Abstract
Excessive intake of food, especially palatable and energy-dense carbohydrates and fats, is largely responsible for the growing incidence of obesity worldwide. Although there are a number of candidate antiobesity drugs, only a few of them have been proven able to inhibit appetite for palatable foods without the concurrent reduction in regular food consumption. In this review, we discuss the interrelationships between homeostatic and hedonic food intake control mechanisms in promoting overeating with palatable foods and assess the potential usefulness of systemically administered pharmaceuticals that impinge on the endogenous cannabinoid, opioid, aminergic, cholinergic, and peptidergic systems in the modification of food preference behavior. Also, certain dietary supplements with the potency to reduce specifically palatable food intake are presented. Based on human and animal studies, we indicate the most promising therapies and agents that influence the effectiveness of appetite-modifying drugs. It should be stressed, however, that most of the data included in our review come from preclinical studies; therefore, further investigations aimed at confirming the effectiveness and safety of the aforementioned medications in the treatment of obese humans are necessary.
Keywords: Antiobesity therapy, cannabinoids, food preferences, neuropeptides, neurotransmitters, opioids.
INTRODUCTION
Numerous theories have attempted to explain a socio-medical phenomenon dubbed the “obesity epidemic” in the context of human evolution (for review, see [1]). The rapid increase in the worldwide obesity prevalence over the last decades suggests, however, that changes in lifestyle, especially dietary habits, can be implicated for this trend. Nowadays, a growing number of modern communities live in an obesogenic environment characterized by an extremely easy access to cheap and palatable but high-calorie foods. Additionally, food products are advertized in a suggestive manner using neuromarketing techniques [2], and this, along with the natural attractiveness of sweets and fats, has led to the increased consumption of palatable, energy-dense foods [3]. There is a clear direct relationship between the rising body mass index (BMI) and preference for fatty foods in both genders, sweet foods in women [4], and fat and sweet foods in children [5]. Moreover, a free-choice cafeteria diet similar to the western diet style in humans has been shown to be the most efficient way to induce obesity in laboratory animals [6, 7]. Thus, increased consumption of high-calorie food combined with low physical activity has greatly facilitated the development of the obesity epidemic. This phenomenon is not only characterized by a growing number of obese individuals in general [8, 9] but also by an increasing number of morbidly obese people with the BMI greater than 40 kg/m2 [10, 11] and, in particular, rapidly increasing percentage of obese children [9, 12]. Since excessive body weight is considered to be a serious public health problem resulting in a variety of medical disorders [13], unbeneficial psychological consequences [14], and even in a lower socioeconomic status [15], various strategies are used to counteract these negative trends. Indisputably, the best way to lose weight and maintain normal body weight is to change to a healthier lifestyle. Unfortunately, living in the modern world is not conducive to regularly eating healthy food products and exercising. Furthermore, follow-up studies have indicated that most dieting individuals who managed to lose weight successfully experienced the so-called “yo-yo” effect related to a fast weight regain after the end of a diet [16, 17]. Therefore, other strategies ensuring the maintenance of a reduced body weight have been developed. Currently, operations on the gastrointestinal tract, known as “bariatric” or “metabolic” surgery are considered to be the most effective anti-obesity treatment methods, and are especially recommended for patients with severe obesity with BMI ≥ 40 kg/m2, for those with BMI ≥ 35 kg/m2and significant comorbidities, for those with “diabesity” (i.e., diabetes mellitus coexisting with obesity), or possibly for patients with metabolic syndrome [18, 19]. Although a variety of beneficial effects including fast weight loss and improvement in metabolic parameters have been found in patients after bariatric surgery [20, 21], this operation may cause long-term clinical complications such as nutritional (mainly mineral) deficiencies and difficult to predict changes in drug absorption [22, 23]. Also, despite this radical intervention on the digestive system, some patients who underwent this surgery failed to lose weight [24] or regained weight lost shortly after the operation [25]. These patients may be subjected, therefore, to the revisional bariatric surgery that increases the risk of postoperative complications [26, 27] and mortality [28]. Other patients with excessive weight, in turn, cannot be qualified for weight-loss operations because of medical contraindications or simply because of their reluctance to take on the risk of surgery. Hence, pharmacological treatment would be another option for them and for those who experienced cyclic weight loss and weight gain after dieting.
HOMEOSTATIC AND HEDONIC HUNGER – THE TWO FACES OF FOOD INTAKE CONTROL
It is generally accepted that the amount of food consumed is determined by two control mechanisms [29]. The first one is related to the regulation of energy balance homeostasis and is driven mainly by the hypothalamic feeding centers (i.e., arcuate nucleus, lateral hypothalamic area, mediobasal hypothalamic area, paraventricular nucleus) [30]. They receive either direct or the brainstem dorsal vagal complex-mediated information from the periphery about the nutritional status [31]. Chemical signals such as absorbed nutrients and gastrointestinal (ghrelin, cholecystokinin – CCK, glucagon-like peptide-1 – GLP-1, and peptide YY - PYY), adipose tissue (leptin) and pancreatic (insulin) hormones are the carriers of this information [32-34]. This metabolic mechanism operates through the sensation of “homeostatic” hunger or satiety. The second mechanism regulating food intake is related to the brain reward system and consists of the corticolimbic (e.g., the hippocampus, amygdala, prefrontal and insular cortex) and mesolimbic (e.g., the ventrotegmental area, nucleus accumbens, and striatum) structures. They use dopamine, opioids, and endocannabinoids as neurotransmitters [35]. The corticolimbic system is responsible for “liking” food (i.e., pleasure related to palatable food consumption reflected by characteristic behavior such as licking), and the mesolimbic system is related to “wanting” food (i.e., appetite and motivation to eat reflected by a desire to work for food) [35, 36].
Brain areas involved in the homeostatic and hedonic regulatory mechanisms interact closely with each other to establish energy homeostasis in the body. This is possible due to numerous neural and functional connections between these two systems [2, 37] and prompts the view that they actually act as a single integrated system [38, 39]. In support of this hypothesis is the fact that brain structures involved in the reward-related mechanism express genes that encode similar feeding-related peptides [38] and respond to the same peripheral signals as those involved in homeostatic regulation, e.g., portal blood glucose [40] and hormones such as leptin, insulin, ghrelin, GLP-1, PYY, and CCK [41, 42] (Fig. 1). Interestingly, brain regions involved in both metabolic- (i.e., hypothalamic nuclei) and reward-related (i.e., the nucleus accumbens) food intake control were shown to be activated concurrently when rats pressed the lever to obtain sucrose [43]. Apparently, the changes in the nutritional status and energy balance in the body evoke a simultaneous response of the homeostatic and hedonic centers that, in turn, act in concert to affect feeding behavior through specific, aforementioned mechanisms. Indeed, the feeling of hunger, which is considered to be rather unpleasant, strongly activates food-seeking behavior and leads to consumption of food portions suitable for restoration of energy balance. On the one hand, consumption of an adequate amount of food is associated with both the sensation of pleasure and satiety that cause cessation of eating. Hence, the hedonic food intake control supports the homeostatic regulation. In addition, changes in the nutritional status may reinforce or attenuate the rewarding properties of food. A hungry individual would eat food that may not be palatable, whilst the satiated one would refuse to eat even highly palatable food [35]. Often, however, the hedonic mechanism especially triggered by unlimited access to palatable though high-calorie foods overcomes the metabolic cues, a phenomenon referred to as “hedonic hunger” in contrast to “homeostatic hunger”. While the latter leads to restoration of energy homeostasis, overstimulation of the former results in overeating and gaining excessive weight. Food craving may be an evolutionarily-favored behavior [44] especially in populations that have experienced long-term periods of starvation in the past [1]. This genetically fixed behavior may be potentiated by learned environmental cues acting as conditioned stimuli that encourage people to reach for food even when they do not feel hungry [45]. At the individual level, storing up energy as fat leads to obesity that, in turn, has been found to enhance appetite for more energy-dense foods in rats and humans [46-48]. Moreover, when obesity-prone rats were prevented from access to the palatable, high-fat and high-sugar diet, they exhibited the withdrawal symptoms similar to those seen in drug-addicted animals [49]. Interestingly, research done on humans also indicated a positive correlation between the BMI and craving for palatable foods [50]. Hence, obesity in both humans and animals likely drives a positive feedback mechanism that finally leads to (possibly) permanent functional changes in neural circuits responsible for the food intake regulation (for review, see [51]). These changes manifest as an increased orexigenic drive to consume palatable, high-energy foods (Fig. 1). In addition to the dysregulated consummatory behavior, obese individuals present with enhanced appetitive behavior that is directed towards food acquisition [52]. The altered function of brain areas responsible for the appetitive phase of ingestive behavior, as revealed in neuroimaging studies of overweight individuals, may result from repeated consumption of palatable foods [36, 41, 53, 54]. In this way, overconsumption becomes a fixed habit that shares some similarities with drug or alcohol addiction [51, 55-57], and which is driven by different brain areas than those responsible for satisfying hunger [58].
Fig. (1).
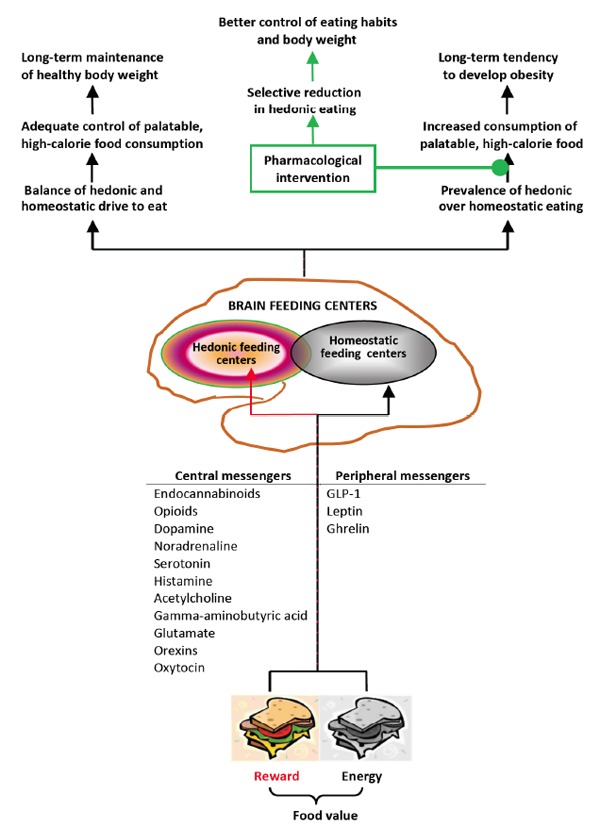
Central and peripheral messengers conveying information about food value to the brain feeding centers, and the relationship between body weight changes and balanced or imbalanced hedonic and homeostatic drive to eat.
SATIETY-ANOTHER PLAYER IN A CO-OP GAME OF SURVIVAL
Satiety is commonly considered as a term opposite to hunger. From the physiological point of view, satiety may be defined as the lack of hunger and feeling of fullness after eating the amount of food sufficient to restore energy balance. Although closely related to appetitive behavior, satiety is driven by brain centers and neurotransmitters different from those responsible for hunger control. Also, the same peripheral stimuli differentially affect the activity of hunger and satiety centers. For example, the hypothalamic arcuate nucleus, one of the best-known feeding centers consists of both “anorexigenic” (i.e., satiety-stimulating) and “orexigenic” (i.e., hunger-stimulating) neurons. They use, respectively, the alpha-melanocyte stimulating hormone (α-MSH) and neuropeptide Y to communicate with neurons in other feeding centers. Hormonal (insulin, leptin) and metabolic (glucose, amino acids, and fatty acids) factors released after food intake stimulate the former and inhibit the latter group of neurons that is associated with increased satiety rates. The feeling of satiety is, however, largely subjective and may be affected by the individual’s convictions and experiences as to the nutritional value of the just-eaten meal [59]. Moreover, evidence has accumulated that obese people are less sensitive to internal satiety signals [60], where leptin resistance is the most striking example of such dysregulated feedback mechanism. An important issue for the present review is that the feeling of satiety not only depends on the caloric content but also on the type and proportions of macronutrients in a meal with proteins having the highest satiating power and, hence, potential to support weigh loss [59]. It should be also noted that fat- and sugar- but not protein-rich foods are believed to have addictive properties [61]. Furthermore, according to the protein leverage hypothesis [62], too little protein intake might result in a “compensatory” overconsumption of carbohydrates and fats that thus contribute to obesity [63]. Consequently, a high-protein breakfast was demonstrated to reduce evening snacking; this indicates that increased protein intake may help with beneficial alteration in eating habits [64]. However, the latest reports suggest that excessive intake of some animal proteins may be an important risk factor for obesity [65], especially when associated with the consumption of carbohydrate-rich foods [66].
PHARMACOLOGICAL MODIFICATION OF HUNGER AND APPETITE
Many pharmaceuticals used for scientific purposes have been known to affect food consumption. However, considering the homeostatic and hedonic faces of food intake control discussed above as well as the role of excessive consumption of palatable, high-calorie foods in the obesity development and maintenance, it seems that the drug that impinges on both mechanisms would be the most promising solution. Ideally, the anti-obesity medication should not only be safe and decrease food consumption and body weight but also change eating habits without affecting other functions related to the activity of the reward system, such as mood and emotions. Such a drug would also be expected to decrease primarily the appetite for palatable, high-calorie foods (i.e., fat and sweets) and to a much lesser extent reduce the intake of foods considered “healthy” (i.e., containing all nutrients that are necessary for life but not as caloric and often not as attractive as sweet and fatty foods). Hence, the purpose of this study is to review pharmaceuticals that have been found to selectively reduce appetite for palatable, obesogenic foods when administered peripherally in humans and animals (Table 1).
Table 1.
Peripherally-applied pharmaceutical drugs found to selectively inhibit hedonic eating.
| Physiological Messengers | Pharmacological Intervention | Drug | Species | References |
|---|---|---|---|---|
| Endocannabinoids Opioids Dopamine Gamma-aminobutyric acid Glutamate Glucagon-like peptide-1 Oxytocin |
CB1 receptor
inhibition MOR receptor inhibition D2/D3 receptor stimulation Reuptake inhibition GABAB receptor stimulation mGluR5 receptor inhibition NMDA receptor inhibition GLP-1 receptor stimulation Oxytocin receptor stimulation |
Rimonabant (SR 141716) AM 251, rimonabant Naltrexone Naltrexone Quinpirole Methylphenidate Baclofen MTEP Memantine Exendin-4 Liraglutide Liraglutide Oxytocin |
Marmosets ♀ Rats Rats Humans Rats Rats Rats Baboons Rats Rats Rats Humans Humans |
[73] [77, 78] [93] [104, 105] [127] [148] [253, 257] [267] [183] [82] [182] [307] [366] |
CB1 receptor – cannabinoid 1 receptor; MOR receptor – mu opioid receptor; D receptor – dopamine receptor; GABAB receptor – gamma-aminobutyric acid B receptor; mGluR5 receptor – metabotropic glutamate receptor 5; NMDA receptor – N-methyl-D-aspartate receptor; GLP-1 receptor – glucagon-like peptide-1 receptor
PHARMACOLOGICAL INHIBITION OF THE ENDOCANNABINOID SYSTEM AND PALATABLE FOOD INTAKE
The two major endocannabinoids anandamide (N-arachidonoylethanolamide, AEA) and 2-arachidonoylglycerol (2-AG) are produced both centrally and peripherally and affect feeding mainly via the cannabinoid 1 (CB1) receptor. The role of cannabinoids and their antagonists as modulators of metabolic and hedonic food intake has been reviewed extensively by Kirkham [44], Di Marzo [67], and Silvestri [68] and has been updated recently by Cristino [69] and Jager [70] in terms of the involvement of the endocannabinoid system in the reward-motivated feeding. The most important conclusions arising from the studies cited in these review articles emphasize a role for endocannabinoids as orexigenic factors that primarily increase motivation to eat through activation of the nucleus accumbens neurons and interaction with dopaminergic, opioidergic and ghrelin signaling pathways. Stimulation of the CB1 receptor by its synthetic agonist, ACEA (N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide) injected intraperitoneally to rats given free access to a 3-component diet containing protein, fat, and carbohydrates resulted exclusively in an increase in carbohydrate consumption indicating that endocannabinoids may specifically influence the appetite for macronutrients [71]. Moreover, in the periphery, endocannabinoids were found to enhance specifically the taste cell response to sweeteners [72]. For the present review, however, the most important issue is that the pharmacological blockade of the CB1 receptor was found to reduce appetite for palatable foods in laboratory animals maintained on a free-choice diet that best reflects natural conditions conducive to overeating in humans. Indeed, SR 141716 (rimonabant), a CB1 receptor antagonist, given orally to primate monkeys (i.e., marmosets) reduced intake of sweet food and increased intake of standard chow [73]. On the contrary, rimonabant injected intramuscularly to baboons representing another primate species reduced evenly intake of candies and normal food thus suggesting that the blockade of the CB1 receptor did not affect appetite for specific foods in these animals [74]. Similar results were obtained in obese diabetic humans treated with rimonabant for six months. Although the drug significantly reduced overall calorie intake and body weight in these patients, it did not change the relative proportion of macronutrients consumed, including sugar [75]. Rimonabant was found, however, to attenuate the neural response to the sight and flavor of chocolate in healthy volunteers treated with the drug for seven days, as observed with functional magnetic resonance imaging (fMRI) [76].
The data obtained in experiments performed on rodents that were allowed free choice between normal and palatable foods were also conflicting. The CB1 receptor antagonists AM 251 and rimonabant injected intraperitoneally to female rats with optional access to standard chow and sweet foods reduced the intake of the latter only [77]. Similarly, rimonabant reduced selectively sucrose feeding and drinking in male Wistar rats [78]. Consistently with these findings, AM 251 markedly diminished motivation to obtain chocolate-flavored pellets in female rats that were taught to work (i.e., press the lever) for food, while it did not change their responding to standard pellets [79]. Also, rimonabant attenuated the rewarding value of a palatable drink [80] and addictive-like eating behavior In mice [81]. Thus, blocking the CB1 receptor has the potential to reduce not only consummatory but also appetitive (i.e., food-seeking) behavior. On the other hand, the results obtained in our laboratory indicated that AM 251 was not able to reduce selectively food consumption in male Wistar rats that were offered a choice between sweet and normal rat chow [82, 83]. This discrepancy may result from different experimental conditions (e.g., restricted vs. non-restricted access to sucrose-rich food in the study by Arnone et al. [78] and Radziszewska et al. [82], respectively) as well as from different sensitivity of male and female rats to the CB1 receptor antagonists, an event found originally in mice [84]. Moreover, AM 251 used at doses comparable to those that specifically decreased sweet food intake in female rats in the aforementioned study by Mathes et al. [77] equally reduced the consumption of standard and sweet food in female mice [85]. Hence, animals, even within similar taxonomic groups, differ as to their response to the CB1 receptor inhibitors. Although it is not known exactly what the cause of these differences is, the work by Brand et al. [86] seems to provide possible explanation. They reported that two strains of rats commonly used in laboratories, i.e., Wistar and Fischer344 rats, exhibited significant differences in the intake and motivation to obtain sweetened milk and in the sensitivity to rimonabant as a drug inhibiting palatable food intake. These variations could be likely attributed to congenital differences in expression of certain proteins (e.g., CB1 receptor protein) that are pivotal for the endocannabinoid system function found in brains of both rat strains. It seems therefore that similar yet still hypothetical alterations in the endocannabinoid system activity occurring between various animal species/strains/genders might account for the above-described differences in the CB1 receptor antagonists’ efficacy to specifically inhibit appetite for sweet foods. It should be noted that rimonabant was initially approved in Europe for treating obesity but was withdrawn from sale due to adverse psychiatric side effects. Nevertheless, CB1 receptor antagonists could be considered as candidate medications with the potency to selectively reduce appetite for sweets in individuals susceptible to these drugs provided that safe and free of side effects CB1 antagonists will be developed.
PHARMACOLOGICAL INHIBITION OF THE OPIOID SYSTEM AND PALATABLE FOOD INTAKE
Opioid peptides (e.g., endorphins, enkephalins, dynorphins) and their receptors (mu [MOR], kappa [KOR] and delta [DOR] opioid receptors) were detected in various brain structures including those involved in homeostatic and hedonic food intake control (for review, see [87, 88]). It is of particular importance for the present study to note that opioids principally influence intake of sweet and fatty foods [89]. In a review on the role of opioids in the food reward system, Peciña and Smith [90] indicated that MOR stimulation by opioid agonists increased both pleasure related to consumption of palatable foods (“liking”) and motivation to obtaining them (“wanting”) and suggested that inhibition of MOR activity by opioid antagonists would be a potential treatment method for eating disorders resulting from dysregulation of the brain reward system. Consistently, the MOR (i.e., naloxone and naltrexone) but not KOR and DOR antagonists injected intraperitoneally were shown to decrease saccharin [91] and sweet-fat mixture [92] consumption in the rat. Naltrexone was also found to selectively decrease the consumption of sweet food under a free-choice (i.e., chocolate cookies versus chow) paradigm in rats [93]. These findings were extended by Olszewski et al. [94] who demonstrated that naloxone, when used at low doses, selectively reduced intake of sweet chow in rats with scheduled access to either sweet or standard chow. Subsequent studies indicated, however, that naloxone decreased similarly consumption of chocolate cookies and standard diet or a sucrose and starch diet when they were presented simultaneously to the rat [95]. Moreover, Glass et al. [96] demonstrated that naloxone injected into rats offered a choice between a high-carbohydrate or high-fat diet decreased intake of the diet they preferred initially. In contrast, another report [97] showed that naltrexone injected subcutaneously within rats given a choice between sucrose and starch solutions increased the percentage of sucrose intake by reduction in starch intake independently of the basal sucrose/starch preference. Furthermore, a recent study [98] did not show any differences in food preferences in naloxone-treated rats maintained on a cafeteria diet containing ingredients found in typical snacks. Also, naloxone facilitated the extinction of the learned operant behavior of rats to obtain standard and sweet pellets in a similar way [99]. Hence, animal studies do not show clear evidence for efficacy of the MOR blockade in the selective suppression of palatable foods consumption. The above-described discrepancies might be explained, at least in part, by large differences in the susceptibility to naltrexone found between inbred mice strains in terms of the drug’s ability to suppress sucrose intake [100]. Similar differences in the saccharin consumption were reported between the Lewis and Fischer rat strains treated with naloxone under two different experimental regimes [101]. Collectively, these data indicate that unidentified genetic variations may determine effectiveness of opioid antagonists as drugs that modify appetite for palatable foods in laboratory animals and, likely, humans.
Recent human studies demonstrated that MOR antagonists attenuated the response of certain brain structures, such as the amygdale, dorsal anterior cingulate cortex, and caudate, to the sight and taste of palatable foods,as reflected by fMRI [102, 103]. Consistently, naltrexone-treated individuals reported diminished pleasantness related to the consumption of sweet, fatty, and high-protein foods and consumed less fat and proteins as compared with placebo [104, 105]. Importantly, taste preferences and ingestion of cookies and chocolate was mostly reduced by naloxone in binge eaters [106, 107]. Based on results obtained in numerous studies, Lee and Fujioka [108] suggested, however, that this drug failed to reduce body weight in obese humans when administered as monotherapy. Hence, considering the above-described inconsistencies in the experimental data obtained from animal and human studies, usefulness of opioid antagonists in reducing selectively consumption of tasty, high-calorie foods seems to be disputable.
PHARMACOLOGICAL INTERVENTION IN THE NEUROTRANSMITTER SYSTEMS AND PALATABLE FOOD INTAKE
Neurotransmitters known to be involved in food intake regulation include monoamines, such as dopamine, noradrenaline, serotonin, histamine, acetylcholine, gamma-aminobutyric acid, and glutamate. They all have been shown to affect food intake by interaction with the reward-related mechanisms [109], and at least some of them are now considered as targets for anti-obesity therapy [110].
DOPAMINE
Dopamine is of particular interest for this review because it is the principle neurotransmitter of the brain reward system involved in the hedonic control of food intake [111]. Dopamine-producing neurons in the brain arise from the substantia nigra in the brainstem and the ventral tegmental area in the midbrain, and project to various brain areas related to the reward system [112, 113] where dopamine receptors are also located [114]. Here, dopamine interacts with peripheral satiety signals (e.g., leptin and GLP-1) [115-117] to affect palatable food intake. Mesolimbic [118-121] and corticolimbic [122] dopamine signaling pathways were demonstrated to affect sucrose solution preference in rodents through enhancement of motivation (“wanting”) to obtain it [123-125]. Dopamine-dependent modulation of the rewarding value of food is mediated presumably through the dopamine D2 receptor [126]. Nonetheless, a D1 receptor agonist, SKF 38393, injected subcutaneously was shown to significantly increase palatable food (chocolate biscuits) and decrease normal rat chow ingestion, while quinpirole, a D2/D3 receptor agonist, caused the opposite effects, i.e., decreased intake of chocolate biscuits but increased normal chow consumption in rats maintained on a free-choice diet [127]. Hence, dopamine may differentially affect food intake; this has been confirmed by other studies demonstrating that dopamine might either stimulate or inhibit food intake depending on the brain area [128] and, possibly, receptor subtype. Although it has not been confirmed by all studies [129-131], a number of studies suggest that abnormal dopamine signaling related to decreased D2 receptor availability [132-134] and/or dopamine secretion [135, 136] found in the brain reward system (especially the striatal regions) of obese humans and animals might result in attenuated reinforcing properties of food. Whether this is a primary (i.e., genetically determined) or secondary (i.e., resulting from overeating and obesity) disorder remains a matter of debate. Nevertheless, to compensate for decreased pleasure from eating, subjects with that deficit will eat more food than is necessary for energy balance maintenance [132, 137] and thus continuously gain weight, creating a vicious cycle. Therefore, pharmacological intervention in the D2 receptor-dependent dopamine signaling might be a potential tool for breaking that cycle.
Studies on the effects of drugs/narcotics that impinge on the dopaminergic system provided further Interesting data about the potential usefulness of these substances in modifying appetite for palatable foods. Amphetamine has been long known for its anti-obesity effect but, because of its addictive properties and serious adverse effects, it cannot be used to treat obesity. Amphetamine affects food intake likely by increasing release of catecholamines including dopamine in the brain [138], particularly in the mesolimbic, mesocortical, and nigrostriatal pathways [139]. In humans, oral amphetamine decreased consumption of all basic nutrients (i.e., fat, carbohydrates and protein) when subjects were allowed to self-select lunch but did not affect sweet food intake when they could choose between foods categorized by nonsweet/sweet taste [140, 141]. Similar results were obtained in non-human primates (i.e., baboons) treated with intramuscular amphetamine [74]. Also, the drug did not affect appetitive behavior in these animals [142]. Methylphenidate, another psychostimulant that acts primarily as a dopamine reuptake inhibitor, is used to treat the attention-deficit hyperactivity disorder (ADHD) but may have potency to reduce selectively appetite for high-calorie foods. A single dose of methylphenidate given to obese adolescents [143], young adults [144], and obese males [145] reduced food intake during a subsequent meal especially due to the diminished energy intake from fat and carbohydrates. On the other hand, chronic methylphenidate treatment resulted in a reduced intake of all nutrients in ADHD individuals [146]. Importantly, this therapy resulted in a significant weight loss [146, 147]. Using a rat model of binge eating disorder to determine the methylphenidate effect on feeding behavior, it was shown that the drug concurrently increased the normal chow intake and decreased sugar intake thus indicating that methylphenidate might be targeted therapy for the aforementioned disorder in humans [148]. A separate category of drugs that, apart from treating psychiatric disorders, may affect appetite are antipsychotic medications (neuroleptics). Chronic treatment with haloperidol, a dopamine receptor antagonist, did not modify food selection [149], although a single haloperidol dose decreased the “sham” intake of sucrose solution [150] and changed reward-related behavior by increasing percent choice of smaller but immediately available to larger but delayed sucrose portions in rats [151]. Risperidone, a D2 receptor antagonist injected chronically to rats, reduced fat and increased protein intake without affecting body weight [152]. Raclopride, another D2 receptor antagonist, showed different (i.e., stimulatory or inhibitory) effects on sweet-fat mixture consumption in rats depending on sucrose content and availability (daily versus intermittent) [92]. Interestingly, a recent report demonstrated that blocking the D1 and D2 receptor with ecopipam and haloperidol, respectively, reduced willingness to work for sucrose while leaving sucrose intake, preference, and hedonic impact intact in rats [153]. Hence, these pharmaceutical drugs are a viable candidate for reducing human appetitive response to palatable foods. Clozapine and quetiapine, second generation antipsychotic drugs that interact with a variety of monoamine receptors including dopaminergic receptors, have been found, however, to enhance preferences for a high-fat high-sugar diet in rats [154] and dietary saturated fat in humans [155]. Thus, determination of whether or not pharmacological intervention in the dopaminergic system activity is a promising approach to selectively reduce calorie intake from palatable foods requires further investigations, likely due to the ambiguous impact of dopamine itself on food intake.
NORADRENALINE
Noradrenaline is involved principally in learning and cognition. These processes also underlie the control of food intake by the brain’s reward system [156]. Noradrenergic pathways in the prefrontal cortex, which is responsible for planning and decision making, were demonstrated to affect dopamine release induced by palatable food intake in the nucleus accumbens in mice [157], thus indicating that these two transmitter systems interact to control food consumption. Also, noradrenaline affects food consumption through the effect on the hypothalamus, which receives noradrenergic inputs from the brainstem. Acting through different receptors, noradrenaline either enhances or inhibits food consumption [158]. Palatable food ingestion is associated with an increase in noradrenaline content in various hypothalamic nuclei and in the amygdale [159], indicating that this neurotransmitter may be involved in the regulation of rewarding food consumption. This was supported by studies demonstrating that infusion of noradrenaline into the paraventricular nucleus of the hypothalamus resulted in a profound increase in carbohydrate intake compared with other macronutrients [160]. Similar results were obtained in rats injected peripherally with clonidine, an alpha-2 adrenergic receptor agonist [161], whilst LY368975, a selective inhibitor of noradrenaline reuptake, was demonstrated to inhibit sweetened milk ingestion in rats [162]. Bupropion, a noradrenaline/dopamine reuptake inhibitor, currently approved for the treatment of depression and reduction of the withdrawal symptoms after smoking cessation, is now investigated as a component of the combined bupropion/naltrexone therapy for obesity. Unfortunately, the data on possible effects of bupropion on food preferences are scarce. In one study, the effect of bupropion on the macronutrient choice in abstinent women after smoking cessation was examined and no differences in macronutrient intake were found [163]. On the other hand, the results of another study performed on individuals who quit smoking and were treated with bupropion indicated that this medication decreased food reward [164]. Interestingly, the bupropion-dependent decrease in food intake likely occurred due to the effect on both the hypothalamic feeding centers and the reward system and was enhanced by the concurrent administration of naltrexone [165]. A recent study [166] using fMRI demonstrated that the combined bupropion/naltrexone therapy attenuated the activation of the hypothalamus and stimulated the regions involved in the inhibitory self-control in response to food cues, thus supporting the view that administration of drugs impinging on both the metabolic and hedonic control of food intake would be the most promising anti-obesity therapy. There is no data, however, on the effects of this combination therapy on food preferences in humans and animals.
SEROTONIN (5-HYDROXYTRYPTAMINE, 5-HT)
Like dopamine, serotonin is a key neurotransmitter of the brain reward system. It acts via different receptors categorized into 7 receptor families (5-HT1-7) that are further subdivided into numerous receptor subtypes located in various brain areas responsible for both homeostatic and hedonic food intake control (for review, see [167, 168]). Serotonin effects on eating behavior depend on the receptor type and receptor localization. It has been demonstrated that central 5-Ht1A, 5-Ht1B (whose human counterpart is the 5-HT1D receptor), 5-Ht2C, 5-HT4 and 5-Ht6 receptors are involved in food intake control and, at least some of them, may be potential targets for obesity treatment [169, 170]. It should be noted, however, that there are significant discrepancies as to the effects of stimulation and blockade/genetic knockout of these receptors on energy balance [167].
An early study [171] demonstrated that peripherally injected serotonin selectively inhibited fat consumption in the rat. Further reports using systemically administered pharmaceuticals modifying the serotonergic system activity provided further evidence that these drugs may change macronutrient selection in rodents and humans. Diabetic individuals reduced fat and carbohydrate consumption after oral administration of 5-hydroxy-tryptophan, the precursor of serotonin [172]. Similarly, stimulation of 5-HT receptors by mCPP, a nonselective serotonin receptor agonist, decreased carbohydrate intake [173]. In contrast, fluoxetine (Prosac), a selective serotonin uptake inhibitor, which is used to treat depression and eating disorders such as bulimia, was shown to decrease fat and protein consumption while leaving carbohydrate intake unchanged in male and female rats [174, 175]. Fluoxetine was shown, however, to decrease preference to carbohydrates and diminish intake of fats and protein in starved Zucker rats [176]. When rats were allowed to choose between the standard chow and a carbohydrate supplement, fluoxetine and 2,5-dimethoxy-4-iodoamphetamine (DOI; a 5-HT2A/B/C receptor agonist) but not fenfluramine, mCPP, RU24969 (a 5-HT1A/5-HT1B receptor agonist), and MK212 (a 5-HT1C receptor agonist) reduced intake of carbohydrates more than the consumption of standard diet [177]. Another selective serotonin reuptake inhibitor, paroxetine, was found, however, to diminish the appetitive response to both palatable (sucrose-containing) and non-palatable (quinine-containing) fluids in rats [178]; this suggests that enhanced serotonergic transmission attenuated both positive and negative reward system-related reactions. In humans, fluoxetine decreased intake of all macronutrients [179, 180], thus indicating a nonselective action of this drug on nutrient intake. Similarly, sibutramine, a serotonin/noradrenalin uptake inhibitor, was demonstrated to reduce comparably intake of fat, carbohydrate and protein diets in male and female rats [181]. These results are consistent with the subsequent study where sibutramine was found to decrease the consumption of both palatable, high-sugar high-fat food and standard chow in rats offered a two-choice diet [182], but they are in contrast to other results that demonstrated the suppressory effect of sibutramine on lard consumption and, interestingly, the concurrent stimulation of standard chow consumption in rats allowed to chose between both feeds [183]. Importantly, these effects were achieved at drug doses similar to those administered in a previous study [179] (7.5 vs. 5 mg/kg, respectively). Sibutramine was also shown to reduce motivation to obtain sweet pellets as well as decrease the amount of sweet pellets or sweetened high fat chow eaten under a free-feeding paradigm in rats [184, 185]. Despite the above-described discrepancies regarding this drug's ability to preferentially inhibit palatable food consumption, sibutramine seemed to have a potency to modify appetite and to significantly reduce body weight. Unfortunately, sibutramine was demonstrated to produce serious adverse cardiovascular effects and had to be withdrawn from the pharmaceutical market. Since other abovementioned selective serotonin reuptake inhibitors (i.e., fluoxetine and paroxetine) significantly increased body weight when used for a long period of time [186], they cannot be applied as appetite-reducing drugs.
Fenfluramine, yet another pharmaceutical that activates the serotonergic system by stimulation serotonin release and inhibition of its uptake, was found to decrease primarily fat consumption in the rat [176, 187, 188]. On the other hand, dexfenfluramine, a fenfluramine isomer, selectively reduced the intake of carbohydrates and fat in one human study [189] but did not affect macronutrient selection [190] or sweet food intake [140] in the others. Other clinical studies suggested that dexfenfluramine like fenfluramine reduced mostly fat intake (for review, see [191]). Also, dexfenfluramine decreased liking of sucrose solution in female rats during estrous [192], demonstrating that sex hormones might modify the reward system response to this drug, and reduced sweet candy consumption in both male and female baboons [193]. This effect, however, was associated with the parallel reduction in standard food intake and did not alter food-seeking behavior in these animals, indicating that the drug did not affect motivation to obtain palatable food. Hence, the selective effect of fenfluramine/dexfenfluramine on palatable food consumption has not been proven conclusively. Importantly, both fenfluramine and dexfenfluramine turned out to be very effective in weight reduction both in humans and animals, and, therefore, there were high hopes for their use as antiobesity medications. Unfortunately, clinical studies revealed that they caused numerous severe side effects [194], which resulted in the withdrawal of approval to use these drugs. For the same reason, they cannot be used as appetite modifying drugs. Nevertheless, fenfluramine was shown to cause appetite inhibition via the 5-HT1B and 5-Ht2C receptors [146], and further studies demonstrated that pharmacological agents targeting these receptors may diminish the rewarding value of palatable foods. mCPP, which causes hypophagia specifically via the 5-HT2C receptors [195], was demonstrated to reduce motivation (“wanting”) for palatable food in mice [80]. Similar results were obtained in rats treated with another selective 5-HT2C receptor agonist, WAY 163909 [196]. Lorcaserin, a 5-HT2 receptor agonist with high affinity to the 5-HT2C receptor, is devoid of adverse effects characteristic of other drugs impinging on the serotonergic system and has been approved currently for marketing by the U.S. Food and Drug Administration (FDA) as one of few antiobesity drugs. Lorcaserin affects eating behavior possibly by attenuation of the rewarding value of food [197]. Since there are no other data on the effect of this drug on food preferences in humans and animals, the possible impact of lorcaserin on highly palatable versus normal diet consumption remains to be investigated. This problem is of special interest, due to the high efficacy of the drug on body weight reduction. The data on the effects of pharmacological modification of the activity of other 5-HT receptors on food choice behavior are also scarce. DOI, the aforementioned 5-HT2A/B/C receptor agonist, and 8-OH-DPAT, a 5-Ht1A receptor agonist, were demonstrated to enhance preference to glucose and saccharin (a non-caloric sweetener) containing solution over “pure” glucose solution [198]. Hence, the modification of food choice behavior mediated by the above-mentioned serotonin receptors involves a strong motivational component. In the other study, 8-OH-DPAT was found to increase the intake of a high-fat diet over a low-fat diet in Osborne-Mendel rats, i.e., enhanced consumption of food that was initially preferred by these rats whilst the same drug increased intake of a low-fat diet in S5B/P1 rats that initially showed no preference for any of these foods [199]. Thus, unidentified genetic differences between the animals prone (i.e., Osborne-Mendel) and resistant (i.e., S5B/P1) to diet-induced obesity may affect the response of to the 5-HT1A receptor agonist in respect with food choice behavior. Interestingly, mCPP, the aforementioned serotonin agonist influencing food intake primarily vie the 5-HT2C receptor, decreased intake of both diets in Osborne-Mendel and S5B/P1 rats under the same experimental conditions [200], further supporting the view that particular serotonin receptors may differently affect food intake and food preferences. In conclusion, it seems that serotonin signaling via the 5-Ht1A receptor increases appetite for palatable foods through strengthening motivation and, therefore, blockade of this receptor might result in decreased consumption of such foods. This statement, however, is only hypothetical because to date no studies have been performed on the effect of the 5-HT1A receptor antagonists on food preferences. It is known, nevertheless, that those pharmaceuticals reduce food intake without causing adverse effects in mice [201], indicating that they could be potential candidates for medications modifying food-choice behavior. A review of previously published experimental data suggests that pharmacological blockade of the 5-HT6 receptor is effective in appetite and body weight reduction [202], but, to our best knowledge, there are no full paper studies on the possible effects of 5-HT6 receptor antagonists on food choice in animals or humans.
HISTAMINE
Histamine, a histidine-derived monoamine, is known to reduce food intake through the central H1 receptor in the ventromedial hypothalamus [203, 204]. The knowledge about the possible impact of this amine on food choice and appetite for palatable foods is poor. Animal studies demonstrated that the hypothalamic histaminergic system responds differentially to various tastants including saccharin [205], indicating that histamine may be involved in taste perception. Histamine could affect palatable food consumption indirectly by inhibiting the mesolimbic dopamine pathways [203]. A single dose of betahistine (an H1 receptor agonist and H3 receptor antagonist) was not found, however, to affect hunger and satiety in obese women [206]. Considering that histamine was demonstrated to be released from the tuberomammillary nucleus where histamine-producing neurons are located during the appetitive but not consumatory phase of feeding in the rat, this neurotransmitter seems to cause transient anorexia associated with food-seeking behavior rather than satiety [207]. Consistently, histamine might be involved in the control of food anticipatory behavior [204] and, therefore, participate in motivation to obtain palatable food. Interestingly, a significant increase in palatable, high-calorie fat emulsion intake was achieved due to the concurrent blockade of H1, 5-HT2A/2C and muscarinic receptors, while the respective antagonists administered individually had no effect on food intake in rats [208]. This proves that the histaminergic, serotonergic, and cholinergic systems act in concert to regulate appetite for palatable foods, and, therefore, the simultaneous pharmacological intervention in these systems would result in changes in appetite that are more pronounced than appetite modifications by individual neurotransmitter systems. Therefore, modulation of histaminergic transmission might be a promising tool for appetite modification, but further studies are needed to check whether such modulation would result in the selective suppression of palatable food consumption.
CHOLINERGIC TRANSMISSION, NICOTINE, AND TOBACCO SMOKING
Acetylcholine has been proven to affect feeding through muscarinic M3, nicotinic β4, and, likely, α7 receptors in the hypothalamic neurons where this neurotransmitter is co localized with POMC [209-212]. Specifically, acetylcholine decreases food intake due to activation of the POMC neurons through α3β4 nicotinic receptors [210]. It should be noted, however, that different subtypes of nicotinic receptors have been identified in a variety of brain areas involved in the control of energy balance (for review, see [213]). Hence, acetylcholine may participate in food intake control at multiple levels of organization of feeding centers. For example, acetylcholine is considered to be a satiety signal when secreted within the nucleus accumbens, and its imbalanced release in this area may be linked with food addiction withdrawal symptoms [214]. In general, acetylcholine alters the reward system-related food intake using muscarinic receptors [215-218]. Importantly, both the muscarinic (scopolamine) and nicotinic (mecamylamine) antagonists, when injected peripherally to rats, were able to disrupt the incentive motivation to obtain food reward [219, 220], indicating that systemically administered pharmaceuticals impinging on the cholinergic system might be used to modify the rewarding value of food. Previous studies demonstrated that acute and repeated intraperitoneal injections of 18-methoxycoronaridine, an α3β4 nicotinic antagonist, resulted in a selective inhibition of sucrose solution intake in female [221, 222] but not male [223] rats. The latter, however, in contrast to females, did not develop obesity when maintained on a high-sucrose diet, indicating that the blockade of the α3β4 nicotinic acetylcholine receptor was most efficient in the selective inhibition of appetite for sweets in subjects originally preferring this kind of food.
Most reports that address the effects of cholinergic transmission modification on appetite for palatable foods, however, concern the effect of nicotine, a psychoactive constituent of tobacco smoke, on food intake and food preferences in humans and animals. A relationship between cigarette smoking and body weight has long been known. Active smokers maintain lower body weight than non-smokers, and smoking cessation results in a fast weight gain in both men and women [224]. The body weight-reducing effect of smoking is attributed to the nicotine-induced potentiating effects on energy expenditure and inhibiting effects on appetite [224], while weight gain following smoking cessation may be related to the increased rewarding value of food [164]. Smoking and nicotine may also modify brain mechanisms that control food preferences. The hypothalamic response to sweet milkshake, as revealed by the fMRI, was greater in smokers than in the age-, sex-, and BMI-matched non-smokers, while the respective response to tasteless solution was similar between these two groups of individuals [225]. Women who were current smokers preferred sweet foods more than women who did not smoke [226]. Similarly, male smokers consumed more sucrose than nonsmokers [227]. Furthermore, smokers were found to be less sensitive to food-related stimuli [228] and to consume fewer sucrose-containing foods than nonsmokers [229]. Hence, the results of studies on effects of tobacco smoke on food preferences are inconsistent. Exposure to nicotine in utero and/or via maternal nursing resulted in an increased motivation to obtain sucrose [230] associated with the permanent changes in nicotinic receptor expression in the brain reward system in adolescent rats [231]. Exposure of adult rats to nicotine also increased food-cue reactivity as measured by sucrose self-administration [232]. In agreement with the above facts, humans whose mothers smoked during gestation showed the preference to carbohydrates over proteins [233] and increased fat intake [234]. In conclusion, most of the data indicate that continuous smoking and prenatal nicotine/tobacco smoke exposure increases attractiveness of palatable, high-calorie foods through the effect on the brain reward system in humans and animals. This might, to some extent, explain the observation based on a large cohort study that heavy smokers tended to gain more weight, adjusted for age and body weight at baseline, than light smokers or nonsmokers [235]. On the other hand, smoking cessation and resulting nicotine withdrawal also augments the rewarding value of food, especially sweets and fats [236-238]. Furthermore, women who quit smoking and received nicotine replacement therapy tended to eat more fats and carbohydrates but gained less weight than those who were not subjected to nicotine therapy [239]. Likely, in these cases, eating palatable foods became a substitute for a habit of reaching for a cigarette, but nicotine-induced enhancement of energy expenditure prevented the body weight increment. Currently, to our best knowledge, there are no reports on the possible effects of the use of electronic nicotine delivery systems, commonly known as e-cigarettes, on food intake and preferences. Considering the variety of cholinergic receptors and neurotransmitters that acetylcholine may interact with and an evident paucity of studies on pharmacological modification of appetite for palatable foods by peripherally administered cholinergic agonists/antagonists, it is difficult to determine whether it will be possible to change eating behavior using such pharmaceuticals.
GAMMA-AMINOBUTYRIC ACID (GABA)
GABA receptors, categorized into GABAA and GABAB classes that are widely distributed in the brain of mammals, are responsible for synaptic inhibition [240]. At the level of the lateral hypothalamus, separate subsets of GABA-ergic neurons control appetitive and consummatory behavior [241]. In addition, GABA is involved in food intake control both in the arcuate nucleus of the hypothalamus and ventral tegmental dopamine system where it acts as a neuro-transmitter suppressing anorexigenic signaling pathways [242]. Similarly, GABA, acting via the GABAA receptor, is likely an orexigenic signal within the ventromedial hypothalamus [243], but GABAA receptor inhibition with bicuculline increased ingestion of sweet milk and selective intake of fat when infused into the anterolateral hypothalamus and the medial ventral pallidum, respectively [244, 245]. There are, however, no studies on the effects of peripherally-injected bicuculline on palatable food intake. Genetic ablation of GABAB receptors in the hypothalamic POMC neurons failed to influence food intake [246] while the peripherally administered GABAB agonist, baclofen, was able to influence food consumption both in humans and animals. Baclofen decreased food intake when administered to obese mice and humans [247, 248], and attenuated both appetitive and consummatory behavior in baboons [249] but increased the short-term food consumption in lean mice [250] and rats [251, 252]. Interestingly, the results of many animal studies suggest that baclofen may specifically decrease appetite for certain palatable, high-calorie foods. The drug reduced intake of fatty food and increased the consumption of standard food in rats subjected to a binge-eating protocol [253]. Baclofen also decreased motivation to obtain fat-reach food in rats after the history of binge eating [254]. Subsequent studies demonstrated that the drug clearly inhibited consumption of fat, but its effect on standard food intake was ambiguous, i.e., baclofen either did not change, decreased, or increased concomitantly the consumption of normal chow [255-258]. Baclofen was not able, however, to suppress the consumption of sucrose solution, and, furthermore, it increased sweet-fat food consumption [256]. On the other hand, baclofen (administered at doses similar to those in the above studies) was shown to suppress intake of fat-sucrose mixtures regardless of whether they were available intermittently or daily [92]. Importantly, the effectiveness of this drug in suppressing palatable food intake could be abolished by the thickening agent added to the foodstuff [259]. The selective suppressory effect of baclofen on fat- and/or sugar-containing feed could be enhanced by naltrexone [260]. Taking into account the above facts, baclofen might be considered as a candidate drug to limit craving for palatable foods under the condition that its effectiveness will be confirmed in human studies.
GLUTAMATE
Glutamate is the most abundant excitatory neuro-transmitter in the central nervous system. Glutamate is involved in food intake control via the metabotropic glutamate receptor mGlu5 [261]. Recently, variations in genes responsible for glutamate signaling have been demonstrated to be crucial for human obesity pathogenesis [262], and drugs impinging on glutamatergic neurons have gained attention as potential medications for treatment binge eating disorder [263]. The results of studies conducted so far indicated that glutamate released within the hypothalamus and pharmacological modification of NMDA and AMPA glutamatergic receptors’ activity in the reward-system regions affected palatable food intake [264, 265] by increasing selectively carbohydrate consumption in rats allowed to choose between 3 diets, each of which contained one basic macronutrient [266]. Consistently, the blockade of NMDA and mGluR5 receptors by systemically injected memantine and MTEP, respectively, decreased sweet pellet consumption in baboons maintained on a binge-eating protocol but only the latter drug showed the selective effect, i.e., reduced candy but not regular chow intake [267]. Under similar experimental conditions, memantine used in a higher dose decreased the lard and, interestingly, increased regular chow consumption in rats [183]. Since no relevant side effects have been reported for this drug, it may be assumed as a candidate for selective appetite suppression especially in binge eaters. Interestingly, stimulation of the mGlu2/3 receptor with its agonist LY379268 via intraperitoneal injection attenuated sucrose seeking in rats [268], indicating that alterations in glutamate receptor activity may change various aspects of feeding behavior.
Regardless of the fact that glutamate as a neuro-transmitter is synthesized in the brain, glutamic acid is ubiquitously present in dietary proteins [269]. Glutamate blood levels, however, increase only slightly after consumption of meals, even when enriched with this compound, because of a very efficient intestinal breakdown [270]. Furthermore, the blood-brain barrier protects the brain from the excessive influx of circulating glutamate [271]. Nevertheless, peripheral glutamate may directly affect feeding centers lacking this barrier such as the hypothalamic arcuate nucleus [272] or indirectly influence the brain feeding centers such as the lateral hypothalamus via stimulation of vagal afferents in the digestive system [273]. Of special relevance for this review is, however, the effect of monosodium glutamate, an important umami tastant that signals the presence of proteins in the digestive system, on appetite. Monosodium glutamate is commonly added to food to improve its taste. As such, this compound increases appetite and therefore food intake [274, 275], but its long-term use is not associated with higher energy intake or a significant body weight increase among nursing home elderly [276], in mice [277, 278], and rats [278, 279]. This might result from the simultaneous stimulating effect of glutamate on appetite and satiety, leading to the increased pleasantness of meals during ingestion and subsequent enhancement of satiety [274]. In agreement with this finding, addition of monosodium glutamate to nutritionally valuable foods increased their intake and reduced the subsequent consumption of sweet desserts and snacks [280, 281] but did not alter the consumption of the subsequent “main” meal [282]. Hence, a simple procedure of flavoring dishes with a popular tastant might help to change eating habits in the more desirable direction. Furthermore, monosodium glutamate was shown to decrease body weight in rats fed with high-fat high-sugar diets due to increased energy expenditure [273, 283], but this beneficial effect has not been reported in humans. Importantly, Luscombe-Marsh et al. [284] suggested that the addition of monosodium glutamate to a meal may contribute to increased energy intake at a second course. Although the use of monosodium glutamate in a diet is considered to be safe [285], it should be remembered that the excessive administration of this compound may be potentially dangerous for the central nervous system neurons due to the known neurotoxicity of this amino acid neurotransmitter [272], and the parenteral injection of monosodium glutamate to neonatal rodents is one of methods used to induce obesity in animal studies.
PHARMACOLOGICAL INTERVENTION IN THE ADIPOSE TISSUE-GUT-BRAIN AXIS AND PALATABLE FOOD INTAKE
The adipose tissue and the alimentary tract synthesize and secrete hormones that influence feeding centers in the brain. Except ghrelin, all of these hormones (e.g., GLP-1, galanin, CCK, and leptin) produce satiating effects and reduce food intake. Recently, a lot of evidence collected from preclinical studies indicated that some of these hormones may modify intake of rewarding foods, and, therefore, they have potential to diminish appetite for palatable foods.
GLUCAGON-LIKE PEPTIDE-1 (GLP-1)
GLP-1, one of the most studied peptides of the gut-brain axis, is a well-known anorectic and body weight reducing agent. GLP-1 analogs such as exenatide and liraglutide, which are more robust than the natural hormone, have been approved for diabetes treatment. The role of peripheral and central GLP-1 in the food intake control in humans and animals has been extensively reviewed recently [286-289]. An increasing number of reports suggest that GLP-1 analogs may be used as anti-obesity medications [287, 290-292] and, consequently, liraglutide has recently been approved by the FDA for obesity treatment. Furthermore, research on the central effects of GLP-1 indicate that this peptide decreases the rewarding value, palatability and appetitive responses to foods in animals [117, 293-296] and humans [288, 297]; therefore, this suggests that GLP-1 agonists might be useful as appetite suppressants that especially decrease appetite for palatable foods. Many studies reported that peripherally administered GLP-1 agonists inhibited high-fat and high-sugar food consumption [298-303] but few addressed the issue of whether these drugs may be useful in the selective inhibition of sugar and fat intake. An early study in this field indicated that exendin-4 (a potent GLP-1 agonist) injected intraperitoneally decreased high-protein diet consumption without changing intake of high-carbohydrate food in rats [304]. On the other hand, liraglutide did not modify intake of basic nutrients, although it did decrease overall calorie intake in humans [305]. Other studies demonstrated, however, that exenatide attenuated sweet taste reactivity in obese rats [306], and this might contribute to the decreased preference to high-fat/high-sugar diet seen in liraglutide-treated rats [182] and humans [307]. It is of special interest that apart from the decrease in palatable food consumption the drug was shown to increase the regular chow intake in rats [182] and to change the eating habit to a healthier pattern in humans [308]. Similar results, i.e., a decrease in palatable food intake and simultaneous increase in regular food intake, were obtained in rats co-injected with GLP-1 and an inhibitor of dipeptidyl peptidase IV (an enzyme responsible for GLP-1 degradation, inagliptin) maintained on a two-choice diet consisting of standard chow and a high-fat/high-sugar chow [309]. Exendin-4 and AM 251 is another drug combination that involves the long-lasting GLP-1 analogue and has been demonstrated to inhibit selectively high-sucrose diet intake in rats [82]. Although the GLP-1 agonist used in that study was able, as such, to decrease sweet food consumption, the blockade of the CB1 receptor by AM 251 significantly enhanced this effect. Surprisingly, the effect of co-injection of exendin-4 and WIN 55, 212-2, a CB1 receptor agonist, on the selective inhibition of palatable food intake was even more spectacular and resulted not only in almost complete suppression of high-sucrose food consumption but also in an increase in the standard food consumption and reduction in body weight [82]. Hence, administration of GLP-1 agonists, regardless of whether alone or in combination with other drugs that potentiate their action, seems to be a promising approach aimed at specifically reducing appetite for palatable, high-calorie foods. It should be noted, however, that some distressing gastrointestinal side effects related to GLP-1 agonists may limit usefulness of such drugs [310].
LEPTIN
The role of leptin as a hormone, which inhibits hunger by impacting both homeostatic and hedonic feeding centers and therefore has the potential to be an antiobesity medication, has been investigated repeatedly in recent years [311-316]. Briefly, leptin, via the specific receptor LepRb (referred to as ObRb in animals) located in hypothalamic and brainstem feeding centers and mesolimbic reward circuits, was demonstrated to decrease food intake, food palatability, appetitive activities, and to induce satiety and reduce body weight in humans and animals. It is of special notice, however, that exogenous, peripherally administered leptin may influence food preferences. Leptin added to (and, presumably, naturally occurring in) maternal milk, which the pups were fed with, modified food preferences in adult rats by diminishing fat intake [317]. Leptin and its analog, metreleptin, decreased the reward value of visual food stimuli in subjects with the congenital and acquired leptin deficiency [318, 319]. This phenomenon might account for attenuated sweet cravings seen in metreleptin-treated women after Roux-en-Y gastric bypass [320] and in leptin-injected mice [321]. Consistently, leptin infused to Wistar rats with normal body weight decreased the consumption of sweet carbohydrates and proteins [322], while in obese leptin-deficient women, leptin treatment resulted in a proportional reduction in the intake of all basic macronutrient including sugar [323]. Leptin was not able to decrease the consumption of a three-choice (i.e., standard, high-sugar and high-fat chow) diet, although it decreased the intake of two-choice (i.e., chow and high-sugar or chow and high-fat) diets, thus indicating that the former feeding paradigm caused leptin resistance [324]. It is worth notice, however, that leptin-induced reduction in the total caloric intake in chow and high-sugar diet-fed rats found in that study resulted from the decreased chow but not sugar intake, which is in agreement with the observation made in a study by Wierucka-Rybak et al. [83]. Thus, the usefulness of leptin as a selective suppressant of high palatable food consumption seems to be ambiguous. What is more, due to leptin resistance, when administered as monotherapy, this hormone failed to reduce food intake and body weight in obese individuals, but further studies indicated that combined therapies involving leptin and other anorectic hormones had greater potential for weight loss [325] and, possibly, for selective inhibition of palatable food consumption.
GHRELIN
The gastric hormone ghrelin (its active form is acyl ghrelin, a product of acylation of the peptide by an enzyme ghrelin O-acyl transferase) [326] and ghrelin-related peptides such as desacyl ghrelin have recently gained much attention due to their ability to regulate glucose and energy homeostasis including reward-motivated feeding (see [327-330] for review). Accordingly, the growth hormone secretagogue (GHS) receptor, i.e., the ghrelin receptor, was detected in the brain centers responsible for both homeostatic and hedonic feeding in humans and animals [331-333]. Genetic deletion of the GHS receptor resulted in resistance to diet-induced adiposity in mice, thus confirming a role for ghrelin in obesity pathogenesis [334]. A significant majority of studies on ghrelin action on eating behavior have been concerned with evaluating the effects of centrally applied hormone. They indicated that ghrelin increased motivation for palatable food and, importantly, regulated independently homeostatic and rewarding aspects of food intake, thus affecting differentially the consumption of palatable and bland food (for review, see [335, 336]). Consequently, the peptide increased motivation to obtain palatable food without affecting regular chow consumption. Thus, rats and mice with pharmacological or genetic ablation of the GHS receptor-reduced intake of rewarding food (i.e., sugar pellets, peanut butter, or chocolate drink) but not of regular chow intake in a free choice or operant paradigm [337, 338]. Similarly, the ghrelin O-acyl transferase-null mice with subsequent acyl ghrelin deficiency decreased consumption of the high-fat chow presented after normal chow to previously fasted animals (a procedure referred to as a “dessert effect test”) as compared with their wild-type littermates [339]. These findings suggest that manipulation in the peripheral ghrelin signaling system might be a potential tool for the selective inhibition of reward-motivated eating. On the other hand, the lack of ghrelin, acyl ghrelin, or GHS receptor did not result in body weight/food intake reduction when the mice were fed a no-choice high-fat diet [340]. Since the free-choice protocol employed in the study by Davis et al. [339] seems to better correspond to natural conditions leading to overeating than the non-choice diet in the study by Sun et al. [340], the pharmacological blockade of the ghrelin system may still be a promising method for suppression of excessive appetite for palatable foods. To be effective in the suppression of appetite, however, ghrelin antagonists should be able to cross the blood-brain barrier [341]. In favor of this approach, a GHS receptor antagonist, JMV2959, injected intraperitoneally to rats was shown to antagonize the changes in the activity of brain areas involved in both metabolic and hedonic food intake control induced by ghrelin revealed by fMRI [342]. This drug was also demonstrated to reduce intake of sweet-tasting solutions containing either sucrose or saccharine in rodents [343], whilst, consistently, intraperitoneally injected ghrelin increased saccharine but not bland food consumption in mice [344]. On the contrary, JMV 2959 injected intraperitoneally to the prairie vole did not affect either sucrose preference or consumption [345]. In summary, most studies indicate that ghrelin antagonists are worthy of further research as drugs to specifically inhibit appetite for palatable, especially sweet, foods. Since ghrelin was shown to interact with other orexigenic (i.e., dopamine, endocannabinoids, and orexins) [330, 346-348] as well as anorexigenic (i.e., leptin, GLP-1) [349, 350] substances to influence food intake, studies on combined treatment with the respective antagonists would be also of interest.
OTHER NEUROPEPTIDES, NUTRACEUTICS, AND DIETARY SUPPLEMENTS INHIBITING PALATABLE FOOD CONSUMPTION
OREXIN
Orexins A and B (hypocretins 1 and 2), peptide neurotransmitters produced in the lateral hypothalamus, promote hyperphagia and stimulate the reward seeking behavior [351]. Orexin knockout mice were shown to consume less sucrose solution than the wild-type mice [352], and, consistently, the orexin 1 receptor antagonist SB-334867 decreased sucrose self-administration in food restricted rats, although it did not affect sucrose-seeking behavior [353, 354]. Pharmacological blockade of the orexin 1 receptor by ACT-335827 resulted, however, in a significant increase in the standard and simultaneous decrease in high-fat high-sugar food consumption in rats. This effect was associated with weight gain, and no beneficial changes in the metabolic profile of ACT-335827-treated obese rats were found. Therefore, the authors concluded that the therapy based on inactivation of orexin 1 receptor would be of minor significance as an obesity treatment method [355].
OXYTOCIN
A hypothalamic neurohormone oxytocin has long been known to be involved in the regulation of reproductive functions and social behavior. Recently, however, oxytocin has been stressed as an anorexigenic agent limiting the intake of palatable foods [356]. Indeed, peripherally administered oxytocin reduced food intake in humans [357] and animals [358-361]. Rodent studies indicated that endogenous oxytocin was an inhibitor of carbohydrate (especially sucrose) but not fat intake [362-364] that decreased sweet taste sensitivity [365]. Importantly, these findings were confirmed by human studies reporting the suppressory effect of intranasal oxytocin on snack, i.e., chocolate cookies intake without affecting hunger rating and consumption of the (basic) test meal [366]. Although further long-term studies are necessary to confirm the usefulness of oxytocin treatment for the selective inhibition of appetite for high-sugar foods, the results of studies published so far in this field seem to be very promising.
BROWN RICE
γ-Oryzanol is a mixture of ferulic acid esters present in the bran of brown rice that was recently found to have anti-diabetic and anti-obesity properties [367, 368]. Moreover, addition of brown rice to the laboratory chow decreased significantly not only body weight but also the preference for high-fat diet in mice maintained on a free-choice procedure [369], thus indicating that supplementation of meals with brown rice (or, possibly, γ-oryzanol) has a potency to change appetite for palatable food. Nevertheless, more studies are needed to verify the usefulness of such therapy.
PLANT EXTRACTS
A series of recent studies indicate that plant extracts might be helpful in reducing appetite for palatable foods. A plant extract made of the common kidney bean Phaseolus vulgaris was shown to reduce motivation to obtain chocolate-flavored beverage in rats [370] and consumption of sweet, palatable cookies and beverages in mice [371]. The latter effect, however, was not specific because the P. vulgaris extract also reduced the regular chow intake [371]. On the other hand, morning, pre-meal consumption of spinach extract was found to decrease desire and, consequently, intake of sweet and fat snacks in overweight women, which is associated with body weight reduction [372, 373]. Hence, these preliminary studies suggest a new and simple method of supplementation a diet with natural compounds derived from the easily available plants that have a potential to reduce not only homeostatic but also hedonic hunger.
FATTY ACIDS
The jejunal infusion of linoleic acid, one of the long-chain fatty acids, resulted in a selective reduction of high-fat diet consumption in rats that could choose between a high-fat and high-carbohydrate diet [374]. Obese women whose diet was supplemented with oil rich in docosahexaenoic acid, an omega-3 fatty acid, decreased carbohydrate and fat intake and showed tendency to lower body weight [375]. Hence, these preliminary studies indicate that diet supplementation with (at least some) fatty acids may be another promising direction of research on appetite-modifying medications.
Despite the rapidly increasing number of studies on the effects of gut microbiota modifying agents (probiotics and prebiotics) on calorie intake and obesity development, we were not able to find any reports on their effects on food preferences in humans or animals.
SUMMARY AND PROSPECTS FOR FUTURE RESEARCH
In this review, we focused on the discussion of research studies investigating the possible usefulness of currently available pharmaceuticals in changing food preferences. Feeding behavior is controlled by homeostatic and hedonic centers that act in concert to induce hunger/satiety and “liking”/”wanting” of food, respectively. The non metabolic cues, however, that are driven mostly by food palatability may overhelm the homeostatic control and lead to overeating. Due to “additive-like” properties and high energy content, dietary sweet carbohydrates and fats are responsible primarily for increasing obesity rates worldwide. Therefore, we have searched especially for systemically active pharmaceuticals that selectively inhibited appetite for such rewarding high-calorie foods without reducing bland food consumption in a free-choice protocol when at least two dietary components (i.e., sweet/fat and bland food) were available. Many of these drugs could inhibit not only the consummatory but also appetitive behavior. The review of results of animal and human studies revealed several drugs that satisfy the abovementioned conditions with varying degrees. The most promising treatments include CB1 receptor antagonists, methylphenidate, baclofen, GLP-1 agonists, and oxytocin. Additionally, lorcaserin and two combined therapies with bupropion/naltrexone and exendin-4/AM251 as well as dietary supplements sodium glutamate and fatty acids have been found to have potency to reduce rewarding food intake, but their effectiveness needs to be confirmed by further studies. The main problems we encountered when discussing the results of research studies were different (and sometimes difficult to compare) methodologies used to estimate the effects of a given treatment on food preferences and a relative paucity of animal studies enabling the estimation of drugs’ effects under a free choice paradigm. Another important issue that may hinder an objective evaluation of results obtained is different individual sensitivity to pharmaceuticals caused by not completely identified factors, including sex hormones, genetic differences in enzyme activity, or receptor sensitivity to pharmaceuticals. It seems, therefore, that future studies aimed at finding successful therapeutic agents that modify food preference behavior must also take account of these issues.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
There is no conflict of interest. None of the authors have any relationships with the pharmaceutical industry, including the current or past employment or grants or honoraria received.
REFERENCES
- 1.Genné-Bacon E.A. Thinking evolutionarily about obesity. Yale J. Biol. Med. 2014;87:99–112. [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud H-R. The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc. 2012;71:478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.la Fleur S.E., Serlie M.J. The interaction between nutrition and the brain and its consequences for body weight gain and metabolism; studies in rodents and men. Best Pract. Re.s Clin. Endocrinol. Metab. 2014;28:649–659. doi: 10.1016/j.beem.2014.06.001. http://dx.doi.org/10.1016/j.beem.2014.06.001 . [DOI] [PubMed] [Google Scholar]
- 4.Deglaire A., Méjean C., Castetbon K., Kesse-Guyot E., Hercberg S., Schlich P. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (The Nutrinet-Santé study). Eur. J. Clin. Nutr. 2015;69:40–46. doi: 10.1038/ejcn.2014.139. [DOI] [PubMed] [Google Scholar]
- 5.Lanfer A., Knof K., Barba G., Veidebaum T., Papoutsou S., de Henauw S., Soós T., Moreno L.A., Ahrens W., Lissner L. Taste preferences in association with dietary habits and weight status in European children: results from the IDEFICS study. Int. J. Obes. 2012;36:27–34. doi: 10.1038/ijo.2011.164. [DOI] [PubMed] [Google Scholar]
- 6.Sampey B.P., Vanhoose A.M., Winfield H.M., Freemerman A.J., Muehlbauer M.J., Fueger P.T., Newgard C.B., Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.la Fleur S.E., Luijendijk M.C., van der Zwaal E.M., Brans M.A., Adan R.A. The snacking rat as model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int. J. Obes. 2014;38:643–649. doi: 10.1038/ijo.2013.159. [DOI] [PubMed] [Google Scholar]
- 8.Stevens G.A., Singh G.M., Lu Y., Danaei G., Lin J.K., Finucane M.M., Bahalim A.N., McIntire R.K., Gutierrez H.R., Cowan M., Paciorek C.J., Farzadfar F., Riley L., Ezzati M. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., Abraham J.P., Abu-Rmeileh N.M., Achoki T., AlBuhairan F.S., Alemu Z.A., Alfonso R., Ali M.K., Ali R., Guzman N.A., Ammar W., Anwari P., Banerjee A., Barquera S., Basu S., Bennett D.A., Bhutta Z., Blore J., Cabral N., Nonato I.C., Chang J.C., Chowdhury R., Courville K.J., Criqui M.H., Cundiff D.K., Dabhadkar K.C., Dandona L., Davis A., Dayama A., Dharmaratne S.D., Ding E.L., Durrani A.M., Esteghamati A., Farzadfar F., Fay D.F., Feigin V.L., Flaxman A., Forouzanfar M.H., Goto A., Green M.A., Gupta R., Hafezi-Nejad N., Hankey G.J., Harewood H.C., Havmoeller R., Hay S., Hernandez L., Husseini A., Idrisov B.T., Ikeda N., Islami F., Jahangir E., Jassal S.K., Jee S.H., Jeffreys M., Jonas J.B., Kabagambe E.K., Khalifa S.E., Kengne A.P., Khader Y.S., Khang Y.H., Kim D., Kimokoti R.W., Kinge J.M., Kokubo Y., Kosen S., Kwan G., Lai T., Leinsalu M., Li Y., Liang X., Liu S., Logroscino G., Lotufo P.A., Lu Y., Ma J., Mainoo N.K., Mensah G.A., Merriman T.R., Mokdad A.H., Moschandreas J., Naghavi M., Naheed A., Nand D., Narayan K.M., Nelson E.L., Neuhouser M.L., Nisar M.I., Ohkubo T., Oti S.O., Pedroza A., Prabhakaran D., Roy N., Sampson U., Seo H., Sepanlou S.G., Shibuya K., Shiri R., Shiue I., Singh G.M., Singh J.A., Skirbekk V., Stapelberg N.J., Sturua L., Sykes B. L., Tobias M., Tran B.X., Trasande L., Toyoshima H., van de Vijver S., Vasankari T.J., Veerman J.L., Velasquez-Melendez G., Vlassov V.V., Vollset S.E., Vos T., Wang C., Wang X., Weiderpass E., Werdecker A., Wright J.L., Yang Y.C., Yatsuya H., Yoon J., Yoon S.J., Zhao Y., Zhou M., Zhu S., Lopez A.D., Murray C.J., Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelstein E.A., Khavjou O.A., Thompson H., Trogdon J.G., Pan L., Sherry B., Dietz W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Sturm R., Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int. J. Obes. 2013;37:889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Onis M., Blössner M., Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. http://dx.doi.org/10.3945/ajcn.2010.29786 . [DOI] [PubMed] [Google Scholar]
- 13.Polikandrioti M., Stefanou E. Obesity disease. Health Sci. J. 2009;3:132–138. [Google Scholar]
- 14.Puhl R.M., King K.M. Weight discrimination and bullying. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:117–127. doi: 10.1016/j.beem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Conley D., Glauber R. Gender, body mass, and socioeconomic status: new evidence from the PSID. Adv. Health Econ. Health Serv. Res. 2007;17:253–275. doi: 10.1016/S0731-2199(06)17010-7. [DOI] [PubMed] [Google Scholar]
- 16.Field A.E., Austin S.B., Taylor C.B., Malspeis S., Rosner B., Rockett H.R., Gillman M.W., Colditz G.A. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics. 2003;112:900–906. doi: 10.1542/peds.112.4.900. http://dx.doi.org/10.1542/peds.112.4.900 . [DOI] [PubMed] [Google Scholar]
- 17.Bacon L.1., Aphramor L. Weight science: evaluating the evidence for a paradigm shift. [Accessed June 16, 2015];Nutr. J. 2011 10:9. doi: 10.1186/1475-2891-10-9. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3041737/pdf/1475-2891-10-9.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos-Leví A.M., Rubio Herrera M.A. Metabolic surgery: quo vadis? Endocrinol. Nutr. 2014;61:35–46. doi: 10.1016/j.endonu.2013.04.006. http://dx.doi.org/10.1016/j.endonu.2013.04.006 . [DOI] [PubMed] [Google Scholar]
- 19.Neff K.J., le Roux C.W. Bariatric surgery: the indications in metabolic disease. Dig. Surg. 2014;31:6–12. doi: 10.1159/000351440. [DOI] [PubMed] [Google Scholar]
- 20.Sesti G., Folli F., Perego L., Hribal M.L., Pontiroli A.E. Effects of weight loss in metabolically healthy obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. PLoS One. 2011;8(6):e17737. doi: 10.1371/journal.pone.0017737. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3050899/pdf/pone.0017737.pdf. (Accessed June 16, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svane M.S., Madsbad S. Bariatric surgery - effects on obesity and related co-morbidities. Curr. Diabetes Rev. 2014;10:208–214. doi: 10.2174/1573399810666140616144141. [DOI] [PubMed] [Google Scholar]
- 22.Edholm D., Näslund I., Anders Karlsson F., Rask E., Sundbom M. Twelve-year results for revisional gastric bypass after failed restrictive surgery in 131 patients. Surg. Obes. Relat. Dis. 2014;10:44–48. doi: 10.1016/j.soard.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Stein J., Stier C., Raab H., Weiner R. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment. Pharmacol. Ther. 2014;40:582–609. doi: 10.1111/apt.12872. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu H., Annaberdyev S., Motamarry I., Kroh M., Schauer P.R., Brethauer S.A. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes. Surg. 2013;23:1766–1773. doi: 10.1007/s11695-013-1012-1. [DOI] [PubMed] [Google Scholar]
- 25.Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg. Obes. Relat. Dis. 2014;10:1220–1225. doi: 10.1016/j.soard.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.McKenna D., Selzer D., Burchett M., Choi J., Mattar S.G. Revisional bariatric surgery is more effective for improving obesity-related co-morbidities than it is for reinducing major weight loss. Surg. Obes. Relat. Dis. 2013;10:654–659. doi: 10.1016/j.soard.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Brethauer S.A., Kothari S., Sudan R., Williams B., English W.J., Brengman M., Kurian M., Hutter M., Stegemann L., Kallies K., Nguyen N.T., Ponce J., Morton J.M. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg. Obes. Relat. Dis. 2014;10:952, 972. doi: 10.1016/j.soard.2014.02.014. http://dx.doi.org/10.1016/j.soard.2014.02.014 . [DOI] [PubMed] [Google Scholar]
- 28.Hussain A., El-Hasani S. Bariatric emergencies: current evidence and strategies of management. World J. Emerg. Surg. 2013;8:58. doi: 10.1186/1749-7922-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rui L. Brain regulation of energy balance and body weight. Rev. Endocr. Metab. Disord. 2013;14:387–407. doi: 10.1007/s11154-013-9261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneeberger M., Gomis R., Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014;220:T25–T46. doi: 10.1530/JOE-13-0398. [DOI] [PubMed] [Google Scholar]
- 31.Chambers A.P., Sandoval D.A., Seeley R.J. Integration of satiety signals by the central nervous system. Curr. Biol. 2013;23:R379–R388. doi: 10.1016/j.cub.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Näslund E., Hellström P.M. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol. Behav. 2007;92:256–262. doi: 10.1016/j.physbeh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Neunlist M., Schemann M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. J. Physiol. 2014;592:2959–2965. doi: 10.1113/jphysiol.2014.272948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobrino Crespo C., Perianes Cachero A., Puebla Jiménez L., Barrios V., Arilla Ferreiro E. Peptides and food intake. Front. Endocrinol. 2014;5:58. doi: 10.3389/fendo.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egecioglu E., Skibicka K.P., Hansson C., Alvarez-Crespo M., Friberg P.A., Jerlhag E., Engel J.A., Dickson S.L. Hedonic and incentive signals for body weight control. Rev. Endocr. Metab. Disord. 2011;12:141–151. doi: 10.1007/s11154-011-9166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berridge K.C., Ho C.Y., Richard J.M., DiFeliceantonio A.G. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro D.C., Cole S.L., Berridge K.C. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olszewski P.K., Cedernaes J., Olsson F., Levine A.S., Schiöth H.B. Analysis of the network of feeding neuroregulators using the Allen Brain Atlas. Neurosci. Biobehav. Rev. 2008;32:945–956. doi: 10.1016/j.neubiorev.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams D.L. Neural integration of satiation and food reward: Role of GLP-1 and orexin pathways. Physiol. Behav. 2014;136:194–199. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaere F., Akaoka H., De Vadder F., Duchampt A., Mithieux G. Portal glucose influences the sensory, cortical and reward systems in rats. Eur. J. Neurosci. 2013;38:3476–3486. doi: 10.1111/ejn.12354. [DOI] [PubMed] [Google Scholar]
- 41.Burger K.S., Berner L.A. A functional neuroimaging review of obesity, appetitive hormones and ingestive behavior. Physiol. Behav. 2014;136:121–127. doi: 10.1016/j.physbeh.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeeni N., Nadkarni N., Bell J.D., Even P.C., Fromentin G., Tome D., Darcel N. Peripherally injected cholecystokinin-induced neuronal activation is modified by dietary composition in mice. Neuroimage. 2010;50:1560–1565. doi: 10.1016/j.neuroimage.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 43.Figlewicz D.P., Bennett-Jay J.L., Kittleson S., Sipols A.J., Zavosh A. Sucrose self-administration and CNS activation in the rat. Am. J. Physiol. 2011;300:R876–R884. doi: 10.1152/ajpregu.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirkham T.C. Endocannabinoids and the non-homeostatic control of appetite. In: Kendall D., Alexander S., editors. Behavioral Neurobiology of the Endocannabinoid System, Current Topics in Behavioral Neurosciences. Berlin, Heidelberg: Springer‐Verlag; 2009. pp. 231–253. [DOI] [PubMed] [Google Scholar]
- 45.Kanoski S.E. Cognitive and neuronal systems underlying obesity. Physiol. Behav. 2012;106:337–344. doi: 10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mela D.J., Sacchetti D.A. Sensory preferences for fats: relationships with diet and body composition. Am. J. Clin. Nutr. 1991;53:908–915. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 47.Shin A.C., Townsend R.L., Patterson L.M., Berthoud H.R. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am. J. Physiol. 2011;301:R1267–R1280. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berthoud H.R., Zheng H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012;107:527–532. doi: 10.1016/j.physbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickering C., Alsiö J., Hulting A.L., Schiöth H.B. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology (Berl.) 2009;204:431–443. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- 50.Burton P., Smit H.J., Lightowler H.J. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–197. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Alsiö J., Olszewski P.K., Levine A.S., Schiöth H.B. Feed-forward mechanisms: addiction-like behavioral and molecular adaptations in overeating. Front. Neuroendocrinol. 2012;33:127–139. doi: 10.1016/j.yfrne.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Ransleyb J.K., Donnellya J.K., Bothama H., Kharaa T.N., Greenwoodb D.C., Cade J.E. Use of supermarket receipts to estimate energy and fat content of food purchased by lean and overweight families. Appetite. 2003;41:141–148. doi: 10.1016/S0195-6663(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 53.De Silva A., Salem V., Matthews P.M., Dhillo W.S. The use of functional MRI to study appetite control in the CNS. [Accessed October 13, 2015];Exp. Diabetes Res. 2012 2012:764017. doi: 10.1155/2012/764017. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3376546/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Val-Laillet D., Aarts E., Weber B., Ferrari M., Quaresima V., Stoeckel L.E., Alonso-Alonso M., Audette M., Malbert C.H., Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avena N.M., Rada P., Hoebel B.G. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen C.M. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:109–122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hebebrand J., Albayrak Ö., Adan R., Antel J., Dieguez C., de Jong J., Leng G., Menzies J., Mercer J.G., Murphy M., van der Plasse G., Dickson S.L. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Rolls E.T. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 2015;127-128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Chambers L., McCrickerd K., Yeomans M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015;41:149–160. doi: 10.1016/j.tifs.2014.10.007. [DOI] [Google Scholar]
- 60.Llewellyn C.H., Trzaskowski M., van Jaarsveld C.H., Plomin R., Wardle J. Satiety mechanisms in genetic risk of obesity. JAMA Pediatr. 2014;168:338–344. doi: 10.1001/jamapediatrics.2013.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Journel M., Chaumontet C., Darcel N., Fromentin G., Tomé D. Brain responses to high-protein diets. Adv. Nutr. 2012;3:322–329. doi: 10.3945/an.112.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpson S.J., Raubenheimer D. Obesity: the protein leverage hypothesis. Obes. Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 63.Krebs J.R. The gourmet ape: evolution and human food preferences. Am. J. Clin. Nutr. 2009;90:707S–711S. doi: 10.3945/ajcn.2009.27462B. [DOI] [PubMed] [Google Scholar]
- 64.Leidy H.J., Ortinau L.C., Douglas S.M., Hoertel H.A. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am. J. Clin. Nutr. 2013;97:677–688. doi: 10.3945/ajcn.112.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alkerwi A., Sauvageot N., Buckley J.D., Donneau A.F., Albert A., Guillaume M., Crichton G.E. The potential impact of animal protein intake on global and abdominal obesity: evidence from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Public Health Nutr. 2015;22:1–8. doi: 10.1017/s1368980014002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith J.D., Hou T.1., Ludwig D.S., Rimm E.B., Willett W., Hu F.B., Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am. J. Clin. Nutr. 2015;101:1216–1224. doi: 10.3945/ajcn.114.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Marzo V., Ligresti A., Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int. J. Obes. 2009;33(Suppl. 2):S18–S24. doi: 10.1038/ijo.2009.67. [DOI] [PubMed] [Google Scholar]
- 68.Silvestri C., Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Cristino L.1., Becker T., Di Marzo V. Endocannabinoids and energy homeostasis: An update. Biofactor. 2014;40:389–397. doi: 10.1002/biof.1168. [DOI] [PubMed] [Google Scholar]
- 70.Jager G., Witkamp R.F. The endocannabinoid system and appetite: relevance for food reward. Nutr. Res. Rev. 2014;27:172–185. doi: 10.1017/S0954422414000080. [DOI] [PubMed] [Google Scholar]
- 71.Escartín-Pérez R.E., Cendejas-Trejo N.M., Cruz-Martínez A.M., González-Hernández B., Mancilla-Díaz J.M., Florán-Garduño B. Role of cannabinoid CB1 receptors on macronutrient selection and satiety in rats. Physiol. Behav. 2009;96:646–650. doi: 10.1016/j.physbeh.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida R., Niki M., Jyotaki M., Sanematsu K., Shigemura N., Ninomiya Y. Modulation of sweet responses of taste receptor cells. Semin. Cell Dev. Biol. 2013;24:226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Simiand J., Keane M., Keane P.E., Soubrié P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav. Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- 74.Foltin R.W., Haney M. Effects of the cannabinoid antagonist SR141716 (rimonabant) and d-amphetamine on palatable food and food pellet intake in non-human primates. Pharmacol. Biochem. Behav. 2007;86:766–773. doi: 10.1016/j.pbb.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heppenstall C., Bunce S., Smith J.C. Relationships between glucose, energy intake and dietary composition in obese adults with type 2 diabetes receiving the cannabinoid 1 (CB1) receptor antagonist, rimonabant. Nutr. J. 2012;11:50. doi: 10.1186/1475-2891-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horder J., Harmer C., Cowen P., McCabe C. Reduced neural response to reward following 7 days treatment with the cannabinoid CB1 antagonist rimonabant in healthy volunteers. Int. J. Neuropsychopharmacol. 2010;13:1103–1113. doi: 10.1017/S1461145710000453. [DOI] [PubMed] [Google Scholar]
- 77.Mathes C.M., Ferrara M., Rowland N.E. Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. Am. J. Physiol. 2008;295:R67–R75. doi: 10.1152/ajpregu.00150.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnone M., Maruani J., Chaperon F., Thiébot M.H., Poncelet M., Soubrié P., Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl.) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 79.Droste S.M., Saland S.K., Schlitter E.K., Rodefer J.S. AM 251 differentially effects food-maintained responding depending on food palatability. Pharmacol. Biochem. Behav. 2010;95:443–448. doi: 10.1016/j.pbb.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 80.Ward S.J., Lefever T.W., Jackson C., Tallarida R.J., Walker E.A. Effects of a cannabinoid1 receptor antagonist and serotonin2C receptor agonist alone and in combination on motivation for palatable food: a dose-addition analysis study in mice. J. Pharmacol. Exp. Ther. 2008;325:567–576. doi: 10.1124/jpet.107.131771. [DOI] [PubMed] [Google Scholar]
- 81.Mancino S., Burokas A.1., Gutiérrez-Cuesta J., Gutiérrez-Martos M., Martín-García E., Pucci M., Falconi A., D'Addario C., Maccarrone M., Maldonado R. Epigenetic and proteomic expression changes promoted by eating addictive-like behavior. Neuropsychopharmacology. 2015;129 doi: 10.1038/npp.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Radziszewska E., Wolak M., Bojanowska E. >Concurrent pharmacological modification of cannabinoid-1 and glucagon-like peptide-1 receptor activity affects feeding behavior and body weight in rats fed a free-choice, high-carbohydrate diet. Behav. Pharmacol. 2014;25:53–60. doi: 10.1097/FBP.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 83.Wierucka-Rybak M., Wolak M., Bojanowska E. The effects of leptin in combination with a cannabinoid receptor 1 antagonist, AM 251, or cannabidiol on food intake and body weight in rats fed a high-fat or a free-choice high sugar diet. J. Physiol. Pharmacol. 2014;65:487–496. [PubMed] [Google Scholar]
- 84.Ward S.J., Walker E.A. Sex and cannabinoid CB1 genotype differentiate palatable food and cocaine self-administration behaviors in mice. Behav. Pharmacol. 2009;20:605–613. doi: 10.1097/FBP.0b013e328331ba30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathes C.M., Ferrara M., Rowland N.E. Selection of a palatable dietary option is not preferentially reduced by cannabinoid CB1 receptor antagonist AM251 in female C57Bl/6J mice. Pharmacol. Biochem. Behav. 2009;94:119–123. doi: 10.1016/j.pbb.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 86.Brand T., Spanagel R., Schneider M. Decreased reward sensitivity in rats from the Fischer344 strain compared to Wistar rats is paralleled by differences in endocannabinoid signaling. PLoS One. 2012;7:e31169. doi: 10.1371/journal.pone.0031169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Merrer J., Becker J.A., Befort K., Kieffer B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nogueiras R., Romero-Picó A., Vazquez M.J., Novelle M.G., López M., Diéguez C. The opioid system and food intake: homeostatic and hedonic mechanisms. Obes. Facts. 2012;19:196–207. doi: 10.1159/000338163. [DOI] [PubMed] [Google Scholar]
- 89.Taha S.A. Preference or fat? Revisiting opioid effects on food intake. Physiol. Behav. 2010;100:429–437. doi: 10.1016/j.physbeh.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peciña S., Smith K.S. Hedonic and motivational roles of opioids in food reward: implications for overeating disorders. Pharmacol. Biochem. Behav. 2010;97:34–46. doi: 10.1016/j.pbb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 91.Davis C.M., Stevenson G.W., Cañadas F., Ullrich T., Rice K.C., Riley A.L. Discriminative stimulus properties of naloxone in Long-Evans rats: assessment with the conditioned taste aversion baseline of drug discrimination learning. Psychopharmacology (Berl.) 2009;203:421–429. doi: 10.1007/s00213-008-1233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong K.J., Wojnicki F.H., Corwin R.L. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol. Biochem. Behav. 2009;92:528–536. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooper S.J., Turkish S. Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacol. Biochem. Behav. 1989;33:17–20. doi: 10.1016/0091-3057(89)90422-X. [DOI] [PubMed] [Google Scholar]
- 94.Olszewski P.K., Shaw T.J., Grace M.K., Höglund C.E., Fredriksson R., Schiöth H.B., Levine A.S. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides. 2009;30:226–233. doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giraudo S.Q., Grace M.K., Welch C.C., Billington C.J., Levine A.S. Naloxone's anorectic effect is dependent upon the relative palatability of food. Pharmacol. Biochem. Behav. 1993;46:917–921. doi: 10.1016/0091-3057(93)90222-F. [DOI] [PubMed] [Google Scholar]
- 96.Glass M.J., Grace M., Cleary J.P., Billington C.J., Levine A.S. Potency of naloxone's anorectic effect in rats is dependent on diet preference. Am. J. Physiol. 1996;271:R217–R221. doi: 10.1152/ajpregu.1996.271.1.R217. [DOI] [PubMed] [Google Scholar]
- 97.Bonacchi K.B., Ackroff K., Touzani K., Bodnar R.J., Sclafani A. Opioid mediation of starch and sugar preference in the rat. Pharmacol. Biochem. Behav. 2010;96:507–514. doi: 10.1016/j.pbb.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gugusheff J.R., Ong Z.Y., Muhlhausler B.S. Naloxone treatment alters gene expression in the mesolimbic reward system in 'junk food' exposed offspring in a sex-specific manner but does not affect food preferences in adulthood. Physiol. Behav. 2014;133:14–21. doi: 10.1016/j.physbeh.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Norris J.N., Pérez-Acosta A.M., Ortega L.A., Papini M.R. Naloxone facilitates appetitive extinction and eliminates escape from frustration. Pharmacol. Biochem. Behav. 2009;94:81–87. doi: 10.1016/j.pbb.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 100.Dym C.T., Pinhas A., Ginzberg M., Kest B., Bodnar R.J. Genetic variance contributes to naltrexone-induced inhibition of sucrose intake in inbred and outbred mouse strains. Brain Res. 2007;1135:136–145. doi: 10.1016/j.brainres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Desko A.G., Cobuzzi J.L., Riley A.L. Naloxone-induced taste aversions in opiate-naïve Lewis and Fischer 344 rat strains. Drug Alcohol Depend. 2012;122:152–155. doi: 10.1016/j.drugalcdep.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 102.Rabiner E.A., Beaver J., Makwana A., Searle G., Long C., Nathan P.J., Newbould R.D., Howard J., Miller S.R., Bush M.A., Hill S., Reiley R., Passchier J., Gunn R.N., Matthews P.M., Bullmore E.T. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol. Psychiatry. 2011;16:826–835. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murray E., Brouwer S., McCutcheon R., Harmer C.J., Cowen P.J., McCabe C. Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology (Berl.) 2014;231:4323–4335. doi: 10.1007/s00213-014-3573-7. [DOI] [PubMed] [Google Scholar]
- 104.Yeomans M.R., Gray R.W. Selective effects of naltrexone on food pleasantness and intake. Physiol. Behav. 1996;60:439–446. doi: 10.1016/S0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 105.Langleben D.D., Busch E.L., O'Brien C.P., Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology (Berl.) 2012;220:559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drewnowski A., Krahn D.D., Demitrack M.A., Nairn K., Gosnell B.A. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol. Behav. 1992;51:371–379. doi: 10.1016/0031-9384(92)90155-U. [DOI] [PubMed] [Google Scholar]
- 107.Drewnowski A., Krahn D.D., Demitrack M.A., Nairn K., Gosnell B.A. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am. J. Clin. Nutr. 1995;61:1206–1212. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- 108.Lee M.W., Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin. Pharmacother. 2009;10:1841–1845. doi: 10.1517/14656560903048959. [DOI] [PubMed] [Google Scholar]
- 109.Fernandes M.F., Sharma S., Hryhorczuk C., Auguste S., Fulton S. Nutritional controls of food reward. Can. J. Diabetes. 2013;37:260–268. doi: 10.1016/j.jcjd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Kennett G.A., Clifton P.G. New approaches to the pharmacological treatment of obesity: can they break through the efficacy barrier? Pharmacol. Biochem. Behav. 2010;97:63–83. doi: 10.1016/j.pbb.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 111.Volkow N.D., Wang G.J., Baler R.D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Björklund A., Dunnett S.B. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 113.Taber K.H., Black D.N., Porrino L.J., Hurley R.A. Neuroanatomy of dopamine: reward and addiction. J. Neuropsychiatry Clin. Neurosci. 2012;24:1–4. doi: 10.1176/appi.neuropsych.24.1.1. [DOI] [PubMed] [Google Scholar]
- 114.Levey A.I., Hersch S.M., Rye D.B., Sunahara R.K., Niznik H.B., Kitt C.A., Price D.L., Maggio R., Brann M.R., Ciliax B.J. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc. Natl. Acad. Sci. USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N., Maratos-Flier E., Flier J.S. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Hommel J.D., Trinko R., Sears R.M., Georgescu D., Liu Z.W., Gao X.B., Thurmon J.J., Marinelli M., DiLeone R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 117.Mietlicki-Baase E.G., Ortinski P.I., Rupprecht L.E., Olivos D.R., Alhadeff A.L., Pierce R.C., Hayes M.R. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hajnal A., Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/S0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 119.Shimura T., Kamada Y., Yamamoto T. Ventral tegmental lesions reduce overconsumption of normally preferred taste fluid in rats. Behav. Brain Res. 2002;134:123–130. doi: 10.1016/S0166-4328(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 120.Martínez-Hernández J., Lanuza E., Martínez-García F. Selective dopaminergic lesions of the ventral tegmental area impair preference for sucrose but not for male sexual pheromones in female mice. Eur. J. Neurosci. 2006;24:885–893. doi: 10.1111/j.1460-9568.2006.04944.x. [DOI] [PubMed] [Google Scholar]
- 121.Tõnissaar M., Herm L., Rinken A., Harro J. Individual differences in sucrose intake and preference in the rat: circadian variation and association with dopamine D2 receptor function in striatum and nucleus accumbens. Neurosci. Lett. 2006;403:119–124. doi: 10.1016/j.neulet.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 122.Anderberg R.H., Anefors C., Bergquist F., Nissbrandt H., Skibicka K.P. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol. Behav. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 123.Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 124.Peciña S., Cagniard B., Berridge K.C., Aldridge J.W., Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J. Neurosci. 2003;23:9395–93402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith K.S., Berridge K.C., Aldridge J.W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baik J.H. Dopamine signaling in food addiction: role of dopamine D2 receptors. BMB Rep. 2013;46:519–526. doi: 10.5483/BMBRep.2013.46.11.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cooper S.J., Al-Naser H.A. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 128.van Gestel M.A., Kostrzewa E., Adan R.A., Janhunen S.K. Pharmacological manipulations in animal models of anorexia and binge eating in relation to humans. Br. J. Pharmacol. 2014;171:4767–4784. doi: 10.1111/bph.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cosgrove K.P., Veldhuizen M.G., Sandiego C.M., Morris E.D., Small D.M. Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse. 2015;69:195–202. doi: 10.1002/syn.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karlsson H.K., Tuominen L., Tuulari J.J., Hirvonen J., Parkkola R., Helin S., Salminen P., Nuutila P., Nummenmaa L. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J. Neurosci. 2015;35:3959–3965. doi: 10.1523/JNEUROSCI.4744-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Weijer B.A., van de Giessen E., Janssen I., Berends F.J., van de Laar A., Ackermans M.T., Fliers E., la Fleur S.E., Booij J., Serlie M.J. Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia. 2014;57:1078–1080. doi: 10.1007/s00125-014-3178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van de Giessen E., Celik F., Schweitzer D.H., van den Brink W., Booij J. 5. Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J. Psychopharmacol. 2014;28:866–873. doi: 10.1177/0269881114531664. [DOI] [PubMed] [Google Scholar]
- 133.Volkow N.D., Wang G.J., Telang F., Fowler J.S., Thanos P.K., Logan J., Alexoff D., Ding Y.S., Wong C., Ma Y., Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang G.J., Volkow N.D., Logan J., Pappas N.R., Wong C.T., Zhu W., Netusil N., Fowler J.S. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/S0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 135.Geiger B.M., Behr G.G., Frank L.E., Caldera-Siu A.D., Beinfeld M.C., Kokkotou E.G., Pothos E.N. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang G.J., Tomasi D., Convit A., Logan J., Wong C.T., Shumay E., Fowler J.S., Volkow N.D. BMI modulates calorie-dependent dopamine changes in accumbens from glucose intake. PLoS One. 2014;9:e101585. doi: 10.1371/journal.pone.0101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stice E., Burger K.S., Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA Allele. J. Neurosci. 2015;35:10316–10324. doi: 10.1523/JNEUROSCI.3607-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seger D. Cocaine, metamfetamine, and MDMA abuse: the role and clinical importance of neuroadaptation. Clin. Toxicol. 2010;48:695–708. doi: 10.3109/15563650.2010.516263. [DOI] [PubMed] [Google Scholar]
- 139.Schep L.J., Slaughter R.J., Beasley D.M. The clinical toxicology of metamfetamine. Clin. Toxicol. 2010;48:675–694. doi: 10.3109/15563650.2010.516752. [DOI] [PubMed] [Google Scholar]
- 140.Goodall E., Feeney S., McGuirk J., Silverstone T. A comparison of the effects of d- and l-fenfluramine and d-amphetamine on energy and macronutrient intake in human subjects. Psychopharmacology (Berl.) 1992;106:221–227. doi: 10.1007/BF02801976. [DOI] [PubMed] [Google Scholar]
- 141.Foltin R.W., Kelly T.H., Fischman M.W. Effect of amphetamine on human macronutrient intake. Physiol. Behav. 1995;58:899–907. doi: 10.1016/0031-9384(95)00149-D. [DOI] [PubMed] [Google Scholar]
- 142.Foltin R.W. Effects of dietary and pharmacological manipulations on appetitive and consummatory aspects of feeding in non-human primates. Appetite. 2005;45:110–120. doi: 10.1016/j.appet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 143.Danilovich N., Mastrandrea L.D., Cataldi L., Quattrin T. Methylphenidate decreases fat and carbohydrate intake in obese teenagers. Obesity (Silver Spring) 2014;22:781–785. doi: 10.1002/oby.20574. [DOI] [PubMed] [Google Scholar]
- 144.Goldfield G.S., Lorello C., Cameron J., Chaput J.P. Gender differences in the effects of methylphenidate on energy intake in young adults: a preliminary study. Appl. Physiol. Nutr. Metab. 2011;36:1009–1013. doi: 10.1139/h11-098. [DOI] [PubMed] [Google Scholar]
- 145.Leddy J.J., Epstein L.H., Jaroni J.L., Roemmich J.N., Paluch R.A., Goldfield G.S., Lerman C. Influence of methylphenidate on eating in obese men. Obes. Res. 2004;12:224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- 146.Durá-Travé T., Gallinas-Victoriano F. Caloric and nutrient intake in children with attention deficit hyperactivity disorder treated with extended-release methylphenidate: analysis of a cross-sectional nutrition survey. JRSM Open. 2014;5:2042533313517690. doi: 10.1177/2042533313517690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Levy L.D., Fleming J.P., Klar D. Treatment of refractory obesity in severely obese adults following management of newly diagnosed attention deficit hyperactivity disorder. Int. J. Obes. 2009;33:326–334. doi: 10.1038/ijo.2009.5. [DOI] [PubMed] [Google Scholar]
- 148.Bello N.T., Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res. Bull. 2006;70:422–429. doi: 10.1016/j.brainresbull.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 149.Minet-Ringuet J., Even P.C., Guesdon B., Tomé D., de Beaurepaire R. Effects of chronic neuroleptic treatments on nutrient selection, body weight, and body composition in the male rat under dietary self-selection. Behav. Brain Res. 2005;163:204–211. doi: 10.1016/j.bbr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 150.Schneider L.H., Gibbs J., Smith G.P. D-2 selective receptor antagonists suppress sucrose sham feeding in the rat. Brain Res. Bull. 1986;17:605–611. doi: 10.1016/0361-9230(86)90231-5. [DOI] [PubMed] [Google Scholar]
- 151.Boomhower S.R., Rasmussen E.B. Haloperidol and rimonabant increase delay discounting in rats fed high-fat and standard-chow diets. Behav. Pharmacol. 2014;25:705–716. doi: 10.1097/FBP.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fell M.J., Anjum N., Dickinson K., Marshall K.M., Peltola L.M., Vickers S., Cheetham S., Neill J.C. The distinct effects of subchronic antipsychotic drug treatment on macronutrient selection, body weight, adiposity, and metabolism in female rats. Psychopharmacology. 2007;194:221–231. doi: 10.1007/s00213-007-0833-9. [DOI] [PubMed] [Google Scholar]
- 153.Pardo M., López-Cruz L., San Miguel N., Salamone J.D., Correa M. Selection of sucrose concentration depends on the effort required to obtain it: studies using tetrabenazine, D1, D2, and D3 receptor antagonists. Psychopharmacology (Berl.) 2015;232:2377–2391. doi: 10.1007/s00213-015-3872-7. [DOI] [PubMed] [Google Scholar]
- 154.Smith G.C., Vickers M.H., Cognard E., Shepherd P.R. Clozapine and quetiapine acutely reduce glucagon-like peptide-1 production and increase glucagon release in obese rats: implications for glucose metabolism and food choice behaviour. Schizophr. Res. 2009;115:30–40. doi: 10.1016/j.schres.2009.07. [DOI] [PubMed] [Google Scholar]
- 155.Henderson D.C., Sharma B., Fan X., Copeland P.M., Borba C.P., Freudenreich O., Cather C., Evins A.E., Goff D.C. Dietary saturated fat intake and glucose metabolism impairments in nondiabetic, nonobese patients with schizophrenia on clozapine or risperidone. Ann. Clin. Psychiatry. 2010;22:33–42. [PubMed] [Google Scholar]
- 156.Fallon S., Shearman E., Sershen H., Lajtha A. Food reward-induced neurotransmitter changes in cognitive brain regions. Neurochem. Res. 2007;32:1772–1782. doi: 10.1007/s11064-007-9343-8. [DOI] [PubMed] [Google Scholar]
- 157.Ventura R., Morrone C., Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. USA. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adan R.A., Vanderschuren L.J., la Fleur S.E. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol. Sci. 2008;29:208–217. doi: 10.1016/j.tips.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 159.Savard P., Mérand Y., Leblanc J., Dupont A. Limitation of access to highly palatable foods increases the norepinephrine content of many discrete hypothalamic and amygdaloidal nuclei of rat brain. Life Sci. 1983;33:2513–2519. doi: 10.1016/0024-3205(83)90160-1. [DOI] [PubMed] [Google Scholar]
- 160.Leibowitz S.F., Weiss G.F., Yee F., Tretter J.B. Noradrenergic innervation of the paraventricular nucleus: specific role in control of carbohydrate ingestion. Brain Res. Bull. 1985;14:561–567. doi: 10.1016/0361-9230(85)90105-4. [DOI] [PubMed] [Google Scholar]
- 161.Chafetz M.D., Byrne K.S., King B.M. Effects of clonidine on self-selection of nutrients. Physiol. Behav. 1989;46:999–1002. doi: 10.1016/0031-9384(89)90204-7. [DOI] [PubMed] [Google Scholar]
- 162.Gehlert D.R., Dreshfield L., Tinsley F., Benvenga M.J., Gleason S., Fuller R.W., Wong D.T., Hemrick-Luecke S.K. The selective norepinephrine reuptake inhibitor, LY368975, reduces food consumption in animal models of feeding. J. Pharmacol. Exp. Ther. 1998;287:122–127. [PubMed] [Google Scholar]
- 163.Levine M.D., Cheng Y., Kalarchian M.A., Perkins K.A., Marcus M.D. Dietary intake after smoking cessation among weight-concerned women smokers. Psychol. Addict. Behav. 2012;26:969–973. doi: 10.1037/a0028948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lerman C., Berrettini W., Pinto A., Patterson F., Crystal-Mansour S., Wileyto E.P., Restine S.L., Leonard D.G., Shields P.G., Epstein L.H. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl.) 2004;174:571–577. doi: 10.1007/s00213-004-1823-9. [DOI] [PubMed] [Google Scholar]
- 165.Billes S.K., Sinnayah P., Cowley M.A. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol. Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 166.Wang G.J., Tomasi D., Volkow N.D., Wang R., Telang F., Caparelli E.C., Dunayevich E. Effect of combined naltrexone and bupropion therapy on the brain's reactivity to food cues. Int. J. Obes. 2014;38:682–688. doi: 10.1038/ijo.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lam D.D., Garfield A.S., Marston O.J., Shaw J., Heisler L.K. Brain serotonin system in the coordination of food intake and body weight. Pharmacol. Biochem. Behav. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 168.Hayes D. J1.; Greenshaw, A.J. 5-HT receptors and reward-related behaviour: a review. Neurosci. Biobehav. Rev. 2011;35:1419–1449. doi: 10.1016/j.neubiorev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 169.Garfield A.S., Heisler L.K. Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. 2009;587:49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Voigt J.P., Fink H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015;277:14–31. doi: 10.1016/j.bbr.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 171.Kanarek R.B., Dushkin H. Peripheral serotonin administration selectively reduces fat intake in rats. Pharmacol. Biochem. Behav. 1988;31:113–122. doi: 10.1016/0091-3057(88)90321-8. [DOI] [PubMed] [Google Scholar]
- 172.Cangiano C., Laviano A., Del Ben M., Preziosa I., Angelico F., Cascino A., Rossi-Fanelli F. Effects of oral 5-hydroxy-tryptophan on energy intake and macronutrient selection in non-insulin dependent diabetic patients. Int. J. Obes. Relat. Metab. Disord. 1998;22:648–654. doi: 10.1038/sj.ijo.0800642. [DOI] [PubMed] [Google Scholar]
- 173.Antonatos S., Galanopoulou P. Effects of mu-CPP and mesulergine on dietary choices in deprived rats: possible mechanisms of their action. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:112–119. doi: 10.1016/j.pnpbp.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 174.Heisler L.K., Kanarek R.B., Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol. Biochem. Behav. 1997;58:767–773. doi: 10.1016/S0091-3057(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 175.Heisler L.K., Kanarek R.B., Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol. Biochem. Behav. 1999;63:377–385. doi: 10.1016/s0091-3057(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 176.Duhault J., Lacour F., Espinal J., Rolland Y. Effect of activation of the serotoninergic system during prolonged starvation on subsequent caloric intake and macronutrient selection in the Zucker rat. Appetite. 1993;20:135–144. doi: 10.1006/appe.1993.1015. [DOI] [PubMed] [Google Scholar]
- 177.Lawton C.L., Blundell J.E. 5-HT manipulation and dietary choice: variable carbohydrate (Polycose) suppression demonstrated only under specific experimental conditions. Psychopharmacology (Berl.) 1993;112:375–382. doi: 10.1007/BF02244936. [DOI] [PubMed] [Google Scholar]
- 178.Mathes C.M., Gregson J.R., Spector A.C. The selective serotonin reuptake inhibitor paroxetine decreases breakpoint of rats engaging in a progressive ratio licking task for sucrose and quinine solutions. Chem. Senses. 2013;38:211–220. doi: 10.1093/chemse/bjs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Foltin R.W., Haney M., Comer S.D., Fischman M.W. Effect of fluoxetine on food intake of humans living in a residential laboratory. Appetite. 1996;27:165–181. doi: 10.1006/appe.1996.0043. [DOI] [PubMed] [Google Scholar]
- 180.Ward A.S., Comer S.D., Haney M., Fischman M.W., Foltin R.W. Fluoxetine-maintained obese humans: effect on food intake and body weight. Physiol. Behav. 1999;66:815–821. doi: 10.1016/S0031-9384(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 181.LeBlanc M., Thibault L. Effect of sibutramine on macronutrient selection in male and female rats. Physiol. Behav. 2003;80:243–252. doi: 10.1016/j.physbeh.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 182.Hansen G., Jelsing J., Vrang N. Effects of liraglutide and sibutramine on food intake, palatability, body weight and glucose tolerance in the gubra DIO-rats. Acta Pharmacol. Sin. 2012;33:194–200. doi: 10.1038/aps.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Popik P., Kos T., Zhang Y., Bisaga A. Memantine reduces consumption of highly palatable food in a rat model of binge eating. Amino Acids. 2011;40:477–485. doi: 10.1007/s00726-010-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pratt W.E., Connolly M.E. Contrasting effects of systemic and central sibutramine administration on the intake of a palatable diet in the rat. Neurosci. Lett. 2010;484:30–34. doi: 10.1016/j.neulet.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 185.van der Zwaal E.M. Janhunen, S.K.; Luijendijk, M.C.; Baclesanu, R.; Vanderschuren, L.J; Adan, R.A.; La Fleur, S.E. Olanzapine and sibutramine have opposing effects on the motivation for palatable food. Behav. Pharmacol. 2012;23:198–204. doi: 10.1097/FBP.0b013e3283512ca1. [DOI] [PubMed] [Google Scholar]
- 186.Ravindran P.P., Zang W., Renukunta S., Mansour R., Denduluri S. Effect of comedication of bupropion and other antidepressants on body mass index. Ther. Adv. Psychopharmacol. 2015;5:158–165. doi: 10.1177/2045125315577057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smith B.K., York D.A., Bray G.A. Chronic d-fenfluramine treatment reduces fat intake independent of macronutrient preference. Pharmacol. Biochem. Behav. 1998;60:105–114. doi: 10.1016/S0091-3057(97)00549-2. [DOI] [PubMed] [Google Scholar]
- 188.Jourdan D., Piec I., Gaulier J.M., Lacassie E., Alliot J. Effect of fenfluramine on caloric intake and macronutrient selection in Lou/c rats during aging. Neurobiol. Aging. 2003;24:67–76. doi: 10.1016/S0197-4580(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 189.Drent M.L., Zelissen P.M., Koppeschaar H.P., Nieuwenhuyzen Kruseman A.C., Lutterman J.A., van der Veen E.A. The effect of dexfenfluramine on eating habits in a Dutch ambulatory android overweight population with an overconsumption of snacks. Int. J. Obes. Relat. Metab. Disord. 1995;19:299–304. [PubMed] [Google Scholar]
- 190.Breum L., Møller S.E., Andersen T., Astrup A. Long-term effect of dexfenfluramine on amino acid profiles and food selection in obese patients during weight loss. Int. J. Obes. Relat. Metab. Disord. 1996;20:147–153. [PubMed] [Google Scholar]
- 191.Blundell J.E., Lawton C.L. Serotonin and dietary fat intake: effects of dexfenfluramine. Metabolism. 1995;44(Suppl. 2):33–37. doi: 10.1016/0026-0495(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 192.Atchley D.P., Weaver K.L., Eckel L.A. Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats. Physiol. Behav. 2005;86:265–271. doi: 10.1016/j.physbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 193.Foltin R.W., Danysz W., Bisaga A. A novel procedure for assessing the effects of drugs on satiation in baboons: effects of memantine and dexfenfluramine. Psychopharmacology (Berl.) 2008;199:583–592. doi: 10.1007/s00213-008-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim G.W., Lin J.E., Blomain E.S., Waldman S.A. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin. Pharmacol. Ther. 2014;95:53–66. doi: 10.1038/clpt.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Prinssen E.P., Koek W., Kleven M.S. The effects of antipsychotics with 5-HT(2C) receptor affinity in behavioral assays selective for 5-HT(2C) receptor antagonist properties of compounds. Eur. J. Pharmacol. 2000;388:57–67. doi: 10.1016/S0014-2999(99)00859-6. [DOI] [PubMed] [Google Scholar]
- 196.Cunningham K.A., Fox R.G., Anastasio N.C., Bubar M.J., Stutz S.J., Moeller F.G., Gilbertson S.R., Rosenzweig-Lipson S. Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61:513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Higgins G.A., Silenieks L.B., Rossmann A., Rizos Z., Noble K., Soko A.D., Fletcher P.J. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;377:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Blasio A., Narayan A.R., Kaminski B.J., Steardo L., Sabino V., Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT2A/C and 5-HT1A receptor agonists. Psychopharmacology (Berl.) 2012;219:377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.White C.L., Kashima K., Bray G.A., York D.A. Effect of a serotonin 1-A agonist on food intake of Osborne-Mendel and S5B/P1 rats. Physiol. Behav. 2000;68:715–722. doi: 10.1016/S0031-9384(99)00243-7. [DOI] [PubMed] [Google Scholar]
- 200.White C.L., Ishihara Y., York D.A., Bray G.A. Effect of meta-chlorophenylpiperazine and cholecystokinin on food intake of Osborne-Mendel and S5B/P1 rats. Obesity (Silver Spring) 2007;15:624–631. doi: 10.1038/oby.2007.579. [DOI] [PubMed] [Google Scholar]
- 201.Dill M.J., Shaw J., Cramer J., Sindelar D.K. 5-HT1A receptor antagonists reduce food intake and body weight by reducing total meals with no conditioned taste aversion. Pharmacol. Biochem. Behav. 2013;112:1–8. doi: 10.1016/j.pbb.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 202.Sargent B.J. Henderson, A.J. Targeting 5-HT receptors for the treatment of obesity. Curr. Opin. Pharmacol. 2011;11:52–58. doi: 10.1016/j.coph.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 203.Teff K.L., Kim S.F. Atypical antipsychotics and the neural regulation of food intake and peripheral metabolism. Physiol. Behav. 2011;104:590–508. doi: 10.1016/j.physbeh.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Torrealba F., Riveros M.E., Contreras M., Valdes J.L. Histamine and motivation. [Accessed June 22, 2015];Front Syst Neurosci. 2012 6:51. doi: 10.1016/j.bbr.2005.06.004. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3389384/pdf/fnsys-06-00051.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Treesukosol Y., Ishizuka T., Yamamoto C., Senda K., Tsutsumi S., Yamatodani A., Yamamoto T. Hypothalamic histamine release by taste stimuli in freely moving rats: possible implication of palatability. Behav. Brain Res. 2005;164:67–72. doi: 10.1016/j.bbr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 206.Ali A.H., Yanoff L.B., Stern E.A., Akomeah A., Courville A., Kozlosky M., Brady S.M., Calis K.A., Reynolds J.C., Crocker M.K., Barak N., Yanovski J.A. Acute effects of betahistine hydrochloride on food intake and appetite in obese women: a randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2010;92:1290–1297. doi: 10.3945/ajcn.110.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Valdés J.L., Sánchez C., Riveros M.E., Blandina P., Contreras M., Farías P., Torrealba F. The histaminergic tuberomammillary nucleus is critical for motivated arousal. Eur. J. Neurosci. 2010;31:2073–2085. doi: 10.1111/j.1460-9568.2010.07241.x. [DOI] [PubMed] [Google Scholar]
- 208.Hartfield A.W., Moore N.A., Clifton P.G. Serotonergic and histaminergic mechanisms involved in intralipid drinking? Pharmacol. Biochem. Behav. 2003;76:251–258. doi: 10.1016/S0091-3057(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 209.Yamada M., Miyakawa T., Duttaroy A., Yamanaka A., Moriguchi T., Makita R., Ogawa M., Chou C.J., Xia B., Crawley J.N., Felder C.C., Deng C.X., Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 210.Mineur Y.S., Abizaid A., Rao Y., Salas R., DiLeone R.J., Gündisch D., Diano S., De Biasi M., Horvath T.L., Gao X.B., Picciotto M.R. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.van Dijk G., Evers S.S., Guidotti S., Thornton S.N., Scheurink A.J., Nyakas C. The lateral hypothalamus: a site for integration of nutrient and fluid balance. Behav. Brain Res. 2011;221:481–487. doi: 10.1016/j.bbr.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 212.McFadden K.L., Cornier M.A., Tregellas J.R. The role of alpha-7 nicotinic receptors in food intake behaviors. Front. Psychol. 2014;5:553. doi: 10.3389/fpsyg.2014.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zoli M., Picciotto M.R. Nicotinic regulation of energy homeostasis. Nicotine Tob. Res. 2012;14:1270–1290. doi: 10.1093/ntr/nts159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Avena N.M., Rada P.V. Cholinergic modulation of food and drug satiety and withdrawal. Physiol. Behav. 2012;106:332–336. doi: 10.1016/j.physbeh.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pratt W.E., Kelley A.E. Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav. Neurosci. 2004;118:730–739. doi: 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- 216.Sharf R., McKelvey J., Ranaldi R. Blockade of muscarinic acetylcholine receptors in the ventral tegmental area prevents acquisition of food-rewarded operant responding in rats. Psychopharmacology (Berl.) 2006;186:113–121. doi: 10.1007/s00213-006-0352-0. [DOI] [PubMed] [Google Scholar]
- 217.Perry M.L., Andrzejewski M.E., Bushek S.M., Baldo B.A. Intra-accumbens infusion of a muscarinic antagonist reduces food intake without altering the incentive properties of food-associated cues. Behav. Neurosci. 2010;124:44–54. doi: 10.1037/a0018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shabani S., Foster R., Gubner N., Phillips T.J., Mark G.P. Muscarinic type 2 receptors in the lateral dorsal tegmental area modulate cocaine and food seeking behavior in rats. Neuroscience. 2010;170:559–569. doi: 10.1016/j.neuroscience.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dickson S.L., Hrabovszky E., Hansson C., Jerlhag E., Alvarez-Crespo M., Skibicka K.P., Molnar C.S., Liposits Z., Engel J.A., Egecioglu E. Blockade of central nicotine acetylcholine receptor signaling attenuate ghrelin-induced food intake in rodents. Neuroscience. 2010;171:1180–1186. doi: 10.1016/j.neuroscience.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 220.Ostlund S.B., Kosheleff A.R., Maidment N.T. Differential effects of systemic cholinergic receptor blockade on Pavlovian incentive motivation and goal-directed action selection. Neuropsychopharmacology. 2014;39:1490–1497. doi: 10.1038/npp.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Taraschenko O.D., Rubbinaccio H.Y., Maisonneuve I.M., Glick S.D. 18-methoxycoronaridine: a potential new treatment for obesity in rats? Psychopharmacology (Berl.) 2008;201:339–350. doi: 10.1007/s00213-008-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McCallum S.E., Taraschenko O.D., Hathaway E.R., Vincent M.Y., Glick S.D. Effects of 18-methoxycoronaridine on ghrelin-induced increases in sucrose intake and accumbal dopamine overflow in female rats. Psychopharmacology (Berl.) 2011;215:247–256. doi: 10.1007/s00213-010-2132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Taraschenko O.D., Maisonneuve I.M., Glick S.D. Resistance of male Sprague-Dawley rats to sucrose-induced obesity: effects of 18-methoxycoronaridine. Physiol. Behav. 2011;102:126–131. doi: 10.1016/j.physbeh.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Audrain-McGovern J., Benowitz N.L. Cigarette smoking, nicotine, and body weight. Clin. Pharmacol. Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Geha P.Y., Aschenbrenner K., Felsted J., O' Malley S.S., Small D,M. Altered hypothalamic response to food in smokers. Am. J. Clin. Nutr. 2013;97:15–22. doi: 10.3945/ajcn.112.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pepino M.Y., Mennella J.A. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol. Clin. Exp. Res. 2007;31:1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nuttens M.C., Romon M., Ruidavets J.B., Arveiler D., Ducimetiere P., Lecerf J.M., Richard J.L., Cambou J.P., Simon C., Salomez J.L. Relationship between smoking and diet: the MONICA-France project. J. Intern. Med. 1992;231:349–356. doi: 10.1111/j.1365-2796.1992.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 228.Machulska A., Zlomuzica A., Adolph D., Rinck M., Margraf J. “A cigarette a day keeps the goodies away”: smokers show automatic approach tendencies for smoking-but not for food-related stimuli. PLoS One. 2015;10:e0116464. doi: 10.1371/journal.pone.0116464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Suliburska J., Bogdański P. Smoking and dietary intake in the patients with metabolic diseases and nervous system disorders. Przegl. Lek. 2012;69:769–772. [in Polish]. [PubMed] [Google Scholar]
- 230.Lacy R.T., Hord L.L., Morgan A.J., Harrod S.B. Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend. 2012;124:299–306. doi: 10.1016/j.drugalcdep.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen H., Parker S.L., Matta S.G., Sharp B.M. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur. J. Neurosci. 2005;22:380–388. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- 232.Grimm J.W., Ratliff C., North K., Barnes J., Collins S. Nicotine increases sucrose self-administration and seeking in rats. Addict. Biol. 2012;17:623–633. doi: 10.1111/j.1369-1600.2012.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ayres C., Silveira P.P., Barbieri M.A., Portella A.K., Bettiol H., Agranonik M., Silva A.A., Goldani M.Z. Exposure to maternal smoking during fetal life affects food preferences in adulthood independent of the effects of intrauterine growth restriction. J. Dev. Orig. Health Dis. 2011;2:162–167. doi: 10.1017/S204017441100016X. [DOI] [PubMed] [Google Scholar]
- 234.Lee K.W., Abrahamowicz M., Leonard G.T., Richer L., Perron M., Veillette S., Reischl E., Bouchard L., Gaudet D., Paus T., Pausova Z. Prenatal exposure to cigarette smoke interacts with OPRM1 to modulate dietary preference for fat. J. Psychiatry Neurosci. 2015;40:38–45. doi: 10.1503/jpn.130263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chiolero A., Faeh D., Paccaud F., Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am. J. Clin. Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 236.Caan B., Coates A., Schaefer C., Finkler L., Sternfeld B., Corbett K. Women gain weight 1 year after smoking cessation while dietary intake temporarily increases. J. Am. Diet. Assoc. 1996;96:1150–1155. doi: 10.1016/S0002-8223(96)00296-9. [DOI] [PubMed] [Google Scholar]
- 237.Spring B., Pagoto S., McChargue D., Hedeker D., Werth J. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol. Biochem. Behav. 2003;76:351–360. doi: 10.1016/j.pbb.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 238.Stadler M., Tomann L., Storka A., Wolzt M., Peric S., Bieglmayer C., Pacini G., Dickson S.L., Brath H., Bech P., Prager R., Korbonits M. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite. Eur. J. Endocrinol. 2014;170:219–217. doi: 10.1530/EJE-13-0590. [DOI] [PubMed] [Google Scholar]
- 239.Allen S.S., Hatsukami D., Brintnell D.M., Bade T. Effect of nicotine replacement therapy on post-cessation weight gain and nutrient intake: a randomized controlled trial of postmenopausal female smokers. Addict. Behav. 2005;30:1273–1280. doi: 10.1016/j.addbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 240.Young A.B., Chu D. Distribution of GABAA, and GABAB, receptors in mammalian brain: potential targets for drug development. Drug Dev. Res. 1990;21:161–167. doi: 10.1002/ddr.430210303. [DOI] [Google Scholar]
- 241.Jennings J.H., Ung R.L., Resendez S.L., Stamatakis A.M., Taylor J.G., Huang J., Veleta K., Kantak P.A., Aita M., Shilling-Scrivo K., Ramakrishnan C., Deisseroth K., Otte S., Stuber G.D. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cansell C., Denis R.G., Joly-Amado A., Castel J., Luquet S. Arcuate AgRP neurons and the regulation of energy balance. Front. Endocrinol. 2012;3:169. doi: 10.3389/fendo.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kamatchi G.L., Rathanaswami P. Inhibition of deprivation-induced food intake by GABA(A) antagonists: roles of the hypothalamic, endocrine and alimentary mechanisms. J. Clin. Biochem. Nutr. 2012;51:19–26. doi: 10.3164/jcbn.11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Covelo I.R., Patel Z.I., Luviano J.A., Stratford T.R., Wirtshafter D. Manipulation of GABA in the ventral pallidum, but not the nucleus accumbens, induces intense, preferential, fat consumption in rats. Behav. Brain Res. 2014;270:316–325. doi: 10.1016/j.bbr.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kelly J., Alheid G.F., Newberg A., Grossman S.P. GABA stimulation and blockade in the hypothalamus and midbrain: effects on feeding and locomotor activity. Pharmacol. Biochem. Behav. 1977;7:537–541. doi: 10.1016/0091-3057(77)90250-7. [DOI] [PubMed] [Google Scholar]
- 246.Ito Y., Banno R., Shibata M., Adachi K., Hagimoto S., Hagiwara D., Ozawa Y., Goto M., Suga H., Sugimura Y., Bettler B., Oiso Y., Arima H. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J. Neurosci. 2013;33:17166–17173. doi: 10.1523/JNEUROSCI.0897-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sato I1., Arima H., Ozaki N., Ozaki N., Watanabe M., Goto M., Shimizu H., Hayashi M., Banno R., Nagasaki H., Oiso Y. Peripherally administered baclofen reduced food intake and body weight in db/db as well as diet-induced obese mice. FEBS Lett. 2007;581:4857–4864. doi: 10.1016/j.febslet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 248.Arima H., Oiso Y. Positive effect of baclofen on body weight reduction in obese subjects: a pilot study. Intern. Med. 2010;49:2043–2047. doi: 10.2169/internalmedicine.49.3918. [DOI] [PubMed] [Google Scholar]
- 249.Foltin R.W. Baclofen decreases feeding in non-human primates. Pharmacol. Biochem. Behav. 2005;82:608–614. doi: 10.1016/j.pbb.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 250.Ebenezer I.S., Prabhaker M. The effects of intraperitoneal administration of the GABA(B) receptor agonist baclofen on food intake in CFLP and C57BL/6 mice. Eur. J. Pharmacol. 2007;569:90–93. doi: 10.1016/j.ejphar.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 251.Higgs S., Barber D.J. Effects of baclofen on feeding behaviour examined in the runway. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:405–408. doi: 10.1016/j.pnpbp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 252.Patel S.M., Ebenezer I.S. The effects of chronic intraperitoneal administration of the GABA B receptor agonist baclofen on food intake in rats. Eur. J. Pharmacol. 2008;593:68–72. doi: 10.1016/j.ejphar.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 253.Buda-Levin A., Wojnicki F.H., Corwin R.L. Baclofen reduces fat intake under binge-type conditions. Physiol. Behav. 2005;86:176–184. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wojnicki F.H., Roberts D.C., Corwin R.L. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol. Biochem. Behav. 2006;84:197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rao R.E., Wojnicki F.H., Coupland J., Ghosh S., Corwin R.L. Baclofen, raclopride, and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacol. Biochem. Behav. 2008;89:581–590. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Berner L.A., Bocarsly M.E., Hoebel B.G., Avena N.M. Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behav. Pharmacol. 2009;20:631–634. doi: 10.1097/FBP.0b013e328331ba47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang Y. 1; Wilt, D.C.; Wojnicki, F.H.; Babbs, R.K.; Coupland, J.N.; Corwin, R.L. Fat emulsion composition alters intake and the effects of baclofen. Appetite. 2011;57:628–634. doi: 10.1016/j.appet.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wojnicki F.H., Brown S.D., Corwin R.L. Factors affecting the ability of baclofen to reduce fat intake in rats. Behav. Pharmacol. 2014;25:166–172. doi: 10.1097/FBP.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 259.Wojnicki F.H., Charny G., Corwin R.L. Baclofen-induced reductions in optional food intake depend upon food composition. Appetite. 2013;64:62–70. doi: 10.1016/j.appet.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Avena N.M., Bocarsly M.E., Murray S., Gold M.S. Effects of baclofen and naltrexone, alone and in combination, on the consumption of palatable food in male rats. Exp. Clin. Psychopharmacol. 2014;22:460–467. doi: 10.1037/a0037223. [DOI] [PubMed] [Google Scholar]
- 261.Bradbury M., Campbell U., Giracello D., Chapman D., King C., Tehrani L., Cosford N.D., Anderson J., Varney M.A. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J. Pharmacol. Exp. Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- 262.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., Croteau-Chonka D.C., Esko T., Fall T., Ferreira T., Gustafsson S., Kutalik Z., Luan J., Mägi R., Randall J.C., Winkler T.W., Wood A.R., Workalemahu T., Faul J.D., Smith J.A., Hua Zhao J., Zhao W., Chen J., Fehrmann R., Hedman Å.K., Karjalainen J., Schmidt E.M., Absher D., Amin N., Anderson D., Beekman M., Bolton J.L., Bragg-Gresham J.L., Buyske S., Demirkan A., Deng G., Ehret G.B., Feenstra B., Feitosa M.F., Fischer K., Goel A., Gong J., Jackson A.U., Kanoni S., Kleber M.E., Kristiansson K., Lim U., Lotay V., Mangino M., Mateo Leach I., Medina-Gomez C., Medland S.E., Nalls M.A., Palmer C.D., Pasko D., Pechlivanis S., Peters M.J., Prokopenko I., Shungin D., Stančáková A., Strawbridge R.J., Ju Sung Y., Tanaka T., Teumer A., Trompet S., van der Laan S.W., van Setten J., Van Vliet-Ostaptchouk J.V., Wang Z., Yengo L., Zhang W., Isaacs A. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Brownley K.A., Peat C.M., La Via M., Bulik C.M. Pharmacological approaches to the management of binge eating disorder. Drugs. 2015;75:9–32. doi: 10.1007/s40265-014-0327-0. [DOI] [PubMed] [Google Scholar]
- 264.Stratford T.R., Swanson C.J., Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav. Brain Res. 1998;93:43–50. doi: 10.1016/S0166-4328(97)00140-X. [DOI] [PubMed] [Google Scholar]
- 265.Guyenet S.J., Matsen M.E., Morton G.J., Kaiyala K.J., Schwartz M.W. Rapid glutamate release in the mediobasal hypothalamus accompanies feeding and is exaggerated by an obesogenic food. Mol. Metab. 2013;2:116–122. doi: 10.1016/j.molmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rosa C.B., Goularte J.F., Trindade N.A., De Oliveira A.P., Rasia-Filho A.A. Glutamate microinjected in the posterodorsal medial amygdala induces subtle increase in the consumption of a three-choice macronutrient self-selection diet in male rats. Anat. Rec. 2011;294:1226–1232. doi: 10.1002/ar.21419. [DOI] [PubMed] [Google Scholar]
- 267.Bisaga A.1., Danysz W., Foltin R.W. Antagonism of glutamatergic NMDA and mGluR5 receptors decreases consumption of food in baboon model of binge-eating disorder. Eur. Neuropsychopharmacol. 2008;18:794–802. doi: 10.1016/j.euroneuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bossert J.M., Poles G.C., Sheffler-Collins S.I., Ghitza U.E. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav. Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 269.Tapiero H., Mathé G., Couvreur P., Tew K.D., II Glutamine and glutamate. Biomed. Pharmacother. 2002;56:446–457. doi: 10.1016/S0753-3322(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 270.Tsai P., Huang P.C. Circadian variations in plasma and erythrocyte glutamate concentrations in adult men consuming a diet with and without added monosodium glutamate. J. Nutr. 2000;130(4S Suppl):1002S–1004S. doi: 10.1093/jn/130.4.1002S. [DOI] [PubMed] [Google Scholar]
- 271.Hawkins R.A. The blood-brain barrier and glutamate. Am. J. Clin. Nutr. 2009;90:867S–874S. doi: 10.3945/ajcn.2009.27462BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hermanussen M., Tresguerres J.E.F. Overweight, appetite control, and the role of glutamate and excess nutritional protein during child development. [Accessed May 30, 2015];Hum. Ontogent. 2007 1:23–35. http://onlinelibrary.wiley.com/doi/10.1002/huon.200700004/abstract . [Google Scholar]
- 273.Torii K., Uneyama H., Nakamura E. Physiological roles of dietary glutamate signaling via gut-brain axis due to efficient digestion and absorption. J. Gastroenterol. 2013;48:442–451. doi: 10.1007/s00535-013-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Masic U., Yeomans M.R. Umami flavor enhances appetite but also increases satiety. Am. J. Clin. Nutr. 2014;100:532–538. doi: 10.3945/ajcn.113.080929. [DOI] [PubMed] [Google Scholar]
- 275.Dermiki M., Prescott J., Sargent L.J., Willway J., Gosney M.A., Methven L. Novel flavours paired with glutamate condition increased intake in older adults in the absence of changes in liking. Appetite. 2015;90:108–113. doi: 10.1016/j.appet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 276.Essed N.H., van Staveren W.A., Kok F.J., de Graaf C. No effect of 16 weeks flavor enhancement on dietary intake and nutritional status of nursing home elderly. Appetite. 2007;48:29–36. doi: 10.1016/j.appet.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 277.Ren X., Ferreira J.G., Yeckel C.W., Kondoh T., de Araujo I.E. Effects of ad libitum ingestion of monosodium glutamate on weight gain in C57BL6/J mice. Digestion. 2011;83(Suppl. 1):32–36. doi: 10.1159/000323405. [DOI] [PubMed] [Google Scholar]
- 278.Tordoff M.G., Aleman T.R., Murphy M.C. No effects of monosodium glutamate consumption on the body weight or composition of adult rats and mice. Physiol. Behav. 2012;107:338–345. doi: 10.1016/j.physbeh.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 279.Boutry C., Bos C., Matsumoto H., Even P., Azzout-Marniche D., Tome D., Blachier F. Effects of monosodium glutamate supplementation on glutamine metabolism in adult rats. Front. Biosci. 2011;3:279–290. doi: 10.2741/e243. [DOI] [PubMed] [Google Scholar]
- 280.Bellisle F. Experimental studies of food choices and palatability responses in European subjects exposed to the Umami taste. Asia Pac. J. Clin. Nutr. 2008;17(Suppl. 1):376–379. [PubMed] [Google Scholar]
- 281.Imada T., Hao S.S., Torii K., Kimura E. Supplementing chicken broth with monosodium glutamate reduces energy intake from high fat and sweet snacks in middle-aged healthy women. Appetite. 2014;79:158–165. doi: 10.1016/j.appet.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 282.Carter B.E., Monsivais P., Perrigue M.M., Drewnowski A. Supplementing chicken broth with monosodium glutamate reduces hunger and desire to snack but does not affect energy intake in women. Br. J. Nutr. 2011;106:1441–1448. doi: 10.1017/S0007114511001759. [DOI] [PubMed] [Google Scholar]
- 283.Kondoh T., Torii K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague-Dawley rats. Physiol. Behav. 2008;95:135–144. doi: 10.1016/j.physbeh.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 284.Luscombe-Marsh N.D., Smeets A.J., Westerterp-Plantenga M.S. The addition of monosodium glutamate and inosine monophosphate-5 to high-protein meals: effects on satiety, and energy and macronutrient intakes. Br. J. Nutr. 2009;102:929–937. doi: 10.1017/S0007114509297212. [DOI] [PubMed] [Google Scholar]
- 285.Brosnan J.T., Drewnowski A., Friedman M.I. Is there a relationship between dietary MSG and [corrected] obesity in animals or humans? Amino Acids. 2014;46:2075–2087. doi: 10.1007/s00726-014-1771-6. [DOI] [PubMed] [Google Scholar]
- 286.Punjabi M., Arnold M., Geary N., Langhans W., Pacheco-López G. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol. Behav. 2011;105:71–76. doi: 10.1016/j.physbeh.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 287.De Silva A., Bloom S.R. Gut hormones and appetite control: A Focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6:10–20. doi: 10.5009/gnl.2012.6.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Trapp S., Richards J.E. The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr. Opin. Pharmacol. 2013;13:964–969. doi: 10.1016/j.coph.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.van Bloemendaal L., IJzerman R.G., Ten Kulve J.S., Barkhof F., Konrad R.J., Drent M.L., Veltman D.J., Diamant M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 290.Iepsen E.W., Torekov S.S., Holst J.J. Therapies for inter-relating diabetes and obesity - GLP-1 and obesity. Expert Opin. Pharmacother. 2014;15:2487–2500. doi: 10.1517/14656566.2014.965678. [DOI] [PubMed] [Google Scholar]
- 291.Ladenheim E.E. Liraglutide and obesity: a review of the data so far. Drug Des. Devel. Ther. 2015;9:1867–1875. doi: 10.2147/DDDT.S58459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Martin K.A., Mani M.V., Mani A. New targets to treat obesity and the metabolic syndrome. Eur. J. Pharmacol. 2015. [Accessed June 22, 2015)]. http://www.sciencedirect.com/science/article/pii/S0014299915004574# . [DOI] [PMC free article] [PubMed]
- 293.Dickson S.L., Shirazi R.H., Hansson C., Bergquist F., Nissbrandt H., Skibicka K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dossat A.M., Diaz R., Gallo L., Panagos A., Kay K., Williams D.L. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am. J. Physiol. 2013;304:E1314–E1320. doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Alhadeff A.L., Grill H.J. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. 2014;307:R465–R470. doi: 10.1152/ajpregu.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Alhadeff A.L., Baird J.P., Swick J.C., Hayes M.R., Grill H.J. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014:2233–2243. doi: 10.1038/npp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.van Bloemendaal L., Veltman D.J., Ten Kulve J.S., Groot P.F., Ruhé H., Barkhof F., Sloan J.H., Diamant M., IJzerman R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by GLP-1 receptor activation in humans. Diabetes Obes. Metab. 2015. [Accessed June 24, 2015]. http://onlinelibrary.wiley.com/doi/10.1111/dom.12506/pdf . [DOI] [PubMed]
- 298.Mack C.M., Moore C.X., Jodka C.M., Bhavsar S., Wilson J.K., Hoyt J.A., Roan J.L., Vu C., Laugero K.D., Parkes D.G., Young A.A. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int. J. Obes. 2006;30:1332–1340. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- 299.Lamont B.J., Drucker D.J. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes. 2008;57:190–198. doi: 10.2337/db07-1202. [DOI] [PubMed] [Google Scholar]
- 300.Washington M.C., Raboin S.J., Thompson W., Larsen C.J., Sayegh A.I. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Res. 2010;1344:124–133. doi: 10.1016/j.brainres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 301.Hayes M.R., Kanoski S.E., Alhadeff A.L., Grill H.J. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 2011;19:1342–1349. doi: 10.1038/oby.2011.50. [DOI] [PubMed] [Google Scholar]
- 302.Yang Y., Moghadam A.A., Cordner Z.A., Liang N.C., Moran T.H. Long term exendin-4 treatment reduces food intake and body weight and alters expression of brain homeostatic and reward markers. Endocrinology. 2014;155:3473–3483. doi: 10.1210/en.2014-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Swick J.C., Alhadeff A.L., Grill H.J., Angarita P., Lee S.M., Roh H., Baird J.P. Parabrachial nucleus contributions to glucagon-like-peptide-1 agonist-induced hypophagia. Neuropsychopharmacology. 2015;40:2001–2014. doi: 10.1038/npp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Peters C.T., Choi Y.H., Brubaker P.L., Anderson G.H. A glucagon-like peptide-1 receptor agonist and an antagonist modify macronutrient selection by rats. J. Nutr. 2001;131:2164–2170. doi: 10.1093/jn/131.8.2164. [DOI] [PubMed] [Google Scholar]
- 305.Flint A., Kapitza C., Zdravkovic M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes Obes. Metab. 2013;15:958–962. doi: 10.1111/dom.12108. [DOI] [PubMed] [Google Scholar]
- 306.Zhang X.J., Wang Y.Q., Long Y., Wang L., Li Y., Gao F.B., Tian H.M. Alteration of sweet taste in high-fat diet induced obese rats after 4 weeks treatment with exenatide. Peptides. 2013;47:115–123. doi: 10.1016/j.peptides.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 307.Inoue K., Maeda N., Kashine S., Fujishima Y., Kozawa J., Hiuge-Shimizu A., Okita K., Imagawa A., Funahashi T., Shimomura I. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc. Diabetol. 2011;10:109. doi: 10.1186/1475-2840-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fujishima Y., Maeda N., Inoue K., Kashine S., Nishizawa H., Hirata A., Kozawa J., Yasuda T., Okita K., Imagawa A., Funahashi T., Shimomura I. Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight, eating behavior, and glycemic control, in Japanese obese type 2 diabetes. Cardiovasc. Diabetol. 2012;11:107. doi: 10.1186/1475-2840-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hansen H.H., Hansen G., Paulsen S., Vrang N., Mark M., Jelsing J., Klein T. The DPP-IV inhibitor linagliptin and GLP-1 induce synergistic effects on body weight loss and appetite suppression in the diet-induced obese rat. Eur. J. Pharmacol. 2014;741:254–263. doi: 10.1016/j.ejphar.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 310.Lean M.E., Carraro R., Finer N., Hartvig H., Lindegaard M.L., Rössner S., Van Gaal L., Astrup A. NN8022-1807 Investigators. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int. J. Obes. 2014;38:689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Pénicaud L., Meillon S., Brondel L. Leptin and the central control of feeding behavior. Biochimie. 2012;94:2069–2074. doi: 10.1016/j.biochi.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 312.Dalamaga M., Chou S.H., Shields K., Papageorgiou P., Polyzos S.A., Mantzoros C.S. Leptin at the intersection of neuro- endocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18:29–42. doi: 10.1016/j.cmet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 313.Murray S., Tulloch A., Gold M.S., Avena N.M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 2014;10:540–552. doi: 10.1038/nrendo.2014.91. [DOI] [PubMed] [Google Scholar]
- 314.Park H.K., Ahima R.S. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sáinz N., González-Navarro C.J., Martínez J.A., Moreno-Aliaga M.J. Leptin signaling as a therapeutic target of obesity. Expert Opin. Ther. Targets. 2015;19:893–909. doi: 10.1517/14728222.2015.1018824. [DOI] [PubMed] [Google Scholar]
- 316.Kanoski S.E., Alhadeff A.L., Fortin S.M., Gilbert J.R., Grill H.J. Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology. 2014;39:605–613. doi: 10.1038/npp.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sánchez J., Priego T., Palou M., Tobaruela A., Palou A., Picó C. Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology. 2008;149:733–740. doi: 10.1210/en.2007-0630. [DOI] [PubMed] [Google Scholar]
- 318.Farooqi I.S., O'Rahilly S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 2014;223:T63–T70. doi: 10.1530/JOE-14-0480. [DOI] [PubMed] [Google Scholar]
- 319.Farr O.M., Fiorenza C., Papageorgiou P., Brinkoetter M., Ziemke F., Koo B.B., Rojas R., Mantzoros C.S. Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. J. Clin. Endocrinol. Metab. 2014;99:E2529–E2238. doi: 10.1210/jc.2014-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Conroy R., Febres G., McMahon D.J., Thorner M.O., Gaylinn B.D., Conwell I., Aronne L., Korner J. Recombinant human leptin does not alter gut hormone levels after gastric bypass but may attenuate sweet cravings. Int. J. Endocrinol. 2014;2014:120286. doi: 10.1155/2014/120286. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3980779/pdf/IJE2014-120286.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Domingos A.I., Vaynshteyn J., Voss H.U., Ren X., Gradinaru V., Zang F., Deisseroth K., de Araujo I.E., Friedman J. Leptin regulates the reward value of nutrient. Nat. Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wetzler S., Dumaz V., Goubern M., Tomé D., Larue-Achagiotis C. Intraperitoneal leptin modifies macronutrient choice in self-selecting rats. Physiol. Behav. 2004;83:65–72. doi: 10.1016/S0031-9384(04)00311-7. [DOI] [PubMed] [Google Scholar]
- 323.Licinio J., Milane M., Thakur S., Whelan F., Yildiz B.O., Delibasi T., de Miranda P.B., Ozata M., Bolu E., Depaoli A., Wong M.L. Effects of leptin on intake of specific micro- and macronutrients in a woman with leptin gene deficiency studied off and on leptin at stable body weight. Appetite. 2007;49:594–599. doi: 10.1016/j.appet.2007.03.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.van den Heuvel J.K., Eggels L., van Rozen A.J., Luijendijk M.C., Fliers E., Kalsbeek A., Adan R.A., la Fleur S.E. Neuropeptide Y and leptin sensitivity is dependent on diet composition. J. Neuroendocrinol. 2014;26:377–385. doi: 10.1111/jne.12155. [DOI] [PubMed] [Google Scholar]
- 325.Crujeiras A.B., Carreira M.C., Cabia B., Andrade S., Amil M., Casanueva F.F. Leptin resistance in obesity: An epigenetic landscape. [Accessed October 12, 2015];Life Sci. 2015 pii:S0024–3205(15)00259-3. doi: 10.1016/j.lfs.2015.05.003. http://www.sciencedirect.com/science/article/pii/S0024320515002593 . [DOI] [PubMed] [Google Scholar]
- 326.Delporte C. Structure and physiological actions of ghrelin. [Accessed June 22, 2015];Scientifica. 2013 2013:518909. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Menzies J.R., Skibicka K.P., Leng G., Dickson S.L. Ghrelin, reward and motivation. Endocr. Dev. 2013;25:101–111. doi: 10.1159/000346058. [DOI] [PubMed] [Google Scholar]
- 328.Pradhan G., Samson S.L., Sun Y. Ghrelin: much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:619–624. doi: 10.1097/MCO.0b013e328365b9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Heppner K.M., Tong J. Mechanisms in endocrinology: regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. Eur. J. Endocrinol. 2014;171:R21–R32. doi: 10.1530/EJE-14-0183. [DOI] [PubMed] [Google Scholar]
- 330.Perello M., Dickson S.L. Ghrelin signaling on food reward: a salient link between the gut and the mesolimbic system. J. Neuroendocrinol. 2015;27:424–434. doi: 10.1111/jne.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Guan X.M., Yu H., Palyha O.C., McKee K.K., Feighner S.D., Sirinathsinghji D.J., Smith R.G., Van der Ploeg L.H., Howard A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997;48:23–29. doi: 10.1016/S0169-328X(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 332.Menyhért J., Wittmann G., Hrabovszky E., Szlávik N., Keller E., Tschöp M., Liposits Z., Fekete C. Distribution of ghrelin-immunoreactive neuronal networks in the human hypothalamus. Brain Res. 2006;1125:31–36. doi: 10.1016/j.brainres.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 333.Harrold J.A., Dovey T., Cai X.J., Halford J.C., Pinkney J. Autoradiographic analysis of ghrelin receptors in the rat hypothalamus. Brain Res. 2008;1196:59–64. doi: 10.1016/j.brainres.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 334.Zigman J.M., Nakano Y., Coppari R., Balthasar N., Marcus J.N., Lee C.E., Jones J.E., Deysher A.E., Waxman A.R., White R.D., Williams T.D., Lachey J.L., Seeley R.J., Lowell B.B., Elmquist J.K. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Labarthe A., Fiquet O., Hassouna R., Zizzari P., Lanfumey L., Ramoz N., Grouselle D., Epelbaum J., Tolle V. Ghrelin-derived peptides: a link between appetite/reward, GH axis, and psychiatric disorders? [Accessed June 22, 2015];Front. Endocrinol. 2014 5:163. doi: 10.3389/fendo.2014.00163. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4209873/pdf/fendo-05-00163.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lockie S.H., Andrews Z.B. The hormonal signature of energy deficit: Increasing the value of food reward. Mol. Metab. 2013;2:329–336. doi: 10.1016/j.molmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Egecioglu E., Jerlhag E., Salomé N., Skibicka K.P., Haage D., Bohlooly-Y M., Andersson D., Bjursell M., Perrissoud D., Engel J.A., Dickson S.L. Ghrelin increases intake of rewarding food in rodents. Addict. Biol. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Skibicka K.P., Hansson C., Alvarez-Crespo M., Friberg P.A., Dickson S.L. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 339.Davis J.F., Perello M., Choi D.L., Magrisso I.J., Kirchner H., Pfluger P.T., Tschoep M., Zigman J.M., Benoit S.C. GOAT induced ghrelin acylation regulates hedonic feeding. Horm. Behav. 2012;62:598–604. doi: 10.1016/j.yhbeh.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sun Y., Ahmed S., Smith R.G. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell. Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.McCoull W., Barton P., Brown A.J., Bowker S.S., Cameron J., Clarke D.S., Davies R.D., Dossetter A.G., Ertan A., Fenwick M., Green C., Holmes J.L., Martin N., Masters D., Moore J.E., Newcombe N.J., Newton C., Pointon H., Robb G.R., Sheldon C., Stokes S., Morgan D. Identification, optimization, and pharmacology of acylurea GHS-R1a inverse agonists. J. Med. Chem. 2014;57:6128–6140. doi: 10.1021/jm500610n. [DOI] [PubMed] [Google Scholar]
- 342.Sárvári M., Kocsis P., Deli L., Gajári D., Dávid S., Pozsgay Z., Hegedűs N., Tihanyi K., Liposits Z. Ghrelin modulates the fMRI BOLD response of homeostatic and hedonic brain centers regulating energy balance in the rat. PLoS One. 2014;9:e97651. doi: 10.1371/journal.pone.0097651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Landgren S., Simms J.A., Thelle D.S., Strandhagen E., Bartlett S.E., Engel J.A., Jerlhag E. The ghrelin signalling system is involved in the consumption of sweets. PLoS One. 2011;6:e18170. doi: 10.1371/journal.pone.0018170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Disse E., Bussier A.L., Veyrat-Durebex C., Deblon N., Pfluger P.T., Tschöp M.H., Laville M., Rohner-Jeanrenaud F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol. Behav. 2010;101:277–281. doi: 10.1016/j.physbeh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 345.Stevenson J., Buirkle J.M., Buckley L.E., Young K.A., Albertini K.M., Bohidar A.E. GHS-R1A antagonism reduces alcohol but not sucrose preference in prairie voles. Physiol. Behav. 2015;147:23–29. doi: 10.1016/j.physbeh.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 346.Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A.B., Fekete C., Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. [Accessed June 22, 2015];PLoS One. 2008 3:e1797. doi: 10.1371/journal.pone.0001797. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258435/pdf/pone.0001797.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Perello M., Sakata I., Birnbaum S., Chuang J.C., Osborne-Lawrence S., Rovinsky S.A., Woloszyn J., Yanagisawa M., Lutter M., Zigman J.M. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kanoski S.E., Fortin S.M., Ricks K.M., Grill H.J. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hsu T.M., Hahn J.D., Konanur V.R., Lam A., Kanoski S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40:327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kanoski S.E., Hayes M.R., Greenwald H.S., Fortin S.M., Gianessi C.A., Gilbert J.R., Grill H.J. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Xu T.R., Yang Y., Ward R., Gao L., Liu Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 2013;25:2413–2423. doi: 10.1016/j.cellsig.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 352.Matsuo E., Mochizuki A., Nakayama K., Nakamura S., Yamamoto T., Shioda S., Sakurai T., Yanagisawa M., Shiuchi T., Minokoshi Y., Inoue T. Decreased intake of sucrose solutions in orexin knockout mice. J. Mol. Neurosci. 2011;43:217–224. doi: 10.1007/s12031-010-9475-1. [DOI] [PubMed] [Google Scholar]
- 353.Cason A.M., Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl.) 2013;228:499–507. doi: 10.1007/s00213-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Cason A.M., Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in female rats. Neuropharmacology. 2014;86:97–102. doi: 10.1016/j.neuropharm.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Steiner M.A., Sciarretta C., Pasquali A., Jenck F. The selective orexin receptor 1 antagonist ACT-335827 in a rat model of diet-induced obesity associated with metabolic syndrome. Front. Pharmacol. 2013;4:165. doi: 10.3389/fphar.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sabatier N., Leng G., Menzies J. Oxytocin, feeding, and satiety. Front. Endocrinol. 2013;4:35. doi: 10.3389/fendo.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Lawson E.A., Marengi D.A., DeSanti R.L., Holmes T.M., Schoenfeld D.A., Tolley C.J. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015;23:950–956. doi: 10.1002/oby.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Morton G.J., Thatcher B.S., Reidelberger R.D., Ogimoto K., Wolden-Hanson T., Baskin D.G., Schwartz M.W., Blevins J.E. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. 2012;302:E134–E144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Blevins J.E., Graham J.L., Morton G.J., Bales K.L., Schwartz M.W., Baskin D.G., Havel P.J. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am. J. Physiol. 2014;2015(308):R431–R438. doi: 10.1152/ajpregu.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Iwasaki Y., Maejima Y., Suyama S., Yoshida M., Arai T., Katsurada K., Kumari P., Nakabayashi H., Kakei M., Yada T. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am. J. Physiol. 2015;308:R360–R369. doi: 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- 361.Maejima Y., Rita R.S., Santoso P., Aoyama M., Hiraoka Y., Nishimori K., Gantulga D., Shimomura K., Yada T. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-fos induction in limited brain areas. Neuroendocrinology. 2015;101:35–44. doi: 10.1159/000371636. [DOI] [PubMed] [Google Scholar]
- 362.Miedlar J.A., Rinaman L., Vollmer R.R., Amico J.A. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am. J. Physiol. 2007;293:R1063–R1068. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- 363.Olszewski P.K., Klockars A., Olszewska A.M., Fredriksson R., Schiöth H.B., Levine A.S. Molecular, immunohistochemical, and pharmacological evidence of oxytocin's role as inhibitor of carbohydrate but not fat intake. Endocrinology. 2010;151:4736–4744. doi: 10.1210/en.2010-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Herisson F.M., Brooks L.L., Waas J.R., Levine A.S., Olszewski P.K. Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. Neuroreport. 2014;25:909–914. doi: 10.1097/WNR.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 365.Sinclair M.S., Perea-Martinez I., Abouyared M., St John S.J., Chaudhari N. Oxytocin decreases sweet taste sensitivity in mice. Physiol. Behav. 2015;141:103–110. doi: 10.1016/j.physbeh.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ott V., Finlayson G., Lehnert H., Heitmann B., Heinrichs M., Born J., Hallschmid M. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. doi: 10.2337/db13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kozuka C., Yabiku K., Takayama C., Matsushita M., Shimabukuro M. Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: recent studies on brown rice and γ-oryzanol. Obes. Res. Clin. Pract. 2013;7:e165–e172. doi: 10.1016/j.orcp.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 368.Shimabukuro M., Higa M., Kinjo R., Yamakawa K., Tanaka H., Kozuka C., Yabiku K., Taira S., Sata M., Masuzaki H. Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br. J. Nutr. 2014;111:310–320. doi: 10.1017/S0007114513002432. [DOI] [PubMed] [Google Scholar]
- 369.Kozuka C., Yabiku K., Sunagawa S., Ueda R., Taira S., Ohshiro H., Ikema T., Yamakawa K., Higa M., Tanaka H., Takayama C., Matsushita M., Oyadomari S., Shimabukuro M., Masuzaki H. Brown rice and its component, γ-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes. 2012;61:3084–3093. doi: 10.2337/db11-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zaru A., Maccioni P., Riva A., Morazzoni P., Bombardelli E., Gessa G.L., Carai M.A., Colombo G. Reducing effect of a combination of Phaseolus vulgaris and Cynara scolymus extracts on operant self-administration of a chocolate-flavoured beverage in rats. Phytother. Res. 2013;27:944–947. doi: 10.1002/ptr.4814. [DOI] [PubMed] [Google Scholar]
- 371.Loi B., Fantini N., Colombo G., Gessa G.L., Riva A., Bombardelli E., Morazzoni P., Carai M.A. Reducing effect of an extract of Phaseolus vulgaris on food intake in mice - focus on highly palatable foods. Fitoterapia. 2013;85:14–19. doi: 10.1016/j.fitote.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 372.Montelius C., Erlandsson D., Vitija E., Stenblom E.L., Egecioglu E., Erlanson-Albertsson C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green- plant membranes for three months in overweight women. Appetite. 2014;81:295–304. doi: 10.1016/j.appet.2014.06.101. [DOI] [PubMed] [Google Scholar]
- 373.Stenblom E.L., Egecioglu E., Landin-Olsson M., Erlanson-Albertsson C. Consumption of thylakoid-rich spinach extract reduces hunger, increases satiety and reduces cravings for palatable food in overweight women. Appetite. 2015;91:209–219. doi: 10.1016/j.appet.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 374.Ogawa N., Ito M., Yamaguchi H., Shiuchi T., Okamoto S., Wakitani K., Minokoshi Y., Nakazato M. Intestinal fatty acid infusion modulates food preference as well as calorie intake via the vagal nerve and midbrain-hypothalamic neural pathways in rats. Metabolism. 2012;61:1312–1320. doi: 10.1016/j.metabol.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 375.Harden C.J., Dible V.A., Russell J.M., Garaiova I., Plummer S.F., Barker M.E., Corfe B.M. Long-chain polyunsaturated fatty acid supplementation had no effect on body weight but reduced energy intake in overweight and obese women. Nutr. Res. 2014;34:17–24. doi: 10.1016/j.nutres.2013.10.004. [DOI] [PubMed] [Google Scholar]


