Abstract
Background and Aims Mounting concerns about balancing food security with the environmental impacts of agro-chemical use underpin the need to better understand the mechanisms by which crop plants, particularly during the vulnerable seedling stage, attract or repel herbivores.
Methods The feeding preferences of the mollusc Helix aspersa were determined for several oilseed rape (Brassica napus) cultivars and a rank order of acceptability was established. This was compared with glucosinolate concentrations and volatile organic compound (VOC) profiles to determine whether seedling acceptability to molluscs was linked to either form of defence.
Key Results While VOC profiles for each oilseed rape cultivar could be separated by canonical discriminant analysis and associated with mollusc feeding preferences, glucosinolate profiles were unrelated to snail feeding behaviour. A mixture of monoterpenes (α-pinene, β-myrcene and δ-3-carene) was identified as a putative attractant, while a blend of the green leaf volatiles 3-hexen-1-ol, 3-hexen-1-ol acetate and the monoterpene α-terpinene was identified as a putative repellent mix. Added to the VOC profile of oilseed rape seedlings, the ‘repellent’ mix reduced mollusc selection, while the ‘attractant’ mix had no effect.
Conclusions Despite the widespread assumption that seedling selection by generalist herbivores is governed by chemical defence and taste, we show that olfactory cues may be more important. Oilseed rape may be atypical of wild plants, but our ability to identify repellent volatile organic compounds that can influence snail olfactory selection points to new methods for crop protection using modified VOC profiles during the vulnerable seedling stage.
Keywords: Brassica napus L., Brassicaceae, crop protection, food security, green leaf volatiles, Helix aspersa Müller, monoterpenes, plant volatiles, seedling herbivory, solid-phase microextraction
INTRODUCTION
Human population growth, coupled with the impact of a changing climate on crop yield, has the potential to destabilize global food security (Godfray et al., 2010). In addition, the likely impacts of anthropogenic climate change on agricultural pest numbers and distributions (Bebber et al., 2013) may increase already significant annual global crop losses to pathogens and herbivores (Oerke, 2006) and necessitate increased agro-chemical inputs to maintain productivity. This option is undesirable for many reasons, particularly given the development of pest resistance and imposition of further carbon costs (Alyokhin et al., 2014). Pesticides have adverse effects on key non-target species such as pollinators and cause wider environmental contamination (Clarke et al., 2009; Whitehorn et al., 2012). Consequently there is a pressing need to achieve food security whilst minimizing the further adverse environmental impact of agro-chemical use (Godfray et al., 2010).
Fundamental to this goal is an understanding of how and why pests are attracted to crop plants and how they are able to overcome natural defences. Initial location of host plants by pest species in agricultural and forestry systems often relies on detection of volatile organic compounds (VOCs), rather than visual cues (Leather, 1987; Bruce et al., 2005). Once a plant has been located, other cues including taste (Newland, 1998) or physical characteristics such as toughness play a part in determining acceptance or rejection (Hanley et al., 2007). Generalist herbivores are often attracted to plants by VOCs, but may be repelled by specific VOCs, or constitutive chemical or structural defences (Bruce, 2014). Specialist herbivores, by contrast, are often attracted to specific VOCs, sometimes using the very cues that repel generalists, frequently employing evolved mechanisms to metabolize or sequester defensive toxins produced by the plant. For example, larva of the small cabbage white butterfly (Pieris rapae) prefer food plants containing glucosinolates (Renwick and Lopez, 1999), while the monarch butterfly (Danaus plexippus) can locate and then sequester cardiac glycosides from milkweed species (Rothschild et al., 1975).
The likelihood and consequences of herbivore attack on plants are more serious for seedlings than for mature plants, typically because most seedlings are less well defended against, and thus more susceptible to, herbivore attack than their mature counterparts (Barton and Hanley, 2013). The fact that removal of even small amounts of plant tissue is proportionally more damaging, and harder to recover from, means that seedlings, whether chemically distasteful or not, can ill afford to suffer herbivore attack (Hanley et al., 2004). Even if tissue loss to herbivores at the seedling stage does not result in immediate mortality, significant negative repercussions for plant growth and reproductive potential may be apparent many weeks or even months later (Hanley and Fegan, 2007; Hanley, 2012; Barton, 2013). Thus, it may be advantageous for a seedling to advertize its chemical defences before damage occurs.
Although many different invertebrates and vertebrates are responsible for seedling losses, the principal seedling herbivores in temperate agriculture are slugs and snails (Moens and Glen, 2002; Strauss et al., 2009). While regarded as generalists (Iglesias and Castillejo, 1999), terrestrial gastropods can be highly selective when presented with a choice of food plants (Hanley, 2004). Although a link between seedling chemical defences and mollusc selection is often suggested (Hanley, 2004; Elger et al., 2009), there is in fact little direct evidence to support this assumption (but see Hanley and Lamont, 2001) and the reasons for seedling selection remain poorly understood (Barton and Hanley, 2013). Recent evidence for temperate grassland plants implies, however, that molluscs use seedling volatiles to discriminate between species (Hanley et al., 2011, 2013) supporting the suggestion that the detection of VOCs by pest species may contribute to the location and selection of crop seedlings (see also Carroll et al., 2006, 2008). Initial selection of food plants by slugs and snails is by olfaction using the sensory epithelia of the two posterior tentacles (Friedrich and Teyke, 1998) and molluscs are known to respond to the VOCs fenchone, carvone, ρ-cymene, β-caryophyllene, D-limonene, γ-coniceine, β-pinene and β-myrcene (Frank et al., 2002; Birkett et al., 2004). Glucosinolates, the constitutive defence chemicals contained in oilseed rape, may also drive gustatory selection by molluscs (Falk et al., 2014; Moshgani et al., 2014).
Despite the increased interest in seedling–herbivore interactions (Barton and Hanley, 2013), no study to date has attempted to link herbivore food plant selection with variation in seedling constitutive chemical defence and volatile chemistry. In addition to offering a valuable contribution to our wider understanding of plant–herbivore interactions, a more complete understanding of variation in seedling defence may be vital in our attempts to protect crops from pest attack over the coming decades. The aim of this study was therefore to elucidate whether selection of cultivars of the commonly cultivated crop plant oilseed rape by a generalist mollusc herbivore (the snail Helix aspersa) is linked to variation in the expression of seedling VOC profiles and glucosinolate defence. We test the following hypotheses:
That H. aspersa exhibit consistent feeding preferences between different seedlings (i.e. different oilseed rape cultivars)
That selection of oilseed rape seedlings by snails is linked to glucosinolate and VOC profiles
That seedlings with effective glucosinolate defences advertise defensive capability with distinct VOC profiles, to deter potentially fatal ‘sampling’ by herbivores
That repellent VOC profiles identified for low-acceptability cultivars can be used to manipulate snail olfactory preferences.
To this end we quantified the feeding preferences of molluscs for seedlings of 13 oilseed rape cultivars against a lettuce standard to yield an acceptability index (AI) and compared this with glucosinolate and volatile profiles from six cultivars, chosen to span the range of measured AI. To alter the attractiveness of oilseed rape, we added putative repellent or attractant volatiles to the profile of oilseed rape seedlings and tested snail preferences in a y-tube olfactometer to determine the feasibility of using VOC signals to influence mollusc food plant selection.
MATERIALS AND METHODS
Study species
Oilseed rape (Brassica napus L.) is widely grown for food, bioenergy oils and as cattle feedstock (Moens and Glen, 2002). The crop accounted for 715 000 ha (18 % by area) of agricultural land use in the UK (DEFRA, 2014) and in 2012, 63·7 million tonnes was grown worldwide, the largest producers being Canada and China (DEFRA, 2012; USDA, 2014). Severe damage of oilseed rape seedlings by molluscs necessitates the use of metaldehyde-based molluscicides (Garthwaite et al., 2013) with resulting ecotoxicological effects on non-target species (Birkett et al., 2004; Nicholls, 2014) and contamination of drinking water (Clarke et al., 2009). Oilseed rape has been the subject of breeding programmes over many decades to reduce its toxicity as an animal feed, consequently reducing concentrations of its main secondary defence metabolite, glucosinolates (Moens and Glen, 2002). This may have consequences for crop protection, especially due to cultivar-specific variation in glucosinolate profiles (Glen et al., 1990; but see Giamoustaris and Mithen, 1995). Mature plants have a well-resolved VOC profile comprising monoterpenes, green leaf volatiles (GLVs), aromatics and isothiocyanates (Blight et al., 1997; McEwan and Macfarlane, 1998), but nothing is known about seedling defences.
Although slugs, particularly Deroceras spp., are the most common mollusc pest species of UK arable ecosystems (Moens and Glen, 2002; Birkett et al., 2004) we used the snail Helix aspersa Müller in our experiments due to ease of collection and culture. As generalist herbivores, H. aspersa and Deroceras reticulatum have broadly similar feeding preferences and patterns of seedling selection (Hanley, 1995).
Snail feeding preference
The oilseed rape cultivars used in these experiments were ‘Amulet’, ‘Carnival’, ‘Fashion’, ‘Kumily’, ‘Tamarin’ (seeds supplied by Senova Ltd, Great Abington, UK), ‘Avatar’, ‘Cracker’, ‘Sesame’, ‘Thorin’ (LS Plant Breeding, Impington, UK), ‘Agatha’, ‘Astrid’ and ‘Cubic’ (Grainseed Ltd, Eye, UK). Oilseed rape seeds were germinated in 90-mm-diameter Petri dishes containing two layers of 90-mm Whatman No. 1 filter paper, 5 mL of distilled water and maintained in an incubator at 18 °C on a 12 : 12-h light–dark cycle. Following radicle appearance, two seedlings from the same oilseed rape cultivar (cv.) were planted 45 mm apart in 50-mm plastic pots containing John Innes No. 2 compost. These plants were planted with two 1-week-old lettuce seedlings (‘Little Gem’) such that the seedlings were arranged in a square with each species at opposite corners. Lettuce cultivated in the same way as oilseed rape was used to ascertain the relative acceptability of the ‘test’ oilseed rape plants with reference to a standard ‘index’ (Fenner et al., 1999), but due to differences in development time they required an extra week to attain a similar relative size as 7-d-old oilseed rape at growth stage 1·0 (cotyledons only) to 1·1 (cotyledons plus one leaf) (Sylvester-Bradley, 1985). Seedlings were then cultivated for a further 7 d at 18 °C (12 : 12-h light–dark cycle) to reach growth stage 1·2. Four replicate pots for each oilseed rape cultivar were sunk into the corners of large plastic propagator trays (375 × 230 × 60 mm deep) filled with John Innes No. 2 compost, such that the top of each pot was flush with the level of the compost (Hanley and Sykes, 2009). This arrangement was replicated ten times for each oilseed rape cultivar.
Several hundred individual snails were collected around Plymouth, UK (or for subsequent experiments, Southampton), retained in large aerated plastic containers in a constant environment (16 ± 2 °C, 12 : 12-h day–night) containing compost (Westland Horticulture Ltd, Dungannon, UK), wood and cuttlefish shells, and fed a mixed diet of lettuce and carrot. Snails were kept under this regime for 4 months before use in behavioural assays and no individual was reused in any trial. Snails were starved for 4 d prior to the start of any experiment. Two snails of uniform size (approx. 30 mm in diameter) were added to each tray and retained overnight (approx. 16 h) under a clear plastic propagator lid sprayed with water. To determine AI, above-ground seedling tissue loss was quantified for each replicate tray by counting the number of seedlings removed by snails and adding to this an estimate of the proportion of tissue removed from remaining seedlings. Damage was scored as: whole seedling eaten − 1; one leaf eaten − 0·5; half a leaf eaten − 0·25; small hole − 0·02. The combined damage score was used to calculate the AI as follows (modified from Hanley and Sykes, 2009):
where A = acceptability index; DO = oilseed rape damage score and DL = lettuce damage score.
Seedling glucosinolate content
Six cultivars (‘Agatha’, ‘Astrid’, ‘Avatar’, ‘Cracker’, ‘Cubic’ and ‘Thorin’) were selected for further work as they spanned the full AI range. To test the hypothesis that glucosinolate profiles would match snail selection preferences and VOC profiles, we quantified the glucosinolate concentrations of growth stage 1·1 oilseed rape seedlings, and compared with AI by conducting a canonical discriminant analysis (CDA) before testing the resultant discriminant functions (DFs) for correlation (van Dam and Poppy, 2008). Seeds from the six oilseed rape cultivars were germinated as described above and after radicle emergence, 20 seedlings from each cultivar were transplanted into 90-mm pots containing John Innes No.2 compost. These were grown on in a glasshouse under natural light, at 25 ± 1 °C and watered daily. All seedlings were harvested by cutting the stem just above the soil and immediately flash frozen in liquid nitrogen, before storage at − 80 °C. Samples were dried in batches of four for 48 h using an Edwards Modulyo freeze-drier. Each sample was pulverized in a Retsch MM300 mill (Retsch GmbH, Haan, Germany) and weighed on a Mettler-Toledo AB54 balance (Mettler-Toledo Ltd, Leicester, UK) before glucosinolate extraction. Then, 50·0 mg of dry material was placed in a 2 ml Eppendorf tube and extracted as in van Dam et al. (2004). Correction factors for detection at 229 nm from Buchner (1987) and Brown et al. (2003) were used to calculate the concentrations of the different types of glucosinolates based on the reference curve for sinigrin.
Glucosinolates were identified based on retention time, UV spectrum, LC-MS analysis of selected reference samples, and the following reference standards obtained from Phytoplan (Heidelberg, Germany): glucoiberin (3-methylsulfenylpropylGSL), glucoerucin (4-methylthiobutylGSL), progoitrin (2-hydroxy-3-butenylGSL), sinigrin (2-propenylGSL), gluconapin (3-butenylGSL), glucobrassicanapin (4-pentenylGSL), glucobrassicin (indol-3-ylmethylGSL), sinalbin (4-hydoxybenzylGSL), glucotropaeolin (benzylGSL) and gluconasturtiin (2-phenylethylGSL).
VOC collection and GC-MS
Volatiles were collected from each of the six oilseed rape cultivars to establish cultivar-specific variation and examine any relationship with AI. For each cultivar, 20 seedlings were grown in seven pots (90-mm-diameter pots as described above). In preliminary trials we found that seedlings at growth stage 1·0–1·1 did not produce detectable levels of VOCs, so were allowed to grow until they reached growth stage 1·1–1·2. Seedlings were gently removed from their pots, soil carefully washed away to avoid damage, and up to 140 seedlings per replicate placed together in a 200-mL glass beaker (Fisher Scientific, Loughborough, UK) with 100 mL of distilled water. Initial trials had established that while this eliminated volatiles from the soil and the pot, it did not alter the VOC profile of the plants (Supplementary Data, Fig. S1). All collections took place within an environment-controlled room (ECR) at 23 °C. Each beaker was placed inside a 46 × 56-cm polyester (PET) oven bag (Lakeland, Cumbria, UK) with one corner cut off, through which a Teflon tube was inserted before being tied shut (Stewart-Jones and Poppy, 2006). Air was drawn from the ECR air inlet via Tygon tubing (Saint-Gobain S.A., Paris, France), passed through an activated charcoal filter and pumped into the bag at a rate of 1000 mL min−1 using a Neuberger KNDC B pump (Neuberger, Freiburg, Germany). Three samples and one control (a bag containing a beaker with distilled water) were taken simultaneously, under two racks of compact fluorescent bulbs, giving approx. 200 μmol photons m−2 s−1 of photosynthetically active radiation at canopy height, equivalent to an overcast day. The open end of the bag was tied around a manual solid-phase microextraction (SPME) fibre holder (Supelco Inc., Bellefont, PA, USA) until the bag inflated, after which the SPME holder was removed. The bag was left for 1 h with the pump running to completely purge unfiltered air and allow the plants to acclimatize. The SPME holder was then replaced, the bag allowed to fully inflate and the SPME fibre exposed (Blue PDMS/DVB 65-μm fibre 57310-U, all from Lot no. 40981, Supelco). The airflow to each bag was reduced to 100 mL min−1 to maintain positive pressure, preventing contamination over the 4 h of VOC collection. As the volume of each tied and inflated bag was approx. 14 litres, this small airflow resulted in only one change of air every 140 min over a 240-min collection time. The SPME fibres were never saturated with any particular VOC. The maximum amount collected was a total ion current (TIC) of around 160 000, five to ten times less than typically collected when running standards. Initial trials also established that the proportion of each volatile in the profile remained constant as collection time was increased, so there was no effect of volatiles being excluded from a saturated fibre due to a long collection time (Supplementary Data Fig. S2).
VOCs were detected using a Hewlett Packard HP 6890 gas chromatography (GC) system fitted with an HP Innowax column (polyethylene glycol, 30·0 m × 250 μm i.d. × 0·25 μm film) coupled to an HP 6890 mass selective detector (MS) (Hewlett Packard Inc., Palo Alto, CA, USA) run in EI mode. Immediately following collection, the VOCs were thermally desorbed from the fibre in the injector port. The GC system was operated in splitless mode, with helium carrier gas at 7·45 p.s.i. and the inlet temperature at 250 °C. The oven was maintained at 50 °C for the first 2 min, then increased by 5 °C min−1 for 4 min, followed by 10 °C min−1 for 17 min, ending at 240 °C. The GC-MS device was controlled by HP ChemStation version B.02.05 software (Hewlett Packard) and data analysis by HP Enhanced Data Analysis version B.01.00. This protocol proved sufficiently sensitive to collect and identify a sample from 8 ng each of α-pinene and d-limonene diluted in hexane and introduced to an oven bag on filter paper (Whatman, GE Healthcare, Little Chalfont, UK). The peak TIC for these test samples (5000) was at the lower end of the range of the samples taken from the oilseed rape plants (approx. 4000–160 000). VOCs were initially identified using the NIST database, and confirmed by comparing retention time with standards (Sigma-Aldrich Ltd, Poole, UK).
Modified VOC profile olfactory assay
Olfactory preferences of snails were tested using a glass y-tube olfactometer (5 cm diameter; main tube 20 cm long; arm length 10 cm; angle between arms 90°). Twenty stage 1·1 (cotyledons and one true leaf) to 1·2 (second true leaf) oilseed rape seedlings (Sylvester-Bradley, 1985), ‘Astrid’ or ‘Agatha’ (representing the least attractive and a moderately attractive cultivar, respectively), cultivated as above in 90-mm-diameter pots, were in each of the entrainment jars, together with a filter paper with 2·5 μL of hexane. Hexane has no effect on feeding behaviour in a mollusc (Clark et al., 1997). In the treatment jar, the hexane contained three VOCs at a concentration such that 10 ng of each was present; this concentration was around the middle of the estimated range for the detected volatiles. On the basis that none of our cultivars released single VOCs and that phytophagous insects respond to blends of VOCs (Bruce et al., 2005) we used a blend of three VOCs rather than single volatiles. In the putative attractive mix, these were α-pinene, β-myrcene and δ-3-carene; in the putative repellent mix, they were cis-1-hexen-3-ol, cis-1-hexen-3-ol acetate and α-terpinene.
Air was pumped at 250 mL min−1 through a charcoal filter using a KNDC B pump (Neuberger) through a 100-mL Dreschel bottle containing activated charcoal, deionized water in another and then to one of two glass entrainment jars (10 × 24 cm) containing the plant material. Air and VOCs then flowed from each entrainment jar to one arm of the y-tube. Airflow was maintained at 150 mL min−1 using a Platon NGX regulator (CT Platon, Domont, France). All apparatus was connected using 8-mm-diameter Teflon tubing (Fisher Scientific) and all glassware supplied by Soham Scientific (Ely, UK). Three minutes were allowed for the VOCs to reach the y-tube before snails were placed in the main tube. They were deemed to have made a choice once their shell had completely entered one of the arms and they were travelling towards the end. Each treatment was tested using 36 snails, allowed to choose without time restriction although snails were timed through the olfactometer. All equipment was thoroughly washed between trials to remove mucus and rinsed with acetone to remove any remaining VOCs. The y-tube was flipped between runs and the position of test plants was varied to eliminate potential positional bias.
Statistical analysis
Snail feeding preference for different oilseed rape cultivars (AI) was analysed by ANOVA using the arcsine square-root transformed AI score, and subsequently by a series of pairwise t-tests using a Bonferroni correction in R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). A modelling approach was first used to establish whether any factor other than cultivar was significant.
To test whether glucosinolate content varied between oilseed rape cultivars, a series of one-way ANOVAs were conducted in R 3.0.2, and correlation between individual glucosinolate and snail preferences was tested using Pearson’s product-moment correlations with Bonferroni corrections applied. As each sample contains multiple glucosinolates, each of which may vary independently, a CDA was also carried out on the entire glucosinolate data set using IBM SPSS Statistics 21 (IBM Corp., New York, USA), to identify which glucosinolates contributed most to differences between cultivars (van Dam and Poppy, 2008). To test Hypothesis 2, that snail feeding preferences are linked to a suite of identifiable defensive chemicals, any significant DFs from the CDA were tested for correlation with the AI using Spearman’s rank correlation in R 3.0.2.
For the VOCs, the cut-off point for integration was set at a TIC area of 2500. Peaks that were artefacts of the SPME fibre were also discarded. As more than one SPME fibre was employed for volatile collection, the total abundance of VOCs could not be compared directly. Therefore, the arcsine square-root transformed proportions of the MS-TIC peak area of each VOC out of the total VOCs for that sample were the responses used in a CDA carried out in IBM SPSS Statistics 21. As with glucosinolate profiles, any significant DFs were tested for correlation with AI values.
To test Hypothesis 4, that addition of VOCs could alter the attractiveness of oilseed rape to snails, a generalized linear model (GLM) with binomial errors was carried out on the modified VOC profile assay data to determine if cultivar or any positional factor had affected snail choice. As none of these factors was significant and the sample size was small, snail choice was analysed using separate two-tailed exact binomial tests to determine whether snails preferred the modified plant volatile profiles or unmodified controls. As the positive and negative mix tests were entirely independent, no Bonferroni correction was applied. The more stringent two-tailed test was employed, allowing no prior assumptions about the direction of preference. All analysis of the modified profile assays was carried out using R 3.0.2. In all of the above analyses except for the binomial choice assays, data were tested for a normal distribution using Shapiro–Wilks tests in R 3.0.2, and transformed if necessary.
Results
Snail feeding preferences
Snails consistently preferred oilseed rape over lettuce (ANOVA, F1,258 = 126·6, P < 0·001) and a rank order of preference for cultivars was established (Fig. 1), with AI values ranging from the least attractive (‘Astrid’), with an AI of 0·61 ± 0·09 (n trials = 10) to the most preferred, ‘Thorin’ (0·78 ± 0·06). Overall, of 897 seedlings attacked by snails, only six (0·67 %) were not consumed entirely.
Fig. 1.
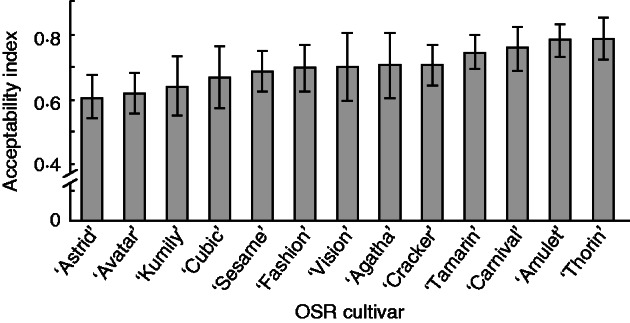
Mean (± 1 s.e.) seedling acceptability to snails (Helix aspersa) of 13 oilseed rape (Brassica napus) cultivars determined as a proportion of the amount of damage suffered relative to a lettuce (Lactuca sativa) control.
Glucosinolate content and snail feeding preferences
Five glucosinolates were detected from the six oilseed rape cultivars investigated (Table 1): progoitrin (PRO), 4-hydroxyglucobrassicin (4OH), 4-methoxyglucobrassicin (4MeO), neo-glucobrassicin (NEO) and glucobrassicin (GBC). Of these, only PRO (ANOVA, F5,16 = 3·661, P = 0·021) and NEO (ANOVA, F5,16 = 5·867, P = 0·003) differed significantly between cultivars. GBC dominated with concentrations ranging from 0·23 ± 0·02 μmol (g d. wt)−1 (‘Astrid’) to 0·73 ± 0·21 μmol (g d. wt)−1 (‘Thorin’) for PRO, and 0·34 ± 0·03 to 0·75 ± 0·09 μmol (g d. wt)−1 for GBC. Other compounds rarely exceeded 0·2 μmol (g d. wt)−1 (Table 1). A CDA was performed and, while this effectively separated the cultivars on the basis of their glucosinolate profile (with 95 % of cases correctly categorized), snail feeding preferences were not correlated with any DF; nor were they correlated with any individual glucosinolate, indole glucosinolates or total glucosinolates. Thus, we conclude that the order of snail gustatory selection of oilseed rape cultivars was not related to their glucosinolate profile.
Table 1.
Glucosinolate (GSL) concentrations in growth stage 1.1–1.2 seedling oilseed rape (Brassica napus) cultivars
| Oilseed rape cultivar | n | Glucosinolate concentration ± 1 s.e. [μmol (g d. wt)−1] |
||||||
|---|---|---|---|---|---|---|---|---|
| PRO | 4OH | GBC | 4MeO | NEO | Total indoles | Total GSLs | ||
| ‘Agatha’ | 3 | 0·327 ± 0·047 | 0·097 ± 0·009 | 0·534 ± 0·042 | 0·039 ± 0·002 | 0·158 ± 0·013 | 0·829 ± 0·065 | 1·156 ± 0·111 |
| ‘Astrid’ | 4 | 0·294 ± 0·053 | 0·070 ± 0·015 | 0·349 ± 0·031 | 0·032 ± 0·003 | 0·042 ± 0·003 | 0·493 ± 0·048 | 0·787 ± 0·083 |
| ‘Avatar’ | 4 | 0·559 ± 0·048 | 0·133 ± 0·030 | 0·752 ± 0·094 | 0·040 ± 0·005 | 0·087 ± 0·014 | 1·012 ± 0·126 | 1·571 ± 0·167 |
| ‘Cracker’ | 3 | 0·279 ± 0·080 | 0·133 ± 0·031 | 0·434 ± 0·023 | 0·030 ± 0·005 | 0·072 ± 0·005 | 0·669 ± 0·049 | 0·948 ± 0·128 |
| ‘Cubic’ | 4 | 0·236 ± 0·022 | 0·101 ± 0·033 | 0·584 ± 0·116 | 0·032 ± 0·005 | 0·073 ± 0·007 | 0·772 ± 0·146 | 1·008 ± 0·159 |
| ‘Thorin’ | 4 | 0·737 ± 0·213 | 0·201 ± 0·101 | 0·473 ± 0·121 | 0·048 ± 0·012 | 0·073 ± 0·024 | 0·795 ± 0·257 | 1·532 ± 0·469 |
PRO, progoitrin; 4OH, 4-hydroxyglucobrassicin; GBC, glucobrassicin; 4MeO, 4-methoxyglucobrassicin; NEO, neo-glucobrassicin.
VOCs and mollusc preferences
Twenty-four different VOCs were collected from the six oilseed rape cultivars and were mainly classed as monoterpenes and GLVs (Table 2). Most are well described in oilseed rape, with the exception of the ten unknown compounds, here labelled as Umono A–C (thought to be monoterpenes, from examination of their retention time on the GC column, and the size of fragments in the spectrum obtained from the MS) and unidentified compounds Ucomp A–G. The seedlings produced relatively low levels of volatiles overall, and these unknown compounds were detected in such small quantities that it was not possible to make a consistent identification. Some compounds were detected in only one sample per cultivar. Monoterpenes dominated the VOC profile of most of the cultivars, particularly α-pinene, α -phellanderene, δ-3-carene, D-limonene and γ-terpinene. There was also a substantial proportion of GLVs, notably cis-1-hexen-3-ol acetate (particularly dominant in ‘Astrid’) and cis-1-hexen-3-ol. Although proportions of several VOCs differed between cultivars (Table 2), there was no correlation between any one VOC and the AI preferences.
Table 2.
Volatile organic compounds (VOCs) detected in six different cultivars of growth stage 1.1–1.2 oilseed rape (Brassica napus) seedlings by GC-MS
| VOC | OSR cultivar |
|||||
|---|---|---|---|---|---|---|
| ‘Agatha’ | ‘Astrid’ | ‘Avatar’ | ‘Cracker’ | ‘Cubic’ | ‘Thorin’ | |
| Acetone | 8·00 ± 8·00 | n.d. | 1·90 ± 1·75 | 3·81 ± 3·43 | n.d. | 0·11 ± 0·11 |
| Ucomp F | 1·24 ± 1·24 | n.d. | 0·19 ± 0·19 | n.d. | n.d. | 1·48 ± 1·31 |
| α-Pinene** | 8·13 ± 1·23 | 1·82 ± 0·89 | 4·95 ± 0·39 | 8·12 ± 2·54 | 13·75 ± 3·09 | 5·64 ± 0·92 |
| α-Phellanderene | 15·17 ± 4·04 | 4·66 ± 2·09 | 19·55 ± 4·20 | 15·64 ± 6·68 | 15·10 ± 6·48 | 16·71 ± 2·28 |
| Ucomp G | n.d. | n.d. | 0·38 ± 0·24 | n.d. | n.d. | 0·40 ± 0·23 |
| Umono C | 1·43 ± 0·85 | n.d. | 3·22 ± 1·09 | 2·33 ± 1·34 | 1·12 ± 1·12 | 2·17 ± 0·80 |
| β-Myrcene | 0·77 ± 0·77 | n.d. | 1·36 ± 1·10 | 6·63 ± 5·79 | 2·39 ± 1·85 | 1·41 ± 0·59 |
| δ-3-Carene** | 22·71 ± 3·94 | 4·10 ± 2·21 | 18·41 ± 2·36 | 16·10 ± 3·84 | 20·49 ± 3·00 | 18·32 ± 3·47 |
| α-Terpinene*** | n.d. | n.d. | 2·18 ± 0·46 | n.d. | n.d. | 1·38 ± 0·80 |
| d-Limonene* | 23·97 ± 4·29 | 6·04 ± 2·53 | 31·75 ± 4·02 | 19·47 ± 8·86 | 20·66 ± 5·67 | 25·14 ± 3·87 |
| Eucalyptol | n.d. | n.d. | 0·74 ± 0·43 | n.d. | n.d. | 0·25 ± 0·14 |
| Ucomp C* | n.d. | n.d. | n.d. | n.d. | n.d. | 0·20 ± 0·12 |
| γ-Terpinene | 1·91 ± 1·14 | 0·51 ± 0·51 | 5·66 ± 1·14 | 2·14 ± 1·29 | 1·41 ± 0·87 | 4·66 ± 1·88 |
| Octadiene | 1·85 ± 1·85 | 1·70 ± 1·14 | 0·44 ± 0·31 | 0·31 ± 0·31 | 1·69 ± 1·32 | 3·27 ± 1·72 |
| ρ-Cymene | 0·72 ± 0·72 | 0·51 ± 0·51 | 1·34 ± 0·48 | n.d. | 0·37 ± 0·37 | 1·72 ± 0·65 |
| Umono A | n.d. | n.d. | 0·43 ± 0·25 | n.d. | n.d. | 0·51 ± 0·30 |
| Umono B | n.d. | n.d. | 0·25 ± 0·25 | 1·98 ± 1·22 | 0·25 ± 0·25 | 0·19 ± 0·19 |
| 3-Hexen-1-ol acetate*** | 1·09 ± 1·09 | 75·27 ± 8·65 | 4·98 ± 4·83 | 17·51 ± 8·37 | 10·45 ± 6·56 | 7·35 ± 3·71 |
| Anisole | 0·45 ± 0·45 | n.d. | 0·36 ± 0·36 | n.d. | n.d. | 0·25 ± 0·15 |
| 3-Hexen-1-ol | n.d. | 0·65 ± 0·40 | 0·12 ± 0·12 | n.d. | n.d. | n.d. |
| Ucomp E | 5·86 ± 4·77 | 2·70 ± 2·70 | 0·71 ± 0·46 | 2·08 ± 0·79 | 3·68 ± 3·22 | 5·60 ± 3·78 |
| Ucomp A | 2·44 ± 2·44 | 1·04 ± 1·04 | 0·16 ± 0·16 | 1·51 ± 0·92 | 1·07 ± 1·07 | 1·68 ± 1·39 |
| Ucomp D | 1·55 ± 1·55 | 0·55 ± 0·55 | 0·55 ± 0·42 | 0·68 ± 0·68 | 2·54 ± 0·88 | 0·78 ± 0·28 |
| Ucomp B* | 2·71 ± 1·06 | 0·47 ± 0·47 | 0·36 ± 0·23 | 1·70 ± 0·61 | 5·03 ± 1·53 | 0·79 ± 0·58 |
Asterisks next to names indicate whether proportions were significantly different across cultivars (*P < 0·05; **P < 0·01; ***P < 0·001); figures indicate the percentage of that cultivar’s total made up by that VOC, ±1 s.e.; n.d. = not detected. Ucomp A–G indicate unknown compound; Umono A–C indicate unknown monoterpenes.
The CDA on seedling VOCs reduced the data set to five DFs, of which DF1 and DF2 were significant (Fig. 2). The analysis successfully categorized 100 % of the samples into their correct cultivar, while DF1 accounted for 82·3 % of the variation and DF2 explained 15·2 %. The structure matrices for DF1 and DF2 (Table 3) show the correlation between individual VOCs and that discriminant factor. VOCs with a more positive value are likely to be at a higher level in samples with more positive values of that DF, and the opposite is true for negative values. Thus, DF1 represents an axis with more GLVs at the negative end and monoterpenes at the positive end, but with some exceptions, while DF2 does not split by chemical category as easily, although there were more GLVs negatively correlated than positively correlated.
Fig. 2.
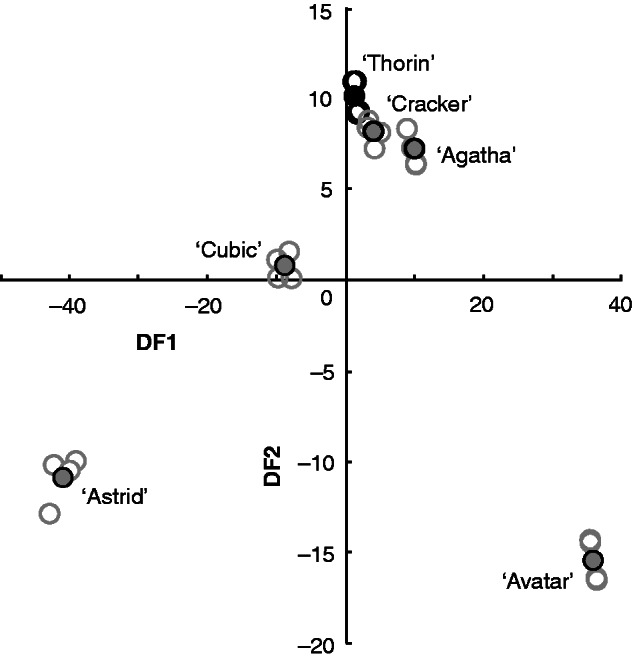
Discriminant factor 1 (DF1) against DF2 from a canonical discriminant analysis on relative proportions of 24 volatile organic chemicals released by six cultivars of oilseed rape (Brassica napus) seedlings at growth stage 1·1–1·2. Group centroids are represented by closed circles, individual samples (n = 4) by open circles of the same colour, as standard error bars are too small to be shown. The analysis classified each sample into its correct cultivar with 100 % accuracy.
Table 3.
Structure matrices for discriminant factor 1 (DF1) and DF2 for volatile organic compounds (VOCs) from six cultivars of growth stage 1·1–1·2 oilseed rape (Brassica napus) seedlings
| VOC | DF1 | VOC | DF2 |
|---|---|---|---|
| Ucomp D | 0·233 | Ucomp B | 0·141 |
| Ucomp B | 0·188 | Ucomp D | 0·062 |
| Ucomp A | 0·042 | α-Pinene | 0·061 |
| α-Terpinene | 0·041 | δ-3-Carene | 0·056 |
| δ-3-Carene | 0·038 | Ucomp A | 0·044 |
| d-Limonene | 0·034 | Ucomp C | 0·037 |
| Umono C | 0·024 | β-Myrcene | 0·022 |
| γ-Terpinene | 0·024 | Ucomp F | 0·022 |
| α-Phellanderene | 0·022 | Umono B | 0·020 |
| Eucalyptol | 0·021 | α-Phellanderene | 0·016 |
| α-Pinene | 0·019 | Octadiene | 0·014 |
| Umono A | 0·017 | d-Limonene | 0·013 |
| Ucomp G | 0·017 | Acetone | 0·011 |
| ρ-Cymene | 0·011 | Anisole | 0·006 |
| Anisole | 0·011 | Umono C | 0·004 |
| Acetone | 0·010 | γ-Terpinene | −0·009 |
| β-Myrcene | 0·007 | Umono A | −0·011 |
| Ucomp F | 0·007 | ρ-Cymene | −0·012 |
| Umono B | 0·006 | Ucomp G | −0·012 |
| Ucomp C | 0·001 | Ucomp E | −0·015 |
| Octadiene | −0·006 | Eucalyptol | −0·030 |
| Ucomp E | −0·051 | 3-Hexen-1-ol | −0·052 |
| 3-Hexen-1-ol acetate | −0·069 | α-Terpinene | −0·058 |
| 3-Hexen-1-ol | −0·680 | 3-Hexen-1-ol acetate | −0·086 |
A more positive value (on a scale of 1 to −1) indicates a stronger positive correlation between that VOC and the DF; a more negative value indicates a negative correlation, while a value close to zero indicates little correlation.
There was no relationship between DF1 and AI (rho = 0·2, P = 0·7139, d.f. = 4), but their DF2 values were significantly correlated with AI (rho = 0·943, P = 0·0167, d.f. = 4). VOCs most positively associated with DF2 (Table 3) are likely to be the most attractive to snails, while negatively associated compounds may be repellent (Fig. 3). Thus, we conclude that oilseed rape seedling acceptability to snails is strongly related to VOCs (Hypothesis 2).
Fig. 3.
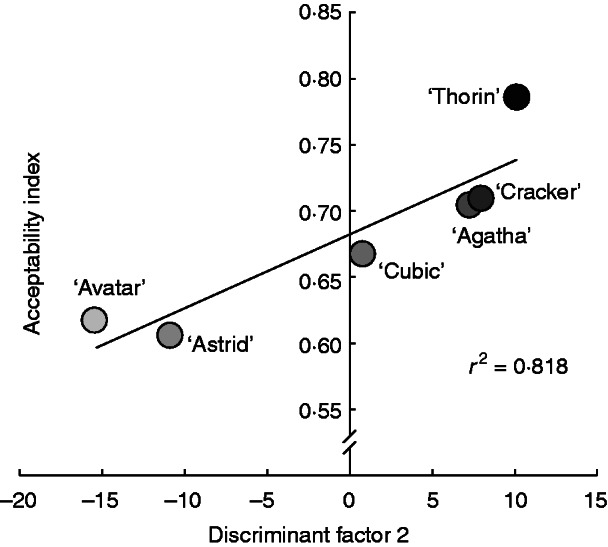
Relationship between discriminant factor 2 from a canonical discriminant analysis on relative proportions of 24 volatile organic chemicals released by six cultivars of oilseed rape (Brassica napus) seedlings at growth stage 1·1–1·2, and gustatory preferences of snails (Helix aspersa), calculated as oilseed rape seedling consumption relative to a standard index species, lettuce (Lactuca sativa).
Glucosinolates and VOCs
To test the hypothesis that VOC profiles would reflect defensive chemicals (i.e. glucosinolates), we conducted a series of pairwise correlation tests. The monoterpene ρ-cymene was positively correlated with PRO (r2 = 0·89, P = 0·004) and 4MeO (r2 = 0·90, P = 0·004), while PRO was also correlated with Ucomp G (r2 = 0·92, P = 0·003) and Umono A (r2 = 0·95, P = 0·001). These VOCs were not identified as attractive or repellent by the CDA (Table 3) and thus there was only limited support for this hypothesis.
Modified VOC profile assay
The putative attractant volatiles identified following CDA of the VOCs (‘positive mix’) were α-pinene, β-myrcene and δ-3-carene; the ‘negative mix’ contained cis-1-hexen-3-ol, cis-1-hexen-3-ol acetate and α-terpinene. Positive or negative treatments did not affect the time taken to choose (GLM, z = 0·797, P = 0·42, d.f. = 69). The positive mix attracted only slightly more (53 %, n = 36) of the snails than did controls, indicating that there was no difference in the attractiveness of the treatments [P (two tailed) = 0·868], but snails avoided the negative mix, with only 31 % (n = 35) choosing it over the control [P (two-tailed) = 0·041]. This finding adds further weight to our conclusion and confirms that snail preferences are influenced by (repellent) VOCs, supporting Hypothesis 4 that VOCs can be used to manipulate seedling selection by snails.
DISCUSSION
Molluscs exhibit strong preferences for seedlings of different plant species (Fenner et al., 1999; Hanley, 2004; Barlow et al., 2013) but the reasons underpinning selection remain unclear and the link with chemical defences is inferred rather than established (Barton and Hanley, 2013). Terrestrial molluscs are known to discriminate between the odours of macerated seedlings (Hanley et al., 2011) and also avoid food laced with volatiles that they find unpleasant; consequently, the fact that we show that snail selection of oilseed rape seedlings is highly correlated with cultivar-specific VOC profiles is perhaps unsurprising. In addition to the fact that this study is the first to show this effect for intact seedlings, it is interesting that we were unable to locate any relationship between seedling selection by snails and cultivar-specific variation in glucosinolates. Thus, seedling acceptability in oilseed rape was determined primarily by olfactory selection (VOC profile) rather than gustatory cues, likely to be a much more effective way of signalling defensive capabilities to herbivores than a process that relies on potentially lethal tissue loss at such an early ontogenetic stage (Hanley and Fegan, 2007; Hanley, 2012).
Nonetheless, the lack of any relationship between glucosinolates and AI was unexpected, especially given that many studies have detected a deterrent effect for (or reduced performance of) molluscs when presented with plants high in glucosinolates (Moshgani et al., 2014: but see Moyes et al., 2000). This may be due to the low glucosinolate levels present in young seedlings; indeed, the glucosinolate concentration in our samples was around 10 % of those reported for mature cultivated and wild brassica plants (Gols et al., 2008; Moshgani et al., 2014) and selective breeding has also reduced natural glucosinolate levels (Moens and Glen, 2002). Given that biochemical constraints on young seedlings imposed by the need to develop resource-acquiring organs (true-leaves and roots) are thought to limit the ability to independently synthesize chemical defences (Boege and Marquis, 2005), it is likely that cotyledon-stage defence is the result of maternal provisioning alone. Indeed, Cole (1980) reported a positive association between glucosinolate concentrations and plant age in oilseed rape during early ontogeny. Consequently, the spectrum of possible defences available for young seedlings is limited (including a likely inability to deploy induced defences) and low glucosinolate concentrations in oilseed rape are unsurprising. Given that Helix is also able to produce a sulfatase enzyme capable of detoxifying glucosinolates (Wittstock et al., 2000), snails are probably equipped to tolerate the low level of glucosinolates present in young oilseed rape seedlings.
Cultivar-specific variation in VOC profiles therefore remains as the strongest candidate to explain oilseed rape seedling selection by snails. The relationship between VOCs and seedling acceptability to snails we established enabled us to predict which VOCs would be attractant or repellent, and demonstrate that mollusc feeding behaviour can be modified by the addition of repellent VOC blends. Many studies with gastropods have shown that single chemicals are insufficient to elicit any behavioural response (but see Chase, 1982), but like many other invertebrate herbivores (Webster et al., 2008) molluscs show greater response to volatiles presented as a blend (Fink et al., 2006), a situation that most closely mirrors natural field conditions (Ache and Young, 2005). The VOC profiles of the least attractive oilseed rape cultivars were actually characterized by GLVs, a group of plant VOCs indicative of herbivore damage (Shiojiri et al., 2006; Fürstenau et al., 2012) and whose increased concentrations with age have previously been linked to reduced olfactory selection of Plantago lanceolata by Helix (Hanley et al., 2013). GLV repellent capabilities were further evidenced here by the fact that the ‘negative’ VOC mix contained two GLVs (cis-1-hexen-3-ol and cis-1-hexen-3-ol acetate) and altered snail behaviour greatly. Given that we found no relationship between the expression of these two GLVs and glucosinolate profiles across our oilseed rape cultivars, it seems likely that these VOCs form the basis of early seedling defence in oilseed rape [like Schiestl (2014), we also failed to identify any isothiocyanates in seedling VOC profiles, the most plausible candidates to advertise glucosinolates to would-be herbivores]. It is interesting that GLVs are derived from the same oxylipin pathway leading to jasmonic acid synthesis (Kombrink, 2012; Scala et al., 2013), leaving open the possibility that they might be perceived by herbivores as a signal that a plant’s defences are activated. The other main component of our ‘negative’ blend, α-terpinene, is a taxonomically widespread volatile (Pherobase, 2014) and has previously been identified as a herbivore repellent (for whitefly: Bleeker et al., 2009; and weevils: Wang et al., 2009). Taken together these volatiles fulfil the criteria suggested by Bleeker et al. (2009) for accepting a blend of volatiles as actively repellent.
Crop plants are bred for various desirable characteristics, but most often priority is given to increased yield and disease resistance over traits favouring herbivore resistance. We have demonstrated, however, that distinct VOC profiles already produced by different oilseed rape cultivars could be harnessed to lessen the attractiveness of this crop to molluscs during early establishment. Enhancement of repellent oilseed rape volatiles might be achieved by conventional plant breeding techniques (Bleeker et al., 2009) and these may be especially effective if used in tandem with other crop protection methods: for instance, a ‘push–pull’ system, where pests may be repelled by VOCs from the crop at the same time as being pulled towards nearby attractive plants as part of an integrated pest management strategy (Cook et al., 2007; Kergunteuil et al., 2015). At a time when increasing demands for food security (Godfray et al., 2010) are in conflict with concern over pesticide use (Whitehorn et al., 2012), we show that for one major crop species at least, plant protection could be developed without ecotoxic side effects.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Figure S1: Comparison of volatiles collected from oilseed rape seedlings ‘Avatar’ with soil carefully washed away and from ‘Avatar’ seedlings left in pots; Figure S2: Peak area TIC collected on SPME fibres over increasing collection time.
ACKNOWLEDGEMENTS
We thank Jane Akerman, Chelsea White-Hall, Mike Cotton and Sebastian Krosse for technical assistance, Dr Inka Lusebrink and Dr Robbie Girling for invaluable advice, Senova Ltd, LS Plant Breeding Ltd and Grainseed Ltd for generously donating oilseed rape seeds, and two anonymous referees for comments on an earlier version of the manuscript. This work was supported by the Leverhulme Trust (grant number RPG-083 to M.E.H., P.L.N. and G.M.P.) and the Seale-Hayne Educational Trust (to M.E.H.).
LITERATURE CITED
- Ache BW, Young JM. 2005. Olfaction: diverse species, conserved principles. Neuron 48: 417–430. [DOI] [PubMed] [Google Scholar]
- Alyokhin A, Mota-Sanchez D, Baker M, et al. 2014. The Red Queen in a potato field: integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Management Science 71: 343–356. [DOI] [PubMed] [Google Scholar]
- Barlow SE, Close AJ, Port GR. 2013. The acceptability of meadow plants to the slug Deroceras reticulatum and implications for grassland restoration. Annals of Botany 112: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE. 2013. Ontogenetic patterns in the mechanisms of tolerance to herbivory in Plantago. Annals of Botany 112: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Hanley ME. 2013. Seedling–herbivore interactions: insights into plant defence and regeneration patterns. Annals of Botany 112: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebber DP, Ramotowski MAT, Gurr SJ. 2013. Crop pests and pathogens move polewards in a warming world. Nature Climate Change 3: 985–988. [Google Scholar]
- Birkett M, Dodds C, Henderson I, et al. 2004. Antifeedant compounds from three species of Apiaceae active against the field slug, Deroceras reticulatum (Muller). Journal of Chemical Ecology 30: 563–576. [DOI] [PubMed] [Google Scholar]
- Bleeker PM, Diergaarde PJ, Ament K, et al. 2009. The role of specific tomato volatiles in tomato–whitefly interaction. Plant Physiology 151: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight M, Métayer M, Delègue M-H, Pickett J, Marion-Poll F, Wadhams L. 1997. Identification of floral volatiles involved in recognition of oilseed rape flowers, Brassica napus by honeybees, Apis mellifera. Journal of Chemical Ecology 23: 1715–1727. [Google Scholar]
- Boege K, Marquis RJ. 2005. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology & Evolution 20: 441–448. [DOI] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62: 471–481. [DOI] [PubMed] [Google Scholar]
- Bruce TJA. 2014. Glucosinolates in oilseed rape: secondary metabolites that influence interactions with herbivores and their natural enemies. Annals of Applied Biology 164: 348–353. [Google Scholar]
- Bruce TJA, Wadhams LJ, Woodcock CM. 2005. Insect host location: a volatile situation. Trends in Plant Science 10: 269–274. [DOI] [PubMed] [Google Scholar]
- Buchner R. 1987. Approach to determination of HPLC response factors for glucosinolates In: JP Wathelet, ed. Glucosinolates in rapeseed. Dordrecht: Martinus Nijhoff Publishers, 50–58. [Google Scholar]
- Carroll MJ, Schmelz EA, Meagher RL, Teal PEA. 2006. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. Journal of Chemical Ecology 32: 1911–1924. [DOI] [PubMed] [Google Scholar]
- Carroll MJ, Schmelz EA, Teal PEA. 2008. The attraction of Spodoptera frugiperda neonates to cowpea seedlings is mediated by volatiles induced by conspecific herbivory and the elicitor inceptin. Journal of Chemical Ecology 34: 291–300. [DOI] [PubMed] [Google Scholar]
- Chase R. 1982. The olfactory sensitivity of snails, Achatina fulica. Journal of Comparative Physiology 148: 225–235. [Google Scholar]
- Clark SJ, Dodds CJ, Henderson IF, Martin AP. 1997. A bioassay for screening materials influencing feeding in the field slug Deroceras reticulatum (Müller) (Mollusca: Pulmonata). Annals of Applied Biology 130: 379–385. [Google Scholar]
- Clarke J, Wynn S, Twining S, et al. 2009. Pesticide availability for cereals and oilseeds following revision of Directive 91/414/EEC; effects of losses and new research priorities. Research Review No. 70, Home Grown Cereals Authority, 1–131.
- Cole RA. 1980. Volatile components produced during ontogeny of some cultivated crucifers. Journal of the Science of Food and Agriculture 31: 549–557. [Google Scholar]
- Cook SM, Khan ZR, Pickett JA. 2007. The use of push–pull strategies in integrated pest management. Annual Review of Entomology 52: 375–400. [DOI] [PubMed] [Google Scholar]
- van Dam NM, Poppy GM. 2008. Why plant volatile analysis needs bioinformatics – detecting signal from noise in increasingly complex profiles. Plant Biology 18: 29–37. [DOI] [PubMed] [Google Scholar]
- van Dam NM, Witjes L, Svatoš A. 2004. Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytologist 161: 801–810. [DOI] [PubMed] [Google Scholar]
- DEFRA. 2012. Agriculture in the United Kingdom 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/208436/auk-2012–25jun13.pdf (last accessed 10 June 2014).
- DEFRA. 2014. Structure of the agricultural industry in England and the UK at June. https://www.gov.uk/government/statistical-data-sets/structure-of-the-agricultural-industry-in-england-and-the-uk-at-june (last accessed 18 July 2014).
- Elger A, Lemoine DG, Fenner M, Hanley ME. 2009. Plant ontogeny and chemical defence: older seedlings are better defended. Oikos 118: 767–773. [Google Scholar]
- Falk KL, Kästner J, Bodenhausen N, et al. 2014. The role of glucosinolates and the jasmonic acid pathway in resistance of Arabidopsis thaliana against molluscan herbivores. Molecular Ecology 23: 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner M, Hanley ME, Lawrence R. 1999. Comparison of seedling and adult palatability in annual and perennial plants. Functional Ecology 13: 546–551. [Google Scholar]
- Fink P, von Elert E, Jüttner F. 2006. Volatile foraging kairomones in the littoral zone: attraction of an herbivorous freshwater gastropod to algal odors. Journal of Chemical Ecology 32: 1867–1881. [DOI] [PubMed] [Google Scholar]
- Frank T, Bieri K, Speiser B. 2002. Feeding deterrent effect of carvone, a compound from caraway seeds, on the slug Arion lusitanicus. Annals of Applied Biology 141: 93–100. [Google Scholar]
- Friedrich A, Teyke T. 1998. Identification of stimuli and input pathways mediating food-attraction conditioning in the snail, Helix. Journal of Comparative Physiology A 183: 247–254. [Google Scholar]
- Fürstenau B, Rosell G, Guerrero A, Quero C. 2012. Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. Journal of Chemical Ecology 38: 378–388. [DOI] [PubMed] [Google Scholar]
- Garthwaite DG, Behmer S, Barker I, Parrish G, Smith L, Pietravalle S. 2013. Pesticide usage survey report 250: arable crops in the United Kingdom 2012. York: Food & Environment Research Agency, DEFRA. [Google Scholar]
- Giamoustaris A, Mithen R. 1995. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Annals of Applied Biology 126: 347–363. [Google Scholar]
- Glen DM, Jones H, Fieldsend JK. 1990. Damage to oilseed rape seedlings by the field slug Deroceras reticulatum in relation to glucosinolate concentration. Annals of Applied Biology 117: 197–207. [Google Scholar]
- Godfray HCJ, Beddington JR, Crute IR, et al. 2010. Food security: the challenge of feeding 9 billion people. Science 327: 812–818. [DOI] [PubMed] [Google Scholar]
- Gols R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA. 2008. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. Journal of Chemical Ecology 34: 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME. 1995. The influence of molluscan herbivory on seedling regeneration in grassland. PhD thesis, University of Southampton, UK.
- Hanley ME. 2004. Seedling herbivory and the influence of plant species richness in seedling neighbourhoods. Plant Ecology 170: 35–41. [Google Scholar]
- Hanley ME. 2012. Seedling defoliation, plant growth and flowering potential in native- and invasive-range Plantago lanceolata populations. Weed Research 52: 252–259. [Google Scholar]
- Hanley ME, Fegan E. 2007. Timing of cotyledon damage affects growth and flowering in mature plants. Plant, Cell & Environment 30: 812–819. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB. 2001. Herbivory, serotiny and seedling defence in Western Australian Proteaceae. Oecologia 126: 409–417. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. 2009. Impacts of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany 103: 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvill B. 2004. Early plant growth: identifying the end point of the seedling phase. New Phytologist 163: 61–66. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. 2007. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics 8: 157–178. [Google Scholar]
- Hanley ME, Collins SA, Swann C. 2011. Advertising acceptability: is mollusc olfaction important in seedling selection? Plant Ecology 212: 727–731. [Google Scholar]
- Hanley ME, Girling RD, Felix AE, Olliff ED, Newland PL, Poppy GM. 2013. Olfactory selection of Plantago lanceolata by snails declines with seedling age. Annals of Botany 112: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J, Castillejo J. 1999. Field observations on feeding of the land snail Helix aspersa Müller. Journal of Molluscan Studies 65: 411–423. [Google Scholar]
- Kergunteuil A, Dugravot S, Danner H, van Dam N, Cortesero A. 2015. Characterizing volatiles and attractiveness of five Brassicaceous plants with potential for a ‘Push-Pull’ strategy toward the cabbage root fly, Delia radicum. Journal of Chemical Ecology 41: 330–339. [DOI] [PubMed] [Google Scholar]
- Kombrink E. 2012. Chemical and genetic exploration of jasmonate biosynthesis and signaling paths. Planta 236: 1351–1366. [DOI] [PubMed] [Google Scholar]
- Leather SR. 1987. Pine monoterpenes stimulate oviposition in the pine beauty moth, Panolis flammea. Entomologia Experimentalis et Applicata 43: 295–297. [Google Scholar]
- McEwan, Macfarlane S. 1998. Identification of volatile organic compounds emitted in the field by oilseed rape (Brassica napus ssp. oleifera) over the growing season. Clinical & Experimental Allergy 28: 332–338. [DOI] [PubMed] [Google Scholar]
- Moens R, Glen DM. 2002. Agriolimacidae, Arionidae and Milacidae as pests in West European oilseed rape In: G Barker, ed. Terrestrial molluscs as crop pests. Wallingford, UK: CABI International, 425–440. [Google Scholar]
- Moshgani M, Kolvoort E, de Jong TJ. 2014. Pronounced effects of slug herbivory on seedling recruitment of Brassica cultivars and accessions, especially those with low levels of aliphatic glucosinolates. Basic and Applied Ecology 15: 607–615. [Google Scholar]
- Moyes C, Collin H, Britton G, Raybould A. 2000. Glucosinolates and differential herbivory in wild populations of Brassica oleracea. Journal of Chemical Ecology 26: 2625–2641. [Google Scholar]
- Newland PL. 1998. Avoidance reflexes mediated by contact chemoreceptors on the legs of locusts. Journal of Comparative Physiology A 183: 313–324. [Google Scholar]
- Nicholls CJ. 2014. Implications of not controlling slugs in oilseed rape and wheat in the UK. HGCA Research Review 79: 1–9. [Google Scholar]
- Oerke E-C. 2006. Crop losses to pests. Journal of Agricultural Science 144: 31–43. [Google Scholar]
- Pherobase. 2014. The Pherobase: Database of Pheromones and Semiochemicals. http://www.pherobase.com (last accessed 12 September 2014).
- Renwick JAA, Lopez K. 1999. Experience-based food consumption by larvae of Pieris rapae: addiction to glucosinolates? Entomologia Experimentalis et Applicata 91: 51–58. [Google Scholar]
- Rothschild M, Euw JV, Reichstein T, Smith DAS, Pierre J. 1975. Cardenolide storage in Danaus chrysippus (L.) with additional notes on D. plexippus (L.). Proceedings of the Royal Society of London. Series B. Biological Sciences 190: 1–31. [Google Scholar]
- Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC. 2013. Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. International Journal of Molecular Sciences 14: 17781–17811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP. 2014. Correlation analyses between volatiles and glucosinolates show no evidence for chemical defense signalling in Brassica rapa. Frontiers in Ecology and Evolution 2: 1–10. [Google Scholar]
- Shiojiri K, Kishimoto K, Ozawa R, et al. 2006. Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences 103: 16672–16676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Jones A, Poppy GM. 2006. Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. Journal of Chemical Ecology 32: 845–864. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Stanton ML, Emery NC, et al. 2009. Cryptic seedling herbivory by nocturnal introduced generalists impacts survival, performance of native and exotic plants. Ecology 90: 419–429. [DOI] [PubMed] [Google Scholar]
- Sylvester-Bradley R. 1985. Revision of a code for stages of development in oilseed rape (Brassica napus L.). Aspects of Applied Biology 10: 395–400. [Google Scholar]
- USDA. 2014. Oilseeds: World Markets and Trade. http://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (last accessed 10 June 2014).
- Wang JL, Li Y, Lei CL. 2009. Evaluation of monoterpenes for the control of Tribolium castaneum (Herbst) and Sitophilus zeamaise Motschulsky. Natural Product Research 23: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Webster B, Bruce T, Dufour S, et al. 2008. Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. Journal of Chemical Ecology 34: 1153–1161. [DOI] [PubMed] [Google Scholar]
- Whitehorn PR, O’Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336: 351–352. [DOI] [PubMed] [Google Scholar]
- Wittstock U, Fisher M, Svendsen I, Halkier BA. 2000. Cloning and characterization of two cDNAs encoding sulfatases in the Roman snail, Helix pomatia. IUBMB Life 49: 71–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


