Abstract
Nitric oxide, bicarbonate, natriuretic peptides (ANP, BNP and CNP), guanylins, uroguanylins and guanylyl cyclase activating proteins (GCAPs) activate a family of enzymes variously called guanyl, guanylyl or guanylate cyclases that catalyze the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP) and pyrophosphate. Intracellular cyclic GMP is a second messenger that modulates: platelet aggregation, neurotransmission, sexual arousal, gut peristalsis, blood pressure, long bone growth, intestinal fluid secretion, lipolysis, phototransduction, cardiac hypertrophy and oocyte maturation. This review briefly discusses the discovery of cGMP and guanylyl cyclases, then nitric oxide, nitric oxide synthase and soluble guanylyl cyclase are described in slightly greater detail. Finally, the structure, function, and regulation of the individual mammalian single membrane-spanning guanylyl cyclases GC-A, GC-B, GC-C, GC-D, GC-E, GC-F and GC-G are described in greatest detail as determined by biochemical, cell biological and gene-deletion studies.
Keywords: Signal transduction, Hormones, Enzymes, cGMP
1. Introduction
Cyclic GMP was first purified and identified in rat urine in 1963 by Ashman and colleagues [1]. The enzymes that catalyze the conversion of GTP into cGMP were discovered 6 years later by three separate groups [2–4]. The cGMP synthesizing activity was initially called guanyl cyclase, then guanylate cyclase and in recent literature it is most often referred to as guanylyl cyclase. The Nomenclature Committee of the International Union of Biochemistry and Molecular Biology indicates that the accepted name is guanylate cyclase or GTP pyrophosphatelyase, EC 4.6.1.2. The reaction catalyzed is: divalent metal bound GTP→cGMP+PPi. The divalent metal cofactor under biologic conditions is Mg2+, but many early investigations used Mn2+ in the presence of non-ionic detergent in order to artificially stimulate the enzymes because the biologic activators of the respective enzymes were not known. As a result of these initial studies, it was determined that the enzyme could be separated into distinct soluble and particular forms with unique kinetic properties and tissue distributions [5, 6]. However, the true diversity of the family was not fully appreciated until molecular cloning identified cDNAs that encoded individual enzymes.
Mammals express four soluble (α1, α2, β1 and β2) guanylyl cyclase subunits and seven bona fide single membrane-spanning forms known as GC-A, GC-B, GC-C, GC-D, GC-E, GC-F and GC-G (see Table 1 for ligands and “knockout” phenotypes associated with each cyclase). However, GC-D and GC-G are pseudogenes in humans [7]. The soluble forms exist as heterodimers and the transmembrane members are homodimers, except for GC-C, which appears to be a homotrimer. The minimal guanylyl cyclase catalytic unit is a dimer [8, 9]. The soluble forms contain an amino terminal heme-binding, dimerization and carboxyl-terminal catalytic domains. The membrane-spanning forms contain an extracellular ligand-binding, transmembrane, kinase homology, dimerization and carboxyl-terminal catalytic domains (see Fig. 1 for topology of each cyclase). The latter domain is the most conserved between family members and is homologous to the catalytic domain of adenylyl cyclase [10, 11].
Table 1.
Guanylyl cyclases, tissue distribution, cognate ligand and effect of inactivation. Abbreviations are: ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CNP, C-type natriuretic peptide; CO, carbon monoxide; CO2/HCO3, carbon dioxide/bicarbonate; GCAPs, guanylyl cyclase activator proteins; Gn, guanylin; Hb, hemoglobin; NO, nitric oxide; Sta, heat-stable enterotoxin; Uro, uroguanylin.
| Guanylyl cyclase | Tissue expression | Ligand | Effect of inactivation |
|---|---|---|---|
| GC-A | Lung, kidney, adrenal, vascular smooth muscle, endothelium, heart, adipose | ANP, BNP | Hypertension, cardiac hypertrophy and cardiac fibrosis |
| GC-B | Bone, vascular smooth muscle, lung, brain, heart, liver, uterus and follicle | CNP | Acromesomelic dysplasia type Maroteaux dwarfism, female sterility, axon bifurcation in spinal cord |
| GC-C | Intestinal epithelium | Guanylin, uroguanylin and bacterial heat-stable enterotoxin | Increased colonic epithelial cell proliferation |
| GC-D | Olfactory bulb | CO2/HCO3 | Loss of guanylin- and uroguanylin-dependent olfactory signaling |
| GC-E | Retina, pineal gland | GCAPs | Cone dystrophy, Leber congenital amaurosis type 1, blindness |
| GC-F | Retina | GCAPs | No obvious phenotype |
| GC-G | Lung, intestine, skeletal muscle, testes | CO2/HCO3 | Increased deleterious responses to renal ischemia/reperfusion injury |
| Soluble alpha 1 | Platelets, lung, brain, vasculature | NO, CO | Loss of NO-dependent platelet aggregation |
| Soluble alpha 2 | Brain, vasculature | NO, CO | No obvious effect |
| Soluble beta 1 | Platelets, lung, brain, vasculature | NO, CO | Hypertension, loss of NO-dependent platelet aggregation, male sterility and severely reduced intestinal motility |
| Soluble beta 2 | Kidney and liver | ? | ? |
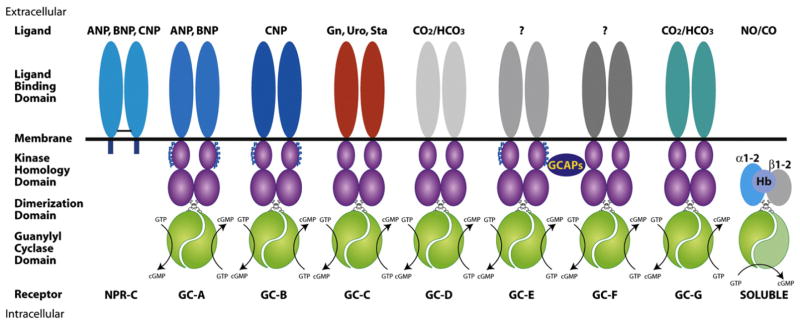
Fig. 1.
Schematic of mammalian guanylyl cyclases and their ligands. Seven transmembrane and four soluble guanylyl cyclases are expressed in mammals. The structure and function of each member is discussed in the text. Similarity of extracellular domain color represents primary amino acid sequence similarity between receptors. The blue “P” indicates known phosphorylation sites. The black bar between NPR-C subunits indicates intermolecular disulfide bond. Abbreviations are: ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CNP, C-type natriuretic peptide; CO, carbon monoxide; CO2/HCO3, carbon dioxide/bicarbonate; GCAPs, guanylyl cyclase activator proteins; Gn, guanylin; Hb, hemoglobin; NO, nitric oxide; NPR-C, natriuretic peptide receptor C; Sta, heat-stable enterotoxin; Uro, uroguanylin.
Interestingly, Caenorhabdiis elegans are predicted to have at least 26 membrane-spanning and 7 soluble guanylyl cyclases based on genome analysis [12], but humans only express five membrane spanning and 3 soluble subunits, which may indicate the human forms are more pleotropic than the enzymes found in worms. Multimembrane-spanning guanylyl cyclases similar to adenylyl cyclases were identified in Dictyostelium discoidium, Plasmodium falciparum, Paramecium tetraurelia and Tetrahymena pyriformis. These guanylyl cyclases probably evolved from adenylyl cyclase based upon topology [13]. One guanylyl cyclase was identified in cyanobacteria [14] and the crystal structure of the catalytic domain of this enzyme was solved [15]. Additionally, the crystal structure of an inactive soluble guanylyl cyclase from the green algae Chlamydomonas reinhardtii was solved [16].
According to the structures described above, the guanylyl cyclase catalytic domain contains a central seven-stranded β sheet surrounded by several α helices in a wreath-like structure that is characteristic of class III nucleotide cyclases. The central cleft of the momodimeric form is at the dimer interface and contains two symmetric active sites, which is consistent with positive cooperative substrate-velocity curves observed for particulate cyclases [15]. Each monomer contributes unique residues to the catalytic site. Joubert and colleagues created a mutant form of the catalytic domain of rat GC-A by making single amino acid substitutions on each monomer to inactive only one GTP binding site and observed linear kinetics, consistent with mammalian GCs adopting a similar structure to the cyanobacterial GC [17]. Mutagenesis studies converting human GC-E and rat soluble guanylyl cyclase to adenylyl cyclases are consistent with mammalian guanylyl cyclases adopting similar structures and catalytic mechanisms as adenylyl cyclases [10, 11]. No structure of an active mammalian guanylyl cyclase has been solved to date.
2. Soluble guanylyl cyclase, nitric oxide and nitric oxide synthase
The primary and best-studied endogenous activator of soluble guanylyl cyclase (sGC) is nitric oxide (NO), which was originally describe as endothelium-derived relaxing factor for its potent ability to relax blood vessels in response to vasodilators like acetylcholine or bradykinin [18]. NO is synthesized by nitric oxide synthase (NOS), which catalyzes a NADPH-dependent 5-electron oxidation of L-arginine by O2 to produce NO and citrulline.
There are three genetically distinct isoforms of NOS: a constitutively expressed and calcium-activated endothelial form (eNOS or NOS3), a neuronal isozyme (nNOS or NOS1) and a cytokine- or endotoxin-induced, calcium-independent form (iNOS or NOS2) that is highly expressed in macrophages and neutrophils. Endothelium produced NO migrates into blood and surrounding vascular smooth muscle cells to inhibit platelet aggregation and stimulate vasorelaxation, respectively. Blood pressures in mice lacking endothelial NOS are about 20 mm of Hg higher than pressures in wild type littermates [19, 20]. Neuronal NOS also causes vasodilation and may be a retrograde messenger in the long-term potentiation process involved in the formation of memories. Mice with disruptions in exon two of nNOS have enlarged pyloric sphincters and increased aggressive behavior, but normal blood pressure [21, 22]. The main role of inducible NOS is to kill invading microorganisms by producing high levels of NO, which combine with superoxide to form peroxynitrite. Thus, the immune function of NO is independent of guanylyl cyclase.
Soluble GCs are found in most tissues. Lung, brain, kidney and vascular tissue are rich in sGC. In humans, two α and two β subunits have been identified, but the function of the β2 is uncertain. The best-characterized heterodimers are the α1/β1 and the α2/β1 forms. β1 is 619 residues and contains an evolutionarily conserved aminoterminal heme prosthetic group binding domain of about 200 residues, a dimerization region and a carboxyl-terminal guanylyl cyclase domain of about 250 residues. The β2 subunit has an additional 86 carboxyl-terminal amino acids compared to the β1 isoform that contains a consensus CAAX sequence for possible isoprenylation or carboxymethylation [23]. In contrast to the other subunits, β2 was reported to form an active homodimer [24]. The human α1 subunit is 717 amino acids and is about 34% homologous to the β1 subunit [25]. The α2 subunit is 48% identical to the α1 subunit at the amino acid level [26]. α1 is the major subunit in platelets and lung but only accounts for about half the NO-dependent guanylyl cyclase activity in the brain [27]. Although α2 only represents 6% of the soluble cyclase activity in the vasculature, it is sufficient to yield maximum smooth muscle relaxation in response to NO in mice lacking α1 [27].
His-105 at the amino terminus of the β1 subunit is the axial ligand of the pentacoordinated reduced iron center of heme [28]. His-105 and a heme prosthetic group containing reduced iron are required for NO activation of the enzyme. NO activates sGC by binding to the sixth position of the heme ring, which breaks the bond between the axial histidine and iron to form a 5-coordinated ring with NO in the fifth position. Carbon monoxide can also activate sGC by binding heme to form a 6-coordinated complex. However, since NO activates the enzyme 100 to 200 fold whereas CO only activates it about 4-fold. It is unclear if CO signals in a physiologic significance manner.
Genetic disruption of the α1 subunit leads to the loss of NO-dependent platelet aggregation but has a normal vasorelaxation response [27]. Disruption of the β1 subunit [29] leads to the loss of both these responses with blood pressures being elevated 26 mm of Hg in the knockout compared to wild type animals [29]. In addition, the homozygous knockout animals exhibited severely reduced parastalsis leading to gastrointestinal obstruction similar to that observed in animals lacking cGMP dependent protein kinase I, (PKGI/cGKI) [30]. The male mice lacking the β1 subunit are infertile [30]. In conclusion, sGC appears to be the sole mediator of the major cardiovascular and sexual arousal effects of NO [31].
2.1. GC-A
In contrast to soluble GC, transmembrane GCs are activated by peptides. The archetypical transmembrane guanylyl cyclase is GC-A, which is also called NPR-A or NPR1. It is activated by atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) [32]. Mature ANP is a 28 amino acid disulfide-linked peptide released from atrial granules [33, 34]. Synthetic human ANP called Carperitide is used to treat congestive heart failure in Japan. BNP is found in atrial granules as well but it is released in greater concentrations from the ventricles due to increased mass. A synthetic version of human BNP called Nesiritide is approved for the treatment of congestive heart failure in the US. Genetic ablation of ANP results in hypertensive mice with cardiac hypertrophy [35, 36]. Disruption of BNP in mice increases ventricular fibrosis but does not affect blood pressure [37].
GC-A is highly expressed in kidney, lung, adrenal, vasculature, brain, liver, endothelial and adipose tissues [38, 39]. It is expressed at lower levels in the heart [40]. GC-A null mice exhibit cardiac hypertrophy, high blood pressure and ventricular fibrosis [41, 42]. The extracellular domain of rat GC-A contains three intramolecular disulfide bonds and five N-linked glycosylation sites [43]. In the basal state, GC-A exists as a homodimer and ligand binding does not result in further oligomerization [44]. Crystalligraphic studies indicate that the rat GC-A extracellular domain dimer binds ANP asymmetrically because ANP has no internal symmetry. The dimerization interface between the two monomers is in the membrane distil region as is a buried chloride ion that was suggested to regulate renal chloride secretion [45, 46]. ANP binds to GC-A in a 1:2 stoichiometry and activates the receptor by causing an intermolecular twist with little intramolecular conformational change [47]. GC-A is phosphorylated on five serines and two threonines within the aminoterminal portion of its kinase homology domain in the unstimulated state [48]. Phosphorylation of GC-A is required for hormonal activation, and dephosphorylation inhibits the receptor [49]. ATP also increases the enzymatic activity GC-A, but the mechanism of the activation is controversial. Some groups report increases in maximal velocity [50, 51], whereas we find that it decreases the Michaelis constant for GTP [52, 53].
2.2. GC-B
GC-B, which is also called NPR-B or NPR2, is structurally similar to GC-A but is activated in a paracrine manner by CNP, which exists in 22 and 53 amino acid forms that are structurally similar to ANP and BNP [32, 54]. The most obvious effects of CNP are the stimulation of endochondrial ossification required for long bone growth and oocyte maturation required for female reproduction [55, 56]. Mice lacking CNP are dwarfs and less than half survive postnatal development [57]. Chondrocyte overexpression of CNP rescued growth in a mouse model of the most common type of human dwarfism [58]. Human patients with translocations of portions of chromosome 2 of the CNP gene were shown to have elevated CNP levels and skeletal overgrowth associated with a Marfanoid-like phenotype [59, 60].
GC-B is abundantly expressed in brain, lung, bone, heart and ovary tissue [61–63]. It is also expressed at relatively high levels in fibroblast and vascular smooth muscle cells. Like GC-A, it contains three intramolecular disulfide bonds and is highly glycosylated on asparagine residues. GC-B is highly phosphorylated and dephosphorylation is associated with receptor inhibition [64, 65]. ATP increases the enzymatic activity of GC-B by reducing the Michaelis constant an order of magnitude [52, 53].
Two loss-of-function mouse models exist for GC-B. In one model the exons that encode the carboxyl-terminal half of the extracellular domain and transmembrane segment were deleted by homologous recombination [66]. These mice are dwarfs and the females are sterile, which is consistent with CNP stimulating oocyte maturation [56]. The heterozygous mice were significantly shorter than the wild-type animals. The other mouse model has a spontaneous mutation of an arginine for a highly conserved leucine in the catalytic domain. This cn/cn mouse that contains two defective alleles also displays dwarfism, although female sterility was not noted [67]. Several homozygous loss of function mutations have been identified in human patients that cause acromesomelic dysplasia, type Maroteaux, a rare form of short-limbed dwarfism [68]. Individuals with one normal and one abnormal allele display normal limb proportions but are statistically shorter than the average person from their respective populations [69]. GC-B is the primary particulate guanylyl cyclase in the failed heart, but the cardiac cellular expression of GC-B is unclear [40]. GC-B and PKGI were also shown to be essential for sensory axon bifurcation in the spinal cord of mice [70].
2.3. GC-C
Guanylyl cyclase C (GC-C) has similar topology to GC-A and GC-B but contains about a 60 amino acid carboxyl terminal extension that renders the receptor detergent insoluble. GC-C is highly concentrated at the apical membrane of intestinal epithelial cells and is the target for small peptides secreted from pathogenic bacteria called heat stable enterotoxins (Sta) that contain three stabilizing disulfide bonds. Hence, it is sometimes referred to as the heat stable enterotoxin receptor or simply, StaR. Sta activation of GC-C leads to intracellular cGMP elevations, PKGII-dependent phosphorylation of the cystic fibrosis transmembrane regulator and increased Cl− secretion in the gut. Lumenal ion secretion increases intestinal water content, which results in diarrhea and severe dehydration that can lead to death, especially in infants.
Guanylin and uroguanylin are endogenous peptide ligands for GC-C. Guanylin is a 15 amino acid peptide that contains two intramolecular disulfide bonds. Uroguanylin is a 19 amino acid peptide with conserved disulfide bonds to guanylin [71]. Human guanylin and uroguanylin are 53% identical. Both peptides compete for binding with Sta to the extracellular domain of GC-C. Mice lacking functional guanylin display increased colonic epithelial cell proliferation but no change in blood pressure or sodium excretion [72]. In contrast, mice lacking uroguanylin display decreased ability to excrete an enteral NaCl load as well as salt-independent hypertension [73].
The extracellular domain of GC-C contains many N-linked glycosylation sites [74–76] and multiple intramolecular disulfide bonds [77]. GC-C exists as a higher ordered structure; dimer [78] or trimer forms have been reported [79]. ATP increases the ligand-dependent guanylyl cyclase activity of GC-C, possibly by stabilizing the active form of the receptor [78, 80, 81]. Unlike GC-A and GC-B, GC-C is not basally phosphorylated [78], but treatment of cells with phorbol esters increases the phosphate content and activity of GC-C, consistent with protein kinase C-dependent regulation [78, 82]. Mice lacking functional GC-C are resistant to heat stable enterotoxin infection and have increased proliferation of intestinal epithelia cells [83–85]. Recent work indicates that GC-C is a marker [86] and possible therapeutic target for colon cancer [87–89]. A modified version of Sta called Linaclotide is in phase three clinical trials for the treatment of chronic constipation and irritable bowel syndrome [90].
2.4. GC-D
GC-D was molecularly cloned from rat olfactory epithelium [91], and subsequently colocalized by immunofluorescence detection with phosphodiesterase 2 in mouse olfactory cilia [92] and with phosphodiesterase 2 and the selective cGMP gated nucleotide channel, CNGA3, in neurons that project to the necklace glomeruli of the olfactory bulb in rats [93]. GC-D positive cells completely lack essential components of the well-known cAMP olfactory signal transduction system. Recently, uroguanylin or guanylin but not Sta were shown to elicit an excitatory calcium elevation signal in GC-D positive neurons. The response to both compounds was abolished in mice lacking either GC-D or CNGA3 [94]. Whether guanylin or uroguanylin directly bind GC-D is not known. However, since GC-D positive cells do not respond to Sta, this seems unlikely [94]. GC-D is activated by carbon dioxide or bicarbonate [95, 96] and participates in the socially transmitted food preference response in mice [97].
2.5. GC-E and GC-F
Cyclic GMP is an essential regulator of phototransduction; a process where rods and cones in the retina convert photons into electrical signals producing vision. In the presence of light, photons bind the seven membrane-spanning protein, rhodopsin, which leads to transducin-dependent activation of phosphodiesterase 6 and reduced intracellular cGMP concentrations. As a result, cGMP-gated sodium and calcium channels close, causing hyperpolarization. In the dark, rhodopsin signaling is reduced and cGMP degradation is slowed. This in combination with cyclase activation (see below) causes the opening of cGMP-gated cation channels, elevations of intracellular sodium and calcium concentrations and cell depolarization, which causes photorecovery.
Early studies indicated that guanylyl cyclase activity in the retina was inhibited by calcium [98] through a factor that was separated from the cyclase by a high salt wash [99]. Two single membrane-spanning guanylyl cyclases GC-E and GC-F, also known as RetGC-1 and RetGC-2, respectively, were cloned [100–102]. GC-E is expressed in the retina and pineal gland, whereas GC-F is only expressed in the retina [100–102]. Both retinal cyclases contain the same signature domains as GC-A, GC-B and GC-C but unlike these known hormone receptors, no extracellular activators of GC-E or GC-F have been identified [100–102]. Instead, small (20–24 kDa), soluble, intracellular calcium-binding molecules called guanylyl cyclase activator proteins (GCAPs) regulate GC-E and GC-F [103–106]. In vertebrate retinas, at least two GCAPs (GCAP1 and GCAP2) are present. Both are fatty acylated on their amino termini and contain four calcium binding EF-hand domains, although only three are functional. GCAPs are constitutively associated with GC-E, but only activate the cyclase under low calcium conditions. When intracellular calcium levels rise, GCAPs inhibit cyclase activity. Like GC-A and GC-B, GC-E contains multiple serine and threonine phosphorylation sites in the aminoterminal portion of the kinase homology domain, but unlike natriuretic peptide receptors, phosphorylation is not required for activation of GC-E by its peptide activators [107]. ATP also increases GC-E activity and an azido ATP analog was cross-linked to the receptor [108–110]. Inactivation of GC-E in mice caused suppression of a and b waves of electroretingrams from dark-adapted null mice and cone dystrophy but had no effect on rods [111]. Inactivating GC-E/ RetGC-1 mutations in humans cause Leber congenital amaurosis type 1, an autosomal recessive disease characterized by early onset rod and cone dystrophy and blindness [112, 113]. Inactivation of GC-F in mice causes no electroretinographic effects, but mice lacking both retinal cyclases have unstable and nonfunctional cones and rods [114]. No mutations in human GC-F/RetGC-2 have been reported. Functional inactivation of GCAP1 and GCAP2 results in mice that lack calcium-dependent retinal guanylyl cyclase regulation and a delayed recovery of the photoresponse [115]. Expression of GCAP1 in a double GCAP knockout background restores wild type flash responses in rods and cones, suggesting that GCAP1 is sufficient for normal retina photoregulation [116, 117]. Several missense mutations within GCAP1 are associated with autosomal dominant cone dystrophy in humans [118].
2.6. GC-G
GC-G was initially amplified by PCR from a rat small intestine cDNA library with degenerate primers common to single membrane spanning guanylyl cyclases [119]. Additional cloning and amplification techniques based on cDNA and gene structures were required to assemble a full-length clone [120]. Comparison of the extracellular domain of GC-G indicated that it is most similar to natriuretic peptide receptors. Expression in Cos7 and 293 cells revealed elevated basal guanylyl cyclase activity that was not increased by ANP, BNP, CNP or Sta. Northern hybridization analysis revealed high GC-G mRNA expression in lung, intestine and skeletal muscle. Cloning of the apparent mouse homolog of GC-G revealed high expression in the testes [121]. Mice lacking functional GC-G were not histologically different from wild type animals. Despite apparent low expression in the kidney, serum creatinine and urea levels were reduced in GC-G knockout mice compared to wild type animals in response to renal ischemia/reperfusion injury [122]. Finally, a recent report indicated that GC-G is activated by bicarbonate in the Grueneberg ganglion olfactory subsystem [123]. Importantly, a recombinant GST-fusion protein expressing the catalytic domain of GC-G was activated by NaHCO3 in a concentration dependent manner, which is consistent with direct binding of HCO3 or CO2 to the catalytic domain of GC-G.
3. Conclusion and perspectives
Guanylyl cyclases are ubiquitous enzymes that regulate critical functions in bacteria to humans. In mammals there are seven mGCs and activators of all members have now been identified. Mutations in the genes that encode mGCs or pathologic activation of these enzymes or their activators are associated with diseases of the cardiovascular, skeletal, intestinal or visual systems. Understanding the regulation of mGCs is important because several family members are drug targets. For example, GC-A is the target of two drugs (Carperitide and Nesiritide) approved for the treatment of congestive heart failure as well as a new chimeric natriuretic peptide called CD-NP that had favorable therapeutic effects in a recent clinical study [124, 125]. GC-B is the target for drugs in development for the treatment of skeletal diseases and glaucoma, and GC-C is the target of linaclotide, a drug that is currently in phase III clinical trials for the treatment of chronic constipation and irritable bowel syndrome. A novel, yet untested, approach to activating these receptors is to target the allosteric ATP binding site with small, cell-permeable ATP analogs. Alternatively, blocking GC-C activity with competitive or mixed inhibitors as was recently demonstrated for GC-A and GC-B may lead to a viable drug for the treatment or prevention of heat-stable enterotoxin infection [126].
Acknowledgments
I thank Deborah Dickey and Jerid Robinson for their helpful comments on this manuscript.
References
- 1.Ashman DF, Lipton R, Melicow MM, Price TD. Biochemical and Biophysical Research Communications. 1963;11:330. doi: 10.1016/0006-291x(63)90566-7. [DOI] [PubMed] [Google Scholar]
- 2.Hardman JG, Sutherland EW. Journal of Biological Chemistry. 1969;244(23):6363. [PubMed] [Google Scholar]
- 3.Schultz G, Bohme E, Munske K. Life Sciences. 1969;8(24):1323. doi: 10.1016/0024-3205(69)90189-1. [DOI] [PubMed] [Google Scholar]
- 4.White AA, Aurbach GD. Biochimica et Biophysica Acta. 1969;191(3):686. doi: 10.1016/0005-2744(69)90362-3. [DOI] [PubMed] [Google Scholar]
- 5.Chrisman TD, Garbers DL, Parks MA, Hardman JG. Journal of Biological Chemistry. 1975;250(2):374. [PubMed] [Google Scholar]
- 6.Kimura H, Murad F. Journal of Biological Chemistry. 1974;249(21):6910. [PubMed] [Google Scholar]
- 7.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298(5600):1912. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Ruoho AE, Rao VD, Hurley JH. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13414. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson DK, Garbers DL. Journal of Biological Chemistry. 1995;270(1):425. doi: 10.1074/jbc.270.1.425. [DOI] [PubMed] [Google Scholar]
- 10.Sunahara RK, Beuve A, Tesmer JJ, Sprang SR, Garbers DL, Gilman AG. Journal of Biological Chemistry. 1998;273(26):16332. doi: 10.1074/jbc.273.26.16332. [DOI] [PubMed] [Google Scholar]
- 11.Tucker CL, Hurley JH, Miller TR, Hurley JB. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):5993. doi: 10.1073/pnas.95.11.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N, Harris TW, Antoshechkin I, Bastiani C, Bieri T, Blasiar D, Bradnam K, Canaran P, Chan J, Chen CK, Chen WJ, Cunningham F, Davis P, Kenny E, Kishore R, Lawson D, Lee R, Muller HM, Nakamura C, Pai S, Ozersky P, Petcherski A, Rogers A, Sabo A, Schwarz EM, Van Auken K, Wang Q, Durbin R, Spieth J, Sternberg PW, Stein LD. Nucleic Acids Research. 2005;33:D383. doi: 10.1093/nar/gki066. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder JU, Schultz JE. Molecular and Cellular Biochemistry. 2002;230(1–2):149. [PubMed] [Google Scholar]
- 14.Ochoa De Alda JA, Ajlani G, Houmard J. Journal of Bacteriology. 2000;182(13):3839. doi: 10.1128/jb.182.13.3839-3842.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch A, Leipelt M, Russwurm M, Steegborn C. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(41):15720. doi: 10.1073/pnas.0808473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winger JA, Derbyshire ER, Lamers MH, Marletta MA, Kuriyan J. BMC Structural Biology. 2008;8:42. doi: 10.1186/1472-6807-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joubert S, McNicoll N, De Lean A. Biochemical Pharmacology. 2007;73(7):954. doi: 10.1016/j.bcp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Furchgott RF, Zawadzki JV. Nature. 1980;288(5789):373. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 19.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Nature. 1995;377(6546):239. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 20.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(23):13176. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Cell. 1993;75(7):1273. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 22.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Nature. 1995;378(6555):383. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 23.Yuen PS, Potter LR, Garbers DL. Biochemistry. 1990;29(49):10872. doi: 10.1021/bi00501a002. [DOI] [PubMed] [Google Scholar]
- 24.Koglin M, Vehse K, Budaeus L, Scholz H, Behrends S. Journal of Biological Chemistry. 2001;276(33):30737. doi: 10.1074/jbc.M102549200. [DOI] [PubMed] [Google Scholar]
- 25.Giuili G, Scholl U, Bulle F, Guellaen G. FEBS Letters. 1992;304(1):83. doi: 10.1016/0014-5793(92)80594-7. [DOI] [PubMed] [Google Scholar]
- 26.Harteneck C, Wedel B, Koesling D, Malkewitz J, Bohme E, Schultz G. FEBS Letters. 1991;292(1–2):217. doi: 10.1016/0014-5793(91)80871-y. [DOI] [PubMed] [Google Scholar]
- 27.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. The Journal of Clinical Investigation. 2006;116(6):1731. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedel B, Humbert P, Harteneck C, Foerster J, Malkewitz J, Bohme E, Schultz G, Koesling D. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(7):2592. doi: 10.1073/pnas.91.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7699. doi: 10.1073/pnas.0609778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. EMBO Journal. 1998;17(11):3045. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. The New England Journal of Medicine. 1992;326(2):90. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 32.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, Imura H. Endocrinology. 1992;130(1):229. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 33.Potter LR. Pharmacology and Therapeutics. 2011;130(1):71. doi: 10.1016/j.pharmthera.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Handbook of Experimental Pharmacology. 2009;191:341. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Science. 1995;267(5198):679. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 36.John SW, Veress AT, Honrath U, Chong CK, Peng L, Smithies O, Sonnenberg H. American Journal of Physiology. 1996;271(1 Pt 2):R109. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 37.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4239. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryan PM, Smirnov D, Smolenski A, Feil S, Feil R, Hofmann F, Lohmann S, Potter LR. Biochemistry. 2006;45(4):1295. doi: 10.1021/bi051253d. [DOI] [PubMed] [Google Scholar]
- 39.Potter LR, Abbey-Hosch S, Dickey DM. Endocrine Reviews. 2006;27(1):47. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 40.Dickey DM, Flora DR, Bryan PM, Xu X, Chen Y, Potter LR. Endocrinology. 2007;148(7):3518. doi: 10.1210/en.2007-0081. [DOI] [PubMed] [Google Scholar]
- 41.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Nature. 1995;378(6552):65. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 42.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14730. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potter LR, Hunter T. Journal of Biological Chemistry. 2001;276(9):6057. doi: 10.1074/jbc.R000033200. [DOI] [PubMed] [Google Scholar]
- 44.Chinkers M, Wilson EM. Journal of Biological Chemistry. 1992;267(26):18589. [PubMed] [Google Scholar]
- 45.Ogawa H, Qiu Y, Philo JS, Arakawa T, Ogata CM, Misono KS. Protein Science : A Publication of the Protein Society. 2010;19(3):544. doi: 10.1002/pro.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC. Nature. 2000;406(6791):101. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa H, Qiu Y, Ogata CM, Misono KS. Journal of Biological Chemistry. 2004;279(27):28625. doi: 10.1074/jbc.M313222200. [DOI] [PubMed] [Google Scholar]
- 48.Potter LR, Hunter T. Molecular and Cellular Biology. 1998;18(4):2164. doi: 10.1128/mcb.18.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potter LR, Garbers DL. Journal of Biological Chemistry. 1992;267(21):14531. [PubMed] [Google Scholar]
- 50.Kurose H, Inagami T, Ui M. FEBS Letters. 1987;219(2):375. doi: 10.1016/0014-5793(87)80256-9. [DOI] [PubMed] [Google Scholar]
- 51.Wong SK, Ma CP, Foster DC, Chen AY, Garbers DL. Journal of Biological Chemistry. 1995;270(51):30818. doi: 10.1074/jbc.270.51.30818. [DOI] [PubMed] [Google Scholar]
- 52.Antos LK, Abbey-Hosch SE, Flora DR, Potter LR. Journal of Biological Chemistry. 2005;280(29):26928. doi: 10.1074/jbc.M505648200. [DOI] [PubMed] [Google Scholar]
- 53.Antos LK, Potter LR. American Journal of Physiology, Endocrinology and Metabolism. 2007;293(6):E1756. doi: 10.1152/ajpendo.00321.2007. [DOI] [PubMed] [Google Scholar]
- 54.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Science. 1991;252(5002):120. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 55.Yasoda A, Ogawa Y, Suda M, Tamura N, Mori K, Sakuma Y, Chusho H, Shiota K, Tanaka K, Nakao K. Journal of Biological Chemistry. 1998;273(19):11695. doi: 10.1074/jbc.273.19.11695. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Science. 2010;330(6002):366. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4016. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K. Nature Medicine. 2004;10(1):80. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 59.Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, Garofalo S, Tavella S, Lerone M, Zuffardi O, Bader M, Ravazzolo R, Gimelli G. Human Mutation. 2007;28(7):724. doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- 60.Moncla A, Missirian C, Cacciagli P, Balzamo E, Legeai-Mallet L, Jouve JL, Chabrol B, Le Merrer M, Plessis G, Villard L, Philip N. Human Mutation. 2007 Dec 28;12(12):1183. doi: 10.1002/humu.20611. [DOI] [PubMed] [Google Scholar]
- 61.Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. Cell. 1989;58(6):1155. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 62.Nagase M, Katafuchi T, Hirose S, Fujita T. Journal of Hypertension. 1997;15(11):1235. doi: 10.1097/00004872-199715110-00007. [DOI] [PubMed] [Google Scholar]
- 63.Chrisman TD, Schulz S, Potter LR, Garbers DL. Journal of Biological Chemistry. 1993;268(5):3698. [PubMed] [Google Scholar]
- 64.Potter LR. Biochemistry. 1998;37(8):2422. doi: 10.1021/bi972303k. [DOI] [PubMed] [Google Scholar]
- 65.Potter LR, Hunter T. Journal of Biological Chemistry. 1998;273(25):15533. doi: 10.1074/jbc.273.25.15533. [DOI] [PubMed] [Google Scholar]
- 66.Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17300. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuji T, Kunieda T. Journal of Biological Chemistry. 2005;280(14):14288. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- 68.Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, Kant S, Cole T, Morton J, Cormier-Daire V, Faivre L, Lees M, Kirk J, Mortier GR, Leroy J, Zabel B, Kim CA, Crow Y, Braverman NE, van den Akker F, Warman MLA. American Journal of Human Genetics. 2004;75(1):27. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Journal of Clinical Endocrinology and Metabolism. 2006;91(4):1229. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt H, Stonkute A, Juttner R, Schaffer S, Buttgereit J, Feil R, Hofmann F, Rathjen FG. The Journal of Cell Biology. 2007;179(2):331. doi: 10.1083/jcb.200707176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, et al. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(22):10464. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. American Journal of Pathology. 2002;161(6):2169. doi: 10.1016/S0002-9440(10)64494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB. The Journal of Clinical Investigation. 2003;112(8):1244. doi: 10.1172/JCI18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghanekar Y, Chandrashaker A, Tatu U, Visweswariah SS. Biochemical Journal. 2004;379(Pt 3):653. doi: 10.1042/BJ20040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa M, Hidaka Y, Wada A, Hirayama T, Shimonishi Y. European Journal of Biochemistry. 1999;263(2):338. doi: 10.1046/j.1432-1327.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 76.Vaandrager AB, Schulz S, De Jonge HR, Garbers DL. Journal of Biological Chemistry. 1993;268(3):2174. [PubMed] [Google Scholar]
- 77.Hasegawa M, Matsumoto-Ishikawa Y, Hijikata A, Hidaka Y, Go M, Shimonishi Y. The Protein Journal. 2005;24(5):315. doi: 10.1007/s10930-005-6752-x. [DOI] [PubMed] [Google Scholar]
- 78.Vaandrager AB, van der Wiel E, de JHR. Journal of Biological Chemistry. 1993;268(26):19598. [PubMed] [Google Scholar]
- 79.Vaandrager AB, van der Wiel E, Hom ML, Luthjens LH, de Jonge HR. Journal of Biological Chemistry. 1994;269(23):16409. [PubMed] [Google Scholar]
- 80.Bhandari R, Srinivasan N, Mahaboobi M, Ghanekar Y, Suguna K, Visweswariah SS. Biochemistry. 2001;40(31):9196. doi: 10.1021/bi002595g. [DOI] [PubMed] [Google Scholar]
- 81.Gazzano H, Wu HI, Waldman SA. Infection and Immunity. 1991;59(4):1552. doi: 10.1128/iai.59.4.1552-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crane JK, Shanks KL. Molecular and Cellular Biochemistry. 1996;165(2):111. doi: 10.1007/BF00229472. [DOI] [PubMed] [Google Scholar]
- 83.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. American Journal of Pathology. 2007;171(6):1847. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mann EA, Jump ML, Wu J, Yee E, Giannella RA. Biochemical and Biophysical Research Communications. 1997;239(2):463. doi: 10.1006/bbrc.1997.7487. [DOI] [PubMed] [Google Scholar]
- 85.Schulz S, Lopez MJ, Kuhn M, Garbers DL. The Journal of Clinical Investigation. 1997;100(6):1590. doi: 10.1172/JCI119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carrithers SL, Parkinson SJ, Goldstein S, Park P, Robertson DC, Waldman SA. Gastroenterology. 1994;107(6):1653. doi: 10.1016/0016-5085(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 87.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(14):7846. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, Hajnoczky G, Terzic A, Waldman SA. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2695. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, Boddupalli SS, Currie MG, Forte LR. Cancer Research. 2000;60(18):5151. [PubMed] [Google Scholar]
- 90.Roque MV, Camilleri M. Expert Review of Gastroenterology & Hepatology. 2011;5(3):301. doi: 10.1586/egh.11.30. [DOI] [PubMed] [Google Scholar]
- 91.Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3571. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(7):3388. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(19):10595. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(36):14507. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo D, Zhang JJ, Huang XY. Biochemistry. 2009;48(20):4417. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):2041. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P, Wennemuth G, Biel M, Zufall F, Kelliher KR. Current biology: CB. 2010;20(16):1438. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lolley RN, Racz E. Vision Research. 1982;22(12):1481. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- 99.Koch KW, Stryer L. Nature. 1988;334(6177):64. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 100.Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5535. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shyjan AW, de Sauvage FJ, Gillett NA, Goeddel DV, Lowe DG. Neuron. 1992;9(4):727. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- 102.Yang RB, Foster DC, Garbers DL, Fulle HJ. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(2):602. doi: 10.1073/pnas.92.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. Neuron. 1994;12(6):1345. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 104.Gorczyca WA, Gray-Keller MP, Detwiler PB, Palczewski K. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(9):4014. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS, et al. Neuron. 1994;13(2):395. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 106.Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. Journal of Biological Chemistry. 1995;270(42):25200. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 107.Bereta G, Wang B, Kiser PD, Baehr W, Jang GF, Palczewski K. Journal of Biological Chemistry. 2010;285(3):1899. doi: 10.1074/jbc.M109.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aparicio JG, Applebury ML. Journal of Biological Chemistry. 1996;271(43):27083. doi: 10.1074/jbc.271.43.27083. [DOI] [PubMed] [Google Scholar]
- 109.Laura RP, Dizhoor AM, Hurley JB. Journal of Biological Chemistry. 1996;271(20):11646. doi: 10.1074/jbc.271.20.11646. [DOI] [PubMed] [Google Scholar]
- 110.Yamazaki A, Yu H, Yamazaki M, Honkawa H, Matsuura I, Usukura J, Yamazaki RK. Journal of Biological Chemistry. 2003;278(35):33150. doi: 10.1074/jbc.M303678200. [DOI] [PubMed] [Google Scholar]
- 111.Yang RB, Robinson SW, Xiong WH, Yau KW, Birch DG, Garbers DL. Journal of Neuroscience. 1999;19(14):5889. doi: 10.1523/JNEUROSCI.19-14-05889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tucker CL, Ramamurthy V, Pina AL, Loyer M, Dharmaraj S, Li Y, Maumenee IH, Hurley JB, Koenekoop RK. Molecular Vision. 2004;10:297. [PubMed] [Google Scholar]
- 113.Hanein S, Perrault I, Gerber S, Tanguy G, Barbet F, Ducroq D, Calvas P, Dollfus H, Hamel C, Lopponen T, Munier F, Santos L, Shalev S, Zafeiriou D, Dufier JL, Munnich A, Rozet JM, Kaplan J. Human Mutation. 2004;23(4):306. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- 114.Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. Journal of Biological Chemistry. 2007;282(12):8837. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9948. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pennesi ME, Howes KA, Baehr W, Wu SM. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6783. doi: 10.1073/pnas.1130102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Howes KA, Pennesi ME, Sokal I, Church-Kopish J, Schmidt B, Margolis D, Frederick JM, Rieke F, Palczewski K, Wu SM, Detwiler PB, Baehr W. EMBO Journal. 2002;21(7):1545. doi: 10.1093/emboj/21.7.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palczewski K, Sokal I, Baehr W. Biochemical and Biophysical Research Communications. 2004;322(4):1123. doi: 10.1016/j.bbrc.2004.07.122. [DOI] [PubMed] [Google Scholar]
- 119.Schulz S, Green CK, Yuen PS, Garbers DL. Cell. 1990;63(5):941. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 120.Schulz S, Wedel BJ, Matthews A, Garbers DL. Journal of Biological Chemistry. 1998;273(2):1032. doi: 10.1074/jbc.273.2.1032. [DOI] [PubMed] [Google Scholar]
- 121.Kuhn M, Ng CK, Su YH, Kilic A, Mitko D, Bien-Ly N, Komuves LG, Yang RB. Biochemical Journal. 2004;379(Pt 2):385. doi: 10.1042/BJ20031624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin H, Cheng CF, Hou HH, Lian WS, Chao YC, Ciou YY, Djoko B, Tsai MT, Cheng CJ, Yang RB. Journal of the American Society of Nephrology. 2008;19(2):339. doi: 10.1681/ASN.2007050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chao YC, Cheng CJ, Hsieh HT, Lin CC, Chen CC, Yang RB. The Biochemical Journal. 2010;432(2):267. doi: 10.1042/BJ20100617. [DOI] [PubMed] [Google Scholar]
- 124.Dickey DM, Burnett JC, Jr, Potter LR. Journal of Biological Chemistry. 2008;283:35003. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC., Jr Journal of Clinical Pharmacology. 2009;49(6):668. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robinson JW, Lou X, Potter LR. British Journal of Pharmacology. 2011;164:499. doi: 10.1111/j.1476-5381.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]