Abstract
Chitosan is one of the most abundant carbohydrate biopolymers in the world, and chitosan oligosaccharide (COS), which is prepared from chitosan, is a plant immunity regulator. The present study aimed to validate the effect of COS on inducing resistance to tobacco mosaic virus (TMV) in Arabidopsis and to investigate the potential defence-related signalling pathways involved. Optimal conditions for the induction of TMV resistance in Arabidopsis were COS pretreatment at 50 mg/L for 1 day prior to inoculation with TMV. Multilevel indices, including phenotype data, and TMV coat protein expression, revealed that COS induced TMV resistance in wild-type and jasmonic acid pathway- deficient (jar1) Arabidopsis plants, but not in salicylic acid pathway deficient (NahG) Arabidopsis plants. Quantitative-PCR and analysis of phytohormone levels confirmed that COS pretreatment enhanced the expression of the defence-related gene PR1, which is a marker of salicylic acid signalling pathway, and increased the amount of salicylic acid in WT and jar1, but not in NahG plants. Taken together, these results confirm that COS induces TMV resistance in Arabidopsis via activation of the salicylic acid signalling pathway.
Chitin is an abundant, water insoluble biopolymer, which is found mainly in the hard outer skeleton of marine animals, as well as in mushrooms and yeasts1,2. Chitosan is the product of chitin deacetylation and has limited application due to its insolubility in both organic solvents and water. Chitosan oligosaccharide (COS), derived from the enzymatic hydrolysis of chitosan, overcomes this limitation1,3. To date, COS has been shown to have a wide range of biological applications, including use as a health food4, a plant growth stimulator5,6, feed additive7, and an antimicrobial agent8.
The most noteworthy application of COS is its use as a regulator of plant immunity9. In 1980, Hadwiger first reported that COS could induce plant immunity10. Since then, COS has been considered as a potent elicitor of plant immunity that is used in many plants, including tobacco11, camellia6, wheat12, oilseed rape13, tomato14, soybean15, and grapevine16,17. However, the mechanism of COS-induced immunity in plants, especially the signalling processes involved, remains unclear.
Salicylic acid (SA) and jasmonic acid (JA) are two plant hormones essential for defence signal transduction18,19,20, which signal through two different pathways. SA mediates systemic acquired resistance (SAR), while JA mediates induced systemic resistance (ISR). SA and JA signalling pathways influence each other through a complex network of synergistic and antagonistic interactions19,21.
The results of previous studies have shown that COS induces the production of plant hormones, especially JA, in plants. Following COS treatment, the JA content in tomato22, rice23, and oilseed rape13 increased, suggesting that COS activates plant immunity through the JA signalling pathway in some plant systems. However, other studies have reported the reverse effect. For example, Obara et al. found that methyl-salicylic acid (MeSA) accumulates in rice leaves 7 h after COS treatment24.
Tobacco mosaic virus (TMV) is a plant virus used to study plant disease, and SA and JA signalling pathways have both been shown to be involved in plant defence following TMV infection. In tobacco and tomato, SA treatment was found to increase resistance to TMV, which resulted in reduced viral accumulation and delayed appearance of disease symptoms25,26. In addition, exogenous application of jasmonic acid methyl ester reduced local resistance to TMV and permitted systemic viral movement27. However, other reports on TMV-infected tobacco, showed that the expression of genes associated with the JA-mediated defence pathway were significantly upregulated after 1 d, whereas genes associated with the SA-mediated defence pathway were significantly upregulated after 5 days. Thus, both SA and JA are required for systemic resistance against TMV in tobacco28. We previously confirmed that COS could induce resistance to TMV in plants9,29,30; however, the signalling pathway involved in this resistance is unknown.
Mutants with defective signalling pathways are important experimental models, especially in the study of SA and JA in plant resistance31,32,33. Jar1, an ethyl methane sulfonate mutant first obtained by Staswick et al., contains a mutation in AT2G4637031, which encodes jasmonic acid-amido synthetase JAR1. Jar1 plants have reduced sensitivity to JA, and have been widely used as a JA signalling-deficient mutant since 199233,34,35,36. NahG plants, in which the bacterial nahG gene has been introduced, encode salicylate hydroxylase, which degrades SA. These plants accumulate very little SA and therefore have a defective SA pathway. This mutant was first introduced by Delaney et al. in 1994, and has been widely used in studies of plant defence and signalling37,38,39,40,41.
In the present study, multilevel indices, including phenotype data, TMV coat protein (TMV-CP) gene expression, and TMV-CP levels were investigated to confirm the effect of COS on inducing resistance to TMV in Arabidopsis. Furthermore, jar1 and NahG Arabidopsis mutants were employed to explore the signalling pathways involved in COS-induced resistance to TMV.
Results
COS induces resistance to TMV in Arabidopsis
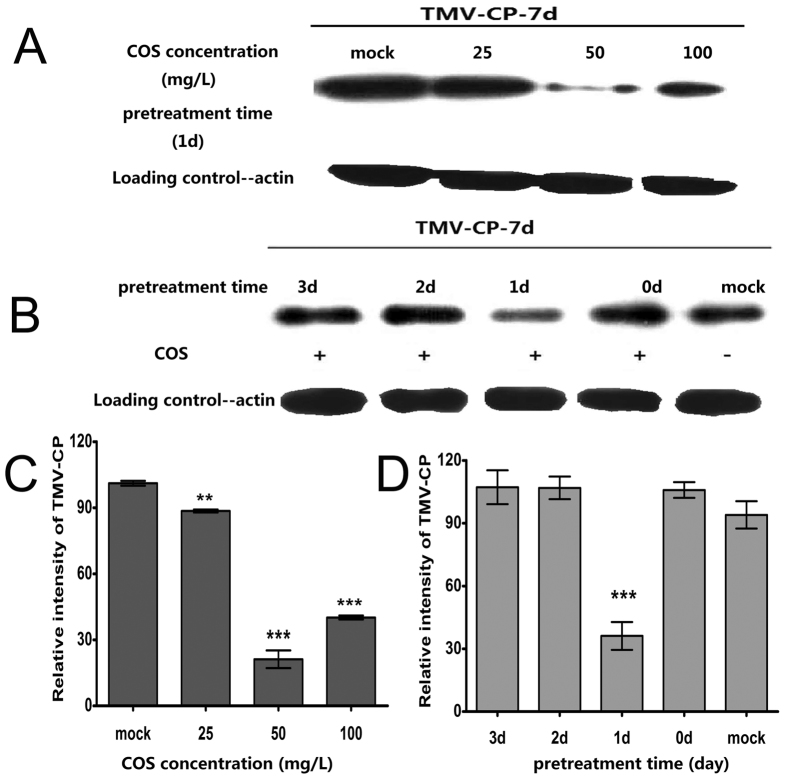
The most effective concentration of COS, and the optimal pretreatment time, for resistance to TMV were studied in wild Arabidopsis plants. The TMV-CP levels in inoculated leaves showed that COS could induce TMV resistance in Arabidopsis (Fig. 1). The most effective COS concentration was 50 mg/L (Fig. 1A,C) and the optimal pretreatment time was 1 day (Fig. 1B,D).
Figure 1. The most effective COS concentration and pretreatment time.
TMV-CP was detected by western-blot using anti-TMV antibody.(A) The amount of TMV-CP in leaves pretreated 1d before TMV inoculation with different concentrations of COS. (B) The amount of TMV-CP in leaves pretreated with 50 mg/L COS with different pretreatment time. All samples were inoculated TMV for 7d, more than 30 Arabidopsis plants were used in each groups. These were a representative experiment and were independently repeated three times using different samples from independent treatment. The same amount of protein was loaded on SDS-PAGE gels, detected by anti-plant actin antibody, which served as the protein loading control. Full-length blots were uploaded as supplementary information (Supplementary Fig. A,B). The detection of TMV-CP and loading control were carried out under the same experimental conditions. (C,D) The relative TMV-CP levels in (A) and (B) were quantified by scanning densitometry using image j program. The quantitative densitometry values (histograms) which normalized to the loading controls were expressed as mean ± SD of three independent experiments. Asterisks indicate significant differences (**P < 0.01; ***P < 0.001).
The effectiveness of COS on defence signalling pathway mutants
In order to explore the signalling pathways involved in COS-induced resistance to TMV in Arabidopsis, WT, JA signalling-deficient mutant (jar1) and SA signalling-deficient mutant (NahG, sid2) plants were used.
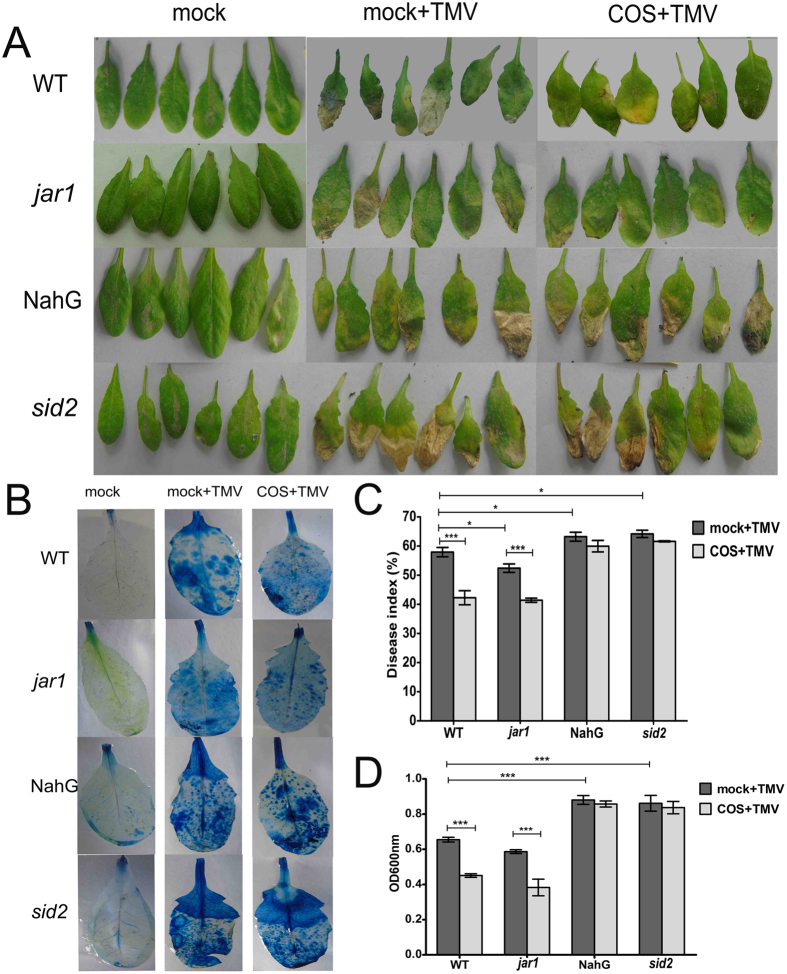
The symptoms of TMV infected leaves showed multiple necrotic lesions (Fig. 2A). The areas of necrotic lesions on infected leaves were evaluated (Fig. 2B). The symptoms on NahG leaves (63.2% ± 2.7%) and sid2 (64.2% ± 2.2%) were more severe than those on WT (57.8% ± 2.7%) and jar1 (52.4% ± 2.5%) leaves after 7-days TMV infection (Fig. 2B). In COS-pretreated plants, the TMV symptoms on WT (42.2 ± 4.2%) and jar1 (41.4% ± 1.3%) leaves were less severe, but no significant effect was observed on NahG (61.0% ± 3.4%) and leaves sid2 (61.6% ± 0.3%). These results showed that COS could induce resistance to TMV in Arabidopsis, and that COS was more effective in WT and jar1 plants.
Figure 2. The effect of COS treatment on different mutants.
Mock means pretreated Arabidopsis with water, and then inoculated with PBS by gently rubbing the leaf surface using Carborundum (silicon carbide). Mock + TMV or COS + TMV means pretreated Arabidopsis with water or 50 mg/L COS, and then inoculated with TMV by gently rubbing the leaf surface using Carborundum (silicon carbide). (A) The necrotic lesion on leaves after inoculated with TMV for 7d in different mutants. (B) The amount of cell death in rosette leaves of Arabidopsis which inoculation with TMV 7d was stained with Evans blue. (C) The disease index which evaluated the necrotic lesion on leaves of different mutants. Values are the means ± SD from three independent measurements. Asterisks indicate significant differences (*P < 0.05, **P < 0.01; ***P < 0.001). (D) The amount of cell death in rosette leaves, by measured the absorption under 600 nm using spectrophotometer. Values are the means ± SD from three independent measurements. Asterisks indicate significant differences (**P < 0.01, ***P < 0.001).
Cell death in the rosette leaves of Arabidopsis following a 7-day inoculation with TMV was observed by Evans blue staining. Increased levels of cell death were observed in NahG leaves than in WT and jar1 leaves. Cell death in WT and jar1 leaves was not observed in the COS pretreated group, but little effect was observed in NahG and sid2 plants (Fig. 2C,D). These data suggested that COS pretreatment could inhibit cell death in leaves during TMV infection in a SA-dependent manner.
In addition, mRNA and proteins levels of TMV-CP were determined in inoculated leaves. The q-PCR (Fig. 3C) and western blot data (Fig. 3A,B) showed that the expression of TMV-CP mRNA and protein in NahG leaves was much higher than that in WT and jar1 leaves. In COS pretreated plants, the expression of TMV-CP mRNA and protein was markedly decreased in WT and jar1 leaves, but was not obviously changed in NahG leaves.
Figure 3. The expression of TMV-CP in rosette leaves of different Arabidopsis mutants.
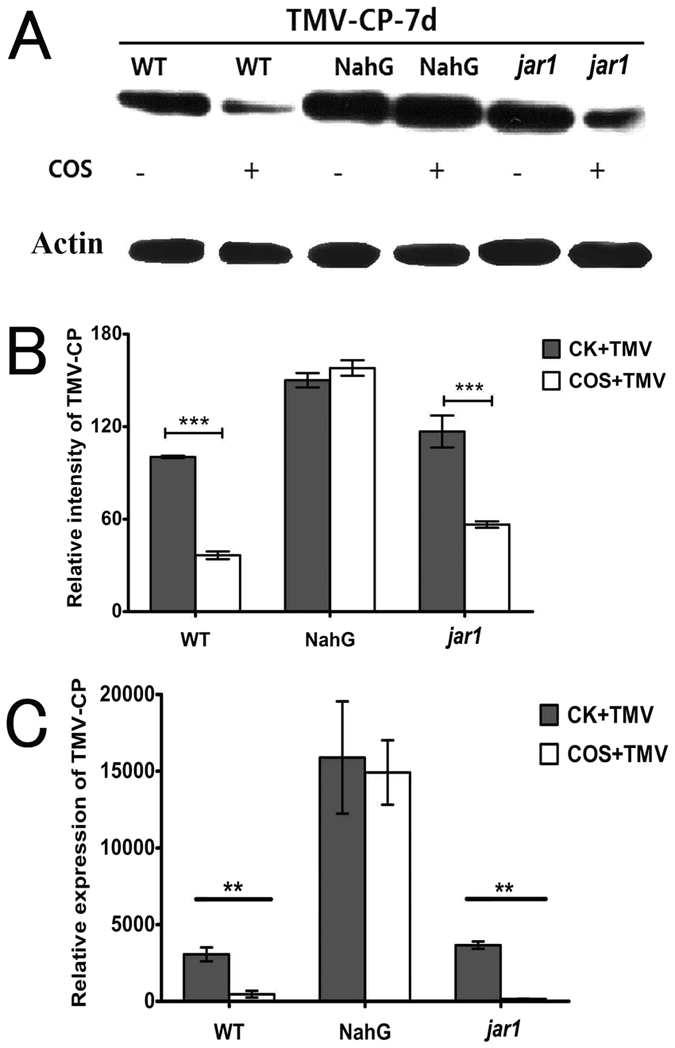
(A) The amount of TMV-CP in leaves was detected by western-blot. All samples were inoculated TMV for 7d, more than 30 Arabidopsis plants were used in each groups. This was a representative experiment and was independently repeated three times using different samples from independent treatment. The same amount of protein was loaded on SDS-PAGE gels, detected by anti-plant actin antibody, which served as the protein loading control. Full-length blots were uploaded as supplementary information (Supplementary Fig. C). The detection of TMV-CP and loading control were carried out under the same experimental conditions. (B) The relative TMV-CP levels in (A), which was quantified by scanning densitometry using image j program. The quantitative densitometry values (histograms) which normalized to the loading controls were expressed as mean ± SD of three independent experiments. Asterisks indicate significant differences (***P < 0.001). (C) The expression of TMV-CP in leaves was detected by q-PCR, using actin8 as reference gene. Values were presented as the means ± SD from three independent measurements. Asterisks indicate significant differences (**P < 0.01).
Taken together, these data confirm that COS pretreatment induced resistance to TMV in WT and jar1 plants, but did not have an obvious effect in NahG plants, implying that COS induces TMV resistance via the SA signalling pathway.
Expression of defence-related genes
To explore the role of SA and JA signalling pathways in COS-induced resistance to TMV, we examined the expression of signalling pathway marker genes, including PR1 (a SA pathway marker) and PDF1.2 (a JA pathway marker) in leaves following infection with TMV for 7 days.
In WT Arabidopsis, TMV infection (WT + mock + TMV) promoted a 4.16-, and 8.28-fold increase in the expression of PR1 and PDF1.2, respectively, compared with the control group (WT + mock) (Fig. 4A). Following COS pretreatment (WT + COS + TMV), PDF1.2 expression was inhibited but the expression of PR1 was increased 5.37-fold compared with that observed in the WT + mock + TMV group.
Figure 4. The relative expression of PR1 and PDF1.2 in different mutants detected by q-PCR.
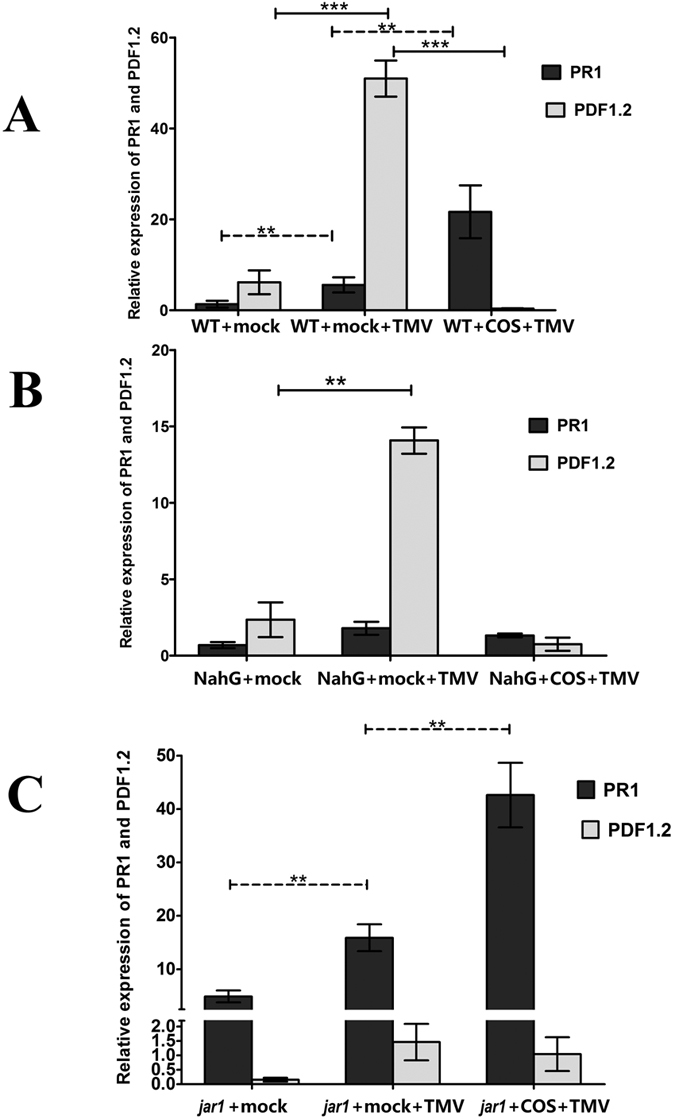
Mock + TMV or COS + TMV means Arabidopsis pretreated with water or 50 mg/L COS, then inoculated with TMV by gently rubbing the leaf surface using Carborundum (silicon carbide). Mock means pretreated Arabidopsis with water, then inoculated with PBS. (A) The expression of PR1 and PDF1.2 in WT. (B) The expression of PR1 and PDF1.2 in NahG mutant. (C) The expression of PR1 and PDF1.2 in jar1 mutant. All data were measured using actin8 as reference gene. Values were presented as means ± SD from three independent measurements. Asterisks indicate significant differences (**P < 0.01; ***P < 0.001).
In NahG plants, which have a defective SA signalling pathway, the expression of PR1 (NahG + mock) was much lower compared with that in the related WT control (2.09-fold lower, WT + mock) and jar1 (7.10-fold lower, jar1 + mock) groups. In NahG plants, TMV infection (NahG + mock + TMV) resulted in up-regulation of PDF1.2, which was increased 5.99-fold compared with the control (NahG + mock) (Fig. 4B). In the COS pretreated group (NahG + COS + TMV), PR1 expression was not enhanced compared with that in the WT and jar1 plants, since SA signalling is defective in NahG plants; however, the expression of PDF1.2 was still inhibited (Fig. 4B).
In jar1 plants, which have defective JA signalling, the level of PDF1.2 expression was much lower compared with the WT and NahG plants. The expression of PR1 in jar1 plants (jar1 + mock) was higher than that in WT plants (3.66-fold increase, WT + mock), while the expression of PDF1.2 was decreased 39.61-fold. In jar1 mutant plants, TMV infection (jar1 + mock + TMV) resulted in the upregulation of PR1 expression, which increased 2.55-fold compared to the control plants (jar1 + mock) (Fig. 4C). In the COS pretreated group (jar1 + COS + TMV), the expression of PR1 was enhanced 7.31-fold compared to the control group (jar1 + mock) (Fig. 4C).
In conclusion, expression of the signalling pathway defence marker PR1 was upregulated following COS pretreatment in WT and jar1 plants, which suggests that COS-induced TMV-resistance in Arabidopsis is mainly dependent on the SA signalling pathway.
COS induced changes in SA and JA level in Arabidopsis
To further confirm that COS induces resistance to TMV in Arabidopsis via the SA signalling pathway, the levels of SA and JA were determined in inoculated leaves. The SA content was clearly enhanced in COS-pretreated WT (WT + COS + TMV) and jar1 (jar1 + COS + TMV) plants, with increases of 10.01- and 4.43-fold observed, respectively, compared with the mock + TMV groups (WT + mock + TMV, jar1 + mock + TMV) (Fig. 5A), which is consistent with the expression of PR1 shown in Fig. 4. The SA content in NahG plants was much lower than that in the related WT and jar1 plants, and COS pretreatment had no effect on expression in NahG plants (Fig. 5A).
Figure 5. The relative level of SA and JA in different mutants.
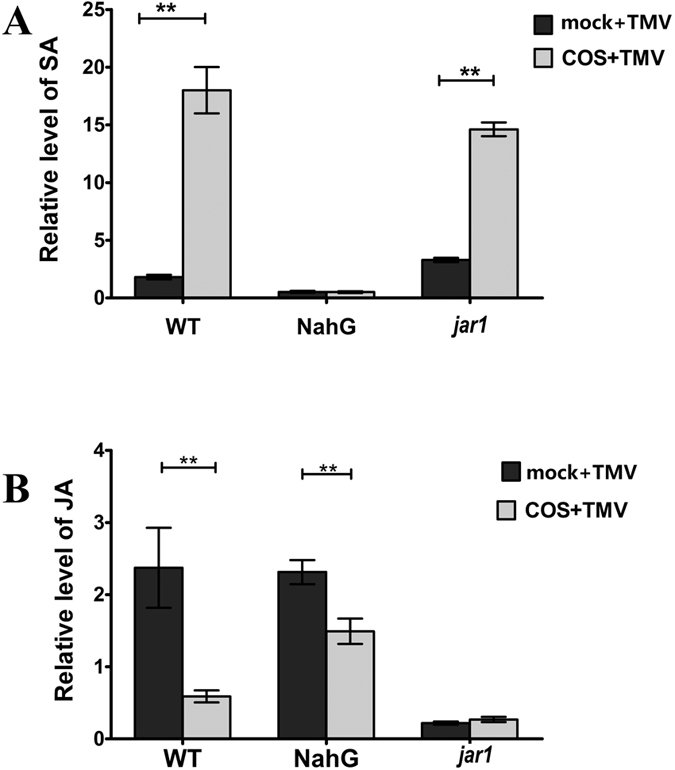
mock + TMV or COS + TMV means Arabidopsis pretreated with water or 50 mg/L COS, then inoculated with TMV by gently rubbing the leaf surface using Carborundum (silicon carbide). (A) The content of SA in different mutants. (B) The JA accumulation in different mutants. WT + mock were used as the control group. Values were presented as means ± SD from three independent measurements. Asterisks indicate significant differences (**P < 0.01).
TMV infection increased the JA content in WT and NahG plants, whereas in COS pretreated plants, the JA content was decreased due to an increase in the level of SA (Fig. 5B). The JA content in jar1 was much lower than that in WT and NahG (Fig. 5B) plants, since the jasmonic acid-amido synthetase JAR1 was dysfunctional in jar1 mutants. Phytohormone data suggested that COS induces resistance to TMV in Arabidopsis, in a manner that is dependent on the SA signalling pathway.
COS induced NO production in Arabidopsis rosette leaves
NO is an important signalling messenger involved in plant immunity. We found that COS induced NO production in epidermal cells in WT, NahG, and jar1 mutant plants (Fig. 6A). To further quantify changes in NO production following COS treatment in different mutants, the NO content in leaves was measured. After the rosette leaves were sprayed with 50 mg/L COS, the NO content in leaves of different mutants (WT, NahG, jar1) increased 2.41-, 3.06-, 2.90-fold, respectively, compared to that in the mock-treated control group.
Figure 6. The NO production in rosette leaves of Arabidopsis.
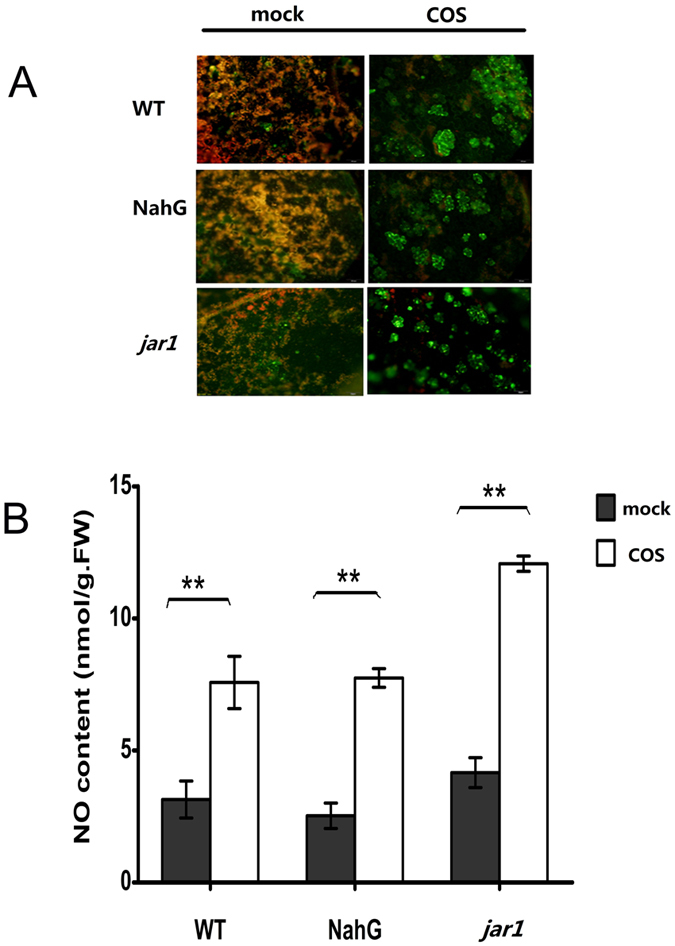
(A) The NO production (green) in epidermal cells of different Arabidopsis mutants after COS treatment, observed by using fluorescence microscopy. (B) The NO content in rosette leaves measured by Griess reagent. Values are the means ± SD from three independent measurements. Asterisks indicate significant differences (**P < 0.01).
In conclusion, COS induced NO production in Arabidopsis leaves independent of the SA and JA signalling pathways.
Discussion
COS has been shown to elicit plant defence responses to a broad spectrum of pathogens in many plant species, including tobacco, rapeseed, rice, and grapevine11,16,30,42,43. The duration and concentration of pretreatment are important for the effect of COS on plant defence. In a previous study, plants pretreated with COS prior to pathogen infection were get better control efficacy than COS treatment after pathogen infection9. The optimal duration of COS pretreatment differs for different plant-pathogen interactions. For example, the optimal duration of COS pretreatment is 1 day for tobacco-TMV interactions29, and 3 days for rapeseed-S. sclerotiorum interactions44. The reason for this difference may be because COS signal transduction, and the plant response to different diseases occur differently in different plants45. As a broad-spectrum plant elicitor, low concentrations of COS (25–100 mg/L) were most effective at activating plant disease defences12,46. Thus, in the present study, the optimum pretreatment time (0–3 days) and concentration (0–100 mg/L) were explored using TMV-CP protein levels as the disease evaluation index. We found that COS could induce TMV resistance in Arabidopsis and that the most effective concentration was 50 mg/L with a pretreatment duration of 1 day prior to inoculation. These data confirm that COS is an effective elicitor of plant immunity in TMV-infected Arabidopsis.
The hypersensitive response (HR) occurs during induced disease resistance in plants. HR is characterized by rapid cell death at the site of pathogen invasion, commonly resulting in the formation of necrotic lesions in which the pathogen is thought to localize, thereby inhibiting its multiplication and spread47. Thus, the area of the necrotic lesion correlates with the amount of virus present on the leaves, to some extent. Data obtained in the present study regarding phenotype data (Fig. 2) were consistent with those obtained by q-PCR (Fig. 3C) and western blotting (Fig. 3A,B) for TMV-CP in rubbed leaves. These data confirm that COS induced TMV resistance in Arabidopsis, which was more obvious in WT and jar1 plants. We used these four data from different levels to get this conclusion, so this research work was more systemic and convinced than former works5,6,13,17. Taken together, the results of the present study confirm that COS induces TMV resistance to Arabidopsis via the SA signalling pathway.
The results of a previous study suggested that SA plays a critical role in plant defence against virus invasion. In compatible host-virus interactions, SA reduces viral replication and restricts cell-to-cell and systemic movement, and several defence-related genes are induced by SA-dependent signalling48,49. Exogenously applied SA increased resistance to TMV in tomato, and TMV infection increased the endogenous SA content26. Activation of SA biosynthesis or signalling in many plant-pathogen interactions has been shown to inhibit JA biosynthesis or signalling21,50. Exogenously applied methyl jasmonate, a methyl ester of JA, reduced local resistance to TMV and permitted systemic viral movement in tobacco plants27. Thus, the results of previous studies suggested that SA functions as a positive regulator of TMV resistance. This conclusion is consistent with our findings that COS induces TMV resistance in Arabidopsis by upregulating the expression of PR1 and increasing the level of SA.
Previous studies have shown that several genes associated with JA synthesis and regulation are induced by COS in Brassica napus45 and that the JA content in tomato22, rice23, and rapeseed13 is increased. Those results suggested that COS defence signalling is mainly mediated by activation of the JA signal pathway under some plant-disease interactions. However, the results of the present study showed that COS activated the SA signalling pathway, suggesting that COS induces plant defences via different signalling pathways during different plant-disease interactions. This is a common phenomenon for many plant elicitors.
For example, harpin, the product of the hrpN gene of Erwinia amylovora, is an effective elicitor that induces plant responses in many plants51,52,53. Harpin induced disease (Peronospora parasitica and Pseudomonas syringae) resistance in Arabidopsis through activation of the SA signalling pathway54. However, in harpin-treated Phalaenopsis orchids, JA signalling was activated55. This phenomenon was also found for flg22, another effective elicitor that induces plant defence responses. Flg22 treatment induced SA accumulation and increased the expression of typical SA-related genes56,57 in Arabidopsis. Flg22-triggered oxidative bursts have been shown to depend on SA signalling58. In addition, flg22 induced Arabidopsis resistance to Golovinomyces cichoracearum through SA-mediated signalling59. However, analysis of Arabidopsis mutants showed that JA, ethylene, and SA signalling all contribute positively to flg22-triggered immunity to a virulent bacterial strain, Pseudomonas syringae DC300060.
Those results suggested that the type of plant disease is important for the elicitor to activate a specific defence signal pathway. Usually, biotrophic and hemi-biotrophic pathogens are more sensitive to SA-dependent responses, whereas necrotrophic pathogens and herbivorous insects are commonly resisted by JA-dependent defence mechanisms61,62. Elicitors signal through appropriate signalling pathways in order to resist different diseases; thus, the effect of the elicitor becomes broad spectrum.
The q-PCR data presented in this study (Fig. 4) showed that the expression of PR1 was higher in jar1 plants compared with WT plants (3.66-fold increase), and COS enhanced PR1 expression to a greater level in jar1 mutants (1.93-fold increase), implying that the resistance to TMV was stronger in jar1 plants. Indeed, from the disease index (Fig. 2C), the effect of COS on TMV disease control was more obvious in jar1 plants. However, from the cell death level (Fig. 2D) and the expression of TMV-CP (Fig. 3), the disease control in jar1 was not enhanced compared with WT (no significant difference). Furthermore, the SA content (Fig. 5A) was not completely consistent with the expression of PR1 (Fig. 4). Signalling networks in plants are highly complex; therefore, further research is needed.
Once COS signals reach the cytoplasm, they activate a complicated signalling network, which requires further study. The results of the present study suggest that COS induces TMV resistance in Arabidopsis, mainly through the SA signalling pathway. Whether an early signalling molecule, such as NO is involved in COS-induced resistance, and whether it acts in a SA-dependent manner, remains unknown. To address this, we measured NO production in different Arabidopsis mutants following COS pretreatment. In a previous study, COS promoted NO production in epidermal cells of B. napus leaves shortly after treatment. NO was generated within 5 min of treatment, lasted for more than 30 min, and declined after 2 h13. We found that the NO content in rosette leaves of different Arabidopsis plants (WT, NahG, jar1) increased following 15-min COS treatment (Fig. 6). Those results suggested that COS induced NO production in epidermal cells of Arabidopsis in a SA- and JA-independent manner, implying that NO may be acting upstream of the SA and JA signalling pathways.
In conclusion, COS induces resistance to TMV in Arabidopsis at an optimal pretreatment dose of 50 mg/L for 1 day before inoculation with TMV. Furthermore, from the phenotype, TMV-CP expression, defence gene expression, and phytohormone results, we confirm that COS induces TMV resistance via the SA signalling pathway. From this and previous findings, we conclude that COS induces different signalling pathways involved in plant defence according to the type of plant disease.
Materials and Methods
Reagents
COS with a degree of polymerization (DP) from 2 to 10 and a degree of deacetylation (DD) of 95% was obtained from Dalian GlycoBio (Dalian, China). Reagents for use in molecular biology studies were purchased from TaKaRa Biotechnology (Dalian, China). SA and JA standards were obtained from sigma. The remaining chemicals were all of analytical grade and were purchased from Chinese companies.
Plant materials and treatment
Arabidopsis ecotype Columbia (WT) and mutants including jar1 (JA-defective mutant) and NahG (express the bacterial nahG gene encoding salicylate hydroxylase to degradation SA), sid2 (SA-deficient mutant, a generous gift from Dr. Lin Hao’s Laboratory, Shenyang Normal University) were used in this experiment. The seed germination medium was composed of half-strength MS basal salts, with 1% sucrose and 0.8% agar, pH 5.8. All of the seeds were sterilized and planted on the agar plates. After cold treatment, the plates were oriented vertically in the growth chamber under 12 h of light, 12 h of dark, 22 °C, 55% humidity, with a light intensity of ~120 μmol m−2s−1 for germination. The seedlings were transplanted in plastic pots containing a mixture of vermiculite and sterilized loamy soil (1:1, v/v) on the 7th day after germination, then grown in the growth chamber for 30 days, before being used in this experiment.
To determine the most effective treatment method, WT plants were pretreated by spraying different concentrations of COS (0, 25, 50, 100 mg/L) one day before inoculation with TMV to search for the most effective COS concentration. WT plants were then pretreated with COS for different durations (3, 2, 1, 0 day), and then inoculated with TMV to identify the best pretreatment time. Each group contained more than 30 Arabidopsis plants, which were divided equally between three parallel experiments. The experiments were repeated at least three times.
To identify the major signalling pathway involved in COS-induced resistance to TMV, the WT, NahG, sid2 and jar1 plants were evenly assigned into control group (mock) (sprayed with water 1 day before inoculation with 50 mM phosphate buffer (PBS)), COS group (sprayed with COS 1 day before inoculation with PBS), mock + TMV group (sprayed with water 1 day before inoculation with TMV), COS + TMV group (sprayed with COS 1 day before inoculation with TMV). Each group contained more than 30 Arabidopsis plants, divided equally into three parallel experiments. The experiments were repeated at least three times.
TMV inoculation
TMV was kept in our lab and multiplied in tobacco. TMV was extracted from infected tobacco leaves by homogenization in 50 mM phosphate buffer (pH 5.5) (1 g/5 mL), then centrifuged at 2000 × g for 6 min. The supernatant extract was used for mechanical inoculation29.
Rosette leaves of Arabidopsis plants were used for TMV inoculation. Inoculation was performed by gently rubbing the leaf surface using carborundum (silicon carbide) with viral suspension or 50 mM phosphate buffer (pH 5.5). Plants were then inoculated at 22 °C with 12 h of light at 120 μmol m−2 s−1.
Symptoms were assessed 7 days after TMV infection as follows: first, counting the leaves number in each level (the percentage of necrotic lesions areas on leaves(S)): 0 < S < 0.25, 1 level; 0.25 < S < 0.5, 2 level; 0.5 < S < 0.75, 3 level; 0.75 < S < 1, 4 level. Then, the following formula was used to obtain symptom data: disease index (%) = (∑level × leaves per level/total number of leaves × the highest level) × 100. Inoculated leaves were sampled 7 days after TMV infection, weighed, frozen in liquid nitrogen, and kept in −80 °C until use.
SDS-PAGE and immunoblotting
Plant material was ground in liquid nitrogen, resuspended in 200 μL 20 mM PBS (20 mM Na2HPO4, and 20 mM NaH2PO4, pH 7.4) per 100 mg of plant material in an ice bath, and centrifuged. Part of the supernatant was used to determine protein concentration, by the Bradford method using bovine serum albumin as a standard (Bradford 1976). The remaining supernatant was immediately mixed with SDS-PAGE loading buffer, denatured in boiling water for 5 min, and the same amount of protein was subjected to two SDS-PAGE (10%) gels. Western blotting was performed using polyvinylidene difluoride membranes, blocked with 5% (w/v) non-fat dry milk in Tris-buffered saline (20 mM Tris-HCl, pH 8.0, and 150 mM NaCl) with 0.05% Tween 20.
One membrane was probed with an anti-TMV-CP antibody (1:3000; a generous gift from Dr. Huaifang Li’s Laboratory, China Agricultural University). Bound primary antibody was detected with ECL reagent after incubation with a goat anti-rabbit antibody (1:2000; Santa Cruz). Another membrane was probed with an anti-plant actin antibody (1:1500; Earthox), and goat anti-mouse antibody (1:2000; Earthox) as a second antibody. Actin was detected under the same conditions, and served as a protein loading control. Each data point was normalized against the corresponding actin data point. To obtain quantitative results, immunoblots were analysed by densitometry using Image J software (National Institutes of Health, Bethesda, MD, USA).
Measurement of cell death
Evans blue staining was used as a marker of cell death, as follows. The leaves incubated with TMV for 7 days were soaking in 0.05% Evans blue for 24 h, washed with water, dried with filter paper, and then soaked in boiled anhydrous alcohol and glycerol (9:1 V/V) for 30 min to remove the chlorophyll. The leaves were imaged with a digital camera, and the blue areas on leaves represent cell death63.
To estimate the level of cell death in Arabidopsis thaliana leaves caused by TMV infection, the leave tissues that had been stained with Evans blue were added to 4 mL 1% SDS water solution to extract Evans blue for 3 days. The tubes were centrifuged at 9000 × g for 3 min. The supernatant was removed and the optical density was determined at 600 nm using a spectrophotometer64.
Assay of nitric oxide production
NO production was detected using the fluorescent indicators DAF-2DA13 with some modification. The epidermis was peeled carefully from the surface of rosette leaves and then incubated in MES/KCl buffer (10 mM MES/KOH, 50 mM KCl, 100 μM CaCl2, pH 6.5) for 30 min in the light. Then, the epidermis was incubated in Tris/KCl buffer (10 mM Tris, 50 mM KCl, pH 7.2) containing 5 μmol/L DAF-2DA (sigma) for 30 min at room temperature in the dark. After excess DAF-2DA was removed by washing three times with fresh Tris/KCl buffer, the epidermis was placed in Tris/KCl buffer containing COS for 15 min, Tris/KCl buffer as contrast. NO production was detected using fluorescence microscopy with an excitation wavelength of 430–485 nm and an emitting wavelength of 515 nm.
The NO content in leaves of different Arabidopsis mutants was determined using Griess reagent (Beyotime Biotech) according to the manufacturer’s instructions. Rosette leaves of WT, NahG, and jar1 plants were sprayed with COS or water, incubated for 15 min, picked and weighed, then quickly frozen in liquid nitrogen for subsequent NO content determination. The experiments were repeated at least three times for each treatment.
Quantitative PCR (q-PCR) Analysis of Gene Expression
Total RNA for q-PCR analysis was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and then quantified by ScanDrop100 (AnalytikJenaAG, German). RNA of the same quality was reverse transcribed into cDNA with AMV Reverse Transcriptase (Takara). Dilutions of cDNA were used as templates in q-PCR (qTOWER 2.2, AnalytikJenaAG, German) using a SYBR Green kit (Bio-Rad) with gene-specific primers designed using Premier 5.0 (Table 1). Relative expression levels were calculated using the 2−ΔΔCt method65, and were all normalized to the expression of actin in each group.
Table 1. Primers used for quantitative PCR.
| Gene names | Locus ID | Forward | Reverse |
|---|---|---|---|
| Atactin8 | AT1G49240 | CACATGCTATCCTCCGTCTC | CACTTGTCCGTCGGGTAAT |
| TMV-CP | --------- | ATTGTCATCAGCGTGGGC | TGCTGTGACTAGCGGGTCTA |
| AtPR1 | AT2G14610 | AATGCTCAAGATAGCCCACAAG | AATAAGTCACCGCTACCCCAG |
| AtPDF1.2 | AT5G44420 | TCACCCTTATCTTCGCTGCTC | ATGTCCCACTTGGCTTCTCG |
SA and JA Measurements
Quantification of JA and SA in inoculated Arabidopsis leaves was performed using a liquid chromatography-electrospray ionisation tandem mass spectrometry method as previously described66 with some modification. Chromatography conditions were modified by using a C18 column (Thermo, 250 × 4.6 mm), and an elution gradient of 5 ml/L formic acid (A) and acetonitrile (B) was set at a flow rate of 1 mL/min. Elution program (t min [%A:%B]) was: 0 min (90:10), 25 min (20:80), 26 min (90:10), and 35 min (90:10). The temperature of the column oven was 30 °C and the injection volume was 10 μL. Mass spectrometry conditions were modified by using Q-trap 5500 (AB SCIEX, USA) with different collision energy.
Additional Information
How to cite this article: Jia, X. et al. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Sci. Rep. 6, 26144; doi: 10.1038/srep26144 (2016).
Supplementary Material
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31370811) and the National Key Technology Support Program (2013BAB01B01), H.Y was supported by CAS Youth Innovation Promotion Association (2015144).
Footnotes
Author Contributions H.Y. designed the experiments; X.C.J., Q.S.M., H.H.Z. and W.X.W. planted the Arabidopsis for experiment, inoculated TMV and sampled; X.C.J. and Q.S.M. carried out experiments and analysed experimental results. X.C.J. interpreted the data and wrote the manuscript; H.Y. revised and approved the final manuscript.
References
- Kim S. K. & Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohyd Polym. 62, 357–368, doi: 10.1016/j.carbpol.2005.08.012 (2005). [DOI] [Google Scholar]
- Nitschke J., Altenbach H. J., Malolepszy T. & Molleken H. A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohyd Res. 346, 1307–1310, doi: 10.1016/j.carres.2011.03.040 (2011). [DOI] [PubMed] [Google Scholar]
- Yin H., Du Y. G. & Zhang J. Z. Low Molecular Weight and Oligomeric Chitosans and Their Bioactivities. Curr Top Med Chem. 9, 1546–1559 (2009). [DOI] [PubMed] [Google Scholar]
- Chantarasataporn P. et al. Water-based oligochitosan and nanowhisker chitosan as potential food preservatives for shelf-life extension of minced pork. Food Chem. 159, 463–470, doi: 10.1016/j.foodchem.2014.03.019 (2014). [DOI] [PubMed] [Google Scholar]
- Chatelain P. G., Pintado M. E. & Vasconcelos M. W. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 215, 134–140, doi: 10.1016/j.plantsci.2013.11.009 (2014). [DOI] [PubMed] [Google Scholar]
- Li S. J. & Zhu T. H. Biochemical response and induced resistance against anthracnose (Colletotrichum camelliae) of camellia (Camellia pitardii) by chitosan oligosaccharide application. Forest Pathol. 43, 67–76, doi: 10.1111/j.1439-0329.2012.00797.x (2013). [DOI] [Google Scholar]
- Swiatkiewicz S., Swiatkiewicz M., Arczewska-Wlosek A. & Jozefiak D. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J Anim Physiol an N. 99, 1–12, doi: 10.1111/Jpn.12222 (2015). [DOI] [PubMed] [Google Scholar]
- Yang L. Y., Zhao P., Wang L., Filippus I. & Meng X. H. Synergistic effect of oligochitosan and silicon on inhibition of Monilinia fructicola infections. J Sci Food Agr. 90, 630–634, doi: 10.1002/Jsfa.3860 (2010). [DOI] [PubMed] [Google Scholar]
- Yin H., Zhao X. M. & Du Y. G. Oligochitosan: A plant diseases vaccine-A review. Carbohyd Polym. 82, 1–8 (2010). [Google Scholar]
- Hadwiger L. A. & Beckman J. M. Chitosan as a Component of Pea-Fusarium-Solani Interactions. Plant Physiol. 66, 205–211, doi: 10.1104/Pp.66.2.205 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Y., Wang W. X., Yin H., Zhao X. M. & Du Y. G. Oligochitosan induces programmed cell death in tobacco suspension cells. Carbohyd Polym. 87, 2270–2278, doi: 10.1016/j.carbpol.2011.10.059 (2012). [DOI] [Google Scholar]
- Wang M. Y. et al. Effects of chitosan oligosaccharides on the yield components and production quality of different wheat cultivars (Triticum aestivum L.) in Northwest China. Field Crop Res. 172, 11–20, doi: 10.1016/j.fcr.2014.12.007 (2015). [DOI] [Google Scholar]
- Yin H. et al. Chitosan Oligosaccharides-Triggered Innate Immunity Contributes to Oilseed Rape Resistance against Sclerotinia Sclerotiorum. Int J Plant Sci. 174, 722–732, doi: 10.1086/669721 (2013). [DOI] [Google Scholar]
- Zhang P. Y. & Chen K. S. Age-Dependent Variations of Volatile Emissions and Inhibitory Activity Toward Botrytis cinerea and Fusarium oxysporum in Tomato Leaves Treated with Chitosan Oligosaccharide. J Plant Biol. 52, 332–339, doi: 10.1007/s12374-009-9043-9 (2009). [DOI] [Google Scholar]
- Valdes-Lopez O. et al. Genotypic variation of gene expression during the soybean innate immunity response. Plant Genet Resour-C. 12, S27–S30, doi: 10.1017/S1479262114000197 (2014). [DOI] [Google Scholar]
- Cabrera J. C., Messiaen J., Cambier P. & Van Cutsem P. Size, acetylation and concentration of chitooligosaccharide elicitors determine the switch from defence involving PAL activation to cell death and water peroxide production in Arabidopsis cell suspensions. Physiol Plantarum. 127, 44–56, doi: 10.1111/j.1399-3054.2006.00677.x (2006). [DOI] [Google Scholar]
- Falcon A. B. et al. The effect of size and acetylation degree of chitosan derivatives on tobacco plant protection against Phytophthora parasitica nicotianae. World J Microb Biot. 24, 103–112, doi: 10.1007/s11274-007-9445-0 (2008). [DOI] [Google Scholar]
- Loake G. & Grant M. Salicylic acid in plant defence-the players and protagonists. Curr Opin Plant Biol. 10, 466–472, doi: 10.1016/j.pbi.2007.08.008 (2007). [DOI] [PubMed] [Google Scholar]
- Koornneef A. & Pieterse C. M. J. Cross talk in defense signaling. Plant Physiol. 146, 839–844, doi: 10.1104/pp.107.112029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M. & Ruelland E. Magical mystery tour: Salicylic acid signalling. Environ Exp Bot. 114, 117–128, doi: 10.1016/j.envexpbot.2014.07.003 (2015). [DOI] [Google Scholar]
- Zhang S. et al. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J Exp Bot. 66, 1951–1963, doi: 10.1093/Jxb/Eru538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares S. H., Syrovets T., Weiler E. W. & Ryan C. A. Oligogalacturonides and Chitosan Activate Plant Defensive Genes through the Octadecanoid Pathway. P Natl Acad Sci USA. 92, 4095–4098, doi: 10.1073/pnas.92.10.4095 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakwal R., Tamogami S., Agrawal G. K. & Iwahashi H. Octadecanoid signaling component “burst” in rice (Oryza sativa L.) seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochem Biophys Res Commun. 295, 1041–1045, doi: 10.1016/S0006-291x(02)00779-9 (2002). [DOI] [PubMed] [Google Scholar]
- Obara N., Hasegawa M. & Kodama O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci Biotech Bioch. 66, 2549–2559 (2002). [DOI] [PubMed] [Google Scholar]
- Nie X. Salicylic Acid Suppresses Potato virus Y Isolate N:O-Induced Symptoms in Tobacco Plants. Phytopathology. 96, 255–263, doi: 10.1094/phyto-96-0255 (2006). [DOI] [PubMed] [Google Scholar]
- Liao Y. W. K. et al. Salicylic acid binding of mitochondrial alpha-ketoglutarate dehydrogenase E2 affects mitochondrial oxidative phosphorylation and electron transport chain components and plays a role in basal defense against tobacco mosaic virus in tomato. New Phytol. 205, 1296–1307, doi: 10.1111/Nph.13137 (2015). [DOI] [PubMed] [Google Scholar]
- Oka K., Kobayashi M., Mitsuhara I. & Seo S. Jasmonic acid negatively regulates resistance to Tobacco mosaic virus in tobacco. Plant Cell Physiol. 54, 1999–2010, doi: 10.1093/Pcp/Pct137 (2013). [DOI] [PubMed] [Google Scholar]
- Zhu F. et al. Salicylic Acid and Jasmonic Acid Are Essential for Systemic Resistance Against Tobacco mosaic virus in Nicotiana benthamiana. Mol Plant Microbe In. 27, 567–577, doi: 10.1094/Mpmi-11-13-0349-R (2014). [DOI] [PubMed] [Google Scholar]
- Zhao X. M., She X. P., Du Y. G. & Liang X. M. Induction of antiviral resistance and stimulary effect by oligochitosan in tobacco. Pestic Biochem Phys. 87, 78–84, doi: 10.1016/j.pestbp.2006.06.006 (2007). [DOI] [Google Scholar]
- Chen Y. F. et al. Functions of oligochitosan induced protein kinase in tobacco mosaic virus resistance and pathogenesis related proteins in tobacco. Plant Physiol Bioch. 47, 724–731, doi: 10.1016/j.plaphy.2009.03.009 (2009). [DOI] [PubMed] [Google Scholar]
- Eshraghi L. et al. Defence Signalling Pathways Involved in Plant Resistance and Phosphite-Mediated Control of Phytophthora Cinnamomi. Plant Mol Biol Rep. 32, 342–356, doi: 10.1007/s11105-013-0645-5 (2014). [DOI] [Google Scholar]
- Niu D. et al. Bacillus cereus AR156 activates PAMP-triggered immunity and induces a systemic acquired resistance through a NPR1-and SA-dependent signaling pathway. Biochem bioph res co. 469, 120–125, doi: 10.1016/j.bbrc.2015.11.081 (2016). [DOI] [PubMed] [Google Scholar]
- Fujioka K. et al. Protection induced by volatile limonene against anthracnose disease in Arabidopsis thaliana. J Gen Plant Pathol. 81, 415–419, doi: 10.1007/s10327-015-0621-z (2015). [DOI] [Google Scholar]
- Staswick P. E., Su W. P. & Howell S. H. Methyl Jasmonate Inhibition of Root-Growth and Induction of a Leaf Protein Are Decreased in an Arabidopsis-Thaliana Mutant. P Natl Acad Sci USA. 89, 6837–6840, doi: 10.1073/pnas.89.15.6837 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarie S. et al. Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 56, 2158–2168, doi: 10.1093/pcp/pcv127 (2015). [DOI] [PubMed] [Google Scholar]
- de Ollas C., Arbona V. & Gomez-Cadenas A. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ. 38, 2157–2170, doi: 10.1111/pce.12536 (2015). [DOI] [PubMed] [Google Scholar]
- Delaney T. P. A Central Role of Salicylic-Acid in Plant-Disease Resistance (Vol 266, Pg 1247, 1994). Science. 266, 1793–1793 (1994). [DOI] [PubMed] [Google Scholar]
- Scott I. M., Ward J. L., Miller S. J. & Beale M. H. Opposite variations in fumarate and malate dominate metabolic phenotypes of Arabidopsis salicylate mutants with abnormal biomass under chilling. Physiol Plantarum. 152, 660–674, doi: 10.1111/ppl.12210 (2014). [DOI] [PubMed] [Google Scholar]
- Hackmann C. et al. Salicylic acid-dependent and -independent impact of an RNA-binding protein on plant immunity. Plant Cell Environ. 37, 696–706, doi: 10.1111/pce.12188 (2014). [DOI] [PubMed] [Google Scholar]
- Wan J. R. et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell. 20, 471–481, doi: 10.1105/tpc.107.056754 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrina Ramonell*, M. B.-L., Serry Koh, Jinrong Wan, Herb Edwards, Gary Stacey, and Shauna Somerville. Loss-of-Function Mutations in Chitin Responsive Genes Show Increased Susceptibility to the Powdery Mildew Pathogen Erysiphe cichoracearum. Plant Physiol.138, 1027–1036, (2005). [DOI] [PMC free article] [PubMed]
- Kendra D. F., Christian D. & Hadwiger L. A. Chitosan Oligomers from Fusarium-Solani Pea Interactions, Chitinase Beta-Glucanase Digestion of Sporelings and from Fungal Wall Chitin Actively Inhibit Fungal Growth and Enhance Disease Resistance. Physiol Mol Plant P. 35, 215–230, doi: 10.1016/0885-5765(89)90052-0 (1989). [DOI] [Google Scholar]
- Hadwiger L. A., Ogawa T. & Kuyama H. Chitosan Polymer Sizes Effective in Inducing Phytoalexin Accumulation and Fungal Suppression Are Verified with Synthesized Oligomers. Mol Plant Microbe In. 7, 531–533, doi: 10.1094/Mpmi-7-0531 (1994). [DOI] [PubMed] [Google Scholar]
- Yin H., Bai X. F. & Du Y. G. The primary study of oligochitosan inducing resistance to Sclerotinia sclerotiorum on Brassica napus. J Biotechnol. 136, S600–S601, doi: 10.1016/j.jbiotec.2008.07.1217 (2008). [DOI] [Google Scholar]
- Yin H., Li S., Zhao X., Du Y. & Ma X. cDNA microarray analysis of gene expression in Brassica napus treated with oligochitosan elicitor. Plant Physiol Biochem. 44, 910–916, doi: 10.1016/j.plaphy.2006.10.002 (2006). [DOI] [PubMed] [Google Scholar]
- Guo W. H. et al. Measurement of oligochitosan-tobacco cell interaction by fluorometric method using europium complexes as fluorescence probes. Talanta. 78, 977–982, doi: 10.1016/j.talanta.2009.01.020 (2009). [DOI] [PubMed] [Google Scholar]
- Kaneda T. et al. The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. Embo J. 28, 926–936, doi: 10.1038/emboj.2009.39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G., Rodriguez M. C., Manacorda C. A. & Asurmendi S. Transgenic Expression of Tobacco mosaic virus Capsid and Movement Proteins Modulate Plant Basal Defense and Biotic Stress Responses in Nicotiana tabacum. Mol Plant Microbe In. 25, 1370–1384, doi: 10.1094/Mpmi-03-12-0075-R (2012). [DOI] [PubMed] [Google Scholar]
- Rodriguez M. C., Conti G., Zavallo D., Manacorda C. A. & Asurmendi S. TMV-Cg Coat Protein stabilizes DELLA proteins and in turn negatively modulates salicylic acid-mediated defense pathway during Arabidopsis thaliana viral infection. Bmc Plant Biol. 14, doi: 10.1186/S12870-014-0210-X (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J. S., Humphrey P. T. & Whiteman N. K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17, 260–270, doi: 10.1016/j.tplants.2012.02.010 (2012). [DOI] [PubMed] [Google Scholar]
- Wei Z. M. et al. Harpin, Elicitor of the Hypersensitive Response Produced by the Plant Pathogen Erwinia-Amylovora. Science. 257, 85–88, doi: 10.1126/science.1621099 (1992). [DOI] [PubMed] [Google Scholar]
- Reboutier D. et al. The HrpN(ea) harpin from Erwinia amylovora triggers differential responses on the nonhost Arabidopsis thaliana cells and on the host apple cells. Mol Plant Microbe In. 20, 94–100, doi: 10.1094/Mpmi-20-0094 (2007). [DOI] [PubMed] [Google Scholar]
- Che Y. Z. et al. A novel antimicrobial protein for plant protection consisting of a Xanthomonas oryzae harpin and active domains of cecropin A and melittin. Microb Biotechnol. 4, 777–793, doi: 10.1111/j.1751-7915.2011.00281.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. S., Delaney T. P., Bauer D. W. & Beer S. V. Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 20, 207–215, doi: 10.1046/j.1365-313x.1999.00595.x (1999). [DOI] [PubMed] [Google Scholar]
- Chuang H. W., Chang P. Y. & Syu Y. Y. Harpin Protein, an Elicitor of Disease Resistance, Acts as a Growth Promoter in Phalaenopsis Orchids. J Plant Growth Regul. 33, 788–797, doi: 10.1007/s00344-014-9425-1 (2014). [DOI] [Google Scholar]
- Mishina T. E. & Zeier J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 50, 500–513, doi: 10.1111/j.1365-313X.2007.03067.x (2007). [DOI] [PubMed] [Google Scholar]
- Tsuda K., Sato M., Glazebrook J., Cohen J. D. & Katagiri F. Interplay between MAMP-triggered and SA-mediated defense responses (vol 53, pg 763, 2008). Plant J. 55, 1061–1061, doi: 10.1111/j.1365-313X.2008.03589.x (2008). [DOI] [PubMed] [Google Scholar]
- Yi S. Y., Shirasu K., Moon J. S., Lee S. G. & Kwon S. Y. The Activated SA and JA Signaling Pathways Have an Influence on flg22-Triggered Oxidative Burst and Callose Deposition. Plos One. 9, doi: 10.1371/journal.pone.0088951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. L. et al. Mutant Allele-Specific Uncoupling of PENETRATION3 Functions Reveals Engagement of the ATP-Binding Cassette Transporter in Distinct Tryptophan Metabolic Pathways. Plant Physiol. 168, 814, doi: 10.1104/pp.15.00182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Sato M., Stoddard T., Glazebrook J. & Katagiri F. Network Properties of Robust Immunity in Plants. Plos Genet. 5, doi: 10.1371/journal.pgen.1000772 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A. & Baldwin I. T. Plant responses to insect herbivory: The emerging molecular analysis. Annu Rev Plant Biol. 53, 299–328, doi: 10.1146/annurev.arplant.53.100301.135207 (2002). [DOI] [PubMed] [Google Scholar]
- Thomma B. P. H. J., Penninckx I. A. M. A., Broekaert W. F. & Cammue B. P. A. The complexity of disease signaling in Arabidopsis. Curr Opin Immunol. 13, 63–68, doi: 10.1016/S0952-7915(00)00183-7 (2001). [DOI] [PubMed] [Google Scholar]
- Hirakawa Y., Nomura T., Hasezawa S. & Higaki T. Simplification of vacuole structure during plant cell death triggered by culture filtrates of Erwinia carotovora. J Integr Plant Biol. 57, 127–135, doi: 10.1111/jipb.12304 (2015). [DOI] [PubMed] [Google Scholar]
- Singh V. K. & Upadhyay R. S. Fusaric acid induced cell death and changes in oxidative metabolism of Solanum lycopersicum L. Bot Stud. 55, doi: 10.1186/S40529-014-0066-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−ΔΔC) method. Methods. 25, 402–408, doi: 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
- de Sa M. et al. A liquid chromatography/electrospray ionisation tandem mass spectrometry method for the simultaneous quantification of salicylic, jasmonic and abscisic acids in Coffea arabica leaves. J Sci Food Agr. 94, 529s36, doi: 10.1002/Jsfa.6288 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.