Abstract
Obesity is causally linked with the development of cardiovascular disorders. Accumulating evidence indicates that cardiovascular disease is the “collateral damage” of obesity-driven adipose tissue dysfunction that promotes a chronic inflammatory state within the organism. Adipose tissues secrete bioactive substances, referred to as adipokines, which largely function as modulators of inflammation. The microenvironment of adipose tissue will affect the adipokine secretome, having actions on remote tissues. Obesity typically leads to the upregulation of pro-inflammatory adipokines and the downregulation of anti-inflammatory adipokines, thereby contributing to the pathogenesis of cardiovascular diseases. In this review, we focus on the microenvironment of adipose tissue and how it influences cardiovascular disorders, including atherosclerosis and ischemic heart diseases, through the systemic actions of adipokines.
Keywords: Obesity, Adipokine, Adipose Tissue, Atherosclerosis, Myocardial Infarction
INTRODUCTION
The prevalence of obesity, defined as a body mass index ≥ 30kg/m2, is now recognized worldwide as a major health problem, reaching epidemic proportions probably as a consequence of changes in food composition and exacerbated by sedentary lifestyles in Western societies1–3. Large epidemiological studies have conclusively demonstrated that obesity is associated with increased mortality mostly due to augmented risk of cardiovascular (CV) death4. Moreover, the increasing prevalence of obesity is changing the etiology of cardiovascular diseases (CVD), which in many individuals can be viewed as the consequence of dysfunctional changes within the adipose tissues. Obesity induces a complex remodeling of adipose tissue, which expands to accommodate the excessive caloric intake and markedly changes its structure and cellular composition. It is widely accepted that this obesity-associated remodeling generates a systemic pro-inflammatory state, which is mediated by an imbalanced production of adipocyte-derived cytokines (adipokines) that directly and indirectly affect the CV system. In this Review article, we summarize the pathophysiological mechanisms underlying adipose tissue remodeling and dysfunction in obese individuals, and how this affects the production of adipokines and ultimately contributes to CVD.
Adiposopathy, regional adiposity and CV risk
While adipose tissue quantity (volume) is undoubtedly linked to CV risk, recent human data indicate that differences in fat tissue “quality”, which can be examined directly by immunohistochemistry or non-invasively by computed tomography (CT) radiodensity attenuation imaging, are closely linked to insulin resistance, cardiometabolic risk and all-cause mortality, independent of total fat volume5–8. These data demonstrate that abnormalities at the adipose tissue level may be key factors that regulate systemic metabolism and drive cardiometabolic disease (CMD), independent of body mass index. These qualitative abnormalities in fat, which have been recently termed ‘adiposopathy’ or “sick fat”9, are a growing area of research interest and may in part explain the clinical observation of metabolically healthy obesity. While animal models of obesity tend to generate fairly uniform phenotypes, the degree of adipose tissue dysfunction in obese humans exhibits significant heterogeneity with lower degrees of adiposopathy being associated with more favorable systemic metabolic profiles and vascular function8, 10–13. This inter-individual variability in adipose tissue ‘quality’ may be related, in part, to differences in lifestyle, as physical activity has effects on adipose tissue physiology and CMD risk14, 15.
Differences in adipose tissue ‘quality’ are also closely linked with the observation that distinct fat depots have different impacts on the propensity to develop CMD. Numerous clinical studies using adiposity measures such as waist circumference and waist-to-hip ratio as markers of central obesity as well as cross-sectional abdominal imaging, have established clear links between overall fat burden and systemic CMD, with generally stronger associations for visceral adiposity7, 8, 16–26. It is now recognized that expansion of visceral fat is strongly associated with increased cardiometabolic risk8, 16, 17, 26–29, whereas expansion of subcutaneous fat has a minor contribution or, in some cases, even decreases the risk of metabolic dysfunction16, 17, 30, 31. Thus, it has been hypothesized that visceral fat exhibits lower ‘quality” than subcutaneous depots, exhibiting specific properties that are linked to a higher cardiometabolic risk. Subcutaneous fat comprises approximately 80% of total body fat mass, while abdominal visceral adipose tissue accounts for 5–20%32. Despite visceral fat not being the predominate white adipose tissue (WAT) depot, inflammatory markers including IL-6, CRP, and TNF-α tend to circulate at higher concentrations in subjects with abdominal compared with peripheral obesity33–36, and visceral fat has been shown to be a significant source of circulating FFA and IL-6 levels37, 38. Although arterial disease tends to worsen with increasing overall weight burden in adults and children18, 39, CT or MRI studies of fat compartments identify visceral fat volume to be more highly associated with systemic endothelial dysfunction compared to subcutaneous fat20, 21. In addition, gene expression analyses of human specimens suggest a more atherogenic gene expression profile in visceral fat, characterized by greater expression of pro-inflammatory, oxidative stress-related and anti-angiogenic genes40–46. Visceral and subcutaneous adipose depots arise from different origins during development47, 48, and this may in part explain the propensity for visceral fat to develop differing metabolic, inflammatory, angiogenic, and lipolytic properties that contribute to CMD compared to subcutaneous.
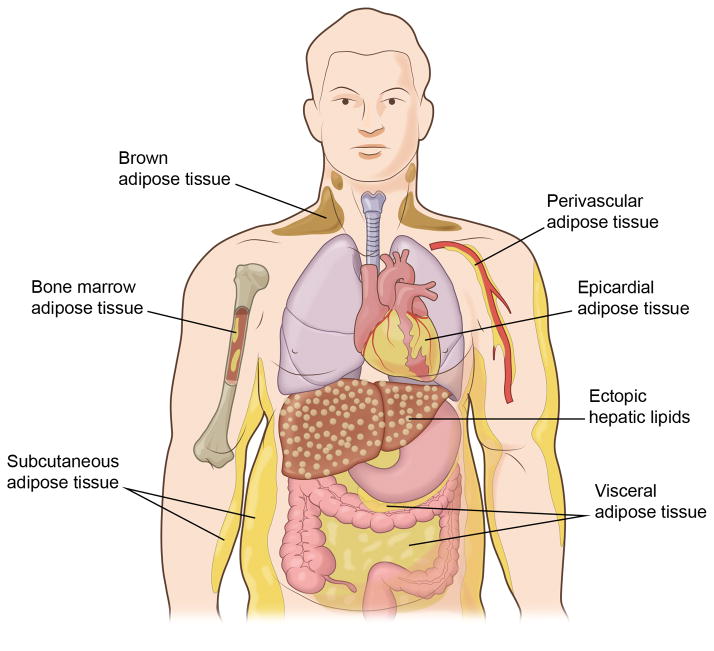
In addition to the subcutaneous and visceral fat depots, adipocytes are associated with many organs and tissues including heart, kidney and bone marrow and the degree of adiposity can vary with obesity and aging (Figure 1). Recently, the possibility of functionally significant brown adipose tissue (BAT) depots in adults has become of interest. BAT is primarily located beneath the clavicle, and it has a thermogenic function and it oxidizes rather than stores fat. Historically, BAT received little attention because it was thought to exist only in human infants, rodents, etc. to maintain body temperature. However, it is now recognized that some adults contain appreciable levels of BAT, and that its oxidative function declines with obesity and advanced age49, 50. Intriguingly, rodent studies have suggested that BAT may contribute significantly to overall systemic metabolic control due to its potentially high oxidative capacity51. Compared to WAT, BAT contains abundant mitochondria that are uncoupled, due to the expression of UCP1, and highly vascularized to accommodate the greater demand for oxygen. Interestingly, the phenotype of perivascular adipose tissue (PVAT), that surrounds the major blood vessels, appears to be intermediate between that of WAT and BAT, and its degree of “browning” varies in different vascular beds52–55. These morphological differences between PVAT depots suggest that it may contribute to the phenotypic variability between distinct vascular regions and their different susceptibility to atherosclerosis and other vascular disorders. In this regard, it is conceivable that differences in adipokine section by these various adipose tissue depots can selectively affect organ function via paracrine mechanisms.
Figure 1.
Adipose tissue depots occur throughout the body. Studies suggest that visceral adipose tissue accumulation is a major risk factor for cardio-metabolic disease, whereas subcutaneous fat appears to be neutral or protective. Other adipose tissue depots of note include the epicardium, the perivascular space, and bone marrow, but the functional significance of these tissues is largely unknown. Brown adipose tissue occurs in the supraclavicular and paraspinal regions. In contrast to white adipose tissue, brown adipose tissue is very metabolically active and it functions to utilize fuel to produce heat. In addition, ectopic lipid can accumulate in tissues, such as liver, in metabolically dysfunctional organisms.
CHANGES IN THE MICROENVIRONMENT OF THE ADIPOSE TISSUE ASSOCIATED WITH OBESITY
Adiposopathy in obese individuals is ultimately the consequence of a dysfunctional remodeling of the adipose tissue. Therefore, understanding both quantitative and qualitative aspects of this adipose tissue remodeling is of utmost importance to comprehend how obesity contributes to CVD.
Adipose tissue expansion
The mechanisms by which adipose depots expand in response to an excessive caloric intake represent a crucial determinant of the risk of metabolic dysfunction and CVD. This expansion is mediated by an increase in adipocyte numbers (hyperplasia) and/or an enlargement of adipocyte size (hypertrophy). It has been classically accepted that hyperplasia allows a “healthy” expansion of the adipose tissue, since it is mediated by the formation of functional adipocytes from progenitor cells (adipogenesis). In contrast, adipocyte hypertrophy typically leads to lipid-laden, dysfunctional adipocytes that undergo cell death and contribute to adipose tissue inflammation, dysfunction and associated pathologies. As discussed above, different adipose tissue depots contribute differentially to disease processes, and this may be connected to a dysfunctional expansion of the different fat depots. It has been proposed that subcutaneous fat in many human individuals exhibits limited expandability due to a deficient adipogenic capacity, which leads to subcutaneous adipocyte enlargement (hypertrophic obesity) and ultimately promotes the storage of fat in visceral and other ectopic depots56. In this regard, it is noteworthy that several genetic modifications have been shown to improve insulin sensitivity in obese mice by inducing subcutaneous adipose tissue expansion without increasing adipocyte size57, 58, highlighting the therapeutic potential of strategies aimed at promoting adipogenic/hyperplastic growth of subcutaneous fat as a mean of preventing the metabolic and CV complications of obesity.
Recent studies with a mouse strain that allows adipocyte tracing in vivo (AdipoChaser mice) have provided detailed insight into the mechanism and dynamics of adipose tissue expansion in obese mice59. These studies showed that visceral adipose tissue expansion in diet-induced obese mice is initially mediated by adipocyte hypertrophy, which is followed by a massive increase in adipogenesis after prolonged high-fat diet (i.e., 2 months). In contrast, subcutaneous adipose tissue expansion was shown to be mostly mediated by adipocyte hypertrophy, with minimal de novo adipogenesis regardless of the time of HFD exposure. Hence, at least in this depot, mouse models may mimic the conditions of human hypertrophic obesity. However, while these studies represent excellent examples of the application of mouse genetics to cardiometabolic research, they must be interpreted with caution given the many differences between the different mouse and human adipose tissue depots. For example, while in humans the prototypical visceral depot is omental fat, this depot is essentially absent in mice. Conversely, perigonadal fat is the most typical visceral depot in mice, but it does not have a truly equivalent depot in humans, and does not drain blood into the portal circulation, in contrast to human visceral depots. Thus, the extent to which the dynamics of fat depot expansion in mice mimics the processes involved in human obesity are unclear.
Immune cell infiltration
Regardless of the mechanisms of adipose tissue expansion, in most cases chronic excessive caloric intake eventually leads to adipocyte dysfunction, and this is paralleled by quantitative and qualitative changes in the cellular composition of adipose tissue. Immune cells are of particular relevance in this regard. Chronic, low-grade inflammation is a major hallmark of the obese adipose tissue, and it is now known that, at least in mice, almost every immune cell type can be found in the adipose tissue under one experimental condition or another. Total numbers of T cells, B cells, macrophages, neutrophils, and mast cells are increased in visceral adipose tissue of obese individuals and/or dietary obese mice. In contrast, the number of eosinophils and specific subsets of T cells – T helper type 2 (Th2) cells, regulatory T (Treg) cells – remain static or are decreased in the obese adipose tissue60.
Macrophages are the most abundant immune cell in the adipose tissue of obese individuals, and their recruitment and proliferation upon high calorie feeding is generally associated with adipose tissue inflammation and insulin resistance61–63. In addition, the phenotype of adipose tissue macrophages (ATMs) is markedly different in obese and lean mice. Macrophages resident in the adipose tissue of lean organisms tend to express genes associated with a M2-like or “alternatively activated” phenotype (e.g. the mannose receptor CD206), whereas ATMs in obese organisms typically express genes associated with a M1-like or “classically activated” phenotype (e.g. CD11c)64. The M1/M2 concept is an artificial binary classification of the inflammatory status of macrophage, and it should be noted that in vivo macrophages exist along the M1/M2 spectrum and frequently have mixed phenotypes. This is particularly evident in ATMs, which frequently exhibit a complex phenotype due to the simultaneous exposure to a variety of stimuli65–67. In spite of this, the M1/M2-like dichotomy is a useful starting point to understand the biology of ATMs. Stimulation with T helper 1 (Th1)-type cytokines, including interferon-γ induces an M1 phenotype in macrophages, that leads to increased production of pro-inflammatory cytokines, such as TNF-α, and higher levels of reactive oxygen and nitrogen intermediates. This class of macrophages is typically associated with inflammation and tissue destruction. On the other hand, stimulation with Th2-type cytokines (e.g. IL-4, IL-13) leads to M2 macrophages, which preferentially express anti-inflammatory cytokines, such as IL-10, and are typically associated with wound healing, angiogenesis and the resolution of inflammation. It is believed that M1-like macrophages promote insulin resistance, whereas M2-like macrophages protect against obesity-induced adipose tissue inflammation and insulin resistance68. Supporting this notion, ablation of CD11c-positive, M1-like cells normalizes insulin sensitivity in obese mice69. Consistently, an increased content of CD11c-positive macrophages has been associated with insulin resistance in obese human individuals70. The mechanisms accountable for ATM phenotypic shifting in obesity are still unclear, but are probably linked to changes in both immune cells in the adipose tissue71–74 and myeloid progenitors in the bone marrow75, 76. The M2 phenotype of resident macrophages within the lean adipose tissue is believed to be maintained by the local production of Th2-type cytokines by eosinophils71, and other immune cells abundant in the lean adipose tissue, such as CD4+ Foxp3+ Treg cells and TH2-polarized T cells, that preserve adipose tissue function and insulin sensitivity73, 77. Under conditions of obesity, the accumulation of CD8+ effector T cells and CD4+ Th1 cells in the adipose tissue leads to a predominance of Th1 signals that promote the recruitment and M1-like activation of macrophages, contributing to adipose tissue inflammation72, 73. Pro-inflammatory cytokine production by effector T cells and Th1 cells is promoted by B cells recruited to the obese adipose tissue, which also contribute to M1 macrophage activation apparently through the production of pathogenic immunoglobulins74. Additional lymphocyte subsets such as Th17 or NKT cells may also play important roles in modulating macrophage phenotype and adipose tissue inflammation (reviewed in 78).
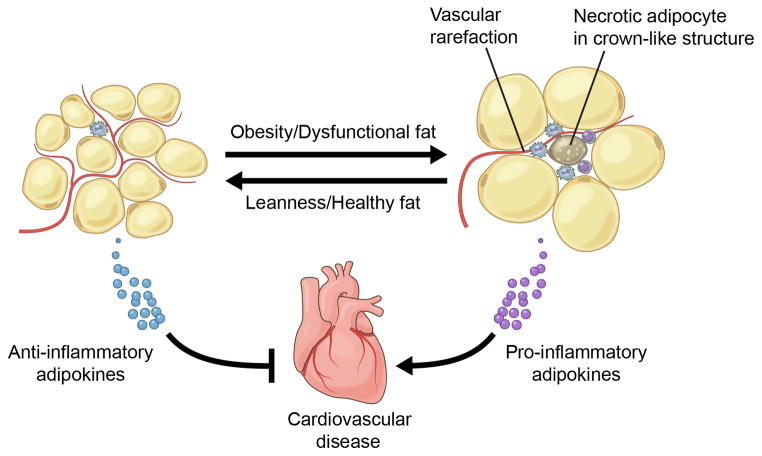
In addition to quantitative and phenotypic changes, obesity also changes the location of macrophages within the adipose tissue. While ATMs are typically dispersed in lean individuals, in metabolically dysfunctional organisms they tend to accumulate in “crown-like” structures (CLS), defined as clusters of lipid-scavenging macrophages that surround free lipid droplets of dead adipocytes both in animal models and obese patients79, 80 (Figure 2). Importantly, this condition appears to contribute to adipose tissue dysfunction, since the number of CLS correlates with adipose tissue inflammation and insulin resistance in metabolic syndrome patients11, 79. Consistently, obese subjects lacking CLS exhibit better metabolic function, diminished inflammatory gene expression in adipose tissue, and reduced CV risk than body mass-matched individuals with CLS11.
Figure 2.
Functional adipose tissue (left), predominantly found in lean organisms, tends to express anti-inflammatory adipokines that protect against cardiovascular disease. In contrast, excess adipose tissue expansion promotes dysfunction (right), leading to the expression of pro-inflammatory adipokines that promote cardiovascular disease. Dysfunctional adipose tissue is characterized by enlarged adipocytes, vascular rarefaction, increased inflammatory cell infiltrate and the appearance of crown-like structures.
In addition to macrophages, other myeloid cells, such as neutrophils and mast cells, contribute to adipose tissue dysfunction in obesity. Neutrophils accumulate rapidly in the adipose tissue after HFD feeding81–83, and they appear to promote macrophage recruitment and adipose tissue inflammation via neutrophil elastase secretion82, 83. Similarly, mast cells have been reported to accumulate in obese adipose tissue, and studies in mast-cell deficient mice suggest a role for this cell-type in obesity-associated metabolic dysfunction84.
Impaired vascular structure and function
Several studies in humans and animal models have shown that obesity induces capillary rarefaction in adipose tissue, and this has been associated with metabolic dysfunction40, 85–88. Thus, it is widely accepted that obesity leads to reduced adipose tissue capillarization, which may limit nutrient delivery and contribute to adipocyte dysfunction and insulin resistance. Recent studies with genetically-engineered mice have provided evidence of a causal role of adipose tissue vascularization in obesity-associated metabolic dysfunction. Experiments with mice overexpressing vascular endothelial growth factor A (VEGF-A) in adipocytes show that increased VEGF-mediated angiogenesis in adipose tissue can attenuate some of the metabolic effects of diet-induced obesity, such as insulin resistance and hepatic steatosis89–91. Conversely, adipocyte-restricted deletion of VEGF-A results in diminished adipose tissue vascularization, which leads to increased adipose tissue inflammation and systemic metabolic dysfunction51, 91, further supporting the noxious effects of reduced adipose tissue vascularity in obesity.
However, a major limitation of the above mentioned studies is that the current mouse genetic reagents generally do not permit depot-specific ablation or overexpression of candidate angiogenic regulators in adipose tissue. In this regard, a recent study compared the consequences of VEGF ablation (and obesity) on capillarization and hypoxia in WAT and BAT51. Whereas, VEGF-deficiency led to similar declines in capillarization in WAT and BAT, the effects on WAT dysfunction, assessed by measures of hypoxia, inflammation and mitochondrial status were marginal compared to the impact of VEGF ablation on these parameters in BAT. In contrast, VEGF deficiency in BAT led to robust mitochondrial dysfunction and loss, leading the tissue to take on a “whitened” phenotype due to the accumulation of lipid droplets. Notably, adenovirus-mediated delivery of VEGF to BAT could reverse the systemic metabolic effects of VEGF ablation. VEGF-mediated rescue of the vascular deficit in BAT can also improve metabolic parameters in models of diet-induced obesity51, 92. The differential effect of reduced capillarization in white versus BAT is consistent with the greater respiratory capacity of BAT, thereby increasing its tendency to undergo hypoxic stress in response to obesity or genetic VEGF ablation. While these data highlight the importance of angiogenesis in BAT with consequences on systemic metabolic function in the murine system, the question of whether the status of BAT can affect CVD processes should be evaluated by future studies. Furthermore, whereas it is well established that BAT activity contributes significantly to overall systemic metabolism in rodents93, it is not clear whether brown fat can serve a similar function in adult humans or whether it is a vestigial tissue.
Clinical studies have focused mainly on WAT and suggest that expanding fat may “outgrow” its blood supply possibly owing to deficient angiogenesis that triggers a cycle of ischemia, hypoxia, necrosis and inflammation within the adipose milieu86, 87, 94, 95. Capillary dropout and deficient vascularization develop in obese humans, particularly in visceral fat, and are associated with inflammation and whole body metabolic dysfunction40, 86, 87, 95–97. In contrast, subcutaneous fat exhibits higher capillary density and angiogenic capacity compared to the visceral depot40, 90, 98–100. Microarrays studies show significant differences in gene transcripts associated with angiogenesis between visceral and subcutaneous fat in obese humans40. Pro-angiogenic ANGPTL-4 is down-regulated in visceral fat and may play an important role96. Additionally, an anti-angiogenic splice variant of VEGF, VEGF-A165b, is expressed at higher levels in human visceral fat compared to subcutaneous fat and is linked to impaired tissue angiogenesis98. Blood levels of VEGF-A165b are elevated in obese compared to lean subjects and decrease after bariatric surgery weight loss. This observation has potential clinical implications as systemic upregulation of anti-angiogenic agents and other mediators in obesity raises the possibility of their contribution to vascular disease and ischemia beyond the adipose environment. In this regard, a possible role of VEGF-A165b in mechanisms of peripheral arterial disease in animal models and humans was recently described101. It is thus becoming increasingly clear that qualitative features of adipose tissue, including its vascularity, could play an important role in the pathogenesis of obesity-induced cardiometabolic complications. However, whether modulation of adipose tissue angiogenesis in either white or brown fat could alter clinical consequences of human obesity remains an open question.
In addition to capillary rarefaction, obesity also leads to endothelial cell activation in the adipose tissue, which further contributes to the recruitment of immune cells. Endothelial cells within the adipose tissue of obese mice express higher levels of adhesion molecules such as P-selectin, E-selectin, and intercellular adhesion molecule 1 (ICAM-1). Moreover, administration of anti-ICAM-1 antibody to obese mice prevents macrophage infiltration into adipose tissue102. Collectively, these data illustrate the importance of a pathological interplay that can exist between adipose and vascular tissues. In fact, there is evidence from human studies that inflammatory cytokines over-expressed in fat impair vasoregulatory and anti-atherogenic properties leading to vasomotor dysfunction of the local microvasculature41 as well as systemic vessels11, 12, 41. Clinical studies utilizing videomicroscopy and culture myograph techniques to study physiological properties of microvessels within human fat have demonstrated profound abnormalities in endothelial vasomotor dysfunction of obese individuals, particularly in visceral fat41, 42, 103–110. In experiments that examined paired subcutaneous and visceral adipose tissue biopsy samples from obese subjects during planned bariatric surgery, endothelium-dependent, acetylcholine-mediated vasodilation was severely impaired in visceral compared to subcutaneous arterioles41. The degree of vasomotor impairment is profound and consistent across varying systemic metabolic phenotypes and endothelial agonists such as bradykinin, shear stress and insulin107. Vessels from obese fat even exhibit paradoxical vasoconstriction, consistent with severe endothelial dysfunction107. In these vessels, responses to sodium nitroprusside and papaverine (endothelial-independent vasodilators) are generally preserved, indicating functional impairment specifically at the level of the vascular endothelium early in the disease state. Complementary studies demonstrate impairment in eNOS phosphorylation at the activating site serine 1177 in vascular endothelial cells isolated from fat suggesting abnormalities in NO bioactivity as a significant contributing mechanism42. Adipose microvascular dysfunction appears specific to the obese state as arterioles isolated from visceral tissue of lean subjects exhibit preserved vasomotor function109, 110 while extreme microenvironmental perturbations are observed in visceral obesity.
There are likely multiple mechanisms that negatively regulate vascular function in visceral fat. Cytokine-driven inflammation likely plays a key role, as the adipose secretome and transcriptome is markedly pro-inflammatory in visceral depots. Experimental studies in mice demonstrate that transplantation of inflamed visceral fat accelerates atherosclerosis in Apo-E knockout mice111. Adipose expression of inflammatory mediators correlates inversely with acetylcholine-mediated vasodilation of human microvessels41, 42. Endothelial cells isolated from visceral fat display enhanced expression of inflammatory mediators such as CCL-5, IL-6, TNF-α and TLR-441. More direct evidence that inflammatory mechanisms are involved is provided by clinical studies that demonstrate vascular inflammation by histology and the reversal of microvascular dysfunction following treatment with IL-6 and TNF-α antagonists106, 110. However, other pathogenic processes that involve oxidative stress, mitochondrial dysfunction and endoplasmic-reticulum stress are likely to contribute to vascular diathesis. Recent data demonstrate evidence of impaired NO-dependent vasodilation, mitochondrial hyperpolarization, and increased mitochondrial superoxide production in the adipose tissue of type-2 diabetic subjects108. Moreover, increased expression of cyclooxygenase (COX)-mediated vasoconstrictor prostanoids might also contribute to endothelial dysfunction, supporting a role of the eicosanoid/cyclooxygenase pathway in obesity-linked disease42. Since the vasodilator responses and eNOS phosphorylation status in the adipose microvasculature have been shown to correlate with CV risk factors and systemic brachial arterial responses, further investigation into the vascular microenvironment of adipose tissue will likely provide translational clues relevant to systemic vascular disease mechanisms103, 105, 112.
Adipose tissue fibrosis
Within the adipose tissue of lean organisms, adipocytes are surrounded by extracellular matrix (ECM) that provides mechanical support and participates in cell signaling. With the development of obesity, there is a general increase in the synthesis of several ECM components, in particular collagen VI, which leads to adipose tissue fibrosis and is associated with impaired metabolic function in mice113. In obese human individuals adipose tissue fibrosis is increased in both subcutaneous and visceral depots114–116. Obesity-induced adipose tissue fibrosis is due, at least in part, to hypoxia-induced upregulation of hypoxia-inducible factor 1α (HIF1α)117, 118. Interestingly, HIF1α activation does not contribute to an angiogenic response in this context, but instead promotes adipose tissue fibrosis. Mechanistically, the features that lead to these divergent tissue-specific actions of HIF1α are not understood. Recent studies are uncovering additional mechanisms that modulate adipose tissue fibrosis in obesity. Endotrophin, a cleavage product of the α3 subunit of collagen VI that is secreted by adipocytes, has been shown to promote adipose tissue fibrosis and systemic metabolic dysfunction in obese mice119. In addition, PDGFRα signaling has been reported to oppose adipogenic differentiation of adipose tissue progenitors and to favor the generation of profibrotic cells that contribute to WAT fibrosis120. Whether profibrotic changes in adipose tissue contribute to the increased CV risk associated with obesity remains to be established. Thus, uncovering the causes and consequences of adipose tissue fibrosis is an area that deserves further attention.
ADIPOKINES AND CARDIOVASCULAR DISEASE
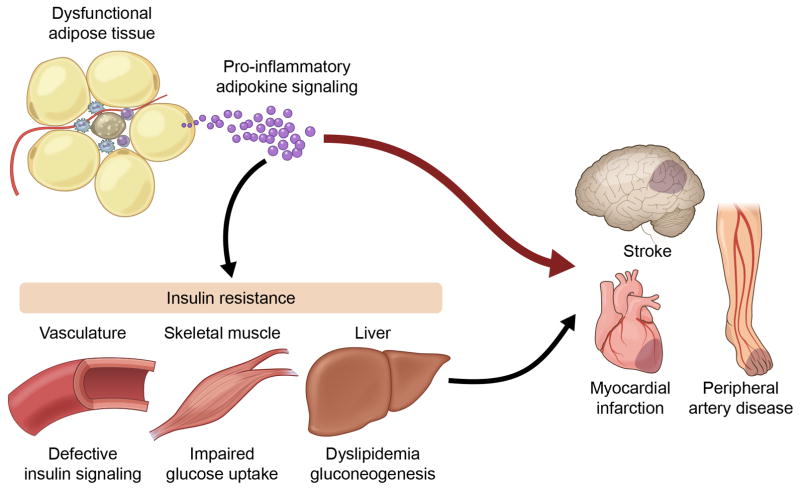
In addition to energy storage, adipose tissue is now recognized as an important factor in the regulation of many systemic, pathological processes through the secretion of multiple bioactive proteins referred to as adipokines. Although from a strict point of view these terms should be restricted to adipocyte-derived secreted proteins with immunomodulating actions, they are now widely used with a broader meaning to include any protein secreted by the adipose tissue – either by adipocyte or non-adipocyte cells- that is able to act as a modulator of immune, metabolic and/or CV functions. It is now widely accepted that dysfunctional adipose tissue remodeling leads to an unbalanced production of adipokines that contributes to the systemic pro-inflammatory state associated with obesity and has important adverse actions on the CV system121, 122, particularly in the obese state where adipose tissue mass can range from 30% to more than 50% of total body mass. In addition to their direct effects on pathophysiological processes in the CV system, adipokines can affect CV risk indirectly by modulating metabolism in liver, skeletal muscle and heart (Figure 3). Adipokines can also promote insulin resistance in microvessels within the adipose tissue and in other vessels, contributing to endothelial dysfunction and thereby increasing CV risk. However, these indirect actions of adipokines will not be discussed in detail here.
Figure 3.
Obesity leads to adipose tissue dysfunction, triggering the release of pro-inflammatory adipokines which can directly act on cardiovascular tissues to promote disease. The adipokine imbalance can also affect the function of metabolically important tissues and the microvasculature, promoting insulin resistance and indirectly contributing to CVD.
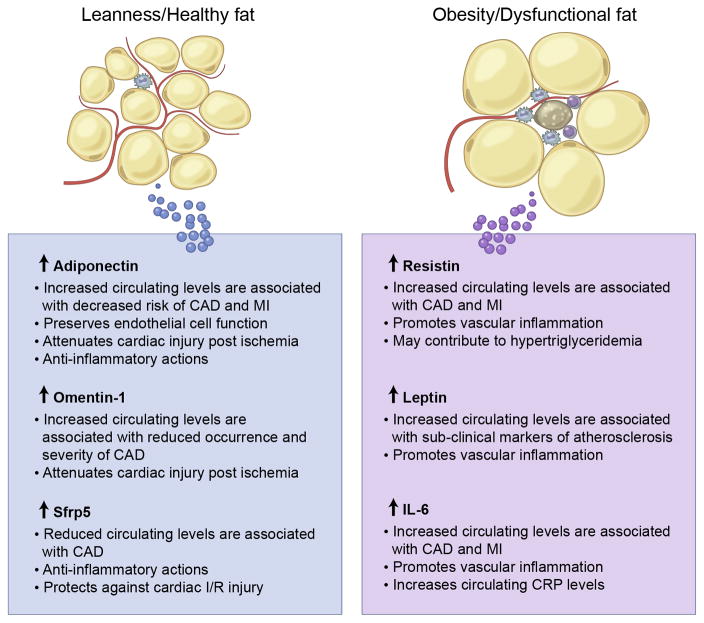
Since the identification of adipsin in 1987123, the list of adipokines has expanded vastly. Notably, the majority of adipokines are proinflammatory. Examples include leptin, TNFα, IL-6 and resistin. In contrast, relatively few adipokines are anti-inflammatory. Examples include adiponectin, omentin-1, CTRP9, and Sfrp5. Most adipokines have been identified in visceral and/or subcutaneous adipose tissue, which seem to produce different profiles of secreted proteins46, 124–126, which may play a role in the above-discussed different contribution of these fat depots to cardiometabolic risk. However, in addition to these depots, the body exhibits other smaller fat depots in association with multiple organs, including heart, kidneys, bone marrow, lungs and blood vessels (Figure 1). In addition to conventional fat depots, ectopic lipid deposition in liver, skeletal muscle and heart occurs in metabolically dysfunctional organisms. Although the production of adipokines by spatially distinct fat depots has been less investigated in general, it must be noted that it could have important implications in CV and metabolic diseases since adipokines secreted by these depots may act in a localized manner to stimulate neighboring organs. Indeed, a mounting body of evidence coming from human and animal studies suggests that obesity modulates the phenotype of PVAT52, 53 and that these changes directly influence vascular function and the development of vascular pathologies127–134. These studies open the question of whether increased CV risk associated with an adipokine imbalance is due to a paracrine mechanism, i.e. the local release of pro-inflammatory factors from epicardial adipose tissue and PVAT, or an endocrine mechanism secondary to changes in serum adipokines levels. While both mechanisms probably contribute to obesity-associated CVD, mouse studies based on PVAT transplantation suggest that the anatomical location of PVAT is critical in some pathophysiological settings127. However, it remains to be established whether different PVAT depots, that display a varying degree of brown fat characteristics, exhibit distinct profiles of adipokine secretion. Furthermore, while the known adipokines are secreted by WAT depots, the role of BAT as a secretory organ remains largely unexplored.
CARDIOVASCULAR ACTIONS OF SELECT ADIPOKINES
Leptin
The adipokine leptin is an adipose tissue-specific secreted hormone encoded by the ob gene, which was identified in genetically obese ob/ob mice through positional cloning135. Leptin is highly expressed by adipocytes, and circulating leptin levels increase in parallel to adipose tissue mass135. Other tissues, such as the heart, have also been reported to express and secrete leptin to some extent136. Leptin exerts important metabolic actions by suppressing appetite and increasing energy expenditure135. Accordingly, leptin-deficient mice exhibit increased appetite and associated obesity and insulin resistance, which are reversed upon leptin administration137, 138. However, obese humans and rodents have elevated levels of leptin (hyperleptinemia) without the expected anorexic responses137, suggesting that leptin resistance commonly occurs in obesity. Many lines of evidence suggest that hyperleptinemia contributes to CVD. Leptin has pro-inflammatory actions in many immune cell types including monocytes/macrophages139–142, neutrophils143, NK cells144, and T cells145, 146. In addition, it exhibits several pro-atherogenic actions. For example, leptin increases ROS production in endothelial cells147, 148. In VSMCs it promotes the expression of MMP-2, a metalloproteinase linked to atherosclerotic plaque vulnerability149. In addition, leptin facilitates cholesterol accumulation in macrophages150, 151.
Despite this body of evidence suggesting a pathogenic role for leptin in CVD, animal studies have given rise to inconsistent results regarding its role in atherosclerosis development. LDL-receptor knockout (LDLR-KO) mice that are also leptin-deficient develop more extensive atherosclerotic lesions than single LDLR-KO controls, likely due to the confounding effects of exacerbated insulin resistance and the general worsening of the circulating lipids profile caused by leptin deficiency-associated obesity and hyperphagia152, 153. Studies in apoE-KO mice, an atherosclerosis model that is less prone to obesity and insulin resistance154, have also generated conflicting results. In one study, leptin deficiency was found to suppress atherosclerosis development in apoE-KO mice fed an atherogenic diet, supporting the pro-atherogenic role of leptin155. In contrast, apoE-KO mice lacking the long isoform of the leptin receptor have been reported to exhibit hastened atherosclerosis regardless of the type of dietary regime156. When considering these conflicting results, it must be noted that the interpretation of these studies is difficult due to the secondary metabolic defects that result from hyperphagia in mice deficient in leptin or the leptin receptor (e.g. hyperglycemia, hyperinsulinemia, and insulin resistance). To overcome this limitation and explore the consequences of hyperleptinemia in organisms with intact leptin signaling, some studies have investigated the effects of exogenous leptin delivery on atherosclerosis development in non-obese mice. Supporting the pro-atherogenic role of leptin, two independent studies found that recombinant leptin administration aggravates atherosclerosis in apo-E-KO mice without affecting blood lipids levels155, 157 and one study found that it strongly promotes plaque calcification158. Taleb et al. investigated atherosclerosis development in leptin-deficient, LDLR-KO mice and LDLR-KO controls matched according to circulating cholesterol levels to evaluate the actions of leptin independently of its anorexic and metabolic actions159. In this experimental setting, leptin-deficient mice exhibited markedly reduced atherosclerosis, coinciding with an attenuated Th1 immune response and improved Treg cell function. Overall, these results support the notion that leptin plays a major pathogenic, pro-inflammatory role in atherosclerosis. Importantly, many human studies support this hypothesis. Some studies have shown a significant correlation between circulating leptin levels and markers of subclinical atherosclerosis such as coronary artery calcification160 and intima-media thickness of the common carotid artery161, 162. Similarly, several independent reports have shown that circulating leptin levels are a potent predictor of the risk of cardiac ischemic events163–167. However, this has not been replicated in other studies168, and one study found markedly different results, reporting that low plasma leptin predicted CV mortality in women169. This latter report is in line with a number of experimental studies suggesting a cardioprotective role of leptin after MI, at least in part, through prevention of cardiomyocyte apoptosis170–174. Overall, most of the evidence from animal and human studies generally suggests a scenario where hyperleptinemia in obese individuals promotes atherosclerosis and thereby increases the risk of cardiac ischemic events, but also exerts some local protective actions in the cardiac tissue by attenuating tissue damage post-ischemia. Whether any of these protective actions are subjected to leptin resistance in obese individuals remains unanswered.
Interleukin 6
Interleukin 6 (IL-6) is a pleiotropic cytokine with complex roles in metabolic and CVD. IL-6 is known to be secreted by several tissues and can act in a local fashion. However, adipose tissue is a major source of this protein, capable of producing high levels of this protein in the blood. Therefore, IL-6 can be considered an adipokine with endocrine actions. It has been estimated that as much as one third of total circulating IL-6 originates from adipose tissue175, where it can be secreted by both adipocytes and non-adipocyte cells, including pre-adipocytes and macrophages124, 176, 177. Importantly, expression and secretion of IL-6 are 2 to 3 times greater in visceral compared to subcutaneous adipose tissue in humans124 and indexes of visceral adiposity associated with CV risk correlate with increased circulating levels of IL-633, 178.
IL-6-induced cell signaling is typically classified as either classic or trans-signaling, and it can lead to different cell responses. In classic signaling, IL-6 stimulates target cells via a membrane bound IL-6 receptor (IL6R), which upon ligand binding forms a complex with the signaling receptor protein gp130. Few cell types express membrane bound IL6R, whereas essentially all cells exhibit gp130 on the cell surface. While the cells that only express gp130 are not responsive to IL-6 alone, they can be stimulated, via trans-signaling, by a complex of IL-6 bound to a naturally occurring soluble form of IL6R (sIL6R), markedly expanding the spectrum of IL-6 actions and target cells.
IL-6 has been widely accepted to act as a pro-inflammatory cytokine since the discovery of its critical role in mediating the hepatic acute phase response179–181. In addition, IL-6 has direct pro-inflammatory actions in a variety of immune and non-immune cell types, promoting the expression of adhesion molecules in endothelial cells and lymphocytes182, 183, monocyte-to-macrophage differentiation184, antibody production by B cells185 and recruitment of T-cells to sites of injury186. In contrast, IL-6 has also been reported to exert regenerative and anti-inflammatory actions in some settings187–189. IL-6 also appears to play conflicting metabolic roles in different tissues, inducing insulin resistance in hepatocytes190 and endothelial cells191, but increasing insulin sensitivity in skeletal muscle under some conditions191–193.
Similarly, the actions of IL-6 in the CV system are complex and incompletely understood. Human studies have provided compelling evidence supporting the notion that high circulating levels of IL-6 are associated with increased risk of coronary artery disease (CAD) and MI194–197. In addition, Mendelian randomization studies suggest that IL6R signaling contributes to the development of CAD198, 199. However, mouse studies cast some doubts onto the causative role of IL-6 in CVD, although these must be interpreted with caution given that mouse and human IL-6 proteins exhibit only 41% sequence identity. Early reports showed that chronic administration of supraphysiological doses of recombinant mouse IL-6 exacerbate atherosclerosis in apoE-KO mice200. However, systemic inactivation of IL-6 also results in larger atherosclerotic lesions in the apoE-KO model201, 202 and does not seem to affect atherosclerotic plaque size in LDLR-KO mice203. These conflicting results could be due to compensatory activation of other IL-6 family proteins in IL-6-deficient mice. Alternatively, they may reflect the complex and multifaceted actions of this cytokine. In addition to its immunomodulatory actions, IL-6 may have some anti-atherogenic activities by preventing cholesterol deposition in the vessel through increased cholesterol efflux in macrophages204 and HDL translocation through the endothelium205. Therefore, it is possible that IL-6 plays dual roles in atherogenesis, preventing early plaque formation via removal of cholesterol from the vessel wall, but promoting plaque development at later stages by contributing to the perpetuation of vascular inflammation. Supporting this notion, one study found that IL-6 deficiency results in larger plaques, but markedly reduces plaque inflammation201. Regardless of the mechanisms underlying the phenotype of IL-6 deficient mice, recent studies have begun to evaluate the therapeutic potential of pharmacological inhibition of IL-6 signaling in the setting of atherosclerosis. In this regard, post-natal inhibition of IL-6 trans-signaling (by treatment with a fusion protein of soluble gp130 and IgG1-Fc) has been shown to reduce atherosclerosis development and plaque inflammation in LDLR-KO mice206.
The role of IL-6 in pathologic cardiac remodeling after ischemic injury is similarly complex. While human studies have shown a strong association between circulating IL-6 levels and the severity or prognosis of chronic heart failure207–211, causality is uncertain given that mouse studies have generated conflicting data. One study found no effect of genetic IL-6 deficiency or recombinant IL-6 delivery on MI size, left ventricular (LV) remodeling, or mortality after permanent coronary ligation212. In contrast, another study found that a single injection of an IL6-R-blocking antibody after MI suppresses myocardial inflammation, resulting in the amelioration of LV remodeling213. In addition, IL-6 may even exert some cardioprotective actions, since treatment with a combination of recombinant IL-6 and sIL6R inhibits cardiomyocyte apoptosis and reduces infarct size in a rat model of cardiac ischemia/reperfusion (I/R) injury214.
Resistin
Resistin is a secreted protein that is highly expressed by mature adipocytes in rodents and was initially suggested to be a major link between obesity and insulin resistance215. Circulating resistin levels are increased in obese and diabetic mice216, and several loss- and gain-of-function studies in mice have suggested an important role of resistin in obesity-associated metabolic dysfunction through pleiotropic effects on glucose metabolism and insulin sensitivity215, 217–219. However, human studies have yielded conflicting results on the role of resistin in insulin resistance220–226, and have revealed striking differences in resistin expression patterns in rodents and humans. While in rodents resistin is mostly expressed by adipocytes215, 227, the main sources of this protein in humans are monocytes and macrophages228, 229. Regardless of these differences between species, several studies suggest a tight connection between resistin and inflammatory disorders. Human resistin expression in monocytes/macrophages is increased in response to various pro-inflammatory stimuli230–232 and serum resistin levels show a positive correlation with various circulating markers of inflammation, such as C-reactive protein, TNF-α, or IL-6 in different pathophysiological settings233–237. In addition, resistin has been reported to promote monocyte/endothelium interactions238 and pro-inflammatory activation of macrophages239, 240, which suggests an important role in the development of atherosclerosis. Consistently, peri-adventitial resistin gene transfer accelerates plaque development in rabbit models of atherosclerosis241. In addition, a recent study suggested that overexpression of mouse resistin can promote atherosclerosis by an alternative mechanism mediated by central leptin resistance and reduced BAT activity leading to hypertriglyceridemia242. Despite some conflicting studies243–245, human studies also support an import role for resistin in atherosclerotic disorders. Elevated circulating levels of resistin have been reported to be associated with coronary artery calcification246 and CAD247, and to predict the occurrence and severity of CAD in several clinical studies248–252. Furthermore, resistin has been proposed to be an independent risk factor for major CV events in CAD patients253, 254. Although the role of resistin in cardiac ischemic events has not been investigated in animal models, some human studies suggest that it might also play a role in this setting, since high circulating levels of resistin are present in patients with acute coronary syndrome (ACS)251, 255, 256. In addition, resistin expression and secretion by epicardial adipose tissue has been shown to be increased in these patients257.
Adiponectin
Adiponectin is a widely studied adipokine that is very abundantly expressed in plasma (range: 3–30 μg/ml in human)258, 259. The adiponectin peptide contains collagen-like domain followed by a globular domain that is similar to complement factor C1q. Adiponectin exists in blood stream as three major oligomeric complexes: trimers, hexamers and high-molecular weight form258, 259. Plasma adiponectin levels are decreased in obese subjects relative to lean control subjects259, and adiponectin levels negatively correlate with visceral fat accumulation260. Dysfunctional adipocytes produce lower levels of adiponectin but higher levels of pro-inflammatory cytokines, which further inhibit the production of adiponectin in adipocytes. Adiponectin expression by adipocytes is also inhibited by endoplasmic reticulum and oxidant stresses, which are features of adipose tissue dysfunction in obesity.
A number of clinical studies demonstrate that low plasma adiponectin levels are associated with systemic inflammation258, 261 and obesity-linked CV disorders262–266. Plasma adiponectin concentrations are lower in patients with CAD than in age- and BMI-adjusted control subjects263, 264. Circulating adiponectin levels are also reduced in patients with ACS265, and adiponectin levels rapidly decline following acute myocardial infarction266. High plasma adiponectin levels are associated with a decreased risk of MI in healthy men267 and diabetic men268. Low adiponectin has been reported to be an independent risk factor of coronary heart disease in some studies269 but not others270–272. On the contrary, hyperadiponectinemia is associated with mortality in patients with diseases that are associated with cachexia such as heart or respiratory failure273, 274. Adiponectin levels are also elevated in a number of chronic inflammatory and autoimmune diseases275. The upregulation of adiponectin in these severe diseases may represent a compensatory response since animal studies that model these diseases show that adiponectin is protective under these conditions.
Experimental studies have shown that adiponectin exerts anti-inflammatory and vasculoprotective actions in different settings276–287. In mice, lack of adiponectin results in an enhancement of myocardial ischemia-reperfusion injury, which is associated with increased myocardial cell apoptosis and TNF-α production276. Conversely, systemic adenovirus-mediated delivery of adiponectin diminishes infarct size in both APN-KO and wild-type mice. In this model, adiponectin stimulates COX-2 expression and synthesis of prostaglandin E2 (PGE2), a vascular- protective autocoid that inhibits inflammatory cytokine production in cardiac myocytes. Adiponectin-induced expression of COX-2 in myocytes is reduced by inhibition or deletion of sphingosine kinase-1 (SphK-1), or blockade of a sphingosine-1-phosphate (S1P) receptor288, and it has been shown that adiponectin stimulates ceramidase activity in cardiac myocytes and other cell types to promote survival289. In addition to its effects on COX-2 expression, adiponectin protects the myocardium from ischemic injury through its ability to activate AMPK signaling278, 279, 287. Adiponectin also protects from ischemia-reperfusion injury through inhibition of peroxynitrite-induced oxidative and nitrosative stresses290. In extension of these genetic models, delivery of recombinant adiponectin protein can protect the heart in murine models of I/R injury276. Notably, one study showed that intracoronary injection of adiponectin protein improved cardiac function after ischemia-reperfusion in a pig model using similar instrumentation and standard of care as in patients291.
Whereas experimental studies examining the effects of adiponectin on ischemic heart disease have been consistent in documenting a protective effect, adiponectin’s role in atherogenesis is less clear. A series of studies show that adiponectin modulates macrophage function promoting an anti-inflammatory phenotype that would be consistent with an anti- atherogenic role. For example, adiponectin suppresses lipopolysaccharide-stimulated TNF-α production292, 293, inhibits Toll-like receptor-mediated NF-κB activation294, and enhances the production of the anti-inflammatory cytokine IL-10 in cultured macrophages293, 295. Consistently, adiponectin promotes macrophage polarization towards an anti-inflammatory phenotype296, and facilitates the rapid removal of apoptotic debris from the body which is critical in preventing pathological inflammation and immune system dysfunction297. Adiponectin also inhibits macrophage-to-foam cell transformation and reduces intracellular cholesteryl ester content in human macrophages by suppressing expression of class A scavenger receptor (SR-A)298.
Consistent with the above-mentioned in vitro findings, overproduction of circulating adiponectin inhibits the formation of atherosclerotic lesions and decreases mRNA levels of SR-A, TNF-α and VCAM-1 in the vascular wall in apoE-knockout mice, suggesting that adiponectin attenuates atherogenesis through anti-inflammatory actions on macrophages and vascular endothelial cells299, 300. Adenovirus-mediated overexpression of adiponectin also attenuates angiotensin II-accelerated atherosclerosis301. Conversely, one study showed that adiponectin deficiency in ApoE-knockout mice exacerbates atherogenesis and accelerates T lymphocyte accumulation in atheromata302. In contrast, an extensive study reported that neither adiponectin overexpression nor deficiency has any effects on atherosclerotic lesion formation in either ApoE-KO or LDLR-KO mice when fed either a normal chow or a high cholesterol diet303. Thus, additional studies are required to determine whether adiponectin has a significant atheroprotective role in vivo.
Cardiovascular disease and adiponectin receptors
While a large number of studies have shown that adiponectin acts as a CV-protective adipokine in many systems, the receptor-mediated signaling systems that confer these protective actions are understudied. Early, it was reported that the beneficial actions of adiponectin on metabolic function and AMPK signaling pathway is mediated through combined signaling through its cell surface receptors AdipoR1 and AdipoR2304. However, subsequent studies suggest that AdipoR1 and AdipoR2 have opposing actions: AdipoR1-deficiency in mice leads to metabolic dysfunction, whereas AdipoR2-deficiency actually promotes resistance to obesity and insulin resistance305–307. The roles of AdipoR1 and AdipoR2 in CV tissues have mostly been deduced from cell culture studies. For example, in vitro studies in cardiac myocytes have shown that both AdipoR1 and AdipoR2 mediate the anti-hypertrophic effects of adiponectin308. Similarly, AdipoR1 has been shown to mediate the pro-angiogenic actions of adiponectin in cultured endothelial cells277. Relatively few studies have analyzed the roles of adiponectin receptors in the CV system using in vivo models. Functional evidence for receptor involvement in vivo would involve documentation that receptor-deficiency has a similar phenotype as adiponectin-deficiency and that the receptor-deficient mice would be impaired in their response to exogenously-administered adiponectin. In this regard, it was recently shown in a murine model of peripheral artery disease that AdipoR2-deficiency impairs the revascularization process (as does adiponectin-deficiency), and eliminates the enhanced revascularization response to exogenous adiponectin305. In contrast, AdipoR1-deficiency led to a dysfunctional metabolic phenotype, suggesting that the in vivo vascular and metabolic effects of adiponectin diverge at the level of the AdipoR1/2 receptors.
When considering receptors it is important to reconcile the unusual properties of adiponectin as a ligand. For example, adiponectin levels are 1000-fold greater than most growth factors and cytokines259, raising questions about receptor affinity and occupancy. Adiponectin also has an unusual structure that comprises a globular head and collagenous tail that is similar to the collectin family of proteins, including C1q, mannose binding lectin and lung surfactant proteins, that contribute to innate immune system regulation by functioning as “pattern recognition receptors” via low affinity interactions with various macromolecules309. Like other collectin family proteins, adiponectin preferentially forms higher order multimers, including dodecamers with a molecular mass in excess of 400 kDa, presumably to allow multivalent associations with low affinity targets. Studies have shown that adiponectin exhibits collectin-like properties, including the ability to opsonize apoptotic cells and facilitate their clearance297, 310, and it has been shown that adiponectin can bind to C1q in serum311. Thus, one would not expect a simple binary, ligand/receptor-occupancy model to account for the interaction between adiponectin and the AdipoRs or other candidate receptor molecules. In light of these considerations, studies have documented that adiponectin is highly localized to the heart and the vascular endothelium through an interaction with T-cadherin, a GPI-anchored cell surface glycoprotein312–314. Data from mouse studies have shown that T-cadherin-deficiency leads to marked elevations in the level of circulating adiponectin, ostensibly because of its release from tissue depots. T-cadherin-deficiency in mice also blocks the salutary actions of exogenously administered adiponectin on ischemia-reperfusion injury and remodeling following pressure-overload in the heart313, and on adiponectin-stimulated revascularization in a murine model of peripheral artery disease314. Thus, T-cadherin plays a key role in mediating the CV effects of adiponectin although it lacks a transmembrane signaling domain. Hypothetically, T-cadherin may function as a co-receptor molecule involved in the localization and presentation of adiponectin, or a particular configuration of adiponectin, to AdipoR1/2, potentially explaining how adiponectin can function to activate receptor-mediated signaling pathways in addition to its low affinity, pattern recognition activities.
CTRPs
C1q/TNF-related proteins (CTRPs) are conserved paralogs of adiponectin that contain collagen tail domain and a globular C1q-like domain at the C-terminus315. Recent studies demonstrate that, like adiponectin, some CTRPs act as adipokines that exert cardioprotective effects. Examples include CTRP3 and CTRP9, which are primarily expressed in adipose tissue and whose expression is downregulated in obese states316–319.
CTRP9, which has the highest amino acid sequence similarity to adiponectin (45%)320, has been shown to have protective actions in the CV system. Systemic delivery of CTRP9 protein reduces myocardial infarct size and apoptosis following myocardial infarction or ischemia-reperfusion injury in mice317, 321. In vitro, treatment of cardiac myocytes with CTRP9 protein attenuates hypoxia-reoxygenation-induced apoptosis via an AMPK-dependent pathway involving AdipoR1317. CTRP9 is also effective in reducing myocardial infarct size, apoptosis and oxidative stress in diabetic mice after ischemia-reperfusion322. Consistently, recent studies with CTRP9-deficient mice have shown that CTRP9 promotes cardiac function and myocyte survival and diminishes fibrosis following myocardial infarction in an AdipoR1 and AMPK-dependent manner323. Because circulating CTRP9 levels are reduced in mice after ischemia-reperfusion or myocardial infarction317, 321, replenishment of CTRP9 could be beneficial in the context of ischemic heart.
CTRP3 has a 28% amino acid identity with adiponectin320, and supplementation of this adipokine has been reported to improve cardiac function and reduce fibrosis in mice after myocardial infarction, which is accompanied by increased capillary density and decreased apoptosis in ischemic areas of the heart324, 325. In vitro, CTRP3 inhibits TGF-β-induced profibrotic gene expression in cardiac fibroblasts325 and promotes cardiac myocyte survival and VEGF-A expression through its ability to activate an Akt/HIF-1α-dependent pathway. In humans, circulating CTRP3 levels are negatively correlated with several markers of systemic inflammation and cardiometabolic risk326.
Omentin
Omentin-1, also referred to as intelectin-1, was identified as a soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall327. Human omentin-1 is abundantly expressed in human visceral adipose tissue328. Omentin-1 is detectable in human blood, and circulating omentin levels are reduced in obese subjects329 and in patients with impaired glucose tolerance and type 2 diabetes330. Furthermore, circulating omentin-1 levels negatively correlate with multiple cardiometabolic risk factors such as increased waist circumferences, dyslipidemia, elevated blood pressure, and glucose intolerance331. Recent clinical studies also suggest the relationship between omentin-1 and CV disorders. Circulating omentin levels are markedly lower in patients with CAD than in age-matched control subjects332–334.. Another study demonstrated the inverse correlation between serum omentin-1 levels and the presence and severity of CAD in patients with metabolic syndrome335. In healthy men, omentin-1 levels negatively correlate with carotid intima/media thickness336, which is a marker for subclinical atherosclerosis.
Experimental studies also support the notion that omentin-1 exerts protective actions on the CV system337–340. Systemic administration of omentin-1 attenuates cardiac injury following ischemia-reperfusion in mice through Akt- and AMPK-dependent mechanisms341. In vitro, omentin-1 has been shown to suppress TNFα-induced inflammatory responses in vascular endothelial cells via an AMPK-eNOS pathway342. More recently, omentin overexpression has been reported to attenuate atherosclerosis in hyperlipidemic mice343. Overall, it is plausible that low levels of omentin-1 can contribute to the development of CV dysfunction in obese individuals.
Sfrp5
Secreted frizzled-related protein 5 (Sfrp5) was identified as an adipokine that exerts salutary effects on metabolic function with anti-inflammatory properties344. Sfrp5 is expressed abundantly in WAT in lean mice, and it is down-regulated in severely obese rodents, such as 20-week-old ob/ob mice. Mechanistically, Sfrp proteins are known to function as soluble modulators that sequester Wnt proteins in the extracellular space and prevent their binding to receptors. In this context, Sfrp5 appears to function as an inhibitor of Wnt5a-mediated non-canonical Wnt signaling, which contributes to pro-inflammatory cytokine production via JNK activation44, 344, 345. Although some conflicting data have been reported regarding the magnitude of Sfrp5 secretion by human WAT346, 347, an increasing body of evidence suggests that Sfrp5 is dynamically regulated in humans. Several studies have shown that circulating levels of Sfrp5 are reduced in obese individuals, particularly in those exhibiting clear evidence of metabolic dysfunction, such as impaired glucose tolerance and insulin resistance346, 348–350. Consistently, human Sfrp5 transcript levels in visceral adipose tissue decrease with obesity351. In marked contrast, one study found a positive association between increased serum Sfrp5 levels and high HOMA-IR, an index of insulin resistance, in humans352. An additional study failed to replicate Sfrp5 downregulation in human obesity, but strikingly it showed that caloric restriction-induced weight loss increases serum concentration of Sfrp5353. Taken together, these studies suggest that Sfrp5 is downregulated in obesity-associated metabolic dysfunction in humans, although further investigations are still required to corroborate this notion.
Sfrp5 may also affect the development of obesity-linked CVD. A recent study demonstrated that genetic Sfrp5 ablation exacerbates cardiac I/R injury in mice, coinciding with increased inflammation and cardiomyocyte death345. In addition, in a murine model of peripheral artery disease, Sfrp5-deficiency promoted the influx of Wnt5a-positive cells into the ischemic limb and impaired revascularization101. The role of Sfrp5 in atherosclerosis remains unknown at this time, but a number of studies suggest a potential atheroprotective action of this adipokine. A recent clinical study demonstrated that low levels of serum Sfrp5 are associated with CAD354. Furthermore, Sfrp5 may affect atherosclerosis development by inhibiting Wnt5a, which is expressed in murine and human atherosclerotic lesions355, 356. It has been suggested that Wnt5a contributes to endothelial dysfunction in diabetic patients357 and promotes inflammatory reactions in macrophages and endothelial cells44, 355, 358. Thus, it is plausible that Sfrp5 attenuates inflammatory response to Wnt5a in the vasculature, but additional studies will be required to clarify the role of Sfrp5 in the regulation of atherosclerosis development.
CONCLUSION
An increasing body of evidence supports the evolving concept that quantity, location and quality of adipose tissue are critical factors in shaping cardiometabolic phenotypes in obese humans but specific pathogenic mechanisms and their relative contributions remain incompletely understood. adipose tissue communicate with remote organs, including heart and vasculature, through the release of various adipokines. In mouse models and many human individuals’ obesity leads to adipose tissue dysfunction or adiposopathy, particularly in visceral fat depots, which is mediated by dysfunctional tissue remodeling that involves adipocyte hypertrophy, exacerbated inflammation, increased fibrosis and impaired vascular function and structure. This ultimately creates an imbalance in adipokine levels (Figure 4), which contributes to a chronic, low grade systemic inflammatory reaction that is central to the initiation and progression of metabolic and CV complications. While some adipokines have been highly studied and have shown to be causally linked to various disease processes, new adipokine candidates continue to be discovered and elucidated. In light of the fact that a third of the world’s population is currently overweight or obese, and this proportion is expected to increase in the coming decades, studies of adipokine biology should provide a better understanding of the pathogenesis of CVD. As our understanding of adipokine biology and obesity-induced adiposopathy increases, the major challenge will reside in translating this information into new prognostic and therapeutic approaches to limit CV risk in obese individuals.
Figure 4.
Selected anti- and pro-inflammatory adipokines with summaries of their regulation and actions in the cardiovascular system.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Kenneth Walsh is supported by National Institutes of Health Grants grants HL126141, HL081587, and HL131006. Dr. Noyan Gokce is supported by National Institutes of Health Grants HL081587, HL126141, and HL114675.
Nonstandard Abbreviations and Acronyms
- AdipoR1, AdipoR2
adiponectin receptor 1, 2
- AdipoRs
adiponectin receptors
- Akt
Also known as Protein kinase B. Ak refers to the mouse strain and t to thymoma.
- AMPK
adenosine monophosphate-activated protein kinase
- ANGPTL-4
angiopoietin-like 4
- APN-KO
adiponectin knockout
- Apo-E, apoE
apolipoprotein E
- ATMs
adipose tissue macrophages
- BAT
brown adipose tissue
- BMI
body mass index
- CAD
coronary artery disease
- CD (CD4, CD8, CD11)
cluster of differentiation (4, 8, 11)
- CLS
“crown-like” structures
- CMD
cardiometabolic disease
- COX
cyclooxygenase
- CRP
C-reactive protein
- CT
computed tomography
- CTRPs (CTRP3, CTRP9)
C1q/tumor necrosis factor-related proteins (3, 9)
- CV
cardiovascular
- CVD
cardiovascular disease
- ECM
extracellular matrix
- eNOS
endothelial nitrous oxide synthase
- FFA
free fatty acids
- gp130
glycoprotein 130
- GPI
glycosylphosphatidylinositol
- HDL
high-density lipoprotein
- HFD
high fat diet
- HIF1α
hypoxia-inducible factor 1 alpha
- HOMA-IR
homeostatic model assessment-insulin resistance
- ICAM-1
intercellular adhesion molecule 1
- IgG1-Fc
immunoglobulin 1-fragment, crystallizable
- IL (IL-4, IL-6, IL-10, IL-13)
interleukin (4, 6, 10, 13)
- IL6R
interleukin 6 receptor
- JNK
c-Jun N-terminal kinase
- Ldlr/LDLR
low-density lipoprotein-receptor
- LV
left ventricular
- MI
myocardial infarction
- MMP-2
matrix metallopeptidase 2
- MRI
magnetic resonance imaging
- NF-κB
nuclear factor-kappa B
- NK cells
natural killer cells
- NKT
natural killer T cells
- NO
nitric oxide
- PDGFRα
platelet-derived growth factor receptor, alpha polypeptide
- PVAT
perivascular adipose tissue
- ROS
reactive oxygen species
- Sfrp5
secreted frizzled-related protein 5
- sIL6R
soluble form of interleukin 6 receptor
- SphK-1
sphingosine kinase-1
- Th1, Th2, Th17
T helper (1, 2, 17)
- TLR-4
toll-like receptor 4
- TNF-α
tumor necrosis factor alpha
- VEGF-A
vascular endothelial growth factor A
- WAT
white adipose tissue
- Wnt (Wnt5a)
Wingless-Type MMTV Integration Site Family (Member 5A)
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Archer E, Shook RP, Thomas DM, Church TS, Katzmarzyk PT, Hebert JR, McIver KL, Hand GA, Lavie CJ, Blair SN. 45-Year trends in women's use of time and household management energy expenditure. PLoS One. 2013;8:e56620. doi: 10.1371/journal.pone.0056620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubes G. The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. BMJ. 2013;346:f1050. doi: 10.1136/bmj.f1050. [DOI] [PubMed] [Google Scholar]
- 4.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–50. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 6.Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, Murabito JM, Hoffmann U, Fox CS. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100:227–34. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762–71. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–47. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, Beale E, Xie C, Greenberg AS, Allayee H, Goran MI. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes. 2011;60:2802–9. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–9. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, Gokce N. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A, Joseph L, Apovian CM, Gokce N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes. 2012;2:e30. doi: 10.1038/nutd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92:157–91. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 15.Lavie CJ, De Schutter A, Milani RV. Healthy obese versus unhealthy lean: the obesity paradox. Nat Rev Endocrinol. 2015;11:55–62. doi: 10.1038/nrendo.2014.165. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–60. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arkin JM, Alsdorf R, Bigornia S, Palmisano J, Beal R, Istfan N, Hess D, Apovian CM, Gokce N. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. 2008;101:98–101. doi: 10.1016/j.amjcard.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–83. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, Yoshizumi M, Toba K, Ouchi Y. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disord. 1998;22:477–84. doi: 10.1038/sj.ijo.0800620. [DOI] [PubMed] [Google Scholar]
- 21.Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, O'Donnell CJ, Vasan RS, Mitchell GF, Hoffmann U, Fox CS, Benjamin EJ. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 2009;17:2054–9. doi: 10.1038/oby.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–65. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 23.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 24.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 26.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, Lopez-Jimenez F. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med. 2015 doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D'Agostino RB, Sr, O'Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–8. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–20. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 29.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 30.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–75. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, Seidell JC, Health ABCS. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 33.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13:674–82. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 35.Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA, Katsilambros N. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism. 1999;48:1332–5. doi: 10.1016/s0026-0495(99)90277-9. [DOI] [PubMed] [Google Scholar]
- 36.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–7. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 40.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–94. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol. 2012;32:467–73. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farb MG, Tiwari S, Karki S, Ngo DT, Carmine B, Hess DT, Zuriaga MA, Walsh K, Fetterman JL, Hamburg NM, Vita JA, Apovian CM, Gokce N. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity (Silver Spring) 2014;22:349–55. doi: 10.1002/oby.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010;18:879–83. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 44.Fuster JJ, Zuriaga MA, Ngo DT, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–48. doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledoux S, Queguiner I, Msika S, Calderari S, Rufat P, Gasc JM, Corvol P, Larger E. Angiogenesis associated with visceral and subcutaneous adipose tissue in severe human obesity. Diabetes. 2008;57:3247–57. doi: 10.2337/db07-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–9. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 47.Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–75. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–81. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–93. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124:2099–112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–9. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–64. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–52. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–37. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gustafson B, Smith U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis. 2015;241:27–35. doi: 10.1016/j.atherosclerosis.2015.04.812. [DOI] [PubMed] [Google Scholar]
- 57.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–49. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–71. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kratz M, Coats BR, Hisert KB, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–8. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 67.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–30. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 73.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, Martinez-Santibanez G, Lumeng CN. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3:664–75. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–35. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ip BC, Hogan AE, Nikolajczyk BS. Lymphocyte roles in metabolic dysfunction: of men and mice. Trends Endocrinol Metab. 2015;26:91–100. doi: 10.1016/j.tem.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–8. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 81.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Mansuy-Aubert V, Zhou QL, Xie X, et al. Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17:534–48. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 86.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–5. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–28. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 89.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–9. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 92.Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, Scherer PE. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab. 2014;3:474–83. doi: 10.1016/j.molmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013;1831:943–9. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 95.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 96.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–64. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227–35. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 98.Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA, Hess DT, Walsh K, Gokce N. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation. 2014;130:1072–80. doi: 10.1161/CIRCULATIONAHA.113.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Villaret A, Galitzky J, Decaunes P, Esteve D, Marques MA, Sengenes C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumie A. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–63. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–54. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 101.Kikuchi R, Nakamura K, MacLauchlan S, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–71. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–21. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens. 2012;25:528–34. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115:525–32. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grassi G, Seravalle G, Scopelliti F, Dell'Oro R, Fattori L, Quarti-Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 2010;18:92–8. doi: 10.1038/oby.2009.195. [DOI] [PubMed] [Google Scholar]
- 106.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 107.Grizelj I, Cavka A, Bian JT, Szczurek M, Robinson A, Shinde S, Nguyen V, Braunschweig C, Wang E, Drenjancevic I, Phillips SA. Reduced flow-and acetylcholine-induced dilations in visceral compared to subcutaneous adipose arterioles in human morbid obesity. Microcirculation. 2015;22:44–53. doi: 10.1111/micc.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2012;32:2531–9. doi: 10.1161/ATVBAHA.112.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Virdis A, Duranti E, Rossi C, Dell'Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J. 2015;36:784–94. doi: 10.1093/eurheartj/ehu072. [DOI] [PubMed] [Google Scholar]
- 110.Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, Salvetti A, Pinchera A, Taddei S. Vascular generation of tumor necrosis factor-alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–47. doi: 10.1016/j.jacc.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 111.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karki S, Farb MG, Ngo DT, Myers S, Puri V, Hamburg NM, Carmine B, Hess DT, Gokce N. Forkhead box O-1 modulation improves endothelial insulin resistance in human obesity. Arterioscler Thromb Vasc Biol. 2015;35:1498–506. doi: 10.1161/ATVBAHA.114.305139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guglielmi V, Cardellini M, Cinti F, Corgosinho F, Cardolini I, D'Adamo M, Zingaretti MC, Bellia A, Lauro D, Gentileschi P, Federici M, Cinti S, Sbraccia P. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes. 2015;5:e175. doi: 10.1038/nutd.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clement K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990–8. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33:904–17. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga J, Ploug T, An Z, Scherer PE. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, Olson LE. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 2015;29:1106–19. doi: 10.1101/gad.260554.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–9. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–5. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 124.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 125.Dahlman I, Elsen M, Tennagels N, Korn M, Brockmann B, Sell H, Eckel J, Arner P. Functional annotation of the human fat cell secretome. Arch Physiol Biochem. 2012;118:84–91. doi: 10.3109/13813455.2012.685745. [DOI] [PubMed] [Google Scholar]
- 126.Roca-Rivada A, Alonso J, Al-Massadi O, Castelao C, Peinado JR, Seoane LM, Casanueva FF, Pardo M. Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. J Proteomics. 2011;74:1068–79. doi: 10.1016/j.jprot.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 127.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–11. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 128.Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225:99–104. doi: 10.1016/j.atherosclerosis.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 129.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–78. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–61. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Margaritis M, Antonopoulos AS, Digby J, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–21. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 133.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–63. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 134.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–9. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 136.Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol. 2004;287:H2877–84. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- 137.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 138.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 139.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 140.Surmi BK, Atkinson RD, Gruen ML, Coenen KR, Hasty AH. The role of macrophage leptin receptor in aortic root lesion formation. Am J Physiol Endocrinol Metab. 2008;294:E488–95. doi: 10.1152/ajpendo.00374.2007. [DOI] [PubMed] [Google Scholar]
- 141.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem Biophys Res Commun. 2009;384:311–5. doi: 10.1016/j.bbrc.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 142.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–8. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caldefie-Chezet F, Poulin A, Vasson MP. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2003;37:809–14. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 144.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 145.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 146.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–8. [PubMed] [Google Scholar]
- 148.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 149.Li L, Mamputu JC, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–34. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- 150.Hongo S, Watanabe T, Arita S, Kanome T, Kageyama H, Shioda S, Miyazaki A. Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am J Physiol Endocrinol Metab. 2009;297:E474–82. doi: 10.1152/ajpendo.90369.2008. [DOI] [PubMed] [Google Scholar]
- 151.O'Rourke L, Gronning LM, Yeaman SJ, Shepherd PR. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277:42557–62. doi: 10.1074/jbc.M202151200. [DOI] [PubMed] [Google Scholar]
- 152.Hasty AH, Shimano H, Osuga J, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–8. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- 153.Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, Stengel D, Ninio E, Navab M, Mackness B, Mackness M, Holvoet P. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107:1640–6. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- 154.Wang J, Perrard XD, Perrard JL, Mukherjee A, Rosales C, Chen Y, Smith CW, Pownall HJ, Ballantyne CM, Wu H. ApoE and the role of very low density lipoproteins in adipose tissue inflammation. Atherosclerosis. 2012;223:342–9. doi: 10.1016/j.atherosclerosis.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chiba T, Shinozaki S, Nakazawa T, Kawakami A, Ai M, Kaneko E, Kitagawa M, Kondo K, Chait A, Shimokado K. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008;196:68–75. doi: 10.1016/j.atherosclerosis.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 156.Wu KK, Wu TJ, Chin J, Mitnaul LJ, Hernandez M, Cai TQ, Ren N, Waters MG, Wright SD, Cheng K. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 2005;181:251–9. doi: 10.1016/j.atherosclerosis.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 157.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:e119–22. doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 158.Zeadin M, Butcher M, Werstuck G, Khan M, Yee CK, Shaughnessy SG. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:2069–75. doi: 10.1161/ATVBAHA.109.195255. [DOI] [PubMed] [Google Scholar]
- 159.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clement K, Holvoet P, Tedgui A, Mallat Z. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–8. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 160.Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, Rader DJ, Kimmel SE. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–8. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 161.Ciccone M, Vettor R, Pannacciulli N, Minenna A, Bellacicco M, Rizzon P, Giorgino R, De Pergola G. Plasma leptin is independently associated with the intima-media thickness of the common carotid artery. Int J Obes Relat Metab Disord. 2001;25:805–10. doi: 10.1038/sj.ijo.0801623. [DOI] [PubMed] [Google Scholar]
- 162.Atabek ME, Kurtoglu S, Demir F, Baykara M. Relation of serum leptin and insulin-like growth factor-1 levels to intima-media thickness and functions of common carotid artery in children and adolescents with type 1 diabetes. Acta Paediatr. 2004;93:1052–7. doi: 10.1111/j.1651-2227.2004.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 163.Soderberg S, Ahren B, Jansson JH, Johnson O, Hallmans G, Asplund K, Olsson T. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246:409–18. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 164.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, Somers VK. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100:234–9. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wallerstedt SM, Eriksson AL, Niklason A, Ohlsson C, Hedner T. Serum leptin and myocardial infarction in hypertension. Blood Press. 2004;13:243–6. doi: 10.1080/08037050410021405. [DOI] [PubMed] [Google Scholar]
- 166.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 167.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–24. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 168.Couillard C, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ, Despres JP. Leptinemia is not a risk factor for ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Diabetes Care. 1998;21:782–6. doi: 10.2337/diacare.21.5.782. [DOI] [PubMed] [Google Scholar]
- 169.Piemonti L, Calori G, Mercalli A, Lattuada G, Monti P, Garancini MP, Costantino F, Ruotolo G, Luzi L, Perseghin G. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–9. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 170.McGaffin KR, Moravec CS, McTiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circ Heart Fail. 2009;2:676–83. doi: 10.1161/CIRCHEARTFAILURE.109.869909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McGaffin KR, Witham WG, Yester KA, Romano LC, O'Doherty RM, McTiernan CF, O'Donnell CP. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 2011;89:60–71. doi: 10.1093/cvr/cvq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McGaffin KR, Zou B, McTiernan CF, O'Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res. 2009;83:313–24. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S, Davidson SM, Hausenloy DJ, Yellon DM. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–70. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 176.Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–7. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 177.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 178.Cartier A, Lemieux I, Almeras N, Tremblay A, Bergeron J, Despres JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93:1931–8. doi: 10.1210/jc.2007-2191. [DOI] [PubMed] [Google Scholar]
- 179.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 181.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–50. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, Rose-John S, von Andrian UH, Baumann H, Evans SS. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7:1299–308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 183.Chen Q, Wang WC, Bruce R, Li H, Schleider DM, Mulbury MJ, Bain MD, Wallace PK, Baumann H, Evans SS. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity. 2004;20:59–70. doi: 10.1016/s1074-7613(03)00358-3. [DOI] [PubMed] [Google Scholar]
- 184.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 185.Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–94. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 188.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–30. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 191.Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, Kingwell BA, Clark MG, Rattigan S, Febbraio MA. Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-alpha expression. Diabetes. 2009;58:1086–95. doi: 10.2337/db08-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–97. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 193.Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab. 2005;289:E251–7. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- 194.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 195.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P, Group PS. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–61. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 197.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 198.Interleukin-6 Receptor Mendelian Randomisation Analysis Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–7. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 201.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJ, Drexler H. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 202.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–20. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 203.Song L, Schindler C. IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis. 2004;177:43–51. doi: 10.1016/j.atherosclerosis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 204.Frisdal E, Lesnik P, Olivier M, Robillard P, Chapman MJ, Huby T, Guerin M, Le Goff W. Interleukin-6 protects human macrophages from cellular cholesterol accumulation and attenuates the proinflammatory response. J Biol Chem. 2011;286:30926–36. doi: 10.1074/jbc.M111.264325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Robert J, Lehner M, Frank S, Perisa D, von Eckardstein A, Rohrer L. Interleukin 6 stimulates endothelial binding and transport of high-density lipoprotein through induction of endothelial lipase. Arterioscler Thromb Vasc Biol. 2013;33:2699–706. doi: 10.1161/ATVBAHA.113.301363. [DOI] [PubMed] [Google Scholar]
- 206.Schuett H, Oestreich R, Waetzig GH, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:281–90. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 207.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 208.Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77:723–7. doi: 10.1016/s0002-9149(97)89206-5. [DOI] [PubMed] [Google Scholar]
- 209.MacGowan GA, Mann DL, Kormos RL, Feldman AM, Murali S. Circulating interleukin-6 in severe heart failure. Am J Cardiol. 1997;79:1128–31. doi: 10.1016/s0002-9149(96)00063-x. [DOI] [PubMed] [Google Scholar]
- 210.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–8. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 211.Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–82. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- 212.Fuchs M, Hilfiker A, Kaminski K, Hilfiker-Kleiner D, Guener Z, Klein G, Podewski E, Schieffer B, Rose-John S, Drexler H. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. FASEB J. 2003;17:2118–20. doi: 10.1096/fj.03-0331fje. [DOI] [PubMed] [Google Scholar]
- 213.Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, Matsubara H, Nakata T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res. 2010;87:424–30. doi: 10.1093/cvr/cvq078. [DOI] [PubMed] [Google Scholar]
- 214.Matsushita K, Iwanaga S, Oda T, Kimura K, Shimada M, Sano M, Umezawa A, Hata J, Ogawa S. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab Invest. 2005;85:1210–23. doi: 10.1038/labinvest.3700322. [DOI] [PubMed] [Google Scholar]
- 215.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 216.Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671–9. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 217.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–8. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 218.Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083–90. doi: 10.2337/db05-0615. [DOI] [PubMed] [Google Scholar]
- 219.Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–9. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen BH, Song Y, Ding EL, Roberts CK, Manson JE, Rifai N, Buring JE, Gaziano JM, Liu S. Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care. 2009;32:329–34. doi: 10.2337/dc08-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–16. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino RB, Sr, Wilson PW, Meigs JB. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–72. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–56. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 224.Menzaghi C, Coco A, Salvemini L, Thompson R, De Cosmo S, Doria A, Trischitta V. Heritability of serum resistin and its genetic correlation with insulin resistance-related features in nondiabetic Caucasians. J Clin Endocrinol Metab. 2006;91:2792–5. doi: 10.1210/jc.2005-2715. [DOI] [PubMed] [Google Scholar]
- 225.Youn BS, Yu KY, Park HJ, Lee NS, Min SS, Youn MY, Cho YM, Park YJ, Kim SY, Lee HK, Park KS. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:150–6. doi: 10.1210/jc.2003-031121. [DOI] [PubMed] [Google Scholar]
- 226.Utzschneider KM, Carr DB, Tong J, Wallace TM, Hull RL, Zraika S, Xiao Q, Mistry JS, Retzlaff BM, Knopp RH, Kahn SE. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330–3. doi: 10.1007/s00125-005-1932-y. [DOI] [PubMed] [Google Scholar]
- 227.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276:11252–6. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 228.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 229.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 230.Lu SC, Shieh WY, Chen CY, Hsu SC, Chen HL. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002;530:158–62. doi: 10.1016/s0014-5793(02)03450-6. [DOI] [PubMed] [Google Scholar]
- 231.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–90. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 232.Kunnari AM, Savolainen ER, Ukkola OH, Kesaniemi YA, Jokela MA. The expression of human resistin in different leucocyte lineages is modulated by LPS and TNFalpha. Regul Pept. 2009;157:57–63. doi: 10.1016/j.regpep.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 233.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450–7. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 234.Qi Q, Wang J, Li H, Yu Z, Ye X, Hu FB, Franco OH, Pan A, Liu Y, Lin X. Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur J Endocrinol. 2008;159:585–93. doi: 10.1530/EJE-08-0427. [DOI] [PubMed] [Google Scholar]
- 235.Fargnoli JL, Sun Q, Olenczuk D, Qi L, Zhu Y, Hu FB, Mantzoros CS. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. Eur J Endocrinol. 2010;162:281–8. doi: 10.1530/EJE-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bo S, Gambino R, Pagani A, Guidi S, Gentile L, Cassader M, Pagano GF. Relationships between human serum resistin, inflammatory markers and insulin resistance. Int J Obes (Lond) 2005;29:1315–20. doi: 10.1038/sj.ijo.0803037. [DOI] [PubMed] [Google Scholar]
- 237.Kunnari A, Ukkola O, Paivansalo M, Kesaniemi YA. High plasma resistin level is associated with enhanced highly sensitive C-reactive protein and leukocytes. J Clin Endocrinol Metab. 2006;91:2755–60. doi: 10.1210/jc.2005-2115. [DOI] [PubMed] [Google Scholar]
- 238.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–40. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 239.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 240.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 241.Cho Y, Lee SE, Lee HC, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 242.Asterholm IW, Rutkowski JM, Fujikawa T, Cho YR, Fukuda M, Tao C, Wang ZV, Gupta RK, Elmquist JK, Scherer PE. Elevated resistin levels induce central leptin resistance and increased atherosclerotic progression in mice. Diabetologia. 2014;57:1209–18. doi: 10.1007/s00125-014-3210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–23. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 244.Lim S, Koo BK, Cho SW, Kihara S, Funahashi T, Cho YM, Kim SY, Lee HK, Shimomura I, Park KS. Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study. Atherosclerosis. 2008;196:398–404. doi: 10.1016/j.atherosclerosis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 245.Luc G, Empana JP, Morange P, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Evans A, Kee F, Bingham A, Machez E, Ducimetiere P. Adipocytokines and the risk of coronary heart disease in healthy middle aged men: the PRIME Study. Int J Obes (Lond) 2010;34:118–26. doi: 10.1038/ijo.2009.204. [DOI] [PubMed] [Google Scholar]
- 246.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–9. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 247.Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ, Nasir K, Criqui MH, Cushman M, McClelland RL, Allison MA. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239:101–8. doi: 10.1016/j.atherosclerosis.2014.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M, Nakamura H, Ohsuzu F. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379–80. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 249.Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764–71. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 250.Hu WL, Qiao SB, Hou Q, Yuan JS. Plasma resistin is increased in patients with unstable angina. Chin Med J (Engl) 2007;120:871–5. [PubMed] [Google Scholar]
- 251.Wang H, Chen DY, Cao J, He ZY, Zhu BP, Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin Med Sci J. 2009;24:161–6. doi: 10.1016/s1001-9294(09)60082-1. [DOI] [PubMed] [Google Scholar]
- 252.Sinan UY, Canbolat IP, Baydar O, Oktay V, Imre G, Kocas C, Abaci O, Coskun U, Bostan C, Kilickesmez KO, Yildiz A, Kaya A, Gurmen T, Yigit Z. Relationship between increased serum resistin level and severity of coronary artery disease. Angiology. 2014;65:239–42. doi: 10.1177/0003319713502718. [DOI] [PubMed] [Google Scholar]
- 253.Momiyama Y, Ohmori R, Uto-Kondo H, Tanaka N, Kato R, Taniguchi H, Arakawa K, Nakamura H, Ohsuzu F. Serum resistin levels and cardiovascular events in patients undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2011;18:108–14. doi: 10.5551/jat.6023. [DOI] [PubMed] [Google Scholar]
- 254.Krecki R, Krzeminska-Pakula M, Peruga JZ, Szczesniak P, Lipiec P, Wierzbowska-Drabik K, Orszulak-Michalak D, Kasprzak JD. Elevated resistin opposed to adiponectin or angiogenin plasma levels as a strong, independent predictive factor for the occurrence of major adverse cardiac and cerebrovascular events in patients with stable multivessel coronary artery disease over 1-year follow-up. Med Sci Monit. 2011;17:CR26–32. doi: 10.12659/MSM.881325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lubos E, Messow CM, Schnabel R, Rupprecht HJ, Espinola-Klein C, Bickel C, Peetz D, Post F, Lackner KJ, Tiret L, Munzel T, Blankenberg S. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Atherosclerosis. 2007;193:121–8. doi: 10.1016/j.atherosclerosis.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 256.Chu S, Ding W, Li K, Pang Y, Tang C. Plasma resistin associated with myocardium injury in patients with acute coronary syndrome. Circ J. 2008;72:1249–53. doi: 10.1253/circj.72.1249. [DOI] [PubMed] [Google Scholar]
- 257.Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, Sinagra G, Zingone B, Alfieri O, Ferrero E, Maseri A, Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–53. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 258.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 259.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 260.Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–81. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- 261.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Mohlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–7. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 262.Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–42. doi: 10.1080/08037050410021397. [DOI] [PubMed] [Google Scholar]
- 263.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 264.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y Osaka CADSGCad. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 265.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, Kataoka T, Kamimori K, Shimodozono S, Kobayashi Y, Yoshiyama M, Takeuchi K, Yoshikawa J. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–33. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kojima S, Funahashi T, Sakamoto T, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 268.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–9. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 269.Ai M, Otokozawa S, Asztalos BF, White CC, Cupples LA, Nakajima K, Lamon-Fava S, Wilson PW, Matsuzawa Y, Schaefer EJ. Adiponectin: an independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis. 2011;217:543–8. doi: 10.1016/j.atherosclerosis.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lindsay RS, Resnick HE, Zhu J, Tun ML, Howard BV, Zhang Y, Yeh J, Best LG. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–6. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 271.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–83. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 272.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–9. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 273.Walkey AJ, Rice TW, Konter J, Ouchi N, Shibata R, Walsh K, deBoisblanc BP, Summer R. Plasma adiponectin and mortality in critically ill subjects with acute respiratory failure. Crit Care Med. 2010;38:2329–34. doi: 10.1097/CCM.0b013e3181fa0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 275.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–30. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 276.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–99. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–13. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 280.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–24. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 283.Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 284.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–23. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 285.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–9. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 287.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–17. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 288.Ikeda Y, Ohashi K, Shibata R, Pimentel DR, Kihara S, Ouchi N, Walsh K. Cyclooxygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 2008;582:1147–50. doi: 10.1016/j.febslet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 291.Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, Shintani S, Walsh K, Ouchi N, Murohara T. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166–73. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 293.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 294.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 295.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 296.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–86. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–63. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 299.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 300.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 301.van Stijn CM, Kim J, Barish GD, Tietge UJ, Tangirala RK. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PLoS One. 2014;9:e86404. doi: 10.1371/journal.pone.0086404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–25. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 303.Nawrocki AR, Hofmann SM, Teupser D, Basford JE, Durand JL, Jelicks LA, Woo CW, Kuriakose G, Factor SM, Tanowitz HB, Hui DY, Tabas I, Scherer PE. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2010;30:1159–65. doi: 10.1161/ATVBAHA.109.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 305.Parker-Duffen JL, Nakamura K, Silver M, Zuriaga MA, MacLauchlan S, Aprahamian TR, Walsh K. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. J Biol Chem. 2014;289:16200–13. doi: 10.1074/jbc.M114.548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Linden D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 307.Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, Sullivan JM, Reifel-Miller A. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–92. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- 308.Fujioka D, Kawabata K, Saito Y, Kobayashi T, Nakamura T, Kodama Y, Takano H, Obata JE, Kitta Y, Umetani K, Kugiyama K. Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am J Physiol Heart Circ Physiol. 2006;290:H2409–16. doi: 10.1152/ajpheart.00987.2005. [DOI] [PubMed] [Google Scholar]
- 309.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 310.Galvan MD, Hulsebus H, Heitker T, Zeng E, Bohlson SS. Complement protein C1q and adiponectin stimulate Mer tyrosine kinase-dependent engulfment of apoptotic cells through a shared pathway. J Innate Immun. 2014;6:780–92. doi: 10.1159/000363295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nakatsuji H, Kobayashi H, Kishida K, Nakagawa T, Takahashi S, Tanaka H, Akamatsu S, Funahashi T, Shimomura I. Binding of adiponectin and C1q in human serum, and clinical significance of the measurement of C1q-adiponectin / total adiponectin ratio. Metabolism. 2013;62:109–20. doi: 10.1016/j.metabol.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 312.Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15:3256–64. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–52. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288:24886–97. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–77. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Deng W, Li C, Zhang Y, Zhao J, Yang M, Tian M, Li L, Zheng Y, Chen B, Yang G. Serum C1q/TNF-related protein-3 (CTRP3) levels are decreased in obesity and hypertension and are negatively correlated with parameters of insulin resistance. Diabetol Metab Syndr. 2015;7:33. doi: 10.1186/s13098-015-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287:18965–73. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285:39691–701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower Circulating C1q/TNF-Related Protein-3 (CTRP3) Levels Are Associated with Obesity: A Cross-Sectional Study. PLoS One. 2015;10:e0133955. doi: 10.1371/journal.pone.0133955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ouchi N, Walsh K. Cardiovascular and metabolic regulation by the adiponectin/C1q/tumor necrosis factor-related protein family of proteins. Circulation. 2012;125:3066–8. doi: 10.1161/CIRCULATIONAHA.112.114181. [DOI] [PubMed] [Google Scholar]
- 321.Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, Wang Y, Su H, Wang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation. 2013;128:S113–20. doi: 10.1161/CIRCULATIONAHA.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol. 2013;108:315. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kambara T, Shibata R, Ohashi K, Matsuo K, Hiramatsu-Ito M, Enomoto T, Yuasa D, Ito M, Hayakawa S, Ogawa H, Aprahamian T, Walsh K, Murohara T, Ouchi N. C1q/Tumor Necrosis Factor-Related Protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-dependent mechanism. Mol Cell Biol. 2015;35:2173–85. doi: 10.1128/MCB.01518-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125:3159–69. doi: 10.1161/CIRCULATIONAHA.112.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, Li L, Wu LL. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J Mol Med (Berl) 2015 doi: 10.1007/s00109-015-1309-8. [DOI] [PubMed] [Google Scholar]
- 326.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Choi DS, Baik SH, Bluher M, Youn BS, Choi KM. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One. 2013;8:e55744. doi: 10.1371/journal.pone.0055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tsuji S, Yamashita M, Nishiyama A, Shinohara T, Li Z, Myrvik QN, Hoffman DR, Henriksen RA, Shibata Y. Differential structure and activity between human and mouse intelectin-1: human intelectin-1 is a disulfide-linked trimer, whereas mouse homologue is a monomer. Glycobiology. 2007;17:1045–51. doi: 10.1093/glycob/cwm075. [DOI] [PubMed] [Google Scholar]
- 328.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–61. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 329.de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 330.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 331.Shibata R, Ouchi N, Takahashi R, Terakura Y, Ohashi K, Ikeda N, Higuchi A, Terasaki H, Kihara S, Murohara T. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr. 2012;4:37. doi: 10.1186/1758-5996-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Shibata R, Ouchi N, Kikuchi R, Takahashi R, Takeshita K, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis. 2011;219:811–4. doi: 10.1016/j.atherosclerosis.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 333.Onur I, Oz F, Yildiz S, Oflaz H, Sigirci S, Elitok A, Pilten S, Karaayvaz EB, Cizgici AY, Kaya MG, Onur ST, Sahin I, Dinckal HM. Serum omentin 1 level is associated with coronary artery disease and its severity in postmenopausal women. Angiology. 2014;65:896–900. doi: 10.1177/0003319713511322. [DOI] [PubMed] [Google Scholar]
- 334.Zhong X, Zhang HY, Tan H, Zhou Y, Liu FL, Chen FQ, Shang DY. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32:873–8. doi: 10.1038/aps.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Shang FJ, Wang JP, Liu XT, Zheng QS, Xue YS, Wang B, Zhao LY. Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers. 2011;16:657–62. doi: 10.3109/1354750X.2011.622789. [DOI] [PubMed] [Google Scholar]
- 336.Shibata R, Takahashi R, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T, Ouchi N. Association of a fat-derived plasma protein omentin with carotid artery intima-media thickness in apparently healthy men. Hypertens Res. 2011;34:1309–12. doi: 10.1038/hr.2011.130. [DOI] [PubMed] [Google Scholar]
- 337.Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–72. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 338.Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, Ogawa H, Murohara T, Ouchi N. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. 2012;287:408–17. doi: 10.1074/jbc.M111.261818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Greulich S, Chen WJ, Maxhera B, et al. Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studies. PLoS One. 2013;8:e59697. doi: 10.1371/journal.pone.0059697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Matsuo K, Shibata R, Ohashi K, et al. Omentin functions to attenuate cardiac hypertrophic response. J Mol Cell Cardiol. 2015;79:195–202. doi: 10.1016/j.yjmcc.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 341.Kataoka Y, Shibata R, Ohashi K, et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol. 2014;63:2722–33. doi: 10.1016/j.jacc.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 342.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–43. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 343.Hiramatsu-Ito M, Shibata R, Ohashi K, et al. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv282. [DOI] [PubMed] [Google Scholar]
- 344.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–7. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Nakamura K, Sano S, Fuster JJ, Kikuchi R, Shimizu I, Ohshima K, Katanasaka Y, Ouchi N, Walsh K. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia-reperfusion injury. J Biol Chem. 2015 doi: 10.1074/jbc.M115.693937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Prats-Puig A, Soriano-Rodriguez P, Carreras-Badosa G, Riera-Perez E, Ros-Miquel M, Gomila-Borja A, de Zegher F, Ibanez L, Bassols J, Lopez-Bermejo A. Balanced duo of anti-inflammatory SFRP5 and proinflammatory WNT5A in children. Pediatr Res. 2014;75:793–7. doi: 10.1038/pr.2014.29. [DOI] [PubMed] [Google Scholar]
- 347.Ehrlund A, Mejhert N, Lorente-Cebrian S, Astrom G, Dahlman I, Laurencikiene J, Ryden M. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab. 2013;98:E503–8. doi: 10.1210/jc.2012-3416. [DOI] [PubMed] [Google Scholar]
- 348.Hu Z, Deng H, Qu H. Plasma SFRP5 levels are decreased in Chinese subjects with obesity and type 2 diabetes and negatively correlated with parameters of insulin resistance. Diabetes Res Clin Pract. 2013;99:391–5. doi: 10.1016/j.diabres.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 349.Hu W, Li L, Yang M, Luo X, Ran W, Liu D, Xiong Z, Liu H, Yang G. Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J Clin Endocrinol Metab. 2013;98:290–8. doi: 10.1210/jc.2012-2466. [DOI] [PubMed] [Google Scholar]
- 350.Tan X, Wang X, Chu H, Liu H, Yi X, Xiao Y. SFRP5 correlates with obesity and metabolic syndrome and increases after weight loss in children. Clin Endocrinol (Oxf) 2014;81:363–9. doi: 10.1111/cen.12361. [DOI] [PubMed] [Google Scholar]
- 351.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Activation of noncanonical Wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab. 2014;99:E1407–17. doi: 10.1210/jc.2014-1191. [DOI] [PubMed] [Google Scholar]
- 352.Carstensen M, Herder C, Kempf K, Erlund I, Martin S, Koenig W, Sundvall J, Bidel S, Kuha S, Roden M, Tuomilehto J. Sfrp5 correlates with insulin resistance and oxidative stress. Eur J Clin Invest. 2013;43:350–7. doi: 10.1111/eci.12052. [DOI] [PubMed] [Google Scholar]
- 353.Schulte DM, Muller N, Neumann K, Oberhauser F, Faust M, Gudelhofer H, Brandt B, Krone W, Laudes M. Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS One. 2012;7:e32437. doi: 10.1371/journal.pone.0032437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Miyoshi T, Doi M, Usui S, Iwamoto M, Kajiya M, Takeda K, Nosaka K, Nakayama R, Okawa K, Takagi W, Nakamura K, Hirohata S, Ito H. Low serum level of secreted frizzled-related protein 5, an anti-inflammatory adipokine, is associated with coronary artery disease. Atherosclerosis. 2014;233:454–9. doi: 10.1016/j.atherosclerosis.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 355.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, Song K, Lee I. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185:1274–82. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 356.Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–70. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 357.Breton-Romero R, Feng B, Holdbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, Gokce N, Fuster JJ, Walsh K, Hamburg N. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.115.306578. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–10. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.