Abstract
Purpose
Planning studies to compare x-ray and proton techniques and to select the most suitable technique for each patient have been hampered by the nonequivalence of several aspects of treatment planning and delivery. A fair comparison should compare similarly advanced delivery techniques from current clinical practice and also assess the robustness of each technique. The present study therefore compared volumetric modulated arc therapy (VMAT) and single-field optimization (SFO) spot scanning proton therapy plans created using a simultaneous integrated boost (SIB) for dose escalation in midesophageal cancer and analyzed the effect of setup and range uncertainties on these plans.
Methods and Materials
For 21 patients, SIB plans with a physical dose prescription of 2 Gy or 2.5 Gy/fraction in 25 fractions to planning target volume (PTV)50Gy or PTV62.5Gy (primary tumor with 0.5 cm margins) were created and evaluated for robustness to random setup errors and proton range errors. Dose–volume metrics were compared for the optimal and uncertainty plans, with P<.05 (Wilcoxon) considered significant.
Results
SFO reduced the mean lung dose by 51.4% (range 35.1%-76.1%) and the mean heart dose by 40.9% (range 15.0%-57.4%) compared with VMAT. Proton plan robustness to a 3.5% range error was acceptable. For all patients, the clinical target volume D98 was 95.0% to 100.4% of the prescribed dose and gross tumor volume (GTV) D98 was 98.8% to 101%. Setup error robustness was patient anatomy dependent, and the potential minimum dose per fraction was always lower with SFO than with VMAT. The clinical target volume D98 was lower by 0.6% to 7.8% of the prescribed dose, and the GTV D98 was lower by 0.3% to 2.2% of the prescribed GTV dose.
Conclusions
The SFO plans achieved significant sparing of normal tissue compared with the VMAT plans for midesophageal cancer. The target dose coverage in the SIB proton plans was less robust to random setup errors and might be unacceptable for certain patients. Robust optimization to ensure adequate target coverage of SIB proton plans might be beneficial.
Summary.
Comparing spot-scanning proton therapy single-field optimization plans with volumetric modulated arc therapy plans indicated that single-field optimization can achieve significant sparing of normal tissue for midesophageal cancer compared with volumetric modulated arc therapy. However, the boost volume dose coverage in the simultaneous integrated boost proton plans appears less robust to setup errors. Robust optimization to ensure adequate target coverage of simultaneous integrated boost proton plans might be beneficial.
Introduction
Radiation therapy (RT) is an important component in the management of esophageal cancer, for both preoperative and definitive treatment, although the 5-year survival rates in the United Kingdom have been only 12% (1). A meta-analysis of preoperative chemoradiation therapy suggested a radiation dose–response relationship for improved pathologic remission (2) and has provoked interest in RT dose escalation to improve outcomes 3, 4, 5.
Advanced RT techniques such as intensity modulated RT (IMRT) 3, 4 and volumetric modulated arc therapy (VMAT) (6) offer opportunities for increasing the dose to the tumor, although normal tissue sparing for some patients might be limited by the anatomy (5). Proton therapy is thought to improve sparing of normal tissues. Preliminary clinical outcomes with passive scattering proton therapy (PSPT) suggest that 50.4 Gy (relative biological effectiveness (RBE)) can be safely delivered with concurrent chemotherapy 7, 8. Also, the results of a planning study have suggested that using 2 or 3 proton beams with single-field optimization (SFO) spot scanning proton therapy for esophageal cancer could improve lung and heart sparing compared with photon RT (9).
Nonetheless, planning studies to compare x-ray and proton techniques and to select the most suitable technique for each patient have been hampered by the nonequivalence of several aspects of treatment planning and delivery. A fair comparison should compare similarly advanced delivery techniques from current clinical practice and should also assess the robustness of each technique. Therefore, we chose to compare SFO proton plans and VMAT plans 10, 11. SFO plans using pencil beam scanning have been shown to generate a more robust target dose distribution for brain and spine, prostate, and head and neck tumors than multifield-optimized intensity modulated proton therapy plans, when the dose distributions are obtained without robust optimization 12, 13.
The principal factor affecting plan robustness is setup error due to interfraction variations in patient position. Daily cone beam computed tomography (CT) image guidance is routinely used for x-ray RT, and although on-line volumetric image guidance for proton therapy is not yet feasible in clinical practice, daily beam's eye view images would allow patient positioning with bony anatomy or fiducial markers as a reference (14). Additional uncertainty is present for proton beams, arising from the uncertainty in CT number and conversion to proton stopping power, which can modify the proton range in the patient (15).
The present study therefore examined the robustness of the VMAT and SFO dose distributions for a range of patients with midesophageal cancer, assessing the effect of setup error and additional proton range uncertainty on the dose–volume metrics for the target and normal tissues.
Methods and Materials
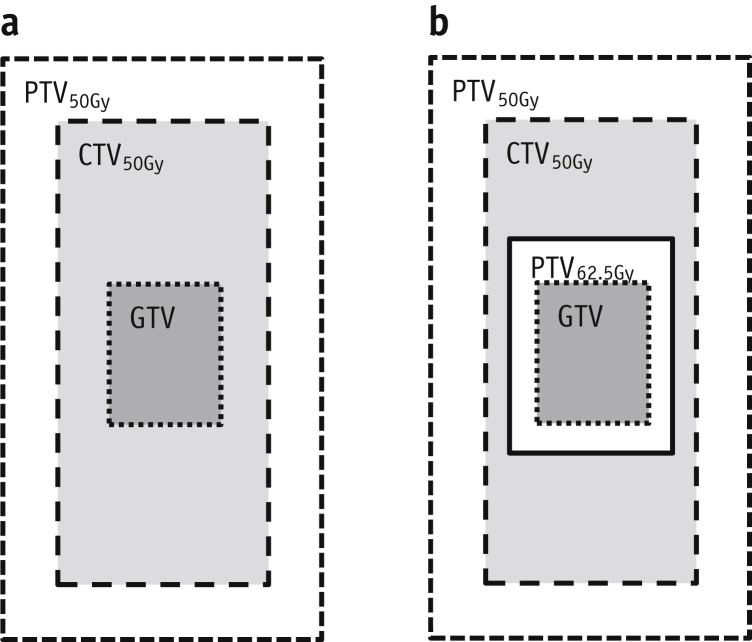
A subset of 21 patients with midthoracic esophageal cancer was selected from the SCOPE1 clinical trial (ISRCTN 47718479) database (in which the mean planning target volume (PTV) for the entire cohort was 334 cm3). The 21 patients selected as representative had a PTV range of 140 to 591 cm3 and a mean PTV of 327 cm3. The SCOPE protocol standard margins (16) were reapplied to the trial-derived gross tumor volumes (GTVs). The GTV was increased manually 2 cm superoinferiorly along the esophageal axis, with an additional 1 cm radial margin to create the clinical target volume (CTV50Gy). An additional 1 cm margin was applied to create the planning target volume (PTV50Gy), which was prescribed a dose of 2 Gy/fraction for 25 fractions. The boost volume CTV62.5Gy was considered identical to the GTV, with PTV62.5Gy created by adding an isotropic 0.5 cm margin. This was prescribed a simultaneous integrated boost dose of 2.5 Gy/fraction (Fig. 1). A 0.5 cm margin is the minimum value calculated from random and systematic errors recorded when portal imaging of bony anatomy is used for image guidance in esophageal cancer (17). This margin allows for dose falloff from 62.5 to 50 Gy and minimizes the volume of tissue irradiated to >50 Gy. Visual assessment of normal tissue contours was performed, with data imported into Eclipse, version 13 (Varian, Palo Alto, CA). Two treatment plans were then created for each patient: a RapidArc (VMAT) plan using two 360° arcs of 6 MV and a spot scanning proton therapy plan (SFO) of 70 to 250 MeV using the 3-field beam arrangement described by Welsh et al (9) with single-field optimization (Fig. 2, top row). Both plans were created in physical dose (Gy; ie, proton RBE fixed at a constant value of 1.1), and the beams were optimized using all the constraints listed in Table 1, to achieve the correct target coverage where possible. For proton plans, a proximal and distal range margin (range 0.3-0.5 cm) was added to the PTV50Gy. A PTV62.5Gy median dose (D50%) was used for plan normalization in the standard plans and showed no statistically significant difference between the VMAT and SFO plans. Plan robustness was evaluated for both VMAT and SFO techniques, using the plan uncertainty tool provided in Eclipse, version 13. This tool simulates setup and range errors and recalculates a new dose distribution for each perturbed plan. Setup errors of x (left to right) ±0.5 cm, y (craniocaudal) ±0.7 cm, and z (anteroposterior) ±0.5 cm were used to generate 6 perturbed plans, representing the maximum expected interfraction setup error for VMAT and SFO. These simulations encompassed the random setup error observed in online image guidance protocols for esophageal cancer (17). For the proton plans, the effect of a range error of ±3.5% was simulated separately (18). Dose–volume metrics for the total dose (62.5 Gy in 25 fractions) for the nominal VMAT and SFO plans were compared across all patients using the Wilcoxon signed rank test in SPSS, version 20.0.0 (IBM Corp, Armonk, NY), with P<.05 considered significant. For each patient, the robustness of the VMAT and SFO plans to the setup errors and the SFO plans to range error were compared as the dose per fraction.
Fig. 1.
Target volumes and dose prescription for (a) standard and (b) simultaneous integrated boost plans. Abbreviations: CTV = clinical target volume; GTV = gross tumor volume; PTV = planning target volume.
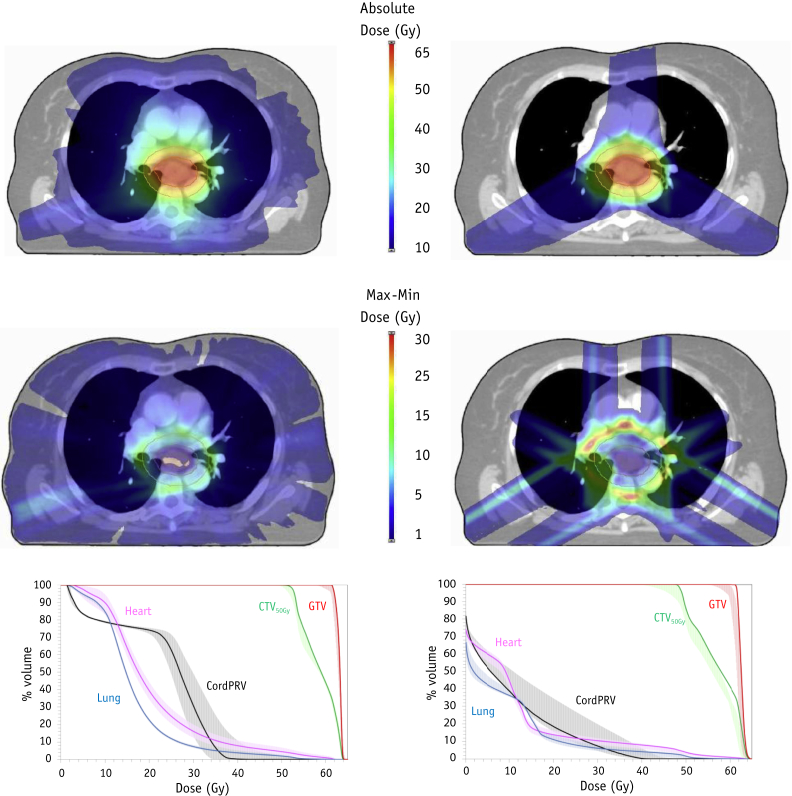
Fig. 2.
(Top) Example of dose distribution for 1 patient for volumetric arc therapy (VMAT) (Left) and single-field optimization (SFO) (Right) plans. (Middle) The maximum (Max) to minimum (Min) dose difference for the uncertainty plans for VMAT (Left) and SFO (Right). (Bottom) Dose–volume histogram showing the dose to the target volumes and organs-at-risk for VMAT (Left) and SFO (Right). Shaded regions indicate the envelope of maximum and minimum dose from the uncertainty plans.
Table 1.
Dose–volume constraints used for analysis of treatment plans for midesophageal cancer
| Dose–volume metric data |
|---|
| PTV50Gy |
| V95% (47.5 Gy) > 95% |
| PTV62.5Gy |
| V95% (59.4 Gy) > 95% |
| Dmax (0.1 cm3) <107% (66.9 Gy) |
| Lung |
| Mean dose <20 Gy |
| V20Gy <25% |
| Heart |
| Mean dose <25 Gy |
| V30Gy <45% |
| CordPRV (5 mm margin) |
| Dmax (0.1 cm3) <40 Gy (45 Gy permitted) |
Abbreviations: CordPRV = spinal cord planning risk volume; Dmax = maximum dose; PTV = planning target volume.
Results
Nominal plans
The VMAT plans met the dose constraints for 16 of 21 patients. The mean heart dose was exceeded for 3 patients (patients 7, 11, and 17), and the lung V20Gy limit was not met for patients 16 and 21. For patient 21 (with the largest PTV of 590.8 cm3), it was challenging to achieve the desired PTV50Gy V95% because of the overlap with lung tissue, although coverage of the CTV50Gy was adequate for this patient, a D98% of 49.1 Gy (Table E1; available online at www.redjournal.org). Except for this patient, the PTV50Gy V95% was 95.8% to 99.9%. For the nominal VMAT plans across all 21 patients, the mean heart dose was 21.2 Gy (median; range 14.4-29.8), mean lung dose was 13.6 Gy (range 8.4-18.1), lung V20 was 15.6% (range 5.8%-29.7%), and maximum cord dose (0.1 cm3) was 33.3 Gy (range 21.5-36.5). The dose–volume metrics for each patient are provided in Table E1 (available online at www.redjournal.org).
The nominal SFO plans were able to achieve all dose constraints for 20 of the 21 patients (Table E1; available online at www.redjournal.org). The mean heart dose was just exceeded for patient 11 (25.3 Gy), for whom significant overlap of the PTV contour with the heart was present. The target dose coverage was acceptable for all patients, with a D98% of CTV50Gy >95% of the prescribed dose (PD), a median CTV50Gy D98% of 48.9 Gy (range 48.3-49.9), and median GTV D98% of 62.6 Gy (range 62.4-62.9). The proton plans showed a reduced dose to the normal tissue, with a mean median lung value of 6.3 Gy (range 2.5-11.4), lung V20 of 6.6% (range 2.5%-17.1%), and maximum cord dose of 23.7 Gy (range 0.1-39.3; Table E1; available online at www.redjournal.org). The higher cord dose of patient 16 allowed greater lung sparing than the VMAT plan. Across all patients, the SFO mean lung dose was only 50.7% (Fig. 3) of dose delivered using photons (median; range 23.9%-64.9%), and this result was statistically significant (Z = −4.01; P<.001). The SFO mean heart dose was 12.7 Gy (median; range 8.2-25.3), only 59.8% (range 49.1%-84.9%) of the VMAT value (Z = 4.01; P<.001), and the low dose to the heart contour was also reduced, with a significant reduction in the heart V5 for all patients.
Fig. 3.
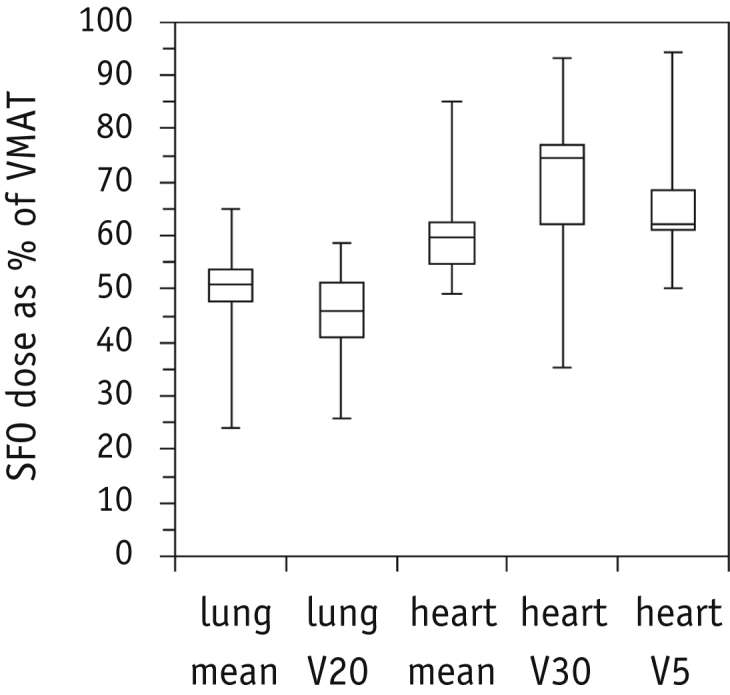
Percentage of dose–volume metric of proton plans compared with photon plans for organs-at-risk. Median and interquartile range are shown in the box plot, with the maximum and minimum values shown as error bars limits. Abbreviations: SFO = single-field optimization; VMAT = volumetric arc therapy.
VMAT and SFO plan robustness
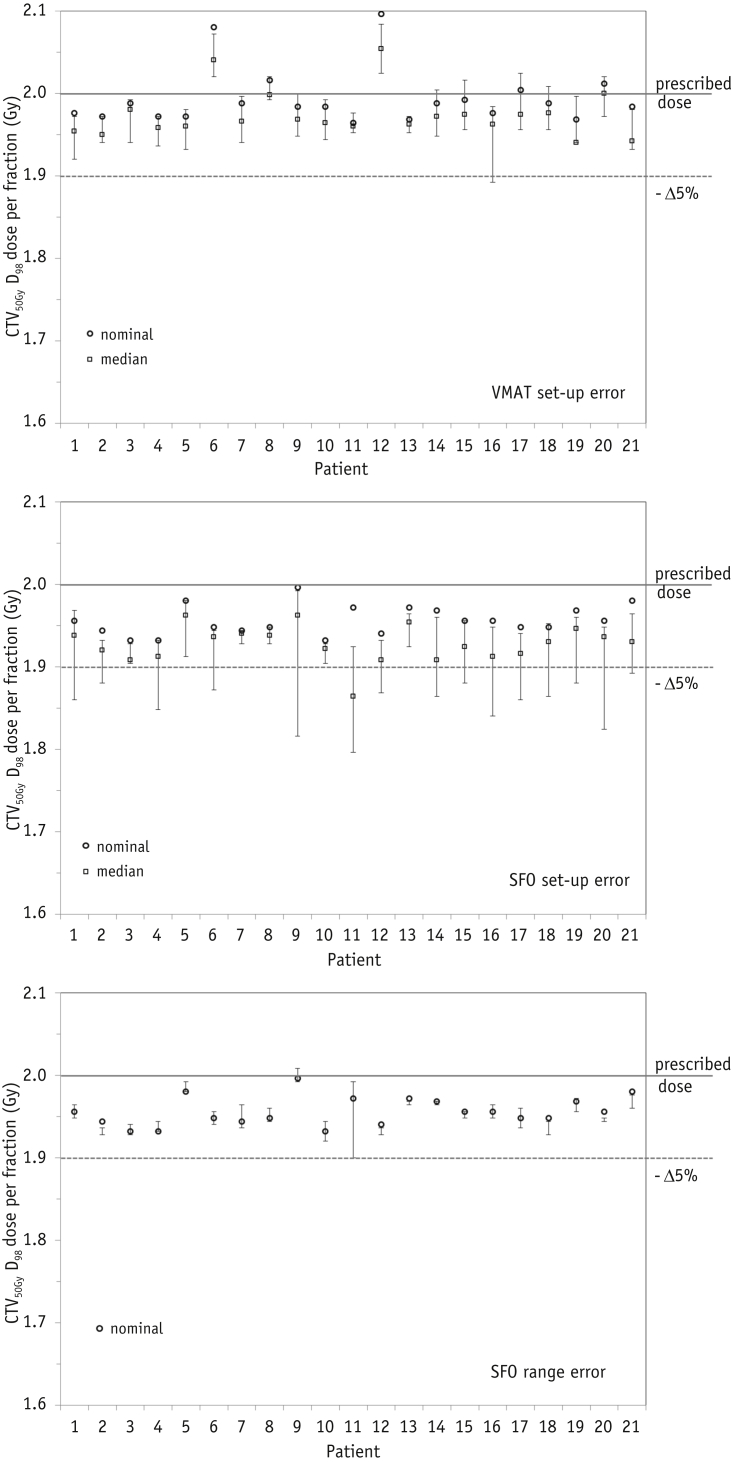
The CTV50Gy coverage in the presence of setup errors was somewhat reduced (Fig. 4), although for VMAT plans, only 1 patient (patient 16) showed possible dose-per-fraction deterioration of >5% of the PD. This patient had a large overlap (2.5%) of lung tissue in the PTV. For SFO, instances of PD deterioration >5% were observed in 15 of 21 patients. These were most commonly related to displacements 5 mm to the left (9 patients) or 7 mm superiorly (9 patients). Although the absolute minimum SFO dose was always lower (by 0.6%-7.8% of the PD) for each patient than with VMAT. The median perturbed SFO dose was only >5% deterioration level for patient 11, because the proximity of the target and heart generated sharp dose gradients. Proton plan range robustness was acceptable: for all patients, the CTV D98 was 95.0% to 100.4% of the PD. The lowest range perturbed dose coverage was again for patient 11.
Fig. 4.
Clinical target volume (CTV) D98 dose per fraction for each patient for the nominal plans (circles), with perturbed plans' median dose (squares) and maximum/minimum values (error bars). (Top) Volumetric arc therapy (VMAT) setup error. (Middle) Single-field optimization (SFO) setup error. (Bottom) SFO range error. The prescribed dose and 5% loss in dose per fraction are indicated by horizontal lines.
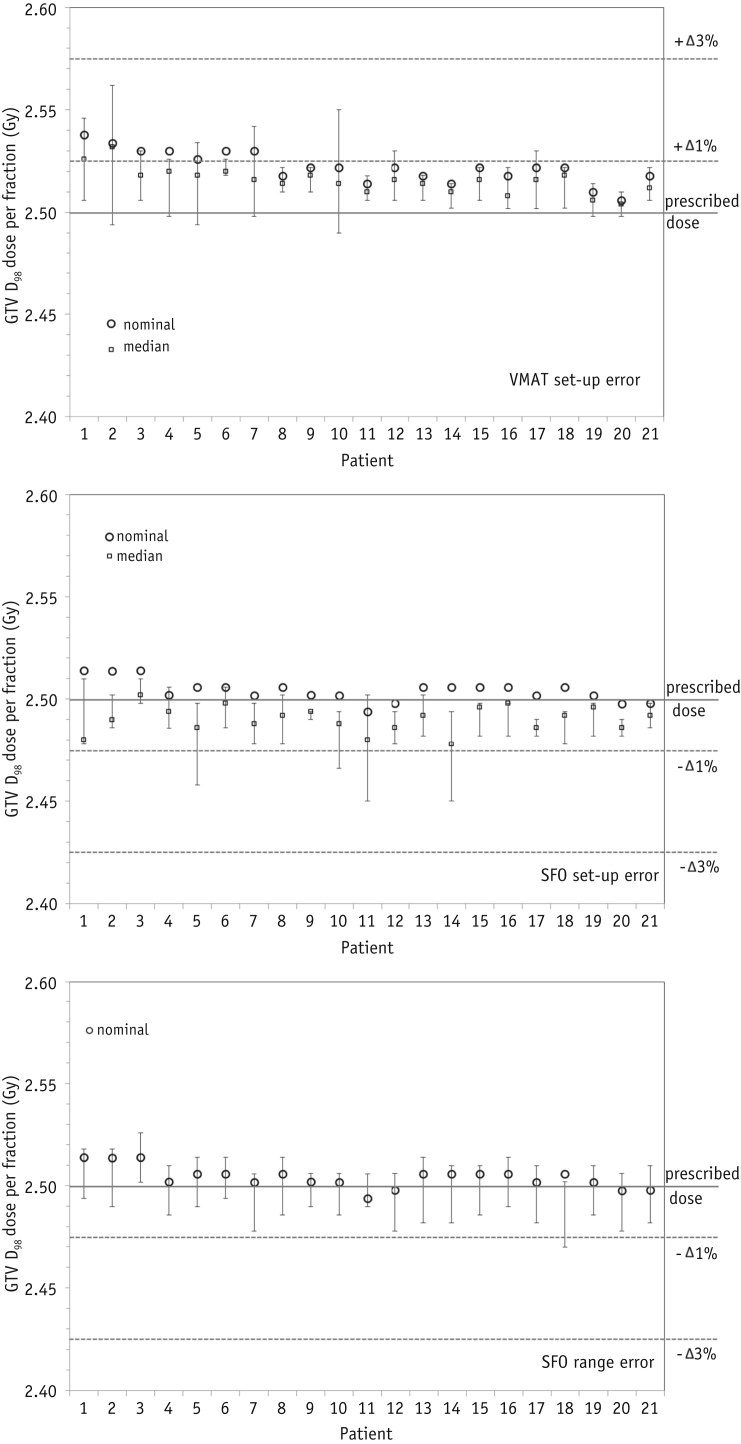
GTV D98 plan robustness is illustrated in Figure 5. The VMAT and SFO plans were normalized such that the PTV62.5Gy median dose equaled the PD, although the VMAT plan GTV dose was slightly greater than the SFO dose. Setup error in the VMAT plans gave a minimum GTV D98 value of 99.6% to 102.5% the PD, where the median dose reduction from the nominal plan was 0.6% of the PD (range 0.3%-1.6%). The minimum doses were observed for 5-mm displacement right or posteriorly. The setup error in the SFO plans led to a dose of 0.4% to 2% of the PD. The largest dose deteriorations occurred in patients 5 and 14, with 5 mm lateral displacements. Patient 11 (with the sharp dose gradients around the target to reduce the cardiac dose) again had potentially lower minimum GTV D98 coverage than that of the other patients. The minimum GTV D98 values with the SFO setup errors were lower than the VMAT values by 0.3% to 2.2% of the prescribed GTV dose.
Fig. 5.
Gross target volume (GTV) D98 dose per fraction for each patient for the nominal plans (circles), with perturbed plans' median dose (squares) and maximum/minimum values (error bars). (Top) Volumetric arc therapy (VMAT) setup error. (Middle) Single-field optimization (SFO) setup error. (Bottom) SFO range error. The prescribed dose and 1% and 3% change in dose per fraction are indicated by horizontal lines.
The range errors in the SFO plans resulted in smaller GTV dose deterioration. The values were within 1% of the PD for all but 1 patient, with a median deterioration per fraction of 0.02 Gy (0.8% of the PD).
The reduction in dose to the GTV can be translated to a reduction in the predicted tumor control probability (TCP) using the parameters from Geh et al (2). For the nominal VMAT plans, the TCP was a median of 55.6% (range 54.4%-56.8%); for the SFO technique, the median TCP was 55.1% (range 54.8%-55.3%). When the setup error was included, the TCP for the VMAT plans decreased by 0.3% to 1.5% (median 0.8%), and for perturbed SFO plans, the TCP was decreased by a median of 1.8% (range 1.1%-2.9%), depending on the patient.
For all VMAT plans, even when setup errors were considered, the maximum dose (0.1 cm3) to the spinal cord was always less than the required 1.8 Gy/fraction (equivalent to 45 Gy over the entire treatment course), and also always less than the recommended 1.6 Gy/fraction (40 Gy over the treatment course; Figure E1; available online at www.redjournal.org). For the SFO plans, the spinal cord dose was less than the 1.6-Gy dose per fraction limit for all nominal plans and for all but 1 patient for all the perturbed plans. However, patient 16, with a nominal plan spinal cord dose of 1.57 Gy, showed a possible spinal cord dose maximum of 1.8 Gy when the setup error was 7 mm anterior. If this setup error were reproduced at every fraction across the entire treatment course, the resultant dose would be 45.1 Gy.
The doses to lung and heart delivered with setup uncertainties were virtually unchanged for all patients (ie, the same number of patients undergoing VMAT would still not meet the mean heart dose constraint [patients 7, 11, and 17] or lung V20Gy limit [patients 16 and 21]). The lung and heart dose–volume parameters also showed no clinically significant change for the SFO plans with setup error uncertainties.
Discussion
Our comparison of RT techniques for midesophageal cancer indicated that SFO offers improved cardiopulmonary sparing and equivalent target coverage compared with VMAT. Previous studies have indicated that arc therapy and fixed-field IMRT produce esophageal cancer treatment plans of similar quality 19, 20. The SFO plans significantly reduce the dose to normal tissues, with a median 50% decrease in the mean dose to lung tissue, leading to an expected reduced risk of radiation pneumonitis in these patients. These findings suggest that SFO plans would be favorable for all patients, in particular, for those patients in whom dose escalation with photons is not possible using the current dose constraints. The mean dose to the whole heart was also reduced, with an absolute reduction per patient of 1.9 to 18.7 Gy. Data from a retrospective analysis of breast cancer survivors suggested a 7% reduction in the risk of ischemic heart disease for every 1-Gy decrease in the mean heart dose (21). Also, a significant reduction occurred with SFO in the heart V5, which was identified as a dosimetric risk factor in the recent Radiation Therapy Oncology Group 0617 trial (22). However, the volume of heart receiving a greater dose (range V40Gy-V60Gy) was similar with both techniques, indicating that in cases in which patient anatomy causes close proximity or overlap of organs-at-risk (OARs) with the target volumes, proton therapy will not spare these regions any better than will advanced photon therapy. A recent study of 3-dimensional conformal RT for esophageal cancer suggested that ventricular segments encompassed by the 45-Gy isodose were associated with an increased risk of myocardial ischemia (23). This underlines the need for a correlation of cardiac toxicity with the dose–volume parameters for heart substructures. Knowledge of the safe dose thresholds for cardiac structures will allow for better optimization and comparison of RT plans in the future.
Plan robustness was compared by simulating random setup errors, with a systematic 3.5% range error for the proton plans. Under these conditions, CTV50Gy and GTV D98 coverage was acceptable for VMAT and for SFO when a 3.5% range error was taken into account. Random setup error in SFO plans causes a reduction in the dose to the CTV and GTV, for which the magnitude of the dose deterioration is highly dependent on patient anatomy and the direction of the displacement. The errors we simulated were unlikely to be reproduced at every fraction during the treatment course and can be considered as overly pessimistic; some findings have indicated that dose differences are washed out if the treatment course is sufficiently fractionated (24). However, for some patients, strategies to ensure sufficient robustness of the target dose with proton plans would be necessary. This could include optimizing the beam angles for OAR sparing and target robustness for each patient or the use of field-specific margins (25). Large systematic setup errors or range errors >3.5% could reduce the apparent robustness of the SFO planning approach we used, and alternative strategies would be required. Robustness can also be included in the optimization of spot-scanning proton beams (26), although for several of the patients in the present study, the close proximity of OARs and target volumes meant that robust optimization of the dose distribution would likely be challenging. A clinical decision regarding any modification of the target volume margins or OAR sparing would then be required on a patient-by-patient basis.
Patient intrafraction motion owing to respiration could also affect the comparison of the delivered and planned dose distribution for both techniques. Respiration modifies the tumor position and the water-equivalent thickness (WET) of the beam path during the breathing cycle. Because no 4-dimensional (4D)-CT images were available for the patients in the SCOPE database, we measured the WET on a 4D-CT scan of a typical patient with esophageal cancer from our clinic, which showed WET changes of <8 mm along the beam axis crossing the diaphragmatic region. Analysis of the WET variation to select the proton beam angle (using a 4D-CT data set) has been suggested by Chang et al (27) to produce robust SFO plans for thoracic tumors. A planning study using 4D-CT data from lung cancer patients and comparing passive scattering proton therapy (PSPT) and IMRT showed that the dosimetric differences for PSPT were no worse those than for IMRT (28). A similarly rigorous comparison for spot scanning would require detailed knowledge of the time-dependent spot delivery and the interplay with the patient's respiratory cycle. In this context, rescanning methods can conserve target coverage for lung tumors, even for tumor motion of ≤12 mm (29). More significant interfractional dosimetric changes might occur with changes in the baseline respiration amplitude or with changes in gastrointestinal organ filling, which would require careful image-guided verification of the patient anatomy and adaptive replanning during the treatment course.
Inaccuracies in the dose calculation algorithm (in particular, in the presence of heterogeneities) could alter the predicted dose distribution 30, 31, 32. For our study, the maximum heart dose or dose fall-off around the PTV might have been underestimated; however, the GTV coverage and mean lung dose should have been sufficiently accurate. Greater accuracy for dose calculations, in particular for thoracic tumor sites, can be achieved using Monte Carlo and should be introduced for routine treatment planning for both photons and protons.
We compared the physical dose distributions; however, the RBE varies with tissue type and along the path of the proton beam. The net effect might be an underestimation of the RBE (and biological dose), which will be small in the proximal region but can be large in the distal end of the beam, and could significantly underestimate the hot spots in the normal tissues surrounding the tumor (33). For our plan comparison, although the biological lung dose might have been slightly greater than predicted in the proton plans, the advantage in lung sparing was still very clear. In contrast, the dose to heart tissue close to the target could have been underestimated in the proton plans.
Conclusions
SFO plans achieve significant sparing of lung tissue for all patients with midesophageal cancer compared with VMAT. Further understanding of the mechanism relating the dose to heart toxicity is required, although tumors located adjacent to the heart will always pose a planning challenge, irrespective of the RT modality. The proton plan dose coverage of CTV50Gy and GTV was less robust to random setup errors than were the photon plans, although this was highly patient dependent and can be offset by the potential for OAR sparing. More advanced optimization strategies to ensure adequate target coverage of simultaneous integrated boost proton plans might be required to compensate for large systematic setup errors or range errors >3.5%.
Footnotes
Conflict of interest: S.W. and M.P. are supported by Cancer Research UK (CRUK) (Grant C5255/A15935). S.W. received a travel grant from CRUK (Grant C50792/A18034). M.H. received a Medical Research Council Fellowship (Grant MC_PC_12001/2). The remaining authors have no conflicts of interest.
Supplementary material for this article can be found at www.redjournal.org.
Supplementary Data
References
- 1.Quaresma M., Coleman M.P., Rachet B. 40-Year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: A population-based study. Lancet. 2015;385:1206–1218. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 2.Geh J.I., Bond S.J., Bentzen S.M. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: Evidence of a radiation and chemotherapy dose response. Radiother Oncol. 2006;78:236–244. doi: 10.1016/j.radonc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Roeder F., Nicolay N., Nguyen T. Intensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancer. Radiat Oncol. 2014;9:191. doi: 10.1186/1748-717X-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh J., Palmer M.B., Ajani J.A. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys. 2012;82:468–474. doi: 10.1016/j.ijrobp.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren S., Partridge M., Carrington R. Radiobiological determination of dose escalation and normal tissue toxicity in definitive chemoradiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2014;90:423–429. doi: 10.1016/j.ijrobp.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins M.A., Bedford J.L., Warrington A.P. Volumetric modulated arc therapy planning for distal oesophageal malignancies. Br J Radiol. 2012;85:44–52. doi: 10.1259/bjr/25428720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echeverria A.E., McCurdy M., Castillo R. Proton therapy radiation pneumonitis local dose-response in esophagus cancer patients. Radiother Oncol. 2013;106:124–129. doi: 10.1016/j.radonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin S.H., Komaki R., Liao Z. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:e345–e351. doi: 10.1016/j.ijrobp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh J., Gomez D., Palmer M.B. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: A dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81:1336–1342. doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luhr A., Lock S., Roth K. Concept for individualized patient allocation: ReCompare—Remote comparison of particle and photon treatment plans. Radiat Oncol. 2014;9:59. doi: 10.1186/1748-717X-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roelofs E., Engelsman M., Rasch C. Results of a multicentric in silico clinical trial (ROCOCO): Comparing radiotherapy with photons and protons for non-small cell lung cancer. J Thorac Oncol. 2012;7:165–176. doi: 10.1097/JTO.0b013e31823529fc. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X.R., Poenisch F., Li H. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol. 2014;9:202. doi: 10.1186/1748-717X-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan E.M., Liu W., Wu R. Preliminary evaluation of multifield and single-field optimization for the treatment planning of spot-scanning proton therapy of head and neck cancer. Med Phys. 2013;40:081709. doi: 10.1118/1.4813900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S., Zenklusen S., Knopf A.C. Current status and future prospects of multi-dimensional image-guided particle therapy. Radiol Phys Technol. 2013;6:249–272. doi: 10.1007/s12194-013-0199-0. [DOI] [PubMed] [Google Scholar]
- 15.Park P.C., Cheung J.P., Zhu X.R. Statistical assessment of proton treatment plans under setup and range uncertainties. Int J Radiat Oncol Biol Phys. 2013;86:1007–1013. doi: 10.1016/j.ijrobp.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurt C.N., Nixon L.S., Griffiths G.O. SCOPE1: A randomised phase II/III multicentre clinical trial of definitive chemoradiation, with or without cetuximab, in carcinoma of the oesophagus. BMC Cancer. 2011;11:466. doi: 10.1186/1471-2407-11-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins M.A., Aitken A., Hansen V.N. Set-up errors in radiotherapy for oesophageal cancers—Is electronic portal imaging or conebeam more accurate? Radiother Oncol. 2011;98:249–254. doi: 10.1016/j.radonc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Moyers M.F., Miller D.W., Bush D.A. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 19.Van Benthuysen L., Hales L., Podgorsak M.B. Volumetric modulated arc therapy vs. IMRT for the treatment of distal esophageal cancer. Med Dosim. 2011;36:404–409. doi: 10.1016/j.meddos.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Lin C.Y., Huang W.Y., Jen Y.M. Dosimetric and efficiency comparison of high-dose radiotherapy for esophageal cancer: Volumetric modulated arc therapy versus fixed-field intensity-modulated radiotherapy. Dis Esophagus. 2014;27:585–590. doi: 10.1111/dote.12144. [DOI] [PubMed] [Google Scholar]
- 21.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 22.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gayed I.W., Liu H.H., Yusuf S.W. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47:1756–1762. [PubMed] [Google Scholar]
- 24.Lowe M., Albertini F., Aitkenhead A. Incorporating the effect of fractionation in the robustness analysis of proton plans to setup errors. Proceedings to the 54 Annual Meeting for the Particle Therapy Cooperative Group (PTCOG) and the 2 Annual Meeting of PTCOG – North America, Summer 2015. Int J Particle Ther. 2015;2:55–365. [Google Scholar]
- 25.Pflugfelder D., Wilkens J.J., Oelfke U. Worst case optimization: A method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2008;53:1689–1700. doi: 10.1088/0031-9155/53/6/013. [DOI] [PubMed] [Google Scholar]
- 26.Stuschke M., Kaiser A., Pottgen C. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiother Oncol. 2012;104:45–51. doi: 10.1016/j.radonc.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Chang J.Y., Li H., Zhu X.R. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90:809–818. doi: 10.1016/j.ijrobp.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matney J., Park P.C., Bluett J. Effects of respiratory motion on passively scattered proton therapy versus intensity modulated photon therapy for stage III lung cancer: Are proton plans more sensitive to breathing motion? Int J Radiat Oncol Biol Phys. 2013;87:576–582. doi: 10.1016/j.ijrobp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Huth I., Wegner M. Clinical evaluation of Varian probeam rescanning performance for lung tumour treatments. Proceedings to the 54 Annual Meeting for the Particle Therapy Cooperative Group (PTCOG) and the 2 Annual Meeting of PTCOG. Int J Particle Ther. 2015;2:55–365. [Google Scholar]
- 30.Van Esch A., Tillikainen L., Pyykkonen J. Testing of the analytical anisotropic algorithm for photon dose calculation. Med Phys. 2006;33:4130–4148. doi: 10.1118/1.2358333. [DOI] [PubMed] [Google Scholar]
- 31.Kroon P.S., Hol S., Essers M. Dosimetric accuracy and clinical quality of Acuros XB and AAA dose calculation algorithm for stereotactic and conventional lung volumetric modulated arc therapy plans. Radiat Oncol. 2013;8:149. doi: 10.1186/1748-717X-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassberger C., Daartz J., Dowdell S. Quantification of proton dose calculation accuracy in the lung. Int J Radiat Oncol Biol Phys. 2014;89:424–430. doi: 10.1016/j.ijrobp.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wedenberg M., Toma-Dasu I. Disregarding RBE variation in treatment plan comparison may lead to bias in favor of proton plans. Med Phys. 2014;41:091706. doi: 10.1118/1.4892930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.