Abstract
Background
Dysfunction of the dorsolateral prefrontal cortex (DLPFC) and parahippocampal region along with poor working memory are common neurophysiological and behavioral features associated with schizophrenia and normal aging. It is, however, unknown whether the associated patterns of neural activation differ between these two groups when their cognitive performance is closely matched in a pairwise manner. The authors sought to pinpoint common and differential pathophysiological features that accompany comparable working memory impairments in schizophrenia and healthy aging.
Methods
Fifty-three subjects were scanned using H215O PET regional cerebral blood flow measurements during working memory. Seventeen medication-free patients with schizophrenia were individually matched for working memory performance with seventeen healthy aging subjects. Brain activation of the two index groups were compared to each other and to nineteen young healthy individuals.
Results
Patients with schizophrenia showed right DLPFC hypoactivation, both when compared to age-matched controls, and after direct comparison with working memory performance-matched elderly subjects. Moreover, both groups with working memory deficits shared an inability to suppress parahippocampal and anterior medial prefrontal cortex activation.
Conclusions
These results provide new insights into the mechanisms by which impaired working memory performance can arise by showing that both common (parahippocampal/anterior medial PFC) and differential (DLPFC) pathophysiological features accompany similar cognitive impairments. The aging data also demonstrate that poor performance is not necessarily accompanied by the DLPFC hypofunction that was seen in schizophrenia. Finally, these results more closely link the DLPFC functional abnormalities in schizophrenia to the pathophysiology of the disorder, rather than to poor performance per se.
Keywords: aging, schizophrenia, working memory, dorsolateral prefrontal cortex, neuroimaging
Common neurophysiological and behavioral features, such as dysfunction of the dorsolateral prefrontal cortex (DLPFC) and the parahippocampal region, along with poor working memory, are associated with both schizophrenia and normal aging. In schizophrenia, decades of research in a number of different disciplines, including neuroimaging, neuropsychological and neuropathological studies have demonstrated that the prefrontal cortex plays an important role. In functional neuroimaging studies of schizophrenia, one of the most well-replicated findings is prefrontal dysfunction, particularly during cognitive conditions involving the dorsolateral prefrontal cortex (DLPFC) (1–4). During working memory in schizophrenia, both decreased (2, 5–7) and increased (8, 9) DLPFC activation have been described, discrepancies that some have attributed to differences in performance and/or task difficulty (3,10). The mechanism and meaning of this DLPFC dysfunction has remained unclear, in part, because patients typically perform more poorly than healthy subjects, an observation that could potentially affect the imaging findings.
To circumvent this problem, a number of elegant event-related fMRI designs in which analyses are limited to correct trials only (2,3), as well as parametrically designed cognitive control paradigms that include control conditions in which patients perform as well than patients, have been performed (11–14). Yet, these research strategies studying patients whose working memory performance is at or near normal do not address the mechanism of cognitive failure inherent to the disease (4,8,12,14,15).
A different strategy, comparing patients with schizophrenia to other groups who also perform poorly, has the potential to clarify mechanisms of brain dysfunction by identifying common and unique pathophysiological features. Additionally, this approach can provide information about the role of the patients’ performance in the physiological findings, as well as about the role of the DLPFC in the cognitive impairment of schizophrenia. In particular, if DLPFC hypofunction is due to poor performance per se, we would expect to observe it in any poor performing group. Here we sought to compare patients with schizophrenia to healthy aging subjects since: (i) they share overlapping features of cognitive impairment on tasks linked to prefrontal cortex, and (ii) both groups show neurophysiological changes in the DLPFC and the hippocampal formation (16–21). The specificity of the neurofunctional findings in the two groups has not been adequately tested because previous findings have been based mainly on analyses of each index group compared separately to a control group, and because medications have often been a confounding factor.
The goal of the present study was to directly compare working-memory related brain activity in medication-free patients with schizophrenia and healthy aging subjects, matched on a pairwise basis for cognitive performance. This approach is of particular interest in the context of age- and schizophrenia-related alterations because it may provide a more complete understanding of the relationships between cerebral changes occurring in normal aging and in schizophrenia, while keeping constant performance levels and addressing the mechanism of cognitive failure.
Methods and materials
Participants
Fifty-three right-handed participants consisting of three groups of subjects provided written consent after complete description of the study in accordance with the NIH IRB and Radiation Safety Committee. Two index groups, a cohort of patients with DSM-IV–diagnosed schizophrenia (n=17) and a cohort of healthy older subjects (n=17) (Table 1), were compared to each other and to a young control group (n=19), matched in age with patients (t-test, P=0.21). Without reference to the PET data, index groups were chosen from larger cohorts to be closely matched in a pairwise manner for 2-back working memory performance (Fig. 1). Subjects were carefully screened with physical examination, history, and structural MRIs to rule out neurostructural abnormalities and risk factors for cerebrovascular diseases, with particular attention to those that might accompany the aging process. With the exception of the primary diagnosis in the schizophrenia group, participants were free of neurological, psychiatric, and medical illness and were taking no medications that affect rCBF. Patients with schizophrenia were withdrawn from all medication at least two weeks prior to the experiment, and all participants abstained from caffeine and nicotine for four hours prior to the scanning sessions. Since all patients with schizophrenia had been previously treated with neuroleptics, it cannot be excluded that long term effects of medications could affect our neuroimaging results. Participants were trained on the task and familiarized with scanning procedures prior to the study.
Table 1.
Demographic and Characteristics of all participants
| Subject Characteristics | Patients with Schizophrenia (n = 17) | Healthy Aging Subjects (n = 17) | Control Subjects (n = 19) |
|---|---|---|---|
| Age (Years) | 30.7 (22–43<comma> SD 6.3) | 67.5 (54–79<comma> SD 7.7) | 27.5 (20–36<comma> SD 5) |
| Men | 10 | 9 | 10 |
| % Correct<comma> 2-Back Working Memory Performance | 53% (SD 13) | 49% (SD 14) | 78% (SD 15) |
| % Correct<comma> 0-Back Working Memory Performance | 93% (SD 14) | 98.9% (SD 1.1) | 96.8% (SD 5.7) |
| Mean Duration of Illness | 9 yrs (SD 7) | ||
| PANSS Positive Score | 19 (SD 8.4) | ||
| PANSS Negative Score | 18.8 (SD 9.8) | ||
| Global PANSS Score | 18.9 (SD 11.6) | ||
| Total PANSS Score | 37.6 (SD 22.9) | ||
| 2-Back RT (Correct Trials) | 886 (SD 538) msec | 783.6 (SD 168) msec | |
| 0-Back RT (Correct Trials) | 860 (SD 494) msec | 632 (SD 123) msec | |
| Education Level | 12.9 (SD 1.2) yrs | 16.4 (SD 3.9) yrs | 18.3 (2.7) yrs |
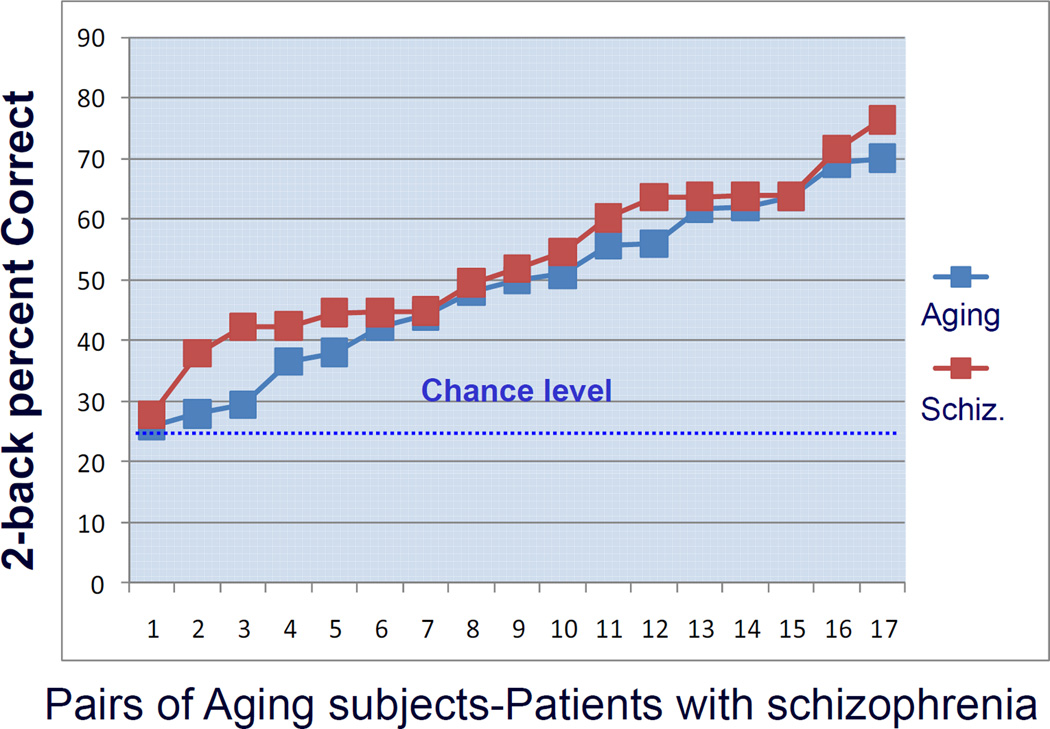
Figure 1. Behavioral results.
Pairwise match between patients and healthy aging subjects: 2-back performance accuracy. See text for statistical information.
Working-memory task
We used a version of the n-back working-memory task previously validated in our group as a consistent and robust activator of DLPFC and deactivator of the parahippocampal region in healthy subjects (Supp. Fig. 1). Briefly, subjects are presented with a diamond shape containing four circles, one in each corner. A single number appears at random in one of the four positions, with the same number (e.g. 2) always appearing at the same spatial location. Stimuli are presented for 1.5 s, and each trial lasts two seconds. Subjects press one of four buttons, using their right thumb, on a diamond shaped response button box held in their right hand. In the 0-back sensorimotor control condition, subjects press the button corresponding to the current number. In the 2-back (working memory) condition, subjects press the button corresponding to the number presented two trials before. Thus, the 2-back condition requires subjects simultaneously to encode the current number/position presented, to retrieve the number/position seen two trials back, and to press the corresponding button. This task encompasses processes involved in maintenance of previous information, monitoring, updating, retrieving, and temporally and dynamically linking the contents of working memory. Chance level performance on this version of the N-back task is 25%.
Data acquisition
Imaging data were acquired while subjects lay supine in a GE Advance, 3D PET camera (GE Medical Systems, Milwaukee, USA) using an IV bolus of 10 mCi of H215O for each of 14 regional cerebral blood flow (rCBF) scans. The 0-back and 2-back tasks were each performed during seven scans in alternating order. Head position was maintained using individually-fitted thermoplastic face masks. The scans occurred at 6-minute intervals and PET data were collected for 60 seconds during 90 seconds of stimulus presentation, allowing 45 trials.
Image data processing
SPM99 software (http://www.fil.ion.ucl.ac.uk/spm), which is well-suited to the analysis of PET data, was used for all aspects of image processing. Images were corrected for attenuation and reconstructed into 32 planes (resolution 6.5 mm FWHM), and background activity was subtracted. After registration, images were anatomically normalized to a study-specific PET template composed of the average of all subjects in the study to preclude potential confounds due to structural variation between the three groups. Data were smoothed with an isotropic Gaussian kernel filter of 10 mm3 (FWHM) and scan to scan variation in global counts was removed using proportional scaling to a global mean CBF of 50.
Statistical analysis
rCBF Activation during Working Memory (2–back versus 0-back)
To examine the main effect of task, rCBF during 2-back was compared to that during 0-back with a two-step random effects analysis across all control subjects. This random-effects analysis involved two steps: (1) a first-level voxel-wise comparison was calculated for each subject across all runs to produce one contrast image for each subject; (2) the contrast images from each individual were entered into a one-sample t-test performed across all subjects.
To identify differential pathophysiology in schizophrenia and normal aging, we next compared working memory-related brain activation between the two index groups as well as between each index group and the control group separately by using a one-way ANOVA with 3 groups using a significance threshold of P<0.001, uncorrected, and a cluster corrected threshold of P<0.05.
To identify pathophysiology that was common across schizophrenia and aging, we also performed a conjunction analysis to localize alterations in rCBF activation that were seen in both index groups relative to the control group. The formal conjunction analysis carried out was computed by first performing the comparison 2 back>0 back in patients with schizophrenia > controls (threshold of P<0.001) and then by masking the results inclusively with the results of the same contrast (2 back>0 back) in healthy aging subjects > controls.
Results
Behavior
Performance accuracy in the 2-back working memory task of older subjects and patients with schizophrenia was matched closely in a pair-wise manner (Fig. 1). The mean percent correct of the aging subjects was 49% (SD 14), the mean percent correct of the patients with schizophrenia was 53% (SD 13) and that of the control group was 78% (SD 15). Although both index groups performed better than the 25% chance level on the 2-back task, they performed significantly worse than healthy young controls (aging versus controls: F(1,34)=27.6, P<0.0001; schizophrenia versus controls: F(1,34)=35.4, P<0.00001). On the 0-back control condition, neither aging subjects (mean performance correct 98%, SD 1), nor patients with schizophrenia (mean performance correct 93%, SD 14) differed significantly from controls (mean performance correct 97%, SD 6), nor between each other (all P>0.1). Reaction times for correct 2-back trials did not differ between older subjects and patients with schizophrenia: mean RTs for aging subjects=783.6 ms (SD 169); mean RTs for patients=886.1 ms (SD 538) (P=0.51, unpaired-test with unequal variance of the 2 samples).
Activation analyses
Brain regions activated in controls
In healthy young controls, the 2-back versus 0-back comparison, revealed activity in a large bilateral prefronto-parietal network (DLPFC: x,y,z= 46,44,20; Z-score=6.2; x,y,z= −36,36,20, Z-score=4.8; Fronto-polar cortex: x,y,z= 36,56,8; Z-score=5.8; x,y,z= −32,56,12; Z-score=3.9; Intra-parietal region: x,y,z= 38,−52,40, Z-score=6.8; x,y,z= −42,−46,32, Z-score=6.75) that also included the thalami (x,y,z= 10,−18,−4, Z-score=8.3; x,y,z= −10,−22,−4, Z-score=3.4) and the cerebellar hemispheres (x,y,z= −38,−56,−44, Z-score=6.45; x,y,z= 42,−60,−36, Z-score=5.7) (Supp. Fig. 2, top). Conversely, relative deactivation during the 2-back task relative to the 0-back task was observed in a bilateral network that included extensive temporal (x,y,z= −52,2,−32, Z-score=6.6; x,y,z= 66,−8,−32, Z-score=6.3) and anterior medial frontal cortex areas (x,y,z=8,64,−8, Z-score=6.7) as well as parahippocampus (x,y,z=26,−8,−32, Z-score=8.1; x,y,z= −18,−12,−32, Z-score=8.4) and posterior cingulate cortex (x,y,z= 4,−48,24, Z-score=6.9), consistent with the default network (Supp. Fig. 2, bottom).
Interactions between groups and conditions
Patients with schizophrenia versus controls (2–back minus 0-back)
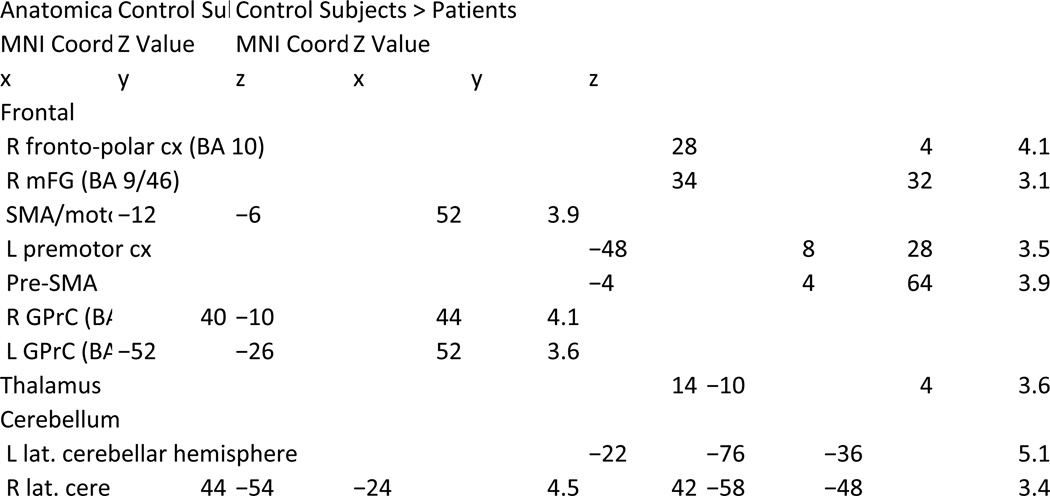
Compared to controls, patients with schizophrenia showed reduced activation in regions activated by the controls, including the right DLPFC and fronto-polar cortex, the left premotor cortex, the pre-SMA, the thalamus and cerebellum bilaterally (Fig. 2a, left, Table 3). In contrast, patients with schizophrenia showed higher activation than controls in regions deactivated by the controls, including the anterior medial prefrontal cortex, the posterior cingulate cortex and the left superior temporal gyrus (Fig. 2a, right; Fig. 3b; Table 2). Importantly, patients also deactivated the parahippocampal region less than controls.
Figure 2. Comparisons of index groups to controls.
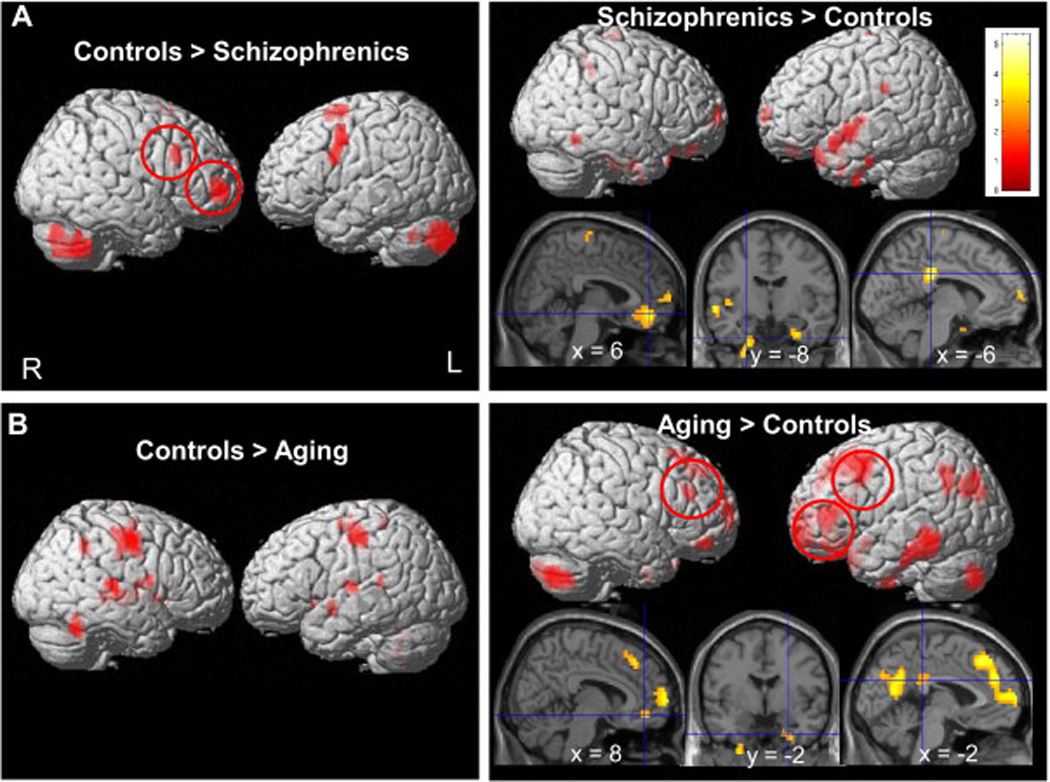
Brain regions significantly activated by 2-back relative to the 0-back conditions overlaid onto a 3D rendered brain for: (a) patients with schizophrenia<controls (top left), patients with schizophrenia>controls (top right); (b) healthy aging<controls (left) and healthy aging>controls (right). Patients with schizophrenia showed less activation in the right DLPFC than controls while healthy aging subjects overactivated the left DLPFC (normally less recruited than the right DLPFC in controls). Results are displayed at p<.005 for visualization.
Table 3.
Regions of Activation Observed in the 2 Back > 0 Back Contrast Comparing Control Subjects > Aging and Control Subjects > Patients
Figure 3.
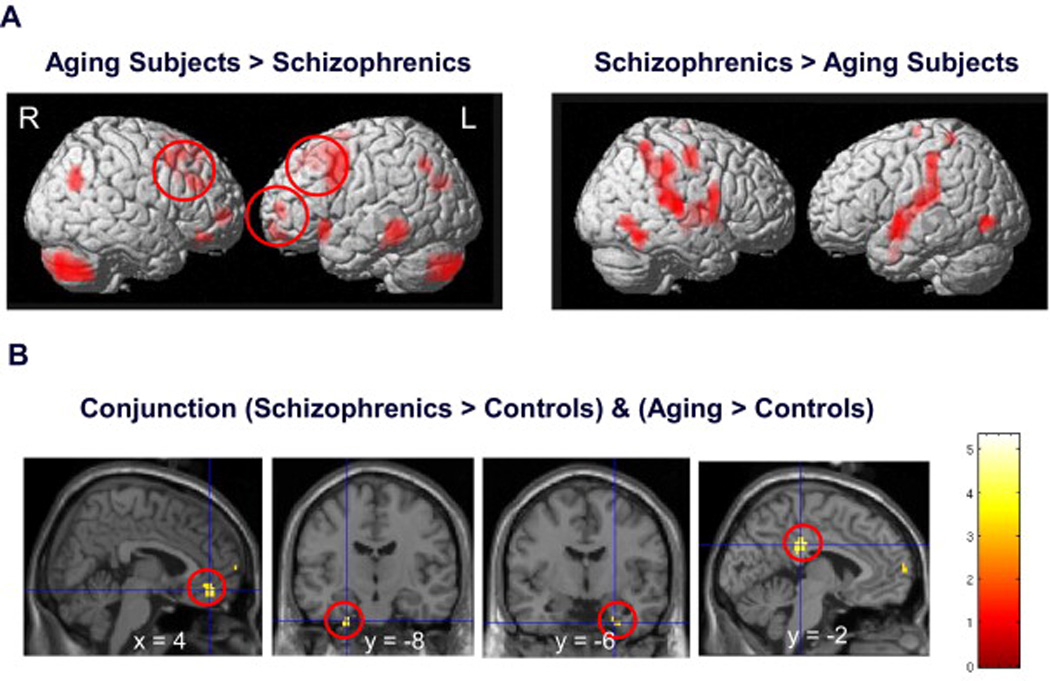
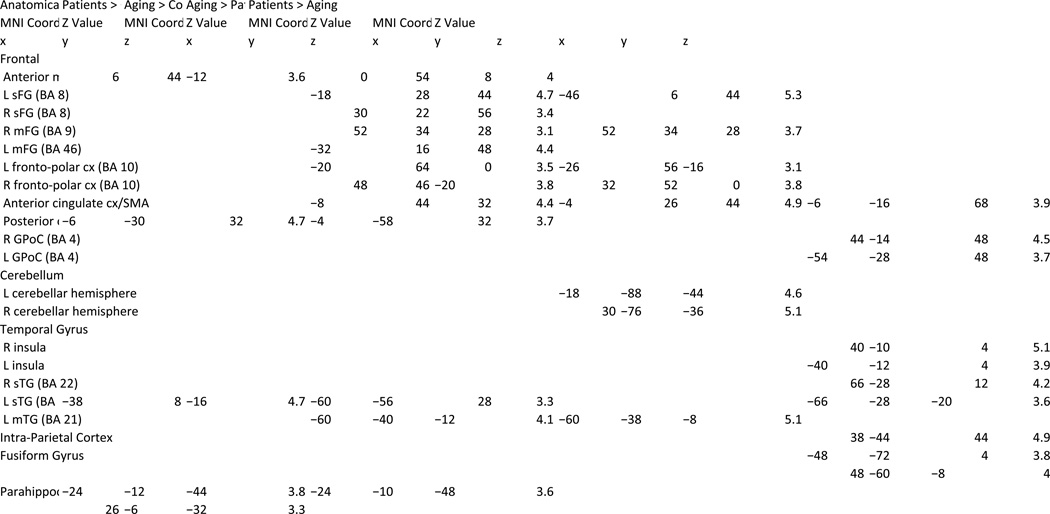
(a) Direct comparison of patients with schizophrenia and healthy aging subjects. Data for brain regions significantly activated by 2-back relative to the 0-back conditions overlaid onto a 3D rendered brain for aging subjects>patients with schizophrenia and patients with schizophrenia>aging. Healthy aging subjects overactivated the left lateral PFC compared to patients with schizophrenia, whereas patients with schizophrenia did not activate any PFC region more than aging subjects. (b) Common findings in aging and schizophrenia. Conjunction analysis of patients with schizophrenia and healthy aging subjects overlaid on saggital and coronal slices. Decreased deactivation of the anterior medial PFC, parahippocampal region and posterior cingulate cortex were observed in both patients with schizophrenia and healthy aging subjects.
Table 2.
Regions of Activation Observed in the 2-Back > 0-Back Contrast Compared Between Groups
Healthy aging subjects versus controls (2–back minus 0-back)
Compared to controls (and in contrast to the schizophrenia vs. control results), healthy aging subjects exhibited higher activity in the lateral PFC bilaterally, particularly robust in the left hemisphere, the bilateral superior frontal gyri, bilateral fronto-polar cortices, the anterior cingulate cortex, the left middle temporal gyrus, the left superior temporal cortex (Fig. 2b, right). Like the patients with schizophrenia, aging subjects, compared to controls, also showed less deactivation in the left parahippocampal region, the anterior medial prefrontal cortex and the posterior cingulate cortex. Moreover, older subjects showed less activation in the motor cortex bilaterally, the SMA/medial frontal gyrus and the cerebellum as compared to controls (Fig. 2b, left).
Healthy aging subjects versus patients with schizophrenia (2–back minus 0-back)
Direct comparison between the aging group and performance-matched patients with schizophrenia revealed that older subjects showed higher activation in the right DLPFC, left premotor cortex, and anterior cingulate cortex. More robust activation in aging was also found in the fronto-polar cortices bilaterally, right inferior frontal cortices, left inferior temporal gyrus and cerebellar hemispheres bilaterally (Fig. 3a, left).
In contrast, there were no prefrontal regions where activation for patients with schizophrenia exceeded that of aging subjects. Higher activity in patients was observed only in the SMA/motor part of the ACC, bilateral post-central gyri, posterior insula bilaterally, right intra-parietal region, inferior and superior temporal gyri and fusiform region (Fig. 3a, right).
Pathophysiology common to patients with schizophrenia and healthy aging subjects
The formal conjunction analysis revealed that, in comparison to the control group, the two index groups shared decreased deactivation of the anterior medial PFC (x,y,z=4, 42, −12, Z=4.2), the parahippocampal gyrus region bilaterally (x,y,z= −24, −12, −40, Z=4.1; x,y,z= 28, −4, −36, Z=3.8) and the posterior cingulate cortex (x,y,z= −4, −32, 32, Z=4.7) (Fig. 3b). Moreover, patients with schizophrenia and aging subjects shared hyperactivation in the left superior temporal cortex (x,y,z= −38, 10, −16, Z=4.8).
Discussion
The results of this study demonstrate that working memory impairments that are similar at the behavioral level in patients with schizophrenia and healthy aging subjects are reflected in both common and distinct neurophysiological features. Specifically, these two index groups share decreased deactivation of the parahippocampal gyrus region and the anterior medial PFC but differ in the observed patterns of prefrontal cortex dysfunction. Compared to young, healthy controls, patients with schizophrenia were hypofrontal (Fig. 2a, left), whereas performance-matched older subjects over-activated the lateral PFC, particularly in the left hemisphere (Fig. 2b). These differences in patterns of brain activation were further emphasized by direct comparison between the index groups (Fig. 3a), and, importantly, cannot be explained on the basis of differences in cognitive performance. These observations suggest an intrinsic distinction between the neurobiological substrates of the working memory impairment in schizophrenia and normal aging. They further support the notion that DLPFC hypoactivation in schizophrenia is not due to poor task performance per se, but, rather, reflects a fundamental neurophysiological characteristic of the disease as observed by PET. Our findings of both reduced DLPFC activation and reduced deactivation of the default mode network (including the anterior medial prefrontal cortex, posterior cingulate cortex and temporal region) in schizophrenia could reflect a relative inefficiency of resource allocation between functionally competitive large-scale neurocognitive systems (22–24).
Differential findings in patients with schizophrenia and healthy aging
Patients with schizophrenia showed underactivation of the right DLPFC and bilateral frontopolar cortices relative to both young controls and performance-matched older adults (Fig. 2a, left; Fig. 3a, right). This result showing PFC dysfunction in medication-free patients with schizophrenia extends numerous previous neuroimaging studies on working memory and executive functions in this patient group (2, 5–8, 12–14, 19). The failure to activate the frontopolar cortex in patients may reflect an impairment in retrieving information from working memory or inappropriate use of strategies involving switching processes since this brain region is frequently implicated in working memory (25), memory retrieval (26) and when combining working memory with task switching processes (27, 28). As noted previously, while the majority of neuroimaging studies of working memory in schizophrenia, particularly those using PET, have reported reduced DLPFC activity compared to healthy young controls (2,5,6,29), there are also a number of reports of hyperfrontality (8,9). Differences in the directionality of the findings (i.e., too much or too little prefrontal recruitment; 7) may be due to a number of discrepancies across studies, such as different clinical symptoms, stage of illness and medication status in the patients; interindividual differences in strategy or behavioral performance (30), methodological differences in imaging modality or task demands; capacity constraints of task load (8,11,12,15), and possibly the engagement of dysfunctional and compensatory macro-circuits (14). It cannot be excluded that use of different cognitive strategies might also influence the pattern of brain differences observed between patients and aging subjects. One hypothesis is that even when schizophrenic patients are able to keep up with processing demands, they do so less efficiently than controls, and this ‘working harder to keep up’ necessitates the recruitment of greater and/or less focused cortical activity (31). It has been proposed that there is an inverted U-shaped function between working memory load and DLPFC activation, such that increasing task demands are associated with increasing activation, which falls off after the subject’s working memory capacity is exceeded (12,14). This curve would be shifted to the left in schizophrenia, causing patients to show more activation than controls at low task demands, and to reach their maximum capacity earlier, consequently, showing less activation.
Supporting this view, intact or even relatively increased prefrontal cortical activation has been found in patients whose working memory performance is near normal (4,8,11,12,14,15). Although this approach has the advantage of ruling out confounds such as lack of effort or poor performance per se, such studies do not address the important question of the neurobiological mechanism by which patient cognitively fail. Instead, those results suggest that when patients are able to keep up with working memory processing demands, they tend to do so less efficiently by engaging greater cerebral metabolic activity or a less focused cortical response (4,8,9,12,14,15). In contrast our study examined two groups in whom working memory performance is impaired, and we matched schizophrenic patients with healthy aging subjects based on poor working memory performance in a pairwise fashion. Our analyses demonstrated hypoactivation of the DLPFC in the group with schizophrenia, and not in the aging group. Because all subjects in this study were unmedicated, the observed impairment in DLPFC function in schizophrenia cannot be attributed to concurrent neuroleptic treatment. Our findings not only demonstrate that DLPFC circuits are implicated in the pathophysiology of schizophrenia, they, also extend to the comparison with older healthy subjects previous findings comparing patients with schizophrenia and affective disorders, where the former were found to perform worse in executive function tasks and to have more DLPFC activation deficits (4,17,29,32).
In addition to the differential pattern of prefrontal cortex recruitment compared to aging subjects, patients with schizophrenia, as compared to controls, showed relatively greater activity in the SMA, bilateral insula, temporal cortices and in the intra-parietal region, complementing similar previous overactivation reported in a meta-analysis of executive functions in schizophrenia (7). These regions may be associated with a compensatory response and/or may be recruited for implementing alternate strategies to support task performance.
Conversely, and in contrast to patients with schizophrenia, when compared to young subjects, healthy aging subjects showed greater activity in the lateral PFC, particularly in the left hemisphere. This over-recruitment in healthy aging subjects is consistent with previous functional neuroimaging studies reporting that older adults activate other frontal regions more than younger adults during demanding cognitive control tasks, including verbal and spatial working memory tasks and verbal encoding and retrieval from episodic memory (21,33,34).
Commonalities between patients and healthy aging
Patients with schizophrenia and healthy aging subjects shared decreased deactivation of the anterior medial prefrontal cortex (mPFC), the parahippocampal gyrus region and the posterior cingulate cortex (Fig. 3b). These results are consistent with previous reports that the parahippocampal region, which comprises the entorhinal, perirhinal and parahippocampal cortices is sensitive to the effect of aging (35). This brain region reciprocally connects the hippocampus and the neocortex and is critical for normal learning and memory (36). The pattern of reduced deactivation observed in the parahippocampal region in patients and healthy aging subjects may be due to inappropriate use of long-term memory encoding during our working memory paradigm. Moreover, the similar pattern of reduced deactivation observed in the anterior mPFC and posterior cingulate cortex in the two index groups is consistent with previous reports showing that these brain regions are part of the ‘default mode network’, less deactivated in healthy aging than in young controls (37–39), and are also less deactivated in patients as compared to healthy young controls during a working memory paradigm (23,24,40). These functional changes in the parahippocampal region and the anterior mPFC shared by the two index groups may either reflect a compensatory process or a deficit in cognitive control with deficient resource allocation to the task at hand.
The reduced activation of the DLPFC and reduced deactivation of the parahippocampal gyrus we observed in patients with schizophrenia may reflect the disturbed functional connectivity between the prefrontal cortex and the medial-temporal lobe region during working memory performance (23). Such inappropriate interaction between the DLPFC and medial-temporal lobe is also present in heathy aging subjects performing working memory paradigms (41,42).
Another common physiological feature that could act in parallel to the observed distributed cerebral changes in schizophrenia and aging compared to young controls is related to the dopaminergic system, which undergoes age-related degeneration (43) and is also known to be dysregulated in schizophrenia (44). Dopamine is not only critical for modulating PFC and working memory function but also for regulating episodic memory (45), dependent upon the parahippocampal formation. A recent multimodal neuroimaging study combining fMRI and measures of midbrain dopamine synthesis with FDOPA PET reported an age-related change in the direction of the relationship (from a positive to a negative correlation) between midbrain dopamine synthesis and prefrontal activity, suggesting an age-dependent dopaminergic tuning mechanism for prefrontal reward processing (46). Together, these findings support the idea that dysfunctional dopaminergic modulation in aging and schizophrenia may impact the working memory system which depends on the functional interplay between prefrontal and parahippocampal regions.
Conclusion
Our results clarify mechanisms of brain dysfunction by identifying common and distinct pathophysiological features in schizophrenia and healthy aging. Patients with schizophrenia and healthy aging subjects matched for performance were studied using the exact same experimental methods and research design. Our results demonstrate that, in these two very different populations, the same impairments at the behavioral level are reflected in both common and differential neurophysiological features. The most prominent commonality was that these two groups both failed to suppress activation of the parahippocampal gyrus and anterior medial PFC during working memory. The main difference between the two similarly performing groups was found in the dorsolateral prefrontal cortex: patients with schizophrenia hypoactivated the right dorsolateral prefrontal cortex, while normally aging individuals overactivated this region, thereby differentiating the neural mechanisms underlying the working memory deficits that characterize these two groups of subjects. These findings demonstrate that poor working memory performance per se in schizophrenia is not the cause, but rather the result of the patients’ dysfunctional prefrontal cortices. Our results also offer new insights into the various mechanisms by which cognitive failure in the working memory domain can arise.
Supplementary Material
Acknowledgments
We thank Rosanna Olsen for help recruiting and testing patients.
This work was supported by the NIMH Intramural Research Program.
Footnotes
Financial Disclosures. All authors have no financial disclosures to report and no conflicts of interest.
References
- 1.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35(1):258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62(10):1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- 3.Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108(1–3):143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- 6.Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, Skudlarski P, Goldman-Rakic PS, Krystal JH. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64(12):1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 9.Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 10.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 12.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 13.Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(Suppl 1):i171–i181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 14.Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163(11):1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey NF, Koning HA, Welles P, Cahn W, van der Linden JA, Kahn RS. Excessive recruitment of neural systems subserving logical reasoning in schizophrenia. Brain. 2002;125(Pt 8):1793–1807. doi: 10.1093/brain/awf188. [DOI] [PubMed] [Google Scholar]
- 16.Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Duzel E. Ageing and early-stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130(Pt 9):2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, Johnstone EC, Lawrie SM. Prefrontal function and activation in bipolar disorder and schizophrenia. Am J Psychiatry. 2008;165(3):378–384. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- 18.Persson J, Nyberg L. Altered brain activity in healthy seniors: what does it mean? Prog Brain Res. 2006;157:45–56. doi: 10.1016/s0079-6123(06)57004-9. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher P. The missing link: a failure of fronto-hippocampal integration in schizophrenia. Nat Neurosci. 1998;1(4):266–267. doi: 10.1038/1078. [DOI] [PubMed] [Google Scholar]
- 20.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia [see comments] Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 21.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62(4):379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 24.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38(8):1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 25.Nyberg L, Forkstam C, Petersson KM, Cabeza R, Ingvar M. Brain imaging of human memory systems: between-systems similarities and within-system differences. Brain Res Cogn Brain Res. 2002;13(2):281–292. doi: 10.1016/s0926-6410(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23(9):3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreher JC, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3(9):e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbalat G, Chambon V, Franck N, Koechlin E, Farrer C. Organization of cognitive control within the lateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 2009;66(4):377–386. doi: 10.1001/archgenpsychiatry.2009.10. [DOI] [PubMed] [Google Scholar]
- 29.Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53(5):376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 30.Glabus MF, Horwitz B, Holt JL, Kohn PD, Gerton BK, Callicott JH, Meyer-Lindenberg A, Berman KF. Interindividual differences in functional interactions among prefrontal, parietal and parahippocampal regions during working memory. Cereb Cortex. 2003;13(12):1352–1361. doi: 10.1093/cercor/bhg082. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 32.Berman KF, Doran AR, Pickar D, Weinberger DR. Is the mechanism of prefrontal hypofunction in depression the same as in schizophrenia? Regional cerebral blood flow during cognitive activation. Br J Psychiatry. 1993;162:183–192. doi: 10.1192/bjp.162.2.183. [DOI] [PubMed] [Google Scholar]
- 33.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 34.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122(Pt 5):963–979. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- 36.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 37.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19(6):1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 41.Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14(4):364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 42.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A. 2008;105(39):15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.