Abstract
Women who developed gestational diabetes (GDM) represent a high risk population for hypertension later in life. The role of diet in the progression of hypertension among this susceptible population is unknown. We conducted a prospective cohort study of 3,818 women with a history of GDM in the Nurses’ Health Study II. These women were followed-up from 1989 to 2011. Incident hypertension was identified through self-administered questionnaires that were validated previously by medical record review. Adherence scores for the alternative Healthy Eating Index-2010 (AHEI-2010), the Mediterranean diet (aMed) and the Dietary Approaches to Stop Hypertension (DASH) were computed for each participant. Cox proportional hazard models were used to evaluate the associations between dietary scores and hypertension while adjusting for major risk factors for hypertension. We documented 1,069 incident hypertension cases during a median of 18.5 years of follow-up. After adjustment for major risk factors for hypertension including body mass index, AHEI, aMed and DASH scores were significantly inversely associated with the risk of hypertension; hazard ratio (HR) and 95% confidence interval (CI) comparing the extreme quartiles (highest vs. lowest) was 0.76 (0.61 to 0.94, P for linear trend=0.03) for AHEI score, 0.72 (0.58 to 0.90, P for trend=0.01) for DASH score and 0.70 (0.56 to 0.88, P for trend=0.002) for aMed score. Adherence to a healthful dietary pattern was related to a lower subsequent risk of developing hypertension among women with a history of gestational diabetes.
Keywords: nutrition, dietary pattern, hypertension, gestational diabetes, women
Introduction
Hypertension is one of the most prevalent and preventable risk factors for cardiovascular and kidney diseases, and is one of the leading causes of death in the United States1. Approximately 78 million US adults are currently hypertensive2, 3. Nationally representative survey data show a prevalence of hypertension of 30.8%, which has plateaued since 19994. Decreasing the prevalence of hypertension has been identified as a key public health priority by the American Heart Association, which has proposed as a target decreasing hypertension prevalence to 26.9% by 20204, 5. Similarly, the Centers for Disease Control and Prevention has set hypertension prevention as its top priority action to prevent a million heart attacks and strokes by the year 20176. To reduce hypertension and its related public health burden4,7, it is important to identify modifiable risk factors for the early prevention of hypertension, particularly among high risk populations.
Approximately 7% (ranging from 1% to 14%) of all pregnancies in the United States are complicated by Gestational diabetes mellitus (GDM), is associated with an elevated risk for hypertension8. We have previously reported that the cumulative incidence of hypertension for women with a history of GDM was 26% higher than those who did not have GDM even 16 years after the index pregnancy8. Thus, women with a history of GDM represent a high risk population for hypertension that could benefit from early prevention. While there is extensive literature on how lifestyle factors may influence blood pressure in the general population4, 9–11, no information is currently available on the role of diet and lifestyle in the development of hypertension specifically in this susceptible population. Only one small randomized clinical trial has investigated the effects of DASH diet in pregnancy women with gestational diabetes but did not have hypertension as endpoint12, 13. It is currently unknown whether and to what extent following a DASH diet after GDM affect subsequent hypertension risk. The DASH and Mediterranean diet were inversely associated with blood pressure in the general population14, 15, but whether these healthy dietary patterns could be helpful in a high risk population such as women with a history of GDM is unknown. To address these gaps, we prospectively examined the associations between long-term adherence to three healthy diets with subsequent risk of hypertension among women with a history of GDM, specifically the Dietary Approach to Stop Hypertension (DASH) diet, the alternative Mediterranean diet (aMED), and the Alternative Healthy Eating Index (AHEI). We also assessed whether the associations were potentially mediated through changes in body mass index (BMI).
Methods
Study Population
The study population is composed of women with a history of GDM in the Nurses’ Health Study II (NHSII), as part of the ongoing Diabetes & Women’s Health Study16. The NHSII is an ongoing prospective cohort study established in 1989. It consists of 116,430 female nurses, aged 24–44 years at baseline. Study participants report their lifestyle information and medical history through a self-administered questionnaire every two years. Dietary information is collected every 4 years using a validated semi-quantitative food frequency questionnaire (FFQ)17.
GDM diagnosed by a physician was ascertained by self-report on each biennial questionnaire through 2001. Investigators ceased the update of occurrence of gestational diabetes in 2001 as most women had passed reproductive age. In a previous validation study consisted of a subgroup of the Nurses’ Health Study II cohort, about 94% of self-reported gestational diabetes events were confirmed by medical records18. Physicians were most likely to use the National Diabetes Data Group criteria and among both cases and non-cases in the NHS cohort, there was a high level of gestational diabetes surveillance. From a supplemental questionnaire sent to a random sample of parous women who did not report gestational diabetes (n=114), 83% of them reporting undergoing a 50 g glucose screening test during pregnancy and 100% reported frequent prenatal urine glucose screening.
Among the 94,166 women who reported a pregnancy, we excluded women with a history of cardiovascular diseases or cancer at baseline (n=895), multiple gestations or pregnancies lasting less than 6 months (n=2,274), and those without a history of GDM (n=89,532). We also excluded women with a history of hypertension prior to the diagnosis of GDM, or with missing data on post-pregnancy diet (n=1,465), leaving 3,818 women in the study.
We censored participants during follow-up if they reported a physician diagnosis of hypertension, died or if they were lost to follow-up. Our final analytic sample included 3,818 women with a mean age at GDM of 32 years; 2,017 women had GDM identified through the baseline questionnaire and 1,801 women had GDM identified during the follow-up. This study was approved by the institutional review board of the Harvard T.H. Chan School of Public Health and the Partners Health Care system.
Exposure
A semi-quantitative FFQ was administered every four years to assess the participants’ usual diet over the past year17, 19. Previous validation studies have shown good correlations between FFQ and multiple food records and biomarkers of intake17, 19. For each participant we calculated an adherence score to three dietary patterns as previously described10, 20, 21: the alternative healthy eating index 2010 (AHEI), Dietary Approaches to Stop Hypertension (DASH) and the alternative Mediterranean diet (aMed). AHEI 2010, based on a comprehensive literature review of foods, nutrients and components that are consistently associated with a lower risk of chronic disease (cardiovascular disease and cancer) in the general population, emphasizes high consumption of nuts, legumes, whole grains, fish, polyunsaturated fatty acids (PUFAs), and lower intake of sugar sweetened beverages10. The DASH diet was originally developed for blood pressure reduction and emphasizes a low consumption of sodium, animal protein and sweets, and encourages high intakes of fruits, vegetables, legumes and nuts, and moderate low-fat dairy products22. The alternative Mediterranean diet is rich in fruits, vegetables, fish, and whole grains, moderate in dairy products and alcohol, high in fat (mainly from monounsaturated fat), olive oil, and low in meat and meat products11, 23.
We modeled different dietary patterns using cumulative average level of dietary variables and stopped updating after participants developed intermediate outcomes that could change dietary habits such as diabetes, coronary heart disease, stroke, cancer, angina, and coronary artery bypass grafting. Dietary scores were divided into quartiles with highest quartiles representing the most healthful diet.
Outcome
The outcome was newly diagnosed hypertension as reported in follow-up questionnaires among women with a history of GDM. Every two years, study participants returned self-administered questionnaires indicating whether they had clinically diagnosed hypertension24. In a validation study among a random sample of NHS II participants, self-reported hypertension had a sensitivity of 94% and specificity of 85% when compared against medical records24.
Covariates
We adjusted for major socio-demographic information, family history of hypertension, lifestyle and clinical covariates which were collected and updated with biennial questionnaires since 1989. Self-reported weight was highly correlated with measured weight (r = 0.97) in a previous validation study25. Physical activity and sedentary behaviors were repeatedly assessed in 1989, 1991, 1997, 2001, 2005 and 2009. Participants were asked to report the average amount of time spent per week doing various activities. Weekly energy expenditure in metabolic equivalent (MET)-hours was calculated. In a previous validation study26, the correlation of physical activity reported on questionnaires was 0.79 when compared to prospectively collected 1-week recalls and 0.62 when compared to prospectively collected physical activity diaries. BMI at age 18 was collected in the 1989 questionnaires, which has been validated in a previous study with relatively good validity8, 27–29. Hypertensive disorder in pregnancy was defined as self-reported pregnancy induced hypertension, pre-eclampsia, or toxemia.
Statistical analysis
Participants were followed from the date of the first questionnaire when GDM was reported to the date of the questionnaire first reporting hypertension diagnosed by a physician, death, or end of follow-up in June, 2011, whichever came first. Cox proportional hazard models with time varying covariates were used. In Model 1, we adjusted for age. In model 2 we further adjusted for major risk factors for hypertension selected a priori, including parity (1, 2, 3, 4+), age at first birth (12–24, 25–29, ≥ 30), race/ethnicity (Caucasian, African-American, Hispanic, Asian, others), family history of diabetes (yes, no), oral contraceptive use (current, former, never), menopausal status (premenopausal, postmenopausal), cigarette smoking (current, former, never), alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥ 15 g/d, for DASH score only), total energy intake (kcals/d, quartiles), physical activity (MET hrs/week, quartiles), birth weight (<5.4, 5.5–6.9, 7–8.4, 8.5–9.9, 10+ lbs), hypertensive disorder of pregnancy(yes, no), family history of hypertension (yes, no), aspirin use (yes, no), acetaminophen use (yes, no), other anti-inflammatory medication use (yes, no), BMI at age 18 (<19.9, 20.0–21.9, 22.0–24.9, 25.0–29.9, 30+ kg/m2). Model 3 was further adjusted for updated BMI at follow-up (≤ 22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, 35+ kg/m2).
To evaluate the robustness of the findings, we conducted a series of sensitivity analyses, including restricting the analysis to women with a GDM identified at baseline questionnaire cycle (prevalent GDM, n=2,017), women with GDM identified during the follow-up from 1991 until 2001 only (incident GDM, n=1,801), and among Caucasian women only. We performed stratified analyses by family history of hypertension (yes, no), baseline obesity status (BMI≥30 kg/m2, yes, no), physical activity level (Q 3 and 4 vs. Q 1 and 2), and history of GDM (yes, no). We also additionally adjusted for duration of lactation.
We plotted the cumulative incidence of hypertension among women by extreme quartiles of dietary quality. Using mediation analysis method (difference approach)30, we calculated the proportions of the associations between healthful dietary patterns with risk of hypertension mediated through BMI change after GDM. BMI change was calculated as the difference between BMI at baseline and at the end of follow-up. Since BMI change was a significant mediator for the association with AHEI score, we further plotted the predicted probabilities of hypertension among women with a history of GDM for the joint effects of AHEI scores with baseline BMI, and the joints effects of diet scores with changes in BMI. We calculated predicted probabilities assuming median values for covariates.
Results
We documented a total of 1,069 incident hypertension cases during 27,251 person-years of follow-up among women with a history of GDM. Women with greater adherence to the AHEI, DASH, or aMED diet patterns were less likely to be current smokers (Table 1). They were more likely to be moderate alcohol drinkers, eat more cereal fiber, be more physically active, and less likely to consume trans fat (Table 1). BMI at age 18 was similar across quartiles of AHEI, DASH and aMED scores. The median duration of time to the development of hypertension since the index GDM pregnancy was 17 years.
Table 1.
Baseline characteristics of study sample by quartiles of dietary scores among women with a history of gestational diabetes
| Characteristics | AHEI | DASH | aMED | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Number of participants | 870 | 654 | 706 | 747 | 581 | 720 |
| Median score | 37 | 61 | 18 | 30 | 2 | 6 |
| Age (years) | 35.8(4.4) | 37.5(4.0) | 36.3(4.3) | 36.7(4.3) | 36.2(4.3) | 36.7(4.2) |
| Race, white (%) | 94 | 91 | 93 | 92 | 93 | 91 |
| Family history of diabetes (%) | 29 | 23 | 26 | 24 | 28 | 24 |
| Family history of hypertension, % | 54 | 45 | 52 | 47 | 52 | 51 |
| Baseline BMI (kg/m2) | 26.7(6.2) | 24.8(4.9) | 26.8(6.5) | 25.8(5.7) | 27.0(6.4) | 25.8(6.0) |
| BMI at age 18 (kg/m2) | 21.1(3.3) | 21.0(2.8) | 21.3(3.4) | 21.2(3.1) | 21.4(3.4) | 20.9(2.9) |
| Current smoking (%) | 12 | 8 | 19 | 7 | 16 | 7 |
| Total physical activity, MET-hrs/wk | 13.5(19.6) | 22.9(25) | 12.5(18.3) | 21.9(25.4) | 13.2(19.9) | 22.1(25.8) |
| Vigorous activity, MET-hrs/wk | 8.0 (15.4) | 14.7(19.8) | 7.5(14.4) | 14.3(20.6) | 7.9(15.7) | 14.4(20.1) |
| Walking, MET-hrs/wk | 4.7(7.7) | 7.4(9.6) | 4.4(7.2) | 6.9(9.1) | 4.6(7.7) | 7.0(9.7) |
| TV watching, hrs/wk | 10.2(8.8) | 7.3(7.6) | 9.6(8.7) | 8.2(8.1) | 9.1(8.3) | 8.5(8.3) |
| Age at first birth | 26.7(4.6) | 27.9(5.0) | 26.5(4.8) | 27.8(4.8) | 26.7(4.9) | 27.5(4.6) |
| Pregnancy induced hypertensive disorder, % | 4 | 5 | 4 | 4 | 4 | 5 |
| Parity status, number of children, % | ||||||
| 1 | 20 | 27 | 19 | 24 | 20 | 22 |
| 2 | 44 | 47 | 48 | 45 | 47 | 46 |
| 3 | 24 | 21 | 22 | 23 | 23 | 24 |
| 4+ | 12 | 5 | 11 | 8 | 10 | 8 |
| Current use of oral contraceptives (%) | 9 | 7 | 9 | 5 | 7 | 6 |
| Menopause (%) | 3 | 2 | 3 | 3 | 3 | 2 |
| Total calories (kcal/d) | 2006(555) | 1815(559) | 1639(504) | 2160(532) | 1596(497) | 2213(525) |
| Carbohydrates (% energy) | 49.0(7.0) | 50.6(7.8) | 46.5(7.6) | 52.4(6.4) | 46.4(7.7) | 51.5(6.3) |
| Total protein (% energy) | 18.3(3.0) | 20.7(3.8) | 18.9(3.4) | 20.2(3.3) | 19.7(3.9) | 19.6(3.1) |
| Total fat (% energy) | 33.7(5.1) | 29.9(6.0) | 35.1(5.3) | 29.0(5.1) | 34.5(5.5) | 30.2(4.9) |
| SFA (% energy) | 12.3(2.3) | 10.1(2.4) | 12.6(2.4) | 10.2(2.2) | 12.8(2.3) | 10.2(1.9) |
| MUFA (% energy) | 13.1(2.2) | 11.2(2.6) | 13.6(2.3) | 10.9(2.2) | 13.2(2.4) | 11.6(2.2) |
| PUFA (% energy) | 5.3(1.2) | 6.0(1.6) | 5.8(1.3) | 5.4(1.3) | 5.5(1.3) | 5.6(1.3) |
| Trans fat (% energy) | 2.0(0.6) | 1.3(0.4) | 2.0(0.6) | 1.3(0.4) | 1.9(0.6) | 1.5(0.5) |
| P:S ratio | 0.4(0.1) | 0.6(0.2) | 0.5(0.1) | 0.6(0.2) | 0.4(0.1) | 0.6(0.2) |
| Cereal fiber (g/d) | 4.7(2.0) | 6.7(3.2) | 4.7(2.3) | 6.4(2.7) | 4.6(2.3) | 6.3(2.4) |
| Glycemic index | 55.1(3.1) | 52.5(3.4) | 55.7(3.3) | 52.5(3.0) | 54.5(3.7) | 53.4(2.8) |
| Glycemic load | 121.2(20.8) | 120.2(22.5) | 118.1(23.2) | 123(18.9) | 115.4(23.1) | 122.4(18.1) |
| Alcohol (g/d) | 1.6(5.2) | 2.9(4.2) | 2.0(5.0) | 2.2(4.4) | 1.7(4.8) | 2.8(4.1) |
Abbreviations: AHEI, Alternative Healthy Eating Index 2010; DASH, Dietary Approach to Stop Hypertension; aMED, alternative Mediterranean diet; BMI, body mass index; GDM, gestational diabetes mellitus; MET, metabolic equivalent; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids. P:S, ratio of polyunsaturated fatty acids to saturated fatty acids
Values are means (SD) unless otherwise specified.
Figure 1 shows that the cumulative incidence of hypertension among women with AHEI, DASH and aMED scores in the highest quartile was higher than those in the lowest quartile after 18 years of follow up. Moreover, all the three dietary pattern scores were strongly inversely associated with hypertension risk. For AHEI score, we observed a consistent strong inverse association with risk of hypertension. Comparing extreme quartiles, women who consumed a high quality diet after GDM were 35% less likely to develop hypertension (hazard ratio [HR] = 0.65, 95% confidence interval [CI]: 0.53 to 0.81, p for trend=0.0002, Table 2). After further adjustment for BMI, the association didn’t change appreciably (hazard ratio [HR] = 0.76, 95% confidence interval [CI]: 0.61 to 0.94, p for trend=0.03, Table 2). We observed similar results for DASH and aMED scores. The results were also similar when we used simple updated dietary scores (Supplementary Table S1), stopped updating diet after development of intermediate outcomes, restricted the analysis to Caucasian women only, further adjusting for duration of lactation, or restricted the analysis to prevalent or incident GDM cases only (Data not shown). We did not identify significant effect modification by family history of hypertension, baseline obesity status, physical activity, or history of GDM (Supplementary Table S2).
Figure 1. Cumulative incidence of hypertension by extreme quartiles of AHEI, DASH and aMED score among women with a history of GDM.
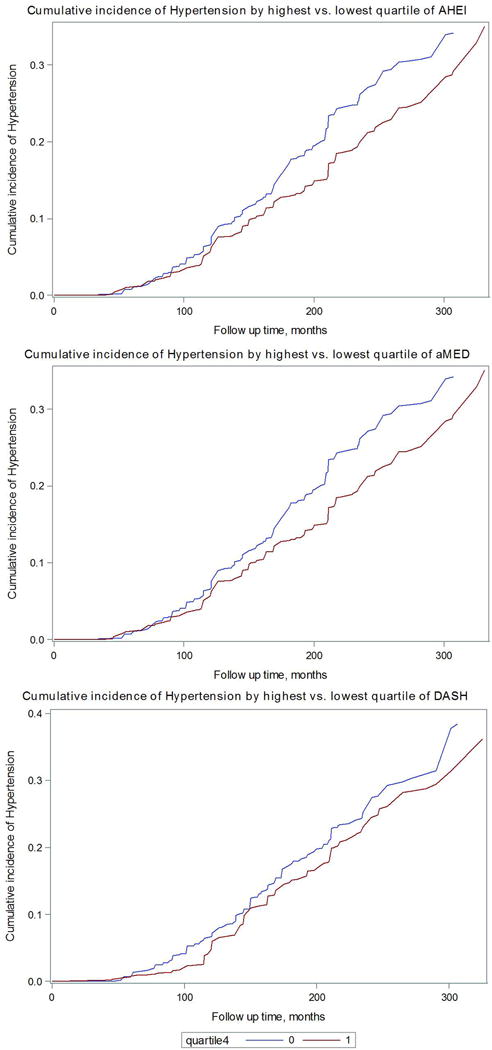
Model adjusted for age, parity (1, 2, 3, 4+), age at first birth (12–24, 25–29, ≥ 30), race/ethnicity (Caucasian, African-American, Hispanic, Asian, others), family history of diabetes (yes, no), oral contraceptive use (current, former, never), menopausal status (premenopausal, postmenopausal), cigarette smoking (current, former, never), alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥ 15 g/d, for DASH score only), total energy intake (kcals/d, quartiles), physical activity (MET hrs/week, quartiles), birth weight (≤5.4, 5.5–6.9, 7–8.4, 8.5–9.9, 10+lbs), hypertensive disorder of pregnancy(yes, no), family history of hypertension (yes, no), aspirin use (yes, no), acetaminophen use (yes, no), other anti-inflammatory medication use (yes, no), BMI at age 18 (≤19.9, 20.0–21.9, 22.0–24.9, 25.0–29.9, 30+ kg/m2), and updated BMI (≤ 22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, 35.0+ kg/m2).
Blue line indicates dietary score quartile 1 (the least healthful diet)
Red line indicates dietary score quartile 4 (the most healthful diet)
AHEI: Alternative Healthy Eating Index
DASH: Dietary Approach to Stop Hypertension
aMED: Mediterranean diet
Table 2.
Relative risk (RR) and its 95% Confidence Interval (CI) of dietary score with subsequent risk of hypertension among women with a history of GDM
| Quartiles of cumulative average dietary score
|
|||||
|---|---|---|---|---|---|
| RR (95%CI) | 1 | 2 | 3 | 4 | P-trend1 |
| AHEI | |||||
| Median | 37.2 | 44.9 | 51.5 | 60.8 | |
| Cases/Person years | 259/157296 | 261/156498 | 263/156043 | 256/155914 | |
| Model 1 | 1.00 | 0.82 (0.68–0.99) |
0.80 (0.66–0.97) |
0.67 (0.55–0.81) |
<.0001 |
| Model 2 | 1.00 | 0.80 (0.65–0.97) |
0.80 (0.66–0.98) |
0.65 (0.53–0.81) |
0.0002 |
| Model 3 | 1.00 | 0.80 (0.65–0.97) |
0.84 (0.69–1.03) |
0.76 (0.61–0.94) |
0.03 |
| DASH | |||||
| Median | 18 | 22 | 25 | 30 | |
| Cases/Person years | 310/157364 | 236/147095 | 287/169868 | 206/151424 | |
| Model 1 | 1.00 | 0.77 (0.64–0.93) |
0.81 (0.68–0.97) |
0.59 (0.49–0.72) |
<.0001 |
| Model 2 | 1.00 | 0.81 (0.66–0.98) |
0.83 (0.68–1.01) |
0.63 (0.51–0.79) |
0.0001 |
| Model 3 | 1.00 | 0.82 (0.67–1.01) |
0.89 (0.73–1.09) |
0.72 (0.58–0.90) |
0.01 |
| aMED | |||||
| Median | 2 | 3 | 5 | 6 | |
| Cases/Person years | 276/142060 | 308/189929 | 234/138389 | 221/155421 | |
| Model 1 | 1.00 | 0.85 (0.71–1.01) |
0.78 (0.65–0.95) |
0.62 (0.51–0.76) |
<.0001 |
| Model 2 | 1.00 | 0.82 (0.68–1.00) |
0.77 (0.62–0.95) |
0.61 (0.48–0.76) |
<.0001 |
| Model 3 | 1.00 | 0.90 (0.74–1.09) |
0.81 (0.65–1.00) |
0.70 (0.56–0.88) |
0.002 |
Model 1: Age adjusted model.
Multivariable model 2: Model 1 further adjusted for age (months), parity (1, 2, 3, 4+), age at first birth (12–24, 25–29, ≥ 30), race/ethnicity (Caucasian, African-American, Hispanic, Asian, others), family history of diabetes (yes, no), oral contraceptive use (current, former, never), menopausal status (premenopausal, postmenopausal), cigarette smoking (current, former, never), alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥ 15 g/d, for DASH score only), total energy intake (kcals/d, quartiles), physical activity (MET hrs/week, quartiles), birth weight (≤5.4, 5.5–6.9, 7–8.4, 8.5–9.9, 10+lbs), hypertensive disorder of pregnancy (yes, no), family history of hypertension (yes, no), aspirin use (yes, no), acetaminophen use (yes, no), other anti-inflammatory medication use (yes, no), BMI at age 18 (≤19.9, 20.0–21.9, 22.0–24.9, 25.0–29.9, 30+ kg/m2).
Multivariable model 3: multivariable model 2 plus additional adjustment for updated BMI (≤22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, 35.0+ kg/m2).
Mediation analysis using the difference method indicated that weight gain after GDM was a significant mediator for the association between AHEI and risk of hypertension. For the AHEI score, 43.9% (95% CI: 12.3% to 75.6%, p=0.006) was mediated through BMI change. For the DASH diet score, 13.8% (95% CI: −18.9% to 46.6%, p=0.4) of the association was mediated through BMI change. For the aMED score, −10.9% (95% CI: −55.4% to 33.6%, p=0.6) was mediated through BMI change. We plotted the predicted incidence rate of hypertension by the joint effects of diet scores and baseline BMI and the joint effects of diet scores and changes in BMI (Figure 2). The probabilities were in general higher for women who gained weight or who were obese throughout the study period compared with those who lost weight during the follow-up.
Figure 2. Joint effects of AHEI score and body mass index on risk of hypertension among women with a history of GDM.
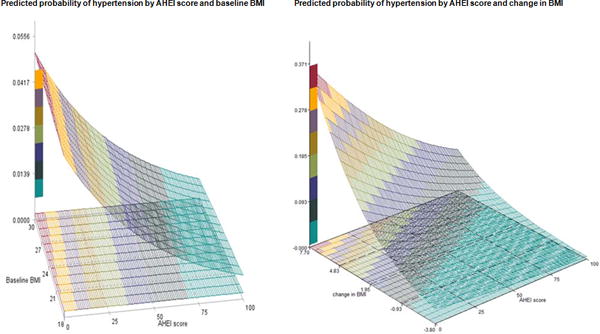
Model adjusted for age, parity (1, 2, 3, 4+), age at first birth (12–24, 25–29, ≥ 30), race/ethnicity (Caucasian, African-American, Hispanic, Asian, others), family history of diabetes (yes, no), oral contraceptive use (current, former, never), menopausal status (premenopausal, postmenopausal), cigarette smoking (current, former, never), alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥ 15 g/d, for DASH score only), total energy intake (kcals/d, quartiles), physical activity (MET hrs/week, quartiles), birth weight (≤5.4, 5.5–6.9, 7–8.4, 8.5–9.9, 10+lbs), hypertensive disorder (yes, no), family history of hypertension (yes, no), aspirin use (yes, no), acetaminophen use (yes, no), other anti-inflammatory medication use (yes, no), BMI at age 18 (≤19.9, 20.0–21.9, 22.0–24.9, 25.0–29.9, 30+ kg/m2), and updated BMI (≤ 22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, 35.0+ kg/m2). (Assume median values for all covariates).
Baseline was defined as the questionnaire cycle when the GDM was first reported.
Unit for baseline BMI and change in BMI was kg/m2.
Y axis represents predicted probability for hypertension. Color from green to red indicates risk of hypertension from low to high.
X axis represents baseline BMI or changes in BMI. For changes in BMI, positive number represents increase in BMI, while negative number represents decrease in BMI.
Z axis represents AHEI score, which ranges from 0 to 100, with 0 indicating worst diet quality and 100 indicating best healthiest diet.
Discussion
In this large long-term prospective cohort of US women with a history of gestational diabetes, we observed an inverse association between adherence to healthful dietary patterns, AHEI, DASH or Mediterranean diet, and risk of hypertension later in life. Comparing extreme categories, women consuming a higher quality diet after GDM had on average a 20% lower risk of subsequent hypertension. Increase in BMI explained around 20–30% of the association between healthful dietary patterns and hypertension.
Although GDM is a pregnancy complication, it has been demonstrated that the majority of women with GDM may have reduced insulin secretion and/or chronic insulin resistance before pregnancy and the glucose intolerance may deteriorate through their life course31. Studies on the associations between dietary and lifestyle factors and the risk of hypertension among women with GDM are sparse. One small randomized clinical trial of 34 women studied intermediate outcomes and reported that among pregnant women with GDM, the DASH diet improved glucose tolerance and lipid profiles12, 13. However, risk of hypertension as the endpoint was not investigated in this study. The role of dietary factors in long-term prevention of hypertension after the index pregnancy remains to be elucidated. Findings from the present study among the high risk population are, in general, in line with studies from the general population where beneficial associations of AHEI, DASH and aMED with the risk of hypertension have been observed15, 22, 32–35.
Compared with the general population, women with GDM are more likely to have a sedentary lifestyle, to be obese and to develop diabetes7,8. Nonetheless, we observed similar effect sizes of the three dietary scores in association with hypertension among women with GDM, compared with the general population. Adjustment for BMI moderately attenuated these findings. Mediation results suggested that moderate effects of diet, particularly AHEI diet score, on hypertension risk reduction were mediated through changes in BMI.
Prevention of hypertension involves reduction in sodium intake, a healthful dietary pattern, and maintaining a healthy body weight36. AHEI, DASH and aMED dietary patterns are rich in fiber, fruits and vegetables, with high intake of magnesium, potassium, and low intake of sodium. The DASH diet has been shown to lower risk of hypertension consistently and was recommended for prevention and treatment of high blood pressure9, 20, 37, 38. The high fiber content from whole grains and legumes, a characteristic from the three dietary patterns, could reduce glucose absorption, improve insulin sensitivity, improve endothelial function, and have anti-inflammatory and antioxidant effects39, 40. Fruits and vegetables contain high potassium and vitamin K, ascorbic acid and antioxidants, which could reduce vascular oxidative stress, endothelial impairment, atrial stiffness, and lower blood pressure41–43. High intake of magnesium and potassium may inhibit sodium reabsorption in the proximal renal tubules, suppress renin secretion, relax smooth muscle, suppress formation of free radicals, and protect against vascular injury36, 44–46. Low fat dairy in the DASH diet also could potentially stimulate secretion of insulinotropic peptides and reduce saturated fat intake47. Long-chain omega-3 PUFA in the AHEI increases the production of vasodilators and modifies membrane phospholipid composition48, 49, lowering blood pressure. Furthermore, the evaluation of dietary patterns focuses on food groups instead of individual components, and takes into account the interactions or synergistic effects between these components.
A limitation of our study was that our participants were mainly Caucasian educated women. Future studies are needed to examine this association in other race/ethnic groups, as previous studies have suggested that Hispanic and African American women have an increased risk of hypertension compared with Caucasian women4, 50. However, the homogeneous race/ethnic background and socio-economic status in our cohort is also a strength as it reduces the possibility of residual confounding. In addition, the biological mechanisms underlying the healthy dietary pattern and hypertension among GDM women are likely to be the same across different race/ethnic groups51–54. Further, in our study, hypertension cases were identified by self-report of a clinician diagnosis in the past two years. However, previous validation study based on medical records review among a subset of random sample of NHS II participants demonstrated a high validity for self-reported hypertension cases24.
Our study is one of the first studies examining the association of different healthful dietary patterns with the risk of hypertension among a high risk population, women who develop GDM in a prior pregnancy. Strengths of our study include a large sample size, long term follow-up, and repeated measurement of dietary and lifestyle information. We also have detailed information on weight, physical activity, birth weight, BMI at age 18 and updated BMI, which allows detailed adjustment for cardiovascular risk factors across the adult lifespan, including both pre-pregnancy and post-pregnancy. We also have detailed covariates adjustment, and similar socio-demographic characteristics in this study sample, which further reduce residual confounding. Our results were robust to a series of sensitivity analyses.
Supplementary Material
Perspectives.
After having gestational diabetes in pregnancy, women who adhered to a healthy dietary pattern experienced substantially lower risk of hypertension. The associations were moderately mediated through changes in body mass index, suggesting that maintaining a healthy body weight is a key factor in preventing hypertension among these high risk women. Findings from the present study indicate that women with a history of GDM may benefit from adhering to a healthy dietary pattern characterized by rich intake in fruits and vegetables, whole grains, and low in red and processed meats, and low in refined grain.
Novelty and Significance.
What is new
Gestational diabetes (GDM), one of the most common pregnancy complications, has short-term and long-term health implications for both women and their offspring. Women with a history of GDM represent a high risk population for hypertension that could benefit from early prevention. Although healthful diet and lifestyle factors may help lower the risk of hypertension in the general population, it is unknown among this susceptible high risk population, whether and how healthful dietary patterns are related to the risk of hypertension. Such information is particularly critical in future development of effective and targeted prevention strategy of hypertension among this high risk population. This study is one of the first studies examining the association of different healthful dietary patterns with the risk of hypertension among a high risk population, women who had a history of GDM. In this prospective study of U.S. women from the Nurses’ Health Study II, we aimed to investigate the association after 18.5 years of follow-up of women who had diabetes during pregnancy. We observed that after gestational diabetes, adherence to a healthy dietary pattern was associated with lower risk of developing hypertension. The associations were moderately mediated through changes in body mass index, suggesting that maintaining a healthy body weight is a key factor in preventing hypertension among these high risk women.
What is relevant
Findings from the present study indicate that women with a history of GDM may benefit from adhering to a healthy dietary pattern characterized by rich intake in fruits and vegetables, whole grains, and low in red and processed meats, and low in refined grain.
Summary
Adherence to a healthful dietary pattern was related to a lower subsequent risk of developing hypertension among women with a history of gestational diabetes.
Acknowledgments
Sources of Funding: The study was support by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract No HHSN275201000020C) and grants DK58845, CA50385, P30 DK46200, and UM1 CA176726 from the National Institutes of Health The funding agency played no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest/Disclosure
We have read and understood policy on declaration of interests and declare no competing interests. No support from any other organization besides National Institute of Health for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. Shanshan Li, Yeyi Zhu, Jorge E. Chavarro, Wei Bao, Deirdre K. Tobias, Sylvia H. Ley, John P. Forman, Aiyi Liu, James Mills, Katherine Bowers, Marin Strøm, Susanne Hansen, Frank B. Hu, Cuilin Zhang have no disclosure.
All authors included in this paper fulfil the criteria of authorship. In addition, there is no one else who fulfils the criteria but has not been included as an author. SL and ZC contributed to the study design, data analysis, and interpretation and manuscript preparations. JEC, WB, DKT, SHL, YZ, JPF, AL, JM, KB, MS, SH, and FBH contributed to the study design and critical review of the manuscript. SL and ZC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere. This work has presented as a poster presentation at the American Heart Association meeting in March, 2015, Baltimore, Maryland.
Data sharing: no additional data available
Copyright/license for publication: The corresponding author grant the exclusive licence on behalf of all authors. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) to exploit all subsidiary rights in the Contribution, v) the inclusion of electronic links from the Contribution to third party material where-ever it may be located; and, vi) license any third party to do any or all of the above. The manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Ethics approval of research: This study was approved by the institutional review board of the Harvard T.H. Chan School of Public Health and the Partners Health Care system. All study participants have provided informed consent.
Transparency declaration: The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported. No other important aspects of the study have been omitted. Any discrepancies from the study as planned have been explained.
References
- 1.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the united states: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Medicine. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Bauman M, King SMC, Fonarow GC, Lawrence W, Williams KA, Sanchez E. An effective approach to high blood pressure control: A science advisory from the american heart association, the american college of cardiology, and the centers for disease control and prevention. Hypertension. 2013;63:878–885. doi: 10.1161/HYP.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushnell C, Mccullough L. Stroke prevention in women: Synopsis of the 2014 American Heart Association/American Stroke Association guideline. Annals of Internal Medicine. 2014;160:853–857. doi: 10.7326/M14-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the united states 1999–2012: Progress toward healthy people 2020 goals. Circulation. 2014;130:1692–1699. doi: 10.1161/CIRCULATIONAHA.114.010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Frieden TR, Berwick DM. The “million hearts” initiative–preventing heart attacks and strokes. The New England Journal Of Medicine. 2011;365 doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas JP, Hingorani AD, Williams D. Tyler 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1772–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 8.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: Findings from a large prospective cohort study. Diabetes Care. 2011;34:1582–1584. doi: 10.2337/dc11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: A scientific statement from the american heart association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 10.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. The Journal Of Nutrition. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: A cultural model for healthy eating. The American Journal Of Clinical Nutrition. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 12.Asemi Z, Samimi M, Tabassi Z, Sabihi SS, Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of dash diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition. 2013;29:619–624. doi: 10.1016/j.nut.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the dietary approaches to stop hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: A randomised clinical trial. The British Journal Of Nutrition. 2013;109:2024–2030. doi: 10.1017/S0007114512004242. [DOI] [PubMed] [Google Scholar]
- 14.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of dietary approaches to stop hypertension (dash) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253–1261. doi: 10.1016/j.numecd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Toledo E, Hu FB, Estruch R, et al. Effect of the mediterranean diet on blood pressure in the predimed trial: Results from a randomized controlled trial. BMC Medicine. 2013;11:207. doi: 10.1186/1741-7015-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Hu FB, Olsen SF, et al. Rationale, design, and method of the diabetes & women’s health study–a study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstetricia et Gynecologica Scandinavica. 2014;93:1123–1130. doi: 10.1111/aogs.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. American Journal Of Epidemiology. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 18.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA: The Journal Of The American Medical Association. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal Of The American Dietetic Association. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 20.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a dash-style diet and risk of coronary heart disease and stroke in women. Archives Of Internal Medicine. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 21.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a mediterranean diet and survival in a greek population. The New England Journal Of Medicine. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. Dash collaborative research group. The New England Journal Of Medicine. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 23.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the mediterranean diet on health: An updated systematic review and meta-analysis. The American Journal Of Clinical Nutrition. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 24.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 27.Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Archives Of Internal Medicine. 2012;172:1566–1572. doi: 10.1001/archinternmed.2012.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troy LM, Michels KB, Hunter DJ, Spiegelman D, Manson JE, Colditz GA, Stampfer MJ, Willett WC. Self-reported birthweight and history of having been breastfed among younger women: An assessment of validity. International Journal Of Epidemiology. 1996;25:122–127. doi: 10.1093/ije/25.1.122. [DOI] [PubMed] [Google Scholar]
- 29.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. International Journal Of Obesity And Related Metabolic Disorders: Journal Of The International Association For The Study Of Obesity. 1995;19:570–572. [PubMed] [Google Scholar]
- 30.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nature Reviews Endocrinology. 2012;8:639–649. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the dash diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The encore study. Archives Of Internal Medicine. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the omniheart randomized trial. JAMA: The Journal Of The American Medical Association. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 34.Núñez-Córdoba JM, Valencia-Serrano F, Toledo E, Alonso A, Martínez-González MA. The mediterranean diet and incidence of hypertension: The seguimiento universidad de navarra (sun) study. American Journal Of Epidemiology. 2009;169:339–346. doi: 10.1093/aje/kwn335. [DOI] [PubMed] [Google Scholar]
- 35.Doménech M, Roman P, Lapetra J, García de la Corte FJ, Sala-Vila A, de la Torre R, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Lamuela-Raventós R-M, Toledo E, Estruch R, Coca A, Ros E. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension. 2014;64:69–76. doi: 10.1161/HYPERTENSIONAHA.113.03353. [DOI] [PubMed] [Google Scholar]
- 36.Sacks FM, Campos H. Dietary therapy in hypertension. New England Journal Of Medicine. 2010;362:2102–2112. doi: 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 37.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA: The Journal Of The American Medical Association. 2009;302:401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toledo E, Carmona-torre F, De A, Alonso A, Puchau B, Zulet MA, Martinez JA, Martinez-Gonzalez MA. Hypothesis-oriented food patterns and incidence of hypertension: 6-year follow-up of the sun (seguimiento universidad de navarra) prospective cohort. Public Health Nutrition. 2010;13:338–349. doi: 10.1017/S1368980009991066. [DOI] [PubMed] [Google Scholar]
- 39.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Medicine. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Gaziano JM, Liu S, Manson JE, Buring JE, Sesso HD. Whole- and refined-grain intakes and the risk of hypertension in women. The American Journal Of Clinical Nutrition. 2007;86:472–479. doi: 10.1093/ajcn/86.2.472. [DOI] [PubMed] [Google Scholar]
- 41.Alonso A, de la Fuente C, Martin-Arnau AM, de Irala J, Martinez JA, Martinez-Gonzalez MA. Fruit and vegetable consumption is inversely associated with blood pressure in a mediterranean population with a high vegetable-fat intake: The seguimiento universidad de navarra (sun) study. The British Journal Of Nutrition. 2004;92:311–319. doi: 10.1079/BJN20041196. [DOI] [PubMed] [Google Scholar]
- 42.Esposito K, Nappo F, Giugliano F, Giugliano G, Marfella R, Giugliano D. Effect of dietary antioxidants on postprandial endothelial dysfunction induced by a high-fat meal in healthy subjects. The American Journal Of Clinical Nutrition. 2003;77:139–143. doi: 10.1093/ajcn/77.1.139. [DOI] [PubMed] [Google Scholar]
- 43.Mullan BA, Young IS, Fee H, McCance DR. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension. 2002;40:804–809. doi: 10.1161/01.hyp.0000039961.13718.00. [DOI] [PubMed] [Google Scholar]
- 44.Houston MC, Harper KJ. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. Journal Of Clinical Hypertension. 2008;10:3–11. doi: 10.1111/j.1751-7176.2008.08575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen H, Odelola OA, Rangaswami J, Amanullah A. A review of nutritional factors in hypertension management. International Journal Of Hypertension. 2013;2013:698940. doi: 10.1155/2013/698940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strom BL, Anderson CM, Ix JH. Sodium reduction in populations: Insights from the institute of medicine committee. JAMA: The Journal Of The American Medical Association. 2013;310:31–32. doi: 10.1001/jama.2013.7687. [DOI] [PubMed] [Google Scholar]
- 47.Elwood P, Pickering J, Givens DI, Gallacher J. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: An overview of the evidence. Lipids. 2010;45:925–939. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinsella JE, Lokesh B, Stone RA. Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: Possible mechanisms. The American Journal Of Clinical Nutrition. 1990;52:1–28. doi: 10.1093/ajcn/52.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Valensi P. Hypertension, single sugars and fatty acids. J Hum Hypertens. 2005;19:S5–S9. doi: 10.1038/sj.jhh.1001954. [DOI] [PubMed] [Google Scholar]
- 50.Bentley-Lewis R, Powe C, Ankers E, Wenger J, Ecker J, Thadhani R. Effect of race/ethnicity on hypertension risk subsequent to gestational diabetes mellitus. The American Journal Of Cardiology. 2014;113:1364–1370. doi: 10.1016/j.amjcard.2014.01.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: A systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2016;15:76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101:587–97. doi: 10.3945/ajcn.114.090688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitt-Glover MC, Hunter JC, Foy CG, Quandt SA, Vitolins MZ, Leng I, Hornbuckle LM, Sanya KA, Bertoni AG. Translating the Dietary Approaches to Stop Hypertension (DASH) diet for use in underresourced, urban African American communities, 2010. Prev Chronic Dis. 2013;10:120088. doi: 10.5888/pcd10.120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turban S, Thompson CB, Parekh RS, Appel LJ. Effects of sodium intake and diet on racial differences in urinary potassium excretion: results from the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial. Am J Kidney Dis. 2013;61:88–95. doi: 10.1053/j.ajkd.2012.08.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


