Abstract
Many signaling pathways converge on the nucleus to regulate critical nuclear events such as transcription, DNA replication and cell cycle progression. While the vast majority of research in this area has focused on signals generated in response to hormones or other soluble factors, the nucleus also responds to mechanical forces. During the past decade or so, much has been learned about how mechanical force can affect transcription, as well as the growth and differentiation of cells. Much has also been learned about how force is transmitted via the cytoskeleton to the nucleus and then across the nuclear envelope to the nuclear lamina and chromatin. In this brief review, we focus on some of the key proteins that transmit mechanical signals across the nuclear envelope.
Introduction
Cells respond to mechanical forces in their environment [1,2]. Forces influence cell division, differentiation, and migration, ultimately affecting processes from morphogenesis to tissue repair. Mechanotransduction - the process by which mechanical stimuli generate cellular signaling events - occurs in all eukaryotic cells and is attributed partly to the structural qualities of the cytoskeleton which behaves as a conductive and viscoelastic material. In this way, the cytoskeleton transmits force and propagates stress within and between cells. Characterizing the elements that sense, transduce, and respond to physical force has implicated adhesion receptors, cytoskeletal elements, and organelles in a structurally integrated network [3,4].
Morphological changes to the nucleus in response to force were observed over 80-years ago [5,6]. Later work showed that forces applied to integrins can lead to rapid (seconds) force transmission to the nucleus [7], resulting in positional and morphological changes to the nucleus itself. The effects of mechanical force on nuclear positioning [8,9], nuclear morphology [10,11], and gene activity (e.g. c-fos, egr-1, iex-1, c-myc) [9,12,13] have also been observed in other contexts. Immediate nuclear responses to force (<30 minutes), such as physical changes to the nuclear lamina [11], repositioning of intranuclear markers [14], and nuclear localization of mechanical response mediators [15,16], suggest that a cell's mechanotransduction pathways coordinate and communicate with the nucleus. On longer time scales (hours-days), the nucleus can alter its stiffness to reflect the stiffness of the cellular microenvironment [17]. Changes in matrix stiffness activates genetic programs to direct development [18], tumorigenesis [19], and stem cell fate [20]. These findings indicate that the nucleus is a critical component of the cell mechanoresponse and provides, at the very least, long term cellular adaptation to force through transcriptional regulation. But how is this accomplished and what effect do mechanical forces have on nuclear function? To understand this further, we examine recent literature regarding the role of the LINC complex in mechanotransduction and nuclear function with emphasis on Nesprin, SUN, and emerin proteins. Although we are interested in how mechanical forces affect chromatin structure and gene transcription, space limitations prevent us from considering these topics in this review.
The nucleus: linking structural form to function
The nucleus contains several stratified and interconnecting elements that bridge the two lipid bilayers of the nuclear envelope to the underlying nucleoskeleton and chromatin. The inner and outer nuclear membranes connect via nuclear pores that mediate communication between the cytoplasmic and nucleoplasmic compartments. The inner nuclear membrane is mechanically supported by the nuclear lamina which consists of filamentous lamin proteins (lamins A, B, and C), and several integral membrane proteins, including LEM-domain containing members, LAP2, emerin, and MAN1 [21]. The nuclear lamina is a dynamic structure that associates with chromatin domains and regulates the global organization of chromatin and gene expression [22,23]. Multiple severe pathologies, known as laminopathies [24], are associated with defects to proteins of the nuclear lamina, underscoring its structural importance to physiology.
Early biochemistry and electron microscopy studies contributed to the notion that the cytoskeleton interconnects with the nuclear lamina [25–28] (Figure 1) but our current molecular understanding stems from studies on nuclear migration [29]. Characterization of two distinct families of proteins that co-localize to the nuclear envelope, namely the SYNE/Nesprin-family [30,31] and SUN-family [32,33], were shown to connect the cytoskeleton and nuclear lamina. Seminal work by Starr and colleagues in C. elegans mutants, anc-1 and unc-84, demonstrated that ANC-1 (homologue of Nesprin 2) associates with actin at its N-terminus while UNC-84 (SUN1/2 homologue) associates at its C-terminus [34]. Furthermore, UNC-84 localizes to the nuclear envelope in a lamin-dependent manner [33]. Thus, a molecular bridge linking the nuclear lamina to the cytoskeleton was defined and shown to be critical for nuclear movement. The term LINC (linker of nucleoskeleton and cytoskeleton) complex was coined for these structures [35] (Figure 2) and later work revealed homologues for its core components from yeast to human.
Figure 1. The nuclear lamina and cytoskeleton are highly interconnected.
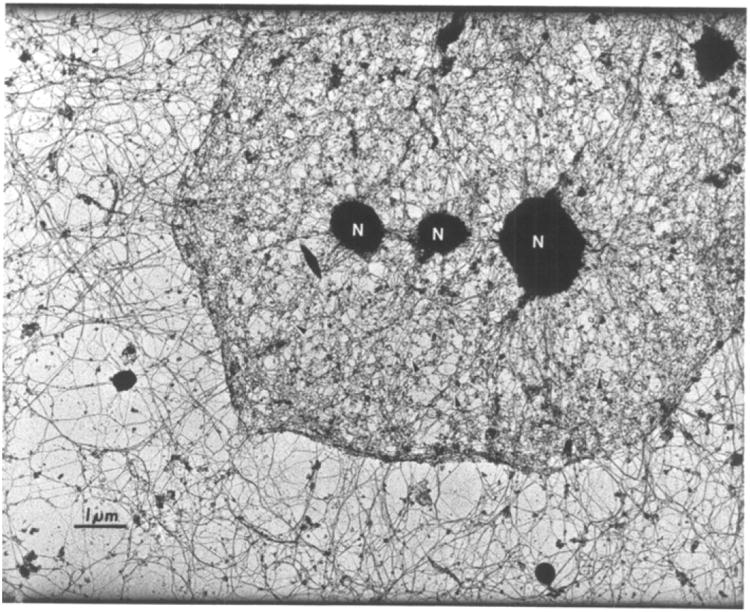
Transmission electron micrograph of a HeLa cell, after removal of membranes and nucleic acids, showing nuclear filaments interconnecting with the cytoskeleton. Reprinted from Cell, vol. 29, Capco DG, Wan KM, Penman S, “The nuclear matrix: three-dimensional architecture and protein composition”, 847-858, 1982, with permission from Elsevier (license#: 3727211229007)
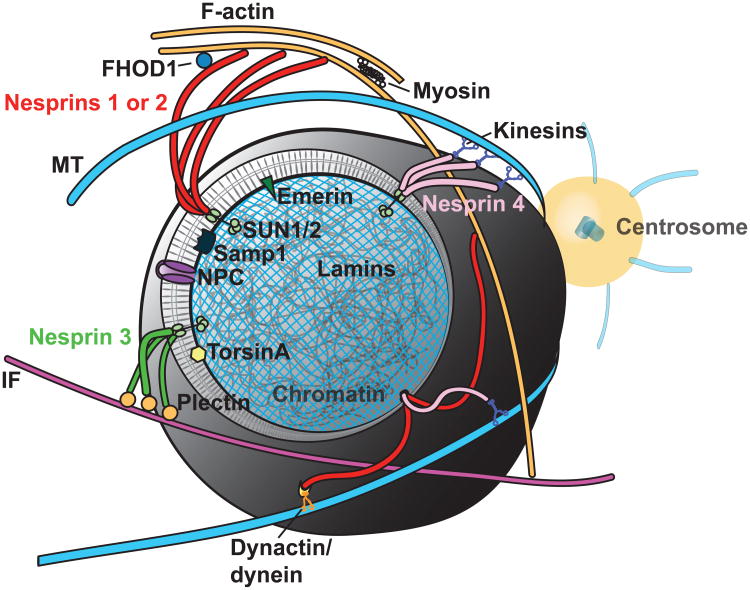
Figure 2. LINC complexes tether the nucleus.
The nuclear envelope is cutaway to expose the nuclear lamina and underlying chromatin. Inner nuclear envelope proteins emerin, SUN1/2, and Samp1 regulate interactions with KASH domain-containing Nesprin family members, 1-4. Nesprins associate with the cytoskeleton directly and indirectly via adaptor proteins and molecular motors.
LINC complexes and nuclear mechanotransduction
Isolated nuclei respond to physical force [36,37], suggesting that sensory, transducing, and responding functions exist within the nucleus itself. Similar to whole cells which have adhesion receptors bridging the extracellular environment to the cytoskeleton, the LINC complex connects across the nuclear membrane, associating filamentous systems on each side. The structural similarity between whole cells and nuclei raises the possibility that force-sensitive signal amplification which in focal adhesions involves proteins like talin and vinculin, may similarly occur at the nuclear membrane. We speculate that the spectrin-repeats of Nesprins may cooperatively unfold under tension, exposing binding sites that promote Nesprin dimerization and recruitment of additional factors, facilitating complex stability and rigidity. In this light, LINC complexes could act as force-sensitive signaling hubs for cytoplasmic proteins and fine-tune nuclear responses to various mechanosensory inputs. A force-sensing mechanism on Nesprins could be locally amplified by the structural changes that occur within the nuclear lamina [36].
LINC complexes are thought to be the primary structure controlling nuclear mechanotransduction but how does nuclear mechanotransduction affect cell function? Driscoll and colleagues recently showed that the LINC complex contributes to the pre-stress state of the cell using mesenchymal stem cells [16]. (Pre-stress derives from stresses generated within and experienced by cells in their environment.) This suggests that LINC complexes regulate cell-wide tension as well as strain transfer to the nucleus. This balance of internal cellular tension is a key component of the cellular tensegrity model proposed by Donald Ingber [4,38]. The major elements that regulate the pre-stress state of the cell (adhesion receptors, actomyosin contractility, and the cytoskeleton) are also important for force transmission to the nucleus [7,39,40]. Not only can the LINC complex influence cell-wide tension but cell-generated tension also regulates nuclear mechanics. Actomyosin contractility changes the stability of the nuclear lamina through rapid [41] and long-term [17] changes to lamin A. Tension on the LINC complex (mimicking actomyosin tension) rapidly increases the stiffness of the nucleus [42] (Figure 3). This can have broader and longer lasting consequences to the pre-stress state of the cell as seen during stem cell differentiation on substrates of different rigidity [20].
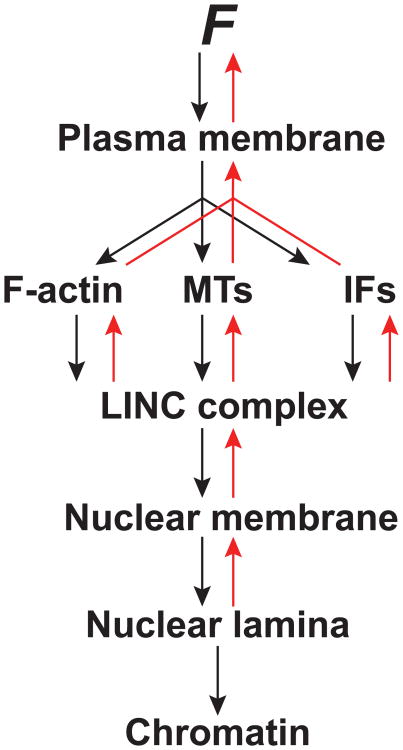
Figure 3. Force transduction to the nucleus and back.
Schematic demonstrating the flow of cellular force from the plasma membrane to the nucleus. Force is manifested as distortion of the plasma membrane or proteins within it. Tension is transmitted to the different systems of the cytoskeleton and transmitted to the nucleus via the LINC complex. In turn, the LINC complex transmits force across the nuclear membrane to the nuclear lamina and to chromatin. The nucleus rapidly responds to force application at the nuclear lamina and this response is conveyed back through these same elements.
Nesprins
Mammals have five Nesprin genes (SYNE 1-4, KASH5) that share a conserved C-terminal KASH (Klarsicht/ANC-1/Syne-1 homology) domain that interacts with SUN proteins. Cytoskeletal force is transduced to LINC complexes via specific Nesprins which bind different cytoskeletal systems. For example, Nesprin 1 and 2 bind actin [34] and connect to microtubules via dynein/dynactin [43] and kinesins [43,44]; Nesprin 3 binds to intermediate filaments through plectin [45]; Nesprin 4 binds to microtubules through kinesin [46]; and KASH5 binds to microtubules through dynein/dynactin [47]. Importantly, Nesprins are complex as they reveal multiple splice-variants which add to their functional repertoire; have adaptable expression patterns making depletion studies difficult; and are thought to interact both physically and functionally with each other [31,48–50]. It is likely that Nesprins 1-3 predominantly mediate force transduction to the nucleus in most cells as they are widely expressed relative to Nesprin 4 and KASH5. Expression of Nesprin 4 appears restricted to sensory hair and secretory epithelial cells (mammary, salivary, pancreas) [46,51] while KASH5 is restricted to reproductive organs [47].
Mechanical tension on isolated nuclei through Nesprin 1 results in stiffening of the nucleus [36]. In this work, nuclear adaptation to force through Nesprin 1 is dependent upon several nuclear lamina proteins, including both SUN1 and 2, emerin, and lamin A/C. Nesprins have been shown to function in cellular responses to force in other systems. For example, loss of Nesprin 1 and 2 in mouse cardiomyocytes causes reduced expression of mechanical response genes after biaxial stretching [52]. In endothelial cells, depletion of Nesprin 1 causes failure to align in response to uniaxial stretch [53], while depletion of Nesprin 3 causes a failure in centrosome reorientation in response to fluid shear [54]. Use of dominant-negative approaches that recapitulate loss of Nesprin-SUN complexes demonstrate force transmission from the cytoskeleton to nucleus is reduced [9]. It was recently shown that nuclear localization of the mechanically responsive transcriptional cofactor, YAP, is dependent upon Nesprin 1G in response to stretch [16]. The LINC complex is also important for NFκB activity in response to stretch [55]. Together, these findings suggest that LINC complexes may regulate other mechanoresponsive transactivators, such as β-catenin [56] and Twist [57]. Nesprin 2 has already been shown to regulate Wnt-ligand induced nuclear translocation of β-catenin [58].
Maintaining nuclear positioning requires force transmission from the cytoskeleton to the nucleus [59,60] and Nesprin loss results in defects to this critical process in many systems [34,61-64][43]. It is difficult to separate nuclear migration defects, which affect cell polarity, migration and other processes, from defects ascribed to mechanotransduction. However, the deregulation of these processes may be attributed to the latter. For example, dorsal actin stress fiber structures that traverse the apical side of the nucleus have been implicated in force transmission through the LINC complex to the nucleus [65] [66]. Photo-ablation of Nesprin-positive stress fibers over the nucleus causes local deformation of the underlying nucleus and nuclear displacement [67,68], suggesting the LINC complex regulates nuclear position by maintaining tension between the cytoskeleton and nucleus. Dynamic mechanical coupling of the nucleus with the cytoskeleton is best seen in 3D migration (see minireview on this subject by McGregor et al. in this issue). Petrie and colleagues discovered that a hydrostatic pressure differential exists between the cell front and back, and that this arises through force generated on the nucleus during cell migration in 3D [69]. In this work, actomyosin contractile force is transmitted to the nucleus via vimentin and Nesprin 3. Depletion of Nesprin 3 caused a concomitant loss of nuclear positioning and intracellular pressure asymmetry during 3D migration.
The importance of Nesprin function in mechanotransduction can also be recognized in human diseases. Patients with Emery-Dreifuss muscular dystrophy (EDMD) exhibit late-onset neuromuscular disorders with mutations in emerin (X-linked form), lamin A/C (autosomal dominant form), or Nesprin 1 and 2 [70]. EDMD leads to increased nuclear fragility and defective mechanosensitive gene responses in highly contractile skeletal and cardiac muscle. In mice, deletion of Nesprin 1 and 2 results in cardiomyopathy as well as impaired gene expression in response to mechanical stimuli [52]. Additionally, mutations in Nesprin 4 have been identified from families that exhibited hereditary hearing-loss [51]. nesprin-4-/- mice showed gradual degradation of the highly mechanosensory outer-hair cells within the cochlear organ. Nuclear positioning defects were concomitant with hair cell degradation.
SUNs
Mammals have five SUN-domain containing proteins (SUNs 1-5). SUN1 and 2 are widely expressed while SUN3-5 appear testis specific [71-73]. Oligomerization of SUN proteins is required for binding with Nesprin KASH domains [74]. Structural evidence shows SUN proteins assemble as a trimer that can interact at a 1:1 SUN:KASH domain stoichiometry [75]. From this work, Sosa and colleagues proposed that a covalent linkage existed between the SUN-KASH domains. Such a link would be strong enough to withstand and enable high levels of force-transmission and might be regulated by TorsinA - a member of the AAA+ superfamily of ATPases [76] (see minireview on this subject by Laudermilch and Schlieker in this issue). Recent work supports this notion as TorsinA ATP-hydrolysis activity is regulated by LAP1 and LULL1 [77]. TorsinA displaces SUN2, Nesprin 2G, and Nesprin 3 from the nuclear envelope but does not affect SUN1 [78]. Differences in SUN1 and 2 regulation and function have been seen elsewhere and are discussed below.
Cells can respond to low magnitude vibrations and Uzer and colleagues have shown that the nucleus is critical for detecting this type of mechanical stimulus [79]. Working with mesenchymal stem cells, they found activation of FAK and Akt pathways by vibration induced RhoA signaling, F-actin remodeling, and repression of adipogenic gene expression. SUN1/2 co-depletion, as well as expression of the DN-KASH domain, disrupted vibration-induced responses [79]. In C. elegans, UNC-84 (SUN1/2 homologue) interacts with lamin to transfer cytoplasmic forces to the nucleus during nuclear migration [80]. Co-depletion of SUN1/2 also blocks nuclear stiffening in response to forces applied to isolated nuclei via Nesprin 1 [36], suggesting that SUN1 and SUN2 can operate separately and may be functionally redundant. This is consistent with SUN1/2 null mice in which Nesprin 1 localization is disrupted but not in either SUN1 or SUN2 expressing cells [81]. Conversely, functional differences have also been proposed. Despite similar affinities to the KASH domain of mini-Nesprin 2G, SUN1 has been shown to be dispensable for Nesprin 2 anchoring while SUN2 was necessary [82]. In C. elegans, UNC-84 may recruit UNC-83 (KASH-domain containing protein) at the nuclear envelope where they mediate force transmission during nuclear migration [83]. UNC-84 is required for proper nuclear envelope architecture in high force-bearing cells [74], consistent with its role as a force transducer in the LINC complex. Lastly, SUN1 protein levels increase in lamin null cells as a result of reduced protein turnover, whereas SUN2 remains unchanged [84]. This suggests that different protein degradation pathways and compensation mechanisms may regulate SUN1 and 2 and could provide insight into how SUN1 contributes to lamin pathologies.
Emerin
Emerin is a ubiquitous integral membrane protein that localizes to the inner nuclear membrane and associates with Nesprin 1/2, SUN1/2, lamin A-C [85–87] and other proteins [88]. Emerin mutations in EDMD [89] and emerin-null fibroblasts exhibit defects in mechanotransduction [13,37]. Emerin becomes tyrosine phosphorylated by Src kinase in response to tension applied to isolated nuclei via Nesprin 1 [36]. This rapid phosphorylation coincides with accumulation of lamin A/C and nuclear reinforcement. Emerin promotes actin polymerization [90], potentially increasing nuclear rigidity as a result of actin polymerization at the nuclear lamina in some situations. However, actin polymerization does not appear to contribute to nuclear stiffening in response to applied force [36]. Interestingly, emerin phosphorylation increases on substrata of increasing stiffness [36] and this is blocked after decreasing whole cell actomyosin contractility through inhibiting myosin-II. This suggests that cellular pre-stress can regulate emerin phosphorylation and nuclear signaling. Furthermore, Emerin regulates mechanoresponsive transcription factors such as Lmo7 and MKL1 [91] and thus may be important in relaying mechanical signals that affect longer term adaptation. MKL1 dissociates from G-actin and translocates to the nucleus upon mitogen and mechanical stimuli [92]. Aberrant MKL1-SRF signaling can be rescued in lamin null and mutant cells by addition of emerin [91]. Taken together, these findings demonstrate emerin's ability to regulate rapid nuclear stiffening, actin cytoskeletal polymerization, and gene activation, though, how emerin function is regulated during these processes is unclear.
Conclusion
Overwhelming evidence demonstrates that the nucleus is integral to mechanotransductive processes in cells. Defects in the LINC complex influence nuclear functions and have far reaching effects on cellular architecture and behavior. But why do mutations in otherwise ubiquitously expressed LINC complex proteins manifest as disease states in specific cell types? Could LINC complex defects be predominantly attributed to changes in the pre-stress state of the cell? Pre-stress states vary by cell-type and continually adjust to the mechanical demands of the microenvironment. LINC complex disruptions are most evident in cells that experience high mechanical strain, such as cardiac and skeletal myocytes. As the LINC complex regulates the pre-stress state in multiple ways, these cell-types may be particularly prone to defects in the LINC complex. Strong evidence for this was recently provided by Cain and colleagues in unc-84 mutants in which nuclear envelope architecture was irregular only in cells that experience high mechanical strain [74]. It is important to remember that dynamic cellular adaptation to mechanical stress is critical for cell homeostasis and is well defined for bone and soft tissue (Wolff's law and Davis' law) and has also been seen in other cell-types [93]. As we continue to explore the role of nuclear mechanotransduction, it will be valuable to address the individual contributions that the proteins of the LINC complex and nuclear lamina make in regulating the pre-stress state of cells and how these changes regulate overall cell behavior.
Acknowledgments
We thank Gunes Uzer and Larry Jacobson for their helpful comments on this manuscript. We apologize to all authors whose work could not be cited due to space constraints. The authors were supported by NIH grant GM029860 (KB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Janmey PA, Wells RG, Assoian RK, McCulloch CA. From tissue mechanics to transcription factors. Differentiation. 2013;86:112–120. doi: 10.1016/j.diff.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N, Tytell JD, Ingber DE. Focus on mechanotransduction perspectives. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 5.Chambers R, Fell HB. Micro-operations on cells in tissue cultures. Proc R Soc B Biol Sci. 1931;109:380–403. [Google Scholar]
- 6.Sauer FC. Mitosis in the neural tube. J Comp Neurol. 1935;62:377–405. [Google Scholar]
- 7.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci. 2009;122:577–586. doi: 10.1242/jcs.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000;269:781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 11.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: Are the pieces now in place? J Cell Biochem. 2008;104:1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 13.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth-Gauthier EA, Alcoser TA, Yang G, Dahl KN. Force-induced changes in subnuclear movement and rheology. Biophys J. 2012;103:2423–2431. doi: 10.1016/j.bpj.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 16**.Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J. 2015;108:2783–2793. doi: 10.1016/j.bpj.2015.05.010. This work shows cytoskeletal tension transmits strain to the nucleus, affecting nuclear shape and cell traction force, and is the first to show strain transfer to the nucleus through Nesprin 1 is essential for YAP nuclear translocation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Nuclear Lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104-1–1240104-15. doi: 10.1126/science.1240104. This study uses proteomics to look at lamin protein levels in different solid tissues and finds collagen levels determine tissue stiffness and lamin A levels strongly correlate with this. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammoto T, Mammoto A, Ingber DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol. 2013;29:27–61. doi: 10.1146/annurev-cellbio-101512-122340. [DOI] [PubMed] [Google Scholar]
- 19.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Barton LJ, Soshnev AA, Geyer PK. Networking in the nucleus: A spotlight on LEM-domain proteins. Curr Opin Cell Biol. 2015;34:1–8. doi: 10.1016/j.ceb.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zullo JM, Demarco IA, Piqué-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell. 2012;149:1474–1487. doi: 10.1016/j.cell.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 23**.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, et al. LBR and Lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–598. doi: 10.1016/j.cell.2013.01.009. This work looks at chromatin organization from LBR and lamin A/C null mice and find these proteins cooperate in tethering peripheral heterochromatin to the nuclear envelope and in regulating gene activity. [DOI] [PubMed] [Google Scholar]
- 24.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–1121. doi: 10.1016/j.cub.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capco DG, Wan KM, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29:847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- 26.Fey EG, Wan KM, Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984;98:1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehto VP, Virtanen I, Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978;272:175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- 28.Berezney R, Coffey D. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974;60:1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- 29.Dupin I, Etienne-Manneville S. Nuclear positioning: Mechanisms and functions. Int J Biochem Cell Biol. 2011;43:1698–1707. doi: 10.1016/j.biocel.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 32.Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 33.Lee KK, Starr D, Cohen M, Liu J, Han M, Wilson KL, Gruenbaum Y. Lamin-dependent localization of UNC-84, a protein required for nuclear migration in Caenorhabditis elegans. Mol Biol Cell. 2002;13:892–901. doi: 10.1091/mbc.01-06-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- 35.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Guilluy C, Osborne LD, Van LL, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. This study is the first to show that tension applied directly through Nesprin 1 on isolated nuclei results in a nuclear stiffening response that is dependent upon nuclear lamina proteins, particularly through emerin phosphoregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowat AC, Lammerding J, Ipsen JH. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys J. 2006;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 39.Hu S, Chen J, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochem Biophys Res Commun. 2005;329:423–428. doi: 10.1016/j.bbrc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 40**.Wu J, Kent IA, Shekhar N, Chancellor TJ, Mendonca A, Dickinson RB, Lele TP. Actomyosin pulls to advance the nucleus in a migrating tissue cell. Biophys J. 2014;106:7–15. doi: 10.1016/j.bpj.2013.11.4489. This paper proposes a model based on experimental data in which actomyosin generated tension between the front and trailing edges of a migrating cell are transmitted through the nucleus and that force transmission to the nucleus is dependent upon an intact LINC complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PCDP, Athirasala A, Kao YRC, Cho S, Harada T, Shin JW, et al. Matrix elasticity regulates lamin-a,c phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. This paper identifies a tension-dependent regulatory circuit in which substrate rigidity couples to myosin-II contractility. In turn, this regulates lamin A/C phosphorylation which regulates myosin-IIA gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guilluy C, Burridge K. Nuclear mechanotransduction: Forcing the nucleus to respond. Nucleus. 2015;6:19–22. doi: 10.1080/19491034.2014.1001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan J, Beck KA. A role for the spectrin superfamily member Syne-1 and kinesin II in cytokinesis. J Cell Sci. 2004;117:619–629. doi: 10.1242/jcs.00892. [DOI] [PubMed] [Google Scholar]
- 45.Wilhelmsen K, Litjens SHM, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morimoto A, Shibuya H, Zhu X, Kim J, Ishiguro KI, Han M, Watanabe Y. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol. 2012;198:165–172. doi: 10.1083/jcb.201204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu W, Schneider M, Neumann S, Jaeger VM, Taranum S, Munck M, Cartwright S, Richardson C, Carthew J, Noh K, et al. Nesprin interchain associations control nuclear size. Cell Mol Life Sci. 2012;69:3493–3509. doi: 10.1007/s00018-012-1034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajgor D, Mellad JA, Autore F, Zhang Q, Shanahan CM. Multiple novel Nesprin-1 and Nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PLoS One. 2012;7:e40098. doi: 10.1371/journal.pone.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duong NT, Morris GE, Lam LT, Zhang Q. Nesprins: tissue-specific expression of epsilon and other short isoforms. PLoS One. 2014;9:e94380. doi: 10.1371/journal.pone.0094380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Banerjee I, Zhang J, Moore-Morris T, Pfeiffer E, Buchholz KS, Liu A, Ouyang K, Stroud MJ, Gerace L, Evans SM, et al. Targeted ablation of Nesprin 1 and Nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet. 2014;10:e1004114. doi: 10.1371/journal.pgen.1004114. This paper is the first to show nuclear morphology and gene expression defects in response to mechanical force in Nesprin 1 and 2 null cardiomyocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin tension exerted on the nucleus through Nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys J. 2010;99:115–123. doi: 10.1016/j.bpj.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan JT, Pfeiffer ER, Thirkill TL, Kumar P, Peng G, Fridolfsson HN, Douglas GC, Starr DA, Barakat AI. Nesprin-3 regulates endothelial cell morphology, perinuclear cytoskeletal architecture, and flow-induced polarization. Mol Biol Cell. 2011;22:4324–4334. doi: 10.1091/mbc.E11-04-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int J Biochem Cell Biol. 2010;42:1717–1728. doi: 10.1016/j.biocel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, Pestell R, Tozeren A, Byers SW. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 56**.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. This paper identifies TWIST in a mechanotransductive pathway that is distinct from YAP/TAZ. Increasing matrix stiffness promotes nuclear translocation of TWIST and transcription of genes that promote epithelial-mesenchymal transition and invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neumann S, Schneider M, Daugherty RL, Gottardi CJ, Eming SA, Beijer A, Noegel AA, Karakesisoglou I. Nesprin-2 interacts with α-catenin and regulates Wnt signaling at the nuclear envelope. J Biol Chem. 2010;285:34932–34938. doi: 10.1074/jbc.M110.119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gundersen GG, Worman HJ. Nuclear positioning. Cell. 2013;152:1376–1389. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- 61.Tsujikawa M, Omori Y, Biyanwila J, Malicki J. Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc Natl Acad Sci U S A. 2007;104:14819–14824. doi: 10.1073/pnas.0700178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- 63.Postel R, Ketema M, Kuikman I, de Pereda JM, Sonnenberg A. Nesprin-3 augments peripheral nuclear localization of intermediate filaments in zebrafish. J Cell Sci. 2011;124:755–764. doi: 10.1242/jcs.081174. [DOI] [PubMed] [Google Scholar]
- 64.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci U S A. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luxton GWG, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Nagayama K, Yahiro Y, Matsumoto T. Apical and basal stress fibers have different roles in mechanical regulation of the nucleus in smooth muscle cells cultured on a substrate. Cell Mol Bioeng. 2013;6:473–481. This paper shows different physical effects on the nucleus upon photo-ablation of apical versus basal stress-fibers, and suggests force transmission to the nucleus occurs predominantly through apical stress-fibers. [Google Scholar]
- 67**.Nagayama K, Yamazaki S, Yahiro Y, Matsumoto T. Estimation of the mechanical connection between apical stress fibers and the nucleus in vascular smooth muscle cells cultured on a substrate. J Biomech. 2014;47:1422–1429. doi: 10.1016/j.jbiomech.2014.01.042. This paper identifies two subtypes of apical stress-fibers over the nucleus and, through photo-ablation, finds tension is transmitted to the nucleus differently between the two. [DOI] [PubMed] [Google Scholar]
- 68**.Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science. 2014;345:1062–1065. doi: 10.1126/science.1256965. This paper is the first to show that actomyosin, Nesprin 3, and vimentin cooperatively move the nucleus like a piston during 3D migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery - Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 70**.Horn HF, Brownstein Z, Lenz DR, Shivatzki S, Dror AA, Dagan-Rosenfeld O, Friedman LM, Roux KJ, Kozlov S, Jeang KT, et al. The LINC complex is essential for hearing. J Clin Invest. 2013;123:740–750. doi: 10.1172/JCI66911. This paper is the first to identify a genetic mutation in Nesprin 4 that results in hereditary deafness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Göb E, Schmitt J, Benavente R, Alsheimer M. Mammalian Sperm Head Formation Involves Different Polarization of Two Novel LINC Complexes. PLoS One. 2010;5:e12072. doi: 10.1371/journal.pone.0012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yassine S, Escoffier J, Nahed RA, Pierre V, Karaouzene T, Ray PF, Arnoult C. Dynamics of Sun5 localization during spermatogenesis in wild type and Dpy19l2 knock-out mice indicates that Sun5 is not involved in acrosome attachment to the nuclear envelope. PLoS One. 2015;10:e0118698. doi: 10.1371/journal.pone.0118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Cain NE, Tapley EC, McDonald KL, Cain BM. The SUN protein UNC-84 is required only in force-bearing cells to maintain nuclear envelope architecture. J Cell Biol. 2014;206:163–172. doi: 10.1083/jcb.201405081. This paper shows that SUN1 is required for normal nuclear envelope morphology only in cells that experience high mechanical strain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–1047. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sosa BA, Kutay U, Schwartz TU. Structural insights into LINC complexes. Curr Opin Struct Biol. 2013;23:285–291. doi: 10.1016/j.sbi.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Zhao C, Brown RS, Chase AR, Eisele MR, Schlieker C. Regulation of Torsin ATPases by LAP1 and LULL1. Proc Natl Acad Sci U S A. 2013;110:E1545–1554. doi: 10.1073/pnas.1300676110. This paper shows that ATP-hydrolysis of TorsinA occurs upon association with nuclear envelope proteins, LAP1 and LULL1, providing another line of evidence that TorsinA may regulate the LINC complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vander Heyden AB, Naismith TV, Snapp EL, Hodzic D, Hanson PI. LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation. Mol Biol Cell. 2009;20:2661–2672. doi: 10.1091/mbc.E09-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79**.Uzer G, Thompson WR, Sen B, Xie Z, Yen SS, Miller S, Bas G, Styner M, Rubin CT, Judex S, et al. Cell mechanosensitivity to extremely low magnitude signals is enabled by a LINCed nucleus. Stem Cells. 2015;33:2063–2076. doi: 10.1002/stem.2004. This paper shows the LINC complex is necessary for activation of FAK and AKT signaling in response to extremely low-magnitude mechanical vibration but not for high-magnitude substrate strain in mesenchymal stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bone CR, Tapley EC, Gorjánácz M, Starr DA. The C. elegans SUN protein UNC-84 interacts with lamin to transfer forces from the cytoplasm to the nucleoskeleton during nuclear migration. Mol Biol Cell. 2014;25:2853–2865. doi: 10.1091/mbc.E14-05-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122:4099–4108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Starr DA, Hermann GJ, Malone CJ, Fixsen W, Priess JR, Horvitz HR, Han M. Unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development. 2001;128:5039–5050. doi: 10.1242/dev.128.24.5039. [DOI] [PubMed] [Google Scholar]
- 84.Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Stewart CL, Jeang KT. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell. 2012;149:565–577. doi: 10.1016/j.cell.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wheeler MA, Davies JD, Zhang Q, Emerson LJ, Hunt J, Shanahan CM, Ellis JA. Distinct functional domains in nesprin-1alpha and nesprin-2beta bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp Cell Res. 2007;313:2845–2857. doi: 10.1016/j.yexcr.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 87.Mislow JMK, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, McNally EM. Nesprin-1α self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- 88.Berk JM, Tifft KE, Wilson KL. The nuclear envelope LEM-domain protein emerin. Nucleus. 2013;4:298–314. doi: 10.4161/nucl.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bione S, Small K, Aksmanovic VM, D'Urso M, Ciccodicola A, Merlini L, Morandi L, Kress W, Yates JR, Warren ST. Identification of new mutations in the Emery-Dreifuss muscular dystrophy gene and evidence for genetic heterogeneity of the disease. Hum Mol Genet. 1995;4:1859–1863. doi: 10.1093/hmg/4.10.1859. [DOI] [PubMed] [Google Scholar]
- 90.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: Evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2004;2:e231. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91**.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. This paper shows that the impaired mechanosensitive transcription factor activity of MKL1 in laminA mutant cells can be rescued by ectopic expression of emerin, suggesting that the altered actin dynamics seen in laminA mutant cells can be recovered by the actin polymerization function of emerin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 93.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]