Abstract
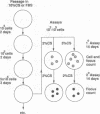
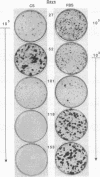
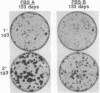
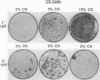
Human cancers undergo protracted complex development from benign to malignant states, as most thoroughly documented in the mole-to-melanoma sequence. The early stages of the sequence tend to redifferentiate into normal tissues; the later stages progress to ever increasing multiplication and malignancy. When placed under the growth constraint of either crowding or low serum concentrations, the NIH 3T3 line of mouse cells readily undergoes transformation, expressed in the development of foci of cells that continue to multiply at confluence when the rest of the population has stopped. If the nontransformed cells are maintained for 3 months by frequent low-density passages in high concentrations of calf serum, they gradually lose the capacity to undergo transformation when the constraints are applied. The same conditions of passage have been used to reverse the transformation, both processes resembling in principle the reversal of the early stages of the mole-to-melanoma sequence. When the frequent low-density passages are made in high concentrations of fetal bovine serum, which supports a slightly lower growth rate than an equal concentration of calf serum, the degree of transformation is gradually increased, so that the foci become more numerous, broader, and thicker, reaching a maximum in successive assays at about 3 months of passaging. A diversity of focal morphologies is sporadically generated in the calf serum passage by exposing the cells to various concentrations of calf serum for 14 days of growth and confluency before assaying them. The dependence of the number, density, and morphology of foci on the environment in which the cells had been grown before assay reinforces the evidence that the transformation is an epigenetic process. The fact that these effects develop in culture gradually over an extended period of time suggests parallels to the characteristically long-term early regression and later progression, as well as the diversity of the mole-to-melanoma sequence, and may also be representative of other cancers.
Full text
PDF
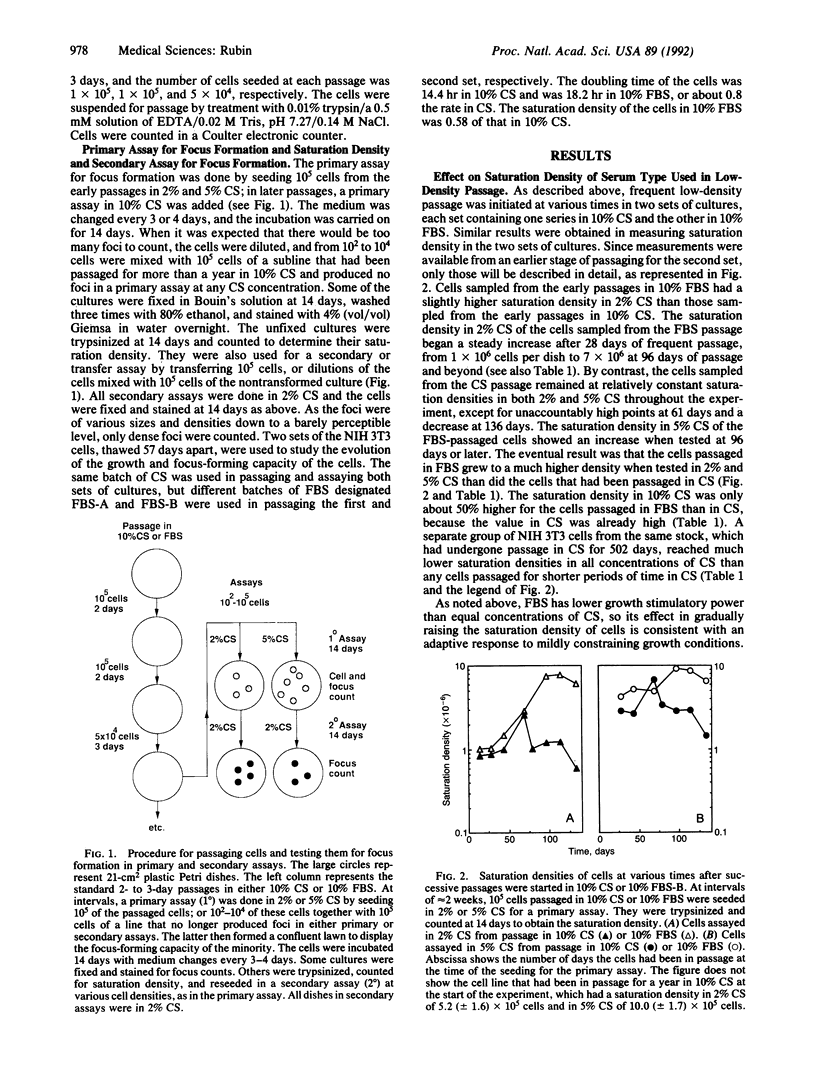
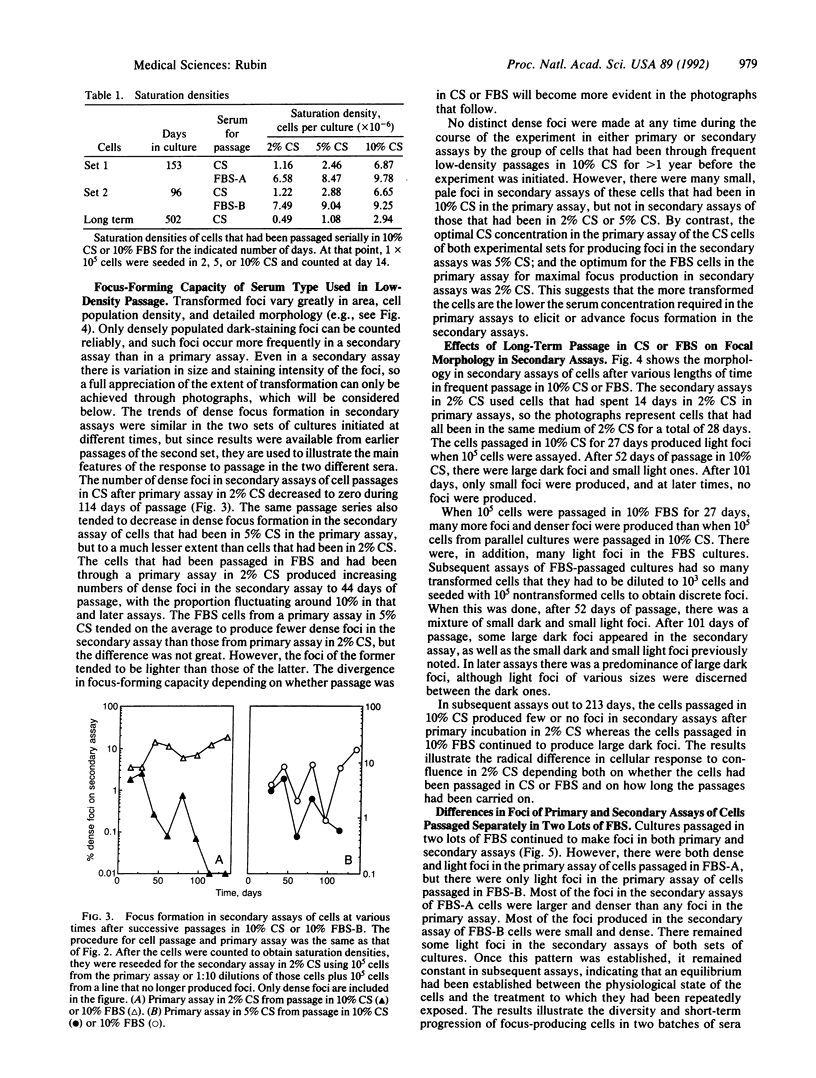
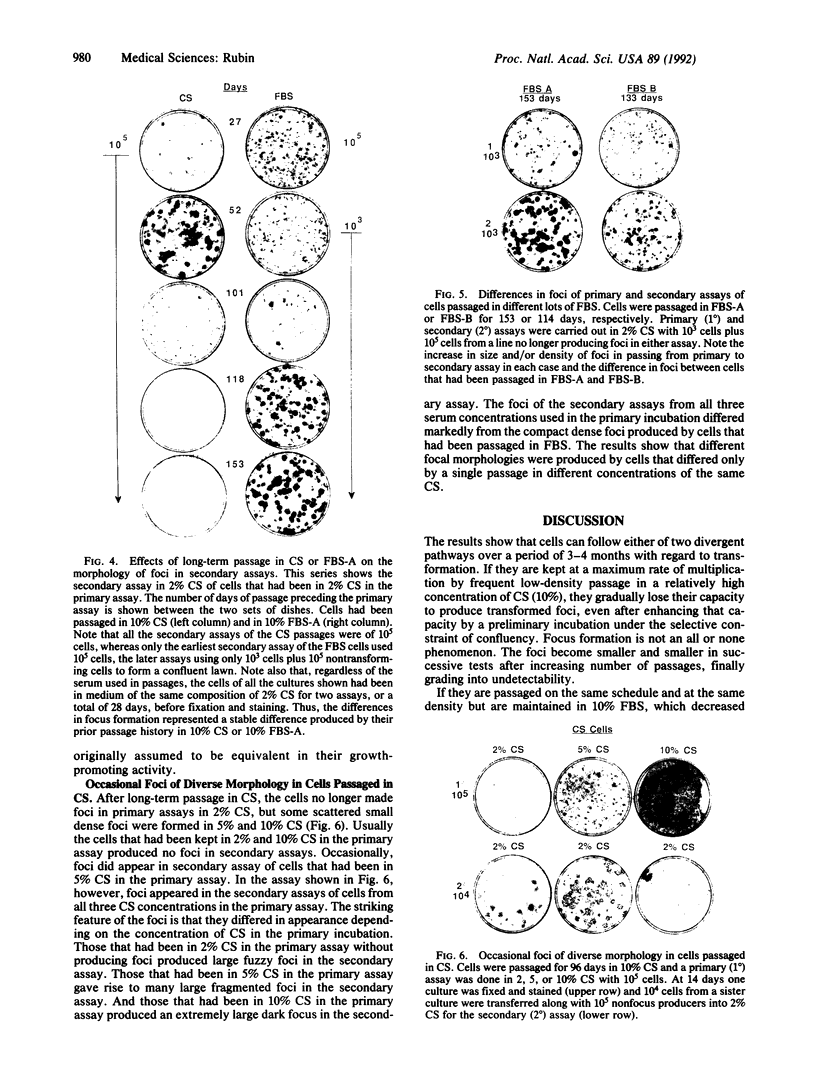

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. H. Tumour progression and the nature of cancer. Br J Cancer. 1991 Oct;64(4):631–644. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Clonal adaptation during carcinogenesis. Biochem Pharmacol. 1990 Jun 15;39(12):1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Farber E., Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991 Jun 1;51(11):2751–2761. [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M., Travis G. L., Ray F. A., Cram L. S. Spontaneous neoplastic evolution of Chinese hamster cells in culture: multistep progression of phenotype. Cancer Res. 1983 Oct;43(10):4822–4827. [PubMed] [Google Scholar]
- Rubin A. L., Arnstein P., Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A. L., Yao A., Rubin H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):482–486. doi: 10.1073/pnas.87.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. The significance of biological heterogeneity. Cancer Metastasis Rev. 1990 Jul;9(1):1–20. doi: 10.1007/BF00047585. [DOI] [PubMed] [Google Scholar]
- Rubin H. Uniqueness of each spontaneous transformant from a clone of BALB/c 3T3 cells. Cancer Res. 1988 May 1;48(9):2512–2518. [PubMed] [Google Scholar]
- Rubin H., Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]