Abstract
We present a systematic investigation of well-characterized, experimentally pure polystyrene (PS) rings with molar mass of 161 000 g/mol in dilute solutions. We measure the ring form factor at θ- and good-solvent conditions as well as in a polymeric solvent (linear PS of roughly comparable molar mass) by means of small-angle neutron scattering (SANS). Additional dynamic light scattering (DLS) measurements support the SANS data and help elucidate the role of solvent quality and solution preparation. The results indicate the increase of ring dimensions as the solvent quality improves. Furthermore, the experimental form factors in both θ-solvent and linear matrix behave as ideal rings and are fully superimposable. The nearly Gaussian conformations of rings in a melt of linear chains provide evidence of threading of linear chains through rings. The latter result has implications for the dynamics of ring–linear polymer mixtures.
Graphical Abstract
I. INTRODUCTION
Ring polymers have always been considered exceptional and fascinating because they have no free ends. Yet, it is established that the presence of free ends in polymers is important for the understanding of their motion. Hence, questions relating to the size and dynamics of ring polymers have been of interest for a long time. Ring polymers are also important in biology. For example, mitochondiral and plasmic DNA are cyclic, and therefore, ring polymers are ideal models for a number of fundamental biophysical problems.1–5,53 The organization of chromatin in the cell nucleus can be understood by drawing analogies to the conformation and dynamics of densely packed rings. Chromatin fibers are packed in vivo at a reasonably high density, like a melt of linear chains. However, the different chromosomes in the nucleus do not intermix, but instead segregate in different stable distinct regions, by analogy with the conformation in a melt of pure nonconcatenated rings. This type of segregated conformation appears typical for the cells of higher eukaryotes, including humans. In addition, the fundamental understanding of structure and dynamics of rings is particularly relevant to applications ranging from DNA separation to enzymology and from protein structure stabilization to drug delivery.3,4 Therefore, a thorough understanding of the physics of ring polymers is necessary for many of their applications. In spite of the progress made to date in both experimental5–7 and simulation8,9 studies of rings, a number of issues remain elusive. For example, the adjustment of a ring conformation to changing environment (e.g., solvent quality or mixture) is very important for understanding its dynamics, yet its detailed investigation remains elusive. In the same light the issue of whether cyclization conditions like e.g. the choice of a θ-solvent or a good solvent, which locks in different fractions of knots, lead to measurable different chain dimensions and according form factor modifications is still to be solved.
Here, we address this fundamental challenge by studying the role of solvent quality on the conformation and size of well-characterized, i.e., critically fractionated rings. We consider polystyrene (PS) rings of weight-average molar mass 161 000 g/mol that were synthesized in cyclohexane, which is a θ-solvent.10 The ring polymer was studied in three different solvents: d8-toluene (a good solvent), d12-cyclohexane (a θ-solvent at 31.5 °C), and a melt of linear chains of comparable molar mass. In the latter case excluded volume interactions are expected to be screened based on Flory’s theorem,11 and this blend is mainly used in order to explore the entropic ring–linear interactions. To support our results, we provide additional experimental evidence with another sample of well-characterized polystyrene rings, synthesized in a THF/heptane mixture, but having a different molar mass of 13 800 g/mol.12 The article is organized as follows: in section II we present the materials and methods and in section III the results and interpretation. The main conclusions are summarized in section IV.
II. EXPERIMENTAL SECTION
II.1. Materials
The PS rings with weight-average molar mass Mw = 161 000 g/mol were synthesized anionically in dilute solution of a θ-solvent cyclohexane, as described in ref 10. This procedure reduces the probability of uncoupled linear chains, although it does not completely eliminate it.13 However, although not explicitly mentioned in ref 10, the ring closure was indeed started in cyclohexane, but the successive addition of the living polymer must have created rapidly a good solvent environment. In particular, the linear difunctional precursor was anionically prepared in a 50/50 mixture of benzene/THF (about 50 mL) at 0 °C with sodium naphthalenide as the initiator. The cyclization for this sample was brought about through stepwise addition of small amounts of the linear precursor solution to a larger amount of cyclohexane (about 100–120 mL) at 25 °C with simultaneous slow introduction of a less than stoichiometric amount of dimethyldichlorosilane as the coupling agent.10,31 Hence, while these initial conditions may be below the θ-temperature, the increasing presence of added benzene and THF should rapidly produce good solvent conditions. However, we have shown that even the possible presence of a few knots does not affect the dynamic6 properties of rings, and our results here indicate that their conformation is Gaussian. The sample was subsequently purified by the liquid chromatography at the critical condition (LCCC) procedure14,5 and characterized by size exclusion chromatography (SEC). Following the above procedure, we obtained an “as pure as currently possible” ring PS sample, coded further as R161. A sample of smaller PS rings with weight-average molar mass 13 800 g/mol was synthesized in a THF/heptane mixture,12 purified by LCCC, characterized by SEC, and coded as R14. In addition, fully hydrogenous and deuterated linear PS samples were obtained by anionic polymerization with sec-BuLi in benzene and characterized by SEC and off-line light scattering. The weight-average molar masses of these two linear samples were virtually identical (250 000 ± 4000 g/mol). All polymers had polydispersity indices Mw/Mn < 1.1.
II.2. Small-Angle Neutron Scattering (SANS)
SANS experiments were performed at the KWS1 spectrometer of the Jülich Centre for Neutron Science at the FRJ-2 research reactor in Forschungszentrum, Jülich, Germany. Data were collected at different sample-to-detector distances to cover a scattering vector range q between 0.002 and 0.2 Å−1 using a neutron wavelength λ = 7 Å and wavelength spread Δλ/λ = 0.20. The scattering vector q is related to the scattering angle θ via q = (4π/λ) sin(θ/2). Two-dimensional recorded scattering intensities were collected in 128 × 128 channels each of 5.2 mm × 5.2 mm and corrected pixel-wise in a standard way for empty cell, background, dark current scattering, and detector sensitivity, after which the radial averaging was performed. Transmissions were measured in situ in forward direction at q = 0 using a neutron monitor inside the beam stop. Absolute intensities [cm−1] were obtained by calibration with a 1.5 mm thick secondary Plexiglas standard. Dilute solutions of ring R161 in d8-toluene (about 0.5 wt %) and d12-cyclohexane (about 1 wt %) were contained in Hellma quartz cells with path lengths 1 mm. The overlap concentrations for rings in these solvents are estimated to be 1.8 and 14.7 wt %, respectively. The cells were held in a brass furnace at a temperature 35 ± 1 °C which implied a minimum temperature of 33 ± 0.5 °C in the sample, based on a calibration curve that takes into account as good as possible temperature gradients over the furnace, the cells, and their contents. This temperature is close to or just above the expected θ-temperature of hydrogenous PS rings in d12-cyclohexane (vide inf ra) within the uncertainties of the experiment. As a consistency check, the θ-temperature was independently redetermined from SANS measurements of the smaller R14 sample in d12-cyclohexane in a concentration range 0.8–2.4 wt % (dilute). Eventual deviations from the above temperature by a few degrees are not expected to affect the SANS results (33 °C < T < 35 °C).15 The blend of hydrogenous rings and deuterated linear chains as well as a reference isotopic blend consisting of pure linear chains was prepared by solution blending with subsequent vacuum drying. At the SANS concentration, no effects of χ-interactions between the components are expected. The blends were press-molded under vacuum at T = 150 °C for 20 min to a thickness of 1 mm. The samples were fixed at a cadmium frame which was positioned right before the outcoming neutron beam.
II.3. Dynamic Light Scattering
The measurements of ring and linear polystyrenes in dilute solution (at concentrations c ≤ 0.5 wt %) in both solvents were carried out using an ALV goniometer setup and an ADLAS Nd:YAG laser operating at λ = 5320 Å. The Brownian motion of the polymer was detected through the concentration fluctuations of solutions at different scattering wavevectors q = (4πn/λ) sin(θ/2), where n is the refractive index of the solvent and θ is the scattering angle. The time autocorrelation function of the scattered intensity G(q,t) was determined with the aid of an ALV-5,000/E fast multi-τ correlator in the time range 10−7–103 s. The measurement consisted of obtaining the intermediate scattering (field) function C(q,t) = [(G(q,t) − 1)/f*]1/2 in the polarized (VV) geometry, where f* is an instrumental factor relating to the coherence area.16 The data were analyzed using the CONTIN program which yielded the distribution of relaxation times. From the q-dependence of the characteristic relaxation time at the peak of a mode’s distribution, the respective diffusion coefficient D was extracted. The hydrodynamic radius Rh was calculated from the measured diffusion coefficient using the Stokes–Einstein–Sutherland relation, Rh = kT/(6πηD), where k is the Boltzmann constant and η is the solvent viscosity.
III. RESULTS AND DISCUSSION
III.1. SANS from Dilute Ring Solution
There are only limited scattering studies of the structure of ring polymers, 12,17–19 due to the limited availability of rings, and especially highly pure rings. The differences in the structure between rings and linear chains are associated with the closed topology of the ring polymer. We use SANS as the ideal microscopic tool to validate the ring shape in three different solvents as well as to verify the absence of quantifiable amounts of linear impurities. For the present R161 sample the contamination after LCCC was detected rheologically and reported to be below 0.1 wt %.5 Whereas such a low percentage of linear chains most probably cannot be detected in SANS, the most sensitive low-to-intermediate q-region in the form factor may be affected. This led to the necessity to focus on the best possible ring samples, both obtained from LCCC fractionation as discussed above. The temperature control of the experiment is crucial for the investigation of the unperturbed polymer dimensions. The θ-temperature of R14 was obtained from temperature-dependent SANS measurements. Figure 1 depicts the extracted second virial coefficient A2 as a function of temperature in d12-cyclohexane. These results were obtained from simultaneous fits of the form factors at three different concentrations well below c*. The extrapolation to A2 = 0 yields a value of θ = 35 ± 1 °C for the R14 ring polymer.15 This result can be rationalized by using literature estimates20–22 and taking into account deuterium composition and structural effects. The θ-temperature of a hydrogenous PS ring in cyclohexane was reported earlier to be 27.7 °C,20 whereas for its linear counterpart a value of 34.5 °C was found. The cyclization thus lowers the θ-temperature by 6.8 °C.23 Considering experimental uncertainties, a temperature decrement of 6.8 ± 0.3 °C due to the ring structure is reasonable. Isotopic changes due to solvent alteration from h12-cyclohexane to its d12-analogue lead to an experimental temperature increment of 3.8 °C, i.e., a θ-temperature of linear PS of 38.3 °C.24 This increment, which depends slightly on molecular weight, was also estimated by Siporska et al.,21,25 who found a similar value, as 3.5 °C. Hence, entirely on the basis of experimental facts,24 we estimate the θ-temperature of the R161 PS ring to be 31.5 ± 1 °C (i.e., close to the extracted value of 35 ± 1 °C for R14 which exhibits a broader θ-range due to the lower molecular weight). For practical reasons, the SANS measurements of R161 in d12-cyclohexane and d8-toluene were performed at the same temperature of 33 ± 1 °C, which is just above its θ-temperature. As such, no precipitation or appreciable collapsing is to be expected. The same temperature was used in ref 26. We note, however, that a systematic study of the isotope effect the θ-decrement upon cyclization as well as the presence of knots7,52 and its molecular weight dependence, although interesting, has not been pursued at this stage as it was not within the scope of the present investigation.
Figure 1.
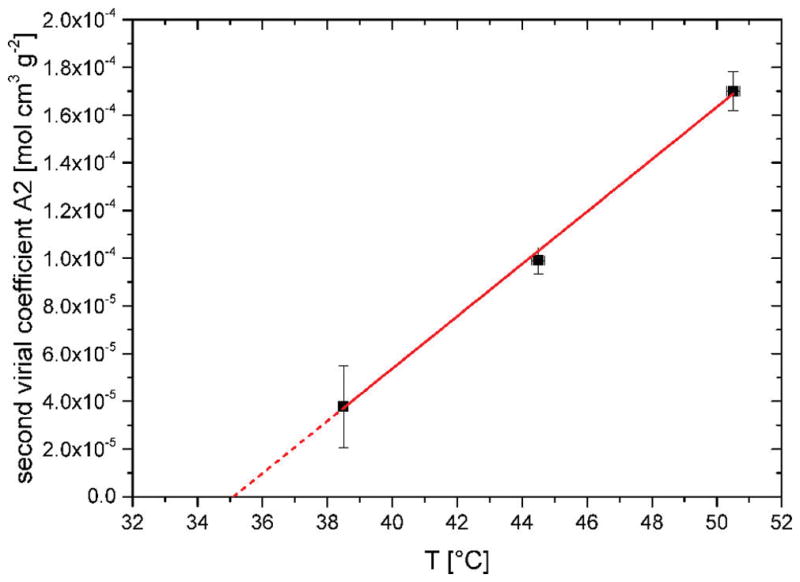
Second virial coefficient of R14 in d12-cyclohexane as a function of temperature, obtained from SANS.14 The average extrapolated θ-temperature is about 35 ± 1 °C. The maximum width of uncertainty is determined from the error bars to be about ±3 °C.
Since in this study we shall modify the extent of excluded volume interactions through the choice of solvent, second virial coefficients A2 will be included in the SANS analysis as described below. First, we consider the form factor of an ideal cyclic polymer without interactions. The mean-square distance between any pair of monomers i and j out of N monomers of the ring with statistical segment length l can be written within the Gaussian approximation as19,27,28
| (1) |
where the subscript “0” represents unperturbed chain dimensions.
Similarly, any mean-square distance between monomers i and j on a linear chain in a good solvent can be estimated within Flory’s mean-field approximation27,29 as
| (2) |
where we define 1 + ε = 2ν, ν being the excluded volume exponent. The ε-notation is chosen for easier comparison to the literature. For cyclic polymers, Bensafi et al.19 presented various expressions for the form factor of dilute rings in good solvents. They differ only in the empirical treatment of the closure condition. For simplicity, we use the Zimm–Bloomfield result30:
| (3) |
Very similar fits are obtained for two other models presented in ref 18. Since the treatment of the closure in good solvents is not analytical and always leads to empirical formulations, we do not concentrate in the present paper on the differences between the approximations, which are beyond the scope of this article. Our focus here is to perform a self-consistent comparison of the size and conformation of rings in different solvents.
The form factor P(q) of ring polymers in the general discrete representation is then given by
| (4) |
and is thus determined by the mean-squared distance between pairs of monomers only. The latter can be simplified as
| (5) |
Figure 2 presents the scattering function I(q) of the polystyrene rings in three different solvents.32 It is characterized by three main features demonstrated for the good solvent case: at low q values the typical Guinier region is observed. Here, the exponential decay of I(q) ~ exp(−(qRg)2) is valid up to q < 2/Rg. At intermediate scattering vectors (q ≥ 3 × 10−2 Å−1) the experimental intensity decays with a steeper q-dependence in a θ-solvent than in the good solvent case. At the highest q values in the so-called Porod region, the statistics of the chain can be identified. For random walk statistics the scattering intensity scales as q−2 whereas in the good solvent limit (like d8-toluene) it is expected to scale as q−1/ν, where ν = 0.59 is the scaling exponent in the good solvent. The increase of intensity in the lowest q range for both θ-solvent and linear polymer matrix is discussed later.
Figure 2.
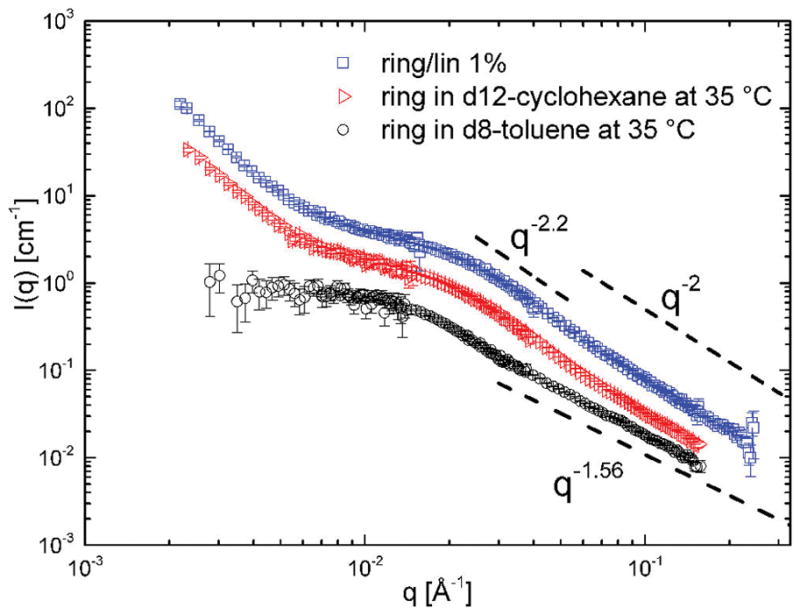
Scattering functions and representative slopes for the overall and internal structure of ring polymers in various solvents at different length scales. The linear polymeric matrix in the ring/linear blend is congruent with the θ-solvent.
The ring nature can be prominently identified from the Kratky representation of the intensities, i.e., from the plot of Iq2 as a function of scattering vector q, e.g., as shown in Figure 3. A peak is expected at qRg ~ 2.05 according to the numerical calculation of a ring form factor in θ-solvent.19 This is consistent with a more compact structure of cyclic polymers in comparison to linear polymer configurations. The peak position allows for a rough size estimate only.
Figure 3.
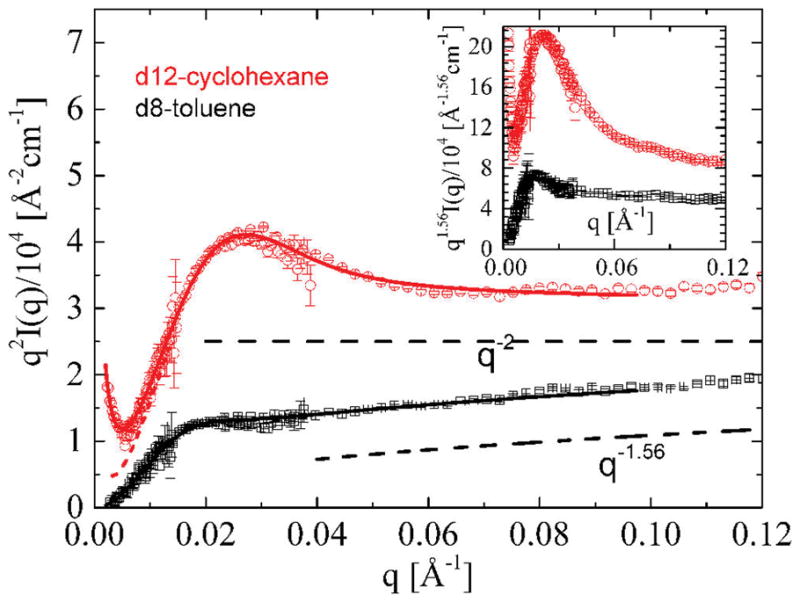
Kratky plot of rings dissolved in different solvents. Inset: a characteristic peak in either solvent is prominent in generalized Kratky plots with scaled ordinate axes Iq1/ν. Solid lines are best fits to the data using eq 4. Dashed lines represent the limiting slopes of I(q) vs q curves of Figure 2. The upturn at the lowest q is due to the parasitic scattering. Its contribution was computed as Aq−B. Dashed line is the form factor calculation without considering the latter.
Having introduced the form factor of an ideal ring polymer, the scattered intensity in different solvents can be evaluated from absolute scattering intensities by including two-body interactions using the second virial coefficient A2. This yields
| (6) |
In eq 6, Δρ2 is the neutrons contrast factor between the solute and the solvent, NA is the Avogadro number, ϕ = c/d is the volume fraction at mass concentration c, d the bulk density, and Vw the molar volume of the (ring) polymer. I(q) is given in [cm−1] and A2 in [cm3 mol/g2]. From inspection of eq 6 it is expected that the forward scattering (at q = 0) is lowered. Moreover, for low q, expanding P(q) yields the classical Guinier expression (A2 = 0) or the more general Zimm approximation (A2 ≠ 0).31
At the lowest range of scattering vector q another distinctly different power law behavior ~q−α with 3 < α < 4 is observed in Figure 2 for both θ-solvent and linear polymer matrix. This q-range excludes any relation to the PS ring dimensions and relates to length scales of the order of 700 Å, if a description by means of a Beaucage approach is assumed.33 The excellent comparison of the experimental extrapolated forward scattering intensities P(q → 0) with the computation on the basis of the volume fraction, Mw, and A2 = 0 following eq 6 points to other origins.34 The low-q/large-scale dynamics in the θ-solvent cyclohexane was investigated by DLS, and the results are discussed in section III.2. Concerning the further form factor analysis from SANS, the parasitic intensity was treated as an incoherently added signal and optimized along with the form factor which is affected by less than 1%. With all these facts, the scattering results can be interpreted as follows:
θ-State
A simple Guinier fitting of the lowest q-data yields a radius of gyration, independent of any assumptions about the shape of the particle and in the absence ofinteractions except for the parasitic (low-q upturn) scattering treatment. In d12-cyclohexane, the chain size of R161 corresponds to Rg,0 = 72.3 ± 0.7 Å. Here, the scattering vector range q < 0.02 Å−1 was used. The best determination is obtained if one considers the full accessed q-range in θ-state, allowing for a residual second virial coefficient parameter to be fitted. Therewith an estimate of A2 = (4 ± 0.2) × 10−4 cm3 mol/g2 and a ring size (Figures 2, 3, and 4) Rg0 = 76 ± 0.5 Å result. This value is in good accordance with the approximate size estimate from the experimental peak in the Kratky plot of our data, which is observed at qpeak ≈ 0.0275 ± 0.002 Å−1, yielding Rg0 ≈ 75 ± 6 Å. The latter is based on an assumption of Gaussian ring conformation. Note that the peak coincides with the crossover of two different q-dependencies as discussed earlier in the context of Figure 2. The ring dimensions are summarized in Table 1.
Figure 4.
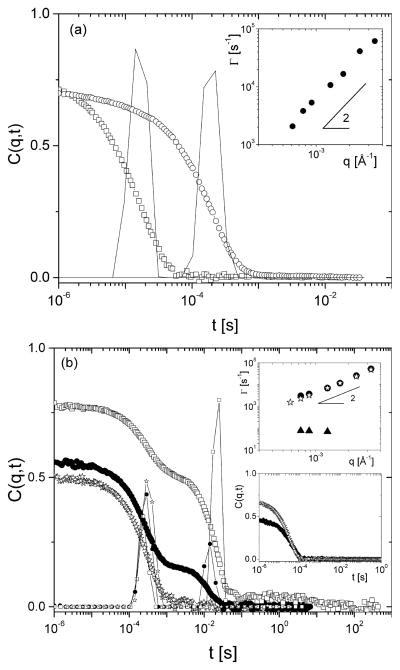
(a) Intermediate light scattering function for a ring PS R161 solution in d8-toluene (0.5 wt %) at 33 °C. Data are shown for the lowest and highest scattering angles, 30° (circles) and 150° (squares). The respective distributions of relaxation times obtained from CONTIN analysis are also shown. Inset: the q-dependence of the extracted characteristic relaxation rate. (b) Intermediate light scattering function for the same R161 solution in d12-cyclohexane (0.012 wt %) and different temperatures and scattering angles, indicated in the plot: 33 °C and 30° (open squares); 40 °C and 30° (bold circles); 50 °C and 30° (open stars). The respective distributions of relaxation times obtained from CONTIN analysis are also shown. Insets: (top) Respective q-dependent characteristic relaxation rates at 40 and 50 °C. The slow mode 40 °C is depicted by bold triangles. (bottom) Intermediate light scattering function for a linear PS (250 000 g/mol) solution in d8-toluene (0.024 wt %) at a scattering angle of 150° and two temperatures, 25 °C (bold symbols) and 50 °C (open symbols).
Table 1.
Extracted Experimental Sizes of R161 PS Ring in Different Solvents
| size | solvent
|
||
|---|---|---|---|
| d12-cyclohexane | d8-toluene | linear PS | |
| Rg,peak [Å] | 75 ± 6 | 105 ± 8 | 75 ± 6 |
| Rg,P(Q) [Å] | 76 ± 0.5 | 108 ± 0.8 | 72 ± 0.7 |
| A2 [cm3 mol g−2] | (4 ± 0.2) × 10−4 | (1.04 ± 0.08) × 10−3 | 0 |
Good Solvent
For rings in good solvent d8-toluene there is no peak in the classical Kratky (i.e., Iq2 vs q) representation. The disappearance of the peak is due to the different excluded volume exponents (0.59 instead of 0.5). However, a pronounced peak reappears at q ≈ 0.02 Å−1 if one plots Iq1.6 instead of Iq2 vs q. A rough estimate of the swollen ring Rg from the peak position (which is solvent dependent) as discussed above yields 105 Å. Likewise, from a Guinier fitting neglecting deliberately the second virial interactions, an apparent Rg = 108 ± 0.7 Å can be derived. The latter analysis is hampered, however, by the nonseparability of the polymer–solvent interactions (A2), as can be easily seen from eq 6 in the Guinier expression of ln[I(q)] vs q2.
Finally, the full form factor of the ring polymer in the respective solvent was evaluated. We have again applied the Bloomfield–Zimm description for the ring19,30 and took into account explicitly non-negligible ring–solvent interactions (A2). The swollen ring in d8-toluene was fitted exactly (as discussed above) in the full q-range, and we obtained Rg = 108 ± 0.8 Å. The best-fit excluded-volume-related exponent was ε = 0.093 ± 0.004, with Rg2 ~ N1+ε, i.e., conforming to earlier studies by Roovers35 and Fetters et al.36 With only one concentration measured due to the minute amount of LCCC-purified ring available, a value of A2 = (1.04 ± 0.08) × 10−3 cm3 mol/g2 > 0 was extracted, which is about 2 orders of magnitude larger than that in cyclohexane. This value differs somewhat from reports in the literature,10 but this may have to do with the uncertainty from an estimation from one only concentration as well as with the present purified R161 sample. On the other hand, it compares reasonably well with the value of 5 × 10−4 cm3 mol/g2, which was extracted from a compilation of Fetters et al.36 for a linear PS of similar molar mass in toluene at 34 °C. By analogy of the linear chain configuration,11 here we consider self-avoiding walks of thermal blobs, each consisting of about gth ≈ 100 monomers. However, the influence of this configuration on the intensity at low q (see eq 1) cannot be neglected and prohibits the use of any simple Guinier or Zimm law. It appears that this correction was not considered in ref 18. This contribution is responsible for a reduction of the intensity to about 1/4, fading away toward higher scattering angle along with the decay of the form factor. Furthermore, for further consistency we compared our fitted ε value with published Mark–Houwink parameters, α, in the literature.35 The Mark–Houwink function relates the product of intrinsic viscosity [η] and molar mass M to the chain hydrodynamic volume Vh:
| (7) |
By identifying (1 + α)/3 = (1 + ε)/2, we obtain ε = (2 + 2α)/3 − 1. Our value of ε = 0.093 ± 0.004 in toluene corresponds to the Mark–Houwink exponent α = 0.64 ± 0.01, which is in reasonable agreement with the reported α= 0.67 at 35 °C for a similar cyclic PS.35 Furthermore, following Bensafi,19 A2 bears additional useful information. With
| (8) |
and by measuring the swelling factor s = Rg,linear/Rg,ring independently, an additional consistency check of our data is possible. Here, we made use of the Gaussian relation of the radii of gyration between identical ideal linear and ring polymers. For the present R161 sample, A2 can be estimated in principle and compared with the experiment.11,37 Moreover, absolutely calibrated neutron scattering data can confirm the results of molecular characterization of R161.10,14 For example, with knowledge of the contrast factor Δρ2 between hydrogenous ring and deuterated solvent, A2 and the form factor P(q) (which is normalized to 1 at q = 0), its weight-average molar mass was found to be Mw = 156 000 ± 2600 g/mol, which is in view of all the efforts to calibrate the neutron intensities in very good agreement with the cited value of 161 000, which is derived from low-angle-light scattering within ±9000 g/mol.35
Furthermore, the swelling factor due to excluded volume interaction of the PS ring in toluene is s = Rg,toluene/Rg,θ = 108/76 = 1.42, using the full form factor fits above.19 If we calculate the g-factor, previously defined in ref 38 as (1/s)2, we obtain (76/108)2 = 0.495 ± 0.02, in fair agreement with Roovers’ value of 0.519.10 Relying on the relations for Rg and molecular weight of linear PS in cyclohexane, Rg = 0.279M0.5 and in toluene, Rg = 0.12M0.596,36,38 the ratio Rg,lin,toluene/Rg,lin,cyclohexane = 0.43M0.096 = 1.36. All radii of gyration are given in Å, and therewith the prefactors have dimension [Å mol1/2/g1/2]. Our experiment thus indicates that the R161 PS ring expanded 5% more than a linear polymer, within the limits of accuracy. Based entirely on these values, from eq 8 A2 in toluene is predicted to be 1.2 × 10−3 cm3 mol/g2, consistent with our earlier analysis.
III.2. DLS Analysis
We performed dynamic light scattering (DLS) measurements with the R161 samples of the SANS experiments in deuterated solvents. The key results are summarized in Figure 4. The DLS data of ring R161 in d8-toluene at a temperature of 33 °C and concentration of 0.5 wt % revealed a clear unimodal single-exponential decay for the intermediate scattering function, with purely diffusive character (inset Figure 4a). A characteristic example of intermediate DLS function is shown in Figure 4a for two scattering angles. The extracted hydrodynamic radius Rh ≈ 70 ± 5 Å conforms to the SANS data. In fact, the ratio Rg/Rh for rings in good solvent is expected to be about 1.25,39 i.e., slightly smaller than the respective of linear chains (1.27). Given the fact that the purpose of the DLS study was different and we did not perform a systematic study over many q-values, the extracted ratio of 1.1 is considered reasonable. However, the situation is very different in d12-cyclohexane (concentration about 1.2 × 10−4 wt %). As can be seen in Figure 4b, a single-exponential decay is observed at temperatures above θ (T = 40 and 50 °C), consistent with the toluene data of Figure 4a and yielding an estimated size of Rh ≈ 80 ± 4 Å. The higher value compared to toluene (at lower temperature) reflects slight experimental issues including a small number of q-values and a far from perfect baseline due to the second mode (apparent at lower temperatures). The main finding, however, is the unambiguous observation that, in contrast with this high-temperature data, a second slow mode is clearly detected at a temperature of 33 °C (close to but just above the best possible θ-temperature estimate for the ring PS but below that of the respective linear PS).40 This mode grows in intensity at lower q’s whereas its relaxation rate remains constant, pointing to dynamic clusters (upper inset of Figure 4b). Here, at a temperature of 33 °C, the fast diffusive mode yields approximately the same Rh, whereas the slow mode is nondiffusive (data not shown). Hence, it bares similarities with the low-q intensity upturn of the respective SANS data at the same temperature. At the same time, it becomes stronger and slower at lower temperatures, pointing to possible tendency for phase separation. What is remarkable, however, is that upon heating this mode melts away, and upon cooling back to 33 °C, it does not reappear at least within 1 h (which exceeds the DLS measurement time). On the basis of this observation, we reflected on the used experimental protocol. It turns out that during preparation of the sample for SANS or DLS measurements a trace amount of ring was diluted to a vial containing a preweighted amount of solvent, at room temperature and under continuous gentle stirring. For d8-toluene that was sufficient. However, this was apparently not the case for d12-cyclohexane where the dissolution was performed below the θ-temperature. This prompted the question about the possible role of macromolecular architecture; hence the same sample preparation protocol was applied to a linear PS (250 000 g/mol, 2.3 × 10−4 wt %) in d12-cyclohexane. In this case, under identical preparation procedures no slow mode was observed below the θ-temperature (lower inset of Figure 4b). Hence, the issue is unsettled, but it is tempting to speculate that the local microstructure plays a role; e.g., some kind of “frozen” fluctuations” and/or aggregates of few remaining linear chains even after critical fractionation might have been present in the sample before heating and then disappeared thereafter. Nevertheless, the DLS results suggest that for the present discussion, the SANS analysis is reliable: the sample protocol allowed sufficient time between dilute solution preparation and measurement. Therefore, one can safely ignore the low-q mode without losing information on the ring conformational properties. The Gaussian ring characteristics are opposite to crumbled or segregated-collapsed conformations, typically expected for temperatures below the critical temperature.
III.3. SANS Ring–Linear Melt Analysis
The interesting and still open question is whether rings in θ-solvent have the same conformations as rings in a blend of linear polymers at the same dilute concentration. Rings in a melt of medium-to-large-sized rings are known to collapse considerably compared to the Gaussian conformation.28,41–45 This was demonstrated by theoretical arguments and computer simulations29,8,42,43,46–50 as well as experimentally by SANS by some of us on a series of blends of H and D PEO rings.28,44,45,51
Upon strong dilution of rings in linear polymers below their overlap concentration cring*, contributions from inter-ring interactions can be excluded and the form factor of rings, dispersed in linear matrix, can be measured directly. Figure 5 illustrates the overlap of the data in both θ-solvent and the linear matrix. The scattering functions are virtually isomorphous and illustrate already the ideal Gaussian statistics in both “solvents” at least at length scales smaller than ~Rg/3. In addition, Figure 5 contains rescaled data for the form factor of the linear matrix chain which is ideal as well. Although the random walk-based Gaussian relation between Rg and the M is well established for linear PS,27 here, it is rechecked for internal consistency. This was done on a dilute isotopic blend of the monodisperse linear hydrogenous (H) and deuterated (D) chains with the same molar masses of 250 000 g/mol of which the D version was also used as the matrix for the PS ring, Rg,linear was found to be 138 Å. This result confirms the relationship Rg = 0.28√M (in Å).36 The prefactor of 0.28 can be computed theoretically from the chain stiffness parameter as (C∞nbl02/6m0)1/2. Here, nb is the number of backbone bonds per monomer, m0 its molecular weight, and l0 the mean bond length. Furthermore, if one considers the R161 ring polymer as an effective linear chain of molar mass Mw,ring/2 = 80 000 g/mol, using the relation above, the Rg of the Gaussian ring PS can be estimated to be 79 Å. The agreement with the size determined before in θ-solution from the full form factor (76 Å) is very reasonable and therefore suggests that a cyclic polymer dispersed in a linear matrix remains nearly Gaussian. This conjecture is supported by results from computer simulations41,43 with blends of rings and linear chains having the same degree of polymerization N. They showed Gaussian statistics and size for ϕring → 0. Despite the close similarity of the overall Rg, the corresponding scattering curve would not lead to a peaked Kratky shape. Note that the expected size of the respective linear chain with Mw,linear = 160 000 g/mol (= Mw,ring) is 112 Å if we use √2 factor in connection with the N/2-mer similarity. For the present analysis and in view of the simulation results for this particular limit of ring in linear chains,43 the Gaussian linear chain dimension Rg,0,linear, as implicit in eq 1, was fitted to the data. This experimental linear analogue (without the closure correction) has Rg,lin = 101.9 ± 0.7 Å. The Gaussian ring then has Rg = Rg,lin/√2 ≈ 72 Å. The comparison with the θ-solvent results within 4% is satisfactory. Note that a random phase approximation (RPA) treatment considering explicitly the linear matrix yields the same Rg = 71.6 ± 0.1 Å for the ring component. The Rg of the linear chain was defined before when the numerical prefactor linking the size and molecular weight was obtained. This result can be considered identical, and therefore single chain characteristics were obtained. Hence, we confirm that the ring in a long linear homopolymer matrix can be considered as Gaussian. The so-obtained ratio Rg,lin/Rg,ring ≈ 102/76 = 1.34 ≈ √2 is in good agreement with results from simulations of dilute symmetric ring–linear blends.41,42 Beside the overall chain dimension, also the full q-dependence of the Gaussian ring form factor is confirmed.
Figure 5.
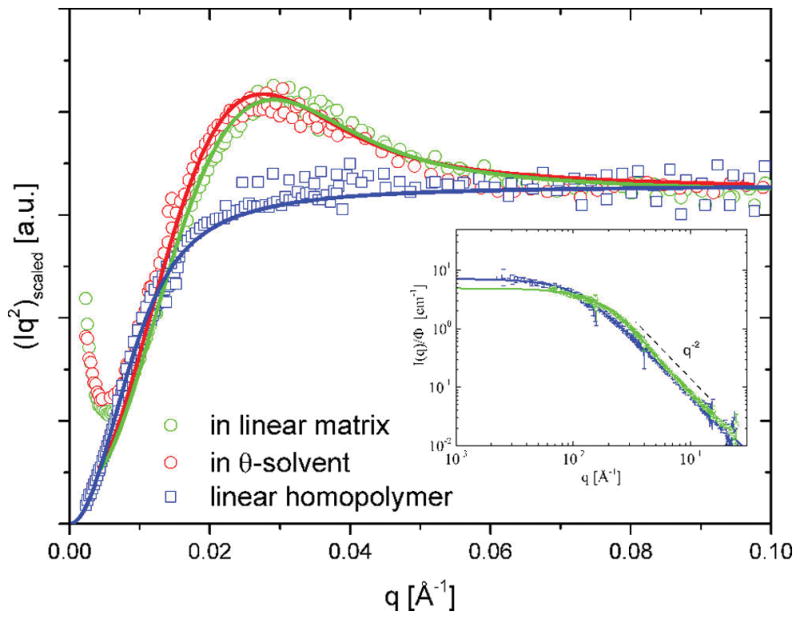
Comparison of the experimental ring form factor in θ- solvent and in a comparable linear matrix. Intensities were normalized to the same concentrations and rescaled to overlap at high scattering vectors. Symbols are explained in the figure legend. Solid lines are best fit curves to the ideal Gaussian ring form factor. A small difference in the radius of gyration, i.e., 72 to 76 Å, can be noticed from the peak positions. For further comparison also the form factor of the linear matrix chain which is a perfect random walk Debye chain is included. The inset shows the direct intensity comparison (I vs q) for latter ideal linear chain blend and the ring–linear blend.
We close with a remark on ring threading by linear chains, which has been invoked to explain the dynamics of ring–linear blends.5,41 The observed (non)swelling from θ-state to a blend of rings and linear by threading is in favor of quasi-ideal ring statistics and does hardly exceed the Gaussian limit within the factor 76/72 =1.05. Although we are aware that our observations are based on the investigation of a single molecular weight and concentration, they are consistent due to the three different solvent cases and constitute a unique data set on the most pure cyclic polymers in three different solvents.
IV. CONCLUSIONS
We have critically examined the quality and conformation of a well-characterized model PS ring polymer by means of the combination of SANS and DLS close to or just above the critical temperature for PS rings in deuterated solvent. The variations of θ-temperature with both architecture and isotope labeling could be accounted for with a comparison with a smaller PS ring. The main results from this work is that we have demonstrated that the cyclic polymer configuration in dilute θ- like state (i.e., θ-solvent or linear homopolymer) is virtually Gaussian, in agreement with available simulations, and that structural parameters could be determined with high accuracy. The homopolymer case as polymeric solvent is particularly important as it confirms the threading of rings by linear chains. Excess parasitic scattering was noted in the unperturbed state in both θ-solution and in a homopolymer polymer mixture of which the origin is not yet fully elucidated, but precipitation of clusters can be excluded. Based on DLS evidence, it is suggested that it does not influence the accuracy of the presented ring conformation data.
Acknowledgments
D.V. thanks Antje Larsen for help with the DLS measurements and Benoit Loppinet for discussions. We acknowledge EU for funding through ITN-214627 (DYNACOP) and Key Action Strengthening the European Research Area, Research Infrastructures (RII3-CT-2003-505925) under the 6th Framework Programme. A.R.B. acknowledges DFG-SPP1568 for a Postdoc position. T.C. acknowledges the support from NRF (2008-0061892 and 2012R1A2A2A01015148). D.V. acknowledges funding from Greek General Secretarial for Research and Technology (ESPA, ARISTEIA-RINGS). P.L. acknowledges the late C. Strazielle of the ICS-Strasbourg for numerous and hearty discussions on knotted rings. M.R. acknowledges financial support from the National Science Foundation under Grants DMR-1309892, DMR-1436201, DMR-121107, and DMR-1122483, the National Institutes of Health under 1-P01-HL108808-01A1, and the Cystic Fibrosis Foundation.
Footnotes
Notes
The authors declare no competing financial interest.
Contributor Information
Sebastian Gooßen, Forschungszentrum Jülich, JCNS-1/ICS-1, Jülich 52425, Germany.
Ana R. Brás, Forschungszentrum Jülich, JCNS-1/ICS-1, Jülich 52425, Germany
Wim Pyckhout-Hintzen, Forschungszentrum Jülich, JCNS-1/ICS-1, Jülich 52425, Germany.
Andreas Wischnewski, Forschungszentrum Jülich, JCNS-1/ICS-1, Jülich 52425, Germany.
Dieter Richter, Forschungszentrum Jülich, JCNS-1/ICS-1, Jülich 52425, Germany.
Michael Rubinstein, Department of Chemistry, University of North Carolina, Chapel Hill, North Carolina 27599-3290, United States.
Jacques Roovers, Institute for Environmental Chemistry, National Research Council of Canada, Ottawa, Canada.
Pierre J. Lutz, Institut Charles Sadron, CNRS UPR 22, University of Strasbourg, 23, rue du Loess, 67034, Strasbourg, France
Youncheol Jeong, Division of Advanced Materials Science and Department of Chemistry, Pohang University of Science & Technology, Pohang 790784, Korea.
Taihyun Chang, Division of Advanced Materials Science and Department of Chemistry, Pohang University of Science & Technology, Pohang 790784, Korea.
Dimitris Vlassopoulos, Institute of Electronic Structure and Laser, Foundation for Research and Technology–Hellas (FORTH), P.O. Box 1527, Heraklion, Crete 71110, Greece. Department of Materials Science and Technology, University of Crete, P.O. Box 2208, Heraklion, Crete 71003, Greece.
References
- 1.Meaburn KJ, Misteli T. Nature. 2007;445:379. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- 2.Jun S, Mulder B. Proc Natl Acad Sci U S A. 2006;103:12388. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cremer T, Cremer M, Dietzel S, Müller S, Solovei I, Fakan S. Curr Opin Cell Biol. 2006;18:307. doi: 10.1016/j.ceb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Chisholm MH, Gallucci JC, Yin H. Proc Natl Acad Sci U S A. 2006;103:15315. doi: 10.1073/pnas.0602662103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapnistos M, Lang M, Vlassopoulos D, Pyckhout-Hintzen W, Richter D, Cho D, Chang T, Rubinstein M. Nat Mater. 2008;7:997. doi: 10.1038/nmat2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquino R, Vasilakopoulos TC, Jeong YC, Lee H, Rogers S, Sakellariou G, Allgaier J, Takano A, Brás AR, Chang T, Gooßen S, Pyckhout-Hintzen W, Wischnewski A, Hadjichristidis N, Richter D, Rubinstein M, Vlassopoulos D. ACS Macro Lett. 2013;2:874. doi: 10.1021/mz400344e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tezuka Y, editor. Topological Polymer Chemistry. Progress of Cyclic Polymers in Syntheses, Properties and Functions. World Scientific; Singapore: 2013. [Google Scholar]
- 8.Halverson JD, Lee WB, Grest GS, Grosberg AY, Kremer KJ. Chem Phys. 2011;134:204904. doi: 10.1063/1.3587137. [DOI] [PubMed] [Google Scholar]
- 9.Halverson JD, Lee WB, Grest GS, Grosberg AY, Kremer KJ. Chem Phys. 2011;134:204905. doi: 10.1063/1.3587138. [DOI] [PubMed] [Google Scholar]
- 10.Roovers J, Toporowski PM. Macromolecules. 1983;16:843. [Google Scholar]
- 11.Rubinstein R, Colby RH. Polymer Physics. Oxford University Press; New York: 2003. [Google Scholar]
- 12.Ederle Y, Naraghi KS, Lutz PJ. Synthesis of Cyclic Macromolecules. In: Cahn RW, Haasen P, Kramer EJ, editors. Synthesis of Polymers. Materials Science and Technology. A Comprehensive Treatment. Chapter 19. Wiley; New York: 1999. pp. 621–647. [Google Scholar]
- 13.Cyclization in θ-solvent enhances the probability of knot formation. However, knots cannot not be detected, let alone quantified, by any known technique yet (see also discussion below). Indirect evidence from comparison of the melt viscosities of PS rings synthesized in good and θ-solvent conditions suggests that, if there are some knots in the latter case, they do not affect the terminal melt dynamics appreciably.6
- 14.Lee HC, Lee H, Lee W, Chang T, Roovers J. Macromolecules. 2000;33:8119. [Google Scholar]
- 15.Gooßen S, et al. Unpublished work. [Google Scholar]
- 16.Berne BJ, Pecora R. Dynamic Light Scattering. Dover; New York: 2000. [Google Scholar]
- 17.Gagliardi S, Arrighi V, Ferguson R, Dagger AC, Semlyen J, Higgins JS. J Chem Phys. 2005;122:064904. doi: 10.1063/1.1849162. [DOI] [PubMed] [Google Scholar]
- 18.Takano A, Ohta Y, Masuoka K, Matsubara K, Nakano T, Hieno A, Itakura M, Kinugasa S, Kawaguchi D, Takahashi Y, Matsushita Y. Macromolecules. 2012;45:369. [Google Scholar]
- 19.Bensafi A, Maschke U, Benmouna M. Polym Int. 2000;49:175. [Google Scholar]
- 20.Takano A, Kushida Y, Ohta Y, Masuoka K, Matsushita Y. Polymer. 2009;50:1300. [Google Scholar]
- 21.Siporska A, Szydlowski J, Rebelo LPN. Phys Chem Chem Phys. 2003;5:2996. [Google Scholar]
- 22.Strazielle C, Benoit H. Macromolecules. 1975;8:203. [Google Scholar]
- 23.Suzuki J, Takano A, Matsushita Y. J Chem Phys. 2011;135:204903. doi: 10.1063/1.3663383. [DOI] [PubMed] [Google Scholar]
- 24.Endo H. J Phys Soc Jpn. 2013;82:SA014. [Google Scholar]
- 25.Whereas this shift is dependent on molar mass, for the range considered here this dependence is rather small,7,10,12 hence not affecting the discussion here.
- 26.Takano A, Ohta Y, Mausoka K, Matsubara K, Nakano T, Hieno A, Itakura M, Takahashi K, Kinugasa S, Kawaguchi D, Takahashi Y, Matsushita Y. Macromolecules. 2012;45:369. [Google Scholar]
- 27.Higgins JS, Benoit HC. Neutron Scattering and Polymers. Clarendon Press; Oxford: 1994. [Google Scholar]
- 28.Brás AR, Pasquino R, Koukoulas T, Tsolou G, Holderer O, Radulescu A, Allgaier J, Mavrantzas VG, Pyckhout-Hintzen W, Wischnewski A, Vlassopoulos D, Richter D. Soft Matter. 2011;7:11169. [Google Scholar]
- 29.Hammouda B. Adv Polym Sci. 1993;106:87. [Google Scholar]
- 30.Bloomfield VA, Zimm BH. J Chem Phys. 1966;41:315. [Google Scholar]
- 31.Casassa EF. J Polym Sci, Part A. 1965;3:605. [Google Scholar]
- 32.Note that we consider the linear matrix of 250 000 g/mol as a polymeric solvent.
- 33.Beaucage G, Kulkarni AS. Macromolecules. 2010;43:532. [Google Scholar]
- 34.It is possible that colloidal salts or other imperfections from the synthesis might be responsible. Note, however, that possible leftover impurities from LCCC can be excluded, as these were not observed in the good solvent solution of the same R161 material. Furthermore, the microscopic holes cannot be fully excluded in the melt since they have the strongest contrast with the deuterated matrix. Moreover, he contribution of the precipitating aggregates if the solution is in a poor solvent below the θ-temperature cannot be excluded.
- 35.Roovers J. J Polym Sci, Polym Phys Ed. 1985;23:1117. [Google Scholar]
- 36.Fetters LJ, Hadjichristidis N, Lindner JS, Mays JW. J Phys Chem Ref Data. 1994;23:619. [Google Scholar]
- 37.André P, Folk SJ, Adam M, Rubinstein M, DeSimone JM. J Phys Chem A. 2004;108:9901. [Google Scholar]
- 38.Douglas JF, Roovers J, Freed KF. Macromolecules. 1990;23:4168. [Google Scholar]
- 39.Burchard W, Schmidt M. Polymer. 1980;21:745. [Google Scholar]
- 40.Note that the measurement time was rather short (within 10 min a stable signal was obtained), and no evidence of precipitation or turbidity was observed.
- 41.Halverson JD, Grest GS, Grosberg AY, Kremer K. Phys Rev Lett. 2012;108:038301. doi: 10.1103/PhysRevLett.108.038301. [DOI] [PubMed] [Google Scholar]
- 42.Iyer BVS, Lele AJ, Shanbhag S. Macromolecules. 2007;40:5995. [Google Scholar]
- 43.Subramamian G, Shanbhag S. Phys Rev E. 2008;77:011801. doi: 10.1103/PhysRevE.77.011801. [DOI] [PubMed] [Google Scholar]
- 44.Brás AR, Gooßen S, Krutyeva M, Radulescu A, Farago B, Allgaier J, Pyckhout-Hintzen W, Wischnewski A, Richter D. Soft Matter. 2014;10:3649. doi: 10.1039/c3sm52717d. [DOI] [PubMed] [Google Scholar]
- 45.Gooßen S, Brás AR, Krutyeva M, Sharp M, Falus P, Feoktystov A, Gasser U, Pyckhout-Hintzen W, Wischnewski A, Richter D. Phys Rev Lett. 2014;113:168302. doi: 10.1103/PhysRevLett.113.168302. [DOI] [PubMed] [Google Scholar]
- 46.Müller M, Wittmer JP, Cates ME. Phys Rev E. 1996;53:5063. doi: 10.1103/physreve.53.5063. [DOI] [PubMed] [Google Scholar]
- 47.Hur K, Winkler RG, Yoon DY. Macromolecules. 2006;39:3975. [Google Scholar]
- 48.Suzuki J, Takano A, Deguchi T, Matsushita Y. J Chem Phys. 2009;131:144902. doi: 10.1063/1.3247190. [DOI] [PubMed] [Google Scholar]
- 49.Obukhov S, Johner A, Baschnagel J, Meyer H, Wittmer JP. Europhys Lett. 2014;105:48005. [Google Scholar]
- 50.Grosberg AY. Soft Matter. 2014;10:560. doi: 10.1039/c3sm52805g. [DOI] [PubMed] [Google Scholar]
- 51.Note that no deuterated PS rings were available for the present study.
- 52.Narros A, Moreno AJ, Likos CN. Macromolecules. 2013;46:3654. doi: 10.1021/ma400308x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halverson JD, Smrek J, Kremer K, Grosberg AY. Rep Prog Phys. 2014;77:022601. doi: 10.1088/0034-4885/77/2/022601. [DOI] [PubMed] [Google Scholar]