Abstract
Covalent intermolecular cross-linking provides collagen fibrils with stability. The cross-linking chemistry is tissue-specific and determined primarily by the state of lysine hydroxylation at specific sites. A recent study on cyclophilin B (CypB) null mice, a model of recessive osteogenesis imperfecta, demonstrated that lysine hydroxylation at the helical cross-linking site of bone type I collagen was diminished in these animals (Cabral, W. A., Perdivara, I., Weis, M., Terajima, M., Blissett, A. R., Chang, W., Perosky, J. E., Makareeva, E. N., Mertz, E. L., Leikin, S., Tomer, K. B., Kozloff, K. M., Eyre, D. R., Yamauchi, M., and Marini, J. C. (2014) PLoS Genet. 10, e1004465). However, the extent of decrease appears to be tissue- and molecular site-specific, the mechanism of which is unknown. Here we report that although CypB deficiency resulted in lower lysine hydroxylation in the helical cross-linking sites, it was increased in the telopeptide cross-linking sites in tendon type I collagen. This resulted in a decrease in the lysine aldehyde-derived cross-links but generation of hydroxylysine aldehyde-derived cross-links. The latter were absent from the wild type and heterozygous mice. Glycosylation of hydroxylysine residues was moderately increased in the CypB null tendon. We found that CypB interacted with all lysyl hydroxylase isoforms (isoforms 1–3) and a putative lysyl hydroxylase-2 chaperone, 65-kDa FK506-binding protein. Tendon collagen in CypB null mice showed severe size and organizational abnormalities. The data indicate that CypB modulates collagen cross-linking by differentially affecting lysine hydroxylation in a site-specific manner, possibly via its interaction with lysyl hydroxylases and associated molecules. This study underscores the critical importance of collagen post-translational modifications in connective tissue formation.
Keywords: collagen, cyclophilin, glycosylation, hydroxylysine (Hyl), molecular chaperone, post-translational modification (PTM), tendon
Introduction
Collagens comprise a large family of structurally related extracellular matrix proteins (1). Among all of the genetic types of collagen identified, fibrillar type I collagen is the most abundant, providing tissues and organs with form and stability. It is a heterotrimeric molecule composed of two α1 chains and one α2 chain forming a long uninterrupted triple helix with short non-helical domains (telopeptide) at both N and C termini. One of the functionally important characteristics of collagen is its unique, sequential post-translational modifications of Lys residues. The modifications include hydroxylation and mono- and diglycosylation of hydroxylysine (Hyl),3 and oxidative deamination of Lys and Hyl in the N- and C-telopeptides followed by extensive covalent intermolecular cross-linking (2). It is now clear that defective Lys modifications of collagen cause and/or are associated with a broad range of connective tissue disorders, including Ehlers-Danlos syndrome type VIA (3), bronchopulmonary dysplasia (4), Kuskokwim syndrome (5), Bruck syndrome (6, 7), fibrosis (8), disuse osteoporosis (9), and cancer progression (10, 11). Our recent finding showed that a switch of collagen cross-link phenotype may regulate tumor cell invasive and metastatic propensities (12).
Hydroxylation of Lys residues in the nascent proα1(I) and proα2(I) chains occurs in the rough endoplasmic reticulum (ER) before the formation of a triple helix procollagen molecule. The reaction is catalyzed by lysyl hydroxylase 1–3 (LH1–3) in the sequence of X-Lys-Gly and X-Lys-Ala/Ser in the helical and telopeptidyl domains of the procollagen α chains, respectively (13). Studies indicate that LH1 mainly functions as a helical LH, and LH2 functions as a telopeptidyl LH (8, 13–15). LH3, although it possesses both LH and glycosyltransferase activities, may mainly function as a galactosylhydroxylysyl glucosyltransferase in type I collagen (16–19). These initial Lys modifications are critical to determine the fate of the final modification, covalent intermolecular cross-linking, and are therefore important for collagen matrix stability. However, the substrate specificity and the regulatory mechanism of LH activity are still not well understood.
Recent studies on recessive osteogenesis imperfecta (OI) have shed light on a control mechanism suggesting that ER-resident chaperones (i.e. cyclophilin B (CypB) and 65-kDa FK506-binding protein (FKBP65)) may regulate specific LH activity directly impacting collagen cross-linking chemistry (20). CypB, encoded by the PPIB gene, is a peptidyl-prolyl cis-trans-isomerase and a component of the prolyl-3-hydroxylase complex. It is a multifunctional protein implicated in recessive OI (21–24), inflammation (25), and cancer (26). Our recent study on Ppib−/− (hereafter referred to as CypB KO) mice (27) provided evidence that CypB deficiency affects collagen cross-linking by diminishing Lys hydroxylation specifically at the helical cross-linking sites in bone type I collagen. This was surprising because the rate of procollagen chain folding in CypB KO cells was impaired, and this, according to the traditional view, would have resulted in Lys overmodification (23). Thus, the decrease in Lys hydroxylation seen in CypB KO bone is due in part to an impaired interaction between CypB and LH1 that facilitates the helical LH activity, as proposed by Ishikawa et al. (28). However, the tissue- and molecular site-specific effect of CypB deficiency on Lys modifications and its mechanisms are still not well defined.
To gain further insight into the function of CypB in Lys post-translational modifications of collagen, fibrillogenesis, and its potential mechanism, we analyzed type I collagen in CypB KO mouse tendon, another type I collagen-rich tissue. Here we report intriguing findings indicating that CypB may differentially regulate Lys modifications at the helical and telopeptidyl cross-linking sites of collagen, which could be important for the development and organization of tendon.
Experimental Procedures
Animal care and experiments were performed in accordance with a protocol approved by the NICHD, National Institutes of Health, animal care and use committee.
Ppib Null Mice
Ppib null mice have recently been generated as a mouse model of recessive OI (27). In this model, Ppib transcripts and CypB protein were not detected in primary cells and tissues.
Histological Evaluation
Wild type (WT), heterozygous (Het), and CypB KO mouse tails were transected at the proximal end, and the histological sections were prepared from the area 3 mm from the proximal end. The specimens were immersed for 3 days with 10% formalin and demineralized with 0.5 m EDTA (pH 7.4) for 2 weeks, immersed in 70% ethyl alcohol, dehydrated through ascending gradations of ethanol, embedded in paraffin, and sectioned into 5-μm-thick slices. After hydration, the slices were stained with hematoxylin and eosin (H&E) and observed under light microscopy (Olympus BX40 microscope, Olympus, Tokyo, Japan). In addition, to evaluate the organization and maturation of tendon collagen matrices, the sections were also stained with 0.1% solution of Sirius Red in saturated aqueous picric acid (Electron Microscopy Sciences, Hatfield, PA) for 60 min, washed with 0.01 n HCl, dehydrated, and mounted. The fascicles of tail tendon were observed under polarized light microscopy (BX40 microscope) and photographed as reported previously (29).
Characterization of Collagen Fibrils by Transmission EM
The specimens were prepared from the same areas as above, fixed with 2.5% EM grade glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.4, for 3 days, and demineralized with 0.5 m EDTA (pH 7.4) for 2 weeks. The samples were then postfixed in potassium ferrocyanide-reduced osmium for 1 h at room temperature. After rinsing with distilled water, the samples were dehydrated with a graded series of ethanol concentrations and embedded in PolyBed-812 epoxy resin (Polysciences, Warrington, PA). Sections of 70-nm thickness were cut, mounted on copper Formvar-carbon filmed grids, and stained with 4% urinary acetate and Reynolds' lead citrate (30). Cross-sectional and longitudinal views of the collagen fibrils were observed using a LEO EM-910 transmission electron microscope operating at 80 kV (Carl Zeiss SMT, Peabody, MA), and images were taken at ×25,000 using a Gatan Orius SC1000 CCD camera with Digital Micrograph 3.11.0 (Gatan, Inc., Pleasanton, CA). For each sample, the diameters of 3,000 fibrils were measured using ImageJ version 1.44p software (National Institutes of Health, Bethesda, MD).
Collagen Preparation for Biochemical Analysis
Tendons were prepared from 2-month-old WT, Het, and CypB KO mouse tails. Both proximal and distal ends of the tail (∼2 mm from each end) were cut off, the skin was removed, and tendon collagen fibers were carefully removed using forceps. All operations were carried out on ice or at 4 °C. The dissected tendons were washed several times with cold phosphate-buffered saline (PBS) and then with cold distilled water by repeated centrifugation at 4,000 × g for 30 min and lyophilized.
Collagen Type Analysis
Lyophilized tendons from WT, Het, and KO mice were heated at 60 °C for 15 min in 50 mm sodium phosphate buffer (pH 7.2), and the supernatants (gelatin) were collected by centrifugation. Collagen types were identified by LC-MS/MS analysis following trypsin digestion with searching the acquired MS/MS spectra against the UniProtKB/Swiss-Prot database (release 2014_08) using ProteinPilot software version 4.0 (AB Sciex, Foster City, CA) as described previously (31). Types I and III collagen were further quantified by multiple reaction monitoring (MRM) mode using stable isotope-labeled collagen (SI-collagen) as an internal standard (32). In brief, SI-collagen was first mixed into the gelatin samples, and protease digestion by sequencing grade trypsin (Promega, Madison, WI; 1:100 enzyme/substrate ratio) was performed in 100 mm Tris-HCl, 1 mm CaCl2 (pH 7.6) at 37 °C for 16 h. The tryptic peptide solutions were subjected to LC-electrospray ionization-MS using a hybrid triple quadrupole/linear ion trap 3200 QTRAP mass spectrometer (AB Sciex) coupled to an Agilent 1200 series HPLC system (Agilent Technologies, Palo Alto, CA). Marker peptides of type I and III collagens were monitored by MRM mode with reverse-phase separation using an Ascentis Express C18 HPLC column (5-μm particle size, length × inner diameter 150 mm × 2.1 mm; Supelco, Bellefonte, PA) (32). Data acquisition and analysis were performed using Analyst version 1.6.2 (AB Sciex). Concentrations of type I and type III collagens were estimated by the peak area ratio of the marker peptides relative to the isotopically heavy peptides derived from SI-collagen.
Site-specific Characterization of Post-translational Modifications of Type I Collagen
The tendon gelatin samples were digested with trypsin (1:100 enzyme/substrate ratio) in 100 mm Tris-HCl, 1 mm CaCl2 (pH 7.6) at 37 °C for 16 h to analyze the Lys and Pro post-translational modifications at the specific molecular sites within the triple helical domain of type I collagen. In parallel, the samples were also digested with recombinant collagenase from Grimontia hollisae (Wako Chemicals, Osaka, Japan; 1:20 enzyme/substrate ratio) (33) in 100 mm Tris-HCl, 5 mm CaCl2 (pH 7.5) at 37 °C for 16 h to analyze Lys hydroxylation at N- and C-telopeptides of type I collagen. The peptide solutions digested with trypsin or collagenase were analyzed by LC-electrospray ionization-MS. Peptides were separated on the Ascentis Express C18 HPLC column at a flow rate of 500 μl/min with a binary gradient as follows: 98% solvent A (0.1% formic acid) for 2.5 min, linear gradient of 2–50% solvent B (100% acetonitrile) for 12.5 min, 90% solvent B for 2.5 min, and 98% solvent A for 2.5 min. Capillary voltage was 4.5 kV, declustering potential was 30 V, heater gas temperature was 700 °C, curtain gas was 40 p.s.i., nebulizer gas was 50 p.s.i., and heater gas was 80 p.s.i. Collision energy was 50 V with a collision energy spread of ±15 V for MS/MS analysis. Peptide identification was performed by searching the acquired MS/MS spectra against the UniProtKB/Swiss-Prot database with ProteinPilot software version 4.0. Site occupancy of Lys hydroxylation/glycosylation (Lys, Hyl, galactosyl-Hyl (G-Hyl), and glucosylgalactosyl-Hyl (GG-Hyl)) and Pro 3-hydroxylation (3-Hyp) were calculated using the ratio of peak areas of peptides containing the respective molecular species as reported previously (27). For Lys hydroxylation at the C-telopeptide domain, the site occupancy was calculated by a ratio of additionally combined peak areas of MS/MS fragments of +16 Da precursor ion derived from those with Lys and Pro hydroxylation, respectively.
Reduction of Collagen with NaB3H4
Dried tendon samples (∼1.0 mg each) were suspended in buffer containing 0.15 m N-trismethyl-2-aminoethanesulfonic acid and 0.05 m Tris-HCl, pH 7.4, and reduced with standardized NaB3H4. The specific activity of the NaB3H4 was determined by the method described previously (34, 35). The reduced samples were washed with cold distilled water several times by repeated centrifugation at 4,000 × g and lyophilized.
Quantification of GG-Hyl, G-Hyl, and Free Hyl by HPLC
Reduced collagen was hydrolyzed with 6 n HCl and subjected to amino acid analysis as described previously (36). The level of total Hyl (GG-Hyl, G-Hyl, and free Hyl) in a collagen molecule was calculated based on the value of 300 residues of Hyp per collagen molecule. To determine the level of glycosylated Hyl, collagen samples were also subjected to base hydrolysis with 2 n NaOH. GG-Hyl, G-Hyl, and free Hyl in the hydrolysates were separated with a specific gradient program on HPLC and quantified as residues/collagen molecule as reported (17).
Cross-link Analysis
Reduced collagen was hydrolyzed with 6 n HCl and subjected to cross-link analysis as described previously (36). Upon reduction, dehydrodihydroxylysinonorleucine (dehydro-DHLNL)/its ketoamine, dehydrohydroxylysinonorleucine (dehydro-HLNL)/its ketoamine, and dehydrohistidinohydroxymerodesmosine (dehydro-HHMD) are reduced to stable secondary amines, DHLNL, HLNL, and HHMD. The reducible cross-links were analyzed as their reduced forms (i.e. DHLNL, HLNL, and HHMD). Hereafter, the terms DHLNL, HLNL, and HHMD will be used for both the unreduced and reduced forms. The levels of immature reducible (DHLNL, HLNL, and HHMD) and mature non-reducible cross-links (Pyr and d-Pyr) were quantified as moles/mole of collagen.
The glycosylated immature reducible cross-links were analyzed employing base hydrolysis of the reduced samples as described above (34). By applying the hydrolysates to the HPLC system, the glycosylated (GG- and G-) and non-glycosylated cross-links were separated. These forms of cross-links were quantified as moles/mole of collagen, as reported previously (18). As for the mature non-reducible cross-link, Pyr, its glycosylated forms were not quantified because it is labile with base hydrolysis (37).
Interaction of CypB with Lysyl Hydroxylases 1–3 and FKBP65
The pLVX-Puro2 plasmid was modified from pLVX-Puro (Clontech) plasmid by adding the SbfI and NotI cutting sites after the XbaI site. The C-terminal 3× FLAG-tagged LH (LH1, LH2, and LH3) cDNAs were cloned into the XbaI and NotI sites on pLVX-Puro2 to generate expression plasmids of LH1-3FLAG, LH2-3FLAG, and LH3-3FLAG. The C-terminal Myc tagged CypB cDNA was cloned into the XhoI and BamHI sites on pLVX-Puro to generate CypB-Myc expression plasmid. The C-terminal HA-HEEL-tagged FKBP65 cDNA was cloned into the XhoI and BamHI sites on pLVX-Puro to generate FKBP65-HA expression plasmid. The HEEL is the ER retention sequence of FKBP65. Mouse P21 cDNA was cloned in frame into the HindIII and SalI sites on pCMV-3Tag-7 plasmid (Agilent) to generate the P21-Myc expression plasmid as a negative control. 293F cells were transfected with different doses (0, 0.1, and 0.5 μg) of Myc-CypB or 0.5 μg of Myc-tagged P21 plasmid and 4 μg of FLAG-LHs or HA-tagged FKBP65 plasmids using Fugene 6 transfection reagent (Promega). At 48 h after transfection, cells were lysed with cell lysis buffer (Cell Signaling Technology), and the total cell lysates were precleared with PBS with protein G-agarose. The lysates were incubated with the Myc antibody (Millipore) on a rotator at 4 °C overnight, and protein G beads were added to pull down the antibody-protein complexes. The beads were washed with PBS supplemented with PMSF five times, and the protein complexes were resolved from the beads by adding 1× SDS loading buffer and boiling for 5 min. The protein samples were subjected to Western blotting analysis to detect the presence of proteins with the FLAG (Sigma), Myc, and HA (Cell Signaling Technology) antibodies.
Statistical Analysis
Statistical analyses were performed using Jmp® version 8.0 software (SAS Institute Inc., Cary, NC). Statistical differences were determined by Kruskal-Wallis one-way analysis of variance and means comparison by Student's t test. The data are presented as means ± S.D., and a p value of <0.05 was considered significant.
Results
Histological Analysis of Collagen Matrix in Tendon
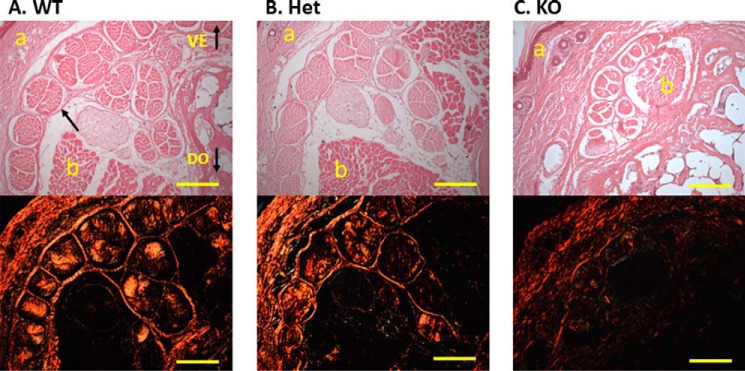
The effect of CypB deficiency on tendon collagen matrix was first examined by light microscopy with H&E and picrosirius red staining (Fig. 1). H&E-stained cross-sections of tails demonstrated that tendon collagen bundles in WT and Het mice were highly organized, and each bundle group contained 10–12 fascicles. In KO mice, however, collagen bundles were significantly smaller than WT and Het, fragmented, and poorly organized, and each bundle contained only 5–7 fascicles. When stained with picrosirius red and observed under polarized light, the collagen matrix of WT and Het mice showed a relatively uniform orange color indicative of thick and tightly packed collagen matrix. However, the KO tendon showed a green and, in some areas, orange/red color, indicating that the collagen fibers are generally thin and are not packed in an orderly fashion.
FIGURE 1.
Histological evaluation of tail tendon. Tail tendons were transected and stained with H&E (top) and picrosirius red (bottom) from wild-type (A), heterozygous (B), and CypB KO (C) mice. CypB KO mouse tendon contained fewer collagen bundles, each consisting of fewer fascicles that are poorly organized and have an abnormal morphology. The collagen fibers in the CypB KO tendon showed a green and, in some areas, orange/red color, whereas those of wild-type and heterozygous mouse tendon show a relatively uniform orange color with picrosirius staining. The arrows indicate the single fascicle in the tendon. VE, ventral side of tendon; DO, dorsal side of tendon; a, dermis; b, muscle. Bar, 200 μm.
Characterization of Collagen Fibrils by Transmission Electron Microscopy
Tendon collagen fibrils were then analyzed by transmission electron microscopy. Cross-sectional and longitudinal views of collagen fibrils and diameter distribution are shown in Fig. 2. Cross-sectional images demonstrated that collagen fibril diameters in CypB KO tendon were markedly smaller than WT and Het and irregular in shape. Longitudinal sections in CypB KO mice also revealed thin fibrils with varied orientation and often showed branching into smaller fibrils, as compared with WT and Het. The fibril diameters were measured from 3,000 randomly selected fibrils of each animal. In CypB KO mice, the mean fibril diameters and their ranges were about one-third the size of fibrils in WT and Het mice (CypB KO, 55 ± 51 nm versus WT (174 ± 76 nm) and Het (176 ± 90 nm); p < 0.001). The results suggest aberrant lateral growth of fibrils and/or incorporation of collagen molecules into fibrils in CypB KO mice. Sections from three animals in each group were examined, and all showed similar patterns.
FIGURE 2.
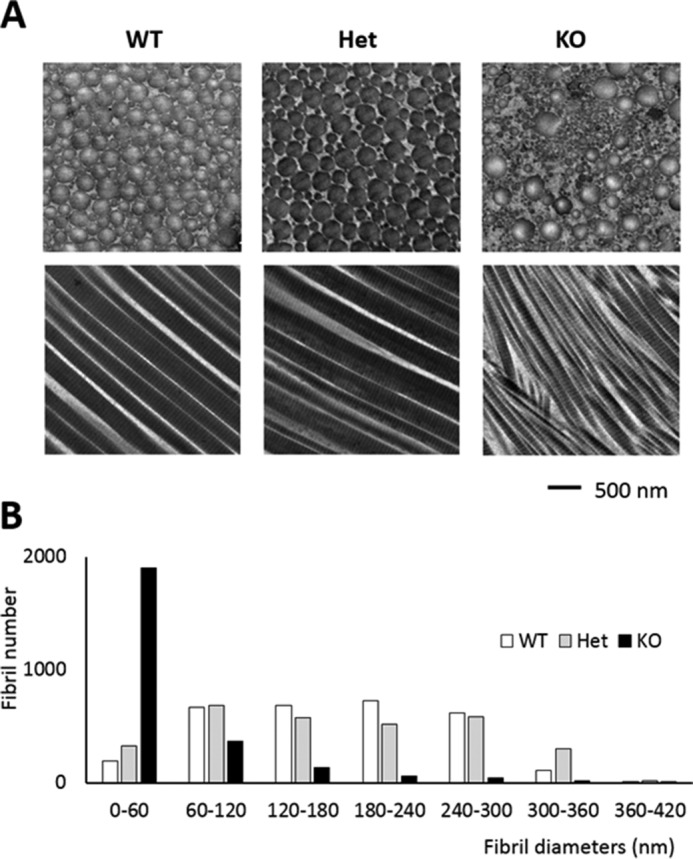
Ultrastructural analysis of collagen fibrils in tail tendon by transmission electron microscopy. A, cross-sectional views of the tail tendon collagen fibrils from WT, Het, and CypB KO mice. KO tendon fibrils are irregular in shape and markedly smaller in diameter. Longitudinal sections in KO showed an axial twist and branch into smaller fibrils. Scale bar, 500 nm. B, diameter distribution measured from cross-sections. Three thousand fibrils in each group were measured and plotted.
Collagen Type
Collagen type was first analyzed by LC-MS/MS analysis using tryptic digests of gelatinized tendon samples. Almost all identified peptides were derived from type I collagen in the tendon samples, and a minute amount of peptide from type III collagen was identified only in CypB KO mice (data not shown). Type V collagen was not identified in any of the samples. Collagen types I and III were further analyzed by MRM analysis using SI-collagen as an internal standard (32). Although type III collagen is a very minor collagen type in tendon, MRM peaks of marker peptides of type III collagen were detected in all of the samples by this method. This highly sensitive MS analysis revealed that type III collagen content was slightly increased in KO tendon (5.3%) compared with WT (0.4%) and Het (1.9%). Thus, the tendon collagen is predominantly type I collagen.
Lysine Hydroxylation and Hydroxylysine Glycosylation
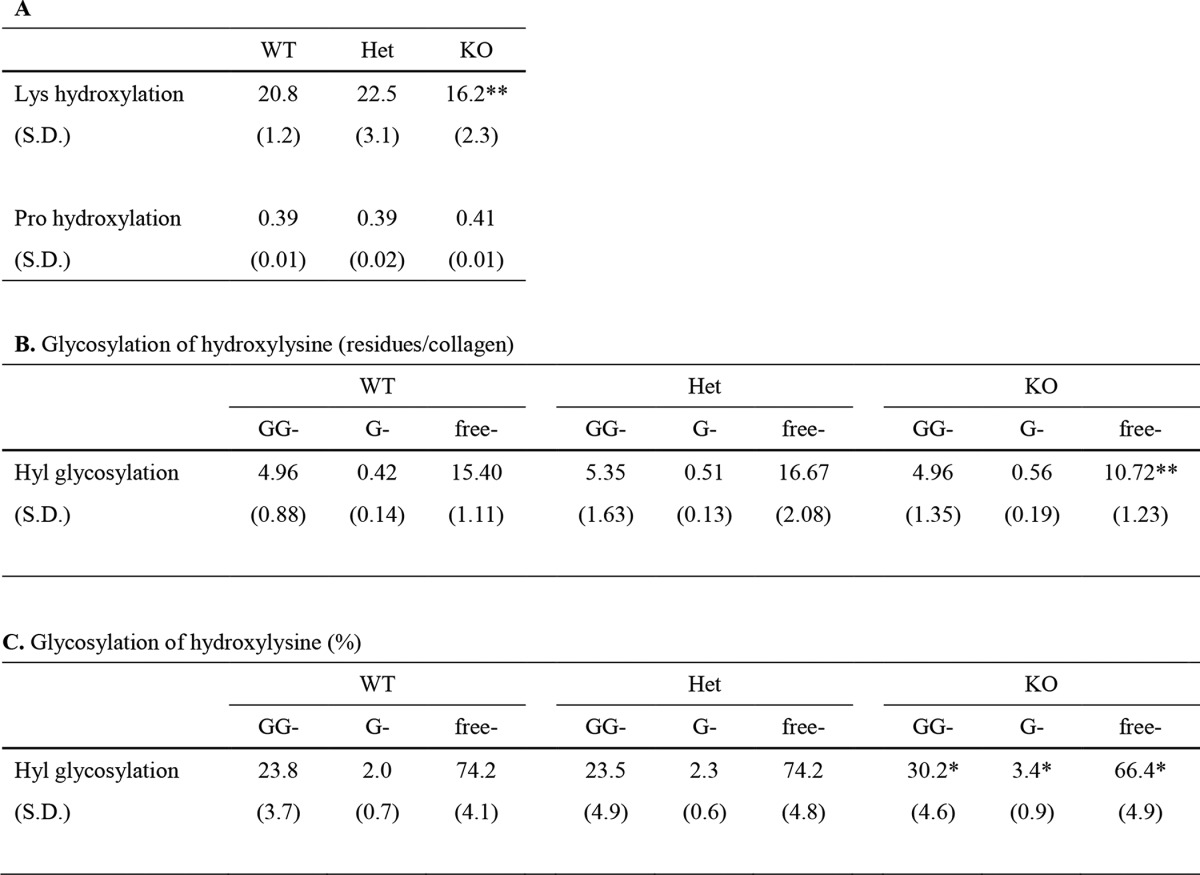
Levels of Lys hydroxylation and mono- (G-) and diglycosylation (GG-) of Hyl residues of collagen were quantified by HPLC as reported (17). In CypB KO mouse collagen, the extent of Lys hydroxylation was moderately but significantly decreased by 22–28% as compared with those from WT and Het mice (p < 0.001) (Table 1A). Because type I collagen represents the majority of collagen (see above), this indicates that the level of Lys hydroxylation in the helical domain of type I collagen molecule is lower in KO mouse tendon. Although the decrease in Lys hydroxylation was not as striking as in KO skin collagen (27), it is still distinct from KO bone collagen, in which the extent of Lys hydroxylation was slightly higher than in WT and Het (27). The level of Pro hydroxylation in KO collagen was comparable with those of WT and Het (Table 1A), which is the same as in bone and skin collagen (27). This suggests that CypB does not affect 4-hydroxylation of Pro in type I collagen. The results on Hyl glycosylation revealed that there was a significant decrease in the level of free Hyl in KO collagen in comparison with those of WT and Het (p < 0.001), whereas the levels of both GG- and G-Hyl were comparable among the three groups (Table 1B). However, when calculated as percentages of GG-Hyl, G-Hyl, and free Hyl in total Hyl (Table 1C), both GG- (30.2%) and G-Hyl (3.4%) in KO collagen were slightly but significantly higher than in WT (23.8% GG-Hyl (p < 0.01) and 2.0% G-Hyl (p < 0.01)) and Het (23.5% GG-Hyl (p < 0.01) and 2.3% G-Hyl (p < 0.01)). The percentage of free Hyl in KO collagen (66.4%) was significantly lower compared with those of WT (74.2%) and Het (74.2%) (p < 0.01 in WT, and p < 0.01 in Het) (Table 1C).
TABLE 1.
Hydroxylation of lysine and proline, glycosylation of hydroxylysine (residues/mole of collagen), and percentages of glycosylation in total hydroxylysine of WT, Het, and CypB KO mouse tendon collagen
Values represent mean ± S.D. (n = 10) of triplicate analysis of the hydrolysates. A, Lys hydroxylation was calculated as Hyl/collagen based on the value of 300 residues of Hyp/collagen. Pro hydroxylation was calculated by Hyp/(Pro + Hyp). B, glycosylation of hydroxylysine was calculated as residues/collagen. free, nonglycosylated Hyl. C, glycosylation of hydroxylysine (%) represents the relative levels of GG-Hyl, G-Hyl, and free Hyl (GG-Hyl + G-Hyl + free-Hyl = 100%).
*Significantly different from both WT and Het (p < 0.01).
**Significantly different from both WT and Het (p < 0.001).
Alterations of Collagen Post-translational Modifications at Specific Molecular Loci
The distributions of Pro 3-hydroxylation and Lys hydroxylation/glycosylation at specific sites within the triple helical region of type I collagen were semiquantitatively estimated by LC-MS analysis using tryptic digests of gelatinized tendon samples (Table 2). The values of WT and Het were almost identical to each other with no statistical difference. In CypB KO tendon, a significant reduction of Pro 3-hydroxylation at α1(I) Pro-986 was observed (82.1% for WT, 84.4% for Het, and 17.0% for KO), consistent with our previous report for bone and skin (27). Only slightly lower 3-hydroxylation was observed at α2(I) Pro-707 (76.3% for WT, 82.1% for Het, and 67.5% for KO). The Pro 3-hydroxylation at α1(I) Pro-707 was not affected by the absence of CypB (43.2% for WT, 47.1% for Het, and 44.9% for KO).
TABLE 2.
Summary of site-specific modification analysis by mass spectrometry of non-cross-linked, hydroxylated (this table shows Pro hydroxylation), and glycosylated residues in mice tail tendon type I collagen
| Site occupancy |
||||
|---|---|---|---|---|
| WT | Het | KO | ||
| % | % | % | ||
| α1(I) Pro-986 | Pro | 17.9 ± 1.2 | 15.6 ± 0.7 | 83.0 ± 2.7a,b |
| 3-Hyp | 82.1 ± 1.2 | 84.4 ± 0.7 | 17.0 ± 2.7a,b | |
| α1(I) Pro-707 | Pro | 56.8 ± 3.5 | 52.9 ± 2.9 | 55.1 ± 6.1 |
| 3-Hyp | 43.2 ± 3.5 | 47.1 ± 2.9 | 44.9 ± 6.1 | |
| α2(I) Pro-707 | Pro | 23.7 ± 4.9 | 17.9 ± 1.7 | 32.5 ± 8.8b |
| 3-Hyp | 76.3 ± 4.9 | 82.1 ± 1.7 | 67.5 ± 8.8b | |
| α1(I) Lys-87 | Lys | 9.0 ± 0.6 | 3.6 ± 0.3 | 38.5 ± 5.0a,b |
| Hyl | 66.9 ± 2.4 | 68.5 ± 2.3 | 39.1 ± 1.6a,b | |
| G-Hyl | 4.9 ± 1.3 | 6.3 ± 1.0 | 4.0 ± 0.3b | |
| GG-Hyl | 19.2 ± 1.5 | 21.7 ± 1.4 | 18.4 ± 3.4 | |
| α1(I) Lys-174 | Lys | 58.7 ± 0.8 | 59.5 ± 3.0 | 54.1 ± 3.4 |
| Hyl | 37.7 ± 0.7 | 37.2 ± 2.5 | 38.0 ± 3.0 | |
| G-Hyl | 2.0 ± 0.1 | 1.9 ± 0.4 | 4.9 ± 0.4a,b | |
| GG-Hyl | 1.6 ± 0.1 | 1.5 ± 0.2 | 2.9 ± 0.4a,b | |
| α1(I) Lys-219 | Lys | 83.5 ± 1.4 | 82.6 ± 1.1 | 73.8 ± 3.6a,b |
| Hyl | 16.5 ± 1.4 | 17.4 ± 1.1 | 26.2 ± 3.6a,b | |
| α1(I) Lys-603 | Lys | 33.6 ± 0.4 | 34.5 ± 2.1 | 32.9 ± 3.2 |
| Hyl | 66.4 ± 0.4 | 65.5 ± 2.1 | 67.1 ± 3.2 | |
| α2(I) Lys-87 | Lys | 4.4 ± 0.4 | 4.9 ± 0.4 | 44.7 ± 2.1a,b |
| Hyl | 95.6 ± 0.4 | 95.1 ± 0.4 | 55.3 ± 2.1a,b | |
| α2(I) Lys-174 | Lys | 25.5 ± 3.0 | 31.9 ± 7.9 | 80.0 ± 1.2a,b |
| Hyl | 70.7 ± 4.3 | 65.6 ± 6.9 | 16.5 ± 0.9a,b | |
| G-Hyl | 3.4 ± 1.3 | 2.0 ± 0.6 | 1.9 ± 0.4 | |
| GG-Hyl | 0.3 ± 0.1 | 0.5 ± 0.4 | 1.5 ± 0.9 | |
| α2(I) Lys-219 | Lys | 67.0 ± 0.2 | 69.7 ± 3.3 | 55.7 ± 2.5a,b |
| Hyl | 32.2 ± 0.2 | 29.7 ± 3.2 | 41.6 ± 2.6a,b | |
| G-Hyl | 0.4 ± 0.1 | 0.3 ± 0.1 | 1.2 ± 0.2a,b | |
| GG-Hyl | 0.4 ± 0.1 | 0.3 ± 0.0 | 1.5 ± 0.4a,b | |
a p < 0.05 between WT and KO.
b p < 0.05 between Het and KO.
Using the tryptic digests, we then analyzed Lys modifications in the triple helical region of type I collagen. These modifications were found to be in a site-specific manner in the absence of CypB. The extent of Lys hydroxylation in WT and Het type I collagen was also almost identical at all sites analyzed (p > 0.1 at all of the sites). In KO tendon type I collagen, significant underhydroxylation was observed at α1 Lys-87 (66.9% for WT, 68.5% for Het, and 39.1% for KO), α2 Lys-87 (95.6% for WT, 95.1% for Het, and 55.3% for KO), and α2-Lys-174 (70.7% for WT, 65.6% for Het, and 16.5% for KO). However, Lys hydroxylation at α1 Lys-174, α1 Lys-219, α1 Lys-603, and α2 Lys-219 was unchanged or slightly increased in CypB KO type I collagen. Although the extent of overall glycosylation in tendon type I collagen is markedly lower than that from skin and bone (32), the effects of CypB KO on glycosylation patterns were found to be site-specific. When calculated as percentages of GG-, G-, and free-Hyl in total Hyl (Table 3), GG-Hyl at α1-87, the major glycosylation site, was significantly higher in CypB KO collagen compared with in WT and Het, whereas G-Hyl at this site showed no difference. Other sites (i.e. α1-174, α2-174, and α2-219) were glycosylated at lower levels compared with α1-87, but the relative abundances of both GG- and G-Hyl in CypB KO collagen were higher than in WT and Het. Glycosylation at α1-759 that was identified at a very low level in bone (27) was not detected in any of the tendon samples.
TABLE 3.
Glycosylation of hydroxylysine residues estimated by mass spectrometry of non-cross-linked, glycosylated peptides
Hyl + G-Hyl + GG-Hyl = 100%.
| Site occupancy |
||||
|---|---|---|---|---|
| WT | Het | KO | ||
| % | % | % | ||
| α1(I) Lys-87 | Hyl | 73.5 ± 2.4 | 71.0 ± 1.9 | 63.6 ± 2.2a,b |
| G-Hyl | 5.4 ± 1.1 | 6.5 ± 0.9 | 6.6 ± 0.5 | |
| GG-Hyl | 21.1 ± 1.3 | 22.5 ± 1.2 | 29.8 ± 2.6a,b | |
| α1(I) Lys-174 | Hyl | 91.3 ± 0.1 | 91.7 ± 0.6 | 82.9 ± 1.2a,b |
| G-Hyl | 4.8 ± 0.1 | 4.6 ± 0.6 | 10.8 ± 0.7a,b | |
| GG-Hyl | 3.9 ± 0.2 | 3.7 ± 0.2 | 6.3 ± 0.5a,b | |
| α2(I) Lys-174 | Hyl | 94.9 ± 1.7 | 96.4 ± 0.8 | 82.6 ± 2.0a,b |
| G-Hyl | 4.7 ± 1.7 | 2.9 ± 0.5 | 9.7 ± 1.6a,b | |
| GG-Hyl | 0.4 ± 0.1 | 0.6 ± 0.4 | 7.7 ± 3.5 | |
| α2(I) Lys-219 | Hyl | 97.4 ± 0.2 | 98.0 ± 0.1 | 93.9 ± 1.0a,b |
| G-Hyl | 1.3 ± 0.4 | 0.9 ± 0.1 | 2.7 ± 0.4a,b | |
| GG-Hyl | 1.3 ± 0.2 | 1.1 ± 0.0 | 3.3 ± 0.7a,b | |
a p < 0.05 between WT and KO.
b p < 0.05 between Het and KO.
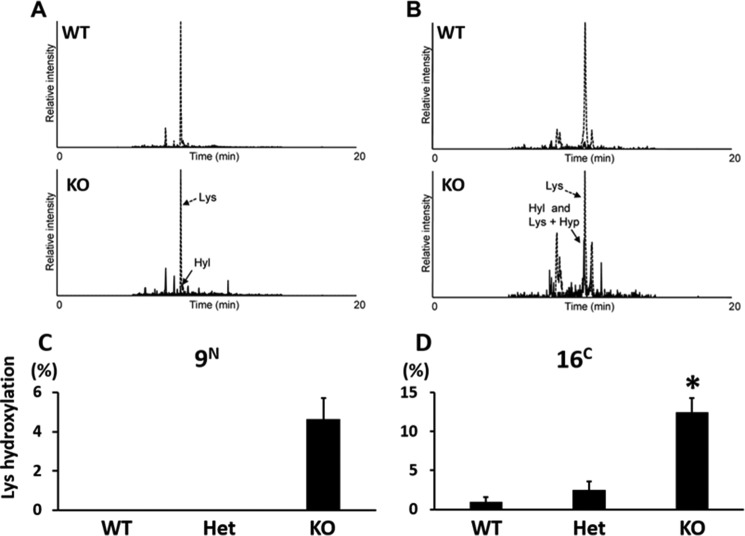
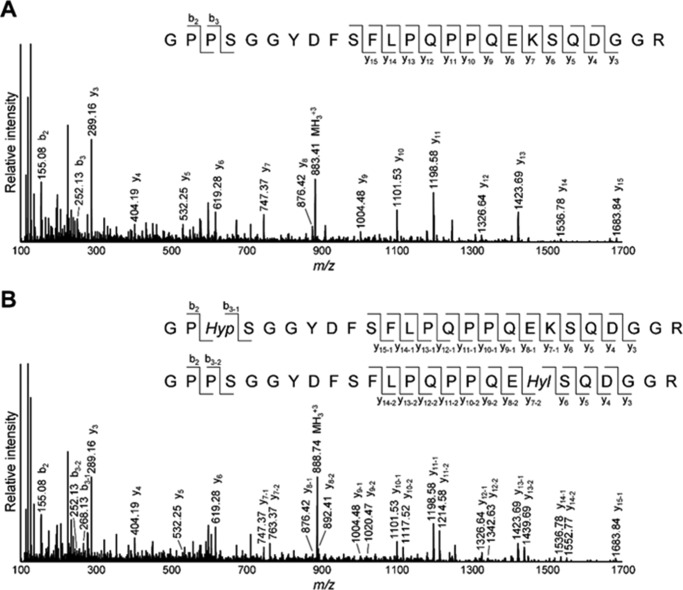
We then analyzed Lys hydroxylation in the telopeptides of type I collagen after digestion by G. hollisae collagenase (33). With this treatment, the triple helical region of collagen was cleaved mostly into the tripeptides (i.e. Gly-X-Y), the majority of which flowed through the reversed phase column (C18) used for LC-MS. The N- and C-telopeptides of type I collagen retained in the column were then eluted and analyzed. We identified α1(I) N-telopeptide (GYDEKSAGVSVP) and heterogeneous α1(I) C-telopeptide (GPPSGGYDFSFLPQPPQEKSQDGGR, -RY, and -RYY), although α2(I) N-telopeptide was not detected by the LC-MS analysis, probably because of its involvement in the intra- and/or intermolecular cross-linking. In the case of α1(I) N-telopeptide, because Grimontia collagenase consistently generated the shorter peptide from the telopeptide region compared with Clostridium collagenase generating pQMSYGYDEKSAGVSVPGPM (pQ indicates pyroglutamic acid) (38), Lys hydroxylation can be simply estimated by an increase in the mass of 16 Da in the MS scan (Fig. 3A). Whereas Hyl-containing α1(I) N-telopeptide was not detected in WT and Het tendon, ∼5% Lys hydroxylation at this site was observed in CypB KO tendon (Fig. 3, A and C). α1(I) C-telopeptide generated by Grimontia collagenase was found to contain Hyp in addition to Hyl by MS/MS analysis (Fig. 4). Thus, we estimated Lys hydroxylation at the C-telopeptide using both MS scan and MS/MS fragmentation of the +16 Da species (Figs. 3B and 4B). It was found that the level of Lys hydroxylation in the C-telopeptide in KO was also significantly increased (12.4%) in comparison with WT (0.9%) and Het (2.5%) (Fig. 3D). The absence of CypB seemed to have a larger impact on the Lys hydroxylation in the C-telopeptide. The higher Lys hydroxylation in the telopeptides is consistent with the generation of Hylald-derived cross-links in KO tendon collagen (see below).
FIGURE 3.
Lysine hydroxylation in the N- and C-telopeptides of tendon type I collagen. Gelatinized tendon samples were digested by Grimontia collagenase and subjected to LC-MS analysis. A, monoisotopic extracted ion chromatograms of α1(I) N-telopeptide (GYDEKSAGVSVP, z = 2; m/z 604.79–605.29 for Lys and m/z 612.79–613.29 for Hyl). B, monoisotopic extracted ion chromatograms of α1(I) C-telopeptide (GPPSGGYDFSFLPQPPQEKSQDGGR, z = 3; m/z 883.41–883.74 for Lys and m/z 888.74–889.07 for Hyl and Lys + Hyp). Peptide containing Hyl and Hyp (m/z 894.08–894.41, z = 3) was under the detection limit. C, Lys hydroxylation at α1(I) N-telopeptide estimated from the peak area ratio of extracted ion chromatograms shown in A. D, Lys hydroxylation at α1(I) C-telopeptide. The site occupancy was estimated by multiplicatively combining the peak area ratio of extracted ion chromatograms shown in B and that of y11–1 ion (Lys + Hyp, m/z 1198.58) and y11–2 ion (Hyl, m/z 1214.58) generated by MS/MS fragmentation of the m/z 888.74 peak (Fig. 4B). Error bars, S.D.
FIGURE 4.
LC-MS/MS analysis of collagenase-digested α1(I) C-telopeptide. A, MS/MS spectrum of α1(I) C-telopeptide containing Lys (m/z 883.41, z = 3) from CypB KO tendon. B, MS/MS spectrum of α1(I) C-telopeptide containing Hyl and Lys + Hyp (m/z 888.74, z = 3) from CypB KO tendon.
Collagen Cross-link Analysis
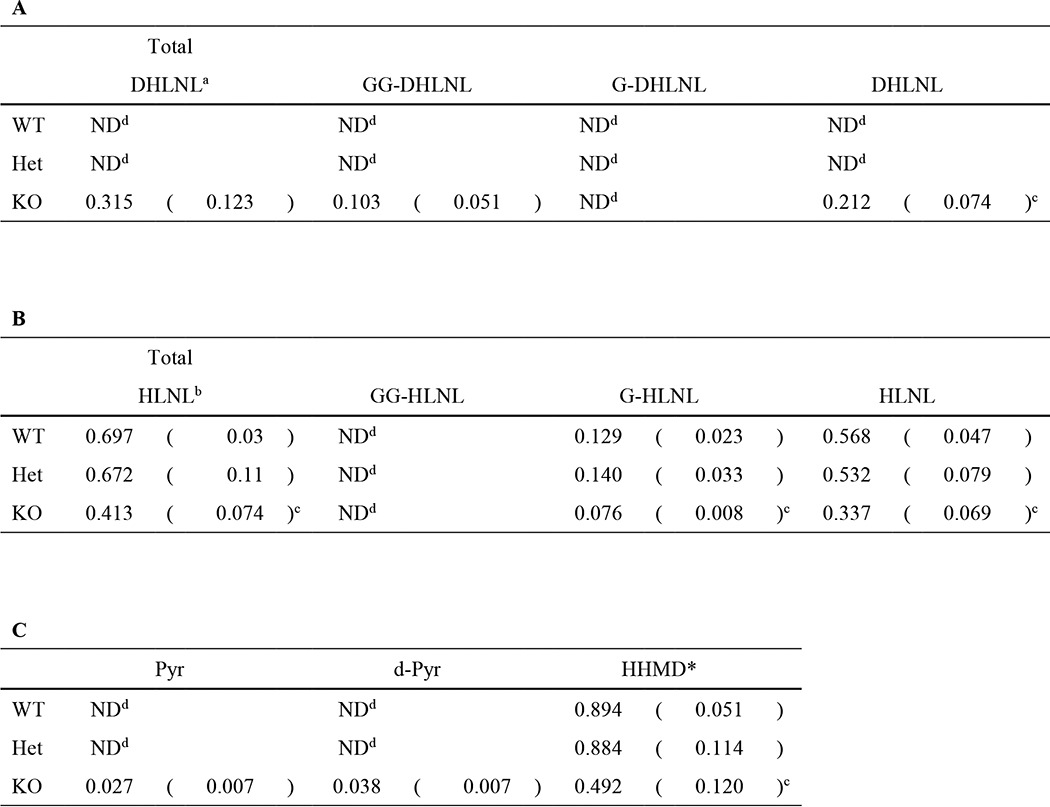
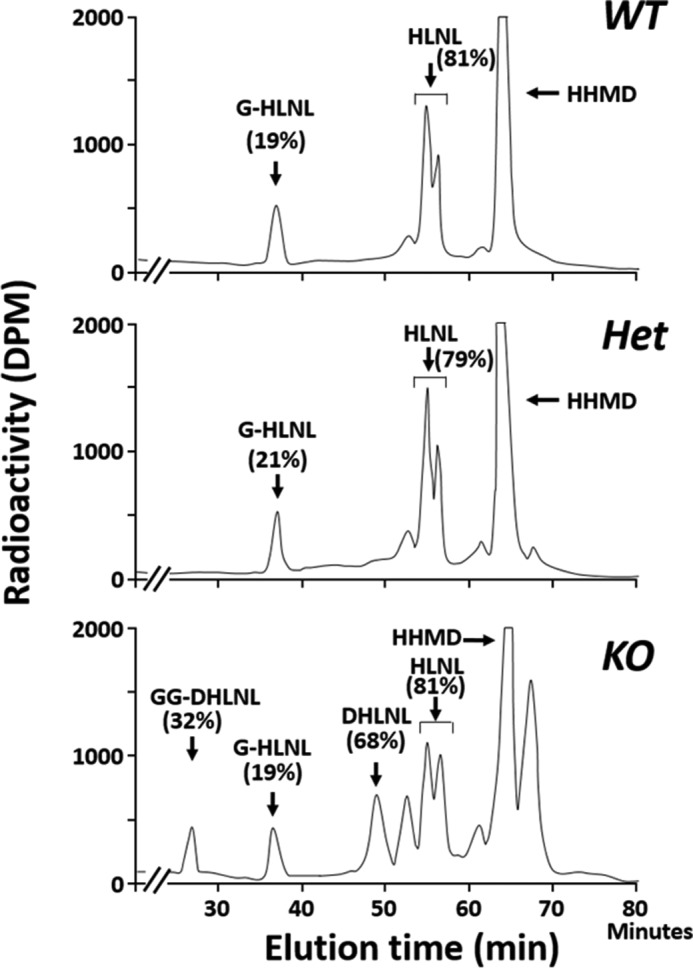
Fig. 5 shows the typical chromatographic patterns of collagen cross-links obtained from the acid hydrolysates of reduced WT, Het, and KO tail tendon samples. In WT and Het, there were essentially two Lysald-derived reducible cross-links, a bifunctional cross-link, HLNL, and a tetravalent cross-link, HHMD, consistent with the cross-linking pattern of mouse tail tendon collagen reported previously (39). In KO tendon, both of these cross-links were significantly diminished (i.e. HLNL, 0.697 ± 0.03 in WT, 0.672 ± 0.11 in Het, and 0.413 ± 0.074 mol/mol of collagen in KO, p < 0.001; for HHMD, 0.894 ± 0.051 in WT, 0.884 ± 0.114 in Het, and 0.492 ± 0.120 mol/mol of collagen in KO, p < 0.001, respectively) (Table 4). However, the most striking finding was that significant amounts of the Hylald-derived reducible cross-link, DHLNL (0.32 mol/mol collagen), and non-reducible cross-links, Pyr (0.03 mol/mol collagen), and d-Pyr (0.04 mol/mol collagen), all of which were absent from WT and Het, were identified in KO (Figs. 5 and 6). These results strongly indicate that, in the absence of CypB, the telopeptidyl Lys is significantly hydroxylated, converted to Hylald, and incorporated into cross-linking (Table 1). The low ratio of Pyr/d-Pyr in KO mice tendon indicates underhydroxylation of the helical Lys residues involved in the cross-linking.
FIGURE 5.
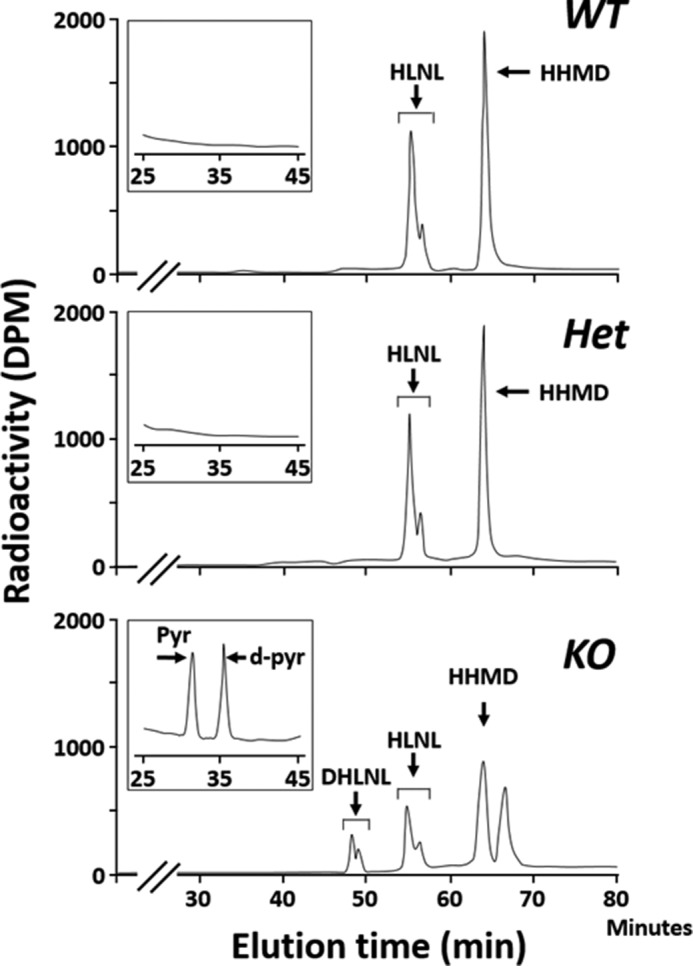
Typical chromatographic patterns of collagen cross-links from the acid hydrolysates of reduced tail tendon obtained from WT (top), Het (middle), and CypB KO (bottom). In WT and Het, cross-links were composed of HLNL and HHMD. However, in KO, DHLNL, Pyr, and d-Pyr were formed in addition to HLNL and HHMD.
TABLE 4.
Levels of immature reducible cross-links (HLNL, DHLNL, and its glycosylation) and mature non-reducible cross-links (Pyr, d-Pyr, and HHMD)
Values represent mean moles/mole of collagen (S.D.) from three independent samples. A, immature cross-links (DHLNL and its glycosylation); B, immature cross-links (HLNL and its glycosylation); C, non-reducible cross-links (Pyr, d-Pyr, and HHMD). *, HHMD was not glycosylated.
a Total DHLNL = GG-DHLNL + G-DHLNL + non-glycosylated DHLNL.
b Total HLNL = GG-HLNL + G-HLNL + non-glycosylated HLNL.
c Significantly different from both WT and Het.
d ND, not detected.
FIGURE 6.
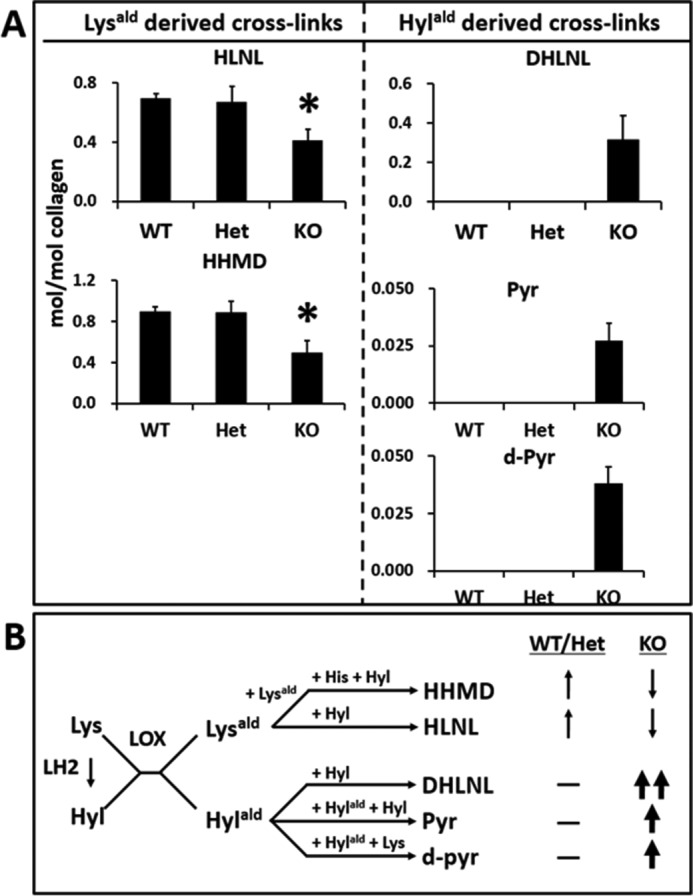
Quantitative cross-link analysis (A) and the major cross-linking pathways found (B). A, Lysald-derived (HLNL and HHMD) and Hylald-derived cross-links (DHLNL, Pyr, and d-Pyr) are shown. Cross-links are expressed as moles per mole of collagen (mol/mol collagen). In CypB KO tendon collagen, HLNL and HHMD were significantly lower than in WT and Het (p < 0.001). Hylald-derived cross-links were identified only in KO tendon. B, the major cross-linking pathways in mouse tail tendon collagen. LOX, lysyl oxidase.
Fig. 7 shows the typical chromatographic pattern of the base hydrolysates obtained from reduced WT, Het, and KO tendon. The percentages of glycosylated (G- and GG-) and non-glycosylated forms of DHLNL and HLNL are also indicated. The amount of HHMD cross-link did not change with base hydrolysis, indicating that the Hyl residue involved in this cross-link is not glycosylated. In KO mouse tendon, the relative amounts of glycosylated and non-glycosylated HLNL were comparable with WT and Het tendon (i.e. ∼20% glycosylated and ∼80% free form in all three groups). Another divalent cross-link, DHLNL, identified only in KO, was only found to be in the GG (32%) or free (68%) form. The residue α1-87 (α2-87 in rodent type I collagen) is not glycosylated (17), and the major glycosylation site (Table 3) is also the major helical cross-linking site to form DHLNL (α1-16C × α1-87) (40). The ratio of GG-DHLNL to free DHLNL indeed corresponds well to that of Hyl at α1-87 (Table 3) and is consistent with the increased Lys hydroxylation in the C-telopeptide (Fig. 3D). The relative levels of glycosylated and non-glycosylated forms of DHLNL and HLNL are also shown in Table 4. In KO tendon, there were significant decreases in the level of free HLNL and G-HLNL (p < 0.001) compared with those from WT and Het tendons (Table 4).
FIGURE 7.
Typical chromatographic patterns of collagen cross-links of the base hydrolysates. Shown are WT (top), Het (middle), and CypB KO (bottom) mice. The amounts of GG-, G-, and free DHLNL and HLNL are shown in percentages (GG-DHLNL + DHLNL = 100%, G-HLNL + HLNL = 100%). In CypB KO tendon, the relative amounts of glycosylated (G-) and non-glycosylated HLNL were comparable with WT and Het tendon. The DHLNL in CypB KO tendon was found to be the diglycosylated or non-glycosylated form. No glycosylation was detected for HHMD.
Interaction between CypB and Lysyl Hydroxylases 1–3 and FKBP65
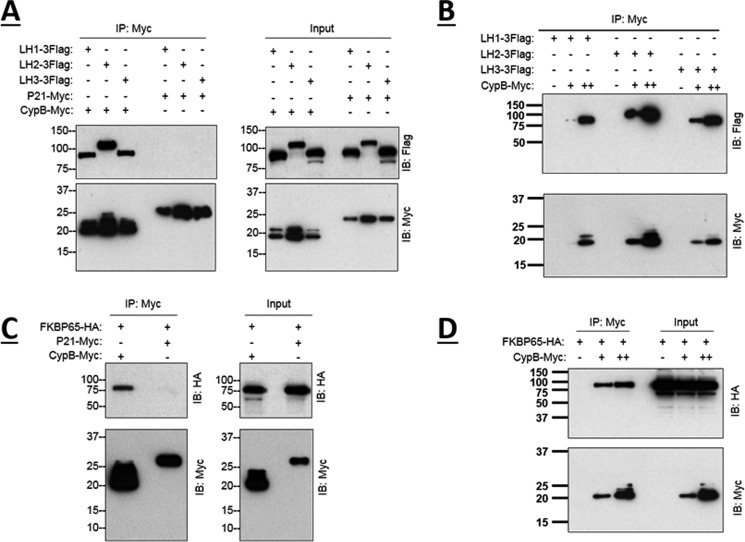
The data above demonstrated that loss of CypB differentially affected Lys hydroxylation between the telopeptidyl and helical domains of the collagen molecule in tendon. This suggests that CypB may regulate more than one LH function. Thus, to test whether or not CypB interacts with different LH isoforms, we coexpressed CypB- or P21-Myc and LH1-3FLAG in 293F cells. After immunoprecipitating CypB with Myc antibody, we detected the associated LHs with FLAG antibody in a dose-dependent manner, whereas there was a lack of interaction between LHs and P21 (Fig. 8, A and B). This finding clearly indicated that CypB interacted with not only LH1 but also LH2 and -3. Because loss of CypB increased the telopeptidyl Lys hydroxylation and, thus, probably LH2 activity, we also investigated the potential interaction between CypB and the LH2 chaperone, FKBP65. For this, we coexpressed CypB- or P21-Myc and FKBP65-HA in 293F cells, immunoprecipitated with Myc antibody, and blotted for FKBP65 with HA antibody (Fig. 8, C and D). The data also showed that the interaction of CypB with FKBP65 was observed in a dose-dependent manner. No interaction was detected between P21 and FKBP65. These data suggest that CypB may regulate the function of LHs by forming a complex with LHs and/or the LH2 chaperone, FKBP65.
FIGURE 8.
CypB binds to LHs and FKBP65. Binding assay in a cell culture system. 293F cells were transiently co-transfected with CypB or P21-Myc and LHs-FLAG or FKBP65-HA plasmids and subjected to co-immunoprecipitation (IP). After 48 h, the cells were lysed, and CypB or p21 protein complexes were immunoprecipitated from the lysates with the anti-Myc antibody or normal mouse IgG as a negative control. The pulldown of CypB or P21 was confirmed by Western blotting analysis with anti-Myc antibody, and the pull-down of LHs or FKBP65 proteins was confirmed with an anti-FLAG or HA antibody. A, interaction between CypB or P21 and LH1, -2, and -3. B, interaction between CypB and LHs by increasing concentrations of CypB. C, interaction between CypB or P21 and FKBP65. D, interaction between CypB and FKBP65 by increasing concentrations of CypB.
Discussion
In this study, we demonstrated that the absence of CypB resulted in a remarkable change in collagen cross-linking chemistry, defective fibrillogenesis, and tendon tissue degeneration. All of the biochemical and histological analyses done in this study showed that there is no significant phenotypic difference between WT and Het.
CypB KO mice were generated as a mouse model of recessive OI (27). CypB is a component of the Pro-3 hydroxylation (P3H) complex that catalyzes 3-hydroxylation of Pro-986 in the α1 chain and Pro-707 in the α2 chain of type I collagen during procollagen biosynthesis (27). The deficiency of any component of the P3H1 complex leads to recessive OI (24, 41, 42). In the present study, Pro-986 of the α1 chain is 82–84% 3-hydroxylated in WT/Het mouse tendon, and it diminished to ∼17% in KO, indicating that CypB is an important contributor to this modification but not essential. Distinct from P3H2 KO mouse tendon (43), 3-hydroxylation of Pro at α1-707 was not affected in CypB KO tendon collagen. The extent of 3-hydroxylation of Pro at α2-707 in mouse tendon is significantly higher than in bone and skin collagen (27); however, the decrease in this modification at this position in CypB KO was moderate. A recent study has shown that P3H2 expression in tendon is significantly higher than P3H1 or P3H3 (44). In addition, the 3-Hyp at Pro-707 of both α1 and α2 chain was significantly underhydroxylated in tendon type I collagen in P3H2 null mice (43). Thus, it is likely that P3H2 can hydroxylate this site without requiring CypB in tendon collagen.
The biosynthesis of fibrillar type I collagen requires a number of specific post-translational modifications, which are critical for tissue stability (2). Hydroxylation of specific Lys residues catalyzed by LHs is an important modification because it determines the fate of the cross-linking pathway and provides the glycosylation sites in type I collagen (2, 17, 18). Mutations in the LH-encoding genes lead to various connective diseases, but it has also become clear that defects in specific ER chaperones/foldases result in aberrant LH functions, suggesting that these ER proteins also control the functionality of LHs.
By analyzing skin and tendon type I collagen in American quarter horses with hyperelastosis cutis caused by a CypB missense mutation, Ishikawa et al. was the first to report that CypB directly interacts with LH1 and may facilitate its function (28). An interesting finding in their report is that the extent of suppression in Lys hydroxylation of collagen was significantly different between the two tissues examined. Recently, we have reported that bone type I collagen of CypB KO mice showed significantly diminished Lys hydroxylation at the helical cross-linking sites, suggesting an altered CypB-LH1 interaction (27). However, except at a limited number of specific sites, overall Lys hydroxylation in the KO bone collagen was not diminished by the absence of CypB but instead slightly increased (27). Skin type I collagen was quite different. In this tissue, Lys hydroxylation in the whole type I collagen molecule was severely diminished, revealing less than one-fifth of that in WT/Het. This value implies that there is only about one Hyl residue per α chain in the KO skin type I collagen molecule. We now have evidence that this extremely low level of Lys hydroxylation in skin collagen produces abnormal collagen cross-links (data not shown). Because primary cells from bone and skin of CypB KO mice abundantly express LH1 at both transcript and protein levels, this tissue-specific effect on Lys hydroxylation predicts additional regulatory mechanisms of LH functions in different tissues. Detailed biochemical analyses of type I collagen in various tissues are critically important to gain insight into such mechanisms.
The current study of the KO tendon collagen had a number of surprises. First, there was severe degeneration of tendon collagen matrices in KO, and the fibrils were markedly small, disorganized, and non-uniform. The fibrillar phenotype is similar to that seen in horse tendon with hyperelastosis cutis (28). Second, the effect on Lys hydroxylation in KO tendon collagen was distinct from both bone and skin collagen. Whereas KO bone collagen had slightly higher Lys hydroxylation compared with WT/Het, the extent of Lys hydroxylation in KO tendon collagen was diminished (by ∼22–28%) but not as severe as in skin (>80%). Because type I collagen is the predominant type in KO, WT, and Het tendon (≥95%), this demonstrates that tendon type I collagen in KO represents another distinct molecular phenotype. The mechanism by which Lys hydroxylation is controlled in a tissue-specific manner is still unclear but could possibly be due to the specific interaction of CypB with other ER proteins (see below). Third, and most significantly, collagen cross-linking in KO tendon was quite different from WT/Het. In mouse tail tendon collagen, the predominant (if not all) collagen cross-links are known to be the Lysald-derived cross-links forming HLNL (Lysald × Hyl) and HHMD (Lysald × Lysald × His × Hyl), as seen in WT/Het (39). However, unique to KO tendon collagen, significant amounts of Hylald-derived cross-links, such as DHLNL (Hylald × Hyl), Pyr (Hylald × Hylald × Hyl), and d-Pyr (Hylald × Hylald × Lys) were formed. This indicates that, although overall helical Lys hydroxylation was diminished, the telopeptidyl Lys hydroxylation was increased by the absence of CypB. The total number of aldehydes involved in cross-linking was not significantly affected, suggesting that lysyl oxidase activity was not affected by the loss of CypB. Thus, the main effect of CypB on cross-linking is on the particular cross-linking pathway utilized rather than on the abundance of cross-links. Fourth, CypB seems to interact with all LHs and LH2 chaperone, FKBP65. The interaction between CypB and LH1 has been reported (28, 45); however, its interaction with other LHs is not known. The finding that CypB interacts with all LHs and an LH2 chaperone, FKBP65 (Fig. 8), suggests that CypB may regulate not only LH1 but also LH2 and -3 activity. Considering the fact that significant amounts of the Hylald-derived cross-links are formed in KO, whereas they are absent from WT/Het, it is tempting to speculate that CypB may negatively control LH2 function as a telopeptidyl LH with LH2 and/or through its chaperone FKBP65. Because LH2 is expressed ubiquitously in mice, including tendon (46), but the Hylald-derived cross-links are essentially absent from this tissue, it is possible that, in the normal tendon, LH2 function could be suppressed by LH2-associated molecules in ER, possibly including CypB. Thus, in the absence of CypB, LH2 function could be enhanced, resulting in the formation of the Hylald-derived cross-links. Further studies are necessary to validate this hypothesis. At all glycosylation sites analyzed, the relative abundance of glycosylated Hyl per total Hyl was generally higher in KO than in WT/Het (Table 3). It is of interest to note that the relative abundance of GG-Hyl in total Hyl at the major glycosylation site, α1-87, was significantly higher in KO than WT/Het, although that of G-Hyl was unaffected. Because CypB appears to interact with LH3 (Fig. 8), CypB may also regulate LH3 function as a glucosyltransferase (17) at specific sites. The delay in collagen folding from the absence of CypB (27) could also be a contributing factor to such overglucosylation. Although we confirmed the interaction between CypB and LHs/FKBP65 by co-immunoprecipitation using a whole cell extract, this does not prove the direct protein-protein interaction. A comprehensive characterization of the interaction, such as direct binding, binding kinetics, and determination of the binding sites, may provide further insights into the molecular mechanisms and biological significance of the CypB-controlled collagen post-translational modification.
In CypB KO mice, whereas the extent of decrease in Lys hydroxylation in the helical domain varies depending on the tissues (i.e. bone, skin, and tendon), a significant decrease of this modification at the helical cross-linking sites (i.e. α1/2-87) appears to be a common molecular phenotype in all of these three tissues. The reason for this specificity is not clear at present. Considering the fact that these are the major glycosylation sites of type I collagen (17, 40), Lys hydroxylation at these sites may occur in the context of sequential modifications involving multiple enzymes, i.e. LH1, glycosyltransferases such as a glycosyltransferase 25 domain 1 (GLT25D1) and LH3, a complex process in which CypB may be required.
One unresolved question is whether the phenotypes seen in CypB KO tendon (i.e. aberrant organization, degenerative collagen bundles, small/non-uniform fibrils) are due to the diminished P3H or altered Lys modifications, including cross-linking. A hypothesis on the role of P3H proposed by Weis et al. (47) is that it helps to fine tune the supramolecular assembly of collagen fibrils by providing inter-triple helical hydrogen bonding. Thus, the lack of 3-Hyp may impair fibrillogenesis. A study on tendon from horses with hyperelastosis cutis may provide additional insight. In this model, the level of P3H was not significantly affected, yet the aberrant fibrillar morphology is remarkably similar to that seen in CypB KO tendon. This suggests that the fibrillar phenotype is caused by defects other than P3H. In addition, we also reported that, when LH2 is overexpressed, the resultant collagen fibrils become small in diameter (48). These results suggest that the phenotype seen in CypB KO tendon might be due mainly to altered Lys modifications rather than diminished P3H. Another possibility is that the altered post-translational modifications of collagen in CypB KO may change its interaction with collagen-associated small leucine-rich proteoglycans, such as decorin, biglycan, and fibromodulin, resulting in defective fibrils (49, 50).
In conclusion, this study demonstrated that CypB modulates collagen cross-linking chemistry by differentially affecting Lys hydroxylation in the helical and telopeptidyl domains of tendon type I collagen, possibly via its specific interaction with LH isoforms and associated molecules, FKBP65. The deficiency of CypB causes aberrant cross-linking and defective tendon tissue formation. These results underscore the critical role of this ER protein in collagen stability and assembly.
Author Contributions
M. T. and Y. T. conducted most biochemical and mass spectrometric analyses, data analyses, and interpretation. Y. C., G. H. F., and J. M. K. conducted co-immunoprecipitation assays and their interpretation. W. A. C. and J. C. M. generated KO mice, provided tissues, and contributed to writing the paper. S. S. helped with initial characterization of cross-links in tendon. M. N. and N. S. conducted histological analyses. S. H. helped with interpretation of MS data. M. Y. conceived the idea for the project, interpreted all data, and wrote the paper with M. T., Y. T., and the other co-authors.
Acknowledgment
We thank Dr. Y. Mochida at Boston University for assisting the preliminary binding studies.
This work was supported in part by National Institute of Health Grants, National Institutes of Health-NIAMS R21AR060978, NCI R01CA105155. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Hyl
- hydroxylysine
- CypB
- cyclophilin B
- ER
- endoplasmic reticulum
- LH
- lysyl hydroxylase
- OI
- osteogenesis imperfecta
- FKBP65
- 65-kDa FK506-binding protein
- Het
- heterozygous
- MRM
- multiple reaction monitoring
- G-
- galactosyl-
- GG-
- glucosylgalactosyl-
- Hyp
- hydroxyproline
- DHLNL
- dihydroxylysinonorleucine
- HLNL
- hydroxylysinonorleucine
- HHMD
- histidinohydroxymerodesmosine
- Pyr
- pyridinoline
- d-Pyr
- deoxypyridinoline
- Lysald
- lysine aldehyde
- Hylald
- hydroxylysine aldehyde
- SI-collagen
- stable isotope-labeled collagen.
References
- 1. Ricard-Blum S. (2011) The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamauchi M., and Sricholpech M. (2012) Lysine post-translational modifications of collagen. Essays Biochem. 52, 113–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeowell H. N., and Walker L. C. (2000) Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol. Genet. Metab. 71, 212–224 [DOI] [PubMed] [Google Scholar]
- 4. Witsch T. J., Turowski P., Sakkas E., Niess G., Becker S., Herold S., Mayer K., Vadász I., Roberts J. D. Jr., Seeger W., and Morty R. E. (2014) Deregulation of the lysyl hydroxylase matrix cross-linking system in experimental and clinical bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 306, L246–L259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes A. M., Duncan G., Weis M., Paton W., Cabral W. A., Mertz E. L., Makareeva E., Gambello M. J., Lacbawan F. L., Leikin S., Fertala A., Eyre D. R., Bale S. J., and Marini J. C. (2013) Kuskokwim syndrome, a recessive congenital contracture disorder, extends the phenotype of FKBP10 mutations. Hum. Mutat. 34, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bank R. A., Robins S. P., Wijmenga C., Breslau-Siderius L. J., Bardoel A. F. J., van der Sluijs H. A., Pruijs H. E., and TeKoppele J. M. (1999) Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: Indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc. Natl. Acad. Sci. U.S.A. 96, 1054–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ha-Vinh R., Alanay Y., Bank R. A., Campos-Xavier A. B., Zankl A., Superti-Furga A., and Bonafé L. (2004) Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am. J. Med. Genet. A 131, 115–120 [DOI] [PubMed] [Google Scholar]
- 8. van der Slot A. J., Zuurmond A. M., Bardoel A. F., Wijmenga C., Pruijs H. E., Sillence D. O., Brinckmann J., Abraham D. J., Black C. M., Verzijl N., DeGroot J., Hanemaaijer R., TeKoppele J. M., Huizinga T. W., and Bank R. A. (2003) Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J. Biol. Chem. 278, 40967–40972 [DOI] [PubMed] [Google Scholar]
- 9. Shiiba M., Arnaud S. B., Tanzawa H., Kitamura E., and Yamauchi M. (2002) Regional alterations of type I collagen in rat tibia induced by skeletal unloading. J. Bone Miner. Res. 17, 1639–1645 [DOI] [PubMed] [Google Scholar]
- 10. Gilkes D. M., Bajpai S., Wong C. C., Chaturvedi P., Hubbi M. E., Wirtz D., and Semenza G. L. (2013) Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol. Cancer Res. 11, 456–466 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Eisinger-Mathason T. S., Zhang M., Qiu Q., Skuli N., Nakazawa M. S., Karakasheva T., Mucaj V., Shay J. E., Stangenberg L., Sadri N., Pursé E., Yoon S. S., Kirsch D. G., and Simon M. C. (2013) Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 3, 1190–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y., Terajima M., Yang Y., Sun L., Ahn Y.-H., Pankova D., Puperi D. S., Watanabe T., Kim M. P., Blackmon S. H., Rodriguez J., Liu H., Behrens C., Wistuba I. I., Minelli R., et al. (2015) Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J. Clin. Invest. 125, 1147–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myllylä R., Wang C., Heikkinen J., Juffer A., Lampela O., Risteli M., Ruotsalainen H., Salo A., and Sipilä L. (2007) Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3). J. Cell. Physiol. 212, 323–329 [DOI] [PubMed] [Google Scholar]
- 14. Takaluoma K., Lantto J., and Myllyharju J. (2007) Lysyl hydroxylase 2 is a specific telopeptide hydroxylase, while all three isoenzymes hydroxylate collagenous sequences. Matrix Biol. 26, 396–403 [DOI] [PubMed] [Google Scholar]
- 15. Pornprasertsuk S., Duarte W. R., Mochida Y., and Yamauchi M. (2004) Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J. Bone Miner. Res. 19, 1349–1355 [DOI] [PubMed] [Google Scholar]
- 16. Schegg B., Hülsmeier A. J., Rutschmann C., Maag C., and Hennet T. (2009) Core glycosylation of collagen is initiated by two β(1-O)galactosyltransferases. Mol. Cell. Biol. 29, 943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sricholpech M., Perdivara I., Nagaoka H., Yokoyama M., Tomer K. B., and Yamauchi M. (2011) Lysyl hydroxylase 3 glucosylates galactosylhydroxylysine residues in type I collagen in osteoblast culture. J. Biol. Chem. 286, 8846–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sricholpech M., Perdivara I., Yokoyama M., Nagaoka H., Terajima M., Tomer K. B., and Yamauchi M. (2012) Lysyl hydroxylase 3-mediated glucosylation in type I collagen: molecular loci and biological significance. J. Biol. Chem. 287, 22998–23009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heikkinen J., Risteli M., Wang C., Latvala J., Rossi M., Valtavaara M., and Myllylä R. (2000) Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J. Biol. Chem. 275, 36158–36163 [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa Y., Boudko S., and Bachinger H. P. (2015) Ziploc-ing the structure: triple helix formation is coordinated by rough endoplasmic reticulum resident PPIases. Biochim. Biophys. Acta 1850, 1983–1993 [DOI] [PubMed] [Google Scholar]
- 21. Pyott S. M., Schwarze U., Christiansen H. E., Pepin M. G., Leistritz D. F., Dineen R., Harris C., Burton B. K., Angle B., Kim K., Sussman M. D., Weis M., Eyre D. R., Russell D. W., McCarthy K. J., et al. (2011) Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. Hum. Mol. Genet. 20, 1595–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Dijk F. S., Nesbitt I. M., Zwikstra E. H., Nikkels P. G., Piersma S. R., Fratantoni S. A., Jimenez C. R., Huizer M., Morsman A. C., Cobben J. M., van Roij M. H., Elting M. W., Verbeke J. I., Wijnaendts L. C., Shaw N. J., et al. (2009) PPIB mutations cause severe osteogenesis imperfecta. Am. J. Hum. Genet. 85, 521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forlino A., Cabral W. A., Barnes A. M., and Marini J. C. (2011) New perspectives on osteogenesis imperfecta. Nat. Rev. Endocrinol. 7, 540–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes A. M., Carter E. M., Cabral W. A., Weis M., Chang W., Makareeva E., Leikin S., Rotimi C. N., Eyre D. R., Raggio C. L., and Marini J. C. (2010) Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N. Engl. J. Med. 362, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcant A., Denys A., Melchior A., Martinez P., Deligny A., Carpentier M., and Allain F. (2012) Cyclophilin B attenuates the expression of TNF-α in lipopolysaccharide-stimulated macrophages through the induction of B cell lymphoma-3. J. Immunol. 189, 2023–2032 [DOI] [PubMed] [Google Scholar]
- 26. Choi J. W., Schroeder M. A., Sarkaria J. N., and Bram R. J. (2014) Cyclophilin B supports Myc and mutant p53-dependent survival of glioblastoma multiforme cells. Cancer Res. 74, 484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cabral W. A., Perdivara I., Weis M., Terajima M., Blissett A. R., Chang W., Perosky J. E., Makareeva E. N., Mertz E. L., Leikin S., Tomer K. B., Kozloff K. M., Eyre D. R., Yamauchi M., and Marini J. C. (2014) Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta. PLoS Genet. 10, e1004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishikawa Y., Vranka J. A., Boudko S. P., Pokidysheva E., Mizuno K., Zientek K., Keene D. R., Rashmir-Raven A. M., Nagata K., Winand N. J., and Bächinger H. P. (2012) Mutation in cyclophilin B that causes hyperelastosis cutis in American quarter horse does not affect peptidylprolyl cis-trans isomerase activity but shows altered cyclophilin B-protein interactions and affects collagen folding. J. Biol. Chem. 287, 22253–22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grzesik W. J., Cheng H., Oh J. S., Kuznetsov S. A., Mankani M. H., Uzawa K., Robey P. G., and Yamauchi M. (2000) Cementum-forming cells are phenotypically distinct from bone-forming cells. J. Bone Miner. Res. 15, 52–59 [DOI] [PubMed] [Google Scholar]
- 30. Reynolds E. S. (1963) The use of lead citrate high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taga Y., Kusubata M., Ogawa-Goto K., and Hattori S. (2012) Development of a novel method for analyzing collagen O-glycosylations by hydrazide chemistry. Mol. Cell. Proteomics 10.1074/mcp.M111.010397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taga Y., Kusubata M., Ogawa-Goto K., and Hattori S. (2014) Stable isotope-labeled collagen: a novel and versatile tool for quantitative collagen analyses using mass spectrometry. J. Proteome Res. 13, 3671–3678 [DOI] [PubMed] [Google Scholar]
- 33. Teramura N., Tanaka K., Iijima K., Hayashida O., Suzuki K., Hattori S., and Irie S. (2011) Cloning of a novel collagenase gene from the Gram-negative bacterium Grimontia (Vibrio) hollisae 1706B and its efficient expression in Brevibacillus choshinensis. J. Bacteriol. 193, 3049–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamauchi M., Katz E. P., and Mechanic G. L. (1986) Intermolecular cross-linking and stereospecific molecular packing in type I collagen fibrils of the periodontal ligament. Biochemistry 25, 4907–4913 [DOI] [PubMed] [Google Scholar]
- 35. Yamauchi M., Katz E. P., Otsubo K., Teraoka K., and Mechanic G. L. (1989) Cross-linking and stereospecific structure of collagen in mineralized and nonmineralized skeletal tissue. Connect. Tissue Res. 21, 159–167; discussion 168–169 [DOI] [PubMed] [Google Scholar]
- 36. Yamauchi M., and Shiiba M. (2008) Lysine hydroxylation and cross-linking of collagen. Methods Mol. Biol. 446, 95–108 [DOI] [PubMed] [Google Scholar]
- 37. Eyre D. (1987) Collagen cross-linking amino acids. Methods Enzymol. 144, 115–139 [DOI] [PubMed] [Google Scholar]
- 38. Lietman C. D., Rajagopal A., Homan E. P., Munivez E., Jiang M. M., Bertin T. K., Chen Y., Hicks J., Weis M., Eyre D., Lee B., and Krakow D. (2014) Connective tissue alterations in Fkbp10−/− mice. Hum. Mol. Genet. 23, 4822–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mechanic G. L., Farb R. M., Henmi M., Ranga V., Bromberg P. A., and Yamauchi M. (1987) Structural crosslinking of lung connective tissue collagen in the blotchy mouse. Exp. Lung Res. 12, 109–117 [DOI] [PubMed] [Google Scholar]
- 40. Terajima M., Perdivara I., Sricholpech M., Deguchi Y., Pleshko N., Tomer K. B., and Yamauchi M. (2014) Glycosylation and cross-linking in bone type I collagen. J. Biol. Chem. 289, 22636–22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barnes A. M., Chang W., Morello R., Cabral W. A., Weis M., Eyre D. R., Leikin S., Makareeva E., Kuznetsova N., Uveges T. E., Ashok A., Flor A. W., Mulvihill J. J., Wilson P. L., Sundaram U. T., et al. (2006) Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N. Engl. J. Med. 355, 2757–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cabral W. A., Chang W., Barnes A. M., Weis M., Scott M. A., Leikin S., Makareeva E., Kuznetsova N. V., Rosenbaum K. N., Tifft C. J., Bulas D. I., Kozma C., Smith P. A., Eyre D. R., and Marini J. C. (2007) Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat. Genet. 39, 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hudson D. M., Joeng K. S., Werther R., Rajagopal A., Weis M., Lee B. H., and Eyre D. R. (2015) Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations. J. Biol. Chem. 290, 8613–8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pokidysheva E., Zientek K. D., Ishikawa Y., Mizuno K., Vranka J. A., Montgomery N. T., Keene D. R., Kawaguchi T., Okuyama K., and Bächinger H. P. (2013) Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice. J. Biol. Chem. 288, 24742–24752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishikawa Y., and Bächinger H. P. (2013) A molecular ensemble in the rER for procollagen maturation. Biochim. Biophys. Acta 1833, 2479–2491 [DOI] [PubMed] [Google Scholar]
- 46. Hyry M. (2012) Lysyl hydroxylases 1 and 2: characterization of their in vivo roles in mouse and the molecular level consequences of the lysyl hydroxylase 2 mutations found in Bruck syndrome. Doctoral dissertation, University of Oulu Graduate School, Oulu, Finland [Google Scholar]
- 47. Weis M. A., Hudson D. M., Kim L., Scott M., Wu J. J., and Eyre D. R. (2010) Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J. Biol. Chem. 285, 2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pornprasertsuk S., Duarte W. R., Mochida Y., and Yamauchi M. (2005) Overexpression of lysyl hydroxylase-2b leads to defective collagen fibrillogenesis and matrix mineralization. J. Bone Miner. Res. 20, 81–87 [DOI] [PubMed] [Google Scholar]
- 49. Kalamajski S., Liu C., Tillgren V., Rubin K., Oldberg Å., Rai J., Weis M., and Eyre D. R. (2014) Increased C-telopeptide cross-linking of tendon type I collagen in fibromodulin-deficient mice. J. Biol. Chem. 289, 18873–18879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robinson P. S., Huang T.-F., Kazam E., Iozzo R. V., Birk D. E., and Soslowsky L. J. (2005) Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J. Biomech. Eng. 127, 181–185 [DOI] [PubMed] [Google Scholar]