Abstract
Gastrointestinal and vaginal mucosa are major sites of entry in natural HIV infection and therefore the preferred sites to elicit high-avidity CD8+ CTL by vaccination. We directly compare systemic and mucosal immunization in mice after DNA priming and boosting with rgp160 env expressed either in MVA or Ad for their ability to induce mucosal as well as systemic HIV-specific CTL. The optimal CTL response in the gut mucosa was observed after priming with the HIV-1 gp160 env DNA vaccine and boosting with rMVA or rAd encoding the same envelope gene all administered intrarectally (IR). Maximum levels of high-avidity CD8+ T cells were seen in intestinal lamina propria following this regimen. When the prime and boost routes were distinct, the delivery site of the boost had a greater impact than the DNA priming. IM DNA prime and IR rMVA boost were more effective than IR DNA prime and IM rMVA boost for eliciting mucosal CD8+ T-cell avidity. A systemic DNA-prime-followed by systemic rMVA boost induced high levels of high-avidity CD8+ T cells systemically, but responses were undetectable in mucosal sites. A single systemic immunization with rMVA was sufficient to induce high-avidity IFN-γ secreting CD8+ T cells in systemic organs, whereas a single mucosal immunization with rMVA was not sufficient to elicit high-avidity CD8+ T cells in mucosa. Thus, a heterologous mucosal DNA prime-viral vectored boost strategy was needed. The requirement for a heterologous DNA prime-recombinant viral boost strategy for generation of high-avidity CD8+ T cells in mucosal sites in mice may be more stringent than for the induction of high-avidity CD8+ T cells in systemic compartments.
Keywords: Recombinant modified vaccinia virus Ankara, Recombinant adenovirus, Mucosal immunity, CD8+ CTL avidity
Introduction
The AIDS pandemic has claimed over 40 million lives around the world, and vaccination represents a promising approach to the prevention of AIDS, especially in developing countries (Mwau and McMichael, 2003; Nabel et al., 2002; Stover et al., 2007). Mucosal tissues are the major site of natural HIV transmission and the reservoir for HIV replication (Brenchley et al., 2004; Guadalupe et al., 2003; Mehandru et al., 2004; Miller et al., 1989; Veazey et al., 1998; Zhang et al., 1999) and dissemination. It is now well-documented that intestinal CD4+ CCR5+ memory cells are the primary target for the virus and are rapidly depleted in the gut of HIV-infected individuals (Brenchley et al., 2004; Mehandru et al., 2004). The majority of mucosal memory CD4+ T cells coexpress CCR5, the major coreceptor expressed by mucosally transmitted strains of HIV and SIV (Mattapallil et al., 2005). After vaginal SIV challenge, infection is localized to endocervical tissue during the first 2–7 days without dissemination to the systemic circulation (Spira et al.,1996; Zhang et al.,1999). Therefore, it would be desirable to deliver a vaccine that induces resident mucosal CD8+ CTL to rapidly deploy upon infection to limit viral replication and reduce the natural reservoir within local mucosal tissues prior to systemic dissemination (Belyakov et al., 2004a; Belyakov and Berzofsky, 2004; Berzofsky et al., 2004a, 1999). Our previous studies (Belyakov et al., 2001a, 2007a, 2006b) and studies by other groups (Baba et al., 2000; Kaul et al., 2000; Kozlowski et al., 1997), demonstrated a strong precedent for development of a mucosal AIDS vaccine to induce sufficient local immune responses (both mucosal CD8+ CTL and antibodies) to prevent the steady and massive spread of virus from the gut mucosa into the systemic circulation. Current HIV-1 vaccines in Phase I and II clinical trials aim to elicit CD8+ CTL in the hope that they may prevent or reduce initial viral burden following infection and dissemination of virus from mucosal sites into the systemic circulation. An effective T-cell based HIV-1 vaccine would reduce peak viral load in the infected individual and subsequent disease course concomitant with a reduction in transmission rate within the population at risk. Systemic delivery of HIV-1 antigens by DNA prime heterologous viral vector boost regimens has proven immunogenic in humans and capable of eliciting multifunctional CD8+ CTL responses parenterally. Although mucosal responses have been detected following systemic delivery of HIV and SIV vaccines, it is unknown whether mucosal responses generated by systemic delivery of heterologous prime-boost vaccines will prove effective as a defense at mucosal surfaces following infection.
Different forms of heterologous prime-boost vaccines delivered systemically are currently being tested in a number of ongoing preclinical and clinical trials and hold significant promise for eliciting cellular responses and determining the efficacy of T-cell based vaccines (Allen et al., 2000; Amara et al., 2001; Barouch et al., 2000; Belshe et al., 1998; Dale et al., 2006; Gherardi et al., 2003; Hel et al., 2001b; Kent et al., 1998; Neeson et al., 2006; O'Neill et al., 2002; Sharpe et al., 2003; Tritel et al., 2003; Wierzbicki et al., 2002; Wille-Reece et al., 2006). Heterologous prime-boost immunization is a proven strategy that elicits high frequencies of effector and memory T cells, and in particular high-avidity CD8+ CTL that are relevant to vaccine efficacy (Alexander-Miller et al., 1996; Belyakov et al., 2007a, 2007b, 2006b; Derby et al., 2001; Estcourt et al., 2002; Gallimore et al., 1998; Masopust et al., 2006; Ranasinghe et al., 2007). A major question of current vaccine trials is whether the timing and kinetics as well as the magnitude and quality of the response in vaccinated individuals is sufficient to have an impact on initial virus replication in the gut and/or subsequent levels of viral load in peripheral blood.
We have attempted to address this question experimentally by mucosal immunization in small animal models and SHIV protection studies in rhesus macaques (Belyakov et al., 1998a, 2000, 2001a, 2007a, 2006b). In previous studies, we demonstrated that mucosal vaccination of rhesus macaques with an HIV/SIV peptide vaccine was more effective than a systemically delivered vaccine at clearing virus following intrarectal SHIV challenge from the major reservoir of SHIV replication in the intestinal mucosa (Belyakov et al., 2001a). Recently, we showed that a mucosal vaccine capable of inducing high levels of high-avidity mucosal CTLs can delay the appearance of acute-phase peak viremia in macaques challenged intrarectally with pathogenic SHIV, and this outcome correlated better with the magnitude of high-avidity mucosal CTLs than with systemic CTLs (Belyakov et al., 2006b). These data suggest a direct role for resident mucosal effector CD8+ CTL in reducing initial virus burden and altering subsequent disease course following mucosal infection. Furthermore, the preservation of CD4+ T cells in intestinal lamina propria and the reduction of virus in the gut correlated better with high-avidity mucosal CTL induced by the mucosal vaccine (Belyakov et al., 2007a).
These results provide support for the development of mucosal vaccines for HIV-1 that induce local mucosal high-avidity CD8+ CTL. A direct comparison of different vaccine regimens to induce the most optimal high-avidity CD8+ T-cell response in mucosal sites has not been carried out. In this study, we find that the generation of a high level of systemic high-avidity CD8+ T cells is less dependent on a heterologous prime-boost regimen and that a single immunization with a recombinant viral vector is sufficient to induce high levels of systemic high-avidity CD8+ T cells. Systemic prime-boost immunization significantly improved the magnitude of the CD8+ T-cell response but not the quality of these responses. In contrast, for mucosal immunization, the heterologous prime-boost strategy was more critical, at least in mice, in order to induce high levels of mucosal high-avidity CD8+ T cells. Interestingly, a DNA vaccine given intramuscularly can also prime for a mucosal boost. In the current study we attempted to address the avidity issue experimentally by comparing mucosal versus systemic prime-boost immunization in small animal models. However, some additional pre-clinical study in the SIV model in macaques is needed prior to translation of our findings to an HIV clinical trial.
Results
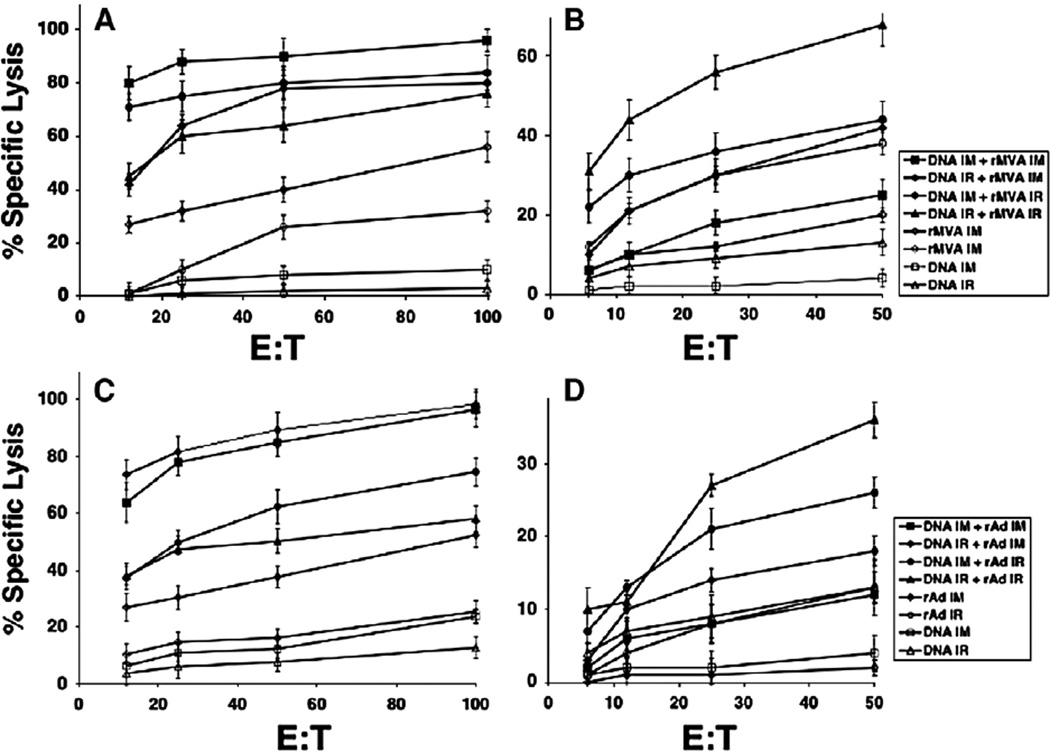
To examine the influence of mucosal compared to systemic prime-boost immunization on development of functional CTL responses, we tested four different prime-boost immunization strategies in a DNA vaccine prime and rMVA or rAd boost, both encoding the HIV Env B protein, for their effectiveness at inducing mucosal (Peyer's patch) as well as systemic (spleen) HIV-specific CTL in mice. DNA priming immunizations and recombinant vector boosts (rMVA or rAd) were administered intrarectally (IR) or intramuscularly (IM) in combinations, including 1) DNA and recombinant vector IM; 2) DNA IR and recombinant vector injected IM; 3) DNA IM and recombinant vector injected IR; 4) DNA and recombinant vector IR. Two additional groups of animals received either the DNA immunization only or a single immunization with recombinant virus. We used 3 consecutive DNA immunizations (50 µg/per mouse per immunization) with 3 weeks interval between each immunization and boosted mice with rMVA (107 pfu) (Figs. 1A, B) or rAd (1010 pfu) (Figs. 1C, D). We found that the systemic prime-boost approach for either virus (rMVA or rAd) induced a high P18-I10 CTL response in the spleen (Figs. 1A, C). However, the HIV-specific CTL response in Peyer's patches of immunized animals was very low after systemic (IM) DNA-MVA prime-boost immunizations (Fig. 1B). Similar results were observed when rAd was used for boosting the DNA prime (Fig. 1D). An intrarectal DNA prime followed by a systemic (IM) MVA or rAd boost induced very high CTL responses in the spleen and a more modest CTL response in Peyer's patches (Fig. 1). Similar to results observed in other studies DNA immunization alone was not effective at eliciting a significant CTL response, and a single immunization with either recombinant vaccinia or adenovirus elicited comparable and weak CTL responses in both spleen and Peyer's patches. Thus, the heterologous prime-boost strategy was effective at eliciting strong CTL in both sites; however, delivery of either the prime or the boost through the mucosal route improved the mucosal response. When the prime and boost routes were distinct, in the majority of cases, the route of the boost had more impact than the route of DNA priming. Thus, IM-IR was more effective than IR-IM for PP responses (p<0.05) (Figs. 1B, D), whereas the opposite was true for splenic responses (p<0.05) (Figs. 1A, C). Therefore, it appears that DNA can cross-prime for a boost by either route.
Fig. 1.
The optimal CD8+ CTL response in the gut mucosa was observed after priming with DNA vaccine and boosting with HIV-1 rMVA or rAd both through the intrarectal route. Groups of BALB/c mice (five per group) were primed IM or IR with HIV DNA 3 times at three-week intervals between each immunization and boosted with HIV-1 rMVA (A, B) or HIV-1 rAd (C, D). Three weeks after the last immunization, SP (A, C) and PP (B, D) cells were stimulated in vitro for 7 days before assay. Cytolytic activity of CTL was measured by a 4-hr assay with 51Cr-labeled targets. 51Cr-labeled P815 targets were tested in the presence or absence of P18-I10 peptide (1 µM). E:T 100:1 ratio means 100 effectors to 1 target. Negative control values were subtracted from experimental lysis. SEM of triplicate cultures were all <5% of the mean.
The optimal CTL response in the gut mucosa was observed when both priming with HIV DNA vaccine and boosting with recombinant MVA were delivered through the intrarectal route (Fig. 1B; solid triangles). Interestingly, although the mucosal CTL response was also optimized when the DNA prime and rAd boost were both delivered intrarectally, the response was significantly less (p<0.01) than that obtained with the DNA prime-recombinant MVA boost (Fig. 1B vs D; filled triangles). It is important to note that a mucosal prime-boost strategy with either recombinant virus also induced a significant, P18-I10-specific CTL response in the systemic circulation as well (Figs. 1A, C; filled triangles) albeit less than a DNA prime viral vector boost delivered by the same IM route. If extrapolatable to humans, these results suggest that a heterologous prime-boost strategy delivered through the mucosal route may be valuable to induce optimal mucosal immunity available as a first line of defense to limit replication of the virus.
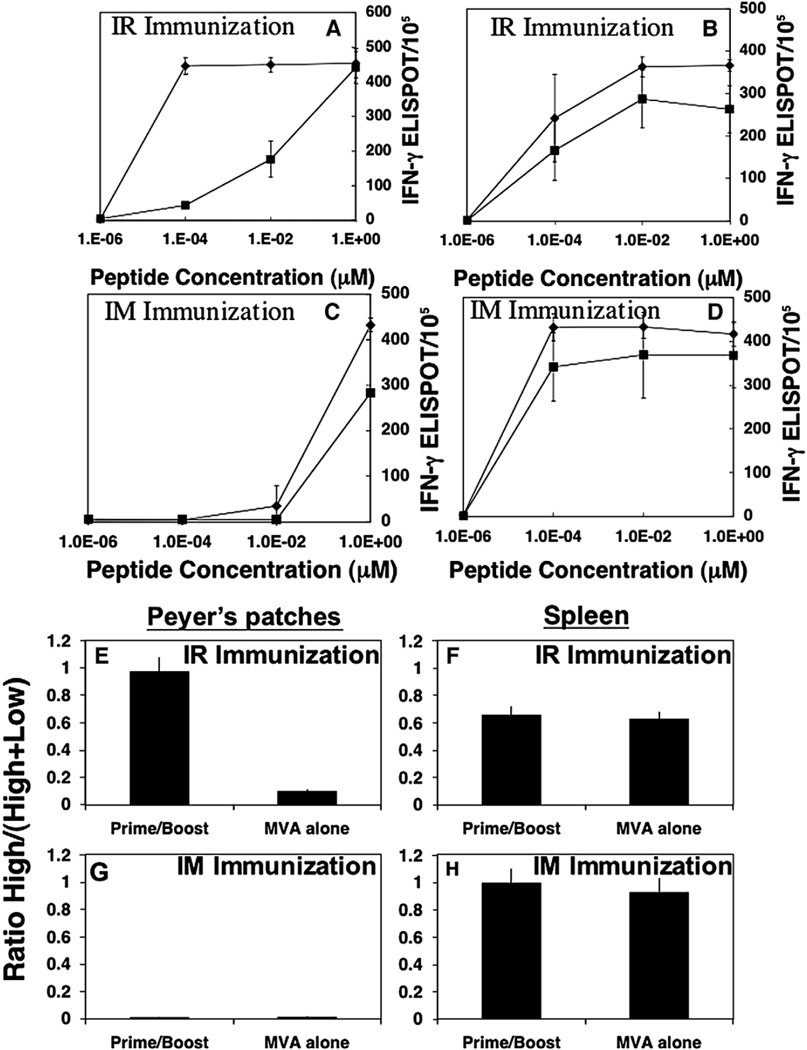
Because we found similar patterns of immunity with both rMVA and rAd vector boosts, we carried out subsequent studies only with rMVA as a boost. Since we and other groups have shown in several different challenge models that high-avidity CTL are most effective at clearing viral challenge in vivo (Alexander-Miller et al., 1996; Belyakov et al., 2007a, 2006b; Derby et al., 2001; Gallimore et al., 1998; Ranasinghe et al., 2007), we examined the influence of mucosal prime-boost vs systemic prime-boost immunization on the development of CD8+ T-cell avidity in mucosal and systemic compartments. We compared the IR to the IM route of immunization for induction of high-avidity CD8+ T cells following a DNA prime and rMVA boost. IR prime-boost immunization induced a higher proportion of high-avidity IFN-γ secreting CTLs in the GALT that could be activated at low P18-I10 peptide concentration compared with IM immunized animals in the same compartment (Fig. 2A vs C; filled diamonds). A significant number of IFN-γ CD8+ CTLs in Peyer's patches after IR prime-boost immunization were observed after activation with high (1 µM) and low (0.0001 µM) concentrations of P18-I10 peptide (Fig. 2A). IR immunization with rMVA alone (filled squares) induced a comparable number of IFN-γ CD8+ T cells in Peyer's patches against the high concentration of peptide (1 µM), but few cells responded by production of IFN-γ to the lower concentration of P18-I10 peptide (0.0001 µM) indicative of high-avidity CD8+ T cells (Fig. 2A). Thus, the mucosal DNA prime-viral vectored boost strategy was necessary for the generation of high-avidity CD8+ T cells in mucosal sites, whereas a single mucosal immunization with the rMVA alone was not sufficient to produce the high-avidity CD8+ T-cell profile. In contrast, the necessity of the DNA prime in a heterologous prime-boost strategy was less apparent for induction of high-avidity CD8+ T cells in the spleen, when the heterologous prime-boost was delivered by the intrarectal route (Fig. 2B). There was no significant difference in the peptide dose response curves seen in the spleen after IR prime-boost immunization or rMVA immunization alone (p>0.05) (Fig. 2B). The magnitude of the CD8+ immune responses in the spleen was slightly greater when animals were given an IR prime-boost compared to a single rMVA immunization (Fig. 2B), but the quality of response was similar in that both groups responded with equal avidity to the lower concentrations of peptide. In marked contrast to the mucosally immunized animals, neither DNA prime rMVA boost nor rMVA alone delivered by the IM route was capable of inducing high-avidity CD8+ T cells at the mucosal site (Fig. 2C). IM prime-boost and rMVA immunization alone induced predominantly low-avidity CD8+ T cells in Peyer's patches. High-avidity systemic (spleen) responses were robust after IM immunization regardless of whether a prime-boost strategy or a single rMVA immunization strategy was employed. The magnitude of CD8+ IFN-γ secreting cells responsive to nanomolar concentrations of P18-I10 peptide was equal in both cases (Fig. 2D). Thus, even a single IM immunization with rMVA is sufficient to induce high-avidity CD8+ T cells in the spleen. Further, the avidity in the spleen was slightly higher after IM than IR immunization. Thus, although it is not surprising that a heterologous prime-boost immunization is more effective than MVA alone, it was not previously appreciated that this difference is much more dramatic after mucosal immunization than after systemic immunization.
Fig. 2.
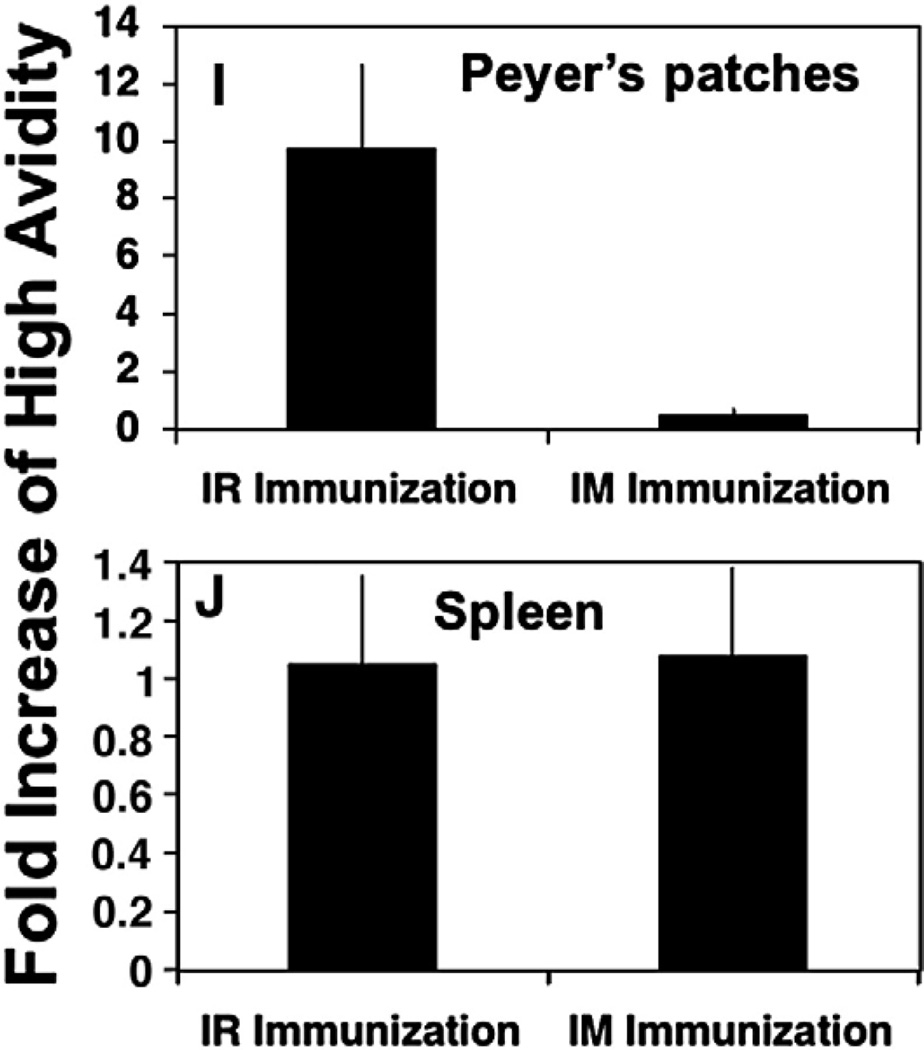
Mucosal prime-boost immunization is essential for high-avidity CD8+ T cells in inductive mucosal sites (Peyer's patches), whereas a single systemic immunization with recombinant vector was sufficient to induce high-avidity CD8+ T cells in systemic sites. (A) CD8+ T-cell activity measured as IFN-γ-producing cells by ELISPOT as a function of peptide concentration for HIV-1 P18-I10 peptide in the PP after IR DNA prime and rMVA boost (closed diamond) or rMVA alone (closed square). T cells were activated with different concentrations of P18-I10 peptides (1, 0.01, 0.0001 µM and no peptide, as indicated). (B) IFN-γ-producing cells as a function of peptide concentration for HIV-1 P18-I10 peptide in the SP after IR immunization with DNA prime and rMVA boost (closed diamond) or rMVA alone (closed square). (C) IFN-γ producing cells as a function of peptide concentration for HIV-1 P18-I10 peptide in the PP after IM immunization with DNA prime and rMVA boost (closed diamond) or rMVA alone (closed square). (D) IFN-γ producing cells as a function of peptide concentration for HIV-1 P18-I10 peptide in the SP after IM immunization with DNA prime and rMVA boost (closed diamond) or rMVA alone (closed square). Error bars represent SD of four mice/group. Ratio high/(high + low) CD8+ T-cell avidity (E–H). (E, G) PP; (F, H) SP. Ratio of the number of IFN-γ+ cells against 0.0001 µM peptide (high-avidity T cells) to the number of IFN-γ-producing cells against 1 µM peptide (total low- and high-avidity T cells). Prevalent high-avidity CD8+ T cells generated after prime-boost immunization, whereas recombinant vector alone was not effective in induction of functionally active CD8+ T cells in mucosal sites (I, J). To quantify the improvement in CD8+ T-cell avidity in Peyer's patches and spleen after prime-boost mucosal vs systemic immunizations, we calculated the fold difference in the avidity ratio after prime-boost immunization vs immunization with rMVA alone in Peyer's patches (I) or spleen (J) after IR vs IM immunizations. rMVA alone was delivered IR for mucosally immunized animals and IM for systemically immunized mice.
To quantify the CD8+ T-cell avidity differences among the groups immunized through different routes in a prime-boost strategy, we calculated the ratio (%) between the number of IFN-γ secreting cells against a low (0.0001 µM) P18-I10 peptide concentration (reflecting high-avidity CD8+ T cells) and the number of IFN-γ secreting cells against a high (1 µM) concentration (reflecting both high- and low-avidity CD8+ T cells) for the CD8+ T cells to the P118-I10 epitope (Figs. 2E–H). A higher ratio indicates the skewing of CD8+ T cells toward high-avidity cells, whereas a lower value indicates skewing toward low-avidity cells. A striking difference was seen in the avidity ratio in Peyer's patches after the IR prime-boost regimen compared to IR rMVA immunization alone (p<0.001) (Fig. 2E). Greater than 95% of antigen-specific cells detected in the Peyer's patch were high avidity following the IR prime-boost strategy whereas only 10% of the total responding cells were high avidity in animals immunized with rMVA alone. In contrast, none of the antigen-specific CD8+ IFN-γ secreting cells detected in the mucosa (PP) following the IM prime-boost immunization regimen were high-avidity CD8+ T cells (Fig. 2G). The avidity ratio in spleen after IR immunization with prime-boost was similar to the avidity ratio after IR immunization with rMVA alone (p>0.05) (Fig. 2F). This fact indicates that the generation of high-avidity CD8+ T cells in mucosal compartment can be highly improved by the DNA prime-boost regimen, whereas generation of high-avidity CD8+ T cells in systemic lymphoid tissue does not appear to require DNA priming. In contrast to the PP noted above (Fig. 2G), a high-avidity ratio was observed in the spleen after IM prime-boost immunization or immunization with rMVA alone (Fig. 2H).
To quantify the improvement in CD8+ T-cell avidity in Peyer's patches and spleen after prime-boost strategy after mucosal vs systemic immunizations, we calculated the fold difference in the avidity ratio after prime-boost immunization vs immunization with rMVA alone in Peyer's patches or spleen after IR vs IM immunizations. CD8+ T-cell avidity in Peyer's patches was increased about 10-fold after IR prime-boost immunization compared to IR immunization with rMVA alone (p<0.01) (Fig. 2I). There were no increases in CD8+ T-cell avidity in Peyer's patches after IM prime-boost immunization compare to IM immunization with rMVA alone (Fig. 2I). Thus, the mucosal prime-boost regimen is clearly more important for generation of high-avidity CD8+ T cells in mucosal sites. However, in the spleen, IM and IR prime-boost immunizations were not significantly more effective in generation of high-avidity CD8+ T cells than rMVA immunization alone (p>0.05) (Fig. 2J).
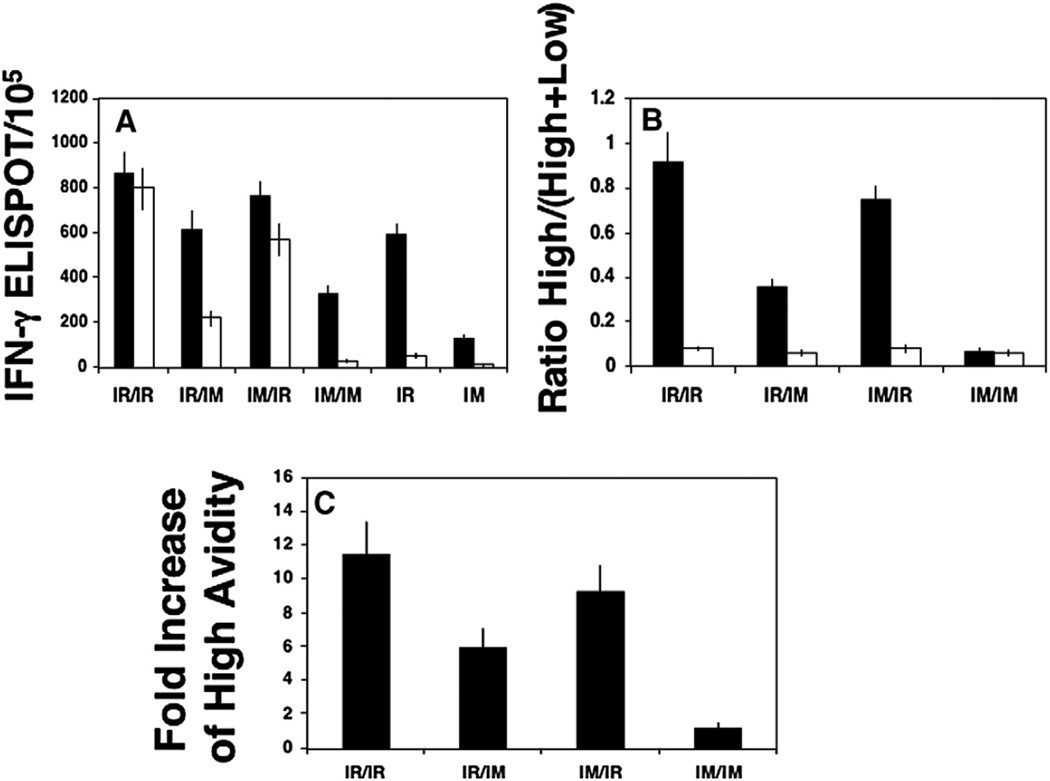
Since we observed that the IR prime-boost regimen was optimum for inducing high-avidity CD8+ T cells at inductive sites of the GALT (Peyer's patches) we next asked the question whether IR prime-boost and mismatched routes of prime-boost immunization also induced a higher proportion of high-avidity IFN-γ secreting CD8+ T cells in the lamina propria as well, since these cells would need to continuously disseminate into the effector site of the GALT to confront an incoming viral infection. To examine CD8+ T cell avidity in the effector site of the mucosa after IR prime-boost, IR prime-IM boost, IM prime-IR boost and IM prime-boost immunizations versus animals immunized with rMVA alone, we evaluated the number of IFN-γ secreting cells in intestinal lamina propria (LP) after activation with high (1 µM) and low (0.0001 µM) concentration of P18-I10 peptide (Fig. 3A). Similar results to those obtained in Peyer's patch (inductive sites) were found. Whereas most antigen-specific effector cells in the lamina propria were high avidity following IR prime-boost, less than 10% of effector cells in the lamina propria induced through the IM prime-boost strategy were high avidity (Fig. 3A). When the prime-boost routes were mismatched, the IR route of the boost rather than the prime had the largest impact on CD8+ T-cell avidity in gut mucosa (Fig. 3A). Thus, IM DNA prime and IR rMVA boost was more effective in generation of high-avidity CD8+ T cells in the lamina propria than IR DNA prime and IM rMVA boost (Fig. 3A). However, this demonstrates surprisingly that even IM DNA priming can cross-prime for high-avidity CD8+ T cells in lamina propria (Fig. 3A). No significant generation of high-avidity CD8+ T cells was observed in the lamina propria after IR immunization with rMVA alone or after IM prime-boost or rMVA alone immunizations (p>0.05) (Fig. 3A). Thus, generation of high-avidity CD8+ T cells in inductive and effectors sites of the intestinal mucosa appears to be highly dependent upon a DNA prime-heterologous boost strategy of immunization.
Fig. 3.
Mucosal prime-boost immunization is most effective for high-avidity CD8+ T cells in a mucosal effector site (lamina propria), whereas IR immunization with recombinant vector alone is not sufficient. (A) CD8+ T-cell activity measured as IFN-γ-producing cells by ELISPOT as a function of peptide concentration for HIV-1 P18-I10 peptide in the LP after IR immunization with DNA prime and rMVA boost or rMVA alone. T cells were activated with 1 µM (closed bar) or 0.0001 µM (open bar) concentration of P18-I10 peptides. (B) Ratio of the number of IFN-γ+ cells against 0.0001 µM peptide (high-avidity T cells) to the number of IFN-γ-producing cells against 1 µM peptide (total low- and high-avidity T cells) after IR or IM immunizations. The avidity ratio in LP after prime-boost immunization — closed bar and rMVA immunization alone — open bar. (C) Fold increase in the avidity ratio after prime-boost (IR/IR and IM/IM) and criss-cross (IR/IM and IM/IR) immunizations vs immunization with rMVA alone in lamina propria. rMVA alone delivered IR or IM and depending on the route of boost in the group of comparison. For IR/IR and IM/IR groups, rMVA was injected IR and for IM/IM and IR/IM, it was given IM.
To quantify this CD8+ T-cell avidity difference in the lamina propria after each prime-boost strategy vs immunization with rMVA alone, we calculated the ratio (%) between the number of IFN-γ secreting cells against a low P18-I10 peptide concentration and the number of IFN-γ secreting cells against a high concentration of the P118-I10 epitope (Fig. 3B). The avidity ratio in LP was significantly higher after IR prime-boost immunization compared to criss-crossed routes, IM prime-boost immunizations or IR rMVA immunization alone (p<0.01) (Fig. 3B). CD8+ T-cell avidity in lamina propria were increased about 12 fold after IR prime-boost immunization, compared to IR immunization with rMVA alone (Fig. 3C). IM prime-IR boost increased lamina propria CD8+ T-cell avidity about 9 fold, whereas IR prime-IM boost increased it about 6 fold (Fig. 3C). However, IM prime-boost vaccination was not sufficient to improve CD8+ T-cell avidity in the effector site of the intestinal mucosa (Fig. 3C). Thus, in contrast to the systemic immune response, generation of functionally active HIV-1-specific CD8+ T cells in the gut mucosa more stringently required the mucosal prime-boost or IM DNA prime and IR rMVA or rAd boost strategy of immunization.
Discussion
The regimen of immunization for generation of functionally active and effective CD8+ CTL responses in mucosal and systemic sites is not well understood (Belyakov et al., 2004a, 2007a; Tatsis et al., 2007). The current study demonstrates that in mice mucosal prime and boost immunizations are more stringently required for the induction of high-avidity CD8+ T-cell responses in inductive and effector sites of gastrointestinal mucosa than for systemic immune responses. Intrarectal immunization with rMVA alone was not effective in generation of the most functional CD8+ T cells in the gut mucosa, whereas high-avidity CD8+ T cells were observed in the spleen after single IR or IM immunizations with rMVA. Thus, we find that an IR prime-boost or IM prime-IR boost strategy is most effective for induction of high-avidity mucosal CD8+ T cells as well as for the magnitude of immune responses at mucosal sites, whereas at systemic sites MVA alone is sufficient to induce high-avidity CD8+ T cells and the prime-boost immunization is important primarily for the magnitude of the immune responses.
Generation of HIV-1-specific CD8+ CTL responses by vaccines may facilitate efficient control of HIV or SIV replication (Amara et al., 2001; Barouch et al., 2000; Shiver et al., 2002). Different combinations of homologous and heterologous prime-boost vaccines are currently being tested in multiple experiments and clinical trials for AIDS, other infectious diseases and cancer and hold significant promise (Ahlers et al., 2003; Allen et al., 2000; Amara et al., 2001; Barouch et al., 2000; Belshe et al., 1998; Berzofsky et al., 2004b; Dale et al., 2006; Gherardi et al., 2003). A sequential vaccination regimen of priming with DNA and boosting with recombinant viral vaccines encoding multiple HIV antigens (Hanke et al., 1998) has proven successful in eliciting robust CTL responses in multiple animal models as well as humans. The prime-boost strategy is capable of inducing broad and high levels of T-cell immunity and ameliorating SIV infection in macaques (Amara et al., 2001; Barouch et al., 2000; Belyakov et al., 2006b; Neeson et al., 2006; Shiver et al., 2002). It has also been shown that a heterologous prime-boost immunization is more effective than a homologous prime-boost vaccination in the magnitude of the T-cell response, but the responses did not always correlate with protection. Recently we evaluated the efficacy of systemic immunization with two human ALVAC-HIV-1 recombinant vaccines expressing Gag, Pol, and gp120 or Gag, Pol, and gp160 in a prime-boost protocol with their homologous vaccine native Env proteins (Pal et al., 2006). The protective efficacy was studied after mucosal challenge with a high-dose of pathogenic neutralization-resistant variant SHIV(KU2) (Pal et al., 2006). Systemic immunization with both vaccine regimens decreased viral load levels not only in blood but unexpectedly also in mucosal sites and protected macaques from peripheral CD4+ T-cell loss (Pal et al., 2006). However, in this study the efficacy of mucosal versus systemic prime-boost regimen was not evaluated.
Plasmid DNA vaccines and rMVA and rAd vaccines are promising HIV-1 vaccine candidates, although delivering either vaccine alone may be inadequate to induce sufficient CD8+ CTL responses for effective viral control (Amara et al., 2001; Barouch et al., 2000; Shiver et al., 2002). Very little data exist on the relative immunogenicity of rMVA or rAd vectors at mucosal sites when administered via mucosal routes or injected by different regimens (with or without DNA prime). Casimiro et al. evaluated several DNA vaccine formulations, a rMVA vector, and a replication-defective adenovirus serotype 5 vector, each expressing the same codon-optimized HIV-1 gag gene for immunogenicity in rhesus monkeys (Casimiro et al., 2003). It was observed that the DNA vaccine primed T-cell responses most effectively and provided the best overall immune responses after boosting with Ad5-gag (Casimiro et al., 2003). However, the mucosal immune responses after different regimens of immunizations with different recombinant vectors have not been compared. rAd and rMVA vectors were used in prime-boost vaccine regimens to address the effect of repeated immunizations on transgene product-specific CD8+ cell frequencies in systemic (spleen, blood, lymph nodes, and peritoneal lavage) and mucosal (mesenteric lymph nodes, intestinal epithelium, and Peyer's patches) lymphoid tissues (Tatsis et al., 2007). It was found that multiple dose vaccine regimens have increased functionally active transgene-specific T cells and improve the levels of activated T cells in the gut mucosa (Tatsis et al., 2007). The recent study demonstrates that a single oral immunization with a chimpanzee derived adenoviral vector expressing HIV gag was inefficient at eliciting responses in the mesenteric lymph nodes and Peyer's patches, while a single intramuscular administration elicited strong systemic and detectable mucosal responses (Lin et al., 2007). However, the different routes of mucosal vaccination (IR route for example) and functional avidity of mucosal CD8+ T cells were not characterized in this study. Our study demonstrated that a mucosal prime-boost or intramuscular DNA mucosal vector boost with rMVA or rAd in which the DNA priming is mucosal or systemic but the viral vector boost is mucosal, is effective in generation of mucosal HIV specific CD8+ CTL in gut mucosal sites. However, IM vaccination was less effective in induction of antigen-specific CD8+ T cells in the intestine.
Optimal routes and numbers of vaccinations for generation of functionally active and effective CD8+ CTL are not well understood especially for mucosal CTL. A recent study by Masopust et al. demonstrated that memory T-cell phenotype differed substantially depending on the number of immunizations. Prime-boost vaccination strategy resulted in the generation of effector memory CD8+ T cells with preferential accumulation in nonlymphoid tissues (Masopust et al., 2006). In another prime-boost study by Evans et al. (2003) it was shown that oral immunization with recombinant Salmonella and boosting with rMVA induced a high level of the intestinal homing receptor α4β7 on peripheral blood lymphocytes and improved control of virus replication following a rectal challenge with SIVmac239. Thus, protection against rectal or vaginal challenges was dependent on robust mucosal immunity, and mucosal routes of vaccination generated these protective immune responses.
Our results suggest several possible explanations for the efficacy of criss-crossed prime-boost. Mixed prime-boost immunization strategies may activate T cells in mucosal inductive sites with different efficacy with acquisition of different levels of expression of the integrin α4β7 and the chemokine receptor CCR9. These molecules are important for their subsequent localization to the small intestine. DCs are important for the induction of tissue tropic effector T-cell subsets (Johansson-Lindbom and Agace, 2007). For example, MLN or PP DC's are necessary and sufficient for the generation of CCR9+α4β7+CD62L− gut-homing T cells in vitro and mucosal DCs (not systemic DC) were able to convert dietary vitamin A to retinoic acid, which in turn induced T-cell expression of CCR9 and α4β7 (Iwata et al., 2004; Johansson-Lindbom and Agace, 2007; Saurer et al., 2007). After systemic DNA prime, a strong mucosal boosting immunization (with rMVA vector) may provide TLR ligands that activate mucosal DCs and induce a strong proliferation of T-cell precursors derived from systemic DNA priming or result in release of chemokines that attract T cells to the mucosal site. Also a strong rMVA boosting will help migration (“migratory gut environment”) of activated T cells from inductive lymphoid tissues to the effector non-lymphoid mucosal sites. In any case, with the recognized need for both mucosal and systemic CTL, a criss-cross heterologous prime-boost strategy may be an effective way to achieve both goals.
Functional impairment of antigen-specific CD8+ CTL has been associated with clinical AIDS progression (Acierno et al., 2006; Appay et al., 2000; Hel et al., 2001a; McKay et al., 2002). CD8+ CTL that can be activated by low concentrations of peptide are defined as high-avidity CD8+ CTL, while those that recognize peptide/MHC only at high antigen concentration are termed low-avidity CD8+ CTL (Alexander-Miller et al., 1996; Belyakov et al., 2006b; Snyder et al., 2003). It is very clear now that high-avidity CD8+ CTL are essential for elimination of tumor (Yee et al., 1999; Zeh et al., 1999) and more effective in vivo in protection against viral infection (Alexander-Miller et al., 1996; Belyakov et al., 2007a, 2006b; Derby et al., 2001; Estcourt et al., 2002; Gallimore et al., 1998). Recently we demonstrated that a mucosal prime-boost vaccine inducing high levels of high-avidity mucosal CTLs can have an impact on dissemination of intrarectally administered pathogenic SHIV-ku2 and such protection correlates better with mucosal than systemic CTLs and particularly with levels of high-avidity mucosal CTLs (Belyakov et al., 2006b). In a recent study by Ranasinghe et al. the avidity of HIV-specific CD8+ CTL generated by mucosal and systemic poxvirus prime-boost vaccines were evaluated. It was demonstrated that mucosal HIV-1 recombinant homologous poxvirus prime-boost immunization induces high-avidity CD8+ T cells with high expression of granzyme B mRNA in genital mucosa (Ranasinghe et al., 2007). The I.N. prime-I.M. boost group elicited a higher number of CTL-expressing granzyme B mRNA from the genitomucosal sites compared with the I.M. prime-boost regime. Also CTL generated after both I.N. or I.M. vaccination expressed Th2 cytokine IL-4 mRNA, although lower numbers were observed after I.N. prime-boost immunization (Ranasinghe et al., 2007). Similarly we found an influence of the route of immunization on generation of functionally active CD8+ T cells (Belyakov et al., 2007a). We compared the effect of mucosal and systemic immunization for induction of high-avidity CD8+ CTL at the local sites of immunization vs distally in mice and macaques (Belyakov et al., 2007a). The optimum vaccine strategy for the induction of high-avidity CTL at both mucosal and systemic sites has to our knowledge never been demonstrated before, and now requires some additional pre-clinical testing in SIV model in macaques.
The current study demonstrates that, for generation of functionally active mucosal CD8+ T cells in mice, a heterologous mucosal prime-boost regiment is most effective. A single mucosal immunization with a recombinant vector is not sufficient for induction of a high level of high-avidity CD8+ T cells in the intestine, whereas it is at systemic sites. The requirement for a heterologous prime-boost strategy for generation of high-avidity CD8+ T cells in mucosal sites is much more stringent than for induction of high-avidity CD8+ T cells in systemic lymphoid tissues. Further, we find that systemic DNA priming is sufficient to induce high-avidity mucosal CD8+ T cells if the heterologous viral vector boost is delivered mucosally, in a criss-cross strategy.
Materials and methods
Mice
Female BALB/c mice were purchased from the Frederick Cancer Research Center (Frederick, MD).
Recombinant viruses
MVA/HXB2env was constructed as follows: the HXB2 env gene, truncated by 104 amino acids to yield a C-terminal sequence of SIRLVNGS, was cloned into shuttle plasmid pLW24. This plasmid directs recombination into deletion III of MVA utilizing the P7.5 early/late promoter. The recombinant modified vaccinia Ankara (rMVA) was produced using standard techniques (Earl et al., 1998; Wyatt et al., 2008). Live immunostaining using rabbit sera R2144 produced against HIV env, was used for plaque screening.
Recombinant adenovirus (rAd) was constructed as follows: the clade B Env cDNA is a gp140 chimera derived from HXB2 with transposition of the V3 region from HIV-1 Bal with mutations in the cleavage site, fusion peptide, and interhelical domain (DCFI) and prepared as previously described (Chakrabarti et al., 2002). The insert was introduced into a rAd5 vector and prepared by standard methods (Huang et al., 2001; Letvin et al., 2004). Virus was purified by cesium chloride centrifugation and stored at 1010 particles per ml in phosphate-buffered saline.
Vaccine and immunizations
Six groups of 5 BALB/c mice per group were immunized in different combinations by different routes in a heterologous DNA prime viral vector boost strategy. Group 1) DNA and recombinant vector IM; 2) DNA given IR and recombinant vector injected IM; 3) DNA given IM and recombinant vector injected IR; 4) DNA and recombinant vector IR. Group 5) Animals received DNA immunization only and Group 6 and 7) a single immunization with either recombinant vaccinia virus or recombinant adenovirus. For the DNA immunizations mice were injected IM with 50 µg of plasmid DNA in the quadriceps muscle at 3 week intervals between each immunization, for a total of three injections and boosted once with recombinant MVA (107 pfu) or recombinant Adenoviral vector (1010pfu). For IR immunization plasmid DNA (50 µg) was complexed with the cationic lipid DOTAP (50 µg) by vigorous mixing by vortex. For recombinant viral immunization, virus was diluted to the appropriate titer (pfu) in sterile phosphate-buffered saline (PBS), and 150 µl of the virus inoculum injected IR through an umbilical catheter inserted about 4 cm deep while mice were under inhalation anesthesia (methoxyflurane; Pitman-Moore, Inc., Mundelein, IL) (Belyakov et al., 1999, 1998c). Mice were boosted with rMVA (1 × 107 pfu) or the replication-deficient adenoviral vector (1010 pfu) encoding each antigen injected IR or IM. The DNA-rMVA or DNA-rAd boost animals were injected with 50 µg DNA IR or IM as described above, followed by the rMVA or rAd boost 3 weeks after the third plasmid DNA injection. The optimum immunodominant CTL epitope P18-I10 peptide (RGPGRAFVTI) from the V3 loop of the HIV-1IIIB envelope protein presented by H-2Dd in BALB/c mice (Takahashi et al., 1988) was synthesized on an automated peptide synthesizer (Symphony Multiplex; Rainin, Boston, Mass) and purified to >95% purity by RP HPLC on a C18 column or purchased from Neo MPS (San Diego).
Cell purification
Three weeks after the last immunization, antigen-specific T cells were isolated from Peyer's patch (PP), lamina propria (LP), and spleen (SP). The PP were carefully excised from the intestinal wall and dissociated into single cells by using collagenase type VIII, 300 U/ml (Sigma) as described previously (Belyakov et al., 1998b). Our data showed that the predominant CD3+ T cells isolated from PP of normal mice were CD4+ (25%), while CD3+ CD8+ T cells were less frequent (8%). Collagenase type VIII did not alter expression of CD3, CD4, or CD8 splenic T cells treated with this enzyme. LP lymphocyte isolation was performed as described previously, typically recovering over 2 × 106 viable LP lymphocytes per mouse (Belyakov et al., 1998b). The large and small intestines were dissected from individual mice and mesenteric and connective tissues carefully removed. Fecal material was flushed from the lumen with RPMI 1640 medium. After the PP were removed from the intestinal wall, the intestines were opened longitudinally, cut into short segments, and washed extensively in RPMI 1640 medium containing 2% fetal bovine serum. To remove the epithelial cell layer, tissues were placed into 100 ml of 1 µM EDTA and incubated twice (first for 40 min and then for 20 min) at 37 °C with stirring. Following EDTA treatment, tissues were washed in complete RPMI 1640 medium for 10 min at room temperature and then placed into 50 ml of RPMI medium containing 10% FCS and incubated for 15 min at 37 °C with stirring. The tissues and medium were transferred to a 50 ml tube and shaken vigorously for 15 sec, and then the medium containing epithelial cells was removed. This mechanical removal of cells was repeated twice more, by using fresh medium each time, to completely remove the epithelial cell layer. Histologic examination revealed that the structure of the villi and LP were preserved. To isolate LP lymphocytes, tissue were cut into small pieces and incubated in RPMI 1640 medium containing collagenase type VIII, 300 U/ml (Sigma) for 50 min at 37 °C with stirring. Supernatants containing cells were collected, washed and then resuspended in complete RPMI 1640 medium. This collagenase dissociation procedure was repeated two times and the isolated cells pooled and washed again. Cells were passed through a cotton wool column to remove dead cells and tissue debris and then layered onto a discontinuous gradient containing 75% and 40% Percoll (Pharmacia). After centrifugation (4 °C, 600×g, 20 min), the interface layer between the 75% and 40% Percoll was carefully removed and washed with incomplete medium. SP were aseptically removed and single cell suspensions prepared by gently teasing them through sterile screens. The erythrocytes were lysed in Tris-buffered ammonium chloride and the remaining cells washed extensively in RPMI 1640 medium containing 2% fetal bovine serum (Belyakov et al., 2003).
CTL assay
We measured the CD8+ CTL response by 51Cr-release assay, 7 days after in vitro stimulation with P18-I10-peptide in the spleen and Peyer's patches. Immune cells from SP, PP were cultured with or without the P18-I10 peptide (1 µM) at 5×106/ml in 12-well culture plates in complete T cell medium: RPMI 1640 medium containing 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 mg/ml), and 5×10−5 M 2-mercaptoethanol. After 3 days we added medium containing 10% ConA-stimulated spleen cell supernatant (T-stim; Collaborative Biomedical Products, Bedford, MA) as a source of IL-2. Specific T cells were found to increase 3–7 fold during the 7-day culture. Cytolytic activity of CD8+ CTL was measured by a 4-h assay with 51Cr-labeled P815 targets tested in the presence or absence of P18-I10 peptide (1 µM). For testing the peptide specificity of CTL, 51Cr-labeled P815 targets were pulsed for 2 h with peptide at the beginning of the assay (Ahlers et al., 2001). The percent specific 51Cr release was calculated as 100×(experimental release-spontaneous release)/(maximum release-spontaneous release). Maximum release was determined from supernatants of cells that were lysed by addition of 5% Triton X-100. Spontaneous release was determined from target cells incubated without added effector cells (Belyakov et al., 2004b).
IFN-γ ELISPOT assay performed on mouse splenocytes, PP and LPL
Lymphoid cells were cultured in the wells of a 96-well ELISPOT plate in a volume of 200 µl, at 1 × 105 lymphoid cells/well. Splenocytes from naive BALB/c mice were used as stimulator cells (2 × 105/well), pulsed in complete media for at least 1 h with different concentrations of P18-I10 peptide (RGPGRAFVTI), washed with complete media twice, gamma-irradiated (3000R) and then added to cultures containing lymphocytes from the spleen, PP and LP isolated from immunized mice. The plates were incubated for at least 18 h at 37 °C in 5% CO2 and air. Overnight incubation of T cells with peptide allows the functional assessment of CD8+ T cells (which cannot be detected ex vivo from freshly isolated cells) without changing the absolute number of antigen-specific CD8+ T cells as measured by tetramer staining or the repertoire of TCR analyzed with mAb (Belyakov et al., 2001b). After that, the plates were washed thoroughly with PBS–0.05% Tween 20 (Sigma) followed by incubation with rat anti-mouse IFN-γ biotinylated monoclonal R4-6A2 (2 µg/ml; Mabtech) in PBS–0.5% BSA for 3 h at 37 °C. After incubation with secondary mAbs, plates were washed with PBS–0.05% Tween 20, and incubated with alkaline phosphatase substrate prepared from VECTASTAIN Elite ABC Kit (Vector Laboratories, Inc) for 1 h at room temperature. At the last stage, plates were washed with PBS–0.05% Tween 20 followed by PBS and developed with BD™ ELISPOT AEC Substrate Set (Pharmingen). Spots were counted on an AID ELISPOT Reader (Cell Technology, Inc.) (Belyakov et al., 2007a). Background levels of IFN-γ-producing cells measured on total lymphoid cell populations with no antigen stimulation were substracted from the experimental groups as previously described (Belyakov et al., 2006a).
Statistical analysis
Statistical comparisons were performed using Student's t-tests (Kuznetsov et al., 2004). The pre-determined level of significance was set at a p value of <0.05.
Acknowledgments
We thank Dr. David Margulies for critical comments on the manuscript and helpful suggestions. We thank Drs. Linda Wyatt and Patricia Earl for providing MVA/HXB2env, and Drs. Ling Xu, Zhi-Yong Yang, and Wing Kong for generating and providing rAd vectors. This work was carried out with the support of the intramural programs of the Center for Cancer Research, National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, NIH.
References
- Acierno PM, Schmitz JE, Gorgone DA, Sun Y, Santra S, Seaman MS, Newberg MH, Mascola JR, Nabel GJ, Panicali D, Letvin NL. Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 2006;176(9):5338–5345. doi: 10.4049/jimmunol.176.9.5338. [DOI] [PubMed] [Google Scholar]
- Ahlers JD, Belyakov IM, Thomas EK, Berzofsky JA. High affinity T-helper epitope induces complementary helper and APC polarization, increased CTL and protection against viral infection. J. Clin. Invest. 2001;108:1677–1685. doi: 10.1172/JCI13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers JD, Belyakov IM, Berzofsky JA. Cytokine, chemokine and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Current Molecular Medicine. 2003;3:285–301. doi: 10.2174/1566524033479843. [DOI] [PubMed] [Google Scholar]
- Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high or low avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, Vogel TU, Fuller DH, Mothe BR, Steffen S, Boyson JE, Shipley T, Fuller J, Hanke T, Sette A, Altman JD, Moss B, McMichael AJ, Watkins DI. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 2000;164(9):4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ, Rowland-Jones SL. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192(1):63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 2000;6(2):200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Craiu A, Kuroda MJ, Schmitz JE, Zheng XX, Santra S, Frost JD, Krivulka GR, Lifton MA, Crabbs CL, Heidecker G, Perry HC, Davies ME, Xie H, Nickerson CE, Steenbeke TD, Lord CI, Montefiori DC, Strom TB, Shiver JW, Lewis MG, Letvin NL. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. U. S. A. 2000;97(8):4192–4197. doi: 10.1073/pnas.050417697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe RB, Gorse GJ, Mulligan MJ, Evans TG, Keefer MC, Excler JL, Duliege AM, Tartaglia J, Cox WI, McNamara J, Hwang KL, Bradney A, Montefiori D, Weinhold KJ. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. Aids. 1998;12(18):2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004;20(3):247–253. doi: 10.1016/s1074-7613(04)00053-6. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Ahlers JD, Brandwein BY, Earl P, Kelsall BL, Moss B, Strober W, Berzofsky JA. The Importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal-viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Invest. 1998a;102(12):2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, Berzofsky JA. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. U. S. A. 1998b;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Wyatt LS, Ahlers JD, Earl P, Pendleton CD, Kelsall BL, Strober W, Moss B, Berzofsky JA. Induction of mucosal CTL response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing HIV 89.6 envelope protein. J. Virol. 1998c;72:8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Moss B, Strober W, Berzofsky JA. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Ahlers JD, Clements JD, Strober W, Berzofsky JA. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific cytotoxic T lymphocytes. J. Immunol. 2000;165:6454–6462. doi: 10.4049/jimmunol.165.11.6454. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins D, Allen TM, Sette A, Altman J, Woodward R, Markham P, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 2001a;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Wang J, Koka R, Ahlers JD, Snyder JT, Tse R, Cox J, Gibbs JS, Margulies DH, Berzofsky JA. Activating CTL precursors to reveal CTL function without skewing the repertoire by in vitro expansion. Eur. J. Immunol. 2001b;31:3557–3566. doi: 10.1002/1521-4141(200112)31:12<3557::aid-immu3557>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. U. S. A. 2003;100(16):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Ahlers JD, Berzofsky JA. Mucosal AIDS vaccines: current status and future directions. Expert Rev. Vaccines. 2004a;3((4), Suppl):65–73. doi: 10.1586/14760584.3.4.s65. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTL and protective immunity by migration of primed skin dendritic cells. J. Clin. Invest. 2004b;113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Klinman D, Berzofsky JA. Enhancement of CD8+ T cell immunity in the lung by CpG ODN increases protective efficacy of a modified vaccinia Ankara vaccine against lethal poxvirus infection even in CD4-deficient host. J. Immunol. 2006a;177:6336–6343. doi: 10.4049/jimmunol.177.9.6336. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal P, Clements JD, Lewis MG, Strober S, Franchini G, Berzofsky JA. Impact of vaccine-induced mucosal high avidity CD8+ CTLs in delay of AIDS-viral dissemination from mucosa. Blood. 2006b;107(8):3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J. Immunol. 2007a;178:7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Kozlowski S, Mage M, Ahlers JD, Boyd LF, Margulies DH, Berzofsky JA. Role of {alpha}3 domain of class I MHC molecules in the activation of high- and low-avidity CD8+ CTLs. Int. Immunol. 2007b;19(12):1413–1420. doi: 10.1093/intimm/dxm111. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. Approaches to improve engineered vaccines for human immunodeficiency virus (HIV) and other viruses that cause chronic infections. Immunol. Rev. 1999;170:151–172. doi: 10.1111/j.1600-065x.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Ahlers J, Janik J, Morris J, Oh S, Terabe M, Belyakov IM. Progress on new vaccine strategies against chronic viral infections. J. Clin. Invest. 2004a;114:450–462. doi: 10.1172/JCI22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J. Clin. Invest. 2004b;113(11):1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003;77(11):6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, King SR, Montefiori DC, Nabel GJ. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 2002;76(11):5357–5368. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CJ, Thomson S, De Rose R, Ranasinghe C, Medveczky CJ, Pamungkas J, Boyle DB, Ramshaw IA, Kent SJ. Prime-boost strategies in DNA vaccines. Methods Mol. Med. 2006;127:171–197. doi: 10.1385/1-59745-168-1:171. [DOI] [PubMed] [Google Scholar]
- Derby MA, Alexander-Miller MA, Tse R, Berzofsky JA. High avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low avidity CTL. J. Immunol. 2001;166:1690–1697. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. In: BR, Ausubel FM, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: 2 Greene Publishing Associates & Wiley Interscience; 1998. [Google Scholar]
- Estcourt MJ, Ramsay AJ, Brooks A, Thomson SA, Medveckzy CJ, Ramshaw IA. Prime-boost immunization generates a high frequency, high-avidity CD8(+) cytotoxic T lymphocyte population. Int. Immunol. 2002;14(1):31–37. doi: 10.1093/intimm/14.1.31. [DOI] [PubMed] [Google Scholar]
- Evans DT, Chen LM, Gillis J, Lin KC, Harty B, Mazzara GP, Donis RO, Mansfield KG, Lifson JD, Desrosiers RC, Galan JE, Johnson RP. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 2003;77(4):2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi MM, Najera JL, Perez-Jimenez E, Guerra S, Garcia-Sastre A, Esteban M. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes. J. Virol. 2003;77(12):7048–7057. doi: 10.1128/JVI.77.12.7048-7057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 2003;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke T, Blanchard TJ, Schneider J, Hannan CM, Becker M, Gilbert SC, Hill AVS, Smith GL, McMichael A. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine. 1998;16:439–445. doi: 10.1016/s0264-410x(97)00226-0. [DOI] [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Kelsall BL, Tsai WP, Letvin NL, Parks RW, Tryniszewska E, Picker L, Lewis MG, Edghill-Smith Y, Moniuszko M, Pal R, Stevceva L, Altman J, Allen TM, Watkins D, Torres JV, Berzofsky JA, Belyakov IM, Strober W, Franchini G. Impairment of Gag-specific D8+ T-cell function in mucosal and sytemic compartments of SIVmac251- and SHIVKU2-infected macaques. J. Virol. 2001a;75:11483–11495. doi: 10.1128/JVI.75.23.11483-11495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hel Z, Tsai WP, Thornton A, Nacsa J, Giuliani L, Tryniszewska E, Poudyal M, Venzon D, Wang X, Altman J, Watkins DI, Lu W, von Gegerfelt A, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Potentiation of simian immunodeficiency virus (SIV)-specific CD4(+) and CD8(+) T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J. Immunol. 2001b;167(12):7180–7191. doi: 10.4049/jimmunol.167.12.7180. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kong WP, Nabel GJ. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 2001;75(10):4947–4951. doi: 10.1128/JVI.75.10.4947-4951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald KS, Bwayo JJ, McMichael AJ, Rowland-Jones SL. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 1998;72(12):10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 1997;65(4):1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov VA, Stepanov VS, Berzofsky JA, Belyakov IM. Assessment of the relative therapeutic effects of vaccines on virus load and immune responses in small groups at several time points: efficacy of mucosal and subcutaneous polypeptide vaccines in rhesus macaques exposed to SHIV. J. Clin. Virol. 2004;31(Suppl 1):S69–S82. doi: 10.1016/j.jcv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Huang Y, Chakrabarti BK, Xu L, Seaman MS, Beaudry K, Korioth-Schmitz B, Yu F, Rohne D, Martin KL, Miura A, Kong WP, Yang ZY, Gelman RS, Golubeva OG, Montefiori DC, Mascola JR, Nabel GJ. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 2004;78(14):7490–7497. doi: 10.1128/JVI.78.14.7490-7497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SW, Cun AS, Harris-McCoy K, Ertl HC. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine. 2007;25(12):2187–2193. doi: 10.1016/j.vaccine.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177(2):831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- McKay PF, Schmitz JE, Barouch DH, Kuroda MJ, Lifton MA, Nickerson CE, Gorgone DA, Letvin NL. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 2002;168(1):332–337. doi: 10.4049/jimmunol.168.1.332. [DOI] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Alexander NJ, Sutjipto S, Lackner AA, Gettie A, Hendrickx AG, Lowenstine LJ, Jennings M, Marx PA. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwau M, McMichael AJ. A review of vaccines for HIV prevention. J. Gene Med. 2003;5(1):3–10. doi: 10.1002/jgm.343. [DOI] [PubMed] [Google Scholar]
- Nabel G, Makgoba W, Esparza J. HIV-1 diversity and vaccine development. Science. 2002;296(5577):2335. doi: 10.1126/science.296.5577.2335. [DOI] [PubMed] [Google Scholar]
- Neeson P, Boyer J, Kumar S, Lewis MG, Mattias L, Veazey R, Weiner D, Paterson Y. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response characterized by high levels of alpha4beta7 integrin and an effector memory phenotype. Virology. 2006;354(2):299–315. doi: 10.1016/j.virol.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E, Martinez I, Villinger F, Rivera M, Gascot S, Colon C, Arana T, Sidhu M, Stout R, Montefiori DC, Martinez M, Ansari AA, Israel ZR, Kraiselburd E. Protection by SIV VLP DNA prime/protein boost following mucosal SIV challenge is markedly enhanced by IL-12/GM-CSF co-administration. J. Med. Primatol. 2002;31(4–5):217–227. doi: 10.1034/j.1600-0684.2002.02008.x. [DOI] [PubMed] [Google Scholar]
- Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, Hudacik L, Rose N, Nacsa J, Y ES, Belyakov IM, Berzofsky JA, Markham P, Letvin NL, Tartaglia J, Franchini G Washington Parks, R. Systemic Immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-Cell loss and reduces both systemic and mucosal SHIVKU2 RNA levels. J. Virol. 2006;80(8):3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe C, Turner SJ, McArthur C, Sutherland DB, Kim JH, Doherty PC, Ramshaw IA. Mucosal HIV-1 pox virus prime-boost immunization induces high-avidity CD8+ T cells with regime-dependent cytokine/granzyme B profiles. J. Immunol. 2007;178(4):2370–2379. doi: 10.4049/jimmunol.178.4.2370. [DOI] [PubMed] [Google Scholar]
- Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 2007;179(6):3504–3514. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- Sharpe S, Hanke T, Tinsley-Bown A, Dennis M, Dowall S, McMichael A, Cranage M. Mucosal immunization with PLGA-microencapsulated DNA primes a SIV-specific CTL response revealed by boosting with cognate recombinant modified vaccinia virus Ankara. Virology. 2003;313(1):13–21. doi: 10.1016/s0042-6822(03)00282-4. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Snyder JT, Alexander-Miller MA, Berzofsky JA, Belyakov IM. Molecular mechanisms and biological significance of CTL avidity. Current HIV Research. 2003;1:287–294. doi: 10.2174/1570162033485230. [DOI] [PubMed] [Google Scholar]
- Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183(1):215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover J, Bollinger L, Hecht R, Williams C, Roca E. The impact of an AIDS vaccine in developing countries: a new model and initial results. Health Aff. (Millwood) 2007;26(4):1147–1158. doi: 10.1377/hlthaff.26.4.1147. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Cohen J, Hosmalin A, Cease KB, Houghten R, Cornette J, DeLisi C, Moss B, Germain RN, Berzofsky JA. An immunodominant epitope of the HIV gp160 envelope glycoprotein recognized by class I MHC molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1988;85:3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology. 2007;367(1):156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritel M, Stoddard AM, Flynn BJ, Darrah PA, Wu CY, Wille U, Shah JA, Huang Y, Xu L, Betts MR, Nabel GJ, Seder RA. Prime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responses. J. Immunol. 2003;171(5):2538–2547. doi: 10.4049/jimmunol.171.5.2538. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A, Kiszka I, Kaneko H, Kmieciak D, Wasik TJ, Gzyl J, Kaneko Y, Kozbor D. Immunization strategies to augment oral vaccination with DNA and viral vectors expressing HIV envelope glycoprotein. Vaccine. 2002;20(9–10):1295–1307. doi: 10.1016/s0264-410x(01)00480-7. [DOI] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006;203(5):1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LS, Belyakov IM, Earl PL, Berzofsky JA, Moss B. Enhanced cell surface expression, immunogenicity and genetic stability resulting from a spontaneous truncation of HIV Env expressed by a recombinant MVA. Virology. 2008;372(2):260–272. doi: 10.1016/j.virol.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- Zhang ZQ, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]