Abstract
Twenty-five years ago a ‘new’ protein was identified from cancers that caused hypercalcemia. It was credited for its ability to mimic parathyroid hormone, and hence was termed parathyroid hormone-related protein (PTHrP). Today it is recognized for its widespread distribution, its endocrine, paracrine, and intracrine modes of action driving numerous physiologic and pathologic conditions with a central role in organogenesis. The multiple biological activities within a complex molecule with paracrine modulation of adjacent target cells present boundless possibilities. The protein structure of PTHrP has been traced, dissected and deleted comprehensively and conditionally, yet numerous questions lurk in its past that will carry into the future. Issues of the variable segments of the protein including the enigmatic nuclear localization sequence are only recently being clarified. Aspects of PTHrP production and action in the menacing condition of cancer are emerging as dichotomies that may represent intended temporal actions of PTHrP. Relative to PTH, the hormone regulating calcium homeostasis, PTHrP ‘controls the show’ locally at the PTH/PTHrP receptor throughout the body. Great strides have been made in our understanding of PTHrP actions, yet years of exciting investigation and discovery are imminent.
Keywords: PTHrP, PTH, paracrine, hypercalcemia, bone, cartilage
Historical Perspective
Parathyroid hormone-related protein (PTHrP) seemed almost to come out of nowhere, produced by certain cancers, mimicking PTH action and causing the complication of hypercalcemia. Fuller Albright in 1941 (1), when discussing a patient with renal carcinoma, a solitary metastasis and hypercalcemia, suggested that some tumors might cause hypercalcemia by secreting PTH or something very like it. As with many of his predictions he was ultimately proved correct, but not before the passing of a few decades during which the concept of “ectopic PTH production” by cancers was promulgated as the cause of non-metastatic hypercalcemia (2;3). Doubts began to appear in the 1970’s, when improved radioimmunoassays for PTH indicated that the immunoreactivity in tumor or plasma of patients with cancer differed from authentic PTH (4–7), and with some assays PTH immunoreactivity could not be detected at all (8). From this background three excellent clinical studies put beyond reasonable doubt the biochemical similarity between primary hyperparathyroidism and this syndrome of humoral hypercalcemia of malignancy (9–11). By that time, rapid, sensitive, robust biological assays of PTH had developed and extracts and culture supernatants of hypercalcemic animal and human tumors were found to contain PTH-like adenylate cyclase responses in osteoblast and kidney targets (12–14). This paved the way for purification of PTHrP from a human lung cancer cell line (15), a breast cancer (16) and a renal cancer cell line (17). The cloning of its cDNA (18;19) showed 8 of the first 13 residues of PTHrP identical to those in PTH, any remaining identities no more than expected by chance, and the structural requirements for full biological activity of PTHrP contained within the first 34 amino acids, as was known to be the case with PTH (20). These findings explained the biochemical similarities between syndromes of PTH excess and non-metastatic hypercalcemia in cancer, signaling the discovery of an evolutionary relationship between these two molecules, most likely derived from a common ancestor and evolving from a gene duplication event. The PTHrP gene had a more complex structure than that of PTH, but with similar intron-exon boundaries, and the marked conservation of the PTHrP amino acid sequence in human, rat, mouse, chicken and canine up to position 111 indicated that important functions are likely to reside in this region. This was the beginning of the rise of PTHrP to a position of great interest; yet in many circles it still plays a secondary role in the family of parathyroid hormones.
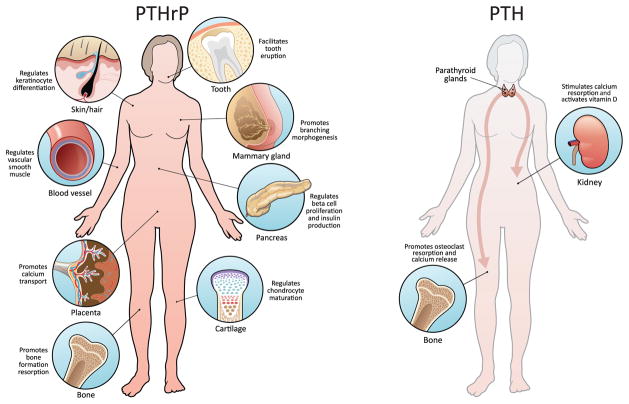
Soon after its discovery, it became apparent, that far from being simply an evolutionary relic that mimics PTH action in non – metastatic cancer, PTHrP has major roles in other aspects of cancer, in development, and in normal physiological functions in post-natal life. At the time the receptor was discovered, it was evident that this receptor functioned to relay both PTH and PTHrP biological activity (21), yet knock out of this PTH/PTHrP receptor (PPR) highlighted the importance of PTHrP (22;23). Indeed, PTHrP is the master regulator with widespread paracrine actions (Figure 1), and as illustrated for example by the pharmacological use of PTH as an anabolic agent in the treatment of osteoporosis where PTH administration actually mimics PTHrP actions locally in the bone microenvironment (24). Still, many questions remain unanswered. This perspective piece will briefly consider aspects of PTHrP function in development and disease and pose outstanding questions that despite 25 years of inquiry still linger.
Figure one. PTHrP Paracrine actions.
PTHrP has numerous paracrine actions in physiologic homeostasis including roles in keratinocytes/hair follicles, cartilage, vascular smooth muscle, bone, mammary gland development, tooth eruption, pancreas, and others not depicted. In comparison, PTH has relatively fewer direct physiologic targets via its endocrine mode of operation in bone and kidney.
PTHrP – evolutionary insights
It has long been accepted that PTH and PTHrP arose from gene duplication, PTH located on human chromosome 11 and PTHrP on chromosome 12. If conservation across species is evidence of gene importance, PTHrP prevails – fugu fish PTH has 44% identity with human PTH whereas fugu fish PTHrP bears 53% similarity with human PTHrP (25). At the amino acid level, chicken PTH shares 88% similarity with human, and chicken PTHrP shares 91% similarity with human (26). The first 111 amino acids of PTHrP are extraordinarily conserved among many species. Interestingly, PTH appears to play a paracrine role in lower vertebrates but evolved in higher vertebrates with a more restricted yet vital endocrine role. In fish, PTH produced in the gills is responsible for local calcium regulation; whereas via evolution to tetrapods the parathyroid gland assumed an endocrine role as calcium requirements shifted from an aquatic to a terrestrial environment (27). PTHrP, having a fairly simple gene structure in lower vertebrates acquired a more complex structure with added exons and alternative promoters with progression to humans, and in parallel picked up a stronger paracrine emphasis (26;28).
PTHrP – more than an endocrine factor
A great surge of interest and research activity came with the finding that PTHrP is normally produced in many tissues and acts in those sites in a paracrine manner. There are only three identified circumstances in which PTHrP species are present in the circulation and act in an endocrine manner: 1) the humoral hypercalcemic syndrome, in which PTHrP is produced by tumors and circulates to the bone to stimulate bone resorption (29;30), 2) lactation, in which PTHrP is made in the breast and reaches the circulation (31), and 3) fetal life, where PTHrP regulates maternal-to-fetal placental calcium transport (32;33). There remains to the present time no convincing evidence of biologically relevant circulating PTHrP levels otherwise in normal humans. Hence, the vast majority of PTHrP actions, unlike PTH are paracrine in nature.
There are three splice variant isoforms of PTHrP rendering PTHrP 1-139, 1-173, or 1-141 with transcriptional regulation from three distinct promoters (34). The multiple products of post-translational processing including glycosylation, the short half-life of PTHrP mRNA, and the multiple biological activities contained within PTHrP, equip it ideally to function as a paracrine effector with a developmental focus (35–38). Together with the obvious susceptibility of PTHrP to post-translational modification through proteolysis (39), and the generation of several constituent peptides, this increased complexity highlights the leading role of PTHrP, yet leaves questions that remain unanswered to this day. Why are there 3 PTHrP splice variants in humans but not in other mammals? What is the extent of biologically relevant PTHrP peptide fragments, and how do they function? Biologically active PTHrP peptide fragments functioning independent of the N-terminus raise the question – are there yet unidentified receptors ?
Skeletal actions
The physiological importance of PTHrP in the skeleton was evident with deletion of PTHrP resulting in death in mice immediately after birth from respiratory failure, attributed to defective rib cage formation (40). Here PTHrP stands out from PTH, whose later gene deletion resulted in a comparatively mild phenotype (41). Multiple defects in skeletal development confirmed the importance of PTHrP in fetal bone development (42). Whereas haploinsufficient PTHrP (+/−) mice are phenotypically normal at birth, by three months of age they have low bone mass, with a marked decrease in trabecular thickness and connectivity and an abnormally high number of adipocytes in the bone marrow (43). PTHrP +/− mice have compromised recruitment of bone marrow precursors and increased osteoblast apoptosis compared to wild-type mice. Importantly, this phenotype was recapitulated in transgenic mice with osteoblast-specific knockout of PTHrP thereby confirming the role of osteoblast-derived PTHrP in the process of bone formation (24). These mice also demonstrated reduced osteoclast formation likely due to impaired ability of PTHrP null osteoblasts to support osteoclast formation. Confounding work in this area, is the nature of PTHrP, its low-abundance mRNA and protein products that have been difficult to identify by conventional immunohistochemical approaches. Coincident expression of PTHrP mRNA and protein was noted in both chondrocytes and osteoblasts in endochondral bone formation in the mouse, and both also in preosteoblasts and actively synthesizing osteoblasts in a regenerating bone model in the rabbit (44). On the other hand, using a PTHrP-lacZ knock-in mouse, Chen and colleagues were not able to demonstrate osteoblast derived PTHrP production suggesting osteoblast-derived PTHrP would not drive local bone formation (45).
In the growth plate, chondrocyte maturation is tightly regulated by a paracrine PTHrP/Indian hedgehog (Ihh) signaling loop. PTHrP produced by the distal perichondrium interacts with the PPR expressed in the proliferative and perhypertrophic zones of the growth plate. These findings support a key role of PTHrP in controlling the pace of growth plate development via preventing premature differentiation of chondrocytes into prehypertrophic and hypertrophic chondrocytes (46). More recently, findings have extended to articular joints where evidence suggests PTHrP is produced in response to loading and functions in a similar Ihh signaling loop to support articular cartilage maintenance (47).
The favored current concept is that PTH the hormone, regulates calcium homeostasis in development and maturity. PTHrP the local factor, on the other hand, directs growth plate development by controlling chondrocyte proliferation and differentiation, while of these two proteins postnatally, PTHrP is the main factor generated locally in bone and acting through the PPR in bone remodeling, without normally contributing to the maintenance of serum calcium levels. These studies also highlight emerging evidence and pose questions – Does anabolic PTH essentially co-opt PTHrP physiologic actions? Might we regard the use of PTH in skeletal anabolic therapy as an attempt to reproduce the local action of PTHrP?
Placental calcium transport
A role for PTHrP action in the placenta is highlighted by its ability to promote trans-placental calcium transport in sheep (32), with PTHrP (67–86) and (38–94) the most active peptides (48;49), and no action of amino-terminal PTHrP. Studies in genetically manipulated mice confirm that PTHrP controls placental calcium transport to bring about mineralization of the fetal skeleton (50), with the main PTHrP source being the placenta.
Smooth muscle relaxation
A particularly instructive example of the paracrine actions of PTHrP is found in the smooth muscle beds of the vasculature. It had been known since the 1920’s that injection of parathyroid extract in animals results in dose-dependent increases in blood flow through a range of vascular beds, and decreases in blood pressure (51–54). When PTHrP was discovered it became clear that this was not the normal function of PTH but instead, a local physiological role of PTHrP, produced in smooth muscle beds of the stomach and intestine, uterus, urinary bladder, and arterial vessels, acting in all those tissues as a muscle relaxant (36;55;56). PTHrP expressed in smooth muscle acts rapidly to relax the vasculature (57) through an endothelium – independent mechanism, and vasoconstrictors such as angiotensin II induced a rapid rise in PTHrP production (58). Thus increased PTHrP production following vasoconstriction could provide a mechanism to limit or reverse this effect through the relaxing action of PTHrP on smooth muscle. The paracrine production and action of PTHrP in local vascular beds comes into action as required physiologically. On the other hand, when PTH is administered systemically, with simultaneous activation in many sites, the response of general vasodilatation and decline in blood pressure is not surprising. Yet, where does PTHrP stand in the hierarchy of paracrine vasoactive peptides?
Mammary gland development
Although the neonatal lethality of PTHrP−/− mice initially presented difficulty in identifying tissue specificity, rescue of these mice was achieved by directing PTHrP production to cartilage with use of the collagen II promoter, allowing study of the effect of the PTHrP null phenotype on several other organs (59). In the case of the breast, “rescued” PTHrP null mice show failure of early breast ductal development, providing strong evidence of a further paracrine role for PTHrP in promoting branching morphogenesis (59). With such dramatic expressions of PTHrP involvement in early breast development, it is perhaps not surprising that PTHrP emerges as a factor important in breast cancer biology yet a recent study provides evidence against a PTHrP role in post-natal breast development (60). The discovery of RANKL production by primitive ductal cells, acting on RANK in mammary stem cells to promote their expansion (61) highlights the need for further investigation of the role of PTHrP and RANKL in the breast.
Teeth and skin
Further evidence supporting a prominent role for PTHrP in development came with the PTHrP type II collagen promoter rescue. These mice lack tooth eruption, a cardinal sign of defective osteoclastogenesis (62). PTHrP is produced by cells of the enamel organ during development (63) and receptors for PTHrP exist in the bone surrounding the developing tooth, and also in the dental follicle and in cementoblasts lining the tooth root surface (64–66). Philbrick and colleagues used a cytokeratin, K14-PTHrP transgene, to show that replacement of PTHrP in the enamel epithelium restores tooth eruption (62). Since osteoclasts do not express PPR receptors, this supported the developmental role of PTHrP to drive osteoclasts necessary for clearing the path for the erupting tooth through a paracrine/juxtacrine interaction. Murine studies were validated with human analyses of loss of function of the PPR revealing ankylosed and distorted tooth development (67).
Yet another tissue/organ site of PTHrP paracrine actions was identified in the hair follicle with reciprocal expression of the PPR in the dermal components. Overexpression of PTHrP led to premature termination of anagen and entrance to catagen in the hair cycle (68). PTHrP expression was identified to be high in late anagen and thought to participate in the hair cycle but not necessarily be essential for hair cycle progression. Similarly, studies of PTHrP expression in keratinocytes found temporal dependent production of PTHrP with a reduction as keratinocytes differentiate (69;70) suggesting a regulatory loop similar to that found in cartilage development.
Pancreas
In the pancreas, islet cells were found to produce PTHrP as well as bear the PPR, and PTHrP provides a robust increase in intracellular calcium in beta cells (71). All four endocrine cell types, alpha, beta, delta, and pancreatic polypeptide cells produce PTHrP (72). Overexpression of PTHrP as well as PTHrP 1-36 administration increases beta cell proliferation via cell-cycle specific activation (73). PTHrP overexpression in beta cells results in islet hyperplasia and insulin-mediated hypoglycemia associated with reduced apoptosis (74). This effect was also shown via exogenous administration of PTHrP 1-36 supporting a local mediated action at the PPR. PTHrP also increases beta cell production of insulin suggesting its consideration in therapeutic strategies to improve islet growth and function.
PTHrP in other locations
Beyond the organ - focused investigations discussed above, PTHrP has been detected in nearly every tissue/organ in the body. Early reports of PTHrP and PPR expression in the heart, brain, skeletal muscle, bladder, lungs, bile ducts, immune system, liver, uterus, testes, as well as most endocrine organs including the pituitary and thyroid gland C-cells (36;75) leave unanswered questions years later as to the tissue-specific significance of PTHrP in health and disease. However, many of the early studies in this area did not have PTHrP knockout mice available as negative controls, and did not use in situ hybridization to detect PTHrP mRNA. To this day, challenges surrounding the specificity of PTHrP immunohistochemistry still need to be overcome.
PTHrP – the intracrine factor
A most intriguing early finding was the discovery that PTHrP attains a nuclear/nucleolar location through a specific transport process, and is likely to exert some of its functions from that site. Nucleolar localization of PTHrP through a defined sequence in the mid-region is associated with enhanced chondrocyte survival following prolonged periods of serum starvation (76;77). Expression of PTHrP is cell cycle-specific in smooth muscle cells (78) and keratinocytes (78;79), and its mRNA highest at the G1 phase, when localized to the nucleolus (80), with cyclin-dependent kinase phosphorylation of T85 resulting in exclusion of PTHrP from the nucleus (81).
Within the PTHrP sequence there are nucleus (CcN) and nucleolus localization motifs, with the former being similar to that described for the archetypal CcN-containing protein, SV40 T-antigen (82). The mechanism of nuclear import requires PTHrP interaction with importinβ and GTP-Ran expression (83–85), and cyclin-dependent kinase phosphorylation of T85 results in exclusion of PTHrP from the nucleus (81). A nuclear targeting sequence that inhibits apoptosis exists at PTHrP (87–107) (76;86–88), and PTHrP (109–139) is involved in its nuclear export (89). Evidence supports direct binding of PTHrP to RNA through a distinct motif in the NTS (90) and further points to PTHrP as likely to exert important functions from its nuclear site. PTHrP appears so far to be the only protein classed at least in some circumstances as a hormone, which possesses a CcN motif and displays differential cellular localization (nuclear/nucleolar versus cytoplasmic). There must be some important purpose behind the evolutionary conservation of this property, and prompts the question, what is the significance of nuclear entry of PTHrP in the many tissues in which PTHrP is considered to play a local role?
The impact of other biological activities exerted by domains within PTHrP was exemplified in two studies in mice, in one of which knock-in of PTHrP (1–84), lacking both the nuclear localization sequence (NLS) and C-terminal region while retaining the bioactive amino-terminal, resulted in multiple abnormalities and early lethality in mice (91). Homozygous mice exhibited skeletal growth retardation and osteopenia associated with reduced proliferation and increased apoptosis of osteoblasts as well as early senescence with altered expression patterns and subcellular distribution of proliferative- and senescence-related genes in multiple tissues. A further knock-in of PTHrP (1–66) excluding a significant part of the mid-region resulted in an even more severe phenotype and highlighted the role of PTHrP in stem cells as well as later lineage cell commitment (92). These genetic studies in mice show that many of the actions of PTHrP are not mediated by the amino-terminal region, and among the generalized abnormalities, absence of the mid-region, NLS and C-terminal region result in greatly impaired commitment and survival of osteogenic and hematopoietic precursors.
PTHrP and Cancer
The significance of PTHrP in cancer was not confined to the humoral hypercalcemic syndrome. Breast cancer was one of the original sources of PTHrP (16). Hypercalcemic breast cancer patients with metastatic bone disease have elevated plasma PTHrP levels (30), and 60% of primary breast cancers and 90% of bone metastases are positive for PTHrP by immunohistochemistry (93;94). From this arose the concept that PTHrP production in the bone marrow by breast cancer cells promotes bone resorption, thus favoring tumor establishment and expansion. Extensive experimental evidence was produced in support of this (95;96), including prevention and treatment of tumor growth by inhibiting bone resorption, using bisphosphonates or neutralizing monoclonal antibodies against PTHrP (95;97). All of this accorded with the “seed and soil” hypothesis developed by Stephen Paget (98), which depicted bone as the favorable soil for the “seed” of breast cancer. A major contributor to this co-operation is tumor production of PTHrP, together with the several other cancer-derived factors that influence bone metastasis establishment, including prostaglandins and cytokines (96) and of factors favoring the homing and/or adherence of cancer cells to bone (99). Although this emphasizes PTHrP involvement in breast cancer, increasing evidence implicates it in prostate, lung, renal, colon, lymphoid and other cancers (100–104).
The history and findings need to be considered when evaluating the place of PTHrP in the pathogenesis of bone metastasis formation and progression. What is the relative importance of PTHrP compared to other tumor derived factors and the type of cancer (e.g. breast vs. prostate and their different bone phenotypes)? When a PTHrP-producing tumor metastasizes to bone, how does this locally produced factor compare in its biologic impact with other tumor-derived factors? Is there a very early role for PTHrP as an endocrine/tumor-derived circulating factor in conditioning the bone microenvironment, i.e. a pre-metastatic niche? Does PTHrP influence tumor cell dormancy?
Does PTHrP indeed have an entirely independent function, perhaps early in cancer development, of contributing to a less invasive phenotype of the cancer? That suggestion comes from a long-term, prospective study of consecutive patients at a single center, that tumors positive for PTHrP at surgery were independently predictive of improved patient survival, with reduced metastases at all sites, including bone (105;106). Such a mechanism is distinct from the bone resorbing action later in disease, that can explain the association of PTHrP production with bone metastases (107–110). Highlighting the controversy, of two independent studies of genetically induced breast cancer in mice, one concluded that loss of PTHrP expression resulted in poorer outcomes in breast cancer (111), the other concluded the opposite, that PTHrP promotes the initiation and progression of primary tumors (112).
With PTHrP directing an important role in early mammary gland development (59;67;113), it might not be surprising if it were to play a part in early stages of cancer development – but is this concept of PTHrP a credible one - protective at one stage of cancer yet deleterious at another? Indeed PTHrP has been shown to have multiple and opposing roles in other circumstances such as in its ability to both protect and promote apoptosis in osteoblastic cells and pneumocytes (114–116). A similar example of a dual action is TGFβ, which acts early as a tumor suppressor by inhibiting proliferation of epithelial, endothelial and hematopoietic cells. Refractoriness to these effects develops later, and over expression of TGFβ leads to a microenvironment conducive to tumor growth (117–119). Confirmation of a dual role for PTHrP requires further clinical and basic study, with the critical question: What are the temporal implications of PTHrP in tumorigenesis; are there different and contrasting early and later actions?
Summary
After 25 years, what does PTHrP really do? Can we regard PTH use as a pharmacological agent that is simply a surrogate for what PTHrP does physiologically? We can only speculate about the nature of the PTHrP molecule that gains access through a paracrine mechanism to its adjacent target cells, and the likelihood of multiple biological activities within the molecule presents complex possibilities. Assuming that full-length PTHrP is secreted, is that the predominant form that interacts with target cells locally, or does its susceptibility to proteolytic breakdown yield shorter products, even in that local environment? PTHrP was designated as ‘related’ to PTH, but it is certainly not a distant cousin. Although knowledge of PTH actions far preceded our knowledge of PTHrP, over the past 25 years PTHrP has emerged as the key regulator of normal physiology as well as pathophysiologic events. Whereas PTH actions center on its role in calcium metabolism, the multifactorial nature of PTHrP will continue to give years of exciting investigation.
Acknowledgments
The authors acknowledge critical reading of the manuscript by Thomas J. Rosol.
Footnotes
Authors’ roles – Both authors were involved in the design of this perspective, in drafting the manuscript, in revising the manuscript content, and in approving the final version. Both authors take responsibility for all aspects of the article’s content.
Disclosures
The authors have no conflicts of interest relative to this perspective. Support was provided by the NIH DK53904 and CA093900 (LKM), the NHMRC and Victorian Government OIS Program (TJM).
References
- 1.Albright F. Case records of the Massachusetts General Hospital - Case 39061. N Engl J Med. 1941;225:789–796. doi: 10.1056/NEJM196404092701510. [DOI] [PubMed] [Google Scholar]
- 2.Omenn GS, Roth SI, Baker WH. Hyperparathyroidism associated with malignant tumors of nonparathyroid origin. Cancer. 1969;24:1004–1011. doi: 10.1002/1097-0142(196911)24:5<1004::aid-cncr2820240520>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Martin TJ, Atkins D. Biochemical regulators of bone resorption and their significance in cancer. Essays Med Biochem. 1979;4:49–82. [Google Scholar]
- 4.Melick RA, Martin TJ, Hicks JD. Parathyroid hormone production and malignancy. Br Med J. 1972;2:204–205. doi: 10.1136/bmj.2.5807.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roof BS, Carpenter B, Fink DJ, Gordan GS. Some thoughts on the nature of ectopic parathyroid hormones. Am J Med. 1971;50:686–691. doi: 10.1016/0002-9343(71)90124-0. [DOI] [PubMed] [Google Scholar]
- 6.Benson RCJ, Riggs BL, Pickard BM, Arnaud CD. Immunoreactive forms of circulating parathyroid hormone in primary and ectopic hyperparathyroidism. J Clin Invest. 1974;54:175–181. doi: 10.1172/JCI107739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riggs BL, Arnaud CD, Reynolds JC, Smith LH. Immunologic differentiation of primary hyperparathyroidism from hyperparathyroidism due to nonparathyroid cancer. J Clin Invest. 1971;50:2079–2083. doi: 10.1172/JCI106701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell D, Singer FR, Murray TM, Minkin C, Potts JR. Nonparathyroid humoral hypercalcemia in patients with neoplastic diseases. New England Journal of Medicine. 1973;289:176–181. doi: 10.1056/NEJM197307262890403. [DOI] [PubMed] [Google Scholar]
- 9.Stewart AF, Horst R, Deftos LJ, Cadman EC, Lang R, Broadus AE. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and nonhumoral groups. New England Journal of Medicine. 1980;303:1377–1383. doi: 10.1056/NEJM198012113032401. [DOI] [PubMed] [Google Scholar]
- 10.Kukreja SC, Shemerdiak WP, Lad TE, Johnson PA. Elevated nephrogenous cyclic AMP with normal serum parathyroid hormone levels in patients with lung cancer. J Clin Endocrinol Metab. 1980;51:167–169. doi: 10.1210/jcem-51-1-167. [DOI] [PubMed] [Google Scholar]
- 11.Rude RK, Sharp CF, Fredericks RS, Oldham SB, Elbaum N, Link J, Irwin L, Singer FR. Urinary and nephrogenous adenosine 3′,5′-monophosphate in the hypercalcemia of malignancy. J Clin Endocrinol Metab. 1981;52:765–771. doi: 10.1210/jcem-52-4-765. [DOI] [PubMed] [Google Scholar]
- 12.Rodan SB, Insogna KL, Vignery AM, Stewart AF, Broadus AE, D’souza SM, Bertolini DR, Mundy GR, Rodan GA. Factors associated with humoral hypercalcemia of malignancy stimulate adenylate cyclase in osteoblastic cells. J Clin Invest. 1983;72:1511–1515. doi: 10.1172/JCI111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart AF, Insogna KL, Goltzman D, Broadus AE. Identification of adenylate cyclase-stimulating activity and cytochemical glucose-6-phosphate dehydrogenase-stimulating activity in extracts of tumors from patients with humoral hypercalcemia of malignancy. Proc Natl Acad Sci U S A. 1983;80:1454–1458. doi: 10.1073/pnas.80.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strewler GJ, Williams RD, Nissenson RA. Human renal carcinoma cells produce hypercalcemia in the nude mouse and a novel protein recognized by parathyroid hormone receptors. J Clin Invest. 1983;71:769–774. doi: 10.1172/JCI110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall REH, Kemp BE, Suva LJ, Rodda CP, Ebeling PR, Hudson PJ, Zajaz JD, Martin TJ. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA. 1987;84:5048–5052. doi: 10.1073/pnas.84.14.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, Broadus AE, Stewart AF. Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem. 1987;262:7175–7156. [PubMed] [Google Scholar]
- 17.Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC, Rosenblatt M, Niseenson RA. Parathyroid hormone-like protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J Clin Invest. 1987;80:1803–1807. doi: 10.1172/JCI113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suva LJ, Winslow GA, Wettenhall REH, Hammonds RG, Moseley JM, Diefenbach-Jagger H, Rodda CP, Kemp BE, Rodriguez H, Chen EY, Hudson PJ, Martin TJ, Wood WI. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893–897. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 19.Mangin M, Webb AC, Dreyer BE, Posillico JT, Ikeda K, Weir EC, Stewart AF, Bander NH, Milstone L, Barton DE, Francke U, Broadus AE. Identification of a cDNA encoding a parathyroid hormone-like peptide from a human tumor associated with humoral hypercalcemia of malignancy. Proc Natl Acad Sci U S A. 1988;85:597–601. doi: 10.1073/pnas.85.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp BE, Moseley JM, Rodda CP, Ebeling PR, Wettenhall REH, Stapleton D, Diffenbach-Jagger H, Ure F, Michelangeli VP, Simmons HA, Raisz LG, Martin TJ. Parathyroid hormone-related protein of malignancy: active synthetic fragments. Science. 1987;238:1568–1570. doi: 10.1126/science.3685995. [DOI] [PubMed] [Google Scholar]
- 21.Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Hock J, Potts JT, Kronenberg HM, Segre GV. A G protein-linked receptor for parathryoid hormone and parathryoid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 22.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LHK, Ho C, Mulligan RC, Abou-Samra AB, Jueppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 23.Lanske B, Divieti P, Kovacs CS, Pirro A, Landis WJ, Krane SM, Bringhurst FR, Kronenberg HM. The parathyroid hormone (PTH)/PTH-related peptide receptor mediates actions of both ligands in murine bone. Endocrinology. 1998;139:5194–5204. doi: 10.1210/endo.139.12.6361. [DOI] [PubMed] [Google Scholar]
- 24.Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest. 2005;115:2402–2411. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danks JA, Ho PM, Notini AJ, Katsis F, Hoffmann P, Kemp BE, Martin TJ, Zajac JD. Identification of a parathyroid hormone in the fish fugu rubripes. J Bone Miner Res. 2003;18:1326–1331. doi: 10.1359/jbmr.2003.18.7.1326. [DOI] [PubMed] [Google Scholar]
- 26.Pinheiro PLC, Cardoso JCR, Gomes AS, Fuentes J, Power DM, Canário AVM. Gene structure, transcripts and calciotropic effects of the PTH family of peptides in Xenopus and chicken. BMC Evolutionary Biology. 2010;10:373. doi: 10.1186/1471-2148-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okabe M, Graham A. The origin of the parathyroid gland. Proc Natl Acad Sci U S A. 2004;101:17716–17719. doi: 10.1073/pnas.0406116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Ibrahim AS, Tay B, Richardson SJ, Bell J, Walker TI, Brenner S, Venkatesh B, Danks JA. Parathyroid hormone gene family in a cartilaginous fish, the elephant shark (callorhinshus milii) J Bone Miner Res. 2010;25:2613–2623. doi: 10.1002/jbmr.178. [DOI] [PubMed] [Google Scholar]
- 29.Burtis WJ, Fodero JP, Gaich G, Debeyssey M, Stewart AF. Preliminary characterization of circulating amino- and carboxy-terminal fragments of parathyroid hormone-related peptide in humoral hypercalcemia of malignancy. J Clin Endocrinol Metab. 1992;75:1110–1114. doi: 10.1210/jcem.75.4.1400879. [DOI] [PubMed] [Google Scholar]
- 30.Grill V, Ho P, Body JJ, Johanson N, Lee SC, Krukreja SC, Moseley JM, Martin TJ. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309–1315. doi: 10.1210/jcem-73-6-1309. [DOI] [PubMed] [Google Scholar]
- 31.Grill V, Hillary J, Ho PM, Law FM, MacIsaac RJ, Macisaac IA, Moseley JM, Martin TJ. Parathyroid hormone-related protein: a possible endocrine function in lactation. Clinical Endocrinology (Oxf) 1992;37:405–410. doi: 10.1111/j.1365-2265.1992.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 32.Rodda CP, Kubota M, Heath JA, Ebeling PR, Moseley JM, Care AD, Caple IW, Martin TJ. Evidence for a novel parathyroid hormone-related protein in fetal lamb parathyroid glands and sheep placenta: comparisons with a similar protein implicated in humoral hypercalcemia of malignancy. J Endocrinology. 1998;117:261–271. doi: 10.1677/joe.0.1170261. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A. 1996;93:15233–15238. doi: 10.1073/pnas.93.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard V, Luchin A, Brena RM, Plass C, Rosol TJ. Quantitative Evaluation of Alternative Promoter Usage and 3′ Splice Variants for Parathyroid Hormone-Related Protein by Real-Time Reverse Transcription-PCR Assay. Clin Chem. 2003;49:1398–1402. doi: 10.1373/49.8.1398. [DOI] [PubMed] [Google Scholar]
- 35.Sellers RS, Luchin AI, Richard V, Brena RM, Lima D, Rosol TJ. Alternative splicing of parathyroid hormone-related protein mRNA: expression and stability. J Mol Endocrinol. 2004;33:227–241. doi: 10.1677/jme.0.0330227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiological Reviews. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 37.Orloff JJ, Ganz MB, Nathanson MH, Moyer NS, Kats Y, Mitnick M, Behal A, Gasalla-Herraiz J, Isales CM. A midregion parathyroid hormone-related peptide mobilizes cytosolic calcium and stimulates formation of inositol trisphosphate in a squamous carcinoma cell line. Endocrinology. 1996;137:5376–5385. doi: 10.1210/endo.137.12.8940360. [DOI] [PubMed] [Google Scholar]
- 38.Wu TL, Soifer NE, Burtis WJ, Milstone LM, Stewart AF. Glycosylation of parathyroid hormone-related peptide secreted by human epidermal keratinocytes. J Clin Endocrinol Metab. 1991;73:1002–1007. doi: 10.1210/jcem-73-5-1002. [DOI] [PubMed] [Google Scholar]
- 39.Orloff JJ, Reddy D, de Papp AE, Yang KH, Soifer NE, Stewart AF. Parathyroid hormone-related protein as a prohormone: posttranslational processing and receptor interactions. Endocr Rev. 1994;15:40–60. doi: 10.1210/edrv-15-1-40. [DOI] [PubMed] [Google Scholar]
- 40.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VLJ, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes and Development. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 41.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. Journal of Clinical Investigation. 2002;109:1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol. 1994;126:1611–1623. doi: 10.1083/jcb.126.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amizuka N, Karaplis AC, Henderson JE, Warshawsky H, Lipman ML, Matsuki Y, Ejiri S, Tanaka M, Izumi N, Ozawa H, Goltzman D. Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Developmental Biology. 1996;175:166–176. doi: 10.1006/dbio.1996.0104. [DOI] [PubMed] [Google Scholar]
- 44.Kartsogiannis V, Moseley J, McKlevie B, Chou ST, Hards DK, Ng KW, Martin TJ, Zhou H. Temporal expression of PTHrP during endochondral bone formation in mouse and intramembranous bone formation in an in vivo rabbit model. Bone. 1997;21:385–392. doi: 10.1016/s8756-3282(97)00180-4. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Macica CM, Dreyer BE, Hammond VE, Hens JR, Philbrick WM, Broadus AE. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006;21:113–123. doi: 10.1359/JBMR.051005. [DOI] [PubMed] [Google Scholar]
- 46.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg H. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macica C, Liang G, Nasiri A, Broadus AE. Genetic evidence of the regulatory role of parathyroid hormone-related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis Rheum. 2011;63:3333–3343. doi: 10.1002/art.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Care AD, Abbas SK, Pickard DW, Barri M, Drinkhill M, Findlay JBC, White IR, Caple IW. Stimulation of ovine placental transport of calcium and magnesium by mid-molecule fragments of human prarthyroid hormone-related protein. Exp Physiol. 1990;75:605–608. doi: 10.1113/expphysiol.1990.sp003437. [DOI] [PubMed] [Google Scholar]
- 49.Wu TL, Vasavada RC, Yang K, Massfelder T, Ganz M, Abbas SK, Care AD, Stewart AF. Structural and physiologic characterization of the mid-region secretory species of parathyroid hormone-related protein. J Biol Chem. 1996;271:24371–24378. doi: 10.1074/jbc.271.40.24371. [DOI] [PubMed] [Google Scholar]
- 50.Simmonds CS, Kovacs CS. Role of parathyroid hormone (PTH) and PTH-related protein (PTHrP) in regulating mineral homeostasis during fetal development. Crit Rev Eukaryot Gene Expr. 2010;20:235–273. doi: 10.1615/critreveukargeneexpr.v20.i3.40. [DOI] [PubMed] [Google Scholar]
- 51.Charbon GA. A rapid and selective vasodilator effect of parathyroid hormone. European Journal of Pharmacology. 1968;3:275–278. doi: 10.1016/0014-2999(68)90144-1. [DOI] [PubMed] [Google Scholar]
- 52.Charbon GA, Hulstaert PF. Augmentation of arterial hepatic and renal flow by extracted and synthetic parathyroid hormone. Endocrinology. 1974;96:621–626. doi: 10.1210/endo-95-2-621. [DOI] [PubMed] [Google Scholar]
- 53.Wang HH, Drugge ED, Yen YC, Blumenthal MR, Pang PK. Effects of synthetic parathyroid hormone on hemodynamics and regional blood flows. Eur J Pharmacol. 1984;97:209–215. doi: 10.1016/0014-2999(84)90452-7. [DOI] [PubMed] [Google Scholar]
- 54.Mok LLS, Nickols GA, Thompson JC, Cooper CW. Parathyroid hormone as a smooth muscle relaxant. Endocr Rev. 1989;10:420–436. doi: 10.1210/edrv-10-4-420. [DOI] [PubMed] [Google Scholar]
- 55.Qian J, Lorenz JN, Maeda S, Sutliff RL, Weber C, Nakayama T, Colbert MC, Paul RJ, Fagin JA, Clemens TL. Reduced blood pressure and increased sensitivity of the vasculature to parathyroid hormone-related protein (PTHrP) in transgenic mice overexpressing the PTH/PTHrP receptor in vascular smooth muscle. Endocrinology. 1999;140:1826–1833. doi: 10.1210/endo.140.4.6645. [DOI] [PubMed] [Google Scholar]
- 56.Martin TJ, Moseley JM, Williams ED. Parathyroid hormone-related protein: hormone and cytokine. J Endocrinology. 1997;154:S23–S37. [PubMed] [Google Scholar]
- 57.Roca-Cusache A, Dipette DJ, Nickols GA. Regional and systemic hemodynamic effects of parathyroid hormone-related protein: preservation of cardiac function and coronary and renal flow with reduced blood pressure. J Pharmacol Exp Ther. 1991;256:110–118. [PubMed] [Google Scholar]
- 58.Pirola CJ, Wang HM, Kamyar A, Wu S, Enomoto H, Sharifi B, Forrester JS, Clemens TL, Fagin JA. Angiotensin II regulates parathyroid hormone-related protein expression in cultured rat aortic smooth muscle cells through transcriptional and post-transcriptional mechanisms. J Biol Chem. 1993;268:1987–1994. [PubMed] [Google Scholar]
- 59.Wysolmerski JJ, McCaughern-Carucci JF, Daifotis AG, Broadus AE, Philbrick WM. Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development. 1995;121:3539–3547. doi: 10.1242/dev.121.11.3539. [DOI] [PubMed] [Google Scholar]
- 60.Boras-Granic K, VanHouten J, Hiremath M, Wysolmerski JJ. Parathyroid hormone-related protein is not required for normal ductal or alveolar development in the post-natal mammary gland. PLoS One. 2011;6:e27278. doi: 10.1371/journal.pone.0027278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 62.Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC. Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci U S A. 1998;95:11846–11851. doi: 10.1073/pnas.95.20.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck F, Tucci J, Russell A, Senior PV, Ferguson MW. The expression of the gene coding for parathyroid hormone-related protein (PTHrP) during tooth development in the rat. Cell Tissue Reseach. 1995;280:283–290. doi: 10.1007/BF00307800. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang H, McCauley LK, Berry JE, D’Errico JA, Strayhorn CL, Somerman MJ. Response of immortalized murine cementoblasts/periodontal ligament cells to parathyroid hormone and parathyroid hormone-related protein in vitro. Arch Oral Biol. 2000;45:293–303. doi: 10.1016/s0003-9969(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 65.Ouyang H, McCauley LK, Berry JE, Saygin NE, Tokiyasu Y, Somerman MJ. PTHrP regulates extracellular matrix gene expression in cementoblasts and inhibits cementoblast-mediated mineralization, in vitro. J Bone Miner Res. 2000;15:2140–2153. doi: 10.1359/jbmr.2000.15.11.2140. [DOI] [PubMed] [Google Scholar]
- 66.Tenorio D, Hughes FJ. An immunohistochemical investigation of the expression of parathyroid hormone receptors in rat cementoblasts. Arch Oral Biol. 1996;41:299–305. doi: 10.1016/0003-9969(95)00113-1. [DOI] [PubMed] [Google Scholar]
- 67.Wysolmerski JJ, Cormier S, Philbrick WM, Dann P, Zhang JP, Roume J, Delezoide AL, Silve C. Absence of functional type 1 parathyroid hormone (PTH)/PTH-related protein receptors in humans is associated with abnormal breast development and tooth impaction. Journal of Clinical Endocrinology & Metabolism. 2001;86:1788–1794. doi: 10.1210/jcem.86.4.7404. [DOI] [PubMed] [Google Scholar]
- 68.Wysolmerski JJ, Broadus AE, Zhou J, Fuchs E, Milstone LM, Philbrick WM. Overexpression of parathyroid hormone-related protein in the skin of transgenic mice interferes with hair follicle development. Proc Natl Acad Sci U S A. 1994;91:1133–1137. doi: 10.1073/pnas.91.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werkmeister JR, Merryman JI, McCauley LK, Horton JE, Capen CC, Rosol TJ. Parathyroid hormone-related protein production by normal human keratinocytes in vitro. Exp Cell Res. 1993;208:68–74. doi: 10.1006/excr.1993.1223. [DOI] [PubMed] [Google Scholar]
- 70.Foley J, Longely BJ, Wysolmerski JJ, Dreyer BE, Broadus AE, Philbrick WM. PTHrP regulates epidermal differentiation in adult mice. J Invest Dermatol. 1998;111:1122–1128. doi: 10.1046/j.1523-1747.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- 71.Vasavada RC, Cavaliere C, D’Ercole AJ, Dann P, Burtis WJ, Madlener AL, Zawalich K, Zawalich W, Philbrick W, Stewart AF. Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem. 1996;271:1200–1208. doi: 10.1074/jbc.271.2.1200. [DOI] [PubMed] [Google Scholar]
- 72.Drucker DJ, Asa SL, Henderson J, Goltzman D. The parathyroid hormone-like peptide gene is expressed in the normal and neoplastic human endocrine pancreas. Mol Endocrinol. 2011;3:1589–1595. doi: 10.1210/mend-3-10-1589. [DOI] [PubMed] [Google Scholar]
- 73.Guthalu Kondegowda N, Johshi-Gokhale S, Harb G, Williams K, Zhang XY, Takane KK, Zhang P, Scott DK, Stewart AF, Garcia-Ocana A, Vasavada RC. Parathyroid hormone-related protein enhances human β-cell proliferation and function with associated induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes. 2010;59:3131–3138. doi: 10.2337/db09-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cebrian A, Garcia-Ocana A, Takane KK, Sipula D, Stewart AR, Vasavada RC. Overexpression of parathryoid hormone-related protein inhibits pancreatic b-cell death in vivo and in vitro. Diabetes. 2002;51:3003–3013. doi: 10.2337/diabetes.51.10.3003. [DOI] [PubMed] [Google Scholar]
- 75.Ureña P, Kong XF, Abou-Samra AB, Jüppner H, Kronenberg HM, Potts JR, Segre GV. Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology. 1993;133:617–623. doi: 10.1210/endo.133.2.8393771. [DOI] [PubMed] [Google Scholar]
- 76.Henderson JE, Amizuka N, Warshawsky H, Biasotto D, Lanske BM, Goltzman D, Karaplis AC. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Molecular and Cellular Biology. 1995;15:4064–4075. doi: 10.1128/mcb.15.8.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aarts MM, Davidson D, Corluka A, Petroulakis E, Guo J, Bringhurst FR, Galipeau J, Henderson JE. Parathyroid hormone-related protein promotes quiescence and survival of serum-deprived chondrocytes by inhibiting rRNA synthesis. J Biol Chem. 2001;276:37943. doi: 10.1074/jbc.M105510200. [DOI] [PubMed] [Google Scholar]
- 78.Okano K, Pirola CJ, Wang HM, Forrester JS, Fagin JA, Clemens TL. Involvement of cell cycle and mitogen-activated pathways in induction of parathyroid hormone-related protein gene expression in rat aortic smooth muscle cells. Endocrinology. 1995;136:1782–1789. doi: 10.1210/endo.136.4.7895691. [DOI] [PubMed] [Google Scholar]
- 79.Lam MH, Olsen SL, Rankin WA, Ho P, Martin TJ, Gillespie MT, Moseley JM. PTHrP and cell division:expression and localization of PTHrP in a keratinocyte cell line (HaCaT) during the cell cycle. J Cell Physiol. 1997;173:433–446. doi: 10.1002/(SICI)1097-4652(199712)173:3<433::AID-JCP16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 80.Lam MH, Thomas RJ, Martin TJ, Gillespie MT, Jans DA. Nuclear and nucleolar localization of parathyroid hormone-related protein. Immunol Cell Biol. 2000;78:395–402. doi: 10.1046/j.1440-1711.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 81.Lam MH, House CM, Tiganis T, Mitchelhill KI, Sarcevic B, Cures A, Ramsay R, Kemp BE, Martin TJ, Gillespie MT. Phosphorylation at the cyclin-dependent kinases site (Thr85) of parathyroid hormone-related protein negatively regulates its nuclear localization. J Biol Chem. 1999;274:18559–18566. doi: 10.1074/jbc.274.26.18559. [DOI] [PubMed] [Google Scholar]
- 82.Jans DA. The regulation of protein transport to the nucleus by phosphorylation. Biochem J. 2011;311(Pt3):705–716. doi: 10.1042/bj3110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lam MH, Briggs LJ, Hu W, Martin TJ, Gillespie MT, Jans DA. Importin beta recognizes parathyroid hormone-related protein with high affinity and mediates its nuclear import in the absence of importin alpha. J Biol Chem. 1999;274:7391–7398. doi: 10.1074/jbc.274.11.7391. [DOI] [PubMed] [Google Scholar]
- 84.Jans DA, Thomas RJ, Gillespie MT. Parathyroid hormone-related protein (PTHrP): a nucleocytoplasmic shuttling protein with distinct paracrine and intracrine roles. Vitam Horm. 2003;66:345–384. doi: 10.1016/s0083-6729(03)01010-0. [DOI] [PubMed] [Google Scholar]
- 85.Cingolani G, Bednenko J, Gillespie MT, Gerace L. Molecular basis for the recognition of a nonclassical nuclear localization signal by importin b. Molecular Cell. 2002;10:1345–1353. doi: 10.1016/s1097-2765(02)00727-x. [DOI] [PubMed] [Google Scholar]
- 86.De Miguel F, Fiaschi-Taesch NM, Lopez-Talavera JC, Takane KK, Massfelder T, Helwig JJ, Stewart AF. The C-terminal region of PTHrP, in addition to the nuclear localization signal, is essential for the intracrine stimulation of proliferation in vascular smooth muscle cells. Endocrinology. 2001;142:4096–4105. doi: 10.1210/endo.142.9.8388. [DOI] [PubMed] [Google Scholar]
- 87.Aarts MM, Rix A, Guo J, Bringhurst FR, Henderson JE. The nucleolar targeting signal (NTS) of parathyroid hormone related protein mediates endocytosis and nucleolar translocation. J Bone Miner Res. 1999;14:1493–1503. doi: 10.1359/jbmr.1999.14.9.1493. [DOI] [PubMed] [Google Scholar]
- 88.Dougherty KM, Blomme EAG, Koh AJ, Henderson JE, Pienta KJ, Rosol TJ, McCauley LK. Parathyroid hormone related protein (PTHrP) as a growth regulator of prostate carcinoma. Cancer Res. 1999;59:6015–6022. [PubMed] [Google Scholar]
- 89.Pache JC, Burton DW, Deftos LJ, Hastings RH. A carboxyl leucine-rich region of parathyroid hormone-related protein is critical for nuclear export. Endocrinology. 2007;147:990–998. doi: 10.1210/en.2005-0663. [DOI] [PubMed] [Google Scholar]
- 90.Aarts MM, Levy D, He B, Stregger S, Chen T, Richard S, Henderson JE. Parathyroid hormone-related protein interacts with RNA. J Biol Chem. 1999;274:4832–4838. doi: 10.1074/jbc.274.8.4832. [DOI] [PubMed] [Google Scholar]
- 91.Miao D, Su H, He B, Gao J, Xia Q, Zhu M, Gu Z, Goltzman D, Karaplis AC. Severe growth retardation and early lethality in mice lacking the nuclear localization sequence and C-terminus of PTH-related protein. Proc Natl Acad Sci U S A. 2008;105:20309–20314. doi: 10.1073/pnas.0805690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toribio RE, Brown HA, Novince CM, Marlow B, Hernon K, Lanigan LG, Hildreth BE, Werbeck JL, Shu ST, Lorch G, Carlton M, Foley J, Boyaka P, McCauley LK, Rosol TJ. The midregion, nuclear localization sequence and C terminus of PTHrP regulate skeletal development, hematopoiesis, and survival in mice. FASEB J. 2010;24:1947–1957. doi: 10.1096/fj.09-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Southby J, Kissin MW, Danks JA, Hayman JA, Moseley JM, Henderson MA, Bennett RC, Martin TJ. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res. 1990;50:7710–7716. [PubMed] [Google Scholar]
- 94.Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ. Localization of parathyroid hormone-related protein in breast cancer metastasis: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059–3061. [PubMed] [Google Scholar]
- 95.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nature Reviews: Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 97.Yoneda T, Michigami T, Yi B, Williams PJ, Niewolna M, Hiraga T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 2000;88:2988. doi: 10.1002/1097-0142(20000615)88:12+<2979::aid-cncr13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 98.Paget S. The distribution of secondary growths in cancer of the bones. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 99.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 100.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nature Reviews Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Loberg RD, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deftos LJ, Barken I, Burton DW, Hoffman RM, Geller J. Direct evidence that PTHrP expression promotes prostate cancer progression in bone. Biochemical & Biophysical Research Communications. 2005;327:468–472. doi: 10.1016/j.bbrc.2004.11.162. [DOI] [PubMed] [Google Scholar]
- 103.Sourbier C, Massfelder T. Parathyroid hormone-related protein in human renal cell carcinoma. Cancer Letters. 2006;240:170–182. doi: 10.1016/j.canlet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 104.Shu ST, Dirksen WP, Lanigan LG, Martin CK, Thudi NK, Werbeck JL, Fernandez SA, Hildreth BE, Rosol TJ. Effects of parathyroid hormone-related protein and macrophage inflammatory protein-1a in Jurkat T-cells on tumor formation in vivo and expression of apoptosis regulatory genes in vitro. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2011.626883. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Spillane JB, Hopper JL, Martin TJ. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66:2250–2256. doi: 10.1158/0008-5472.CAN-05-2814. [DOI] [PubMed] [Google Scholar]
- 106.Henderson MA, Danks JA, Moseley JM, Slavin JL, Harris TL, McKinlay MR, Hopper JL, Martin TJ. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. Journal of the National Cancer Institute. 2001;93:234–237. doi: 10.1093/jnci/93.3.234. [DOI] [PubMed] [Google Scholar]
- 107.Kissin MW, Henderson MA, Danks JA, Hayman JA, Bennett RC, Martin TJ. Parathyroid hormone related protein in breast cancers of widely varying prognosis. Eur J Surg Oncol. 1993;19:134–142. [PubMed] [Google Scholar]
- 108.Bundred NJ, Walker RA, Ratcliffe WA, Warwick J, Morrison JM, Ratcliffe JG. Parathyroid hormone related protein and skeletal morbidity in breast cancer. Eur J Cancer. 1992;28:690–692. doi: 10.1016/s0959-8049(05)80127-3. [DOI] [PubMed] [Google Scholar]
- 109.Liapis H, Crouch EC, Grosso LE, Kitazawa S, Wick MR. Expression of parathyroid like protein in normal, proliferative and neoplastic human breast tissues. Am J Pathol. 1993;143:1169–1178. [PMC free article] [PubMed] [Google Scholar]
- 110.Bouizar Z, Spyratos F, De Vernejoul MC. The parathyroid hormone-related protein (PTHrP) gene: use of downstream TATA promoter and PTHrP 1-139 coding pathways in primary breast cancers vary with the occurrence of bone metastasis. J Bone Miner Res. 1999;14:406–414. doi: 10.1359/jbmr.1999.14.3.406. [DOI] [PubMed] [Google Scholar]
- 111.Fleming NI, Trivett MK, George J, Slavin JL, Murray WK, Moseley JM, Anderson RL, Thomas DM. Parathyroid hormone-related protein protects against mammary tumor emergence and is associated with monocyte infiltration in ductal carcinoma in situ. Cancer Res. 2009;69:7473–7479. doi: 10.1158/0008-5472.CAN-09-0194. [DOI] [PubMed] [Google Scholar]
- 112.Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J Clin Invest. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.VanHouten J, Dann P, Stewart AF, Watson C, Pollack M, Karaplis A, Wysolmerski JJ. Mammary-specific deletion of PTHrP reduces bone turnover and preserves bone mass during lactation. J Clin Invest. 2003;112:1429–1436. doi: 10.1172/JCI19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen H, Demiralp B, Schneider A, Koh AJ, Silve C, Wang CY, McCauley LK. Parathyroid Hormone and Parathyroid Hormone Related Protein Exert Both Pro- and Anti-apoptotic Effects in Mesenchymal Cells. J Biol Chem. 2002;277:19374–19381. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- 115.Hastings RH, Quintana RA, Sandoval R, Duey D, Yvette R, Burton DW, Deftos LJ. Proapoptotic effects of parathyroid hormone-related protein in type II pneumocytes. Am J Respir Cell Mol Biol. 2003;29:733–742. doi: 10.1165/rcmb.2002-0314OC. [DOI] [PubMed] [Google Scholar]
- 116.Turner PR, Mefford S, Christakos S, Nissenson RA. Apoptosis mediated by activation of the G protein coupled receptor. Mol Endocrinol. 2000;14:214–254. doi: 10.1210/mend.14.2.0417. [DOI] [PubMed] [Google Scholar]
- 117.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nature Reviews Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 118.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 119.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, Wakefield LM. TGF-beta switches from tumor supporessor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]