Abstract
First seen as a storage organ, the white adipose tissue (WAT) is now considered as an endocrine organ. WAT can produce an array of bioactive factors known as adipokines acting at physiological level and playing a vital role in energy metabolism as well as in immune response. The global effect of adipokines in metabolic activities is well established, but their impact on the physiology and the pathophysiology of the central nervous system (CNS) remains poorly defined. Adipokines are not only produced by the WAT but can also be expressed in the CNS where receptors for these factors are present. When produced in periphery and to affect the CNS, these factors may either cross the blood brain barrier (BBB) or modify the BBB physiology by acting on cells forming the BBB. Adipokines could regulate neuroinflammation and oxidative stress which are two major physiological processes involved in neurodegeneration and are associated with many chronic neurodegenerative diseases. In this review, we focus on four important adipokines (leptin, resistin, adiponectin, and TNFα) and one lipokine (lysophosphatidic acid—LPA) associated with autotaxin, its producing enzyme. Their potential effects on neurodegeneration and brain repair (neurogenesis) will be discussed. Understanding and regulating these adipokines could be an interesting lead to novel therapeutic strategy in order to counteract neurodegenerative disorders and/or promote brain repair.
Keywords: Diabetes, Obesity, White adipose tissue, Adipocytokines, Central nervous system, Neuroinflammation, Neurodegeneration, Neurogenesis
Background
Obesity and type 2 diabetes mellitus (T2DM) are main health issues in our modern societies and constitute very important public health challenges [1–3]. The World Health Organization (WHO) reported that worldwide obesity has more than doubled since 1980 and more than 1.9 billion adults were overweight in 2014 [2]. One result from excess body weight and physical inactivity is the dramatic development of type 2 diabetes that WHO has predicted to be the seventh leading cause of death in 2030 [3–5]. In parallel, 35.6 million people display dementia and 7.7 million new cases are reported every year, Alzheimer’s disease (AD) being the main cause of dementia [6, 7]. An increasing number of data recently highlights that metabolic syndrome, notably obesity and type 2 diabetes, are correlated with an increased risk to develop dementia and/or neurodegenerative diseases such as AD, as well as neurological and neurovascular disorders [8–11]. Consequently, adiposity has been proposed as an independent factor favoring the development of AD [12–14]. However, breaking the paradigm, recent studies show that underweight people (BMI < 20 kg/m2) display higher risk of dementia while very obese people (BMI > 40 kg/m2) have lower dementia risk than healthy weight people [15]. Similarly, a decrease in BMI from mid-life to late-life has been correlated with an increased risk of dementia [16]. Interestingly, it has been suggested that the misexpression of adipose-derived factors called adipokines or adipocytokines may disrupt directly or indirectly brain homeostasis and functions.
In this review, we aimed at first describing the links between adiposity, adipokines levels, and neurological disorders. Furthermore, adipokine signaling in the central nervous system (CNS), highlighting their potential effects on cognition, neurogenesis, and brain functioning, has also been explored. Finally, the possibilities of adipokines to disturb brain physiology and functions through blood brain barrier disruption resulting from increased inflammation and oxidative stress have been discussed.
White adipose tissue: not just energy storage
White adipose tissue secretes adipokines
White adipose tissue (WAT) was originally described to store energy in the form of triglycerides. However, since the discovery of the leptin hormone in 1994, WAT is also recognized as a major endocrine organ secreting a wide variety of biologically active factors collectively called adipokines or adipocytokines [17, 18]. To date, about hundred adipokines constituting the adipokinome have been documented to be released from white adipocytes [19]. The most studied adipokines are leptin, adiponectin, apelin, resistin, monocytes, and macrophage chemotactic protein 1 (MCP1), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-alpha (TNFα), and transforming growth factor (TGFβ). In addition to adipokines, lipid-derived factors (sometimes referred as lipokines) such as the lysophosphatidic acid are also important mediators produced by the fat tissue [20, 21]. Pro-inflammatory factors include adipokines such as leptin, TNFα, and IL-6, while anti-inflammatory ones include adiponectin and the secreted frizzled-related protein 5 (sFRP5) [20, 22, 23]. Adipokines exert pleiotropic effects on different tissues such as the lung, skeletal muscle, heart, liver, and blood vessels and regulate numerous physiological functions such as appetite, energy expenditure, insulin sensitivity and secretion, fat distribution, lipid and glucose metabolism, endothelial function, blood pressure, hemostasis, neuroendocrine functions, and also immunity [18, 21, 24–28]. The data generated over the last 20 years considerably change our view on adipose tissue as WAT plays a wide-ranging role in metabolic regulation and physiological homeostasis [17, 18].
Adipokines and diseases: focus on neurological disorders and diseases
The dysregulation of adipokine production and/or levels has been correlated with several diseases and could notably promote and/or result in obesity-linked metabolic disorders [27, 29]. Thus, low plasmatic leptin concentrations are associated with an increased risk for cardiovascular diseases [30]. In contrast, higher plasmatic adiponectin levels seem to be associated with decreased risk for developing type 2 diabetes mellitus (T2DM) [31]. Other data also show relationships between MCP-1 serum levels and insulin resistance, as diabetic patients exhibit highest MCP-1 levels [32]. In the same line of evidences, it appears that the inflammatory status of WAT in obese patients might be a key player linking high WAT mass to insulin resistance. Interestingly, an increasing number of studies reported links between metabolic disorders (i.e., T2DM and obesity) and brain homeostasis and functioning [9, 12, 33–39]. Initial studies demonstrated that a higher body mass index (BMI) and/or waist-to-hip ratio in middle-aged individuals is associated with a reduction in the whole brain volume. Indeed, over the last decade, a number of magnetic resonance imaging (MRI) and computed tomography (CT) studies also reported alterations in brain morphology of overweight/obese individuals [40–42]. Studies documented a link between abdominal fat and reduced brain volume in healthy middle-aged adults notably the temporal lobe volume and the hippocampus [43, 44]. In a cross-sectional study of normal elderly individuals showing no sign of cognitive deficit, tensor-based morphometry also unveiled atrophy in the white and gray matter of the frontal lobes, anterior cingulate gyrus, hippocampus, and thalamus in both male and female subjects with a high BMI (BMI > 30) as compared to individuals with a normal BMI (18.5–25) [45]. Upon further investigation, the brain volume reduction in gray and white matter was found to be associated with a common variant of the fat mass and obesity-associated (FTO) gene [46]. In addition, a growing body of studies also show that obesity in mid-life is a predictor of mild cognitive impairment with aging and altered executive function and short-term memory compared to normal weight counterparts [39, 47–49]. Such data were also confirmed in rodents for which high fat diets result in impaired cognitive functions including a decrease in memory performance, learning, and executive functions [39, 50, 51]. Furthermore, during the development of obesity in rodent models, it appears that neurochemical changes occurs in the brain altering cognition processes, reward neurocircuitry, and stress responsiveness [52]. Consequently, numerous studies described association between rich diets (sugar and/or fat) and cognitive defects in rodents and humans [39, 52], and it seems that such effects of diets could occur through the disruption of neurovascular function [52–54]. In addition, a linkage has been demonstrated between overweight, neuroinflammation, and neurodegenerative diseases namely AD, Parkinson’s disease (PD), and autoimmune nervous system diseases such as multiple sclerosis [9, 12, 33–38]. Similarly, T2DM is associated to impaired cognition, especially learning and memory deficits such as shown in rodents and humans while such effects are rarely observed in type 1 diabetes [55, 56]. This is peculiarly interesting given that T2DM patients are mostly overweight or obese compared to type 1 diabetic. In a recent study, working on 80 T2DM patients and 80 healthy controls demonstrated a cortical and subcortical atrophy and that cognition impairment was correlated with reduced hippocampal CA1 size in the diabetic group [57]. Diabetes is associated with an increased risk of AD and vascular dementia, supported by increasing oxidative stress and inflammation and impaired insulin and amyloid metabolisms [56, 58–62]. T2DM patients also display lower cerebral blood flow and neural slowing on recordings of sensory-evoked potentials [56]. Numerous studies performed on rodents also show an impact of diabetes on neurogenesis, depression, and cognition [63].
Taken together, these data show that obesity and diabetes have negative effects on brain structures and/or functions. It also raises the question about the roles of adipokines in such neurological disorders. A possible explanation could be that abnormal adipokine concentrations, such as increase pro-inflammatory adipokines TNFα, resistin, leptin, IL-1β, and also IL-6, could influence the blood brain barrier integrity and disrupt brain homeostasis through oxidative stress and inflammation [12, 20]. In the following part, we aim to describe the effects of some adipokines in the brain, regarding their transport in the central nervous system and their signaling.
Adipokines and targets in the brain
In this part, we focus on specific adipokines (leptin, resistin, adiponectin, TNFα) and also on a lipokine of interest, the lysophosphatidic acid (LPA) and their targets in the brain as well as their potential impact on brain inflammation and functions (Fig. 1).
Fig. 1.
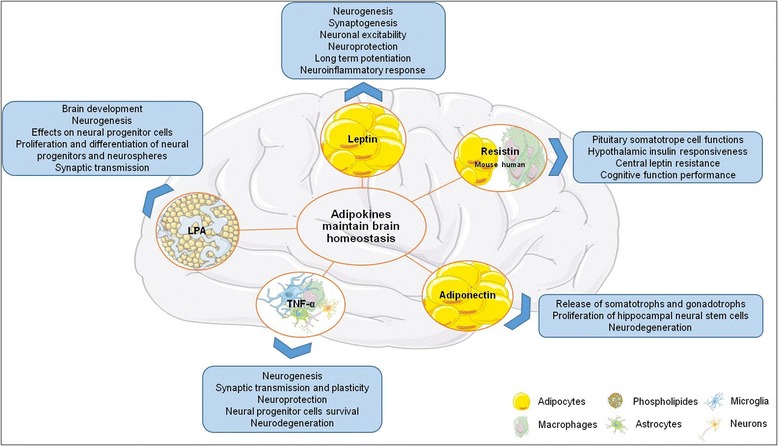
Effects of the main adipokines on brain homeostasis/functions. LPA lisophosphatidic acid, TNFα tumor necrosis factor-α
Leptin
Leptin is probably the most studied adipose-derived hormones. Leptin which is mainly produced by adipocytes exerts its effects both peripherally and centrally [17, 21, 24, 64]. This adipokine plays a key role in regulating energy intake and expenditure, metabolism, and behavior by directly acting on the CNS. Mice invalidated for leptin (ob/ob mice) display obesity, insulin resistance, and hyperphagia showing notably the impact of this adipose-derived hormone on feeding behavior [65]. Peripheral leptin exerts its central effect through its binding at the level of choroid plexus leading to its transport across the blood brain barrier [66–69]. Such a transport involved leptin receptors and probably other mechanisms that are still poorly understood [9]. However, some studies have shown that leptin could be also locally and de novo produced in the brain, in the cerebellum, the cortex, and the hypothalamus [70–73], suggesting other specific and local functions for leptin than those previously described. Leptin receptors belong to the family of cytokine receptors, and at least five different isoforms have been identified in mouse: Ob-Ra to Ob-Re [65, 74]. In the CNS, leptin receptors (Ob-R or LepR) were first identified in choroid plexus and in the hypothalamus [75, 76]. Among all Ob-R isoforms, only the full-length isoform (Ob-Rb) appears to fully transduce the activation signal at least in the brain and is essential for leptin’s weight-reducing effects [65, 74]. Ob-Rb is expressed in the hypothalamic nuclei notably in the arcuate nucleus (ARC), the dorsomedial nucleus (DMH), the paraventricular nucleus (PVN), the ventromedial hypothalamic nucleus (VMH), and the lateral hypothalamic nucleus (LH) [65, 77, 78] but is also detected in the neocortex, the hippocampus, the hindbrain (nucleus of the solitary tract), the ventral tegmental area, the medulla, and the cerebellum [77, 79–83]. In addition, a weaker expression was also detected by in situ hybridization in the hippocampus and the thalamus [77]. The expression of leptin receptors and leptin mRNAs is documented in the mouse brain and notably in the main neurogenic niches, the subventricular zone of the lateral ventricles, and the dentate gyrus of the hippocampus (Allen Brain Atlas [http://www.brain-map.org], [84]). This work clearly illustrates the expression of leptin receptors in the cortex, along the ventricular walls and also in the hippocampus. Leptin is expressed in the same regions at lower levels. In the hypothalamus, the primary leptin targets are the orexigenic agouti-related peptide (AgRP) neurons and the anorexigenic pro-opiomelanocortin (POMC) neurons that are involved in feeding behavior. Thus, in the CNS, leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus [85]. The appetite-stimulating effects of AgRP/NPY are inhibited by leptin in the arcuate nucleus avoiding the release of orexigenic factors [86, 87]. Furthermore, leptin receptors were also expressed in glutamatergic and GABAergic neurons [78, 88, 89]. Vong and colleagues (2011) have shown that the main effects of leptin are mediated by GABAergic neurons and only barely by glutamatergic neurons [88]. However, it was recently demonstrated that glutamate release mediates leptin action on energy expenditure [89]. We realize now that the effects of leptin on these different neuronal types and brain nuclei are not so easy to understand as originally thought. In homeostatic conditions, leptin inhibits food intake, and in extra-hypothalamic sites, leptin acts on neurogenesis, synaptogenesis, neuronal excitability, and neuroprotection [9, 90, 91]. Leptin was also shown to improve cognition and mood in depressed and anxious animal models, notably by improving long-term potentiation [9]. Leptin levels negatively correlated with the development of Alzheimer’s disease in lean humans [91, 92], and leptin signaling seems to be dysregulated in Alzheimer’s disease brains [93]. Interestingly, there are also positive correlations between plasma levels of leptin and body weight [94, 95].
Resistin
Resistin (or adipose tissue-specific secretory factor: ADSF or C/EBP-epsilon-regulated myeloid-specific secreted cysteine-rich protein: XCP1) is a cysteine-rich adipose-derived peptide hormone, encoded by the RETN gene, and known for its implication in inflammatory processes [20, 96]. Its expression increases in parallel to adiposity [97–99] and is strongly related to insulin resistance in obese rodents [100]. Interestingly, in humans, resistin is mainly expressed and secreted by macrophages while adipocytes are the main source in rodents [100]. Resistin is known to play a key role in the CNS notably by regulating pituitary somatotrope cell functions [101], affecting hypothalamic and peripheral insulin responsiveness, thermogenesis, and feeding behavior, and also by enhancing renal sympathetic nerve activity [102–104]. However, the resistin receptor and the molecular mechanisms sustaining such effects are poorly understood and mainly unexplored until recently. Although resistin receptor has not been clearly identified, some potential candidate receptors have been proposed in different cell types such as an isoform of decorin (a small proteoglycan associated with collagen fibrils) in adipose progenitor cells, tyrosine kinase-like orphan receptor-1 (ROR1) in 3T3-L1 cells [105] or IGF-1R in fibroblast [106]. Nevertheless, it has been shown that resistin administration modulates or activates several signaling pathway involving Gs protein-dependent mechanisms, the adenylate cyclase/cAMP/protein kinase A pathway, the phosphatidylinositol 3-kinase/Akt pathway, the protein kinase C, and extracellular Ca2+ signaling through L-type voltage-sensitive Ca2+ [102, 107]. Such puzzling data strongly suggest that resistin could potentially interact with different receptors depending on tissue and cell types. Furthermore, resistin also regulates the synthesis and secretion of the pro-inflammatory cytokines TNFα and IL-6 through nuclear factor-κB-dependent pathway in macrophage [108–110]. Recently, Toll-like receptor 4 (TLR-4) receptors were identified as potential receptor for resistin in the hypothalamus, leading to the activation of JNK and p38/MAPK pathways [111]. Interestingly, resistin was also reported to be expressed in the hypothalamus and the cortex and to inactivate hypothalamic neurons [112–114]. In the rat brain, resistin is de novo produced suggesting specific roles for this local synthesis [114]. Resistin gene expression in the brain of mouse is reported in the cortex along the walls of the lateral ventricles and also in the hippocampus (Allen Brain Atlas [http://www.brain-map.org], [84]). In rat, traumatic brain injury (TBI) increased resistin mRNA expression in the ipsilateral cortex without any effects on the contralateral hemisphere. However, resistin expression is upregulated after TBI in the ipsi- and contralateral hippocampus [73]. One explanation is that given TBI compromises the integrity of the blood brain barrier, it could result in the changes in gene expression in the contralateral side of the hippocampus by exposing the brain to circulating factors of peripheral origin (Brown et al., 2008). The relatively rapid increase of resistin expression following TBI (at 12 h post-injury), is in contrast to the delayed upregulation of resistin in hypoxic ischemic mouse brain (>7 days) [115]. Thus, resistin could participate in the acute responses to cerebral damage probably through inflammatory mechanisms. A recent study suggested that resistin was not related to cognitive function performance [116].
Adiponectin
Adiponectin was first characterized in 1995 in 3T3-L1 adipocyte differentiation [117]. It is one of the most abundant adipokines considering its concentration in plasma relative to many other hormones [118, 119]. Adiponectin self-associates into larger structures forming homotrimers that also self-associate and form hexamers or dodecamers. A globular fraction, named globular adiponectin, resulting from the cleavage of the full-length monomer, was also documented [120]. Adiponectin is mainly synthesized and secreted by adipocytes. However, it is now well admitted that adiponectin is expressed at the mRNA and/or protein level by the placenta, the liver, epithelial cells, osteoblasts, myocytes, and also by pituitary cells [114, 119, 121]. Interestingly, some studies documented adiponectin transcript expression in the diencephalon of chicken [114, 122] and in the human pituitary [121]. In the pituitary, adiponectin could have a role in the release of somatotrophs and gonadotrophs [119]. It also modulates a wide range of metabolic processes such as body-weight regulation, glucose regulation, insulin sensitivity, lipid catabolism (fatty acid oxidation), endothelial function, and also anti-atherogenic process [119, 123–126]. Such effects are mediated by three different receptor types: adiponectin receptor 1 (Adipo-R1), adiponectin receptor 2 (Adipo-R2), and T-cadherin (CDH13) and involved different signaling pathways including AMPK, p38-MAPK, JNK, PPAR-α, and NF-kB. These receptors appear to be widely expressed in the mammalian brain including mouse, rat, pork and human. Their expression was documented in different brain structures such as the pituitary, the hypothalamus, and in cortical and subcortical neurons [97, 119, 121, 127–131]. In their review, Thundyil and colleagues (2012) documented adiponectin receptor expression in the central nervous system showing that Adipo-R1 is mainly expressed in the hypothalamus, the brainstem, and the pituitary gland while Adipo-R2 seems to be mostly expressed in the cortex. Furthermore, Adipo-R1 is strongly expressed in neurons and to a lesser extent in astrocytes while Adipo-R2 is figured to be only weakly expressed in astrocytes and neurons [119]. Adiponectin gene expression is widely expressed in the cortex and the hippocampus. Concerning T-cadherin receptor, it seems to be temporally and spatially expressed in different neuronal populations during axon growth [132]. Furthermore, T-cadherin showed broad expression in the cerebral cortex, basal ganglia, amygdala, and hippocampus in the developing postnatal telencephalon of marmoset (Callithrix jacchus) [133]. In mouse, CDH13 was also expressed by projection neurons within the main and accessory olfactory bulbs. Interestingly, adiponectin deficiency is associated with exaggerated inflammatory response in critical illness or septic patients [134–136]. Recently, Adipo-R1 and Adipo-R2 expression was described in both U373 MG (human glioblastoma astrocytoma cell line) and primary human astrocytes [137]. It also appears that adiponectin induces a pro-inflammatory response in human astrocytes, increasing notably IL-6 and MCP-1 through NF-κB, p38MAPK, and ERK1/2 pathways (Wan et al., 2014). In contrast, adiponectin was described to inhibit pro-inflammatory signal, notably by suppressing IL-6 release from blood brain barrier (BBB) endothelial cells [138]. It results that adiponectin indirectly modulates inflammatory signaling across the BBB by negatively modulating IL-6 and TNFα release. In vitro experiment of hippocampal neurons reveals that adiponectin exerts neuroprotective effects through AMPK pathway [139]. Such neuroprotective effects of adiponectin are further reinforced by the fact that knock-out mice for adiponectin exhibit more brain damages after ischemic stroke to controls [140]. This neuroprotective action is mediated through an endothelial nitric oxide synthase (eNOS)-dependent mechanism [140].
Tumor necrosis factor α
Many pro-inflammatory factors are produced in activated WAT, such as TNFα, IL-1, and PGE2. We choose to describe in more depth the prototype inflammatory cytokine TNFα. TNFα is a pro-inflammatory adipokine well-known for its role in chronic peripheral and central inflammation [9, 141]. TNFα is primarily produced as a transmembrane protein that self-associated into stable homotrimers [142, 143]. Such homotrimers could be cleaved by the TNFα-converting enzyme (TACE, also called ADAM17), allowing the release of secreted form of TNFα [144]. In WAT, TNFα is produced by macrophages as well as by adipocytes, and its expression is increased at the mRNA and protein levels in obese and in T2DM models [145]. TNFα actions are mediated by two receptors: TNF-R1 (TNF-RSF1a) and TNF-R2 (TNF-RSF1b). TNF-R1 is expressed in most tissues and can be fully activated by both the membrane-bound and soluble trimeric forms of TNF, while TNF-R2 is found in a limited cell types including cell of the immune system, oligodendrocytes, and certain neuron subtypes and responds to the membrane-bound form of the TNF homotrimer [9]. TNF-R1 and TNF-R2 are also expressed in the cortex, the subventricular zone of the lateral ventricle, and the hippocampus (Allen Brain Atlas [http://www.brain-map.org], [84]). In homeostatic conditions, the TNFα gene expression is low. However, in stress conditions (infection, trauma, pathologies), TNFα level can increase dramatically. As most information regarding TNF signaling is derived from TNF-R1, the role of TNF-R2 is likely underestimated. In rodents, TNFα has been shown to be transported across the BBB, but to be also locally produced by microglia, astrocytes and neurons in the brain [146–148]. In the CNS, TNFα acts through TNF-receptors on neurons and astrocytes regulating a wide range of cellular processes such as cell survival [9, 149, 150]. Actually, TNFα exhibits pleiotropic effects with positive and negative outcomes on the brain. On the one hand, TNFα is considered as the prototypic inflammatory cytokine and elevated levels of TNF have been described in many neurodegenerative situations [151, 152]. For instance, we have demonstrated the role of TNF in chemically induced neurodegeneration [153]. On the other hand, inhibition of TNF in inflammatory peripheral diseases induced CNS side effects including demyelination and neuropathies, suggesting a positive role for TNF maintaining the homeostasis in the CNS [154]. It acts on neurogenesis, synaptic transmission, and plasticity [9]. Thus, TNFα was described for its neuroprotective roles on hippocampal neurons by suppressing the accumulation of reactive oxygen species (ROS) and by maintaining intracellular levels of calcium [155]. In addition, it modulates glutamatergic transmission [156]. Furthermore, TNFα favors neural progenitor cell survival by mediating anti-apoptotic signals via TNF-R2 [157]. In rat, TNFα appears to promote the survival of stroke-generated hippocampal and striatal neurons [158]. In addition, TNFα knock-out mice show cognitive impairment (i.e., significant poorer learning, retention, and spatial learning), suggesting a strong role for TNFα on these mechanisms [159]. However, TNFα also exhibits a dark face, as reported in numerous other studies. It is notably involved in myelin damages [160], in favoring glutamate excitotoxicity [161], in inhibition of long-term potentiation in Cornu Ammonis area 1 (CA1) and in the dentate gyrus of the rat hippocampus [150, 162, 163] and in decreasing neurogenesis [164, 165].
Altogether, these data established that the role of TNFα is complex. TNFα could exhibit multiple faces exerting neuroprotective versus neurotoxic roles, pro- versus anti-neurogenic effects according to the conditions (concentrations, physiological, or pathological conditions…). Neuroinflammation and metabolic disorders such as obesity could act on these mechanisms through an excess of TNFα secretion.
Lysophosphatidic acid
Among the factors secreted by the adipose tissue, there are many lipids from the lipokine family such as prostaglandin E2 (PGE2), anandamide, and also lysophosphatidic acid (LPA). LPA is a bioactive signaling phospholipid acting on a wide range of biological processes including cell growth, migration, and morphology [166]. LPA is detected in several biological fluids and tissues including the brain [167]. It is synthesized from different enzymatic activities involving notably phospholipase A1 and A2, monoacylglycerol kinase, but the main enzyme leading to LPA synthesis is autotaxin [168]. Autotaxin is a multifunctional phosphodiesterase that converts lysophospholipids into LPA through its lysophospholipase D activity. To date, LPA effects are mediated through five G protein coupled receptors. However, additional receptors have been identified for their potential responsiveness to LPA [168–170]. Using knock-out mice for the five most known LPA receptors (LPA-R), it was shown that LPA plays key roles on inflammation [171], angiogenesis [172], reproduction [173–175], brain development, and neurogenesis [176, 177]. Indeed, LPA exerts pleomorphic effects on neural progenitor cells from cortex, and notably calcium-mediated conductance [178]. In the nervous system, neural progenitor cells, neurons, oligodendrocytes, Schwann cells, astrocytes, and microglia have been documented for expressing different subsets of LPA receptors [168]. It partially explains why LPA exerts a wide variety of effects on these different cell types. Thus, LPA can favor proliferation and differentiation of neural progenitor cells as shown by treatment on ex vivo embryonic brain slice cultures resulting in an increase cell survival and differentiation [179]. Furthermore, LPA has been shown to promote proliferation and differentiation in neurospheres [180, 181]. LPA also displays effect on cell morphology and neurite formation in both neural progenitor cells and neurons [168]. It exhibits both cell death and survival properties on neurons possibly due to differences in LPA concentration or signaling through different receptors [182–184]. For instance, it induces apoptosis and necrosis in hippocampal neurons [182]. LPA also exerts various effects on glial and microglial cells, by modulating intracellular calcium levels in oligodendrocytes, astrocytes, and microglia [168]. It notably favors astrocytes and microglia proliferation in vitro [185, 186]. Overexpression of autotaxin in microglia and by consequences, increased levels of LPA, protects the cells from an oxidative stress by increasing the level of catalase [187] and decreases the inflammatory response at least partially through an upregulation of IL-10 [188]. Interestingly, following brain injury, in human postmortem brains, LPA receptors 1–3 and autotaxin are only weakly expressed while LPA-R2 is increased and autotaxin transcripts are decreased. Such data also reinforce the fact that LPA signaling is involved in neurotrauma [189]. During embryogenesis, LPA-R1 was detected in neural progenitors reinforcing a potential role of LPA/LPA-R1 signaling in neurogenesis [190]. In addition, LPAR-1 knock-out reduces brain cell proliferation, differentiation, and cell survival in the mouse dentate gyrus, consequently strongly impairing neurogenesis [176]. Autotaxin is widely expressed in the brain of mouse notably in neurogenic niches while LPA-R1 displays a lower and more discrete expression (Allen Brain Atlas [http://www.brain-map.org], [84]).
Blood brain barrier, adipokines, inflammation, and oxidative stress
The effects and actions of adipokines on the CNS are dependent on their capacity to interact with the cells of the BBB and eventually to enter the CNS (Fig. 2). As mentioned above about the TNFα, some adipokines including leptin can cross the BBB [9]. Concerning adiponectin, its capacity to cross the BBB is questionable. In one study, the results based on the measurement of radioactive labeled adiponectin and concentration in the cerebrospinal fluid indicate that adiponectin does not cross the BBB but will affect the cells of the BBB and indirectly affect the CNS acting via AMPK pathway [9, 138]. In another work using adiponectin KO mice and recombinant adiponectin, it is shown that adiponectin crosses the BBB [191]. As the conclusion, these two publications proposed opposite results concerning the possibility of adiponectin to cross the BBB, and more conclusive results will be needed to clarify this point.
Fig. 2.
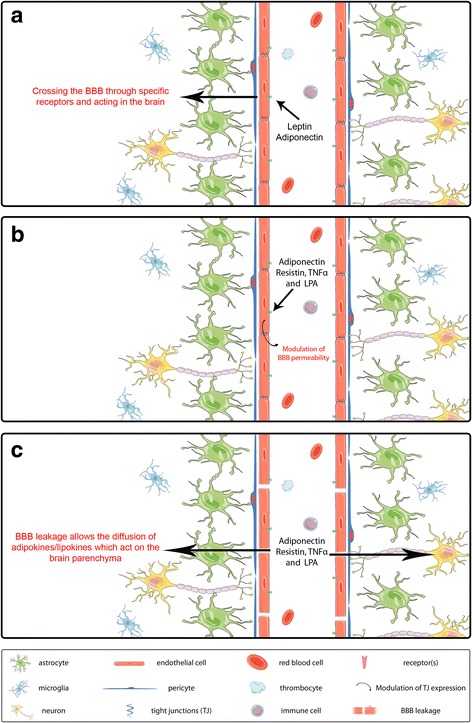
Adipokines and LPA interactions with the blood brain barrier and the central nervous system. The BBB is composed of endothelial cells (displaying tight junctions), pericytes and astrocytes. a In physiological conditions, some adipokines such as leptin and TNFα can cross the BBB through different mechanisms and act on the central nervous system. b Adipokines can also activate endothelial cell receptors resulting in the modulation of the expression of tight junctions and in the modulation of the BBB permeability. In adiponectin case, one study reports its crossing through the BBB [191], while another indicates that it does not cross the BBB [138]. Both possibilities are shown. In inflammatory conditions, the BBB is leaking and could allow an increase passage of adipokines and LPA into the CNS, leading to an increase of oxidative stress and neurodegeneration (c)
The BBB is a key player in the adipokine signaling from the periphery to the CNS. Inflammation and oxidative stress associated with pathologies impact neurodegeneration and neurogenesis but also affect the BBB.
In this review, we highlighted the striking correlations between metabolic syndrome and the prevalence of neurological disorders and dementia including AD. White adipose tissue was not initially envisioned as a source of inflammatory factors. However, it is now well accepted that WAT is a key player in the development of a chronic low-grade inflammation associated with adiposity and consequently obesity [192, 193] with elevated production of pro-inflammatory cytokines, such as TNFα, IL-6, and IL-1 [194, 195]. In contrast, loss of WAT is associated with a decrease in inflammation markers [196, 197]. Interestingly, chronic and low-grade inflammation has been proposed to negatively favor neurodegenerative diseases through the disruption of the BBB. Indeed, the blood brain barrier is a key interface linking systemic inflammation, neuroinflammation, and neurodegeneration [198], inflammatory factors being a main cause of the BBB disruption [199]. For instance, studies established positive correlations between mid-life adiposity in women with disruption of BBB integrity, showing that overweight/obesity could favor the onset of vascular disorders increasing BBB permeability later in life [200]. In the same line of evidence, rats fed with Western diet, known for promoting diabetes and obesity, display a leakier BBB due to the decreased expression of tight junctions [201]. Kanoski and colleagues have also shown that a primary cerebral target following BBB disruption is the hippocampus, well-known for its involvement in cognitive processes [201]. This is of peculiar interest given that AD patients display hippocampal atrophy and disruption of fronto-hippocampal connections early in the course of the disease [202–204]. This is further reinforced by the fact that AD in human and rodent models is strongly linked to an increased permeability of the BBB [205, 206]. Consequently, the chronic low-grade inflammation that takes place in obese and diabetic people could negatively favor brain inflammation and degeneration through BBB disruption.
Some interesting links exist between dietary factors displaying anti-inflammatory properties, inflammation, and disease outcomes. For instance, polyphenols such as flavonoids and curcumin and spices such as cinnamon have been suggested to decrease pro-inflammatory cytokines, inflammation, and cardiovascular diseases and type 2 diabetes which are known to be risk factors for AD (for a recent review on this topic see ref [207]).
Conclusions
While the causal nature of all the processes leading to neurodegeneration has not been definitively established, it is widely accepted that neuroinflammation and oxidative stress responses occur with clinical manifestation of the disease. In this review, we described the impact of pro-inflammatory adipokines (TNFα and leptin) on brain homeostasis and functions. In addition, pro-inflammatory adipokines play a major role in the production of reactive oxygen species (ROS) [208, 209]. Due to its ability to secrete adipokines that promote ROS production, WAT has been regarded as an independent factor provoking oxidative stress [210–212]. Exposure to obesity for a long time in a host system downregulates and depletes the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx); these enzymes being found to be significantly lowered compared with healthy persons which in turn lead to the development of obesity-related health problems [213]. In addition to this, levels of vitamin A and levels of serum antioxidants, such as vitamin E, vitamin C, and β-carotene, as well as glutathione, are also decreased in obesity [214]. When compared to the normal or lean individuals, obese individuals exhibit high levels of biomarkers of oxidative damage and inflammation such as C-reactive protein, LDL oxidation, and triglyceride levels [215]. Thus, apart from inflammation, which is quite well-known to be one of the critical factors that damages the brain, ROS production which exceeds the antioxidant defenses in the host system is another factor that can also result in brain damages [216]. Cytokines produced by the monocytes and macrophages in WAT are the potent stimulators for the production of reactive oxygen (ROS) and nitrogen species (RNS) which generates oxidative stress. Adipose tissue also has the secretory capacity of angiotensin II, which stimulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. NADPH oxidase comprises the major route for ROS production in adipocytes [217]). Thus, obesity results in an increased oxidative stress status that can lead to neural dysfunction and death [218, 219]. It has been reported that obesity may induce systemic oxidative stress and, in turn, oxidative stress is associated with an irregular production of adipokines, which contributes to the development of the metabolic syndrome [220]. In parallel, oxidative stress is implicated in numerous neurological diseases and/or disorders such as AD, PD, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), cerebral ischemia/reperfusion injury, and TBI, promoting neurodegeneration [221]. An increasing number of studies using in vitro models and knock-out animals demonstrate that oxidative stress disrupts the BBB permeability [221–223].
Taken together, these data suggest that in pathological conditions, adipokines released by WAT promote inflammation and ROS production that may disrupt the BBB permeability and could directly or indirectly act on different brain structures, the hippocampus being one of the most sensitive areas. It could explain why metabolic syndrome is associated with hippocampus atrophy and an increase risk to develop dementia such as AD. One main issue in people suffering from metabolic syndrome should be to struggle against inflammation and reduce oxidative stress in order to decrease their potential effects on brain neurodegeneration and their adverse effects.
Funding
This work was supported by the grants from Conseil Régional de La Réunion and Europe (CPER/FEDER). ACD is funded by a fellowship from University of La Réunion. AP was funded by a fellowship from “Conseil Régional de La Réunion.”
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CLH and ND contributed equally to this work. AP carried out the writing of the study. ACD and ND contributed to the figures. CLH conceived the study. CLH and ND participated in its coordination and helped to draft the manuscript. AP, ACD, RA, RP, ND, and CLH contributed to the revision and edition of the study. All authors read and approved the final manuscript.
Contributor Information
Avinash Parimisetty, Email: maverikavinash@gmail.com.
Anne-Claire Dorsemans, Email: anne-claire.dorsemans@univ-reunion.fr.
Rana Awada, Email: awada-rana@hotmail.com.
Palaniyandi Ravanan, Email: ravanan.p@vit.ac.in.
Nicolas Diotel, Email: nicolas.diotel@univ-reunion.fr.
Christian Lefebvre d’Hellencourt, Phone: (+262) 262 93 88 16, Email: Christian.Lefebvre-d-Hellencourt@univ-reunion.fr.
References
- 1.Awada R, Parimisetty A, Lefebvre d’Hellencourt C. Influence of obesity on neurodegenerative diseases. Neurodegener Dis. 2013;Chapter 16:381–401. [Google Scholar]
- 2.World Health Organisation W: obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 22 Mar 2016.
- 3.World Health Organisation W: diabetes. http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed 22 Mar 2016.
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–5. [DOI] [PubMed]
- 6.Reisberg B, Burns A, Brodaty H, Eastwood R, Rossor M, Sartorius N, et al. Diagnosis of Alzheimer’s disease. Report of an International Psychogeriatric Association Special Meeting Work Group under the cosponsorship of Alzheimer’s Disease International, the European Federation of Neurological Societies, the World Health Organization, and the World Psychiatric Association. Int Psychogeriatr. 1997;9 Suppl 1:11–38. [DOI] [PubMed]
- 7.World Health Organisation W: dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed 22 Mar 2016.
- 8.Nguyen S, Major K, Demonet JF, Smith C, Rubli E, Humbert M, et al. [Diabetes and dementia: the dangerous liaisons?]. Rev Med Suisse. 2014;2090-2092(10):2094–6. [PubMed]
- 9.Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 2014;24:1982-1999. [DOI] [PMC free article] [PubMed]
- 10.Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13:913–23. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson DR, Backman K, Waern M, Ostling S, Guo X, Zandi P, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–66. [DOI] [PMC free article] [PubMed]
- 12.Letra L, Santana I, Seica R. Obesity as a risk factor for Alzheimer’s disease: the role of adipocytokines. Metab Brain Dis. 2014;29:563–8. doi: 10.1007/s11011-014-9501-z. [DOI] [PubMed] [Google Scholar]
- 13.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–18. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 15.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:431–6. [DOI] [PubMed]
- 16.Tolppanen AM, Ngandu T, Kareholt I, Laatikainen T, Rusanen M, Soininen H, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–9. [DOI] [PubMed]
- 17.Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol. 2013;33:2–13. doi: 10.1016/j.semnephrol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 19.Chaldakov GN. The adipobiology of disease. In: Immunology, endocrine & metabolic agents in medicinal chemistry (formerly current medicinal chemistry—immunology, endocrine and metabolic agents), vol. Volume 7, Number 2, April 2007. 2007. p. 105–5.
- 20.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 22.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. [DOI] [PubMed]
- 23.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI200317797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–39. doi: 10.1079/PNS200194. [DOI] [PubMed] [Google Scholar]
- 25.Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 27.Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–45. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Bluher M. Adipokines—removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3:230–40. doi: 10.1016/j.molmet.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011;217:503–8. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. [DOI] [PubMed]
- 32.Herder C, Baumert J, Thorand B, Koenig W, de Jager W, Meisinger C, et al. Chemokines as risk factors for type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984-2002. Diabetologia. 2006;49:921–9. [DOI] [PubMed]
- 33.Lee EB. Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci. 2011;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5:713–20. doi: 10.1016/S1474-4422(06)70526-9. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Tian B. Metabolic syndrome: an important risk factor for Parkinson’s disease. Oxid Med Cell Longev. 2014;2014:729194. doi: 10.1155/2014/729194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianfrancesco MA, Acuna B, Shen L, Briggs FB, Quach H, Bellesis KH, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract. 2014;8:e435–47. [DOI] [PMC free article] [PubMed]
- 37.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 1792;2009:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring). 2008;16:119–24. [DOI] [PubMed]
- 42.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, Massaro JM, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–44. [DOI] [PMC free article] [PubMed]
- 44.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–81. doi: 10.1212/01.WNL.0000141850.47773.5F. [DOI] [PubMed] [Google Scholar]
- 45.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–64. [DOI] [PMC free article] [PubMed]
- 46.Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, et al. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A. 2010;107:8404–9. [DOI] [PMC free article] [PubMed]
- 47.Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–14. [DOI] [PubMed]
- 48.Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis. 2009;5:547–52. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II cohort study. Am J Clin Nutr. 2009;89:601–7. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res. 2011;217:134–41. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009;23:4353–60. [DOI] [PubMed]
- 52.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2015;58:36-45. [DOI] [PubMed]
- 53.Lynch CM, Kinzenbaw DA, Chen X, Zhan S, Mezzetti E, Filosa J, et al. Nox2-derived superoxide contributes to cerebral vascular dysfunction in diet-induced obesity. Stroke. 2013;44:3195–201. [DOI] [PMC free article] [PubMed]
- 54.Li W, Prakash R, Chawla D, Du W, Didion SP, Filosa JA, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1001–8. [DOI] [PMC free article] [PubMed]
- 55.Zhou H, Liu J, Ren L, Liu W, Xing Q, Men L, et al. Relationship between [corrected] spatial memory in diabetic rats and protein kinase Cgamma, caveolin-1 in the hippocampus and neuroprotective effect of catalpol. Chin Med J (Engl). 2014;127:916–23. [PubMed]
- 56.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–9. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 57.Zhang YW, Zhang JQ, Liu C, Wei P, Zhang X, Yuan QY, et al. Memory dysfunction in type 2 diabetes mellitus correlates with reduced hippocampal CA1 and subiculum volumes. Chin Med J (Engl). 2015;128:465–71. [DOI] [PMC free article] [PubMed]
- 58.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–90. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 59.Haan MN. Therapy insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2:159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 60.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–91. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 61.Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7:373–80. doi: 10.1007/s11910-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 62.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 63.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346–62. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 65.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 66.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 67.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab. 2000;278:E1158–65. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 68.Devos R, Richards JG, Campfield LA, Tartaglia LA, Guisez Y, van der Heyden J, et al. OB protein binds specifically to the choroid plexus of mice and rats. Proc Natl Acad Sci U S A. 1996;93:5668–73. [DOI] [PMC free article] [PubMed]
- 69.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–41. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson M, Morash B, Ur E. The brain is a source of leptin. Front Horm Res. 2000;26:106–25. doi: 10.1159/000061018. [DOI] [PubMed] [Google Scholar]
- 71.Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–8. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 72.Brown R, Imran SA, Belsham DD, Ur E, Wilkinson M. Adipokine gene expression in a novel hypothalamic neuronal cell line: resistin-dependent regulation of fasting-induced adipose factor and SOCS-3. Neuroendocrinology. 2007;85:232–41. doi: 10.1159/000104248. [DOI] [PubMed] [Google Scholar]
- 73.Brown R, Thompson HJ, Imran SA, Ur E, Wilkinson M. Traumatic brain injury induces adipokine gene expression in rat brain. Neurosci Lett. 2008;432:73–8. doi: 10.1016/j.neulet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorska E, Popko K, Stelmaszczyk-Emmel A, Ciepiela O, Kucharska A, Wasik M. Leptin receptors. Eur J Med Res. 2010;15(Suppl 2):50–4. doi: 10.1186/2047-783X-15-S2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 76.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. [DOI] [PubMed]
- 77.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–6. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 78.Yi CX, Meyer CW, Jastroch M. Leptin action in the brain: How (and when) it makes fat burn. Mol Metab. 2013;2:63–4. doi: 10.1016/j.molmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71:187–95. [DOI] [PubMed]
- 80.Savioz A, Charnay Y, Huguenin C, Graviou C, Greggio B, Bouras C. Expression of leptin receptor mRNA (long form splice variant) in the human cerebellum. Neuroreport. 1997;8:3123–6. doi: 10.1097/00001756-199709290-00023. [DOI] [PubMed] [Google Scholar]
- 81.Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545:933–44. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011;121:2413–21. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. [DOI] [PMC free article] [PubMed]
- 84.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. [DOI] [PubMed]
- 85.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. [DOI] [PubMed]
- 86.Baskin DG, Hahn TM, Schwartz MW. Leptin sensitive neurons in the hypothalamus. Horm Metab Res. 1999;31:345–50. doi: 10.1055/s-2007-978751. [DOI] [PubMed] [Google Scholar]
- 87.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–94. [DOI] [PubMed]
- 88.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu Y, Kim ER, Zhao R, Myers MG, Jr, Munzberg H, Tong Q. Glutamate release mediates leptin action on energy expenditure. Mol Metab. 2013;2:109–15. doi: 10.1016/j.molmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64:1808–12. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paz-Filho G, Wong ML, Licinio J. Leptin levels and Alzheimer disease. JAMA. 2010;303:1478. doi: 10.1001/jama.2010.436. [DOI] [PubMed] [Google Scholar]
- 93.Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128:162–72. [DOI] [PMC free article] [PubMed]
- 94.Fleisch AF, Agarwal N, Roberts MD, Han JC, Theim KR, Vexler A, et al. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. J Clin Endocrinol Metab. 2007;92:948–54. [DOI] [PMC free article] [PubMed]
- 95.Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing risk factors for obesity between childhood and adolescence: I. Birth weight, childhood adiposity, parental obesity, insulin, and leptin. Pediatrics. 2002;110:299–306. doi: 10.1542/peds.110.2.299. [DOI] [PubMed] [Google Scholar]
- 96.Wang H, Chu WS, Hemphill C, Elbein SC. Human resistin gene: molecular scanning and evaluation of association with insulin sensitivity and type 2 diabetes in Caucasians. J Clin Endocrinol Metab. 2002;87:2520–4. doi: 10.1210/jcem.87.6.8528. [DOI] [PubMed] [Google Scholar]
- 97.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5. [DOI] [PubMed]
- 98.Lee JH, Bullen JW, Jr, Stoyneva VL, Mantzoros CS. Circulating resistin in lean, obese, and insulin-resistant mouse models: lack of association with insulinemia and glycemia. Am J Physiol Endocrinol Metab. 2005;288:E625–32. doi: 10.1152/ajpendo.00184.2004. [DOI] [PubMed] [Google Scholar]
- 99.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–71. [DOI] [PubMed]
- 100.Park HK, Ahima RS. Resistin in rodents and humans. Diabetes Metab J. 2013;37:404–14. doi: 10.4093/dmj.2013.37.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Broglio C, Gómez A, Durán E, Ocaña FM, Jiménez-Moya F, Rodríguez F, et al. Hallmarks of a common forebrain vertebrate plan: specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res Bull. 2005;66:277–81. [DOI] [PubMed]
- 102.Kosari S, Camera DM, Hawley JA, Stebbing M, Badoer E. ERK1/2 in the brain mediates the effects of central resistin on reducing thermogenesis in brown adipose tissue. Int J Physiol Pathophysiol Pharmacol. 2013;5:184–9. [PMC free article] [PubMed] [Google Scholar]
- 103.Kosari S, Rathner JA, Badoer E. Central resistin enhances renal sympathetic nerve activity via phosphatidylinositol 3-kinase but reduces the activity to brown adipose tissue via extracellular signal-regulated kinase 1/2. J Neuroendocrinol. 2012;24:1432–9. doi: 10.1111/j.1365-2826.2012.02352.x. [DOI] [PubMed] [Google Scholar]
- 104.Yi CX, Tschop MH. Brain-gut-adipose-tissue communication pathways at a glance. Dis Model Mech. 2012;5:583–7. doi: 10.1242/dmm.009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez-Solana B, Laborda J, Baladron V. Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol Endocrinol. 2012;26:110–27. doi: 10.1210/me.2011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Böstrom EA, Svensson M, Andersson S, Jonsson IM, Ekwall AK, Eisler T, et al. Resistin and insulin/insulin-like growth factor signaling in rheumatoid arthritis. Arthritis Rheum. 2011;63:2894–904. [DOI] [PubMed]
- 107.Rodriguez-Pacheco F, Vazquez-Martinez R, Martinez-Fuentes AJ, Pulido MR, Gahete MD, Vaudry H, et al. Resistin regulates pituitary somatotrope cell function through the activation of multiple signaling pathways. Endocrinology. 2009;150:4643–52. [DOI] [PubMed]
- 108.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 109.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 110.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 111.Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, et al. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 2013;62:102–14. [DOI] [PMC free article] [PubMed]
- 112.Brown R, Wiesner G, Ur E, Wilkinson M. Pituitary resistin gene expression is upregulated in vitro and in vivo by dexamethasone but is unaffected by rosiglitazone. Neuroendocrinology. 2005;81:41–8. doi: 10.1159/000084873. [DOI] [PubMed] [Google Scholar]
- 113.Morash BA, Willkinson D, Ur E, Wilkinson M. Resistin expression and regulation in mouse pituitary. FEBS Lett. 2002;526:26–30. doi: 10.1016/S0014-5793(02)03108-3. [DOI] [PubMed] [Google Scholar]
- 114.Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86:191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- 115.Wiesner G, Brown RE, Robertson GS, Imran SA, Ur E, Wilkinson M. Increased expression of the adipokine genes resistin and fasting-induced adipose factor in hypoxic/ischaemic mouse brain. Neuroreport. 2006;17:1195–8. doi: 10.1097/01.wnr.0000224776.12647.ba. [DOI] [PubMed] [Google Scholar]
- 116.Miralbell J, Lopez-Cancio E, Lopez-Oloriz J, Arenillas JF, Barrios M, Soriano-Raya JJ, et al. Cognitive patterns in relation to biomarkers of cerebrovascular disease and vascular risk factors. Cerebrovasc Dis. 2013;36:98–105. [DOI] [PubMed]
- 117.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 118.Matsuzawa Y. Adiponectin: identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler Suppl. 2005;6:7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–27. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. [DOI] [PubMed]
- 121.Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89:38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- 122.Maddineni S, Metzger S, Ocon O, Hendricks G, 3rd, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250–6. doi: 10.1210/en.2005-0254. [DOI] [PubMed] [Google Scholar]
- 123.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/S1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 124.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70. [DOI] [PubMed]
- 125.Stefan N, Stumvoll M. Adiponectin—its role in metabolism and beyond. Horm Metab Res. 2002;34:469–74. doi: 10.1055/s-2002-34785. [DOI] [PubMed] [Google Scholar]
- 126.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 127.Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–702. [DOI] [PMC free article] [PubMed]
- 128.Hoyda TD, Fry M, Ahima RS, Ferguson AV. Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol. 2007;585:805–16. doi: 10.1113/jphysiol.2007.144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thundyil J, Tang SC, Okun E, Shah K, Karamyan VT, Li YI, et al. Evidence that adiponectin receptor 1 activation exacerbates ischemic neuronal death. Exp Transl Stroke Med. 2010;2:15. [DOI] [PMC free article] [PubMed]
- 130.Repunte-Canonigo V, Berton F, Cottone P, Reifel-Miller A, Roberts AJ, Morales M, et al. A potential role for adiponectin receptor 2 (AdipoR2) in the regulation of alcohol intake. Brain Res. 2010;1339:11–7. [DOI] [PMC free article] [PubMed]
- 131.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. [DOI] [PubMed]
- 132.Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991;7:391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- 133.Matsunaga E, Nambu S, Oka M, Iriki A. Differential cadherin expression in the developing postnatal telencephalon of a New World monkey. J Comp Neurol. 2013;521:4027–60. doi: 10.1002/cne.23389. [DOI] [PubMed] [Google Scholar]
- 134.Venkatesh B, Hickman I, Nisbet J, Cohen J, Prins J. Changes in serum adiponectin concentrations in critical illness: a preliminary investigation. Crit Care. 2009;13:R105. doi: 10.1186/cc7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hillenbrand A, Knippschild U, Weiss M, Schrezenmeier H, Henne-Bruns D, Huber-Lang M, et al. Sepsis induced changes of adipokines and cytokines—septic patients compared to morbidly obese patients. BMC Surg. 2010;10:26. [DOI] [PMC free article] [PubMed]
- 136.Hillenbrand A, Weiss M, Knippschild U, Wolf AM, Huber-Lang M. Sepsis-induced adipokine change with regard to insulin resistance. Int J Inflam. 2012;2012:972368. doi: 10.1155/2012/972368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wan Z, Mah D, Simtchouk S, Klegeris A, Little JP. Globular adiponectin induces a pro-inflammatory response in human astrocytic cells. Biochem Biophys Res Commun. 2014;446:37–42. doi: 10.1016/j.bbrc.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 138.Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–7. [PubMed]
- 139.Qiu G, Wan R, Hu J, Mattson MP, Spangler E, Liu S, et al. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age (Dordr). 2011;33:155–65. [DOI] [PMC free article] [PubMed]
- 140.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–23. [DOI] [PubMed]
- 141.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62. [DOI] [PMC free article] [PubMed]
- 142.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 143.Tang P, Hung MC, Klostergaard J. TNF cytotoxicity: effects of HER-2/neu expression and inhibitors of ADP-ribosylation. Lymphokine Cytokine Res. 1994;13:117–23. [PubMed] [Google Scholar]
- 144.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. [DOI] [PubMed]
- 145.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 146.Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, et al. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–6. [DOI] [PubMed]
- 147.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 148.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–52. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 150.Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–70. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- 151.Clark IA, Vissel B. A neurologist’s guide to TNF biology and to the principles behind the therapeutic removal of excess TNF in disease. Neural Plast. 2015;2015:358263. doi: 10.1155/2015/358263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 2015;2015:610813. doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harry GJ, Funk JA, Lefebvre d’Hellencourt C, McPherson CA, Aoyama M. The type 1 interleukin 1 receptor is not required for the death of murine hippocampal dentate granule cells and microglia activation. Brain Res. 2008;1194:8–20. doi: 10.1016/j.brainres.2007.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22. doi: 10.1016/j.neuroscience.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 155.Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A. 1995;92:9328–32. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–5. [DOI] [PubMed]
- 157.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–81. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 158.Heldmann U, Thored P, Claasen JH, Arvidsson A, Kokaia Z, Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp Neurol. 2005;196:204–8. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 159.Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF- and its receptors. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1056–64. doi: 10.1002/ajmg.b.30712. [DOI] [PubMed] [Google Scholar]
- 160.Selmaj K, Raine CS. Tumor necrosis factor mediates myelin damage in organotypic cultures of nervous tissue. Ann N Y Acad Sci. 1988;540:568–70. doi: 10.1111/j.1749-6632.1988.tb27175.x. [DOI] [PubMed] [Google Scholar]
- 161.Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–9. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- 162.Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–26. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 163.Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 164.Lan X, Chen Q, Wang Y, Jia B, Sun L, Zheng J, et al. TNF-alpha affects human cortical neural progenitor cell differentiation through the autocrine secretion of leukemia inhibitory factor. PLoS ONE. 2012;7:e50783. [DOI] [PMC free article] [PubMed]
- 165.Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–12. [DOI] [PMC free article] [PubMed]
- 166.Frisca F, Sabbadini RA, Goldshmit Y, Pebay A. Biological effects of lysophosphatidic acid in the nervous system. Int Rev Cell Mol Biol. 2012;296:273–322. doi: 10.1016/B978-0-12-394307-1.00005-9. [DOI] [PubMed] [Google Scholar]
- 167.Tokumura A. Metabolic pathways and physiological and pathological significances of lysolipid phosphate mediators. J Cell Biochem. 2004;92:869–81. doi: 10.1002/jcb.20147. [DOI] [PubMed] [Google Scholar]
- 168.Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 169.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, et al. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–28. [DOI] [PubMed]
- 170.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 171.Zhao Y, Natarajan V. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochim Biophys Acta. 1831;2013:86–92. doi: 10.1016/j.bbalip.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen Y, Ramakrishnan DP, Ren B. Regulation of angiogenesis by phospholipid lysophosphatidic acid. Front Biosci (Landmark Ed) 2013;18:852–61. doi: 10.2741/4130. [DOI] [PubMed] [Google Scholar]
- 173.Ye X, Skinner MK, Kennedy G, Chun J. Age-dependent loss of sperm production in mice via impaired lysophosphatidic acid signaling. Biol Reprod. 2008;79:328–36. doi: 10.1095/biolreprod.108.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. [DOI] [PMC free article] [PubMed]
- 175.Ye X, Chun J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab. 2010;21:17–24. doi: 10.1016/j.tem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matas-Rico E, Garcia-Diaz B, Llebrez-Zayas P, Lopez-Barroso D, Santin L, Pedraza C, et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci. 2008;39:342–55. [DOI] [PMC free article] [PubMed]
- 177.Estivill-Torrus G, Llebrez-Zayas P, Matas-Rico E, Santin L, Pedraza C, De Diego I, et al. Absence of LPA1 signaling results in defective cortical development. Cereb Cortex. 2008;18:938–50. [DOI] [PubMed]
- 178.Dubin AE, Herr DR, Chun J. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J Neurosci. 2010;30:7300–9. doi: 10.1523/JNEUROSCI.6151-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–9. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- 180.Fukushima N, Shano S, Moriyama R, Chun J. Lysophosphatidic acid stimulates neuronal differentiation of cortical neuroblasts through the LPA1-G(i/o) pathway. Neurochem Int. 2007;50:302–7. doi: 10.1016/j.neuint.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 181.Svetlov SI, Ignatova TN, Wang KK, Hayes RL, English D, Kukekov VG. Lysophosphatidic acid induces clonal generation of mouse neurospheres via proliferation of Sca-1- and AC133-positive neural progenitors. Stem Cells Dev. 2004;13:685–93. doi: 10.1089/scd.2004.13.685. [DOI] [PubMed] [Google Scholar]
- 182.Holtsberg FW, Steiner MR, Keller JN, Mark RJ, Mattson MP, Steiner SM. Lysophosphatidic acid induces necrosis and apoptosis in hippocampal neurons. J Neurochem. 1998;70:66–76. doi: 10.1046/j.1471-4159.1998.70010066.x. [DOI] [PubMed] [Google Scholar]
- 183.Zheng ZQ, Fang XJ, Zhang Y, Qiao JT. Neuroprotective effect of lysophosphatidic acid on AbetaP31-35-induced apoptosis in cultured cortical neurons. Sheng Li Xue Bao. 2005;57:289–94. [PubMed] [Google Scholar]
- 184.Zheng ZQ, Fang XJ, Qiao JT. Dual action of lysophosphatidic acid in cultured cortical neurons: survival and apoptogenic. Sheng Li Xue Bao. 2004;56:163–71. [PubMed] [Google Scholar]
- 185.Keller JN, Steiner MR, Holtsberg FW, Mattson MP, Steiner SM. Lysophosphatidic acid-induced proliferation-related signals in astrocytes. J Neurochem. 1997;69:1073–84. doi: 10.1046/j.1471-4159.1997.69031073.x. [DOI] [PubMed] [Google Scholar]
- 186.Moller T, Contos JJ, Musante DB, Chun J, Ransom BR. Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J Biol Chem. 2001;276:25946–52. doi: 10.1074/jbc.M102691200. [DOI] [PubMed] [Google Scholar]
- 187.Awada R, Rondeau P, Gres S, Saulnier-Blache JS, Lefebvre d’Hellencourt C, Bourdon E. Autotaxin protects microglial cells against oxidative stress. Free Radic Biol Med. 2012;52:516–26. doi: 10.1016/j.freeradbiomed.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 188.Awada R, Saulnier-Blache JS, Gres S, Bourdon E, Rondeau P, Parimisetty A, et al. Autotaxin downregulates LPS-induced microglia activation and pro-inflammatory cytokines production. J Cell Biochem. 2014;115:2123–32. [DOI] [PMC free article] [PubMed]
- 189.Frugier T, Crombie D, Conquest A, Tjhong F, Taylor C, Kulkarni T, et al. Modulation of LPA receptor expression in the human brain following neurotrauma. Cell Mol Neurobiol. 2011;31:569–77. [DOI] [PMC free article] [PubMed]
- 190.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–83. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111:15810–5. [DOI] [PMC free article] [PubMed]
- 192.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 194.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58. [DOI] [PubMed]
- 195.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 196.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–69. [DOI] [PubMed]
- 197.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 198.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mauro C, De Rosa V, Marelli-Berg F, Solito E. Metabolic syndrome and the immunological affair with the blood-brain barrier. Front Immunol. 2014;5:677. doi: 10.3389/fimmu.2014.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–50. doi: 10.1111/j.1365-2796.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 201.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–19. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wisse LE, Reijmer YD, ter Telgte A, Kuijf HJ, Leemans A, Luijten PR, Koek HL, Geerlings MI, Biessels GJ, Utrecht Vascular Cognitive Impairment Study G. Hippocampal disconnection in early Alzheimer's disease: a 7 tesla MRI study. J Alzheimers Dis. 2015;45:1247-1256. [DOI] [PubMed]
- 203.Remy F, Vayssiere N, Saint-Aubert L, Barbeau E, Pariente J. White matter disruption at the prodromal stage of Alzheimer’s disease: relationships with hippocampal atrophy and episodic memory performance. Neuroimage Clin. 2015;7:482–92. doi: 10.1016/j.nicl.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Whitwell JL. Progression of atrophy in Alzheimer’s disease and related disorders. Neurotox Res. 2010;18:339–46. doi: 10.1007/s12640-010-9175-1. [DOI] [PubMed] [Google Scholar]
- 205.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–14. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–70. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 207.Gardener SL, Rainey-Smith SR, Martins RN. Diet and inflammation in Alzheimer’s disease and related chronic diseases: a review. J Alzheimers Dis. 2015;50:301–34. doi: 10.3233/JAD-150765. [DOI] [PubMed] [Google Scholar]
- 208.Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15:1–10. doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]
- 209.Laurikka A, Vuolteenaho K, Toikkanen V, Rinne T, Leppanen T, Tarkka M, et al. Adipocytokine resistin correlates with oxidative stress and myocardial injury in patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2014;46:729–36. [DOI] [PubMed]
- 210.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. [DOI] [PMC free article] [PubMed]
- 211.Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–30. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–32. [DOI] [PMC free article] [PubMed]
- 213.Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, et al. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem. 2002;35:627–31. [DOI] [PubMed]
- 214.Vincent HK, Vincent KR, Bourguignon C, Braith RW. Obesity and postexercise oxidative stress in older women. Med Sci Sports Exerc. 2005;37:213–9. doi: 10.1249/01.MSS.0000152705.77073.B3. [DOI] [PubMed] [Google Scholar]
- 215.Pihl E, Zilmer K, Kullisaar T, Kairane C, Magi A, Zilmer M. Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int J Obes (Lond) 2006;30:141–6. doi: 10.1038/sj.ijo.0803068. [DOI] [PubMed] [Google Scholar]
- 216.Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morrow JD. Is oxidant stress a connection between obesity and atherosclerosis? Arterioscler Thromb Vasc Biol. 2003;23:368–70. doi: 10.1161/01.ATV.0000063107.86298.FD. [DOI] [PubMed] [Google Scholar]
- 218.Rodriguez-Rodriguez A, Egea-Guerrero JJ, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014;21:1201–11. doi: 10.2174/0929867321666131217153310. [DOI] [PubMed] [Google Scholar]
- 219.Ruszkiewicz J, Albrecht J. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem Int. 2015;88:66-72. [DOI] [PubMed]
- 220.Esposito K, Ciotola M, Schisano B, Misso L, Giannetti G, Ceriello A, et al. Oxidative stress in the metabolic syndrome. J Endocrinol Invest. 2006;29:791–5. [DOI] [PubMed]
- 221.Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta. 1822;2012:822–9. doi: 10.1016/j.bbadis.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–36. [DOI] [PMC free article] [PubMed]
- 223.Enciu AM, Gherghiceanu M, Popescu BO. Triggers and effectors of oxidative stress at blood-brain barrier level: relevance for brain ageing and neurodegeneration. Oxid Med Cell Longev. 2013;2013:297512. doi: 10.1155/2013/297512. [DOI] [PMC free article] [PubMed] [Google Scholar]


