Abstract
Objectives
To compare the clinical effectiveness of the intravesical administration of combined hyaluronic acid and chondroitin sulfate (HA+CS) versus current standard management in adult women with recurrent urinary tract infections (RUTIs).
Setting
A European Union-based multicentre, retrospective nested case–control study.
Participants
276 adult women treated for RUTIs starting from 2009 to 2013.
Interventions
Patients treated with either intravesical administration of HA+CS or standard of care (antimicrobial/immunoactive prophylaxis/probiotics/cranberry).
Primary and secondary outcome measures
The primary outcome was occurrence of bacteriologically confirmed recurrence within 12 months. Secondary outcomes were time to recurrence, total number of recurrences, health-related quality of life and healthcare resource consumption. Crude and adjusted results for unbalanced characteristics are presented.
Results
181 patients treated with HA+CS and 95 patients treated with standard of care from 7 centres were included. The crude and adjusted ORs (95% CI) for the primary end point were 0.77 (0.46 to 1.28) and 0.51 (0.27 to 0.96), respectively. However, no evidence of improvement in terms of total number of recurrences (incidence rate ratio (95% CI), 0.99 (0.69 to 1.43)) or time to first recurrence was seen (HR (95% CI), 0.99 (0.61 to 1.61)). The benefit of intravesical HA+CS therapy improves when the number of instillations is ≥5.
Conclusions
Our results show that bladder instillations of combined HA+CS reduce the risk of bacteriologically confirmed recurrences compared with the current standard management of RUTIs. Total incidence rates and hazard rates were instead non-significantly different between the 2 groups after adjusting for unbalanced factors. In contrast to what happens with antibiotic prophylaxis, the effectiveness of the HA+CS reinstatement therapy improves over time.
Trial registration number
Keywords: Antimicrobial Resistance, Hyaluronic acid, chondroitin sulphate
Strengths and limitations of this study.
These real-world data show that bladder instillations of combined hyaluronic acid and chondroitin sulfate may reduce the risk of bacteriologically confirmed urinary tract infections versus current standard management.
However, if the recurrence occurs, there is no evidence of benefit in terms of total number or time to first recurrence.
The number of instillations seems to be an important marker of success for this non-antimicrobial therapy.
Owing to the retrospective observational design, these findings need confirmation from prospective and preferably randomised studies.
Background
Urinary tract infection (UTI) is a major healthcare concern in women with an annual incidence of 30 per 1000.1 Nearly 33% of women will have had at least one UTI episode, with characteristics of acute cystitis, requiring antimicrobial therapy by the age of 24 years and as many as 60% of women reporting having had a UTI in their lifetime.2 3
UTIs have a propensity to recur;4 5 evidence shows that between 24% and 50% of initial episodes are followed by a second infection within 6 months.6–9 A widely accepted definition of recurrent UTIs (RUTIs) is two or more UTI episodes over 6 months, or three or more episodes over 12 months.10 On a population scale, the high incidence and prevalence of RUTIs results in considerable healthcare costs; at the individual level, the impact of this condition on health-related quality of life (HRQoL) is not negligible.11–13
The pathogenesis of RUTI involves colonisation of the vagina with uropathogenic bacteria and subsequent migration per urethra to the bladder. About 68–77% of recurrences caused by Escherichia coli involve strains genetically indistinguishable from those that caused previous infections.14
The diagnosis is often made on clinical presentation with local genitourinary symptoms of dysuria, frequency, and urgency or hesitancy appearing suddenly.14 However, urine culture is useful in women presenting with RUTI to confirm the diagnosis, direct antimicrobial therapy and exclude infection from an overactive bladder or interstitial cystitis.15 Evidence-based clinical practice guidelines recommend empiric initial therapy for acute management or continuous antimicrobial therapy or self-initiated therapy and prophylaxis, either antimicrobial or non-antimicrobial based.16 17
The choice of specific strategy for care depends on the number of recurrences experienced per year, the patient's preferences and careful review of modifiable risk factors.14 18 As the second most common reason for prescribing antibiotics (following otitis media), there is currently increasing concern about empiric use of these agents due to increased antimicrobial resistance (AMR). Antibiotic use selects for resistant pathogens: a major risk factor for an antibioticresistant UTI is prior antibiotic use.5 In an international survey investigating the prevalence and susceptibility of pathogens causing cystitis, 10.3% of E. coli isolates were resistant to at least three different classes of antimicrobial agents, including ampicillin (48.3%), trimethoprim/sulfamethoxazole (29.4%) and nalidixic acid (18.6%).19
Non-antimicrobial prevention strategies have become popular in the age of increasing antimicrobial use and resistance. However, no probiotic agent has been approved for therapeutic use and the potential benefit of cranberry in terms of product type (solid vs liquid), dosing and optimal patient population remains to be elucidated.14 18 A new therapy based on the reinstatement of the glycosaminoglycan (GAG) bladder epithelium has recently been proposed for the treatment of RUTIs.20 This GAG layer consists of non-sulfated, for example, hyaluronic acid (HA), and sulfated, for example, heparan sulfate and heparin, chondroitin sulfate (CS), dermatan sulfate and keratan sulfate, GAGs. Limited evidence has shown the preventive activity of intravesical GAG substation therapy (with HA alone or with HA+CS) on recurrence of infections in patients with recurrent bacterial cystitis.21 However, large-scale studies are needed to underline the benefit of this therapy.22
Therefore, we decided to perform a European retrospective multicentre study to compare the clinical effectiveness of the intravesical administration of combined HA+CS (ialuril, IBSA Institut Biochimique SA) versus current standard management of RUTIs in adult women.
Methods
Study design
This was a European Union (EU) based multicentre, retrospective nested case–control comparison of individual patient data collected from electronic medical records and/or administrative databases available at the participating institutions. Centres using the intravesical administration of combined HA+CS, in the countries where ialuril was already registered and on the market (ialuril received a CE mark for this indication in 2009), were identified and invited to take part in the study.
Study population
All patients treated with either HA+CS or standard of care at the participating centres, high volume organisations with specific expertise in the treatment of UTIs, starting from 2009, were included if they were women, aged 18–75 years, diagnosed with RUTIs, defined as at least three episodes of uncomplicated UTIs accompanied by clinical symptoms and documented by urine culture with the isolation of >103 CFU/mL of an identified pathogen in the past 12 months. Uncomplicated UTI is defined as an infection in a person with a normal urinary tract and function.17 Women with complicated UTIs (ie, individuals with functional or structural abnormalities of the genitourinary tract) were excluded. Within Europe, patients at first diagnosis of RUTIs are offered an approach based on behavioural changes, antimicrobial prophylaxis or aspecific non-antimicrobial prevention. However, several women refuse to take antimicrobials over an extended period of time; hence, intravesical administration of HA+CS is intended for women refractory or not satisfied with first-line management of RUTIs. On the basis of a previous cohort study,11 we estimated that 208 patients were needed to observe a 50% difference in the proportions of patients recurring between the two groups within 12 months with 90% power and an α-level of 0.05.
Groups and interventions
Patients were treated with intravesical administration of combined HA 1.6% and CS 2.0%. The recommended scheme is one instillation per week for the first month, followed by one instillation every 2 weeks for the second month and one instillation per month afterwards until stable remission of the symptoms; however, different patterns are seen in clinical practice. These patients were compared with patients treated with antimicrobial prophylaxis (continuous or postcoital), or immunoactive prophylaxis or prophylaxis with probiotics or prophylaxis with cranberry, or a combination of these,17 as recommended by the European Association of Urology.
Study outcomes
The primary outcome for this study was the occurrence of objective UTI recurrence, defined as the occurrence of at least one bacteriologically confirmed UTI within 12 months after treatment initiation for RUTIs. According to current clinical guidelines, in non-pregnant women, urine culture is recommended in symptomatic patients only. Information about clinically confirmed recurrences was also sought, although they are not reported in this manuscript as they are assumed to be less objective than the bacteriologically confirmed ones. Patients who developed a UTI while on the HA+CS instillation protocol were treated according to clinical guidelines with antibiotics but could continue the instillations afterwards. After the first bacteriologically confirmed recurrence, the time to first recurrence was recorded, as well as the number of additional UTIs. The secondary outcome measures were the time to recurrence (defined as the time from the start of the treatment until the occurrence of the first objective recurrence); the total number of recurrences; HRQoL as assessed through the Short Form 36 (SF-36)23 or Euro QoL 5D (EQ-5D)24 questionnaires. Dutch,25 Italian26 and UK27 tariffs were used to estimate utility values from the EQ-5D questionnaires in the Netherlands, Italy and Slovakia, respectively. Information about healthcare resource consumption was also collected. A cost analysis was planned and will be the subject of a future publication.
Data collection
General patient demographic characteristics, diagnosis and treatment information were collected on the basis of a predefined form designed on the input obtained from collaborating centres during a workshop held in July 2013. An intuitive electronic system was implemented (Advice Pharma Ltd) to record and store data on a secure remote server provider.
Statistical analyses
Continuous baseline characteristics are presented as the median and IQR or mean and SD, as appropriate. For proportions, absolute and relative frequencies are reported. The Wilcoxon-Mann-Whitney test or Student t test was used for continuous and ordinal variables baseline differences, whereas the χ2 test was used for proportions. In our primary analyses, we applied logistic, Poisson and Cox regression for objective recurrence, number of recurrences and time to recurrence, respectively. Results were presented as crude and adjusted OR, incidence rate ratio (IRR) and HR, respectively, with their 95% CIs. Adjusting variables were age, body mass index (BMI), employment and menopause status, postcoital infections, dyspareunia, Female Sexual Function Index (FSFI) and severity of RUTI. A prespecified sensitivity analysis was conducted to investigate the impact of adherence to HA+CS treatment on clinical outcomes considering patients who had ≥5 instillations. Pairwise deletion was used to deal with missing data. All significance tests were two-tailed at the 0.05 significance level. All the analyses were conducted using Stata SE StataCorp LP 11.
Results
Overall, 276 patients treated for RUTIs at seven European centres from January 2009 up to December 2013 were included in the analyses. Of these, 181 women were treated with HA+CS intravesical administration and 95 women received standard management of RUTIs. The numerical imbalance was probably due to the participating organisations being tertiary referral centres for patients who are not satisfied with standard management of RUTIs. A flow diagram reporting the number of patients at each stage of the study is shown in figure 1. The baseline sociodemographic and clinical characteristics of patients are reported in table 1. Given the non-experimental nature of the study, the distribution of several characteristics was not homogeneous between the two groups; in particular, women treated with HA+CS were older, with a higher BMI and probability of dyspareunia.
Figure 1.
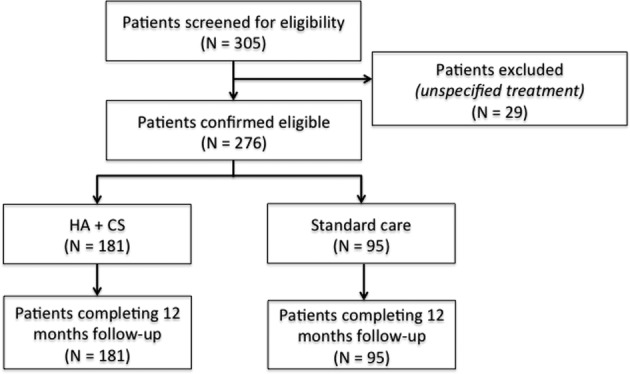
Flow diagram describing numbers of individuals at each stage of study. HA+CS, hyaluronic acid and chondroitin sulfate.
Table 1.
Baseline characteristics
| Characteristics | HA+CS (N=181) | Standard care (N=95) | p Value |
|---|---|---|---|
| Age—mean (SD) | 55.24 (17.33) | 48.84 (21.16) | 0.017 |
| Employed—n (%) | 83 (46) | 62 (65) | 0.003 |
| Partner—n (%) | 148 (82) | 72 (76) | 0.172 |
| Sexually active—n (%) | 114 (63) | 60 (63) | 0.931 |
| Menopause—n (%) | 69 (28) | 49 (51) | 0.038 |
| BMI—mean (SD) | 26.20 (6.37) | 24.56 (5.03) | 0.019 |
| Postcoital infections—n (%) | 31 (17) | 29 (31) | 0.012 |
| Dyspareunia—n (%) | 40 (22) | 5 (5) | <0.001 |
| Severity RUTI—median (IQR)* | 4 (2–5) | 4 (3–4) | 0.316 |
| Prophylaxis—n (%) | |||
| Antimicrobial prophylaxis continuous | NA | 42 (44.21) | |
| Antimicrobial prophylaxis postcoital | NA | 19 (20) | |
| Intermittent immunoactive prophylaxis | NA | 28 (29.47) | |
| On demand immunoactive therapy | NA | 1 (1.05) | |
| Others | NA | 5 (5.26) | |
| FSFI—mean (SD) | 5.35 (8.71) | 3.13 (5.43) | 0.018 |
| EQ-5D—median (IQR) | 0.69 (0.24–0.73)† | 0.69 (0.28–0.81)‡ | 0.696 |
| SF-36 PCS—median (IQR) | 60 (54.5–70)§ | 60 (53–67.5)¶ | 0.500 |
| SF-36 MCS—median (IQR) | 60 (54–70)** | 60 (53–67.5)¶ | 0.551 |
*According to the European Association of Urology Guidelines on Urological Infections where 1 is low severity cystitis and 6 is extreme severity including organ failure.21
†N=90 patients.
‡N=29 patients.
§N=72 patients.
¶N=60 patients.
**N=73 patients.
BMI, body mass index; EQ-5D, Euro QoL 5D 3 level; FSFI, Female Sexual Function Index; HA+CS, hyaluronic acid and chondroitin sulfate; NA, not applicable; RUTI, recurrent urinary tract infection; SF-36 MCS, Short Form 36 mental component score; SF-36 PCS, Short Form 36 physical component score.
Primary analyses
In the HA+CS group, 55.7% of patients showed bacteriologically confirmed recurrences, whereas 62.1% had such recurrence in the standard of care group (p=0.313). However, the adjusted OR (95% CI) for developing a bacteriologically confirmed recurrence within 12 months was 0.51 (0.27 to 0.96), meaning that, other characteristics being equal, there is a 49% reduced risk of developing a recurrence in patients treated with HA+CS compared with standard care (table 2). When the number of recurrences is considered, in the HA+CS group there were 121 bacteriologically confirmed recurrences in 61.5 person-years, whereas in the standard treatment group there were 59 bacteriologically confirmed recurrences in 51.1 person-years (p=0.001). However, we observed an adjusted IRR (95% CI) of 0.99 (0.69 to 1.43), showing non-significant differences in the incidence rates between the group treated with HA+CS and the control. Similar results were obtained from the univariate and multivariate Cox regression models used to estimate the HR (95% CI) for the time to first bacteriologically confirmed recurrence, with an unadjusted estimate of 0.99 (0.61 to 1.61) (table 2). Although the median time to first recurrence was higher in the standard care group (169.5 days (IQR, 72.5–341.5) vs 320 days (IQR, 179–365); p value <0.001) during the 12-month follow-up, we observed the distribution of recurrences at separate follow-up times, and noted that the incident proportion of patients who developed the recurrence versus those still at risk was lower in the HA+CS group in the latest part of follow-up, after 8 months (table 3). All patients were alive at the 12-month follow-up. There were 14 all-cause hospitalisations in the HA+CS group and 1 in the control group.
Table 2.
Bacteriologically confirmed recurrence, total number of recurrences and time to first recurrence between HA+CS versus standard of care treated patients
| Outcome | OR (95% CI) | Adjusted* OR (95% CI) |
|---|---|---|
| Bacteriologically confirmed recurrence | 0.77 (0.46 to 1.28) | 0.51 (0.27 to 0.96) |
| IRR (95% CI) | Adjusted* IRR (95% CI) | |
| Total number of bacteriologically confirmed recurrence | 1.73 (1.27 to 2.37) | 0.99 (0.69 to 1.43) |
| HR (95% CI) | Adjusted* HR (95% CI) | |
| Time to first bacteriologically confirmed recurrence | 1.66 (1.09 to 2.54)† | 0.99 (0.61 to 1.61) |
*Adjusted for age, BMI, employment and menopause status, postcoital infections, dyspareunia, FSFI and severity of RUTI.
†Log-rank test p value 0.018.
BMI, body mass index; FSFI, Female Sexual Function Index; HA+CS, hyaluronic acid and chondroitin sulfate; IRR, incidence rate ratio; RUTI, recurrent urinary tract infection.
Table 3.
Incidence of bacteriologically confirmed recurrences during 12-month follow-up
| Incidence of bacteriologically confirmed recurrences (days) | HA+CS (%) | Standard care (%) | p Value |
|---|---|---|---|
| 0–90 | 15.3 | 12.7 | 0.610 |
| 91–180 | 15.6 | 12.9 | 0.628 |
| 181–240 | 10.7 | 3.7 | 0.132 |
| 241–365 | 16.3 | 30.8 | 0.043 |
HA+CS, hyaluronic acid and chondroitin sulfate.
HRQoL and resources consumption
In a subset of patients, a measure of the HRQoL as measured through the SF-36 or EQ-5D 3 level questionnaires was available at baseline and after 12 months of follow-up. There was no evidence of better improvement in HRQoL in the HA+CS group compared with control with SF-36 results, whereas when EQ-5D data were considered, the HA+CS group seemed to have received a higher benefit in terms of HRQoL than the control (see online supplementary table S1).
bmjopen-2015-009669supp_table1.pdf (10.9KB, pdf)
There is a general reduction in physical units of health resources (ie, medical visits, laboratory and imaging tests) consumed by the two groups before the treatment and during the follow-up (see online supplementary table S2) without significant differences between the two groups.
bmjopen-2015-009669supp_table2.pdf (109KB, pdf)
Sensitivity analyses
We repeated all primary analyses and considered different exposure intensity (ie, number of intravesical administrations received) in the HA+CS group. All findings consistently show the additional benefit gained by patients when the number of instillations increases, possibly revealing the importance of adherence to this medical device therapy for the treatment of RUTIs (table 4). As a post hoc subgroup analysis, we repeated primary analyses in non-sexually active patients only and obtained similar patterns of results as in the whole sample, although with loss of statistical significance.
Table 4.
Sensitivity analysis—impact of number of intravesical administration of HA+CS on clinical outcomes
| Bacteriologically confirmed recurrence | OR (95% CI) | Adjusted* OR (95% CI) |
|---|---|---|
| ≥5 Instillations (n=156) vs standard of care | 0.81 (0.48 to 1.36) | 0.52 (0.27 to 0.99) |
| ≥6 Instillations (n=134) vs standard of care | 0.69 (0.40 to 1.18) | 0.47 (0.25 to 0.91) |
| ≥7 Instillations (n=82) vs standard of care | 0.63 (0.34 to 1.14) | 0.43 (0.21 to 0.88) |
| Total number of bacteriologically confirmed recurrence | IRR (95% CI) | Adjusted* IRR (95% CI) |
| ≥5 Instillations vs standard of care | 1.82 (1.33 to 2.49) | 1.05 (0.73 to 1.52) |
| ≥6 Instillations vs standard of care | 1.64 (1.18 to 2.28) | 0.97 (0.66 to 1.40) |
| ≥7 Instillations vs standard of care | 1.46 (1 to 2.13) | 0.90 (0.60 to 1.36) |
| Time to first bacteriologically confirmed recurrence | HR (95% CI) | Adjusted* HR (95% CI) |
| ≥5 Instillations vs standard of care | 1.72 (1.12 to 2.65) | 1.04 (0.64 to 1.71) |
| ≥6 Instillations vs standard of care | 1.55 (0.99 to 2.41) | 0.96 (0.58 to 1.57) |
| ≥7 Instillations vs standard of care | 1.33 (0.79 to 2.22) | 0.85 (0.49 to 1.47) |
*Adjusted for age, BMI, employment and menopause status, postcoital infections, dyspareunia, FSFI and severity of RUTI.
BMI, body mass index; FSFI, Female Sexual Function Index; HA+CS, hyaluronic acid and chondroitin sulfate; IRR, incidence rate ratio; RUTI, recurrent urinary tract infection.
Discussion
In this European multicentre retrospective observational study, we compared bacteriologically confirmed recurrence rates at the 12 month follow-up after the initiation of intravesical administration of HA+CS versus standard of care for the treatment of RUTIs. After adjusting for unbalanced confounding factors between the two groups, we observed that the HA+CS patients had a 49% reduction (OR 0.51, 95% CI 0.27 to 0.96) in the risk of a bacteriologically confirmed recurrence, whereas there was no statistical evidence for a difference in the incidence and hazard rates of such recurrences between the two groups.
Four clinical studies28–31 have been performed to investigate the efficacy and tolerability of intravesically administered GAG for RUTI prophylaxis, all showing that HA alone or HA+CS instillations reduce the number of UTIs per patient per year at no increased risk of severe adverse events and prolong the time interval between RUTI episodes, with a high rate of patients being free of recurrence at the end of the study period. In particular, two randomised control trials (RCTs) studies compared HA+CS administration to either placebo29 or long-term antibiotic prophylaxis using sulfamethoxazole 200 mg and trimethoprim 40 mg.30 Damiano et al report a decrease in the UTI rate per patient of 77% (95% CI 72.3 to 80.8) in the experimental versus placebo group, whereas De Vita and colleagues report the mean±SD number of recurrent cystitis per patient per year as 1±1.2 vs 2.3±1.4 in HA+CS and antibiotic treated patients, respectively. Despite the prospective and randomised design, these trials were limited by the small sample size (ie, they included 57 and 28 patients, respectively) and the single centre setting that considerably reduces the generalisability of the findings. While waiting for definitive RCT evidence clarifying the comparative effectiveness profile of this therapy in support of its adoption, our observational study design provides useful information around its effectiveness in real-world practice.
In this respect, our study involved seven centres across three European countries and 276 patients, thus providing important additional evidence with respect to current treatment options for RUTIs. Furthermore, the non-experimental observational design allows for a closer representation of the routine clinical practice of the use of the HA+CS reinstatement therapy as compared with standard of care in place at high volume university hospitals, that is on purpose defined as very broad given the variety of recommended strategies17 and general scarce adherence to clinical guidelines.32
On the other hand, the retrospective design limited the availability of data to that previously collected at the centres. Contacting patients ex-post to gather additional data was not applicable (eg, as in the case of HRQoL assessment) or not helpful, given the potential significant recall bias introduced by delayed reporting. The issue of missing data was dealt with by assuming that they were missing at random (ie, given the observed data, data are missing independently of unobserved data, ie, missing data do not depend on the level of their outcome) and applying pairwise deletion. In this regard, we performed two additional analyses, first by restricting the primary analyses to all-complete-cases (ie, no missing values in outcomes and adjusting variables) and providing similar results to those presented here (data not shown). Second, for all outcomes and adjusting variables, we tested through Fisher's exact test whether proportions of missing values was different between HA+CS and Standard of Care groups. No significant difference was observed with the exception of the resource consumption where the number of missing values was higher in the HA+CS group.
Data on uropathogens and AMR within the groups were unfortunately not available from this database, although we know that the most commonly prescribed antibiotics were ciprofloxacin (13.2% of all prescriptions), cefuroxime (6.9%), fosfomycin (6.9%), nitrofurantoin (6.4%) and E. coli bacterial extract (OM-89, 4.8%).
HRQoL assessment in routine practice is still uncommon, as indicated by the significant proportion of missing information (up to 73% in the control group) on this outcome. However, our findings are in line with previous reports showing that the GAG replacement treatment in women with RUTIs had a positive impact on patients' quality of life, reducing the symptoms and improving the maximum cystometric capacity.29 30
RUTIs in women are a common condition, associated with significant morbidity and burden for the whole society. In a study of 684 women aged 18–70 years with UTI, participants reported an average of 3.83 symptom days, 2.89 restricted-activity days, and 3.13 days during which they were unwell.33 In another study, patients reported 1.2 days on which they were not able to attend classes or work, and 0.4 days in bed.34 New effective prevention strategies are needed; in particular, non-antimicrobial approaches would be desirable for several reasons. First of all, a prolonged antibiotic use as a prophylactic approach to RUTI increases the risk of side effects, including vaginal and oral candidiasis, and gastrointestinal symptoms.10 This, in turn, lowers patients' compliance and therefore the effectiveness of the treatment.35 However, the most worrying effect of the antibiotic use (and misuse) is the exacerbation of AMR.36 Recently, a UK commissioned report on health and macroeconomic consequences of AMR estimated 10 million extra deaths a year and costs up to €90 trillion for the global economy by 2050 if this problem is not tackled properly.37 Although this first report might not be as scientifically rigorous or informed by evidence as possible,38 it brought renewed interest in the worldwide AMR crisis. The war against the spread of drug-resistant microbes is attracting considerable attention as well as investment from all major governments and research organisations in the EU and beyond.39 40 The way forward outlined by all of these major research initiatives includes establishing appropriate funding and rewards to subsidise access to and development of new antibiotic agents, preservation of existing drugs antimicrobial activity through prescription tailored to diagnosis, prioritisation and controlled access, and identification of novel approaches and therapies for microbial diseases.
The case of GAG reinstatement therapy is a good example of an innovative approach to prevent and manage bacterial urinary infections, a medical device intervention as opposed to a drug. In contrast to antibiotic therapy, which aims at eradicating pathogens, treatment with HA+CS targets bacterial adherence to the bladder mucosa by physically recovering a damaged GAG layer that facilitates bacterial adherence and, therefore, RUTIs. Although patients who benefit from the treatment in the first place might decide to undertake a higher number of instillations compared with patients who do not benefit immediately, the different mechanism of action could explain the apparent reduction in the incidence of UTIs in the group treated with HA+CS instillations compared with standard care when considering later time intervals (table 3). While antibiotics are immediately effective, although subject and conducive to resistance, GAG layer administration is progressively restoring the epithelium that will protect women from future uropathogen infections. On the other hand, catheterisation-induced UTIs might represent an unintended consequence of this procedure. Previous reports29 30 have highlighted good tolerability and safety of the intervention that must be performed under sterile conditions by nurses trained in the procedure. As regards the economic profile of the two alternative approaches, it has been reported that the cost of HA+CS could be even five times higher than the cost for a 6-month antibiotic prophylaxis. However, this consideration corresponds to a very restrictive, if not naïve, cost-analysis as it is well known that all direct healthcare costs and consequences, including those for the wider society, as would be containment of drug-resistance spreading, should be taken into account when assessing the cost-effectiveness profiles of health technologies. Future methodologically sound economic evaluation studies are recommended to compare the societal or payer value of the two treatment strategies.
Conclusions
In order to treat and prevent RUTI, there is a need for effective and safe alternative strategies for antimicrobial therapy. Our study showed that in a real-world setting, bladder instillation of combined HA+CS may reduce the risk of bacteriologically confirmed recurrences compared with the current standard management in this study population. Total incidence rates and hazard rates were instead non-significantly different between the two groups. The number of HA+CS instillations seems to be an important marker of success for intravesical administration therapy. Furthermore, in contrast to what happens with antibiotic prophylaxis, owing to side effects and development of resistance, the effectiveness of GAG reinstatement therapy improves over time, with an even better expected comparative effectiveness profile in the long run.
Although firm conclusions are difficult due to the retrospective observational design, these findings highlight the relevance of additional prospective and randomised studies in this area and the promising role of the HA+CS reinstatement therapy for prevention and treatment of RUTI in an era of worryingly increased AMR.
Footnotes
Twitter: Follow Oriana Ciani at @OrianaCiani
Contributors: OC, RT and ML designed the study. EA, MR, RL, EC, MDB, GM, EF, TR, MB and GG contributed to the data collection. OC analysed the data and drafted the manuscript. All the authors commented on and approved the final version of the manuscript.
Funding: This study was funded by an unrestricted grant from the TETI Association—study group for urogenital diseases. Members of the association were involved in the data collection and revised the manuscript.
Competing interests: None declared.
Ethics approval: The study protocol was reviewed and approved at the coordinating centre by an Independent Ethics Committee at the Department of Urology, University of Perugia.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data set is available by emailing the corresponding author.
References
- 1.Laupland KB, Ross T, Pitout JD et al. Community-onset urinary tract infections: a population-based assessment. Infection 2007;35:150–3. 10.1007/s15010-007-6180-2 [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002;113(Suppl 1A):5S–13S. 10.1016/S0002-9343(02)01054-9 [DOI] [PubMed] [Google Scholar]
- 3.Foxman B, Barlow R, D'Arcy H et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000;10:509–15. 10.1016/S1047-2797(00)00072-7 [DOI] [PubMed] [Google Scholar]
- 4.Hooton TM, Scholes D, Hughes JP et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 1996;335:468–74. 10.1056/NEJM199608153350703 [DOI] [PubMed] [Google Scholar]
- 5.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol 2010;7:653–60. 10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- 6.Sen A. Recurrent cystitis in non-pregnant women. Clin Evid 2008;07:801. [PMC free article] [PubMed] [Google Scholar]
- 7.Ikaheimo R, Siitonen A, Heiskanen T et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996;22:91–9. 10.1093/clinids/22.1.91 [DOI] [PubMed] [Google Scholar]
- 8.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health 1990;80:331–3. 10.2105/AJPH.80.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foxman B, Gillespie B, Koopman J et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol 2000;151:1194–205. 10.1093/oxfordjournals.aje.a010170 [DOI] [PubMed] [Google Scholar]
- 10.Albert X, Huertas I, Pereiró II et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004;(3):CD001209 10.1002/14651858.CD001209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciani O, Grassi D, Tarricone R. An economic perspective on urinary tract infection: the ‘costs of resignation’. Clin Drug Investig 2013;33:255–61. 10.1007/s40261-013-0069-x [DOI] [PubMed] [Google Scholar]
- 12.Renard J, Ballarini S, Mascarenhas T et al. Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: a prospective, observational study. Infect Dis Ther 2015;4:125–35. 10.1007/s40121-014-0054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermingham SL, Ashe JF. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int 2012;110(Pt C):E830–6. 10.1111/j.1464-410X.2012.11337.x [DOI] [PubMed] [Google Scholar]
- 14.Gupta K, Trautner BW. Diagnosis and management of recurrent urinary tract infections in non-pregnant women. BMJ 2013;346:f3140 10.1136/bmj.f3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arya LA, Northington GM, Asfaw T et al. Evidence of bladder oversensitivity in the absence of an infection in premenopausal women with a history of recurrent urinary tract infections. BJU Int 2012;110:247–51. 10.1111/j.1464-410X.2011.10766.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta K, Hooton TM, Naber KG et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103–20. 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 17.Grabe M, Bartoletti R, Bjerklund Johansen T et al. Guidelines on urological infections. Eur Assoc Urol 2015. http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf [Google Scholar]
- 18.Shepherd AK, Pottinger PS. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med Clin North Am 2013;97:737–57, xii 10.1016/j.mcna.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 19.Schito GC, Naber KG, Botto H et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents 2009;34:407–13. 10.1016/j.ijantimicag.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 20.Lazzeri M, Montorsi F. The therapeutic challenge of “chronic cystitis”: search well, work together, and gain results. Eur Urol 2011;60:78–80. 10.1016/j.eururo.2011.03.039 [DOI] [PubMed] [Google Scholar]
- 21.De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for recurrent bacterial cystitis in adult women: a meta-analysis. Int Urogynecol J 2013;24:545–52. 10.1007/s00192-012-1957-y [DOI] [PubMed] [Google Scholar]
- 22.Madersbacher H, van Ophoven A, van Kerrebroeck PE. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans—a review. Neurourol Urodyn 2013;32:9–18. 10.1002/nau.22256 [DOI] [PubMed] [Google Scholar]
- 23.Brazier JE, Harper R, Jones NM et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 25.Lamers LM, McDonnell J, Stalmeier PF et al. The Dutch tariff: results and arguments for an effective design for national EQ-5D valuation studies. Health Econ 2006;15:1121–32. 10.1002/hec.1124 [DOI] [PubMed] [Google Scholar]
- 26.Scalone L, Cortesi PA, Ciampichini R et al. Italian population-based values of EQ-5D health states. Value Health 2013;16:814–22. 10.1016/j.jval.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108. 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 28.Constantinides C, Manousakas T, Nikolopoulos P et al. Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU Int 2004;93:1262–6. 10.1111/j.1464-410X.2004.04850.x [DOI] [PubMed] [Google Scholar]
- 29.Damiano R, Quarto G, Bava I et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol 2011;59:645–51. 10.1016/j.eururo.2010.12.039 [DOI] [PubMed] [Google Scholar]
- 30.De Vita D, Giordano S. Effectiveness of intravesical hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: a randomized study. Int Urogynecol J 2012;23:1707–13. 10.1007/s00192-012-1794-z [DOI] [PubMed] [Google Scholar]
- 31.Lipovac M, Kurz C, Reithmayr F et al. Prevention of recurrent bacterial urinary tract infections by intravesical instillation of hyaluronic acid. Int J Gynaecol Obstet 2007;96:192–5. 10.1016/j.ijgo.2006.11.025 [DOI] [PubMed] [Google Scholar]
- 32.Taur Y, Smith MA. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin Infect Dis 2007;44:769–74. 10.1086/511866 [DOI] [PubMed] [Google Scholar]
- 33.Little P, Merriman R, Turner S et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ 2010;340:b5633 10.1136/bmj.b5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med 2002;113(Suppl 1A):14S–19S. 10.1016/S0002-9343(02)01055-0 [DOI] [PubMed] [Google Scholar]
- 35.Hooton TM, Levy SB. Antimicrobial resistance: a plan of action for community practice. Am Fam Physician 2001;63:1087–98. [PubMed] [Google Scholar]
- 36.Fauci AS, Marston lD. The perpetual challenge of antimicrobial resistance. JAMA 2014;311:1853–4. 10.1001/jama.2014.2465 [DOI] [PubMed] [Google Scholar]
- 37.Review on Antimicrobial Resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. http://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
- 38.[No authors listed] Antimicrobial resistance: in terms politicians understand. Lancet 2014;384:2173 10.1016/S0140-6736(14)62412-0 [DOI] [PubMed] [Google Scholar]
- 39.Woolhouse M, Farrar J. Policy: an intergovernmental panel on antimicrobial resistance. Nature 2014;509:555–7. 10.1038/509555a [DOI] [PubMed] [Google Scholar]
- 40.Geoghegan-Quinn M. Funding for antimicrobial resistance research in Europe. Lancet 2014;384:1186 10.1016/S0140-6736(14)61723-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2015-009669supp_table1.pdf (10.9KB, pdf)
bmjopen-2015-009669supp_table2.pdf (109KB, pdf)


