Abstract
Obesity is gaining acceptance as a serious primary health burden that impairs the quality of life because of its associated complications, including diabetes, cardiovascular diseases, cancer, asthma, sleep disorders, hepatic dysfunction, renal dysfunction, and infertility. It is a complex metabolic disorder with a multifactorial origin. Growing evidence suggests that oxidative stress plays a role as the critical factor linking obesity with its associated complications. Obesity per se can induce systemic oxidative stress through various biochemical mechanisms, such as superoxide generation from NADPH oxidases, oxidative phosphorylation, glyceraldehyde auto-oxidation, protein kinase C activation, and polyol and hexosamine pathways. Other factors that also contribute to oxidative stress in obesity include hyperleptinemia, low antioxidant defense, chronic inflammation, and postprandial reactive oxygen species generation. In addition, recent studies suggest that adipose tissue plays a critical role in regulating the pathophysiological mechanisms of obesity and its related co-morbidities. To establish an adequate platform for the prevention of obesity and its associated health risks, understanding the factors that contribute to the cause of obesity is necessary. The most current list of obesity determinants includes genetic factors, dietary intake, physical activity, environmental and socioeconomic factors, eating disorders, and societal influences. On the basis of the currently identified predominant determinants of obesity, a broad range of strategies have been recommended to reduce the prevalence of obesity, such as regular physical activity, ad libitum food intake limiting to certain micronutrients, increased dietary intake of fruits and vegetables, and meal replacements. This review aims to highlight recent findings regarding the role of oxidative stress in the pathogenesis of obesity and its associated risk factors, the role of dysfunctional adipose tissue in development of these risk factors, and potential strategies to regulate body weight loss/gain for better health benefits.
Introduction
Obesity, the primary health burden of the 21st century, is a chronic disease that affects the quality of individual life physiologically, economically, and psychologically, irrespective of cultural, financial, or ethnic background.1 An excess amount of body fat not only reduces the quality of life but also increases both healthcare-associated costs and the risk of death.2 Obesity is associated with the development of a large number of health disorders, including diabetes, cardiovascular complications, cancer, asthma, sleep disorders, hepatic dysfunction, renal dysfunction, and infertility.3,4
The World Health Organization (WHO) defines overweight as a body mass index (BMI) of 25.0 to 29.9 kg/m2 and obesity as a BMI of ≥30 kg/m2.5 However, as a defining parameter, BMI has certain limitations as it does not distinguish the difference between lean mass and fat nor does it identify fat distribution. Recent studies have shown that obesity-associated risk factors depend not on excess body weight per se, but rather on the regional distribution of the excess body fat.6 In light of this, it is now well recognized that abdominal fat is a significant risk factor for obesity-associated diseases; in fact, visceral fat accumulation stimulates pro-oxidant and proinflammatory states.7
Epidemiological, clinical, and animal studies have reported the role of oxidative stress in the pathogenesis of obesity and its associated risk factors.8 Oxidative stress could trigger obesity by stimulating the deposition of white adipose tissue (WAT) and altering food intake; both cell culture and animal studies have demonstrated that oxidative stress can cause an increase in preadipocyte proliferation, adipocyte differentiation, and the size of mature adipocytes.9–11 Reactive oxygen species (ROS) have been found to be involved in the control of body weight by exerting different effects on hypothalamic neurons, which control satiety and hunger behavior.12 Obesity per se can also induce systemic oxidative stress through multiple biochemical mechanisms, such as superoxide generation from NADPH oxidases (NOX), oxidative phosphorylation, glyceraldehyde auto-oxidation, protein kinase C (PKC) activation, and polyol and hexosamine pathways.8,13 Other factors that also contribute oxidative stress to obesity include hyperleptinemia,14 tissue dysfunction,13 low antioxidant defense,15 chronic inflammation,7 and postprandial ROS generation.16
A broad range of strategies are recommended to reduce the increasing prevalence of obesity, including regular physical activity, ad libitum food intake, meal replacements, micronutrient supplementation, and dietary intake of fruits and vegetables. It is well documented that weight reduction decreases oxidation markers, increases antioxidant defenses, and reduces obesity-associated pathological risk factors.17 Dietary consumption of foods rich in monounsaturated fatty acids, ω-3 polyunsaturated fatty acids, antioxidants, micronutrients, phytochemicals, and probiotics has been found to be helpful in maintaining body weight and reducing the incidence of metabolic diseases.18,19 Data from observational and human interventional studies suggest that consumption of multiple nutrients rather than a single dietary component is beneficial in reducing obesity and its associated pathologies.20,21
This review gives information about the recent findings regarding the role of oxidative stress in the pathogenesis of obesity and its associated risk factors, the role of dysfunctional adipose tissue in the development of obesity-associated risk factors, the etiology of obesity, and potential strategies that could regulate body weight loss/gain for better health benefits.
Oxidative Stress, Obesity, and Its Associated Heath Risks
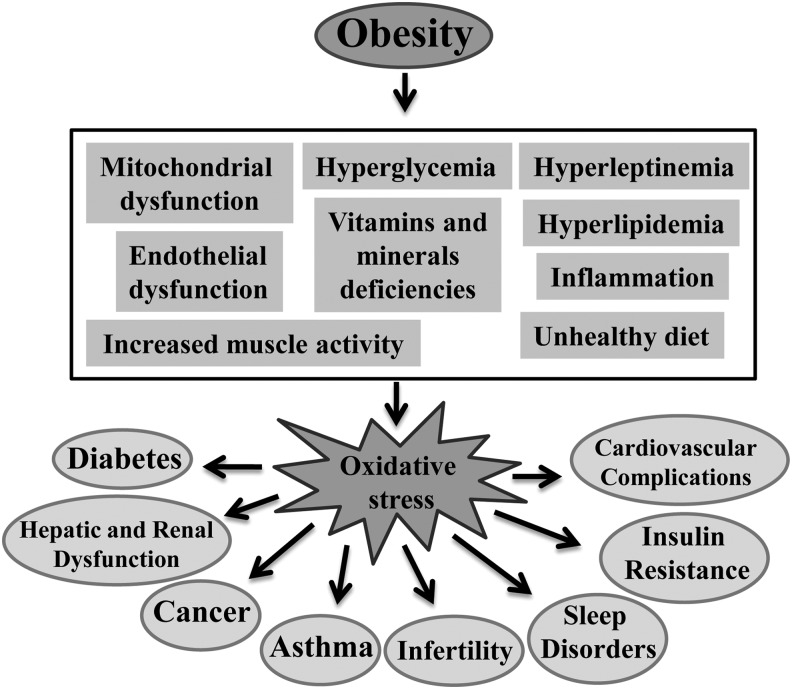
Oxidative stress plays an important role in the development of co-morbidities in obesity. Over the last few years, evidence of obesity-induced oxidative stress in humans has been reported in the literature.22 The possible contributors to oxidative stress in obesity include hyperglycemia,23 elevated tissue lipid levels,24 vitamin and mineral deficiencies,25,26 chronic inflammation,7 hyperleptinemia,27 increased muscle activity to carry excessive weight,28 endothelial dysfunction,29 impaired mitochondrial function,30 and type of diet.31 Malondialdehyde (MDA), F-2 isoprostanes (F2-IsoP), 8-iso Prostaglandin F2α (8-isoPGF2α), and protein carbonylation are well-known plasma, serum, or urine oxidative stress biomarkers. A significant positive correlation has been observed between BMI and oxidative stress biomarkers.22 Activities of the antioxidant enzymes, Cu-Zn superoxide dismutase (SOD) and glutathione peroxidase (GPx), were found to be lower in the erythrocytes of obese subjects compared to those of nonobese controls.32,33 Ferric reducing antioxidant power (FRAP) and total antioxidant status (TAS) have been used as comprehensive measures of radical quenching capacity by antioxidants in plasma. Several studies have reported lower plasma levels of FRAP and TAS in obese subjects compared to those seen in nonobese controls.22,34,35 Obesity-induced oxidative stress causes the development of various pathological events, including insulin resistance and diabetes, cardiovascular complications, sleep disorders, asthma, oncological problems, reproduction, rheumatological problems, and liver failure.3,4 In this study, we will discuss in detail about the role of various contributing factors in developing oxidative stress in obesity. In addition, we will also discuss the role of oxidative stress underlying the development of different health risks associated with obesity (Fig. 1).
FIG. 1.
Conditions generating oxidative stress in the pathogenesis of obesity and the role of oxidative stress in the development of obesity associated health risks.
Conditions generating oxidative stress in obesity
Hyperglycemia and oxidative stress in obesity
Obesity is directly associated with insulin resistance and hyperglycemia. Intracellular glucose overload increases the glycolytic pathway and the tricarboxylic acid cycle, leading to the overproduction of NADH and FADH2; the resulting increase in proton gradient across the mitochondrial inner membrane causes electron leakage at complex III, leading to superoxide production. The free radical thus inhibits glyceraldehyde-3-phosphate dehydrogenase and thereby redirects upstream metabolites into four alternative pathways36: (1) glucose is shifted to the polyol pathway; (2) fructose-6-phosphate is shifted to the hexosamine pathway; (3) triose phosphates produce methylglyoxal, the main precursor of advanced glycation end products (AGE); and (4) dihydroxyacetone phosphate is converted to diacylglycol, which activates the PKC pathway. Activation of these alternative pathways induces oxidative/nitrosative stress either by enhancing free radical production or by impairing antioxidant defenses. Activation of the polyol pathway causes NADPH depletion and increases the conversion of glucose to sorbitol, which activates several stress genes and causes oxidative stress, as evidenced in several animal studies.37 Formation of glucosamine-6-phosphate in the hexosamine pathway inhibits thioredoxin activity and induces oxidative and endoplasmic reticulum (ER) stress; AGE and PKC stimulate the production of ROS/RNS by activating NOX and NF-kB.38,39 Activation of NOX enzymes increases the production of superoxide radicals (O2•−) by catalyzing the reduction of oxygen using NADPH as an internal electron donor.40 Glucose auto-oxidation also produces reactive oxidants similar to hydroxyl and superoxide radicals.23 AGE binds to specific cell surface receptors causing the modification of postreceptor signaling and promotes further generation of ROS.41 Activation of NF-kB drives transcription of adhesion molecules (E-selectin, intercellular adhesion molecule-1, and endothelin-1), proinflammatory cytokines [tumor necrosis factor-α (TNF-α) and interleukin (IL)-6], iNOS, and microRNAs involved in adipogenesis, inflammation, and oxidative stress.42
Elevated lipid levels and oxidative stress in obesity
Obesity is associated with an increase in plasma free fatty acids (FFA) as well as excessive fat storage in WAT. Elevated plasma FFA promote the generation of O2•− in the mitochondrial electron transport chain by inhibiting the translocation of adenine nucleotides.43 FFA stimulate the production of reactive intermediates through PKC-dependent activation of NOX in cultured vascular cells.44 Conjugated fatty acids are susceptible to oxidation, stimulate the formation of radicals, and enhance the accumulation of oxidative by-products.9 The susceptibility of lipids to oxidative modification is shown by the higher concentrations of 4-hydroxynonenal (4-HNE) per unit of intramuscular triglycerides in obese patients.45 Higher concentrations of lipid molecules in the obese may also present an enlarged target for oxidative modification by ROS.46 In a study with several animal models of obesity, Furukawa et al. found that accumulation of excessive fat in WAT caused an increase in lipid peroxidation in the WAT itself.9 In the animal studies, it was observed that obesity increased the NOX activity and decreased the mRNA expression and the activities of antioxidant enzymes such as SOD, catalase (CAT), and GPx in WAT.9 Dietary intake of specific lipids also induces systematic oxidative stress. Consumption of conjugated linolenic acid increased the urinary concentration of 8-epi PGF2α in middle-aged men with abdominal obesity.47
Vitamin and mineral deficiencies and oxidative stress in obesity
Adequate intracellular antioxidant defenses are necessary to maintain the antioxidant–pro-oxidant balance in tissues. Deficiencies in vitamins and minerals can also contribute to the development of an impaired antioxidant defense in the pathogenesis of obesity.25,26 Plasma levels of α-tocopherol or β-carotene expressed per unit of plasma low-density lipoprotein (LDL) are well-known biomarkers for estimating the antioxidant protection within circulating lipids. An increase in BMI has been found to be related to low levels of carotenoids, vitamin C, and vitamin E.48–50 The Coronary Artery Risk Development in Young Adults (CARDIA) study reported an inverse relationship between BMI and the concentration of total serum carotenoids (α-carotene, β-carotene, α-cryptoxanthin, and zeaxanthin/lutein).51 In the National Health and Exanimation survey, obese children showed levels of serum β-carotene lower than those seen in normal control subjects.25 For instance, in one study, it was observed that obese girls had lower plasma levels of α-tocopherol/LDL and β-carotene/LDL than those seen in nonobese girls.52 Aasheim and Bohmer reported that most obese patients have profound reductions in vitamin levels, especially vitamins A, B6, C, D, and E.53 The multicenter prospective population study in Europe (EPIC) showed that the plasma vitamin C level was inversely related to central fat distribution.54 Obese adults (BMI >50) also had lower plasma levels of vitamin E/triglycerides compared to nonobese adults (BMI <30).55 Lower selenium and zinc levels have been observed in obese children, especially in children with central obesity.25,56 In addition, morbidly obese patients also show magnesium, selenium, iron, and zinc deficiencies.57 These results suggest that in the obese population, inadequate concentrations of vitamins and minerals cause the observed impaired antioxidant defense.
Chronic low-grade inflammation and oxidative stress in obesity
Obesity is described as a state of chronic low-grade inflammation, which is another important source of oxidative stress in obesity.58 Elevated levels of plasma and uriary F2-IsoPs, a biomarker of oxidative stress, have been found in a number of inflammatory diseases, such as Crohn's disease59 and rheumatic diseases.60 TNF-α, IL-6, and IL-1 are the most well-known mediators of the early inflammatory response. Other cytokines that are frequently seen in inflammation include IL-8, IFN-γ, IL-18, and IL-1ra (IL-1 receptor antagonist).61 Both TNF-α and IL-6 increase the activities of NOX and the production of superoxide anion.62,63
Hyperleptinemia and oxidative stress in obesity
Plasma leptin concentrations are associated with the amount of adipose tissue.64 Obesity is associated with elevated plasma leptin levels.65 Leptin plays an important role in obesity-induced oxidative stress.66 The hormone leptin activates NOX and induces the production of reactive intermediates such as H2O2 and hydroxyl radical.67 In a rodent model, leptin injection caused higher levels of plasma and urinary lipid hydroperoxide, MDA, isoprostane, and protein carbonyl content compared to levels seen in nontreated controls.68 In addition, leptin also stimulates the proliferation of monocytes and macrophages and thus promotes the production of proinflammatory cytokines.68,69 Exposure of monocyte-derived macrophages to leptin induces the PKC activity and macrophage lipoprotein lipase activity.70 Leptin also reduces the activity of the cellular antioxidant paranoxase-1 (PON-1); this reduction is related to increased levels of plasma and urinary 8-isoPGF2α and plasma levels of MDA and hydroperoxides.68
Increased muscle activity and oxidative stress in obesity
In obesity, increased muscle activity can generate excessive free radicals through the activation of metabolic pathways, including increased electron transport chain activity and conversion of hypoxanthine to urate.71 In addition, rapid electron transfer during increased respiration may cause some electrons to leak from the electron transport chain.72 For this reason, among obese individuals, the rate of cellular respiration and oxygen consumption may be exacerbated in muscle tissue during physical activities.34 It has been observed that, during the same amount of load-bearing walking activity, obese persons have a 38% higher oxygen consumption than do nonobese individuals and these values were found to be correlated with postexercise lipid hydroperoxide values.34 Obese individuals are also mechanically less efficient during exercise and this insufficiency contributes to the increased energy expenditure for a given exercise load.22 An increase in mitochondrial respiration for energy production is associated with higher levels of lipid hydroperoxide in obese people.73 During exercise, increased concentrations of hypoxanthine have been reported in obese people; the conversion of hypoxanthine to urate is associated with the formation of superoxide anion.71
Endothelial dysfunction and oxidative stress in obesity
The vascular endothelium is an important site for several enzymatic sources of oxidant generation, including NOX, xanthine oxidase, and NO synthase.74 Activation of NOX is a major player in the production of endothelial O2•−.75 Xanthine oxidase also reacts with O2 to form O2•− and H2O2.76 Production of excessive O2•− leads to rapid reaction with NO to form ONOO• and thus reduces NO bioavailability and causes nitrosylation of proteins.29 The enzyme NO synthase also stimulates the formation of excessive O2•− and ONOO• by catalyzing the electron transport from NADPH to another heme group.77 The activities of these oxidant producing enzymes can be modified by other cytokines and hormones such as those present in the renin–angiotensin system. It has been observed that in angiotensin II-induced hypertension, the production of endothelial O2•− is significantly elevated as a consequence of increased NADPH-oxidase activity.78 Obesity is associated with higher concentrations of the hormones in the renin–angiotensin system.79 Increased concentrations of angiotensin II may promote oxidative stress in vasculature through several mechanisms, including activation of NOX, formation of O2•−, and production of H2O2.80,81 Elevated intraluminal pressure from the hypertension associated with obesity may also stimulate the formation of O2•− and ONOO•.82
Impaired mitochondrial function and oxidative stress in obesity
Mitochondrial dysfunction has been implicated in the pathogenesis of a variety of diseases, including obesity and its associated risk factors.83 During the adipocyte differentiation process, mitochondrial biogenesis and activity increase rapidly, suggesting a critical role for mitochondria in this organelle.84 Mitochondria play a central role in ATP production, energy expenditure, and disposal of ROS.83 The process of oxidative phosphorylation in mitochondria is very efficient; however, a small excess of electrons cause a reduction of oxygen resulting in the formation of potentially toxic-free radicals.7 In addition to this, under certain conditions, protons can be reintroduced into the mitochondrial matrix through different uncoupling proteins, leading to an alteration in the regulation of free radical production in mitochondria.30 Excessive energy substrate causes mitochondrial dysfunction, which has been linked to the dysregulated secretion of adipokines,85 defects in fatty acid oxidation,86 increased production of ROS,86 and alteration of glucose homeostasis.87
Role of diet type in inducing oxidative stress in obesity
Diet is another possible contributing factor in the generation of ROS during the pathogenesis of obesity and its associated risk factors. Consumption of a high-fat diet may alter oxygen metabolism. It has been observed that consumption of a diet high in fat and carbohydrates induces significant oxidative stress and inflammation in persons with obesity.16 Lower dietary intake of protective phytochemicals rich in antioxidants (β-carotene, vitamin E and C, etc.) may cause an inadequate antioxidant defense.88 Dietary intake of antioxidant phytochemicals is inversely associated with degree of adiposity,89 BMI, and lipid peroxidation.88 Serum levels of dietary antioxidants and levels of trace minerals (zinc, selenium, etc.), which are cofactors for antioxidant enzymes, were found to be lower in obese people compared to those seen in nonobese individuals.49,89,90
Role of oxidative stress in obesity-associated health risks
Obesity, oxidative stress, and type 2 diabetes
In vivo studies. Both obesity and diabetes are major public health problems throughout the world and are associated with significant, potentially life-threatening co-morbidities. Results from metabolic and epidemiological studies provide strong evidence that the increasing prevalence of obesity is closely associated with the increase in type 2 diabetes.91,92 Some experts call this dual epidemic diabesity.93 However, while it has been observed that not all subjects with type 2 diabetes are obese and that, conversely, many obese subjects do not have diabetes, most subjects with type 2 diabetes are overweight or obese. According to the guidelines of the American Diabetes Association, the diagnosis criteria of diabetes mellitus include the following: (1) A1C ≥6.5% or fasting plasma glucose after an 8-hr fast ≥126 mg/dL, or 2-hr postload glucose ≥200 mg/dL during an OGTT, or symptoms of diabetes mellitus and a random plasma glucose concentration ≥200 mg/dL. Several studies in the literature reported a linear association between BMI and type 2 diabetes94,95 and it has been observed that the risk of type 2 diabetes increases as BMI increases above 23.96 Very recently, in a systematic review of 18 weight-related diseases, the investigators found that diabetes is at the top of the risk list: men with BMI ≥30 had a 7-fold and women with BMI ≥30 had a 12-fold higher risk of developing type 2 diabetes compared with men and women in the normal weight ranges (BMI ≤25).97 In addition to total body fat, distribution of fat and the relative proportions of lipids in various insulin-sensitive tissues (liver, skeletal muscle, and adipose) also play an important role in this pathophysiology.98 Abdominal obesity is one the most important, and most dangerous, forms of obesity. Accumulation of intra-abdominal or visceral fat is major feature of metabolic syndrome and is associated with a higher incidence of diabetes and cardiovascular disease (CVD) risk factors.99,100 Waist circumference (WC) as a measure of abdominal obesity has been considered as a better predictor of the risk of developing type 2 diabetes.101 Insulin resistance coupled with pancreatic β-cell dysfunction is a critical factor for the development of type 2 diabetes.
Mechanistic studies—Oxidative stress and β-cell dysfunction. Glucotoxicity and lipotoxicity have been recognized as the major contributors to β-cell dysfunction in the pathogenesis of type 2 diabetes.102 Pancreatic β-cells have a relatively low expression of many antioxidant enzymes, including catalase and GPx, which makes them susceptible to ROS-induced damage.103 In addition, overexpression of catalase or GPx can reverse these effects and protect β-cells.104,105 Hou et al. demonstrated that chronic exposure to high glucose and/or circulating FFA increases ROS production and decreases insulin content and glucose-stimulated insulin secretion of β-cells.106 ROS has been shown to reduce insulin gene expression and insulin secretion, probably through post-translational repression of two key transcriptional factors, musculoaponeurotic fibrosarcoma protein A (MafA) and pancreatic duodenal homeobox-1 (PDX-1), which bind to the promoter region of the insulin gene.107,108 Superoxide-induced activation of uncoupling protein 2 (UCP2) lowers ATP levels and negatively regulates glucose-stimulated insulin secretion.109–111 UCP2 knockout mice do not experience hyperglycemia- and obesity-induced loss of glucose responsiveness.111
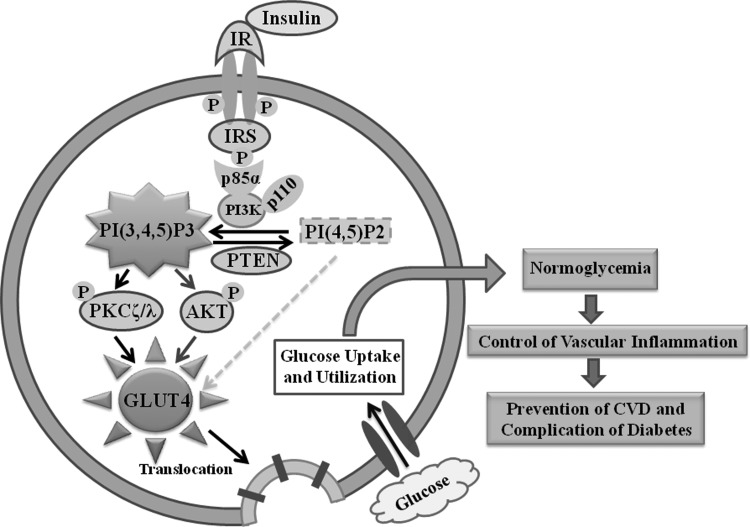
Mechanistic studies—Oxidative stress and insulin resistance. The insulin signaling cascade is initiated by the binding of insulin with its receptor (IR), which undergoes receptor autophosphorylation and enhanced tyrosine kinase activity. Subsequent phosphorylation of insulin receptor substrate (IRS) on tyrosine residues stimulates the downstream signaling cascade of glucose metabolism112 (Fig. 2). In contrast, serine/threonine phosphorylation of the IR and IRS provides a negative regulatory mechanism by opposing their tyrosine phosphorylation.113 Elevated ROS production has been shown to activate the stress-sensitive serine/threonine kinase, c-jun-N-terminal kinase (JNK), which in turn causes phosphorylation of IRS at serine residues and thus attenuates insulin signaling.114 Although the precise mechanism by which ROS activates JNK remains uncertain, it has been shown that H2O2 can inhibit thioredoxin by modification of its cysteine residues and cause full activation of apoptosis signal-regulating kinase 1 (ASK1), which in turn activates JNK.115,116 H2O2 has also been shown to activate JNK through inhibition of mitogen-activated protein kinase (MAPK) phosphatase by oxidation of the cysteine.106 Intake of a high-fat diet has been shown to increase mitochondrial ROS production and direct the cellular redox environment to a more oxidized state in skeletal muscle of rodents or humans.9,117,118 Reduction in mitochondrial ROS production by antioxidant treatment or overexpression of catalase or MnSOD has been found to prevent high-fat diet-induced insulin resistance in mice.117 These findings suggest that elevated mitochondrial ROS production by excessive metabolic flux promotes insulin resistance. Transgenic mice [overexpressing peroxisome proliferator-activated receptor-alpha (PPARα)] engineered to increase fatty acid β-oxidation develop severe insulin resistance and glucose intolerance despite being protected from diet-induced obesity.119 However, genetically engineered mice (AIF, apoptosis inducing factor knockout) with reduced mitochondrial oxidative phosphorylation are protected from obesity and diabetes.120 These observations suggest that reduction in mitochondrial oxidative phosphorylation prevents insulin resistance. In addition to post-translational modification of the insulin signaling pathway, oxidative stress also influences glucose metabolism through the regulation of transcriptional factors. The FoxO family of Forkhead transcription factor regulates gluconeogenesis, adipocyte differentiation, and β-cell proliferation.121–123 FoxO1 is a negative regulator of insulin sensitivity. Oxidative stress can activate FoxO through the formation of the cysteine thiol disulfide-dependent complex with p300/CBP acetyl transferase and subsequent acetylation of FoxO.124 FoxO also regulates various biological functions, such as cell cycle arrest, antioxidant response, DNA repair, and apoptosis.125 Deletion of FoxO1 in diabetic mice reverses the diabetic phenotype.123
FIG. 2.
Insulin stimulated signaling cascade of glucose metabolism. Binding of insulin with its receptor (IR) undergoes receptor autophosphorylation and enhances its kinase activity. Subsequent binding of IRS (insulin receptor substrate) with the p85 regulatory subunit of PI3K (phosphoinositide 3-kinase) upregulates the synthesis of PtdIns(3,4,5)P3 utilizing PtdIns(4,5)P2 as a substrate. The phosphatase, PTEN (phosphatase and tensin homolog deleted on chromosome 10) dephosphorylates PtdIns(3,4,5)P3 at the 3′-position. Formation of PtdIns(3,4,5)P3 activates downstream effector protein molecules, Akt (serine/threonine protein kinase) and PKCζ/λ (protein kinase C zeta/lambda). This causes the translocation of GLUT (glucose transporter) from intracellular site to the plasma membrane followed by glucose uptake and utilization by the cells leading to normoglycemia, control of vascular inflammation, and prevention of CVD (cardiovascular diseases) and the complications of diabetes.
Obesity, oxidative stress, and CVD
In vivo studies: Obesity is a major contributor to the development of fatal and nonfatal CVD such as coronary artery disease, stroke, peripheral artery disease, cardiomyopathy, and congestive heart failure.126,127 Excessive body weight is directly associated with a number of cardiovascular risk factors, including hypertension, dyslipidemia, and hemostatic and rheological factors.128 Numerous studies in the literature have reported a clear association between the risk of coronary heart disease (CHD) and a modest increase in BMI.129,130 In a 16-year follow-up study among middle-aged US women (35 to 55 years), it was demonstrated that a small increase in BMI (>23 but <25) was associated with a 50% increase in risk for nonfatal or fatal CHD.130 Among the male US population, those aged 40–65 years with a BMI >23 but <29 had a 72% increased risk of CHD after adjusting for other coronary risk factors.129
Mechanistic studies. Oxidative stress plays an important role in the development of CVD risk factors among the obese population. Low levels of circulating high-density lipoprotein (HDL), increased clearance of HDL particles, higher levels of postprandial triglycerides, and elevated levels of LDL induce ROS generation in the endothelium.131 Elevated ROS can directly cause damage to lipids, proteins, or DNA molecules and thereby modulate intracellular signaling cascades, such as MAPK and redox-sensitive transcription factors.131 ROS-mediated changes in lipid expression, formation of oxidized lipid products, such as oxidized LDL (Ox-LDL) particles, and activation of macrophages induce the formation of atherosclerotic lesions. Paraoxonase-1 (PON-1) is a HDL-attached extracellular esterase that contributes to the antiatherogenic, antioxidant, and anti-inflammatory properties of HDL.132 A decrease in PON-1 is a risk factor for CVD and has recently been found to be associated with obesity.133 Activation of the NOX system and renin–angiotensin system and stimulation of proinflammatory cytokine release also induce ROS production and the progression of vascular disease. Elevated ROS in the blood stream causes migration of monocytes/macrophages and apoptosis of endothelial cells, which promotes vascular inflammation and injury. Endothelium NO causes vascular relaxation, and a decrease in endothelium NO release reduces vasodilation, which can favor the development of hypertension.134 Various studies have reported the role for oxidative stress in the development of hypertension.134 Elevated ROS as well as lower NO levels and high levels of F2-isoprostanes induce vasoconstriction and platelet hyperactivity.135 Ox-LDL induces adipocyte proliferation either directly or indirectly by increasing the accumulation of fatty acids in adipocytes,136 by inducing the expression of lipoprotein lipase,136 or by increasing the infiltration of monocytes/macrophages.137 Ox-LDL-induced alteration in adipokine secretion can also induce CVD complications.138 Higher Ox-LDL in obese subjects may be due to lower serum activity of antioxidant enzymes139 or reduced PON-1 levels.132
Obesity, oxidative stress, and carcinogenesis
In vivo studies. Recently, it has been observed that obesity is a major risk factor for cancer.140 A number of prospective epidemiological studies have demonstrated a direct association between being overweight and developing cancer, although obesity alone does not cause an increase in cancer risk in all tissues by the same extent.140–142 The International Agency for Research into Cancer (IARC)143 and the World Cancer Research Fund (WRCF) reports144 concluded that common cancers in obese people are endometrial, esophageal, adenocarcinoma, colorectal, postmenopausal breast, pancreas, prostate, and renal. There are also some less common malignancies associated with obesity, such as thyroid cancer,145 leukemia, non-Hodgkin's lymphoma, and multiple myeloma.146
Mechanistic studies. Oxidative stress is one of the leading causes of DNA damage with the formation of modified bases and mutations of tumor suppressor genes, which is considered to be the most critical factor in carcinogenesis.147 Obesity-induced inflammation is thought to be a critical link between obesity and cancer. Inflammation-induced increased production of free radicals and subsequent development of oxidative stress create a microenvironment favorable to tumor development in obese persons.148 Adiponectin functions as a negative regulator of obesity-related carcinogenesis, and hypoadiponectinemia is a known risk factor for tumorigenesis.149
Obesity, oxidative stress, and asthma
In vivo studies: Both obesity and asthma have a considerable impact on public health.150–153 Various cross-sectional studies in different countries show an increased prevalence of asthma among obese adults compared to its occurrence in the normal-weight population, suggesting that obesity could increase the risk of asthma.150 The relationship between obesity and asthma has been observed consistently regardless of the ethnic origin of the studies' population.150,154–157 In a prospective study with the highest number of subjects and with the longest follow-up (135,000 Norwegians, follow-up for 21 years), the incidence of asthma increased 10% and 7% per unit increase in BMI in men and women, respectively.158
Mechanistic studies. Oxidative stress plays an important role in the pathogenesis of asthma.159,160 Children with asthma have higher plasma levels of MDA and 8-isoprostanes compared to those of healthy controls.161 Asthmatic subjects with more obstructed airways have a higher degree of oxidative stress.162 Obesity-mediated oxidative stress may affect the lung function of asthmatic subjects by airway inflammation and thus reduce the effectiveness of inhaled corticosteroids.163 There are several pathways by which obesity may increase airway oxidative stress, such as adipokine imbalance, obstructive sleep apnea (OSA), and reduced antioxidant defense.163
Obesity, oxidative stress, and sleep disorders
In vivo studies. Insomnia, OSA, and sleep-related movement disorders are the three most prevalent types of sleep disorders, as characterized by the International Classification of Sleep Disorders.164 All of these sleep disorders cause a decrease in sleep duration and quality and this has been associated with an increase in body weight and adiposity.165,166 People with obesity are significantly more likely to report insomnia or difficulty sleeping.167 Significant sleep apnea is present among ∼40% of obese individuals; ∼70% of OSA patients are obese.168 Several cross-sectional studies have reported an association between an increase in body weight and the risk of OSA.169 Similarly, weight loss in OSA patients has been found to significantly decrease apnea frequency.170,171
Mechanistic studies. Oxidative stress in OSA patients has been found to be associated with central obesity rather than intermittent hypoxia or respiratory disturbances.172 Among obese OSA patients, expression of NF-κB is higher than that observed in obese OSA-free subjects, suggesting that elevated levels of proinflammatory cytokines upregulate NF-κB in neutrophils and monocytes of sleep apnea patients, which upregulates ROS production and causes oxidative stress in this pathophysiology.173
Obesity, oxidative stress, and liver dysfunction
In vivo studies. Obesity is associated with an increased risk of hepatic dysfunction, known as nonalcoholic fatty liver disease (NAFLD), which is characterized by an increase in intrahepatic lipid accumulation due to increased inflow of FFA and/or de novo lipogenesis.174,175 A serious consequence of NAFLD is nonalcoholic steatohepatitis (NASH), which can progress to liver fibrosis, cirrhosis, and eventually hepatocellular carcinoma. The prevalence of NAFLD increases with the increase in BMI.176 Among nonobese subjects, the rates of occurrence of steatosis and steatohepatitis were ∼15% and 3%, respectively; however, in subjects with BMI between 30 and 39.9, the prevalence rates were 65% and 20%, respectively, and in extremely obese subjects with BMI ≥40, the rates were 85% and 40%, respectively.175 Racial/ethnic background and genetic variation in specific genes can influence the relationship between BMI and NAFLD.177–179 NAFLD has become an important public health issue because it is associated with a high risk of developing type 2 diabetes, cardiovascular complications, and dyslipidemia.180 An increase in intrahepatic triglyceride content is associated with alterations in glucose, fatty acids, and lipoprotein metabolism, leading to hepatic insulin resistance in association with serious cardiovascular dysfunction.
Mechanistic studies. Oxidative stress has been implicated in the pathogenesis of NAFLD/NASH.181 Mitochondrial, peroxisomal, microsomal, and ER oxidative stress plays an important role in the pathogenesis of NAFLD.182 Elevated fatty acid catabolism in hepatocytes causes excessive electron flux in the mitochondrial electron transport chain, which impairs the oxidative capacity of the mitochondria and stimulates the peroxisomal and microsomal pathways of fat oxidation. The consequent overproduction of ROS and reactive aldehyde derivatives causes oxidative stress and cell death by reducing cellular ATP, NAD, and glutathione levels and causing DNA, lipid, and protein damage.183 ER stress-induced cell death is mediated through calcium perturbations, ROS production, and activation of JNK-dependent signaling cascade. Hyperglycemia is also an important contributor to hepatic lipid accumulation; apart from the stimulation of increased ROS production and the consequent development of oxidative stress, it also causes activation of carbohydrate responsive element-binding protein (ChREBP), which stimulates the transcription of L-type pyruvate kinase (L-PK) and various lipogenic genes.184
Obesity, oxidative stress, and renal dysfunction
In vivo studies: Although obesity has long been recognized as an independent risk factor for CVD and diabetes mellitus, recent studies in the literature have reported that obesity is also an important risk factor for chronic kidney diseases (CKD).185–187 In 1974, Weisinger et al. first reported an association between massive obesity and nephrotic syndrome.188 Subsequent studies in the literature demonstrated that obesity could induce renal dysfunction, namely, obesity-related glomerulopathy (ORG).189–191 In the Framingham Offspring cohort study, a single-unit increase in BMI was found to be associated with a 20% increase in kidney disease over a period of 20 years of follow up.192 In a large-scale clinicopathologic study, Kambham et al. found a progressive increase in ORG from 0.2% in the years between 1986 and 1990 to 2% in the years between 1996 and 2000.191 Kambham et al. suggest that this 10-fold increase in incidence of ORG over 15 years is a newly emerging epidemic.191
Mechanistic studies. Oxidative stress has been increasingly linked to a higher incidence of CKD.193 A significant imbalance between pro-oxidants and antioxidants has been observed among patients with renal dysfunction.194 Serum TAS is inversely associated with the glomerular filtration rate (GFR).195 Impaired lymphocytic function has been described in chronic renal failure.196 Tepel et al. demonstrated that elevated ROS production may cause impaired lymphocytic function in patients with renal dysfunction.194
Obesity, oxidative stress, and infertility
In vivo studies: Elevated body weight can influence various aspects of reproduction in both men and women, from sexual activity to conception.197 The relationship between obesity and reproductive disturbances in women was recognized a long time ago.198 More recently, in the Nurses' Health Study, a classic U-shaped relationship was observed between obesity and infertility, primarily anovulatory infertility; women with BMIs between 20 and 24 showed the lowest infertility, but infertility increased with both lower and higher BMIs.199 Several other cross-sectional and prospective studies have reported similar findings.200,201 Furthermore, weight loss in obese women improves fertility and increases the likelihood of ovulation and conception.202,203 A study by Hammoud et al. showed an increased incidence of low sperm count (from 5.3% to 15.6%) and poor sperm motility (from 4.5% to 13.3%) in obese men compared to those in normal weight men.204 Chavarro et al. also found major differences in reproductive hormone levels with increases in body weight.205 The effect of obesity on the development of sexual dysfunction has also been observed among obese men and women compared with normal weight individuals.206–209
Mechanistic studies. Oxidative stress also plays a significant role in infertility. Elevated steroid production in the growing follicle causes an increase in P450, resulting in ROS formation. Oxidative stress in the follicular fluid environment of ovaries is detrimental in several ways, causing poor development of both oocytes and embryos, and adversely affecting the overall outcome of pregnancy.210–213 Oxidative stress is associated with several female reproductive diseases, such as endometriosis, polycystic ovary syndrome (PCOS), and preeclampsia.214 In addition to the effects of obesity on female fertility, its effect on male infertility is also gaining attention worldwide.215 Various studies reported a decrease in sperm count and concentration, sperm motility, DNA fragmentation, and normal sperm morphology among obese men.215 Recent evidence suggests that ROS-mediated damage to sperm is a significant contributing factor to male infertility.216–218 ROS causes male infertility through two principal mechanisms: first, ROS damage the sperm membrane, which in turn reduces the sperm's motility and ability to fuse with the oocyte, and second, ROS can directly damage sperm DNA, compromising the paternal genomic contribution to the embryo.219
Obesity and Dysfunctional Adipose Tissue
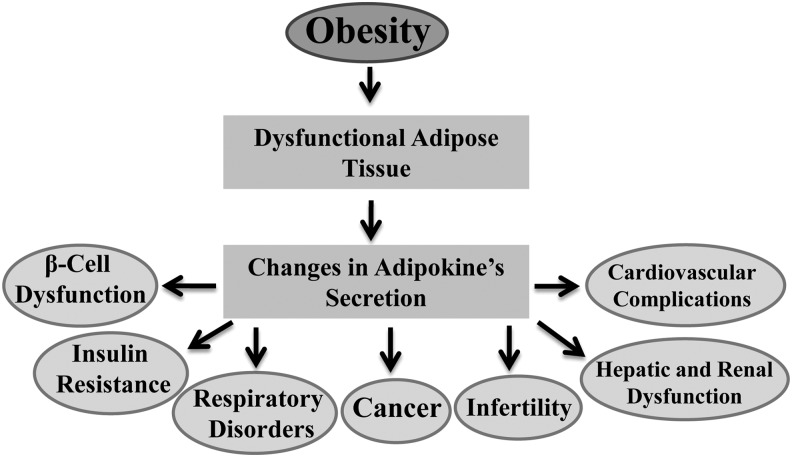
In the human body there are two types of adipose tissue, brown adipose tissue (BAT) and WAT, which differ structurally and functionally.220 BAT, mainly found in small and discrete regions of newborn humans, is responsible for the production of heat by a process called thermogenesis.220 However, more recently, a significant amount of BAT has also been found in adults.221 WAT, in contrast, is the predominant form of adipose tissue found in adults and is responsible for fat storage in the body.220 In addition to energy storage, it is now well established that WAT also functions as an endocrine organ that secretes various bioactive substances, commonly known as adipokines, involved in glucose metabolism (e.g., adiponectin, resistin), lipid metabolism [e.g., cholesteryl ester transfer protein (CETP)], inflammation [e.g., TNF-α; IL-6, IL-8, C-reactive protein (CRP), monocyte chemoattractive protein (MCP-1)], coagulation [plasminogen activator inhibitor-1 (PAI-1)], blood pressure regulation (e.g., angiotensinogen, angiotensin II), and feeding behavior (leptin).222–224 Obesity is mainly characterized by an increase in body fat or WAT and is associated with marked changes in the secretory function of WAT.225 In humans, it has been observed that the tissue expression, as well as the circulating concentration of many adipokines, increases with an increase in adiposity, as is the case for leptin, TNF-α, IL-6, IL-8, CRP, MCP-1, PAI-1, heptoglobin, and angiotensinogen.226 An exception is adiponectin, the concentration of which is inversely related to body weight.226 Similar findings have also been observed in animal studies.65 The deposition of excess fat in the WAT perturbs its normal endocrine function, causing dysregulated expression of secreted factors, which has been linked to the pathogenesis of various obesity-related diseases.226–228 Herein we discuss the role of dysfunctional adipose tissue in the development of obesity-associated health risks (Fig. 3).
FIG. 3.
Role of dysfunctional adipose tissue in the development of obesity associated health risks.
Role of dysfunctional adipose tissue in obesity-associated health risks
Adipokines and type 2 diabetes
During the progression from normal weight to obesity, changes in the circulating levels of adipocyte-derived factors play an important role in the pathogenesis of β-cell dysfunction and insulin resistance.229–231 It has been observed that some adipokines have beneficial effects, whereas others have detrimental properties; however, the overall contribution of the changed concentrations of adipokines is highly dependent on the balance between these effects and the interactions between the adipokines, which act on the β-cell and insulin-sensitive organs such as the liver and muscle tissues by means of a number of intersecting intracellular signaling cascades.229–231
Effects of adipokines on the β-cell function: The biological effects of adipokines such as leptin, adiponectin, TNF-α, and IL-6 on β-cell function, including insulin synthesis and secretion, cell survival, and cell death, have been well documented in the literature.230,231 Leptin inhibits the secretion of insulin from human and murine islets, as well as the pancreatic β-cell lines, through the activation of KATP channels,232–234 reduction in the cellular concentration of cAMP,235 alteration in the PLC-PKC pathway,236 and activation of the PI3K pathway.230,236 Leptin also reduces insulin mRNA expression by inhibiting the action of the insulin promoter and suppresses β-cell apoptosis through reduction of triglyceride accumulation,237,238 inhibiting NO production,230,239 and activating the antiapoptosis transcriptional factor, Bcl-2.240
The protective effects provided to β-cells by adiponectin have been reported in the literature. A defect in insulin secretion has been observed in adiponectin knockout mice.241 Globular domain adiponectin (gAd) is the functional adiponectin protein. The gAd transgenetic ob/ob mice (a cross between the gAd transgenetic mice and leptin-deficient ob/ob mice) exhibit higher plasma insulin levels, along with increased insulin sensitivity, compared to those seen in ob/ob litter-mates, indicating that adiponectin protects β-cells.242,243 A direct effect of adiponectin has also been observed when gAd strongly inhibited palmitic acid- and cytokine-induced suppression of glucose, stimulated insulin secretion, and attenuated apoptosis of INS-1 cells.244 Adiponectin also inhibits the accumulation of lipids through the activation of AMP protein kinase (AMPK) pathways in both rodent pancreatic β-cells and MIN6 cells.245
Data derived from various cell culture studies showed that TNF-α decreased glucose-induced insulin secretion from β-cells.246,247 TNF-α increases expression of Ca2+-independent adhesion molecules, which perturbs the segregation between β-cells and non-β-cells; this alteration in the islet architecture may influence insulin secretion.248 Activation of caspases and reduction in Bcl-2 expression have also been reported to play an important role in TNF-α-mediated apoptosis in β-cells.249–252 A line of studies in the literature demonstrated the beneficial effect of IL-6 on insulin secretion from pancreatic islets.253–255 Involvement of the PLC-IP3-dependent pathway plays an important role in IL-6-induced insulin secretion from pancreatic β-cells.253 Moreover, IL-6 also increased the insulin secretion and the expression of preproinsulin mRNA through the Ca2+-dependent pathway.254
Role of adipokines on insulin resistance. The adipokine leptin enhances the action of insulin on both the inhibition of hepatic glucose production and on glucose uptake,256 which may perhaps explain the role of insulin in relation to insulin sensitivity.257 It has been observed that plasma leptin concentrations are independently associated with insulin sensitivity.258 The effect of leptin on insulin sensitivity in the liver and skeletal muscle is mediated through phosphorylation and activation of AMPK and inhibition of the activity of acetyl-CoA carboxylase.259,260 Leptin-induced stimulation of the PI3K signaling pathway appears to be important for the regulation of glucose metabolism.261 Leptin has also been shown to be effective in regulating feeding behavior through the central nervous system.262 Leptin-deficient mice (ob/ob) show abnormally increased feeding, obesity, and insulin resistance, and the administration of leptin to these ob/ob mice reverses these changes.263 However, high levels of leptin have been observed among obese individuals without any anorexic responses, indicating the occurrence of leptin resistance.263
Plasma adiponectin is another positive regulator of insulin sensitivity.264 A significant negative correlation has been observed between age- and sex-adjusted HOMA IR levels and plasma adiponectin levels in a cross-sectional study of 2356 individuals (1998–2001).265 It was observed that high-molecular-weight adiponectin may have a more insulin-sensitizing effect compared to its monomeric form.266 Like leptin, adiponectin also enhances insulin sensitivity through the activation of AMPK.267 Adiponectin also affects hepatic glucose production by regulating the mRNA expression of two essential gluconeogenesis enzymes: phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6PD).267 Obesity-associated downregulation of adiponectin levels is a mechanism that might explain how obesity can cause insulin resistance and diabetes.267
TNF-α, the most widely studied cytokine, plays an important role in the modulation of insulin resistance. Deletion of TNF-α or the TNF-α-receptor resulted in significantly improved insulin sensitivity in both diet-induced obese mice and leptin-deficient ob/ob mice, suggesting a role for TNF-α in obesity-associated insulin resistance.268 In the Framingham Offspring Study, it was observed that age- and sex-adjusted HOMA IR levels were positively associated with fasting plasma TNF-α levels.265 In humans, adipose tissue TNF-α expression is associated with BMI, percentage of body fat, and hyperinsulinemia; a decrease in body weight reduces TNF-α levels.269
IL-6 is emerging as one of the potential mediators linking obesity-derived chronic inflammation with insulin resistance. Chronic exposure to IL-6 induces hepatic insulin resistance270,271 and reduces glucose uptake in adipocytes.272 Circulating IL-6 levels are elevated in obese and insulin-resistant subjects.273,274 It can be speculated that a persistent increase in levels of IL-6, such as those that occur in a state of inflammation such as obesity and type 2 diabetes, may trigger insulin resistance. It has been observed that IL-6 can modulate insulin resistance through several distinct pathways, including JNK1-mediated serine phosphorylation of IRS1, IκB-mediated activation of NF-κB, and induction of suppressor of cytokine signaling-3 (SOCS-3).275
Adipokines and cardiovascular dysfunction
Abnormal production of the adipose tissue-derived factors, adipokines, has been implicated in the pathogenesis and progression of atherosclerosis, endothelial cell dysfunction, and altered expression of proangiogenic/proatherogenic factors such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs), leading to structural and functional changes in the endothelium.276,277 In obese humans, elevated angiotensinogen is associated with the development of hypertension.278 A decrease in body weight reduces the expression of WAT angiotensinogen expression and is thought to contribute to the concomitant fall in blood pressure.279
Various epidemiological studies demonstrate that reduced adiponectin levels correlate with increased risk of CVD in obese individuals.280,281 Many experimental studies demonstrate that adiponectin ameliorates the progression of macrovascular disease and this is consistent with its correlation with improved vascular outcomes in epidemiological studies.281,282 The mechanisms of adiponectin signaling vary among its cellular sites of action. In endothelial cells, adiponectin protects cells from high glucose or TNF-α-induced inflammation through the activation of AMP-activated protein kinase and cyclic AMP-dependent protein kinase signaling cascades.281,283,284 In the myocardium, adiponectin-mediated protection from ischemia–reperfusion injury is linked to COX2-mediated suppression of TNF-α signaling, inhibition of apoptosis by the activation of AMPK signaling pathways.281 Ox-LDL-induced alteration in adipokine secretion can also induce CVD complications.138
Numerous studies demonstrate the association between circulating levels of leptin and CVD.285,286 Although many paradoxical effects have been reported, for the most part, elevated circulating leptin levels have been observed in the majority of obese individuals,287,288 while a lack of leptin (as observed in the genetic animal model of leptin deficiency) has been associated with hypertension, atherosclerosis, stroke, and myocardial infraction.285,286 Recently, it has been documented that circulating leptin levels significantly correlate with heart failure among obese individuals without pre-existing CHD.289 The effects of leptin on the cardiovascular system are not straight forward enough to definitively summarize, and it is important to consider other parameters such as aging, degree of hyperleptinemia, and the coexistence of other factors, which may also have an impact on cardiovascular function.
A degree of cross talk has been observed between resistin and other cytokines: a linear relationship with leptin and a reciprocal relationship with adiponectin. Elevated plasma levels of resistin correlate with proatherogenic inflammatory markers,290 increased cardiovascular risk, endothelial dysfunction (through promotion of adhesion molecules, endothelial migration, and proliferation), unstable angina, and poor prognosis with regard to coronary artery disease.291,292 Resistin induces the proliferation of human aortic smooth muscle cells through the PI3K/AKT and MAPK (p42/44) signaling pathways.293 Resistin also enhances the migration and proliferation of endothelial cells through the activation of p38 and p44/42 MAPKs.294 Moreover, in isolated coronary artery rings, resistin can induce endothelial dysfunction.295 Resistin has been shown to increase transcriptional events, leading to increased expression of several proinflammatory cytokines, including IL-1, IL-6, IL-12, and TNF-α in an NF-kB-mediated pathway.296,297
Adipokines and cancer
Various epidemiological studies demonstrate as association between obesity and a range of various cancer types, although the mechanisms by which obesity induces or promotes tumorigenesis vary from one cancer site to another.298 These include insulin resistance and chronic hyperinsulinemia, a low-grade chronic inflammation, and increased bioavailability of steroid hormones.298 Elevated leptin levels have been implicated in the pathogenesis of certain human cancers, including esophageal and hepatocellular carcinomas.299 Leptin-induced stimulation of insulin-like growth factor-1 (IGF-1) and other growth hormone secretagogues may increase cellular proliferation and/or dedifferentiation,300 and enhance angiogenesis, all of which may promote neoplasia.301 Leptin also acts as a promoting factor for hepatocellular carcinoma,302 as well as inhibiting apoptosis in esophageal cancer.303 Adiponectin, in contrast, reduces the expression of adhesion molecules and provides some protection against carcinogenesis by inhibiting cancer cell growth and tumor-associated angiogenesis.304,305 Lower levels of circulating adiponectin have been observed in patients with various types of cancers and may contribute to their severity.304,305 Hyperinsulinemia, a pathological event common to obesity, has been associated directly with colon cancer in obese humans.306 The inflammatory adipokines associated with obesity also contribute to the development of pancreatic adenocarcinoma.298
Adipokines and respiratory disorders
Obesity is associated with both sleep disorders and asthma, an association that might be partially explained by the physical impairment caused by the presence of fat on both the chest wall and diaphragm.307 However, abdominal obesity is also associated with an increased prevalence of asthma.308 It has been reported that both asthma and obesity were independently and synergistically associated with systemic inflammation.309
Adipokines and asthma. Many of the proinflammatory cytokines, whose levels are elevated in the plasma of obese subjects, also play an important role in the pathogenesis of asthma.310 For instance, TNF-α can increase airway inflammation311 and enhance airway contractility,312 both TNF-α and IL-6 can modulate T-helper 2 cell immunity,153,313 and leptin may facilitate airway hyper-responsiveness.314
Adipokines and sleep disorders. In normal subjects, secretion of adipokines such as TNF-α and IL-6 in a circadian rhythm is involved in the regulation of physiological sleep patterns.315–318 Increased secretion of IL-6 is associated with excessive daytime sleepiness and fatigue,319 while a decrease in overall secretion of IL-6 is associated with a good night's sleep and a sense of well-being the next day.315 Both TNF-α and IL-6 are elevated in sleep apnea independently of obesity.320 In a recent study it was observed that sleep disorder breathing is associated with elevated levels of CRP, independent of age, BMI, WC, or body fat percentage, suggesting an association between low-grade inflammation and sleep disorders.321
Adipokines and liver dysfunction
Animals lacking leptin due to leptin gene mutation or leptin receptor gene mutation are obese, insulin resistant, and have hepatic steatosis.322,323 Leptin injections reduce their fatty livers and metabolic abnormalities324 through the activation of hepatic stellate cells and the modulation of Kupffer cell function. In obese NAFLD patients, plasma leptin levels are elevated and directly correlated with the severity of hepatic steatosis,325 suggesting that there exists a state of leptin resistance in obesity.326
Various studies have suggested a link between lower circulating levels of adiponectin and increased liver fat content and the degree of hepatic insulin resistance.327 Administration of adiponectin decreased hepatic insulin resistance in a mouse model of obesity and diabetes through the activation of the AMPK signaling pathway.327 Local production of IL-6 and TNF-α by Kupffer cells has been proposed to play an important role in the pathogenesis of NAFLD.328 Both IL-6 and TNF-α are potent inhibitors of adiponectin expression and high levels of these cytokines in obesity and NAFLD explain this relationship.329 IL-6 causes hepatic insulin resistance by the inhibition of IRS phosphorylation mediated by activating the SOCS-3 protein.271,330
Adipokines and renal dysfunction
Altered levels of adipokines, including leptin, adiponectin, resistin, and visfatin, are associated with obesity-related renal dysfunction because they increase the risk of developing albuminuria by alteration of the GFR and modulation of renal tubule function.331
Alteration in circulating leptin concentrations plays an important role in the development of renal dysfunction. Elevated levels of leptin cause hypertrophy in glomerular mesangial cells through the activation of the PI3K and ERK1/2 pathways332; glomerular mesangial hypertrophy increases the amount of filtered protein and albumin.333,334 In addition, elevated leptin increases the accumulation of collagen and the secretion of TGF-β1 from glomerular endothelial cells. An increase in TGF-β1 is associated with thickening of the basement membrane, leading to the development of glomerulosclerosis.335 Elevated leptin also increases the expression of matrix metalloproteinase-2 (MMP-2) in renal mesangial cells.336 Patients with end-stage renal disorders have an estimated 4- to 7.5-fold higher plasma leptin concentrations compared to that of healthy control subjects, suggesting a link between elevated leptin levels and the development of CKD in the absence of obesity.337,338
Hypoadiponectinemia has been associated with the development of renal dysfunction and CKD.339,340 It has been reported that adiponectin is an independent predictor of CKD.339,340 Hypoadiponectinemia causes an increase in tubular inflammation by decreasing tubular AMPK activation and causing an accumulation of MCP-1.341 Mice with hypoadiponectinemia treated with adiponectin exhibit podocyte fusion leading to an improvement in glomerular podocyte foot processes through activation of AMPK.340
The circulating resistin level is also linked to the album-to-creatinine ratio and the GFR.342,343 Plasma resistin levels are elevated in uremia, primarily because of reduced renal clearance and inflammation.344 An increased resistin level is associated with a decrease in GFR, which may be due to the activation of macrophages and enhanced inflammatory responses.342,343 Visfatin, a new adipokine, is significantly associated with inflammation/endothelial dysfunction in CKD.345 Elevated visfatin levels have been observed in diabetic patients with CKD.346 Visfatin has been shown to increase the secretion of the inflammatory cytokines such as TNF-α, IL-6, and IL-1β.345 Exposure to elevated levels of visfatin in glomerular mesangial cells increased the mRNA expression of both renin and angiotensinogen; changes in renin and angiotensinogen levels are linked with alteration in GFR leading to renal dysfunction.347
Adipokines and infertility
Recent studies in the literature have reported that alterations in adipokine levels, or in their mechanism of action, are associated with fertility impairment and pregnancy diseases.348,349 An increase in serum leptin levels has been observed among infertile obese men and women.350 Excess levels of leptin have a deleterious effect on sperm production and the formation of androgens by Leydig cells.351 Increased serum leptin concentrations have been observed in women with PCOS compared to those of weight-matched control subjects,352 but these observations are dependent on ethnicity, heterogeneity of the PCOS group, or the different sampling methods used to get fat biopsies in the various studies.349 Adiponectin, in contrast, is described as a beneficial adipokine in reproduction.353 Women diagnosed with PCOS also have reduced adiponectin levels, independent of obesity.354,355 Genomic studies support the observation that hypoadiponectinemia is a causative agent for PCOS.354,355 Various experimental studies demonstrate that adiponectin can directly induce ovarian gene expression.356,357
Potential Strategies to Reduce the Pathogenesis of Obesity
A broad range of strategies are recommended to reduce the prevalence of obesity. Physical activity remains the most common therapy for the treatment of obesity and its associated health risks. There are several ways by which increased physical activity could be beneficial in reducing and preventing obesity.358 Physical activity increases total energy expenditure and decreases total body fat, including fat around the waist, which can help people to reach and maintain a state of energy balance, as long as they are not taking in more food to compensate for the lost calories. Oh et al. reported that exercise training reduced the serum levels of inflammation and oxidative stress markers, ferritin and thiobarbituric acid reactive substances, and significantly increased the adiponectin levels among a total of 108 subjects who completed a 12-week exercise training program.359
Ad libitum food intake limiting to certain micronutrients is another important strategy for the regulation of obesity because of its relation to energy balance.360 Ad libitum low-fat diets, high-protein diets, and low-carbohydrate diets are the proposed tools for facilitating weight loss.361,362 Controlled food intake with variety in the composition of macronutrients reduces a patient's feelings of being restricted to a particular diet, which might improve the weight loss program. A few long-term trials have demonstrated that maintenance of weight loss with ad libitum dietary programs achieved significant results compared to conventional energy-restricted diets.363,364 The CARMEN trial also observed greater loss of weight and fat mass by the long-term intake of ad libitum low-fat, high-carbohydrate diets.365,366
Beside ad libitum food intake, a healthy dietary pattern is also necessary to regulate obesity and its associated health risks. However, adoption of healthy dietary habits is very difficult in an obesogenic environment, in which palatable, inexpensive, high-fat, and energy-dense foods are easily available. Dietary intake of macronutrients is gaining attention as an important factor in the regulation of obesity and its associated risk factors. A number of nutritional intervention studies using diets supplemented or enriched with different micronutrients have shown a variety of results regarding weight loss and weight management. Dietary supplementation with calcium has been shown to play an important role in the regulation of energy metabolism and obesity risk.367,368 Supplementation with high calcium inhibited lipogenesis, stimulated lipolysis, lipid oxidation, and thermogenesis and thus prevented diet-induced obesity in mice.369 One meta-analysis concluded that dietary calcium has the potential to increase fecal fat excretion, which is relevant for its potential contribution to weight loss.370 Potassium and magnesium are two other well-known dietary micronutrients that could favor a decrease in blood pressure371 and are the main components of the DASH diet, which is more effective for weight loss and metabolic variables.372,373 Chromium, an essential trace element, is present in a wide variety of foods, including eggs, cereal, nuts, and vegetables.374 A few clinical studies have reported the weight-lowering effect of chromium supplementation.375,376
Dietary consumption of more fruits and vegetables is also associated with a decrease in the prevalence of obesity.377 The role of an increased consumption of fruits and vegetables in the prevention of overweight and obesity is linked to several features: high water content, relatively low-energy density, and high dietary fiber content.378 Water has the greatest impact on energy density because it increases the weight of food without increasing calories and thus decreases energy density.379 Consumption of whole apples (2.9% fiber) was associated with a higher satiety rating compared with the consumption of apple puree or fiber-free apple juice.380 Similarly, whole oranges (2.5% fiber) versus orange juice (fiber free) and whole grapes (1.3% fiber) versus grape juice (fiber free) also confirmed that whole fruits provide more satiety than juice.381 Dietary fibers, specially viscous dietary fibers, have also been shown to increase postprandial satiety and to decrease subsequent hunger in short-term studies.382 In a review summarizing the effects of high- versus low-fiber diet interventions, it has been observed that participants on the high-fiber diets lost significantly more weight than those on the low-fiber diets.382 Studies investigating the influence of vegetables on feeling full reported that adding at least 200 g of vegetables (carrot and spinach) to meals with equal calories enhanced the feeling of being full, suggesting a correlation between the dietary water and fiber content and the total weight of the meal.383–385 These analyses support the importance of high water and fiber-rich foods, such as fruits and vegetables, in weight regulation.
Meal-replacement strategy may be one important pathway to combat against the prevalence of obesity.386–388 Weight loss programs based on meal replacement for one or two meals per day with a product of defined nutrient and calorie content have shown to improve compliance with an energy-restricted diet as well as weight management in overweight and obese individuals.387,388 Smeets et al. showed that a high-protein replacement meal was more satiating and had a higher thermogenic effect compared to a low-protein replacement meal.389
Certain pharmacological treatments have also been recommended in the management of obesity. Antiobesity medications are categorized according to their mode of action, such as inhibitors of fat absorption, inhibitors of the endocannabinoid system, or modifiers of the central nervous system.390,391 Orlistat is a pancreatic lipase inhibitor that binds to lipase in gut lumen and prevents the hydrolysis and absorption of ∼30% of the dietary fat contained in a meal.392 Sibutramine, a selective inhibitor of neurotransmitter (serotonin and norepinephrine) reuptake, acts centrally to reduce food intake.392 Phentermine is a sympathomimetic amine that promotes the release of catecholamine.393 Common adverse effects of these drugs include several gastrointestinal adverse effects, diarrhea, insomnia, anxiety, and other effects on the central nervous system.393 Due to the numerous adverse side effects and the related safety issues, many antiobesity medications, including drugs in the field of pharmacotherapy, have been withdrawn even after licensing.394
BAT is emerging as an important antiobesity tissue in humans.395,396 An experimental rodent study demonstrated the beneficial role of brown fat against diet-induced obesity.397 Transgenic mice with decreased brown fat develop glucose intolerance and insulin resistance398 and are susceptible to diet-induced obesity, diabetes, and hyperlipidemia,399 suggesting a protective role of brown fat against energy-dense diet-induced obesity in rodents. β3-Adrenergic receptors (β3-AR) are found predominantly in BAT and treatment with β3-AR selective agonists caused a significant increase in energy expenditure and a decrease in obesity in rodents.400 The occurrence of BAT in adult humans varies from 2% to 100%.401,402 Possible BAT-oriented therapeutic treatments to combat obesity, aimed at increasing energy expenditure, might work in one of two ways: first, by stimulating the activity of already existing BAT and, second, by upregulating the occurrence of brown adipocytes by inducing the specific gene expression program of brown fat cells through specific molecular switches.396 Wijers et al. reported a link between BAT activity and diet-induced thermogenesis in humans.403 Recent data suggest the role of thyroid hormone in the regulation of BAT thermogenic activity through modulation of hypothalamic fat metabolism.404
Conclusion
Obesity is the one of the most prevalent metabolic disorders of the 21st century. The rising epidemic of obesity is a serious healthcare catastrophe due to its strong association with several major downstream health consequences such as diabetes, cardiovascular complications, cancer, asthma, sleep disorders, hepatic dysfunction, renal dysfunction, and infertility. Oxidative stress has been suggested as a critical factor linking obesity with its associated complications. There are various biochemical mechanisms by which obesity can induce systemic oxidative stress, such as activation of NOX, oxidative phosphorylation, glyceraldehyde auto-oxidation, PKC activation, polyol and hexosamine pathways, hyperleptinemia, low antioxidant defense, chronic inflammation, and postprandial ROS generation. The deposition of excess fat in the WAT inhibits its normal endocrine function, leading to the dysregulated expression of secreted factors, elevated plasma lipid levels, increased formation of reactive intermediates, impaired mitochondrial function, inadequate cellular antioxidant defense, and development of oxidative stress. This has been linked to the pathogenesis of a variety of obesity-related diseases. The development of obesity is characterized by the interplay of nature and nurture. Moreover, current epidemiological studies indicate that a major cause of the global obesity problem lies in alterations in dietary and physical activity patterns, while genetic and metabolic studies reveal that there are individuals who are more susceptible to weight gain than others. On the basis of the currently identified predominant determinants of obesity, various effective strategies are recommended to regulate or manage the prevalence of obesity, including regular physical activity, ad libitum food intake limiting to certain micronutrients, increased dietary intake of fruits and vegetables, and activation of brown fat. Furthermore, while some pharmacological therapeutics have also been found to help regulate obesity, most of the commonly used pharmacotherapies are associated with serious adverse health effects. At present, modification of life style, increased physical activity and adoption of a healthy diet, including more fruits, vegetables, and balanced micronutrients, have been suggested as beneficial strategies to overcome or regulate the prevalence of obesity and its associated risk factors.
Acknowledgments
The authors are supported by grants from the NIH RO1 AT007442 and the Malcolm Feist Endowed Chair in Diabetes. This study is also funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center, Shreveport. The authors thank Ms. Georgia Morgan for the excellent editing of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zhang ZY, Wang MW. Obesity, a health burden of a global nature. Acta Pharmacol Sin 2012;33:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollin IS, Kral BG, Shattuck T, et al. High prevalence of cardiometabolic risk factors in women considered low risk by traditional risk assessment. J Womens Health (Larchmt) 2008;17:947–953 [DOI] [PubMed] [Google Scholar]
- 3.Jung RT. Obesity as a disease. Br Med Bull 1997;53:307–321 [DOI] [PubMed] [Google Scholar]
- 4.Pi-Sunyer FX. Health implications of obesity. Am J Clin Nutr 1991;53:1595S–1603S [DOI] [PubMed] [Google Scholar]
- 5.Sikaris KA. The clinical biochemistry of obesity. Clin Biochem Rev 2004;25:165–181 [PMC free article] [PubMed] [Google Scholar]
- 6.Despres JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990;10:497–511 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci 2011;12:3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savini I, Catani MV, Evangelista D, et al. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int J Mol Sci 2013;14:10497–10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Lee YJ, Choi H, et al. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 2009;284:10601–10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi M, Dusting GJ, Peshavariya H, et al. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 2013;22:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: Roles for ROS. Trends Endocrinol Metab 2009;20:78–87 [DOI] [PubMed] [Google Scholar]
- 13.Serra D, Mera P, Malandrino MI, et al. Mitochondrial fatty acid oxidation in obesity. Antioxid Redox Signal 2013;19:269–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol 2012;39:168–178 [DOI] [PubMed] [Google Scholar]
- 15.Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: The ATTICA study. Nutr Metab Cardiovasc Dis 2007;17:590–597 [DOI] [PubMed] [Google Scholar]
- 16.Patel C, Ghanim H, Ravishankar S, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 2007;92:4476–4479 [DOI] [PubMed] [Google Scholar]
- 17.Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–1196 [DOI] [PubMed] [Google Scholar]
- 18.Arora T, Singh S, Sharma RK. Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition 2013;29:591–596 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Castejon M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol Res 2011;64:438–455 [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Matute P, Crujeiras AB, Fernández-Galilea M, and Prieto-Hontoria P. Compounds with antioxidant capacity as potential tools against several oxidative stress related disorders: fact or artifact? In: Oxidative stress and diseases, Lushchak VI. and Gospodaryov DV, eds. InTech: Rijeka, Croatia; 2012, 544–580 [Google Scholar]
- 21.Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–418 [DOI] [PubMed] [Google Scholar]
- 23.Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc Diabetol 2002;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltowski J, Wojcicka G, Gorny D, et al. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol 2000;51:883–896 [PubMed] [Google Scholar]
- 25.Ortega RM, Rodriguez-Rodriguez E, Aparicio A, et al. Young children with excess of weight show an impaired selenium status. Int J Vitam Nutr Res 2012;82:121–129 [DOI] [PubMed] [Google Scholar]
- 26.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr 1999;134:160–165 [DOI] [PubMed] [Google Scholar]
- 27.Bouloumie A, Marumo T, Lafontan M, et al. Leptin induces oxidative stress in human endothelial cells. FASEB J 1999;13:1231–1238 [PubMed] [Google Scholar]
- 28.Salvadori A, Fanari P, Fontana M, et al. Oxygen uptake and cardiac performance in obese and normal subjects during exercise. Respiration 1999;66:25–33 [DOI] [PubMed] [Google Scholar]
- 29.Wheatcroft SB, Williams IL, Shah AM, et al. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 2003;20:255–268 [DOI] [PubMed] [Google Scholar]
- 30.Martinez JA. Mitochondrial oxidative stress and inflammation: An slalom to obesity and insulin resistance. J Physiol Biochem 2006;62:303–306 [DOI] [PubMed] [Google Scholar]
- 31.Khan NI, Naz L, Yasmeen G. Obesity: An independent risk factor for systemic oxidative stress. Pak J Pharm Sci 2006;19:62–65 [PubMed] [Google Scholar]
- 32.Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes Relat Metab Disord 2002;26:1159–1164 [DOI] [PubMed] [Google Scholar]
- 33.Ozata M, Mergen M, Oktenli C, et al. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem 2002;35:627–631 [DOI] [PubMed] [Google Scholar]
- 34.Vincent HK, Morgan JW, Vincent KR. Obesity exacerbates oxidative stress levels after acute exercise. Med Sci Sports Exerc 2004;36:772–779 [DOI] [PubMed] [Google Scholar]
- 35.Fenkci V, Fenkci S, Yilmazer M, et al. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 2003;80:123–127 [DOI] [PubMed] [Google Scholar]
- 36.Pennathur S, Heinecke JW. Mechanisms of oxidative stress in diabetes: Implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci 2004;9:565–574 [DOI] [PubMed] [Google Scholar]
- 37.Evans JL, Goldfine ID, Maddux BA, et al. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev 2002;23:599–622 [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Meco MT, Moscat J. The atypical PKCs in inflammation: NF-kappaB and beyond. Immunol Rev 2012;246:154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piperi C, Adamopoulos C, Dalagiorgou G, et al. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab 2012;97:2231–2242 [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Schmeisser A, Garlichs CD, et al. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: Role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res 1999;44:215–222 [DOI] [PubMed] [Google Scholar]
- 41.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991;40:405–412 [DOI] [PubMed] [Google Scholar]
- 42.Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem 2012;68:701–711 [DOI] [PubMed] [Google Scholar]
- 43.Bakker SJ, IJzerman RG, Teerlink T, et al. Cytosolic triglycerides and oxidative stress in central obesity: The missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis 2000;148:17–21 [DOI] [PubMed] [Google Scholar]
- 44.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000;49:1939–1945 [DOI] [PubMed] [Google Scholar]
- 45.Russell AP, Gastaldi G, Bobbioni-Harsch E, et al. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: A case of good vs. bad lipids? FEBS Lett 2003;551:104–106 [DOI] [PubMed] [Google Scholar]
- 46.Vincent HK, Powers SK, Dirks AJ, et al. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord 2001;25:378–388 [DOI] [PubMed] [Google Scholar]
- 47.Basu S, Riserus U, Turpeinen A, et al. Conjugated linoleic acid induces lipid peroxidation in men with abdominal obesity. Clin Sci (Lond) 2000;99:511–516 [DOI] [PubMed] [Google Scholar]
- 48.Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009;90:1252–1263 [DOI] [PubMed] [Google Scholar]
- 49.Reitman A, Friedrich I, Ben-Amotz A, et al. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity. Isr Med Assoc J 2002;4:590–593 [PubMed] [Google Scholar]
- 50.Kaidar-Person O, Person B, Szomstein S, et al. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part A: Vitamins. Obes Surg 2008;18:870–876 [DOI] [PubMed] [Google Scholar]
- 51.Andersen LF, Jacobs DR, Jr., Gross MD, et al. Longitudinal associations between body mass index and serum carotenoids: The CARDIA study. Br J Nutr 2006;95:358–365 [DOI] [PubMed] [Google Scholar]
- 52.Kuno T, Hozumi M, Morinobu T, et al. Antioxidant vitamin levels in plasma and low density lipoprotein of obese girls. Free Radic Res 1998;28:81–86 [DOI] [PubMed] [Google Scholar]
- 53.Aasheim ET, Bohmer T. Low preoperative vitamin levels in morbidly obese patients: A role of systemic inflammation? Surg Obes Relat Dis 2008;4:779–780 [DOI] [PubMed] [Google Scholar]
- 54.Canoy D, Wareham N, Welch A, et al. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am J Clin Nutr 2005;82:1203–1209 [DOI] [PubMed] [Google Scholar]
- 55.Myara I, Alamowitch C, Michel O, et al. Lipoprotein oxidation and plasma vitamin E in nondiabetic normotensive obese patients. Obes Res 2003;11:112–120 [DOI] [PubMed] [Google Scholar]
- 56.Weisstaub G, Hertrampf E, Lopez de Romana D, et al. Plasma zinc concentration, body composition and physical activity in obese preschool children. Biol Trace Elem Res 2007;118:167–174 [DOI] [PubMed] [Google Scholar]
- 57.Kaidar-Person O, Person B, Szomstein S, et al. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part B: Minerals. Obes Surg 2008;18:1028–1034 [DOI] [PubMed] [Google Scholar]
- 58.Skalicky J, Muzakova V, Kandar R, et al. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med 2008;46:499–505 [DOI] [PubMed] [Google Scholar]
- 59.Cracowski JL, Bonaz B, Bessard G, et al. Increased urinary F2-isoprostanes in patients with Crohn's disease. Am J Gastroenterol 2002;97:99–103 [DOI] [PubMed] [Google Scholar]
- 60.Basu S, Whiteman M, Mattey DL, et al. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis 2001;60:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margioris AN. Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care 2009;12:129–137 [DOI] [PubMed] [Google Scholar]
- 62.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–783 [DOI] [PubMed] [Google Scholar]
- 63.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: Redox-dependent execution. FASEB J 2006;20:1589–1598 [DOI] [PubMed] [Google Scholar]
- 64.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292–295 [DOI] [PubMed] [Google Scholar]
- 65.German AJ, Ryan VH, German AC, et al. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 2010;185:4–9 [DOI] [PubMed] [Google Scholar]
- 66.Milagro FI, Campion J, Martinez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity (Silver Spring) 2006;14:1118–1123 [DOI] [PubMed] [Google Scholar]
- 67.Fortuno A, Bidegain J, Baltanas A, et al. Is leptin involved in phagocytic NADPH oxidase overactivity in obesity? Potential clinical implications. J Hypertens 2010;28:1944–1950 [DOI] [PubMed] [Google Scholar]
- 68.Beltowski J, Wojcicka G, Jamroz A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: The possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis 2003;170:21–29 [DOI] [PubMed] [Google Scholar]
- 69.Parola M, Marra F. Adipokines and redox signaling: Impact on fatty liver disease. Antioxid Redox Signal 2011;15:461–483 [DOI] [PubMed] [Google Scholar]
- 70.Maingrette F, Renier G. Leptin increases lipoprotein lipase secretion by macrophages: Involvement of oxidative stress and protein kinase C. Diabetes 2003;52:2121–2128 [DOI] [PubMed] [Google Scholar]
- 71.Saiki S, Sato T, Kohzuki M, et al. Changes in serum hypoxanthine levels by exercise in obese subjects. Metabolism 2001;50:627–630 [DOI] [PubMed] [Google Scholar]
- 72.Ji LL. Exercise, oxidative stress, and antioxidants. Am J Sports Med 1996;24:S20–S24 [PubMed] [Google Scholar]
- 73.Vincent HK, Vincent KR, Bourguignon C, et al. Obesity and postexercise oxidative stress in older women. Med Sci Sports Exerc 2005;37:213–219 [DOI] [PubMed] [Google Scholar]
- 74.Wolin MS, Ahmad M, Gupte SA. The sources of oxidative stress in the vessel wall. Kidney Int 2005;67:1659–1661 [DOI] [PubMed] [Google Scholar]
- 75.Konior A, Schramm A, Czesnikiewicz-Guzik M, et al. NADPH oxidases in vascular pathology. Antioxid Redox Signal 2014;20:2794–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaminski KA, Bonda TA, Korecki J, et al. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int J Cardiol 2002;86:41–59 [DOI] [PubMed] [Google Scholar]
- 77.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res 2000;87:840–844 [DOI] [PubMed] [Google Scholar]
- 78.Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996;97:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egan BM, Greene EL, Goodfriend TL. Insulin resistance and cardiovascular disease. Am J Hypertens 2001;14:116S-125S [DOI] [PubMed] [Google Scholar]
- 80.Schiffrin EL. Beyond blood pressure: The endothelium and atherosclerosis progression. Am J Hypertens 2002;15:115S-122S [DOI] [PubMed] [Google Scholar]
- 81.Dandona P, Kumar V, Aljada A, et al. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: Evidence of an antiinflammatory action. J Clin Endocrinol Metab 2003;88:4496–4501 [DOI] [PubMed] [Google Scholar]
- 82.Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol 2002;283:H2160–H2168 [DOI] [PubMed] [Google Scholar]
- 83.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 2010;17:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bogacka I, Xie H, Bray GA, et al. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 2005;54:1392–1399 [DOI] [PubMed] [Google Scholar]
- 85.Koh EH, Park JY, Park HS, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 2007;56:2973–2981 [DOI] [PubMed] [Google Scholar]
- 86.Gao CL, Zhu C, Zhao YP, et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol 2010;320:25–33 [DOI] [PubMed] [Google Scholar]
- 87.Sutherland LN, Capozzi LC, Turchinsky NJ, et al. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: Potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab 2008;295:E1076–E1083 [DOI] [PubMed] [Google Scholar]
- 88.Vincent HK, Bourguignon CM, Taylor AG. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J Hum Nutr Diet 2010;23:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallstrom P, Wirfalt E, Lahmann PH, et al. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr 2001;73:777–785 [DOI] [PubMed] [Google Scholar]
- 90.Decsi T, Molnar D, Koletzko B. Reduced plasma concentrations of alpha-tocopherol and beta-carotene in obese boys. J Pediatr 1997;130:653–655 [DOI] [PubMed] [Google Scholar]
- 91.Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab 2005;19:649–663 [DOI] [PubMed] [Google Scholar]
- 92.Hussain A, Hydrie MZI, Claussen B, Asghar S. Type 2 Diabetes and obesity: A review. J Diabetol 2010;2:1–7 [Google Scholar]
- 93.Kalra S. Diabesity. J Pak Med Assoc 2013;63:532–534 [PubMed] [Google Scholar]
- 94.Schienkiewitz A, Schulze MB, Hoffmann K, et al. Body mass index history and risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84:427–433 [DOI] [PubMed] [Google Scholar]
- 95.Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–969 [DOI] [PubMed] [Google Scholar]
- 96.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132:501–513 [DOI] [PubMed] [Google Scholar]
- 97.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiss R. Fat distribution and storage: How much, where, and how? Eur J Endocrinol 2007;157 Suppl 1:S39–S45 [DOI] [PubMed] [Google Scholar]
- 99.National Institutes of Health. The practical guide: Identification, evaluation, and treatent of overweight and obesity in adults. Bethesda, MD: National Institute of Health; 2000, NIH Publication Number 00-4084 [Google Scholar]
- 100.Bjorntorp P. Abdominal fat distribution and disease: An overview of epidemiological data. Ann Med 1992;24:15–18 [DOI] [PubMed] [Google Scholar]
- 101.Wei M, Gaskill SP, Haffner SM, et al. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans—a 7-year prospective study. Obes Res 1997;5:16–23 [DOI] [PubMed] [Google Scholar]
- 102.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806 [DOI] [PubMed] [Google Scholar]
- 103.Tiedge M, Lortz S, Drinkgern J, et al. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997;46:1733–1742 [DOI] [PubMed] [Google Scholar]
- 104.Tanaka Y, Tran PO, Harmon J, et al. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A 2002;99:12363–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gurgul E, Lortz S, Tiedge M, et al. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes 2004;53:2271–2280 [DOI] [PubMed] [Google Scholar]
- 106.Hou N, Torii S, Saito N, et al. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology 2008;149:1654–1665 [DOI] [PubMed] [Google Scholar]
- 107.Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem 2005;280:11107–11113 [DOI] [PubMed] [Google Scholar]
- 108.Olson LK, Sharma A, Peshavaria M, et al. Reduction of insulin gene transcription in HIT-T15 beta cells chronically exposed to a supraphysiologic glucose concentration is associated with loss of STF-1 transcription factor expression. Proc Natl Acad Sci U S A 1995;92:9127–9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002;415:96–99 [DOI] [PubMed] [Google Scholar]
- 110.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 2001;105:745–755 [DOI] [PubMed] [Google Scholar]
- 111.Krauss S, Zhang CY, Scorrano L, et al. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest 2003;112:1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med 2004;10:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zick Y. Ser/Thr phosphorylation of IRS proteins: A molecular basis for insulin resistance. Sci STKE 2005;2005:pe4. [DOI] [PubMed] [Google Scholar]
- 114.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 115.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997;275:90–94 [DOI] [PubMed] [Google Scholar]
- 116.Imoto K, Kukidome D, Nishikawa T, et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes 2006;55:1197–1204 [DOI] [PubMed] [Google Scholar]
- 117.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008;118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finck BN, Bernal-Mizrachi C, Han DH, et al. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 2005;1:133–144 [DOI] [PubMed] [Google Scholar]
- 120.Pospisilik JA, Knauf C, Joza N, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007;131:476–491 [DOI] [PubMed] [Google Scholar]
- 121.Kim JJ, Li P, Huntley J, et al. FoxO1 haploinsufficiency protects against high-fat diet-induced insulin resistance with enhanced peroxisome proliferator-activated receptor gamma activation in adipose tissue. Diabetes 2009;58:1275–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab 2005;2:153–163 [DOI] [PubMed] [Google Scholar]
- 123.Nakae J, Biggs WH, 3rd, Kitamura T, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 2002;32:245–253 [DOI] [PubMed] [Google Scholar]
- 124.Dansen TB, Smits LM, van Triest MH, et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol 2009;5:664–672 [DOI] [PubMed] [Google Scholar]
- 125.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 2007;8:440–450 [DOI] [PubMed] [Google Scholar]
- 126.Poirier P, Eckel RH. Obesity and cardiovascular disease. Curr Atheroscler Rep 2002;4:448–453 [DOI] [PubMed] [Google Scholar]
- 127.Chrostowska M, Szyndler A, Hoffmann M, et al. Impact of obesity on cardiovascular health. Best Pract Res Clin Endocrinol Metab 2013;27:147–156 [DOI] [PubMed] [Google Scholar]
- 128.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009;53:1925–1932 [DOI] [PubMed] [Google Scholar]
- 129.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol 1995;141:1117–1127 [DOI] [PubMed] [Google Scholar]
- 130.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med 1995;333:677–685 [DOI] [PubMed] [Google Scholar]
- 131.Ceriello A, Taboga C, Tonutti L, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: Effects of short- and long-term simvastatin treatment. Circulation 2002;106:1211–1218 [DOI] [PubMed] [Google Scholar]
- 132.Krzystek-Korpacka M, Patryn E, Hotowy K, et al. Paraoxonase (PON)-1 activity in overweight and obese children and adolescents: Association with obesity-related inflammation and oxidative stress. Adv Clin Exp Med 2013;22:229–236 [PubMed] [Google Scholar]
- 133.Ferretti G, Bacchetti T, Masciangelo S, et al. HDL-paraoxonase and membrane lipid peroxidation: A comparison between healthy and obese subjects. Obesity (Silver Spring) 2010;18:1079–1084 [DOI] [PubMed] [Google Scholar]
- 134.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004;44:248–252 [DOI] [PubMed] [Google Scholar]
- 135.Anfossi G, Russo I, Massucco P, et al. Impaired synthesis and action of antiaggregating cyclic nucleotides in platelets from obese subjects: Possible role in platelet hyperactivation in obesity. Eur J Clin Invest 2004;34:482–489 [DOI] [PubMed] [Google Scholar]
- 136.Merkel M, Heeren J, Dudeck W, et al. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J Biol Chem 2002;277:7405–7411 [DOI] [PubMed] [Google Scholar]
- 137.Nishimura S, Manabe I, Nagasaki M, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007;56:1517–1526 [DOI] [PubMed] [Google Scholar]
- 138.Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol 2013;24:4–11 [DOI] [PubMed] [Google Scholar]
- 139.Wang B, Trayhurn P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflugers Arch 2006;452:418–427 [DOI] [PubMed] [Google Scholar]
- 140.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc 2008;67:128–145 [DOI] [PubMed] [Google Scholar]
- 142.Louie SM, Roberts LS, Nomura DK. Mechanisms linking obesity and cancer. Biochim Biophys Acta 2013;1831:1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.International Agency for Research on Cancer. Weight control and physical activity. In: Vainio H, Bianchini F. (eds). IARC Handbook of Cancer Prevention, Vol. 6, Lyon, France: IARC Press; 2002: pp. 1–315 [Google Scholar]
- 144.World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective, 2nd edition. Washington, DC: American Institute for Cancer Research; 2007 [Google Scholar]
- 145.Kitahara CM, Platz EA, Freeman LE, et al. Obesity and thyroid cancer risk among U.S. men and women: A pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev 2011;20:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lichtman MA. Obesity and the risk for a hematological malignancy: Leukemia, lymphoma, or myeloma. Oncologist 2010;15:1083–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kang DH. Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues 2002;13:540–549 [DOI] [PubMed] [Google Scholar]
- 148.Crujeiras AB, Diaz-Lagares A, Carreira MC, et al. Oxidative stress associated to dysfunctional adipose tissue: A potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res 2013;47:243–256 [DOI] [PubMed] [Google Scholar]
- 149.Wang Y, Lam KS, Xu A. Adiponectin as a negative regulator in obesity-related mammary carcinogenesis. Cell Res 2007;17:280–282 [DOI] [PubMed] [Google Scholar]
- 150.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005;115:897–909; quiz 910. [DOI] [PubMed] [Google Scholar]
- 151.Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci 2014;1311:31–41 [DOI] [PubMed] [Google Scholar]
- 152.Boulet LP. Asthma and obesity. Clin Exp Allergy 2013;43:8–21 [DOI] [PubMed] [Google Scholar]
- 153.Delgado J, Barranco P, Quirce S. Obesity and asthma. J Investig Allergol Clin Immunol 2008;18:420–425 [PubMed] [Google Scholar]
- 154.Luder E, Ehrlich RI, Lou WY, et al. Body mass index and the risk of asthma in adults. Respir Med 2004;98:29–37 [DOI] [PubMed] [Google Scholar]
- 155.Celedon JC, Palmer LJ, Litonjua AA, et al. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med 2001;164:1835–1840 [DOI] [PubMed] [Google Scholar]
- 156.Chen Y, Dales R, Krewski D, et al. Increased effects of smoking and obesity on asthma among female Canadians: The National Population Health Survey, 1994–1995. Am J Epidemiol 1999;150:255–262 [DOI] [PubMed] [Google Scholar]
- 157.Kim S, Camargo CA., Jr. Sex-race differences in the relationship between obesity and asthma: The behavioral risk factor surveillance system, 2000. Ann Epidemiol 2003;13:666–673 [DOI] [PubMed] [Google Scholar]
- 158.Nystad W, Meyer HE, Nafstad P, et al. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol 2004;160:969–976 [DOI] [PubMed] [Google Scholar]
- 159.Cho YS, Moon HB. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res 2010;2:183–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sahiner UM, Birben E, Erzurum S, et al. Oxidative stress in asthma. World Allergy Organ J 2011;4:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ercan H, Birben E, Dizdar EA, et al. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol 2006;118:1097–1104 [DOI] [PubMed] [Google Scholar]
- 162.Ochs-Balcom HM, Grant BJ, Muti P, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr 2006;60:991–999 [DOI] [PubMed] [Google Scholar]
- 163.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol (1985) 2010;108:754–759 [DOI] [PubMed] [Google Scholar]
- 164.Yaturu S, Davis J. Prevalence of decreased vitamin D levels is high among veterans with diabetes and/or CKD. ISRN Endocrinol 2011;2011:109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lopez-Garcia E, Faubel R, Leon-Munoz L, et al. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr 2008;87:310–316 [DOI] [PubMed] [Google Scholar]
- 166.Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med 2006;166:1775–1782 [DOI] [PubMed] [Google Scholar]
- 168.Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med 1994;154:1705–1711 [PubMed] [Google Scholar]
- 169.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med 2002;165:1217–1239 [DOI] [PubMed] [Google Scholar]
- 170.Smith PL, Gold AR, Meyers DA, et al. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med 1985;103:850–855 [DOI] [PubMed] [Google Scholar]
- 171.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1991;144:494–498 [DOI] [PubMed] [Google Scholar]
- 172.Lee SD, Ju G, Choi JA, et al. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath 2012;16:511–517 [DOI] [PubMed] [Google Scholar]
- 173.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation 2010;121:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marchesini G, Moscatiello S, Di Domizio S, et al. Obesity-associated liver disease. J Clin Endocrinol Metab 2008;93:S74–S80 [DOI] [PubMed] [Google Scholar]
- 175.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2003;124:71–79 [DOI] [PubMed] [Google Scholar]
- 177.Petersen KF, Dufour S, Feng J, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A 2006;103:18273–18277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004;40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 180.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–923 [DOI] [PubMed] [Google Scholar]
- 181.Sumida Y, Niki E, Naito Y, et al. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic Res 2013;47:869–880 [DOI] [PubMed] [Google Scholar]
- 182.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta 2011;412:1297–1305 [DOI] [PubMed] [Google Scholar]
- 183.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 2012;52:59–69 [DOI] [PubMed] [Google Scholar]
- 184.Rahimi RS, Landaverde C. Nonalcoholic fatty liver disease and the metabolic syndrome: Clinical implications and treatment. Nutr Clin Pract 2013;28:40–51 [DOI] [PubMed] [Google Scholar]
- 185.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006;144:21–28 [DOI] [PubMed] [Google Scholar]
- 186.Kramer H, Luke A, Bidani A, et al. Obesity and prevalent and incident CKD: The hypertension detection and follow-up program. Am J Kidney Dis 2005;46:587–594 [DOI] [PubMed] [Google Scholar]
- 187.Reynolds K, Gu D, Muntner P, et al. Body mass index and risk of ESRD in China. Am J Kidney Dis 2007;50:754–764 [DOI] [PubMed] [Google Scholar]
- 188.Weisinger JR, Kempson RL, Eldridge FL, et al. The nephrotic syndrome: A complication of massive obesity. Ann Intern Med 1974;81:440–447 [DOI] [PubMed] [Google Scholar]
- 189.Jennette JC, Charles L, Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep-apnea syndrome. Am J Kidney Dis 1987;10:470–472 [DOI] [PubMed] [Google Scholar]
- 190.Verani RR. Obesity-associated focal segmental glomerulosclerosis: Pathological features of the lesion and relationship with cardiomegaly and hyperlipidemia. Am J Kidney Dis 1992;20:629–634 [DOI] [PubMed] [Google Scholar]
- 191.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int 2001;59:1498–1509 [DOI] [PubMed] [Google Scholar]
- 192.Adelman RD, Restaino IG, Alon US, et al. Proteinuria and focal segmental glomerulosclerosis in severely obese adolescents. J Pediatr 2001;138:481–485 [DOI] [PubMed] [Google Scholar]
- 193.Kao MP, Ang DS, Pall A, et al. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens 2010;24:1–8 [DOI] [PubMed] [Google Scholar]
- 194.Tepel M, Echelmeyer M, Orie NN, et al. Increased intracellular reactive oxygen species in patients with end-stage renal failure: Effect of hemodialysis. Kidney Int 2000;58:867–872 [DOI] [PubMed] [Google Scholar]
- 195.Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis 2006;48:752–760 [DOI] [PubMed] [Google Scholar]
- 196.Descamps-Latscha B, Chatenoud L. T cells and B cells in chronic renal failure. Semin Nephrol 1996;16:183–191 [PubMed] [Google Scholar]
- 197.Pasquali R, Patton L, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes 2007;14:482–487 [DOI] [PubMed] [Google Scholar]
- 198.Stein If LM. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935;29:181–191 [Google Scholar]
- 199.Rich-Edwards JW, Spiegelman D, Garland M, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 2002;13:184–190 [DOI] [PubMed] [Google Scholar]
- 200.Norman RJ, Clark AM. Obesity and reproductive disorders: A review. Reprod Fertil Dev 1998;10:55–63 [DOI] [PubMed] [Google Scholar]
- 201.Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev 2007;8:515–523 [DOI] [PubMed] [Google Scholar]
- 202.Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod 1995;10:2705–2712 [DOI] [PubMed] [Google Scholar]
- 203.Gosman GG, Katcher HI, Legro RS. Obesity and the role of gut and adipose hormones in female reproduction. Hum Reprod Update 2006;12:585–601 [DOI] [PubMed] [Google Scholar]
- 204.Hammoud AO, Wilde N, Gibson M, et al. Male obesity and alteration in sperm parameters. Fertil Steril 2008;90:2222–2225 [DOI] [PubMed] [Google Scholar]
- 205.Chavarro JE, Toth TL, Wright DL, et al. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010;93:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bacon CG, Mittleman MA, Kawachi I, et al. A prospective study of risk factors for erectile dysfunction. J Urol 2006;176:217–221 [DOI] [PubMed] [Google Scholar]
- 207.Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J Urol 2000;163:460–463 [PubMed] [Google Scholar]
- 208.Saigal CS, Wessells H, Pace J, et al. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 2006;166:207–212 [DOI] [PubMed] [Google Scholar]
- 209.Esposito K, Ciotola M, Giugliano F, et al. Association of body weight with sexual function in women. Int J Impot Res 2007;19:353–357 [DOI] [PubMed] [Google Scholar]
- 210.Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008;44:280–287 [DOI] [PubMed] [Google Scholar]
- 211.Jana SK, NB K, Chattopadhyay R, et al. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol 2010;29:447–451 [DOI] [PubMed] [Google Scholar]
- 212.Das S, Chattopadhyay R, Ghosh S, et al. Reactive oxygen species level in follicular fluid—embryo quality marker in IVF? Hum Reprod 2006;21:2403–2407 [DOI] [PubMed] [Google Scholar]
- 213.Pasqualotto EB, Agarwal A, Sharma RK, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril 2004;81:973–976 [DOI] [PubMed] [Google Scholar]
- 214.Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010;7:153–161 [DOI] [PubMed] [Google Scholar]
- 216.Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992;57:409–416 [DOI] [PubMed] [Google Scholar]
- 217.Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl 1993;16:183–188 [DOI] [PubMed] [Google Scholar]
- 218.Shekarriz M, Thomas AJ, Jr., Agarwal A. Incidence and level of seminal reactive oxygen species in normal men. Urology 1995;45:103–107 [DOI] [PubMed] [Google Scholar]
- 219.Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update 2008;14:243–258 [DOI] [PubMed] [Google Scholar]
- 220.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: A mini-review. Gerontology 2012;58:15–23 [DOI] [PubMed] [Google Scholar]
- 221.Fruhbeck G, Becerril S, Sainz N, et al. BAT: A new target for human obesity? Trends Pharmacol Sci 2009;30:387–396 [DOI] [PubMed] [Google Scholar]
- 222.Trayhurn P, Beattie JH. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001;60:329–339 [DOI] [PubMed] [Google Scholar]
- 223.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129–139 [DOI] [PubMed] [Google Scholar]
- 224.Guerre-Millo M. Adipose tissue and adipokines: For better or worse. Diabetes Metab 2004;30:13–19 [DOI] [PubMed] [Google Scholar]
- 225.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008;29:2959–2971 [DOI] [PubMed] [Google Scholar]
- 226.Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta 2013;419:87–94 [DOI] [PubMed] [Google Scholar]
- 227.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ouchi N, Ohashi K, Shibata R, et al. Adipocytokines and obesity-linked disorders. Nagoya J Med Sci 2012;74:19–30 [PMC free article] [PubMed] [Google Scholar]
- 229.Rabe K, Lehrke M, Parhofer KG, et al. Adipokines and insulin resistance. Mol Med 2008;14:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhao YF, Feng DD, Chen C. Contribution of adipocyte-derived factors to beta-cell dysfunction in diabetes. Int J Biochem Cell Biol 2006;38:804–819 [DOI] [PubMed] [Google Scholar]
- 231.Dunmore SJ, Brown JE. The role of adipokines in beta-cell failure of type 2 diabetes. J Endocrinol 2013;216:T37–T45 [DOI] [PubMed] [Google Scholar]
- 232.Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun 1996;224:522–527 [DOI] [PubMed] [Google Scholar]
- 233.Kieffer TJ, Heller RS, Leech CA, et al. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes 1997;46:1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kieffer TJ, Habener JF. The adipoinsular axis: Effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab 2000;278:E1-E14 [DOI] [PubMed] [Google Scholar]
- 235.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest 1998;102:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee JW, Swick AG, Romsos DR. Leptin constrains phospholipase C-protein kinase C-induced insulin secretion via a phosphatidylinositol 3-kinase-dependent pathway. Exp Biol Med (Maywood) 2003;228:175–182 [DOI] [PubMed] [Google Scholar]
- 237.Kulkarni RN, Wang ZL, Wang RM, et al. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 1997;100:2729–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pallett AL, Morton NM, Cawthorne MA, et al. Leptin inhibits insulin secretion and reduces insulin mRNA levels in rat isolated pancreatic islets. Biochem Biophys Res Commun 1997;238:267–270 [DOI] [PubMed] [Google Scholar]
- 239.Okuya S, Tanabe K, Tanizawa Y, et al. Leptin increases the viability of isolated rat pancreatic islets by suppressing apoptosis. Endocrinology 2001;142:4827–4830 [DOI] [PubMed] [Google Scholar]
- 240.Shimabukuro M, Wang MY, Zhou YT, et al. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci U S A 1998;95:9558–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 2002;277:25863–25866 [DOI] [PubMed] [Google Scholar]
- 242.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 2003;278:2461–2468 [DOI] [PubMed] [Google Scholar]
- 243.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–769 [DOI] [PubMed] [Google Scholar]
- 244.Rakatzi I, Mueller H, Ritzeler O, et al. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 2004;47:249–258 [DOI] [PubMed] [Google Scholar]
- 245.Huypens P, Moens K, Heimberg H, et al. Adiponectin-mediated stimulation of AMP-activated protein kinase (AMPK) in pancreatic beta cells. Life Sci 2005;77:1273–1282 [DOI] [PubMed] [Google Scholar]
- 246.Zhang S, Kim KH. TNF-alpha inhibits glucose-induced insulin secretion in a pancreatic beta-cell line (INS-1). FEBS Lett 1995;377:237–239 [DOI] [PubMed] [Google Scholar]
- 247.Tsiotra PC, Tsigos C, Raptis SA. TNFalpha and leptin inhibit basal and glucose-stimulated insulin secretion and gene transcription in the HIT-T15 pancreatic cells. Int J Obes Relat Metab Disord 2001;25:1018–1026 [DOI] [PubMed] [Google Scholar]
- 248.Cirulli V, Halban PA, Rouiller DG. Tumor necrosis factor-alpha modifies adhesion properties of rat islet B cells. J Clin Invest 1993;91:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stephens LA, Thomas HE, Ming L, et al. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology 1999;140:3219–3227 [DOI] [PubMed] [Google Scholar]
- 250.Ishizuka N, Yagui K, Tokuyama Y, et al. Tumor necrosis factor alpha signaling pathway and apoptosis in pancreatic beta cells. Metabolism 1999;48:1485–1492 [DOI] [PubMed] [Google Scholar]
- 251.Dunger A, Cunningham JM, Delaney CA, et al. Tumor necrosis factor-alpha and interferon-gamma inhibit insulin secretion and cause DNA damage in unweaned-rat islets. Extent of nitric oxide involvement. Diabetes 1996;45:183–189 [DOI] [PubMed] [Google Scholar]
- 252.Rabinovitch A, Suarez-Pinzon W, Strynadka K, et al. Transfection of human pancreatic islets with an anti-apoptotic gene (bcl-2) protects beta-cells from cytokine-induced destruction. Diabetes 1999;48:1223–1229 [DOI] [PubMed] [Google Scholar]
- 253.Suzuki T, Imai J, Yamada T, et al. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: Potential involvement of the PLC-IP3-dependent pathway. Diabetes 2011;60:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shimizu H, Ohtani K, Kato Y, et al. Interleukin-6 increases insulin secretion and preproinsulin mRNA expression via Ca2+-dependent mechanism. J Endocrinol 2000;166:121–126 [DOI] [PubMed] [Google Scholar]
- 255.Sandler S, Bendtzen K, Eizirik DL, et al. Interleukin-6 affects insulin secretion and glucose metabolism of rat pancreatic islets in vitro. Endocrinology 1990;126:1288–1294 [DOI] [PubMed] [Google Scholar]
- 256.Barzilai N, Wang J, Massilon D, et al. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest 1997;100:3105–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ebihara K, Ogawa Y, Masuzaki H, et al. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 2001;50:1440–1448 [DOI] [PubMed] [Google Scholar]
- 258.Appleton DJ, Rand JS, Sunvold GD. Plasma leptin concentrations are independently associated with insulin sensitivity in lean and overweight cats. J Feline Med Surg 2002;4:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002;415:339–343 [DOI] [PubMed] [Google Scholar]
- 260.Kahn BB, Alquier T, Carling D, et al. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 261.Morton GJ, Gelling RW, Niswender KD, et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2005;2:411–420 [DOI] [PubMed] [Google Scholar]
- 262.Marti A, Berraondo B, Martinez JA. Leptin: Physiological actions. J Physiol Biochem 1999;55:43–49 [PubMed] [Google Scholar]
- 263.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–770 [DOI] [PubMed] [Google Scholar]
- 264.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab 2013;2:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab 2008;93:3165–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–451 [DOI] [PubMed] [Google Scholar]
- 267.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 268.Uysal KT, Wiesbrock SM, Marino MW, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997;389:610–614 [DOI] [PubMed] [Google Scholar]
- 269.Jellema A, Plat J, Mensink RP. Weight reduction, but not a moderate intake of fish oil, lowers concentrations of inflammatory markers and PAI-1 antigen in obese men during the fasting and postprandial state. Eur J Clin Invest 2004;34:766–773 [DOI] [PubMed] [Google Scholar]
- 270.Klover PJ, Zimmers TA, Koniaris LG, et al. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 2003;52:2784–2789 [DOI] [PubMed] [Google Scholar]
- 271.Senn JJ, Klover PJ, Nowak IA, et al. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002;51:3391–3399 [DOI] [PubMed] [Google Scholar]
- 272.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003;278:45777–45784 [DOI] [PubMed] [Google Scholar]
- 273.Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286–1292 [DOI] [PubMed] [Google Scholar]
- 274.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745–E751 [DOI] [PubMed] [Google Scholar]
- 275.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006;131:934–945 [DOI] [PubMed] [Google Scholar]
- 276.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: The macro- and microcirculation of adipose tissue. FEBS J 2009;276:5738–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007;92:1023–1033 [DOI] [PubMed] [Google Scholar]
- 278.Engeli S, Schling P, Gorzelniak K, et al. The adipose-tissue renin-angiotensin-aldosterone system: Role in the metabolic syndrome? Int J Biochem Cell Biol 2003;35:807–825 [DOI] [PubMed] [Google Scholar]
- 279.Engeli S, Bohnke J, Gorzelniak K, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 2005;45:356–362 [DOI] [PubMed] [Google Scholar]
- 280.Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep 2007;7:25–33 [DOI] [PubMed] [Google Scholar]
- 281.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med 2009;6:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002;106:2767–2770 [DOI] [PubMed] [Google Scholar]
- 283.Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta 2004;344:1–12 [DOI] [PubMed] [Google Scholar]
- 284.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999;100:2473–2476 [DOI] [PubMed] [Google Scholar]
- 285.Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol 2010;7:22–29 [DOI] [PubMed] [Google Scholar]
- 286.Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol 2011;38:905–913 [DOI] [PubMed] [Google Scholar]
- 287.Martin SS, Qasim A, Reilly MP. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol 2008;52:1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Enriori PJ, Evans AE, Sinnayah P, et al. Leptin resistance and obesity. Obesity (Silver Spring) 2006;14 Suppl 5:254S–258S [DOI] [PubMed] [Google Scholar]
- 289.Wannamethee SG, Shaper AG, Whincup PH, et al. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J Am Coll Cardiol 2011;58:1870–1877 [DOI] [PubMed] [Google Scholar]
- 290.Kunnari A, Ukkola O, Paivansalo M, et al. High plasma resistin level is associated with enhanced highly sensitive C-reactive protein and leukocytes. J Clin Endocrinol Metab 2006;91:2755–2760 [DOI] [PubMed] [Google Scholar]
- 291.Norata GD, Ongari M, Garlaschelli K, et al. Plasma resistin levels correlate with determinants of the metabolic syndrome. Eur J Endocrinol 2007;156:279–284 [DOI] [PubMed] [Google Scholar]
- 292.Lubos E, Messow CM, Schnabel R, et al. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Atherosclerosis 2007;193:121–128 [DOI] [PubMed] [Google Scholar]
- 293.Calabro P, Samudio I, Willerson JT, et al. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation 2004;110:3335–3340 [DOI] [PubMed] [Google Scholar]
- 294.Mu H, Ohashi R, Yan S, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res 2006;70:146–157 [DOI] [PubMed] [Google Scholar]
- 295.Dick GM, Katz PS, Farias M 3rd, et al. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am J Physiol Heart Circ Physiol 2006;291:H2997–H3002 [DOI] [PubMed] [Google Scholar]
- 296.Milan G, Granzotto M, Scarda A, et al. Resistin and adiponectin expression in visceral fat of obese rats: Effect of weight loss. Obes Res 2002;10:1095–1103 [DOI] [PubMed] [Google Scholar]
- 297.Silswal N, Singh AK, Aruna B, et al. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun 2005;334:1092–1101 [DOI] [PubMed] [Google Scholar]
- 298.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–591 [DOI] [PubMed] [Google Scholar]
- 299.Redinger RN. The pathophysiology of obesity and its clinical manifestations. Gastroenterol Hepatol (N Y) 2007;3:856–863 [PMC free article] [PubMed] [Google Scholar]
- 300.Kim S, Popkin BM. Commentary: Understanding the epidemiology of overweight and obesity—a real global public health concern. Int J Epidemiol 2006;35:60–67; discussion 81–62. [DOI] [PubMed] [Google Scholar]
- 301.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol Metab 2006;17:328–336 [DOI] [PubMed] [Google Scholar]
- 302.Wang SN, Yeh YT, Yang SF, et al. Potential role of leptin expression in hepatocellular carcinoma. J Clin Pathol 2006;59:930–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ogunwobi O, Mutungi G, Beales IL. Leptin stimulates proliferation and inhibits apoptosis in Barrett's esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology 2006;147:4505–4516 [DOI] [PubMed] [Google Scholar]
- 304.Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am J Clin Nutr 2007;86:s858–s866 [DOI] [PubMed] [Google Scholar]
- 305.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: A link between obesity and cancer. Expert Opin Investig Drugs 2006;15:917–931 [DOI] [PubMed] [Google Scholar]
- 306.Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999;91:1147–1154 [DOI] [PubMed] [Google Scholar]
- 307.Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–643 [DOI] [PubMed] [Google Scholar]
- 308.Brashier B, Salvi S. Obesity and asthma: Physiological perspective. J Allergy (Cairo) 2013;2013:198068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Khan UI, Rastogi D, Isasi CR, et al. Independent and synergistic associations of asthma and obesity with systemic inflammation in adolescents. J Asthma 2012;49:1044–1050 [DOI] [PubMed] [Google Scholar]
- 310.Chung KF, Barnes PJ. Cytokines in asthma. Thorax 1999;54:825–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Thomas PS. Tumour necrosis factor-alpha: The role of this multifunctional cytokine in asthma. Immunol Cell Biol 2001;79:132–140 [DOI] [PubMed] [Google Scholar]
- 312.Sukkar MB, Hughes JM, Armour CL, et al. Tumour necrosis factor-alpha potentiates contraction of human bronchus in vitro. Respirology 2001;6:199–203 [DOI] [PubMed] [Google Scholar]
- 313.Heijink IH, Vellenga E, Borger P, et al. Interleukin-6 promotes the production of interleukin-4 and interleukin-5 by interleukin-2-dependent and -independent mechanisms in freshly isolated human T cells. Immunology 2002;107:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shore SA. Obesity and asthma: Lessons from animal models. J Appl Physiol (1985) 2007;102:516–528 [DOI] [PubMed] [Google Scholar]
- 315.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 1999;84:2603–2607 [DOI] [PubMed] [Google Scholar]
- 316.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004;89:2119–2126 [DOI] [PubMed] [Google Scholar]
- 317.Vgontzas AN, Bixler EO, Lin HM, et al. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 2005;12:131–140 [DOI] [PubMed] [Google Scholar]
- 318.Vgontzas AN, Pejovic S, Zoumakis E, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab 2007;292:E253–E261 [DOI] [PubMed] [Google Scholar]
- 319.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab 1993;77:1690–1694 [DOI] [PubMed] [Google Scholar]
- 320.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem 2008;114:211–223 [DOI] [PubMed] [Google Scholar]
- 321.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep 2007;30:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kaplan ML, Leveille GA. Obesity: Prediction of preobesity among progeny from crosses of ob+ mice. Proc Soc Exp Biol Med 1973;143:925–928 [DOI] [PubMed] [Google Scholar]
- 323.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263–1271 [DOI] [PubMed] [Google Scholar]
- 324.Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007;92:532–541 [DOI] [PubMed] [Google Scholar]
- 325.Margetic S, Gazzola C, Pegg GG, et al. Leptin: A review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 2002;26:1407–1433 [DOI] [PubMed] [Google Scholar]
- 326.Caro JF, Kolaczynski JW, Nyce MR, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: A possible mechanism for leptin resistance. Lancet 1996;348:159–161 [DOI] [PubMed] [Google Scholar]
- 327.Xu A, Wang Y, Keshaw H, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003;112:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2007;13:3540–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Louthan MV, Barve S, McClain CJ, et al. Decreased serum adiponectin: An early event in pediatric nonalcoholic fatty liver disease. J Pediatr 2005;147:835–838 [DOI] [PubMed] [Google Scholar]
- 330.Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 2003;278:13740–13746 [DOI] [PubMed] [Google Scholar]
- 331.Briffa JF, McAinch AJ, Poronnik P, et al. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol 2013;305:F1629–F1636 [DOI] [PubMed] [Google Scholar]
- 332.Lee MP, Orlov D, Sweeney G. Leptin induces rat glomerular mesangial cell hypertrophy, but does not regulate hyperplasia or apoptosis. Int J Obes (Lond) 2005;29:1395–1401 [DOI] [PubMed] [Google Scholar]
- 333.Wohlfarth V, Drumm K, Mildenberger S, et al. Protein uptake disturbs collagen homeostasis in proximal tubule-derived cells. Kidney Int Suppl 2003;S103–S109 [DOI] [PubMed] [Google Scholar]
- 334.Wolf G, Schroeder R, Ziyadeh FN, et al. Albumin up-regulates the type II transforming growth factor-beta receptor in cultured proximal tubular cells. Kidney Int 2004;66:1849–1858 [DOI] [PubMed] [Google Scholar]
- 335.Wolf G, Hamann A, Han DC, et al. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: Potential role in glomerulosclerosis [seecomments]. Kidney Int 1999;56:860–872 [DOI] [PubMed] [Google Scholar]
- 336.Lee MP, Madani S, Sekula D, et al. Leptin increases expression and activity of matrix metalloproteinase-2 and does not alter collagen production in rat glomerular mesangial cells. Endocr Res 2005;31:27–37 [DOI] [PubMed] [Google Scholar]
- 337.Dagogo-Jack S, Ovalle F, Landt M, et al. Hyperleptinemia in patients with end-stage renal disease undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 1998;18:34–40 [PubMed] [Google Scholar]
- 338.Merabet E, Dagogo-Jack S, Coyne DW, et al. Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab 1997;82:847–850 [DOI] [PubMed] [Google Scholar]
- 339.Doumatey AP, Zhou J, Huang H, et al. Circulating adiponectin is associated with renal function independent of age and serum lipids in west africans. Int J Nephrol 2012;2012:730920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008;118:1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Decleves AE, Mathew AV, Cunard R, et al. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 2011;22:1846–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Axelsson J, Bergsten A, Qureshi AR, et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int 2006;69:596–604 [DOI] [PubMed] [Google Scholar]
- 343.Menzaghi C, Salvemini L, Fini G, et al. Serum resistin and kidney function: A family-based study in non-diabetic, untreated individuals. PLoS One 2012;7:e38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Cohen G, Horl WH. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Semin Dial 2009;22:373–377 [DOI] [PubMed] [Google Scholar]
- 345.Malyszko J, Malyszko JS, Mysliwiec M. Visfatin, a new adipocytokine, is predominantly related to inflammation/endothelial damage in kidney allograft recipients. Transplant Proc 2009;41:150–153 [DOI] [PubMed] [Google Scholar]
- 346.Yilmaz MI, Saglam M, Carrero JJ, et al. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant 2008;23:959–965 [DOI] [PubMed] [Google Scholar]
- 347.Huang Q, Guo Y, Zeng H, et al. Visfatin stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Endocr Res 2011;36:93–100 [DOI] [PubMed] [Google Scholar]
- 348.Mitchell M, Armstrong DT, Robker RL, et al. Adipokines: Implications for female fertility and obesity. Reproduction 2005;130:583–597 [DOI] [PubMed] [Google Scholar]
- 349.Chen X, Jia X, Qiao J, et al. Adipokines in reproductive function: A link between obesity and polycystic ovary syndrome. J Mol Endocrinol 2013;50:R21–R37 [DOI] [PubMed] [Google Scholar]
- 350.Farooq R, Lutfullah S, Ahmed M. Serum leptin levels in obese infertile men and women. Pak J Pharm Sci 2014;27:67–71 [PubMed] [Google Scholar]
- 351.Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: Evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999;84:3673–3680 [DOI] [PubMed] [Google Scholar]
- 352.Yildizhan R, Ilhan GA, Yildizhan B, et al. Serum retinol-binding protein 4, leptin, and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertil Steril 2011;96:246–250 [DOI] [PubMed] [Google Scholar]
- 353.Campos DB, Palin MF, Bordignon V, et al. The ‘beneficial’ adipokines in reproduction and fertility. Int J Obes (Lond) 2008;32:223–231 [DOI] [PubMed] [Google Scholar]
- 354.Escobar-Morreale HF, Luque-Ramirez M, San Millan JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev 2005;26:251–282 [DOI] [PubMed] [Google Scholar]
- 355.Carmina E, Orio F, Palomba S, et al. Evidence for altered adipocyte function in polycystic ovary syndrome. Eur J Endocrinol 2005;152:389–394 [DOI] [PubMed] [Google Scholar]
- 356.Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007;133:719–731 [DOI] [PubMed] [Google Scholar]
- 357.Ledoux S, Campos DB, Lopes FL, et al. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 2006;147:5178–5186 [DOI] [PubMed] [Google Scholar]
- 358.Manna P, Sil PC. Arjunolic acid: Beneficial role in type 1 diabetes and its associated organ pathophysiology. Free Radic Res 2012;46:815–830 [DOI] [PubMed] [Google Scholar]
- 359.Oh S, Tanaka K, Warabi E, et al. Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med Sci Sports Exerc 2013;45:2214–2222 [DOI] [PubMed] [Google Scholar]
- 360.Parra D, Ramel A, Bandarra N, et al. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008;51:676–680 [DOI] [PubMed] [Google Scholar]
- 361.Kushner RF, Doerfler B. Low-carbohydrate, high-protein diets revisited. Curr Opin Gastroenterol 2008;24:198–203 [DOI] [PubMed] [Google Scholar]
- 362.Larsen TM, Dalskov S, van Baak M, et al. The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries—a comprehensive design for long-term intervention. Obes Rev 2010;11:76–91 [DOI] [PubMed] [Google Scholar]
- 363.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–2090 [DOI] [PubMed] [Google Scholar]
- 364.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann Intern Med 2004;140:778–785 [DOI] [PubMed] [Google Scholar]
- 365.Raben A, Astrup A, Vasilaras TH, et al. [The CARMEN trial: Increased intake of carbohydrates—simple or complex—and unchanged blood lipids in overweight subjects]. Ugeskr Laeger 2002;164:627–631 [PubMed] [Google Scholar]
- 366.Saris WH, Astrup A, Prentice AM, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: The CARMEN study. The Carbohydrate Ratio Management in European National diets. Int J Obes Relat Metab Disord 2000;24:1310–1318 [DOI] [PubMed] [Google Scholar]
- 367.Teegarden D, White KM, Lyle RM, et al. Calcium and dairy product modulation of lipid utilization and energy expenditure. Obesity (Silver Spring) 2008;16:1566–1572 [DOI] [PubMed] [Google Scholar]
- 368.Major GC, Alarie FP, Dore J, et al. Calcium plus vitamin D supplementation and fat mass loss in female very low-calcium consumers: Potential link with a calcium-specific appetite control. Br J Nutr 2009;101:659–663 [DOI] [PubMed] [Google Scholar]
- 369.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr 2005;24:537s–546s [DOI] [PubMed] [Google Scholar]
- 370.Bendsen NT, Hother AL, Jensen SK, et al. Effect of dairy calcium on fecal fat excretion: A randomized crossover trial. Int J Obes (Lond) 2008;32:1816–1824 [DOI] [PubMed] [Google Scholar]
- 371.Myers VH, Champagne CM. Nutritional effects on blood pressure. Curr Opin Lipidol 2007;18:20–24 [DOI] [PubMed] [Google Scholar]
- 372.Ard JD, Grambow SC, Liu D, et al. The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care 2004;27:340–347 [DOI] [PubMed] [Google Scholar]
- 373.Azadbakht L, Mirmiran P, Esmaillzadeh A, et al. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care 2005;28:2823–2831 [DOI] [PubMed] [Google Scholar]
- 374.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care 2004;27:2741–2751 [DOI] [PubMed] [Google Scholar]
- 375.Pittler MH, Stevinson C, Ernst E. Chromium picolinate for reducing body weight: Meta-analysis of randomized trials. Int J Obes Relat Metab Disord 2003;27:522–529 [DOI] [PubMed] [Google Scholar]
- 376.Onakpoya I, Posadzki P, Ernst E. Chromium supplementation in overweight and obesity: A systematic review and meta-analysis of randomized clinical trials. Obes Rev 2013;14:496–507 [DOI] [PubMed] [Google Scholar]
- 377.Boeing H, Bechthold A, Bub A, et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 2012;51:637–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tetens I, Alinia S. The role of fruit consumption in the prevention of obesity. J Hortic Sci Biotechnol 2009;47–51 [Google Scholar]
- 379.Grunwald GK, Seagle HM, Peters JC, et al. Quantifying and separating the effects of macronutrient composition and non-macronutrients on energy density. Br J Nutr 2001;86:265–276 [DOI] [PubMed] [Google Scholar]
- 380.Haber GB, Heaton KW, Murphy D, et al. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet 1977;2:679–682 [DOI] [PubMed] [Google Scholar]
- 381.Bolton RP, Heaton KW, Burroughs LF. The role of dietary fiber in satiety, glucose, and insulin: Studies with fruit and fruit juice. Am J Clin Nutr 1981;34:211–217 [DOI] [PubMed] [Google Scholar]
- 382.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev 2001;59:129–139 [DOI] [PubMed] [Google Scholar]
- 383.Gustafsson K, Asp NG, Hagander B, et al. Effects of different vegetables in mixed meals on glucose homeostasis and satiety. Eur J Clin Nutr 1993;47:192–200 [PubMed] [Google Scholar]
- 384.Gustafsson K, Asp NG, Hagander B, et al. Satiety effects of spinach in mixed meals: Comparison with other vegetables. Int J Food Sci Nutr 1995;46:327–334 [DOI] [PubMed] [Google Scholar]
- 385.Gustafsson K, Asp NG, Hagander B, et al. Dose-response effects of boiled carrots and effects of carrots in lactic acid in mixed meals on glycaemic response and satiety. Eur J Clin Nutr 1994;48:386–396 [PubMed] [Google Scholar]
- 386.Astrup A. Dietary management of obesity. JPEN J Parenter Enteral Nutr 2008;32:575–577 [DOI] [PubMed] [Google Scholar]
- 387.Cheskin LJ, Mitchell AM, Jhaveri AD, et al. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: A controlled clinical trial. Diabetes Educ 2008;34:118–127 [DOI] [PubMed] [Google Scholar]
- 388.Li Z, Hong K, Saltsman P, et al. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: Relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr 2005;59:411–418 [DOI] [PubMed] [Google Scholar]
- 389.Smeets AJ, Soenen S, Luscombe-Marsh ND, et al. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr 2008;138:698–702 [DOI] [PubMed] [Google Scholar]
- 390.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev 2003;4:CD004094. [DOI] [PubMed] [Google Scholar]
- 391.Carter R, Mouralidarane A, Ray S, et al. Recent advancements in drug treatment of obesity. Clin Med 2012;12:456–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Bray GA, Ryan DH. Drug treatment of the overweight patient. Gastroenterology 2007;132:2239–2252 [DOI] [PubMed] [Google Scholar]
- 393.Vetter ML, Faulconbridge LF, Webb VL, et al. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol 2010;6:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapies for obesity: Past, current, and future therapies. J Obes 2011;2011:179674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chechi K, Nedergaard J, Richard D. Brown adipose tissue as an anti-obesity tissue in humans. Obes Rev 2014;15:92–106 [DOI] [PubMed] [Google Scholar]
- 396.Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: Mechanisms and potential therapeutic targets. Circulation 2012;125:2782–2791 [DOI] [PubMed] [Google Scholar]
- 397.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979;281:31–35 [DOI] [PubMed] [Google Scholar]
- 398.Hamann A, Benecke H, Le Marchand-Brustel Y, et al. Characterization of insulin resistance and NIDDM in transgenic mice with reduced brown fat. Diabetes 1995;44:1266–1273 [DOI] [PubMed] [Google Scholar]
- 399.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 1996;137:21–29 [DOI] [PubMed] [Google Scholar]
- 400.Arch JR, Kaumann AJ. Beta 3 and atypical beta-adrenoceptors. Med Res Rev 1993;13:663–729 [DOI] [PubMed] [Google Scholar]
- 401.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 402.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab 2007;92:4299–4305 [DOI] [PubMed] [Google Scholar]
- 404.Lopez M, Varela L, Vazquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 2010;16:1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]