Abstract
STUDY QUESTION
Are significant abnormalities in outward (K+) conductance and resting membrane potential (Vm) present in the spermatozoa of patients undertaking IVF and ICSI and if so, what is their functional effect on fertilization success?
SUMMARY ANSWER
Negligible outward conductance (≈5% of patients) or an enhanced inward conductance (≈4% of patients), both of which caused depolarization of Vm, were associated with a low rate of fertilization following IVF.
WHAT IS KNOWN ALREADY
Sperm-specific potassium channel knockout mice are infertile with defects in sperm function, suggesting that these channels are essential for fertility. These observations suggest that malfunction of K+ channels in human spermatozoa might contribute significantly to the occurrence of subfertility in men. However, remarkably little is known of the nature of K+ channels in human spermatozoa or the incidence and functional consequences of K+ channel defects.
STUDY DESIGN, SIZE AND DURATION
Spermatozoa were obtained from healthy volunteer research donors and subfertile IVF and ICSI patients attending a hospital assisted reproductive techniques clinic between May 2013 and December 2015. In total, 40 IVF patients, 41 ICSI patients and 26 normozoospermic donors took part in the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Samples were examined using electrophysiology (whole-cell patch clamping). Where abnormal electrophysiological characteristics were identified, spermatozoa were further examined for Ca2+ influx induced by progesterone and penetration into viscous media if sufficient sample was available. Full exome sequencing was performed to specifically evaluate potassium calcium-activated channel subfamily M α 1 (KCNMA1), potassium calcium-activated channel subfamily U member 1 (KCNU1) and leucine-rich repeat containing 52 (LRRC52) genes and others associated with K+ signalling. In IVF patients, comparison with fertilization rates was done to assess the functional significance of the electrophysiological abnormalities.
MAIN RESULTS AND THE ROLE OF CHANCE
Patch clamp electrophysiology was used to assess outward (K+) conductance and resting membrane potential (Vm) and signalling/motility assays were used to assess functional characteristics of sperm from IVF and ICSI patient samples. The mean Vm and outward membrane conductance in sperm from IVF and ICSI patients were not significantly different from those of control (donor) sperm prepared under the same conditions, but variation between individuals was significantly greater (P< 0.02) with a large number of outliers (>25%). In particular, in ≈10% of patients (7/81), we observed either a negligible outward conductance (4 patients) or an enhanced inward current (3 patients), both of which caused depolarization of Vm. Analysis of clinical data from the IVF patients showed significant association of depolarized Vm (≥0 mV) with low fertilization rate (P= 0.012). Spermatozoa with electrophysiological abnormities (conductance and Vm) responded normally to progesterone with elevation of [Ca2+]i and penetration of viscous medium, indicating retention of cation channel of sperm (CatSper) channel function.
LIMITATIONS, REASONS FOR CAUTION
For practical, technical, ethical and logistical reasons, we could not obtain sufficient additional semen samples from men with conductance abnormalities to establish the cause of the conductance defects. Full exome sequencing was only available in two men with conductance defects.
WIDER IMPLICATIONS OF THE FINDINGS
These data add significantly to the understanding of the role of ion channels in human sperm function and its impact on male fertility. Impaired potassium channel conductance (Gm) and/or Vm regulation is both common and complex in human spermatozoa and importantly is associated with impaired fertilization capacity when the Vm of cells is completely depolarized.
STUDY FUNDING/COMPETING INTEREST(S)
The majority of the data were obtained using funding from MRC project grants (#MR/K013343/1, MR/012492/1). Additional funding was provided by NHS Tayside, TENOVUS, Chief Scientist Office NRS Fellowship and University of Abertay. The authors declare that there is no conflict of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: patch clamp electrophysiology, potassium channel, spermatozoa, IVF, male infertility, Slo1, Slo3, sperm dysfunction, CatSper
Introduction
The membrane potential (Vm) of animal cells is typically polarized (negatively charged inside), primarily by the activity of K+ channels. In spermatozoa, as in many cell types, Vm modulates the activity of membrane ion channels and transporters, including the sperm-specific Ca2+-permeable channel CatSper and voltage-gated proton channel Hv1 (Darszon et al., 1999, 2011; Lishko et al., 2012). Maintenance and modulation of Vm is therefore pivotal to sperm function and characterizing the expression and regulation of membrane K+ channels is potentially crucial to understanding the function of spermatozoa from both normal and subfertile men.
Mammalian sperm potassium channels are formed by proteins of the potassium calcium-activated channel (KCN) family. In mouse, sperm slowpoke homologue 3 (Slo3), a sperm-specific protein, forms the primary K+ channel. The pharmacology and biophysical properties of mouse sperm K+ channels are accurately reconstituted by co-expression of recombinant Slo3 [approved name potassium calcium-activated channel subfamily U member 1 (KCNU1)] and the auxiliary subunit LRRC52 [leucine-rich-repeat-containing protein (Navarro et al., 2007; Santi et al., 2010; Zeng et al., 2011, 2013, 2015)]. Mice null for Slo3 or LRRC52 have markedly reduced fertility (Santi et al., 2010; Zeng et al., 2015) and sperm from these animals show functional impairments including depolarized values of Vm, a failure to hyperpolarize during capacitation, reduced motility and lack of hyperactivation (Santi et al., 2010; Zeng et al., 2015).
These observations suggest that malfunction of K+ channels in human spermatozoa might contribute significantly to the occurrence of subfertility in men. However, patch clamp studies have shown that the complement of ion channels, their regulation and their functional significance differ significantly between human and mouse sperm (Miller et al., 2015). Pharmacological studies on human sperm suggest that Slo1 is expressed and involved in setting Vm (Mannowetz et al., 2013; Lopez-Gonzalez et al., 2014), although some characteristics of currents measured by patch clamp resemble Slo3 (Mannowetz et al., 2013; Brenker et al., 2014; Mansell et al., 2014). However, remarkably little is known of the nature of K+ channels in human spermatozoa or the incidence and functional consequences of K+ channel defects.
To address the question of the functional importance of K+ channels in human spermatozoa and the potential contribution of their malfunction to subfertility, we have used whole-cell patch clamp electrophysiology to assess the biophysical characteristics of spermatozoa from semen samples provided by 80 men attending for fertility treatment. This unique approach has allowed us to assess (i) the proportion of men undergoing treatment whose sperm show abnormalities of K+ channel expression/regulation of Vm (compared with sperm from normal, ‘healthy’ donors) and (ii) the association between abnormalities of K+ channel expression/regulation of Vm and fertilizing capacity in IVF. In this, the first study of this type, we report the prevalence, characteristics and importantly the functional consequences of abnormalities of K+ channel function and maintenance of Vm in human spermatozoa.
Materials and Methods
Experimental design
Single whole-cell sperm patch clamp recordings were made on the same day of patient treatment allowing contemporaneous assessment of K+ channel function in cells from the same ejaculate as that used for IVF. This was also the case for the majority of the ICSI patients; however, in some cases, patients were recruited as part of an IVF failed fertilization research clinic. Patch-clamp electrophysiology has been employed to characterize sperm membrane currents under quasi-physiological conditions (Mansell et al., 2014). We employed the identical strategy in order to maximize the information obtained from each patient cell. Outward potassium currents were evoked by imposing a depolarizing voltage ramp from −92 to 68 mV. To assess the functional consequences of channel function, comparison with IVF fertilization rates was made. In specific patients of interest (those with channel conductance abnormalities) and, where sufficient semen sample was available, patient samples were also assessed for Ca2+ influx in response to progesterone and viscous media penetration test. Additionally, genetic screening was performed only on patient samples with conductance abnormalities who also provided informed consent.
Patients displaying significant electrophysiological abnormalities and/or examined in more detail are referred to by a single letter (A, C, D, K, X, Y).
Experimental solutions
Standard extracellular solution: NaCl, 135 mM; KCl, 5 mM; CaCl2, 2 mM; MgSO4, 1 mM; HEPES, 20 mM; glucose, 5 mM; Na pyruvate, 1 mM; lactic acid, 10 mM; pH adjusted to 7.4 with NaOH which brought [Na+] to 154 mM. Standard pipette solution: NaCl, 10 mM; KCl, 18 mM; K gluconate, 92 mM; MgCl2, 0.5 mM, CaCl2, 0.6 mM; EGTA, 1 mM; HEPES, 10 mM; pH adjusted to 7.4 using KOH which brought [K+] to 114 mM and [Ca2+]i to 0.1 µM. Recordings from cells from Patient K in which effects of elevating [Ca2+]i were tested were obtained from a second sample using the following solutions. Pipette solution: 145 mM; KMeSO4, 5 mM; HEPES, 4 mM; KCl, 1 mM; BAPTA, 1 mM; EDTA, 1 mM; EGTA, 1.7 mM; CaCl2 pH 7.4 with KOH (final [Ca2+]i = 0.1 µM which is sufficient to inhibit monovalent CatSper currents (Lishko et al., 2011). High calcium pipette solution: 145 mM; KMeSO4, 5 mM; HEPES, 4 mM; KCl, 1 mM; HEDTA, 1 mM; CaCl2 pH 7.4 with KOH (final [Ca2+]i = 50 µM). Bath solution (mM): 140; KMeSo4, 45; HEPES, 0.1 CaCl2 pH 7.4 with KOH. [Ca2+] in buffered solutions was calculated using MaxChelator (Maxchelator.stanford.edu).
Selection of patients and preparation of spermatozoa
Patients were selected for IVF according to clinical indications and semen quality: e.g. normal sperm concentration and motility (WHO, 2010) and ∼1 × 106 progressively motile cells post-preparation (Williams et al., 2015). Patients having ICSI were those with male factor infertility undergoing ICSI for the first time due to poor quality semen and/or from patients recalled following IVF treatment affected by at least one cycle total failed fertilization/very low fertilization.
Semen samples were obtained from volunteer donors with no known fertility problems with normal sperm concentration and motility. Donor samples were obtained by masturbation after 48–72 h of sexual abstinence. In accordance with established WHO criteria, all donors were shown to produce normal semen characteristics (i.e. ≥32% progressive motility; ≥40% total motility; ≥15 × 106 cells/ml). Sperm cells were isolated using a 40–80% discontinuous density gradient procedure (Alasmari et al., 2013a,b; Tardif et al., 2014). Briefly, after 30 min of liquefaction at 37°C, up to 2 ml of semen was loaded on top of a colloidal silica suspension (Percoll, Sigma Aldrich, UK) made of 80 and 40% layers (1.5 ml each). The density gradient was then centrifuged at 300g for 20 min. Cells were washed in Quinn's Advantage Sperm Washing Media (SWM) (500g, 10 min) and resuspended in Quinn's Advantage™ Fertilization Media supplemented with human serum albumin (5%). Cells were left to capacitate at 37°C/5% CO2 for a minimum of 3 h prior to recording, which was a procedure similar to that used to prepare spermatozoa for IVF. For conditions similar to ICSI, once spermatozoa were washed, they were incubated in SWM at 37°C.
In the Assisted Conception Unit, commercially available media was used for sperm preparation. The spermatozoa were separated from semen by density gradient centrifugation (40:80%) using PureSperm™ (Nidacon, Molndal, Sweden) diluted with Quinns Advantage SWM, a HEPES-buffered solution (Cooper Surgical Inc., USA). After centrifugation, the pellet was washed by centrifugation at 500g for 10 min in 4 ml of SWM. The supernatant was discarded and the pellet resuspended in Quinns Advantage Fertilization Media (Cooper Surgical Inc.). For ICSI, once cells were washed, they were incubated in SWM at 37°C. Cells surplus to requirement were made available for research.
Patient D provided a second sample for research only 11 days after IVF treatment which was used for electrophysiological/computer-assisted sperm analysis (CASA) analysis. The mean electrophysiological data are presented from the treatment and research sample from this patient. Patients D and K had a vasectomy reversal.
Electrophysiology
The biophysical properties of individual sperm were recorded under whole cell conditions (Lishko et al., 2011; Mansell et al., 2014) using borosilicate glass pipettes (10–15 MOhms) filled with standard pipette solution. Gigaohm seals were formed at the back of the head region and suction applied to achieve a whole-cell configuration. It is of note that flagellar beating was observed in all cells selected. Sperm were perfused with standard extracellular solution designed to maintain physiologically relevant ion concentrations (Mansell et al., 2014). To investigate cell conductance and reversal potential, a depolarizing ramp protocol was imposed (−92 to 68 mV) over 2500 ms and membrane potential was held at −92 mV between test pulses. Analysis was done on the average response of currents evoked by successive depolarizations on an individual spermatozoon. Data were sampled at 2 kHz and filtered at 1 kHz (PClamp 10 software, Axon Instruments, USA). Post-recording analysis was conducted as described previously to adjust for liquid junction potential and normalize for cell size (Mansell et al., 2014). Reversal potentials (Erev) were calculated by regression analysis of membrane current over the imposed potential range where the membrane current crosses the x-axis (i.e. I = 0). Resting membrane potential (Vm) is inferred from Erev. All control data are taken from cells from at least three different donors. Membrane conductance (Gm) were derived by regression analysis of data recorded from 20 to 68 mV.
Assessment of [Ca2+]i signals
Approximately 4 million cells were prepared and assessed as previously described using a FLUOstar microplate reader (BMG Labtech Offenburg, Germany) (Alasmari et al., 2013a). After recording for an initial control period, progesterone (3.6 μM) was added to the well. Progesterone-induced increments in the ratio of emission intensities (at 340 and 380 nm excitation) were used to quantify changes in [Ca2+]i concentration (Alasmari et al., 2013a,b).
Viscous media penetration test method
The penetration into viscous media test was performed as previously described (Alasmari et al., 2013a,b). After a minimum of 3 h of capacitation, prepared sperm were adjusted to ∼10–20 × 106/ml before addition of 3.6 µM progesterone/vehicle. Following 1 h incubation at 37°C and 5% CO2, cell numbers at a penetration depth of 1 cm were counted at four different focal planes using a Hamilton Thorne CASA system (Alasmari et al., 2013a), and were normalized to values from parallel, untreated controls.
Fertilization rate at IVF
Oocytes were considered normally fertilized when two pronuclei (2PN) and two distinct or fragmented polar bodies were observed. Following IVF, the fertilization rate was calculated from the number of oocytes normally fertilized divided by the total number of inseminated oocytes. The fertilization rate was calculated where four or more mature oocytes (metaphase II) were present. Low fertilization was defined as where <25% of 4 or more metaphase II oocytes were normally fertilized.
Ethical approval
Written consent was obtained from each patient in accordance with the Human Fertilization and Embryology Authority (HFEA) Code of Practice (version 8) under local ethical approval (13/ES/0091) from the Tayside Committee of Medical Research Ethics B. Similarly, volunteer sperm donors were recruited in accordance with the HFEA Code of Practice (version 8) under the same ethical approval.
Genetic screening
Genetic screening was only performed on patient samples with conductance abnormalities who also provided informed consent. Analysis was performed on Patients D and K. DNA extracted from blood was subjected to whole-exome sequencing as previously described (Williams et al., 2015). Single-nucleotide polymorphism (SNP) and insertion or the deletion of bases (Indel) identification was performed using a standard Genome Analysis Tool Kit (GATK) workflow following bowtie alignment (McKenna et al., 2010; DePristo et al., 2011; Van der Auwera et al., 2013). To identify mutations in loci that may influence potassium conductance, we used the Sting database (http://string-db.org/) to identify a list of candidates. Mutations with an allele frequency >0.05 (as per 1000 Genomes, http://www.1000genomes.org/) were excluded.
Data analysis
Currents evoked by successive voltage ramps were pooled in order to obtain an average response from each spermatozoon. Statistical analysis was carried out to compare parameters between donor sperm and sperm from individual patients when possible. For plotted ramp current–voltage (I–V) relationships, the mean current per millivolt was calculated and mean ± SEM are presented. Data were analysed using Microsoft Excel™ or GraphPad Prism™ (version 5, GraphPad Software Inc.). The normality of Vm data was confirmed both by comparison of frequency distributions with normal distribution generated from sample mean and standard deviation and by generation of quantile–quantile (Q–Q) plots. Statistical significance was determined using the F-test (Variance ratio), Student’s unpaired t-test or analysis of variance/Dunnets post hoc test as appropriate. Data are presented as mean ± SEM with P< 0.05 indicative of statistical significance. *P< 0.05, **P< 0.01, ***P< 0.001.
Results
Voltage ramp-induced currents in donors and patients
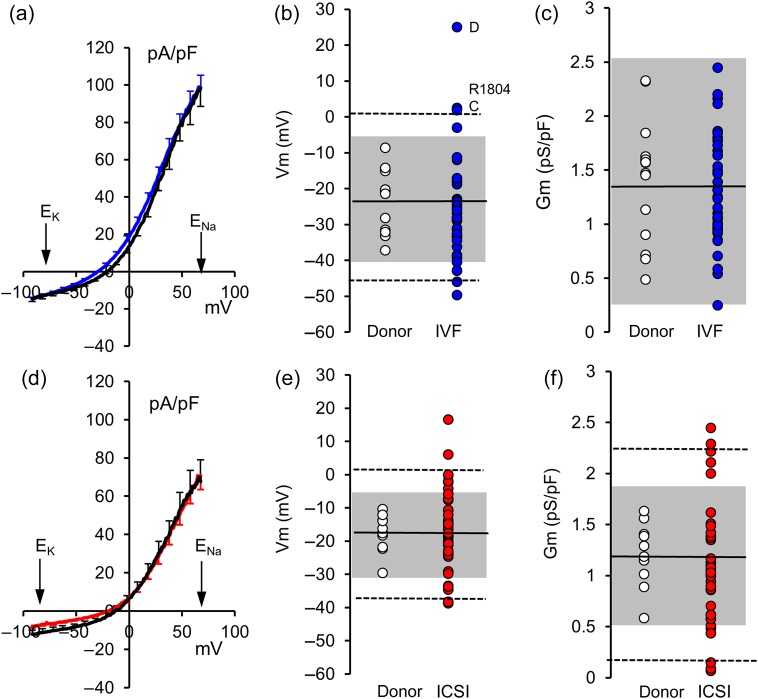
To compare voltage ramp-induced currents in donor and patient sperm, a mean I–V (current–voltage) curve was calculated for each individual. These were then combined to produce I–V plots for donors and for patients (IVF and ICSI). Consistent with previous findings (Mansell et al., 2014), application of voltage ramps (−92 to 68 mV) to donor cells prepared under capacitating conditions (16 donors, 49 cells) elicited modest inward currents at voltages negative to −20 mV and strong outward currents at depolarized voltages. The mean response of spermatozoa from IVF patients (40 patients, 117 cells) to application of voltage ramps was similar (Fig. 1a). To quantify the characteristics of voltage ramp-induced currents, we determined zero current potentials (Vm, reversal potential for the recorded currents) and conductance between 20 and 68 mV (Gm) for every cell and used these to calculate mean values in each individual. Consistent with the similarity of I–V plots, neither Vm (donor = −22.7 ± 2.0 mV; patient = −26.1 ± 2.3 mV; P= 0.30) nor Gm (donor = 1.41 ± 0.13 nS/pF; patient = 1.30 ± 0.08 nS/pF; P= 0.47) differed significantly between the donor control (n = 16) and IVF patient (n = 40) groups (Fig. 1b and c). However, among IVF patients, there was much greater variation of Vm between individuals (Fig. 1b; variance ratio = 3.12; P= 0.020).
Figure 1.
(a) Mean ramp-induced I–V relationship for IVF patients (blue; n = 40 patients) and control donor samples prepared under the same (capacitating) conditions (black; n = 16 donors). Error bars show ±1 SEM. Distribution of Vm (b) and Gm (c) for IVF patients (blue symbols; n = 40) and donor samples prepared under the same (capacitating) conditions (open symbols; n = 16). Black line shows donor mean, dashed lines and grey shading show 99 and 95% limits for two-tailed T-distribution of donor samples. Labelled points show patients illustrated in Fig. 3a and c. (d) Mean ramp-induced I–V relationship for ICSI patients (red; n = 41 patients) and donor control samples prepared under similar (non-capacitating) conditions (black; n = 10 donors). Error bars show ±1 SEM. (e and f) Distribution of values of Vm and Gm, respectively, for ICSI patients (red symbols; n = 41 patients) and donor samples prepared under similar (non-capacitating) conditions (open symbols; n = 10 donors). Black line shows donor mean, dashed lines and grey shading show 99 and 95% limits for two-tailed T-distribution of donor samples.
Data from ICSI patients and donor controls (cells prepared under similar conditions) were analysed in the same way. For the majority of ICSI patients, very few cells were available and in most cases, records from only one cell were obtained. As with spermatozoa from IVF patients, the I–V curves for donors (10 donors, 27 cells) and ICSI patients (41 patients, 50 cells) were similar (Fig. 1d) and neither mean Vm (donor = −17.7 ± 1.8 mV, n = 10; patient = −16.5 ± 1.9 mV; n = 41; P= 0.67) nor mean Gm (donor = 1.21 ± 0.10 nS/pF; patient = 1.06 ± 0.09 nS/pF; P= 0.29) differed significantly. However, similarly to IVF patients, variability of Vm between individuals was significantly greater in the ICSI patient group than the control donor group where cells were prepared under the same conditions (variance ratio = 4.33; P= 0.024).
Abnormal currents in IVF and ICSI patients
For most IVF and ICSI patients, the mean I–V curve resembled those seen in cells from controls (similarly prepared donor cells), but with more variable amplitude and reversal potential. Patients were classified as ‘abnormal’ when the mean Vm and/or Gm fell in the outer 5% of the two-tailed T-distribution calculated from the control (donor) data (Fig. 1). Nine out of 40 IVF patients (22%) had cells with abnormal Vm (including both depolarized and hyperpolarized cells) and 16 out of 41 ICSI patients (40%) were classified as abnormal by Vm and/or Gm (Fig. 1; Table I).
Table I.
Summary of key parameters for spermatozoa from IVF and ICSI patients where values of Vm and/or membrane conductance (Gm; derived by regression analysis of data recorded from 20 to 68 mV) lay in the outer 5% (*), 1% (**) or 0.1% (***) of the two-tailed T-distribution calculated from values for donor control samples prepared under the same conditions (shown in the ‘P’ column).
| Code | Treatment | Donors/cells | Vm (mV) | P-value | Gm (nS/pF) | P-value |
|---|---|---|---|---|---|---|
| Capacitated control donors | 16 donors | −22.7 ± 2.0 | — | 1.41 ± 0.13 | ||
| R1843 | IVF | 1 cell | −49.67 | ** | 2.45 | |
| R1798 | IVF | 1 cell | −46.05 | * | 0.92 | |
| R1856 | IVF | 3 cells | −42.92 | * | 1.83 | |
| A | IVF | 8 cells | −42.85 | * | 2.19 | |
| R1870 | IVF | 2 cells | −40.45 | * | 0.69 | |
| R1802 | IVF | 2 cells | −3.05 | * | 0.54 | |
| C | IVF | 5 cells | 1.82 | ** | 1.31 | |
| R1804 | IVF | 2 cells | 2.41 | ** | 1.25 | |
| D | IVF | 13 cells | 25.01 | *** | 0.25 | * |
| Non-capacitated control donors | 10 donors | −17.7 ± 1.8 | — | 1.21 ± 0.10 | ||
| R1414 | ICSI | 1 cell | −38.8 | ** | 0.48 | * |
| R1848 | ICSI | 1 cell | −38.27 | ** | 2.29 | ** |
| R1788 | ICSI | 1 cell | −33.77 | * | 2.11 | * |
| R1729 | ICSI | 1 cell | −34.62 | * | 2.22 | * |
| R1754 | ICSI | 1 cell | −33.54 | * | 1.08 | |
| A1 | ICSI | 1 cell | −24.75 | 0.44 | * | |
| R9999 | ICSI | 1 cell | −24.17 | 2.45 | ** | |
| R1873 | ICSI | 1 cell | −14.89 | 2.00 | * | |
| X | ICSI | 1 cell | −12.20 | 0.09 | ** | |
| R1640 | ICSI | 1 cell | −4.32 | * | 1.05 | |
| R1632 | ICSI | 1 cell | −2.30 | * | 1.02 | |
| R1777 | ICSI | 1 cell | −0.26 | * | 1.03 | |
| R1943 | ICSI | 1 cell | −2.02 | * | 1.42 | |
| Y | ICSI | 3 cells | −0.08 | * | 0.15 | ** |
| K | ICSI | 8 cells | 5.98 | ** | 0.08 | ** |
| R1819 | ICSI | 1 cell | 16.54 | *** | 0.61 | |
Rows for ‘capacitated control donors’ and ‘non-capacitated control donors’ show mean ± SEM for donor cells prepared under identical conditions to patient samples. Capacitated refers to samples where cells following preparation were incubated in Quinn's Advantage™ Fertilization Media and left to capacitate at 37°C/5% CO2 for a minimum of 3 h prior to recording. Non-capacitated refers to cells where, following preparation, they were incubated in SWM prior to recording to simulate conditions used in preparation of cells for ICSI (see the Materials and methods section).
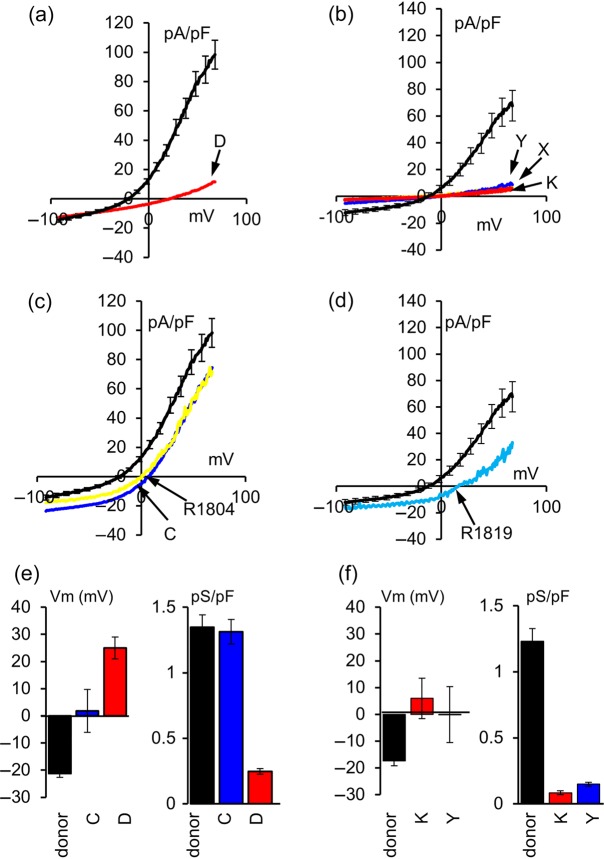
In most cases, examination of I–V traces categorized as abnormal showed no marked abnormality consistent with loss or gross changes in characteristics of the conductances expressed. For instance, in five of the IVF patients, Vm was more negative than −40 mV, but both outward conductance and inward current at negative potentials were within the normal range (Table I). In these cells, activation of outward current apparently occurred at more negative voltages (Fig. 2). However, among the patients showing depolarization of Vm, two types of abnormality were discernible.
Figure 2.
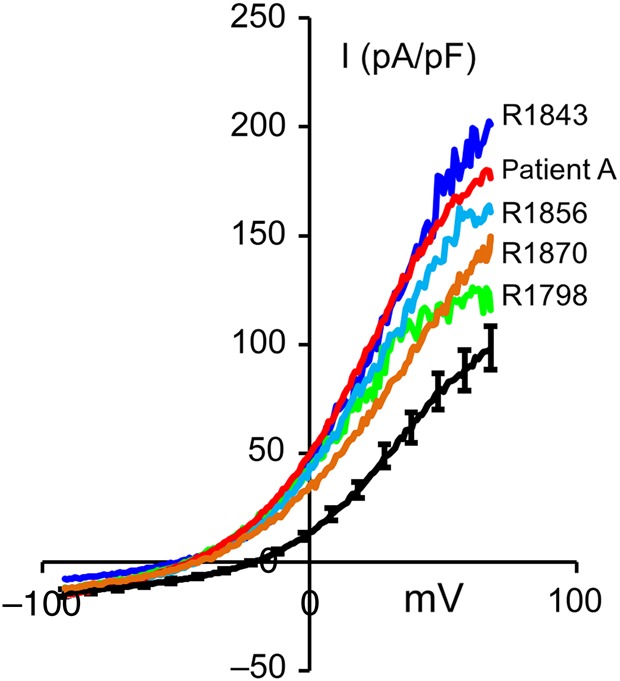
I–V traces from five IVF patients with strongly hyperpolarized Vm (<−40 mV). Black trace shows mean ± SEM I–V for donor cells (controls) prepared under identical conditions (See the Materials and Methods section).
Depolarization associated with low outward conductance
Both IVF and ICSI populations included patients with very low outward conductance. Cells from IVF Patient D (n = 13 cells; Fig. 3a) had a conductance of 0.25 ± 0.02 nS/pF (P< 0.001 compared with mean Gm for capacitated donor cells) and positive Vm (mean=+25.0 ± 4.0 mV; Table I; Fig. 3e). In three of the abnormal ICSI patients [Y (3 cells), K (8 cells) and X (1 cell)], a similar pattern was seen. Outward conductance in cells from these patients was negligible (≈0.1 pA/pF; P< 0.005) compared with the mean Gm for donor cells prepared under similar conditions (Table I; Fig. 3b and f). In a separate series of experiments, we investigated the effects of high [Ca2+]i (50 µM) on K+ currents in cells from Patient K (see the Materials and Methods section). Whereas patching of cells from Patient K with pipettes containing 50 μM Ca2+ backfill failed to increase the outward conductance over that seen using 100 nM Ca2+ backfill [Gm = 0.06 ± 0.01 (n = 4) and 0.10 ± 0.01 (n = 5) with [Ca2+]i = 50 μM and 100 nM Ca2+, respectively; P= 0.06], recordings from donor cells, carried out in parallel, showed an increase in conductance from 0.47 ± 0.08 (n = 6 cells) with backfill containing 100 nM Ca2+ to 1.11 ± 0.03 (n = 4 cells) with 50 μM Ca2+ (P < 0.001).
Figure 3.
Abnormal current–voltage relationships in spermatozoa from patients. Mean I–V relationships for (a) one IVF patient (Patient D) and (b) three ICSI patients (Patients Y, X and K) in which outward conductance was negligible. Black plot shows the mean I–V (±SEM) for donor samples prepared under the same conditions. The mean I–V relationships for (c) two IVF patients (Patients C and R1804) and (d) one ICSI patients (Patient R1819) in which outward conductance was ‘normal’, but increased inward conductance caused depolarization of Vm. Black lines show mean I–V (±SEM) for donor samples prepared under the same conditions (see the Materials and Methods section). (e) Mean ± SEM Vm (left panel) and Gm (right panel) for cells from IVF Patients C (n = 5 cells) and D (n = 13 cells) compared with mean of all control donor cells prepared under the same conditions (n = 49 cells). (f) Mean ± SEM Vm (left panel) and Gm (right panel) for cells from ICSI Patients K (n = 8 cells) and Y (n = 3 cells) compared with the mean of all control donor cells prepared under the same conditions (n = 27 cells).
Depolarization associated with enhanced inward current
Sperm from two IVF patients (C and 1804) were depolarized [+1.8 ± 7.8 mV (SEM) and +2.4 mV, respectively], but conductance was normal (Table I; Fig. 3c and e). In Patient C, where records from five cells were obtained, membrane current at EK (−79 mV; the potential at which there is no net flow of K+ ions) was highly variable and significantly larger than that for donor sperm (Im = −22.2 ± 6.5 pA/pF; n = 5 compared with −13.3 ± 0.9 pA/pF, n = 49, P< 0.01, Supplementary data, Fig. S1). Scatter plotting of single-cell conductance (Gm) versus Vm showed a positive relationship for this patient (P < 0.1; Supplementary data, Fig. S2), consistent with a major contribution of Na+ or non-selective permeability to the cell–cell variation in conductance. Sufficient sample from this patient was available for assessment of viscous medium penetration, a CatSper-dependent behaviour (Alasmari et al., 2013b; Luo et al., 2015; Williams et al., 2015). Performance was comparable to donor sperm, showing a clear stimulatory effect of progesterone (data not shown). One ICSI patient (R1819; single cell) showed a similar I–V relationship with a conductance that was within the normal range but positive Vm (Fig. 3d).
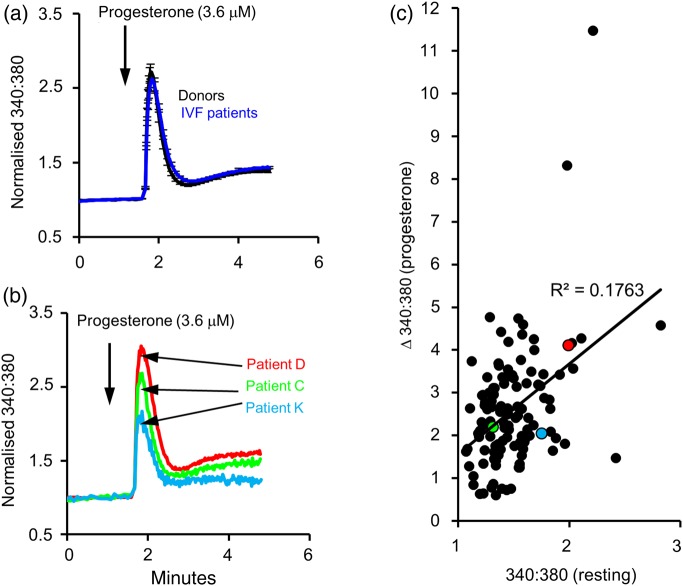
Progesterone-activated [Ca2+]i responses in cells from abnormal IVF patients
K+ channel function in sperm influences Vm and thus may regulate gating of CatSper. To test this, we investigated the [Ca2+]i response of IVF patients to progesterone (3.6 μM), which directly activates the CatSper channel. Stimulation of cell suspensions with progesterone induced a biphasic increase in [Ca2+]i that was virtually identical in donors (n = 45) and IVF patients (n = 124) (P= 0.44; Alasmari et al., 2013a; Williams et al., 2015). An initial [Ca2+]i transient, which peaked within 15 s and decayed over the following minute, was followed by a slowly developing plateau phase (Fig. 4a). In three of the abnormal patients (Patients C, D and K), there was sufficient sample available to record the [Ca2+]i signal induced by application of progesterone. Although resting potentials of spermatozoa from these patients were clearly abnormal, resting [Ca2+]i and progesterone-induced [Ca2+]i responses were similar to those of donor sperm and sperm from IVF patients (Fig. 4; P> 0.5).
Figure 4.
Progesterone-stimulated [Ca2+]i signals appear normal in patients. (a) Mean ± SEM [Ca2+]i (fura-2 340:380 ratio normalized to control period) in donor samples (black line, n= 45) and IVF patients (red line; n = 124). (b) Mean [Ca2+]i in two IVF (Patients C and D)and one ICSI patient (Patient K) in which electrophysiological investigation identified abnormalities in membrane conductance and/or Vm. In both panels, the arrow shows when progesterone (3.6 μM) was added to the cells. (c) The relationship between the fura-2 ratio before stimulation [340:380 (resting)] and the size of the subsequent progesterone-induced increase in the ratio [Δ340:380 (progesterone)] in control donors (black symbols) and in Patients C (green symbol), D (red symbol) and K (pale blue symbol). Both the resting [Ca 2+]I signal and the responses of these donors fall within the normal range.
Genetic analysis of Patients D and K
Analysis of the exome sequences of Patients D and K revealed no candidate causative mutations in KCNU1 (Slo3), KCNMA1 (Slo1) or LRRC52. While no rare variants were detected, Patient K was heterozygous for SNPs rs17407838 (LRRC52, D209E, MAF A = 0.0585) and rs28608091 (KCNU1, W768R, MAF C = 0.3798). Patch clamp analysis of heterologously expressed KCNU1 constructs carrying the rs28608091 mutation indicated that it did not alter the electrophysiological properties of the channel (Supplementary data, Fig. S3). A broader analysis was performed using a candidate gene list (Supplementary data, Table SI) also failed to identify any homozygous, or compound heterozygous, candidate mutations (Supplementary data, Table SII).
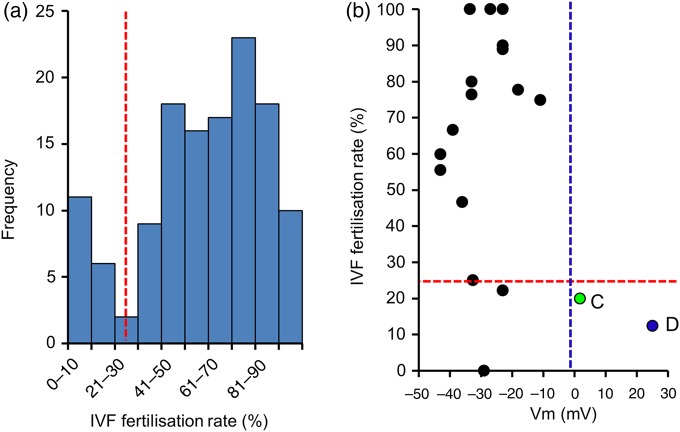
Biophysical abnormality and fertilizing potential of spermatozoa IVF
A key question to address is the functional consequences of these abnormalities. Since IVF patient cells used for electrophysiology were aliquots from the ejaculate used on the day of IVF treatment, we were able to directly compare biophysical characteristics with fertilization success in the same ejaculate (8–20 oocytes per patient). Compiled fertilization data from a large sample of IVF patients (n = 126) at the clinic showed a bimodal distribution (Fig. 5a) with a major peak at 70–80% (normal group) but also a discrete group of patients with abnormally poor fertilization (0–25%). Using the IVF data from 19 patients where we had current records from ≥3 cells (providing an acceptable estimate of population characteristics), we investigated the relationship between mean Vm and fertilization rate. Complete depolarization of Vm (≥0 mV; Patients C and D) was significantly associated with the group showing poor fertilization rates (≤25%) (Fig. 5b; P = 0.016; χ2 contingency).
Figure 5.
Highly depolarized membrane potential is associated with poor fertilization rate in IVF. (a) The distribution of fertilization rates (% fertilized) for 126 IVF patients. Two separate groups can be identified (separated by dashed red line). Most patients achieved ≥25% fertilization but a ‘poor’ subgroup, with fertilization rate <25% is also present. (b) The relationship between mean membrane potential and fertilization rate for 19 patients for whom multiple recordings and fertilization rate data were obtained. (Patients C and D are labelled.) Dashed lines delineate categories used for χ2 contingency analysis: red line shows poor and normal fertilization groups (<25 and ≥25%); blue line shows ‘normal’ Vm and highly depolarized (<0 and ≥0 mV). P = 0.012.
Discussion
In the present study, we have used the whole-cell recording technique to characterize the biophysical properties of sperm from 81 subfertile patients undergoing IVF/ICSI. This is by far the most extensive such study ever undertaken. In both IVF and ICSI patients, the mean I–V relationship was similar to that for donor controls. However, the variability of biophysical characteristics (Vm and Gm) between individuals was much greater among patients than controls (donors) and in a significant minority of patients (20–40%), one or more biophysical characteristics were abnormal. In patients recommended for ICSI treatment, multiple abnormalities of spermatogenesis are common, manifested as reduced sperm count, morphology and/or motility (Sakkas et al., 2015). The detection of ion channel malfunction in these men is therefore perhaps not surprising, although the high incidence of biophysical abnormalities is nevertheless striking. However, patients selected for IVF had effectively ‘normal’ semen parameters (WHO, 2010) and the occurrence of biophysical abnormalities in up to 20% of these men supports the hypothesis that ion channel malfunction may contribute significantly to idiopathic male infertility.
Characteristics of currents in patient spermatozoa
Although the occurrence of greater biophysical variability in spermatozoa of patients must reflect, in part, the presence of some patients with clear abnormalities of current characteristics (see below), there also appeared to be an ‘exaggeration’ of the innate variability in conductance and reversal potential that was present in the control (donor) group. Outward currents recorded under the quasi-physiological conditions used here are carried primarily by flux through a conductance that is poorly K+ selective with GK:GNa ≈7 (Mansell et al., 2014). This channel shows <25% activation when Vm < 0 mV and although reversal potentials for cells prepared under both capacitating and non-capacitating conditions are closer to EK (−79 mV) than ENa (+69 mV), it is clear that the flux of ions other than K+ contributes to resting Vm. Since incubating cells under IVF capacitating conditions results in a change in current characteristics, it is possible that uncoupling and/or poor regulation of the changes in channel function that occur during capacitation may result in the observed increase in biophysical variability in the patient population.
Patients with abnormal currents
Examination of I–V records revealed at least two types of significant current abnormality. In four patients (ICSI patients X, Y, K and IVF Patient D), outward conductance was very low or negligible. Cells in these samples were depolarized and in Patients D and K (13 cells and 8 cells, respectively), the mean Vm was markedly positive (+25 and +6 mV, respectively), resembling Vm after block of K+ conductance with quinidine or bupivacaine (Mansell et al., 2014). In spermatozoa from Patient K, we investigated the effect of elevating intracellular Ca2+ to 50 μM, which enhances K+ currents (Mannowetz et al., 2013; Brenker et al., 2014). This manipulation did not ‘rescue’ resting Vm or outward conductance. It is noteworthy that although most of these patients are in the ICSI group, Patients X and Y had basic semen characteristics (≥15 million cells/ml and ≥25% motility) that would be classed as close to normal by WHO standards (WHO, 2010). We conclude that in these four patients, expression and/or function of K+ conductance is severely impaired.
A second, characteristic abnormality (seen in two IVF patients and one ICSI patient) was the occurrence of an increased inward current such that Vm was depolarized, despite the presence of outward conductance within the normal range. In IVF Patient C, where recordings from five cells were obtained, inward current at EK (−79 mV; where there is no net K+ flux) was highly variable and significantly greater than in controls. It appears that an inward leak conductance (probably Na+) contributed excessively to determination of Vm in these patients. Evidence for expression of epithelial sodium channels (ENaC) and voltage-gated sodium channels (NaV) in human spermatozoa has been reported (Kong et al., 2009; Pinto et al., 2009; Escoffier et al., 2012; Cejudo-Roman et al., 2013), but to date, no such currents have been shown in electrophysiological examination of ejaculated cells. If these channels function primarily in spermatogenic cells, incomplete and/or impaired spermatogenesis (or epididymal maturation) might result in over-expression of these conductances in ejaculated cells of these patients.
Biophysical abnormalities and failure of sperm at IVF
A key question is, what are the functional consequences of these abnormalities? Fertilization rates of IVF patients were distributed bi-modally with a ‘normal’ group which typically achieved 70–80% oocyte fertilization and a subset where fertilization was 0–25%. Of the 19 IVF patients where currents were recorded from ≥3 cells (allowing assessment of population characteristics), only Patients C and D fell in the low fertility group (20% and 12.5% IVF fertilization rate, respectively). Spermatozoa from these patients differed fundamentally from control donor spermatozoa in their biophysical characteristics. Both were strongly depolarized, with the mean Vm > 0 mV, but whereas Patient D was effectively devoid of outward current, in Patient C, the positive Vm reflected the presence of a large inward (probably Na+) leak (see above). χ2 analysis showed that this association of positive membrane potential with the low IVF fertilization rate was non-random (P < 0.02). These data suggest that a strongly depolarized membrane potential (≥0 mV), irrespective of the underlying lesion(s), has a significant adverse effect on fertilizing ability.
A surprising finding was that although major, functionally significant (poor fertilizing potential) abnormalities of outward conductance and membrane potential regulation were detected in sperm from some patients, these sperm appeared normal in other assessments employed in this study. For instance, it has been hypothesized that sperm membrane potential and its regulation might be expected to influence CatSper function (Brenker et al., 2014), yet the progesterone-induced [Ca2+]i signal in patients (C, D and K) resembled those of controls (donors) both in ‘normal’ patients (as described previously; Alasmari et al., 2013a) and in patients with significantly abnormal conductance and/or depolarized Vm. This implies that there is only a weak interaction between Vm and CatSper function. Similarly, sperm from Patient C penetrated viscous media to the same degree as controls (donor spermatozoa) both in an unstimulated state and stimulated with the CatSper agonist progesterone. Additionally, despite the apparent loss of K+ conductance and positive Vm recorded from cells of Patient D, kinematic parameters of sperm motility from this patient (assessed by CASA; data not shown) appeared normal, both in terms of mean value and distribution of single-cell values. Why might these spermatozoa perform normally in these tests even with highly abnormal Vm? One possibility is that the assays employed here are inadequate to detect the subtle interactions between membrane potential, CatSper activity and K+ channel function (Brenker et al., 2014). For instance, CatSper conductance in the absence of progesterone is low even at very depolarized potentials (Lishko et al., 2011), but the saturating concentrations of progesterone used here may produce robust [Ca2+]i signals in human sperm irrespective of Vm. Consequently, future studies on sperm with impaired regulation of Vm should address more subtle aspects of sperm function such as dose-dependence of the action of progesterone, the Ca2+-sensitivity of the K+ channel in human spermatozoa and its potential interplay with CatSper (Brenker et al., 2014). It will be interesting specifically to investigate long-term patterns of [Ca2+]i and motility following CatSper activation in K+-channel null/impaired cells.
We can only speculate as to the nature of the abnormalities(s) that result in altered currents reported here. The characteristics of Patient C and D with reduced fertilization rate are similar to the abnormalities shown in LRRC52 KO mice with significantly reduced but not absent fertilization rates (Zeng et al., 2015). No genetic abnormalities in SLO1, SLO3 or LRCC52 genes were shown in Patient D (Patient C did not give permission for genetic analysis). It is possible that in the absence of any overt genetic abnormality, a defect(s) in testicular and/or epididymal sperm maturation and/or potential processing in the mature cell during capacitation may be present. However, although impaired localization and/or assembly of the SLO1/SLO3/LRCC52 complex is a likely explanation for loss of function, detailed proteomic studies combined with high quality co-localization and imaging will be required to address this.
In this study, we assessed the presence and prevalence of abnormalities in membrane conductance and maintenance of Vm in spermatozoa from 81 patients attending for IVF (normal in their semen characteristics, WHO, 2010) and ICSI treatment. In most cases, these spermatozoa had a Vm and current profile similar to that seen in healthy volunteer donors, although there was greater variation in both outward conductance and Vm. This is consistent with a shift in channel regulation due to changes in spermatogenesis and/or epididymal maturation. In a surprisingly large number of patients, Vm and/or conductance were significantly abnormal, including a subset (5–10% of both IVF and ICSI patients) where currents were grossly abnormal. Where these abnormalities resulted in a strongly depolarized (+ve) Vm, the cells were functionally compromised. Overall, these data suggest that (i) impaired K+ conductance and/or regulation of Vm in spermatozoa is a relatively common feature both in ICSI patients and in men classified as suitable for IVF and (ii) such lesions, particularly when they cause loss or reversal of membrane polarization, may contribute to a significant loss of fertilizing potential. This knowledge could be helpful for the development of novel diagnostic tools to assess semen quality using membrane permeable voltage-sensitive dyes.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Acknowledgements
The authors are very grateful to all members of the Assisted Conception Unit at Ninewells Hospital for their invaluable assistance in obtaining donor and patient samples for research and training purposes, in particular the embryologists (Kath, Ellen, Sylvia, Anne, Mandy and Philip), lab practitioners (Ola, Hannah, Laura, Steven) and nurses. We are also grateful to all the patients and donors who took part in this study. The authors acknowledge other members of the laboratory for their continual helpful advice and comments, and Evelyn Barratt for assisting with the recruitment of patients and donors. We also acknowledge Yuriy Kirichok from UCSF for his invaluable assistance in developing the patch clamping in Dundee, Christopher J. De Jonge, University Minnesota, for critical review of the manuscript and Melissa Miller, University of California, Berkeley with assistance in the genomic sequence analysis.
Authors’ roles
S.G.B. conducted the overwhelming majority of the patch clamp experiments with S.A.M. conducting several on specific patients and controls. S.M.D.S., M.R. and H.L.W. were involved in the recruiting, recalling and consenting of patients and donors and preparation of some patient samples and donors for research and initial screening of individuals for ion channel defects. S.G.B, S.M.W. and S.J.P. performed the detailed analysis of the electrophysiological data. K.A.S. and P.V.L. were responsible for the genetic analysis. P.V.L and S.A.M were responsible for the in vitro mutagenesis. S.J.P., S.G.B. S.M.W. and C.L.R.B. were involved in the design of the study and obtained funding for the experiments. The initial, interim and final manuscript was drafted by S.G.B., S.J.P and C.L.R.B. All authors contributed to the construction, writing and editing of the manuscript. All authors approved the final manuscript.
Funding
We acknowledge the significant contribution of the Medical Research Council project grants (MR/K013343/1, MR/012492/1) in making this study possible (S.G.B., S.J.P., C.L.R.B.) and University of Abertay (sabbatical for S.G.B.). Additional funding was provided by the Infertility Research Trust (C.L.R.B.), Wellcome Trust (C.L.R.B., S.J.P.) and TENOVUS SCOTLAND (S.M.D.S.), Chief Scientist Office/NHS research Scotland (S.M.D.S). P.V.L. was supported by NIH grant R01GM111802 (NIGMS), Alfred P. Sloan Award, R21HD081403 (NICHD) and Pew Scholars Award. Funding to pay the Open Access publication charges for this article was provided by MRC (University of Dundee).
Conflict of interest
C.L.R.B. is the editor-in-chief of Molecular Human Reproduction and Chair of the World Health Organsiation Expert Working Group on Diagnosis of Male infertility (2012–2016).
References
- Alasmari W, Barratt CL, Publicover SJ, Whalley KM, Foster E, Kay V, Martins da Silva S, Oxenham SK. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod 2013a;28:866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, Ramalho-Santos J, Kirkman-Brown J, Michelangeli F, Publicover S et al. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J Biol Chem 2013b;288:6248–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Zhou Y, Muller A, Echeverry F, Trotschel C, Poetsch A, Xia X, Bonigk W, Lingle C, Kaupp U et al. Slo3 in human sperm—a K+ channel activated by Ca2+. ELife 2014;3:e01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejudo-Roman A, Pinto FM, Subiran N, Ravina CG, Fernandez-Sanchez M, Perez-Hernandez N, Perez R, Pacheco A, Irazusta J, Candenas L. The voltage-gated sodium channel nav1.8 is expressed in human sperm. PLoS One 2013;8:e76084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Labarca P, Nishigaki T, Espinosa F. Ion channels in sperm physiology . Physiol Rev 1999;79:481–510. [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Trevino CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 2011;91:1305–1355. [DOI] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data . Nat Genet 2011;43:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffier J, Krapf D, Navarrete F, Darszon A, Visconti PE. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J Cell Sci 2012;125:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XB, Ma HG, Li HG, Xiong CL. Blockade of epithelial sodium channels improves sperm motility in asthenospermia patients. Int J Androl 2009;32:330–336. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011;471:387–391. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 2012;74:453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez I, Torres-Rodriguez P, Sanchez-Carranza O, Solis-Lopez A, Santi CM, Darszon A, Trevino CL. Membrane hyperpolarization during human sperm capacitation. Mol Hum Reprod 2014;20:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Li N, He YQ, Weng SQ, Wang T, Zou QX, Zeng XH. Emodin inhibits human sperm functions by reducing sperm [Ca] and tyrosine phosphorylation. Reprod Toxicol 2015;51C:14–21. [DOI] [PubMed] [Google Scholar]
- Mannowetz N, Naidoo N, Choo S-AS, Smith JF, Lishko PV. Slo1 is the principal potassium channel of human spermatozoa. eLife 2013; doi:10.7554/eLife.01009. [DOI] [PMC free article] [PubMed]
- Mansell SA, Publicover SJ, Barratt CL, Wilson SM. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol Hum Reprod 2014;20:392–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Mansell SA, Meyers SA, Lishko PV. Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium 2015;58:105–113. [DOI] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential . Proc Natl Acad Sci USA 2007;104:7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto FM, Ravina CG, Fernandez-Sanchez M, Gallardo-Castro M, Cejudo-Roman A, Candenas L. Molecular and functional characterization of voltage-gated sodium channels in human sperm. Reprod Biol Endocrinol 2009;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas D, Ramalingam M, Garrido N, Barratt CL. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update 2015;21:711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Martinez-Lopez P, de la Vega-Beltran JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 2010;584:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Madamidola OA, Brown SG, Frame L, Lefievre L, Wyatt PG, Barratt CL, Martins Da Silva SJ. Clinically relevant enhancement of human sperm motility using compounds with reported phosphodiesterase inhibitor activity. Hum Reprod 2014;29:2123–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 2013;11:11.10.1.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HL, Mansell S, Alasmari W, Brown SG, Wilson SM, Sutton KA, Miller MR, Lishko PV, Barratt CL, Publicover SJ et al. Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa. Hum Reprod 2015;30:2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2010) WHO laboratory manual for the examination and processing of human semen. 5th edn. Cambridge University Press. [Google Scholar]
- Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA 2011;108:5879–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Navarro B, Xia XM, Clapham DE, Lingle CJ. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J Gen Physiol 2013;142:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Yang C, Xia XM, Liu M, Lingle CJ. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility . Proc Natl Acad Sci USA 2015;112:2599–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.