ABSTRACT
Polysaccharide utilization loci (PUL) within the genomes of resident human gut Bacteroidetes are central to the metabolism of the otherwise indigestible complex carbohydrates known as “dietary fiber.” However, functional characterization of PUL lags significantly behind sequencing efforts, which limits physiological understanding of the human-bacterial symbiosis. In particular, the molecular basis of complex polysaccharide recognition, an essential prerequisite to hydrolysis by cell surface glycosidases and subsequent metabolism, is generally poorly understood. Here, we present the biochemical, structural, and reverse genetic characterization of two unique cell surface glycan-binding proteins (SGBPs) encoded by a xyloglucan utilization locus (XyGUL) from Bacteroides ovatus, which are integral to growth on this key dietary vegetable polysaccharide. Biochemical analysis reveals that these outer membrane-anchored proteins are in fact exquisitely specific for the highly branched xyloglucan (XyG) polysaccharide. The crystal structure of SGBP-A, a SusD homolog, with a bound XyG tetradecasaccharide reveals an extended carbohydrate-binding platform that primarily relies on recognition of the β-glucan backbone. The unique, tetra-modular structure of SGBP-B is comprised of tandem Ig-like folds, with XyG binding mediated at the distal C-terminal domain. Despite displaying similar affinities for XyG, reverse-genetic analysis reveals that SGBP-B is only required for the efficient capture of smaller oligosaccharides, whereas the presence of SGBP-A is more critical than its carbohydrate-binding ability for growth on XyG. Together, these data demonstrate that SGBP-A and SGBP-B play complementary, specialized roles in carbohydrate capture by B. ovatus and elaborate a model of how vegetable xyloglucans are accessed by the Bacteroidetes.
IMPORTANCE
The Bacteroidetes are dominant bacteria in the human gut that are responsible for the digestion of the complex polysaccharides that constitute “dietary fiber.” Although this symbiotic relationship has been appreciated for decades, little is currently known about how Bacteroidetes seek out and bind plant cell wall polysaccharides as a necessary first step in their metabolism. Here, we provide the first biochemical, crystallographic, and genetic insight into how two surface glycan-binding proteins from the complex Bacteroides ovatus xyloglucan utilization locus (XyGUL) enable recognition and uptake of this ubiquitous vegetable polysaccharide. Our combined analysis illuminates new fundamental aspects of complex polysaccharide recognition, cleavage, and import at the Bacteroidetes cell surface that may facilitate the development of prebiotics to target this phylum of gut bacteria.
INTRODUCTION
The human gut microbiota influences the course of human development and health, playing key roles in immune stimulation (1), intestinal cell proliferation (2), and metabolic balance (3, 4). This microbial community is largely bacterial, with the Bacteroidetes, Firmicutes, and Actinobacteria comprising the dominant phyla (5, 6). The ability to acquire energy from carbohydrates of dietary or host origin is central to the adaptation of human gut bacterial species to their niche. More importantly, this makes diet a tractable way to manipulate the abundance and metabolic output of the microbiota toward improved human health. However, there is a paucity of data regarding how the vast array of complex carbohydrate structures are selectively recognized and imported by members of the microbiota, a critical process that enables these organisms to thrive in the competitive gut environment. The human gut bacteria Bacteroidetes share a profound capacity for dietary glycan degradation, with many species containing >250 predicted carbohydrate-active enzymes (CAZymes), compared to 50 to 100 within many Firmicutes and only 17 in the human genome devoted toward carbohydrate utilization (7). A remarkable feature of the Bacteroidetes is the packaging of genes for carbohydrate catabolism into discrete polysaccharide utilization loci (PUL) (8), which are transcriptionally regulated by specific substrate signatures (9–11). The archetypal PUL-encoded system is the starch utilization system (Sus) (Fig. 1B) of Bacteroides thetaiotaomicron (12, 13). The Sus includes a lipid-anchored, outer membrane endo-amylase, SusG (14, 15); a TonB-dependent transporter (TBDT), SusC, which imports oligosaccharides with the help of an associated starch-binding protein, SusD (13, 16, 17); two additional carbohydrate-binding lipoproteins, SusE and SusF (18); and two periplasmic exo-glucosidases, SusA and SusB, which generate glucose for transport into the cytoplasm (19). The importance of PUL as a successful evolutionary strategy is underscored by the observation that Bacteroidetes such as B. thetaiotaomicron and Bacteroides ovatus devote ~18% of their genomes to these systems (11). Moving beyond seminal genomic and transcriptomic analyses, the current state-of-the-art PUL characterization involves combined reverse-genetic, biochemical, and structural studies to illuminate the molecular details of PUL function (20–23).
FIG 1 .
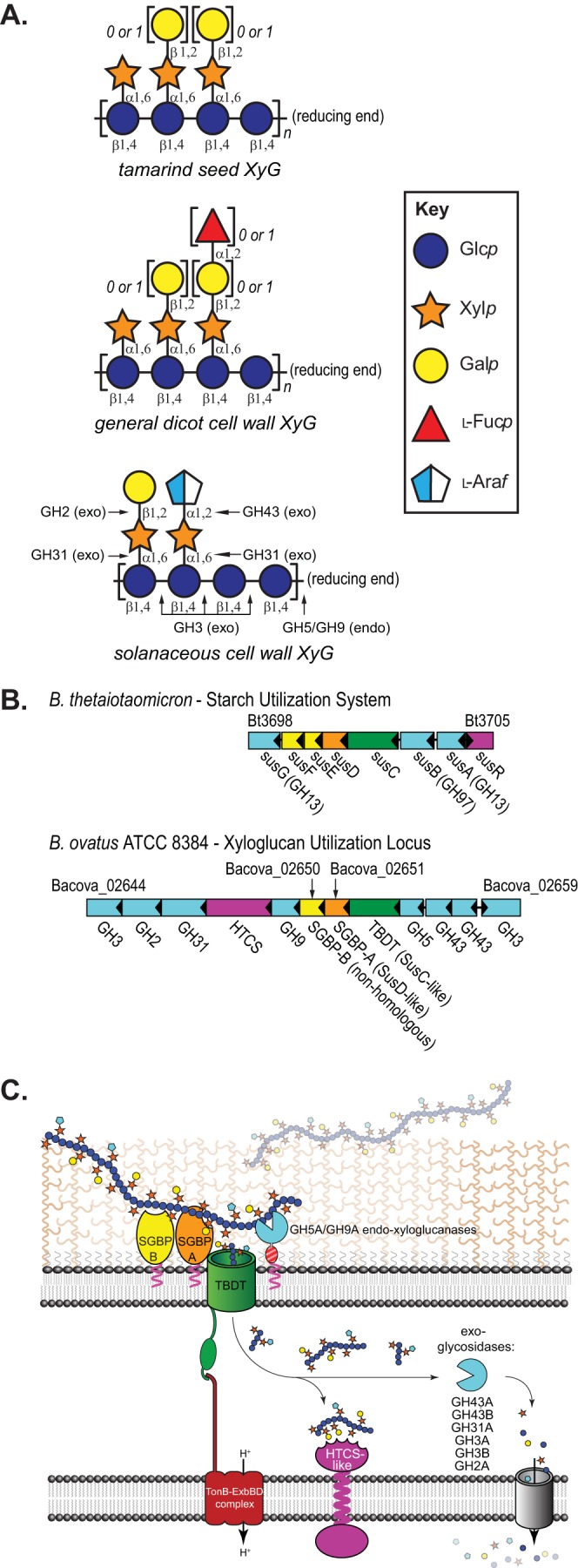
Xyloglucan and the Bacteroides ovatus xyloglucan utilization locus (XyGUL). (A) Representative structures of common xyloglucans (60) using the Consortium for Functional Glycomics Symbol Nomenclature (http://www.functionalglycomics.org/static/consortium/Nomenclature.shtml). Cleavage sites for BoXyGUL glycosidases (GHs) are indicated for solanaceous xyloglucan. (B) BtSus and BoXyGUL. (C) Localization of BoXyGUL-encoded proteins in cellular membranes and concerted modes of action in the degradation of xyloglucans to monosaccharides. The location of SGBP-A/B is presented in this work; the location of GH5 has been empirically determined, and the enzymes have been placed based upon their predicted cellular location (23).
We recently reported the detailed molecular characterization of a PUL that confers the ability of the human gut commensal B. ovatus ATCC 8483 to grow on a prominent family of plant cell wall glycans, the xyloglucans (XyG) (23). XyG variants (Fig. 1A) constitute up to 25% of the dry weight of common vegetables (24, 25). Analogous to the Sus locus, the xyloglucan utilization locus (XyGUL) encodes a cohort of carbohydrate-binding, -hydrolyzing, and -importing proteins (Fig. 1B and C). The number of glycoside hydrolases (GHs) encoded by the XyGUL is, however, more expansive than that by the Sus locus (Fig. 1B), which reflects the greater complexity of glycosidic linkages found in XyG vis-à-vis starch (10). Whereas our previous study focused on the characterization of the linkage specificity of these GHs (23), a key outstanding question regarding this locus is how XyG recognition is mediated at the cell surface.
In the archetypal starch utilization system of B. thetaiotaomicron, starch binding to the cell surface is mediated at eight distinct starch-binding sites distributed among four surface glycan-binding proteins (SGBPs): two within the amylase SusG, one within SusD, two within SusE, and three within SusF (15, 16, 18). The functional redundancy of many of these sites is high: whereas SusD is essential for growth on starch, combined mutations of the SusE, SusF, and SusG binding sites are required to impair growth on the polysaccharide (16, 26). Bacteroidetes PUL ubiquitously encode homologs of SusC and SusD, as well as proteins whose genes are immediately downstream of susD, akin to susE/F, and these are typically annotated as “putative lipoproteins” (8, 27). The genes coding for these proteins, sometimes referred to as “susE/F positioned,” display products with a wide variation in amino acid sequence and which have little or no homology to other PUL-encoded proteins or known carbohydrate-binding proteins (28, 29). As the Sus SGBPs remain the only structurally characterized cohort to date, we therefore wondered whether such glycan binding and function are extended to other PUL that target more complex and heterogeneous polysaccharides, such as XyG.
We describe here the detailed functional and structural characterization of the noncatalytic SGBPs encoded by Bacova_02651 and Bacova_02650 of the XyGUL, here referred to as SGBP-A and SGBP-B, to elucidate their molecular roles in carbohydrate acquisition in vivo. Combined biochemical, structural, and reverse-genetic approaches clearly illuminate the distinct, yet complementary, functions that these two proteins play in XyG recognition as it impacts the physiology of B. ovatus. These data extend our current understanding of the Sus-like glycan uptake paradigm within the Bacteroidetes and reveals how the complex dietary polysaccharide xyloglucan is recognized at the cell surface.
RESULTS AND DISCUSSION
SGBP-A and SGBP-B are cell-surface-localized, xyloglucan-specific binding proteins.
SGBP-A, encoded by the XyGUL locus tag Bacova_02651 (Fig. 1B), shares 26% amino acid sequence identity (40% similarity) with its homolog, B. thetaiotaomicron SusD (16), and similar homology with the SusD-like proteins encoded within syntenic XyGUL identified in our earlier work (23). In contrast, SGBP-B, encoded by locus tag Bacova_02650, displays little sequence similarity to the products of similarly positioned genes in syntenic XyGUL nor to any other gene product among the diversity of Bacteroidetes PUL (18, 27). Whereas sequence similarity among SusC/SusD homolog pairs often serves as a hallmark for PUL identification, the sequence similarities of downstream genes encoding SGBPs are generally too low to allow reliable bioinformatic classification of their products into protein families, let alone prediction of function (30). Hence, there is a critical need for the elucidation of detailed structure-function relationships among PUL SGBPs, in light of the manifold glycan structures in nature.
Immunofluorescence of formaldehyde-fixed, nonpermeabilized cells grown in minimal medium with XyG as the sole carbon source to induce XyGUL expression (23), reveals that both SGBP-A and SGBP-B are presented on the cell surface by N-terminal lipidation, as predicted by signal peptide analysis with SignalP (Fig. 2). Here, the SGBPs very likely work in concert with the cell-surface-localized endo-xyloglucanase B. ovatus GH5 (BoGH5) (23) to recruit and cleave XyG for subsequent periplasmic import via the SusC-like TBDT of the XyGUL (Fig. 1B and C).
FIG 2 .
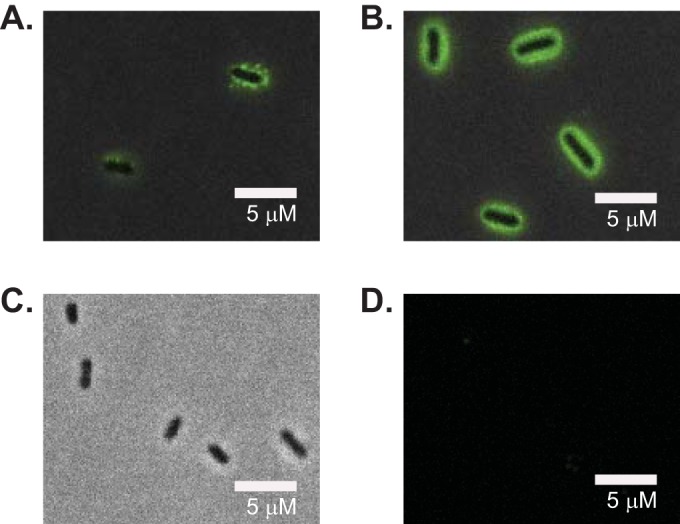
SGBP-A and SGBP-B visualized by immunofluorescence. Formalin-fixed, nonpermeabilized B. ovatus cells were grown in minimal medium plus XyG, probed with custom rabbit antibodies to SGBP-A or SGBP-B, and then stained with Alexa Fluor 488 goat anti-rabbit IgG. (A) Overlay of bright-field and FITC images of B. ovatus cells labeled with anti-SGBP-A. (B) Overlay of bright-field and FITC images of B. ovatus cells labeled with anti-SGBP-B. (C) Bright-field image of ΔSGBP-B cells labeled with anti-SGBP-B antibodies. (D) FITC images of ΔSGBP-B cells labeled with anti-SGBP-B antibodies. Cells lacking SGBP-A (ΔSGBP-A) do not grow on XyG and therefore could not be tested in parallel.
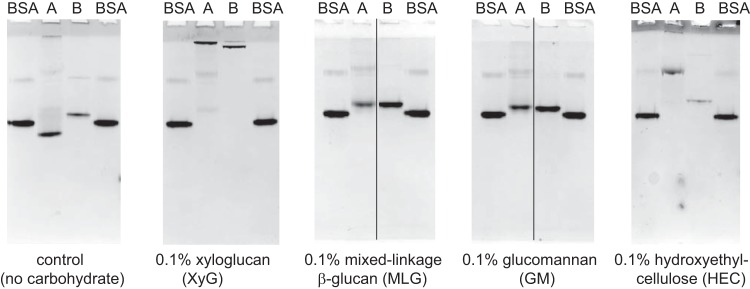
In our initial study focused on the functional characterization of the glycoside hydrolases of the XyGUL, we reported preliminary affinity PAGE and isothermal titration calorimetry (ITC) data indicating that both SGBP-A and SGBP-B are competent xyloglucan-binding proteins (affinity constant [Ka] values of 3.74 × 105 M−1 and 4.98 × 104 M−1, respectively [23]). Additional affinity PAGE analysis (Fig. 3) demonstrates that SGBP-A also has moderate affinity for the artificial soluble cellulose derivative hydroxyethyl cellulose [HEC; a β(1 → 4)-glucan] and limited affinity for mixed-linkage β(1→3)/β(1→4)-glucan (MLG) and glucomannan (GM; mixed glucosyl and mannosyl backbone), which together indicate general binding to polysaccharide backbone residues and major contributions from side-chain recognition. In contrast, SGBP-B bound to HEC more weakly than SGBP-A and did not bind to MLG or GM. Neither SGBP recognized galactomannan (GGM), starch, carboxymethylcellulose, or mucin (see Fig. S1 in the supplemental material). Together, these results highlight the high specificities of SGBP-A and SGBP-B for XyG, which is concordant with their association with XyG-specific GHs in the XyGUL, as well as transcriptomic analysis indicating that B. ovatus has discrete PUL for MLG, GM, and GGM (11). Notably, the absence of carbohydrate-binding modules in the GHs encoded by the XyGUL (23) implies that noncatalytic recognition of xyloglucan is mediated entirely by SGBP-A and -B.
FIG 3 .
SGBP-A and SGBP-B preferentially bind xyloglucan. Affinity electrophoresis (10% acrylamide) of SGBP-A and SGBP-B with BSA as a control protein. All samples were loaded on the same gel next to the BSA controls; thin black lines indicate where intervening lanes were removed from the final image for both space and clarity. The percentage of polysaccharide incorporated into each native gel is displayed.
The vanguard endo-xyloglucanase of the XyGUL, BoGH5, preferentially cleaves the polysaccharide at unbranched glucosyl residues to generate xylogluco-oligosaccharides (XyGOs) comprising a Glc4 backbone with variable side-chain galactosylation (XyGO1) (Fig. 1A; n = 1) as the limit of digestion products in vitro (23); controlled digestion and fractionation by size exclusion chromatography allow the production of higher-order oligosaccharides (e.g., XyGO2) (Fig. 1A; n = 2). ITC demonstrates that SGBP-A binds to XyG polysaccharide and XyGO2 (based on a Glc8 backbone) with essentially equal affinities, while no binding of XyGO1 (Glc4 backbone) was detectable (Table 1; see Fig. S2 and S3 in the supplemental material). Similarly, SGBP-B also bound to XyG and XyGO2 with approximately equal affinities, although in both cases, Ka values were nearly 10-fold lower than those for SGBP-A. Also in contrast to SGBP-A, SGBP-B also bound to XyGO1, yet the affinity for this minimal repeating unit was poor, with a Ka value of ca. 1 order of magnitude lower than for XyG and XyGO2. Together, these data clearly suggest that polysaccharide binding of both SGBPs is fulfilled by a dimer of the minimal repeat, corresponding to XyGO2 (cf. Fig. 1A). The observation by affinity PAGE that these proteins specifically recognize XyG is further substantiated by their lack of binding for the undecorated oligosaccharide cellotetraose (Table 1; see Fig. S3). Furthermore, SGBP-A binds cellohexaose with ~770-fold weaker affinity than XyG, while SGBP-B displays no detectable binding to this linear hexasaccharide. To provide molecular-level insight into how the XyGUL SGBPs equip B. ovatus to specifically harvest XyG from the gut environment, we performed X-ray crystallography analysis of both SGBP-A and SGPB-B in oligosaccharide-complex forms.
TABLE 1 .
Summary of thermodynamic parameters for wild-type SGBP-A and SGBP-B obtained by isothermal titration calorimetry at 25°Ca
| Carbohydrate |
Ka (M−1) |
ΔG (kcal ⋅ mol−1) |
ΔH (kcal ⋅ mol−1) |
TΔS (kcal ⋅ mol−1) |
||||
|---|---|---|---|---|---|---|---|---|
| SGBP-A | SGBP-B | SGBP-A | SGBP-B | SGBP-A | SGBP-B | SGBP-A | SGBP-B | |
| XyGb | (4.4 ± 0.1) × 105 | (5.7 ± 0.2) × 104 | −7.7 | −6.5 | −14 ± 3 | −14 ± 2 | −6.5 | −7.6 |
| XyGO2c | 3.0 × 105 | 2.0 × 104 | −7.5 | −5.9 | −17.2 | −17.6 | −9.7 | −11.7 |
| XyGO1 | NBd | (2.4 ± 0.1) × 103 | NB | −4.6 | NB | −4.4 ± 0.2 | NB | 0.2 |
| Cellohexaose | 568.0 ± 291.0 | NB | −3.8 | NB | −16 ± 8 | NB | −12.7 | NB |
| Cellotetraose | NB | NB | NB | NB | NB | NB | NB | NB |
Shown are average values ± standard errors from two independent titrations, unless otherwise indicated.
Binding thermodynamics for XyG based on the concentration of the binding unit, XyGO2.
Values from a single titration.
NB, no binding observed.
SGBP-A is a SusD homolog with an extensive glycan-binding platform.
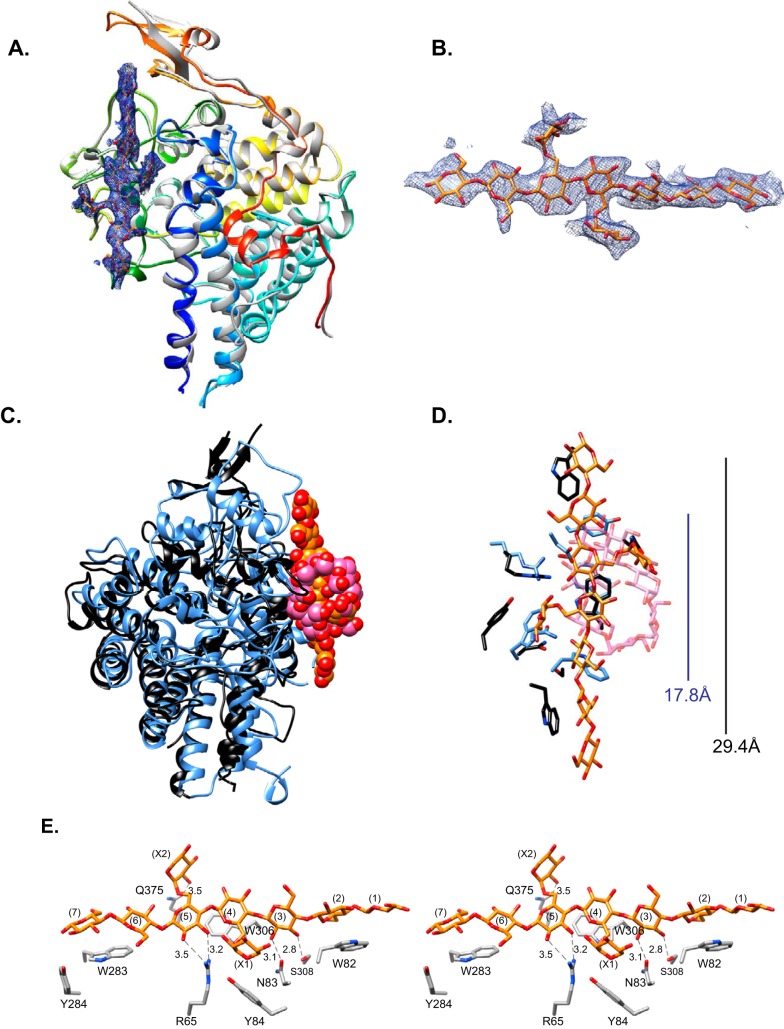
As anticipated by sequence similarity, the high-resolution tertiary structure of apo-SGBP-A (1.36 Å, Rwork = 14.7%, Rfree = 17.4%, residues 28 to 546) (Table 2) displays the canonical “SusD-like” protein fold dominated by four tetratrico-peptide repeat (TPR) motifs that cradle the rest of the structure (Fig. 4A) (31). Specifically, SGBP-A overlays B. thetaiotaomicron SusD (BtSusD) with a root mean square deviation (RMSD) value of 2.2 Å for 363 Cα pairs, which is notable given the 26% amino acid identity (40% similarity) between these homologs (Fig. 4C). Cocrystallization of SGBP-A with XyGO2 generated a substrate complex structure (2.3 Å, Rwork = 21.8%, Rfree = 24.8%, residues 36 to 546) (Fig. 4A and B; Table 2) that revealed the distinct binding-site architecture of the XyG binding protein. The SGBP-A:XyGO2 complex superimposes closely with the apo structure (RMSD of 0.6 Å) and demonstrates that no major conformational change occurs upon substrate binding; small deviations in the orientation of several surface loops are likely the result of differential crystal packing. It is particularly notable that although the location of the ligand-binding site is conserved between SGBP-A and SusD, that of SGBP-A displays an ~29-Å-long aromatic platform to accommodate the extended, linear XyG chain (see reference 32 for a review of XyG secondary structure), versus the shorter, ~18-Å-long, site within SusD that complements the helical conformation of amylose (16) (Fig. 4C and D).
TABLE 2 .
X-ray data collection and refinement statistics
| Parameter | Value(s) fora: |
|||
|---|---|---|---|---|
| SGBP-A apo | SGBP-A/XyGO2 | SGBP-B/XyGO2 | SGBP-B (CD)/XyGO2 | |
| PDB ID no. | 5E75 | 5E76 | 5E7G | 5E7H |
| Resolution (Å) | 21.48–1.36 (1.409–1.36) | 56.13–2.3 (2.382–2.3) | 39.19–2.37 (2.455–2.37) | 30.69–1.57 (1.626–1.570) |
| Space group | P21 | I422 | R32 | P6122 |
| Unit cell dimensions, a, b, c (Å) | 52.8, 81.4, 57.7; β = 107.85° | 131.5, 131.5, 188 | 207.4, 207.4, 117.9 | 87.1, 87.1, 201.6 |
| No. of reflections | ||||
| Total | 355,272 (26,772) | 1,068,014 (102,923) | 324,544 (32,355) | 1,366,812 (129,645) |
| Unique | 99,136 (9,762) | 36,775 (3,625) | 39,362 (3,898) | 62,808 (6,068) |
| Multiplicity | 3.6 (2.7) | 29.0 (28.4) | 8.2 (8.3) | 21.8 (21.4) |
| Completeness (%) | 99.71 (98.82) | 99.63 (99.42) | 99.96 (100.00) | 98.4 (96.98) |
| Mean I/σ〈I〉 | 15.57 (2.29) | 24.93 (6.71) | 20.98 (2.36) | 38.52 (5.03) |
| Wilson B-factor | 11.91 | 31.14 | 43.91 | 17.86 |
| Rmerge | 0.04759 (0.4513) | 0.1428 (0.7178) | 0.09159 (1.197) | 0.05559 (0.7748) |
| CC1/2b | 0.999 (0.759) | 0.999 (0.982) | 0.999 (0.794) | 1.000 (0.933) |
| CC*c | 1.000 (0.929) | 1.000 (0.995) | 1.000 (0.941) | 1.000 (0.982) |
| Rwork | 0.1468 (0.2597) | 0.2178 (0.2788) | 0.1975 (0.3018) | 0.1560 (0.2008) |
| Rfree | 0.1738 (0.2632) | 0.2482 (0.2978) | 0.2260 (0.3219) | 0.1712 (0.2019) |
| No. of non-hydrogen atoms | ||||
| All | 4,562 | 4,319 | 3,678 | 2,328 |
| Macromolecules | 4,079 | 3,974 | 3,425 | 1,985 |
| Ligands | 39 | 116 | 127 | 25 |
| Water | 444 | 229 | 126 | 318 |
| No. of protein residues | 506 | 492 | 446 | 260 |
| RMSD | ||||
| Bond length (Å) | 0.008 | 0.007 | 0.005 | 0.009 |
| Bond angle (°) | 1.15 | 0.96 | 0.87 | 1.18 |
| Ramachandran statistics | ||||
| Favored (%) | 98 | 95 | 97 | 98 |
| Outliers (%) | 0 | 0.41 | 0.23 | 0 |
| Clash score | 0.5 | 2.13 | 0.86 | 1.27 |
| Avg B-factors | ||||
| All | 16.1 | 53.2 | 53 | 25.4 |
| Macromolecules | 15.2 | 53.5 | 52.5 | 22.9 |
| Ligands | 24.7 | 61 | 71.1 | 47 |
| Solvent | 24.4 | 42.9 | 47.6 | 39 |
Numbers in parentheses are for the highest-resolution shell.
CC1/2, Pearson correlation coefficient between the average intensities of each subset.
CC*, Pearson correlation coefficient for correlation between the observed data set and true signal.
FIG 4 .
Molecular structure of SGBP-A (Bacova_02651). (A) Overlay of SGBP-A from the apo (rainbow) and XyGO2 (gray) structures. The apo structure is color ramped from blue to red. An omit map (2σ) for XyGO2 (orange and red sticks) is displayed. (B) Close-up view of the omit map as in panel A, rotated 90° clockwise. (C) Overlay of the Cα backbones of SGBP-A (black) with XyGO2 (orange and red spheres) and BtSusD (blue) with maltoheptaose (pink and red spheres), highlighting the conservation of the glycan-binding site location. (D) Close-up of the SGBP-A (black and orange) and SusD (blue and pink) glycan-binding sites. The approximate length of each glycan-binding site is displayed, colored to match the protein structures. (E) Stereo view of the xyloglucan-binding site of SGBP-A, displaying all residues within 4 Å of the ligand. The backbone glucose residues are numbered from the nonreducing end; xylose residues are labeled X1 and X2. Potential hydrogen-bonding interactions are shown as dashed lines, and the distance is shown in angstroms.
Seven of the eight backbone glucosyl residues of XyGO2 could be convincingly modeled in the ligand electron density, and only two α(1→6)-linked xylosyl residues were observed (Fig. 4B; cf. Fig. 1). Indeed, the electron density for the ligand suggests some disorder, which may arise from multiple oligosaccharide orientations along the binding site. Three aromatic residues—W82, W283, W306—comprise the flat platform that stacks along the naturally twisted β-glucan backbone (Fig. 4E). The functional importance of this platform is underscored by the observation that the W82A W283A W306A mutant of SGBP-A, designated SGBP-A*, is completely devoid of XyG affinity (Table 3; see Fig. S4 in the supplemental material). Dissection of the individual contribution of these residues reveals that the W82A mutant displays a significant 4.9-fold decrease in the Ka value for XyG, while the W306A substitution completely abolishes XyG binding. Contrasting with the clear importance of these hydrophobic interactions, there are remarkably few hydrogen-bonding interactions with the ligand, which are provided by R65, N83, and S308, which are proximal to Glc5 and Glc3. Most surprising in light of the saccharide-binding data, however, was a lack of extensive recognition of the XyG side chains; only Y84 appeared to provide a hydrophobic interface for a xylosyl residue (Xyl1).
TABLE 3 .
Summary of thermodynamic parameters for site-directed mutants of SGBP-A and SGBP-B obtained by ITC with XyG at 25°Ca
| Protein name |
Ka |
ΔG (kcal ⋅ mol−1) | ΔH (kcal ⋅ mol−1) | TΔS (kcal ⋅ mol−1) | |
|---|---|---|---|---|---|
| Fold changeb | M−1 | ||||
| SGBP-A(W82A W283A W306A) | ND | NB | NB | NB | NB |
| SGBP-A(W82A)c | 4.9 | 9.1 × 104 | −6.8 | −6.3 | 0.5 |
| SGBP-A(W306) | ND | NB | NB | NB | NB |
| SGBP-B(230–489) | 0.7 | (8.6 ± 0.20) × 104 | −6.7 | −14.9 ± 0.1 | −8.2 |
| SGBP-B(Y363A) | 19.7 | (2.9 ± 0.10) × 103 | −4.7 | −18.1 ± 0.1 | −13.3 |
| SGBP-B(W364A) | ND | Weak | Weak | Weak | Weak |
| SGBP-B(F414A) | 3.2 | (1.80 ± 0.03) × 104 | −5.8 | −11.4 ± 0.1 | −5.6 |
Shown are average values ± standard deviations from two independent titrations, unless otherwise indicated. Binding thermodynamics are based on the concentration of the binding unit, XyGO2. Weak binding represents a Ka of <500 M−1. ND, not determined; NB, no binding.
Ka fold change = Ka of wild-type protein/Ka of mutant protein for xyloglucan binding.
Values from a single titration.
SGBP-B has a multimodular structure with a single, C-terminal glycan-binding domain.
The crystal structure of full-length SGBP-B in complex with XyGO2 (2.37 Å, Rwork = 19.9%, Rfree = 23.9%, residues 34 to 489) (Table 2) revealed an extended structure composed of three tandem immunoglobulin (Ig)-like domains (domains A, B, and C) followed at the C terminus by a novel xyloglucan-binding domain (domain D) (Fig. 5A). Domains A, B, and C display similar β-sandwich folds; domains B (residues 134 to 230) and C (residues 231 to 313) can be superimposed onto domain A (residues 34 to 133) with RMSDs of 1.1 and 1.2 Å, respectively, for 47 atom pairs (23% and 16% sequence identity, respectively). These domains also display similarity to the C-terminal β-sandwich domains of many GH13 enzymes, including the cyclodextrin glucanotransferase of Geobacillus stearothermophilus (Fig. 5B). Such domains are not typically involved in carbohydrate binding. Indeed, visual inspection of the SGBP-B structure, as well as individual production of the A and B domains and affinity PAGE analysis (see Fig. S5 in the supplemental material), indicates that these domains do not contribute to XyG capture. On the other hand, production of the fused domains C and D in tandem (SGBP-B residues 230 to 489) retains complete binding of xyloglucan in vitro, with the observed slight increase in affinity likely arising from a reduced potential for steric hindrance of the smaller protein construct during polysaccharide interactions (Table 3). While neither the full-length protein nor domain D displays structural homology to known XyG-binding proteins, the topology of SGBP-B resembles the xylan-binding protein Bacova_04391 (PDB 3ORJ) encoded within a xylan-targeting PUL of B. ovatus (22) (Fig. 5C). The structure-based alignment of these proteins reveals 17% sequence identity, with a core RMSD of 3.6 Å for 253 aligned residues. While there is no substrate-complexed structure of Bacova_04391 available, the binding site is predicted to include W241 and Y404 (22), which are proximal to the XyGO binding site in SGBP-B. However, the opposing, clamp-like arrangement of these residues in Bacova_04391 is clearly distinct from the planar surface arrangement of the residues that interact with XyG in SGBP-B (described below).
FIG 5 .
Multimodular structure of SGBP-B (Bacova_02650). (A) Full-length structure of SGBP-B, color coded by domain as indicated. Prolines between domains are indicated as spheres. An omit map (2σ) for XyGO2 is displayed to highlight the location of the glycan-binding site. (B) Overlay of SGBP-B domains A, B, and C (colored as in panel A), with a C-terminal Ig-like domain of the G. stearothermophilus cyclodextrin glucanotransferase (PDB 1CYG [residues 375 to 493]) in green. (C) Cα overlay of SGBP-B (gray) and Bacova_04391 (PDB 3ORJ) (pink). (D) Close-up omit map for the XyGO2 ligand, contoured at 2σ. (E) Stereo view of the xyloglucan-binding site of SGBP-B, displaying all residues within 4 Å of the ligand. The backbone glucose residues are numbered from the nonreducing end, xylose residues are shown as X1, X2, and X3, potential hydrogen-bonding interactions are shown as dashed lines, and the distance is shown in angstroms.
Inspection of the tertiary structure indicates that domains C and D are effectively inseparable, with a contact interface of 396 Å2. Domains A, B, and C do not pack against each other. Moreover, the five-residue linkers between these first three domains all feature a proline as the middle residue, suggesting significant conformational rigidity (Fig. 5A). Despite the lack of sequence and structural conservation, a similarly positioned proline joins the Ig-like domains of the xylan-binding Bacova_04391 and the starch-binding proteins SusE and SusF. We speculate that this is a biologically important adaptation that serves to project the glycan binding site of these proteins far from the membrane surface. Any mobility of SGBP-B on the surface of the cell (beyond lateral diffusion within the membrane) is likely imparted by the eight-residue linker that spans the predicted lipidated Cys (C28) and the first β-strand of domain A. Other outer membrane proteins from various Sus-like systems possess a similar 10- to 20-amino-acid flexible linker between the lipidated Cys that tethers the protein to the outside the cell and the first secondary structure element (15, 16, 33). Analogously, the outer membrane-anchored endo-xyloglucanase BoGH5 of the XyGUL contains a 100-amino-acid, all-β-strand, N-terminal module and flexible linker that imparts conformational flexibility and distances the catalytic module from the cell surface (23).
XyG binds to domain D of SGBP-B at the concave interface of the top β-sheet, with binding mediated by loops connecting the β-strands. Six glucosyl residues, comprising the main chain, and three branching xylosyl residues of XyGO2 can be modeled in the density (Fig. 5D; cf. Fig. 1A). The backbone is flat, with less of the “twisted-ribbon” geometry observed in some cello- and xylogluco-oligosaccharides (34–36). The aromatic platform created by W330, W364, and Y363 spans four glucosyl residues, compared to the longer platform of SGBP-A, which supports six glucosyl residues (Fig. 5E). The Y363A site-directed mutant of SGBP-B displays a 20-fold decrease in the Ka for XyG, while the W364A mutant lacks XyG binding (Table 3; see Fig. S6 in the supplemental material). There are no additional contacts between the protein and the β-glucan backbone and surprisingly few interactions with the side-chain xylosyl residues, despite that fact that ITC data demonstrate that SGBP-B does not measurably bind the cellohexaose (Table 1). F414 stacks with the xylosyl residue of Glc3, while Q407 is positioned for hydrogen bonding with the O4 of xylosyl residue Xyl1. Surprisingly, an F414A mutant of SGBP-B displays only a mild 3-fold decrease in the Ka value for XyG, again suggesting that glycan recognition is primarily mediated via contact with the β-glucan backbone (Table 3; see Fig. S6). Additional residues surrounding the binding site, including Y369 and E412, may contribute to the recognition of more highly decorated XyG, but precisely how this is mediated is presently unclear. Hoping to achieve a higher-resolution view of the SGBP-B–xyloglucan interaction, we solved the crystal structure of the fused CD domains in complex with XyGO2 (1.57 Å, Rwork = 15.6%, Rfree = 17.1%, residues 230 to 489) (Table 2). The CD domains of the truncated and full-length proteins superimpose with a 0.4-Å RMSD of the Cα backbone, with no differences in the position of any of the glycan-binding residues (see Fig. S7A in the supplemental material). While density is observed for XyGO2, the ligand could not be unambiguously modeled into this density to achieve a reasonable fit between the X-ray data and the known stereochemistry of the sugar (see Fig. S7B and C). While this may occur for a number of reasons in crystal structures, it is likely that the poor ligand density even at higher resolution is due to movement or multiple orientations of the sugar averaged throughout the lattice.
SGBP-A and SGBP-B have distinct, coordinated functions in vivo.
The similarity of the glycan specificity of SGBP-A and SGBP-B presents an intriguing conundrum regarding their individual roles in XyG utilization by B. ovatus. To disentangle the functions of SGBP-A and SGBP-B in XyG recognition and uptake, we created individual in-frame deletion and complementation mutant strains of B. ovatus. In these growth experiments, overnight cultures of strains grown on minimal medium plus glucose were back-diluted 1:100-fold into minimal medium containing 5 mg/ml of the reported carbohydrate. Growth on glucose displayed the shortest lag time for each strain, and so lag times were normalized for each carbohydrate by subtracting the lag time of that strain in glucose (Fig. 6; see Fig. S8 in the supplemental material). A strain in which the entire XyGUL is deleted displays a lag of 24.5 h during growth on glucose compared to the isogenic parental wild-type (WT) Δtdk strain, for which exponential growth lags for 19.8 h (see Fig. S8D). It is unknown whether this is because cultures were not normalized by the starting optical density (OD) or viable cells or reflects a minor defect for glucose utilization. The former seems more likely as the growth rates are nearly identical for these strains on glucose and xylose. The ΔXyGUL and WT Δtdk strains display normalized lag times on xylose within experimental error, and curiously some of the mutant and complemented strains display a nominally shorter lag time on xylose than the WT Δtdk strain. Complementation of the ΔSGBP-A strain (ΔSGBP-A::SGBP-A) restores growth to wild-type rates on xyloglucan and XyGO1, yet the calculated rate of the complemented strain is ~72% that of the WT Δtdk strain on XyGO2; similar results were obtained for the SGBP-B complemented strain despite the fact that the growth curves do not appear much different (see Fig. S8C and F). The reason for this observation on XyGO2 is unclear, as the ΔSGBP-B mutant does not have a significantly different growth rate from the WT on XyGO2. Therefore, we limit our discussion to those mutants that displayed the most obvious defects in growth on particular substrates.
FIG 6 .
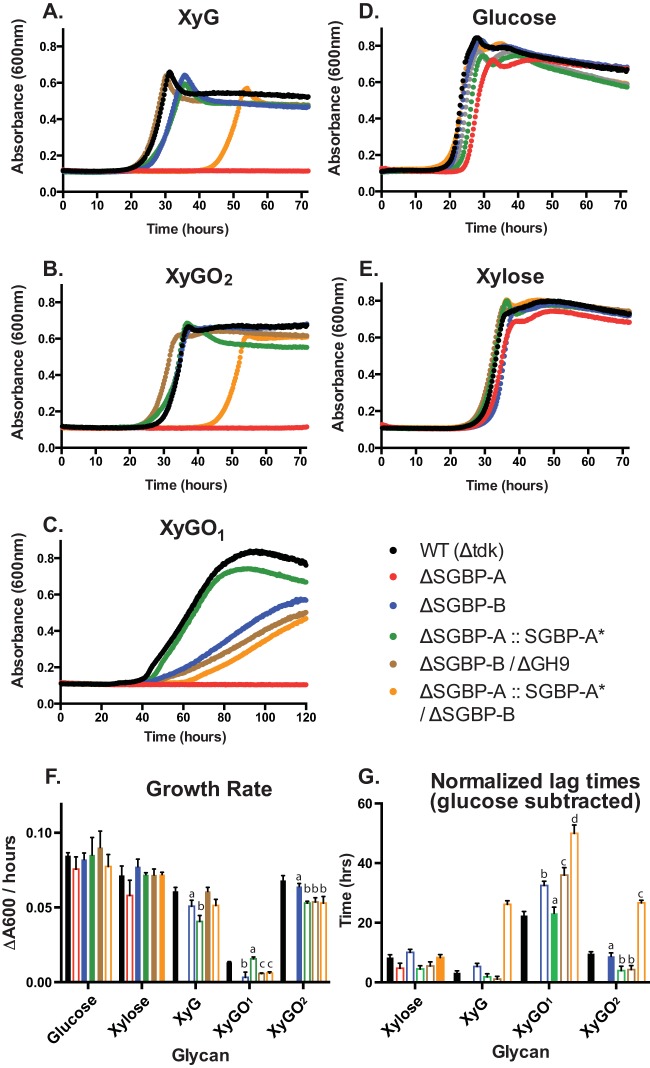
Growth of select XyGUL mutants on xyloglucan and oligosaccharides. B. ovatus mutants were created in a thymidine kinase deletion (Δtdk) mutant as described previously (23). SGBP-A* denotes the Bacova_02651 (W82A W283A W306A) allele, and the GH9 gene is Bacova_02649. Growth was measured over time in minimal medium containing (A) XyG, (B) XyGO2, (C) XyGO1, (D) glucose, and (E) xylose. In panel F, the growth rate of each strain on the five carbon sources is displayed, and in panel G, the normalized lag time of each culture, relative to its growth on glucose, is displayed. Solid bars indicate conditions that are not statistically significant from the WT Δtdk cultures grown on the indicated carbohydrate, while open bars indicate a P value of <0.005 compared to the WT Δtdk strain. Conditions denoted by the same letter (b, c, or d) are not statistically significant from each other but are significantly different from the condition labeled “a.” Complementation of ΔSGBP-A and ΔSBGP-B was performed by allelic exchange of the wild-type genes back into the genome for expression via the native promoter: these growth curves, quantified rates and lag times are displayed in Fig. S8 in the supplemental material.
The ΔSGBP-A (ΔBacova_02651) strain (cf. Fig. 1B) was completely incapable of growth on XyG, XyGO1, and XyGO2, indicating that SGBP-A is essential for XyG utilization (Fig. 6). This result mirrors our previous data for the canonical Sus of B. thetaiotaomicron, which revealed that a homologous ΔsusD mutant is unable to grow on starch or malto-oligosaccharides, despite normal cell surface expression of all other PUL-encoded proteins (16, 26). More recently, we demonstrated that this phenotype is due to the loss of the physical presence of SusD; complementation of ΔsusD with SusD*, a triple site-directed mutant (W96A W320A Y296A) that ablates glycan binding, restores B. thetaiotaomicron growth on malto-oligosaccharides and starch when sus transcription is induced by maltose addition (26). Similarly, the function of SGBP-A extends beyond glycan binding. Complementation of ΔSGBP-A with the SGBP-A* (W82A W283A W306A) variant, which does not bind XyG, supports growth on XyG and XyGOs (Fig. 6; ΔSGBP-A::SGBP-A*), with growth rates that are ~70% that of the WT. In previous studies, we observed that carbohydrate binding by SusD enhanced the sensitivity of the cells to limiting concentrations of malto-oligosaccharides by several orders of magnitude, such that the addition of 0.5 g/liter maltose was required to restore growth of the ΔsusD::SusD* strain on starch, which nonetheless occurred following an extended lag phase (26). In contrast, the ΔSGBP-A::SGBP-A* strain does not display an extended lag time on any of the xyloglucan substrates compared to the WT (Fig. 6). The specific glycan signal that upregulates BoXyGUL is currently unknown. From our present data, we cannot eliminate the possibility that the glycan binding by SGBP-A enhances transcriptional activation of the XyGUL. However, the modest rate defect displayed by the SGBP-A::SGBP-A* strain suggests that recognition of XyG and product import is somewhat less efficient in these cells.
Intriguingly, the ΔSGBP-B strain (ΔBacova_02650) (cf. Fig. 1B) exhibited a minor growth defect on both XyG and XyGO2, with rates 84.6% and 93.9% that of the WT Δtdk strain. However, growth of the ΔSGBP-B strain on XyGO1 was 54.2% the rate of the parental strain, despite the fact that SGBP-B binds this substrate ca. 10-fold more weakly than XyGO2 and XyG (Fig. 6; Table 1). As such, the data suggest that SGBP-A can compensate for the loss of function of SGBP-B on longer oligo- and polysaccharides, while SGBP-B may adapt the cell to recognize smaller oligosaccharides efficiently. Indeed, a double mutant, consisting of a crippled SGBP-A and a deletion of SGBP-B (ΔSGBP-A::SGBP-A*/ΔSGBP-B), exhibits an extended lag time on both XyG and XyGO2, as well as XyGO1. Taken together, the data indicate that SGBP-A and SGBP-B functionally complement each other in the capture of XyG polysaccharide, while SGBP-B may allow B. ovatus to scavenge smaller XyGOs liberated by other gut commensals. This additional role of SGBP-B is especially notable in the context of studies on BtSusE and BtSusF (positioned similarly in the archetypal Sus locus) (Fig. 1B), for which growth defects on starch or malto-oligosaccharides have never been observed. Beyond SGBP-A and SGBP-B, we speculated that the catalytically feeble endo-xyloglucanase GH9, which is expendable for growth in the presence of GH5, might also play a role in glycan binding to the cell surface (23). However, combined deletion of the genes encoding GH9 (encoded by Bacova_02649) and SGBP-B does not exacerbate the growth defect on XyGO1 (Fig. 6; ΔSGBP-B/ΔGH9).
The necessity of SGBP-B is elevated in the SGBP-A* strain, as the ΔSGBP-A::SGBP-A*/ ΔSGBP-B mutant displays an extended lag during growth on XyG and xylogluco-oligosaccharides, while growth rate differences are more subtle. The precise reason for this lag is unclear, but recapitulating our findings on the role of SusD in malto-oligosaccharide sensing in B. thetaiotaomicron, this extended lag may be due to inefficient import and thus sensing of xyloglucan in the environment in the absence of glycan binding by essential SGBPs. Our previous work demonstrates that B. ovatus cells grown in minimal medium plus glucose express low levels of the XyGUL transcript (23). Thus, in our experiments, we presume that each strain, initially grown in glucose, expresses low levels of the XyGUL transcript and thus low levels of the XyGUL-encoded surface proteins, including the vanguard GH5. Presumably without glycan binding by the SGBPs, the GH5 protein cannot efficiently process xyloglucan, and/or the lack of SGBP function prevents efficient capture and import of the processed oligosaccharides. It may then be that only after a sufficient amount of glycan is processed and imported by the cell is XyGUL upregulated and exponential growth on the glycan can begin. We hypothesize that during exponential growth the essential role of SGBP-A extends beyond glycan recognition, perhaps due to a critical interaction with the TBDT. In the BtSus, SusD and the TBDT SusC interact (37), and we speculate that this interaction is necessary for glycan uptake, as suggested by the fact that a ΔsusD mutant cannot grow on starch (16, 26), but a ΔsusD::SusD* strain regains this ability if a transcriptional activator of the sus operon is supplied (26). Likewise, such cognate interactions between homologous protein pairs such as SGBP-A and its TBDT may underlie our observation that a ΔSGBP-A mutant cannot grow on xyloglucan. However, unlike the Sus, in which elimination of SusE and SusF does not affect growth on starch, SGBP-B appears to have a dedicated role in growth on small xylogluco-oligosaccharides.
Conclusions.
The ability of gut-adapted microorganisms to thrive in the gastrointestinal tract is critically dependent upon their ability to efficiently recognize, cleave, and import glycans. The human gut, in particular, is a densely packed ecosystem with hundreds of species, in which there is potential for both competition and synergy in the utilization of different substrates. Recent work has elucidated that Bacteroidetes cross-feed during growth on many glycans; the glycoside hydrolases expressed by one species liberate oligosaccharides for consumption by other members of the community (38, 39). Thus, understanding glycan capture at the cell surface is fundamental to explaining, and eventually predicting, how the carbohydrate content of the diet shapes the gut community structure as well as its causative health effects. Here, we demonstrate that the surface glycan binding proteins encoded within the BoXyGUL play unique and essential roles in the acquisition of the ubiquitous and abundant vegetable polysaccharide xyloglucan. Yet, a number of questions remain regarding the molecular interplay of SGBPs with their cotranscribed cohort of glycoside hydrolases and TonB-dependent transporters.
A particularly understudied aspect of glycan utilization is the mechanism of import via TBDTs (SusC homologs) (Fig. 1), which are ubiquitous and defining components of all PUL (28, 40). PUL-encoded TBDTs in Bacteroidetes are larger than the well-characterized iron-targeting TBDTs from many Proteobacteria and are further distinguished as the only known glycan-importing TBDTs coexpressed with an SGBP (41). A direct interaction between the BtSusC TBDT and the SusD SGBP has been previously demonstrated (13, 37), as has an interaction between the homologous components encoded by an N-glycan-scavenging PUL of Capnocytophaga canimorsus (42). Our observation here that the physical presence of the SusD homolog SGBP-A, independent of XyG-binding ability, is both necessary and sufficient for XyG utilization further supports a model of glycan import whereby the SusC-like TBDTs and the SusD-like SGBPs must be intimately associated to support glycan uptake (Fig. 1C). It is yet presently unclear whether this interaction is static or dynamic and to what extent the association of cognate TBDT/SGBPs is dependent upon the structure of the carbohydrate to be imported. On the other hand, there is clear evidence for independent TBDTs in Bacteroidetes that do not require SGBP association for activity. For example, it was recently demonstrated that expression of nanO, which encodes a SusC-like TBDT as part of a sialic-acid-targeting PUL from B. fragilis, restored growth on this monosaccharide in a mutant strain of E. coli (43). In this instance, coexpression of the susD-like gene nanU was not required, nor did the expression of the nanU gene enhance growth kinetics. Similarly, the deletion of BT1762 encoding a fructan-targeting SusD-like protein in B. thetaiotaomicron did not result in a dramatic loss of growth on fructans (44). Thus, the strict dependence on a SusD-like SGBP for glycan uptake in the Bacteroidetes may be variable and substrate dependent. Furthermore, considering the broader distribution of TBDTs in PUL lacking SGBPs (sometimes known as carbohydrate utilization containing TBDT [CUT] loci; see reference 45 and reviewed in reference 40) across bacterial phyla, it appears that the intimate biophysical association of these substrate-transport and -binding proteins is the result of specific evolution within the Bacteroidetes.
Equally intriguing is the observation that while SusD-like proteins such as SGBP-A share moderate primary and high tertiary structural conservation, the genes for the SGBPs encoded immediately downstream (Fig. 1B [sometimes referred to as “susE positioned”]) encode glycan-binding lipoproteins with little or no sequence or structural conservation, even among syntenic PUL that target the same polysaccharide. Such is the case for XyGUL from related Bacteroides species, which may encode either one or two of these predicted SGBPs, and these proteins vary considerably in length (23). The extremely low similarity of these SGBPs is striking in light of the moderate sequence conservation observed among homologous GHs in syntenic PUL. This, together with the observation that these SGBPs, as exemplified by BtSusE and BtSusF (26) and the XyGUL SGBP-B of the present study, are expendable for polysaccharide growth, implies a high degree of evolutionary flexibility to enhance glycan capture at the cell surface. Because the intestinal ecosystem is a dense consortium of bacteria that must compete for their nutrients, these multimodular SGBPs may reflect ongoing evolutionary experiments to enhance glycan uptake efficiency. Whether organisms that express longer SGBPs, extending further above the cell surface toward the extracellular environment, are better equipped to compete for available carbohydrates is presently unknown. However, the natural diversity of these proteins represents a rich source for the discovery of unique carbohydrate-binding motifs to both inform gut microbiology and generate new, specific carbohydrate analytical reagents (46).
In conclusion, the present study further illuminates the essential role that surface-glycan binding proteins play in facilitating the catabolism of complex dietary carbohydrates by Bacteroidetes. The ability of our resident gut bacteria to recognize polysaccharides is the first committed step of glycan consumption by these organisms, a critical process that influences the community structure and thus the metabolic output (i.e., short-chain fatty acid and metabolite profile) of these organisms. A molecular understanding of glycan uptake by human gut bacteria is therefore central to the development of strategies to improve human health through manipulation of the microbiota (47, 48).
MATERIALS AND METHODS
Protein production and purification.
The gene fragments corresponding to Bacova_02650 (encoding SGBP-B residues 34 to 489) and Bacova_02651 (encoding SGBP-A residues 28 to 546) were amplified from Bacteroidetes ovatus ATCC 8483 genomic DNA by PCR using forward primers, including NdeI restriction sites, and reverse primers, including XhoI. The gene products were ligated into a modified version of pET-28a (EMD Biosciences) containing a recombinant tobacco etch virus (rTEV) protease recognition site (pET-28aTEV) preceding an N-terminal 6-His tag for affinity purification. The expression vector (pET-28TEV) containing SGBP-B was used for subsequent cloning of the domains A (residues 34 to 133), B (residues 134 to 229), and CD (residues 230 to 489). The pET-28TEV vector expressing residues 28 to 546 of SGBP-A was utilized for carbohydrate-binding experiments and crystallization of the apo structure. To obtain crystals of SGBP-A with XyGO2, the DNA sequence coding for residues 36 to 546 was PCR amplified from genomic DNA for ligation-independent cloning into the pETite N-His vector (Lucigen, Madison, WI) according to the manufacturer’s instructions. The N-terminal primer for pETite N-His insertion contained a TEV cleavage site immediately downstream of the complementary 18-bp overlap (encoding the His tag) to create a TEV-cleavable His-tagged protein. The site-directed mutants of SGBP-A and SGBP-B in pET-28TEV were created using the QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. The sequences of all primers to generate these constructs are displayed in Table S1 in the supplemental material.
The plasmids containing the SGBP-A and SGBP-B genes were transformed into Escherichia coli BL21(DE3) or Rosetta(DE3) cells. For native protein expression, cells were cultured in Terrific Broth containing kanamycin (50 µg/ml) and chloramphenicol (20 µg/ml) at 37°C to the mid-exponential phase (A600 of ≈0.6), induced by the addition of 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG), and then incubated for 2 days at 16°C or 1 day at 20°C. Cells were harvested by centrifugation and frozen at −80°C prior to protein purification. For selenomethionine-substituted SGBP-B, the pET-28TEV-SGBP-B plasmid was transformed into E. coli Rosetta(DE3)/pLysS and plated onto LB supplemented with kanamycin (50 µg/ml) and chloramphenicol (20 µg/ml). After 16 h of growth at 37°C, colonies were harvested from the plates, used to inoculate 100 ml of M9 minimal medium supplemented with kanamycin (30 µg/ml) and chloramphenicol (20 µg/ml), and then grown at 37°C for 16 h. This overnight culture was used to inoculate a 2-liter baffled flask containing 1 liter of Molecular Dimensions SelenoMet premade medium supplemented with 50 ml of the recommended sterile nutrient mix, chloramphenicol, and kanamycin. Cultures were grown at 37°C to an A600 of ≈0.45 before adjusting the temperature to 20°C and supplementing each flask with 100 mg each of l-lysine, l-threonine, and l-phenylalanine and 50 mg each of l-leucine, l-isoleucine, l-valine, and l-selenomethionine (49). After 20 additional minutes of growth, the cells were induced with 0.5 mM IPTG, and cultures were grown for an additional 48 h.
For the purification of native and selenomethionine-substituted protein, cells were thawed and lysed via sonication in His buffer (25 mM NaH2PO4, 500 mM NaCl, 20 mM imidazole, pH 7.5) and purified via immobilized nickel affinity chromatography (His-Trap; GE Healthcare) using a gradient of 20 to 300 mM imidazole, according to the manufacturer’s instructions. The His tag was removed by incubation with TEV protease (1:100 molar ratio relative to protein) at room temperature for 2 h and then overnight at 4°C while being dialyzed against His buffer. The cleaved protein was then repurified via nickel affinity chromatography to remove undigested target protein, the cleaved His tag, and His-tagged TEV protease. Purified proteins were dialyzed against 20 mM HEPES–100 mM NaCl (pH 7.0) prior to crystallization and concentrated using Vivaspin 15 (10,000-molecular-weight-cutoff [MWCO]) centrifugal concentrators (Vivaproducts, Inc.).
Glycans.
Xyloglucan from tamarind seed, β-glucan from barley, and konjac glucomannan were purchased from Megazyme. Starch, guar, and mucin were purchased from Sigma. Hydroxyethyl cellulose was purchased from AMRESCO. Carboxymethyl cellulose was purchased from Acros Organics. Xylogluco-oligosaccharides XyGO1 and XyGO2 for biophysical analyses were prepared from tamarind seed XyG according to the method of Martinez-Fleites et al. (61) with minor modifications. XyGO2 for cocrystallization was purchased from Megazyme (O-XGHDP).
Affinity gel electrophoresis.
Affinity PAGE was performed as described previously (50), with minor modification. Various polysaccharides were used at a concentration of 0.05 to 0.1% (wt/vol), and electrophoresis was carried out for 90 min at room temperature in native 10% (wt/vol) polyacrylamide gels. BSA was used as noninteracting negative-control protein.
ITC.
Isothermal titration calorimetry (ITC) of glycan binding by the SGPB-A mutants was performed using the TA Nano isothermal titration calorimeter calibrated to 25°C. Proteins were dialyzed against 20 mM HEPES–100 mM NaCl (pH 7.0), and sugars were prepared using the dialysis buffer. The protein (45 to 50 µM) was placed in the sample cell, and the syringe was loaded with 2.5 to 4 mg/ml XyG polysaccharide. Following an initial injection of 0.5 µl, 26 subsequent injections of 2 µl were performed with stirring at 350 rpm, and the resulting heat of reaction was recorded. Data were analyzed using the Nano Analyze software. All other ITC experiments were performed using a MicroCal VP-ITC titration calorimeter calibrated to 25°C. Proteins were dialyzed into 20 mM HEPES–100 mM NaCl (pH 7.0), and polysaccharides were prepared using the dialysis buffer. Proteins (micromolar concentrations) were placed in the sample cell, and a first injection of 2 µl was performed followed by 24 subsequent injections of 10 µl of 2 to 20 mM oligosaccharide (cellotetraose, cellohexaose, XyGO1, or XyGO2) or 1 to 2.5 mg/ml XyG polysaccharide. The solution was stirred at 280 rpm, and the resulting heat of reaction was recorded. Data were analyzed using the Origin software program.
DSC.
Structural integrity of the SGBP-B mutants was verified by differential scanning calorimetry (DSC). DSC studies were performed on a MicroCal VP-DSC (Malvern Instruments). Experiments were carried out in 50 mM HEPES (pH 7.0) at a scan rate of 60°C/h. All samples (40 µM protein) were degassed for 7 min with gentle stirring under vacuum prior to being loaded into the calorimeter. Background excess thermal power scans were obtained with buffer in both the sample and reference cells and subtracted from the scans for each sample solution to generate excess heat capacity versus temperature thermograms.
The melting temperature decreased from 57.8 ± 0.9°C for the wild-type SGBP-B protein to 54.6 ± 0.1°C for the Y363A mutant, 54.2 ± 0.1°C for the W364A mutant, and 52 ± 1°C for the F414A mutant. All proteins were therefore in their stable folded state for the ITC measurements (see Fig. S9 in the supplemental material).
Bacteroides ovatus mutagenesis.
Gene deletions and complementations were performed via allelic exchange in a Bacteroides ovatus thymidine kinase gene (Bacova_03071) deletion (Δtdk) derivative strain of ATCC 8483 using the vector pExchange-tdk, as previously described (23). Primers for the construction of B. ovatus mutants are listed in Table S1. The B. ovatus Δtdk strain and the B. ovatus ΔXyGUL mutant were a generous gift from Eric Martens, University of Michigan Medical School.
Bacteroides growth experiments.
All Bacteroides ovatus culturing was performed in a Coy anaerobic chamber (85% N2, 10% H2, 5% CO2) at 37°C. Prior to growth on minimal medium plus the carbohydrates indicated (Fig. 6; see Fig. S8 in the supplemental material), each strain was grown for 16 h from a freezer stock in tryptone-yeast extract-glucose (TYG) medium (51) and then back diluted 1:100 into Bacteroides minimal medium supplemented with 5 mg/ml glucose, as previously described (52). After growth for 24 h, cultures were back-diluted 1:100 into Bacteroides minimal medium supplemented with 5 mg/ml of glucose, xylose, XyG, XyGO1, or XyGO2. Growth experiments were performed in replicates of 12 (glucose, xylose, and xyloglucan) or 5 (XyGO1 and XyGO2) as 200-µl cultures in 96-well plates. Plates were loaded into a Biostack automated plate handling device coupled to a Powerwave HT absorbance reader (both devices from Biotek Instruments, Winooski, VT). Absorbance at 600 nm (A600; i.e., optical density at 600 nm [OD600]) was measured for each well at 20-min intervals. Data were processed using Gen5 software (BioTek) and Microsoft Excel. Growth was quantified in each assay by first identifying a minimum time point (Amin) at which A600 had increased by 15% over a baseline reading taken during the first 500 min of incubation. Next, we identified the time point at which A600 reached its maximum (Amax) immediately after exponential growth. The growth rate for each well was defined by (Amax − Amin)/(Tmax − Tmin), where Tmax and Tmin are the corresponding time values for each absorbance. To account for variations in inoculum density, for each strain, the lag time (Tmin) on glucose was subtracted from the lag time for the substrate of interest; in all cases, cultures had shorter lag times on glucose than other glycans.
Immunofluorescence.
Custom rabbit antibodies to recombinant SGBP-A and SGBP-B were generated by Cocalico Biologicals, Inc. (Reamstown, PA). The B. ovatus ATCC 8483 Δtdk and ΔSGBP-B strains were grown in 1 ml minimal Bacteroides medium (11) supplemented with 5 mg/ml tamarind xyloglucan to an A600 of ≈0.6 and then harvested via centrifugation (7,000 × g for 3 min) and washed twice with phosphate-buffered saline (PBS). Cells were resuspended in 0.25 ml PBS, and 0.75 ml of 6% formalin in PBS was added. Cells were incubated with rocking at 20°C for 1.5 h and then washed twice with PBS. Cells were resuspended in 0.5 to 1 ml blocking solution (2% goat serum, 0.02% NaN3 in PBS) and incubated for 16 h at 4°C. Cells were centrifuged and resuspended in 0.5 ml of a 1/100 dilution of custom rabbit antibody sera in blocking solution and incubated by rocking for 2 h at 20°C. Cells were washed with PBS and then resuspended in 0.4 ml of a 1/500 dilution of Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies) in blocking solution and incubated with rocking for 1 h at 20°C. Cells were washed three times with an excess of PBS and then resuspended in 20 µl of PBS plus 1 µl of ProLong Gold antifade (Life Technologies). Cells were spotted on 0.8% agarose pads and imaged at the Center for Live Cell Imaging at the University of Michigan Medical School, using an Olympus IX70 inverted confocal microscope. Images were processed with Metamorph Software.
Crystallization and data collection.
All X-ray diffraction data for both native and selenomethionine-substituted protein crystals were collected at the Life Science Consortium (LS-CAT) at the Advance Photon Source at Argonne National Laboratory in Argonne, IL. The native protein SGBP-B (residues 34 to 489) was concentrated to an A280 of 12.25 prior to crystallization and mixed with 10 mM XyGO2 (Megazyme, O-XGHDP). Hanging drop vapor diffusion was performed against mother liquor consisting of 1.1 to 1.5 M ammonium sulfate and 30 to 70 mM sodium cacodylate (pH 6.5). To decrease crystal nucleation, 0.3 ml of paraffin oil was overlaid on top of 0.5 ml of mother liquor yielding diffraction-quality crystals within 2 weeks. Selenomethionine-substituted crystals of SGBP-B were generated using the same conditions as the native crystals. Crystals of the truncated SGBP-B (domains CD, residues 230 to 489) were obtained by mixing concentrated protein (A600 of 20.6) with 10 mM XyGO2 for hanging drop vapor diffusion against a solution containing 2 M sodium formate and 0.1 M sodium acetate (pH 4.6). All SGBP-B crystals were flash-frozen prior to data collection by briefly soaking in a solution of 80% mother liquor–20% glycerol plus 10 mM xylogluco-oligosaccharide. Data were processed and scaled using HKL2000 and Scalepack (53). SAD phasing from a selenomethionine-substituted protein crystals was used to determine the structure of SGBP-B. The AutoSol (54) and Autobuild (55) algorithms within the Phenix (56) suite of programs were used to locate and refine the selenium positions and automatically build an initial model of the protein structure, respectively. Successive rounds of manual model building and refinement in Coot (57) and Phenix, respectively, were utilized to build a 2.7-Å model of the selenomethionine-substituted protein, which then was placed in the unit cell of the native data set. Additional rounds of manual model building and refinement were performed to complete the 2.37-Å structure of SGBP-B with XyGO2. The structure of the truncated protein (CD domains, residues 230 to 489) was solved via molecular replacement with Phaser (58) using the CD domains of the full-length protein as a model.
The native protein SGBP-A (residues 28 to 546) was concentrated to an A280 of 28.6 and crystallized via hanging drop vapor diffusion from the Morpheus crystal screen (Molecular Dimensions). Crystals formed in well A1 (30 mM MgCl2, 30 mM CaCl2, 20% polyethylene glycol [PEG 500], 10% PEG 20K, 0.1 M imidazole-MES [morpholinethanesulfonic acid], pH 6.5), and were flash-frozen in liquid nitrogen without additional cryoprotectant. The truncated SGBP-A (residues 36 to 546) concentrated to an A280 of 38.2 yielded crystals with 10 mM XyGO2 via hanging drop vapor diffusion against 1.2 to 1.8 M sodium citrate (pH 6.15 to 6.25), and were flash-frozen in a cryoprotectant solution of 80% mother liquor–20% ethylene glycol with the glycan. Data were processed and scaled using HKL2000 and Scalepack (53). The structure of the apo protein was solved via molecular replacement with BALBES (59) using the homologous structure PDB 3JYS, followed by successive rounds of automatic and manual model building with Autobuild and Coot. The structure of SGBP-A with XyGO2 was solved via molecular replacement with Phaser (58) and refined with Phenix (56). X-data collection and refinement statistics are presented in Table 2.
SUPPLEMENTAL MATERIAL
Affinity PAGE with glycans that are not recognized by SGBP-A and SGBP-B. Affinity electrophoresis (10% acrylamide) of SGBP-A and SGBP-B with BSA as a control protein. The percentage of polysaccharide incorporated into each native gel is displayed. Download
Representative isothermal titration calorimetry (ITC) results for SGBP-A and SGBP-B titrations with XyG and XyGO. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 10-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. Concentrations of the protein and glycan are indicated. (A) SGBP-A with XyG; (B) SGBP-B with XyG; (C) SGBP-A with XyGO1; (D) SGBP-B with XyGO1; (E) SGBP-A with XyGO2; (F) SGBP-B with XyGO2. Download
Representative isothermal titration calorimetry (ITC) results for SGBP-A and SGBP-B with cello-oligosaccharides. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 10-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. The concentrations of the protein and glycan are indicated in the figure. (A) SGBP-A with cellotetraose; (B) SGBP-B with cellotetraose; (C) SGBP-A with cellohexaose; (D) SGBP-B with cellohexaose. Download
Representative isothermal titration calorimetry (ITC) results for the SGBP-A site-directed mutants with XyG. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 2-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. Concentrations of the protein and glycan are indicated in figure. (A) SGBP-A(W82A W283A W306A); (B) SGBP-A(W82A); (C) SGBP-A(W306A). Download
Affinity PAGE with glycans that are not recognized by SGBP-A and SGBP-B. Affinity electrophoresis (10% acrylamide, native and 0.1% XyG) of SGBP-A and SGBP-B full-length (residues 34 to 489) and truncated proteins expressing domain A (residues 34 to 133), domain B (residues 134 to 229), or domains CD (residues 230 to 489). BSA was included as a control protein. Download
Representative isothermal titration calorimetry (ITC) results for SGBP-B (domains CD) and the full-length site-directed mutants with XyG. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 10-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. The concentrations of the protein and glycan are indicated in the figure. (A) SGBP-B(230–489); (B) SGBP-B(Y363A); (C) SGBP-B(W364A); (D) SGBP-B(F414A). Download
Structure of domains CD of SGBP-B. (A) Overlay of the full-length SGBP-B (pink) with the 1.57-Å structure of the CD domains (gray). Residues involved in binding XyG are shown as sticks. (B) Representative (2Fo − Fc) electron density for the glycan-binding residues from the CD domains’ structure to demonstrate the overall quality of the electron density map. (C) Omit map (2σ) of the electron density observed for the XyGO2 ligand. However, the ligand was not included in the final model as an unambiguous fit between the density and the ligand could not be achieved. Shown is the XyGO2 from the full-length structure for reference. Download
Growth of select XyGUL mutants on xyloglucan and oligosaccharides. Growth of the B. ovatus wild type, the ΔXyGUL mutant, and complemented SGBP-A (ΔSGBP-A::SGBP-A) and SGBP-B (ΔSGBP-B::SGBP-B) in (A) glucose, (B) xylose, (C) XyG, (D) XyGO1, and (E) XyGO2. Complementation was performed by allelic exchange of the genes back into the genome for expression via the native promoter. In panel F, the growth rate of each strain on the five carbon sources is displayed, and in panel G, the normalized lag time of each culture, relative to its growth on glucose, is displayed. Solid bars indicate conditions that are not statistically significant from the WT Δtdk cultures grown on the indicated carbohydrate, while open bars indicate a P value of <0.005 compared to the WT Δtdk strain. Conditions denoted by the same letter (b, c, or d) are not statistically significant from each other but are significantly different from the condition labeled “a.” Complementation of ΔSGBP-A and ΔSBGP-B was performed by allelic exchange of the wild-type genes back into the genome for expression via the native promoter. Download
Differential scanning calorimetry (DSC) thermograms. Shown are results with 40 μmol of SGBP-B, from both the wild type and mutants, as indicated, after instrument baseline (buffer scan) subtraction and concentration normalization. All samples were taken in 50 mM HEPES (pH 7.0). Samples were scanned from 10 to 70°C at a scan rate of 60°C/h. Thermograms have been shifted on the y axis for greater clarity. Download
List of primers used in this study. Restriction sites are underlined. Mutated codons are in boldface.
ACKNOWLEDGMENTS
We thank Eric C. Martens and his laboratory at the University of Michigan Medical School for the use of their anaerobic chamber and plate reader for kinetic growth assays, as well as for the providing the B. ovatus Δtdk strain and B. ovatus ΔXyGUL strain for genetic manipulation and growth experiments, respectively. We thank Eric C. Martens and Darrell W. Cockburn at the University of Michigan Medical School for helpful discussions on experimental design and data interpretation.
Work in Vancouver was initially supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada and UBC Faculty funding, followed by Canadian Institutes for Health Research Operating Grants (FRN: MOP-137134 and MOP-142472). Infrastructure support was provided by the Canadian Foundation for Innovation and the British Columbia Knowledge Development Fund. Work in Michigan was supported by the University of Michigan Medical School Host Microbiome Initiative. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (grant 085P1000817). None of the funders had any role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Tauzin AS, Kwiatkowski KJ, Orlovsky NI, Smith CJ, Creagh AL, Haynes CA, Wawrzak Z, Brumer H, Koropatkin NM. 2016. Molecular dissection of xyloglucan recognition in a prominent human gut symbiont. mBio 7(2):e02134-15. doi:10.1128/mBio.02134-15.
REFERENCES
- 1.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Sommer F, Nookaew I, Sommer N, Fogelstrand P, Bäckhed F. 2015. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol 16:62. doi: 10.1186/s13059-015-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. doi: 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding T, Schloss PD. 2014. Dynamics and associations of microbial community types across the human body. Nature 509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. 2013. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, Henrissat B, Coutinho PM, Minx P, Latreille P, Cordum H, Van Brunt A, Kim K, Fulton RS, Fulton LA, Clifton SW, Wilson RK, Knight RD, Gordon JI. 2007. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol 5:e156. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 10.Martens EC, Kelly AG, Tauzin AS, Brumer H. 2014. The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J Mol Biol 426:3851–3865. doi: 10.1016/j.jmb.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, Gordon JI. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tancula E, Feldhaus MJ, Bedzyk LA, Salyers AA. 1992. Location and characterization of genes involved in binding of starch to the surface of Bacteroides thetaiotaomicron. J Bacteriol 174:5609–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shipman JA, Berleman JE, Salyers AA. 2000. Characterization of four outer membrane proteins involved in binding starch to the cell surface of Bacteroides thetaiotaomicron. J Bacteriol 182:5365–5372. doi: 10.1128/JB.182.19.5365-5372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shipman JA, Cho KH, Siegel HA, Salyers AA. 1999. Physiological characterization of SusG, an outer membrane protein essential for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol 181:7206–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koropatkin NM, Smith TJ. 2010. SusG: A unique cell-membrane-associated alpha-amylase from a prominent human gut symbiont targets complex starch molecules. Structure 18:200–215. doi: 10.1016/j.str.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Koropatkin NM, Martens EC, Gordon JI, Smith TJ. 2008. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16:1105–1115. doi: 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves AR, D’Elia JN, Frias J, Salyers AA. 1996. A Bacteroides thetaiotaomicron outer membrane protein that is essential for utilization of maltooligosaccharides and starch. J Bacteriol 178:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron EA, Maynard MA, Smith CJ, Smith TJ, Koropatkin NM, Martens EC. 2012. Multidomain carbohydrate-binding proteins involved in Bacteroides thetaiotaomicron starch metabolism. J Biol Chem 287:34614–34625. doi: 10.1074/jbc.M112.397380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Elia JN, Salyers AA. 1996. Contribution of a neopullulanase, a pullulanase, and an alpha-glucosidase to growth of Bacteroides thetaiotaomicron on starch. J Bacteriol 178:7173–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hehemann JH, Kelly AG, Pudlo NA, Martens EC, Boraston AB. 2012. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc Natl Acad Sci U S A 109:19786–19791. doi: 10.1073/pnas.1211002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, Rogowski A, Hamilton BS, Chen R, Tolbert TJ, Piens K, Bracke D, Vervecken W, Hakki Z, Speciale G, Munōz-Munōz JL, Day A, Pena MJ, McLean R, Suits MD, Boraston AB, Atherly T, Ziemer CJ, Williams SJ, Davies GJ, Abbott DW, Martens EC, Gilbert HJ. 2015. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517:165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Basle A, Morland C, Day AM, Zheng H, Rogers TE, Thompson P, Hawkins AR, Yadav MP, Henrissat B, Martens EC, Dupree P, Gilbert HJ, Bolam DN. 2015. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun 6:7481. doi: 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, Klinter S, Pudlo NA, Urs K, Koropatkin NM, Creagh AL, Haynes CA, Kelly AG, Cederholm SN, Davies GJ, Martens EC, Brumer H. 2014. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 506:498–502. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDougall GJ, Morrison IM, Stewart D, Hillman JR. 1996. Plant cell walls as dietary fibre: range, structure, processing and function. J Sci Food Agric 70:133–150. [Google Scholar]
- 25.Schultink A, Liu L, Zhu L, Pauly M. 2014. Structural diversity and function of xyloglucan sidechain substituents. Plants 3:526–542. doi: 10.3390/plants3040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron EA, Kwiatkowski KJ, Lee BH, Hamaker BR, Koropatkin NM, Martens EC. 2014. Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis. mBio 5:e01441-01414. doi: 10.1128/mBio.01441-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terrapon N, Lombard V, Gilbert HJ, Henrissat B. 2015. Automatic prediction of polysaccharide utilization loci in Bacteroidetes species. Bioinformatics 31:647–655. doi: 10.1093/bioinformatics/btu716. [DOI] [PubMed] [Google Scholar]
- 28.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. 2009. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem 284:24673–24677. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolam DN, Sonnenburg JL. 2011. Mechanistic insight into polysaccharide use within the intestinal microbiota. Gut Microbes 2:86–90. doi: 10.4161/gmic.2.2.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellrott K, Jaroszewski L, Li W, Wooley JC, Godzik A. 2010. Expansion of the protein repertoire in newly explored environments: human gut microbiome specific protein families. PLoS Comput Biol 6:e1000798. doi: 10.1371/journal.pcbi.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolam DN, Koropatkin NM. 2012. Glycan recognition by the Bacteroidetes Sus-like systems. Curr Opin Struct Biol 22:563–569. doi: 10.1016/j.sbi.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q, Rutland MW, Teeri TT, Brumer H. 2007. Xyloglucan in cellulose modification. Cellulose 14:625–641. doi: 10.1007/s10570-007-9109-0. [DOI] [Google Scholar]
- 33.Koropatkin N, Martens EC, Gordon JI, Smith TJ. 2009. Structure of a SusD homologue, BT1043, involved in mucin O-glycan utilization in a prominent human gut symbiont. Biochemistry 48:1532–1542. doi: 10.1021/bi801942a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Schantz L, Håkansson M, Logan DT, Nordberg-Karlsson E, Ohlin M. 2014. Carbohydrate binding module recognition of xyloglucan defined by polar contacts with branching xyloses and CH-Pi interactions. Proteins 82:3466–3475. doi: 10.1002/prot.24700. [DOI] [PubMed] [Google Scholar]
- 35.Luís AS, Venditto I, Temple MJ, Rogowski A, Baslé A, Xue J, Knox JP, Prates JA, Ferreira LM, Fontes CM, Najmudin S, Gilbert HJ. 2013. Understanding how noncatalytic carbohydrate binding modules can display specificity for xyloglucan. J Biol Chem 288:4799–4809. doi: 10.1074/jbc.M112.432781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukimoto K, Takada R, Araki Y, Suzuki K, Karita S, Wakagi T, Shoun H, Watanabe T, Fushinobu S. 2010. Recognition of cellooligosaccharides by a family 28 carbohydrate-binding module. FEBS Lett 584:1205–1211. doi: 10.1016/j.febslet.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Cho KH, Salyers AA. 2001. Biochemical analysis of interactions between outer membrane proteins that contribute to starch utilization by Bacteroides thetaiotaomicron. J Bacteriol 183:7224–7230. doi: 10.1128/JB.183.24.7224-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakoff-Nahoum S, Coyne MJ, Comstock LE. 2014. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol 24:40–49. doi: 10.1016/j.cub.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elhenawy W, Debelyy MO, Feldman MF. 2014. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio 5:e00909-14. doi: 10.1128/mBio.00909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemsworth GR, Déjean G, Davies GJ, Brumer H. 2016. Learning from microbial strategies for polysaccharide degradation. Biochem Soc Trans 44:94–108. doi: 10.1042/BST20150180. [DOI] [PubMed] [Google Scholar]
- 41.Noinaj N, Guillier M, Barnard TJ, Buchanan SK. 2010. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol 64:43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renzi F, Manfredi P, Mally M, Moes S, enö P, Cornelis GR. 2011. The N-glycan glycoprotein deglycosylation complex (Gpd) from Capnocytophaga canimorsus deglycosylates human IgG. PLoS Pathog 7:e1002118. doi: 10.1371/journal.ppat.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phansopa C, Roy S, Rafferty JB, Douglas CW, Pandhal J, Wright PC, Kelly DJ, Stafford GP. 2014. Structural and functional characterization of NanU, a novel high-affinity sialic acid-inducible binding protein of oral and gut-dwelling Bacteroidetes species. Biochem J 458:499–511. doi: 10.1042/BJ20131415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. 2010. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell 141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, Denancé N, Vasse J, Lauber E, Arlat M. 2007. Plant carbohydrate scavenging through tonB-dependent receptors: a feature shared by phytopathogenic and aquatic bacteria. PLoS One 2:e224. doi: 10.1371/journal.pone.0000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert HJ, Knox JP, Boraston AB. 2013. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opin Struct Biol 23:669–677. doi: 10.1016/j.sbi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Gordon JI. 2012. Honor thy gut symbionts redux. Science 336:1251–1253. doi: 10.1126/science.1224686. [DOI] [PubMed] [Google Scholar]
- 48.Hutkins RW, Krumbeck JA, Bindels LB, Cani PD, Fahey G Jr, Goh YJ, Hamaker B, Martens EC, Mills DA, Rastal RA, Vaughan E, Sanders ME. 2016. Prebiotics: why definitions matter. Curr Opin Biotechnol 37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. 1993. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol 229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 50.Freelove AC, Bolam DN, White P, Hazlewood GP, Gilbert HJ. 2001. A novel carbohydrate-binding protein is a component of the plant cell wall-degrading complex of Piromyces equi. J Biol Chem 276:43010–43017. doi: 10.1074/jbc.M107143200. [DOI] [PubMed] [Google Scholar]
- 51.Holdeman LV, Cato ED, Moore WEC. 1977. Anaerobe laboratory manual. Virginia Polytechnic Institute and State University Anaerobe Laboratory, Blacksburg, VA. [Google Scholar]
- 52.Martens EC, Chiang HC, Gordon JI. 2008. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4:447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326. [DOI] [PubMed] [Google Scholar]
- 54.Terwilliger TC, Adams PD, Read RJ, McCoy AJ, Moriarty NW, Grosse-Kunstleve RW, Afonine PV, Zwart PH, Hung LW. 2009. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr D Biol Crystallogr 65:582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terwilliger TC, Grosse-Kunstleve RW, Afonine PV, Moriarty NW, Zwart PH, Hung LW, Read RJ, Adams PD. 2008. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr D Biol Crystallogr 64:61–69. doi: 10.1107/S090744490705024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58:1948–1954. doi: 10.1107/S0907444902016657. [DOI] [PubMed] [Google Scholar]
- 57.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 58.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long F, Vagin AA, Young P, Murshudov GN. 2008. BALBES: a molecular-replacement pipeline. Acta Crystallogr D Biol Crystallogr 64:125–132. doi: 10.1107/S0907444907050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuomivaara ST, Yaoi K, O’Neill MA, York WS. 2015. Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr Res 402:56–66. doi: 10.1016/j.carres.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Fleites C, Guerreiro CI, Baumann MJ, Taylor EJ, Prates JA, Ferreira LM, Fontes CM, Brumer H, Davies GJ. 2006. Crystal structures of Clostridium thermocellum xyloglucanase, XGH74A, reveal the structural basis for xyloglucan recognition and degradation. J Biol Chem 281:24922–24933. doi: 10.1074/jbc.M603583200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Affinity PAGE with glycans that are not recognized by SGBP-A and SGBP-B. Affinity electrophoresis (10% acrylamide) of SGBP-A and SGBP-B with BSA as a control protein. The percentage of polysaccharide incorporated into each native gel is displayed. Download
Representative isothermal titration calorimetry (ITC) results for SGBP-A and SGBP-B titrations with XyG and XyGO. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 10-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. Concentrations of the protein and glycan are indicated. (A) SGBP-A with XyG; (B) SGBP-B with XyG; (C) SGBP-A with XyGO1; (D) SGBP-B with XyGO1; (E) SGBP-A with XyGO2; (F) SGBP-B with XyGO2. Download
Representative isothermal titration calorimetry (ITC) results for SGBP-A and SGBP-B with cello-oligosaccharides. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 10-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. The concentrations of the protein and glycan are indicated in the figure. (A) SGBP-A with cellotetraose; (B) SGBP-B with cellotetraose; (C) SGBP-A with cellohexaose; (D) SGBP-B with cellohexaose. Download
Representative isothermal titration calorimetry (ITC) results for the SGBP-A site-directed mutants with XyG. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 2-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. Concentrations of the protein and glycan are indicated in figure. (A) SGBP-A(W82A W283A W306A); (B) SGBP-A(W82A); (C) SGBP-A(W306A). Download
Affinity PAGE with glycans that are not recognized by SGBP-A and SGBP-B. Affinity electrophoresis (10% acrylamide, native and 0.1% XyG) of SGBP-A and SGBP-B full-length (residues 34 to 489) and truncated proteins expressing domain A (residues 34 to 133), domain B (residues 134 to 229), or domains CD (residues 230 to 489). BSA was included as a control protein. Download
Representative isothermal titration calorimetry (ITC) results for SGBP-B (domains CD) and the full-length site-directed mutants with XyG. All titrations were performed in 20 mM HEPES–100 mM NaCl (pH 7.0) and at 25°C. In each case, the upper graph shows the raw heat signal for the 10-µl injections of carbohydrate into protein, and the bottom graph shows the integrated heats and, where appropriate, fits to a 1:1 binding model. The concentrations of the protein and glycan are indicated in the figure. (A) SGBP-B(230–489); (B) SGBP-B(Y363A); (C) SGBP-B(W364A); (D) SGBP-B(F414A). Download
Structure of domains CD of SGBP-B. (A) Overlay of the full-length SGBP-B (pink) with the 1.57-Å structure of the CD domains (gray). Residues involved in binding XyG are shown as sticks. (B) Representative (2Fo − Fc) electron density for the glycan-binding residues from the CD domains’ structure to demonstrate the overall quality of the electron density map. (C) Omit map (2σ) of the electron density observed for the XyGO2 ligand. However, the ligand was not included in the final model as an unambiguous fit between the density and the ligand could not be achieved. Shown is the XyGO2 from the full-length structure for reference. Download
Growth of select XyGUL mutants on xyloglucan and oligosaccharides. Growth of the B. ovatus wild type, the ΔXyGUL mutant, and complemented SGBP-A (ΔSGBP-A::SGBP-A) and SGBP-B (ΔSGBP-B::SGBP-B) in (A) glucose, (B) xylose, (C) XyG, (D) XyGO1, and (E) XyGO2. Complementation was performed by allelic exchange of the genes back into the genome for expression via the native promoter. In panel F, the growth rate of each strain on the five carbon sources is displayed, and in panel G, the normalized lag time of each culture, relative to its growth on glucose, is displayed. Solid bars indicate conditions that are not statistically significant from the WT Δtdk cultures grown on the indicated carbohydrate, while open bars indicate a P value of <0.005 compared to the WT Δtdk strain. Conditions denoted by the same letter (b, c, or d) are not statistically significant from each other but are significantly different from the condition labeled “a.” Complementation of ΔSGBP-A and ΔSBGP-B was performed by allelic exchange of the wild-type genes back into the genome for expression via the native promoter. Download
Differential scanning calorimetry (DSC) thermograms. Shown are results with 40 μmol of SGBP-B, from both the wild type and mutants, as indicated, after instrument baseline (buffer scan) subtraction and concentration normalization. All samples were taken in 50 mM HEPES (pH 7.0). Samples were scanned from 10 to 70°C at a scan rate of 60°C/h. Thermograms have been shifted on the y axis for greater clarity. Download
List of primers used in this study. Restriction sites are underlined. Mutated codons are in boldface.