Abstract
Objective
To identify a unifying cardiac pathophysiology that explains the cardiac pathology in SCD.
Background
Cardiopulmonary complications, the leading cause of adult mortality in sickle cell disease (SCD), are associated with heart chamber dilation, diastolic dysfunction, elevated tricuspid regurgitant jet velocity (TRV) and pulmonary hypertension (PH). However, no unifying cardiac pathophysiology has been identified to explain these findings.
Methods
In a two-part study, we first examined SCD patients who underwent screening echocardiography during steady state at our institution. We then conducted a meta-analysis of cardiac studies in SCD.
Results
In the 134 SCD patients studied (median age 11 years), a significant enlargement of the left atrial volume was present (z-score 3.1, P=0.002), shortening fraction (SF) was normal (37.6 ± 4.7%), and lateral and septal ratios of mitral velocity to early diastolic velocity of the mitral annulus (E/e′) were severely abnormal in 8% and 14% of patients, respectively, indicating impaired diastolic function. Both TRV and lateral E/e′ correlated with enlarged left atrial volume in SCD (P=0.003 and P=0.006, respectively). Meta-analysis of 68 studies confirmed significant left atrial diameter enlargement in SCD patients compared to controls, evidence of diastolic dysfunction and enlarged left ventricular end-diastolic dimension with normal SF. The majority of patients with catheter-confirmed PH had mild pulmonary venous hypertension consistent with restrictive cardiac physiology.
Conclusions
Patients with SCD have a unique cardiomyopathy with restrictive physiology that is superimposed on hyperdynamic physiology, and is characterized by diastolic dysfunction, left atrial dilation and normal systolic function. This results in mild, secondary, pulmonary venous hypertension and elevated TRV. Sudden death is common in other forms of restrictive cardiomyopathy. Our finding of this unique restrictive cardiomyopathy may explain the increased mortality and sudden death seen in patients with SCD with mildly elevated TRV.
Keywords: sickle cell disease, cardiomyopathy, restrictive physiology, pulmonary hypertension
Introduction
Sickle cell disease (SCD) refers to a group of inherited blood disorders caused by abnormal hemoglobin, sickle hemoglobin (HbS), that polymerizes upon deoxygenation. With newborn screening and comprehensive care, most individuals with SCD born in developed countries survive into adulthood, albeit with significantly shortened life expectancy. Cardiopulmonary complications are the most common cause of death in adults with SCD(1). Heart chamber dilation, diastolic dysfunction, and elevated pulmonary arterial pressures (PAP) are commonly reported findings in SCD, but a unifying pathophysiologic explanation has not been recognized. Many studies have characterized the pulmonary hypertension (PH) in patients with SCD, advancing the concept that increased PAP results from pulmonary vascular endothelial dysfunction(2–4). Mildly increased PAP occurs in ~30% of adults and children with SCD when estimated by an increased (>2.5 m/sec) tricuspid regurgitant jet velocity (TRV) on echocardiography(2,5,6). However, the gold standard for the diagnosis of PH, right heart catheterization (RHC), demonstrates that PH is less prevalent than TRV-based estimates. Only 30% of patients with increased TRV have PH confirmed by RHC. In addition, the degree of PH, which is often mild in individuals with SCD, does not readily explain the full extent of cardiac morbidity and frequency of early mortality in SCD(7). Nevertheless, TRV, pulmonary pressure and diastolic dysfunction are clearly associated with increased mortality and sudden death in SCD(2,7,8). The causes of cardiac-related mortality in the absence of apparently clinically significant PH have remained unclear.
Here we describe a consistent pattern of cardiac pathophysiology in children and adults with SCD at our institution, confirm this pattern in a comprehensive meta-analysis of the published cardiac literature in SCD, challenge the conventional wisdom about the cardiopulmonary pathophysiology of SCD, and propose an explanation for its association with sudden death. Specifically, we find that patients with SCD have a unique cardiomyopathy with superimposed restrictive and hyperdynamic physiology that has not been previously recognized. Similar to other forms of restrictive cardiomyopathy (RCM), the SCD-related cardiomyopathy with restrictive physiology can explain the mild elevations in TRV without the need to invoke pulmonary arterial endothelial dysfunction.
Methods
Study Design
We conducted a combined retrospective and prospective study of patients with SCD treated at Cincinnati Children’s Hospital Medical Center, who underwent clinical screening echocardiography at steady-state (no acute SCD events 3 weeks before or afterwards), between 2007–2010 using similar protocols. Of the 134 patients aged 3–22 years, 102 had homozygous SCD (Hb SS), 21 had sickle-hemoglobin C disease (Hb SC), 2 had sickle-β0-thalassemia, and 9 had sickle-β+-thalassemia. Laboratory data were also obtained at time of echocardiography. The Institutional Review Board approved this research protocol and waived the requirement for written informed consent.
Trans-thoracic Echocardiography was performed using 2-dimensional, M-mode, pulse-wave Doppler, color-flow Doppler, and tissue Doppler imaging with a GE Vivid 7 (Milwaukee, Wisconsin) ultrasound system. Images and views were acquired according to the American Society of Echocardiography standards. Pulsed-wave Doppler was used to measure mitral valve inflow velocity peak at early filling (E) and late filling (A) in a standard manner. Tissue Doppler imaging was used to determine mitral valve annular velocities in early (e′) and late diastole (a′) at both the septal (septal e′) and lateral annulus (lateral e′). Details of echocardiographic measurement and methods are described in Supplementary Methods.
Systematic Review for Meta-analysis
MEDLINE, PubMed, Ovid and Google Scholar databases were searched for the following terms: sickle cell disease, sickle cell anemia and sickle cell in combination with each of the following: heart, cardiac, pulmonary hypertension, echocardiography, echocardiogram, cardiac MRI, cardiac MR, CMR, cardiomyopathy, catheterization and hemodynamic. Articles that contained 2-D-, M-mode echocardiographic, Doppler, tissue Doppler, cardiac catheterization or CMR data in SCD patients were retrieved for full text review. Studies that lacked quantitative data (mean, standard deviation [SD], or number of observations) in both SCD and control groups were excluded from the final quantitative analysis. However, these excluded articles were reviewed separately. An overview of the selection process is shown in figure S1.
Statistical Analysis
Continuous and categorical variables were expressed as mean±SD. Student’s t-tests were used to compare continuous variables and χ2 tests were used for categorical variables across groups. Variables were assessed for normality using the Shapiro-Wilk test. Associations between approximately normally distributed continuous variables were calculated using Pearson correlation coefficient, otherwise Spearman rank correlation coefficients were used. Significance of echocardiographic z-scores was estimated using area under the standard normal distribution curve tables for each z-score value.
For the meta-analysis, cochran’s Q test was used to detect heterogeneity and I2 was used to estimate the amount of heterogeneity among studies(9). The 95% confidence intervals for individual studies and random-effects WM and WMD were determined using relevant quantiles of the standard normal distribution. Because of the degree of heterogeneity among studies, statistical significance was determined based on the results of random-effects approach (10). All statistical analyses were performed using Prism 6.0 (GraphPad Software, Inc.) and Excel (Microsoft, Inc.). Differences were considered statistically significant at P<0.05.
Results
Local patient characteristics
The characteristics of 134 SCD patients, mainly children and young adults, are shown in Table S1 (supplementary data). Median age was 11 years (range 3–22years). Twenty-six patients (19%) were ≥18 years. Most (76%) had HbSS.
Patients with SCD have left atrial enlargement
Echocardiographic findings are shown in Table 1. Left atrial volume (LAV) index, left atrial diameter (LAD), left ventricular end-diastolic diameter (LVED), posterior wall thickness (PWT), interventricular septal diameter (IVSD) and indexed left ventricular mass (LVMi) were numerically higher in patients with SCD compared to normal values for age and body size. However, only LAV and LAD were significantly enlarged with an average z-score of 3.1 (P=0.002) and 2.19 (P=0.02), respectively. The two most common abnormalities were left atrial (LA) enlargement (62%) and high LVMi (57%).
Table 1.
The Echocardiographic Characteristics of SCD Patients.
| Echocardiographic measurement | No. of Patients | Value | Z-score | P * | Abnormal (%) |
|---|---|---|---|---|---|
| Left atrial volume index (ml/m2) | 123 | 33.7± 9.9 | 3.1± 2.5 | 0.002 | 76 (62) |
| Left atrial diameter (cm) | 134 | 3.29 ± 0.57 | 2.19 ±1.1 | 0.02 | 77 (57)† |
| Right ventricular diameter (cm) | 134 | 1.90 ± 0.48 | −0.32 ±0.98 | 0.76 | 0 (0)† |
| Interventricular septal diameter (cm) | 134 | 0.79 ± 0.17 | 0.66 ±0.89 | 0.51 | 7 (5)† |
| Left ventricular end-diastolic dimension (cm) | 134 | 4.75 ± 0.64 | 0.79 ±1.15 | 0.43 | 19 (14)† |
| Posterior wall thickness (cm) | 134 | 0.78 ± 0.17 | 1.13 ±1 | 0.26 | 0 (0)† |
| Shortening fraction (%) | 134 | 37.6 ± 4.7 | 0.14 ±1.14 | 0.89 | 6 (4)‡ |
| Indexed left ventricular mass (g/m2.7) | 134 | 44.1 ± 12.3 | N/A | N/A | 77 (57)§ |
| Tricuspid regurgitant jet velocity (m/sec) | 96 | 2.16 ±0.3 | N/A | N/A | 13 (14)II |
N/A: not applicable.
Values expressed as mean ± SD
P value for average z-score (comparison to normal)
Number (percentage) of patients with z-score value>1.96
Number (percentage) of patients with shortening fraction values <29%
Number (percentage) of patients with LVMi values >95th percentile for gender and age.
Number (percentage) of patients with TRV >2.5m/sec.
The LAD was disproportionately enlarged compared to other M-mode chamber measurements. One hundred and twenty-four patients (92.5%) had higher LAD z-scores than LVED z-scores. No patient had mitral regurgitation.
Mean TRV was 2.16±0.3 in 96 patients with measurable TRV. Thirteen patients (13.5%) had TRV≥2.5m/sec, all of whom had HbSS. None had a TRV≥3m/sec. All had normal systolic function (mean shortening fraction [SF]=37.6%). Taken together, these data show that young SCD patients (median age 11 years) have significant and disproportionate LA dilation with normal systolic function. Most (86.5%) do not have increased TRV.
Patients with SCD have diastolic dysfunction
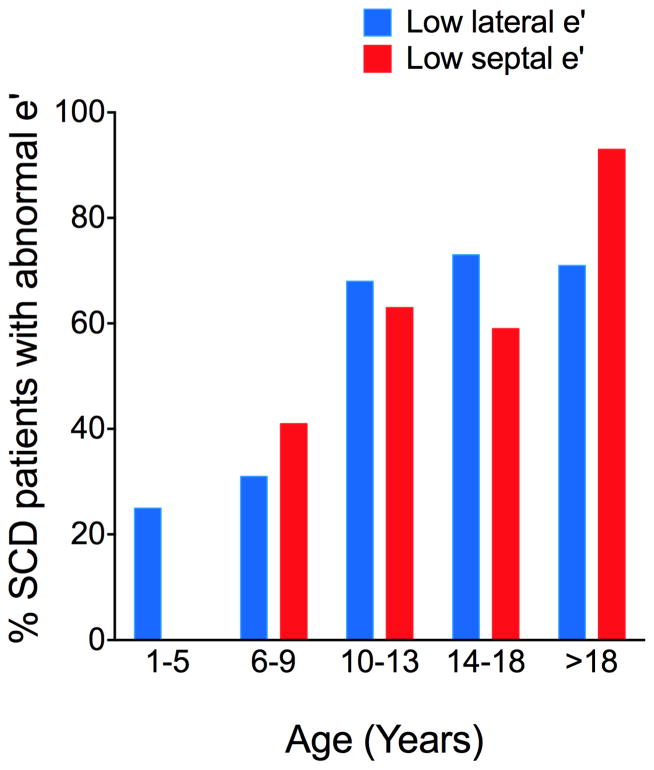
The mitral valve annular velocity e′ correlates with LV relaxation(11). In our cohort, we observed a strong trend toward low lateral and septal e′, consistent with diastolic dysfunction. Notably, lower lateral and septal e′ values were most prevalent in older SCD patients, which is the opposite of that seen in normal pediatric patients, where lateral and septal e′ increases with increasing age(12). In children, the decreasing lateral and septal e′ values with increasing age suggests that diastolic function worsens with age (Figure 1).
Figure 1. The Prevalence of Low Lateral e′ and Septal e′ Increases with Age.
The percentage of SCD with low lateral and septal e′ (z-score<−1) in the age groups: 1–5 years, 6–9 years, 10–13 years, 14–18 years and older than 18 years of age.
A restrictive pattern of diastolic function was observed despite the young age of these patients (Table 2). There was a trend towards increasing E-wave and E/A ratio in SCD patients in general, while 24 patients (18%) and 4 patients (3%) had severely abnormal E and E/A ratio, respectively, consistent with restrictive diastolic dysfunction. In addition, the E/e′ ratio which correlates with LV filling pressure(13) and reported to be abnormal in SCD(4–6), was also severely abnormal in 10 patients (8%) and 18 patients (14%) for lateral and septal E/e′, respectively (Table 2). Together, the pattern of diastolic dysfunction, LA dilation and normal systolic function observed in young patients with SCD is most consistent with a cardiomyopathy with restrictive physiology(14).
Table 2.
Mitral Valve Velocities By Doppler and Tissue Doppler Imaging.
| Echocardiographic measurement | No. of Patients | Value | Average z-score | Abnormal (%)* |
|---|---|---|---|---|
| E (cm/sec) | 134 | 110 ± 18 | 1 ±1.1 | 24 (18) |
| A (cm/sec) | 133 | 59 ± 17 | 0.8 ±1.3 | 22 (17) |
| E/A ratio | 133 | 1.96 ± 0.49 | −0.1 ±0.9 | 4 (3) |
| Lateral E/e′ ratio | 126 | 6.2 ± 1.3 | 0.8 ±0.9 | 10 (8) |
| Septal E/e′ ratio | 126 | 8.1 ± 1.7 | 0.9 ±1.2 | 18 (14) |
Values expressed as mean±SD except *.
Number (percentage) of patients with z-score >1.96
SCD patients with increased TRV have restrictive physiology
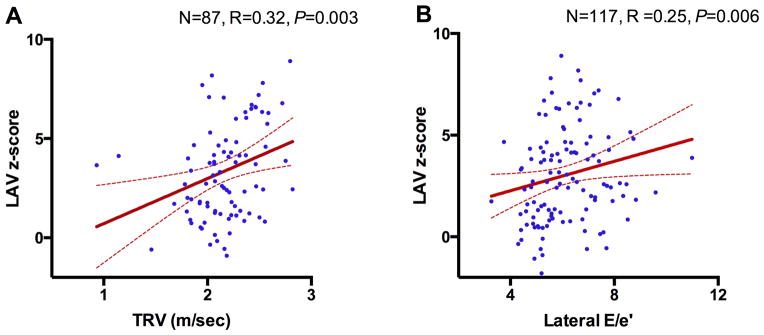
High TRV correlates with increased PAP and increased mortality in adults with SCD(2), postulated to be the result of pulmonary arterial endothelial dysfunction due to intravascular hemolysis(15). However, restrictive physiology also increases PAP and TRV secondary to increased LA pressure(16). To determine the relationship between diastolic function and TRV in this population, we compared two sub-groups based on TRV. SCD patients with TRV≥2.5m/sec in our cohort had significantly larger LA than those with TRV<2.5m/sec (P=0.03 for LAV and P=0.002 for LAD) (Table 3). They also had higher A-wave z-score, which reflects LA-LV pressure gradient during late diastole and is affected by LV compliance and LA contraction(11). The TRV≥2.5m/sec group also had significantly higher lateral E/e′ and lower septal e′/a′ ratios. Septal e′/a′ ratio has been reported to be abnormal and an independent predictor of mortality in SCD(7). The TRV≥2.5m/sec group had numerically higher reticulocytes, leukocytes, platelets, LDH and bilirubin levels, and lower hemoglobin concentrations, but none of these differences was statistically significant. While systolic function was normal in both groups, the echocardiographic estimate of LV filling pressure, lateral E/e′, was significantly associated with TRV and LAV z-score but not with LVED z-score or LVMi (Figure 2, Figure S3 and Table S2), which indicates that patients with TRV≥2.5m/sec have features of restrictive physiology. Next, we examined whether the association between LAV, diastolic dysfunction and TRV is also observed with LVED or LVMi, which are directly related to anemia(17,18). As anticipated, LAV z-score, LVED z-score and LVMi were associated with the degree of anemia, reticulocyte count, LDH, and bilirubin (Table 4). However, high TRV and the diastolic measures E, and lateral E/e′, were only associated with LAV z-score but not with LVED z-score or LVMi (Table 4 and Figure 2). Together, the association of TRV, LAV and diastolic dysfunction is strongly suggestive of distinct SCD-related cardiomyopathy with restrictive physiology superimposed upon anemia-related high-output cardiac physiology. While unlikely, chronic anemia may be contributing to the degree of LA enlargement; however, in the typical patient with significant anemia, a dilated LV is common (due to high-output state), but LA dilation without mitral regurgitation is rare.
Table 3.
Characteristics of SCD Patients According to Tricuspid Regurgitant Jet Velocity.
| Characteristic | TRV < 2.5 m/sec (n=93) | TRV ≥ 2.5 m/sec (n=13) | |||||
|---|---|---|---|---|---|---|---|
| No. | Value | z-score | No. | Value | z-score | P value | |
| Age (yr) | 83 | 11.7 ±4.2 | 13 | 12.7 ±5.5 | 0.47 | ||
| Hemoglobin SS (%) | 83 | 63 (76) | 13 | 13 (100) | <0.0001 | ||
| WBC (103/mm3) | 77 | 10.3 ±3.7 | 12 | 10.5 ±3.3 | 0.86 | ||
| Hemoglobin (g/dL) | 77 | 9.8 ±1.6 | 12 | 9.2 ±1 | 0.2 | ||
| Platelets (103/mm3) | 77 | 402 ±139 | 12 | 434 ±121 | 0.45 | ||
| Reticulocyte count (%) | 78 | 7.8 ±5 | 12 | 10.6 ±3.5 | 0.06 | ||
| LDH (units/L) | 64 | 1267 ±530 | 12 | 1395 ±636 | 0.38 | ||
| Unconjugated bilirubin (mg/dL) | 77 | 2.5 ±3 | 12 | 3.6 ±1.6 | 0.19 | ||
| Shortening fraction (%) | 83 | 37.6 ±4.6 | 13 | 37.7 ±4.5 | 0.97 | ||
| Indexed left ventricular mass (g/m2.7) | 71 | 44.5 ±14.4 | 11 | 44.8 ±11.1 | 0.94 | ||
| Left atrial volume index (ml/m2) | 73 | 32.5±8.9 | 2.7± 2.2 | 12 | 38.8± 12.5 | 4.4 ± 3.2 | 0.03 |
| Left atrial diameter (cm) | 83 | 3.25 ± 0.52 | 2.3 ±1 | 13 | 3.78 ± 0.71 | 3.2 ±1.1 | 0.002 |
| Right ventricular diameter (cm) | 83 | 1.9 ± 0.4 | −0.3 ±1 | 13 | 2.17 ± 0.63 | 0.16 ±1.1 | 0.1 |
| Intraventricular septal diameter (cm) | 83 | 0.79 ± 0.16 | 0.8 ±0.8 | 13 | 0.80 ± 0.29 | 0.6 ±1 | 0.45 |
| Left ventricular end-diastolic dimension (cm) | 83 | 4.74 ± 0.66 | 0.9 ±1 | 13 | 4.89 ± 0.63 | 1.2 ±0.9 | 0.45 |
| Posterior wall thickness (cm) | 83 | 0.78 ± 0.17 | 1.2 ±1 | 13 | 0.79 ± 0.22 | 1.1 ±0.7 | 0.7 |
| E (cm/sec) | 83 | 109 ± 17 | 1 ±1.1 | 13 | 119 ± 15 | 1.6 ±1 | 0.07 |
| A (cm/sec) | 82 | 57 ± 14.7 | 0.6 ±1.2 | 13 | 72 ± 22.9 | 1.7 ±2 | 0.009 |
| E/A ratio | 82 | 2.02 ± 0.5 | −0.03 ±0.9 | 13 | 1.79 ± 0.5 | −0.3 ±1 | 0.31 |
| Lateral e′ (cm/sec) | 78 | 18.5 ± 2.8 | −0.16 ±0.8 | 13 | 18 ± 3.9 | −0.3 ±1.1 | 0.52 |
| Lateral E/e′ ratio | 78 | 0.66 ±0.8 | 13 | 1.32 ±1.4 | 0.04 | ||
| Lateral e′/a′ ratio | 78 | 3.3 ±0.8 | 13 | 3 ±1.2 | 0.43 | ||
| Septal e′/a′ ratio | 78 | 2.5 ±0.6 | 13 | 2.1 ±0.4 | 0.04 | ||
TRV: Tricuspid regurgitant jet velocity.
Figure 2. LAV Association with TRV and Lateral E/e′.
The regression of (A) TRV and LAV z-score, and (B) lateral E/e′ and LAV z-score. Dashed lines represent 95-percent confidence interval.
Table 4.
Association of LAV z-score, LVED z-score and LVMi with Laboratory and Echocardiographic Variables.
| Laboratory or echocardiographic measurement | LAV z-score | LVED z-score | LVMi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | R | P | N | R | P | N | R | P | |
| WBC (103/mm3) | 110 | −0.1 | 0.27 | 120 | −0.03 | 0.71 | 105 | 0.16 | 0.11 |
| Platelets (103/mm3) | 110 | 0.14 | 0.15 | 120 | 0.13 | 0.16 | 105 | 0.02 | 0.84 |
| Hemoglobin (g/dL) | 110 | −0.47 | <0.001 | 120 | −0.4 | <0.001 | 105 | −0.30 | 0.002 |
| Reticulocyte count (%) | 110 | 0.44 | <0.001 | 120 | 0.27 | 0.003 | 105 | 0.33 | 0.001 |
| LDH (units/L) | 96 | 0.46 | <0.001 | 103 | 0.28 | 0.004 | 88 | 0.38 | <0.001 |
| Unconjugated bilirubin (mg/dL) | 112 | 0.27 | 0.005 | 122 | 0.29 | 0.001 | 105 | 0.25 | 0.01 |
| TRV (m/sec) | 87 | 0.32 | 0.003 | 96 | 0.14 | 0.19 | 82 | 0.21 | 0.07 |
| Shortening fraction (%) | 123 | −0.07 | 0.46 | 134 | −0.13 | 0.12 | 114 | −0.13 | 0.17 |
| E wave z-score | 123 | 0.36 | <0.001 | 134 | 0.15 | 0.08 | 114 | 0.10 | 0.31 |
| A wave z-score | 122 | 0.09 | 0.33 | 133 | 0.02 | 0.84 | 113 | 0.16 | 0.09 |
| E/A ratio z-score | 122 | 0.09 | 0.3 | 133 | 0.07 | 0.41 | 113 | −0.13 | 0.18 |
| Lateral E/e′ | 117 | 0.25 | 0.006 | 126 | 0.08 | 0.36 | 107 | 0.16 | 0.10 |
LAV: left atrial volume, LVED: left ventricular end-diastolic dimension, LVMi: indexed left ventricular mass.
Meta-analysis data supports a cardiomyopathy with restrictive physiology in SCD
In order to corroborate our findings, we conducted a meta-analysis of the published echocardiographic, CMR and cardiac catheterization data in patients with SCD. We identified 108 manuscripts for full text review, of which 68 manuscripts were included. Details of meta-analysis article selection and the list of the studies included is provided in figure S1 and supplementary references.
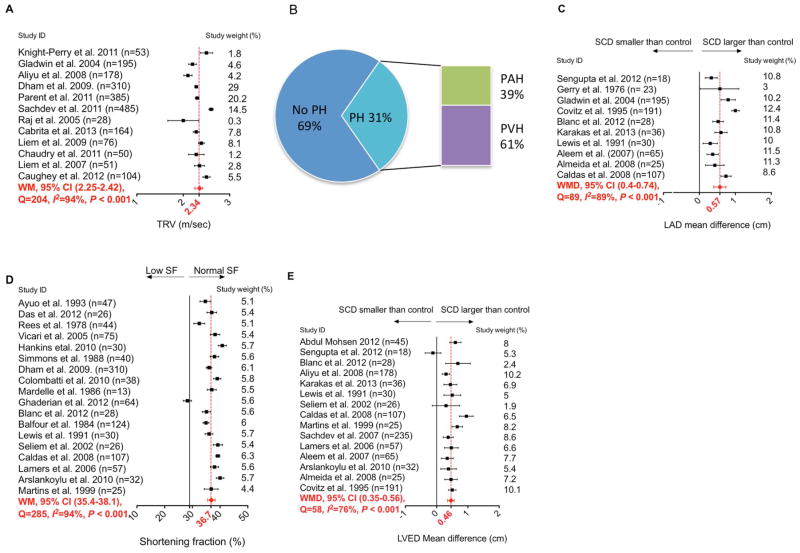
The WM TRV in SCD patients in 12 studies was 2.34m/sec (95%CI 2.25–2.42m/sec) (Figure 3A) (S.ref.8,16,22,27,32–39). Although TRV was associated with PH in SCD, the validity of using TRV as a surrogate measure for PH was not supported by hemodynamic data in some reports(3,19,20). RHC is the gold standard to diagnose PH. In four studies that performed RHC on almost all SCD patients with TRV≥2.5m/sec, only 53 out of 173 patients (31%) had confirmed PH [mean pulmonary artery pressure (mPAP) ≥25 mmHg] (Figure 3B) (S.ref.38,64–66). Thirty-two of these 53 patients with PH (60%) had pulmonary venous hypertension (PVH) defined by pulmonary capillary wedge pressure (PCWP) >15 mmHg, while 40% had pulmonary arterial hypertension (PAH).
Figure 3. Meta-analysis Summarizing the TRV, PH, LAD, SF and LVED in SCD Patients.
(A) Mean TRV values for SCD patients in different studies with weighted mean TRV and 95-percent confidence interval for the included studies. (B) The percentage of SCD patients with RHC-confirmed PH, PAH and PVH in patients that had TRV≥2.5 m/sec. Data are pooled from four different studies. (C) The mean difference between LAD in SCD and control groups in different studies, and the weighted mean difference with 95-percent confidence interval. (D) The mean SF for SCD population in different studies with weighted mean SF and 95-percent confidence interval. (E) The mean difference between LVED in SCD group and control group in different studies and the weighted mean difference with 95-percent confidence interval. Weighted mean and weighted mean difference values in plots A, C, D and E were calculated using a random-effects model. Each point represents mean value spanned by its 95-percent confidence interval.
In patients with RHC-confirmed PH, the WM for mPAP, systolic PAP, diastolic PAP and pulmonary vascular resistance were 33.8 mmHg (range 30–42mmHg), 41.9 mmHg (range 41–58mmHg), 23.3 mmHg (range 19–26mmHg) and 159.2 dyn·s/cm5 (range 137.6–300dyn·s/cm5), respectively (S.ref.38,64–70). These hemodynamic values are remarkably lower than those seen in idiopathic PH(21). Despite the high prevalence of PVH among patients with catheter-confirmed PH, cardiac output was normal to high (WM of 8.4 L/min [range 7.2–10.4 L/min]) in both PAH and PVH patients. Diastolic dysfunction, a common cause of PVH(22), was prevalent in 11% to 77% of SCD patients, depending on the criteria used to define diastolic dysfunction in each study. Despite this variability, nearly all (24 of 26) studies found evidence of diastolic dysfunction in SCD (Table S3) (S.ref.6–31), similar to our younger cohort.
LA enlargement was another common and concordant finding between our data and the meta-analysis. WMD of LAD between SCD patients and controls in 10 studies was 0.57cm (95%CI 0.40–0.74cm) (Figure 3C) (S.ref.9,12,13,21,23,30,35,41,43,44). Even in the studies excluded because they lacked quantitative data or used different measures for LA size, LA was consistently larger in SCD patients compared to controls (S.ref.6–8,16,22,24,26–28,31,40,42,45–47). LA dilation was also seen on electrocardiography (S.ref.13,26,52).
Systolic function was normal in the meta-analysis, with a WM SF of 36.7% (95%CI 35.4–38.1%) (Figure 3D) (S.ref.10,12–14,16,18,19,23,24,29,42,46,47,49,52,58,62,63). A similar finding was observed in studies that used other systolic measures, ejection fraction and stroke volume index (Figure S3 and S4). LVED was larger in SCD patients than controls in the meta-analysis. WMD for LVED was 0.46cm (95%CI 0.35–0.56cm) (Figure 3E) (S.ref.6,8–10,12,13,21,23,24,28–30,41,43,52). The degree of LV enlargement in this meta-analysis was greater than measured in our younger cohort.
In order to address the heterogeneity in meta-analysis results we conducted a meta-regression using the pre-defined variables: age, hemoglobin, date of publication, and origin of study (American or international). These variables were significant in a multivariate model that combines all factors, but not univariately, for LAD and TRV. This model was not significant for LVED or SF. The P values for heterogeneity after accounting for these factors were 0.16 and 0.002 for WMD LAD and WM TRV, respectively, suggesting that other factors contribute the heterogeneity observed in TRV results (Figure S5).
Discussion
In summary, our single-institution data and the meta-analysis support the existence of a unique cardiomyopathy with restrictive physiology in patients with SCD. Like other forms of RCM, the defining features are diastolic dysfunction, LA enlargement and normal systolic function. The standard definition of RCM excludes ventricular dilation to differentiate it from dilated cardiomyopathy; therefore, the SCD-related cardiomyopathy with restrictive physiology does not meet the classic definition of RCM. However, in SCD the LV enlargement is due to the anemia-related hyperdynamic circulation, and unlike dilated cardiomyopathy in which systolic function is decreased, systolic function in SCD is normal. The standard definitions do not account for this distinction. The cardiomyopathy of SCD, therefore, is best characterized as restrictive physiology with superimposed hyperdynamic circulation. This RCM can explain the mild, secondary elevations in PAP and TRV observed in SCD, unlike the severely increased PAP seen in primary PAH. Other forms of RCM are associated with sudden death, especially in times of stress, despite normal or mildly elevated pulmonary pressure. As such, a unique SCD-related cardiomyopathy with restrictive physiology and superimposed hyperdynamic circulation is a unifying explanation for the echocardiographic findings, normal or mildly increased pulmonary pressures (and TRV), and the association with early, sudden mortality.
There is a smaller, separate sub-group of patients with SCD (~3–4%) who have PAH explained by primary pulmonary arterial vascular disease, evidenced, for example, by pulmonary arterial plexiform lesions on autopsy specimens(23), and is plausibly explained by hemolysis-related endothelial dysfunction(15). However, it is not a parsimonious explanation for the broader constellation of features that is better explained by a unique SCD-related cardiomyopathy with restrictive physiology and secondary, mild elevations in pulmonary pressures due to PVH. The use and applicability of the adult diastolic heart failure grading guidelines(11) have not been validated in pediatrics. Indeed, a large proportion of patients with overt RCM are missed when the current guidelines were used to classify their diastolic dysfunction(24). In addition, there is high discordance between different parameters and poor inter-observer agreement in pediatric and adult studies(24,25). Different criteria are likely needed to diagnose and grade diastolic dysfunction in pediatrics. Although grade III/IV or “restrictive” diastolic dysfunction grades were not common in our study, abnormal diastolic function measures were prevalent in our cohort and in the meta-analysis. Diastolic dysfunction and LA enlargement with normal systolic function are seen in nearly every SCD study.
Diastolic dysfunction is an independent risk factor for early mortality in SCD(7,8). In our patients, diastolic dysfunction was associated with LA dilation, which was the most severely and disproportionately enlarged heart chamber. Our data are consistent with previous pediatric studies that showed LA dilation to be more common in young patients with SCD than enlargement of other chambers(26–29). This suggests that LA enlargement precedes enlargement of other chambers in SCD. In addition, LV dilation and LVH are known adaptive responses to anemia(18), and these worsen over time in SCD(17,28,30). Thus, LVED enlargement and LVH were more noticeable in meta-analysis results, compared to our data from a younger cohort of SCD patients than most studies in the meta-analysis. A recent publication described 4-chamber dilation, normal systolic function and diastolic dysfunction in adults with SCD(31) that contrasts with our data in a much younger cohort with milder anemia. With increasing age, LV dilation and LVH are expected to worsen, resulting in progressive 4-chamber dilatation. In a recent meta-analysis of systolic function in SCD, LV systolic function was normal in SCD patients although they had progressive LV dilation over time(32). Together, our data and these studies(31,32) suggest that LA dilation precedes pan-chamber dilation. Indeed, it was these characteristics of our patient population that allowed us to identify the features of cardiomyopathy with restrictive physiology that would otherwise be masked by enlarged ventricles in older patients. Interestingly, we observed a strong correlation between the features of SCD-related cardiomyopathy with restrictive physiology (LA enlargement and diastolic dysfunction) and TRV. The accuracy of defining PH using TRV in SCD has been challenged(3,19,20). Our meta-analysis also confirms these reports and shows that only 12% of SCD patients with TRV≥2.5m/sec have PAH. Importantly, most patients with catheter-confirmed PH were found to have PVH, which may be secondary to restrictive physiology. Recently, Sharma and colleagues showed that PCWP overestimates PAH in SCD and that the real prevalence of PVH may even be higher in SCD patients with PH(33). The typical RCM results in increased LA filling pressures, eventually causing PVH. This is another similarity between SCD-related cardiomyopathy with restrictive physiology and primary RCM(14,34). In addition, the strong association between TRV, LAV and lateral E/e′, which was also noted in our systematic review(4–8,29), further supports that high TRV in SCD is best considered a marker of cardiomyopathy, that predicts mortality and reduced exercise capacity(2,4,20), rather than primary pulmonary arterial pathophysiology.
Another notable similarity between SCD patients and patients with primary RCM is the high incidence of cardiac mortality and sudden death, a known characteristic of RCM, where LA dilation has been correlated with worse outcome(35–37). Sudden death has been reported in about 30% of SCD patients(38,39). The modestly elevated PAP and the mild PH does not directly explain the increased mortality and sudden death in SCD(1). Ischemic injuries to the conduction system, fibrosis and extensive chamber dilatation have been suggested as potential etiologies for arrhythmia in RCM(36), and could also be the causes of sudden cardiac death in SCD. Indeed, ischemic injury of the conduction system(40), and fibrotic and pulmonary vascular changes are also seen in autopsies in patients with primary RCM(35) and SCD(31,41,42). The cause of diastolic dysfunction in SCD is not established. Myocardial fibrosis may be a potential mechanism underlying the restrictive physiology seen in SCD. CMR studies have been inconsistent in detecting the extent of fibrosis in SCD, and microscopic fibrosis, in particular, has not yet been well studied. Most studies have shown small degrees of LV focal fibrosis(31,43–45). Fibrosis was described in autopsy studies in SCD without atherosclerosis(40–42,46). In addition, multiple known pro-fibrotic pathways and signals have been shown to be dysregulated in SCD(47–49). Interestingly, polymorphisms in the pro-fibrotic TGF-β signaling pathway genes have been associated with elevated TRV in SCD(50). This suggests that ventricular stiffness due to fibrosis may contribute to the underlying SCD-related cardiomyopathy with restrictive physiology.
We recognize the limitations of our study, including the absence of a control group, and the lack of hemodynamic data by RHC. It is not ethical to perform this invasive procedure in asymptomatic children with SCD. It is difficult to find a reasonable number of control patients with the same degree of severe life-long anemia that is not confounded by cardiac iron overload or chronic transfusions. In addition, the cross-sectional nature of the study does not permit tenable inference about the rate of progression of cardiomyopathy in SCD or the specific temporal sequence of events. Meta-analyses also have inherent limitations, which include the assumption that all studies were conducted under similar conditions and with the same level of expertise, and the heterogeneity among different studies. In our analysis, results remained statistically significant after using a random effects approach. Some studies were excluded from analysis due to missing data; however, the results of the excluded studies were consistent with the quantitative analysis.
In summary, children and adults with SCD develop a unique cardiomyopathy that is best characterized as restrictive physiology superimposed on a hyperdynamic state. Over time, the LV enlarges and diastolic dysfunction progresses. This unique cardiomyopathy, like other forms of RCM, can explain the secondary, passive mild elevations in TRV and PAP and is consistent with the high rate of cardiopulmonary mortality and sudden death in SCD. Unraveling the causal mechanisms that produce this unique cardiomyopathy could identify therapies to decrease cardiopulmonary morbidity and early mortality in SCD.
Supplementary Material
Figure S1. Search Strategy and Selection of The Cardiac Studies Included in Meta-analysis. (A) Studies reviewed for left atrial and ventricular size measures, systolic function measures, TRV and diastolic function estimates. (B) Studies included and excluded for left atrial size measurements. (C) Studies included and excluded for left ventricular end-diastolic measurements and (D) Studies included and excluded for systolic function analysis.
TDI: tissue Doppler imaging, DD: diastolic dysfunction, TRV: tricuspid regurgitant jet velocity, LA: left atrium, LAD: left atrial diameter, LADI: left atrial diameter index, LAV: left atrial volume, LAVI: left atrial volume index, LVED: Left ventricular end diastolic, LVEDD: left ventricular end diastolic diameter, LVEDV: left ventricular end diastolic volume, LVEDVI: left ventricular end diastolic volume index, SF: shortening fraction, EF: ejection fraction, SV: stroke volume, SVI: stroke volume index.
Figure S2. The association of TRV and lateral E/e′ with LVED z-score and LVMi. The regression of (A) TRV and LVED z-score, (B) TRV and LVMi, (C) lateral E/e′ and LVED z-score, and (D) lateral E/e′ and LVMi. Dashed lines represent 95-percent confidence interval. None of these associations were statistically significant.
Figure S3. Meta-analysis of Ejection Fraction (EF) (%) in SCD Patients. The mean EF values for SCD patient population in different studies with weighted mean EF and 95-percent confidence interval for the reported studies. Each point represents mean value spanned by its 95-percent confidence interval.
Figure S4. Meta-analysis of Stroke Volume Index (SVI) (ml/m2) in SCD Patients. The mean difference between SVI in SCD group and control group of patients in different studies, and the weighted mean difference with 95-percent confidence interval. Each point represents the mean value spanned by its 95-percent confidence interval.
Figure S5. Meta-regression of Left Atrial Diameter and Tricuspid Regurgitant Jet Velocity. Plots of the fitted estimated values for LAD (left) and TRV (right) from a model including age, hemoglobin, date of study and origin of study on x-axis versus left atrial diameter weighted mean difference (left) and tricuspid regurgitant jet velocity weighted mean (right) on y-axis. Each circle on the plot represents a study.
Table S1. Characteristics of SCD Patients
Table S2. Correlation of Lateral E/e′ Ratio with Laboratory and Echocardiographic Variables in SCD Patients.
Table S3. Diastolic Dysfunction in Cardiac Studies in SCD.
Clinical Perspectives.
Competency in medical knowledge (1)
Sickle cell disease (SCD) patients are at risk of early mortality due to cardiopulmonary causes. High TR velocity, diastolic dysfunction and increased PAP are associated with increased mortality in SCD but the pathophysiology behind these features is not known.
Competency in medical knowledge (2)
SCD patients have features of restrictive cardiomyopathy (diastolic dysfunction, LA enlargement and normal systolic function) that can lead to pulmonary venous hypertension and elevated TRV.
Translational outlook
More studies are needed to identify the mechanisms that lead to restrictive physiology in SCD. Understanding the pathophysiology of SCD-related cardiomyopathy with restrictive physiology can lead to targeted therapies that prevent or delay cardiac pathology in SCD.
Acknowledgments
Sources of Funding: This work was supported by NIH grant UO1-HL117709; Excellence in Hemoglobinopathies Research Award (PM, JAT, CTQ, ON, MDT and NB)
We thank Dr. Robert J. Fleck for the helpful discussions and comments, and Ellen Skalski, RN, BS, BSN for helping in data collection.
Abbreviations
- SCD
Sickle cell disease
- PH
Pulmonary hypertension
- PAH
Pulmonary arterial hypertension
- PVH
Pulmonary venous hypertension
- TRV
Tricuspid regurgitant jet velocity
- RHC
right-heart catheterization
- RCM
Restrictive cardiomyopathy
- LAV
left atrial volume
- LVED
left ventricular end diastolic diameter
- SF
Shortening fraction
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 3.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev V, Kato GJ, Gibbs JS, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation. 2011;124:1452–60. doi: 10.1161/CIRCULATIONAHA.111.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey MC, Hinderliter AL, Jones SK, Shah SP, Ataga KI. Hemodynamic characteristics and predictors of pulmonary hypertension in patients with sickle cell disease. Am J Cardiol. 2012;109:1353–7. doi: 10.1016/j.amjcard.2011.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight-Perry JE, de las Fuentes L, Waggoner AD, et al. Abnormalities in Cardiac Structure and Function in Adults with Sickle Cell Disease are not Associated with Pulmonary Hypertension. Journal of the American Society of Echocardiography. 2011;24:1285–1290. doi: 10.1016/j.echo.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrita IZ, Mohammed A, Layton M, et al. The association between tricuspid regurgitation velocity and 5-year survival in a North West London population of patients with sickle cell disease in the United Kingdom. Br J Haematol. 2013;162:400–8. doi: 10.1111/bjh.12391. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Eidem BW, McMahon CJ, Cohen RR, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–47. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 14.Denfield SW, Webber SA. Restrictive cardiomyopathy in childhood. Heart Fail Clin. 2010;6:445–52. viii. doi: 10.1016/j.hfc.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota Y, Shimizu G, Kita Y, et al. Spectrum of restrictive cardiomyopathy: report of the national survey in Japan. Am Heart J. 1990;120:188–94. doi: 10.1016/0002-8703(90)90177-y. [DOI] [PubMed] [Google Scholar]
- 17.Covitz W, Espeland M, Gallagher D, Hellenbrand W, Leff S, Talner N. The heart in sickle cell anemia. The Cooperative Study of Sickle Cell Disease (CSSCD) Chest. 1995;108:1214–9. doi: 10.1378/chest.108.5.1214. [DOI] [PubMed] [Google Scholar]
- 18.Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. 1972;83:415–26. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald M, Fagan K, Herbert DE, Al-Ali M, Mugal M, Haynes J., Jr Misclassification of pulmonary hypertension in adults with sickle hemoglobinopathies using Doppler echocardiography. South Med J. 2012;105:300–5. doi: 10.1097/SMJ.0b013e318256b55b. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39:112–8. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 21.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM. Understanding “diastolic” heart failure. N Engl J Med. 2004;350:1930–1. doi: 10.1056/NEJMp048064. [DOI] [PubMed] [Google Scholar]
- 23.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–43. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 24.Dragulescu A, Mertens L, Friedberg MK. Interpretation of left ventricular diastolic dysfunction in children with cardiomyopathy by echocardiography: problems and limitations. Circ Cardiovasc Imaging. 2013;6:254–61. doi: 10.1161/CIRCIMAGING.112.000175. [DOI] [PubMed] [Google Scholar]
- 25.Unzek S, Popovic ZB, Marwick TH Diastolic Guidelines Concordance I. Effect of recommendations on interobserver consistency of diastolic function evaluation. JACC Cardiovasc Imaging. 2011;4:460–7. doi: 10.1016/j.jcmg.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Blanc J, Stos B, de Montalembert M, Bonnet D, Boudjemline Y. Right ventricular systolic strain is altered in children with sickle cell disease. J Am Soc Echocardiogr. 2012;25:511–7. doi: 10.1016/j.echo.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Caldas MC, Meira ZA, Barbosa MM. Evaluation of 107 patients with sickle cell anemia through tissue Doppler and myocardial performance index. J Am Soc Echocardiogr. 2008;21:1163–7. doi: 10.1016/j.echo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Colombatti R, Maschietto N, Varotto E, et al. Pulmonary hypertension in sickle cell disease children under 10 years of age. Br J Haematol. 2010;150:601–9. doi: 10.1111/j.1365-2141.2010.08269.x. [DOI] [PubMed] [Google Scholar]
- 29.Simmons BE, Santhanam V, Castaner A, Rao KR, Sachdev N, Cooper R. Sickle cell heart disease. Two-dimensional echo and Doppler ultrasonographic findings in the hearts of adult patients with sickle cell anemia. Arch Intern Med. 1988;148:1526–8. doi: 10.1001/archinte.148.7.1526. [DOI] [PubMed] [Google Scholar]
- 30.Lester LA, Sodt PC, Hutcheon N, Arcilla RA. Cardiac abnormalities in children with sickle cell anemia. Chest. 1990;98:1169–74. doi: 10.1378/chest.98.5.1169. [DOI] [PubMed] [Google Scholar]
- 31.Desai AA, Patel AR, Ahmad H, et al. Mechanistic insights and characterization of sickle cell disease-associated cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:430–7. doi: 10.1161/CIRCIMAGING.113.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poludasu S, Ramkissoon K, Salciccioli L, Kamran H, Lazar JM. Left ventricular systolic function in sickle cell anemia: a meta-analysis. J Card Fail. 2013;19:333–41. doi: 10.1016/j.cardfail.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Efird J, Kadali R, et al. Pulmonary artery occlusion pressure may overdiagnose pulmonary artery hypertension in sickle cell disease. Clin Cardiol. 2013;36:524–30. doi: 10.1002/clc.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webber SA, Lipshultz SE, Sleeper LA, et al. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation. 2012;126:1237–44. doi: 10.1161/CIRCULATIONAHA.112.104638. [DOI] [PubMed] [Google Scholar]
- 35.Rivenes SM, Kearney DL, Smith EO, Towbin JA, Denfield SW. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2000;102:876–82. doi: 10.1161/01.cir.102.8.876. [DOI] [PubMed] [Google Scholar]
- 36.Walsh MA, Grenier MA, Jefferies JL, Towbin JA, Lorts A, Czosek RJ. Conduction abnormalities in pediatric patients with restrictive cardiomyopathy. Circ Heart Fail. 2012;5:267–73. doi: 10.1161/CIRCHEARTFAILURE.111.964395. [DOI] [PubMed] [Google Scholar]
- 37.Bayes-Genis A, Vazquez R, Puig T, et al. Left atrial enlargement and NT-proBNP as predictors of sudden cardiac death in patients with heart failure. Eur J Heart Fail. 2007;9:802–7. doi: 10.1016/j.ejheart.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–61. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 39.Manci EA, Culberson DE, Yang YM, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:359–65. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- 40.James TN, Riddick L, Massing GK. Sickle cells and sudden death: morphologic abnormalities of the cardiac conduction system. J Lab Clin Med. 1994;124:507–20. [PubMed] [Google Scholar]
- 41.Martin CR, Johnson CS, Cobb C, Tatter D, Haywood LJ. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428–32. [PMC free article] [PubMed] [Google Scholar]
- 42.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–76. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 43.Bratis K, Kattamis A, Athanasiou K, et al. Abnormal myocardial perfusion-fibrosis pattern in sickle cell disease assessed by cardiac magnetic resonance imaging. Int J Cardiol. 2013;166:e75–6. doi: 10.1016/j.ijcard.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 44.Junqueira FP, Fernandes JL, Cunha GM, et al. Right and left ventricular function and myocardial scarring in adult patients with sickle cell disease: a comprehensive magnetic resonance assessment of hepatic and myocardial iron overload. J Cardiovasc Magn Reson. 2013;15:83. doi: 10.1186/1532-429X-15-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raman SV, Simonetti OP, Cataland SR, Kraut EH. Myocardial ischemia and right ventricular dysfunction in adult patients with sickle cell disease. Haematologica. 2006;91:1329–35. [PubMed] [Google Scholar]
- 46.Baroldi G. High resistance of the human myocardium to shock and red blood cell aggregation (sludge) Cardiologia. 1969;54:271–7. doi: 10.1159/000166261. [DOI] [PubMed] [Google Scholar]
- 47.George A, Pushkaran S, Konstantinidis DG, et al. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121:2099–107. doi: 10.1182/blood-2012-07-441188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohtat D, Thomas R, Du Z, et al. Urinary transforming growth factor beta-1 as a marker of renal dysfunction in sickle cell disease. Pediatr Nephrol. 2011;26:275–80. doi: 10.1007/s00467-010-1677-9. [DOI] [PubMed] [Google Scholar]
- 49.Tharaux PL, Hagege I, Placier S, et al. Urinary endothelin-1 as a marker of renal damage in sickle cell disease. Nephrol Dial Transplant. 2005;20:2408–13. doi: 10.1093/ndt/gfi111. [DOI] [PubMed] [Google Scholar]
- 50.Fertrin KY, Costa FF. Genomic polymorphisms in sickle cell disease: implications for clinical diversity and treatment. Expert Rev Hematol. 2010;3:443–58. doi: 10.1586/ehm.10.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Search Strategy and Selection of The Cardiac Studies Included in Meta-analysis. (A) Studies reviewed for left atrial and ventricular size measures, systolic function measures, TRV and diastolic function estimates. (B) Studies included and excluded for left atrial size measurements. (C) Studies included and excluded for left ventricular end-diastolic measurements and (D) Studies included and excluded for systolic function analysis.
TDI: tissue Doppler imaging, DD: diastolic dysfunction, TRV: tricuspid regurgitant jet velocity, LA: left atrium, LAD: left atrial diameter, LADI: left atrial diameter index, LAV: left atrial volume, LAVI: left atrial volume index, LVED: Left ventricular end diastolic, LVEDD: left ventricular end diastolic diameter, LVEDV: left ventricular end diastolic volume, LVEDVI: left ventricular end diastolic volume index, SF: shortening fraction, EF: ejection fraction, SV: stroke volume, SVI: stroke volume index.
Figure S2. The association of TRV and lateral E/e′ with LVED z-score and LVMi. The regression of (A) TRV and LVED z-score, (B) TRV and LVMi, (C) lateral E/e′ and LVED z-score, and (D) lateral E/e′ and LVMi. Dashed lines represent 95-percent confidence interval. None of these associations were statistically significant.
Figure S3. Meta-analysis of Ejection Fraction (EF) (%) in SCD Patients. The mean EF values for SCD patient population in different studies with weighted mean EF and 95-percent confidence interval for the reported studies. Each point represents mean value spanned by its 95-percent confidence interval.
Figure S4. Meta-analysis of Stroke Volume Index (SVI) (ml/m2) in SCD Patients. The mean difference between SVI in SCD group and control group of patients in different studies, and the weighted mean difference with 95-percent confidence interval. Each point represents the mean value spanned by its 95-percent confidence interval.
Figure S5. Meta-regression of Left Atrial Diameter and Tricuspid Regurgitant Jet Velocity. Plots of the fitted estimated values for LAD (left) and TRV (right) from a model including age, hemoglobin, date of study and origin of study on x-axis versus left atrial diameter weighted mean difference (left) and tricuspid regurgitant jet velocity weighted mean (right) on y-axis. Each circle on the plot represents a study.
Table S1. Characteristics of SCD Patients
Table S2. Correlation of Lateral E/e′ Ratio with Laboratory and Echocardiographic Variables in SCD Patients.
Table S3. Diastolic Dysfunction in Cardiac Studies in SCD.