Abstract
Purpose
Breast cancer stem-like cells (CSCs) are an important therapeutic target as they are predicted to be responsible for tumour initiation, maintenance and metastases. Interleukin-8 (IL-8) is upregulated in breast cancer and associated with poor prognosis. Breast cancer cell line studies indicate that IL-8 via its cognate receptors, CXCR1 and CXCR2, is important in regulating breast CSC activity. We investigated the role of IL-8 in the regulation of CSC activity using patient-derived breast cancers and determined the potential benefit of combining CXCR1/2 inhibition with HER2-targeted therapy.
Experimental design
CSC activity of metastatic and invasive human breast cancers (n=19) was assessed ex vivo using the mammosphere colony forming assay.
Results
Metastatic fluid IL-8 level correlated directly with mammosphere formation (r=0.652; P<0.05; n=10). Recombinant IL-8 directly increased mammosphere formation/self-renewal in metastatic and invasive breast cancers (n=17). IL-8 induced activation of EGFR/HER2 and downstream signalling pathways and effects were abrogated by inhibition of SRC, EGFR/HER2, PI3K or MEK. Furthermore, lapatinib inhibited the mammosphere-promoting effect of IL-8 in both HER2-positive and negative patient-derived cancers. CXCR1/2 inhibition also blocked the effect of IL-8 on mammosphere formation and added to the efficacy of lapatinib in HER2-positive cancers.
Conclusions
These studies establish a role for IL-8 in the regulation of patient-derived breast CSC activity and demonstrate that IL-8/CXCR1/2 signalling is partly mediated via a novel SRC and EGFR/HER2-dependent pathway. Combining CXCR1/2 inhibitors with current HER2-targeted therapies has potential as an effective therapeutic strategy to reduce CSC activity in breast cancer and improve the survival of HER2-positive patients.
Keywords: Breast Cancer, Cancer Stem Cell, HER2, IL-8, CXCR1
INTRODUCTION
Breast cancer stem-like cells (CSCs) are responsible for tumour initiation, maintenance and metastasis. They are defined functionally by their capacity to initiate a tumour in immunocompromised mice, to self-renew giving rise to a new tumour when passaged, and their ability to differentiate into non-self renewing cells which constitute the bulk of the tumour (1). By evading the effects of radiotherapy, chemotherapy and endocrine therapy, breast CSCs are predicted to be responsible for disease recurrence (2-5).
Primary human breast cancers and breast cancer cell lines contain a sub-population of cells characterised by their capacity to survive anoikis in non-adherent conditions and form floating colonies known as mammospheres (6-8). By demonstrating enhanced tumour forming capacity in vivo, we have shown that anoikis resistant cells represent a breast CSC-enriched population (9). Hence the mammosphere culture system can be utilised to investigate factors which regulate CSC activity and assess the efficacy of novel therapeutic agents.
Up to 25% of breast cancers over-express human epidermal growth factor receptor 2 (HER2) which confers a higher rate of recurrence and mortality (10). Studies suggest HER2 over-expression increases CSC self-renewal and invasion (11). Trastuzumab (Herceptin) has improved the survival of HER2 positive patients, possibly due to its ability to reduce the CSC population (11-13). Despite the success of trastuzumab, some HER2 positive breast cancers are resistant to treatment resulting in incurable metastatic disease (14, 15). This has led to the development of agents such as lapatinib, a dual EGFR/HER2 tyrosine kinase inhibitor. Neoadjuvant lapatinib has been shown to inhibit breast CSC activity in HER2 positive tumours, and lapatinib in combination with chemotherapy reduces time to progression of trastuzumab-resistant patients (3, 16).
Although HER2 is an important regulator of CSC self-renewal, factors within the tumour microenvironment are also important and consequently represent novel therapeutic targets. Targeting these factors in combination with HER2 could reduce disease recurrence and improve survival. Inflammation is an established hallmark of cancer and multiple cytokine networks may be important in regulating breast CSCs activity through paracrine and autocrine routes. Interleukin-8 (IL-8), which is known to be upregulated in breast cancer and associated with poor prognosis, has been shown to increase CSC self-renewal in cell line models in vitro (17-19). IL-8 signals via two cell surface G protein-coupled receptors, CXCR1 and CXCR2. Inhibition of these receptors was recently shown to reduce the CSC population, self-renewal and increase the efficacy of docetaxel in reducing tumour size in xenografts (20).
We determined the role of IL-8 in the regulation of breast CSC activity using patient-derived breast cancer cells isolated directly from metastatic ascites and pleural effusions and primary invasive cancers. IL-8 concentrations in metastatic fluid directly correlated with mammosphere formation and IL-8 activation of CXCR1/2 increased patient-derived mammosphere formation and self-renewal ex vivo. CXCR1/2 inhibition decreased mammosphere formation and aldefluor-positivity and added to the efficacy of inhibiting HER2 in HER2 positive cancers. A novel pathway involving transactivation of EGFR/HER2 was mechanistically responsible for the effect of CXCR1/2 activation on CSC activity. Given the importance of HER2 in the regulation of breast CSC activity (11, 12), a pathway driving the activation of this receptor via CXCR1/2 may have important biological and therapeutic implications, especially in tumours which express high levels of IL-8 and other CXCR1/2 activating ligands.
MATERIALS AND METHODS
Patient-derived breast cancers
Nineteen patient-derived breast cancer samples are summarised in Table 1. Ethical approval was granted by the Central Office for Research Ethics Committee (study numbers: 05/Q1402/25 and 05/Q1403/159) and patients gave written informed consent.
Table 1. Summary of metastatic and invasive patient-derived cancers.
Nineteen patient-derived breast cancer samples were used in this study. Primary invasive cancer samples are highlighted in grey. Tumour histology and grade for metastatic samples (Asc and PE) relates to the primary cancer. Samples with hyphenated numbers (e.g. BB3RC46-1 and BB3RC46-2) were obtained from the same patient at different time points. Metastatic fluid IL-8 levels were measured following removal of cellular matter using an ELISA. Asc ascites, B bone, Br brain, CT chemotherapy, duod duodenum, ER oestrogen receptor, ET endocrine therapy, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, Lv liver, Lu Lung, MFE mammosphere forming efficiency, NA not applicable, Neg negative, Neo neoadjuvant, NK not known, Panc pancreas, PE pleural effusion, Pos positive, PR progesterone receptor, T Traztuzumab, UA unavailable.
| Sample | Sample origin |
Tumour histology |
Grade | ER status |
PR status |
HER2 status |
Sites of metastases |
Medical treatment |
MFE (%) |
Metastatic fluid IL-8 level (pg/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| BB3RC36-1 | PE | IDC | 2 | Pos | Pos | Neg | Li and Lu | ET | 1.73 | UA |
| BB3RC36-2 | PE | IDC | 2 | Pos | Pos | Neg | Li and Lu | ET | 1.43 | UA |
| BB3RC38 | Asc | IDC | 3 | Neg | Neg | Neg | B, Lv and Lu | CT | 0.47 | 42.6 |
| BB3RC39 | PE | IDC | 3 | Neg | Neg | Neg | B, Br and Li | CT | 1.95 | 201.1 |
| BB3RC40 | Asc | IDC | 3 | Pos | Pos | Neg | Li | CT, ET | 0.25 | UA |
| BB3RC41 | Asc | IDC | 3 | Pos | Pos | Neg | Li | CT, ET | 1.20 | 135.9 |
| BB3RC42 | PE | IDC | 3 | Pos | Neg | Neg | Lu | CT, ET | 0.27 | 25.2 |
| BB3RC43-1 | Asc | IDC | 3 | Pos | Pos | Pos | Lv and Lu | CT, ET, T | 0.98 | 106.8 |
| BB3RC43-2 | Asc | IDC | 3 | Pos | Pos | Pos | Lv and Lu | CT, ET, T | 0.81 | UA |
| BB3RC43-3 | Asc | IDC | 3 | Pos | Pos | Pos | Li | CT, ET, T | 0.57 | UA |
| BB3RC47 | Asc | IDC | 3 | Pos | Neg | Neg | Li | CT, ET | 0.45 | 54.0 |
| BB3RC48 | Asc | ILC | 2 | Pos | Pos | Neg | Li and Lu | ET | 1.32 | 38.2 |
| BB3RC51 | PE | IDC | 2 | Pos | Pos | Neg | Panc and duod | CT, ET | 1.50 | 94.3 |
| BB3RC53 | Asc | IDC | 2 | Pos | Pos | NK | B, Li | CT, ET | 1.0 | 50.1 |
| BB3RC54 | PE | IDC | 3 | Pos | Pos | NK | B, Li, Lu | CT, ET | 1.51 | 45.1 |
| BB2RC12 | IDC | IDC | 3 | Pos | Neg | Pos | None | Neo CT and T |
0.98 | NA |
| BB2RC13 | IDC | IDC | 3 | Neg | Neg | Neg | None | None | 2.71 | NA |
| BB2RC14 | IDC | IDC | 3 | Neg | Neg | Pos | None | Neo CT and T |
0.30 | NA |
| BB2RC15 | IDC | IDC | 3 | Neg | Neg | Pos | None | Neo CT and T |
1.95 | NA |
Metastatic fluid samples
Fifteen metastatic fluid samples were obtained from 12 patients undergoing palliative drainage of symptomatic ascites or pleural effusions at The Christie, Manchester. Of these, 2 patients provided 2 and 3 samples, respectively. Where more than one fluid sample was obtained from the same patient, these were collected on different days. Oestrogen, progesterone and HER2 receptor status of the primary tumours were reported by the Department of Pathology at The Christie according to established criteria (21, 22). HER2 receptor status was unknown for two patients as they presented prior to the implementation of routine HER2 testing. Eleven patients were treated with multiple cycles of chemotherapy, with or without endocrine therapy and trastuzumab depending on receptor status. One patient presented with advanced metastatic disease and had not received any prior treatment. Thirteen metastatic samples were obtained from patients with ER positive primary invasive cancers and three samples were obtained from a patient with a HER2 positive primary invasive cancer.
Invasive breast cancer samples
Four invasive breast cancer samples were obtained from patients undergoing surgery for invasive ductal carcinoma. Three tumours were HER2 positive; these patients were all treated with neoadjuvant trastuzumab and chemotherapy prior to surgery due to locally advanced disease.
Isolation of breast cancer epithelial cells
Metastatic breast cancer cells were harvested as previously described with minor modifications (9) Invasive breast cancer tissue (1-2cm3) was collected, dissected into 1-2mm3 cubes and digested in media comprising DMEM, 15mM HEPES, 50μg/ml penicillin/streptomycin (Invitrogen) and 10% collagenase/hyaluronidase (Stem Cell Technologies) at 37°C for 16 hours. Digested tissue was filtered sequentially through 100μm, 70μm and 40μm sieves. Red blood cells were removed using Lymphoprep (Axis-Shield) and leukocytes were removed with CD45-negative magnetic sorting according to the manufacturer’s instructions (Miltenyi Biotech).
Mammosphere culture and self-renewal assay
Mammosphere culture was performed as previously described (9). A detailed description of the mammosphere assay protocol for the quantification of breast stem cell activity is described in our recent publication (23). Single cells were plated at 500cells/cm2 and the following treatments were added to the culture media: 100ng/ml recombinant IL-8 (R&D Systems); 100nM SCH563705 (CXCR1/2 inhibitor; Merck, USA) or 1μM lapatinib (GSK, UK). Control cells were treated with 0.01% bovine serum albumin [BSA (Fisher Scientific)] in phosphate buffered saline (PBS) w/v (for IL-8 treatment) and 0.01% dimethyl sulfoxide, DMSO (for SCH563705 and lapatinib treatments). Mammosphere forming efficiency (MFE) was calculated by dividing the number of mammospheres (colonies >60μm in diameter) formed by the number of cells plated and expressed as a percentage. Each experiment was performed in triplicate. To assess self-renewal, mammospheres were counted on day 7, centrifugated (300g), and dissociated into a single cell suspension by incubation for 2 minutes at 37°C in trypsin-EDTA 0.125% (Sigma), followed by mechanical dissociation as described previously (6). Single cells were re-plated at 500cells/cm2 and the number of secondary mammospheres counted after 7 days. Mammosphere self-renewal was calculated by dividing the number of secondary mammospheres formed by the number of primary mammospheres formed. To test clonality, single cells were plated in individual wells and secondary or tertiary mammosphere colonies photographed at 7 (MCF7/HER2-18; Supplementary Figure 1A and B) or 10 days (patient-derived samples; Figure 1E and F).
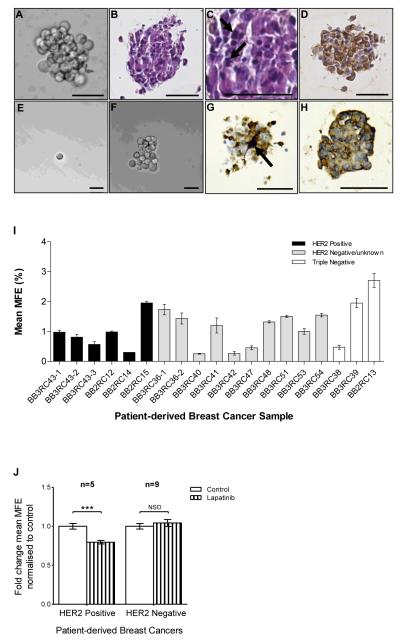
Figure 1. Mammosphere formation from patient-derived breast cancers and effects of EGFR/HER2 inhibition.
A Typical bright-field photomicrograph of a mammosphere (>60μm in diameter) grown from single cells plated at 500 cells per cm2 after 7 days in non-adherent culture. B-D Photomicrographs of 3μm sections of a FFPE patient-derived mammosphere showing B) H&E staining C) pleomorphic nuclei (arrows) and D) pancytokeratin expression (brown). Mammospheres generated after 7 days were dissociated into single cells and re-plated at 1 cell per well; E) Bright-field photomicrograph of a single cell re-plated in a 96 well plate; F) a mammosphere regenerated from a single cell plated per well after 10 days in non-adherent culture. G and H Photomicrographs of 3μm sections of a FFPE patient-derived mammosphere showing G) ALDH1 expression (brown staining), arrow shows intense expression in a single cell and H) CXCR1 expression (brown staining). A-H Scale bars = 50μm. I MFE of 19 independent patient-derived breast cancers. There was no significant difference in MFE between HER2 positive, HER2 negative and triple negative cancers. Columns, mean; bars, SEM of triplicate observations. J MFE of HER2 positive (n=5) and HER2 negative (n=9) cancers after 7 days in non-adherent culture with/without lapatinib (1μM). Controls were treated with vehicle (0.01% DMSO). Columns, fold change in MFE normalised to control; bars, SEM. ***, P <0.001; NSD, no significant difference.
Immunohistochemistry
Patient-derived mammospheres were collected after 7 days, centrifuged at 800g for 2 minutes and fixed in 4% formalin for 10 minutes at room temperature. Mammospheres were washed in PBS and resuspended in 1% high melting-point agarose (37°C), and paraffin-embedded. Formalin-fixed paraffin-embedded (FFPE) patient-derived mammospheres were then cut into 3μm sections as described previously (6). CXCR1 expression was determined using human CXCR1/IL-8 RA antibody (R and D systems, MAB330, clone number 42705) and aldehyde dehydrogenase 1 (ALDH1) expression was assessed using human ALDH1 antibody (BD Biosciences, 611195, clone number 44/ALDH). Antibody binding was detected using Dako Envision Detection System Peroxidase/DAB, Rabbit/Mouse (K5007).
IL-8 Quantification using Enzyme-Linked Immunosorbent Assay (ELISA)
Metastatic fluid was centrifuged at 1000g for 10 minutes at 4°C; the supernatant was collected and IL-8 level determined using a human IL-8 ELISA set (BD Biosciences, 555244) according to manufacturer’s instructions.
Cell line monolayer culture and treatment
The MCF7/HER2-18 cell line was a gift from Professor CC Benz, University of California San Francisco and generated by stable over-expression of HER2 in the parental MCF-7 cell line (24). Cells were maintained in monolayer in complete medium [DMEM, 10% foetal calf serum (FCS) and L-glutamine] as previously described (25) and regularly tested for mycoplasma to verify their negative status.
Mammosphere assay
Subconfluent monolayer cells were enzymatically (trypsin-EDTA 0.125%) and manually (25-g needle) dissociated into a single cell suspension. Cells were plated out (300cells/cm2) in mammosphere culture conditions and the following treatments were added to the culture media: 100ng/ml recombinant IL-8; 100nM SCH563705, 10μM lapatinib, 1-100μM LY294002 (PI3-K inhibitor, Cell Signalling Technologies, 9901), 1-100μM PD98059 (MEK1 inhibitor, Cell Signalling Technologies, 9900) or 10μM 4-amino-3-(4-chlorophenyl)-1-(t-butyl)-1H-pyrazolo[3,4-d]pyrimidine (PP2, inhibitor of SRC-family tyrosine kinases, Sigma-Aldrich, P0042). Control cells were treated with 0.01% BSA in PBS w/v (for IL-8 treatment) and 0.01% DMSO (for SCH563705, lapatinib, LY294002, PD98059 and PP2 treatments). Mammospheres (colonies >60μm in diameter) were counted after 4 days.
CXCR1/2-activated cell signalling
Cells were plated out (2.5×104 cells/cm2) in adherent culture conditions, left for 24 hours and then serum-starved for 48 hours. Media was changed 1 hour prior to treatment to remove any stimulating factors which may have accumulated. Cells were pre-treated with vehicle control (DMSO 0.01%), lapatinib (10μm), SCH563705 (100nM) or PP2 (10μm) and then stimulated with IL-8 (100ng/ml) for 2, 5, 10, 30 and 60 minutes. After the specified time, media was removed, cells were rinsed in ice cold PBS and scraped into lysis buffer Immunoblotting was performed as previously described (26, 27). Membranes were probed for HER2 (Cell Signalling 2242), phospho-HER2Tyr1221/1222 (Cell Signalling 2249), EGFR (Cell Signalling 2232), phospho-EGFR1148 (Cell Signalling 4404), AKT (Cell Signalling 9272), phospho-AKTSer473 (Cell Signalling 4051), ERK1/2 (Cell Signalling 4695), phospho-ERK1/2Thr202/Tyr204 (Cell Signalling 9101) and β-Actin (Sigma A1978) according to the suppliers recommendations.
Short interfering RNA (siRNA) knockdown of HER2
Two predesigned FlexiTube siRNAs to HER2 (siHER2-14) and siHER2-15) and a non-targeting scramble control (All Stars Negative Control) were purchased from Qiagen. MCF7/HER2-18 cells were plated out at 2×105 cells per well (6 well plate) in complete medium and transfected with 30ng siRNA according to the manufacturer’s instructions. After 48 and 72 hours, media was removed, cells were rinsed in ice cold PBS and lysates were collected as described above to determine the extent of protein knockdown by imunoblotting. The effect of HER2 knockdown on CSC activity was determined using the mammosphere assay. Monolayer cells were enzymatically and mechanically dissociated at 48 hours post transfection and plated out in mammosphere culture conditions as above. Cultures were treated with IL-8 (100ng/ml) or vehicle (0.01% BSA in PBS) and MFE was assessed as above. A minimum of three independent experiments each with three technical replicates per experiment was performed for each construct.
Statistical analysis
Throughout this article data are represented as mean ± standard error (SEM) taken over a minimum of three independent experiments with three technical replicates per experiment unless otherwise stated. Statistical differences in MFE between control and treatment conditions were determined using Whitney-U test. Kruskal-Wallis test was used to determine statistical differences between conditions and Conover-Inman post-hoc test used was used to make multiple pairwise comparisons. Statistical differences in mammosphere self-renewal was assessed using Wilcoxon matched pairs test. Differences were considered statistically significant if the two-tailed probability value (P) was ≤ 0.05. Pearson correlation coefficient (r) was used to measure the correlation between metastatic fluid IL-8 level and mean MFE; a two-tailed P ≤ 0.05 was considered statistically significant. Statistical analysis was performed using StatsDirect statistical software Version 2.7.2.
RESULTS
Mammosphere formation from patient-derived breast cancers and effects of EGFR/HER2 inhibition
To assess breast CSC activity in patient-derived breast cancers we used the mammosphere assay as previously described (6, 9). Mammospheres formed after 7 days and a typical bright-field image is shown in Figure 1A. To confirm that the mammospheres were composed of malignant epithelial cells their cellular and nuclear composition was examined histologically. H&E staining showed that mammospheres were composed of cells with pleomorphic nuclei characterised by large, irregular nuclei and prominent nucleoli, which was consistent with the reported nuclear grade of the original tumours (Figure 1B and C), confirmed by a clinical breast cancer pathologist (GL). Pancytokeratin immuostaining confirmed the epithelial origin of the constituent cells in the mammospheres as shown in Figure 1D. Plating of single mammosphere cells confirmed that mammospheres are clonal in origin (Figure 1E and F). Immunostaining for the stem cell marker ALDH1 demonstrated strong expression in a single cell (Figure 1G) while the cytokine receptor CXCR1 was expressed on the membrane of the majority of mammosphere cells (Figure 1H).
Mammosphere forming efficiency (MFE) of individual cancers ranged from 0.3% to 2.7% (Table 1 and Figure 1I). There was no statistical difference in MFE between HER2 positive, HER2 negative and triple negative patient-derived breast cancers. Lapatinib (1μM) treatment resulted in a 20.4% ± 2.3% reduction in MFE in HER2 positive cancers compared to control, (P <0.001), but had no significant effect on MFE in HER2 negative cancers (P = 0.605; Figure 1J). These data demonstrate that the mammosphere colony formation/self-renewal assay can be utilised in patient-derived invasive and metastatic breast cancers and despite prior treatment, HER2 positive mammosphere colonies remain responsive to lapatinib.
CXCR1/2 signalling regulates patient-derived mammosphere formation/self-renewal activity
In order to investigate the involvement of IL-8 in the regulation of mammosphere formation/self-renewal, we analysed metastatic ascites and pleural effusion fluid for IL-8 protein level. IL-8 was detected in all metastatic fluid samples tested (n=10) with a mean IL-8 concentration of 79.2pg/ml (range 25.2 to 201.1pg/ml), see Table 1. There was a significant direct correlation between metastatic fluid IL-8 level and MFE (r = 0.652; P = 0.041) as shown in Figure 2A. These data establish that patients with higher levels of IL-8 in their metastatic fluid have greater ex vivo CSC activity.
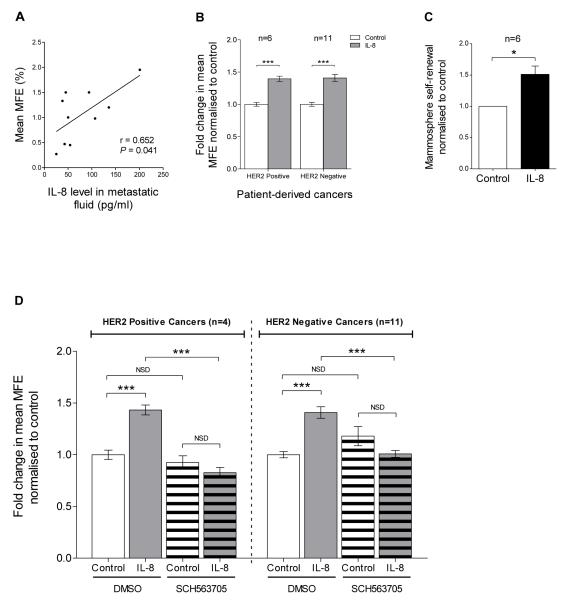
Figure 2. CXCR1/2 signalling regulates patient-derived mammosphere formation/self-renewal activity.
A Correlation between metastatic fluid IL-8 level and MFE, n=10 cancers (Pearson correlation coefficient, r = 0.652; P <0.05). Metastatic fluid IL-8 level was determined following removal of cellular matter using an ELISA. Details of MFE and metastatic fluid IL-8 level for each cancer are summarised in Table 1. B MFE of HER2 positive (n=6) and HER2 negative (n=11) patient-derived breast cancers after 7 days in non-adherent culture with/without IL-8 (100ng/ml). Controls were treated with vehicle. C Secondary mammosphere formation i.e. self-renewal of patient-derived breast cancers (HER2 positive n=2; HER2 negative n=4) treated with IL-8 (100ng/ml) in the first generation. Columns, fold change in mammosphere self-renewal normalised to control; bars, SEM. *, P < 0.05. D MFE of HER2 positive (n=4) and HER2 negative (n=11) cancers treated with either IL-8 (100ng/ml, grey bars), SCH563705 (100nM, white stripped bars), or SCH563705 and IL-8 (grey stripped bars) at the stated respective doses. Controls (white bars) were treated with vehicle. B and D Columns, fold change in MFE normalised to control; bars, SEM. ***, P < 0.001; NSD, no significant difference.
We therefore investigated the effect of IL-8 on patient-derived mammosphere formation/self-renewal. Mammosphere cultures treated with recombinant IL-8 (100ng/ml) resulted in a significant increase in MFE in both HER2 positive (39.6% ± 4.0% increase compared to control, P < 0.001, n=6 cancers) and HER2 negative cancers (40.8% ± 5.5% increase compared to control, P < 0.001, n=11), see Figure 2B. Secondary mammosphere formation of patient-derived breast cancer cells (HER2 positive n=2; HER2 negative n=4) treated with IL-8 (100ng/ml) in the first generation was used to assess self-renewal (51.3% ± 13.2% increase compared to control, P < 0.05, n=6 cancers), see Figure 2C.
The effect of CXCR1/2 inhibition on patient-derived mammosphere formation was subsequently determined using SCH563705, a small molecule CXCR1/2 antagonist (28, 29). SCH563705 (100nM) alone had no significant effect on MFE in HER2 positive (P = 0.454, n=4) or HER2 negative cancers (P = 0.236, n=11), Figure 2D. However, SCH563705 abrogated the effect of IL-8 in both HER2 positive and HER2 negative cancers, Figure 2D. These data demonstrated that IL-8 can directly regulate patient-derived mammosphere formation/self-renewal and this can be blocked by inhibiting CXCR1/2.
CXCR1/2 inhibition adds to the efficacy of EGFR/HER2 inhibition in HER2 positive patient-derived mammospheres
Having demonstrated that HER2 and IL-8 independently regulate patient-derived mammosphere formation activity, we next determined the effect of inhibiting these signalling pathways in combination. As the inhibitory effect of SCH563705 was dependent on exogenous IL-8, all conditions including control were supplemented with recombinant IL-8 (100ng/ml). As above, SCH563705 (100nM) significantly inhibited MFE in HER2 positive and HER2 negative cancers, Figure 3A and B. Lapatinib significantly inhibited MFE in HER2 positive cancers, but also significantly inhibited MFE in HER2 negative cancers suggesting that CXCR1/2 signalling is at least partly dependent on EGFR/HER2 receptor activity, see Figure 3A and B.
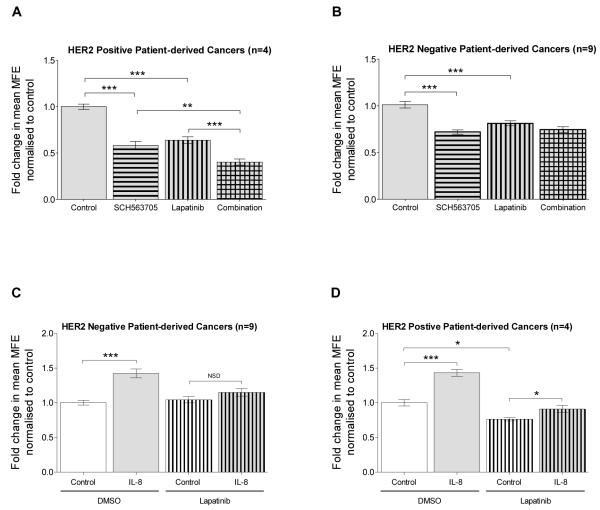
Figure 3. Interaction between CXCR1/2 and HER2 signalling in mammosphere formation.
HER2 positive (n=4) and HER2 negative (n=9) patient-derived breast cancer cells were plated out in non-adherent culture conditions. A and B show the effect of SCH563705 (100nM), lapatinib (1μM) or a combination of lapatinib and SCH563705 at the stated respective doses on MFE in A) HER2 positive B) and HER2 negative breast cancers. All conditions, including control were supplemented with recombinant IL-8 (100ng/ml). C and D show the effect of IL-8 (100ng/ml), lapatinib (1μM) or lapatinib and IL-8 at the stated respective doses on MFE in C) HER2 negative and D) HER2 positive breast cancers. Controls were treated with vehicle. A-D Columns, fold change in MFE normalised to control; bars, SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NSD, no significant difference.
Combination treatment of SCH563705 and lapatinib resulted in a significantly greater reduction in MFE compared to either treatment alone in the HER2 positive cancers (combination versus SCH563705, P < 0.01; combination versus lapatinib, P < 0.001) as shown in Figure 3A. However, combination treatment was no more effective than either treatment alone in the HER2 negative cancers (combination versus SCH563705, P = 0.923; combination versus lapatinib, P = 0.053), see Figure 3B.
IL-8-mediated effects on patient-derived mammosphere formation are EGFR/HER2-dependent
We have shown that in HER2-negative cancers, lapatinib significantly inhibited mammosphere formation under IL-8 supplemented conditions, whereas no significant effect was observed in the absence of IL-8 (Figure 3B and Figure 1J). This suggests that the functional effect of IL-8 was dependent on EGFR/HER2 activity. Indeed, in HER2-negative cancers lapatinib abolished the effect of IL-8 on mammosphere formation as shown in Figure 3C. A similar effect was observed in the HER2 positive cancers; under lapatinib treated conditions, the effect of IL-8 was significantly inhibited compared to control conditions (18.7% ± 6.2% increase in MFE compared to 43.3% ± 4.8% increase; P < 0.05), see Figure 3D. These data establish that the effect of CXCR1/2 activation on patient-derived mammosphere formation activity involves an EGFR/HER2-dependent pathway. The data also suggest that, downstream of CXCR1/2 in HER2-negative cancers, EGFR is activated and/or HER2 is expressed and activated even though the HER2 gene is not amplified. Further inhibition of patient-derived mammosphere formation activity with SCH563705 in combination with lapatinib in HER2 positive cancers indicates that CXCR1/2 signalling is also mediated via an EGFR/HER2-independent pathway.
Transactivation of EGFR/HER2 by CXCR1/2
We next used the HER2-positive breast cancer cell lines, MCF7/HER2-18 (24, 25) and SKBR3, to further explore the role of EGFR/HER2 in CXCR1/2-mediated signalling. Sub-confluent cells grown in monolayer were detached and single cells were plated out (300 cells/cm2) in mammosphere culture conditions. Treatment with IL-8 (100ng/ml) significantly increased MFE and self-renewal (Figure 4A and C and Supplementary Figure 1C, E and F). SCH563705 and lapatinib each decreased MFE similarly to the patient-derived cancers, and abrogated the effect of IL-8 (Figure 4A and Supplementary Figure 1C, G, I and J). SCH563705 also significantly reduced Aldefluor-positivity, further supporting its role in regulating CSC activity (Supplementary Figure 1H).
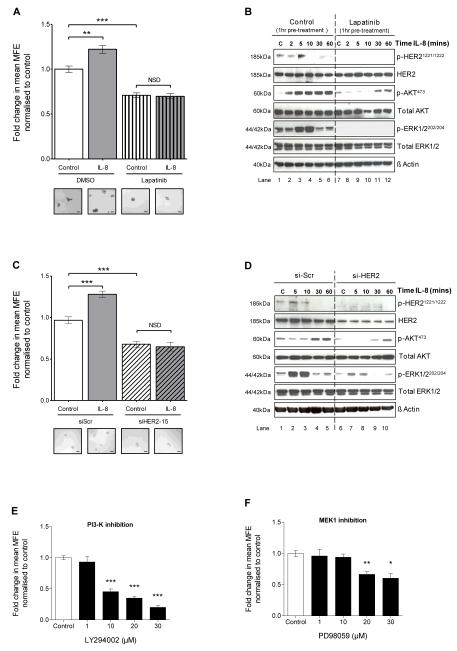
Figure 4. CXCR1/2-mediated effects on mammosphere formation and downstream signalling pathways are dependent on HER2 phosphorylation.
A MCF7/HER2-18 cells were plated out in non-adherent culture conditions and treated with either IL-8 (100ng/ml), lapatinib (10μM) or lapatinib and IL-8 at the stated respective doses. Controls were treated with vehicle. Graph shows the effect of treatments on MFE. Inserts show representative bright-field photomicrographs of mammospheres cultured under each condition; scale bars = 100μm. B MCF7/HER2-18 cells were plated out in adherent culture conditions and pre-treated with either vehicle control (lanes 1-6) or lapatinib (10μM, lanes 7-12) for 1 hour prior to stimulation with IL-8 (100ng/ml) for 2, 5, 10, 30 and 60 minutes. Controls (C) were treated with vehicle for 10 minutes. Immunoblots show the effect of IL-8 on phosphorylation of HER2, AKT and ERK1/2. C MCF7/HER2-18 cells transfected with either scramble control siRNA (siScr), or siRNA to HER2 (siHER2-15) were plated out in non-adherent culture conditions and treated with either IL-8 (100ng/ml) or vehicle control. Graph shows the effect of these treatments on MFE. Inserts show representative bright-field photomicrographs of mammospheres cultured under each condition; scale bars = 100μm. D MCF7/HER2-18 cells were transfected with either siScr (lanes 1-5), or siHER2-15 (lanes 6-10). After 24 hours, cells were serum starved for 48 hours and then stimulated with IL-8 (100ng/ml) for 5, 10, 30 and 60 minutes. Controls (C) were treated with vehicle for 10 minutes. Immunoblots show the effect of IL-8 on phosphorylation of HER2, AKT and ERK1/2. E and F MCF7/HER2-18 cells were plated out in non-adherent culture conditions and treated with increasing doses of LY294002, a PI3-K inhibitor (E) and PD98059, a MEK1 inhibitor (F). Graphs show the effect of treatments on MFE. A, C, E and F Columns, fold change in MFE normalised to control; bars, SEM, n=3 independent experiments. **, P < 0.01; ***, P < 0.001; NSD, no significant difference. B and D blots are representative of 3 independent experiments. β-actin was used as a loading control; molecular weight for each protein is shown.
Next, we investigated the role of EGFR/HER2 in mediating downstream signalling events induced by CXCR1/2 activation by western blotting for EGFR, HER2, AKT and ERK1/2 and their phosphorylated, activated isoforms. MCF7/HER2-18 or SKBR3 cells were incubated with lapatinib (MCF7/HER2-18 10μM; 1.5μM SKBR3) or vehicle control (0.01% DMSO) for 1 hour prior to stimulation with IL-8 (100ng/ml) for up to 60 minutes. Under control conditions, a rapid, transient increase in phosphorylation of HER21221/1222 (MCF7/HER2-18) or EGFR1148 (SKBR3) was observed within 5 minutes of stimulation with IL-8 (Figure 4B and Supplementary Figure 2A). Importantly, inhibiting CXCR1/2 was demonstrated to prevent HER2 phosphorylation (Supplementary Figure 1D). IL-8 also markedly increased phosphorylation of AKT (p-AKT473) and ERK1/2 (p-ERK1/2202/204) within 10 minutes (Figure 4B and Supplementary Figure 2A).
One hour pre-treatment with lapatinib markedly reduced basal levels of p-HER21221/1222 and p-ERK1/2202/204 in MCF7/HER2-18 cells and p-AKT473 and pERK1/2202/204 in SKBR3 cells (Figure 4B and Supplementary Figure 2B). Importantly, lapatinib pre-treatment completely blocked the effect of IL-8 on p-HER21221/1222 and p-ERK1/2202/204 and partially inhibited the effect of IL-8 on p-AKT473 in MCF7/HER2-18 cells (Figure 4B) and inhibited p-ERK1/2202/204 and p-AKT473 in SKBR3 cells (Supplementary Figure 2B). These studies demonstrate that activation of AKT and ERK1/2 signalling pathways by CXCR1/2 is mainly mediated via transactivation of EGFR/HER2 which can be inhibited by lapatinib. The importance of AKT and ERK1/2 signalling pathways was confirmed by targeting the upstream kinases, PI3K and MEK1, using the inhibitors LY294002 and PD98059, respectively. They were both effective in reducing mammosphere formation in MCF7/HER2-18 cells (Figure 4E and F) and the PI3K inhibitor significantly reduced mammosphere formation activity in SKBR3 cells (Supplementary Figure 2C and D).
To validate the role of HER2 in mediating the signalling events induced by CXCR1/2, we targeted HER2 expression using siRNA. First the efficacy of two siRNA constructs targeting HER2 (siHER2-14 and siHER2-15) was assessed by western blotting. A non-targeting scramble sequence was used as a control (siScr). Successful knockdown of HER2 protein level was achieved with siHER2-14 and siHER2-15 at 48 and 72 hours post transfection compared to scramble control siScr (Supplementary Figure 3A). Neither construct had any effect on EGFR gene expression (Supplementary Figure 3B). Mammosphere formation was assessed 48 hours post transfection and a 30.2% ± 3.3% and 34.3% ± 2.5% reduction in MFE was observed with siHER2-14 and siHER2-15 respectively compared to siScr (Supplementary Figure 3C).
Next, the effect of HER2 knockdown on mammosphere formation induced by IL-8 was assessed. IL-8 (100ng/ml) increased MFE in cells transfected with siScr (28.2% ± 3.8% compared to vehicle control, P <0.001), Figure 4C. However, IL-8 had no effect on cells transfected with siHER2-15 as shown in Figure 4C. A similar effect was observed with siHER2-14 construct. These findings validate experiments with lapatinib demonstrating that HER2 function is critical in mediating the effect of CXCR1/2 activation on breast CSC activity.
To corroborate the above functional studies, the effect of IL-8 on HER2 activation and downstream signalling pathways following HER2 knockdown was investigated. MCF7/HER2-18 cells transfected with either siScr or siHER2 (siHER2-15) were stimulated with IL-8 (100ng/ml) for 5, 10, 30 and 60 minutes. Under siScr conditions, IL-8 increased p-HER21221/1222, p-AKT473 and p-ERK1/2202/204 in a time dependent manner, Figure 4D. However, under HER2 knockdown conditions, phosphorylation of HER2 by IL-8 was no longer detectable, and the increase in p-AKT473 and p-ERK1/2202/204 was reduced compared to scramble control conditions as shown in Figure 4D. These effects were similar to those observed with lapatinib (Figure 4B), providing further experimental evidence that activation of AKT and ERK1/2 signalling pathways by CXCR1/2 is mainly mediated via transactivation of HER2. Remaining ERK1/2 phosphorylation where HER2 is knocked down (Figure 4D) compared to Lapatinib treatment (Figure 4B) is hypothesised due to either incomplete loss of HER2 protein or the continued activity of EGFR.
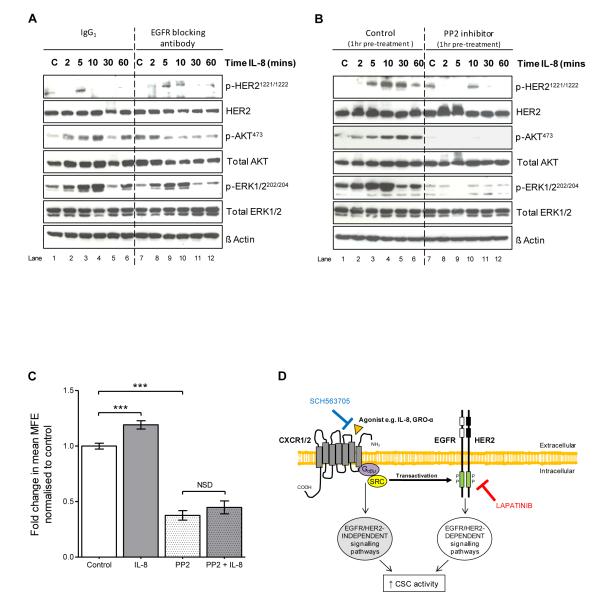
Next, we investigated the mechanism by which HER2 becomes transactivated by CXCR1/2. HER2 is a member of the EGFR family of receptors and whilst few studies have demonstrated transactivation of HER2 by G-protein coupled receptors, a well described mechanism of EGFR transactivation by G-protein coupled receptors involves ligand-dependent activation. Agonist stimulation of G-protein coupled receptors is reported to activate various metalloproteases, such as members of the disintegrin and metalloprotease (ADAM) family, which results in the cleavage and release membrane-bound EGF-like ligands. These soluble ligands can subsequently activate EGFR via a paracrine and/or autocrine manner (30-32).
Unlike EGFR, HER2 is devoid of its own activating ligand, but becomes activated by heterodimerising with EGFR. We therefore hypothesised that HER2 may become activated by heterodimerising with EGFR following ligand-dependent transactivation of EGFR by CXCR1/2 which are both G-protein coupled receptors. This was investigated using an EGFR blocking antibody which prevents EGF-like ligands binding to the ligand binding domain of the receptor. Following dose optimisation, the effect of an EGFR blocking antibody on HER2 activation and downstream signalling pathways induced by CXCR1/2 activation was determined. Adherent MCF7/HER2-18 cells were serum starved for 48 hours then pre-treated with either IgG1 control antibody (5μg/ml) or EGFR ligand blocking antibody (5μg/ml) for 1 hour prior to stimulation with IL-8 (100ng/ml). Phosphorylation of HER2 and ERK1/2 induced by IL-8 was not inhibited by the EGFR blocking antibody as shown in Figure 5A. These findings indicate that transactivation of HER2 by CXCR1/2 is independent of ligand activation of EGFR and therefore likely to be mediated via an intracellular mechanism. We hypothesised that SRC kinase could be involved since it is known to transduce intracellular signals downstream of cytokine receptors and to interact with EGFR/HER2 receptors. We therefore tested the SRC-family kinase inhibitor PP2 for its effects on IL8-induced signalling and established that it effectively blocks HER2, AKT and ERK1/2 phosphorylation in MCF7/HER2-18 cells (Figure 5B). In addition, PP2 abrogates IL8-induced mammosphere formation activity (Figure 5C). These data indicate that SRC-family kinases mediate an intracellular mechanism downstream of CXCR1/2 that transactivates HER2, AKT and ERK1/2 signalling pathways and contribute to mammosphere formation activity (Figure 5D).
Figure 5. Transactivation of HER2 by CXCR1/2 is independent of ligand activation of EGFR, but dependent on SRC kinase activity.
A MCF7/HER2-18 cells were plated out in adherent culture conditions and pre-treated with either IgG1 control antibody (5μg/ml, lanes 1-6) or EGFR ligand blocking antibody (5μg/ml, lanes 7-12) for 1 hour prior to stimulation with IL-8 (100ng/ml) for 2, 5, 10, 30 and 60 minutes. Controls (C) were treated with vehicle for 10 minutes. Immunoblots show the effect of IL-8 on phosphorylation of HER2, AKT, and ERK1/2. B MCF7/HER2-18 cells were plated out in adherent culture conditions and pre-treated with either vehicle control (lanes 1-6) or PP2 (10μM, lanes 7-12) for 1 hour prior to stimulation with IL-8 (100ng/ml) for 2, 5, 10, 30 and 60 minutes. Controls (C) were treated with vehicle for 10 minutes. Immunoblots show the effect of IL-8 on phosphorylation of HER2, AKT, and ERK1/2. C MCF7/HER2-18 cells were plated out in mammosphere culture conditions and treated with either IL-8 (100ng/ml), PP2 (10μM) or PP2 and IL-8 at the stated respective doses. Graph shows the effect of these treatments on MFE. Columns, fold change in MFE normalised to control; bars, SEM, n=3 independent experiments. ***, P < 0.001; NSD, no significant difference. D Putative model of the interaction of CXCR1/2 and HER2 in the regulation of HER2 positive breast CSC activity. Agonist stimulation of CXCR1/2 causes transactivation of HER2 which increases CSC activity via HER2-dependent signalling pathways. Activation of HER2-independent signalling pathways by CXCR1/2 also promotes CSC activity. HER2 inhibition with lapatinib prevents CXCR1/2-mediated transactivation of EGFR/HER2 resulting in a decrease in CSC activity. Blockade of CXCR1/2 with SCH563705 causes a further reduction in CSC activity through inhibition of HER2-independent signalling pathways.
DISCUSSION
We investigated the role of IL-8 in regulating activity of breast CSCs isolated directly from patients, and determined the benefit of combining CXCR1/2 inhibition with HER2-targeted therapy. IL-8 concentration measured in fluid from metastatic ascites and pleural effusions directly correlated with mammosphere formation activity ex vivo. Using patient-derived breast cancers, we report for the first time, that IL-8 is important in regulating mammosphere formation and self-renewal activity. Mechanistically, we demonstrate that IL-8/CXCR1/2-mediated effects are critically dependent on HER2 function and inhibition of CXCR1/2 signalling added to the efficacy of inhibiting HER2 on HER2-positive mammosphere formation activity.
Like normal mammary stem cells, breast CSCs have the capacity to survive in non-adherent culture conditions and form floating colonies known as mammospheres (6, 8, 33). This culture system provides a valuable method of assessing CSC activity in vitro and has been used extensively in breast cancer cell lines. We and others have utilised this technique to investigate factors affecting CSC activity and the efficacy of novel inhibitors in primary pre-invasive, invasive and metastatic breast cancers ex vivo (3, 6, 9). Previous studies have suggested that HER2 positive pre-invasive and invasive breast cancers have a greater proportion of CSCs, as determined by their enhanced capacity to form mammospheres and greater CD24−/CD44+ cell fraction respectively, compared to HER2 negative cancers (3, 6). In contrast, we found no statistical difference in MFE in samples mostly derived from metastatic fluid between HER2-positive and HER2-negative cancers. However, it is possible that our sample size was too limited to observe statistically significant differences between tumour sub-types.
Metastatic fluid contains inflammation-related proteins and diverse cell types including mesothelial cells, macrophages, neutrophils and erythrocytes. We measured IL-8 in ascites and pleural effusion fluid from patients with metastatic breast cancer and found a significant positive correlation between metastatic fluid IL-8 level and mammosphere forming efficiency of tumour cells derived from these fluids cultured ex vivo. Whether increased mammosphere formation was a consequence of metastatic fluid IL-8 level in the patient is unknown, as is whether the source was the tumour cells, other cells in the fluid or relates to systemic IL-8 levels.
SCH563705, a small molecule CXCR1/2 inhibitor, prevented the effect of recombinant IL-8 on mammosphere formation. However when used alone, the compound had no effect on mammosphere formation. These data suggest that whilst CSCs respond to IL-8, they do not secrete it in an autocrine loop. Thus IL-8 probably acts via a paracrine route, secreted by stromal cells and inflammatory cells which comprise the tumour microenvironment (34, 35).
These observations have important clinical implications as there is strong evidence that CSC activity is related to prognostic factors. We and others have previously demonstrated an enrichment of breast CSCs with an increase in tumour grade and high grade tumours are known to be associated with poorer prognosis (6, 36).
Although there is evidence that CXCR1/2 can transactivate EGFR in other tumour types (32, 37-39), there are no previous reports that CXCR1/2 causes transactivation of ErbB family receptors in breast cancer. Our data demonstrate, for the first time, that CXCR1/2 regulates mammosphere formation via a EGFR/HER2-dependent pathway. In both HER2 positive and HER2 negative cancers, the mammosphere promoting effect of IL-8 was inhibited by lapatinib, a dual EGFR/HER2 tyrosine kinase inhibitor. siRNA knockdown of HER2 in MCF7/HER2-18 cells blocked the effect of IL-8 on mammosphere formation. Together these findings establish that HER2 function is essential in mediating the effect of CXCR1/2 activation on breast CSC activity. There may also be a role for HER3 and HER4 in modulating EGFR/HER2 effects downstream of CXCR1/2 but we have yet to address this possibility. A very recent publication reports that HER2/HER3 activity leads to overexpression of IL8, which would potentially increase auto-activation of the HER2 pathway (40).
Mechanistically, we discovered that IL-8 induced transient phosphorylation of EGFR/HER2 and downstream AKT and ERK1/2 signalling pathways. Detectable HER2 phosphorylation varied in its timing from 2-30 minutes (compare Figures 4B, 4D, 5A, 5B and Supplementary Figure 1D) but consistently stimulated downstream AKT and ERK1/2 phosphorylation. These signalling events were dependent on HER2 function as both pharmacological inhibition with lapatinib and genetic knockdown of HER2 inhibited the effect of IL-8 on phosphorylation of HER2, AKT and ERK1/2. These studies indicate that activation of AKT and ERK1/2 signalling pathways by CXCR1/2 occurs via transactivation of HER2. PI3K and/or MEK1 inhibition upstream of AKT and ERK1/2 markedly reduced mammosphere formation activity in our study. This is important as these signalling pathways are critical in regulating breast CSC activity (41).
Given the importance of HER2 in regulating breast CSC activity (11, 12), a pathway driving the activation of this receptor via CXCR1/2 will have important biological consequences. This will be very relevant in ER negative and HER2 positive breast cancers which are reported to have higher levels of IL-8 and this may contribute to their aggressive phenotype (42, 43).
Using an EGFR blocking antibody, we found that phosphorylation of HER2 and ERK1/2 by IL-8/CXCR1/2 was independent of ligand binding to EGFR. As recently demonstrated for CXCR4, another cytokine receptor, we found transactivation of HER2 by CXCR1/2 involves a ligand-independent intracellular signalling mechanism involving SRC kinases (44).
Inhibiting CXCR1/2 added to the efficacy of inhibiting HER2 in our assays, resulting in a greater reduction in mammosphere formation in HER2-positive breast cancers. As the effect of IL-8 was only partially inhibited by blocking HER2 in HER2 positive cancers, we propose that the additional benefit of targeting CXCR1/2 was derived from inhibition of HER2-independent signalling pathways. A putative model of the interaction of CXCR1/2, SRC and HER2 in the regulation of breast CSC activity is shown in Figure 5B. CXCR1/2 signalling is known to involve many signal transduction pathways and some of these, such as focal adhesion kinase, have been implicated in breast CSC maintenance (45). Thus CXCR1/2 inhibition likely prevented the activation of these additional HER2-independent signalling pathways.
In summary, our findings indicate that combining CXCR1/2 inhibition with HER2 inhibition is an effective strategy to target HER2-positive mammosphere formation activity. Interestingly, we also observed that EGFR/HER2 inhibition can prevent CXCR1/2-induced mammosphere formation activity in HER2-negative tumour sub-types. Validation that this would be effective in targeting CSCs requires in vivo studies although it has already shown that inhibition of HER2 or CXCR1/2 alone decreases tumour growth, metastases and CSC self-renewal in xenograft mouse models (12, 20). Phase I clinical trials have demonstrated that Repertaxin, a CXCR1/2 inhibitor, is well tolerated and trials are under development to determine its efficacy in breast cancer (46, 47). Given the emerging importance of IL-8 as a key environmental regulator of breast CSC activity, combining CXCR1/2 inhibitors with current HER2-targeted therapies has the potential as an effective therapeutic strategy to decrease CSC activity and improve the survival of HER2 positive breast cancer patients.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Breast cancer stem-like cells (CSCs) are an important cellular target as they are responsible for tumour initiation, maintenance and metastases. Interleukin-8 (IL-8) has been reported to be upregulated in breast cancer, associated with poor prognosis, and CSCs in breast cell lines are regulated by its receptors, CXCR1/2.
Our research article establishes that IL-8 levels in metastatic fluids correlate directly to breast CSC activity measured ex vivo using patient-derived breast cancer samples. IL-8 stimulation and CXCR1/2 inhibition affects CSC activity in patient-derived samples and we demonstrate mechanistically that CXCR1/2 regulates breast stem cell activity via HER2-dependent and independent pathways. Furthermore, we show that inhibiting CXCR1/2 is useful in combination therapies targeting CSC activity in HER2 positive breast cancer. Overall, we establish that activation of HER2 via CXCR1/2 has important biological and clinical implications, and is likely to be especially relevant in patients expressing high levels of IL-8 and other CXCR1/2 activating ligands.
ACKNOWLEDGMENTS
We thank the patients who provided clinical material for their support of these studies, the Department of Statistics at The Christie for their assistance in data analysis, Rachel Eyre, Hannah Gregson and Rachael Johnson at the Paterson Institute for Cancer Research for technical assistance, GSK for supplying lapatinib and Merck for supplying SCH563705.
GRANT SUPPORT
Royal College of Surgeons of England and Manchester Surgical Research Trust (J.K. Singh), Breast Cancer Campaign (R.B. Clarke and G. Farnie) and Breakthrough Breast Cancer (G. Landberg).
Footnotes
Conflicts of interest: None
REFERENCES
- 1.McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol. 2010;4(5):404–19. doi: 10.1016/j.molonc.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 4.Creighton CJ, Massarweh S, Huang S, Dixon JM, Neumeister VM, Sjolund A, et al. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68(18):7493–501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, et al. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2010;128(1):45–55. doi: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99(8):616–27. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 7.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 9.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70(2):709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 11.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27(47):6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15(6):2010–21. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 14.Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11(4):318–24. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 17.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Research. 2009;69(4):1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissière F, Laune D, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Research. 2007;9(1):R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clinical Cancer Research. 2004;10(21):7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 20.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. Journal of Clinical Investigation. 2010;120(2):485–97. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schwartz JN, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 22.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68. [PubMed] [Google Scholar]
- 23.Shaw FL, Harrison H, Spence K, Ablett MP, Simões BM, Farnie G, et al. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia. 2012;17(2):111–7. doi: 10.1007/s10911-012-9255-3. [DOI] [PubMed] [Google Scholar]
- 24.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24(2):85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 25.Barnes NL, Warnberg F, Farnie G, White D, Jiang W, Anderson E, et al. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007;96(4):575–82. doi: 10.1038/sj.bjc.6603593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad T, Farnie G, Bundred NJ, Anderson NG. The mitogenic action of insulin-like growth factor I in normal human mammary epithelial cells requires the epidermal growth factor receptor tyrosine kinase. J Biol Chem. 2004;279(3):1713–9. doi: 10.1074/jbc.M306156200. [DOI] [PubMed] [Google Scholar]
- 27.Howell SJ, Anderson E, Hunter T, Farnie G, Clarke RB. Prolactin receptor antagonism reduces the clonogenic capacity of breast cancer cells and potentiates doxorubicin and paclitaxel cytotoxicity. Breast Cancer Res. 2008;10(4):R68. doi: 10.1186/bcr2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min SH, Wang Y, Gonsiorek W, Anilkumar G, Kozlowski J, Lundell D, et al. Pharmacological targeting reveals distinct roles for CXCR2/CXCR1 and CCR2 in a mouse model of arthritis. Biochem Biophys Res Commun. 2010;391(1):1080–6. doi: 10.1016/j.bbrc.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Chao J, Taveras AG, Aki C, Dwyer M, Yu Y, Purakkattle B, et al. C(4)-alkyl substituted furanyl cyclobutenediones as potent, orally bioavailable CXCR2 and CXCR1 receptor antagonists. Bioorg Med Chem Lett. 2007;17(13):3778–83. doi: 10.1016/j.bmcl.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291(1):C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 32.Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56(1):25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. International Journal of Cancer. 1997;72(6):937–41. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 36.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140(1):62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55(3):807–20. doi: 10.1002/hep.24739. [DOI] [PubMed] [Google Scholar]
- 38.Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275(10):6868–75. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- 39.Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29(6):275–82. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Aceto N, Duss S, Macdonald G, Meyer DS, Roloff TC, Hynes NE, et al. Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cells via stimulation of interleukin-8 autocrine secretion. Breast Cancer Res. 2012;14(5):R131. doi: 10.1186/bcr3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7(6):e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freund A, Chauveau C, Brouillet J-P, Lucas A, Lacroix M, Licznar A, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22(2):256–65. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Martin A, Colomer R, Menendez JA. Protein array technology to detect HER2 (erbB-2)-induced ‘cytokine signature’ in breast cancer. European Journal of Cancer. 2007;43(7):1117–24. doi: 10.1016/j.ejca.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 44.Cabioglu N, Summy J, Miller C, Parikh NU, Sahin AA, Tuzlali S, et al. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 2005;65(15):6493–7. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 45.Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S, et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69(2):466–74. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121(10):3804–9. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leitner JM, Mayr FB, Firbas C, Spiel AO, Steinlechner B, Novellini R, et al. Reparixin, a specific interleukin-8 inhibitor, has no effects on inflammation during endotoxemia. Int J Immunopathol Pharmacol. 2007;20(1):25–36. doi: 10.1177/039463200702000104. [DOI] [PubMed] [Google Scholar]
- 48.Simões BM, Piva M, Iriondo O, Comaills V, López-Ruiz JA, Zabalza I, et al. Effects of estrogen on the proportion of stem cells in the breast. Breast Cancer Res Treat. 2010;129(1):23–35. doi: 10.1007/s10549-010-1169-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.