Abstract
Breast cancer occurs in approximately 1 in 8 women and 1 in 37 women with breast cancer succumbed to the disease. Over the past decades, new diagnostic tools and treatments have substantially improved the prognosis of women with local diseases. However, women with metastatic disease still have a dismal prognosis without effective treatments. Among different molecular subtypes of breast cancer, the HER2-enriched and basal-like subtypes typically have higher rates of metastasis to the brain. Basal-like metastatic breast tumors frequently express EGFR. Consequently, HER2- and EGFR-targeted therapies are being used in the clinic and/or evaluated in clinical trials for treating breast cancer patients with brain metastases. In this review, we will first provide an overview of the HER2 and EGFR signaling pathways. The roles that EGFR and HER2 play in breast cancer metastasis to the brain will then be discussed. Finally, we will summarize the preclinical and clinical effects of EGFR- and HER2-targeted therapies on breast cancer metastasis.
Keywords: Breast Cancer, Brain Metastasis, EGFR, HER2
2. INTRODUCTION
According to the American Cancer Society, approximately 231,840 new cases of breast cancer will be diagnosed and about 40,290 deaths will occur from breast cancer in 2015 (1). It is also estimated that breast cancer will be the most frequently diagnosed cancer in women in 2015. Of 8 women, one is expected to develop breast cancer in her lifetime while approximately 1 in 37 women with the disease is estimated to succumb to the disease. Approximately 2,430 deaths in women with breast cancer are younger than age 45 and the majority of breast cancer deaths, 37,860, tend to be in women older than 45 years. Although the survival rate for women with non-metastatic breast cancer is 98.5.%, there is a significant drop in survival rates of metastatic breast cancer ranging from 84% to as low as 24% (1).
Metastasis is described as the dissemination of primary cancer cells to a distant organ leading to another tumor. Metastasis is a complex multi-step process involving epithelial-mesenchymal transition (EMT), detachment from the basement membrane, local invasion, intravasation into the circulatory system, extravasation out of the circulatory system into a distant organ, mesenchymal-epithelial transition (MET), and lastly colonization in the distant organ (2).
Approximately 20–30% of breast cancers become metastatic, mainly to the lung, brain, bone, and liver and the metastatic disease contributes to 90% of breast cancer mortality (3–5). Breast cancer metastasis to the brain is observed in approximately 10–20% of women and leads to dismal life expectancy rates of 8–30 months (6–10). This poor prognosis is attributed to the fact that we still do not fully understand the factors driving breast cancer cells to the brain, the biology of brain metastases, or the bidirectional interplay between the breast cancer cells and the brain microenvironment. Due to these gaps of knowledge, we still do not have effective treatments for women with breast cancer brain metastases (BCBM). The standard of care for brain metastases includes surgical resection, stereotactic radiosurgery, and whole brain radiotherapy (WBRT). Individual techniques, or a combination of these techniques, used depends on the number of metastases, size of metastases, anatomical location of metastases, and the presence of neurological symptoms among other factors (11). WBRT has been a mainstay in treatment for brain metastases for many years but recent evidence indicates radiation-induced brain injury and cognitive impairment in patients with extended survival (12,13).
Breast cancers are divided into five different subtypes based on expression levels of hormone receptors (ERα and PR), HER2, cytokeratins (CKs) 5/6, and claudins 3/4/7. The five subtypes are luminal A, luminal B, HER2-enriched, claudin-low, and basal-like (14, 15). Luminal A tumors are positive for ERα and PR, low for Ki67 and are responsive to hormone therapy and chemotherapy. Luminal B tumors are positive for ERα, PR, HER2, and Ki67 with variable response to chemotherapy. HER2-enriched tumors are negative for ERα and PR, but positive for HER2 and Ki67. Claudin-low tumors are triple-negative breast cancer (TNBCs) that are negative for ERα, PR, and HER2 with low expression of Ki67, E-cadherin and claudins 3/4/7. Basal-like tumors tend to be TNBC (approximately 75%) that are positive for CKs, EGFR and Ki67 (16,17). TNBC accounts for 20% of breast cancers and has a high potential to metastasize mainly to the brain (17,18).
In the course of gaining insights in the molecular pathways driving breast cancer progression and metastasis, many lines of evidence indicate a pivotal role that the epidermal growth factor receptor (EGFR) family of receptors plays in regulating the process (19). The EGFR family is comprised of four structurally similar receptors including EGFR (ErbB-1, HER1), HER2 (ErbB-2), HER3 (ErbB-3), and HER4 (ErbB-4) (20–22). Particular interests have been centered on EGFR and HER2 because of their frequent overexpression and/or hyperactivation in breast carcinomas (23–25). EGFR is overexpressed in 15–30% of breast carcinomas and is associated with poor patient outcomes (16, 26–28). Overexpression of HER2 is found in 15%-35% of invasive breast cancers and aberrant HER2 signaling increases the likelihood of developing metastasis resulting in reduced survival (5,26,27,29). It is estimated that HER2 overexpressing TNBC breast cancer subtypes metastasizes to the brain 25–55% of the time (30). There is significant evidence suggesting both EGFR and HER2 are highly expressed in BCBM playing an active role in facilitating brain-specific metastatic spread. Preclinical and clinical studies further demonstrated that EGFR and HER2 are important therapeutic targets for women with BCBM.
3. OVERVIEW OF THE EGFR AND HER2 SIGNALING PATHWAYS
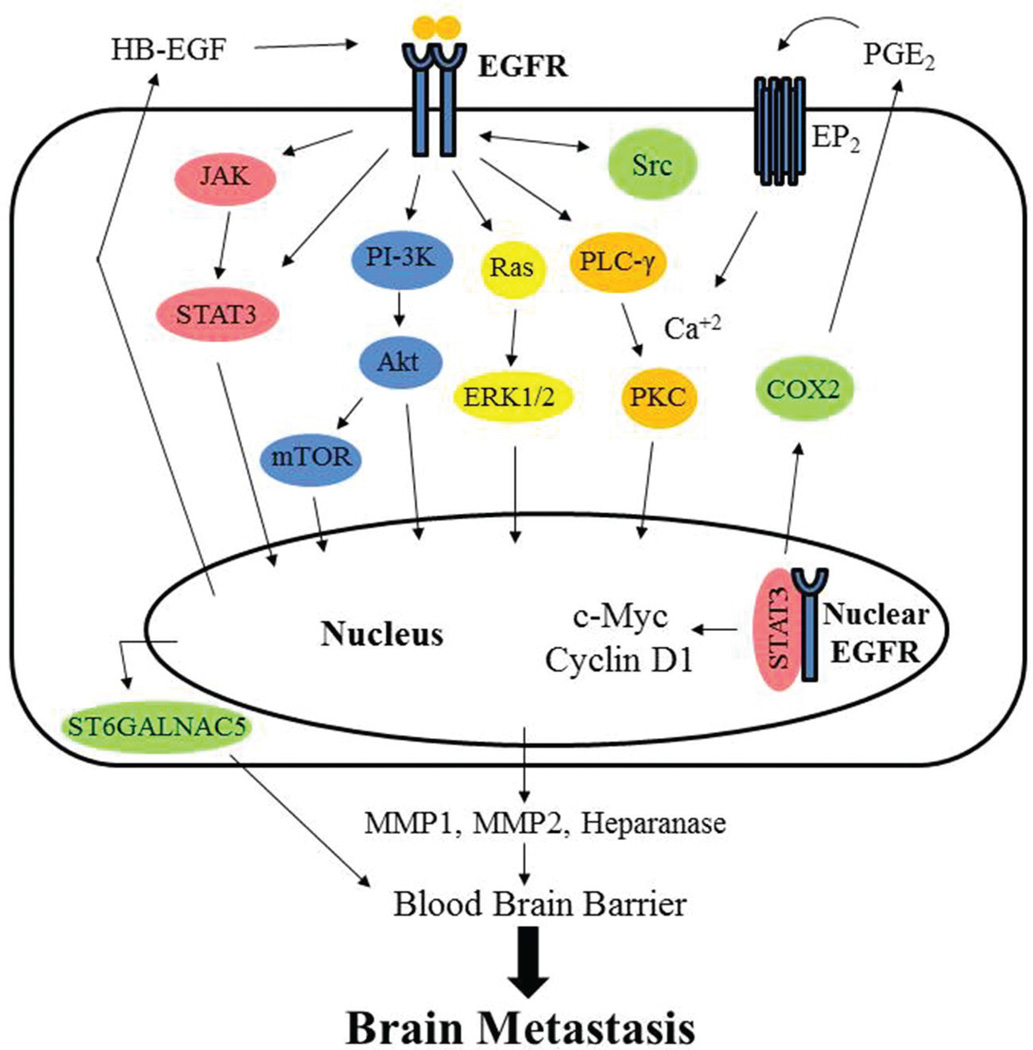
The discovery of epidermal growth factor (EGF) and its receptor EGFR was made more than 40 years ago (31–34). EGFR is a transmembrane receptor tyrosine kinase (RTK) which undergoes homodimerization or heterodimerization and trans-autophosphorylation after ligand binding. The most common ligands of EGFR include EGF, heparin-binding EGF-like growth factor (HB-EGF), and transforming growth factor-α (TGF-α) (32,34). As shown in Figure 1, once phosphorylated and activated, EGFR initiates a number of downstream signaling molecules leading to cell survival, cell growth, and tumor progression (35–39). The signaling transduction cascade begins with EGFR recruitment and binding of adaptor proteins via their SH2 domains allowing for the recruitment and activation of subsequent signaling molecules, including Ras, phosphatidylinositol-3 kinase (PI-3K), phospholipase C-γ (PLC-γ), and Janus-activated kinase (JAK) (40).
Figure 1.
Ligand binding to the receptor initiates hetero- or homodimerization, trans autophosphorylation and subsequent activation of several kinase cascades including RAS-RAF-MEK-MAPK, PI-3K-AKT-mTOR, PLC-γ-Ca2+/PKC, and JAK-STAT3, which promote expression of genes that drive brain metastasis. Additionally, nuclear EGFR, in concert with STAT3, has also been shown to directly upregulate genes that promote brain metastasis.
The traditional Ras mediated pathway can be activated by EGFR leading to the recruitment of the adaptor protein GRB2 and activation of the guanine exchange factor Sos and the small GTP-binding protein Ras. This leads to a kinase cascade that activates RAF, MEK, and ERK allowing for changes in protein activity and gene expression in the nucleus (39, 41,42). The canonical PI-3K pathway includes successive phosphorylation of PI-3K to phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) resulting in activation of the serine/threonine protein kinase B (PKB, Akt) by phosphoinositide-dependent kinase-1 (PDK-1) and mammalian Target of Rapamycin (mTOR). Dephosphorylation of PIP3 by phosphatase and tensin homolog (PTEN), a tumor suppressor, prevents activation of Akt (43). Akt leads to inhibition of apoptosis and cell survival. Cell survival regulation by Akt is mediated by increased glucose uptake through the upregulation of GLUT4 receptors (37,39,43–45). Additionally, activation of the PLC-γ pathway involves binding of PLC-γ to phosphorylated EGFR resulting in the hydrolytic cleavage of PIP2 to yield inositol 1,4,5-triphosphate (IP3) and 1,2-diacylglycerol (DAG). These second messengers are important for intracellular calcium release to activate Ca2+ dependent enzymes, interactions between the lipids and proteins, interactions between phosphosugars and proteins, and protein kinase C (PKC) activation (35,37,39).
Activated EGFR can also directly phosphorylate signal transducer and activator of transcription 3 (STAT3) transcription factor and oncoprotein, leading to their dimerization, translocation to the nucleus, and regulation of gene expression (46, 47). STAT3 belongs to the large STATs family of proteins comprised of seven members STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6. Inactive STATs are located in the cytoplasm and become activated upon phosphorylation by EGFR or by JAK2 at a tyrosine residue. This leads to homo- and hetero-dimerization of two STAT proteins which can then enter the nucleus, bind to specific elements in target gene promoters, and regulate expression of genes important for apoptosis, cell growth, tumor progression and differentiation (36,47–50). Additionally, EGFR can also translocate to the nucleus to regulate gene expression, phosphorylate nuclear proteins, and modulate functions of nuclear proteins (19,51–54). Increased nuclear EGFR is associated with poor clinical outcomes in multiple types of cancer (19,28,55,56). EGFR also undergoes mitochondrial translocation to antagonize therapy-induced apoptosis (57,58). In summary, EGFR overexpression, frequent activation, or activation mutations contribute to tumor progression and therapeutic resistance (42,59–61).
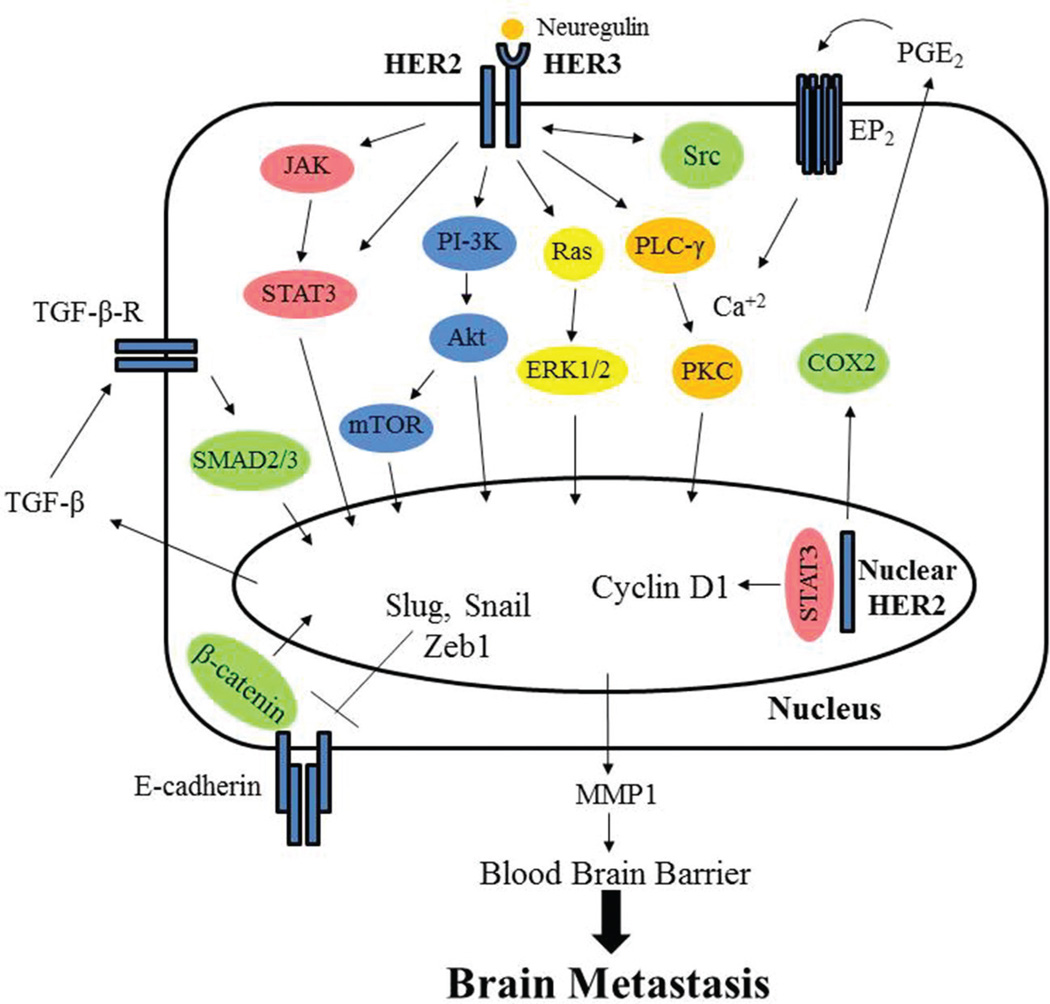
HER2 is a 185 kDa transmembrane protein encoded by the HER2/neu gene (62,63). HER2 is more closely related to EGFR compared to HER3 and HER4 (64).There are no known ligands that bind to HER2 thus far. However, HER2 has a functional tyrosine kinase domain that can be activated upon interactions with ligand-activated EGFR or HER3 (65–67). HER2 is also believed to form homodimers at high concentrations (68). Activated HER2 (hetero- and homo-dimer) proteins initiate phosphorylation events, similar to EGFR, and leads to activation of several signaling pathways, all of which are implicated in breast cancer progression (Figure 2). These pathways include STAT3, RAS-MAPK, and PI-3K which leads to inactivation of proteins triggering apoptosis and upregulation of genes for cell growth allowing the proliferation of tumor cells (50, 69–71). Recently, our laboratory found that HER2 directly interacts with the pro-apoptotic protein, p53 upregulated modulator of apoptosis (PUMA), and phosphorylates PUMA leading to its degradation and tumor cell survival (72). Overexpression of HER2 is found in 15%-35% of invasive breast cancers (5,26,27,29). In addition to breast cancer, HER2 has been found to be overexpressed in other types of cancer (66,73). In summary, HER2 plays a vital role in breast cancer progression (64,74–76).
Figure 2.
HER2 receptor forms a heterodimer with HER3 after ligand binding to HER3. This leads to a kinase cascade of signaling molecules targeted to the nucleus including PI-3K-Akt-mTOR, Ras-Raf-MEK-MAPK, and STAT3 which upregulate expression of genes that activate epithelial-to-mesenchymal transition (EMT) and brain metastasis. Additionally, nuclear HER2 can promote expression of genes driving cell proliferation and brain metastasis.
4. MODEL SYSTEMS FOR STUDYING BRAIN METASTASIS
4.1. In vitro models
While there are many in vitro models to study metastasis in general, there are also several approaches to study brain metastasis specifically, which primarily center on extravasation and colonization of breast cancer cells in the brain microenvironment. One useful model system is a model to mimic the blood brain barrier (BBB). This has been done using a transwell Boyden chamber with brain microvascular endothelial cells lining one side of the chamber and human astrocytes lining the other size of the membrane (77). Fluorescently-labelled breast cancer cells are then seeded in the upper chamber for a defined time period to allow breast cancer cells to pass through the model BBB. In addition to crossing the BBB, others have also studied the interaction between breast cancer cells and astrocytes by using co-culture of breast cancer cells and astrocytes or incubation of breast cancer cell conditioned medium with astrocytes (78). Lastly, organ selective metastatic cell lines, such as MDA-MB-231 brain-specific cells have been developed (79–82). These cells have been used in multiple in vitro systems to compare the brain-selective cells with the parental cells to elucidate mechanisms that drive cells toward the brain.
4.2. In vivo models
Similar to in vitro models, there have been several in vivo models established to study metastasis with some that are specific to brain metastasis. The primary method of determining brain metastasis is using intracardiac injection of breast cancer cells in mice that allows cells to seek out specific organs (most often the bone and brain) for metastatic colonization. This model has been used to confirm in vitro studies on brain metastasis in vivo by expressing or inhibiting proteins that affect the BBB and consequently have an effect on brain colonization by breast cancer cells (77). Brain-selective metastatic cell lines were engineered by intracardiac injection followed by extraction and growth in culture of brain-metastatic cells (79–82). The brain-selective metastatic cells are also frequently used in this model to test the efficacy of targeting therapies to prevent brain metastasis. Together, these models used to study brain metastasis can give clues to the mechanisms driving metastasis toward the brain. The in vivo models primarily lack the influence of the immune system because they are mostly xenograft studies using human breast cancer cells in immunocompromised mice. Further study using a syngeneic mouse model with an intact immune system may give more clues into the specificity of why certain cancer cells choose the brain for colonization. For example, inflammation can have a significant impact on vessel permeability and, therefore, it is likely that inflammation can affect the BBB to influence cancer cell colonization in the brain.
5. ROLE OF EGFR IN BREAST CANCER BRAIN METASTASIS
5.1. Correlative studies
Brain metastasis of breast cancer is observed in approximately 10–20% of breast cancer and the patients survive 16–30 months following diagnosis of metastatic disease (6–9,18). EGFR-expressing basal-like tumors (16,17) have a high likelihood to metastasize to the brain (83,84). IHC and fluorescence in situ hybridization (FISH) of BCBM patient samples showed that 56% expressed ER, 33% expressed PR, 39% expressed EGFR, 89% expressed Ki67, 33% were triple negative and 50% expressed CK5/6 indicating a higher prevalence of brain metastasis in the basal subtype (5,26). The expression of Ki67, a diagnostic marker for proliferation, was previously shown to be correlated to expression of nuclear EGFR (85). Interestingly, primary breast cancer patient samples with no known brain metastasis after 10 years showed only 7% EGFR expression, 29% CK5/6 expression, and 14% were triple-negative (5). Gaedcke et al. demonstrated that EGFR was increased by 40% in brain metastatic tumors compared to primary tumors which showed only 16% of EGFR expression. However, it is important to also note that 75–85% of primary tumors and brain metastatic tumors were shown to have constant EGFR (26,27). A study of BCBM overexpressing HER2 revealed a 5-fold increase in HER2 mRNA levels and a 9 fold increase in EGFR mRNA levels using quantitative-RT-PCR of epithelial cells (86). IHC of matched primary tumors and brain metastases show an average increase in HER3 expression from 29% to 59% as well as increased activation of downstream signaling molecules involved in the MAPK and PI-3K pathways (87). Selection of HER3 expressing tumors leading to brain metastasis may be due to increased levels of neuregulin 1, the ligand for HER3, in the brain compared to other sites in the body. Also, neuregulin 1 is released in the brain under hypoxic conditions which was demonstrated by increased HIF-1α expression in brain metastatic tumors (87).
Additionally, array-comparative genetic hybridization (CGH) that compares patterns of chromosomal aberrations between primary breast cancers and brain metastatic tumors shows that EGFR is overexpressed in 80% of brain metastases compared to only 13% of primary breast tumors (88). Microsatellite analysis for allelic imbalance shows that 64% of brain metastases and 19% primary breast tumors had an allelic imbalance at the PTEN locus in HER2 negative breast cancers (88). Primary breast cancers with metastasis to the brain have significant modifications on chromosome 7 and 10 q, specifically on the loci of EGFR and PTEN. These genetic modifications were mainly observed in TNBCs in both the primary and metastatic tumors indicating that this genetic makeup of the primary tumor may lead to an increased possibility of developing brain metastasis. However, genetic alterations of PI-3K did not show an increased risk of brain metastasis in this TNBC subtype (6). Loss of PTEN leads to the activation of AKT through the PI-3K pathway, downstream of EGFR (60). In many BCBM patients, PI-3K activity is upregulated regardless of the subtype of the primary tumor. Loss of PTEN is also associated with a shorter time for disease recurrence at distant sites including the brain (89). Increased levels of PTEN mutation, leading to loss of function, was observed in triple-negative BCBM and associated with poor overall survival in comparison to triple negative BCBM with functional PTEN. Levels of PTEN expression in matched primary breast tumors and their brain metastasis are also highly correlated and may be utilized as a potential prognostic marker to predict recurrence in the brain (89). Combined, these findings strongly suggest that EGFR plays an important role in brain metastasis.
5.2. Mechanistic studies
Zhang et al. conducted in vitro studies of preferential brain colonizing TNBC cells, transfected to express HER2 and already expressing EGFR, which has been shown to increase levels of heparanase (90). Heparanase degrades heparan sulfate allowing the release of growth factors from the cell surface and extracellular matrix leading to the progression of tumor growth and metastasis (91). Activation of EGFR/HER2 leads to heparanase translocation to the nucleolus where it can increase Topoisomerase 1 activity, an enzyme necessary for gene transcription, allowing increased cell growth (92). Further studies by Zhang et al. show that heparanase effects EGFR and HER2 phosphorylation allowing for an alternate pathway of EGFR activation. Inhibition of heparanase with Roneparstat increased sensitivity of BCBM cells to lapatinib, a dual EGFR/HER2 inhibitor (90).
In vitro, in vivo, and comparative gene expression analysis studies has identified several genes that mediate BCBM (79). Of these genes, heparin-binding EGF (HBEGF), a ligand for EGFR, was identified specifically in brain metastasis compared to the bone and lung. HBEGF was shown to increase migration and invasiveness of cancer cells. Another gene of importance identified was cyclooxygenase-2 (COX2), an enzyme upregulated by nuclear EGFR that synthesizes prostaglandins and plays an important role in permeabilizing the BBB in response to inflammation (77). Lastly, a third gene was identified specific to brain metastatic cancer cells, α2,6-sialyltransferase (ST6GA LNAC5) which allows cell-cell interactions and was shown to aid in penetrating the BBB through adhesion of cancer cells to brain endothelial cells. Knockdown of ST6GALNAC5 and inhibition of EGFR with cetuximab decreased brain metastasis (79). Together, HBEGF, COX2, and ST6GALNAC5 expression in primary breast cancer results in increased extravasation of cancer cells and metastasis to the brain (19, 60, 79).
Once cancer cells have intravasated into the circulation they have to develop mechanisms to survive in the circulatory system and colonize to distant organs. Circulating tumor cells (CTCs) isolated from BMBC patients were shown to overexpress specific proteins for brain metastasis versus lung metastasis such as EGFR, HER2, Heparanase, and Notch1. Expression of these proteins in breast cancer CTCs led to an invasive phenotype and ability to colonize the brain (93). Gene expression analysis and in vitro studies of TNBC and brain metastasis demonstrated that periplakin (PLL), a protein involved in maintaining epithelial cell barriers, and MAPK13 showed higher expression in primary TNBC cells compared to a brain metastatic cell line. Results of silencing PLL or MAPK13 led to increased cell proliferation and decreased cell motility (94). These results suggest that TNBCs may need an increased proliferation ability in order to colonize in the brain.
Matrix metalloproteinases (MMPs) degrade the extracellular matrix where many growth factors are sequestered leading to their release. Degradation of the extracellular matrix (ECM) is important for cancer cell invasion and MMPs have been shown to be upregulated in brain metastatic breast cancer (77,95,96). MMP1, specifically, has recently been shown to target metastatic breast cancer cells to the brain and that MMP1 was upregulated by COX2-mediated prostaglandins (77). We have shown that COX2 is a direct target of nuclear EGFR and EGFRvIII (53) in addition to upregulation of other genes supporting metastasis such as c-Myc (97), cyclin D1 (98), inducible nitric oxide synthase (iNOS) (51), aurora A (99), and breast cancer resistant protein (BCRP) (100). Additionally, in vitro and in vivo studies show that MMP2 expression is directly linked to ERK1/2 (96), a pathway frequently upregulated in EGFR-overexpressing tumors. Thus, MMPs seem to play an important role in targeting metastatic breast cancer cells to the brain.
The BBB is mainly comprised of brain endothelial cells and astrocytes and serves as a protective layer for the brain leading to decreased penetration of many chemotherapeutics. Kim et al. co-cultured mice astrocytes with human breast cancer cells resulting in increased expression of glutathione s-transferase alpha 5 (GSTA5; mediates drug resistance), BCL2L1 (anti-apoptotic Bcl-2 family protein), and TWIST1 (transcription factor that mediates EMT). Interestingly, these genes were only overexpressed in brain metastases but not in the lung metastases indicating the impact of the microenvironment on the cancer cells. Also, GSTA5, BCL2L1, and TWIST1 were shown to be regulated by AKT and MAPK which are downstream signal transducers of EGFR and HER2 (40,81,101). Although EGFR is upstream of many of these pathways, the exact link to EGFR has yet to be shown in regulating these pathways specific to brain metastasis of breast cancer.
EGFR is also significantly involved in glioblastoma (GBM), the most common malignant brain tumor in adults. EGFR is overexpressed or amplified in 40–60% of GBMs (102,103). Additionally, there is an oncogenic EGFR deletion variant termed EGFRvIII that is frequent in GBM (104,105). We, and others, have found that these alterations in EGFR expression or function significantly increase the aggressiveness of GBM (23,53, 57,59,85,106). As mentioned above, it has been shown that there is a 40% increase in EGFR expression in brain metastases compared to primary breast tumors (27). Additionally, non-small cell lung cancer (NSCLC) has a high frequency of activating EGFR gene alterations and a high incidence of metastasis to the brain (107–109). Considering these results it may indicate EGFR drives metastatic tumors toward the brain and/or the brain microenvironment and provides a selective pressure toward EGFR genetic alterations.
6. ROLE OF HER2 IN BREAST CANCER BRAIN METASTASIS
6.1. Correlative studies
Primary breast tumors with modifications in protein expression, gene copy number, or mutations of HER2 lead to increased brain metastasis. Overexpression of HER2 is found in 15%-35% of invasive breast cancers and signaling leads to reduced survival and increases the likelihood of developing metastasis (5,26,27,29). HER2 amplification was observed in 30–35% of metastatic breast cancers with 25–40% of these metastases going to the brain (5, 8,18,26,110). Patients with brain metastasis were generally younger than 50 years and their breast cancers were usually negative for hormone receptors. IHC showed that 37% of BCBM were positive for both ER and HER2 whereas 62% were positive for HER2 (110). The average overall survival for patients with HER2-positive BCBM was 20–30 months (8, 110). IHC of both EGFR and HER2 revealed that 15% of BCBM expressed both EGFR and HER2 (26). Interestingly, 81% of HER2 BCBM patients that underwent surgery along with systemic treatment resulted in a longer overall survival rate implicating promising results for this treatment strategy in HER2-positive brain metastatic patients (18).
Evidence shows that several primary breast cancers, originally negative for HER2, were HER2 positive after metastasizing to the brain (6).Quantitative analysis of matched primary and BCBM tumors for HER2 and truncated HER2 (p95HER2) showed that there was a 2.1 fold increase in HER2 and a 1.5 fold increase in p95HER2 expression from the primary breast tumor to the brain metastasis (111). Additionally, CTCs from BCBM patients were assessed for HER2 expression by antibody based analysis and RT-PCR. Comparison of the primary tumor and the CTCs showed a 30–50% increase of HER2 expression (112,113). Combined, these results indicate that overexpression of HER2 and p95HER2 may increase the ability of breast cancer CTCs to survive in the circulatory system and ultimately metastasize to the brain.
6.2. Mechanistic studies
Understanding the role of HER2 overexpression leading to brain metastasis is important for developing targeted therapies. Increased activation of Src, a tyrosine kinase protein, was shown to enhance cancer cell extravasation and penetration of the BBB in HER2 amplified and TNBC (114). Src activation plays a role in the heterodimerization of HER2 to HER3 allowing for activation of downstream signaling pathways and Src is also a downstream signal transducer of EGFR (114,115). It has also been previously shown that Src and EGFR co-localize on lipid rafts to more effectively relay signals for cell proliferation, growth, and survival (19). Additionally, results of combinatorial treatment with lapatinib and a Src inhibitor decreased progression to BCBM (114, 15).
Gupta et al. demonstrated a role of HER2 in epithelial to mesenchymal transition (EMT), invasion, and metastasis of breast cancer (116). Results showed that increased expression of HER2 allowed for the production of transforming growth factor β (TGFβ) and activation of the TGFβ/SMAD signaling pathway, potentially through the Ras signaling cascade, which ultimately upregulated transcription and activation of Slug, Snail, and ZEB-1 (101,116,117). These proteins are transcription factors that play an important role in EMT by decreasing E-cadherin and cytokeratin-18 expression and increasing N-cadherin expression leading to a mesenchymal phenotype (116,118). Inhibition of HER2 and TGFβ signaling with cucurbitacin (CuB), a triterpenoid steroid, resulted in decreased size of metastatic tumors in the brain, lungs, and liver. Interestingly, pretreatment of mice with CuB before intracardiac injection of highly aggressive stage IV breast cancer cells overexpressing HER2 significantly reduced brain metastasis (116). These findings further support the interaction of HER2 and TGFβ leading to EMT and metastasis (116).
Similar to EGFR, HER2 can directly regulate gene expression (119). HER2 signaling upregulates many pro-metastatic and proliferative pathways and it can also localize to the nucleus to upregulate genes (120–122). Most notably, nuclear HER2 can directly upregulate expression of COX-2 in addition to previously elucidated pathways in which membrane HER2 can upregulate COX-2 (122,123). As mentioned above, COX-2-mediated levels of PGE2 induce expression of MMP1 leading to the BBB being susceptible to brain metastasis (77). Thus, both EGFR and HER2 can directly and indirectly upregulate COX-2, which has been shown to induce brain-specific metastatic targeting.
A decrease in HER2 expression was observed in breast cancer cells overexpressing the transcription factor forkhead box P3 (FOXP3) as well as a decrease in other aggressive metastatic proteins including c-myc and the chemokine receptor CXCR4. It was also shown that expression of FOXP3 was significantly decreased in invasive breast cancer tissue compared to normal breast tissue suggesting that FOXP3 acts as a tumor suppressor and is downregulated in metastatic breast cancers (124).
HER2 may also aid in brain colonization and brain tumor growth. Western blot analysis of triple negative human breast cancer cells with a tendency to metastasize to the brain revealed an increased expression of phosphorylated EGFR, AKT, and ERK compared to MDA-MB-231 cells that tend to colonize the bone (86). Additionally, HER2 transfected cells had a significant 3-fold increase in size of the brain tumor compared to the control (86). While these findings highlight the role of HER2 in colonization and tumor progression in brain metastasis, these studies emphasize the fact that more work is needed in order to elucidate the mechanisms by which HER2-positive breast cancer cells target the brain and how they cross the BBB.
7. EFFECTS OF EGFR- AND HER2-TARGETED THERAPIES ON BREAST CANCER METASTASIS
7.1. Preclinical studies
Although there are several small molecule inhibitors and a monoclonal antibody approved by the FDA for BCBM, the BBB and mutated/alternate signaling pathways of receptor tyrosine kinases leads to increased drug resistance and decreased therapeutic effects. Therefore, it is important to develop novel therapies for the treatment of breast cancer brain metastasis. Gupta et al. demonstrated the therapeutic effects of phenethylisothiocyanate (PEITC), a natural product in cruciferous vegetables in in vitro and in vivo studies using triple negative breast cancer cells overexpressing HER2. Intracardiac injections of breast cancer cells in mice and oral treatment with 10 µmol of PEITC resulted in significantly decreased brain metastasis and reduced metastatic growth by 50–55% (125). Brain sections of treated mice showed decreased expression of HER2 by 90%, EGFR by 50% and vascular endothelia growth factor (VEGF) by 60%. Also, the average survival time increased by 20% in mice treated with PEITC (125). Another approach of targeting brain metastatic breast cancer is through the use of molecularly targeted agents. Fu et al., developed a brain metastatic breast cancer cell-binding peptide (BRBP1) linked to a cell penetrating peptide (TAT) and a pro-apoptotic peptide (KLA) (126). This BRBP1-TAT-KLA was shown to efficiently enter the brain and reduce tumor growth and metastasis of mice with minimal toxicity to the surrounding normal tissues which makes it a great candidate for clinical trials in order to treat metastatic breast cancers (126).
7.2. Clinical studies
There are currently 49 clinical trials targeted for treating breast cancer brain metastasis and nine trials have been completed, as outlined in Table 1. Of the completed trials, five studies used treatments to target EGFR/HER2 or downstream signaling molecules of these receptors. Interestingly, all nine of these studies included the HER2 overexpressing subtype of breast cancer brain metastasis which further enhances the importance of HER2 in breast cancer brain metastasis. Preliminary results of one completed trial using afatinib, a dual inhibitor of EGFR/HER2, indicate improved overall survival of HER2 positive patients with brain metastases (NCT01441596). Results of these early phase completed trials targeting EGFR/HER2 demonstrate tolerability and improved patient outcomes. Therefore, the following trials are ongoing to further asses the efficacy of targeting EGFR and HER2 to reduce brain metastasis or improve survival. There are currently 11 open clinical studies directly targeting BCBM overexpressing EGFR/HER2 or indirectly targeting signaling molecules tightly linked to the EGFR/HER2 pathways. The trial designs are summarized below.
Table 1.
Ouline of clinical trials testing EGFR- and HER2-targeted therapies in breast cancer
| Subtype | Phase | Treatment | Drug target | Results/endpoint | Identifier |
|---|---|---|---|---|---|
| HER2-positive | I | Lapatinib & WBRT then Lapatinib & trastuzumab |
Lapat.: EGFR/HER2 Trastuzumab: HER2 |
1250 mg MTD | NCT00470847 |
| HER2-positive | I | BKM120 & trastuzumab | BKM120: PI3K Trastuzumab: HER2 |
DLT, adverse events | NCT01132664 |
| HER2-positive | II | Trastuzumab & irinotecan | Trastuzumab: HER2 | ORR, stable disease rate | NCT00303992 |
| HER2-positive | I | ANG1005 | n/a | 650 mg/m2 MTD, well tolerated |
NCT00539383 |
| HER2-positive | I | Lapatinib & temozolomide | Lapat.: EGFR/HER2 | Well tolerated, no MTD reached |
NCT00614978 |
| HER2-positive | II | Lapatinib & capecitabine | Lapat.: EGFR/HER2 | 65% ORR | NCT00967031 |
| All subtypes and lung cancer | II | Lapatinib & WBRT | Lapat.: EGFR/HER2 | CNS RR | NCT01218529 |
| HER2-positive | II | Afatinib alone* Afatinib & vinorelbine Investigators choice |
Afat.: EGFR/HER2 | 30% 12 week benefit 34% 12 week benefit 42% 12 week benefit |
NCT01441596 |
| All subtypes, lung cancer, melanoma, and malignant glioma |
I/IIa | Trastuzumab & 2B3-101 2B3-101 alone |
Trastuzumab: HER2 | MTD, safety/tolerability | NCT01386580 |
Identifier: ClinicalTrials.gov; MTD: Maximum tolerated dose; DLT: Dose limiting toxicity; ORR: Objective response rate; RR: Response rate; Lapat.: Lapatinib; Afat.: Afatinib.
Preliminary results show that patients in the afatinib only treated group had an increased overall survival
7.2.1. NCT02154529 (Recruiting)
A combination therapy of KD019 (tesevatinib) and trastuzumab for breast cancer patients with brain metastasis overexpressing HER2 in a phase 1b/2a trial in order to determine the safety and efficacy of this combined treatment. Trastuzumab is a monoclonal antibody against the HER2 receptor which prevents activation and downstream signaling of the HER2 pathway. However, many breast cancers become resistant to trastuzumab through alternate signaling pathways. KD019 is a multi-kinase inhibitor that is orally bioavailable which targets Src, EGFR, HER2, and vascular endothelial growth factor receptor 2 (VEGFR2/KDR) (127). This open label trial, for an estimated 67 patients, contains five arms in phase 1b with 8 mg/kg of trastuzumab every 3 weeks and a range of orally administered KD019 from 150 mg – 400 mg daily. Phase 2a of this study contains two groups and both will be administered 8mg/kg of trastuzumab every 3 weeks in combination with the maximally tolerated dose (MTD) of KD109 daily. The patients in group 1a have HER2 positive breast cancer that progressed to the brain after radiation therapy. The patients in group 2a have HER2 positive breast cancer with no/minimal metastasis to the brain that does not need neurosurgery or radiation therapy immediately. The primary outcome measures are to determine the MTD and to investigate the safety and tolerability.
7.2.2. NCT01921335 (Recruiting)
An open label, randomized, phase I trial of ARRY-380 with trastuzumab for HER2-positive BCBM is being conducted to determine the safety and efficacy of this combined treatment. ARRY-380 is a reversible, small molecule inhibitor of the HER2 receptor that is orally bioavailable and targets the ATP binding region of HER2 (128). This study contains 2 arms and an estimate of 50 patients with HER2-positive breast cancer brain metastases. In both arms patients will be given 6 mg/kg of trastuzumab intravenously every 3 weeks. In the first arm patients will be administered orally 450 mg ARRY-380 twice-daily. In the second arm patients will be administered orally 750mg ARRY-380 once-daily. The primary outcome measure is to define the MTD of ARRY-380 with trastuzumab.
7.2.3. NCT01494662 (Recruiting)
An open label phase II study is being conducted in HER2-positive BCBM patients to determine the efficacy and bioavailability of HKI-272 (neratinib) in the central nervous system. HKI-272 is an irreversible, small molecule, tyrosine kinase inhibitor of HER1, HER2, and HER4 that is orally bioavailable (129). This trial has approximately 105 participants across 3 arms. Arm 1 contains patients with new or progressive brain metastasis who will be given 340 mg of HKI-272 orally once-daily. Participants in arm 2 must have brain metastases, be eligible for neurosurgery and will be administered 240 mg of HKI-272 orally once-daily and a biopsy will be done to determine the concentration of HKI-272 in the sample, in the cerebrospinal fluid, and plasma. Lastly, arm 3 is broken into two parts where participants in arm 3a have no prior treatment with lapatinib. These patients will be given 240 mg of HKI-272 orally once-daily in combination with 750 mg/m2 capecitabine, a 5-FU prodrug that inhibits DNA and RNA synthesis, twice-daily for 14 days with subsequent rest for 7 days. A comparative study will be done in arm 3b where participants that have had prior treatment with lapatinib will undergo a similar regimen. The primary outcome measure is to determine the objective response rate.
7.2.4. NCT02000882 (Recruiting)
A phase II, open label trial of BKM120 (Buparlisib) with capecitabine to treat TNBC brain metastases is being carried out in order to determine the safety and effectiveness of this combined treatment. BKM120 is an orally bioavailable kinase inhibitor of PI3K (130). This trial contains 1 arm and includes approximately 40 TNBC patients with brain metastases that have received at least 3 systemic treatments previously. Patients will be orally administered 100 mg BKM120 daily in combination with 1000 mg/m2 twice-daily for 14 days followed with rest for 7 days. The primary outcome measure of this study is to identify the clinical benefit rate.
7.2.5. NCT00263588
An open label phase II trial is being performed to determine the safety and effectiveness of lapatinib for breast cancer patients with HER2 positive brain metastases after systemic therapy with trastuzumab and cranial radiotherapy. In this single arm study of 242 participants, individuals who have previously undergone cranial radiotherapy, treatment with trastuzumab, and have progressing brain cancer are orally administered 750 mg lapatinib twice-daily. The primary outcome measure is to determine the response rate to lapatinib.
7.2.6. NCT00820222
A phase III, open label, randomized comparison of lapatinib and capecitabine versus trastuzumab and capecitabine is being conducted in grade IV HER2-positive breast cancer patients with previous treatments of anthracyclines or taxanes. This study includes 546 participants and 2 arms to investigate the effect of lapatinib on HER2-positive breast cancer metastasis to the brain. In arm 1 of the trial, patients are given 1250 mg lapatinib once-daily and 2000 mg/m2/day capecitabine for 14 consecutive days in a 21 day cycle. In the second arm of the study, patients are intravenously administered trastuzumab at 6 mg/kg every 3 weeks. Preliminary results indicate 3% of patients had metastasis to the brain as the first site of metastasis in the lapatinib treated group compared to 5% of patients in the Trastuzumab group. Despite a slightly higher clinical benefit in the lapatinib treatment group, the overall survival of these patients was not improved in the lapatinib treated group.
7.2.7. NCT01783756 (Recruiting)
An open label, phase 1b/2 study investigating the combinatorial treatment of lapatinib, everolimus, and capecitabine for breast cancer patients with HER2-positive brain metastases after treatment with trastuzumab is being done to evaluate the safety and effectiveness of the treatment. Everolimus is an inhibitor of mTOR, a downstream signaling molecule of PI3K. In this single-arm trail of approximately 47 participants, individuals are orally administered lapatinib once-daily, everolimus, once-daily, and capecitabine twice-daily for the first 14 days of a 21 day period over 17 cycles. The primary outcome measure of this study is the CNS objective response rate.
7.2.8. NCT01305941
Similarly, a phase II, single-arm, open label study is being done to investigate the safety and effectiveness of the combinatorial treatment of everolimus, trastuzumab, and vinorelbine on breast cancer patients with HER2-positive brain metastasis. Vinorelbine is chemotherapeutic agent that interacts with tubulin to inhibit mitosis (131). Approximately 35 patients will be orally administered 5 mg everolimus once-daily and intravenously 25 mg/m2 vinorelbine along with 2 mg/kg trastuzumab weekly for 3 cycles. The primary outcome measure is the intracranial object response rate.
7.2.9. NCT01622868
An open label, randomized, phase II trial is ongoing to compare whole-brain radiation therapy (WBRT), with or without lapatinib, on breast cancer patients with HER2-positive brain metastases. This study includes approximately 143 patients with at least one non-irradiated brain metastasis of HER2-positive breast cancer across two arms. Participants in arm 1 will receive WBRT once-daily for 5 days per week over 3 weeks for a total of 37.5 Gy (132). Individuals in arm II will receive similar WBRT as in arm I along with 1000 mg lapatinib for 21 days during WBRT and 21 days after WBRT. The primary outcome measure of this study is to examine the complete response rate in the brain.
Taken together these ongoing clinical studies targeted to EGFR and HER2, along with downstream signaling molecules, for breast cancer patients with brain metastases highlights the importance of these receptors in developing therapeutic regimens. Also, further understanding of alternate signaling pathways by EGFR/HER2 leading to drug resistance and metastasis to the brain is needed to further enhance the likelihood of finding an effective therapy.
8. CONCLUSIONS AND FUTURE DIRECTIONS
Metastasis of breast cancer to the brain remains a major health problem associated with increased resistance to current treatment options and exceptionally poor patient survival. To improve the overall survival rates of patients and decrease drug resistance, a more targeted therapy for breast cancer is warranted. Treatment options for patients can be enhanced with a better understanding of tumor biology through expression profiling analysis, mechanistic studies of receptors, and downstream signaling molecules specific to brain metastasis of breast cancer cells. Correlative studies show that EGFR expression is increased in the brain compared to the primary breast tumor, HER2 amplification/mutations led to increased metastases to the brain, HER2 negative primary tumors become positive for HER2 once colonized in the brain, and increased levels of PTEN mutations, a downstream signaling target of EGFR, leads to increased metastases specific to the brain. Several mechanistic studies, linked to EGFR and HER2, reveal specific targets for breast cancer cells to extravasate, permeabilize the BBB, and colonize in the brain. However, the complete mechanistic role of EGFR/HER2 and their downstream targets during each step of metastasis to the brain is not well understood. Several questions are left unanswered including: What allows the change in expression of EGFR/HER2 in the primary tumor versus the brain tumor? What role does EGFR/HER2 play in aiding angiogenesis, escaping anoikis, and evasion of the immune system? What are alternate signaling pathways of EGFR/HER2 that leads to drug resistance of brain tumors? In this review, the importance of EGFR and HER2 signaling leading to breast cancer metastasis to the brain has been identified and further studies are needed to improve treatment for this patient population.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Nola S, Sin S, Bonin F, Lidereau R, Driouch K. A methodological approach to unravel organ-specific breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2012;17(2):135–145. doi: 10.1007/s10911-012-9256-2. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;(10 Suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 5.Shao MM, Liu J, Vong JS, Niu Y, Germin B, Tang P, Chan AW, Lui PC, Law BK, Tan PH, Tse GM. A subset of breast cancer predisposes to brain metastasis. Med Mol Morphol. 2011;44(1):15–20. doi: 10.1007/s00795-010-0495-2. [DOI] [PubMed] [Google Scholar]
- 6.Hohensee I, Lamszus K, Riethdorf S, Meyer-Staeckling S, Glatzel M, Matschke J, Witzel I, Westphal M, Brandt B, Muller V, Pantel K, Wikman H. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183(1):83–95. doi: 10.1016/j.ajpath.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13(6):1644–1647. doi: 10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 8.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, Yood MU, Yardley DA. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 9.Klein A, Olendrowitz C, Schmutzler R, Hampl J, Schlag PM, Maass N, Arnold N, Wessel R, Ramser J, Meindl A, Scherneck S, Seitz S. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 2009;276(2):212–220. doi: 10.1016/j.canlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147(1):103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 11.Lin NU. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 2013;7:307. doi: 10.3332/ecancer.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene-Schloesser D, Payne V, Peiffer AM, Hsu FC, Riddle DR, Zhao W, Chan MD, Metheny-Barlow L, Robbins ME. The peroxisomal proliferator-activated receptor (PPAR) alpha agonist, fenofibrate, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat Res. 2014;181(1):33–44. doi: 10.1667/RR13202.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore ED, Kooshki M, Wheeler KT, Metheny-Barlow LJ, Robbins ME. Differential expression of Homer1a in the hippocampus and cortex likely plays a role in radiation-induced brain injury. Radiat Res. 2014;181(1):21–32. doi: 10.1667/RR13475.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 15.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Brot M, Soares FA, Stiepcich MM, Curcio VS, Gobbi H. (Basal-like breast cancers: clinicopathological features and outcome) Rev Assoc Med Bras. 2009;55(5):529–534. doi: 10.1590/s0104-42302009000500014. [DOI] [PubMed] [Google Scholar]
- 17.Abou-Bakr AA, Eldweny HI. p16 expression correlates with basal-like triple-negative breast carcinoma. Ecancermedicalscience. 2013;7:317. doi: 10.3332/ecancer.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuba S, Ishida M, Nakamura Y, Yamanouchi K, Minami S, Taguchi K, Eguchi S, Ohno S. Treatment and prognosis of breast cancer patients with brain metastases according to intrinsic subtype. Jpn J Clin Oncol. 2014;44(11):1025–1031. doi: 10.1093/jjco/hyu126. [DOI] [PubMed] [Google Scholar]
- 19.Han W, Lo WH. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318(2):124–134. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;4758:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 21.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proceedings of the National Academy of Sciences. 1989;86(23):9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993;90(5):1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo HW. Nuclear mode of the EGFR signaling network: biology, prognostic value, and therapeutic implications. Discov Med. 2010;10(50):44–51. [PMC free article] [PubMed] [Google Scholar]
- 24.Han W, Lo HW. Landscape of EGFR signaling network in human cancers: Biology and therapeutic response in relation to receptor subcellular locations. Cancer Letters. 2012;318(2):124–134. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter R, Lo H-W. Regulation of Apoptosis by HER2 in Breast Cancer. Journal of Carcinogenesis & Mutagenesis. 2013;S7:300. doi: 10.4172/2157-2518.S7-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grupka NL, Lear-Kaul KC, Kleinschmidt-DeMasters BK, Singh M. Epidermal growth factor receptor status in breast cancer metastases to the central nervous system. Comparison with HER-2/neu status. Arch Pathol Lab Med. 2004;128(9):974–979. doi: 10.5858/2004-128-974-EGFRSI. [DOI] [PubMed] [Google Scholar]
- 27.Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH. Predominance of the basal type and HER-2//neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20(8):864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 28.Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF, Hung MC. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005;65(1):338–348. [PubMed] [Google Scholar]
- 29.Roy V, Perez EA. Beyond trastuzumab: small molecule tyrosine kinase inhibitors in HER-2-positive breast cancer. The Oncologist. 2009;14(11):1061–1069. doi: 10.1634/theoncologist.2009-0142. [DOI] [PubMed] [Google Scholar]
- 30.Lim E, Lin NU. Updates on the management of breast cancer brain metastases. Oncology (Williston Park) 2014;28(7):572–578. [PubMed] [Google Scholar]
- 31.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 32.Cohen S, Carpenter G, King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980;255(10):4834–4842. [PubMed] [Google Scholar]
- 33.Cohen S, Carpenter G, King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Prog Clin Biol Res. 1981;66 Pt A:557–567. [PubMed] [Google Scholar]
- 34.Cohen S, Ushiro H, Stoscheck C, Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982;257(3):1523–1531. [PubMed] [Google Scholar]
- 35.Anderson D, Koch CA, Grey L, Ellis C, Moran MF, Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- 36.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proceedings of the National Academy of Sciences. 2001;98(13):7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 38.Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factorreceptorkinase. Proceedings of the National Academy of Sciences. 1996;93(24):13704–13708. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarden Y, Shilo BZ. SnapShot: EGFR signaling pathway. Cell. 2007;131(5):1018. doi: 10.1016/j.cell.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Hui-Wen Lo RLC. Regulation of Apoptosis by HER2 in Breast Cancer. Journal of Carcinogenesis & Mutagenesis. 2013 doi: 10.4172/2157-2518.S7-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 42.Lo HW. Targeting Ras-RAF-ERK and its interactive pathways as a novel therapy for malignant gliomas. Curr Cancer Drug Targets. 2010;10(8):840–848. doi: 10.2174/156800910793357970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrocelli T, Slingerland JM. PTEN deficiency: a role in mammary carcinogenesis. Breast Cancer Res. 2001;3(6):356–360. doi: 10.1186/bcr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Current Opinion in Cell Biology. 1998;10(2):262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung M-C. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3(11):973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 46.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 47.Darnell JE. STATs and Gene Regulation. Science. 1997;277(5332):1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 48.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19(56):6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 49.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20(20):2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 50.Carpenter RL, Lo H-W. STAT3 Target Genes Relevant to Human Cancers. Cancers. 2014;6(2):897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7(6):575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Han W, Carpenter RL, Cao X, Lo HW. STAT1 gene expression is enhanced by nuclear EGFR and HER2 via cooperation with STAT3. Mol Carcinog. 2012 Jun 12; doi: 10.1002/mc.21936. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8(2):232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, McIntush EW, Li LY, Hawke DH, Kobayashi R, Hung MC. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8(12):1359–1368. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 55.Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, Camp R, Rimm D, Burtness BA. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11(16):5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 56.Xia W, Wei Y, Du Y, Liu J, Chang B, Yu YL, Huo LF, Miller S, Hung MC. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog. 2009;48:610–617. doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu H, Cao X, Ali-Osman F, Keir S, Lo HW. EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial translocalization of PUMA and PUMA-mediated apoptosis independent of EGFR kinase activity. Cancer Lett. 2010;294(1):101–110. doi: 10.1016/j.canlet.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao X, Zhu H, Ali-Osman F, Lo HW. EGFR and EGFRvIII undergo stress- and EGFR kinase inhibitor-induced mitochondrial translocalization: a potential mechanism of EGFR-driven antagonism of apoptosis. Mol Cancer. 2011;10:26. doi: 10.1186/1476-4598-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo HW. EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance. Curr Mol Pharmacol. 2010;3(1):37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat. 2006;95(3):211–218. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 61.Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC, Elledge RM. EGFR Expression in Breast Cancer Association with biologic phenotype and clinical outcomes. Cancer. 2010;116(5):1234–1242. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schechter AL, Hung MC, Vaidyanathan L, Weinberg RA, Yang-Feng TL, Francke U, Ullrich A, Coussens L. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science. 1985;229(4717):976–978. doi: 10.1126/science.2992090. [DOI] [PubMed] [Google Scholar]
- 63.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 64.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/neu Oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 65.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Reviews. Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 67.Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. The EMBO Journal. 1995;14(17):4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 69.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 70.Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I. Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signaling pathways. BMC Cancer. 2008;8(1) doi: 10.1186/1471-2407-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH, Schillaci R, Elizalde PV. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol. 2010;30(23):5456–5472. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carpenter RL, Han W, Paw I, Lo HW. HER2 phosphorylates and destabilizes pro-apoptotic PUMA, leading to antagonized apoptosis in cancer cells. PLoS One. 2013;8(11):e78836. doi: 10.1371/journal.pone.0078836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf-Yadlin A, Kumar N, Zhang Y, Hautaniemi S, Zaman M, Kim H-D, Grantcharova V, Lauffenburger DA, White FM. Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Molecular Systems Biology. 2006;2:54–54. doi: 10.1038/msb4100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the HER-2/neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 75.Lemmon MA. The EGF receptor family as therapeutic targets in breast cancer. Breast Disease. 2003;18:33–43. doi: 10.3233/bd-2003-18105. [DOI] [PubMed] [Google Scholar]
- 76.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature Reviews Molecular Cell Biology. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 77.Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, Chan MD, Zhou X, Qasem SA, Pochampally R, Mo YY, Watabe K. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–9854. doi: 10.1074/jbc.M114.602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Reactive astrocytes promote the metastatic growth of breast cancer stemlike cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5(3):384–396. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, Van de Vijver M, Gerald W, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 81.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, Fidler IJ. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13(3):286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massague J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115(1):44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, Abbott RM, Hoog J, Dooling DJ, Koboldt DC, Schmidt H, Kalicki J, Zhang Q, Chen L, Lin L, Wendl MC, McMichael JF, Magrini VJ, Cook L, McGrath SD, Vickery TL, Appelbaum E, Deschryver K, Davies S, Guintoli T, Lin L, Crowder R, Tao Y, Snider JE, Smith SM, Dukes AF, Sanderson GE, Pohl CS, Delehaunty KD, Fronick CC, Pape KA, Reed JS, Robinson JS, Hodges JS, Schierding W, Dees ND, Shen D, Locke DP, Wiechert ME, Eldred JM, Peck JB, Oberkfell BJ, Lolofie JT, Du F, Hawkins AE, O’Laughlin MD, Bernard KE, Cunningham M, Elliott G, Mason MD, Thompson DM, Jr, Ivanovich JL, Goodfellow PJ, Perou CM, Weinstock GM, Aft R, Watson M, Ley TJ, Wilson RK, Mardis ER. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464(7291):999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 85.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. British Journal of Cancer. 2006;94(2):184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D, Vortmeyer AO, Steinberg SM, Aldape K, Steeg PS. Her-2 Overexpression Increases the Metastatic Outgrowth of Breast Cancer Cells in the Brain. Cancer Research. 2007;67(9):4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 87.Da Silva L, Simpson PT, Smart CE, Cocciardi S, Waddell N, Lane A, Morrison BJ, Vargas AC, Healey S, Beesley J, Pakkiri P, Parry S, Kurniawan N, Reid L, Keith P, Faria P, Pereira E, Skalova A, Bilous M, Balleine RL, Do H, Dobrovic A, Fox S, Franco M, Reynolds B, Khanna KK, Cummings M, Chenevix-Trench G, Lakhani SR. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Research: BCR. 2010;12(4):R46–R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wikman H, Lamszus K, Detels N, Uslar L, Wrage M, Benner C, Hohensee I, Ylstra B, Eylmann K, Zapatka M, Sauter G, Kemming D, Glatzel M, Müller V, Westphal M, PanTel K. Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Research: BCR. 2012;14(2):R49–R49. doi: 10.1186/bcr3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adamo B, Deal A, Burrows E, Geradts J, Hamilton E, Blackwell K, Livasy C, Fritchie K, Prat A, Harrell J, Ewend M, Carey L, Miller C, Anders C. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Research. 2011;13(6):R125. doi: 10.1186/bcr3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Ngo JA, Wetzel MD, Marchetti D. Heparanase mediates a novel mechanism in lapatinib-resistant brain metastatic breast cancer()() Neoplasia (New York, N.Y.) 2015;17(1):101–113. doi: 10.1016/j.neo.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nadir Y, Brenner B. Heparanase multiple effects in cancer. Thromb Res. 2014;(133 Suppl 2):S90–S94. doi: 10.1016/S0049-3848(14)50015-1. [DOI] [PubMed] [Google Scholar]
- 92.Zhang L, Sullivan P, Suyama J, Marchetti D. Epidermal growth factor-induced heparanase nucleolar localization augments DNA topoisomerase I activity in brain metastatic breast cancer. Mol Cancer Res. 2010;8(2):278–290. doi: 10.1158/1541-7786.MCR-09-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang L, Ridgway LD, Wetzel MA, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Science translational medicine. 2013;5(180) doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi YK, Woo SM, Cho SG, Moon HE, Yun YJ, Kim JW, Noh DY, Jang BH, Shin YC, Kim JH, Shin HD, Paek SH, Ko SG. Brain-metastatic triple-negative breast cancer cells regain growth ability by altering gene expression patterns. Cancer Genomics Proteomics. 2013;10(6):265–275. [PubMed] [Google Scholar]
- 95.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20(3):161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mendes O, Kim HT, Lungu G, Stoica G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 2007;24(5):341–351. doi: 10.1007/s10585-007-9071-0. [DOI] [PubMed] [Google Scholar]
- 97.Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q, Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS One. 2011;6(5):e19605. doi: 10.1371/journal.pone.0019605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 99.Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, Chuang YH, Lai CH, Chang WC. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008;36(13):4337–4351. doi: 10.1093/nar/gkn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, Chiu PC, Huang WP, Wang YN, Chen CH, Chang WC, Chang WC, Chen AJ, Tsai CH, Hung MC. Nuclear translocation of epidermal growth factor receptor by Akt-dependent phosphorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem. 2011;286(23):20558–20568. doi: 10.1074/jbc.M111.240796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 103.Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8(12):1197–1206. doi: 10.1023/a:1008209720526. [DOI] [PubMed] [Google Scholar]
- 104.Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, Friedman HS, Kwatra MM, Bigner SH, Bigner DD. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87(11):4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89(7):2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14(19):6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hubbs JL, Boyd JA, Hollis D, Chino JP, Saynak M, Kelsey CR. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer. 2010;116(21):5038–5046. doi: 10.1002/cncr.25254. [DOI] [PubMed] [Google Scholar]
- 108.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 109.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 110.Hayashi N, Niikura N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Yamauchi H, Saji S, Iwata H. Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: a multicenter retrospective analysis. Breast Cancer Res Treat. 2015;149(1):277–284. doi: 10.1007/s10549-014-3237-7. [DOI] [PubMed] [Google Scholar]
- 111.Duchnowska R, Sperinde J, Chenna A, Huang W, Weidler JM, Winslow J, Haddad M, Paquet A, Lie Y, Trojanowski T, Mandat T, Kowalczyk A, Czartoryska-Arlukowicz B, Radecka B, Jarosz B, Staszkiewicz R, Kalinka-Warzocha E, Chudzik M, Biernat W, Jassem J. Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, Lattrich C, Lohberg CR, Solomayer E, Rack B, Riethdorf S, Klein C, Schindlbeck C, Brocker K, Kasimir-Bauer S, Wallwiener D, PanTel K. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124(2):403–412. doi: 10.1007/s10549-010-1163-x. [DOI] [PubMed] [Google Scholar]
- 113.Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I, Hilfrich J, Holms F, Tesch H, Eidtmann H, Untch M, von Minckwitz G, PanTel K. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–2645. doi: 10.1158/1078-0432.CCR-09-2042. [DOI] [PubMed] [Google Scholar]
- 114.Zhang S, Huang WC, Zhang L, Zhang C, Lowery FJ, Ding Z, Guo H, Wang H, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 2013;73(18):5764–5774. doi: 10.1158/0008-5472.CAN-12-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, Steeg PS. Effect of Lapatinib on the Outgrowth of Metastatic Breast Cancer Cells to the Brain. JNCI Journal of the National Cancer Institute. 2008;100(15):1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta P, Srivastava SK. HER2 mediated de novo production of TGFbeta leads to SNAIL driven epithelial-to-mesenchymal transition and metastasis of breast cancer. Mol Oncol. 2014;8(8):1532–1547. doi: 10.1016/j.molonc.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grusch M, Petz M, Metzner T, Öztürk D, Schneller D, Mikulits W. The Crosstalk of RAS with the TGF-β Family During Carcinoma Progression and its Implications for Targeted Cancer Therapy. Current cancer drug targets. 2010;10(8):849–857. doi: 10.2174/156800910793357943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chow A, Arteaga CL, Wang SE. When tumor suppressor TGFbeta meets the HER2 (ERBB2) oncogene. J Mammary Gland Biol Neoplasia. 2011;16(2):81–88. doi: 10.1007/s10911-011-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15(21):6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25(24):11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lenferink AE, Busse D, Flanagan WM, Yakes FM, Arteaga CL. ErbB2/neu kinase modulates cellular p27(Kip1) and cyclin D1 through multiple signaling pathways. Cancer Res. 2001;61(17):6583–6591. [PubMed] [Google Scholar]
- 122.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6(3):251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 123.Vadlamudi R, Mandal M, Adam L, Steinbach G, Mendelsohn J, Kumar R. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999;18(2):305–314. doi: 10.1038/sj.onc.1202307. [DOI] [PubMed] [Google Scholar]
- 124.Douglass S, Meeson AP, Overbeck-Zubrzycka D, Brain JG, Bennett MR, Lamb CA, Lennard TW, Browell D, Ali S, Kirby JA. Breast cancer metastasis: demonstration that FOXP3 regulates CXCR4 expression and the response to CXCL12. J Pathol. 2014;234(1):74–85. doi: 10.1002/path.4381. [DOI] [PubMed] [Google Scholar]
- 125.Gupta P, Adkins C, Lockman P, Srivastava SK. Metastasis of Breast Tumor Cells to Brain Is Suppressed by Phenethyl Isothiocyanate in a Novel In vivo Metastasis Model. PLoS ONE. 2013;8(6):e67278. doi: 10.1371/journal.pone.0067278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu B, Long W, Zhang Y, Zhang A, Miao F, Shen Y, Pan N, Gan G, Nie F, He Y, Zhang J, Teng G. Enhanced antitumor effects of the BRBP1 compound peptide BRBP1-TAT-KLA on human brain metastatic breast cancer. Sci Rep. 2015;5:8029. doi: 10.1038/srep08029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mo R, Rao N, Tonra J, Waksal S, Poyurovsky M. Abstract 1827: Inhibition of Src, HER2, and EGFR by the multi-kinase inhibitor KD019 overcomes trastuzumab resistance in breast cancer. Cancer Research. 2014;74(19 Supplement):1827. [Google Scholar]
- 128.Borges VF, Chia SKL, D’Aloisio S, Fernetich G, Sajan B, McSpadden T, Chavira R, Barrett E, Guthrie K, Garrus J, Baetz T, Moulder S. Abstract A050: ARRY-380, an oral HER2 inhibitor: Final phase 1 results and conclusions. Molecular Cancer Research. 2013;11(10 Supplement):A050. [Google Scholar]
- 129.Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, Cortés J, Kiger C, Germa C, Wang K, Martin M, Baselga J, Kim S-B. Safety and Efficacy of Neratinib in Combination With Capecitabine in Patients With Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. Journal of Clinical Oncology. 2014;32(32):3626–3633. doi: 10.1200/JCO.2014.56.3809. [DOI] [PubMed] [Google Scholar]
- 130.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, Goldbrunner M, Baselga J. Phase I, Dose-Escalation Study of BKM120, an Oral Pan-Class I PI3K Inhibitor, in Patients With Advanced Solid Tumors. Journal of Clinical Oncology. 2012;30(3):282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 131.Janni W, Sarosiek T, Karaszewska B, Pikiel J, Staroslawska E, Potemski P, Salat C, Brain E, Caglevic C, Briggs K, Desilvio M, Marini L, Papadimitriou C. A phase II, randomized, multicenter study evaluating the combination of lapatinib and vinorelbine in women with ErbB2 overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2014;143(3):493–505. doi: 10.1007/s10549-013-2828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peereboom DM, Winter KA, Kim IA, De Los Santos JF, Sperduto PW, White JR, Mehta MP. RTOG 1119: Phase II randomized study of whole brain radiotherapy with concurrent lapatinib in patients with brain metastasis from HER2-positive breast cancer--A collaborative study of RTOG and KROG ( NCT01622868) ASCO Meeting Abstracts. 2014;32(15_suppl):TPS664. [Google Scholar]