Abstract
Insulin resistance, obesity, diabetes, dyslipidemia, and nonalcoholic fatty liver are components of the metabolic syndrome, a disease complex that is increasing at epidemic rates in westernized countries. Although proinflammatory cytokines have been suggested to contribute to the development of these disorders, the molecular mechanism is poorly understood. Here we show that overexpression of suppressors of cytokine signaling (SOCS)-1 and SOCS-3 in liver causes insulin resistance and an increase in the key regulator of fatty acid synthesis in liver, sterol regulatory element-binding protein (SREBP)-1c. Conversely, inhibition of SOCS-1 and -3 in obese diabetic mice improves insulin sensitivity, normalizes the increased expression of SREBP-1c, and dramatically ameliorates hepatic steatosis and hypertriglyceridemia. In obese animals, increased SOCS proteins enhance SREBP-1c expression by antagonizing STAT3-mediated inhibition of SREBP-1c promoter activity. Thus, SOCS proteins play an important role in pathogenesis of the metabolic syndrome by concordantly modulating insulin signaling and cytokine signaling.
Type 2 diabetes and the closely related metabolic syndrome associated with central obesity, insulin resistance, hypertension, and dyslipidemia are major causes of morbidity and mortality in westernized countries (1). Nonalcoholic fatty liver, also a component of the metabolic syndrome, is the most common liver abnormality in the U.S. and may lead to hepatic fibrosis, cirrhosis, and death (2). Recent studies have suggested a relationship between the effects of elevated proinflammatory cytokines, such as IL-6 (3) and TNF-α (4), and these diseases (5). The molecular mechanisms underlying this linkage, however, are poorly understood, although TNF-α has been shown to cause insulin resistance by increasing serine phosphorylation of insulin receptor substrate (IRS)-1 (6).
Proinflammatory cytokines also stimulate production of a family of proteins known as suppressors of cytokine signaling [SOCS-1–7 and cytokine inducible src homology 2 domain-containing protein (CIS)] (7) that participate in a negative feedback loop in cytokine signaling (8–10). SOCS-1 and -3 have been shown to bind JAK tyrosine kinase and attenuate its ability to phosphorylate signal transducer and activator of transcription (STAT) proteins (11, 12). Expression of the SOCS proteins is increased by cytokine signaling through activation of STAT- and NF-κB-mediated pathways (8–10, 13). Thus, the negative feedback loop via SOCS proteins is doubly regulated in both phosphorylation- and transcription-dependent manners. Recently, SOCS proteins have been suggested to be involved in insulin/insulin-like growth factor-1 signaling (14, 15). Moreover, it has been shown that SOCS-1 knockout mice have decreased glucose levels, and that cells derived from these mice seem to exhibit enhanced insulin signaling (16), although it is difficult to determine insulin sensitivity in vivo using these mice because they die within 3 weeks of birth (17, 18)
In this study, we show that SOCS-1 and -3 are increased in insulin-resistant obese animals, and that insulin resistance can be induced in vivo by overexpression of SOCS-1 or -3 in liver using adenoviral vectors. Conversely, suppression of SOCS-1, -3, or both in liver partially rescues impaired insulin sensitivity and ameliorates hyperinsulinemia in diabetic db/db mice. More importantly, suppression of SOCS proteins, especially SOCS-3, markedly improves hepatic steatosis. This is due to normalization of the expression of up-regulated sterol regulatory element-binding protein (SREBP)-1 accompanied by restoration of STAT3 phosphorylation.
Materials and Methods
Animals. Eight-week-old female C57BLKS/Jdb/db mice and C57BLKS/J mice were purchased from The Jackson Laboratory. For other studies, 8-week-old male C57BL/6 mice were purchased from Taconic Farms. All animals were housed on a 12-h light/dark cycle and were fed standard rodent chow. All protocols for animal use and death were approved by the Animal Care Use Committee of the Joslin Diabetes Center and Harvard Medical School in accordance with National Institutes of Health guidelines.
RNA Isolation from Mice. Mice were starved overnight, then killed under anesthesia with the tissues removed. Total RNA was isolated from mouse tissues by using an RNeasy kit (Qiagen, Valencia, CA).
Semiquantitative RT-PCR and Northern Blot Analysis. Five hundred nanograms of total RNA was applied to RT-PCR reaction by using the One-Step RT-PCR system (Invitrogen). The primer pairs were: 5′-TCCGATTACCGGCGCATCACG-3′ and 5′-CTCCAGCAGCTCGAAAAGGCA-3′ for SOCS-1; 5′-CACAGCAAGTTTCCCGCCGCC-3′ and 5′-GTGCACCAGCTTGAGTACACA-3′ for SOCS-3; and 5′-ACCACCATGGAGAAGGCCGG-3′ and 5′-CTCAGTGTAGCCCAAGATGC-3′ for GAPDH. The PCR reaction profile was as follows: one cycle at 94°C for 5 min followed by 38 cycles at 94°C for 1 min; 60°C for 30 s and 72°C for 1 min; and finally one cycle at 72°C for 10 min. For Northern blot analysis, we applied 20 μg of the total RNA and used phosphoenolpyruvate carboxykinase, peroxisome proliferator-activated receptor γ-coactivator-1α (PGC-1α), SREBP-1, or fatty acid synthase cDNA fragment as a probe, as described (19, 20).
Generation of Recombinant Adenoviruses. The cDNAs of SOCS-1 and -3 were subcloned between BamHI and EcoRI sites of pCMV-Tag2 vector, respectively, and amplified the full-length SOCS-1 and -3 cDNAs with an N-terminal FLAG tag by using the primer pairs: 5′-GCCGCCACCATGGATTACAAGGAT-3′ and 5′-TCAGATCTGGAAGGGGAAGGAACTCAG-3′ for SOCS-1 and 5′-GCCGCCACCATGGATTACAAGGAT-3′ and 5′-CTAAAGTGGAGCATCATACTGATC-3′ for SOCS-3. After confirming the sequences, we treated the amplified fragments with Klenow enzyme and subcloned them into the SwaI site of the pAdex1CAwt cosmid cassette. The recombinant adenoviruses, Adex1CASOCS-1-FLAG and Adex1CASOCS-3-FLAG, were constructed by homologous recombination between the expression cosmid cassette and parental virus genome, as described (21).
Adenovirus-Mediated Gene Transfer. Eight-week-old male C57BL/6 mice were injected with the adenoviruses at a concentration of 5 × 108 plaque-forming units per gram of body weight in a suspension of 200 μl of PBS through the tail vein, as described (21). Blood samples were obtained on the day before adenoviral injection (day 0) and 5 days after injection (day 5) for measurement of glucose and insulin. Insulin tolerance tests were performed at day 6 and insulin signaling after i.v. insulin injection at day 8.
Antisense Treatment. Two oligonucleotides, designated as AS1 and AS3, were synthesized for antisense treatment against SOCS-1 and -3, respectively. AS1 was designed as a 26-bp single-strand oligonucleotide covering the –5 to ≈+21 region of murine SOCS-1 mRNA: 5′-CACCTGGTTGCGTGCTACCATCCTAC-3′, whereas AS3 was designed covering the –5to ≈+21 region of the murine SOCS-3 mRNA: 5′-AAACTTGCTGTGGGTGACCATGGCGC-3′ (22). Two oligonucleotides, designated as C1 (5′-CAGCTCGTAGCGAGCAACCATCGTAC-3′, a six-base mismatch to AS1), and C3 (5′-AATCTAGCTCTGCGTGAGCATCGCGC-3′, a six-base mismatch to AS3), were also synthesized for controls. All oligonucleotides were synthesized as uniform phosphorothioate chimeric oligonucleotides, with 2′-O-methoxyethyl groups on bases 1–5 and 22–26. To inhibit expression of SOCS-1 and -3 in vivo, db/db mice were treated by i.p. injection with 25 mg/kg of AS1, AS3, C1, C3, or PBS once per week for 2 weeks as described (23).
Metabolic Studies. Blood glucose values were determined by using a Glucometer Elite XL (Bayer, Elkhart, IN), and plasma insulin concentrations were measured by ELISA with mouse insulin as a standard (Crystal Chem, Downers Grove, IL). For the insulin tolerance test, blood samples were obtained by tail bleeding at 0, 15, 30, and 60 min after i.p. injection of 0.75 units/kg insulin (Lilly Research Laboratories, Indianapolis). Plasma triglyceride concentrations from fasted animals were determined by using the GPO-Trinder assay (Sigma). Triglyceride content of the liver was determined by enzymatic measurement (GPQ-Trinder, Sigma) of glycerol and also estimated by Oil red O and hematoxylin staining by using frozen sections of liver (24).
Antibodies. Polyclonal anti-SOCS-1, anti-SOCS-3, anti-STAT3, anti-STAT5, and anti-Akt1/2 antibodies were purchased from Santa Cruz Biotechnology, whereas polyclonal antiphospho-STAT3 and antiphospho-STAT5 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Polyclonal anti-IRS-1, anti-IRS-2, and antiinsulin receptor antibodies were generated as described (19). Monoclonal antiphosphotyrosine antibody was purchased from Upstate Biotechnology (Lake Placid, NY).
In Vivo Insulin Stimulation and Analysis of Insulin Signaling. Mice were starved overnight, anesthetized with pentobarbital, and injected with 5 units of regular human insulin (Lilly) into the inferior vena cava. Five minutes after injection, the liver was removed and frozen in liquid nitrogen. Immunoprecipitation and immunoblot analysis of insulin signaling molecules were performed by using tissue homogenates extracted with buffer A containing 25 mM Tris·HCl, pH 7.4; 10 mM Na3VO4; 100 mM NaF; 50 mM Na4P2O7; 10 mM EGTA; 10 mM EDTA; 5 μg/ml leupeptin; 5 μg/ml aprotinin; 2 mM PMSF; and 1% Nonident-P 40, as described (21).
Immunostaining for SOCS Proteins. After a wash with PBS, 10-μm frozen sections from liver were incubated with 3% normal goat serum in 2.5% Triton X-100/PBS for 2 h for blocking, followed by incubation with anti-SOCS-1 or anti-SOCS-3 antibody in blocking solution at 1:100 dilution for overnight. After six washes with PBS, slides were incubated with Alexa Fluor 488 (Molecular Probes) for SOCS-1 and Alexa Fluor 546 (Molecular Probes) for SOCS-3 in blocking solution at 1:200 for 2 h. After rinsing with PBS, slides were mounted with Slow Fade kit (Molecular Probes) and examined with a fluorescent microscope.
In Vitro Kinase Assays. Tissue homogenates or cells were extracted with buffer A and subjected to immunoprecipitation with antiphosphotyrosine antibody followed by phosphoinositide 3-kinase assay, as described (21). Akt kinase activity in the immunoprecipitates with anti-Akt1/2 antibody was determined by using Crosstide as a substrate (21).
Luciferase Assay. Fao cells were plated at 1 × 105/well in 12-well plates 24 h before transfection. Cells were transfected with 0.5 μg of pGL2 mouse SREBP-1c promoter luciferase vector (25) (kindly provided by H. Shimano, Tsukuba University, Tsukuba, Japan), 0.5 μg of a promoterless Renilla luciferase construct (pRL-null), and 1 μg of pCDA3.1 vector encoding STAT3, STA5b (kindly provided by T. Tanaka, Harvard School of Public Health, Boston), pEF-Boss vector encoding SCOS-1 or -3 (kindly provided by T. Naka, Osaka University, Osaka), or pME18S encoding a mutant STAT3 lacking the C-terminal region [dominant negative (dn)STAT3, kindly provided by A. Miyajima, Institute of Cellular and Molecular Biosciences, Tokyo] by using the Fugene 6 transfection kit (Roche Applied Science). Cells were lysed with passive lysis buffer (Promega) 24 h after transfection. Luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega) following the manufacturer's instructions. Relative light units were determined by quantitation of luminescent signal from the Firefly luciferase normalized with cotransfected Renilla luciferase activity in the same sample.
Results and Discussion
Increased SOCS Proteins in Liver Cause Systemic Insulin Resistance. Several lines of evidence have suggested that insulin resistance in obesity is a component mediated by chronic inflammation associated with elevated cytokines (4, 26, 27), which may induce SOCS proteins in insulin-sensitive tissues. Indeed, in livers of obese diabetic db/db mice, the levels of SOCS-1 and -3 mRNA and proteins are increased ≈2- to 3-fold (Fig. 1 a and b). Similar up-regulation of SOCS mRNAs was also observed in other insulin-resistant models, such as ob/ob mice and mice on a high-fat diet (Fig. 6, which is published as supporting information on the PNAS web site). Thus, SOCS proteins may be a candidate linking between the metabolic syndrome and proinflammatory cytokine signaling.
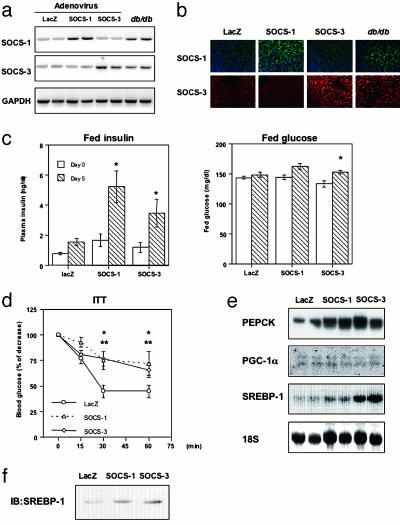
Fig. 1.
Increased SOCS-1 or -3 in liver causes systemic insulin resistance. (a and b) Comparable expressions of SOCS-1 and -3 introduced by adenovirus injection to those in db/db mice. (a) Shown are representative results of RT-PCR using RNA from liver with the indicated treatment. (b) Immunostaining for SOCS-1 (Upper) and SOCS-3 (Lower) in liver. (c) Increased insulin and glucose concentrations caused by overexpression of SOCS-1 or -3 in liver. Each bar represents the mean ± SE (n = 8) of the plasma insulin concentrations (Left) or blood glucose (Right) in random fed state at days 0 and 5 after adenovirus injection. (*, P < 0.05 day 0 vs. 5.) (d) Impaired insulin sensitivity by overexpression of SOCS-1 or -3 estimated by the insulin tolerance test. Results are expressed as mean ± SE (n = 8) of percent of initial glucose level. (*, P < 0.05 LacZ vs. SOCS-1; **, P < 0.05 LacZ vs. SOCS-3 adenovirus treatment.) (e) Amounts of mRNA of phosphoenolpyruvate carboxykinase, PGC-1α, and SREBP-1c in liver treated with SOCS-1 or -3 adenovirus. (f) Increased levels of nuclear SREBP-1 protein in liver overexpressing SOCS proteins. Shown are the representative results of immunoblot analysis with αSREBP-1 using nuclear fractions.
To directly assess the role of increased SOCS proteins in insulin resistance and the metabolic syndrome, mice were injected with adenoviruses encoding either SOCS-1, SOCS-3, or LacZ, respectively. Under these conditions, the levels of expression of SOCS-1 and -3 were within the pathophysiological range and only slightly higher than those in livers of db/db mice (Fig. 1 a and b). β-Galactosidase staining of liver infected with adenovirus encoding LacZ revealed that, using our system, >90% of hepatocytes were infected (data not shown).
Overexpression of either SOCS-1 or -3 in liver by adenoviral infection resulted in significantly increased plasma insulin concentrations (Fig. 1c), with only slight increases in blood glucose levels (Fig. 1c), suggesting insulin resistance in these mice. This was confirmed by a marked impairment in glucose lowering during the insulin tolerance test in mice overexpressing SOCS-1 or -3 (Fig. 1d). This is consistent with findings produced by others and ourselves that SOCS proteins can attenuate insulin signaling by binding to the insulin receptor and reducing their ability to phosphorylate IRS proteins (14, 28, 29). SOCS proteins have also been suggested to interfere with insulin signaling by promoting ubiquitin-mediated degradation of IRS proteins (30).
Consistent with the impaired insulin sensitivity in mice overexpressing SOCS-1 or -3, there was an up-regulation of the mRNA of phosphoenolpyruvate carboxykinase, a key enzyme of gluconeogenesis (Fig. 1e). This occurred without a change in the expression level of peroxisome proliferator-activated receptor γ-coactivator-1α (PGC-1α), one of the key regulators of expression of gluconeogenic enzymes (20). On the other hand, expression of the SREBP-1c, a key regulator of fatty acid synthesis in liver (31), was increased 1.83 ± 0.17 fold (n = 8) in livers overexpressing SOCS-1 and 5.21 ± 0.31 fold (n = 8) in those overexpressing SOCS-3 (Fig. 1e), leading to an increase in levels of SREBP-1 protein in the nuclei of hepatocytes overexpressing SOCS-1 or -3 (Fig. 1f). This increase in SREBP-1c after increased SOCS expression could act as a trigger in the regulation of fatty acid synthesis in liver.
Amelioration of Insulin Resistance by Suppressing SOCS-1 and -3 in the db/db Mouse. To directly address whether SOCS-1 and -3 contribute to insulin resistance, hepatic steatosis, and other metabolic derangement in obese diabetic animals, we treated db/db mice with antisense oligonucleotides against SOCS-1 and -3 (AS1 and AS3). After 2 weeks of treatment with specific antisense oligonucleotides, there was a marked and specific down-regulation of the elevated levels of the expression of the targeted SOCS mRNA. Combination therapy (AS1+AS3) reduced the expression of both isoforms in liver, whereas the control oligonucleotides (C1 and C3) and PBS treatment did not affect expression of either isoform (Fig. 2a Left). Immunostaining using the specific antibody against SOCS-1 or -3 revealed that each antisense treatment specifically reduced the targeted SOCS protein to the level in wild-type mice, whereas combination therapy down-regulated both (Fig. 2a Right). These effects were specific to liver (23); expression of SOCS-1 and -3 in muscle and fat was not significantly altered (data not shown). Because there were no differences in the three control groups (PBS, C1, and C3), in subsequent analyses, these were combined.
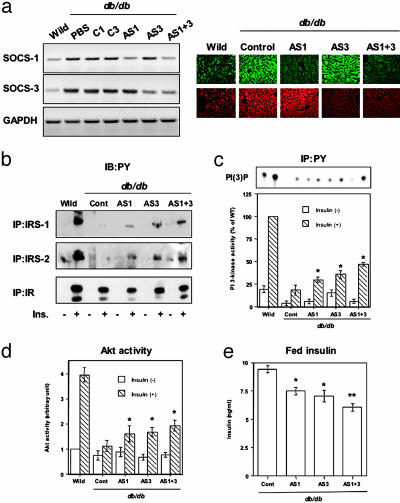
Fig. 2.
Inhibition of the expression of SOCS-1 or -3 in liver ameliorates insulin resistance in db/db mice. (a) Reduced mRNA and protein levels of SOCS-1 and -3 by the specific antisense oligonucleotide treatment (AS1 for SOCS-1, AS3 for SOCS-3 and their specific controls, C1 and C3; AS1 + 3 indicates combination therapy of AS1 and AS3). (Left) Representative results of RT-PCR using RNA from the liver of mouse treated with the indicated material for 2 weeks. (Right) Representative results of immunostaining using liver sections. (b) Tyrosine phosphorylation of IR, IRS-1, and IRS-2 in liver. The immunoprecipitates with antiinsulin receptor (αIR), anti-IRS-1 (αIRS-1), or anti-IRS-2 (αIRS-2) antibodies from liver lysates of the indicated mouse with or without insulin stimulation were immunoblotted with antiphosphotyrosine antibody (αPY). (c) Phosphoinositide 3-kinase activity associated with phosphotyrosine complexes in liver. (Upper) Representative result. (Lower) Each bar represents the mean ± SE (n = 3.) (*, P < 0.05 PBS vs. AS1 or AS3.) (d) Insulin-induced Akt activity in liver. Each bar represents the mean ± SE (n = 3). (*, P < 0.05 PBS vs. AS1 or AS3.) (e) Decreased insulin concentrations caused by suppression of SOCS-1, SOCS-3, or both in liver. Each bar represents the mean ± SE (n = 6–18) of the plasma insulin concentrations in random fed state at day 14 after the first injection of antisense oligonucleotides. (*, P < 0.05 control vs. AS1 or AS3; **, P < 0.05 AS1 or AS3 vs. AS1 + 3.) AS1 + 3 indicates the combination therapy of AS1 and AS3, and control (Cont) includes C1, C3, and PBS groups.
In concordance with the reduction of the SOCS protein in liver, antisense treatment, especially AS3 and the combination of AS1 and AS3, partially restored phosphorylation of both IRS-1 and -2 (Fig. 2b). This is consistent with the greater ability of SOCS-3 to inhibit IRS-1 and -2 phosphorylation (29). By contrast, the impaired phosphorylation of the insulin receptor in db/db mice was unaffected by the reduction of SOCS protein, consistent with the inhibitory mechanisms of SOCS proteins to specifically block IRS phosphorylation (14, 29) and the preexisting down-regulation of the insulin receptor in liver of these obese diabetic mice (32). As a result of the enhancement of phosphorylation of IRS proteins, mice treated with antisense oligonucleotides to each of the SOCS proteins showed modest improvement in phosphoinositide 3-kinase and Akt activity, with combined therapy increasing these to up to 45% of the level of wild-type (Fig. 2 c and d). The residual defects reflect the residual insulin resistance due to reduced insulin receptor signaling and protein in obese animals (32).
As a consequence of reducing SOCS expression after antisense treatment, there was a decrease in the elevated circulating insulin levels in the db/db mice consistent with improved insulin sensitivity (Fig. 2e). This was confirmed by insulin tolerance testing, which showed a significantly improved glucose-lowering effect after suppression of SOCS-3 and a tendency to improvement after reduction of SOCS-1 (Fig. 7, which is published as supporting information on the PNAS web site). Furthermore, the high levels of expression of PGC-1α in the livers of db/db mice that contribute to the increased gluconeogenesis in obese diabetic animals (20) were almost normalized by either single antisense treatment or combined therapy (Fig. 3c). These changes, however, were not sufficient to lower blood glucose concentrations in these severely diabetic mice, in part because the effect of antisense treatment was limited in liver and in part because, even in liver, the insulin receptor signaling was still impaired through other mechanisms, including down-regulation of the insulin receptor itself (32) and SOCS-independent cytokine-mediated pathways (6, 33).
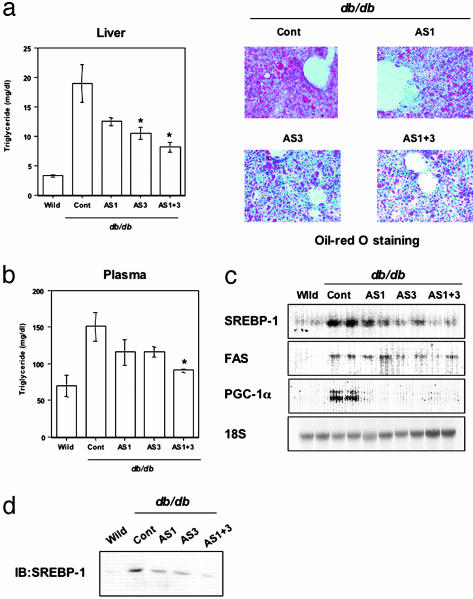
Fig. 3.
Inhibition of the expression of SOCS-1 or -3 in liver improves hepatic steatosis in db/db mice. (a) Decreased lipid accumulation in liver of db/db mouse by suppression of SOCS-1 and -3. In the graph, each bar represents the mean ± SE (n = 6–18; *, P < 0.05 control vs. AS1 + 3). (Right) Representative results of Oil red O staining of liver with the indicated treatment. (b) Reduced concentrations of plasma triglyceride by suppression of SOCS-1 and -3 in db/db mice. Each bar represents the mean ± SE (n = 6–18; *, P < 0.05 control vs. AS3 or AS1 + 3). (c) Decreased expression of SREBP-1 in liver of db/db mouse by suppression of SOCS proteins. Shown are representative results of Northern blot analysis for SREBP-1, fatty acid synthase, or PGC-1α.(d) Decreased levels of nuclear SREBP-1 protein in liver of db/db mouse by suppression of SOCS proteins. Shown are representative results of immunoblot analysis with αSREBP-1 using nuclear fractions. AS1 + 3 indicates the combination therapy of AS1 and AS3, and control (Cont) includes C1, C3, and PBS groups.
Improvement of Hepatic Steatosis and Reduction of Plasma Triglyceride Concentrations by Suppressing SOCS-1 and -3. The most striking effects of SOCS antisense treatment in the db/db mice were on two other aspects of the metabolic syndrome, hepatic steatosis, and hyperlipidemia (34), which aggravate hyperinsulinemia and insulin resistance (35). Thus, control db/db mice exhibited severe hepatic steatosis with a 5.8-fold increase in triglyceride content in liver and more than a doubling of fasting plasma triglyceride levels compared to wild-type mice (Fig. 3 a and b). Antisense treatment, especially with AS3 and AS1+AS3, reduced the elevated lipid content in livers 70% of the way toward normal, as evidenced by measurement of tissue triglyceride content and oil red O staining (Fig. 3a). Likewise, the antisense treatment resulted in reductions of the elevated plasma triglyceride concentrations 45–75% toward normal (Fig. 3b). These changes in hepatic and plasma triglycerides paralleled the changes in the expression of SREBP-1c (Fig. 3c). SREBP-1c was prominently up-regulated in control db/db mice (5.49 ± 0.14-fold increase compared to wild-type, n = 6), and this up-regulation was decreased by treatment with AS1 (2.84 ± 0.36-fold, n = 6) and almost completely normalized by administration of AS3 (1.35 ± 0.15-fold, n = 6) or AS1 + 3 (1.01 ± 0.14-fold, n = 6) (Fig. 3c). The protein levels of nuclear SREBP-1c changed in parallel with the mRNA levels (Fig. 3d). Expression of fatty acid synthase, a target of SREBP-1 (31), also paralleled the changes in SREBP-1c produced by suppression of SOCS proteins (Fig. 3c), suggesting that the lipid content was decreased by antisense treatment through down-regulating SREBP-1c and its downstream target genes.
SOCS-1 and -3 Enhance SREBP-1c Promoter Activity That Is Down-Regulated by STAT3. The mechanism of up-regulation of SREBP-1c expression in insulin-resistant states is poorly understood, although insulin has been suggested to increase SREBP-1c expression in vivo (36). To address how SOCS proteins regulate SREBP-1c expression, we assessed promoter activity of SREBP-1c using well-differentiated Fao rat hepatoma cells. We found that expression of SOCS-1 or -3 enhanced SREBP-1c promoter activity (Fig. 4a) at a time when these same SOCS proteins inhibit insulin signaling, suggesting that SOCS proteins modulate SREBP-1c expression by an insulin-independent mechanism. Indeed, obese animals show abnormal up-regulation of SREBP-1c expression in the fasting state (34), although persistent hyperinsulinemia caused by insulin resistance can also contribute to the up-regulation of SREBP-1c expression by elevating basal insulin signaling.
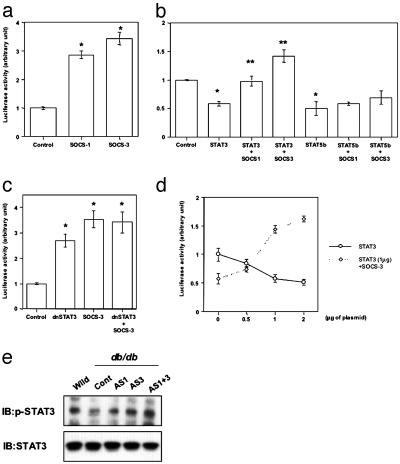
Fig. 4.
SOCS-1 and -3 enhance SREBP-1c promoter activity by attenuating STAT3-mediated inhibition. (a) Enhanced promoter activity of SREBP-1c by SOCS-1 and -3. (b) Inhibition of SREBP-1c promoter activity by STAT3 and STAT5b and antagonization of only STAT3-induced inhibition by SOCS-1 and -3. (c) Up-regulation of SREBP-1c promoter activity by dnSTAT3. Bars represent mean ± SE (n = 4in a–c). [*, P < 0.05 control vs. SOCS-1 or -3 (a), control vs. STAT3 or STAT5b (b), control vs. dnSTAT3 or SOCS-3 or dnSTAT3 + SOCS-3 (c); **, P < 0.05 STAT3 vs. STAT3 = SOCS-1 or STAT3 + SOCS-3]. (d) Dose-dependent inhibition of SREBP-1c promoter activity by STAT3 and dose-dependent antagonization of STAT3-mediated inhibition by SOCS-3. Results are expressed as mean ± SE (n = 4). (e) Restoration of STAT3 phosphorylation in liver of db/db mouse by suppression of SOCS proteins. The immunoprecipitates with anti-STAT3 antibody from liver lysates of the indicated mouse were immunoblotted with antiphospho-STAT3 antibody (αp-STAT3) (Upper) or the same antibody (Lower).
SOCS proteins have been identified as negative regulators of STAT proteins, and the promoter regions of the mouse and rat SREBP-1c genes have at least two potential STAT-binding motifs [TT(N)5AA] at –616 and –540 in the murine sequence. This raises the possibility that STAT proteins could regulate SREBP-1c promoter activity directly or indirectly by modulating expression of other regulatory factors. Indeed, of several STAT proteins tested in transfection experiments, STAT3 and -5b showed inhibitory effects on SREBP-1c promoter activity (Fig. 4b; also see Fig. 8a, which is published as supporting information on the PNAS web site). The STAT3-mediated inhibition was antagonized by coexpression of SOCS-1 or -3, whereas STAT5b-mediated inhibition was little affected (Fig. 4b). Similarly, STAT3-mediated inhibition of SREBP-1c transcription and SOCS-mediated antagonism were also observed in HepG2 human hepatoma cells (Fig. 8b). By contrast, expression of a dominant negative form of STAT3 (dnSTAT3), which inhibits activation of endogenous STAT3, significantly increased SREBP-1c promoter activity. The effect of dnSTAT3 was slightly less than that produced by SOCS-3. Furthermore, the combination of dnSTAT3 and SOCS-3 was not additive, suggesting that SOCS proteins increase SREBP-1c expression, at least in part, through STAT3-mediated inhibition. Indeed, STAT3 inhibited SREBP-1c promoter activity in a dose-dependent manner, and SOCS-3 appeared to competitively attenuate this inhibition (Fig. 4d). Consistent with this, STAT3 phosphorylation was decreased by 50% in liver of the control db/db mouse and was restored by suppression of SOCS proteins (Fig. 4e), whereas there was no alteration in STAT5 phosphorylation among all groups (Fig. 8c).
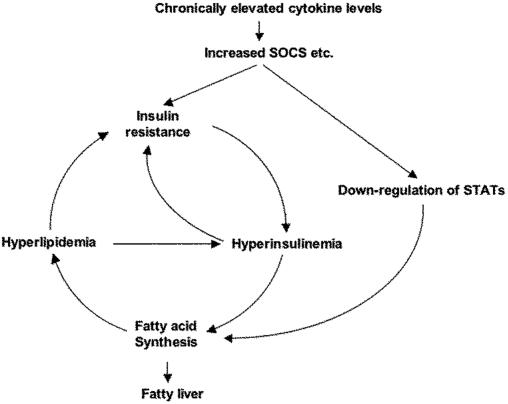
Recently, Kasuga and coworkers (37) have shown that liver-specific STAT3 knockout causes marked increases in SREBP-1 expression and hepatic triglyceride content, and that adenoviral-mediated gene transfer of a constitutively active form of STAT3 in liver of db/db mouse ameliorates hepatic steatosis with down-regulation of SREBP-1 expression. Together with our data, this indicates the importance of SOCS proteins and STAT3 in the regulation of lipid synthesis in liver and a paradigm for integrating several components of the metabolic syndrome, insulin resistance, dyslipidemia, and hepatic steatosis (nonalcoholic fatty liver) in a common regulatory pathway (Fig. 5).
Fig. 5.
Increased SOCS proteins induced by proinflammatory cytokines can be a common path to components of the metabolic syndrome and establish a vicious cycle.
Conclusion
Obese subjects with persistently elevated cytokine levels may have down-regulated STAT3-mediated signaling and insulin signaling by increased SOCS proteins in liver. Because liver is the primary site for insulin clearance (19, 38), insulin resistance caused by increased SOCS proteins in liver may lead to persistent hyperinsulinemia that further exacerbates insulin resistance (19, 38, 39). Thus, increased SOCS proteins concordantly increase fatty acid synthesis by up-regulation of SREBP-1c expression presumably through suppression of STAT3 phosphorylation and persistent hyperinsulinemia even in the fasting state. Overproduction of fatty acid and lipotoxicity results in further insulin resistance (35), creating a vicious cycle leading to the metabolic syndrome (Fig. 5). The present study indicates that the overexpression of SOCS proteins may be a critical step in this vicious cycle, and that reducing expression of SOCS proteins in liver presents a previously undescribed approach to treatment/prevention of hepatic steatosis and several components of the metabolic syndrome associated with diabetes and obesity.
Supplementary Material
Acknowledgments
We thank T. Naka (Osaka University, Osaka) for STAT-induced STAT inhibitor/SOCS expression vectors, T. Kadowaki (University of Tokyo) for the cDNA fragment of SREBP-1 and useful suggestions, B. M. Spiegelman (Dana–Farber Cancer Institute, Boston) for the cDNA fragment of PGC-1α, H. Shimano (University of Tsukuba, Tsukuba, Japan) for SREBP-1c promoter constructs, A. Miyajima (Institute of Cellular and Molecular Bioscience, Tokyo) for a dnSTAT3 expression vector, T. Tanaka (Harvard School of Public Health, Boston) for STAT expression vectors, M. Kaneki (Massachusetts General Hospital, Boston) for helpful suggestions, and E. Fletcher and L. Garcia for technical assistance. This work was supported by National Institutes of Health Grants DK33201 and DK55545, Joslin Diabetes and Endocrinology Research Center Grant DK34834 (to C.R.K.), and a Grant-in-Aid for the 21st Century Center of Excellence Program from the Ministry of Education, Culture, Sports, Science, and Technology (to K.U.)
Abbreviations: SOCS, suppressor of cytokine signaling; IRS, insulin receptor substrate; PGC-1α, peroxisome proliferator-activated receptor γ-coactivator-1α; SREBP, sterol regulatory element-binding protein; STAT, signal transducer and activator of transcription; dn, dominant negative.
References
- 1.Spiegelman, B. M. & Flier, J. S. (2001) Cell 104, 531–543. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini, G., Brizi, M., Bianchi, G., Tomassetti, S., Bugianesi, E., Lenzi, M., McCullough, A. J., Natale, S., Forlani, G. & Melchionda, N. (2001) Diabetes 50, 1844–1850. [DOI] [PubMed] [Google Scholar]
- 3.Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E. & Ridker, P. M. (2001) J. Am. Med. Assoc. 286, 327–334. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. (1993) Science 259, 87–91. [DOI] [PubMed] [Google Scholar]
- 5.Pickup, J. C. & Crook, M. A. (1998) Diabetologia 41, 1241–1248. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil, G. S., Peraldi, P., Budavari, A., Ellis, R., White, M. F. & Spiegelman, B. M. (1996) Science 271, 665–668. [DOI] [PubMed] [Google Scholar]
- 7.Yasukawa, H., Sasaki, A. & Yoshimura, A. (2000) Annu. Rev. Immunol. 18, 143–164. [DOI] [PubMed] [Google Scholar]
- 8.Starr, R., Willson, T. A., Viney, E. M., Murray, L. J., Rayner, J. R., Jenkins, B. J., Gonda, T. J., Alexander, W. S., Metcalf, D., Nicola, N. A. & Hilton, D. J. (1997) Nature 387, 917–921. [DOI] [PubMed] [Google Scholar]
- 9.Naka, T., Narazaki, M., Hirata, M., Matsumoto, T., Minamoto, S., Aono, A., Nishimoto, N., Kajita, T., Taga, T., Yoshizaki, K., et al. (1997) Nature 387, 924–929. [DOI] [PubMed] [Google Scholar]
- 10.Endo, T. A., Masuhara, M., Yokouchi, M., Suzuki, R., Sakamoto, H., Mitsui, K., Matsumoto, A., Tanimura, S., Ohtsubo, M., Misawa, H., et al. (1997) Nature 387, 921–924. [DOI] [PubMed] [Google Scholar]
- 11.Narazaki, M., Fujimoto, M., Matsumoto, T., Morita, Y., Saito, H., Kajita, T., Yoshizaki, K., Naka, T. & Kishimoto, T. (1998) Proc. Natl. Acad. Sci. USA 95, 13130–13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasukawa, H., Misawa, H., Sakamoto, H., Masuhara, M., Sasaki, A., Wakioka, T., Ohtsuka, S., Imaizumi, T., Matsuda, T., Ihle, J. N. & Yoshimura, A. (1999) EMBO J. 18, 1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito, H., Morita, Y., Fujimoto, M., Narazaki, M., Naka, T. & Kishimoto, T. (2000) J. Immunol. 164, 5833–5843. [DOI] [PubMed] [Google Scholar]
- 14.Emanuelli, B., Peraldi, P., Filloux, C., Sawka-Verhelle, D., Hilton, D. & Van Obberghen, E. (2000) J. Biol. Chem. 275, 15985–15991. [DOI] [PubMed] [Google Scholar]
- 15.Zong, C. S., Chan, J., Levy, D. E., Horvath, C., Sadowski, H. B. & Wang, L. H. (2000) J. Biol. Chem. 275, 15099–15105. [DOI] [PubMed] [Google Scholar]
- 16.Kawazoe, Y., Naka, T., Fujimoto, M., Kohzaki, H., Morita, Y., Narazaki, M., Okumura, K., Saitoh, H., Nakagawa, R., Uchiyama, Y., et al. (2001) J. Exp. Med. 193, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starr, R., Metcalf, D., Elefanty, A. G., Brysha, M., Willson, T. A., Nicola, N. A., Hilton, D. J. & Alexander, W. S. (1998) Proc. Natl. Acad. Sci. USA 95, 14395–14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naka, T., Matsumoto, T., Narazaki, M., Fujimoto, M., Morita, Y., Ohsawa, Y., Saito, H., Nagasawa, T., Uchiyama, Y. & Kishimoto, T. (1998) Proc. Natl. Acad. Sci. USA 95, 15577–15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michael, M. D., Kulkarni, R. N., Postic, C., Previs, S. F., Shulman, G. I., Magnuson, M. A. & Kahn, C. R. (2000) Mol. Cell. 6, 87–97. [PubMed] [Google Scholar]
- 20.Yoon, J. C., Puigserver, P., Chen, G., Donovan, J., Wu, Z., Rhee, J., Adelmant, G., Stafford, J., Kahn, C. R., Granner, D. K., et al. (2001) Nature 413, 131–138. [DOI] [PubMed] [Google Scholar]
- 21.Ueki, K., Yamauchi, T., Tamemoto, H., Tobe, K., Yamamoto-Honda, R., Kaburagi, Y., Akanuma, Y., Yazaki, Y., Aizawa, S., Nagai, R. & Kadowaki, T. (2000) J. Clin. Invest. 105, 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkai, U., Prigent-Tessier, A., Tessier, C., Gibori, G. B. & Gibori, G. (2000) Mol. Endocrinol 14, 554–563. [DOI] [PubMed] [Google Scholar]
- 23.Butler, M., McKay, R. A., Popoff, I. J., Gaarde, W. A., Witchell, D., Murray, S. F., Dean, N. M., Bhanot, S. & Monia, B. P. (2002) Diabetes 51, 1028–1034. [DOI] [PubMed] [Google Scholar]
- 24.Laustsen, P. G., Michael, M. D., Crute, B. E., Cohen, S. E., Ueki, K., Kulkarni, R. N., Keller, S. R., Lienhard, G. E. & Kahn, C. R. (2002) Genes Dev. 16, 3213–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amemiya-Kudo, M., Shimano, H., Yoshikawa, T., Yahagi, N., Hasty, A. H., Okazaki, H., Tamura, Y., Shionoiri, F., Iizuka, Y., Ohashi, K., et al. (2000) J. Biol. Chem. 275, 31078–31085. [DOI] [PubMed] [Google Scholar]
- 26.Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L. & Ferrante, A. W., Jr. (2003) J. Clin. Invest. 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu, H., Barnes, G. T., Yang, Q., Tan, G., Yang, D., Chou, C. J., Sole, J., Nichols, A., Ross, J. S., Tartaglia, L. A. & Chen, H. (2003) J. Clin. Invest. 112, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emanuelli, B., Peraldi, P., Filloux, C., Chavey, C., Freidinger, K., Hilton, D. J., Hotamisligil, G. S. & Van Obberghen, E. (2001) J. Biol. Chem. 276, 47944–47999. [DOI] [PubMed] [Google Scholar]
- 29.Ueki, K., Kondo, T. & Kahn, C. R. (2004) Mol. Cell. Biol. 24, 5434–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rui, L., Yuan, M., Frantz, D., Shoelson, S. & White, M. F. (2002) J. Biol. Chem. 277, 42394–42398. [DOI] [PubMed] [Google Scholar]
- 31.Horton, J. D., Goldstein, J. L. & Brown, M. S. (2002) J. Clin. Invest. 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn, C. R. & Roth, J. (1975) Am. J. Clin. Pathol. 63, 656–667. [DOI] [PubMed] [Google Scholar]
- 33.Yuan, M., Konstantopoulos, N., Lee, J., Hansen, L., Li, Z. W., Karin, M. & Shoelson, S. E. (2001) Science 293, 1673–1677. [DOI] [PubMed] [Google Scholar]
- 34.Shimomura, I., Bashmakov, Y. & Horton, J. D. (1999) J. Biol. Chem. 274, 30028–30032. [DOI] [PubMed] [Google Scholar]
- 35.Shulman, G. I. (2000) J. Clin. Invest. 106, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimomura, I., Bashmakov, Y., Ikemoto, S., Horton, J. D., Brown, M. S. & Goldstein, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue, H., Ogawa, W., Ozaki, M., Haga, S., Matsumoto, M., Furukawa, K., Hashimoto, N., Kido, Y., Mori, T., Sakaue, H., et al. (2004) Nat. Med. 10, 168–174. [DOI] [PubMed] [Google Scholar]
- 38.Poy, M. N., Yang, Y., Rezaei, K., Fernstrom, M. A., Lee, A. D., Kido, Y., Erickson, S. K. & Najjar, S. M. (2002) Nat. Genet. 30, 270–276. [DOI] [PubMed] [Google Scholar]
- 39.Shimomura, I., Matsuda, M., Hammer, R. E., Bashmakov, Y., Brown, M. S. & Goldstein, J. L. (2000) Mol. Cell. 6, 77–86. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.