Cdc42 is activated in a unique spatiotemporal manner during cytokinesis due to the localization of its GEFs, Gef1 and Scd1. The fission yeast Gef1 localizes to the actomyosin ring and promotes timely onset of ring constriction. Scd1 localizes to the ingressing membrane to promote septum formation.
Abstract
The Rho-family GTPase Cdc42 regulates cell polarity and localizes to the cell division site. Cdc42 is activated by guanine nucleotide exchange factors (GEFs). We report that Cdc42 promotes cytokinesis via a unique spatiotemporal activation pattern due to the distinct action of its GEFs, Gef1 and Scd1, in fission yeast. Before cytokinetic ring constriction, Cdc42 activation, is Gef1 dependent, and after ring constriction, it is Scd1 dependent. Gef1 localizes to the actomyosin ring immediately after ring assembly and promotes timely onset of ring constriction. Gef1 is required for proper actin organization during cytokinesis, distribution of type V myosin Myo52 to the division site, and timely recruitment of septum protein Bgs1. In contrast, Scd1 localizes to the broader region of ingressing membrane during cytokinetic furrowing. Scd1 promotes normal septum formation, and scd1Δ cells display aberrant septa with reduced Bgs1 localization. Thus we define unique roles of the GEFs Gef1 and Scd1 in the regulation of distinct events during cytokinesis. Gef1 localizes first to the cytokinetic ring and promotes timely constriction, whereas Scd1 localizes later to the ingressing membrane and promotes septum formation. Our findings are consistent with reports that complexity in GTPase signaling patterns enables exquisite precision over the control of cellular processes.
INTRODUCTION
Cytokinesis is the final step in cell division in which the cell separates into two through the formation of an actomyosin–based cytokinetic ring that subsequently constricts, concurrent with membrane ingression (Goyal et al., 2011). Seminal work over the years has given us a good understanding of actomyosin ring assembly (Pollard, 2010; Lee et al., 2012). However, we do not understand what enables concurrent ring constriction and septum formation or how the multiple steps in cytokinesis are precisely coordinated. A recent model indicates that septum ingression, rather than actomyosin ring constriction, provides the force required to overcome internal turgor pressure for membrane furrowing (Proctor et al., 2012). Septum ingression requires the polarized delivery of septum-synthesizing enzymes, the β-glucan synthases, at the division site. Thus polarized delivery of proteins and membrane for proper partitioning of two daughter cells is a critical step in cytokinesis (Wang et al., 2002; Albertson et al., 2005; Boucrot and Kirchhausen, 2007; McCusker and Kellogg, 2012). It is not clear how polarized delivery is regulated at the cell division site and coordinated with actomyosin contractility.
In the fission yeast Schizosaccharomyces pombe, a major regulator of polarized delivery and actin organization in cell growth is the conserved GTPase Cdc42 (Martin et al., 2007; Bendezu et al., 2012). Cdc42 displays remarkable oscillatory behavior that promotes cell morphology and polarity (Das et al., 2012). However, it is not clear whether Cdc42 promotes cytokinesis or what its role may be next to Rho and other well-studied GTPases in this process (Jordan and Canman, 2012; Chircop, 2014). In budding yeast, Cdc42 activity is down-regulated during septum formation and abscission (Atkins et al., 2013; Onishi et al., 2013), which suggests that Cdc42 has a negative role in cytokinesis. Constitutively activated Cdc42 leads to cytokinesis failure in HeLa cells and Drosophila embryos (Dutartre et al., 1996; Crawford et al., 1998). In mouse oocytes, Cdc42 is required for polar body protrusion and asymmetric cytokinesis (Ma et al., 2006; Bielak-Zmijewska et al., 2008; Zhang et al., 2008a; Leblanc et al., 2011; Liu, 2012; Maddox et al., 2012; Dehapiot et al., 2013). In Xenopus embryos, both constitutively active and dominant-negative forms of Cdc42 lead to cytokinetic failure (Drechsel et al., 1997). These reports suggest that Cdc42 needs to be tightly regulated during cytokinesis, similar to what is observed of Cdc42 in polarity establishment.
Cdc42 is active when it is GTP bound and inactive when it is GDP bound (Bos et al., 2007). Cdc42 is activated by guanidine nucleotide exchange factors (GEFs) and inactivated by GTPase-activating proteins (Bos et al., 2007). Fission yeast has only two Cdc42 GEFs, Scd1 and Gef1 (Chang et al., 1994; Coll et al., 2003), making it a simple system with which to understand Cdc42 activation. During polarization, Gef1 promotes growth at the second (new) end in fission yeast (Coll et al., 2003; Das et al., 2012), and Scd1 is required for establishing polarity (Kelly and Nurse, 2011). Although gef1 and scd1 mutants show distinct cellular phenotypes, suggesting distinct roles for each in polarity, a gef1Δscd1Δ double mutant is not viable, suggesting that they are also partially redundant (Coll et al., 2003). During polarization, Scd1 and Gef1 undergo unique regulatory pathways to maintain cell shape and form (Coll et al., 2003; Das et al., 2009, 2015). Scd1 and Gef1 localize to the cell division site, suggesting a role for these GEFs in cytokinesis (Hirota et al., 2003). Gef1 interacts with the Bin/Amphiphysin/Rvs167 (N-BAR) domain–containing protein Hob3, which promotes cytokinesis (Rincon et al., 2007). The roles of Gef1 and Scd1 in cytokinesis are unclear, however, and the relative contributions of each GEF during cytokinesis have not been investigated.
Here we report that, after cytokinetic ring assembly, Cdc42 is activated in a unique spatiotemporal manner at the division site through the distinct functions of Gef1 and Scd1. In addition, the distinct localization pattern of the two GEFs corresponds with their role in cytokinesis. Loss of Gef1-mediated Cdc42 activation leads to a delay in the onset of ring constriction, whereas Scd1 is required for septum formation. Therefore Cdc42 is spatiotemporally activated by two distinct GEFs to promote different events during cytokinesis. These results suggest additional layers of complexity in the regulation of a single GTPase during a complex cellular process. This is likely a general operating principle inherent to this family of regulatory proteins.
RESULTS
Cdc42 is activated at the division site after actomyosin ring assembly
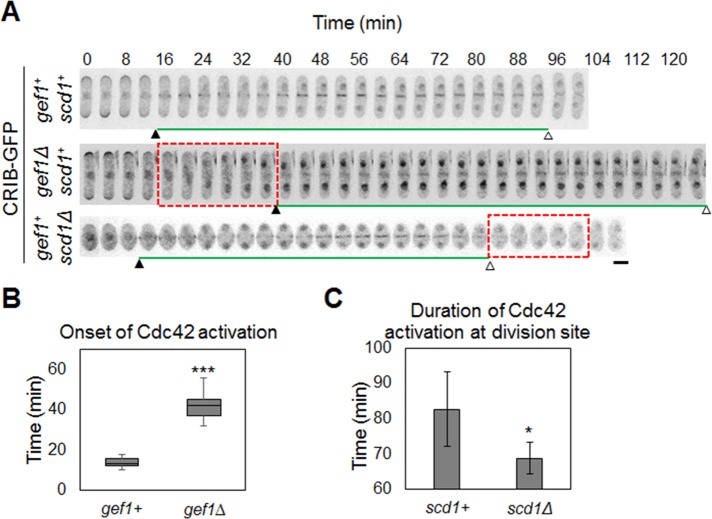
Cdc42 localizes to the cell division site in fission yeast (Merla and Johnson, 2000; Rincon et al., 2007), and previous reports showed that Cdc42 is activated at the division site (Tatebe et al., 2008). To analyze the dynamics of Cdc42 activation at the division site, we studied the localization of a green fluorescent protein (GFP)–tagged Cdc42/Rac interactive-binding peptide ([CRIB] from S. cerevisiae Gic2 protein)–domain bioprobe that is used to detect activated Cdc42 (Tatebe et al., 2008). The CRIB domain specifically binds to GTP-bound (active) Cdc42. CRIB-3xGFP localization at the cell division site was compared with that of spindle pole body (SPB) marker Sad1-mCherry and the cytokinetic ring marker type II myosin light chain Rlc1-Tomato. We found that CRIB-3xGFP localized to the cell division site 12 min after initial SPB separation, when the Rlc1-Tomato ring is assembled, and persisted to the end of cytokinesis (Figure 1 and Supplemental Movie S1). In fission yeast, after actomyosin ring assembly, the ring enters a maturation/dwell phase in which the diameter of the ring stays constant (Laporte et al., 2010). At the end of the maturation/dwell phase, the ring initiates constriction (Laporte et al., 2010). We found that Cdc42 is activated after cytokinetic ring assembly at the onset of the maturation/dwell phase during cytokinesis (Figure 1 and Supplemental Movie S1).
FIGURE 1:
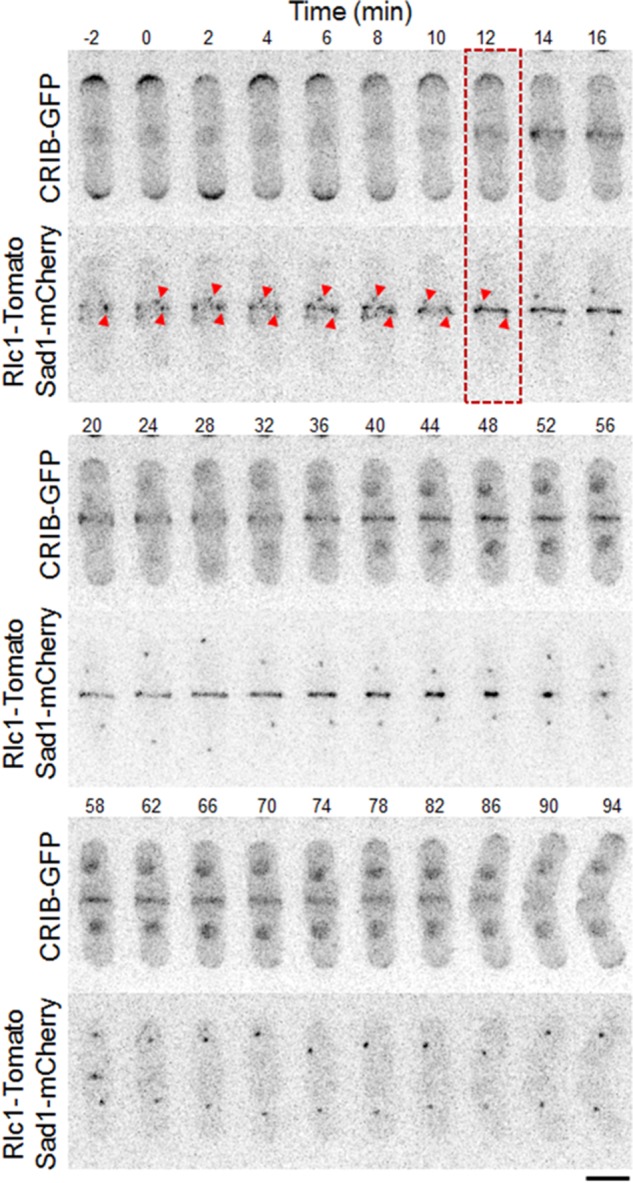
Cdc42 is activated at the site of cell division during cytokinesis. Time-series images showing the appearance and duration of the CRIB-3xGFP signal at the site of cell division. SPB separation by Sad1-mCherry and the cytokinetic ring protein by Rlc1-Tomato (bottom). Red arrowheads show initial stages of SPB marker position. Red box depicts onset of Cdc42 activation. Bar, 5 μm. Time is in minutes.
Next we tested to see whether Gef1 and Scd1 are required for activation of Cdc42 at the cell division site. In gef1Δ cells, Cdc42 activation at the cell division site is significantly delayed (Figure 2, A and B, and Supplemental Movie S2). In gef1Δ cells, CRIB-3xGFP appeared at the cell division site ∼40 ± 4.8 min after SPB marker separation, compared with 13 ± 2.4 min in gef1+ cells (n > 17, p = 1.8E-16; Figure 2, A and B, and Supplemental Movie S2). In scd1Δ cells, CRIB-3xGFP localization was not delayed at the cell division site but disappeared early (Figure 2, A and C, and Supplemental Movie S3). Duration of Cdc42 activation at the cell division site was 82 ± 10.6 min in control cells and 68 ± 4.5 min in scd1Δ cells (n > 8, p = 0.003; Figure 2, A and C, and Supplemental Movie S3). Note that scd1Δ cells display cell polarity defects and are mainly round (Chang et al., 1994). Gef1- and Scd1-mediated Cdc42 activation overlapped at the 40- to 80-min time points (Figure 2A). These results suggest that Gef1 and Scd1 activate Cdc42 sequentially, with significant temporal overlap during cytokinesis.
FIGURE 2:
Cdc42 activation pattern during cytokinesis. (A) Time-series images of CRIB-3xGFP signals during cytokinesis in the indicated cells. Black arrowheads indicate the appearance of CRIB-3xGFP at the site of cell division, and white arrowheads indicate the end of the CRIB-3xGFP signal at the site of cell division. Red box, absence of Cdc42 activation. n > 10. Bar, 5 μm. (B) Quantification of onset of Cdc42 activation cells as indicated (***p < 0.001). (C) Quantification of duration of Cdc42 activation in cells as indicated (*p < 0.05). Error bar, SD. Time is in minutes.
Cdc42 GEFs Gef1 and Scd1 display a distinct spatiotemporal localization pattern during cytokinesis
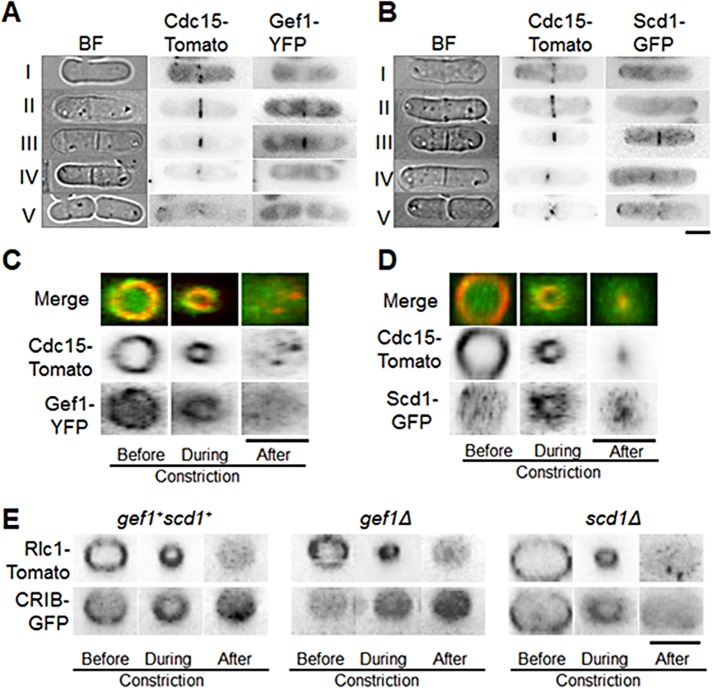
Our findings suggest that after ring assembly, Cdc42 activation at the onset of maturation/dwell phase is likely Gef1 dependent, whereas Scd1 activates Cdc42 in the later stages of cytokinesis. This indicates a distinct temporal pattern for the Cdc42 GEFs at the cell division site. To test this, we studied the localization of Gef1 and Scd1 throughout cytokinesis using Gef1–3x yellow fluorescent protein (3xYFP) and Scd1-3xGFP as markers. Both Gef1 and Scd1 are low- abundance proteins with weak signals and thus are not suitable for time-lapse images (Das et al., 2012). We performed live-cell microscopy in Gef1-3xYFP– or Scd1-3xGFP–expressing cells, with Cdc15-Tomato as a cytokinetic ring marker and bright-field images for septum detection. During ring assembly (Figure 3A, stage I), Gef1-3xYFP was absent from the cell division site. After the formation of the cytokinetic ring (Figure 3A, stage II), we detected Gef1-3xYFP at the cell division site (93% cells, n > 15). In cells containing a constricting Cdc15-Tomato ring (Figure 3A, stage III), Gef1-3xYFP also appeared to undergo constriction. At the end of ring constriction (Figure 3A, stage IV), Gef1-3xYFP was absent from the cell division site. In contrast, we detected Scd1-3xGFP in only a small number of cells in stage II (Figure 3B, 28% cells, n > 15). In stage III, Scd1-3xGFP overlapped with the constricting ring but also extended beyond the Cdc15-Tomato ring and was still visible at the end of constriction in stage IV (Figure 3B). Scd1-3xGFP was not detected at the cell division site during cell separation (Figure 3B, stage V). This indicates that Gef1 localizes to the cell division site immediately after ring formation and is lost as the ring constricts (Figure 3A), whereas Scd1 localizes just before ring constriction and follows the constricting ring (Figure 3B).
FIGURE 3:
Localization of Cdc42 GEFs Gef1 and Scd1 during cytokinesis. (A, B) Cells expressing Cdc15-Tomato with either Gef1-3xYFP or Scd1-3xGFP in the following cytokinetic stages: I, cytokinetic ring assembly; II, after cytokinetic ring assembly; III, cytokinetic ring constriction; IV, end of constriction and septum formation; V, cell separation. (C) Gef1-3xYFP colocalization with Cdc15-Tomato at the cytokinetic ring before, during, and after constriction. (D) Localization of Scd1-3xYFP with Cdc15-Tomato at the cytokinetic ring before, during, and after constriction. (E) CRIB-3xGFP localization in the indicated cells at the site of cell division before, during, and after cytokinetic ring constriction. The images are 3D reconstructed cells rotated to an angle of 90°. Bars, 5 μm.
To analyze localization of Gef1 at the cell division site in greater detail, we compared Gef1-3xYFP with Cdc15-Tomato. Three-dimensional (3D) reconstructed Cdc15-Tomato rings colocalized with Gef1-3xYFP both before and during constriction, suggesting that Gef1 localizes to the cytokinetic ring (Figure 3C). Gef1-3xYFP could not be detected after ring constriction. Further, Gef1-3xYFP did not localize to the site of cell division when cells were treated with latrunculin A (Supplemental Figure S1B). This suggests that Gef1 localization is dependent on the presence of the actomyosin ring. In contrast, Scd1-3xGFP did not colocalize with the Cdc15-Tomato ring before ring constriction (Figure 3D). Scd1-3xGFP appeared to localize to the constricting Cdc15-Tomato ring and to its outer periphery (Figure 3D). Scd1-3xGFP was still detected at the periphery of the constricted ring, suggesting that Scd1 localized to the ingressing membrane that followed the cytokinetic ring. Indeed, after ring constriction, Scd1-3xGFP colocalized with CellMask Orange–stained membrane barrier at the cell division site (Supplemental Figure S2A), suggesting that Scd1 localized to the furrow membrane.
To analyze how this distinct spatiotemporal pattern of Gef1 and Scd1 localization influenced Cdc42 activation at the cell division site, we observed CRIB-3xGFP localization in 3D reconstructed cells. In control cells, CRIB-3xGFP appeared as a ring before cytokinetic ring constriction (Figure 3E). The CRIB-3xGFP ring appeared to localize the outer edge of the Rlc1-Tomato ring. Previous reports indicated that the cytokinetic ring interacts with the membrane (Wachtler et al., 2003; Takeda et al., 2004) and that Cdc42 is a membrane protein (Hall, 2005). It is possible that the GEF at the ring activates Cdc42 at the ring–membrane interface. In agreement with this, CRIB-3xGFP also appeared in the ingressing membrane and at the membrane barrier after constriction (Figure 3E). In gef1Δ cells, CRIB-3xGFP was absent before cytokinetic ring constriction but was visible in the ingressing membrane during constriction and at the membrane barrier after constriction (Figure 3E). In scd1Δ cells, CRIB-3xGFP appeared at the edge of the ring before and during cytokinetic ring constriction but was absent after constriction (Figure 3E). These observations indicate that Cdc42 is activated by its GEFs in a distinct spatiotemporal manner. Gef1 localizes to the cytokinetic ring and activates Cdc42 immediately after cytokinetic ring assembly, whereas Scd1 localizes to the cell division site during cytokinetic ring constriction and activates Cdc42 along the ingressing membrane.
Of interest, in several scd1Δ cells, CRIB-3xGFP was also visible well after ring constriction (Figure 2A) and in the ingressing membrane during constriction (Figure 3E). To address this, we studied Gef1-3xYFP localization in scd1Δ cells. We found that in scd1Δ cells, Gef1-3xYFP shows random localization at the cell membrane and division site (Supplemental Figure S2B). Random Gef1 localization may contribute to Cdc42 activation at the membrane furrow after ring constriction in scd1Δ cells.
Gef1 promotes onset of actomyosin ring constriction
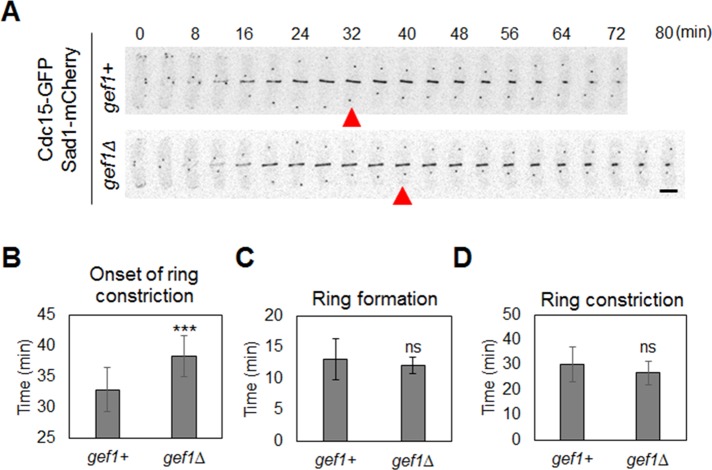
We find that Cdc42 is activated at the division site at the onset of ring maturation during cytokinesis. What is the significance of the maturation phase during cytokinesis in fission yeast? Why does the actomyosin ring not start constriction as soon as it is assembled? One possible explanation is that during maturation, the actomyosin ring and/or the division site prepare to successfully form the membrane furrow (Laporte et al., 2010). Because Cdc42 is activated at the onset of maturation, we posited that it might promote events during maturation. To understand the role of Cdc42 activation during maturation, we studied cytokinetic events in gef1Δ mutants, since these mutants fail to activate Cdc42 at the onset of maturation. We did not find any change in the localization of ring assembly proteins in gef1+ and gef1Δ cells (Supplemental Figure S1A). Further, analysis of different cytokinetic events did not show any delay in ring assembly in gef1Δ mutants (Figure 4C and Supplemental Movie S4). This is in agreement with our observation that Cdc42 activation and Gef1 recruitment at the division site occurred after the actomyosin ring was assembled. Of interest, in the gef1Δ mutants, the duration of the maturation phase was prolonged compared with gef1+ cells, leading to a delay in the onset of ring constriction (Figure 4, A and B, Supplemental Figure S3, and Supplemental Movie S4). We used Cdc15-GFP as a cytokinetic ring marker and Sad1-mCherry as a marker for the SPB. Under normal conditions, the cytokinetic ring forms in ∼13 min after SPB separation (Figure 4, A and C). Ring constriction initiates 33 min after SPB separation, with a 30-min duration (Figure 4, A, B, and D). The time line for cytokinetic events observed in this study is comparable to that reported earlier (Wu et al., 2003). In gef1Δ cells, ring constriction initiated 38.4 ± 3.2 min after SPB separation, compared with 32.9 ± 3.4 min in gef1+ cells (n > 11, p = 0.0006; Figure 4, A and B, and Supplemental Movie S4). We did not observe any change in the duration of actomyosin ring constriction in gef1Δ cells, suggesting that once initiated, ring constriction progressed normally in these cells (Figure 4D and Supplemental Movie S4). Similar results were observed with other cytokinetic ring markers, such as Rlc1-GFP (Supplemental Figure 3).
FIGURE 4:
Gef1 promotes the timely onset of ring constriction. (A) Time-series images of cytokinetic ring represented by Cdc15-GFP in indicated cells during cytokinesis. Sad1-mCherry represents SPB. Red arrowhead indicates the onset of ring constriction. (B) Quantification of timing of onset of ring cytokinetic ring constriction in indicated cells (***p < 0.001). (C) Quantification of timing of cytokinetic ring formation in indicated cells. (D) Quantification of duration of cytokinetic ring constriction in indicated cells; 11 cells. Bar, 5 μm. ns, not significant. Error bars, SD. Time is in minutes.
Because Gef1 is a known activator for Cdc42 (Coll et al., 2003), we tested whether the cytokinetic defect observed in gef1Δ mutants was indeed due to loss of Cdc42 activity. We expressed constitutively active Cdc42, cdc42G12V, in gef1Δ cells. We studied the timing of cytokinetic events in gef1+ and gef1Δ strains expressing cdc42+ or cdc42G12V, using Cdc15-GFP and Sad1-mCherry. Because the cells were grown in minimal media, the cytokinetic events lasted longer than in cells grown in rich media. The onset of cytokinetic ring constriction in cdc42+gef1Δ cells was delayed (44.9 ± 7.2 min) compared with that in cdc42+gef1+ cells (35.8 ± 3.3 min; Supplemental Figure S4, A and B). However, in gef1Δ cells expressing Cdc42G12V, the onset of cytokinetic ring constriction was similar (34.5 ± 7.3 min) to that of cdc42+gef1+ and cdc42G12V gef1+ (34.8 ± 3.6 min) cells (n > 16, p = 0.88; Supplemental Figure S4, A and B). Thus the cytokinetic defect observed in gef1Δ cells can be alleviated via the expression of constitutively active Cdc42. This, together with our findings on Cdc42 activation (Figure 2, A and B), indicates that Gef1 promotes cytokinesis through the activation of Cdc42. Of interest, cells expressing constitutively activated Cdc42 display cytokinetic defects after ring constriction. Cells overexpressing constitutively active Cdc42G12V showed a high septation index (0.71 ± 0.1) compared with control cells (0.18 ± 0.08), indicating a delay in cell separation (n > 450, p = 9.6E-17; Supplemental Figure S4). Thus, whereas constitutively active Cdc42 can promote timely onset of ring constriction, the later stages of cytokinesis are disrupted in these mutants.
The delay in onset of ring constriction in gef1Δ cells could be due to mitotic delays. We analyzed mitotic events in gef1+ and gef1Δ cells by measuring the distance between the SPB markers Sad1-mCherry over time. Onset of anaphase is marked by the separation of the SPB (Nabeshima et al., 1998). No change was detected in the timing of anaphase A and anaphase B in gef1+ and gef1Δ cells (Supplemental Figure S5). Thus delay in the onset of cytokinetic ring constriction in gef1Δ mutants is independent of mitotic events. Therefore these findings suggest that Gef1-dependent Cdc42 activation during maturation is required to promote timely onset of ring constriction.
Gef1 promotes nonmedial actin cables during cytokinesis
To understand the molecular details of the role of Gef1 in cytokinesis, we studied known downstream effectors of Cdc42 during cytokinesis. In budding yeast, Cdc42 has been shown to promote exocyst-mediated delivery (Zhang et al., 2008b; Wu et al., 2010) and recruitment of the septin proteins (Gladfelter et al., 2002, 2005; Caviston et al., 2003). In fission yeast, the exocyst proteins Sec3 and Exo70 require functional Cdc42 for proper localization (Bendezu and Martin, 2011; Estravis et al., 2011; Bendezu et al., 2012). However, we did not see any change in the localization of exocyst proteins or septin proteins in either gef1Δ or scd1Δ mutants, as compared with control cells (Supplemental Figure S6). This suggests that Gef1 and Scd1 function independently of the exocyst complex and septin ring during cytokinesis.
Cdc42 has also been shown to promote actin organization in fission yeast during polarization. We did not observe any change in the actin ring during cytokinesis in gef1Δ, compared with gef1+ cells. However, gef1Δ cells appeared to have fewer actin cables along the long axis of the cell during cytokinesis (Figure 5A). It was reported that during cytokinesis, nonmedial actin cables incorporate into the actomyosin ring (Huang et al., 2012). Athough studies have shown that these nonmedial actin cables are not sufficient to form stable actin rings, it is not clear what precise role these cables perform during cytokinesis (Huang et al., 2012; Coffman et al., 2013). We found that in gef1Δ cells, the number of nonmedial actin cables was reduced compared with gef1+ cells. We counted nonmedial actin cables in gef1+ and gef1Δ cells expressing the actin probe LifeAct-mCherry (Huang et al., 2012). Cells in interphase did not show any change in the number of actin cables (Figure 5, A and B). We next compared cells in the ring phase of cytokinesis. For this, we considered only cells with a distinct actin ring but without a septum (as determined by bright-field imaging). We found an average of 3.5 ± 1 nonmedial actin cables in gef1+ cells compared with 2.6 ± 1.1 cables in gef1Δ cells (n > 23, p = 0.0039; Figure 5B). Septated cells, however, did not show any significant difference in the number of nonmedial actin cables (Figure 5B). Because we observed only a moderate (26%) decrease in the number of nonmedial actin cables in gef1Δ cells, and to eliminate any artifactual errors due the LifeAct-mCherry probe, we confirmed our findings by Alexa Fluor–phalloidin staining of gef1+ and gef1Δ cells. Similar to LifeAct-mCherry cells, phalloidin staining also displayed reduced nonmedial actin cables in gef1Δ cells (Supplemental Figure S7).
FIGURE 5:
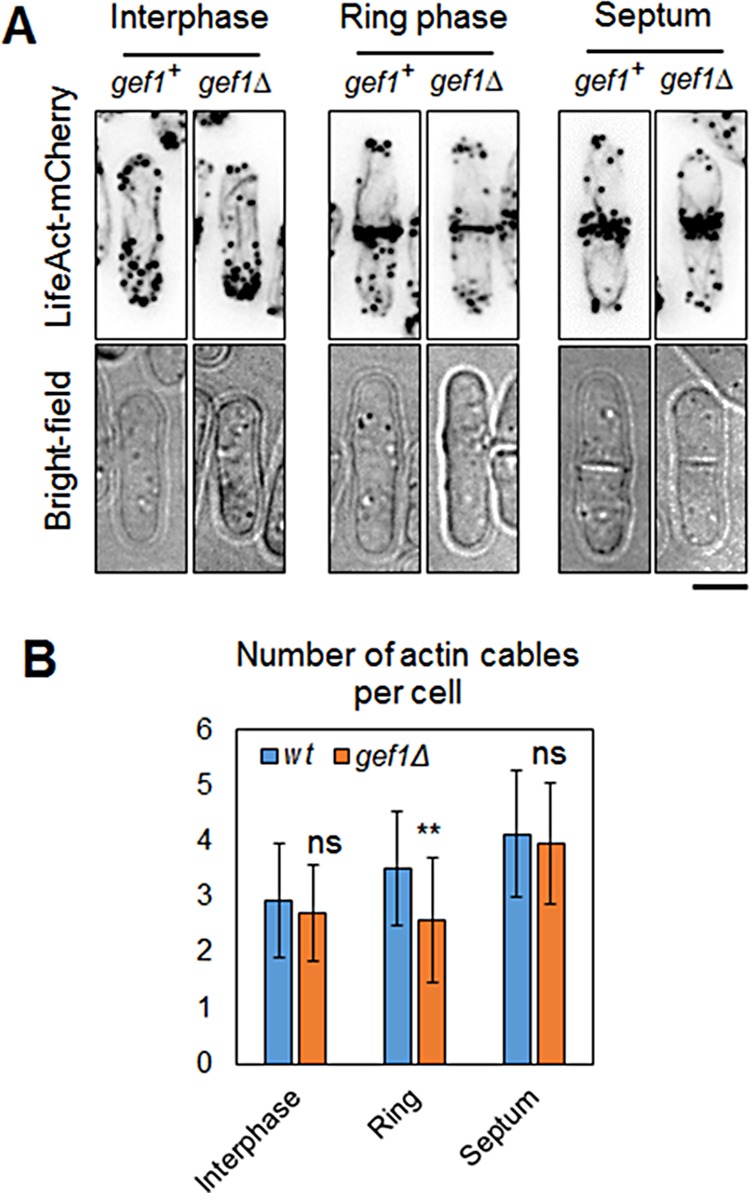
Gef1 is required for nonmedial actin cables during cytokinesis. (A) Actin probe LifeAct-mCherry–expressing gef1+ and gef1Δ cells were analyzed during interphase, the ring phase of cytokinesis, and septation. Cells were visualized under bright field to determine septation. Bar, 5 μm (B) Quantification of nonmedial actin cables as determined in the cells described. n > 23, **p = 0.0039. Error bars, SD. ns, not significant.
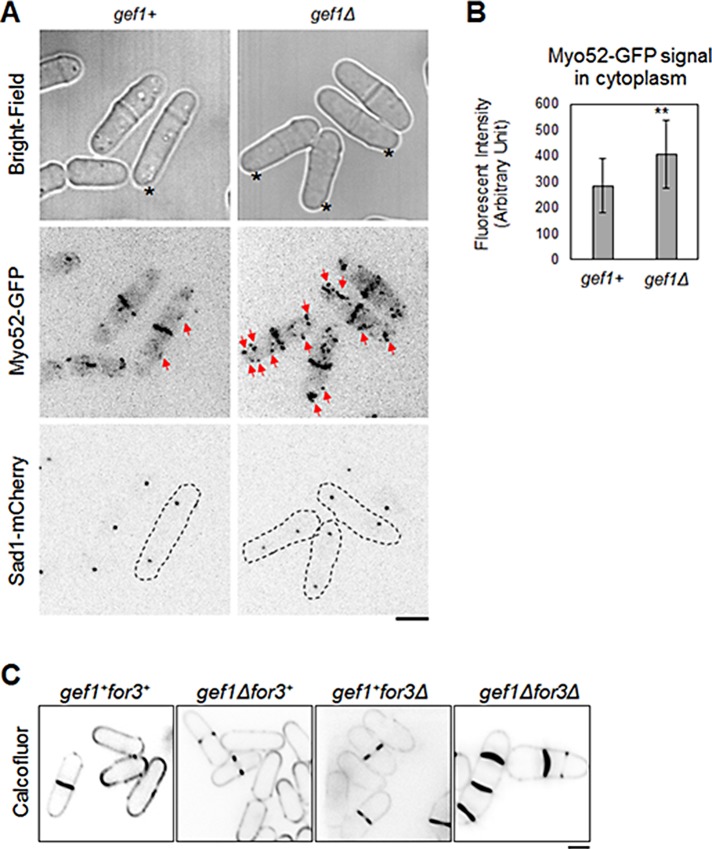
In addition to fewer nonmedial actin cables, gef1Δ cells also displayed more disorganized distribution of the type V myosin motor protein Myo52. The Myo52 motor protein walks along actin cables and carries cargo to the site of delivery (Win et al., 2001). Myo52 promotes delivery of proteins to site of cell division and is involved in the later stages of cytokinesis (Mulvihill et al., 2006). Under normal conditions, Myo52-GFP is localized to the site of cell division during cytokinesis, with very little localization in the cytoplasm (Figure 6A; Win et al., 2001; Mulvihill et al., 2006). In gef1Δ cells, Myo52-GFP is localized to the cell division site, similar to gef1+ cells, but also shows increased patches of Myo52-GFP distribution throughout the cytoplasm (Figure 6A, red arrows). Quantification of Myo52-GFP intensity in the cytoplasm indicates a 42% increase in signal in gef1Δ cells as compared with gef1+ cells (p = 0.0015, n = 22).
FIGURE 6:
Gef1 promotes type V myosin Myo52 localization to the cell division site. (A) Type V myosin Myo52-GFP distribution in gef1+ and gef1Δ cells. Cells with comparable distance of SPB marker Sad1-mCherry were selected. Bright-field images ensured nonseptating cells were selected. Red arrows mark cytoplasmic Myo52-GFP patches. Asterisks mark cells in the ring phase of cytokinesis. (B) Quantification of Myo52-GFP signal in the cytoplasm in gef1+ and gef1Δ cells as described; 22 cells, **p = 0.0015. Error bars, SD. (C) Calcofluor staining of gef1+for3+, gef1Δ, for3Δ, and gef1Δfor3Δ cells grown at 35°C. Bars, 5 μm.
The nonmedial actin cables are polymerized mainly by the formin Cdc12 (Huang et al., 2012). The formin For3 also likely polymerizes actin cables during cytokinesis but to a much smaller extent (Huang et al., 2012). The formins undergo autoinhibition and are activated by Rho GTPases to promote actin assembly (Kovar, 2006; Martin et al., 2007). However, previous reports indicate that Cdc12 is not regulated by this mechanism (Yonetani et al., 2008). The formin For3 has been shown to be activated by Cdc42 to promote actin organization during polarization (Martin et al., 2007). We asked whether Gef1-mediated Cdc42 activation promotes For3-dependent actin organization during cytokinesis. Although For3 is involved in cytokinesis, its functional role in cytokinesis is not clearly understood (Coffman et al., 2013). We studied the genetic relationship between gef1 and for3. If Gef1 functions upstream of For3, we expect to see an epistatic relationship between gef1 and for3. Instead, we find that gef1Δ mutants are sensitive to loss of for3. Both gef1Δ and for3Δ mutants grow normally at 25 and 36°C. We found that, compared with the single mutants, gef1Δfor3Δ double mutants display multiple septa defects at 36°C (Figure 6C). This suggests that For3 and Gef1 function in parallel pathways to promote cytokinesis.
Gef1 is required for recruitment of the septum-synthesizing enzyme Bgs1 to the division site
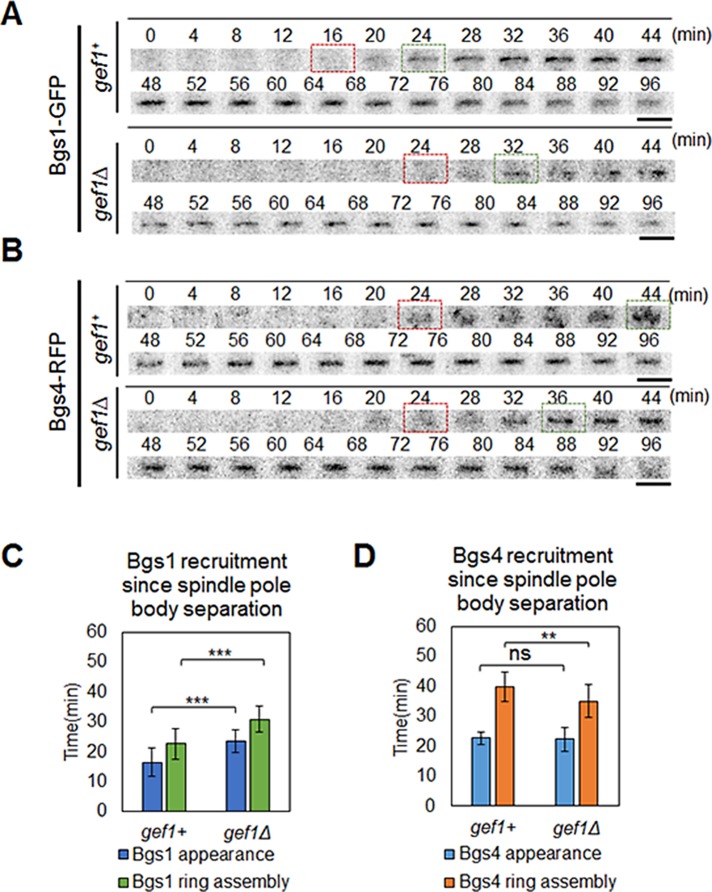
Previous reports showed that Bgs1 is essential for ring constriction, and cells expressing bgs1 temperature-sensitive mutants form a proper actomyosin ring but do not initiate constriction (Liu et al., 1999; Proctor et al., 2012; Cortes et al., 2015). The growing septum provides the force to overcome internal turgor pressure, thereby promoting membrane furrowing and actomyosin ring constriction (Proctor et al., 2012). Bgs1 is recruited to the cell division site in a Myo52-dependent manner (Mulvihill et al., 2006). Because gef1Δ cells displayed a delay in the onset of ring constriction and disorganized Myo52 localization at the division site, we postulated that Gef1 promotes Bgs1 recruitment to the cell division site to promote timely onset of ring constriction. To test this, we studied the recruitment of Bgs1-GFP in gef1+ and gef1Δ cells at the cell division site. We analyzed cells expressing Bgs1-GFP, Rlc1-Tomato, and Sad1-mCherry over time. Similar to previous reports, we find that in gef1+ cells, Bgs1-GFP is recruited to the cell division site 16.5 ± 4.8 min after SPB separation and 5.8 ± 3.1 min after actomyosin ring assembly (Cortes et al., 2015). Thus Bgs1 is recruited to the division site during the maturation phase of cytokinesis before onset of ring constriction. In gef1Δ cells, Bgs1-GFP was recruited to the cell division site 22.7 ± 4.9 min after SPB separation and 13 ± 4 min after actomyosin ring formation (n > 14, p = 2.7E-5; Figure 7, A and C, and Supplemental Movie S5). The delay (∼6.2 min) in Bgs1-GFP recruitment is comparable to the delay in the onset of ring constriction (∼5.5 min) in gef1Δ cells as compared with gef1+ cells. Because Bgs1 is eventually recruited in gef1Δ cells, it is possible that Bgs1 is also recruited by alternate pathways that may include other Rho GTPases or even Scd1.
FIGURE 7:
Gef1 promotes recruitment of Bgs1 but not Bgs4 to the site of cell division. Localization of septum protein (A) Bgs1-GFP and (B) Bgs4-RFP at the site of cell division. Time 0 represents SPB separation. Red box, onset of Bgs1-GFP or Bgs4-RFP recruitment to the site of cell division. Green box, appearance of Bgs1-GFP or Bgs4-RFP as a ring at the division site. Bars, 5 μm. (C) Quantification of time of recruitment of Bgs1-GFP with reference to cytokinetic ring formation as depicted by Rlc1-Tomato; >14 cells. Time in minutes. ***p < 0.001. Error bars, SD. (D) Quantification of time of recruitment of Bgs4-RFP with reference to SPB separation as depicted by Cdc11-GFP; 21 cells. Time in minutes. ns, not significant; **p < 0.01. Error bars, SD.
It is possible that the delay in Bgs1 delivery at the division site was due to an overall delay in the onset of ring constriction and elongation of the maturation phase. In such a scenario, other proteins recruited during the maturation phase would also show a delay in recruitment at the division site. The ring protein Cdc15 is known to increase in level during the maturation phase (Wu and Pollard, 2005). We studied the fold increase in Cdc15-GFP level in gef1+ and gef1Δ cells throughout the ring maturation phase. We did not see a change in the either the level or the timing of Cdc15-GFP recruitment in these cells (Supplemental Figure S8). This suggests that the delayed recruitment of Bgs1 in gef1Δ cells is not due to an overall delay in the maturation phase. In addition, we found that the timing of the delivery of Bgs4 to the cell division site was not affected in gef1Δ cells as compared with gef1+ cells with reference to SPB separation (Figure 7, B and D). In gef1+ cells, Bgs4–red fluorescent protein (RFP) was recruited 22.7 ± 2 min, and in gef1Δ cells, 22.3 ± 4 min, after SPB separation (n > 20, p = 0.67; Figure 7, B and D). This suggests that Bgs1 recruitment is specifically affected by Gef1 at the division site.
Scd1 promotes normal septum formation
Next we studied cytokinetic events in scd1Δ mutants to study the role of Scd1 in cytokinesis. Using Rlc1-Tomato as a ring marker and Sad1-mCherry as a SPB marker, we compared the timing for actomyosin ring assembly, onset of ring constriction, and duration of constriction in scd1+ and scd1Δ cells. There was no change in ring assembly or onset of constriction in scd1+ and scd1Δ cells (Supplemental Figure S9, A–C, and Supplemental Movie S6). However, the duration of ring constriction was longer in scd1Δ cells (44.9 ± 5.8 min) than in scd1+ cells (31.4 ± 3.3 min; n = 14, p = 6.2E-7; Supplemental Figure S9, A and D, and Supplemental Movie S6). One explanation for longer duration of ring constriction could be the wider diameter of scd1Δ cells. Loss of scd1 leads to increased cell width (Kelly and Nurse, 2011), and as a result, the circumference of the actomyosin ring is significantly larger in these cells than in scd1+ cells. To test this, we calculated the rate of ring constriction in scd1+ and scd1Δ cells. Rates of ring constriction were 0.371 ± 0.039 and 0.395 ± 0.035 μm/min in scd1+ and scd1Δ cells, respectively, suggesting that the ring constriction rate is comparable in these cells.
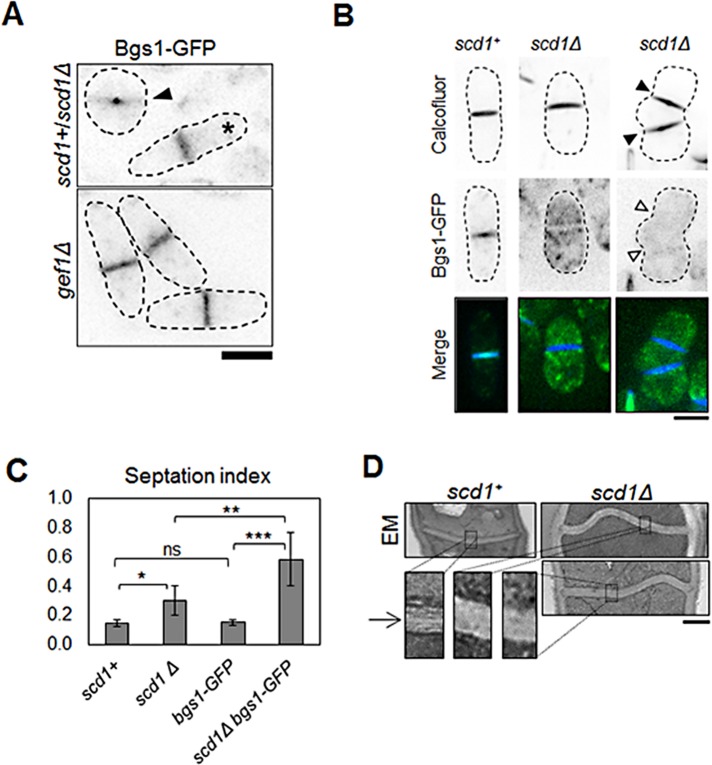
Because Scd1 activated Cdc42 along the membrane during ingression, we hypothesized that Scd1 plays a role in septum formation. Cdc42 is required for Bgs1 delivery to the cell tips (Estravis et al., 2012). During cytokinesis, Bgs1 synthesizes linear β(1,3)-glucans to form the primary septum (Cortes et al., 2002, 2007). We hypothesized that Scd1 promotes Bgs1 recruitment at the site of cell division. We found that Bgs1-GFP signal in scd1Δ cells (n = 21) showed a 48% decrease (p = 0.001) at the septum compared with scd1+ (n = 13; Figure 8A and Supplemental Figure S10A). This suggests that loss of scd1 leads to reduced recruitment of Bgs1 at the septum. Bgs1-GFP is distributed evenly at the cell division site in scd1+ cells (Figure 8A). However, in scd1Δ cells, Bgs1-GFP is localized mainly at the periphery of the ring, with very little signal at the ingressing membrane (Figure 8A). We also compared Bgs1-GFP localization in gef1Δ and scd1Δ cells. Bgs1-GFP is evenly distributed at the cell division site in gef1Δ cells, unlike in scd1Δ cells (Figure 8A). This indicates that Gef1 is required for recruitment of Bgs1 at the periphery of the ring, whereas Scd1 is required for recruitment to the ring periphery and the ingressing membrane.
FIGURE 8:
Scd1 is required for normal septum formation. (A) Cells expressing Bgs1-GFP in scd1+ and scd1Δ cells analyzed in the same field. scd1+ cells are depicted by asterisks, and scd1Δ cells are depicted by arrowheads. Bottom, Bgs1-GFP–expressing gef1Δ cells shown for comparison. Bar, 5 μm. (B) Calcofluor staining of scd1+ and scd1Δ cells expressing Bgs1-GFP. scd1Δ cells with multiple septa (arrowheads). Bar, 5 μm. (C) Quantification of septation index in cells as indicated; >223 cells. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. Error bars, SD. (D) Transmission electron microscopy of septum in scd1+ and scd1Δ cells. Sections of septum (black box) are zoomed at 4× to show primary septum defects. Bar, 500 nm.
We also found that the septation index in scd1+ cells was ∼14 ± 2%, whereas in scd1Δ cells, it was 30 ± 10%, which suggested a defect in cytokinesis. The increased septation index in scd1Δ cells could be due to prolonged ring constriction in these cells. However, the septation index in scd1Δ cells expressing Bgs1-GFP was higher (58 ± 19%) than in either scd1+ or scd1Δ cells (Figure 8C). The septation index in scd1+ cells expressing Bgs1-GFP (15 ± 2%) was similar to that in scd1+ cells alone (Figure 8C). Furthermore, in scd1Δ bgs1-GFP mutants, 45 ± 12% of the septated cells showed multiple septa (n > 286, p = 1.0E-4; Figure 8B and Supplemental Figure S10B). We did not see any multiple septa in scd1+ cells expressing Bgs1-GFP (Figure 8A). Fluorescent tagging of proteins has been reported to cause diminished protein activity in some cases (Cortes et al., 2015). It is possible that fluorescently tagged Bgs1 could have reduced function, rendering these cells more sensitive to loss of scd1. These results suggest that scd1Δ cells are sensitive to the levels of functional Bgs1 and that even minor perturbation of its function can lead to cytokinetic defects.
Because our data indicated that loss of scd1 resulted in septum defects, we studied septum morphology in scd1+ and scd1Δ cells. We performed transmission electron microscopy in scd1+ and scd1Δ cells. scd1+ cells showed fairly straight and even septum, whereas in scd1Δ cells, the septum was buckled and uneven (Figure 8D). Moreover, the septum in scd1Δ cells appeared thicker (360 ± 171 nm) than in scd1+ cells (170 ± 50 nm; n > 16, p < 0.001). In fission yeast, septation involves that formation of the primary septum be followed by the formation of the secondary septum (Cortes et al., 2007). Using electron microscopy, it is possible to observe the primary and secondary septum in normal cells (Cortes et al., 2007). The secondary septum appears darker and flanks the primary septum (Figure 8D). The distinction between primary and secondary septum was not clear in scd1Δ cells compared with scd1+ cells (Figure 8D). Electron microscopy did not show any primary septum defects in gef1Δ cells (Supplemental Figure S11). However, the septum in gef1Δ (369 ± 71 nm, n > 11) cells also appeared thicker, similar to scd1Δ cells.
We find that in scd1Δ cells the ingressing septum has reduced Bgs1 levels but the septum appears thicker than in scd1+ cells. This could be due to increased secondary septum formation in these mutants. The secondary septum is dependent on the β-glucan synthase, Bgs4. We find a small increase in Bgs4 levels in scd1Δ cells. Bgs4-RFP levels increased by 18% in scd1Δ cells compared with scd1+ cells (n > 20, p = 0.04; Supplemental Figure S10, C and D). This supports the idea that scd1Δ cells contain larger amounts of secondary septum. Further studies will determined whether Scd1 indeed promotes primary septum and restricts secondary septum formation.
DISCUSSION
Whereas Cdc42 is a major regulator of cell growth and polarity, its role in cytokinesis is not clear. Here we report that loss of Cdc42 activation leads to cytokinetic defects in fission yeast. During cytokinesis, Cdc42 is activated immediately after actomyosin ring assembly and persists until cell separation. Cdc42 activation is Gef1 dependent just after ring assembly and Scd1 dependent after constriction. Gef1 and Scd1 display unique localization patterns during cytokinesis, resulting in a spatiotemporal activation pattern of Cdc42. Corresponding to their localization patterns, the GEFs promote distinct cytokinetic events. Gef1 localizes to the cytokinetic ring immediately after ring assembly and constricts with the ring. Gef1 promotes timely onset of ring constriction, nonmedial actin cables, type V myosin organization, and timely recruitment of Bgs1. Scd1 localizes to the ingressing membrane and promotes septum formation. Scd1 promotes Bgs1 localization to the septum and is required for normal septum morphology. Loss of scd1 results in primary septum defects, with septum buckling and overall increase in thickness. Cells lacking scd1 did not show any delay in the onset of ring constriction, whereas gef1 mutants did not show aberrant primary septum. Taken together, these findings suggest that the GEFs have unique roles in cytokinesis, as determined by their spatiotemporal localization patterns.
What is the molecular mechanism by which Gef1 and Scd1 regulate cytokinetic events? We find that cells lacking gef1 show a moderate decrease in nonmedial actin cables during the ring phase of cytokinesis. We also find a synthetic genetic interaction between gef1 and for3, suggesting that they function in parallel pathways to promote actin organization. It is possible that Gef1 promotes actin cable stability rather than synthesis during cytokinesis. Of interest, in actin-interacting protein aip1 mutants, actin cables are stable and prominent during cytokinesis and display earlier onset of actomyosin ring constriction (Chen et al., 2015). Further studies will address the role of Gef1 in actin organization during cytokinesis. Reduced actin cables and disrupted distribution of the type V myosin Myo52 in gef1Δ cells could lead to delays in Bgs1 delivery. Indeed, Myo52 promotes Bgs1 recruitment to the division site during cytokinesis (Mulvihill et al., 2006). We also observed reduced Bgs1 levels at the septum in scd1Δ cells. This agrees with previous reports that Cdc42 specifically promotes Bgs1 delivery to the cell tips (Estravis et al., 2012).
Cytokinesis is a highly complex process involving several temporally organized events (Pollard, 2010). In fission yeast, once the actomyosin ring assembles, it does not immediately start constriction but undergoes a maturation phase (Lee et al., 2012). Ring constriction initiates at the end of maturation phase and accompanies membrane furrow ingression and septum formation (Lee et al., 2012). Although the significance of the maturation phase is not clear, proteins required for the later stages of cytokinesis are recruited in this phase (Lee et al., 2012). We find that Cdc42 is not required for actomyosin ring assembly but is required for timely onset of ring constriction. We show that Gef1 at the assembled ring during maturation ensures timely Bgs1 recruitment. We posit that the primary septum built by Bgs1, recruited during maturation, provides the force to overcome the internal turgor pressure to enable ring constriction and membrane furrowing. This supports the idea that the maturation phase prepares the ring/division site for membrane furrowing. Although our data suggest that Gef1 promotes recruitment of Bgs1 to the division site, it is possible that Gef1 also promotes recruitment of other cytokinetic proteins to ensure timely onset of ring constriction. Further studies will address the role of Gef1 in cytokinesis in more details. In most animal cells, actomyosin ring constriction initiates immediately after assembly without a maturation phase. However, in Drosophila embryos, during cellularization, actomyosin ring constriction is biphasic, with a significantly slow initial constriction rate (Royou et al., 2004). This is probably due to membrane expansion events required for furrowing at this stage in the embryo (Figard et al., 2013). Thus it is possible that events similar to ring maturation may be present even in animal cells that require special conditions for successful cytokinesis.
Although Scd1 is required for proper primary septum formation, our data also suggest that Cdc42 activation at the septum restricts overall septum formation, as indicated by thicker and buckling septa in scd1Δ cells. Whereas Cdc42 activation is required for proper ring constriction and septation, excessive activation is detrimental to cytokinesis, as constitutively active Cdc42 shows severe cell separation defects. This is in agreement with reports on budding yeast, in which Cdc42 inhibits abscission during the final stage of cytokinesis (Atkins et al., 2013; Onishi et al., 2013). Taken together, our findings suggest that Cdc42 may act to ensure proper organization of different temporal events during cytokinesis.
Gef1- and Scd1-mediated activation of Cdc42 at the cell division site shows significant temporal overlap (Figure 1B), and gef1Δscd1Δ mutants are inviable (Coll et al., 2003; Hirota et al., 2003). Thus it is possible that Cdc42 activation is essential for cytokinesis and the two GEFs are partially redundant. Given the conserved nature of Cdc42, it is conceivable that Cdc42 is also required for cytokinesis in other eukaryotes. In Caenorhabditis elegans, the PAR proteins, including Cdc42, are required for robust cytokinesis, likely through the regulation of actin organization (Jordan et al., 2016). How are the GEFs spatiotemporally organized, and what is the significance of this in cytokinesis? Why does the cell require different GEFs to activate Cdc42 during the same cellular process? The two GEFs differ structurally, in that Scd1 contains a pleckstrin homology domain required for localization (Endo et al., 2003), whereas Gef1 contains an N-BAR domain (Das et al., 2015). The structural differences in the two GEFs could contribute to distinct localization patterns and/or functions through protein interactions. In addition, the different GEFs may have different kinetics of Cdc42 activation or different feedback mechanisms that could contribute to the spatiotemporal pattern. Indeed, Cdc42 shows an oscillatory activation pattern, which is maintained through feedback mechanisms for the establishment of cell polarity in yeasts (Das et al., 2012; Howell et al., 2012). Distinct patterns of GTPase activation have also been reported to be critical for their function in other eukaryotes (Burkel et al., 2012; Bement and von Dassow, 2014; Vaughan et al., 2014). Our findings indicate a unique GEF-dependent spatiotemporal activation pattern for Cdc42 that corresponds to its function. Pattern organization established by the different regulators may well emerge to be critical for GTPase function.
Understanding the molecular details of GTPase pattern establishment and cellular function in eukaryotes is complicated by the presence of multiple GEFs and GAPs (Miyamoto and Yamauchi, 2010; Shi, 2013; Gadea and Blangy, 2014). The significance of multiple GTPase regulators in promoting different cellular processes is not clear. It is possible that Rho-family GTPases coordinate multiple cellular events through its different regulators. In fission yeast, Cdc42 has only two GEFs, mutants of which show distinct phenotypes during cytokinesis, as reported here. Thus fission yeast provides an advantage for the study of Cdc42 in cytokinesis. Future work is required to understand how Cdc42, activated by different GEFs, mediates distinct functions during cytokinesis and how Cdc42 regulators collaborate to fine-tune its spatiotemporal activation.
MATERIALS AND METHODS
Strains and cell culture
The S. pombe strains used in this study are listed in Supplemental Table S1. All strains were isogenic to the original strain 972. Cells were cultured in yeast extract (YE) medium or Edinburgh minimal medium plus required supplements. Standard techniques were used for genetic manipulation and analysis (Moreno et al., 1991). Unless specified, cells were grown exponentially at 25°C.
Microscopy
All images were acquired at room temperature (23–25°C) with a VT-Hawk two-dimensional array laser scanning confocal microscopy system (Visitech International, Sunderland, UK) with an Olympus IX-83 inverted microscope with a 100×/numerical aperture 1.49 UAPO lens (Olympus, Tokyo, Japan) and electron-multiplying charge-coupled device digital camera (Hamamatsu, Hamamatsu City, Japan).
For still, z-series, and time-lapse images (<5 min), the cells were mounted directly on glass slides with a #1.5 coverslip (Fisher Scientific, Waltham, MA) and imaged immediately. For z-series, images were acquired with a depth interval of 0.4 μm. For time-lapse images >5 min, the cells were placed in 3.5-mm glass-bottom culture dishes (MatTek, Ashland, MA) and overlaid with YE medium plus 1% agar. Ascorbic acid (100 μM) as an antioxidant was added to the culture to minimize fluorescence toxicity to the cell, as reported previously (Frigault et al., 2009). Images were acquired by MetaMorph (Molecular Devices, Sunnyvale, CA) and analyzed by ImageJ (National Institutes of Health, Bethesda, MD). Statistically significant difference between two groups of cells was determined by p value from Student’s t test.
Electron microscopy
Transmission electron microscopy was performed as described previously (Chappell and Warren, 1989). Cells were washed three times in sterile water, fixed for 1 h in 2% potassium permanganate at room temperature, and then harvested by centrifugation, washed three times in sterile water, resuspended in 70% ethanol, and incubated overnight at 4°C. Samples were then dehydrated by sequential washes in 90% ethanol (twice for 15 min) and washed in 100% ethanol (three times for 20 min). The pellet was resuspended in propylene oxide for 10 min, incubated in a 1:1 mixture of propylene oxide and Spurr’s medium for 1 h, and incubated in neat Spurr’s medium for 1 h. This was followed by another change of medium and incubation at 65°C for 1 h. Finally, samples were embedded in Spurr’s medium in a capsule, and resin in the medium was allowed to polymerize at 60°C overnight. Blocks were sectioned with a diamond knife and stained with uranyl acetate and lead citrate. The cells were then examined in a Zeiss Libra 200MC electron microscope (Oberkochen, Germany) at the University of Tennessee Imaging Core facility.
Expression of constitutively active Cdc42
DNA fragment of cdc42G12V was cloned into the vector pjk148 under the thiamine-repressible nmt41 promoter and integrated into the leu1-32 loci in gef1+ and gef1Δ cells. Cells were grown in the absence of thiamine to promote expression of Cdc42G12V. Empty vector pjk148 in gef1+ cells and gef1Δ cells was used as control. Cells expressing Cdc42G12V displayed mixed morphological defects, from round to almost normal, rod-shaped cells. Round cells were not analyzed further to avoid pleotropic effects due to high cdc42G12V levels. We assumed that polarized cells expressed low or moderate levels of Cdc42G12V and used them for further analysis.
Cell staining
To stain the septum and cell wall, cells were stained in YE liquid with 50 μg/ml Calcofluor White M2R (Sigma-Aldrich, St. Louis, MO) at room temperature. Cells were washed with fresh YE liquid once before imaging. For CellMask Orange staining, cells were stained in YE liquid with 5 μg /ml CellMask Orange in dimethyl sulfoxide (Thermo Fisher Scientific) for 5 min at room temperature in the dark. Cells were washed with fresh YE liquid before imaging.
Latrunculin A treatment
Cells were treated with 100 μM latrunculin A in dimethyl sulfoxide (DMSO) in YE medium for 30 min before imaging. Control cells were treated with only 0.1% DMSO in YE medium.
Analysis of Bgs1-GFP and Bgs4-RFP levels at the septum
Cultures of scd1+ and scd1Δ cells expressing Bgs1-GFP or Bgs4-RFP were grown to OD 0.5 at 32°C and then mixed in equal volumes before imaging at 488 nm. Quantification of Bgs1-GFP and Bgs4-RGP in the septa of dividing cells was performed in ImageJ by measuring the fluorescence intensity restricted to fully formed septa (confirmed by bright field). Images were normalized for background fluorescence by subtracting the intensity of a cell-free region within the same image. The mean normalized intensity was calculated for cells of each genotype within the same image. Significance was determined through comparison of each genotype’s mean normalized intensities using a Student’s two-tailed t test assuming unequal variance.
Supplementary Material
Acknowledgments
We thank J. Bembenek, A. Nebenführ, and T. Burch-Smith for discussions; F. Chang, K. Gould, S. Martin, M. Balasubramanian, and J. Q. Wu for strains; and J. Dunlap for electron microscopy.
This work was supported by startup funds from the University of Tennessee and TN-SCORE, a multidisciplinary research program sponsored by the National Science Foundation Experimental Program to Stimulate Competitive Research (EPS-1004083).
Abbreviations used:
- BAR
Bin/Amphiphysin/Rvs167
- CRIB
Cdc42/Rac interactive binding peptide
- DMSO
dimethyl sulfoxide
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- SPB
spindle pole body.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-10-0700) on March 3, 2016.
REFERENCES
- Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Atkins BD, Yoshida S, Saito K, Wu CF, Lew DJ, Pellman D. Inhibition of Cdc42 during mitotic exit is required for cytokinesis. J Cell Biol. 2013;202:231–240. doi: 10.1083/jcb.201301090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement WM, von Dassow G. Single cell pattern formation and transient cytoskeletal arrays. Curr Opin Cell Biol. 2014;26:51–59. doi: 10.1016/j.ceb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu FO, Martin SG. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell. 2011;22:44–53. doi: 10.1091/mbc.E10-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu FO, Vincenzetti V, Martin SG. Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PLoS One. 2012;7:e40248. doi: 10.1371/journal.pone.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel BM, Benink HA, Vaughan EM, von Dassow G, Bement WM. A Rho GTPase signal treadmill backs a contractile array. Dev Cell. 2012;23:384–396. doi: 10.1016/j.devcel.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Longtine M, Pringle JR, Bi E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol Biol Cell. 2003;14:4051–4066. doi: 10.1091/mbc.E03-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, Jung V, Xu HP, Wigler MH. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chappell TG, Warren G. A galactosyltransferase from the fission yeast Schizosaccharomyces pombe. J Cell Biol. 1989;109:2693–2702. doi: 10.1083/jcb.109.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Courtemanche N, Pollard TD. Aip1 promotes actin filament severing by cofilin and regulates constriction of the cytokinetic contractile ring. J Biol Chem. 2015;290:2289–2300. doi: 10.1074/jbc.M114.612978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chircop M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman VC, Sees JA, Kovar DR, Wu JQ. The formins Cdc12 and For3 cooperate during contractile ring assembly in cytokinesis. J Cell Biol. 2013;203:101–114. doi: 10.1083/jcb.201305022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll PM, Trillo Y, Ametzazurra A, Perez P. Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe. Mol Biol Cell. 2003;14:313–323. doi: 10.1091/mbc.E02-07-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JC, Ishiguro J, Duran A, Ribas JC. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J Cell Sci. 2002;115:4081–4096. doi: 10.1242/jcs.00085. [DOI] [PubMed] [Google Scholar]
- Cortes JC, Konomi M, Martins IM, Munoz J, Moreno MB, Osumi M, Duran A, Ribas JC. The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol Microbiol. 2007;65:201–217. doi: 10.1111/j.1365-2958.2007.05784.x. [DOI] [PubMed] [Google Scholar]
- Cortes JCG, Pujol N, Sato M, Pinar M, Ramos M, Moreno B, Osumi M, Ribas JC, Perez P. Cooperation between paxillin-like protein Pxl1 and glucan synthase Bgs1 is essential for actomyosin ring stability and septum formation in fission yeast. PLoS Genet. 2015;11:e1005358. doi: 10.1371/journal.pgen.1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JM, Harden N, Leung T, Lim L, Kiehart DP. Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function. Dev Biol. 1998;204:151–164. doi: 10.1006/dbio.1998.9061. [DOI] [PubMed] [Google Scholar]
- Das M, Drake T, Wiley DJ, Buchwald P, Vavylonis D, Verde F. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science. 2012;337:239–243. doi: 10.1126/science.1218377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Nunez I, Rodriguez M, Wiley DJ, Rodriguez J, Sarkeshik A, Yates JR, 3rd, Buchwald P, Verde F. Phosphorylation-dependent inhibition of Cdc42 GEF Gef1 by 14–3-3 protein Rad24 spatially regulates Cdc42 GTPase activity and oscillatory dynamics during cell morphogenesis. Mol Biol Cell. 2015;26:3520–3534. doi: 10.1091/mbc.E15-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Wiley DJ, Chen X, Shah K, Verde F. The conserved NDR kinase Orb6 controls polarized cell growth by spatial regulation of the small GTPase Cdc42. Curr Biol. 2009;19:1314–1319. doi: 10.1016/j.cub.2009.06.057. [DOI] [PubMed] [Google Scholar]
- Dehapiot B, Carriere V, Carroll J, Halet G. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev Biol. 2013;377:202–212. doi: 10.1016/j.ydbio.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- Dutartre H, Davoust J, Gorvel JP, Chavrier P. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109(pt 2):367–377. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- Endo M, Shirouzu M, Yokoyama S. The Cdc42 binding and scaffolding activities of the fission yeast adaptor protein Scd2. J Biol Chem. 2003;278:843–852. doi: 10.1074/jbc.M209714200. [DOI] [PubMed] [Google Scholar]
- Estravis M, Rincon S, Perez P. Cdc42 regulation of polarized traffic in fission yeast. Commun Integr Biol. 2012;5:370–373. doi: 10.4161/cib.19977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estravis M, Rincon SA, Santos B, Perez P. Cdc42 regulates multiple membrane traffic events in fission yeast. Traffic. 2011;12:1744–1758. doi: 10.1111/j.1600-0854.2011.01275.x. [DOI] [PubMed] [Google Scholar]
- Figard L, Xu H, Garcia HG, Golding I, Sokac AM. The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization. Dev Cell. 2013;27:648–655. doi: 10.1016/j.devcel.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigault MM, Lacoste J, Swift JL, Brown CM. Live-cell microscopy—tips and tools. J Cell Sci. 2009;122:753–767. doi: 10.1242/jcs.033837. [DOI] [PubMed] [Google Scholar]
- Gadea G, Blangy A. Dock-family exchange factors in cell migration and disease. Eur J Cell Biol. 2014;93:466–477. doi: 10.1016/j.ejcb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Bose I, Zyla TR, Bardes ES, Lew DJ. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J Cell Biol. 2002;156:315–326. doi: 10.1083/jcb.200109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Kozubowski L, Zyla TR, Lew DJ. Interplay between septin organization, cell cycle and cell shape in yeast. J Cell Sci. 2005;118:1617–1628. doi: 10.1242/jcs.02286. [DOI] [PubMed] [Google Scholar]
- Goyal A, Takaine M, Simanis V, Nakano K. Dividing the spoils of growth and the cell cycle: the fission yeast as a model for the study of cytokinesis. Cytoskeleton. 2011;68:69–88. doi: 10.1002/cm.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hirota K, Tanaka K, Ohta K, Yamamoto M. Gef1p and Scd1p, the Two GDP-GTP exchange factors for Cdc42p, form a ring structure that shrinks during cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell. 2003;14:3617–3627. doi: 10.1091/mbc.E02-10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AS, Jin M, Wu CF, Zyla TR, Elston TC, Lew DJ. Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell. 2012;149:322–333. doi: 10.1016/j.cell.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Huang Y, Yu H, Subramanian D, Padmanabhan A, Thadani R, Tao Y, Tang X, Wedlich-Soldner R, Balasubramanian MK. Nonmedially assembled F-actin cables incorporate into the actomyosin ring in fission yeast. J Cell Biol. 2012;199:831–847. doi: 10.1083/jcb.201209044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SN, Canman JC. Rho GTPases in animal cell cytokinesis: an occupation by the one percent. Cytoskeleton. 2012;69:919–930. doi: 10.1002/cm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SN, Davies T, Zhuravlev Y, Dumont J, Shirasu-Hiza M, Canman JC. Cortical PAR polarity proteins promote robust cytokinesis during asymmetric cell division. J Cell Biol. 2016;212:39–49. doi: 10.1083/jcb.201510063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FD, Nurse P. Spatial control of Cdc42 activation determines cell width in fission yeast. Mol Biol Cell. 2011;22:3801–3811. doi: 10.1091/mbc.E11-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR. Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol. 2006;18:11–17. doi: 10.1016/j.ceb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Laporte D, Zhao R, Wu JQ. Mechanisms of contractile-ring assembly in fission yeast and beyond. Semin Cell Deve Biol. 2010;21:892–898. doi: 10.1016/j.semcdb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc J, Zhang X, McKee D, Wang ZB, Li R, Ma C, Sun QY, Liu XJ. The small GTPase Cdc42 promotes membrane protrusion during polar body emission via ARP2-nucleated actin polymerization. Mol Hum Reprod. 2011;17:305–316. doi: 10.1093/molehr/gar026. [DOI] [PubMed] [Google Scholar]
- Lee IJ, Coffman VC, Wu JQ. Contractile-ring assembly in fission yeast cytokinesis: recent advances and new perspectives. Cytoskeleton. 2012;69:751–763. doi: 10.1002/cm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ. Polar body emission. Cytoskeleton. 2012;69:670–685. doi: 10.1002/cm.21041. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, McCollum D, Balasubramanian MK. Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics. 1999;153:1193–1203. doi: 10.1093/genetics/153.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Benink HA, Cheng D, Montplaisir V, Wang L, Xi Y, Zheng PP, Bement WM, Liu XJ. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr Biol. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox AS, Azoury J, Dumont J. Polar body cytokinesis. Cytoskeleton. 2012;69:855–868. doi: 10.1002/cm.21064. [DOI] [PubMed] [Google Scholar]
- Martin SG, Rincon SA, Basu R, Perez P, Chang F. Regulation of the formin for3p by cdc42p and bud6p. Mol Biol Cell. 2007;18:4155–4167. doi: 10.1091/mbc.E07-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker D, Kellogg DR. Plasma membrane growth during the cell cycle: unsolved mysteries and recent progress. Curr Opin Cell Biol. 2012;24:845–851. doi: 10.1016/j.ceb.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla A, Johnson DI. The Cdc42p GTPase is targeted to the site of cell division in the fission yeast Schizosaccharomyces pombe. Eur J Cell Biol. 2000;79:469–477. doi: 10.1078/0171-9335-00073. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J. Cellular signaling of Dock family proteins in neural function. Cell Signal. 2010;22:175–182. doi: 10.1016/j.cellsig.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Mulvihill DP, Edwards SR, Hyams JS. A critical role for the type V myosin, Myo52, in septum deposition and cell fission during cytokinesis in Schizosaccharomyces pombe. Cell Motil Cytoskeleton. 2006;63:149–161. doi: 10.1002/cm.20113. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Nakagawa T, Straight AF, Murray A, Chikashige Y, Yamashita YM, Hiraoka Y, Yanagida M. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol Biol Cell. 1998;9:3211–3225. doi: 10.1091/mbc.9.11.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Ko N, Nishihama R, Pringle JR. Distinct roles of Rho1, Cdc42, and Cyk3 in septum formation and abscission during yeast cytokinesis. J Cell Biol. 2013;202:311–329. doi: 10.1083/jcb.201302001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22:50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol. 2012;22:1601–1608. doi: 10.1016/j.cub.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon S, Coll PM, Perez P. Spatial regulation of Cdc42 during cytokinesis. Cell Cycle. 2007;6:1687–1691. doi: 10.4161/cc.6.14.4481. [DOI] [PubMed] [Google Scholar]
- Royou A, Field C, Sisson JC, Sullivan W, Karess R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol Biol Cell. 2004;15:838–850. doi: 10.1091/mbc.E03-06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L. Dock protein family in brain development and neurological disease. Comm Integr Biol. 2013;6:e26839. doi: 10.4161/cib.26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Kawate T, Chang F. Organization of a sterol-rich membrane domain by cdc15p during cytokinesis in fission yeast. Nat Cell Biol. 2004;6:1142–1144. doi: 10.1038/ncb1189. [DOI] [PubMed] [Google Scholar]
- Tatebe H, Nakano K, Maximo R, Shiozaki K. Pom1 DYRK regulates localization of the Rga4 GAP to ensure bipolar activation of Cdc42 in fission yeast. Curr Biol. 2008;18:322–330. doi: 10.1016/j.cub.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan EM, You JS, Elsie Yu HY, Lasek A, Vitale N, Hornberger TA, Bement WM. Lipid domain-dependent regulation of single-cell wound repair. Mol Biol Cell. 2014;25:1867–1876. doi: 10.1091/mbc.E14-03-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtler V, Rajagopalan S, Balasubramanian MK. Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2003;116:867–874. doi: 10.1242/jcs.00299. [DOI] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win TZ, Gachet Y, Mulvihill DP, May KM, Hyams JS. Two type V myosins with non-overlapping functions in the fission yeast Schizosaccharomyces pombe: Myo52 is concerned with growth polarity and cytokinesis, Myo51 is a component of the cytokinetic actin ring. J Cell Sci. 2001;114:69–79. doi: 10.1242/jcs.114.1.69. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Pollard TD. Counting cytokinesis proteins globally and locally in fission yeast. Science. 2005;310:310–314. doi: 10.1126/science.1113230. [DOI] [PubMed] [Google Scholar]
- Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell. 2010;21:430–442. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani A, Lustig RJ, Moseley JB, Takeda T, Goode BL, Chang F. Regulation and targeting of the fission yeast formin cdc12p in cytokinesis. Mol Biol Cell. 2008;19:2208–2219. doi: 10.1091/mbc.E07-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ma C, Miller AL, Katbi HA, Bement WM, Liu XJ. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. J Cell Biol. 2008a;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008b;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.