Abstract
Objective
Interactions between pre-existing differences in mesolimbic function and neuroadaptations induced by consumption of fatty, sugary foods are thought to contribute to human obesity. This study examined basal and cocaine-induced changes in striatal neurotransmitter levels without diet manipulation and D2/D3 dopamine receptor-mediated transmission prior to and after consumption of “junk-foods” in obesity-prone and obesity-resistant rats.
Methods
Microdialysis and liquid chromatography-mass spectrometry were used to determine basal and cocaine-induced changes in neurotransmitter levels in real time with cocaine-induced locomotor activity. Sensitivity to the D2/D3 dopamine receptor agonist quinpirole was examined before and after restricted junk-food exposure. Selectively bred obesity-prone and obesity-resistant rats were used.
Results
Cocaine-induced locomotion was greater in obesity-prone rats versus obesity-resistant rats prior to diet manipulation. Basal and cocaine-induced increases in dopamine and serotonin levels did not differ. Obesity-prone rats were more sensitive to the D2 receptor-mediated effects of quinpirole, and junk-food produced modest alterations in quinpirole sensitivity in obesity-resistant rats.
Conclusions
These data show that mesolimbic systems differ prior to diet manipulation in susceptible versus resistant rats, and that consumption of fatty, sugary foods produce different neuroadaptations in these populations. These differences may contribute to enhanced food craving and an inability to limit food intake in susceptible individuals.
Introduction
Interactions between basal differences in the function of mesolimbic circuits and neuroadaptations induced by weight gain and/or exposure to fatty, sugary foods likely contribute to human obesity (1–3). The mesolimbic system is comprised of projections from dopamine neurons in the ventral tegmental area and glutamatergic projections from hippocampus and prefrontal cortex that converge in the striatum (4). Activation of this system mediates motivational processes that direct behavior toward food, particularly in response to food-associated stimuli in the environment (5,6). Human fMRI studies have found positive relationships between responsivity of striatal circuits to food cues, subsequent weight gain, and difficulty achieving and maintaining weight loss (1,7). In addition, genetic variation in alleles for the D2 dopamine receptor and dopamine transporter has been linked to increased susceptibility to obesity in people (8), and D2 binding is reduced in obesity (9). In rodents, basal dopamine levels in the NAc shell are lower in rats that are susceptible to diet-induced obesity (10–12). These data suggest that both pre-existing and diet/obesity-induced differences in mesolimbic function may contribute to obesity [see also (2,3)].
Consistent with a role for pre-existing differences, we recently found that rats subsequently identified as susceptible to diet-induced obesity show stronger attraction toward food cues prior to any diet manipulation (13). Cue-triggered motivational responses are mediated in large part by the NAc core, whereas consumatory behaviors are more directly mediated by the NAc shell (5,14). Consistent with enhanced attraction to food cues, medium spiny neurons (MSN) in the NAc core are hyperexcitable in obesity-prone rats (15). MSN excitability is strongly influenced by dopamine receptor activity [see (16) for review], and mesolimbic dopamine receptor mRNA expression is enhanced in obesity-prone rats (17). Together these data suggest that striatal function and dopamine receptor-mediated transmission may differ in obesity-prone versus resistant rats prior to diet manipulation. In addition, exposure to sugary, fatty foods alters dopamine receptor expression and function (13,18,19). However, while basal NAc shell dopamine levels have been examined (11), the striatal neurochemical response to mesolimbic activation and the effects of fatty, sugary foods on in vivo dopamine receptor-mediated transmission have not been examined in obesity-prone and obesity-resistant rats.
Here, we examined basal and cocaine-induced changes in striatal neurotransmitter levels in obesity-prone and obesity-resistant rats without diet manipulation using microdialysis paired with recently developed liquid chromatography-mass spectrometry methods. This approach allows for the measurement of 20 neurochemicals, including dopamine and serotonin, in a single sample with 3-min time resolution (20). Locomotor activity was measured simultaneously with neurotransmitter levels. Furthermore, we examined sensitivity to the D2/D3 dopamine receptor (D2/D3R) agonist quinpirole before and after restricted consumption of fatty, sugary “junk-foods.” We found that without diet manipulation, basal and cocaine-evoked increases in dopamine and serotonin levels were similar in obesity-prone and obesity-resistant rats. However, quinpirole was less effective in obesity-prone than obesity-resistant rats. This pattern is consistent with greater D2 receptor-mediated transmission (21). Together, these data suggest that enhanced sensitivity to the locomotor-activating effects of cocaine in obesity-prone rats may be driven by differences in postsynaptic D2 dopamine receptor function and not differences in presynaptic dopamine release in response to cocaine. Furthermore, consumption of junk-food increased sensitivity to the D2R-mediated effects of quinpirole in obesity-resistant rats. Together, these data support the idea that there are fundamental differences in the function of mesolimbic systems prior to diet manipulation and metabolic dysregulation in susceptible individuals, and that consumption of junk-foods may produce different neuroadaptations in obesity-prone versus obesity-resistant populations.
Methods
Subjects
Obesity-prone and obesity-resistant rats were bred in-house; breeders were obtained from Taconic. Male rats were pair-housed on a reverse 12-h light/dark cycle with continuous access to food and water. Testing began 3–4 h after the onset of the dark cycle. Procedures were approved by The University of Michigan Committee on the Use and Care of Animals.
Chemicals and reagents
Chemicals, drugs, and reagents were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. All solutions were made with water and acetonitrile (Burdick & Jackson HPLC grade; VWR; Radnor, PA). aCSF contained (in nM): 145 NaCl, 2.68 KCl, 1.10 MgSO4, 1.22 CaCl2, 0.50 NaH2PO4, and 1.55 Na2HPO4, pH adjusted to 7.4 with 0.1 M NaOH. Stocks (10 mM) of each analyte were diluted in aCSF. Calibration curves were determined using standards: Gly, Ser and Tau (nM): 10, 50, 100, 1,000, 2,000; Asp, Glu, GABA, Hist, Ado, HVA, 5-HIAA, and DOPAC (nM): 1.0, 5.0, 10, 100, 200; ACh, 5-HT, NE, DA, NM, and 3-MT (nM): 0.1, 0.5, 1.0, 10, 20. Internal standards were: Gly, Ser, and Tau: 1 mM; Asp, Glu, GABA, Hist, Ado, HVA, 5-HIAA, and DOPAC: 100 µM; 5-HT, NE, DA, NM, and 3-MT: 10 µM. Standards were derivatized with 13C6 benzoyl chloride using the same procedure as 12C reagent, then diluted 100-fold in 50:50 water:acetonitrile containing 1% sulfuric acid. d4-ACh (50 nM; C/D/N isotopes, Pointe-Claire, Canada) was included in the internal standard to improve ACh quantitation.
Microdialysis and dialysate collection
Microdialysis probes (CMA12 Elite 2 mm, CMA/Microdialysis AB Stockholm, Sweden) were implanted unilaterally into the striatum, with tip located in the NAc core [from bregma: AP: +1.4, ML: ±2.5, DV: −7.8 (22)]. Hemisphere placements were counter-balanced. Probes were stabilized using scull screws (Fine Science Tools, Foster City, CA) and acrylic dental cement (Bosworth Co., Skokie, IL). Probes were implanted 20–24 h before microdialysis sampling to minimize effects of surgery and anesthesia (23,24). Rats were awake and freely moving during experiments. Probes were perfused with aCSF containing radiolabeled dopamine and glutamate (10 nM 13C-dopamine, 500 nM 13C-glutamate; 2 µL/ min, 1 h; 1 µL/min, 1 h) prior to dialysate sample collection. Samples were collected every 3 min and derivatized as previously described (20).
Cocaine-induced locomotor behavior
Locomotor activity was evaluated throughout dialysate collection in chambers equipped with an array of photocell beams (41 × 25.4 × 20.3 cm). After 1-h habituation to the chamber, rats received an injection of saline (1 mL/kg, i.p.) followed 45 min later by a cocaine injection (15 mg/kg, i.p.). Locomotor activity was recorded throughout all testing (3 min bins) and was time locked with dialysate collections (3 min/sample).
HPLC-MS analysis
A Thermo Accela Classic (Waltham, MA) HPLC system with a binary HPLC pump and autosampler was used. A Phenomenex (Torrance, CA) Kinetex biphenyl reverse-phase column (2.1 mm × 100 mm, 1.7 µm, 100 Å pore size) was used for separation. Mobile phase A was 10 mM ammonium formate and 0.15% (v/v) formic acid in water. Mobile phase B was acetonitrile. The mobile phase gradient was: initial, 5% B; 0.1 min, 15% B; 2 min, 40% B; 2.1 min, 100% B; 3 min, 100% B; 3.1 min, 5% B; 3.5 min, 5% B. The flow rate was 450 µL/min, and sample injection volume was 5 µL in “no waste” injection mode; total cycle times were 4.5 min. The autosampler and HPLC column were kept at ambient temperature. A Thermo TSQ Quantum Ultra triple quadrupole mass spectrometer operating in positive MRM mode and a heated electrospray ionization (HESI) probe (at 350°C) were used for detection. Thermo XCalibur software was used to automatically integrate peaks and construct calibration curves based on peak area (Panalyte/PI.S.) versus concentrations of internal standard by linear regression. Glutamate and dopamine concentrations were corrected for in vivo recovery using a stable isotope labeled method. This provides equivalent information to no-net-flux in a fraction of the time and allows for fraction by fraction determination of recovery (25).
Fasted plasma insulin levels and microdialysis probe placement
Rats were fasted for 16 h before euthanasia (Fatal Plus 600 µL; Vortech Pharmaceuticals, Dearborn, MI) and trunk blood collection (1.6 mg/mL, Sarstedt). Plasma was isolated by centrifugation (1,000×g, 4°C, 10 min) and stored (−20°C) for subsequent analysis. Insulin levels were determined by double-antibody radioimmunoassay using an 125I-Human insulin tracer (Linco Research, St. Charles, MO), a rat insulin standard (Novo Nordisk, Plainsboro, NJ), a guinea pig anti-rat insulin first antibody (Linco Research), and a sheep anti-guinea pig gamma globulin-PEG second antibody (Michigan Diabetes Research Core). The limit of sensitivity for this assay was 1 µU/mL. Interassay and intra-assay variability were 11.2% and 3.2%, respectively, at 30.5 µU/mL.
Probe placement was determined from coronal brain sections (50 µm) cut through the forebrain (CM1850 cryostat, Leica Microsystems, Buffalo Grove, IL), mounted onto slides, and stained with cresyl violet.
Sensitivity to quinpirole
Quinpirole can be used to evaluate D2/D3R function. Quinpirole produces a well-characterized inverted U-shaped dose-response function in yawning behavior, such that increases in yawning on the ascending limb are indicative of greater D3R-mediated transmission while reductions in yawning on the descending limb are indicative of greater D2R-mediated transmission (26,27). Yawns were defined as opening of the mouth such that the lower incisors were completely visible (26). All rats were habituated to the testing chamber (41 × 25.4 × 20.3 cm) and injection procedure (30 min in chamber followed by saline; 1 mL/kg, s.c. one/day, 2 days). On the test day, rats were habituated for 30 min followed by administration of five cumulative doses of quinpirole (one injection/30 min; 0.01, 0.032, 0.1, 0.32, 1.0 mg/kg, s.c.). The total number of yawns/10 min was recorded beginning 20 min after each injection. These same rats were then given restricted exposure to junk-food (10 g/day, 14 days) outside the home cage. Restricted exposure outside the home cage was used to ensure that all rats ate the same amount of junk-food. The junk-food was a mash of: Ruffles® Original Potato Chips (40 g), Nabisco Chips Ahoy! Original Chocolate Chip cookies (130 g), Jif® Creamy Peanut Butter (130 g), Nestle Nesquik® Chocolate Flavor Powder (130 g), powdered Lab Diet 5001 (200 g), and water combined using a food processor [180 mL; 19.6% fat, 14% protein, and 58% carbohydrates; 4.5 kcal/g (13)]. Once per day, rats were placed in a standard housing cage and given 10 g of junk-food. Rats remained in the chamber until all junk-food was consumed (<10 min). Rats had free access to standard chow in their home cages. Sensitivity to quinpirole was then reassessed as described above. Tolerance to quinpirole-induced yawning does not develop with repeated testing (19).
Statistical analysis
Two-tailed t-tests or two-way repeated measure ANOVAs were used followed by Sidak’s post-tests or two-tailed t-tests when appropriate (Prism6; GraphPad, San Diego, CA).
Results
Microdialysis probe placement
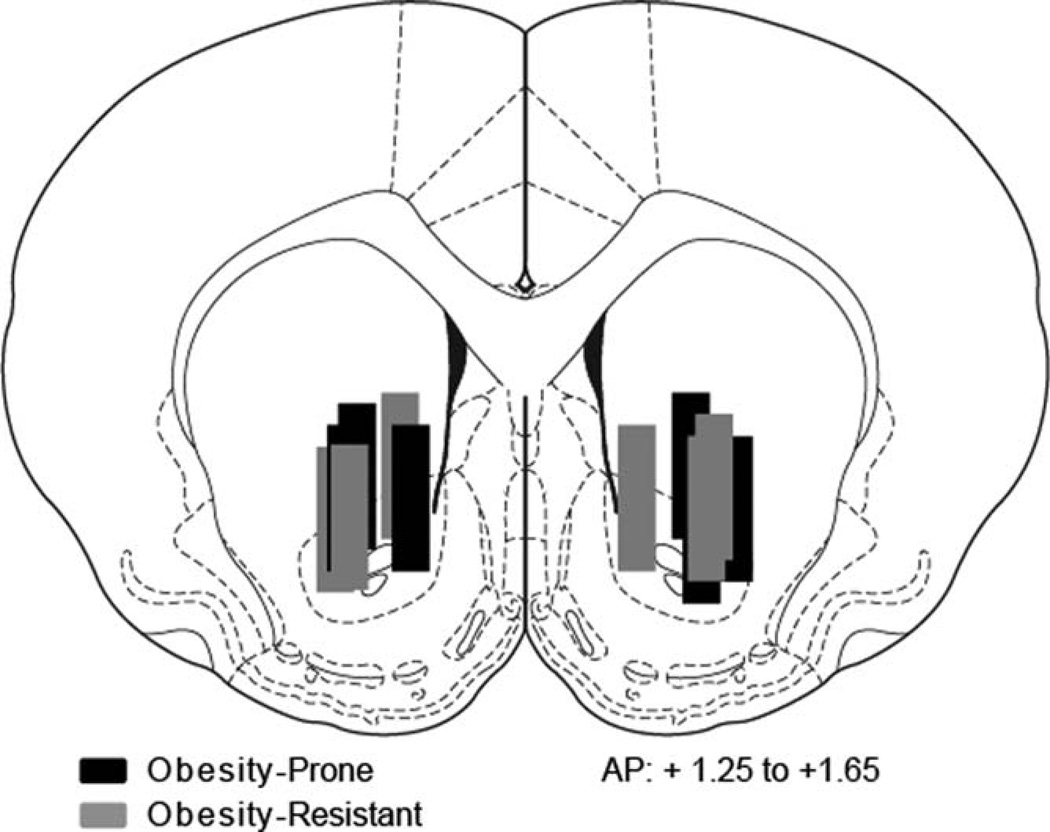
Microdialysis probe placements were between 1.25 and 1.65 mm anterior to bregma with the tip of the probe located in the NAc core and extending into the ventral portion of the caudate putamen. Placements were similar between groups (Figure 1: black, OP N = 6; gray, OR N = 6).
Figure 1.
Microdialysis probe placements. Microdialysis probe tips were located in the nucleus accumbens core with the active region extending into the ventral portion of the caudate putamen between +1.25 and +1.65 from bregma. Placements were similar between obesity-prone (black bars) or obesity-resistant (gray bars) groups.
Locomotor behavior during microdialysis
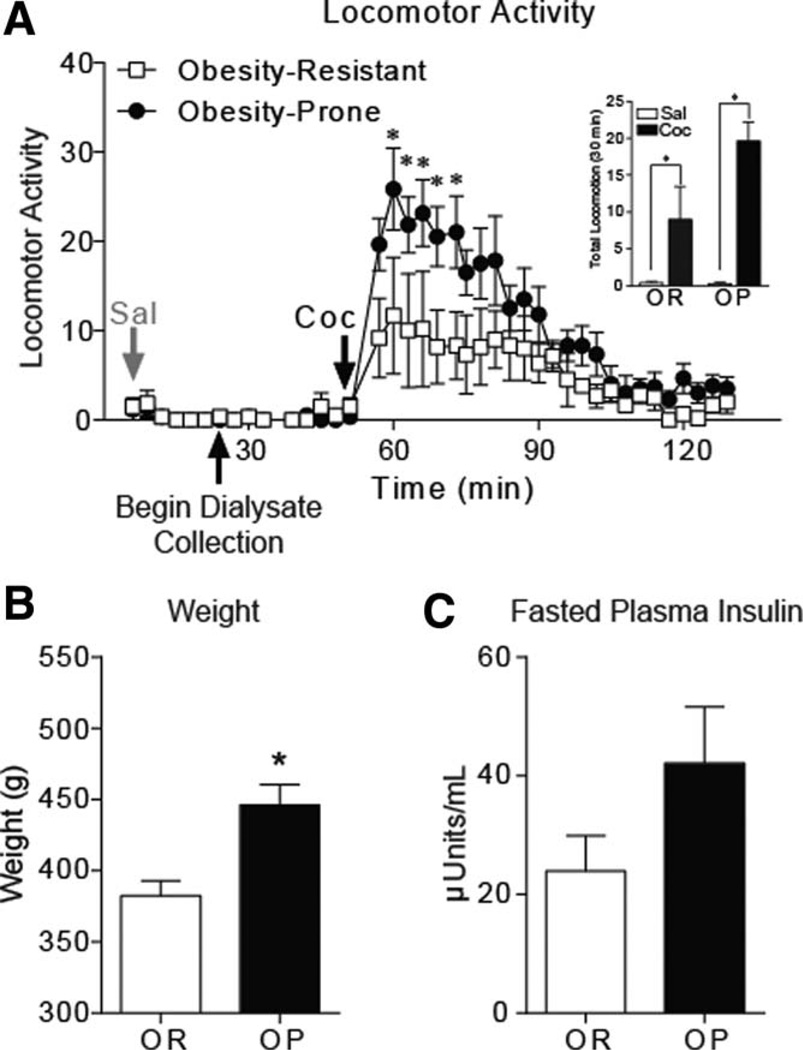
Locomotor activity during habituation and saline injection was similar between groups (Figure 2A). Cocaine-induced locomotion was significantly greater in obesity-prone versus obesity-resistant rats (Figure 2A; two-way RM ANOVA, group × time interaction: F(39, 390) = 2.212, P < 0.001; post-hoc: P < 0.05 from time 60 to 72 min). Cocaine increased locomotor activity in both groups [Figure 2A inset; average locomotor activity (30 min) saline vs. cocaine; two-way ANOVA, main effect: F(1,10) = 29.90, P < 0.001].
Figure 2.
Cocaine induces more robust locomotor activity in obesity-prone versus obesity-resistant rats without diet manipulation. (A) Mean (±SEM) locomotor activity in response to saline and cocaine injection. Within group comparisons of saline versus cocaine-induced locomotion 30 min after injection are shown in the inset. Obesity-prone (OP; N = 6) and obesity-resistant (OR; N = 6) rats showed similar locomotor responses to saline (gray arrow). Obesity-prone rats showed enhanced sensitivity to cocaine-induced locomotion (black arrow, 15 mg/kg i.p.) compared to obesity-resistant rats. In addition, the response to cocaine was greater than the response to saline in both groups. (B) Mean (±SEM) weight on the day of locomotor testing and dialysis collection. Obesity-prone rats were heavier than their obesity-resistant counterparts. (C) Mean (±SEM) fasted plasma insulin levels. Although fasted plasma insulin levels tended to be higher in obesity-prone rats, this difference was not statistically significant. *P < 0.05.
Body weight and fasted insulin
Consistent with previous reports (28), obesity-prone rats given standard chow were heavier than obesity-resistant rats (Figure 2B; t10 = 3.623, P < 0.005). Fasted plasma insulin levels did not differ between groups (Figure 2C; t8 = 1.622, P = 0.1434; OP N = 5, OR N = 5; blood samples not collected from two rats).
Extracellular cocaine and neurotransmitter levels
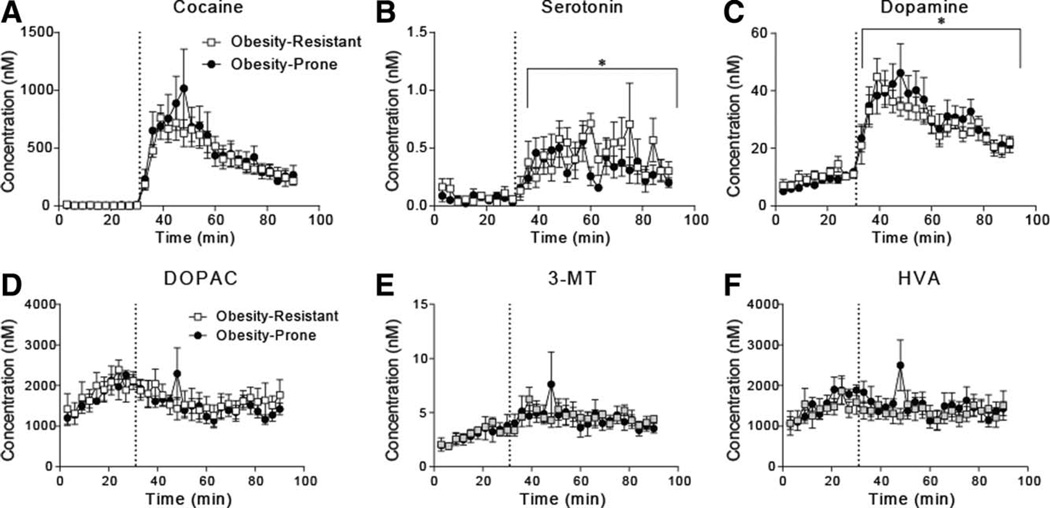
Ten baseline dialysate samples were collected (30 min) prior to cocaine injection (15 mg/kg, i.p.). No differences in basal dopamine were found between groups (Figure 3; time 0–30: two-way RM ANOVA, group × time interaction: F(9,90) = 1.8, P = 0.07; main effect of group: F(1,10) = 1.3, P = 0.27). Baseline levels of all neurotransmitters and their metabolites are provided in Table 1; no group differences were found. Striatal cocaine levels were similar in obesity-prone and obesity-resistant rats (Figure 3A). Cocaine produced increases in dopamine (Figure 3C; two-way RM ANOVA, main effect of cocaine: F(1,10) = 88.35, P < 0.0001) and serotonin levels in both groups (Figure 3B; two-way RM ANOVA, main effect of cocaine: F(1,10) = 36.91, P = 0.0001), but the magnitude of these increases did not differ. No group differences were found for dopamine metabolites (Figure 3D–F). Thus, although cocaine-induced locomotion was enhanced in obesity-prone rats, cocaine-induced increases in dopamine in these same rats did not differ between groups.
Figure 3.
Cocaine evokes similar increases in extracellular dopamine overflow in obesity-prone and obesity-resistant rats without diet manipulation. In all panels, the dotted line indicates when cocaine was given (15 mg/kg, i.p.). (A) Mean (±SEM) concentrations of cocaine reaching the probe after i.p. injection were similar between obesity-prone and obesity-resistant rats. (B) Mean (±SEM) striatal serotonin levels prior to and after cocaine injection. Serotonin levels were similarly increased after cocaine injection in obesity-prone and obesity-resistant rats. (C) Mean (±SEM) striatal dopamine levels prior to and after cocaine injection. (D–F) Mean (±SEM) concentration of the dopamine metabolites DOPAC, 3-MT, and HVA. Striatal dopamine levels were similarly increased after cocaine injection in both obesity-prone and obesity-resistant and no differences in dopamine metabolites were found; *P < 0.05.
TABLE 1.
Basal in vivo dialysate concentration
| Baseline in vivo concentration (nM) | ||
|---|---|---|
| Analyte | Obesity-resistant | Obesity-prone |
| Dopaminea | 11.9 ± 1.46 | 10.3 ± 0.89 |
| DOPA | 0.64 ± 0.33 | 0.65 ± 0.26 |
| DOPAC | 2120 ± 207 | 2120 ± 165 |
| HVA | 1580 ± 225 | 1870 ± 191 |
| 3-MT | 3.59 ± 0.31 | 3.52 ± 0.78 |
| Serotonin | 0.06 ± 0.02 | 0.05 ± 0.01 |
| 5-HIAA | 409 ± 54.0 | 503 ± 54.0 |
| Glutamatea | 874 ± 281 | 638 ± 196 |
| GABA | 40.3 ± 6.16 | 51.1 ± 8.91 |
| Acetylcholine | 0.28 ± 0.05 | 0.30 ± 0.07 |
| Histamine | 3.16 ± 0.37 | 4.29 ± 0.68 |
| Glutamine | 30400 ± 5750 | 34900 ± 5610 |
| Glycine | 1400 ± 391 | 1660 ± 429 |
| Aspartate | 11.3 ± 2.41 | 15.5 ± 3.71 |
| Taurine | 1310 ± 209 | 1460 ± 268 |
| Serine | 4990 ± 1230 | 7660 ± 2470 |
| Adenosine | 2.64 ± 0.67 | 2.37 ± 0.51 |
| Phenylalanine | 649 +/− 64.6 | 720 ± 118 |
| Glucoseb | 158 ± 14.3b | 185 ± 21.0b |
| Tyrosine | 347 ± 112 | 443 ± 115 |
All values given are means ± SEM.
Values are in vivo basal concentrations determined using calibration from 13C-labeled dopamine or glutamate in perfusate. All other concentrations are dialysate concentrations not corrected for recovery.
Values given in µM.
Sensitivity to quinpirole
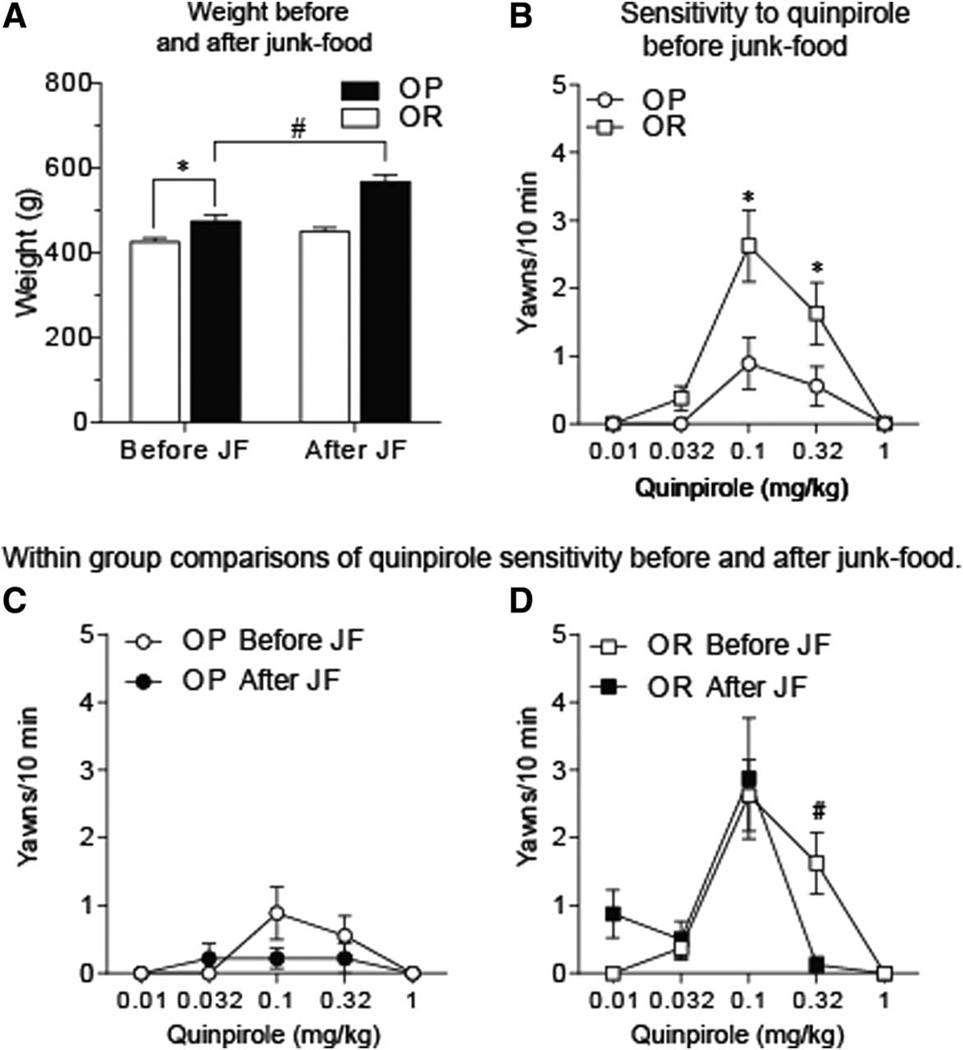
Sensitivity to quinpirole-induced yawning was determined before and after restricted access to junk-food (OP N = 10; OR N = 8). Obesity-prone rats were heavier than obesity-resistant rats before and after junk-food exposure (Figure 4A; two-way RM ANOVA, main effect of group: F(1,16) = 32.1, P = 0.0001). Obesity-prone rats gained weight during junk-food exposure (10 g/day, 14 days), whereas obesity-resistant rats did not (Figure 4A; two-way RM ANOVA, group × time interaction: F(1,16) = 8.6, P < 0.01; OP before junk-food vs. OP after junk-food: P < 0.5). Before junk-food exposure, quinpirole produced an inverted U-shaped dose-response function in yawning in both groups, with the peak effect observed at 0.1 mg/kg (Figure 4B; two-way RM ANOVA, main effect of dose: F(4,75) = 15.64, P < 0.0001). The magnitude of quinpirole-induced yawning was significantly lower in obesity-prone versus obesity-resistant rats (Figure 4B; two-way RM ANOVA, main effect of group: F(1,75) = 10.46, P < 0.0018). There was no relationship between weight and number of yawns induced by 0.1 mg/kg quinpirole (P = 0.23; r2 = 0.095). After restricted junk-food exposure, the dose-response relationship for quinpirole was absent in obesityprone rats, with few responses elicited at any dose tested (Figure 4C; one-way RM ANOVA post-junk-food, main effect of dose: F(2.2,18.2) = 0.57, P = 0.6). In contrast, the dose-response relationship was maintained in obesity-resistant rats after junk-food exposure (Figure 4D; one-way RM ANOVA post-junk-food, main effect of dose: F(1.4,10) = 6.34, P < 0.03), with slight increases in sensitivity to the D2R-mediated effects of quinpirole (Figure 4D: two-way RM ANOVA, test × dose interaction: F(4,28) = 2.46, P = 0.06; OP prevs. post-junk-food 0.32 mg/kg: t7 = 3.55, P < 0.01). In addition, after junk-food exposure, a significant negative correlation was found between weight on the test day and yawning induced by 0.1 mg/kg quinpirole such that the heaviest rats showed the fewest yawns while the lightest rats showed the most yawns (linear regression weight × number of yawns: F(1,15) = 5.327, P < 0.05). Together, these data suggest D2R-mediated transmission is enhanced in obesity-prone versus resistant rats prior to diet manipulation, although changes in D3R-mediated transmission may also contribute, and that restricted junk-food exposure produced slight increases in sensitivity to D2R-mediated effects in obesity-resistant rats.
Figure 4.
Differences in the effects of quinpirole in obesity-prone rats versus obesity-resistant rats. (A) Mean (±SEM) weight before and after junk-food exposure. Obesity-prone rats (OP; N = 10) were heavier than obesity-resistant rats (OR; N = 8) before junk-food exposure (*P < 0.05). In addition, weight was significantly increased after junk-food exposure only in obesity-prone rats (#P < 0.01). (B) Mean (±SEM) number of yawns induced by the D2/D3 agonist quinpirole. Quinpirole-induced yawning was significantly reduced in obesityprone versus obesity-resistant rats, consistent with basal differences in D2/D3R-mediated transmission. (C) Mean (±SEM) number of yawns induced by quinpirole before and after junk-food exposure in the same set of obesity-prone rats. (D) Mean (±SEM) number of yawns induced by quinpirole before and after junk-food exposure in the same set of obesity-resistant rats. The response to quinpirole on the descending limb of the dose-response curve was slightly stronger in obesity-resistant rats after junk-food exposure, consistent with an increase in D2R-mediated transmission.
Discussion
Interactions between pre-existing differences in mesolimbic function and exposure to fatty, sugary foods are thought to contribute to obesity (1,3,13). Here, we examined basal and cocaine-induced increases in striatal neurotransmitter levels and locomotor activity in obesity-prone and obesity-resistant rats without diet manipulation. Although obesity-prone rats showed a stronger locomotor response to cocaine (Figure 2), cocaine-induced increases in striatal dopamine (and serotonin) did not differ between groups. Enhanced sensitivity to cocaine is consistent with greater responsivity of mesolimbic circuits. However, neurochemical data suggest that this may be due to differences in postsynaptic dopamine receptor function, and not differences in presynaptic dopamine release. Consistent with this idea, quinpirole was less effective in obesity-prone than obesity-resistant rats (Figure 4B). This may be attributable to differences in either D2-and/or D3 receptor-mediated transmission, as responses on both the ascending (D3R) and the descending (D2R) limbs of the dose-response function were affected (26,29). When these same rats were subsequently given restricted exposure to junk-food (10 g/day), sensitivity to the D2R-mediated effects of quinpirole increased slightly in obesity-resistant rats (Figure 4C,D). Together, these data suggest that interactions between pre-existing differences in mesolimbic function and consumption of fatty, sugary foods produce distinct neurodaptations in dopamine systems of obesity-prone versus obesity-resistant individuals. These differences may promote enhanced motivation that contributes to obesity (1,7).
The microdialysis technique used here provides high sensitivity and relatively high temporal resolution (20) and including radiolabeled isotopes in the perfusate allows for more precise calculation of basal extracellular concentrations of dopamine and glutamate (25). Previous studies have found reduced basal dopamine levels in the NAc shell of selectively obesity-prone rats (11) and outbred rats identified as susceptible to diet-induced obesity (10,12), but basal differences in dopamine in other striatal regions have not previously been examined. In this study, basal neurotransmitter levels in NAc core and ventral portion of the CPu were similar between groups. This suggests that NAc shell may differ from other striatal regions in obesity-prone rats, although differences between this and previous studies (e.g., sex and normalization method) could also contribute.
The locomotor-activating effects of psychostimulant drugs like cocaine and amphetamine rely largely on dopaminergic transmission within the mesolimbic system (30,31). Thus, enhanced sensitivity to the locomotor-activating effects of cocaine in obesity-prone rats suggests that this system is hyper-responsive or “sensitized.” To our knowledge, no other studies have examined pre-existing differences in cocaine sensitivity in rats susceptible to obesity. However, diet manipulations have been reported to alter psychostimulant sensitivity in outbred rats. Specifically, enhanced locomotor activity has been found in response to systemic psychostimulant injection after access to fatty and sugary diets (13,32,33), though the source of dietary fats may also influence the neurobehavioral response to amphetamine (18). These effects seem to be related to the consumption of fatty, sugary foods rather than to obesity itself, as enhanced sensitivity to amphetamine or cocaine were found in the absence of obesity (13,18,19). Here, consistent with previous studies (28,34), obesityprone rats had slightly higher fasted insulin levels than obesity-resistant rats before junk-food exposure (Figure 2C). Insulin levels in the brain parallel circulating insulin levels (35). In nonobese mice, acute insulin treatment reduces the threshold for dopamine neuron firing and this effect of insulin is attenuated in genetically obese, hyperinsulinemic mice (36). These data suggest that elevations in circulating insulin can influence the function of brain reward systems. Thus, although several factors likely contribute, it is interesting to speculate that the basal differences observed here may be related to mild, chronic elevations of insulin in obesity-prone rats promoting neuroadaptations that enhance the responsivity of mesolimbic systems to cocaine, food, and stimuli paired with food (7,13). Direct tests of this hypothesis should be conducted in the future.
Although cocaine produced robust increases in extracellular striatal dopamine (and moderate increases in serotonin levels), the magnitude of this change was similar between groups. Importantly, cocaine concentration in the brain did not differ between groups, ruling out the possibility of differences in dosing, metabolism, or distribution. It is possible that differences in cocaine-induced dopamine levels may be restricted to more discrete striatal subregions, and/or that enhanced sensitivity to cocaine in obesity-prone rats prior to diet manipulation may be due to differences in postsynaptic dopamine transmission. Consistent with the latter, obesity-prone rats were less sensitive to quinpirole-induced yawning than obesity-resistant rats prior to diet manipulation. Examination of the dose-response relationship suggests less sensitivity to D3R-mediated increases in yawning and greater sensitivity to D2R-mediated decreases in yawning in obesity-prone vs. resistant rats (26,27). These basal differences could be due to differences in receptor expression and/or function. A recent study using the same selectively bred rats as used here found greater D2R gene expression in the NAc of obesity-prone versus resistant rats (17), consistent with basal elevations in D2R-mediated transmission found here. While the effects of D2/D3 receptors on mesolimbic function are complex, the basal differences in sensitivity to quinpirole in obesity-prone versus resistant rats are consistent with greater sensitivity to cocaine-induced locomotion in obesity-prone rats. For example, cocaine-induced locomotor sensitization has been shown to produce a similar pattern of enhanced D2R-mediated effects (37).
While few studies have examined pre-existing differences in dopamine receptor function or expression, rodent and human studies have examined D2R mRNA levels and D2R binding after the onset of diet induced obesity. In rodents, diet-induced obesity and/or exposure to sugary, fatty foods without obesity, decreases striatal D2R mRNA and protein levels (13,18,38), although increases have also been observed (17,39). Similarly, lower levels of D2R binding have been reported in individuals with morbid obesity [BMI 42–60 (9)]. Here, junk-food-induced changes in sensitivity to quinpirole were challenging to evaluate in obesity-prone rats due to floor effects, although the flattening of the dose-response curve after junk-food is consistent with increased sensitivity to D2R-mediated effects. The effect of diet manipulation was clearer in obesity-resistant rats, where junk-food increased sensitivity to D2R-mediated effects. This effect of junk-food is consistent with previous studies showing that exposure to a high fat diet without obesity increases quinpirole sensitivity in outbred rats (19,33). Given that a subpopulation of outbred Sprague-Dawley rats are susceptible to diet-induced obesity (13), the mixed effects of diet-induced obesity on dopamine receptor expression and function may be related to the presence of obesity-prone and obesity-resistant rats in the outbred population.
In summary, prior to diet manipulation obesity-prone rats are hyper-responsive to the locomotor-activating effects of cocaine. This does not appear to be due to differences in cocaine-induced elevations in striatal dopamine but may be due to differences in D2/D3R function and/or number. It is important to note that, as expected, obesity-prone rats were heavier than their obesity-resistant counterparts even without diet manipulation. Thus, basal differences prior to diet manipulation may be influenced by factors accompanying greater weight gain but preceding the development of metabolic dysregulation. In addition, eating junk-food produced slight increases in sensitivity to D2R-mediated effects in obesity-resistant rats. Together, these data support the idea that interactions between predisposition and consumption of fatty, sugary foods produce distinct neuroadaptations in obesity-prone versus obesity-resistant individuals. These differences in the responsivity of mesolimbic circuits may ultimately promote weight gain and contribute to enhanced striatal responsivity to food and food cues in individuals that are susceptible to obesity (1,7,13).
Acknowledgments
Cocaine was provided by the NIDA drug supply program. Dr. Jeremiah Bertz provided consultation for quinpirole-induced yawning measures. Drs. Scott Kanoski and Gregg Collins provided valuable conversations regarding data interpretation.
Funding agencies: PJV is funded by the Biology of Drug Abuse training grant fellowship (T32DA07268). OSM is funded by the Michael J. Fox Foundation for Parkinson’s research. RTK is funded by NIBIB 37 and EB 003320. CRF is supported by DK106188 and a Brain and Behavior Research Foundation NARSAD Young Investigator Award. Studies also utilized the Chemistry Core of the Michigan Diabetes Research and Training Center funded by DK020572 awarded by NIDDK.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300:R1266–R1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neuroscience and biobehavioral reviews. 2012 doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and biobehavioral reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 6.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 8.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science (New York, NY) 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. The Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 10.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiger BM, Behr GG, Frank LE, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiology & behavior. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson MJ, Burghardt PR, Patterson CM, et al. Individual differences in cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oginsky MF, Maust JD, Corthell JT, Ferrario CR. Enhanced cocaine-induced locomotor sensitization and intrinsic excitability of NAc medium spiny neurons in adult but not in adolescent rats susceptible to diet-induced obesity. Psychopharmacology. 2015 doi: 10.1007/s00213-015-4157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu XT. Cocaine withdrawal and neuro-adaptations in ion channel function. Mol Neurobiol. 2007;35:95–112. doi: 10.1007/BF02700626. [DOI] [PubMed] [Google Scholar]
- 17.Valenza M, Steardo L, Cottone P, Sabino V. Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology (Berl) 2015;232:3215–3226. doi: 10.1007/s00213-015-3981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hryhorczuk C, Florea M, Rodaros D, et al. Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacology. 2016;41:811–821. doi: 10.1038/npp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baladi MG, Newman AH, France CP. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology. 2011;217:573–585. doi: 10.1007/s00213-011-2320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song P, Mabrouk OS, Hershey ND, Kennedy RT. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Analytical chemistry. 2012;84:412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology. 2004;174:341–357. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson CJ. The Rat Brain in Stereotaxic Coordinates. 6th. London: Academic Press; 2007. [Google Scholar]
- 23.Di Chiara G, Tanda G, Carboni E. Estimation of in-vivo neurotransmitter release by brain microdialysis: the issue of validity. Behavioural pharmacology. 1996;7:640–657. [PubMed] [Google Scholar]
- 24.Caljon G, Caveliers V, Lahoutte T, et al. Using microdialysis to analyse the passage of monovalent nanobodies through the blood-brain barrier. British journal of pharmacology. 2012;165:2341–2353. doi: 10.1111/j.1476-5381.2011.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hershey ND, Kennedy RT. In vivo calibration of microdialysis using infusion of stable-isotope labeled neurotransmitters. ACS chemical neuroscience. 2013;4:729–736. doi: 10.1021/cn300199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins GT, Witkin JM, Newman AH, et al. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. The Journal of pharmacology and experimental therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baladi MG, Newman AH, France CP. Feeding condition and the relative contribution of different dopamine receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology. 2014;231:581–591. doi: 10.1007/s00213-013-3271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollbrecht PJ, Nobile CW, Chadderdon AM, Jutkiewicz EM, Ferrario CR. Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats. Physiology & behavior. 2015;152:151–160. doi: 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins GT, Newman AH, Grundt P, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and biobehavioral reviews. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes, brain, and behavior. 2009;8:346–355. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire BA, Baladi MG, France CP. Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. European journal of pharmacology. 2011;658:156–159. doi: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baladi MG, Koek W, Aumann M, Velasco F, France CP. Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology. 2012;222:447–457. doi: 10.1007/s00213-012-2663-7. [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. The American journal of physiology. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 35.Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. The Journal of clinical endocrinology and metabolism. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Labouebe G, Karunakaran S, Clee SM, Borgland SL. Effect of insulin on excitatory synaptic transmission onto dopamine neurons of the ventral tegmental area in a mouse model of hyperinsulinemia. Nutrition & diabetes. 2013;3:e97. doi: 10.1038/nutd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology. 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang XF, Zavitsanou K, Huang X, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behavioural brain research. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Alsio J, Olszewski PK, Norback AH, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171:779–787. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]