, luciferase imaging experiments, and flow cytometry assays. In survival assays, Kaplan–Meier curves were plotted and the log rank test was utilized to determine statistical significance. All statistical analyses were performed with the use of Graph Pad Prism software [version 5.01]. A P<0.05 was considered statistically significant. Derived P values are identified as *P<0.05; **P<0.01; ***P<0.001.
Results
oHSV Therapy activates microglia in vivo
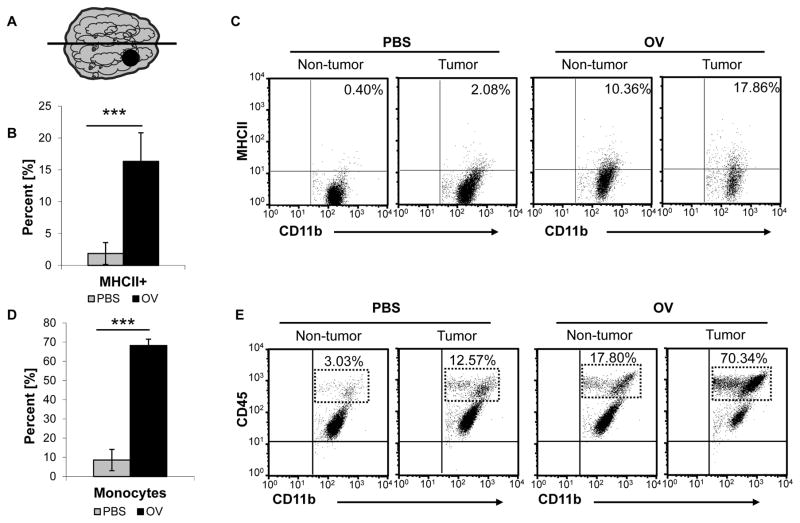
In GB animal models, microglia comprise 13–34% of all viable cells in the tumor (25). Similar ranges are seen in human tumors, and these observations underscore the importance of this cell type in the context of oHSV therapy (26, 27). The ability of microglia in the tumor microenvironment to switch from a glioma supportive role to an anti-viral state following oHSV treatment has not been well studied. To examine changes in microglia activation following oHSV infection in vivo, we treated mice with established U87ΔEGFR intracranial tumors with oHSV or PBS [injection control]. These mice were euthanized 3 days following treatment, and we analyzed the tumor- and non- tumor bearing hemispheres for microglia [CD11b+CD45lo] MHCII expression [Figure 1A]. We observed an 8.75 fold increase in microglia MHCII expression in oHSV treated mice compared to PBS treated animals [P<0.001] [Figure 1B]. Interestingly, oHSV therapy up-regulated mean microglia MHCII expression in both the tumor and non tumor bearing hemispheres of the brain, but this increase was higher in the tumor bearing hemisphere [16.33% MHCII+] compared to the non-tumor bearing hemisphere [8.23% MHCII+] [Figure 1C].
Figure 1.
oHSV treatment increases microglia activation and induces macrophage infiltration into the tumor microenvironment. A. Diagram of mice with intracranial U87ΔEGFR tumors [black dot] treated with 1 x 105 pfu of oHSV [rQNestin34.5] or PBS 7 days post tumor cell implantation. 3 Days post oHSV treatment, the mice were euthanized and the tumor and non-tumor bearing hemispheres were separated via gross dissection [midline drawn between two hemispheres]. B. Quantification of MHCII expression of the tumor bearing hemispheres of PBS and oHSV treated mice. Data shown is mean percent positive ± SD [n= 5/group]. C. Representative scatter plot of MHCII expression on microglia [CD11b+CD45lo] in tumor and non-tumor bearing mice treated with PBS or oHSV. D. Quantification of macrophage infiltration into the tumor bearing hemisphere following oHSV therapy or PBS injection. Data shown are mean percent positive ± SD [n=5/group; P<0.001]. E. Representative scatter plot of macrophage [CD11b+CD45hi] infiltration following oHSV therapy.
oHSV Therapy Increases Macrophage Infiltration into the Brain Tumor Microenvironment
Microglia activation induces the expression of various cytokines and chemokines which can stimulate the migration of immune cells into the CNS (14). Macrophages are important mediators of this innate immune response to viral infection, but the extent of macrophage infiltration into the CNS following oHSV therapy is unknown. To quantify the impact of oHSV induced macrophage migration, we treated mice with established intracranial U87ΔEGFR tumors with oHSV or PBS as described earlier. We observed a 7.96 and 5.70 fold increase in macrophage [CD11b+CD45hi] infiltration into the tumor and non-tumor bearing hemispheres following oHSV infection, respectively [n=5/group; P<0.001 and P<0.05] [Figure 1D–E]. While, oHSV therapy strongly induced macrophage infiltration into both hemispheres, this increase was significantly higher in the tumor bearing hemisphere [P<0.001] [Figure 1D–E]. While macrophages comprised the bulk of the innate immune cell infiltrate, other innate immune cells populations are known are known to migrate into the CNS following viral infection (7, 28). We examined the percoll isolated cell populations for Ly6G+ neutrophils and CD160+ natural killer [NK] cells, and we found few NK cells or neutrophils in the CD11b+CD45+ populations at this time point [Supplemental Figure S1A].
oHSV Therapy Increases Macrophage Activation in the Brain Tumor Microenvironment
oHSV therapy induced significant macrophage infiltration into the brain tumor microenvironment, but the phenotype and activation of these cells remained unknown. Depending on their polarization, macrophages can promote an immune-suppressive or pro-inflammatory tumor microenvironment. The activation status of these infiltrating cells is crucial to understanding how these cells contribute to oHSV therapy for GB. To determine the polarization status of infiltrating macrophages, we evaluated the expression of the classic activation markers CD86, Ly6C, and MHCII. We observed significant increases in the percentages and cell numbers of macrophages [CD11b+CD45hi] positive for CD86+ and LY6C+ following oHSV treatment compared to PBS treatment [P<0.001; P<0.001, respectively] [Figure 2A–B]. While the percentages of MHCII positive macrophages [CD11b+CD45hi] in the tumor environment between oHSV and control treatments did not change, we observed a 9 fold increase in the total numbers of MHCII positive macrophages infiltrating the tumor bearing hemisphere following oHSV therapy compared to control treated mice [P<0.001] [Figure 2C]. The surface expression of these three activation markers increased on macrophages[CD11b+CD45hi] in both the treated and untreated hemispheres, but the treated hemispheres contained higher percentages of macrophages which expressed CD86 and LY6C [Figure 2A–C]. Together, these data suggested the infiltrating macrophages were polarized toward a pro-inflammatory state.
Figure 2.
oHSV treatment increases macrophage [CD11b+CD45hi] activation. A–C. Left- Representative scatter plots of CD86+ [A], LY6C+ [B], and MHCII+ [C] macrophages in the tumor and non-tumor bearing hemispheres following oHSV [rQNestin34.5] or PBS treatment [n=5/group]. Right- Quantification of the percentage and cell numbers of macrophages [CD11b+CD45hi] expressing the indicated marker following oHSV or PBS treatment in the tumor and non-tumor bearing hemisphere [n=5/group]. Quantified data is mean values ± SD.
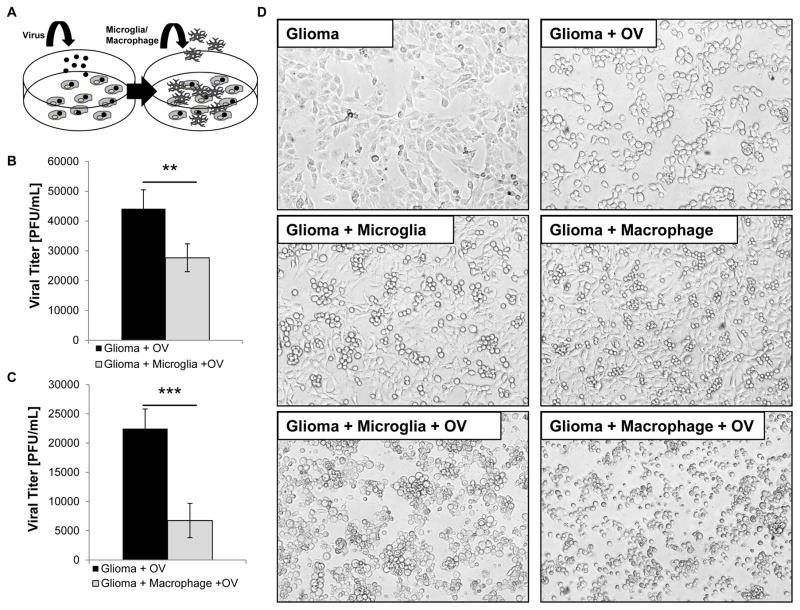
Co-culture of oHSV-Infected Tumor Cells with Microglia or Macrophages Reduces Viral Replication in Vitro
oHSV treatment significantly activated microglia/macrophages in vivo, but the effects of these polarized immune cells on virus replication remained unknown. To determine the functional consequences of this microglia/macrophage activation we developed an in vitro co-culture system. Human glioma cells were infected with oHSV at a MOI of 2, washed to remove unbound virus, and then overlaid with murine macrophages [RAW264.7] or microglia [BV2] [Figure 3A]. To specifically examine the microglia/macrophage response toward infected cells and not towards free virus, the infected cells were cultured for less than 12 hours to prevent the lytic burst of tumor cells and the infection of microglia/macrophages. Compared to infected glioma cells alone, culturing infected cells with microglia or macrophages reduced viral titers by 37.28% and 69.99%, respectively [P<0.01; P<0.001] [Figure 3B–C]. This decrease in virus replication was also accompanied by significant phenotypic changes 12 hours post infection. Uninfected glioma cells were adherent with extensive filopodia. Following infection, these cells became rounded but remained adherent [Figure 3D]. Similarly, uninfected glioma cultured with microglia/macrophages revealed no significant changes in morphology. Interestingly, when infected glioma cells were cultured with macrophages or microglia, the microglia/macrophages surrounded the infected tumor cells and formed tight rosette-like clusters which became non-adherent [Figure 3D; Supplemental Figure S2A–B].
Figure 3.
Microglia and macrophages reduce virus replication in tumor cells in vitro. A. Schematic of microglia/macrophage and tumor cell co-cultures. Tumor cells [oval-shaped cells] were infected with oHSV [rHSVQ1] [black dots] at a MOI of 2. Unbound virus was washed away and microglia or macrophages [branched cells] were overlaid on the infected glioma cells. The cells were cultured for 12 hours and the viral titers were determined by a standard plaque formation assay. B–C Viral titers of glioma cells infected alone or cultured with BV2 microglia [n=4/group] [B.] or RAW264.7 macrophages [n=4/group] [C.]. Data shown is mean virus titer ± SD. D. Representative images of glioma cells cultured with microglia and macrophages with and without oHSV infection 12 hours post infection [n= 4/group].
Macrophage and Microglia Secreted TNFα inhibits virus replication
Culturing infected glioma cells with microglia or macrophages significantly decreased virus replication, but how these cells reduced virus propagation remained to be elucidated. TNFα is a pleiotropic cytokine whose expression is significantly up-regulated in response to viral CNS infections (14, 29). To test if TNFα produced by activated macrophages/microglia could limit viral replication in glioma cells, we determined the levels of TNFα secreted by microglia and macrophages in our co-culture system utilizing a species specific ELISA [Supplemental Figure S3]. Using a murine specific TNFα ELISA, we observed microglia and macrophages produced significant amounts of TNFα in response to infected tumor cells. Compared to uninfected co-cultures, oHSV infection increased macrophage and microglia secreted TNFα by 35.42 and 9.00 fold, respectively [P<0.001; P<0.001] [Figure 4A–B]. Interestingly, we observed a 57.11% and 33.66% decrease in macrophage and microglia secreted TNFα when these cells were cultured with uninfected tumor cells compared to being cultured alone, respectively [Figure 4A–B]. In support of this in vitro data, we also observed a significant increase in murine secreted TNFα in the brain and serum following oHSV treatment in intracranial xenografts [P<0.001; P<0.01, respectively] [Supplemental Figure S4A–B]. This result is concordant with previously published work demonstrating macrophages and microglia produce large amounts of TNFα in response to HSV infection in the CNS (29). Next, we determined if the levels of TNFα produced in these co-cultures were sufficient to reduce virus replication in glioma cells. Treatment of infected cells with 1000 pg/mL or 2000 pg/mL of recombinant human TNFα resulted in 34.29% and 40.73% reductions in virus replication, respectively [P<0.05; P<0.01] [Figure 4C]. Similar results were obtained when infected glioma cells were treated with recombinant murine TNFα [Supplemental Figure S5A]. Visual inspection of infected cells treated with soluble TNFα also revealed surprising morphological changes. oHSV infected glioma cells treated with TNFα became rounded and non-adherent. The cells resembled infected cultures with microglia/macrophages [Figure 4D, Figure 3D and Supplemental Figure S5B]. We did not observe any morphological changes or reductions in cell viability in multiple uninfected glioma cell lines treated with TNFα [Supplemental Figure S6A–B]. Additional experiments with uninfected glioma cells treated with varying doses of TNFα for 60 hours also did not reduce cell proliferation [P<0.001 for all doses] [Supplemental Figure S6C].
Figure 4.
Microglia and macrophage secreted TNFα inhibits virus [rHSVQ1] replication in vitro. A–B. Quantification of TNFα secreted by BV2 microglia [A.] or RAW264.7 macrophages [B.] alone, and when cultured with uninfected or infected U251-T2 glioma cells for 12 hours. Data shown is mean concentration TNFα ± SD. C. Viral titers of glioma cells infected at an MOI of 2 alone, with recombinant human TNFα [1000 or 2000 pg/mL], or with BV2 microglia for 12 hours. Data shown is mean virus titer ± SD D. Representative images of U251-T2 glioma cells infected with oHSV [rHSVQ1] at an MOI of 2 with vehicle [Left] or with TNFα [2000 pg/mL] [Right] for 12 hours. White arrows indicated membrane blebbing in infected cells treated with TNFα. E. Western blot of U251-T2 glioma cells alone, treated with TNFα [5000 pg/mL], infected with oHSV at an MOI of 2, or treated with TNFα and oHSV for 12 hours. Infected U251-T2 glioma cells cultured with BV2 microglia is also shown. Caspase 8, cleaved Caspase 3, Cleaved PARP, and GAPDH are shown. Caspase 8 blot shows full length protein [1], cleaved intermediate protein [2], and active protein [3].
Secreted TNFα induces apoptosis in oHSV infected cells
Macrophage and microglia secreted TNFα significantly reduced virus replication in oHSV infected cells, but the mechanism of TNFα directed virus inhibition remained to be determined. High magnification images of oHSV infected cells treated with TNFα revealed significant changes in cell morphology. Unlike oHSV infected cells alone, the addition of TNFα resulted in significant membrane blebbing [white arrows], cell shrinkage, and a loss of adherence, all features characteristic of cells undergoing apoptosis [Figure 4D]. Based on these observations, we hypothesized TNFα induced apoptosis in infected cells resulting in reduced virus titers. To investigate if TNFα was inducing apoptosis in infected cells, we conducted immunoblot assays for caspase 8, cleaved caspase 3, and cleaved PARP. We observed significant caspase 8, caspase 3, and PARP activation in cells treated with oHSV and TNFα. We did not observe significant activation of these proteins in glioma cells treated with TNFα or oHSV alone [Figure 5E; Supplemental Figure S7].
Figure 5.
Inhibition of microglia/macrophage secreted TNFα increases virus [rHSVQ1] replication in vitro. A. Representative images of U251-T2 glioma cells infected at an MOI of 2 cultured with bone marrow derived macrophages derived from wild-type or TNFα knockout mice for 12 hours. Large representative images are taken at a 4x magnification with the insets taken at a 20x magnification [white arrows indicate blebbing]. B. 12 hour viral titers of cultures described in A. Data shown is mean virus titer ± SD C. Schematic of experimental setup utilizing murine specific TNFα antibodies to block microglia [branched cells] secreted TNFα in co-cultures with infected glioma cells [oval-shaped cells]. D. Quantification of virus titer obtained from infected glioma cells cultured with BV2 microglia with IgG or anti-murine TNFα blocking antibody [1800 ng/ml]. Data shown is mean virus titer ± SD.
Inhibition of macrophage or microglia secreted TNFα increases oHSV replication in vitro
Since macrophage and microglia secreted TNFα reduced virus replication by inducing apoptosis in infected cells, we hypothesized the inhibition of macrophage/microglia produced TNFα would significantly improve virus replication. In order to determine if inhibiting macrophage TNFα was sufficient to rescue virus replication, we conducted co-culture assays with freshly isolated wild type or TNFα knock out [TNFα−/−] bone marrow derived macrophages [BMDM]. Phase contrast microscopy of these cultures revealed significant morphological differences between the two groups. Consistent with our previous results, a majority of the infected glioma cells cultured with wild type BMDMs formed non-adherent clusters and exhibited significant membrane blebbing indicative of apoptosis. In contrast to these observations, infected glioma cells cultured with TNFα−/− BMDMs were adherent and showed substantially less membrane blebbing [Figure 5A]. These observations correlated with changes in virus titers; culturing infected glioma cells with TNFα−/− BMDMs significantly rescued virus replication compared wild type BMDMs [P<0.05] [Figure 5B]. In a similar experiment, we found the addition of murine specific TNFα blocking antibodies rescued the reduction in virus replication in infected glioma cells when cultured with BV2 microglia [P<0.05] [Figure 5C–D].
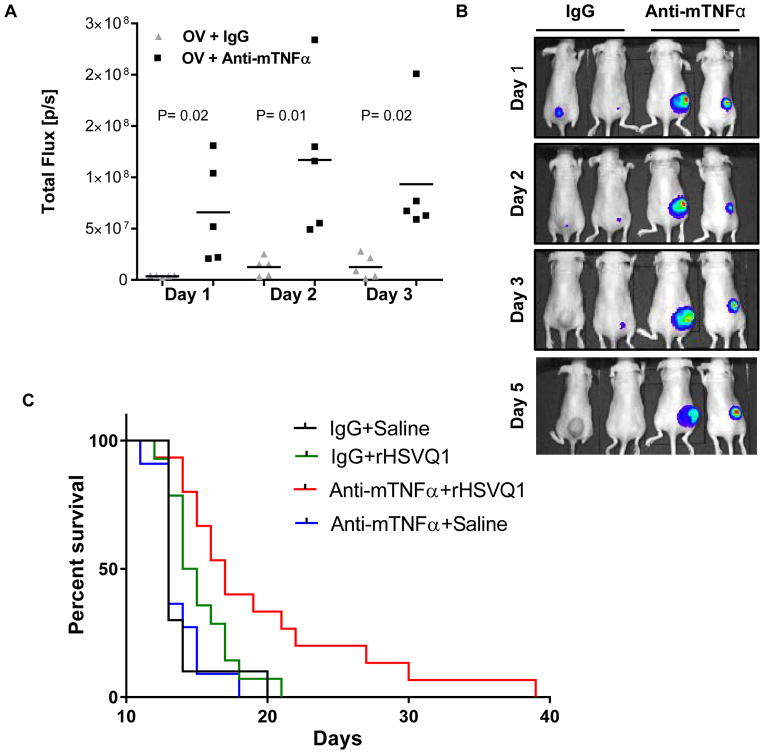
Inhibition of TNFα increases virus replication in vivo
Blockade or knockout of macrophage/microglia secreted TNFα significantly enhanced oHSV replication in vitro. In order to assess the translational relevance of these results for oHSV therapy, we tested if TNFα blockade could enhance virus replication in vivo. In these experiments athymic nude mice were implanted subcutaneously with U87ΔEGFR human GB tumors. When the tumors reached an average volume of 143 mm3 the mice were treated with a single dose of oncolytic virus. Mice were also administered a murine specific TNFα blocking antibody or a control antibody. Mice were given antibody 1 day prior to virus injection, the day of virus administration, and on days 1, 3, and 5 post virus treatment. In these studies, a luciferase expressing oHSV was utilized to visualize virus replication. We observed a significant enhancement in virus propagation in vivo [as measured by luciferase encoded by virus] in mice treated with a TNFα blocking antibody as compared to a control IgG antibody on days 1, 2, and 3 following oHSV administration [n=5/group] [P<0.02; P<0.01; P<0.02, respectively] [Figure 6A–B]. These results suggested the inhibition of TNFα produced by macrophages and the tumor microenvironment was sufficient to increase virus replication in vivo.
Figure 6.
TNFα inhibition increases virus replication and efficacy in vivo. Nude mice with U87ΔEGFR subcutaneous tumors were treated with 1 x 106 pfu of an oHSV expressing luciferase [rHSVQ1-luc]. Murine specific TNFα or isotype control antibodies were administered on days −1, 0, 1, 3, and 5 post oHSV therapy. A. Data shown are quantification of virally expressed luciferase gene activity in U87ΔEGFR subcutaneous tumors treated with control or TNFα blocking antibodies on the days indicated after rHSVQ1-luciferase virus treatment. Data shown is total flux in each mouse [n=5/group]. B. Representative luciferase images of oHSV treated mice with TNFα blocking or isotype control antibodies at the days indicated [n=5/group]. C. Kaplan-Meier survival curve of mice bearing U87ΔEGFR intracranial tumors treated with PBS or 2x105 pfu rHSVQ1 with IgG or TNFα blocking antibody [IgG + Saline n=10; anti-TNFα + Saline n=11; IgG +rHSVQ1 n= 14; anti-TNFα + rHSVQ1 n=15].
Finally, we conducted intracranial GB studies to determine if TNFα blockade could enhance the survival of mice treated with oHSV. In these studies, mice were implanted intracranially with U87ΔEGFR human GB cells and treated with oHSV 8 days later [2x105 pfu rHSVQ1-luc]. The antibody dosing regimen from the subcutaneous tumor experiments was utilized in this study. Mice treated with oHSV and a murine specific TNFα blocking antibody lived significantly than those treated with oHSV and an isotype control antibody [P=0.026], TNFα blocking antibody alone [P=0.0003], or with an isotype control antibody alone [P=0.0003] [Figure 6C]. These results suggested the combination of TNFα blocking antibodies may enhance oHSV therapeutic efficacy for GB.
Discussion
oHSV therapy is a promising treatment modality for GB. The success of oHSV derived therapeutics depends on both the oncolytic destruction of tumor cells and the activation of long-term, anti-tumor immune responses. While the innate immune response is important for activating adaptive responses, the innate responses to oHSV therapy can also inhibit virus replication and oncolytic tumor cell killing. Depletion of macrophages and microglia with clodronate liposomes and CPA has previously been shown to reduce antiviral responses and improve oncolytic virus efficacy for GB (9, 15–20, 30–32). The combination of oncolytic measles virus with CPA is currently being evaluated in a phase I clinical trial for multiple myeloma [ClinicalTrials.gov Identifier: NCT00450814]. Recently, NK cells were shown to help coordinate the innate immune response to oHSV therapy and the depletion of these cells was found to enhance oncolytic virus [OV] efficacy for GB (7). Neutrophils have also been shown to limit OV dissemination in part through the release of neutrophil extracellular traps (33). Collectively, these studies suggest modulating early innate immune responses to achieve the optimal balance between viral replication and inflammation is critical to the clinical success of oHSV therapies.
While microglia and infiltrating macrophages are thought to be the primary mediators of the innate immune response to oHSV infection for GB, the mechanism by which these cells limit virus replication and therapeutic efficacy has not been well studied (16). Here, we quantified the extent of microglia/macrophage activation and infiltration following oHSV treatment. While microglia are the resident immune cells of the CNS, in this study we observed infiltrating macrophages outnumbered microglia more than 2:1 in the tumor microenvironment following oHSV infection. These results suggested monocyte derived macrophages may be the dominant cell type which controls oHSV infection.
While infiltrating macrophages primarily increased in the tumor bearing hemisphere, there was also significant activation and infiltration of immune cells in the contra-lateral hemisphere. These results suggested oHSV infection induced a global inflammatory response in the CNS rather than a localized immune response confined to the tumor. Activation signals such as TNFα are propagated throughout the CNS in response to inflammatory stimuli. While this study focuses on the anti-viral effects of TNFα, in response to virus infection many signals such as IL-1β, IL-6, interferons, and nitric oxide are released in order to control oHSV infection (11). These pro-inflammatory mediators signal in an autocrine and paracrine manner to activate immune cells such as macrophages and enhance their ability to respond to viral infection. oHSV associated inflammation in the non-tumor bearing hemisphere and surrounding healthy brain parenchyma has not been well studied. These observations may have implications in the treatment of brain tumor patients with oHSVs where unchecked inflammation can be detrimental.
In these studies, we observed a significant inflammatory response to viral infection until at least 3 days post treatment. In vivo flow cytometry experiments indicated microglia and infiltrating macrophages were polarized towards an M1, pro-inflammatory state. We demonstrated the anti-viral consequence of microglia and macrophage activation in co-culture studies and found both macrophages and microglia reduced virus replication in glioma cells. Together these results confirmed the anti-viral capabilities of these cell types in modulating oHSV replication in vivo. This data also supports previous studies which identify the anti-viral activity of macrophages and microglia against wild-type Herpes Simplex Virus 1 [HSV-1] infections (34, 35).
In this study, we identified TNFα as a major macrophage/microglia secreted factor which reduces oHSV replication. TNFα is a pleotropic cytokine important for the recruitment and activation of immune cells. Macrophages and microglia are also major producers of TNFα. TNFα is known to limit wild-type HSV replication in the CNS, and it has previously been shown to mediate anti-viral effects in studies with wild-type vesicular stomatitis virus, adenovirus-2, encephalomyocarditis virus, HSV-1, HSV-2, respiratory syncytial virus, and influenza through a variety of mechanisms.(29, 36–43). While TNFα is detrimental to virus replication, TNFα signaling in cancer cells can result in increased tumor cell growth, angiogenesis, invasion, and progression (44–46). Higher levels of the anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 as well as decreased levels of apoptotic proteins such as BAX are commonly observed in recurrent GB and demonstrate the ability of these tumors to resist caspase mediated cell death (47). Consistent with these published studies, we observed TNFα was not toxic to uninfected GB cells in vitro. In infected glioma cells, however, we observed TNFα activated the extrinsic apoptotic pathway resulting in premature cell death, reduced virus replication, and decreased anti-tumor efficacy. The precise mechanism of how the combination TNFα with oHSV induces apoptosis is unclear. While HSV-1 has been shown to inhibit apoptosis, previous work has demonstrated the inability of HSV-1 to prevent apoptosis in infected cells exposed to environmental stimuli such as TNFα (48). TNFα induced cell death was found to be cell-type dependent, and in the case of glioma this process may depend on the expression of pro- and anti-apoptotic proteins within the cells.
Oncolytic HSVs expressing TNFα have previously been tested for their ability to enhance oHSV anti-tumor efficacy (49). In these studies, TNFα expressing viruses did not enhance anti-tumor efficacy in an immune-competent lymphoma model compared to a control oHSV which did not express TNFα. Additionally, in human squamous carcinoma xenografts, the antitumor efficacy of an oHSV expressing high levels of TNFα was significantly less than an oHSV expressing low levels of TNFα. In support of our findings, these results suggest TNFα elicits strong antiviral responses which may be detrimental to oncolytic HSV therapy.
While TNFα blockade lead to increased virus propagation, its effect on toxicity in the context of HSV-1 infections is not clear. Both virus-mediated and immune-mediated mechanisms contribute towards the pathology of HSV-1 infections. In studies with mice infected with wild-type HSV-1, TNFα knockout mice had higher virus titers and were more susceptible to fatal HSV encephalitis than wild type mice. These results highlight the protective, anti-viral functions of TNFα (29, 36). While TNFα is important for controlling virus replication, high levels of TNFα have also been shown to induce blood brain barrier disruption leading to increased inflammation (50). Interestingly, HSV-1 infected mice treated with TNFα blocking antibody showed reduced signs of viral encephalitis and lived longer than those treated with virus alone (51). Thus, a transient blockade of TNFα during virotherapy could increase virus replication and reduce neurotoxicity due to acute inflammation while still allowing for an immune response to eventually clear the infection. Importantly, in our studies we observed no toxicity associated with TNFα antibody administration in combination with our attenuated, oncolytic virus.
Radiation and chemotherapy also induce the production of cytokines such as TNFα (52–54). Oncolytic virotherapy for GB is often administered following tumor resection and concurrently with radiation and chemotherapy. As a result, patients may benefit from the transient use of TNFα inhibitors prior to oHSV administration in order to enhance oncolytic tumor cell killing and reduce CNS inflammation. The TNFα inhibitors etanercept, adalimumab, certolizumab, and golimumab are currently FDA approved for a variety of diseases and could be readily utilized in oHSV clinical trials. These inhibitors may be more effective than general immune suppressants such as high dose myeloablative CPA which can have significant toxicities in patients. The combination of oHSV with TNFα inhibitors could enhance virus replication, reduce TNFα driven tumor proliferation, angiogenesis, and invasion, as well counter the negative effects of chemotherapy/radiation induced inflammation. This transient inhibition of TNFα could then be removed to allow for the activation of long-term, anti-tumor immune responses which may be more potent due to increased virus mediated cell killing and antigen release. In subcutaneous and intracranial tumor studies, we found the inhibition of TNFα secreted by the tumor microenvironment significantly enhanced virus replication and therapeutic efficacy. In these experiments we utilized a TNFα blocking antibody because the current FDA approved TNFα inhibitors are antibody based. While the integrity of the blood-brain-barrier is disrupted in glioblastoma, the ability of therapeutic antibodies to cross the blood-tumor-barrier [BTB] is thought to be limited (55, 56). While we observed up to 9 fold increases in viral luciferase expression in subcutaneous tumors, the therapeutic effect in the intracranial tumor studies was more modest. In addition to antibody penetration into the brain tumor microenvironment following oHSV therapy, we hypothesize the increase in animal survival may have also been through the ability of the antibody to bind TNFα in the serum following oHSV therapy. The future development of specific, soluble TNFα inhibitors which better penetrate the BTB may further increase the anti-tumor efficacy we observed. These experiments support the future use of TNFα inhibitors in combination with oHSV for GB.
Supplementary Material
Translational Relevance.
Glioblastoma is one of the most common and deadly types of primary brain tumors, and patients diagnosed with these tumors have a median survival of only 15 months. Oncolytic herpes simplex viruses [oHSV] represent a promising therapy for glioblastoma, and these viruses are currently being tested in patients for safety and efficacy. Innate immune responses to viral infection are thought to reduce oHSV replication, tumor destruction, and efficacy. In this study, we investigated the anti-viral functions of microglia and macrophages in oHSV therapy for glioblastoma. We identified microglia/macrophage secreted tumor necrosis factor α [TNFα] as a major factor which reduces viral replication through the induction of apoptosis in infected cells. We demonstrated the inhibition of TNFα could significantly enhance virus replication and efficacy in vivo. The results of these studies suggest FDA approved TNFα inhibitors may significantly enhance patient outcomes in oHSV clinical trials.
Acknowledgments
This work was supported in part by: NIH grants R01NS064607, R01CA150153, P30NS045758, P01CA163205 to B. Kaur; and Pelotonia Fellowships to A.C. Jaime-Ramirez and S. Dubin.
Footnotes
No Conflicts of Interest to Disclose
References
- 1.Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer letters. 2013;331:139–46. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Mohyeldin A, Chiocca EA. Gene and viral therapy for glioblastoma: a review of clinical trials and future directions. Cancer J. 2012;18:82–8. doi: 10.1097/PPO.0b013e3182458b13. [DOI] [PubMed] [Google Scholar]
- 4.Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M, et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:1048–55. doi: 10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clinic proceedings. 2014;89:926–33. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6:941–9. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Breckenridge CA, Yu J, Price R, Wojton J, Pradarelli J, Mao H, et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nature medicine. 2012;18:1827–34. doi: 10.1038/nm.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Molecular therapy: the journal of the American Society of Gene Therapy. 2007;15:588–97. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nature medicine. 1999;5:881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 10.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–93. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 11.Lokensgard JR, Cheeran MC, Hu S, Gekker G, Peterson PK. Glial cell responses to herpesvirus infections: role in defense and immunopathogenesis. The Journal of infectious diseases. 2002;186(Suppl 2):S171–9. doi: 10.1086/344272. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–57. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 13.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, et al. Role of microglia in central nervous system infections. Clinical microbiology reviews. 2004;17:942–64. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–26. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer research. 2007;67:9398–406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng KW, Myers R, Greenslade A, Mader E, Greiner S, Federspiel MJ, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene therapy. 2013;20:255–61. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Molecular therapy: the journal of the American Society of Gene Therapy. 2008;16:879–85. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2777–88. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 21.Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–55. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 22.Yoo JY, Haseley A, Bratasz A, Chiocca EA, Zhang J, Powell K, et al. Antitumor efficacy of 34.5ENVE: a transcriptionally retargeted and “Vstat120”-expressing oncolytic virus. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:287–97. doi: 10.1038/mt.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–53. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–61. doi: 10.1097/00006123-200004000-00035. discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 26.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. Journal of neurosurgery. 1979;50:305–11. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 27.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. Journal of neurosurgery. 1979;50:298–304. doi: 10.3171/jns.1979.50.3.0298. [DOI] [PubMed] [Google Scholar]
- 28.Currier MA, Eshun FK, Sholl A, Chernoguz A, Crawford K, Divanovic S, et al. VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:1014–23. doi: 10.1038/mt.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sergerie Y, Rivest S, Boivin G. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. The Journal of infectious diseases. 2007;196:853–60. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- 30.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer research. 2005;65:11255–8. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 31.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Molecular therapy: the journal of the American Society of Gene Therapy. 2006;14:779–88. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. Journal of the National Cancer Institute. 2007;99:1768–81. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 33.Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell host & microbe. 2013;13:169–80. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Morahan PS, Morse SS, McGeorge MG. Macrophage extrinsic antiviral activity during herpes simplex virus infection. The Journal of general virology. 1980;46:291–300. doi: 10.1099/0022-1317-46-2-291. [DOI] [PubMed] [Google Scholar]
- 35.Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–905. [PubMed] [Google Scholar]
- 36.Lundberg P, Welander PV, Edwards CK, 3rd, van Rooijen N, Cantin E. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. Journal of virology. 2007;81:1451–60. doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minami M, Kita M, Yan XQ, Yamamoto T, Iida T, Sekikawa K, et al. Role of IFN-gamma and tumor necrosis factor-alpha in herpes simplex virus type 1 infection. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2002;22:671–6. doi: 10.1089/10799900260100150. [DOI] [PubMed] [Google Scholar]
- 38.Wong GH, Goeddel DV. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature. 1986;323:819–22. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 39.Kulu Y, Kawasaki H, Donahue JM, Kasuya H, Cusack JC, Choi EW, et al. Concurrent chemotherapy inhibits herpes simplex virus-1 replication and oncolysis. Cancer gene therapy. 2013;20:133–40. doi: 10.1038/cgt.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mestan J, Digel W, Mittnacht S, Hillen H, Blohm D, Moller A, et al. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature. 1986;323:816–9. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- 41.Cirino NM, Panuska JR, Villani A, Taraf H, Rebert NA, Merolla R, et al. Restricted replication of respiratory syncytial virus in human alveolar macrophages. The Journal of general virology. 1993;74(Pt 8):1527–37. doi: 10.1099/0022-1317-74-8-1527. [DOI] [PubMed] [Google Scholar]
- 42.Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. Journal of virology. 2002;76:1071–6. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lokensgard JR, Hu S, Sheng W, vanOijen M, Cox D, Cheeran MC, et al. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. Journal of neurovirology. 2001;7:208–19. doi: 10.1080/13550280152403254. [DOI] [PubMed] [Google Scholar]
- 44.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. The American journal of pathology. 1992;140:539–44. [PMC free article] [PubMed] [Google Scholar]
- 45.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer metastasis reviews. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 46.Ryu J, Ku BM, Lee YK, Jeong JY, Kang S, Choi J, et al. Resveratrol reduces TNF-alpha-induced U373MG human glioma cell invasion through regulating NF-kappaB activation and uPA/uPAR expression. Anticancer research. 2011;31:4223–30. [PubMed] [Google Scholar]
- 47.Strik H, Deininger M, Streffer J, Grote E, Wickboldt J, Dichgans J, et al. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. Journal of neurology, neurosurgery, and psychiatry. 1999;67:763–8. doi: 10.1136/jnnp.67.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3931–6. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han ZQ, Assenberg M, Liu BL, Wang YB, Simpson G, Thomas S, et al. Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. The journal of gene medicine. 2007;9:99–106. doi: 10.1002/jgm.999. [DOI] [PubMed] [Google Scholar]
- 50.Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, et al. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. The Journal of pharmacology and experimental therapeutics. 2007;323:488–98. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- 51.Boivin N, Menasria R, Piret J, Rivest S, Boivin G. The combination of valacyclovir with an anti-TNF alpha antibody increases survival rate compared to antiviral therapy alone in a murine model of herpes simplex virus encephalitis. Antiviral research. 2013;100:649–53. doi: 10.1016/j.antiviral.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, et al. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clinical cancer research: an official journal of the American Association for Cancer Research. 1999;5:1517–22. [PubMed] [Google Scholar]
- 53.Kanai R, Rabkin SD, Yip S, Sgubin D, Zaupa CM, Hirose Y, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. Journal of the National Cancer Institute. 2012;104:42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Multhoff G, Radons J. Radiation, inflammation, and immune responses in cancer. Frontiers in oncology. 2012;2:58. doi: 10.3389/fonc.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta neuropathologica. 2000;100:323–31. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 56.Wolburg H, Noell S, Fallier-Becker P, Mack AF, Wolburg-Buchholz K. The disturbed blood-brain barrier in human glioblastoma. Molecular aspects of medicine. 2012;33:579–89. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.