Abstract
The impact of sugar consumption on health continues to be a controversial topic. The objective of this review is to discuss the evidence and lack of evidence that allows the controversy to continue, and why resolution of the controversy is important.
There are plausible mechanisms and research evidence that support the suggestion that consumption of excess sugar promotes the development of cardiovascular disease (CVD) and type 2 diabetes (T2DM) both directly and indirectly. The direct pathway involves the unregulated hepatic uptake and metabolism of fructose, which leads to liver lipid accumulation, dyslipidemia, decreased insulin sensitivity and increased uric acid levels. The epidemiological data suggest that these direct effects of fructose are pertinent to the consumption of the fructose-containing sugars, sucrose and HFCS, which are the predominant added sugars. Consumption of added sugar is associated with development and/or prevalence of fatty liver, dyslipidemia, insulin resistance, hyperuricemia, cardiovascular disease and type 2 diabetes, and many of these associations are independent of body weight gain or total energy intake. There are diet intervention studies in which human subjects exhibited increased circulating lipids and decreased insulin sensitivity when consuming high sugar compared with control diets. Most recently, our group has reported that supplementing the ad libitum diets of young adults with beverages containing 0, 10, 17.5 or 25% of daily energy requirement (Ereq) as high fructose corn syrup (HFCS) increased lipid/lipoprotein risk factors for cardiovascular disease (CVD) and uric acid in a dose response manner. However, un-confounded studies conducted in healthy humans under a controlled, energy-balanced diet protocol that allow determination of the effects of sugar with diets that do not allow for body weight gain are lacking. Furthermore, there are recent reports that conclude that there are no adverse effects of consuming beverages containing up to 30% Ereq sucrose or HFCS, and the conclusions from several meta-analyses suggest that fructose has no specific adverse effects relative to any other carbohydrate.
Consumption of excess sugar may also promote the development the development of CVD and T2DM indirectly by causing increased body weight and fat gain, but this is also a topic of controversy. Mechanistically, it is plausible that fructose consumption causes increased energy intake and reduced energy expenditure due to its failure to stimulate leptin production. Functional magnetic resonance imaging of the brain demonstrates that the brain responds differently to fructose or fructose-containing sugars compared with glucose or aspartame. There are epidemiological studies which show sugar consumption is associated with body weight gain, and there are intervention studies in which consumption of ad libitum high sugar diets promoted increased body weight gain compared with consumption of ad libitum low sugar diets. However, there are no studies in which energy intake and weight gain were compared in subjects consuming high or low sugar, blinded, ad libitum diets formulated to ensure both groups consumed a comparable macronutrient distribution and the same amounts of fiber. There is also little data to determine whether the form in which added sugar is consumed, as beverage or as solid food, affects its potential to promote weight gain.
It will be very challenging to obtain the funding to conduct the clinical diet studies needed to address these evidence gaps, especially at the levels of added sugar that are commonly consumed. Yet, filling these evidence gaps may be necessary for supporting the policy changes that will help to turn the food environment into one that does not promote the development of obesity and metabolic disease.
Keywords: Fructose, sucrose, high fructose corn syrup, diet, food environment, triglyceride, uric acid, metabolic syndrome, cardiovascular disease, type 2 diabetes
Introduction
The impact of added sugar consumption on heath continues to be a controversial topic. In recent counterpoint reviews Bray and Popkin concluded that sugar-sweetened beverages play a role in the epidemics of obesity, metabolic syndrome, and fatty liver disease (1), while Kahn and Sievenpiper concluded that there is no clear or convincing evidence that any dietary or added sugar has a unique or detrimental impact relative to any other source of calories on the development of obesity or diabetes (2). Therefore the objective of this review is to discuss the evidence and lack of evidence that allows the controversy to continue. The evidence is divided into two topics: the direct and the indirect effects of added sugar consumption on the development of metabolic disease. Studies needed to help resolve the controversy will be described, as well as the challenges involved in conducting these studies and reasons they are needed.
The term added sugar consumption in this review refers to sugars not naturally-occurring in foods and these consist mainly of sucrose and high fructose corn syrup (HFCS). It also refers to the sugars added to both beverage and solid foods, even though it cannot be assumed that sugar in solid food and sugar in beverage have equivalent effects. This is discussed later in the review. The term metabolic disease is used to specifically refer to cardiovascular disease (CVD), type 2 diabetes (T2DM), and non-alcoholic fatty liver disease (NAFLD). Metabolic disease was chosen over metabolic syndrome because it is beyond the scope of this review to discuss in detail the evidence and mechanisms related to sugar and its associations with hypertension and central obesity.
The potential for both direct and indirect effects of added sugar consumption on metabolic disease
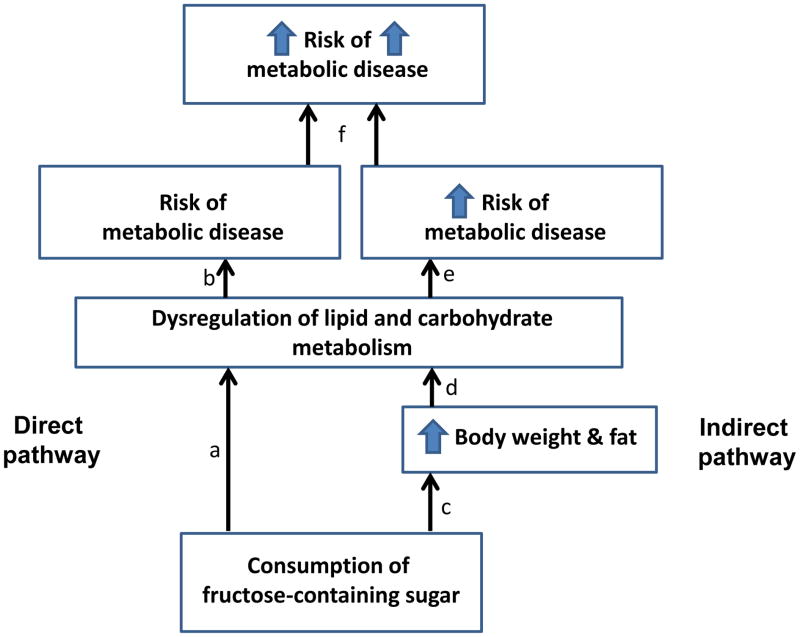
There is evidence to suggest that diets high in added sugar promote the development of metabolic disease both directly and indirectly (Figure 1). Directly, the fructose component in sugar causes dysregulation of lipid and carbohydrate metabolism. Indirectly, sugar promotes positive energy balance, thus body weight and fat gain, which also cause dysregulation of lipid and carbohydrate metabolism. Due to the direct and indirect pathway, we have suggested that risk for metabolic disease is exacerbated when added sugar is consumed with diets that allow for body weight and fat gain (3).
Figure 1. Two pathways by which sugar increases metabolic risk.
Direct pathway: Consumption of sugar leads to dysregulation of lipid and carbohydrate metabolism (a) which increases risk for metabolic disease (b). Indirect pathway: Consumption of sugar promotes body weight and fat gain (c) which leads to dysregulation of lipid and carbohydrate metabolism (d) which increases in risk for metabolic disease (e). Thus, it is possible that risk for metabolic disease is exacerbated when added sugar is consumed with diets that allow for body weight and fat gain (f).
The direct effects of added sugar consumption on the development of metabolic disease
The prevalence of metabolic syndrome, cardiovascular disease and type 2 diabetes are strongly associated with the presence of overweight and obesity. This has led to the widespread belief that diet impacts metabolic disease solely through the effects of excess body weight and fat. The sugar-related industries are campaigning vigorously to reinforce this belief and “educate” the public that the only dietary culprit is excess calories (4, 5). However, if sugar consumption has direct effects that increase risk factors for metabolic disease in the absence of positive energy balance, this assertion is not true, and the public and health care providers need to be informed accordingly.
There is considerable epidemiological evidence suggesting intake of added sugars and/or sugar-sweetened beverages are associated with the presence of unfavorable lipid levels (6–8), insulin resistance (9, 10), fatty liver (11, 12), T2DM (13–17), cardiovascular disease (CVD) (18, 19), metabolic syndrome (20–24), visceral adiposity (25, 26), and hyperuricemia (27–29). For the majority of these studies, the reported associations were not attenuated by adjustment for body mass index (BMI) or total energy intake (6–8, 10, 13–23, 27, 28). Recent evidence from National Health and Nutrition Examination Survey III is especially alarming (30). The adjusted (included adjustment for total energy intake) hazard ratios of CVD mortality across quintiles (Q) of percentage of daily calories consumed from added sugar (Q1: 0–9.59; Q2: 9.6–13.09; Q3: 13.1–16.69; Q4: 16.7–21.29; Q5: ≥21.3% of daily calories) were Q1: 1.00 (reference), Q2: 1.07, Q3: 1.18, Q4: 1.38, and Q5: 2.03 (n=11,733) (30). These data not only suggest that the higher the intake of added sugar, the greater the risk (30); they also show that the average level of added sugar consumption in the US, 15% of daily calories (30), is associated with an 18% increase in risk for CVD mortality.
Is it really possible that, independently of total energy intake, the average level of sugar consumption in this country is increasing risk for CVD death by 18%? This question cannot be answered based on these data alone, since epidemiological data can only demonstrate associations, not cause and effect. To prove added sugar consumption at the US average intake level, or even at levels exceeding the average intake, is independently promoting or contributing to development of CVD or T2DM, we need plausible mechanisms by which added sugar is specifically able to do so and direct experimental evidence from clinical diet intervention studies demonstrating that high sugar diets increase risk factors for CVD or T2DM compared with control diets.
Plausible mechanisms by which consumption of sugar may independently contribute to the development of CVD and T2DM
Our group has reported that subjects consuming fructose-sweetened beverages for ten weeks exhibited increased de novo lipogenesis (DNL), dyslipidemia, and circulating uric acid levels and reduced fatty acid oxidation and insulin sensitivity, while subjects consuming glucose-sweetened beverages did not, despite comparable body weight gain (31–33). These results, our more recent results and the results of many colleagues support the plausibility of the mechanisms, described below and illustrated in Figure 2, by which consumption of added, fructose-containing sugars may mediate or contribute to metabolic disease.
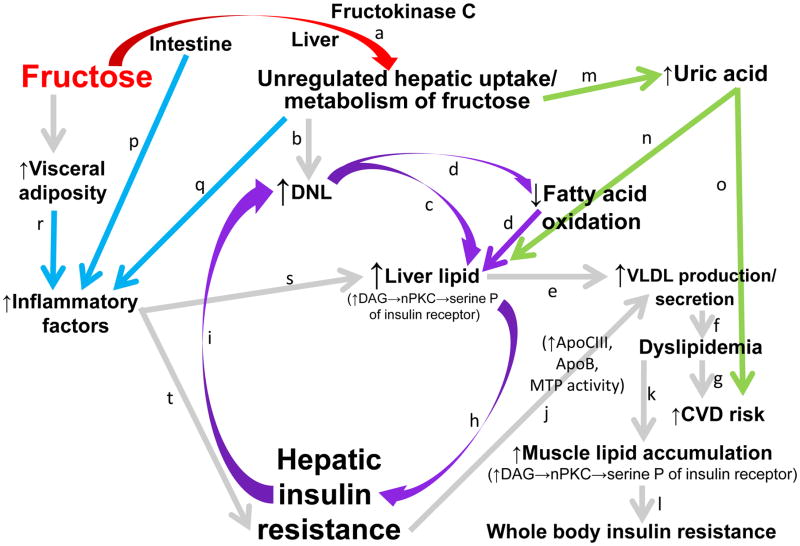
Figure 2. Potential mechanisms by which consumption of fructose affects lipid metabolism and hepatic insulin sensitivity.
The initial phosphorylation of dietary fructose in the liver is largely catalyzed by fructokinase C (a), which is not regulated by hepatic energy status. This results in unregulated fructose uptake and metabolism by the liver. The excess substrate leads to increased de novo lipogenesis (DNL)(b). DNL increases the intra-hepatic lipid supply directly, via synthesis of fatty acids (c), and indirectly, by inhibiting fatty acid oxidation (d). Increased levels of intra-hepatic lipid content promote very low density lipoprotein (VLDL) production and secretion (e). This leads to increased levels of circulating TG and low density lipoprotein cholesterol (dyslipidemia (f)), risk factors for cardiovascular disease (CVD) (g). Increased levels of hepatic lipids may also promote hepatic insulin resistance by increasing levels of diacylglycerol, which may activate novel protein kinase C (nPKC) and lead to serine phosphorylation (serine P) of the insulin receptor and insulin receptor substrate 1 (IRS-1) and impaired insulin action (h). Due to selective insulin resistance, DNL is even more strongly activated in the insulin resistant liver DNL (i), which has the potential to generate a vicious cycle (circular arrows). This cycle would be expected to further exacerbate VLDL production and secretion via increased intra-hepatic lipid supply. Hepatic insulin resistance also exacerbates VLDL production/secretion (j) by increasing apolipoprotein (apo)B availability and apoCIII synthesis, and by up-regulating microsomal triglyceride-transfer protein expression (MTP). This exacerbates and sustains exposure to circulating TG, leading to muscle lipid accumulation (k), impaired insulin signaling, and whole body insulin resistance (l). The fructokinase-catalyzed phosphorylation of fructose to fructose-1-phosphate, which results in conversion of adenosine triphosphate (ATP) to adenosine monophosphate (AMP) and a depletion of inorganic phosphate, leads to uric acid production via the purine degradation pathway (m). High levels of uric acid are associated and may contribute to increased risk for development of fatty liver (n), CVD (o), and metabolic syndrome. Fructose exposure in the intestine (p) and liver (q), and fructose-induced increases of visceral adipose (r) may promote inflammatory responses that further promote liver lipid accumulation (s) and/or impair hepatic insulin signaling (t).
Hepatic glucose metabolism is regulated by insulin and hepatic energy needs, and this allows much of ingested glucose, from starch or a glucose-sweetened beverage, arriving via the portal vein to bypass the liver and reach the systemic circulation. In contrast, the initial phosphorylation of dietary fructose is largely catalyzed by fructokinase, which is not regulated by hepatic energy status. This results in unregulated fructose uptake by the liver, with most of the ingested fructose being metabolized in the liver and very little reaching the systemic circulation (34). The fructose overload in the liver results in excess substrate that leads to increased de novo lipogenesis (DNL) (33). DNL increases the intra-hepatic lipid supply directly (35, 36), via synthesis of fatty acids, and indirectly, by inhibiting fatty acid oxidation (31). Increased levels of intra-hepatic lipid content promote very low density lipoprotein 1 (VLDL1) production and secretion (37), which leads to increased levels of postprandial TG and dyslipidemia (38).
Increased levels of hepatic lipids may also promote hepatic insulin resistance (39), possibly by increasing levels of diacylglycerol (DAG), which activates novel protein kinase C (nPKC) and leads to serine phosphorylation of the insulin receptor and insulin receptor substrate 1 (IRS-1) and impaired insulin action (40). Due to selective insulin resistance, DNL is even more strongly activated in the insulin resistant liver (41). This has the potential to generate a vicious cycle: i.e. DNL increases liver lipid, which increases hepatic insulin resistance, which further increases DNL (Figure 2--circular arrows). This cycle would be expected to further exacerbate VLDL production and secretion by increasing the intra-hepatic lipid supply (37). Hepatic insulin resistance may also indirectly increase VLDL production and secretion by 1) increasing apolipoprotein B (apoB) availability (42, 43), the protein backbone of VLDL; 2) up-regulating microsomal triglyceride-transfer protein expression (41), which catalyzes the assembly of TG and apoB into VLDL; and 3) increasing the production of apolipoprotein CIII (apoCIII) (44). There is evidence to suggest that apoCIII plays a critical role in promoting the second-step incorporation of lipid into VLDL, which converts VLDL2 (smaller, TG-poor particles) into larger, TG-rich VLDL1 particles (45, 46). The overproduction of VLDL1 has been described as the underlying defect that leads to the dyslipidemia that is characteristic of patients with type 2 diabetes and metabolic syndrome (38). Furthermore, apoCIII promotes hypertriglyceridemia by inhibiting both lipoprotein lipase activity and the clearance of TG-rich lipoproteins by hepatic receptors (47).
Thus, there is increased exposure of the vasculature to TG, which can lead to intramyocellular lipid accumulation. Intramyocellular lipid concentrations are correlated with reduced whole body insulin sensitivity in humans (48). It is possible, but not definite (49), that this relationship is mediated by the same mechanism described for the development of hepatic insulin resistance; DAG-mediated activation of nPKC resulting in serine phosphorylation of the insulin receptor or IRS-1 (50). It is also possible that other factors such as inflammation and oxidative stress (51), are contributors to, or possibly mediators of, muscle insulin resistance (52).
The fructokinase-catalyzed phosphorylation of fructose to fructose-1-phosphate, which results in conversion of adenosine triphosphate (ATP) to adenosine monophosphate (AMP) and a depletion of inorganic phosphate, leads to uric acid production via the purine degradation pathway (53). Uric acid is a potential mediator of metabolic disease with most recent studies, but not all (54), showing that it is strongly associated and predictive of metabolic syndrome, fatty liver and CVD (55–57). Our recent data (58) demonstrate that uric acid and apoCIII are, at the very least, strong biomarkers, and possibly mediators, of independent pathways by which consumption of HFCS increases risk factors for CVD.
There is also evidence to suggest that fructose may promote inflammatory responses (59) that can further impair hepatic insulin signaling. Studies in mice and non-human primates show that direct exposure of fructose to the intestine increases intestinal translocation of endotoxin (60, 61). Fructose exposure, compared with glucose exposure, has also been shown to cause activation of c-jun NH2-terminal kinase, increased serine phosphorylation of IRS-1 and reduced insulin-stimulated tyrosine phosphorylation of IRS-1 and IRS-2 in isolated hepatocytes (62).
Thus fructose overload in the liver may mediate a cascade of events by which risk for metabolic disease is increased.
Direct experimental data from diet intervention studies
In addition to plausible mechanisms, we also need direct experimental data from clinical diet intervention studies that show that consumption of added sugar at commonly-consumed levels increases risk factors for metabolic disease compared with a diet containing low amounts of added sugar. There are at least twelve diet intervention studies in humans that document increased risk factors for metabolic disease in human subjects consuming added sugar (specifically sucrose or high fructose corn syrup (HFCS)) (34, 35, 58, 63–73). Yet, despite these studies and the plausible mechanisms to support the epidemiological data, the weight-independent role of sugar consumption at commonly-consumed levels in the epidemics of metabolic disease remains highly controversial. There are 3 major reasons for this:
The diet intervention studies that have investigated the effect of added sugar consumption at the levels that are consumed by most Americans have limitations that preclude their being definitive.
The diet intervention studies that have investigated the effect of added sugar consumption under energy-balanced conditions to allow determination of the weight-independent effects of sugar consumption have limitations that preclude their being definitive.
There is evidence from diet intervention studies that suggests that there are no adverse health effects associated with consumption of added sugar.
1) The diet intervention studies that have investigated the effect of added sugar consumption at the levels that are consumed by most Americans
58% of men and women aged 19–30 years, and 63% of men and women aged 31–50 years consume between 5 and 20% of their daily energy as added sugar (74), and as stated previously, the average intake in US is 15% of energy (30). There are 3 published studies which suggest that consumption of added sugar at 20% Ereq or less can increase risk factors for metabolic disease. In all three of these studies, there were no differences between experimental groups or interventions in body weight gain.
Men and women consuming 1 liter/d of sucrose-sweetened cola (~20%Ereq) for 6 months along with their usual ad libitum diets had increases in liver TG and fasting plasma TG concentrations compared with those who consumed isocaloric amounts of low-fat milk, or iso-volumetric amounts of aspartame-sweetened beverage or water (35). Sucrose consumption also increased visceral fat volume compared with milk consumption, despite comparable body weight gain; and it increased plasma cholesterol concentrations compared with aspartame and water consumption (35).
A group in Switzerland reported that young, healthy men exhibited increased low-density lipoprotein-cholesterol (LDL-C) and small dense LDL-C levels when they consumed 80 g/d sucrose (~13%Ereq) in beverage along with their usual ad libitum diets for 3 weeks compared with when they consumed 80 g/d glucose in beverage (63, 64).
Young men and women consuming the 10% Ereq as HFCS-sweetened beverages along with ad libitum diets had increased levels of nonHDL-C, LDL-C, apoB and postprandial TG compared with their baseline levels (and also compared with the 0% dose group for postprandial TG). The participants consuming 17.5% (and 25%) Ereq as HFCS-sweetened beverages had significant increases in all of these outcomes, plus increased uric acid and postprandial apoCIII, compared with both their baseline levels and the 0% dose group (58).
The obvious limitation of these studies is that the sugar-sweetened beverages were consumed with the subjects’ own usual ad libitum diets for all or part of the study, thus the total amount of added sugar that the participants consumed is unknown. Furthermore, it cannot be stated with certainty that there were no diet variations between the experimental groups or interventions that may have confounded the study results.
2) Diet intervention studies that investigated the weight-independent effect of sugar consumption
There are very few studies conducted under standardized, energy-balanced conditions upon which to base definitive conclusions regarding the effects of sugar consumption when results are not confounded by potential positive energy balance and diet variations between the experimental groups or interventions. In the studies described below, subjects did not exhibit weight gain because they consumed sugar (at ≤30% Ereq) as part of an energy-balanced diet that was prepared/provided as per experimental protocol throughout the entire study.
In a 6-week cross-over study, healthy men who consumed energy-balanced diets containing high (25%Ereq) or low (10% Ereq) amounts of sucrose, exhibited increased levels of total cholesterol and LDL-C during the high sucrose diet (65). However, the authors (study funded by the Dutch Sugar Bureau) attributed the increases of cholesterol and LDL-C to a difference in the saturated fat content of the 2 diets.
Reiser et al. conducted an energy-balanced 6-week crossover study comparing a 30% Ereq sucrose diet with a iso-caloric starch diet in men and women. Fasting TG, cholesterol, (71) glucose and insulin levels, and insulin responses to an oral sucrose load were increased during the sucrose intervention (72). However, 30% Ereq is above the suggested maximal intake level for consumption of added sugars in the Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2010 (75). Also the distribution of energy in the provided diet; 10% Ereq at breakfast, 90% Ereq at dinner; was an atypical meal pattern that could have affected metabolic responses.
Reiser et al. measured the same parameters in participants of a crossover study in which energy-balanced diets containing 5, 18, or 33% Ereq as sucrose were consumed (70). Fasting lipids (69), glucose and insulin, and the glucose and insulin responses to the oral sucrose test (70) were all increased during the 18% and 33% Ereq sucrose diets compared with the 5% Ereq sucrose diet. However, the 24 subjects (36 years, 25 kg/m2) enrolled in this study, were chosen out of 150 potential participants for their exaggerated responses to an oral sucrose test. Also the distribution of energy in the provided diet; 25% at breakfast, 75% at dinner; was not typical of the usual 3 meal/day pattern. And finally, the fat content of the diets in both of the studies conducted by Reiser et al. (69–72) was 41.5–43% Ereq. There are plausible mechanisms by which diets that are high in both sugar and fat may lead to more adverse outcomes than high sugar/low fat diets or high fat/low sugar diets (76).
Most recently, Lewis et al. conducted a randomized crossover study in which older (mean = 46 years), obese (31.7 kg/m2) participants consumed low sucrose (sucrose 5.2%, total sugar 17.1% of daily calories) or high sucrose (sucrose 14.9%, total sugar 30.2% of daily calories—details about non-sucrose sugar are not provided) diets for 6 weeks (66). While there were no differences in peripheral glucose utilization and suppression of endogenous glucose production during a two-step hyperinsulinaemic euglycaemic clamp, fasting and oral glucose tolerance area under the curve (AUC) were higher for both glucose and insulin after the high sucrose diet than after the low.
The results from these 4 studies suggest that added sugar consumption, even at 18% Ereq, increases risk factors for metabolic disease when consumed with a diet that does not allow for weight gain; however these studies have limitations that narrow their generalizability to the typical western diet and/or to healthy adults. These limitations are why Dr. Luc Tappy in his 2012 review stated: “… the results from clinical trials do not support a significant detrimental effect of fructose on metabolic health when consumed as part of a weight-maintaining diet in amounts consistent with the average-estimated fructose consumption in Western countries. However, definitive studies are missing” (77). Three years later, these studies are still missing. This is why Kahn and Sievenpiper are still concluding that “there is no clear or convincing evidence that any dietary or added sugar has a unique or detrimental impact relative to any other source of calories on the development of obesity or diabetes. Sugar is purely a highly palatable source of energy; …” (2).
3. Direct experimental evidence that suggests that there are no adverse health effects associated with consumption of added sugar
The missing studies (77) allow the controversy concerning the independent role of added sugar in the epidemics of metabolic disease to continue. However, the controversy is further fueled by direct experimental evidence, such as described below, that suggests that there is no adverse health effects associated with consumption of added sugar.
Investigators of a recent dose-response study, in which ad libitum diets of men and women were supplemented with beverages containing 8, 18 or 30% Ereq from sucrose or HFCS for 10 weeks (78, 79), have reported that consumption of added sugar does not increase fasting cholesterol and LDL-C (78), and that there were no differences between the three levels of sugar in 24-hour circulating TG and uric acid concentrations (79).
Wang et al. reported that their meta-analysis does not support a uric acid-increasing effect of isocaloric fructose intake in nondiabetic participants (80).
In a more recent meta-analysis, Wang et al. concluded that fructose in isocaloric exchange for other carbohydrate does not increase postprandial TG (81).
These reports share a commonality in that they were industry-funded or were conducted by investigators who have received consulting fees from industries with a strong financial interest in maintaining high levels of sugar consumption. Does this conflict of interest influence the conclusions? Two recent studies that examined whether industry funding or the disclosure of potential conflicts of interest influenced the results of published systematic reviews conducted in the field of sugar-sweetened beverages and weight gain or obesity suggest it may (82, 83). Both studies concluded that reviews with conflicts of interest were more likely to present a conclusion of no or lesser association between sugar-sweetened beverages and weight gain or obesity than those without them (82, 83).
Even so, the discrepancies between the results from the study in which the ad libitum diets of healthy adults were supplemented with beverages containing 8, 18, or 30% Ereq as sucrose or HFCS (78, 79), and the results from our recent study, in which we supplemented the ad libitum diets of healthy adults with beverages containing 0, 10, 17.5 or 25% Ereq as HFCS (58), are startling. As a striking example, Yu et al. reports no differences in the 24-h uric acid AUC “response to the 6 different interventions at baseline or posttesting” (79). In our recent study (58), the participants consuming 25%Ereq HFCS exhibited an increase in the 24-h uric acid AUC that was significant at P = 0.0000006 (paired t test) compared to their baseline levels and at P = 0.00008 (unpaired t test) compared to the increase of uric acid in participants consuming 10% Ereq HFCS. In the 4-group analysis of covariance the significance of both of these comparisons was P < 0.0001.
How is it possible for two similarly-designed studies to yield such discrepant results? Possible reasons that Yu et al. (79) were able to report a null finding include: 1. The vehicle by which the sugar was provided to the participants; 2. The statistical analyses; 3. The monitoring of compliance.
-
Vehicle: Yu et al. provided the sugar in low fat milk and each subject was required to consume three 8-oz servings of milk/day (79) (presumably the servings consumed by the 30% group contained ~50 grams of sugar/8 oz). This is more than 3 times the amount of milk consumed by the average American (84), thus this vehicle very likely resulted in a substantial increase of milk consumption in the majority of the participants. There is no report of a control group who consumed milk without the added sugar, therefore this study is unable to differentiate the effects of increased milk consumption from the effects of increased sugar consumption. This is a very important limitation given that milk consumption is associated with decreased risk of metabolic syndrome (85), CVD and diabetes (86, 87).
The use of milk as a vehicle introduced other limitations including the strong possibility that some of the subjects found the sweetened milk beverages difficult to consume over time due to lactose intolerance. Lactose intolerance may have caused the high dropout rate acknowledged in the paper and may have affected the compliance of those participants who did complete the study.
Two reasons are provided in the paper as to why low-fat milk was used as a vehicle for this study. The first reason provided is, “To enhance participant compliance over the 10-week study” (79). As stated above it would seem more likely that the milk vehicle would undermine compliance in participants with lactose intolerance. It also seems likely that some or most of the participants would find the sweetened milk unpalatable compared to the usual sugar-sweetened sodas and fruit-flavored drinks; especially the high dose beverages that contained 50 grams of sugar/8 oz milk (most sodas contain 35–40 grams sugar/12 oz). Since sweetened milk is normally consumed with a chocolate flavoring, there is the possibility that some participants of this study may have improved the palatability of the sweetened milk by adding cocoa. This would introduce an additional confounder, as approximately 70 human intervention studies indicate that cocoa and cocoa-containing products beneficially affect endothelial function, blood pressure, and cholesterol levels (88).
The second reason provided for the use of the low fat milk vehicle is, “Furthermore, previous investigations that used carbonated soft drinks suffered from the confounding problem of significant inversion of sucrose into its components of fructose and glucose because of the mildly acidic environment of these beverages.” (79) This explanation is inadequate simply because water would have served the same purpose. Sucrose does not hydrolyze in water. Therefore, given its advantages over milk with regard to its being non-caloric and not able to confound metabolic outcomes, it is scientifically inexplicable why water was not used as a vehicle for this study.
Statistical analyses: The statistical model utilized by Yu et al. (79) was a 6 group one-factor (time) ANOVA. With this analysis and 23 subjects per group, the study was powered to detect differences of 1.08 standard deviation or greater with 80% power. This means that only very dramatic differences could be detected between groups. A more suitable analysis could have been utilized; a two-factor ANOVA (type of sugar and dose of sugar), which would take advantage of the 2 by 3 factorial design. With 46 subjects per group (pooled by dose), the investigators could have detected differences of 0.66 standard deviation among the three dosage groups with 80% power. With 69 per group (pooled by sugar), they could detect differences of 0.48 standard deviation between the two sugar types with 80% power. It is not possible to evaluate whether this analysis could have affected the conclusion regarding uric acid because the group data are not reported in the paper (79). The sensitivity of the statistical model would have also been improved by including adjustment for sex. In our study (58), the HFCS-induced increases in 24-h uric acid AUC were higher in men than women (P = 0.008, effect of sex).
Monitoring of compliance: Yu et al. (79) report that compliance to milk consumption was measured with daily dietary logs and state that “tight control” over free living diet consumption was a strength of the study. This is unlikely to be true as inaccurate reporting of food consumption is a well-documented occurrence in dietary research studies (89, 90). Using a biomarker (e.g. riboflavin (58)) in the experimental beverages that can be recovered and measured in urine, and informing the participants of this, provides a more objective index of compliance and also provides motivation for subject compliance.
The reports by Yu et al. (79) and Bravo et al. (78) are on subsets of subjects studied as part of a large randomized control trial (RCT) funded by the Corn Refiners Association for ten million dollars (91). It is the largest RCT (n=352 (92)) yet conducted on the effects of sugar consumption. It has generated several more publications with null findings (93, 94), and will have a marked influence on the conclusions of future meta-analyses. The Principal Investigator of the study, Dr. James Rippe, receives a $41,000/month retainer from the Corn Refiners Association (91). In an interview Dr. Rippe said the corn industry’s payments did not influence the conclusions of his research, “We presented academic research based on the highest gold standard” (91). Yet, the inexplicable use of milk as a vehicle for the study, the lack of a control group, the use of a suboptimal statistical model, and the lack of objective compliance monitoring do not represent gold standard research. Instead they give the appearance that the objective of this industry-sponsored study was not to answer an important public health question, but to generate results that will assure the public that the current level of sugar consumption is safe and maintain the state of controversy.
Meta-analyses
Conclusions from meta-analyses, in which results from all qualifying studies are combined, may have more potential to clarify the role of sugar consumption in the development of metabolic disease than any single study. However the conclusions from recent meta-analyses of intervention studies cover the range from yes to equivocal to no regarding the effects of fructose or sugar consumption on risk factors for metabolic disease. Recent meta-analyses conclude that fructose and/or sugar consumption increase total and LDL-C (95); TG, total and LDL-C, and blood pressure (96), and have significant effects on most components of metabolic syndrome (increased systolic blood pressure, fasting glucose and TG, decreased HDL) (97). Another recent meta-analyses concludes that the available evidence is not sufficiently robust to draw conclusions regarding effects of fructose, HFCS, or sucrose consumption on NAFLD (98). And, as stated earlier recent, Wang et al. concluded that there were no relationships between fructose consumption and levels of uric acid (80) or postprandial TG (81).
Since the diet intervention studies conducted by our group have demonstrated a very marked and consistent effect of fructose or sugar consumption to increase uric acid (32, 58) and postprandial TG (33, 58, 73, 99), it is worth examining the meta-analyses that focused on these two outcomes. Specifically, Wang et al. reported that their meta-analysis does not support a uric acid-increasing effect of isocaloric fructose intake in nondiabetic participants (80). In contrast, the 24-h AUC for uric acid increased in participants in our recently-completed study who consumed either 17.5% (+14%; P = 0.0000002, paired t test, n=22) or 25% Ereq (+19%; P = 0.000000007, paired t test, n=28) of Ereq as fructose-sweetened beverages for 2 weeks (unpublished data). Both groups gained less than 0.1 kg, thus these results are not confounded by weight gain or a hyper-energetic diet. A close examination of some of the studies that met the inclusion criteria for the meta-analyses conducted by Wang et al. (80) may help explain discordance between their conclusion and our results. Of the nine studies that were grouped as ‘isocaloric, no diabetes’, there were four studies in which the effect of fructose consumption to increase circulating uric acid in comparison to the control carbohydrate was zero or less. For two of these studies (100, 101), the comparison or control carbohydrate was sucrose, a fructose-containing sugar that is also likely to increase uric acids levels. Indeed, as already noted, we have recently reported that consumption of beverages containing 17.5 or 25% Ereq as HFCS, the other commonly-consumed fructose-containing added sugar, increased both fasting and 24-h uric acids levels (58). In the other two studies it appears that both the fructose and control diets were energy-restricted (102, 103). Since uric acid production via the purine degradation pathway is increased by hepatic substrate overload leading to generation of excess AMP and a depletion of inorganic phosphate, energy-restricted diets are not likely to lead to increased uric acid levels. Also in the study conducted by Madero et al. the “high” fructose diet consisted of 60 grams “natural” fructose/day from fruit (103). The inclusion of these four studies in a meta-analysis (80) consisting of only 9 total studies grouped as ‘isocaloric, no diabetes’ makes a null conclusion unsurprising.
In a more recent meta-analysis, Wang et al. concluded that fructose in isocaloric exchange for other carbohydrate does not increase postprandial TG (81). Again our recent results suggest otherwise. The 24-h AUC for TG increased in participants who consumed either 17.5% (+16%; P = 0.003, paired t test, n=22) or 25% Ereq (+20%; P = 0.00003, paired t test, n=28) as fructose-sweetened beverages for 2 weeks and gained less than 0.1 kg body weight (unpublished data). Figure 2 of the meta-analysis (81) shows of the five studies of “otherwise healthy” subjects, there were two in which the effect of fructose consumption to increase circulating postprandial TG in comparison to the control carbohydrate was only slightly above zero. Again, for one of these studies (101) the control carbohydrate was sucrose, which would be expected to increase postprandial TG and obscure the effect of fructose. In the other study (104), the postprandial testing period occurred from pre-breakfast to 4-h post-breakfast, which is of insufficient duration to detect an increase in postprandial TG. In our sustained consumption studies with 24-h blood collection protocols (33, 58, 73, 99), we did not observe the effects of fructose/fructose-containing sugars compared with glucose or starch to increase postprandial TG until after lunch, with the most marked differences between the carbohydrates occurring 4–5 hours after dinner. Given the limited number of fructose intervention studies that have reported measures of postprandial TG in healthy humans, the inclusion of these two studies had a marked effect on the null conclusion reported by Wang et al. (81).
Summary - The direct effects of added sugar consumption on the development of metabolic disease
While the epidemiological evidence, the plausibility of the mechanisms, and the results from diet intervention studies provide strong support for a direct causal/contributory role of sugar consumption in the epidemics of metabolic disease, as stated in 2012 by Dr. Tappy (77), definitive studies are missing. These missing studies preclude a resolution to the controversy, while the null findings from industry-funded studies and industry-supported investigators escalate it.
Needed studies
What studies would help resolve the controversy? We need clinical trials, lasting at least 4 weeks (longer would be better) in which healthy subjects consume added sugar as part of energy-balanced diets that are prepared/provided as per experimental protocol throughout the entire study. The optimal study would include a range of added sugar consumption levels including 5% (approximately the daily calorie level recommended by the American Heart Association (105)), 10% (the new daily calorie limit proposed in the Scientific Report of the 2015 Dietary Guideline Advisory Committee (106)), 15% (the average daily calorie level consumed in US (30)), and 25% Ereq (the current upper level recommendation in the 2010 report of the Dietary Guideline Advisory Committee (75)). The diets need to be formulated such that the level of fat is consistent with dietary recommendations and there are no macronutrient, fiber, saturated fat, trans fat differences between groups that can confound results. Compliance needs to be monitored objectively (biomarkers), and also perhaps by utilizing mobile phone technology (107–109).
When will such studies be conducted? This author can only offer the perspective of a US researcher who has pursued funding from NIH to answer questions related to the role of added sugar/diet in the development of metabolic disease utilizing standardized dietary protocols. The hurdles that make obtaining funding for such studies extremely challenging include the expense of these studies, plus the fact that clinical diet intervention studies are often perceived by the NIH reviewers as descriptive, lacking innovation, and even lacking significance.
Expense: It is difficult to design a clinical diet intervention study that is adequately powered and of sufficient duration, and includes a standardized dietary protocol (meaning providing standardized meals, not standardized diet prescriptions), with costs within the usual NIH budget limit ($500,000/year for five years). It has become even more difficult in recent years because NIH is no longer subsidizing essential research expenses, such as facility and nursing costs, through its Clinical and Translational Science Center awards.
Descriptive: Yet, even when a researcher finds a way to budget a diet intervention study within the NIH funding limits, reviewers often reject the proposal because it is descriptive. This means the proposal only seeks to answer a public health question concerning the effect of diet, but does not include the mechanistic studies needed to illuminate the metabolic pathways affected by the diet. In human subjects, these mechanistic studies usually require the use of stable isotopes and mass spectrophotometer analyses. These procedures can easily double or triple the cost of the proposed trial to well beyond the NIH budget limits.
Lack of innovation: An important component by which NIH applications are judged and scored is innovation, eg. does the proposal seek to investigate a new therapeutic target; will it utilize a novel procedure? Due to the ethical constraints regarding what procedure can be performed, studies involving human subject are far less likely to contain innovative aspects than those utilizing animal models. Innovation is likely to be even more limited when the investigator is simply proposing to answer the question: Will Diet A increase risk factors for metabolic disease in human subjects more than Diet B? With only 10–18% of the submitted NIH proposals receiving fundable scores, a proposal that receives a poor innovation score has little or no chance for funding, even if it receives very high scores on all other components.
Lack of significance: An equally important scoring component of NIH proposals is the reviewers’ assessment of the significance of the research with regard to human health. With the prevalence of CVD, T2DM, obesity, and metabolic syndrome causing such public health burdens, one would think that a proposal seeking to answer a public health question that could attenuate these epidemics would score well on the assessment of significance. Our group has had many opportunities to find this is often not true with regard to our proposed investigations of sugar consumption. Ten to fifteen years ago it was often not true because some reviewers were highly skeptical of the hypothesis that dietary sugar could really be a mediator or contributor to our serious public health issues. At that time it could be suggested that this hypothesis had already been disproven, given that John Yudkin had proposed/investigated the same hypothesis in the 1960s through 1980s (110–112). More recently, though, many reviewers have assessed the significance of our proposals as low for the opposite reason. They have stated that everyone, except those associated with the sugar industries, knows that consumption of sugar is bad for health. This change in attitude is validation of the research of John Yudkin (too late to affect him personally) and a testament to the recent investigators conducting research on the adverse metabolic effects of sugar consumption. However, it undermines our ability to demonstrate that added sugar at commonly-consumed levels has a direct, weight independent effect on the development of metabolic disease; and to determine at what levels of consumption this effect occurs.
This is unfortunate because it leaves a void in our scientific knowledge, but it is even more unfortunate from a public health perspective. All of us, whether normal weight or overweight, need to be armed with the knowledge that added sugar at commonly-consumed levels has direct, weight-independent effects on the development of metabolic disease as we continue to face numerous opportunities every day to indulge in palatable, high sugar treats. Parents need this information as they supervise the diets of their children (including normal weight children) and try to instill healthy life-long habits. The belief that our public health crisis is mediated solely through the prevalence of overweight and obesity has clearly not attenuated the crisis. Perhaps the knowledge and understanding that sugar is not simply a source of extra calories (that can be balanced with a little extra exercise later—even though later often never happens), rather it is also a direct contributor to the development of metabolic disease, will be more effective at slowing our epidemics of metabolic disease.
The indirect effects of added sugar consumption on the development of metabolic disease
The prevalence of metabolic syndrome, cardiovascular disease and type 2 diabetes are strongly associated with the presence of overweight and obesity, and there is little argument that this relationship is one of cause (overweight/obesity) and effect (metabolic disease). Therefore, if added sugar consumption promotes body fat gain relative to other macronutrients, this is a second and indirect pathway by which high sugar diets may contribute to the development of metabolic disease. However, the question of whether consumption of added sugar promotes body weight and fat gain is also controversial as indicated by the titles of recent reviews: “Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases” (113), and “Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak” (114).
Again, conflict of interest fuels the controversy (82, 83). However, some of the conflicting conclusion can also be explained by dividing the question; does consumption of added sugar promotes body weight and fat gain; into two separate questions, and realizing that investigators are sometimes not addressing the same question (115–119). The two questions are:
Does consumption of a high sugar diet promote more weight gain than consumption of a low sugar diet that is consumed in iso-caloric quantities?
Does consumption of an ad libitum diet that is high in added sugar promote increased energy intake, and thus increased body weight gain, compared with consumption of an ad libitum diet that is low in sugar?
Question 1 is specifically asking whether sugar (or fructose) has inherent properties that make it more able to promote weight gain than an isocaloric amount of any other food. Investigators who conclude that sugars have no special role in body weight control other than as one of many sources of energy (118) are focusing on Question 1. When these investigators examine a free-living population for evidence of a relationship between sugar consumption and weight gain, total energy intake is a confounder for which they adjust in their statistical model (115).
There is a mechanism by which a high sugar diet could promote more weight gain than consumption of a low sugar diet that is consumed in iso-caloric quantities. Our group has hypothesized that fructose consumption could promote weight gain because it does not stimulate insulin secretion or leptin production (120). Leptin production by adipocytes is regulated by insulin-mediated glucose metabolism (121). Ingestion of fructose does not result in meal-related increases of plasma glucose or insulin concentrations, therefore both short (34, 122) and long-term (123) studies demonstrate that meals accompanied with fructose-sweetened beverages result in reduced circulating leptin concentrations compared with glucose-sweetened beverages. Leptin acts, along with insulin, in the hypothalamus to regulate food intake and energy metabolism via neuropeptide systems including neuropeptide-Y and melanocortins. Accordingly, leptin-deficient patients exhibit increased hunger and impaired satiety (124). Additionally, functional magnetic resonance imaging (MRI) studies have shown that the areas of the brain associated with pleasure and reward are markedly activated when leptin-deficient patients are shown pictures of food, but this activation decreases to the level of normal subjects following 7 days of leptin administration (125). Leptin-responsive neurons also project to pathways which that activates signals to the periphery involved in promoting energy expenditure and fat oxidation. Thus, leptin is a key regulator of energy homeostasis (125). Therefore, it is plausible that, compared to an isocaloric high starch or glucose diet, a high fructose diet could reduce leptin production and circulating leptin concentrations, leading to decreased energy expenditure and increased body weight gain.
There are a several epidemiological studies that report a significant positive relationship between sugar-sweetened beverage consumption and BMI, even with adjustment for total energy intake (126–130). This does suggest that it is possible that consumption of fructose could have effects on body weight that are independent of total energy intake. However, there are no data from diet intervention studies to support this, including the data our own study. Food intake appeared to be similar and weight gain was comparable between the subjects consuming 25% Ereq as fructose- or glucose-sweetened beverages with ad libitum diets for 8 weeks (33). However, in these same subjects, we also observed a fructose-induced decrease in 24-h leptin levels (123), and a decrease in fasting energy expenditure that was not observed with glucose consumption (31). Why then did the subjects consuming fructose fail to show increased body weight gain compared with the subjects consuming glucose? The first and most obvious answer is duration of intervention. The reduction of energy expenditure exhibited by the subjects consuming fructose equated to a gain of 1.6 kg if maintained for one year (31). While this is a clinically significant outcome that could contribute to obesity over time, it would likely not be detectable in an intervention lasting less than one year. Furthermore, the body weight results from our study, and the other studies investigating the effects of fructose on energy intake and weight gain, could be confounded by fructose malabsorption. Consumption of fructose as a monosaccharide can overwhelm the absorptive capacity of the small intestine leading to fructose malabsorption and gastrointestinal distress (131), which can affect energy availability and intake. However, fructose malabsorption is low in normal subjects consuming sucrose or HFCS, because fructose when ingested along with glucose is much more completely absorbed than when ingested as pure fructose (132). Therefore studies comparing the isocaloric consumption of high and low sucrose or HFCS diets on body weight gain are not likely to be confounded by fructose malabsorption. Yet, even so, the costs of such studies, with the duration and power to ensure a clinically relevant answer, is likely to prevent its ever being conducted. Thus it is probable that we will never obtain a definitive answer to the question as to whether a high sugar diet promotes more weight gain than consumption of a low sugar diet that is consumed in iso-caloric quantities.
However, the typical western diet is consumed ad libitum, and the fact that the majority of adults are overweight (in the US, 56% of the women and 67% of men aged 20–39 have BMI ≥25 kg/m2 (133)) provides evidence that this ad libitum diet is being consumed in amounts beyond energy requirement. Therefore Question 2 concerning whether sugar (or fructose) has inherent properties that make it more likely to promote overeating than other macronutrients is far more relevant to the obesity epidemic. For researchers investigating this possibility, total energy intake is not a confounder, but instead an intermediary variable in the relationship between sugar consumption and body weight gain (83).
The answer to this question, whether sugar (or fructose) has inherent properties that make it more likely to promote overeating than other macronutrients, could be as simple as people tend to over-eat sugar because they like the sweet taste. Yet, recent studies on the central effects of sugars in the brain (134–142), made possible by functional magnetic resonance imaging (fMRI) technology, suggest the answer could be more complicated. Luo et al. reported that consumption of a fructose-sweetened beverage resulted in greater brain reactivity to food cues than consumption of a glucose-sweetened beverage in young healthy adults (143). Corroborating these results, fructose versus glucose led to greater hunger and desire for food and a greater willingness to give up long-term monetary rewards to obtain immediate high-calorie foods (143). Stice et al. reported that a high sugar milk shake was more effective at recruiting reward regions of the brain than an equi-caloric high-fat milkshake (142). A high sucrose beverage induced greater activation in the nucleus tractus solitarius than a non-nutritive beverage matched for sweetness in lean and obese women (138). The authors of this study also noted pattern differences between the lean and obese women that may suggest altered interaction between homeostatic and reward networks in obese individuals (138). Gearhardt et al. reported that the neural activation patterns associated with addictive-like eating behavior are similar to those associated with substance dependence (136). In line with this, reward activation to consumption of an ice cream-based chocolate milkshake was reduced in frequent compared with non-frequent consumers of ice cream, which the authors suggest may parallel the tolerance observed in drug addiction (134). Our group reported that women consuming sucrose-sweetened beverage had inhibited responses to stress, which included greater activation in the hippocampus and lowered cortisol levels, compared with women consuming aspartame-sweetened beverages (144). These results offer a potential mechanistic explanation for sugar often being perceived as a stress-relieving or comfort food.
While these fMRI studies hold great promise for illuminating the brain responses to the various macronutrients and eating patterns, sustained diet intervention studies will still be necessary to correlate patterns of activation in the various regions of the brain to long term eating behavior. They are also necessary to simply answer the question whether people tend to consume more energy when consuming sugar. The following studies suggest that they do:
Tordoff et al (145) reported that men and women (BMI just over 25) consuming HFCS-sweetened beverages (~20%E) consumed more energy and gained weight compared with baseline consumption. When the same subjects consumed aspartame-sweetened drinks they consumed less energy and did not gain weight.
Raben et al (146) reported that body weight (+1.6 kg) and fat mass (+1.3 kg) increased in overweight men and women consuming sucrose-sweetened beverages/snacks (~28%E) for 10 weeks and decreased (−1.0 and −0.3 kg, respectively) in men and women consuming comparable beverages/snacks containing non-caloric sweeteners; the between-group differences were highly significant.
Reid et al (147) reported that, compared with baseline, normal-weight women consuming sucrose-sweetened beverages (~21%Ereq) for 4 weeks increased energy intake and women consuming aspartame-sweetened beverages decreased energy intake. The body weight gain was significantly greater in the sucrose group.
Our groups has reported that young men and women consuming the 0 (aspartame),10,17.5 or 25% Ereq as HFCS-sweetened beverages along with ad libitum diets for 2 weeks exhibited a dose response increase in body weight, with the high dose group gaining the most weight (+0.8 kg, P < 0.01 compared with baseline body weight) (58).
These studies however fail to provide definitive evidence to answer the question due to the limitation that the experimental sweetened beverages and snacks were consumed with the subjects’ usual ad libitum diets. Therefore it cannot be known with any degree of certainty that there were not dietary variations between the experimental groups or interventions that could have confounded the results.
There appears to be only one investigation in which ad libitum consumption of a high sugar diet was investigated under controlled dietary conditions. Raben et al. have compared the ad libitum consumption of a high sugar diet (23%Ereq sucrose from both solid food and beverage) with a high complex carbohydrate diet (2%Ereq sucrose), while providing all of the food consumed during the study and maintaining similar proportions of protein and fat during both interventions (148). In this 14-day crossover study, subjects consumed more energy and gained more weight during consumption of the sucrose diet (+0.2 kg) than the complex carbohydrate diet (−0.7 kg) (148). However, as the Principal Investigator of this study points out (149), these results could possibly be explained by the complex carbohydrate diet containing more fiber than the sucrose diet. Another possible confounder was the lack of blinding. While the investigators attempted to match the palatability of the diets as closely as possible, they did not supplement the high complex carbohydrate diet with non-caloric sweeteners. Post-intervention questionnaires indicated that subjects were aware that they were consuming a high sugar or low sugar diet (148). A study by Ng et al. suggests that this is an important issue (139). Using fMRI, they demonstrated that areas of the brain associated with reward valuation were more activated when subjects consumed a milk shake labeled “regular” compared with an identical milk shake labeled “low-fat” (139). Similarly, Crum et al. reported that ghrelin levels of participants decreased following consumption of a 380 calorie milkshake that was labelled “indulgent, 620 calories”, but ghrelin did not decrease following consumption of the identical milkshake when it was labelled “sensible, 140-calories” (150). These findings suggest that preconceptions regarding diet composition, even when they are inaccurate, can affect the brain and ghrelin responses to the diet, and therefore, possibly energy intake as well.
Thus we lack the direct experimental data to definitively answer Question 2: Does consumption of an ad libitum high sugar diet promote increased energy intake, and thus increased body weight gain, compared with consumption of an ad libitum low sugar diet? Clinical trials in which blinded, ad libitum diets, containing high and low amounts of sugar, that have been carefully formulated to ensure that there will be no confounding dietary differences (e.g. dietary fiber, saturated fat) between the study groups are needed. Studies are also needed to compare the effects of sugar consumed in beverages versus solid food. While beverage is the leading single food contributor to sugar consumption, over 60% (151) of added sugar is consumed from solid food sources. This proportion may increase (152) with recent public health initiatives (tax, size limits and warning labels) focusing specifically on decreasing consumption of sugar-sweetened beverage. Yet, nearly all the sustained-consumption sugar intervention studies, with the notable exception being the aforementioned studies by Reiser et al, (69–72) have provided the experimental sugar as either solely or mainly as sugar-sweetened beverage. Sugar-sweetened beverage have been the main public health focus because many acute studies, consisting of liquid or solid food preloads followed by ad libitum consumption of one or two meals, suggest that in comparison to solid food forms, beverages hold weak satiety properties that lead to failure to adjust intake at subsequent eating occasions for energy supplied by the beverages.(153–155) Thus, liquid sugar may promote greater energy intake and weight gain than consuming sugar in solid food. Currently, though, there is only one published, sustained consumption diet intervention study that has compared the effects of consuming the 2 forms of added sugar (sugar-sweetened beverage or in solid food) with usual ad libitum diets on body weight and food intake. In this 4-week crossover study, participants reported greater energy intake while consuming 25% Ereq as sugar-sweetened beverage than as jelly beans (156). Body weight gain, however, was not significantly different between the sugar-sweetened beverage (+0.5 kg) and jelly bean (+0.2 kg) diets. Longer studies with standardized diets, and which provide the added sugar in solid food in a greater variety of more palatable and typically-consumed foods (e.g. sugar-coated cereal, cookies, cake, candy), will provide much-needed data to help address this important question.
It is also possible that sugar in liquid and sugar in solid food may have differential effects that are independent of energy intake and weight gain. Because sugar in liquid is digested and delivered to the liver more quickly than sugar in solid food, it may promote a greater substrate overload (more substrate in a shorter time frame) in the liver. This could lead to greater increases in DNL, hepatic lipid accumulation and postprandial TG in participant consuming sugar-sweetened beverage compared to those consuming sugar in solid food. Evidence from sustained consumption studies to support this is lacking. Metabolic results (i.e. DNL, liver lipid accumulation, postprandial TG levels), which could serve as indicators of increased hepatic overload, have not been reported from the sugar-sweetened beverage versus jelly bean study (156), and there appears to be no other long-term diet intervention studies that have concurrently investigated the effects of consuming added sugar as sugar-sweetened beverage and from solid food.
Obtaining the NIH funding to answer Question 2 or questions about the differences between liquid and solid sugar faces all the same hurdles already outlined; reviewers are likely to consider them expensive, descriptive, lacking innovation, and lacking significance. However, our laboratory has received NIH funding to conduct an 8-week RCT to test the hypothesis that consumption of an ad libitum diet along with 25% Ereq as HFCS-sweetened beverage will increase energy intake and body weight gain compared with consumption of the same ad libitum diet along with aspartame-sweetened beverages. The ad libitum diet will be provided throughout the intervention at 125% Ereq and will be carefully formulated to ensure that all participants will consume a comparable macronutrient distribution. Stable isotopes will be used to assess DNL and VLDL kinetics under meal-fed conditions.
Within this study, we will also test the hypothesis that sugar consumption in the absence of positive energy balance and weight gain has adverse effects on risk factors for metabolic disease by also studying participants who will consume the aspartame-sweetened beverages or the 25% Ereq HFCS-sweetened beverages with energy-balanced meals. This study design will allow us to compare the contribution of sugar with the contribution of energy level and body weight gain to the changes in risk factors, and to compare the effects of sugar versus sugar + weight gain on DNL and VLDL kinetic in non-steady state conditions. However, the answers generated by this study will not be applicable to the commonly-consumed levels of sugar (74) or to sugar consumed in solid food. Our efforts to obtain the funding to study groups consuming sugar in beverage or sugar in solid food at levels ranging from 5 to 20% Ereq under a standardized dietary protocol have, so far, been unsuccessful.
Thus the controversies regarding the role of sugar consumption at commonly-consumed levels in the obesity epidemic and in the epidemics of metabolic disease are likely to continue. Is this so important? The primary objectives of conducting this research are to improve the diet of the general population and attenuate the epidemics of metabolic disease. Maybe this can and will be achieved despite the controversy. However, Petrunoff et al. conducted focus groups to investigate parents’ beliefs regarding providing their pre-school children with high sugar and/or fat snacks that are low in nutrients. They reported that parents mainly believed that these foods can be provided frequently as long as their children are eating a healthy balance of foods (157). On an online survey completed by 3361 US adults 18 years and older, less than 40% of participants identified added sugars as a primary concern when choosing beverages (158). A Gallup poll conducted in July 2014 reported that the number of American who avoid consuming soda has increased by 12% since 2004, however, the number that avoid consuming sugar has only increased from 51 to 52% in the same time period (152). Interestingly, the number that reported consuming sugar has increased from 21 to 27% (152), suggesting that some of the people who now actively avoid soda have decided that sugar in solid food is acceptable. Caregivers of African-American children demonstrated a good understanding of the relationship between an unhealthy diet and obesity and metabolic disease. Yet they also expressed concern that their ability to provide a healthy diet was undermined by child preference for foods higher in fat and sugar, lower pricing of less healthy foods, limited access to healthier food retailers and targeted advertisements (159).
The concerns of these caregivers (159) are validated in an interesting perspective offered by Chandon et al., who described in detail how food marketing has made us fat by providing increased access to ‘continuously cheaper, bigger, and tastier calorie-dense food’ (160). They then contend that researchers have over-estimated the impact that deliberate decision-making has on food intake and have underestimated the impact that peripheral factors and mindless habitual behavior have on food intake (160). In other words they suggest that nutrition and health education has little chance against our palatable and inescapable food environment.
This suggests then that slowing the epidemics of obesity and metabolic disease can only occur through changes in the food environment. One such change is to make ‘cheaper, bigger, and tastier calorie-dense food’ less cheap through soda and junk food taxes. This tactic may prove effective. Mexico implemented a tax on sugar-sweetened beverages (seven cents/liter) and junk food in January 2014. The purpose is to target the epidemics of obesity and T2DM in Mexico, which are among the highest on the planet, by reducing sugar consumption and generating revenue that will be targeted for health programs and providing drinking water in schools. While the early data from Mexico’s National Institute of Public Health suggest in the first three months of 2014, purchases of sugary drinks dropped by 10% compared with the same period in 2013 (161), it will take longer to determine whether the decrease will continue and whether it and the programs funded by the tax will translate into positive health outcomes. In November 2014, a bill to tax sugar-sweetened beverages at one cent/oz was approved by voters by a 3 to 1 margin in Berkeley California, despite an opposition campaign funded by industry for $2.1 million (162).
This represents the highest soda tax to be approved by US voters. Whether voters in other states and cities will follow the lead of the progressive Berkeley voters remains to be seen. A two cents/oz tax did not receive the requisite 2/3 majority from San Francisco voters in the same election; however it did receive 55% of the vote. The American Beverage Association spent over $9 million campaigning against this measure, outspending the pro-tax campaign by 30 to 1 (162).
This is not the first time that efforts to change the food environment were defeated by the deep pockets of the industry. In 2012, the American Beverage Association spent $2.5 million to defeat a soda tax in Richmond California, outspending the pro-tax campaign by 50 to 1 (163). In 2010, the American Beverage Association’s lobby efforts toward getting the Philadelphia City Council to reject the mayor’s proposal to tax sugary drinks at two cents/oz included a donation of $10 million to the Children’s Hospital of Philadelphia to fund research into and prevention of childhood obesity (164). Maryland repealed its snack tax in 1997 when Frito-Lay threatened not to build a planned local plant (165). Louisiana halved and then repealed their soft drink tax in 1997 in response to Coca-Cola’s contracting to build a bottling facility in the state (165).
And this is why we do need to conduct the well-controlled clinical trials to generate the direct experimental data to resolve the controversies regarding the role of sugar consumption in the epidemics of metabolic disease and obesity. Health advocates proposing to improve the food environment will never have money to compete with the deep pockets of industry. They need to be armed with the direct experimental data that definitely demonstrates a causal role of added sugar consumption in the health epidemics.
The current gaps in knowledge allow the industry to arm their lobbyist with statements like:
When the full body of science is evaluated during a major review of scientific literature, experts continue to conclude that sugars intake is not a causative factor in any disease, including obesity (166).
The majority of nutrition experts agree that high fructose corn syrup is safe (167).
When it comes to risk for heart disease, there is nothing unique about the calories from added sugars, or sugar-sweetened beverages for that matter (168).
This allows lawmakers and voters to make immediate financial concerns a priority over long-term health concerns. When we have obtained the definitive evidence that shows that sugar at commonly-consumed levels is an independent and modifiable risk factors for metabolic disease; when we have the definitive evidence that shows that consumption of sugar promotes weight and body fat gain; possibly concerns about the health of our children and the health care costs burden on society will take precedence.
Conclusion
There are epidemiological data, plausible mechanisms and clinical data from diet intervention studies that provide strong support for a direct causal/contributory role of sugar in the epidemics of metabolic disease, and for an indirect causal/contributory role mediated by sugar consumption promoting body weight and fat gain. Yet these are still controversial topics. Clinical diet intervention studies in healthy men and women that definitively demonstrate that sugar consumption at commonly-consumed levels can increase risk factors for metabolic disease in the absence of body weight and fat gain are missing. Also missing are clinical trials in which the effects of an ad libitum high versus low sugar diet on energy intake and body weight gain are compared using a blinded and carefully-formulated dietary protocol that ensures all other dietary variables are comparable between the study groups. The controversy is further fueled by industry-funded studies that report that there are no adverse effects of consuming beverages containing up to 30% Ereq sucrose or HFCS and by the null conclusions of recent meta-analyses. Obtaining the funding to conduct the expensive clinical studies needed to fill the evidence gaps and resolve the controversy will be very challenging. However, obtaining this definitive evidence may be necessary in order to make progress in implementing the policies that will change the food environment into one that does not promote the development of obesity and metabolic disease; especially for implementing policies that may threaten the profits of the sugar and beverage industries.
Footnotes
Dr. Stanhope has no conflicts of interest to report.
Declarations of interest:
The studies conducted by Drs. Havel and Stanhope’s research group were supported with funding from NIH grants R01 HL-075675, 1R01 HL-091333, 1R01 HL-107256 and a Multi-campus Award from the University of California, Office of the President (UCOP #142691). These projects also received support from Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Stanhope is supported by a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958) funded by the National Institute of Child Health and Human Development (NICHD), Office of Research on Women’s Health (ORWH), Office of Dietary Supplements (ODS), and the National Institute of Aging (NIA).
References
- 1.Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care. 2014;37(4):950–6. doi: 10.2337/dc13-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn R, Sievenpiper JL. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: we have, but the pox on sugar is overwrought and overworked. Diabetes Care. 2014;37(4):957–62. doi: 10.2337/dc13-2506. [DOI] [PubMed] [Google Scholar]
- 3.Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24(3):198–206. doi: 10.1097/MOL.0b013e3283613bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Are All Calories Created Equal? An Analysis of the Coca-Cola Company’s Communication in the Fight Against Obesity2014. 2015 Jun 4; Available from: http://www.awpagesociety.com/wp-content/uploads/2014/03/Coca-Cola_CaseStudy.pdf.
- 5.Coca-Cola Joins America’s Beverage Companies and the Alliance for a Healthier Generation in Landmark Partnership to Promote Healthy Lifestyles 2014. 2015 Jun 4; Available from: http://www.coca-colacompany.com/coca-cola-unbottled-old/coca-cola-joins-americas-beverage-companies-and-the-alliance-for-a-healthier-generation-in-landmark-partnership-to-promote-healthy-lifestyles.
- 6.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Jr, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2010;92(4):954–9. doi: 10.3945/ajcn.2010.29478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA. 2010;303(15):1490–7. doi: 10.1001/jama.2010.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123(3):249–57. doi: 10.1161/CIRCULATIONAHA.110.972166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremer AA, Auinger P, Byrd RS. Sugar-Sweetened Beverage Intake Trends in US Adolescents and Their Association with Insulin Resistance-Related Parameters. J Nutr Metab. 2010 doi: 10.1155/2010/196476. Epub 2009 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida M, McKeown NM, Rogers G, Meigs JB, Saltzman E, D’Agostino R, et al. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr. 2007;137(9):2121–7. doi: 10.1093/jn/137.9.2121. [DOI] [PubMed] [Google Scholar]
- 11.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol. 2008;22(10):811–6. doi: 10.1155/2008/810961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–9. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhupathiraju SN, Pan A, Malik VS, Manson JE, Willett WC, van Dam RM, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97(1):155–66. doi: 10.3945/ajcn.112.048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–7. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137(6):1447–54. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 16.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–92. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 18.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125(14):1735–41. S1. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–42. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan TF, Lin WT, Huang HL, Lee CY, Wu PW, Chiu YW, et al. Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents. Nutrients. 2014;6(5):2088–103. doi: 10.3390/nu6052088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denova-Gutierrez E, Talavera JO, Huitron-Bravo G, Mendez-Hernandez P, Salmeron J. Sweetened beverage consumption and increased risk of metabolic syndrome in Mexican adults. Public Health Nutr. 2010;13(6):835–42. doi: 10.1017/S1368980009991145. [DOI] [PubMed] [Google Scholar]
- 22.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 23.Hosseini-Esfahani F, Bahadoran Z, Mirmiran P, Hosseinpour-Niazi S, Hosseinpanah F, Azizi F. Dietary fructose and risk of metabolic syndrome in adults: Tehran Lipid and Glucose study. Nutr Metab (Lond) 2011;8(1):50. doi: 10.1186/1743-7075-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hostmark AT. The Oslo health study: soft drink intake is associated with the metabolic syndrome. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35(5):635–42. doi: 10.1139/H10-059. [DOI] [PubMed] [Google Scholar]
- 25.Odegaard AO, Choh AC, Czerwinski SA, Towne B, Demerath EW. Sugar-sweetened and diet beverages in relation to visceral adipose tissue. Obesity (Silver Spring) 2012;20(3):689–91. doi: 10.1038/oby.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollock NK, Bundy V, Kanto W, Davis CL, Bernard PJ, Zhu H, et al. Greater fructose consumption is associated with cardiometabolic risk markers and visceral adiposity in adolescents. J Nutr. 2012;142(2):251–7. doi: 10.3945/jn.111.150219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batt C, Phipps-Green AJ, Black MA, Cadzow M, Merriman ME, Topless R, et al. Sugar-sweetened beverage consumption: a risk factor for prevalent gout with SLC2A9 genotype-specific effects on serum urate and risk of gout. Ann Rheum Dis. 2014;73(12):2101–6. doi: 10.1136/annrheumdis-2013-203600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, et al. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney international. 2010;77(7):609–16. doi: 10.1038/ki.2009.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin WT, Huang HL, Huang MC, Chan TF, Ciou SY, Lee CY, et al. Effects on uric acid, body mass index and blood pressure in adolescents of consuming beverages sweetened with high-fructose corn syrup. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.121. [DOI] [PubMed] [Google Scholar]
- 30.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added Sugar Intake and Cardiovascular Diseases Mortality Among US Adults. JAMA internal medicine. 2014 doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. 2012;66(2):201–8. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein- 4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9(1):68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5):1562–9. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–9. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 36.Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96(4):727–34. doi: 10.3945/ajcn.112.038695. [DOI] [PubMed] [Google Scholar]
- 37.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–65. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 38.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–36. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 39.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15(5):574–84. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 42.Christian P, Sacco J, Adeli K. Autophagy: Emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta. 2013;1831(4):819–24. doi: 10.1016/j.bbalip.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Fisher EA. The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim Biophys Acta. 2012;1821(5):778–81. doi: 10.1016/j.bbalip.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol. 2012;23(3):206–12. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]
- 45.Sundaram M, Zhong S, Bou Khalil M, Zhou H, Jiang ZG, Zhao Y, et al. Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia. J Lipid Res. 2010;51(6):1524–34. doi: 10.1194/jlr.M005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin W, Sundaram M, Wang Y, Zhou H, Zhong S, Chang CC, et al. Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid precursors within the microsomal lumen. J Biol Chem. 2011;286(31):27769–80. doi: 10.1074/jbc.M110.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng C, Khoo C, Furtado J, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 2010;121(15):1722–34. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–6. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 49.Watt MJ, Hoy AJ. Lipid metabolism in skeletal muscle: generation of adaptive and maladaptive intracellular signals for cellular function. Am J Physiol Endocrinol Metab. 2012;302(11):E1315–28. doi: 10.1152/ajpendo.00561.2011. [DOI] [PubMed] [Google Scholar]
- 50.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–81. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;23(8):391–8. doi: 10.1016/j.tem.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58(5 Suppl):754S–65S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 54.Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, Bavishi C, Lunagaria A, Kottam A, et al. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. European journal of preventive cardiology. 2014 doi: 10.1177/2047487313519346. [DOI] [PubMed] [Google Scholar]
- 55.Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN rheumatology. 2014;2014:852954. doi: 10.1155/2014/852954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai W, Wu X, Zhang B, Miao L, Sun YP, Zou Y, et al. Serum uric acid levels and non-alcoholic fatty liver disease in Uyghur and Han ethnic groups in northwestern China. Arquivos brasileiros de endocrinologia e metabologia. 2013;57(8):617–22. doi: 10.1590/s0004-27302013000800006. [DOI] [PubMed] [Google Scholar]
- 57.Viazzi F, Garneri D, Leoncini G, Gonnella A, Muiesan ML, Ambrosioni E, et al. Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: Insights from the I-DEMAND study. Nutr Metab Cardiovasc Dis. 2014 doi: 10.1016/j.numecd.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 58.Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101(6):1144–54. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab. 2011;96(12):E2034–8. doi: 10.1210/jc.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48(6):983–92. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 61.Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, et al. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr. 2013;98(2):349–57. doi: 10.3945/ajcn.112.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem. 2007;18(1):1–9. doi: 10.1016/j.jnutbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr. 2011;94(2):479–85. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 64.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36(1):150–6. doi: 10.2337/dc12-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Black RN, Spence M, McMahon RO, Cuskelly GJ, Ennis CN, McCance DR, et al. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes. 2006;55(12):3566–72. doi: 10.2337/db06-0220. [DOI] [PubMed] [Google Scholar]
- 66.Lewis AS, McCourt HJ, Ennis CN, Bell PM, Courtney CH, McKinley MC, et al. Comparison of 5% versus 15% sucrose intakes as part of a eucaloric diet in overweight and obese subjects: effects on insulin sensitivity, glucose metabolism, vascular compliance, body composition and lipid profile. A randomised controlled trial. Metabolism. 2013;62(5):694–702. doi: 10.1016/j.metabol.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Marckmann P, Raben A, Astrup A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism. 2000;49(6):731–5. doi: 10.1053/meta.2000.6237. [DOI] [PubMed] [Google Scholar]
- 68.Raben A, Moller BK, Flint A, Vasilaris TH, Christina Moller A, Juul Holst J, et al. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food & nutrition research. 2011;55 doi: 10.3402/fnr.v55i0.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reiser S, Bickard MC, Hallfrisch J, Michaelis OEt, Prather ES. Blood lipids and their distribution in lipoproteins in hyperinsulinemic subjects fed three different levels of sucrose. J Nutr. 1981;111(6):1045–57. doi: 10.1093/jn/111.6.1045. [DOI] [PubMed] [Google Scholar]
- 70.Reiser S, Bohn E, Hallfrisch J, Michaelis OEt, Keeney M, Prather ES. Serum insulin and glucose in hyperinsulinemic subjects fed three different levels of sucrose. Am J Clin Nutr. 1981;34(11):2348–58. doi: 10.1093/ajcn/34.11.2348. [DOI] [PubMed] [Google Scholar]
- 71.Reiser S, Hallfrisch J, Michaelis OEt, Lazar FL, Martin RE, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. I. Effects on levels of fasting blood lipids. Am J Clin Nutr. 1979;32(8):1659–69. doi: 10.1093/ajcn/32.8.1659. [DOI] [PubMed] [Google Scholar]
- 72.Reiser S, Handler HB, Gardner LB, Hallfrisch JG, Michaelis OEt, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. II. Effect on fasting blood insulin, glucose, and glucagon and on insulin and glucose response to a sucrose load. Am J Clin Nutr. 1979;32(11):2206–16. doi: 10.1093/ajcn/32.11.2206. [DOI] [PubMed] [Google Scholar]
- 73.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006. Crit Rev Food Sci Nutr. 2010;50(3):228–58. doi: 10.1080/10408391003626223. [DOI] [PubMed] [Google Scholar]
- 75.DGAC. Report of the Dietary Guidelines Advisory Committee (DGAC) on the Dietary Guidelines for Americans, 2010. 2010 http://wwwcnppusdagov/DGAs2010-DGACReporthtm.
- 76.Stanhope KL. Role of Fructose-Containing Sugars in the Epidemics of Obesity and Metabolic Syndrome. Annu Rev Med. 2012 doi: 10.1146/annurev-med-042010-113026. [DOI] [PubMed] [Google Scholar]
- 77.Tappy L, Mittendorfer B. Fructose toxicity: is the science ready for public health actions? Curr Opin Clin Nutr Metab Care. 2012;15(4):357–61. doi: 10.1097/MCO.0b013e328354727e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2013;38(6):681–8. doi: 10.1139/apnm-2012-0322. [DOI] [PubMed] [Google Scholar]
- 79.Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res. 2013;33(12):1043–52. doi: 10.1016/j.nutres.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 80.Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142(5):916–23. doi: 10.3945/jn.111.151951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang DD, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232(1):125–33. doi: 10.1016/j.atherosclerosis.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 82.Bes-Rastrollo M, Schulze MB, Ruiz-Canela M, Martinez-Gonzalez MA. Financial Conflicts of Interest and Reporting Bias Regarding the Association between Sugar-Sweetened Beverages and Weight Gain: A Systematic Review of Systematic Reviews. PLoS Med. 2013;10(12):e1001578. doi: 10.1371/journal.pmed.1001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massougbodji J, Le Bodo Y, Fratu R, De Wals P. Reviews examining sugar-sweetened beverages and body weight: correlates of their quality and conclusions. Am J Clin Nutr. 2014;99(5):1096–104. doi: 10.3945/ajcn.113.063776. [DOI] [PubMed] [Google Scholar]
- 84.Global Consumption of Dairy Products by Country (Annual) 2013 Dec 7; Version 12 November 2013. Available from: http://www.dairyinfo.gc.ca/index_e.php?s1=dff-fcil&s2=cons&s3=consglo.
- 85.Dugan CE, Fernandez ML. Effects of Dairy on Metabolic Syndrome Parameters: A Review. The Yale journal of biology and medicine. 2014;87(2):135–47. [PMC free article] [PubMed] [Google Scholar]
- 86.Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr. 2014;99(5 Suppl):1235S–42S. doi: 10.3945/ajcn.113.073015. [DOI] [PubMed] [Google Scholar]
- 87.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45(10):925–39. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr. 2013;33:105–28. doi: 10.1146/annurev-nutr-071811-150642. [DOI] [PubMed] [Google Scholar]
- 89.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 90.Rangan A, Allman-Farinelli M, Donohoe E, Gill T. Misreporting of energy intake in the 2007 Australian Children’s Survey: differences in the reporting of food types between plausible, under- and over-reporters of energy intake. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2013 doi: 10.1111/jhn.12182. [DOI] [PubMed] [Google Scholar]
- 91.Lipton E. Rival Industries Sweet-Talk the Public. The New York Times. 2014 [Google Scholar]
- 92.Rippe JM, Angelopoulos TJ. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: what do we really know? Advances in nutrition. 2013;4(2):236–45. doi: 10.3945/an.112.002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lowndes J, Sinnett S, Pardo S, Nguyen VT, Melanson KJ, Yu Z, et al. The effect of normally consumed amounts of sucrose or high fructose corn syrup on lipid profiles, body composition and related parameters in overweight/obese subjects. Nutrients. 2014;6(3):1128–44. doi: 10.3390/nu6031128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lowndes J, Sinnett S, Yu Z, Rippe J. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients. 2014;6(8):3153–68. doi: 10.3390/nu6083153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang YH, An T, Zhang RC, Zhou Q, Huang Y, Zhang J. Very high fructose intake increases serum LDL-cholesterol and total cholesterol: a meta-analysis of controlled feeding trials. J Nutr. 2013;143(9):1391–8. doi: 10.3945/jn.113.175323. [DOI] [PubMed] [Google Scholar]
- 96.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100(1):65–79. doi: 10.3945/ajcn.113.081521. [DOI] [PubMed] [Google Scholar]
- 97.Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30(5):503–10. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 98.Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(3):833–49. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008;100(5):947–52. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crapo PA, Kolterman OG. The metabolic effects of 2-week fructose feeding in normal subjects. Am J Clin Nutr. 1984;39(4):525–34. doi: 10.1093/ajcn/39.4.525. [DOI] [PubMed] [Google Scholar]
- 101.Huttunen JK, Makinen KK, Scheinin A. Turku sugar studies XI. Effects of sucrose, fructose and xylitol diets on glucose, lipid and urate metabolism. Acta odontologica Scandinavica. 1976;34(6):345–51. doi: 10.3109/00016357609004646. [DOI] [PubMed] [Google Scholar]
- 102.Forster H, Heller G. Studies on the significance of carbohydrates in a fully synthetic fat-free diet. Deutsche medizinische Wochenschrift. 1973;98(23):1156–63. doi: 10.1055/s-0028-1106986. [DOI] [PubMed] [Google Scholar]
- 103.Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Perez-Mendez O, et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism. 2011;60(11):1551–9. doi: 10.1016/j.metabol.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Swanson JE, Laine DC, Thomas W, Bantle JP. Metabolic effects of dietary fructose in healthy subjects. Am J Clin Nutr. 1992;55(4):851–6. doi: 10.1093/ajcn/55.4.851. [DOI] [PubMed] [Google Scholar]
- 105.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–20. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 106.DGAC. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015 Available from: http://www.health.gov/dietaryguidelines/2015-scientific-report/
- 107.Daugherty BL, Schap TE, Ettienne-Gittens R, Zhu FM, Bosch M, Delp EJ, et al. Novel technologies for assessing dietary intake: evaluating the usability of a mobile telephone food record among adults and adolescents. Journal of medical Internet research. 2012;14(2):e58. doi: 10.2196/jmir.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Illner AK, Freisling H, Boeing H, Huybrechts I, Crispim SP, Slimani N. Review and evaluation of innovative technologies for measuring diet in nutritional epidemiology. International journal of epidemiology. 2012;41(4):1187–203. doi: 10.1093/ije/dys105. [DOI] [PubMed] [Google Scholar]
- 109.Kerr DA, Pollard CM, Howat P, Delp EJ, Pickering M, Kerr KR, et al. Connecting Health and Technology (CHAT): protocol of a randomized controlled trial to improve nutrition behaviours using mobile devices and tailored text messaging in young adults. BMC public health. 2012;12:477. doi: 10.1186/1471-2458-12-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yudkin J. Metabolic changes induced by sugar in relation to coronary heart disease and diabetes. Nutrition and health. 1987;5(1–2):5–8. doi: 10.1177/026010608700500202. [DOI] [PubMed] [Google Scholar]
- 111.Yudkin J. Dietary factors in arteriosclerosis: sucrose. Lipids. 1978;13(5):370–2. doi: 10.1007/BF02533732. [DOI] [PubMed] [Google Scholar]
- 112.Szanto S, Yudkin J. Plasma lipids, glucose tolerance, insulin levels and body-weight in men after diets rich in sucrose. Proc Nutr Soc. 1969;28(1):11A–2A. [PubMed] [Google Scholar]
- 113.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14(8):606–19. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaiser KA, Shikany JM, Keating KD, Allison DB. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev. 2013;14(8):620–33. doi: 10.1111/obr.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89(1):438–9. doi: 10.3945/ajcn.2008.26980. author reply 9–40. [DOI] [PubMed] [Google Scholar]
- 116.Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am J Clin Nutr. 2008;87(6):1662–71. doi: 10.1093/ajcn/87.6.1662. [DOI] [PubMed] [Google Scholar]
- 117.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cottrell RC, Wittekind A. Conclusions of review of dietary sugars and body weight are unwarranted. Bmj. 2013;346:f1238. doi: 10.1136/bmj.f1238. [DOI] [PubMed] [Google Scholar]
- 119.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Bmj. 2013;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 120.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63(5):133–57. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 121.Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, et al. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139(2):551–8. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 122.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89(6):2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 123.Rezvani R, Cianflone K, McGahan JP, Berglund L, Bremer AA, Keim NL, et al. Effects of sugar-sweetened beverages on plasma acylation stimulating protein, leptin and adiponectin: relationships with metabolic outcomes. Obesity (Silver Spring) 2013;21(12):2471–80. doi: 10.1002/oby.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 125.Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89(3):980S–4S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 126.Balcells E, Delgado-Noguera M, Pardo-Lozano R, Roig-Gonzalez T, Renom A, Gonzalez-Zobl G, et al. Soft drinks consumption, diet quality and BMI in a Mediterranean population. Public Health Nutr. 2011;14(5):778–84. doi: 10.1017/S1368980010002788. [DOI] [PubMed] [Google Scholar]
- 127.Bermudez OI, Gao X. Greater consumption of sweetened beverages and added sugars is associated with obesity among US young adults. Annals of nutrition & metabolism. 2010;57(3–4):211–8. doi: 10.1159/000321542. [DOI] [PubMed] [Google Scholar]
- 128.Bremer AA, Byrd RS, Auinger P. Differences in male and female adolescents from various racial groups in the relationship between insulin resistance-associated parameters with sugar-sweetened beverage intake and physical activity levels. Clin Pediatr (Phila) 2010;49(12):1134–42. doi: 10.1177/0009922810379043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 130.Rhee JJ, Mattei J, Campos H. Association between commercial and traditional sugar-sweetened beverages and measures of adiposity in Costa Rica. Public Health Nutr. 2012;15(8):1347–54. doi: 10.1017/S1368980012001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rao SS, Attaluri A, Anderson L, Stumbo P. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5(8):959–63. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27(10):1161–8. doi: 10.1136/gut.27.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 134.Burger KS, Stice E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. Am J Clin Nutr. 2012;95(4):810–7. doi: 10.3945/ajcn.111.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burger KS, Stice E. Neural responsivity during soft drink intake, anticipation, and advertisement exposure in habitually consuming youth. Obesity (Silver Spring) 2014;22(2):441–50. doi: 10.1002/oby.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of general psychiatry. 2011;68(8):808–16. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Griffioen-Roose S, Smeets PA, Weijzen PL, van Rijn I, van den Bosch I, de Graaf C. Effect of replacing sugar with non-caloric sweeteners in beverages on the reward value after repeated exposure. PLoS ONE. 2013;8(11):e81924. doi: 10.1371/journal.pone.0081924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kilpatrick LA, Coveleskie K, Connolly L, Labus JS, Ebrat B, Stains J, et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology. 2014;146(5):1212–21. doi: 10.1053/j.gastro.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ng J, Stice E, Yokum S, Bohon C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite. 2011;57(1):65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309(1):63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Purnell JQ, Klopfenstein BA, Stevens AA, Havel PJ, Adams SH, Dunn TN, et al. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab. 2011;13(3):229–34. doi: 10.1111/j.1463-1326.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 142.Stice E, Burger KS, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr. 2013;98(6):1377–84. doi: 10.3945/ajcn.113.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luo S, Monterosso JR, Sarpelleh K, Page KA. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc Natl Acad Sci U S A. 2015;112(20):6509–14. doi: 10.1073/pnas.1503358112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, et al. Excessive Sugar Consumption May Be a Difficult Habit to Break: A View From the Brain and Body. J Clin Endocrinol Metab. 2015;100(6):2239–47. doi: 10.1210/jc.2014-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51(6):963–9. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 146.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 147.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr. 2007;97(1):193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- 148.Raben A, Macdonald I, Astrup A. Replacement of dietary fat by sucrose or starch: effects on 14 d ad libitum energy intake, energy expenditure and body weight in formerly obese and never-obese subjects. Int J Obes Relat Metab Disord. 1997;21(10):846–59. doi: 10.1038/sj.ijo.0800494. [DOI] [PubMed] [Google Scholar]
- 149.van Baak MA, Astrup A. Consumption of sugars and body weight. Obes Rev. 2009;10(Suppl 1):9–23. doi: 10.1111/j.1467-789X.2008.00561.x. [DOI] [PubMed] [Google Scholar]
- 150.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2011;30(4):424–9. doi: 10.1037/a0023467. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 151.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005–2010. NCHS data brief. 2013;(122):1–8. [PubMed] [Google Scholar]
- 152.McCarthy J. Americans More Likely to Avoid Drinking Soda Than Before. 2014 Available from: http://www.gallup.com/poll/174137/americans-likely-avoid-drinking-soda.aspx.
- 153.Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc. 2009;109(3):430–7. doi: 10.1016/j.jada.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cassady BA, Considine RV, Mattes RD. Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr. 2012;95(3):587–93. doi: 10.3945/ajcn.111.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tournier A, Louis-Sylvestre J. Effect of the physical state of a food on subsequent intake in human subjects. Appetite. 1991;16(1):17–24. doi: 10.1016/0195-6663(91)90107-4. [DOI] [PubMed] [Google Scholar]
- 156.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 157.Petrunoff NA, Wilkenfeld RL, King LA, Flood VM. ‘Treats’, ‘sometimes foods’, ‘junk’: a qualitative study exploring ‘extra foods’ with parents of young children. Public Health Nutr. 2014;17(5):979–86. doi: 10.1017/S1368980012005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rampersaud GC, Kim H, Gao Z, House LA. Knowledge, perceptions, and behaviors of adults concerning nonalcoholic beverages suggest some lack of comprehension related to sugars. Nutr Res. 2014;34(2):134–42. doi: 10.1016/j.nutres.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 159.Baskin ML, Herbey I, Williams R, Ard JD, Ivankova N, Odoms-Young A. Caregiver perceptions of the food marketing environment of African-American 3–11-year-olds: a qualitative study. Public Health Nutr. 2013;16(12):2231–9. doi: 10.1017/S1368980013001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chandon P, Wansink B. Does food marketing need to make us fat? A review and solutions. Nutr Rev. 2012;70(10):571–93. doi: 10.1111/j.1753-4887.2012.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gallucci M. As Mexico’s Sugary Drink Tax Turns 1 Year Old, US Health Proponents Hope It Can Sway American Voters. 2015 Available from: http://www.ibtimes.com/mexicos-sugary-drink-tax-turns-1-year-old-us-health-proponents-hope-it-can-sway-1779632.
- 162.Evich HB. Berkeley breaks through on soda tax. 2014 Jan 12;2105 Available from: http://www.politico.com/story/2014/11/berkeley-breaks-through-on-soda-tax-112570.html. [Google Scholar]
- 163.Rogers R. Spending on campaign to defeat Richmond soda tax nears $2.5 million 2012. 2015 Jan 12; Available from: http://www.mercurynews.com/breaking-news/ci_21857220/more-than-2-million-spending-richmond-soda-tax.
- 164.Shields J. Big Beverage gives $10 million to CHOP. 2011 Available from: http://www.philly.com/philly/blogs/heardinthehall/118077483.html?c=r.
- 165.Jacobson MF, Brownell KD. Small taxes on soft drinks and snack foods to promote health. American journal of public health. 2000;90(6):854–7. doi: 10.2105/ajph.90.6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.What Does the Science Say? 2015 updated October 31, 2014; cited 2015 January 13, 2015. Available from: http://www.sugar.org/sugar-your-diet/what-does-the-science-say/
- 167.What the Science Says about HFCS. 2015 updated January 5, 2015January 13, 2015. Available from: http://sweetsurprise.com/what-science-says-about-hfcs.
- 168.Beverage Industry Responds To Study On Added Sugar Intake And Cardiovascular Diseases Mortality. 2014 updated February 3, 2014January 14, 2015. Available from: http://www.ameribev.org/news-media/news-releases-statements/more/325/