Abstract
While skeletal muscle mass is an established primary outcome related to understanding cancer cachexia mechanisms, considerable gaps exist in our understanding of muscle biochemical and functional properties that have recognized roles in systemic health. Skeletal muscle quality is a classification beyond mass, and is aligned with muscle’s metabolic capacity and substrate utilization flexibility. This supplies an additional role for the mitochondria in cancer-induced muscle wasting. While the historical assessment of mitochondria content and function during cancer-induced muscle loss was closely aligned with energy flux and wasting susceptibility, this understanding has expanded to link mitochondria dysfunction to cellular processes regulating myofiber wasting. The primary objective of this article is to highlight muscle mitochondria and oxidative metabolism as a biological target of cancer cachexia and also as a cellular regulator of cancer-induced muscle wasting. Initially, we examine the role of muscle metabolic phenotype and mitochondria content in cancer-induced wasting susceptibility. We then assess the evidence for cancer-induced regulation of skeletal muscle mitochondrial biogenesis, dynamics, mitophagy, and oxidative stress. In addition, we discuss environments associated with cancer cachexia that can impact the regulation of skeletal muscle oxidative metabolism. The article also examines the role of cytokine-mediated regulation of mitochondria function regulation, followed by the potential role of cancer-induced hypogonadism. Lastly, a role for decreased muscle use in cancer-induced mitochondrial dysfunction is reviewed.
Keywords: Mitochondria, disuse, cytokine, hypogonadism
1. Introduction
Improving survival and quality of life are inherent goals for successful treatment of cancer patients, and also the expectant translational impact of basic cancer research. Cancer cachexia, the unintentional loss of body weight, has an established adverse effect on these treatment objectives [1–3]. Cachexia development is not associated with all cancers, but the progression of cachexia is directly associated with cancer patient morbidity and mortality. While cachexia has been reported to account for a significant number of cancer deaths [4,5], successful treatment of the cachectic cancer patient remains elusive. The difficulty in treating cancer cachexia parallels the condition’s underlying complex and multifactorial nature, which can vary with the underlying disease severity. Consequently, for biomedical research to either promote the discovery or enhance existing therapeutic approaches to effectively treat cachexia, the facets of body weight loss that are the critical lynch pins for survival need to be further established. There is evidence that cancer-induced weight loss is associated with global endocrine and metabolic abnormalities [6,7], and the disrupted function of many tissues and organs, including the gut, brain, heart, liver, and adipose [8–10]. Nevertheless, for some time, critical importance has been placed on cancer-induced skeletal muscle mass loss [5].
The longstanding interest in skeletal muscle with cancer cachexia appears to be a logical extension of the importance of muscle for health maintenance during aging [11,12]. Skeletal muscle also has an established role for maintaining health during obesity, and in patients with many chronic diseases [11,13,14]. Nonetheless, the mechanistic explanation of how skeletal muscle conveys these health properties is still being revealed. Until recently, research examining cancer cachexia mechanisms in preclinical models placed significant emphasis on skeletal muscle mass as the primary outcome [15]. However, there are considerable gaps in our understanding of muscle biochemical and functional properties that have established roles in either systemic health or life quality. These health consequences are less often investigated. The reality is that skeletal muscle has properties extending beyond mass that can convey health benefits to the cancer patient. To this end, the response of skeletal muscle to increased use may also convey health benefits beyond mass. Skeletal muscle quality is a current method of classifying muscle, and is aligned with muscle’s metabolic capacity and substrate utilization flexibility [16]. Furthermore, skeletal muscle can serve an endocrine function through the secretion of myokines [17,18]. Disruption of the endocrine function or metabolic quality in cachectic muscle could impact health and survival, and also regulate the muscle’s microenvironment [19]. Muscle quality can also be expanded to encompass the regulation of muscle anabolic and catabolic processes, which can regulate metabolic and endocrine functions. The response of skeletal muscle to external stimuli such as inflammation, hormones, and contraction requires integrated cellular signaling pathways involving several organelles and structures. However, the involvement of muscle mitochondria in the regulation of both wasting and metabolic quality has become firmly established [20]. Consequently, the role of the mitochondria in the regulation of cancer-induced muscle wasting has received significant attention during the past several years [20,21].
Mitochondria content aligns with the oxidative metabolic capacity of a myofiber, and has been classically used to define a muscle phenotype, in conjunction with myosin expression and functional properties [22]. However, muscle oxidative metabolism is not a static property; mitochondrial content and function are altered by a host of stimuli, including increased and decreased use, systemic inflammation, and systemic hormonal signaling [23,24]. In addition to cellular ATP production, mitochondria function has an established role in intracellular processes regulating muscle apoptosis, autophagy, and protein turnover [25]. Oxidative metabolism has emerged as a biological target of cancer-induced muscle wasting, and mitochondria loss has been well characterized across many cachectic conditions [26–28]. Interestingly, as little as 5 years ago the discussion surrounding the regulation of cancer cachexia-induced muscle wasting often considered a somewhat narrow role for mitochondria that often aligned with energy flux and wasting susceptibility. A growing body of evidence has successfully expanded this discussion and linked mitochondria dysfunction to cellular processes regulating myofiber wasting [27]. The primary objective of this literature review is to examine the emerging role of skeletal muscle oxidative metabolism as a biological target of cancer cachexia and also as a cellular regulator of cancer-induced muscle wasting. While the review covers concepts from the fields of muscle biology and physiology, content related to wasting is specifically delimited to cancer research using preclinical models and human patients. A description of how cancer cachexia affects muscle mitochondria and oxidative metabolism will be described, but this topic has recently been reviewed [20]. The review will then discuss potential regulators of muscle mitochondria function during the progression of cancer cachexia. Specifically, inflammation, hypogonadism, and muscle use will be scrutinized for the regulation of cancer cachexia-induced mitochondrial changes. Lastly, altered skeletal muscle oxidative metabolism and mitochondrial function, as a regulator of muscle wasting, will be discussed.
2. Skeletal Muscle Oxidative Metabolism and Cancer Cachexia
2.1 Myofiber phenotype’s role in cancer-induced wasting susceptibility
Human skeletal muscle contains myofibers with a heterogeneous mix of oxidative and glycolytic metabolic capacities. The expression level of metabolic enzymes, and substrate storage involving glycogen and lipid abundance, also contribute to differential metabolic capacities. The plasticity of the muscle metabolic phenotype can be a function of innervation and use involving contraction and loading [29,30]. Furthermore, functional parameters related to fatigability and speed of contraction typically mirror the fibers’ metabolic capacity [22]. Sensitivity to many types of atrophic stimuli is also a function of metabolic phenotype. Decreased use (i.e. bed-rest, spaceflight) induces a more rapid atrophy in slow-oxidative muscle fibers than primarily glycolytic muscle [31], while glycolytic fibers demonstrate greater age-induced atrophy and hypoplasia [32]. The majority of studies with rodent cancer cachexia models demonstrate more hindlimb wasting in primarily glycolytic muscle when compared to primarily oxidative muscle [15,33,34]. However, the examination of myofiber cross-section, rather than whole muscle mass, has demonstrated decreases in both glycolytic and oxidative myofibers with cancer cachexia [35–37]. The duration of the cachexia in rodent studies may also be a factor in the wasting outcomes involving oxidative skeletal muscle. The ApcMin/+ mouse, which often undergoes cancer cachexia for several weeks, has consistently demonstrated decreased mass of the oxidative soleus muscle [38–40]. Besides wasting susceptibility, the oxidative soleus muscle has been reported to demonstrate more regeneration and/or necrosis when compared to more glycolytic muscle [40]. Further studies are needed to determine if cancer-induced fatigue and force production impairments are related to oxidative myofiber regeneration with cancer cachexia. The effect of oxidative metabolism on cachexia susceptibility may be linked to the heterogeneous population of mitochondria in red and white muscle [41], but additional research is needed to demonstrate this as a mechanism of glycolytic fiber wasting with cancer. Although clear evidence exists that muscle metabolic phenotype influences the response to many stimuli that alter muscle mass, during late stages of cachexia atrophy of both glycolytic and oxidative muscle has been reported [42]. Further study is needed to establish if decreased use related to inactivity plays a prominent role in oxidative muscle fiber wasting with cancer cachexia. It is also interesting to speculate if decreased muscle use, which corresponds with the sedentary behavior that accompanies cachexia can affect muscle sensitivity to systemic cachectic mediators, such as cytokines.
2.2 Muscle oxidative metabolism capacity and mitochondria content during cancer cachexia
Oxidative metabolism is central to skeletal muscle metabolic homeostasis [20,43] and frequently quantified by mitochondria content, mitochondria respiratory capacity, and the activity of enzymes involved in the Krebs cycle and the electron transport chain (Figure 1). Understanding skeletal muscle’s capacity for oxidative metabolism has been a cornerstone of muscle biology and physiology research for over 40 years [43]. During this time the scientific examination of muscle has extended beyond the classical descriptions of muscle phenotype, and has evolved into mechanistically understanding the regulation of metabolic plasticity related to muscle use, disease, and aging. Initial breakthroughs examining muscle oxidative metabolism established metabolic plasticity with increased and decreased muscle use, which were linked to the capacity for whole body oxygen consumption (fitness) and exercise endurance [23]. While it is established that muscle mitochondria content and function are increased by exercise [44], the mechanistic basis of this plasticity is still being investigated today. As clearly demonstrated by the extensive number of studies published during the past 15 years [43], understanding changes in muscle oxidative metabolism and mitochondria function have become a fundamental focus of aging research investigating sarcopenia, frailty, and quality of life in the elderly. Additionally, there is strong evidence that wasting diseases alter muscle oxidative metabolism [45].
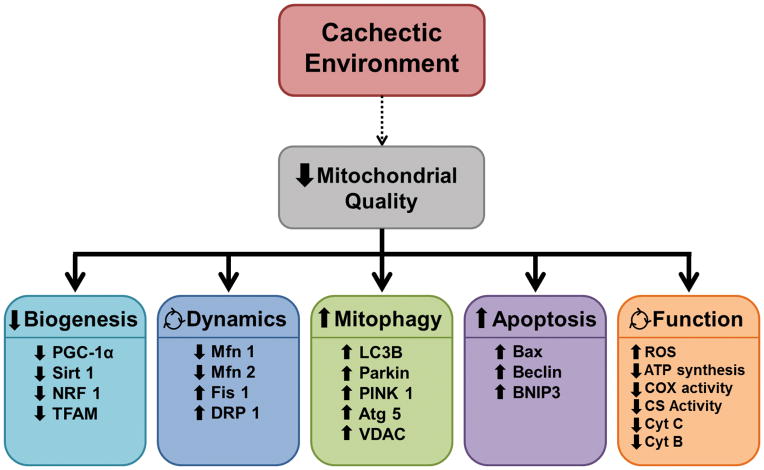
Figure 1. Cancer cachexia associated disruptions in muscle oxidative metabolism.
Mitochondrial biogenesis, dynamics, autophagy (mitophagy), apoptosis, and function are all used to measure mitochondrial quality in order to quantify muscle oxidative capacity. In the cachectic environment there is a decrease in mitochondrial biogenesis quantified by decreased peroxisome proliferator-activated receptor γ coactivator 1 alpha (PGC-1α), sirtuin 1 (Sirt1), nuclear respiratory factor 1 (NRF1), and mitochondria transcription factor A (TFAM). The cachectic environment has also been shown to alter mitochondrial dynamics by increasing mitochondrial fission proteins, fission 1 (Fis1) and dynamin-1-like protein (DRP 1) while decreasing mitochondrial fusion proteins, mitofusion 1 and 2 (Mfn1/2). There is a reduction in present mitochondrial content due to increased mitochondrial autophagy (mitophagy) and apoptosis seen by increased in all isoforms of light chain 3 (LC3), parkin, PTEN-putative kinase 1 (PINK 1), autophagy 5 (Atg 5), voltage-dependent anion channel (VDAC), bcl-2-associated X protein (Bax), beclin, and BCL2/adenovirus E1B 19 kd-interacting protein 3 (BNIP3). While mitochondrial function can be an effect of the previous 4 groups there is also evidence of a direct link to altered mitochondrial function by decreased ATP synthesis, cytochrome c oxidase (COX) activity, citrate synthase, protein and mRNA expression of cytochrome B and C (Cyt B and C), and increase reactive oxygen species (ROS).
The well-described disruption of skeletal muscle oxidative metabolism reported with many cachectic conditions involves muscle mitochondria loss [25–28,46]. Although red and white skeletal muscle differ dramatically in both mitochondria density and the importance of oxidative metabolism, cancer cachexia reduces mitochondrial content and oxidative protein expression in both muscle types in the mouse hindlimb [26]. The loss of muscle oxidative capacity in the later stages of cancer cachexia also corresponds with the development of severe insulin resistance in several rodent models [10,47]. Insulin sensitivity as well as lipid metabolism are impaired in cancer patients with recent weight loss [7]. However, in lung cancer patients exhibiting significant weight loss muscle oxidative capacity has been reported to be preserved [48]. Further study is needed to determine the relationship between muscle oxidative capacity declines and the specific type of cancer; lung cancer can promote rapid weight loss, which could differentially affect oxidative capacity when compared to less aggressive body weight loss. Interestingly in humans, cancer is often accompanied by aging and decreased muscle use, which are not normally accounted for in studies employing animal cancer cachexia models.
Since glycolytic muscle has been reported to be more sensitive to cachectic stimuli, there has been an interest in determining if muscle oxidative metabolism might inherently confer resistance to cancer cachexia. There is clear evidence that hindlimb myofibers from tumor bearing rodents undergo atrophy regardless of the succinate dehydrogenase activity level [26,36,37,49]. Regardless, mitochondria dysfunction is an established regulator of myofiber protein turnover [21,25], and gaps remain in our understanding of whether the fiber metabolic phenotype can produce differential regulation of cellular muscle wasting processes. Outside of the diaphragm muscle [50], load-bearing hindlimb muscle has been studied almost exclusively in highly glycolytic muscle. While heterogeneous populations of mitochondria in red and white muscle have been reported [41], differential responses of these subpopulations to cancer cachexia has not been clearly established. Further examination of myofiber oxidative metabolism and its interaction with cachectic stimuli is certainly warranted; there is likely a high prevalence of oxidative fibers in the aging cancer patient [12]. Additionally, the wasting of oxidative myofibers could be more dramatically impacted by decreased muscle use, which is documented with cancer cachexia in human and rodent studies [38,51–53]. Related to oxidative myofiber sensitivity to cachectic stimuli, the roles and potential regulatory interactions between decreased use, suppressed oxidative metabolism, and mitochondrial dysfunction need to be more firmly established.
An additional line of inquiry has examined if skeletal muscle’s metabolic plasticity can be exploited to prevent cancer-induced muscle wasting. Is there a therapeutic benefit of increasing muscle oxidative metabolism above basal levels? While successful outcomes related to the induction of oxidative capacity could involve muscle mass, blocking metabolic dysfunction could also serve to improve the patient’s survival. While there is encouraging evidence that exercise [47,53–55] and nutraceuticals [56] may be beneficial in conferring resistance to cachexia, the complexity of the systemic responses to these treatments complicates the mechanistic interpretation of the findings. These interventions cannot be directly linked to oxidative metabolism, as they target many systemic parameters and diverse muscle-signaling pathways. Additionally, many of these treatments regulate tumor growth and function that are involved in creating the cachectic milieu [46,54,55,57–59]. Intervention studies often measure tumor size, but this may not account for the tumor’s capacity to create a cachectic environment. A less examined paradigm with clinical significance is the restoration of mitochondrial content in cachectic muscle. Research is needed to establish the constraints, if any, that severe cachexia places on the metabolic plasticity of muscle.
2.3 Mitochondrial biogenesis during cancer cachexia
Myofiber mitochondrial content is subjected to regulation that allows responsiveness to the cellular environment. This cellular metabolic plasticity has been well studied and includes the coordinated processes of mitochondrial biogenesis, fission/fusion, and mitophagy. The disturbance of any of these processes can disrupt muscle metabolism [60]. Mitochondrial biogenesis is required for the maintenance of muscle mitochondria content and function, and the coordinated events essential for this process have been reviewed in detail elsewhere [61,62]. The peroxisome-proliferator gamma-activated receptor (PGC-1) family of co-activators has been described as the ‘master regulators’ of muscle oxidative metabolism. PGC-1α regulates mitochondrial biogenesis by nuclear translocation and activation of oxidative gene transcription [63], and was first identified for its role in brown adipose tissue adaptive thermogenesis [64]. PGC-1α loss reduces mouse muscle mitochondrial content and disrupts mitochondrial function [65], while PGC-1α over-expression increases muscle mitochondrial protein expression [66]. PGC-1α transcriptional control involves mitochondrial proteins, mitochondrial transcription factor A (Tfam), and nuclear respiratory factor-1 (NRF-1) and NRF-2 [62,63]. Several PGC-1 isoforms have also been identified. PGC-1β drives the specific expression of MHC IIX fibers, and is associated with an increase in oxidative phenotype [67]. The PGC-1α4 isoform regulates IGF-1 and myostatin signaling, and is associated with muscle hypertrophy rather than oxidative capacity [68]. There has been substantial interest in understanding if PGC-1α has a regulatory role in cancer-induced muscle wasting. There is strong evidence that wasting and metabolic dysfunction decrease muscle PGC-1α expression in humans and rodents [69–75]. Muscle PGC-1α expression is also decreased with cancer cachexia [26,47,51]. Numerous studies examining PGC-1α overexpression by either in vivo transfection or transgenic mice have found protection from skeletal muscle atrophy due to decreased use [76–79], starvation [78], and cytokine administration [80]. Fewer studies have examined the role of PGC-1α in preventing cancer cachexia induced muscle wasting. PGC-1α overexpression is not sufficient to block Lewis lung carcinoma (LLC)-induced muscle wasting [81]. However, the ability of cachectic muscle to restore mitochondria function after cachectic loss has not been determined. While our knowledge of PGC-1 co-activators continues to advance, it is apparent that PGC-1α controls multiple pathways that regulate mitochondrial content and function in skeletal muscle, and further work is needed to determine how this can both affect and benefit cachectic muscle.
The control of PGC-1α activity occurs through upstream mediators, and provides critical metabolic responsiveness to the cellular environment [43]. Sirtuin 1 (Sirt1) deacetylation of PGC-1α increases its activity, but the function of Sirt1 in cancer-induced muscle wasting has not been clearly established. Sirt1 mRNA expression in cachectic muscle has been reported to be a function of muscle phenotype, being reduced in the cachectic gastrocnemius mouse muscle, but not in the soleus [26]. Interestingly, both the cachectic gastrocnemius and soleus muscles have reduced muscle mitochondria content. AMP-activated protein kinase (AMPK) is a potent regulator of skeletal muscle metabolism, and can be activated by cellular energy status, calcium levels, and cytokine signaling [82,83]. AMPK can regulate mitochondrial content through PGC-1α-dependent mitochondrial biogenesis and ULK1-dependent stimulation of mitophagy [82]. Exercise and pharmacological agents can stimulate AMPK activation and PGC-1α expression in skeletal muscle [84–88]. AMPK can bind to and phosphorylate PGC-1α [86], thereby increasing the activity and transcription of this transcriptional co-activator. While the AMPK-PGC-1α axis can stimulate mitochondrial biogenesis, this signaling is disrupted in mouse models of cancer cachexia. Wasting skeletal muscle from ApcMin/+ mice exhibits chronically elevated AMPK activity, which does not translate to changes in either mitochondrial content or PGC-1α expression [26,47,51]. Interestingly, elevated AMPK is observed in skeletal muscle lacking PGC-1α [89]. Since interleukin-6 (IL-6) can stimulate AMPK and reduce PGC-1α expression in myotubes [47], further research is needed to determine if chronic AMPK activation is a result of circulating inflammatory cytokines or metabolic energy stress in cachectic muscle. Systemic IL-6 inhibition in ApcMin/+ mice can increase mitochondrial content, PGC-1α expression, and mitochondrial protein expression while attenuating the progression of cancer cachexia [47], but a direct regulatory effect has not been established. Understanding the disrupted relationship between AMPK, PGC-1α, and mitochondria biogenesis in cachectic muscle is needed to develop therapies to improve muscle metabolic quality and patient health.
The p38 mitogen-activated protein kinase (MAPK) family plays a critical role in skeletal muscle metabolism. The selective activation of p38 MAPK isoforms (α, β, and γ) can promote distinct cellular metabolic processes. Skeletal muscle p38β MAPK activation can regulate protein catabolism in cachectic muscle [90,91]. p38 MAPK signaling can also regulate muscle PGC-1α activity [92–94]. Activation of p38 MAPK signaling by voluntary exercise or transgenic overexpression can increase muscle PGC-1α gene expression [95]. However, the response to muscle contraction appears specific to p38γ MAPK, as the loss in p38α or p38β MAPK does not affect endurance exercise metabolic adaptation [96], while the loss of muscle p38γ suppresses contraction-induced PGC-1α gene expression [96]. The role of p38γ-PGC-1α regulation of mitochondrial biogenesis with increased contractile activity warrants further investigation to determine if it can be targeted in cachectic muscle.
2.4 Mitochondria dynamics during cancer cachexia
The maintenance of the myofiber mitochondrial network is critically important for adaptation to altered metabolic demands [97,98]. Mitochondrial dynamics involve the coordinated processes of fission and fusion, which can affect mitochondrial function (Figure 2) [99,100]. Mitochondrial fusion expands myofiber mitochondria networks, and fusion proteins 1 and 2 (Mfn1/2) and optic atrophy protein 1 (OPA1) are important regulators of the process [60]. In healthy individuals, mitochondrial fusion is associated with PGC-1α protein expression, citrate synthase activity, and mitochondrial creatine kinase [101]. A reduction in Mfn2 has been observed in muscle from type 2 diabetic [102,103] and obese patients [103]. During the progression of cachexia in mouse hindlimb muscle, Mfn1/2 protein expression is suppressed during the initial stages of cachexia [47], which suggests that altered fusion is an initial event in the cancer-induced disruption of muscle oxidative capacity. Mitochondrial fusion protein expression appears to be IL-6 sensitive. Systemic IL-6 over-expression in vivo decreases muscle mitofusion protein expression in ApcMin/+ mice, whereas IL-6 receptor (IL-6r) antibody administration increases Mfn2 expression in cachectic mouse muscle [47]. Direct effects of IL-6 on Mfn2 expression was shown, as the treatment of primary human muscle cultures by IL-6 reduced Mfn2 gene expression [103]. Mfn2 gene expression is regulated by PGC-1α and PGC-1β [104,105]. PGC-1α overexpression rescues Mfn1/2 expression during unloading-induced atrophy [77]. Extending our understanding of the mechanisms that suppress mitochondrial fusion during cancer cachexia, and determining if this process can serve as a therapeutic target to improve muscle metabolic function are warranted.
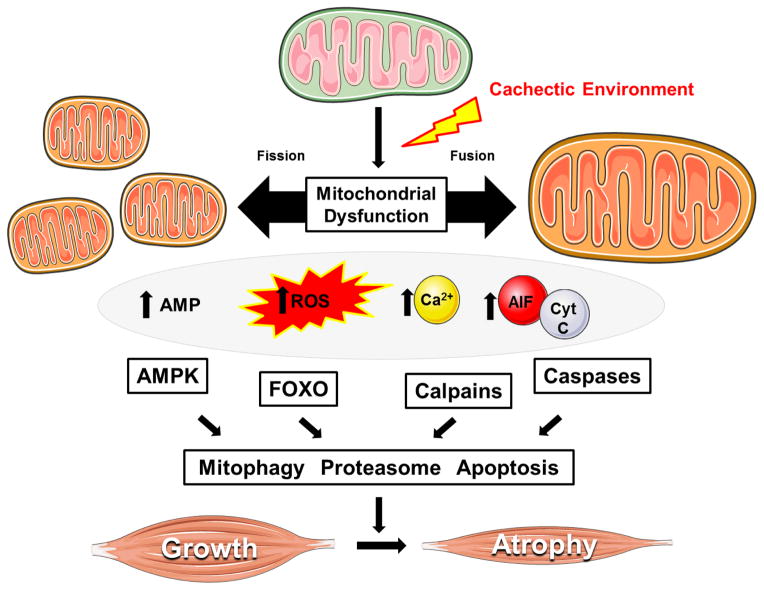
Figure 2. Mechanisms related to skeletal muscle mitochondria dysfunction that can regulate cancer-induced wasting.
The progression of cancer cachexia is associated the disruption of mitochondrial quality (i.e. biogenesis, dynamics, mitophagy), which can lead to the accumulation of dysfunctional mitochondria. Impaired mitochondrial function promotes energetic stress, ROS production, and the cytoplasmic localization of calcium and pro-apoptotic factors. Several key catabolic signaling pathways are activated leading to skeletal muscle atrophy, as well as further impairments in muscle oxidative metabolism. Abbreviations: Adenosine monophospate (AMP). 5′ adenosine monophosphate-activated protein kinase (AMPK). Apoptosis-inducing Factor (AIF). Cytochrome C (Cyt C). Forkhead Box O (FOXO). Figure was made with Servier Medical Art (http://www.servier.com/Powerpoint-image-bank).
Mitochondrial fission, the division of the organelle, is regulated through the expression of dynamin-related protein-1 (DRP1) and Fission protein 1 (Fis1) [60]. Increasing fission results in fragmented mitochondria, while a reduction in fission increases mitochondria networks [106]. Unlike fusion proteins, muscle Fis1 expression is not induced until the more severe stages of cachexia in mice [47]. Increased Fis1 expression in cachectic muscle is not affected by inherent oxidative capacity, being increased in both oxidative and glycolytic muscles [46]. Fis1 expression also appears to be IL-6 sensitive, increasing with systemic IL-6 over-expression in ApcMin/+ mice and decreasing with IL-6r antibody administration during the progression of cachexia [47]. These effects may be due to direct actions of IL-6 on the muscle, as IL-6-treated myotubes increase Fis1 protein expression [47]. Fis1 was also demonstrated to regulate the atrophic process of skeletal muscle [27]. Related to regulation of muscle mass, the over expression of Fis1 has been shown to be pro-apoptotic [107–109], associated with the production of reactive oxygen species [25], and is also capable of activating protein degradation [27]. Muscle apoptosis has been observed in human and rodent models of cancer cachexia [38,110–113]. In ApcMin/+ mice apoptosis was only observed in severely cachectic muscle [38], which coincides with Fis1 expression [26]. Mitochondria fission activates AMPK, which regulates FOXO3 independently of Akt activation [27]. Both denervation- and starvation–induced muscle atrophy activate AMPK, and the knockdown of either AMPK or FOXO can prevent mitochondrial dysfunction and atrophy [27]. Exercise also can affect muscle mitochondrial dynamics, by increasing fission and suppressing fusion [99,114,115]. However, in ApcMin/+ mice exercise attenuated the IL-6 induction of mitochondrial fission and FOXO [47]. While evidence suggest that the regulation of mitochondrial fission and fusion is able to control muscle wasting, as it relates to cancer cachexia, significantly less is understood about the role of mitochondria dynamics and its own regulation. Nonetheless, the restoration of mitochondrial dynamics in cachectic muscle may be a therapeutic target for improving overall function.
2.5 Mitophagy and cancer cachexia
Maintaining mitochondrial quality requires the removal of damaged mitochondria [60]. Autophagy is an essential cellular process for lysosomal-dependent degradation of organelles, and selective removal of damaged or dysfunctional mitochondria is known as mitophagy. This process is linked to mitochondrial dynamics [116]. The molecular components of the autophagy-lysosomal pathway involve several autophagy-related genes (Atgs) [117]. Deletion of Atg7 results in skeletal muscle atrophy, mitochondria abnormalities, and disorganization of sarcomeres [118]. The myofiber requires a coordinated balance between mitochondrial biogenesis and mitophagy, and a cancer-induced disruption in this balance could cause decreased mitochondrial content and the accumulation of dysfunctional mitochondria. Indeed, alterations in mitochondria morphology that indicate dysfunction (i.e., swelling, electron-lucent areas, vesicle-like structures) have been observed in cachectic skeletal muscle [36,119]. Activation of the autophagy-lysosomal system can be observed during the initial stages of weight loss [120–123], and muscle lysosomal enzyme activity has been correlated with weight loss in cancer patients [124]. Rodent cancer cachexia models also demonstrate increased muscle expression of autophagy and lysosomal proteins [46,117,125,126]. Muscle autophagy can be regulated by the FOXO family and mTOR signaling, which are established controllers of muscle mass [27,127]. In ApcMin/+ mice the expression of autophagy proteins is not increased until the muscle is severely cachectic [46]. Interestingly, in cachectic cancer patients there is evidence for the induction of muscle autophagy-lysosomal processes, while activation of the ubiquitin proteasome system in human cancer patients is more equivocal [122,123,128–130]. Also, the link between oxidative metabolism and autophagy is less clear in humans; a decrease in muscle oxidative metabolism has not been reported in some cachectic human cancer patients [48]. As it relates to physical activity, oxidative muscle fibers display higher levels of autophagy flux [131], and exercise can stimulate autophagy [131–133]. Further work is needed to determine if contraction is a stimulus that can restore cachectic muscle’s disrupted balance between mitochondria biogenesis and mitophagy.
2.6 Mitochondria uncoupling and ROS formation during cancer cachexia
Reactive oxygen species (ROS) are molecules that contain an oxygen free radical and can be produced in multiple cellular locations [134]. Superoxide is the most commonly-generated ROS, with hydrogen peroxide being a more stable derivative [134]. ROS are natural byproducts of biochemical reactions and can serve as cellular signaling molecules [135]. While only a small percentage of mitochondrial oxygen is converted to ROS [136,137], this production can increase in response to increased contractile activity, decreased use, or chronic disease [135]. While ROS has a role in cellular signaling during physiological conditions, aberrant ROS production can lead to muscle dysfunction through the oxidation of proteins, lipids, and DNA [138,139]. Muscle atrophy is associated with mitochondrial dysfunction and the production of reactive oxygen species (ROS) [135]. Glycolytic muscle appears to be more susceptible to oxidative stress [41]. Additionally, subsarcollema (SS) mitochondria can produce higher levels of ROS and are preferentially lost when compared to intermyofibrillar (IMF) mitochondria [140,141]. Inflammation can also affect muscle ROS production. Tumor necrosis factor (TNF) is a cytokine that has been associated with increased mitochondrial ROS production [142]. Evidence suggests that muscle oxidative stress is increased in some, but not all rodent models of cancer cachexia [42,110,143,144]. In addition, higher levels of ROS and oxidative stress has been reported in cachectic lung cancer patients [145], and LLC conditioned medium increases ROS production in C2C12 muscle cells [146]. However, determining the contribution of mitochondrial ROS production to the regulation of mechanisms controlling increased catabolic signaling in cachectic muscle warrants further examination.
Mitochondrial uncoupling proteins (UCPs) have been implicated in the control of energy metabolism during cancer cachexia, and may also play a role in the regulation of ROS production in skeletal muscle [147]. Mitochondrial UCPs are membrane proteins that mediate proton leakage and uncouple respiration to produce thermogenesis instead of ATP synthesis [5]. UCP-1 is expressed in adipose cells and has been implicated in ‘browning’ of white adipose tissue during cancer cachexia [148]. In contrast, UCP-2 and -3 have been associated with skeletal muscle wasting [5]. While some researchers have reported increased UCP-2 or UCP-3 expression in cachectic human and rodent skeletal muscle [149–155], others have reported reduced expression [26]. The differential responses observed may be related to the tumor model used, as well as the fasted state of the animal. Skeletal muscle UCP-2 and -3 gene expressions are induced in response to fasting conditions [156,157], which can vary between studies. However, when food intake has been controlled, muscle UCP-2 and -3 gene expression is increased in cachectic muscle [152,154]. Future research is needed to define the significance of muscle UCP expression and its relationship to mitochondrial dysfunction during the progression of cancer cachexia.
3. Cancer Cachexia-Induced Regulation of Skeletal Muscle Oxidative Metabolism
3.1 Cytokine mediated regulation of muscle oxidative metabolism
Systemic inflammation is recognized as a hallmark of cancer cachexia [8], and circulating cytokines are established initiators of the muscle wasting process (Figure 3) [38,158]. Changes in cytokines may be related to the tumor and/or the host response to the tumor [159]. The cellular signaling linking inflammatory mediators to disrupted protein turnover has been widely researched [8,15]. Specific to cancer cachexia, perturbations in cytokines such as IL-6, TNF, TNF-like weak inducer of apoptosis (TWEAK), and myostatin have been implicated in muscle wasting processes [8,160,161]. While often proposed, the evidence directly linking inflammation to cancer cachexia-induced mitochondrial dysfunction is only beginning to emerge [146].
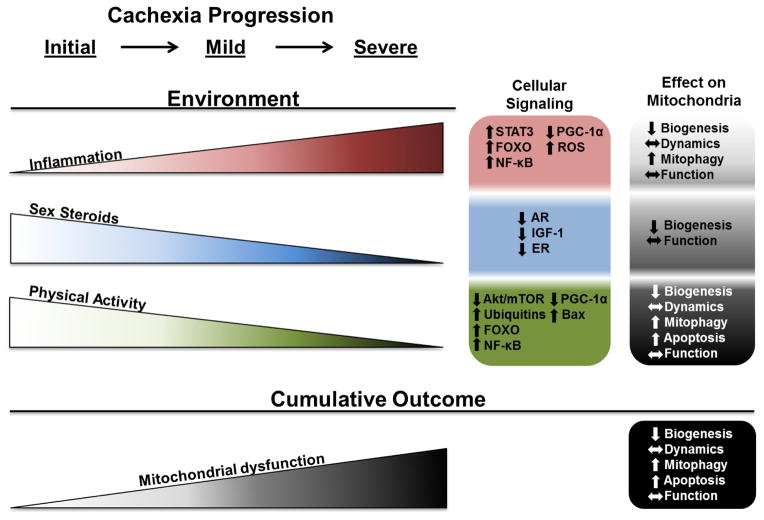
Figure 3. Cancer-induced cachectic environments and their relationship to skeletal muscle mitochondrial dysfunction during the progression of cancer cachexia.
Increases in inflammation and decreases in sex steroids and physical activity disrupt mitochondrial quality throughout the progression of cachexia. Inflammation through systemic interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), TNF-like weak inducer of apoptosis (TWEAK), and myostatin increase throughout cachexia progression result in the activation of STAT3, NF-κB, FOXO, and PGC-1α signaling as well as the generation of reactive oxygen species (ROS). These signaling pathways result in decreased biogenesis, altered dynamics, increased mitophagy, and altered function in cachectic muscle mitochondria. Sex steroids (testosterone and estrogen) and their respective nuclear receptors (androgen and estrogen receptors) decrease throughout cachexia progression. These can negatively regulate anabolic signaling related to insulin-like growth factor 1 (IGF-1). These signaling pathways result in decreased mitochondrial biogenesis and altered mitochondrial function. Physical activity decreases throughout cancer cachexia progression resulting in decreased signaling through Akt/mTOR, and PGC-1α, while increasing signaling through FOXO, NF-κB, Bax, and ubiquitins. Decreased physical activity results in decreased biogenesis, increased mitophagy and apoptosis, and altered mitochondrial dynamics and function. While these factors can work independently, the culmination of systemic factors and decreased use can negatively impact muscle oxidative capacity through the regulation mitochondrial biogenesis, dynamics, mitophagy, apoptosis and function.
IL-6 is a pleiotropic cytokine that has been implicated in the regulation of skeletal muscle metabolism and cachexia in both animal models and cancer patients [48,145,161–163]. IL-6 binding to its receptor complex can activate several intracellular signaling pathways including JAK/STAT signaling. Manipulating systemic IL-6 can affect the disruption of muscle oxidative metabolism during cancer cachexia, but these approaches have not provided direct evidence for the action of IL-6 signaling in myofibers. Systemic IL-6 overexpression disrupts muscle mitochondrial biogenesis and dynamics [47], while IL-6 receptor antibody administration attenuates mitochondrial loss and disrupted oxidative metabolism in cachectic skeletal muscle [26,47,164]. A role for mitochondrial STAT3 in basal cellular respiration has been described [165], and it has been proposed that the accumulation of mitochondrial STAT3 may alter ETC function and ROS production, and produce mitochondrial dysfunction [166]. A role for STAT3 in the disruption of skeletal muscle oxidative metabolism with cancer cachexia has yet to be established. The subcellular localization of STAT3 in non-muscle cells has been implicated in the regulation of autophagy [167], and nuclear STAT3 can promote or suppress target genes regulating autophagy. Interestingly, JAK/STAT3 also has been implicated in the nuclear/cytosolic shutting of FOXO-1 and -3 in CD4+ T cells [168]. While this has not been described in cachectic skeletal muscle, a better understanding of IL-6 and STAT3 regulation of mitophagy in cachectic muscle is warranted.
TNF is an inflammatory cytokine elevated in the circulation with certain cancers and some rodent cancer cachexia models [6,145]. TNF predominantly activates the canonical NF-κB signal transduction pathway, which has been implicated in muscle oxidative metabolism regulation [169]. The canonical NF-κB pathway involves the nuclear localization of the p65/p50 heterodimer complex. This occurs through the degradation of IκBα, which is regulated by IKKβ kinase activity [170]. Activation of the classical signaling pathway impairs mitochondrial biogenesis and oxidative capacity, and alters mitochondrial morphology [169]. In addition, NF-κB signaling has also been shown to suppress muscle mitochondrial gene expression, oxygen consumption, and ATP production [171,172]. Interestingly, non-canonical signaling can also result in mitochondrial NF-κB localization and suppression of mitochondrial gene expression [173]. While NF-κB signaling is activated in multiple rodent models of cancer cachexia [51,174,175], direct actions of this pathway regulating skeletal muscle oxidative metabolism during the progression of cancer cachexia remain to be established.
TWEAK, a member of the TNF superfamily, transduces intracellular signaling through the fibroblast growth factor-inducible 14 (Fn14) receptor [160]. Pathological conditions can increase muscle Fn14 expression to amplify TWEAK signaling [176]. TWEAK-Fn14 signaling is associated with muscle atrophy [177], and the regulation of skeletal muscle oxidative capacity [178,179]. TWEAK inhibits oxidative metabolism in skeletal muscle [178], possibly through the repression of PGC-1α and the activation of the NF-κB [80]. TWEAK loss causes enhanced skeletal muscle mitochondrial content and oxidative capacity [178], whereas TWEAK overexpression decreases mitochondrial density [80]. Interestingly, PGC-1α overexpression can prevent the induction of Fn14 expression during muscle atrophy [80]. Given the interrelationship between NF-κB, TWEAK signaling, and PGC-1α, strategies to target this interaction could have therapeutic potential for rescuing disrupted muscle oxidative metabolism due to cancer.
Myostatin, a transforming growth factor-β (TGF-β) superfamily member, is involved in the regulation of skeletal muscle growth and differentiation [180]. Myostatin binding to ActRIIB phosphorylates downstream effector Smad2/3, and results in the translocation of the Smad2/3 and Smad4 complex to the nucleus, where it regulates the transcriptional suppression of genes responsible for myogenesis [181]. Elevated circulating and muscle myostatin has been reported in tumor-bearing mice [182,183], and myostatin- associated signaling can disrupt protein turnover leading to muscle catabolism [180]. Myostatin has been identified as part of the C26 tumor secretome, and C26-induced cachexia in mice results in a significant reduction in mitochondria content [159]. Additionally, C26-conditioned media can increase ROS production and oxidative stress in C2C12 myotubes [159]. Similar to other cytokines, the direct evidence for myostatin regulation of muscle oxidative metabolism in cachectic muscle is still being established.
3.2 Hypogonadism regulation of muscle oxidative metabolism
Gonad function has a significant role in whole body homeostasis through the production of sex steroids. The capacity for this regulation changes throughout the lifespan, and can also be affected by disease, energy balance, and body composition [6,184,185]. Although hypogonadism is commonly mentioned as an environment associated with cachexia [1], significant gaps remain in our understanding of gonad dysfunction’s regulatory role in cancer-induced muscle wasting. While circulating sex steroids decline with hypogonadism in both the female and male, far more is known about hypogonadism as it relates to cachexia in the male condition. Hypogonadism can indirectly affect muscle wasting through the regulation of other environments related to anemia, insulin resistance, and inflammation, which are also targeted by cancer [184,186,187]. However, circulating sex steroids also have direct effects on many tissues, including muscle and bone [188]. There is clear evidence that estrogen and testosterone have regulatory functions related to skeletal muscle mass, metabolism, and ability to repair from injury [189–192]. Low testosterone in the male can decrease strength and muscle mass in the absence of disease [193,194], and many diseases, including cancer, can decrease circulating testosterone [6,184]. Estrogen deficiency can affect muscle mass retention in the aged female, decrease the ability to recover from atrophy, and adversely affect muscle metabolism and the regulation of protein turnover [189,192,195]. Additionally, since the median age of diagnosis of colon, lung and pancreatic cancers is over 70 years of age, the vast majority of women are post-menopausal when these potentially cachectic cancers are diagnosed [196,197]. Sex hormone therapy in males and females has been widely examined in adults for a range of health benefits, and also to determine the inherent health risks of the specific therapies [198–200]. Beyond effects in patients with conditions related to disease and gonad dysfunction, sex hormone replacement therapy in the aged female and male has been extensively examined [200,201]. The interaction of these therapies with aging has clinical significance for cancer patients, who can exhibit age-induced changes in gonad function at the time of cancer diagnosis [6,184]. Testosterone therapy has convincing effects on muscle mass and strength in both old and young males [202,203]. Estrogen replacement in animal models has demonstrated positive effects for muscle recovery from atrophy, and a growing body of evidence currently supports a role for hormone replacement in muscle mass retention in the post-menopausal female [200]. Beyond being a therapeutic target, further understanding hypogonadism’s mechanistic role in the disruption of muscle protein turnover and oxidative metabolism is certainly warranted, given the known effects of sex steroid loss on muscle, and also the evidence regarding gonadal function in cachectic cancer patients and animal models of cancer cachexia.
While decreased circulating testosterone has not been reported with all types of cancer, lowered total or bioavailable testosterone has been reported in male cancer patients, and also in cachectic cancer patients [6,186,187]. While not widely examined in rodent cancer models, decreased circulating testosterone accompanies the progression of cachexia in the ApcMin/+ mouse, and circulating testosterone levels are correlated with hindlimb muscle loss [204]. However, further work is needed to determine if this effect is related to the specific type of cachexia model or degree of cachexia, as tumor-bearing rats have been reported not to show decreases in circulating testosterone [205]. A complex response that involves muscle phenotype and sex is found when examining skeletal muscle responses to either increased or decreased testosterone levels. Rodent skeletal muscle associated with reproductive functions demonstrates extreme sex steroid sensitivity compared to locomotor hindlimb muscle [206,207]. Additionally, muscles within the rodent hindlimb appear to have different sensitivities to testosterone and estrogen [189,207,208]. Muscle sensitivity to sex hormone levels is affected by muscle androgen and estrogen receptor expression (AR and ER, respectively) [209]. Circulating hormone levels, muscle regeneration, muscle loading, and aging can all affect muscle AR expression [190,191,210,211]. Related to cancer cachexia, muscle AR expression is decreased in cachectic ApcMin/+ mouse muscle [204]. The time course of decreased circulating testosterone and muscle AR expression in male ApcMin/+ mice during the progression of cachexia corresponds to muscle mitochondria loss [47]. Interestingly, independent of disease, orchiectomy can cause a similar reduction in mouse muscle mitochondria content and oxidative metabolism [191,212].
Studies examining overexpression and loss of the AR and ER have established a role for sex hormone signaling in the regulation of muscle metabolism. Similar to overall hormone responsiveness, the studies to date reveal a complex regulation by sex steroid receptors that are affected by both muscle phenotype and sex. AR overexpression increases rat EDL muscle myoglobin expression and mitochondrial enzyme activity [213]. However, muscle fatigue resistance is increased with the global AR [214] or ERβ [215] deletion in a muscle- and sex-specific manner. Myofiber-specific AR loss can increase the percentage of type I oxidative fibers in the soleus muscle, but not the fast-glycolytic EDL muscle [207]. Estrogen signaling through the ERα and ERβ can regulate mitochondrial biogenesis and function through transcriptional regulation of NRF-1 and Tfam [216]. Additionally, ovarian function loss in mice is associated with muscle mitochondrial dysfunction, which is attenuated by estrogen replacement [217]. Emerging regulatory networks have demonstrated how muscle anabolic and catabolic signal transduction pathways are intertwined with mitochondrial function, and cellular sex hormone signaling also overlaps with these processes. Ovariectomy increases the activation of PPARα and lean body mass in rats [218,219], and muscle PPARδ expression, as well as PDK-4, UCP-2, and FOXO1 expressions, are suppressed [220]. In addition to the induction of mTOR signaling [191], testosterone administration can increase muscle PGC-1α and COXIV expression, while AR deletion suppresses their expression [221]. Related to muscle mass and use, muscle AR expression is associated with resistance training responsiveness in humans [222]. Further work is needed to establish if the hypogonadal state during cancer cachexia impedes muscle metabolic plasticity related to increased use and mechanical loading.
3.3 A role for decreased muscle use
Traditionally, cancer-induced environments related to anorexia, inflammation, insulin resistance, hypogonadism, and anemia have played an acknowledged role in the regulation of skeletal muscle wasting [1]. All of these environments have the potential to directly or indirectly regulate skeletal muscle oxidative metabolism. Weakness and fatigue are acknowledged outcomes of cachexia, and are discernable by measurements of decreased strength, oxygen consumption (VO2 max), and physical activity level in the cachectic patient [1]. Interestingly, physical inactivity and decreased muscle use have been well documented with cancer cachexia, but often they are characterized as an outcome of wasting, and not a contributor to the process. While resting energy expenditure has been shown to increase in cachectic cancer patients, total energy expenditure has been reported to decrease, coinciding with decreased physical activity level [52,223]. Rodent models of cancer-induced cachexia have shown a dramatic decrease in voluntary physical activity compared to healthy mice [51,58]. During the progression of cachexia in ApcMin/+ mice the decrease in physical activity precedes weight loss [38]. While physical inactivity and sedentary behavior are established causes of skeletal muscle metabolic dysfunction and atrophy [141,224–226], until recently cancer patient physical inactivity was not clearly acknowledged as a potential contributor to the cachectic muscle phenotype. There are clear gaps in understanding physical inactivity’s contribution to muscle wasting and metabolic dysfunction in the cachectic cancer patient that warrant further investigation.
While numerous and varied definitions have been used to characterize exercise and physical activity, the most physiologically relevant descriptions involve the performance of an activity that increases energy expenditure above the basal level [227]. Increasing energy expenditure might seem counterintuitive for preventing wasting disease; however, physical activity is often associated with an increase in lean body mass [228]. Additionally, a convincing body of research has demonstrated that the health benefits of regular physical activity extend far beyond energy expenditure [229]. In patients with metabolic syndromes, increased physical activity can regulate restoration of metabolic homeostasis [224,230]. Conversely, inactivity is an acknowledged risk factor for decreased health and the development of chronic metabolic disorders [231,232]. The physical activity-induced health benefits and the decrements related to inactivity can be directly related to skeletal muscle metabolic function. Increased physical activity can improve skeletal muscle oxidative metabolism through mitochondria function [233–235] and efficient substrate utilization involving fatty acid oxidation [236] and glucose transport [237]. Increased muscle use can shift muscle towards a more oxidative phenotype [43,238] without necessarily promoting muscle growth [239]. Improving mitochondrial content and function could therefore have significant ramifications on muscle metabolic homeostasis in cachectic skeletal muscle.
Endurance exercise-induced improvements in mitochondria function involve processes related to biogenesis, mitophagy, and mitochondrial dynamics [62,240], which can be disrupted in cachectic muscle [20,47,112]. Conversely, decreased physical activity results in altered signaling pathways leading to loss of skeletal muscle mass and decreased muscle oxidative metabolism [241–244]. PGC-1α is highly responsive to muscle contraction and has been extensively examined for the regulation of oxidative metabolism by physical activity [87,245]. Decreased skeletal muscle use suppresses PGC-1α expression [61,78] and coincides with decreased mitochondrial content and associated protein expression [226]. Additional signaling pathways sensitive to muscle use can regulate oxidative metabolism through PGC-1α, AMPK, FOXO and mTOR interactions [25,170,246]. All of these signaling pathways have demonstrated some degree of disruption in cachectic muscle [180]. While the role exercise on whole body oxidative metabolism is well established, less is known about the effect of muscle contraction on oxidative metabolism in the cachectic patient. However, initial investigations into exercise and muscle contraction during the progression of cachexia in the ApcMin/+ mouse have demonstrated positive outcomes related to the rescue of suppressed muscle anabolic signaling, mitochondrial content, and mitochondrial biogenesis [51,54]. There is a clear rationale for further investigation to determine if cachectic muscle maintains contraction and physical activity-induced metabolic plasticity.
In addition to exercise involving repeated contraction, skeletal muscle phenotype is extremely responsive to increased or decreased loading [247]. Skeletal muscle has well-developed networks that transduce loading conditions to intracellular signaling. A critical pathway in this network is integrin signaling [247]. Muscle sensitivity to stretch is a classic paradigm that demonstrates the potency of mechanical signaling for the induction of growth [248]. Altered mechanical signaling in muscle induced by increased or decreased load can affect both muscle mass and oxidative metabolism. Decreased loading in both humans and rodents induces muscle atrophy that is more rapid in oxidative myofibers [24,249,250]. Several components of load muscle sensitive signaling pathways are altered with cancer cachexia. Protein kinase B/Akt is highly responsive to muscle loading and unloading conditions. Akt can regulate both protein turnover and metabolic regulation in muscle [170,251–254]. Unloading-induced atrophy decreases Akt phosphorylation, which can affect mTOR and FOXO signaling [170]. As discussed earlier, in addition to FOXO and mTOR signaling’s well-described regulation of protein turnover [252], they also regulate mitochondria function and autophagy [253]. Unloading-induced muscle atrophy also activates NF-κB, which has regulatory roles involving both muscle protein degradation and oxidative metabolism [170]. Although mTOR, FOXO and NF-κB have well-described roles in cancer-induced wasting and disuse, the regulation of these pathways and their effectors may differ between conditions [170]. To this end, exercise and stretch can also activate muscle NF-κB signaling [255–257], in addition to activating mTOR by Akt independent signaling [258]. Further research is needed to determine the consequences of simultaneously activating disuse and cachectic signaling pathways in muscle. The effects of countering inactivity with increased muscle use, while the cachectic environment is present, also warrants additional study. Doing so will allow for an improved understanding of the common and stimulus-specific regulatory mechanisms involved in muscle wasting.
4. Conclusion
Muscle oxidative metabolism has an established role in metabolic health, which centers on mitochondria function. Beyond muscle metabolism and substrate utilization, mitochondria maintain skeletal muscle homeostasis through the regulation of protein turnover, autophagy, and apoptosis. As it relates specifically to cancer cachexia, the role of skeletal muscle oxidative metabolism in wasting has recently begun to emerge. In this review, we assessed the growing body of evidence that highlights muscle mitochondria and oxidative metabolism as a biological target of cancer cachexia. While muscle metabolic phenotype can influence the response to cachectic stimuli, both glycolytic and oxidative muscles waste in late stage cancer cachexia. However, further work is needed to establish if the rate of wasting and the susceptibility to cachectic stimuli are influenced by metabolic phenotype. Additionally, further research needs to be established if the mechanisms disrupting protein turnover are differentially regulated in oxidative and glycolytic muscle. While the loss of muscle mitochondrial content and a reduction in overall oxidative metabolic capacity are consistent findings in cachectic rodent muscle, skeletal muscle oxidative capacity changes in human cancer patients requires further investigation. Furthermore, it is unclear if elevating mitochondrial content is sufficient to prevent or reverse cancer-induced muscle wasting associated with impaired mitochondrial function. Next, evidence for the dysfunction of mitochondria related to critical wasting mechanisms in cachectic muscle was examined. There is evidence across different preclinical cancer models that skeletal muscle mitochondrial biogenesis, dynamics, mitophagy, and oxidative stress can all be disrupted in cachectic muscle. Further research will be required to clearly establish these mitochondrial alterations as potential biological targets for treating cancer cachexia. Furthermore, the cancer-induced systemic disruptions causing mitochondria dysfunction need to be better understood. Lastly, we examined how systemic alterations associated with cancer cachexia could impact the regulation of skeletal muscle oxidative metabolism. Current evidence suggests increased catabolic cytokines and decreased sex steroids accompany disrupted muscle oxidative metabolism during the progression of cachexia. Moreover, physical inactivity accompanies skeletal muscle wasting in cachectic rodents and human cancer patients. Related to decreased muscle use, it is not well understood if inactivity can interact with cytokine signaling to amplify the catabolic response in cachectic muscle. While muscle activity is a potent regulator of mitochondrial quality and function, further investigation is warranted to determine the response of cachectic skeletal muscle to increased use, and if increased activity leads to an improved anabolic state. Clearly defining the interactions between muscle use and systemic perturbations on mitochondrial oxidative metabolism will provide greater mechanistic insight to the drivers of cancer-induced muscle wasting. This understanding will have significant implications for future therapeutic treatments in the cachectic cancer patient.
Acknowledgments
This work was supported by National Institutes of Health grant # R01-CA121249 from the National Cancer Institute (JAC), National of Health grant # P20 RR-017698 from the National Center for Research (JAC), SPARC Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina (JPH), and an ACSM Foundation Research Grant from the American College of Sports Medicine Foundation (JPH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315(7117):1219–22. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, Cunningham GR. Hypogonadism in male patients with cancer. Cancer. 2006;106(12):2583–91. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 7.Dodesini AR, Benedini S, Terruzzi I, Sereni LP, Luzi L. Protein, glucose and lipid metabolism in the cancer cachexia: A preliminary report. Acta Oncol. 2007;46(1):118–20. doi: 10.1080/02841860600791491. [DOI] [PubMed] [Google Scholar]
- 8.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–62. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 9.Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8(4):321–7. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. 2011;1812(12):1601–6. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 12.Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–48. doi: 10.1016/S0070-2153(05)68005-2. [DOI] [PubMed] [Google Scholar]
- 13.Gelfi C, Vasso M, Cerretelli P. Diversity of human skeletal muscle in health and disease: contribution of proteomics. J Proteomics. 2011;74(6):774–95. doi: 10.1016/j.jprot.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Choi JW, Joo JI, Wang X, Choi DK, Oh TS, Yun JW. Changes in expression of skeletal muscle proteins between obesity-prone and obesity-resistant rats induced by a high-fat diet. J Proteome Res. 2011;10(3):1281–92. doi: 10.1021/pr101048q. [DOI] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 16.Chomentowski P, Coen PM, Radikova Z, Goodpaster BH, Toledo FG. Skeletal muscle mitochondria in insulin resistance: differences in intermyofibrillar versus subsarcolemmal subpopulations and relationship to metabolic flexibility. J Clin Endocrinol Metab. 2011;96(2):494–503. doi: 10.1210/jc.2010-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103(3):1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 19.He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas-Ahner J, Wang J, Bloomston M, Muscarella P, Nau P, Shah N, Butchbach ME, Ladner K, Adamo S, Rudnicki MA, Keller C, Coletti D, Montanaro F, Guttridge DC. NF-kappaB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest. 2013;123(11):4821–35. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argiles JM, Lopez-Soriano FJ, Busquets S. Muscle wasting in cancer: the role of mitochondria. Curr Opin Clin Nutr Metab Care. 2015;18(3):221–5. doi: 10.1097/MCO.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 21.Romanello V, Sandri M. Mitochondrial biogenesis and fragmentation as regulators of protein degradation in striated muscles. J Mol Cell Cardiol. 2013;55:64–72. doi: 10.1016/j.yjmcc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 23.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(4):831–8. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 24.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol (1985) 1990;68(1):1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Romanello V, Sandri M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr Hypertens Rep. 2010;12(6):433–9. doi: 10.1007/s11906-010-0157-8. [DOI] [PubMed] [Google Scholar]
- 26.White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R201–11. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29(10):1774–85. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol. 2007;170(2):599–608. doi: 10.2353/ajpath.2007.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–39. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin KM, Valdez V, Herrick RE, MacIntosh AM, Roy RR. Biochemical properties of overloaded fast-twitch skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(2):467–72. doi: 10.1152/jappl.1982.52.2.467. [DOI] [PubMed] [Google Scholar]
- 31.Booth FW, Criswell DS. Molecular events underlying skeletal muscle atrophy and the development of effective countermeasures. Int J Sports Med. 1997;18(Suppl 4):S265–9. doi: 10.1055/s-2007-972723. [DOI] [PubMed] [Google Scholar]
- 32.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47(3):B71–6. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Dis Model Mech. 2012;5(4):533–45. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114(3):370–8. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aulino P, Berardi E, Cardillo VM, Rizzuto E, Perniconi B, Ramina C, Padula F, Spugnini EP, Baldi A, Faiola F, Adamo S, Coletti D. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. doi: 10.1186/1471-2407-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontes-Oliveira CC, Busquets S, Toledo M, Penna F, Paz Aylwin M, Sirisi S, Silva AP, Orpi M, Garcia A, Sette A, Ines Genovese M, Olivan M, Lopez-Soriano FJ, Argiles JM. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta. 2013;1830(3):2770–8. doi: 10.1016/j.bbagen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Murphy KT, Struk A, Malcontenti-Wilson C, Christophi C, Lynch GS. Physiological characterization of a mouse model of cachexia in colorectal liver metastases. Am J Physiol Regul Integr Comp Physiol. 2013;304(10):R854–64. doi: 10.1152/ajpregu.00057.2013. [DOI] [PubMed] [Google Scholar]
- 38.Baltgalvis KA, Berger FG, Pena MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol. 2010;109(4):1155–61. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima M, Sato S, Enos RT, Baynes JW, Carson JA. Development of an UPLC mass spectrometry method for measurement of myofibrillar protein synthesis: application to analysis of murine muscles during cancer cachexia. Journal of applied physiology. 2013;114(6):824–8. doi: 10.1152/japplphysiol.01141.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J Appl Physiol. 2005;99(6):2379–87. doi: 10.1152/japplphysiol.00778.2005. [DOI] [PubMed] [Google Scholar]
- 41.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290(3):C844–51. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 42.Barreiro E, de la Puente B, Busquets S, Lopez-Soriano FJ, Gea J, Argiles JM. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005;579(7):1646–52. doi: 10.1016/j.febslet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2):162–84. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–82. [PubMed] [Google Scholar]
- 45.Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta. 2014;1840(4):1276–84. doi: 10.1016/j.bbagen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 46.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One. 2011;6(9):e24650. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle. 2012;2:14. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Op den Kamp CM, Gosker HR, Lagarde S, Tan DY, Snepvangers FJ, Dingemans AM, Langen RC, Schols AM. Preserved muscle oxidative metabolic phenotype in newly diagnosed non-small cell lung cancer cachexia. J Cachexia Sarcopenia Muscle. 2015;6(2):164–73. doi: 10.1002/jcsm.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardee JP, Mangum JE, Gao S, Sato S, Hetzler KL, Puppa MJ, Fix DK, Carson JA. Eccentric Contraction-Induced Myofiber Growth in Tumor-Bearing Mice. J Appl Physiol (1985) 2015 doi: 10.1152/japplphysiol.00416.2015. jap 00416 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R464–77. doi: 10.1152/ajpregu.00231.2013. [DOI] [PubMed] [Google Scholar]
- 51.Puppa MJ, Murphy EA, Fayad R, Hand GA, Carson JA. Cachectic skeletal muscle response to a novel bout of low-frequency stimulation. J Appl Physiol (1985) 2014;116(8):1078–87. doi: 10.1152/japplphysiol.01270.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90(5):996–1002. doi: 10.1038/sj.bjc.6601620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penna F, Busquets S, Pin F, Toledo M, Baccino FM, Lopez-Soriano FJ, Costelli P, Argiles JM. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle. 2011;2(2):95–104. doi: 10.1007/s13539-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc ( Min/+) mouse. J Cachexia Sarcopenia Muscle. 2012;3(2):117–37. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salomao EM, Toneto AT, Silva GO, Gomes-Marcondes MC. Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr Cancer. 2010;62(8):1095–104. doi: 10.1080/01635581.2010.492082. [DOI] [PubMed] [Google Scholar]
- 56.Velazquez KT, Enos RT, Narsale AA, Puppa MJ, Davis JM, Murphy EA, Carson JA. Quercetin supplementation attenuates the progression of cancer cachexia in ApcMin/+ mice. J Nutr. 2014;144(6):868–75. doi: 10.3945/jn.113.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104(4):1137–43. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 58.Toledo M, Penna F, Busquets S, Lopez-Soriano FJ, Argiles JM. Distinct behaviour of sorafenib in experimental cachexia-inducing tumours: the role of STAT3. PLoS One. 2014;9(12):e113931. doi: 10.1371/journal.pone.0113931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colbert LH, Mai V, Tooze JA, Perkins SN, Berrigan D, Hursting SD. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006;27(10):2103–7. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 60.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40(3):159–64. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol (1985) 2001;90(3):1137–57. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 62.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006;209(Pt 12):2265–75. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- 63.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 64.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 65.Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol. 2009;297(1):C217–25. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- 66.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 67.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5(1):35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–31. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282(21):15439–50. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 70.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 71.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skov V, Glintborg D, Knudsen S, Jensen T, Kruse TA, Tan Q, Brusgaard K, Beck-Nielsen H, Hojlund K. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes. 2007;56(9):2349–55. doi: 10.2337/db07-0275. [DOI] [PubMed] [Google Scholar]
- 73.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54(7):1926–33. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 74.Vescovo G, Ravara B, Gobbo V, Angelini A, Dalla Libera L. Skeletal muscle fibres synthesis in heart failure: role of PGC-1alpha, calcineurin and GH. Int J Cardiol. 2005;104(3):298–306. doi: 10.1016/j.ijcard.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 75.Zorzano A, Hernandez-Alvarez MI, Palacin M, Mingrone G. Alterations in the mitochondrial regulatory pathways constituted by the nuclear co-factors PGC-1alpha or PGC-1beta and mitofusin 2 in skeletal muscle in type 2 diabetes. Biochim Biophys Acta. 2010;1797(6–7):1028–33. doi: 10.1016/j.bbabio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol. 2014;592(Pt 20):4575–89. doi: 10.1113/jphysiol.2014.275545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593(8):1981–95. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103(44):16260–5. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vainshtein A, Desjardins EM, Armani A, Sandri M, Hood DA. PGC-1alpha modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle. 2015;5:9. doi: 10.1186/s13395-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hindi SM, Mishra V, Bhatnagar S, Tajrishi MM, Ogura Y, Yan Z, Burkly LC, Zheng TS, Kumar A. Regulatory circuitry of TWEAK-Fn14 system and PGC-1alpha in skeletal muscle atrophy program. FASEB J. 2014;28(3):1398–411. doi: 10.1096/fj.13-242123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Pickrell AM, Zimmers TA, Moraes CT. Increase in muscle mitochondrial biogenesis does not prevent muscle loss but increased tumor size in a mouse model of acute cancer-induced cachexia. PLoS One. 2012;7(3):e33426. doi: 10.1371/journal.pone.0033426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19(7):786–8. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- 85.Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One. 2008;3(10):e3614. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296(2):350–4. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 88.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99(25):15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–35. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Zhang G, Jin B, Li YP. C/EBPbeta mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J. 2011;30(20):4323–35. doi: 10.1038/emboj.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang G, Li YP. p38beta MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPbeta. Skelet Muscle. 2012;2(1):20. doi: 10.1186/2044-5040-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan M, Rhee J, St-Pierre J, Handschin C, Puigserver P, Lin J, Jaeger S, Erdjument-Bromage H, Tempst P, Spiegelman BM. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18(3):278–89. doi: 10.1101/gad.1152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci U S A. 2001;98(17):9713–8. doi: 10.1073/pnas.171184698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8(5):971–82. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 95.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280(20):19587–93. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 96.Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One. 2009;4(11):e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11(9):678–84. doi: 10.1038/embor.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan Z, Lira VA, Greene NP. Exercise Training-induced Regualtion of Mitochondrial Quality. Exerc Sport Sci Rev. 2012;49(3):159–64. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, Wen L, Liu S, Ji LL, Zhang Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800(3):250–6. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 100.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32(2):309–19. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garnier A, Fortin D, Zoll J, N′Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005;19(1):43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 102.Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33(3):645–51. doi: 10.2337/dc09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson H, Manco M, Calvani M, Castagneto M, Palacin M, Mingrone G, Zierath JR, Vidal H, Zorzano A. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54(9):2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 104.Liesa M, Borda-d’Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, Palacin M, Vidal-Puig A, Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS One. 2008;3(10):e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55(6):1783–91. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 106.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15(11):5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77(2):387–97. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- 109.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278(38):36373–9. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 110.Figueras M, Busquets S, Carbo N, Barreiro E, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2004;569(1–3):201–6. doi: 10.1016/j.febslet.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 111.Belizario JE, Lorite MJ, Tisdale MJ. Cleavage of caspases-1, -3, -6, -8 and -9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br J Cancer. 2001;84(8):1135–40. doi: 10.1054/bjoc.2001.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Antunes D, Padrao AI, Maciel E, Santinha D, Oliveira P, Vitorino R, Moreira-Goncalves D, Colaco B, Pires MJ, Nunes C, Santos LL, Amado F, Duarte JA, Domingues MR, Ferreira R. Molecular insights into mitochondrial dysfunction in cancer-related muscle wasting. Biochim Biophys Acta. 2014;1841(6):896–905. doi: 10.1016/j.bbalip.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KC, Argiles JM. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr. 2007;26(5):614–8. doi: 10.1016/j.clnu.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 114.Bo H, Zhang Y, Ji LL. Redefining the role of mitochondria in exercise: a dynamic remodeling. Ann N Y Acad Sci. 2010;1201:121–8. doi: 10.1111/j.1749-6632.2010.05618.x. [DOI] [PubMed] [Google Scholar]
- 115.Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567(Pt 1):349–58. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6(6):439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 117.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18(1):39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 118.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10(6):507–15. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Shum AM, Mahendradatta T, Taylor RJ, Painter AB, Moore MM, Tsoli M, Tan TC, Clarke SJ, Robertson GR, Polly P. Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting. Aging (Albany NY) 2012;4(2):133–43. doi: 10.18632/aging.100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jagoe RT, Redfern CP, Roberts RG, Gibson GJ, Goodship TH. Skeletal muscle mRNA levels for cathepsin B, but not components of the ubiquitin-proteasome pathway, are increased in patients with lung cancer referred for thoracotomy. Clin Sci (Lond) 2002;102(3):353–61. [PubMed] [Google Scholar]
- 121.Op den Kamp CM, Langen RC, Snepvangers FJ, de Theije CC, Schellekens JM, Laugs F, Dingemans AM, Schols AM. Nuclear transcription factor kappa B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr. 2013;98(3):738–48. doi: 10.3945/ajcn.113.058388. [DOI] [PubMed] [Google Scholar]
- 122.Tardif N, Klaude M, Lundell L, Thorell A, Rooyackers O. Autophagic-lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients. Am J Clin Nutr. 2013;98(6):1485–92. doi: 10.3945/ajcn.113.063859. [DOI] [PubMed] [Google Scholar]
- 123.Stephens NA, Skipworth RJ, Gallagher IJ, Greig CA, Guttridge DC, Ross JA, Fearon KC. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle. 2015;6(1):53–61. doi: 10.1002/jcsm.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schersten T, Lundholm K. Lysosomal enzyme activity in muscle tissue from patients with malignant tumor. Cancer. 1972;30(5):1246–51. doi: 10.1002/1097-0142(197211)30:5<1246::aid-cncr2820300516>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 125.McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298(3):C542–9. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer. 2010;126(3):756–63. doi: 10.1002/ijc.24784. [DOI] [PubMed] [Google Scholar]
- 127.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6(6):472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 128.Bossola M, Muscaritoli M, Costelli P, Bellantone R, Pacelli F, Busquets S, Argiles J, Lopez-Soriano FJ, Civello IM, Baccino FM, Rossi Fanelli F, Doglietto GB. Increased muscle ubiquitin mRNA levels in gastric cancer patients. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1518–23. doi: 10.1152/ajpregu.2001.280.5.R1518. [DOI] [PubMed] [Google Scholar]
- 129.Bossola M, Muscaritoli M, Costelli P, Grieco G, Bonelli G, Pacelli F, Rossi Fanelli F, Doglietto GB, Baccino FM. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237(3):384–9. doi: 10.1097/01.SLA.0000055225.96357.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stephens NA, Gallagher IJ, Rooyackers O, Skipworth RJ, Tan BH, Marstrand T, Ross JA, Guttridge DC, Lundell L, Fearon KC, Timmons JA. Using transcriptomics to identify and validate novel biomarkers of human skeletal muscle cancer cachexia. Genome Med. 2010;2(1):1. doi: 10.1186/gm122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27(10):4184–93. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He C, Sumpter R, Jr, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8(10):1548–51. doi: 10.4161/auto.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grumati P, Coletto L, Schiavinato A, Castagnaro S, Bertaggia E, Sandri M, Bonaldo P. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. 2011;7(12):1415–23. doi: 10.4161/auto.7.12.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95(1):1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab. 2012;303(1):E31–9. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29(1):89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 137.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 138.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441(2):523–40. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiol Oncol. 2014;48(3):247–56. doi: 10.2478/raon-2014-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol. 2005;289(4):C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- 141.Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol Respir Environ Exerc Physiol. 1980;48(1):23–8. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- 142.Hennet T, Richter C, Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289( Pt 2):587–92. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Padrao AI, Oliveira P, Vitorino R, Colaco B, Pires MJ, Marquez M, Castellanos E, Neuparth MJ, Teixeira C, Costa C, Moreira-Goncalves D, Cabral S, Duarte JA, Santos LL, Amado F, Ferreira R. Bladder cancer-induced skeletal muscle wasting: disclosing the role of mitochondria plasticity. Int J Biochem Cell Biol. 2013;45(7):1399–409. doi: 10.1016/j.biocel.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 144.Gomes-Marcondes MC, Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. 2002;180(1):69–74. doi: 10.1016/s0304-3835(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 145.Fortunati N, Manti R, Birocco N, Pugliese M, Brignardello E, Ciuffreda L, Catalano MG, Aragno M, Boccuzzi G. Pro-inflammatory cytokines and oxidative stress/antioxidant parameters characterize the bio-humoral profile of early cachexia in lung cancer patients. Oncol Rep. 2007;18(6):1521–7. [PubMed] [Google Scholar]
- 146.McLean JB, Moylan JS, Andrade FH. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front Physiol. 2014;5:503. doi: 10.3389/fphys.2014.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Toime LJ, Brand MD. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol Med. 2010;49(4):606–11. doi: 10.1016/j.freeradbiomed.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–47. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 149.Sanchis D, Busquets S, Alvarez B, Ricquier D, Lopez-Soriano FJ, Argiles JM. Skeletal muscle UCP2 and UCP3 gene expression in a rat cancer cachexia model. FEBS Lett. 1998;436(3):415–8. doi: 10.1016/s0014-5793(98)01178-8. [DOI] [PubMed] [Google Scholar]
- 150.Bing C, Brown M, King P, Collins P, Tisdale MJ, Williams G. Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res. 2000;60(9):2405–10. [PubMed] [Google Scholar]
- 151.Busquets S, Carbo N, Almendro V, Figueras M, Lopez-Soriano FJ, Argiles JM. Hyperlipemia: a role in regulating UCP3 gene expression in skeletal muscle during cancer cachexia? FEBS Lett. 2001;505(2):255–8. doi: 10.1016/s0014-5793(01)02815-0. [DOI] [PubMed] [Google Scholar]
- 152.Busquets S, Almendro V, Barreiro E, Figueras M, Argiles JM, Lopez-Soriano FJ. Activation of UCPs gene expression in skeletal muscle can be independent on both circulating fatty acids and food intake. Involvement of ROS in a model of mouse cancer cachexia. FEBS Lett. 2005;579(3):717–22. doi: 10.1016/j.febslet.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 153.Collins P, Bing C, McCulloch P, Williams G. Muscle UCP-3 mRNA levels are elevated in weight loss associated with gastrointestinal adenocarcinoma in humans. Br J Cancer. 2002;86(3):372–5. doi: 10.1038/sj.bjc.6600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Constantinou C, Fontes de Oliveira CC, Mintzopoulos D, Busquets S, He J, Kesarwani M, Mindrinos M, Rahme LG, Argiles JM, Tzika AA. Nuclear magnetic resonance in conjunction with functional genomics suggests mitochondrial dysfunction in a murine model of cancer cachexia. Int J Mol Med. 2011;27(1):15–24. doi: 10.3892/ijmm.2010.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tzika AA, Fontes-Oliveira CC, Shestov AA, Constantinou C, Psychogios N, Righi V, Mintzopoulos D, Busquets S, Lopez-Soriano FJ, Milot S, Lepine F, Mindrinos MN, Rahme LG, Argiles JM. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol. 2013;43(3):886–94. doi: 10.3892/ijo.2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Boss O, Samec S, Kuhne F, Bijlenga P, Assimacopoulos-Jeannet F, Seydoux J, Giacobino JP, Muzzin P. Uncoupling protein-3 expression in rodent skeletal muscle is modulated by food intake but not by changes in environmental temperature. J Biol Chem. 1998;273(1):5–8. doi: 10.1074/jbc.273.1.5. [DOI] [PubMed] [Google Scholar]
- 157.Boss O, Samec S, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Tissue-dependent upregulation of rat uncoupling protein-2 expression in response to fasting or cold. FEBS Lett. 1997;412(1):111–4. doi: 10.1016/s0014-5793(97)00755-2. [DOI] [PubMed] [Google Scholar]
- 158.Argiles JM, Busquets S, Toledo M, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):263–8. doi: 10.1097/SPC.0b013e3283311d09. [DOI] [PubMed] [Google Scholar]
- 159.Lokireddy S, Wijesoma IW, Bonala S, Wei M, Sze SK, McFarlane C, Kambadur R, Sharma M. Myostatin is a novel tumoral factor that induces cancer cachexia. Biochem J. 2012;446(1):23–36. doi: 10.1042/BJ20112024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Bhatnagar S, Kumar A. The TWEAK-Fn14 system: breaking the silence of cytokine-induced skeletal muscle wasting. Curr Mol Med. 2012;12(1):3–13. doi: 10.2174/156652412798376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38(4):168–76. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97(5):1211–6. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 163.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–48. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fujita J, Tsujinaka T, Yano M, Ebisui C, Saito H, Katsume A, Akamatsu K, Ohsugi Y, Shiozaki H, Monden M. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer. 1996;68(5):637–43. doi: 10.1002/(SICI)1097-0215(19961127)68:5<637::AID-IJC14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 165.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323(5915):793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, Ho MS, Nguyen HN, Friera AM, White KA, McLaughlin JR, Hansen D, Romero JM, Baltgalvis KA, Claypool MD, Li W, Lang W, Yam GC, Gelman MS, Ding R, Yung SL, Creger DP, Chen Y, Singh R, Smuder AJ, Wiggs MP, Kwon OS, Sollanek KJ, Powers SK, Masuda ES, Taylor VC, Payan DG, Kinoshita T, Kinsella TM. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J. 2014;28(7):2790–803. doi: 10.1096/fj.13-244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H, Han W. The role of STAT3 in autophagy. Autophagy. 2015;11(5):729–39. doi: 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oh HM, Yu CR, Dambuza I, Marrero B, Egwuagu CE. STAT3 protein interacts with Class O Forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4(+) T cells. J Biol Chem. 2012;287(36):30436–43. doi: 10.1074/jbc.M112.359661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Remels AH, Gosker HR, Bakker J, Guttridge DC, Schols AM, Langen RC. Regulation of skeletal muscle oxidative phenotype by classical NF-kappaB signalling. Biochim Biophys Acta. 2013;1832(8):1313–25. doi: 10.1016/j.bbadis.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 170.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33(2):155–65. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 171.Guseva NV, Taghiyev AF, Sturm MT, Rokhlin OW, Cohen MB. Tumor necrosis factor-related apoptosis-inducing ligand-mediated activation of mitochondria-associated nuclear factor-kappaB in prostatic carcinoma cell lines. Mol Cancer Res. 2004;2(10):574–84. [PubMed] [Google Scholar]
- 172.Johnson RF, Witzel II, Perkins ND. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-kappaB. Cancer Res. 2011;71(16):5588–97. doi: 10.1158/0008-5472.CAN-10-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cogswell PC, Kashatus DF, Keifer JA, Guttridge DC, Reuther JY, Bristow C, Roy S, Nicholson DW, Baldwin AS., Jr NF-kappa B and I kappa B alpha are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-kappa B. J Biol Chem. 2003;278(5):2963–8. doi: 10.1074/jbc.M209995200. [DOI] [PubMed] [Google Scholar]
- 174.Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8(5):421–32. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 175.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J. 2014;28(2):998–1009. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tajrishi MM, Zheng TS, Burkly LC, Kumar A. The TWEAK-Fn14 pathway: a potent regulator of skeletal muscle biology in health and disease. Cytokine Growth Factor Rev. 2014;25(2):215–25. doi: 10.1016/j.cytogfr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007;21(8):1857–69. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sato S, Ogura Y, Mishra V, Shin J, Bhatnagar S, Hill BG, Kumar A. TWEAK promotes exercise intolerance by decreasing skeletal muscle oxidative phosphorylation capacity. Skelet Muscle. 2013;3(1):18. doi: 10.1186/2044-5040-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sato S, Ogura Y, Tajrishi MM, Kumar A. Elevated levels of TWEAK in skeletal muscle promote visceral obesity, insulin resistance, and metabolic dysfunction. FASEB J. 2015;29(3):988–1002. doi: 10.1096/fj.14-260703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13(3):225–9. doi: 10.1097/mco.0b013e32833862df. [DOI] [PubMed] [Google Scholar]
- 181.Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2(3):143–51. doi: 10.1007/s13539-011-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 183.Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391(3):1548–54. doi: 10.1016/j.bbrc.2009.12.123. [DOI] [PubMed] [Google Scholar]
- 184.Del Fabbro E, Hui D, Nooruddin ZI, Dalal S, Dev R, Freer G, Roberts L, Palmer JL, Bruera E. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010;39(6):1016–24. doi: 10.1016/j.jpainsymman.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 185.Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol. 2003;149(2):145–52. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- 186.Burney BO, Garcia JM. Hypogonadism in male cancer patients. J Cachexia Sarcopenia Muscle. 2012;3(3):149–55. doi: 10.1007/s13539-012-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Burney BO, Hayes TG, Smiechowska J, Cardwell G, Papusha V, Bhargava P, Konda B, Auchus RJ, Garcia JM. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97(5):E700–9. doi: 10.1210/jc.2011-2387. [DOI] [PubMed] [Google Scholar]
- 188.Carson JA, Manolagas SC. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67–78. doi: 10.1016/j.bone.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol (1985) 2006;100(6):2012–23. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- 190.White JP, Baltgalvis KA, Sato S, Wilson LB, Carson JA. Effect of nandrolone decanoate administration on recovery from bupivacaine-induced muscle injury. J Appl Physiol (1985) 2009;107(5):1420–30. doi: 10.1152/japplphysiol.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365(2):174–86. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Spangenburg EE, Geiger PC, Leinwand LA, Lowe DA. Regulation of physiological and metabolic function of muscle by female sex steroids. Med Sci Sports Exerc. 2012;44(9):1653–62. doi: 10.1249/MSS.0b013e31825871fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81(12):4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 194.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83(6):1886–92. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 195.D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280(43):35983–91. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 196.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 197.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2012 2013. 2015 Aug 31; Available from: http://seer.cancer.gov/csr/1975_2012/
- 198.Bhasin S, Tenover JS. Age-associated sarcopenia--issues in the use of testosterone as an anabolic agent in older men. J Clin Endocrinol Metab. 1997;82(6):1659–60. doi: 10.1210/jcem.82.6.4061. [DOI] [PubMed] [Google Scholar]
- 199.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40(1):41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 200.Tiidus PM, Lowe DA, Brown M. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol (1985) 2013;115(5):569–78. doi: 10.1152/japplphysiol.00629.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bhasin S. The brave new world of function-promoting anabolic therapies: testosterone and frailty. J Clin Endocrinol Metab. 2010;95(2):509–11. doi: 10.1210/jc.2009-2550. [DOI] [PubMed] [Google Scholar]
- 202.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82(2):407–13. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 203.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM Task Force ES. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 204.White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open. 2013;2(12):1346–53. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Donatto FF, Neves RX, Rosa FO, Camargo RG, Ribeiro H, Matos-Neto EM, Seelaender M. Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour-bearing rats. Cytokine. 2013;61(2):426–32. doi: 10.1016/j.cyto.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 206.Antonio J, Wilson JD, George FW. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol (1985) 1999;87(6):2016–9. doi: 10.1152/jappl.1999.87.6.2016. [DOI] [PubMed] [Google Scholar]
- 207.Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P, Vanderschueren D. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150(8):3558–66. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- 208.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab. 2009;296(4):E854–61. doi: 10.1152/ajpendo.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69(10):1651–67. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Carson JA, Lee WJ, McClung J, Hand GA. Steroid receptor concentration in aged rat hindlimb muscle: effect of anabolic steroid administration. J Appl Physiol. 2002;93(1):242–50. doi: 10.1152/japplphysiol.01212.2001. [DOI] [PubMed] [Google Scholar]
- 211.Lee WJ, McClung J, Hand GA, Carson JA. Overload-induced androgen receptor expression in the aged rat hindlimb receiving nandrolone decanoate. J Appl Physiol. 2003;94(3):1153–61. doi: 10.1152/japplphysiol.00822.2002. [DOI] [PubMed] [Google Scholar]
- 212.Ibebunjo C, Eash JK, Li C, Ma Q, Glass DJ. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am J Physiol Endocrinol Metab. 2011;300(2):E327–40. doi: 10.1152/ajpendo.00402.2010. [DOI] [PubMed] [Google Scholar]
- 213.Niel L, Shah AH, Lewis GA, Mo K, Chatterjee D, Fernando SM, Hong MH, Chang WY, Vollmayr P, Rosen J, Miner JN, Monks DA. Sexual differentiation of the spinal nucleus of the bulbocavernosus is not mediated solely by androgen receptors in muscle fibers. Endocrinology. 2009;150(7):3207–13. doi: 10.1210/en.2008-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22(8):2676–89. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 215.Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2004;287(6):E1125–31. doi: 10.1152/ajpendo.00098.2004. [DOI] [PubMed] [Google Scholar]
- 216.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105(6):1342–51. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cavalcanti-de-Albuquerque JP, Salvador IC, Martins EL, Jardim-Messeder D, Werneck-de-Castro JP, Galina A, Carvalho DP. Role of estrogen on skeletal muscle mitochondrial function in ovariectomized rats: a time course study in different fiber types. J Appl Physiol (1985) 2014;116(7):779–89. doi: 10.1152/japplphysiol.00121.2013. [DOI] [PubMed] [Google Scholar]
- 218.Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, Thommesen L, Reseland JE. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stunes AK, Westbroek I, Gustafsson BI, Fossmark R, Waarsing JH, Eriksen EF, Petzold C, Reseland JE, Syversen U. The peroxisome proliferator-activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC Endocr Disord. 2011;11:11. doi: 10.1186/1472-6823-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Loss of ovarian function in mice results in abrogated skeletal muscle PPARdelta and FoxO1-mediated gene expression. Biochem Biophys Res Commun. 2010;392(1):1–3. doi: 10.1016/j.bbrc.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Usui T, Kajita K, Kajita T, Mori I, Hanamoto T, Ikeda T, Okada H, Taguchi K, Kitada Y, Morita H, Sasaki T, Kitamura T, Sato T, Kojima I, Ishizuka T. Elevated mitochondrial biogenesis in skeletal muscle is associated with testosterone-induced body weight loss in male mice. FEBS Lett. 2014;588(10):1935–41. doi: 10.1016/j.febslet.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 222.Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8(10):e78636. doi: 10.1371/journal.pone.0078636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275(2 Pt 1):E249–58. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 224.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics. 2007;28(2):146–57. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 226.Eisenberg HA, Hood DA. Blood flow, mitochondria, and performance in skeletal muscle after denervation and reinnervation. J Appl Physiol (1985) 1994;76(2):859–66. doi: 10.1152/jappl.1994.76.2.859. [DOI] [PubMed] [Google Scholar]
- 227.Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–51. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- 228.Lubkowska A, Dudzinska W, Bryczkowska I, Dolegowska B. Body Composition, Lipid Profile, Adipokine Concentration, and Antioxidant Capacity Changes during Interventions to Treat Overweight with Exercise Programme and Whole-Body Cryostimulation. Oxid Med Cell Longev. 2015;2015:803197. doi: 10.1155/2015/803197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hawley JA, Maughan RJ, Hargreaves M. Exercise Metabolism: Historical Perspective. Cell Metab. 2015;22(1):12–7. doi: 10.1016/j.cmet.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 230.Baskin KK, Winders BR, Olson EN. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015;21(2):237–48. doi: 10.1016/j.cmet.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Booth FW, Hawley JA. The erosion of physical activity in Western societies: an economic death march. Diabetologia. 2015;58(8):1730–4. doi: 10.1007/s00125-015-3617-5. [DOI] [PubMed] [Google Scholar]
- 232.Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22(1):4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 233.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–91. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 234.Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001;79(5):386–92. [PubMed] [Google Scholar]
- 235.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–40. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272(20):13255–61. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 237.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47(8):1369–73. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 238.Salmons S, Vrbova G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969;201(3):535–49. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4(2):94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- 240.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281(6):E1340–6. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 241.Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol (1985) 1990;69(1):58–66. doi: 10.1152/jappl.1990.69.1.58. [DOI] [PubMed] [Google Scholar]
- 242.Simard C, Lacaille M, Vallieres J. Enzymatic adaptations to suspension hypokinesia in skeletal muscle of young and old rats. Mech Ageing Dev. 1985;33(1):1–9. doi: 10.1016/0047-6374(85)90104-6. [DOI] [PubMed] [Google Scholar]
- 243.Pierno S, Desaphy JF, Liantonio A, De Bellis M, Bianco G, De Luca A, Frigeri A, Nicchia GP, Svelto M, Leoty C, George AL, Jr, Camerino DC. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain. 2002;125(Pt 7):1510–21. doi: 10.1093/brain/awf162. [DOI] [PubMed] [Google Scholar]
- 244.Remels AH, Pansters NA, Gosker HR, Schols AM, Langen RC. Activation of alternative NF-kappaB signaling during recovery of disuse-induced loss of muscle oxidative phenotype. Am J Physiol Endocrinol Metab. 2014;306(6):E615–26. doi: 10.1152/ajpendo.00452.2013. [DOI] [PubMed] [Google Scholar]
- 245.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546(Pt 3):851–8. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281(37):27643–52. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 247.Carson JA, Wei L. Integrin signaling’s potential for mediating gene expression in hypertrophying skeletal muscle. J Appl Physiol. 2000;88(1):337–43. doi: 10.1152/jappl.2000.88.1.337. [DOI] [PubMed] [Google Scholar]
- 248.Carson JA. The regulation of gene expression in hypertrophying skeletal muscle. Exerc Sport Sci Rev. 1997;25:301–20. [PubMed] [Google Scholar]
- 249.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol. 2004;557(Pt 2):501–13. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol (1985) 1987;63(2):558–63. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 251.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 252.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 253.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–70. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 254.Dupont E, Cieniewski-Bernard C, Bastide B, Stevens L. Electrostimulation during hindlimb unloading modulates PI3K-AKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R408–17. doi: 10.1152/ajpregu.00793.2009. [DOI] [PubMed] [Google Scholar]
- 255.Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34(4):745–53. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- 256.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol (1985) 2007;103(1):388–95. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- 257.Gao S, Carson JA. Lewis Lung Carcinoma Regulation of Mechanical Stretch-Induced Protein Synthesis in Cultured Myotubes. Am J Physiol Cell Physiol. 2015 doi: 10.1152/ajpcell.00052.2015. ajpcell 00052 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006;97(6):1207–16. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]