Abstract
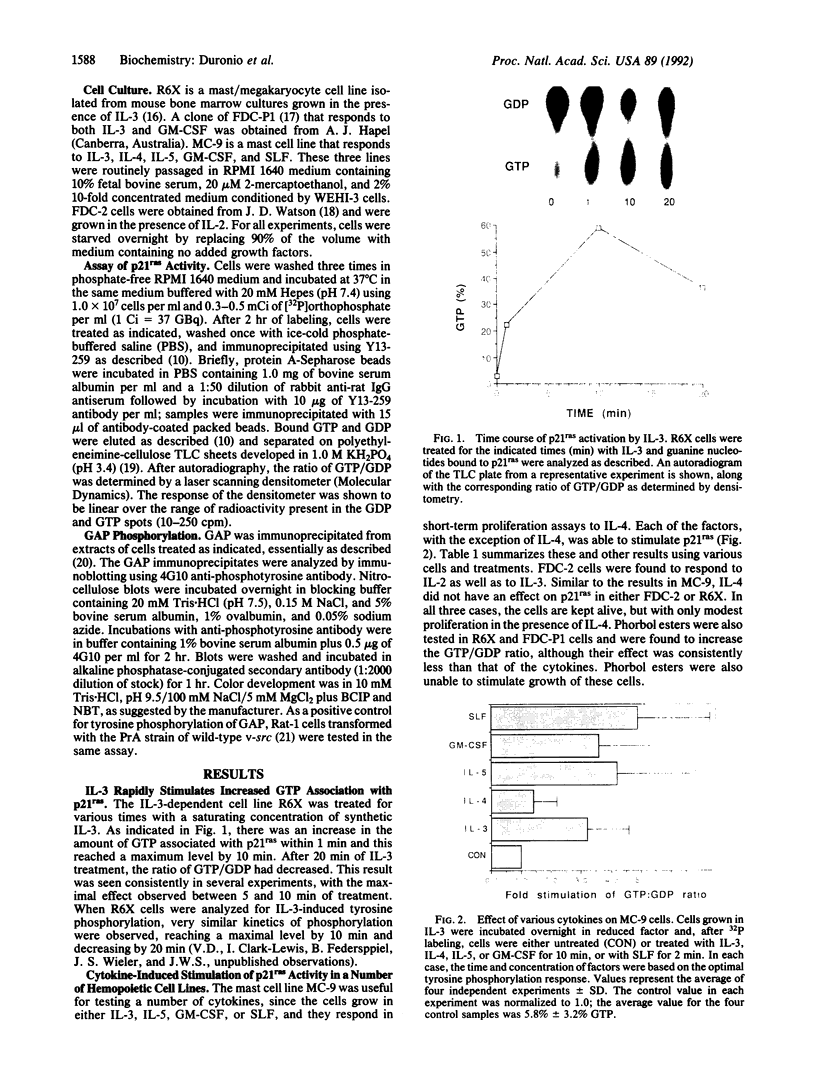
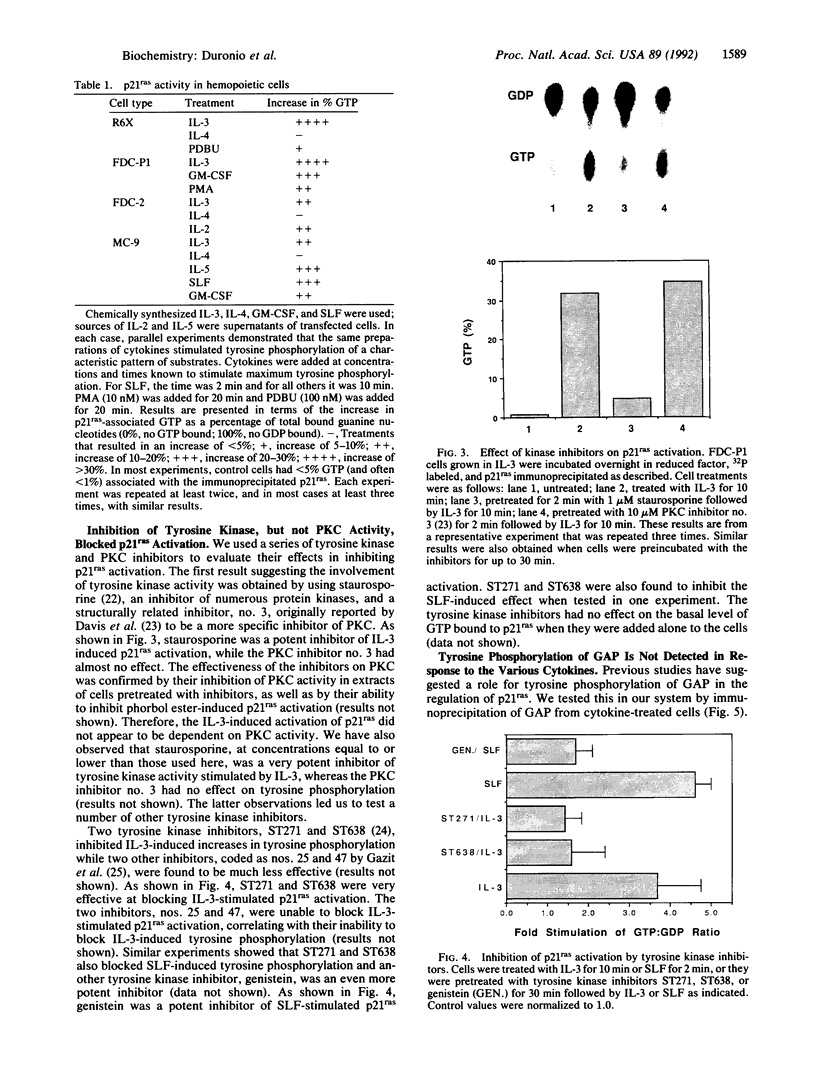
Products of the ras gene family, termed p21ras, are GTP-binding proteins that have been implicated in signal transduction via receptors encoding tyrosine kinase domains. Recent findings have defined a superfamily of hemopoietin receptors that includes receptors for a number of interleukins and colony-stimulating factors. The intracellular portions of these receptors show only restricted homologies, have no tyrosine kinase domain, and provide no clues to the mode of signal transduction. However, in most cases the factors stimulate tyrosine phosphorylation. We demonstrate here that ligand-induced activation of the interleukin (IL)-2, IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor receptors resulted in activation of p21ras in various hemopoietic cell lines. The only cytokine tested that binds to a hemopoietin receptor and that did not activate p21ras was IL-4. Activation of p21ras was also observed in response to Steel factor, which stimulates the endogenous tyrosine kinase activity of the c-kit receptor, as well as with phorbol esters, which activate protein kinase C. Experiments with protein kinase inhibitors implicated tyrosine kinase activity, but not protein kinase C activity, as the upstream signal in p21ras activation via these growth factor receptors. Attempts to demonstrate tyrosine phosphorylation of the p21ras GTPase-activating protein (GAP) were negative, suggesting that phosphorylation of GAP may not be the major mechanism for regulation of p21ras activity by tyrosine kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Lyman S. D., Baird A., Wignall J. M., Eisenman J., Rauch C., March C. J., Boswell H. S., Gimpel S. D., Cosman D. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990 Oct 5;63(1):235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Aebersold R., Ziltener H., Schrader J. W., Hood L. E., Kent S. B. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986 Jan 10;231(4734):134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Kent S. B., Schrader J. W. Purification to apparent homogeneity of a factor stimulating the growth of multiple lineages of hemopoietic cells. J Biol Chem. 1984 Jun 25;259(12):7488–7494. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Riehl R., Wu L., Weinberg R. A. Identification of a nucleotide exchange-promoting activity for p21ras. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5998–6002. doi: 10.1073/pnas.87.15.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. L., Marshall M. S., Gibbs J. B., Stacey D. W., Feig L. A. Preferential inhibition of the oncogenic form of RasH by mutations in the GAP binding/"effector" domain. Cell. 1991 Feb 8;64(3):625–633. doi: 10.1016/0092-8674(91)90246-u. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Garcia Garcia G., Evans G., Michiel D., Linnekin D. Cytokine regulation of protein phosphorylation. Cytokine. 1990 Mar;2(2):77–91. doi: 10.1016/1043-4666(90)90001-a. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S., Scolnick E. M., Dixon R. A., Vogel U. S. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP). J Biol Chem. 1990 Nov 25;265(33):20437–20442. [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Le Gros G. S., Gillis S., Watson J. D. Induction of IL 2 responsiveness in a murine IL 3-dependent cell line. J Immunol. 1985 Dec;135(6):4009–4014. [PubMed] [Google Scholar]
- Martin G. A., Viskochil D., Bollag G., McCabe P. C., Crosier W. J., Haubruck H., Conroy L., Clark R., O'Connell P., Cawthon R. M. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990 Nov 16;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Moran M. F., Polakis P., McCormick F., Pawson T., Ellis C. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol Cell Biol. 1991 Apr;11(4):1804–1812. doi: 10.1128/mcb.11.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottapel R., Reedijk M., Williams D. E., Lyman S. D., Anderson D. M., Pawson T., Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Mol Cell Biol. 1991 Jun;11(6):3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Nakamura S., Kaziro Y. Platelet-derived growth factor stimulates formation of active p21ras.GTP complex in Swiss mouse 3T3 cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5993–5997. doi: 10.1073/pnas.87.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Ziltener H. J., Leslie K. B. Structural homologies among the hemopoietins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2458–2462. doi: 10.1073/pnas.83.8.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi T., Owada M. K., Tatsuka M., Yamashita T., Watanabe K., Kakunaga T. Specific inhibitors of tyrosine-specific protein kinases: properties of 4-hydroxycinnamamide derivatives in vitro. Cancer Res. 1989 May 1;49(9):2374–2378. [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Welham M. J., Wyke J. A. A single point mutation has pleiotropic effects on pp60v-src function. J Virol. 1988 Jun;62(6):1898–1906. doi: 10.1128/jvi.62.6.1898-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. F., Lin B., Tanaka K., Dunn D., Wood D., Gesteland R., White R., Weiss R., Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990 Nov 16;63(4):835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]








