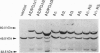
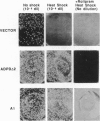
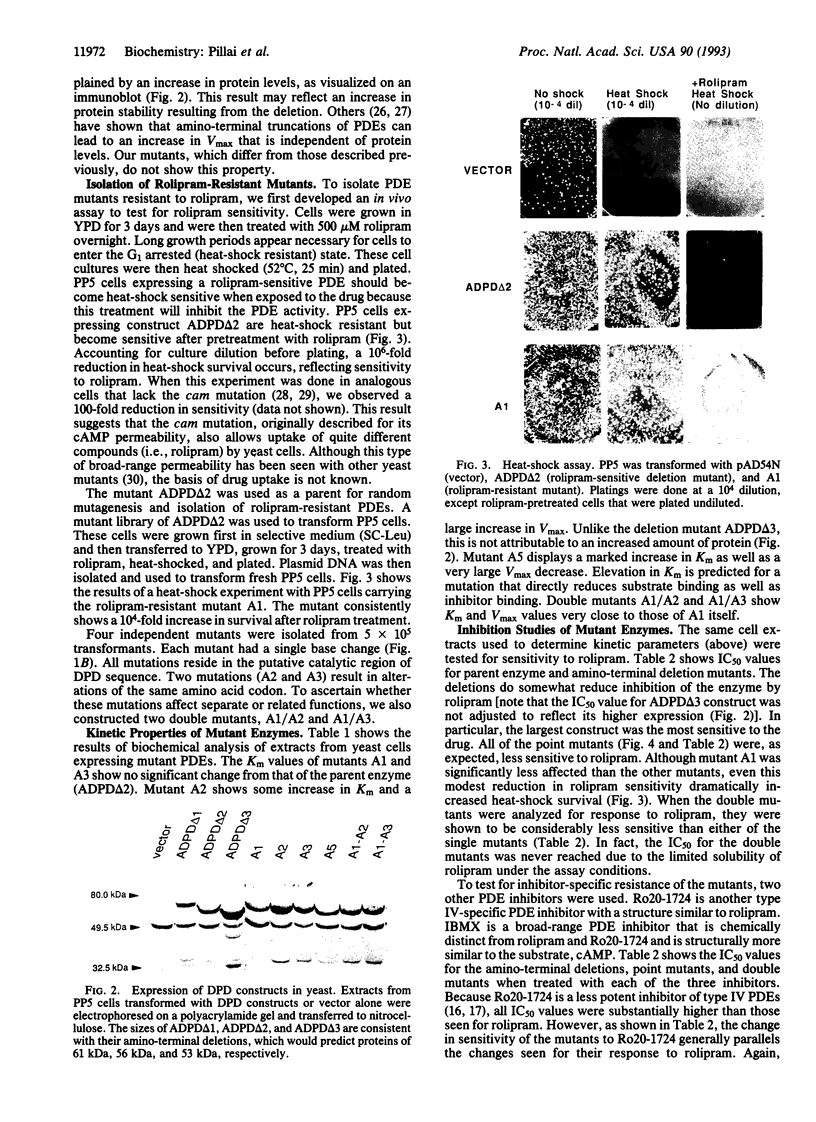
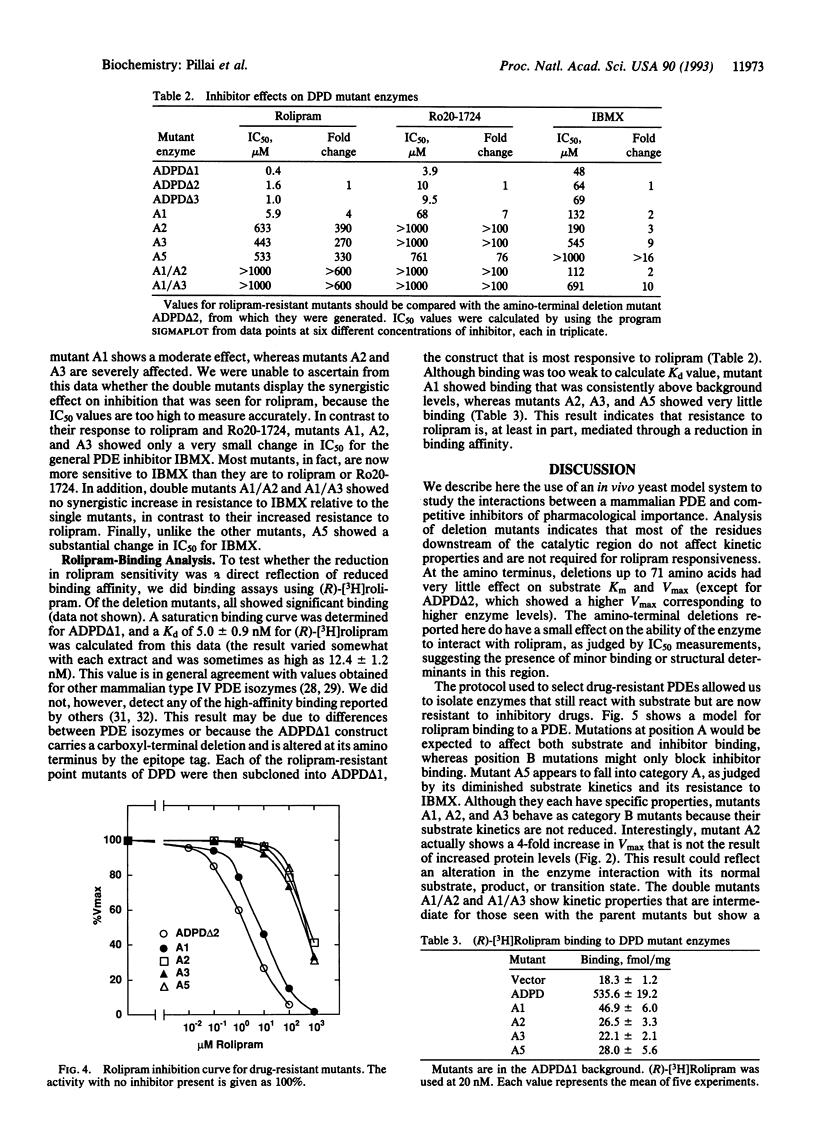
Abstract
Saccharomyces cerevisiae strain PP5 has a phosphodiesterase (PDE) deficiency that results in heat-shock sensitivity due to the intracellular accumulation of cAMP. This strain also carries the cam mutation, which confers permeability to cAMP and, as shown here, to other compounds. Expression of rat type IV PDE in these cells caused them to revert to heat-shock resistance. Treatment of the transformed PP5 cells with rolipram, an antidepressant in humans and a potent inhibitor of type IV PDEs, reinstated sensitivity to heat shock. The biochemical properties of deletion mutants of this PDE were determined, and an active enzyme of minimum length was created. Reversion to heat-shock resistance was then used to select for PDE mutants refractory to the inhibitory effects of rolipram. Four mutants (A1, A2, A3, and A5) were isolated. Each carries a single point mutation; two have mutations in the same codon. Each mutant showed distinct properties, based on analysis of their substrate kinetics and IC50 values for a variety of inhibitors. Mutant A5 had a reduced activity for substrate, mutants A1 and A3 showed no change in substrate kinetics, and mutant A2 displayed an increase in activity. For most mutants, the drug resistance was confined to the class of drug used in the selection. This study shows that it is possible to recreate in yeast cells the susceptibility of mammalian enzymes to pharmacological agents. Our study also demonstrates that such systems can be used to select rare mutants useful in the analysis of drug-protein interactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Beavo J. A. Regulation and function of cyclic nucleotides. Curr Opin Cell Biol. 1992 Apr;4(2):233–240. doi: 10.1016/0955-0674(92)90038-e. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Kadlecek A., Sherbert C. H., Seger D., Sonnenburg W. K., Charbonneau H., Novack J. P., Beavo J. A. Molecular cloning of cDNA encoding a "63"-kDa calmodulin-stimulated phosphodiesterase from bovine brain. J Biol Chem. 1992 Sep 15;267(26):18676–18682. [PubMed] [Google Scholar]
- Casperson G. F., Walker N., Bourne H. R. Isolation of the gene encoding adenylate cyclase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5060–5063. doi: 10.1073/pnas.82.15.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Beier N., Walsh K. A., Beavo J. A. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J., Birchmeier C., Michaeli T., O'Neill K., Riggs M., Wigler M. Isolation and characterization of a mammalian gene encoding a high-affinity cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 May;86(10):3599–3603. doi: 10.1073/pnas.86.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J., Nicolette C., Birchmeier C., Rodgers L., Riggs M., Wigler M. Expression of three mammalian cDNAs that interfere with RAS function in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2913–2917. doi: 10.1073/pnas.88.7.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Takayasu H., Eberwine M., Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Hall I. P. Isoenzyme selective phosphodiesterase inhibitors: potential clinical uses. Br J Clin Pharmacol. 1993 Jan;35(1):1–7. doi: 10.1111/j.1365-2125.1993.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Donald K. A., Griffiths D. E., Donald G. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991 Oct 25;19(20):5791–5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. L., Swinnen J. V., Conti M. Characterization of the structure of a low Km, rolipram-sensitive cAMP phosphodiesterase. Mapping of the catalytic domain. J Biol Chem. 1992 Sep 15;267(26):18929–18939. [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Stith-Coleman I. E., Vaughan M. Proteolytic activation of calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biol Chem. 1985 Jul 25;260(15):9009–9015. [PubMed] [Google Scholar]
- Kramer B., Kramer W., Williamson M. S., Fogel S. Heteroduplex DNA correction in Saccharomyces cerevisiae is mismatch specific and requires functional PMS genes. Mol Cell Biol. 1989 Oct;9(10):4432–4440. doi: 10.1128/mcb.9.10.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S. F., Reardon J. E., Furfine E. S., Kunkel T. A., Bebenek K., Eckert K. A., Kemp S. D., Larder B. A. Biochemical studies on the reverse transcriptase and RNase H activities from human immunodeficiency virus strains resistant to 3'-azido-3'-deoxythymidine. J Biol Chem. 1992 Aug 5;267(22):15789–15794. [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Toh-E A., Ishikawa T., Oshima Y. Cyclic AMP may not be involved in catabolite repression in Saccharomyes cerevisiae: evidence from mutants capable of utilizing it as an adenine source. J Bacteriol. 1982 Apr;150(1):277–285. doi: 10.1128/jb.150.1.277-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. M., Cieslinski L. B., Burman M., Torphy T. J., Livi G. P. A low-Km, rolipram-sensitive, cAMP-specific phosphodiesterase from human brain. Cloning and expression of cDNA, biochemical characterization of recombinant protein, and tissue distribution of mRNA. J Biol Chem. 1993 Mar 25;268(9):6470–6476. [PubMed] [Google Scholar]
- Meacci E., Taira M., Moos M., Jr, Smith C. J., Movsesian M. A., Degerman E., Belfrage P., Manganiello V. Molecular cloning and expression of human myocardial cGMP-inhibited cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3721–3725. doi: 10.1073/pnas.89.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli T., Bloom T. J., Martins T., Loughney K., Ferguson K., Riggs M., Rodgers L., Beavo J. A., Wigler M. Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP phosphodiesterase-deficient Saccharomyces cerevisiae. J Biol Chem. 1993 Jun 15;268(17):12925–12932. [PubMed] [Google Scholar]
- Nikawa J., Sass P., Wigler M. Cloning and characterization of the low-affinity cyclic AMP phosphodiesterase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss J., Wang J. C. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Baichwal V. R. Transcription factor based therapeutics: drugs of the future? Trends Biotechnol. 1993 Jan;11(1):11–18. doi: 10.1016/0167-7799(93)90069-L. [DOI] [PubMed] [Google Scholar]
- Polli J. W., Kincaid R. L. Molecular cloning of DNA encoding a calmodulin-dependent phosphodiesterase enriched in striatum. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11079–11083. doi: 10.1073/pnas.89.22.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass P., Field J., Nikawa J., Toda T., Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. H., Schmiechen R., Brezinski M., Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol. 1986 Aug 7;127(1-2):105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- Sonnenburg W. K., Mullaney P. J., Beavo J. A. Molecular cloning of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase cDNA. Identification and distribution of isozyme variants. J Biol Chem. 1991 Sep 15;266(26):17655–17661. [PubMed] [Google Scholar]
- Sonnenburg W. K., Seger D., Beavo J. A. Molecular cloning of a cDNA encoding the "61-kDa" calmodulin-stimulated cyclic nucleotide phosphodiesterase. Tissue-specific expression of structurally related isoforms. J Biol Chem. 1993 Jan 5;268(1):645–652. [PubMed] [Google Scholar]
- St Clair M. H., Martin J. L., Tudor-Williams G., Bach M. C., Vavro C. L., King D. M., Kellam P., Kemp S. D., Larder B. A. Resistance to ddI and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science. 1991 Sep 27;253(5027):1557–1559. doi: 10.1126/science.1716788. [DOI] [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. The mRNA encoding a high-affinity cAMP phosphodiesterase is regulated by hormones and cAMP. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8197–8201. doi: 10.1073/pnas.86.21.8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J. Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. Pharmacol Ther. 1991;51(1):13–33. doi: 10.1016/0163-7258(91)90039-o. [DOI] [PubMed] [Google Scholar]
- Torphy T. J., Stadel J. M., Burman M., Cieslinski L. B., McLaughlin M. M., White J. R., Livi G. P. Coexpression of human cAMP-specific phosphodiesterase activity and high affinity rolipram binding in yeast. J Biol Chem. 1992 Jan 25;267(3):1798–1804. [PubMed] [Google Scholar]
- Wang M. M., Reed R. R. Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature. 1993 Jul 8;364(6433):121–126. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]