Abstract
Vitamin consumption prior to and during pregnancy has increased as a result of proactive recommendations by health professionals, wide availability of vitamin supplements, and liberal food-fortification policies. Folic acid, alone or in combination with other B vitamins, is the most recommended vitamin consumed during pregnancy because deficiency of this vitamin leads to birth defects in the infant. Folic acid and other B vitamins are also integral components of biochemical processes that are essential to the development of regulatory systems that control the ability of the offspring to adapt to the external environment. Although few human studies have investigated the lasting effects of high vitamin intakes during pregnancy, animal models have shown that excess vitamin supplementation during gestation is associated with negative metabolic effects in both the mothers and their offspring. This research from animal models, combined with the recognition that epigenetic regulation of gene expression is plastic, provides evidence for further examination of these relationships in the later life of pregnant women and their children.
Keywords: folic acid, methyl vitamins, postpartum obesity, pregnancy, vitamins.
INTRODUCTION
Increased consumption of energy-dense foods in today’s obesogenic environment is a significant contributor to the increasing prevalence of obesity.1 Additionally, other dietary patterns have emerged concomitantly with obesity trends in recent decades. Intakes of vitamins in developed countries, particularly in women, continue to increase and may even exceed daily intake recommendations.2–4 In the United States, more than 50% of nonpregnant women consume micronutrient supplements,3 and between 70% and 90% of pregnant women in the United States and Canada report consumption of a multivitamin and/or an additional single-nutrient supplement containing folic acid (vitamin B9).5,6
Methyl-group vitamins, such as folic acid, are active regulators in one-carbon metabolism, and increased consumption of these micronutrients has been proven an effective strategy for reducing adverse pregnancy outcomes, such as neural tube defects, in the offspring.7 Thus, prenatal supplements are widely recommended before and during pregnancy because they are formatted specifically to meet the nutrient requirements of the mother and developing fetus, with particular emphasis on folic acid.8 Folic acid amounts in prenatal supplements may range between 400 µg and 2000 µg, providing up to or beyond the recommended daily allowance (RDA) of 400 µg/d during pregnancy. Women in compliance with these recommendations have also reported better dietary quality, including higher intakes of nutrient-dense and/or folate-rich foods.5 Concomitant use of supplements during pregnancy, in addition to intake from fortified foods, may result in total intakes of some micronutrients that exceed recommendations. Although the negative impact of maternal vitamin deficiency is well understood, the effects of excess but nontoxic consumption of vitamins, specifically methyl vitamins, on metabolic outcomes have not been well described. Moreover, even less is known about the lasting effects of high intakes of vitamins during pregnancy on the later weight and metabolic trajectory of the mother.
This review examines the potential impact of vitamins consumed in excess of recommendations during pregnancy on the offspring and mother. The first sections provide an overview of postpartum obesity and the importance and prevalence of micronutrient intake during pregnancy, with particular emphasis on the functional role of methyl-group vitamins. This is followed by a section describing the potential mechanisms by which maternal vitamin consumption may impact phenotype later in life. The final section addresses the limited amount of human studies along with the more extensive rodent studies showing the effects of excess maternal vitamin intakes on the weight trajectory of the mother and/or offspring later in life.
PREGNANCY AND OBESITY
Global obesity rates have risen dramatically over the last 3 decades, with the number of overweight and obese individuals reaching 2.1 billion in 2013.9 In both developed and developing countries, the prevalence of obesity remains greater in women than in men, and the most rapid weight gain occurs between the ages of 20 and 40 years. These findings are consistent with data analyzed between 1976 and 2008 from the National Health and Nutrition Examination Survey (NHANES) and the Canadian Community Health Survey that showed a substantial increase in the prevalence of obesity in women of reproductive age.10,11 Undoubtedly, the traditional view that childbearing may have long-term consequences on the weight trajectory of the mother is in line with these statistics. The link between pregnancy and obesity was recognized as early as 1862, and in the late 1940s pregnancy was included as a potential endocrinological contributor to the development of obesity.12 However, while excess weight after childbirth has long concerned women in relation to body image, the association between weight retention and adverse health later in life has been given less attention.
Postpartum weight retention is calculated as the difference between weight gain during gestation, weight loss from delivery to 6 weeks post partum, and weight gain from 6 weeks to 1 year post partum. Although the average weight retained during the early postpartum period is small, the variability between women is large, ranging from −12.3 to +26.5 kg.13 In several studies, weight retentions reported are between 3 and 7 kg at 6 weeks post partum, and nearly 25% of women are more than 5 kg heavier than their preconception weight by 1 year post partum.14,15 Women who are unable to return to their preconception weight by the 1 year mark have an increased risk for an adverse cardiometabolic profile, such as increased blood pressure and cholesterol as well as disturbed glucose regulation predisposing them to obesity, diabetes, and cardiovascular disease at an earlier age.16 In addition, greater postpartum weight retention may limit the success of future pregnancies, as pregravid obesity is a high-risk obstetric condition associated with several negative maternal and neonatal outcomes.17
Gestational weight gain is one of the strongest determinates of postpartum weight retention independent of age and ethnicity.18 In healthy weight women, total weight gain recommendations set by the Institute of Medicine during pregnancy range between 12.2 and 18.1 kg (25–35 lbs). Weight gain during pregnancy is attributed to the products of conception (≈35% of total gain), comprising the fetus, placenta, and amniotic fluid, as well as maternal tissue accretion (≈65% of total gain), comprising increased blood volume, uterus, extracellular fluid, and fat stores.19 Fat contributes approximately 30% of the total gestational weight gain and is strongly influenced by environmental factors and lifestyle preferences.15
Diet modification, breastfeeding duration, and physical activity have been the focus of intervention studies to help curb postpartum weight retention. Breastfeeding for the recommended duration and intensity (exclusively for 6 months and up to 12 months) helps facilitate postpartum weight loss and is founded on the premise that more energy is needed to produce more milk.20 Likewise, consumption of nutrient-rich foods low in energy density, along with higher levels of physical activity, is positively associated with lower postpartum weight retention and greater weight loss.21,22 Therefore, it is important to continue to identify risk factors and the related mechanisms of actions predisposing women during the postpartum period to obesity to provide stronger interventions and/or recommendations for a healthy pregnancy and positive long-term outcomes.
MATERNAL NUTRITION AND OFFSPRING HEALTH
Nutritional needs are increased during pregnancy to support fetal growth and adaptations in maternal metabolism.23 Maternal undernutrition, caused by insufficient intakes from macronutrients and/or micronutrients, commonly exist in women from developing nations and is associated with several negative physiological outcomes in the mother and child.24 Maternal nutrient deficiencies increase the risk of preterm delivery, postpartum hemorrhage and anemia, developmental birth defects, and maternal and infant death.25–27 In contrast to the lack of food energy and nutrients available in developing nations, the overall abundance of food energy in developed countries is of concern. For example, maternal obesity is associated with consumption of a high-fat diet during pregnancy and increased susceptibility to maternal complications such as gestational diabetes28 and preeclampsia,29 placing the mother and fetus at risk of unfavorable pregnancy outcomes.
Modifications of the nutritional environment of the mother and fetus can also greatly impact the long-term health trajectory of the child.30 This phenomenon, known as “early life programming,” refers to the process whereby changes in the intrauterine environment at any critical time during fetal development determine the pathologies into adulthood. Experimental animal models of programming have shown a range of nutritional modifications during pregnancy or the early postnatal period to lead to dynamic changes in the epigenome of the offspring and the expression of genes involved in energy homeostatic pathways. As an example, overconsumption of a high-fat diet during pregnancy in rats is associated with permanent alterations within central homeostatic (i.e., hypothalamic) systems that lead to obesity later in the life of the offspring.31 A maternal high-fat diet can also alter methylation of dopamine and opioid genes32 involved in reward-seeking behavior and the implicit (unconscious) “wanting” for food.33 Dysfunction of central intake systems may result in a shift of food preference in the offspring toward energy-dense, palatable choices, contributing to increased adiposity and risk of metabolic diseases later in life.34
In addition to studies of macronutrient status, more recent animal research and, to a lesser extent, studies in humans have been directed toward the effects of changes in maternal micronutrient status and its effect on developmental programming of the offspring.35–39 This shift is justified, as several micronutrients are intimately involved in the metabolic network regulating epigenetic mechanisms such as DNA methylation and subsequent alterations in gene expression.
VITAMIN RECOMMENDATIONS FOR PREGNANT WOMEN: EMPHASIS ON FOLIC ACID
Micronutrient status during pregnancy is well known to affect the development and metabolism of the fetus. As a result, emphasis is placed on ensuring that nutritional needs are met prior to and during pregnancy. The RDAs and tolerable upper limits (highest level of nutrient dose with no likely risks) established by the Institute of Medicine for vitamins for adolescent girls, nonpregnant and nonlactating women, and pregnant women are shown in Table 1. The most substantial increase in requirement is that of the water-soluble B vitamin folate (vitamin B9). The success of folic acid supplementation during pregnancy for treating megaloblastic anemia has been recognized since the classic work of Wills40 in 1931. Folic acid supplementation during the periconceptional period (≈1 month prior and after conception) and maintained throughout pregnancy dramatically reduced the incidence of neural tube defects,41 and mandatory folic acid fortification of cereal products since1998 has further contributed to a reduced prevalence of neural tube defects by up to 50%.7
Table 1.
Recommended dietary allowance and tolerable upper limit values of vitamins for females of reproductive age
| Life stage | Recommended dietary allowancea (upper limitb) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin Ac, µg/d | Vitamin C, mg/d | Vitamin Dd,e, mg/d | Vitamin Ef, mg/d | Vitamin K, mg/d | Thiamin [B1], mg/d | Riboflavin [B2], mg/d | Niacing [B3], mg/d | Pantothenic acid [B5], mg/d | Vitamin B6, mg/d | Folateh [B9], µg/d7 | Vitamin B12, mg/d | Biotin, mg/d | Choline, mg/d | |
| 14–18 y | 700 (2800) | 65 (1800) | 15 (100) | 15 (800) | 75j (ND) | 1.0 (ND) | 1.0 (ND) | 14 (30) | 5j (ND) | 1.2 (80) | 400 (800) | 2.4 (ND) | 25j (ND) | 400j (ND) |
| 19–30 y | 700 (3000) | 75 (2000) | 15 (100) | 15 (1000) | 90j (ND) | 1.1 (ND) | 1.1 (ND) | 14 (35) | 5j (ND) | 1.3 (100) | 400 (1000) | 2.4 (ND) | 30j (ND) | 425j (ND) |
| 31–50 y | 700 (3000) | 75 (2000) | 15 (100) | 15 (1000) | 90j (ND) | 1.1 (ND) | 1.1 (ND) | 14 (35) | 5j (ND) | 1.3 (100) | 400 (1000) | 2.4 (ND) | 30j (ND) | 425j (ND) |
| Pregnancy | 750 (3000) | 85 (2000) | 15 (100) | 15 (1000) | 90j (ND) | 1.4 (ND) | 1.4 (ND) | 18 (35) | 6j (ND) | 1.9 (100) | 600 (1000) | 26 (ND) | 30j (ND) | 450j (ND) |
| Lactation | 1200 (3000) | 120 (2000) | 15 (100) | 15 (1000) | 90j (ND) | 1.4 (ND) | 1.6 (ND) | 17 (35) | 7j (ND) | 2.0 (100) | 500 (1000) | 2.8 (ND) | 35j (ND) | 550j (ND) |
Abbreviation: ND, not determined.
aRepresents the recommended average daily dietary intake level that is sufficient to meet the nutrient requirement of nearly all (97%–98%) healthy individuals in a particular life stage and gender group.
bRepresents the highest level of intake that is likely to pose no risk of adverse health to almost all individuals in the general population. Where upper limit is shown as not determined (ND), there are insufficient data to establish an upper limit, but this does not indicate no potential adverse effects resulting from high intakes.
cAs retinol activity equivalents (RAEs). 1 REA = 1 µg of retinol.
dAs cholecalciferol. 1 µg of cholecalciferol = 40 IU of vitamin D.
eReference values assume minimal sunlight.
fAs α-tocopherol.
gAs niacin equivalents. 1 mg of niacin = 60 mg of tryptophan.
hAs dietary folate equivalents (DFEs): 1 DFE = 1 µg of food folate = 0.6 µg of folic acid as food or supplement = 0.5 µg of supplement taken on an empty stomach.
iUpper limit based on folic acid (synthetic folate) from supplements and/or fortified foods.
jRepresents adequate intakes based on estimates of nutrient intake by a group.
To build and preserve maternal stores and meet the needs of rapidly growing tissues, public health professionals recommend that all women of childbearing age under good compliance with their dietary recommendations receive a minimum of 400 µg of synthetic folic acid each day in addition to dietary folates (naturally occurring folates) obtained from a well-balanced diet or consumed as part of a multivitamin supplement.8 The folic acid content of prenatal supplements ranges between 400 and 2000 µg, and women at risk of deficiency or poor compliance are advised by the Canadian Society of Obstetricians and Gynecologists to consume up to 12.5-fold (5000 µg/d) the RDA of folic acid for optimal protection against neural tube defects.42–44 These recommendations can be met by increasing the intakes of fortified foods and/or taking a multivitamin or a single-nutrient supplement. In addition to folic acid, other B vitamins, such as vitamin B12 (cobalamin) and B6 (pyridoxine), have an important role in supporting maternal and fetal health during pregnancy.26,45–48 Because a combination of B vitamins may better serve to reduce the incidence of birth defects,46,49 vitamins B12 and B6, as a complex or as single-nutrient supplements, are also recommended in higher quantities during this time.
VITAMIN INTAKE DURING PREGNANCY
Although the aim of most research in this area has been to establish adequate intakes during pregnancy, women of childbearing age may be at higher risk of overconsumption of vitamins. Higher intakes of vitamins can be attributed to increased recommendations for vitamin supplementation, consumer belief about the health benefits of vitamins, and the widespread implementation of food-fortification programs.50,51 However, the lasting effects of excess vitamin intakes during human pregnancy on the long-term health of the mother and child have not been elucidated.
The prevalence of vitamin use has increased substantially in the general population within the last 3 decades, with higher usage in women than in men. The proportion of adult women in the United States who reported using dietary supplements increased from 38% in 1971 to 57% in 2000, according to the NHANES I–III.3 In Canada, results from the Canadian Community Health Survey Cycle 2.2 showed that more than 40% of women consume multivitamin supplements52 and 27% of women between the age 19 and 50 years consume an additional folic acid, vitamin B12, or vitamin B6 supplement.53 Several surveys have also shown increased prevalence of vitamin use in women who are pregnant. The National Maternal and Infant Health Survey from 1988 reported that, of the 97% of mothers who had been advised to consume a prenatal vitamin supplement, 83% reported adherence to recommendations during pregnancy.54 In 2000, a survey based on the Pregnancy Risk Assessment Monitoring System further found that up to 41% of women in 19 states consumed a multivitamin supplement more than 4 times per week.55 More recently, data from NHANES 1999–2006 showed an average of 74% of pregnant women consume a multivitamin or multimineral supplement containing folic acid.5 This is also consistent with data from the Behavioral Risk Factor Surveillance System in 2001, which reported 78% of pregnant women to regularly consume multivitamins.51 Epidemiological studies in Canada addressing the prevalence of vitamin use in pregnant women are limited; however, more than 90% of women participating in the first cohort of the Alberta Pregnancy Outcome and Nutrition study in 2012 were found to use a multivitamin or multimineral supplement, and nearly half consumed an additional single-nutrient supplement.6
As a result of the emphasis on increasing vitamin intake during pregnancy, average maternal vitamin intake recommendations in developed countries are not only being met, but also seemingly exceeded. Pregnant women under adequate medical care consume 2.5- to 7-fold the RDA of several micronutrients, including folic acid and vitamins B12 and B6, and even consume beyond the upper limit for folic acid (>1000 µg/d).56,57 In Canada, red blood cell folate deficiency is relatively nonexistent, with 40% of the population showing high blood folate concentrations (>1360 nmol/L) and 78% of women of childbearing age showing levels optimal for reducing the risk of neural tube defects (>900 nmol/L).4 In the Alberta Pregnancy Outcome and Nutrition study, over half of the pregnant women in the first cohort had high levels of red blood cell folate, and less than 1% exhibited vitamin B12 or B6 deficiencies.58 Sufficient vitamin B12 levels (≥148 pmol/L) are also reported in 95% of Canadian men and nonpregnant women,59 and the mean serum B12 status of pregnant women aged 20–39 years in the United States (>300 pmol/L) is considered adequate.60 Vitamin B6 is also consumed in amounts well over adequate (>1.3 mg) by 90% of Canadians aged 19–30 years,61 and in males and females aged 13–54 years from the NHANES III, more than 60% had optimal plasma pyridoxal 5′-phosphate (the biologically active form of vitamin B6) levels.62
While maternal vitamin supplementation has ameliorated negative pregnancy outcomes and birth complications, the increased popularity and use of vitamin supplements has led to concerns about the possible long-term health risks associated with higher intakes. In human studies, high but nontoxic intakes of folic acid have been associated with several types of cancers, including colorectal and prostate cancer in men63,64 and breast cancer in women.65 Thus, there has been concern about folic acid’s possible dual role in carcinogenesis, by which supplementation may inhibit the origination and growth of early tumors but accelerate the progression of preexisting tumors.63
Increasing rates of obesity also reflect the time sequence of food fortification with these vitamins.66 Increased intakes of vitamins, and specifically methyl vitamins, including thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), vitamins B12 and B6, and folic acid have therefore been suggested to be associated with the increased prevalence of weight gain and fat mass in children and adults.66,67 Although this suggestion is based on associations from survey data, the role of vitamins, especially those involved in one-carbon metabolism, add plausibility to the hypothesis.
POTENTIAL MECHANISM OF B VITAMIN−INDUCED OBESITY
Vitamins and one-carbon metabolism
The one-carbon cycle is a metabolic network of interdependent pathways by which one-carbon moieties are transferred to modify numerous biochemical systems, including neurotransmitter synthesis, DNA biosynthesis, gene expression, and metabolism.48,68 The vitamins folate, methionine, choline, riboflavin, and B12 and B6 are involved in one-carbon metabolism and play a central role in the regulation of cell function in all tissues of the body. Folate refers to the various tetrahydrofolate derivatives that naturally occur in food. In contrast, folic acid is the completely oxidized compound used in dietary supplements and food fortification that is not naturally occurring and is more bioactive than folate. As shown in Figure 1A, folic acid enters the one-carbon cycle and is reduced to dihydrofolate and then tetrahydrofolate to form 5,10-methylenetetrahydrofolate. 5,10-Methylenetetrahydrofolate is further reduced to 5-methyltetrahydrofolate (5-MTHF) by methylenetetrahydrofolate reductase in the presence of riboflavin. 5-MTHF is the usable and biologically active form of folate, which can pass through the blood–brain barrier.69 Methylenetetrahydrofolate is also involved in nucleotide synthesis via the conversion of methylenetetrahydrofolate to dihydrofolate by thymidylate synthase–catalyzed conversion of deoxyuridine-5-monophosphate to deoxythimidine-5-monophosphate. 5-Methylenetetrahydrofolate provides the methyl moiety for the conversion of homocysteine to methionine in a reaction catalyzed by a B12–containing methyltransferase. Methionine is then adenosylated to S-adenosylmethionine (SAM), which is the universal methyl donor for methylation reactions that include the methylation of nucleic acids, proteins, phospholipids, and neurotransmitters. After donation of its methyl group, SAM is converted back to homocysteine, which is either metabolized to become the amino acid cysteine via a B6-dependent process, or, through the conversion of choline to betaine, is remethylated for the synthesis of methionine. Thus, B vitamins are critical for proper functioning of the one-carbon cycle. Moreover, because of their role in DNA methylation, their availability is an important determinant of epigenetic regulation of gene expression in several physiological systems.
Figure 1.
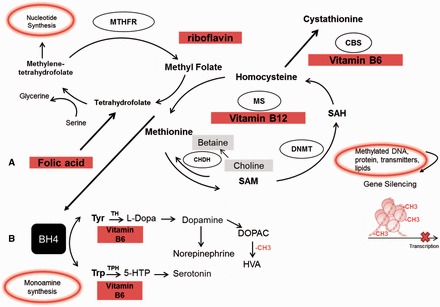
Simplified diagram of methyl vitamins and enzymes involved in (A) one-carbon metabolism and (B) monoamine synthesis. Abbreviations: BH4, tetrahydrobiopterin; CBS, cystathionine β-synthase; CH3, methyl group; CHDH, choline dehydrogenase; DNMT, DNA methyltransferase; DOPAC, 3,4-dihydroxyphenylacetic acid; 5-HTP, 5-hydrooxytryptophan; HVA, homovanillic acid; L-DOPA, L-3,4-dihydroxyphenylalanine; TH, tyrosine-3-hydroxylase; MS, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; SAM, S-adenosylmethionine; TPH, tryptophan-5-hydroxylase; Tyr, tyrosine; Trp, tryptophan.
Epigenetics and DNA methylation
DNA methylation is one of many epigenetic modifications that induce heritable and plastic changes in gene expression as a consequence of environmental influences without alteration in DNA sequence.70 DNA methylation involves the addition of a methyl group (–CH3) to a cytosine at the 5′ position of a cytosine-phosphate-guanine dinucleotide. Regions rich in cytosine-phosphate-guanine are located proximal to the promoter region of a gene, and hypermethylation influences gene silencing primarily by inhibiting the binding of transcription factors.71 Although epigenetic changes are critical to the maintenance and support of cellular development and function,72 epigenetic dysregulation may underlie several diseases in humans, including obesity, diabetes, cardiovascular disease, and neurocognitive impairments.73–75
One of the most well-known birth cohort studies linking early-life nutrition to epigenetic variation and long-term outcome is from the Dutch Famine Cohort (1944–1945). Within this cohort, children who had been prenatally exposed to famine exhibited widespread epigenetic effects,76 including lower DNA methylation of the insulin-like growth factor II gene (an essential gene in regulating growth and development) compared with that in their unexposed, same-sex counterparts. Similarly, in rural Gambia, seasonal variation in nutritional status in pregnant women also affected DNA methylation patterns in neonates.77 Infants born to mothers who conceived during the nutrient-dense rainy season rather than the dry season had higher rates of DNA methylation that were consistent with the nutritional status of the mother. In addition to findings from human studies, a plethora of evidence from animal models has shown that aberrant DNA methylation patterns due to under- or overnutrition in early life modifies expression of critical genes within homeostatic,78–80 nonhomeostatic,32,81 and peripheral82–84 regulatory systems, thereby contributing to long-term negative consequence in metabolic health.
Because folic acid and vitamins B12, and B6 provide enzymatic support for methylation reactions through the one-carbon cycle, excess intakes of dietary methyl donors may result in hypermethylation or, in some cases, hypomethylation of regulatory genes involved in energy homeostasis, thus contributing to negative metabolic effects. The above-recommended intakes of folic acid, for example, may lead to preferential shuttling of one-carbon moieties toward nucleoid synthesis pathways,85 reducing methylation reactions and contributing to dysregulation of energy homeostatic pathways. Furthermore, excess intakes of folic acid increases dihydrofolate, which directly inhibits the production of 5-MTHF and reduces the conversion of homocysteine to methionine, contributing to DNA hypomethylation.86 Imbalances between methyl vitamins may also affect methylation patterns.87 High folic acid intakes may exacerbate inadequacies in methionine synthesis in those with B12 deficiency, further altering the availability of SAM and reducing methylation reactions.
EFFECTS OF B VITAMINS ON FOOD INTAKE REGULATION AND ADIPOSITY
During development in utero, methylation of DNA is a determinant of the function of neuronal pathways regulating physiology, metabolism, and behavior. B vitamins consumed in excess during pregnancy have the potential to contribute to obesity by affecting the development of homeostatic and hedonic food intake regulatory pathways and may also affect fat deposition in the offspring. High methyl vitamin intakes in rats leads to DNA methylation in the hypothalamus that is consistent with changes in gene expression of feeding-related neuropeptides to favor increased food intake, weight gain, and other metabolic disturbances in the offspring.37 Several neurotransmitters, such as dopamine, are also involved in hedonic and homeostatic food intake regulation,33,88 and determinants of neurotransmitter synthesis and activity may affect feeding behavior and body weight homeostasis. For example, in addition to its direct involvement in methylation reactions, 5-MTHF regulates the biosynthesis of the critical cofactor tetrahydrobiopterin, which is required by the rate-limiting enzymes tyrosine hydroxylasenecessary for the synthesis of dopamine and norepinephrine, and tryptophan hydroxylase essential for the synthesis of serotonin68 (Figure 1B). High intakes of 5-MTHF or its precursors (folic acid or folate) enhance dopamine, norepinephrine, and serotonin synthesis via the tetrahydrobiopterin pathway and in turn may affect appetite and body weight homeostasis. Prolonged overproduction of tetrahydrobiopterin, however has been suggested to attenuate neurotransmitter synthesis via the direct and/or indirect generation of oxidative stress89 and could potentially contribute to hypofunctioning of neurotransmitter systems associated with obesity. Vitamin B6 is an independent cofactor for aromatic L-amino acid decarboxylase, an enzyme that catalyzes the reactions of serotonin and dopamine.90,91 Because excess intakes of vitamin B6 enhance dopamine production, they may also modulate dopamine-related components of feeding, including the “wanting” of palatable foods. Furthermore, SAM is critical for the methylation-mediated degradation and, consequently, the turnover rates and concentrations of serotonin, dopamine, and norepinephrine.92 The modulatory effects of excess methyl vitamins on SAM production (either methyl vitamin–enhanced or –reduced SAM biosynthesis) could also negatively affect reinforcing behaviors such as feeding and lead to excess food intake and energy imbalance.
Adiposity may be increased as well by altered regulatory systems in response to high methyl vitamin intake, which favors fat deposition and weight gain. Early rodent studies in the 1930s reported that a combination of B vitamins, including thiamine (12.5 µg), riboflavin (10 µg), choline (1.3 mg), and rice polish concentrate (used as a source of vitamin B6, 300 mg), supplemented in diets enhanced body fat deposition independent of appetite.93 Several subsequent animal studies confirmed the “fat promoting” effects of these vitamins. Although differences exist in the design and execution of these experiments, collectively they provided early support of the role of B vitamins in lipid metabolism. In rats, dietary supplementation with excess vitamin B6 (40 µg) and thiamine (0.1 mg) prevented weight loss, and the combination of thiamine (20 µg), riboflavin (20 µg), pantothenic acid (vitamin B5, 100 µg), and vitamin B6 (40 µg) resulted in a 30% gain of body fat.94 Vitamin B6 alone was also shown to have potent lipotropic effects, as animals receiving B6-deficient diets for 4 weeks suffered weight loss, and those supplemented with B6 (25 µg, positive controls) showed a mean fat gain of up to 12 g.95 In one study, rats were fed either low-fat or high-fat diets with no B6 (B6-deficient group) or with 40 µg of B6 (B6-supplemented group). Those supplemented with B6 had a 3-fold increase in body lipids and net weight gains compared with the B6-deficient group.96
More recent cell culture experiments conducted in 3T3-L1 cells (a cell line derived from mice used to study the differentiation of adipocytes) are consistent with these data. Vitamin B6, along with other water soluble-vitamins, was found to potently stimulate the differentiation of adipocytes and to regulate triglyceride accumulation.97 Vitamin B6 was also shown to directly regulate ligand-dependent corepressors of peroxisome proliferator-activator receptor-γ (PPAR-γ), a master regulator of adipocyte proliferation and differentiation.98 Furthermore, administration of pyridoxal-5′-phosphate (the active form of vitamin B6) independently upregulates the activity of PPAR-γ, similar to the antidiabetic drug thiazolidinedione, thereby promoting adipogenesis.99 Together, these data hint at a plausible link between micronutrients, higher body weight, and adiposity in the human population.
MATERNAL VITAMIN INTAKE AND OBESITY: EVIDENCE FROM HUMAN STUDIES
Few studies in humans have evaluated the relationship between high maternal intakes of vitamins, specifically methyl vitamins, and weight gain in the offspring, and even fewer have examined the effects of high intakes of vitamins on the postpartum maternal phenotype. The limited body of evidence pertaining to vitamin-induced weight gain in the mother–offspring dyad or in the offspring alone is described below and summarized in Table 2.38,100,104–108
Table 2.
Summary of human studies investigating the effects of vitamins on weight gain in mother–offspring dyads or in offspring alone
| Reference | Study design | Maternal exposure | Exposure | Effect on mother | Effect on offspring |
|---|---|---|---|---|---|
| Yajnik et al. (2008)38 |
|
Maternal micronutrient status collected at 18 and 28 weeks of gestation | N/A | N/A |
|
| Mardones-Santander et al. (1988)100 |
|
|
35 weeks of gestation |
|
Fortified products:↑ mean birth weight |
| Changamire et al. (2012) 104 |
|
|
|
|
|
| Asemi et al. (2014)105 |
|
|
20 wk total; daily starting at wk 16 of gestation | Multivitamin supplement:↑ mean GWG at wk 28 and at delivery | Multivitamin/mineral supplement:↑ mean birth weight and head circumference |
| Vaidya et al. (2008)106 |
|
|
Second and third trimesters of pregnancy | N/A |
|
| Stewart et al. (2011)107 |
|
|
Early pregnancy to 3 mo post partum | N/A |
|
| Dougan et al. (2015)108 |
|
Self-recall of prenatal vitamin intake | Regular intake during pregnancy | N/A | No effect on body weight or waist circumference of nurses’ daughters during childhood or early adulthood |
Abbreviations and symbols: GWG, gestational weight gain; HIV, human immunodeficiency virus; N/A, not applicable; NHS, Nurses’ Health Study; RCT, randomized controlled trial; ↑, increased; ↓, decreased.
In developing countries, greater gestational weight gain induced by vitamin supplementation may be beneficial, as it consistently lowers the risk for low birth weight and neonatal death, and correction of deficiency in the mother may also help to re-establish a healthy increase in body weight. However, in women from North America, where higher gestational weight gain and overall energy abundance are significant predisposing factors for obesity,14 vitamin-induced gestational weight gain may be of potential concern for the long-term health of the mother or child. Five of the studies described are randomized control trials in developing countries in which women were supplemented with either the recommended amounts or higher amounts of vitamins during pregnancy, and two are prospective cohort studies that followed children born to mothers in either a developing or a developed country who consumed vitamins during pregnancy.
In Chile, low-income, underweight pregnant women who received a milk-based product fortified with thiamine, niacin, vitamin B6, folic acid, and vitamins A, C, and E had greater gestational weight gain, but no changes in postpartum body weight, compared with those receiving a nonfortified milk product.100 Similarly, mean birth weight of offspring from mothers fed the fortified milk-based product was greater than that of offspring from mothers fed the nonfortified product. In infants, formula feeding is associated with an increased risk of obesity101,109 but does not negatively affect energy expenditure.102,103 These results suggest that added vitamins, rather than decreased energy expenditure, may have a primary role in the effects of enriched milk-based products on weight gain and body fat changes during this time. In a placebo-controlled randomized controlled trial conducted in HIV-negative women from Tanzania, micronutrients supplemented at more than 6-fold the RDA, including folic acid, thiamine, niacin, and vitamins B6, C, and E, resulted in mean increases in gestational weight gain and higher birth weight of the offspring.104 Another study in Iran compared adequate intakes of a multivitamin with a combination of multivitamins and multiminerals in primigravid women aged 18–35 years. This study showed that adequate intakes of multivitamins alone increased both gestational weight gain at 28 weeks and maternal weight at delivery more than the multivitamin/multimineral supplement combination.105 In contrast, newborn mean birth weight was higher in offspring born to mothers who received the multivitamin/multimineral combination, suggesting that the in utero effects of the maternal diet may differentially affect outcomes in the mother vs offspring. Consistent with these results, a randomized controlled trial in Nepal found that supplementation with a multivitamin/multimineral mix containing the RDA of 15 vitamins and minerals led to children with higher body weight and body size at 2.5 years of age.106 Unfortunately, maternal anthropometric measures were not available for comparison. Imbalances between vitamins may also have negative effects on long-term health. In the Pune Maternal Nutrition Study in India, children 6 years of age who were born to mothers with high folate and low vitamin B12 concentrations at 28 weeks of gestation had higher adiposity and measures of insulin resistance.38 In contrast, a subanalysis from a randomized controlled trial in Nepal showed that, although vitamin B12 deficiency during pregnancy was positively associated with risk of insulin resistance in the child, the association was not dependent on folate or other micronutrient status.107
At present, only one human study has reported the potential effects of vitamin intakes during pregnancy on weight gain in the offspring in an affluent setting. A prospective cohort study using mother–daughter dyads from the Nurses’ Health Study II108 found regular prenatal vitamin consumption to have no effect on body weight or waist circumference of daughters throughout the different life stages. Because information on the quantity, quality, and timing of specific supplements used during pregnancy was not assessed, these results may underestimate the effects of higher intakes of vitamins during pregnancy on long-term outcome. Furthermore, data on the later-life phenotype of the mother was not collected, leaving unclear the effect of vitamins on the maternal weight trajectory.
ANIMAL EXPERIMENTS
Although evidence from human studies is lacking, several vitamin supplementation trials using rodent models show vitamins to be an important determinant in the developmental programming of obesity in the offspring and a modifier of the long-term health of the mother. These studies also show that epigenetic mechanisms mediate plasticity of central food intake regulatory systems and that in utero programming by vitamins can be modified by nutritional challenges post weaning. Likewise, the macronutrient composition of the maternal diet during pregnancy (i.e., balanced, high-fat, or high-protein) can also modify the long-term effects of vitamins consumed during this time. Table 3 35–37,110–113,115–120 presents a summary of the evidence from animal studies lending support to the link between vitamins and obesity, as described below.
Table 3.
Summary of animal studies investigating the effects of vitamins on weight gain in mother−offspring dyads or in offspring alone
| Reference | Model | Maternal exposure | Duration of exposure | Postweaning exposure | Results |
|---|---|---|---|---|---|
| Szeto et al. (2008)35 | Wistar rat |
|
Pregnancy |
|
|
| Cho et al. (2013)36 | Wistar rat |
|
Pregnancy |
|
High-folic-acid gestational diet and recommended pup diet: ↑ body weight, 72-h food intake, glucose response to insulin load, and Pomc DNA methylation and ↓ expression of Pomc and 5-Htr 2A genes in hypothalamus |
| Cho et al. (2015)37 | Wistar rat |
|
Pregnancy |
|
|
| Szeto et al. (2009)110 | Wistar rat |
|
Pregnancy |
|
|
| Reza-López et al. (2009)112 | Wistar rat |
|
Pregnancy |
|
|
| Reza-López et al. (2013)113 | Wistar rat |
|
Pregnancy |
|
|
| Szeto et al. (2010)111 | Wistar rat |
|
Pregnancy |
|
Maternal multivitamin: ↓ body weight, food intake, fat mass, protein intake after fasting, serotonergic sensitivity in hypothalamus, and expression of 5-Htr 1A/2A/2C and Pomc mRNA in hypothalamus (weaning) |
| Cho et al. (2013)115 | Wistar rat | High (10-fold) multivitamins | Pregnancy |
|
|
| Huang al. (2014)116 | Mouse C57BL/6J |
|
Pregnancy |
|
|
| Lillycrop et al. (2005)117 | Wistar rat |
|
Pregnancy |
|
|
| Carlin et al. (2013)118 | Mouse C57BL/6J/DBA/2J FI hybrids |
|
Pregnancy and lactation |
|
|
| Reza-López et al. (2011)119 | Wistar rat |
|
Pregnancy |
|
|
| Pannia et al. (2014)120 | Wistar rat |
|
Pregnancy |
|
|
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; F, female;; M, male; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PW, post weaning; RV, recommended vitamin; SBP, systolic blood pressure; . ↑, increased; ↓, decreased.
Wistar rats are an outbred strain and are relevant to obesity in humans because of the multifactorial nature of the disease. In this model, feeding pregnant Wistar rats a high multivitamin (HV) diet containing 10-fold the amounts of the recommended multivitamin (RV; via the AIN-93G diet) content and then an RV diet post weaning produced male, but not female, offspring with higher measures of food intake, body weight, visceral adiposity, and other components of the metabolic syndrome35 than those found in offspring from RV-fed dams. The absence of effect of the HV gestational diet on female offspring when fed an RV diet post weaning may be related to the higher growth rate and greater susceptibility of male offspring to nutritional changes in utero. In addition, the interaction between the HV gestational diet and the postnatal nutritional environment is an important determinant of the development and programming of central intake regulatory systems in the offspring. For example, even though the effects of the HV diet were exacerbated in male offspring, when male and female offspring from HV-fed dams were weaned to an obesogenic diet,110 female offspring exhibited higher food intake and developed obesity more quickly than their male siblings. In contrast, in female offspring born to mothers fed an HV diet and then switched to a high-casein diet post weaning, lower measures of body weight, food intake, macronutrient (protein) selection, serotonin, and expression of the pro-opiomelanocortin gene, as well as greater sensitivity in the hypothalamus, were observed compared with controls.111 The HV gestational diet also led to changes in fatty acid concentrations,112 glucose metabolism, and expression of the peroxisome proliferator-activated receptor (Ppar) gene in the tissue of offspring; these changes were dependent on the sex, age, and diet of the pups post weaning,113 lending support to the influence of pre- and postnatal nutrition in mediating offspring health.
The vitamins most likely responsible for the effects of the HV diet in Wistar rat offspring are those involved in methyl metabolism. Supplementing the gestational diet with HV or high folic acid produced offspring with an obesogenic phenotype compared with offspring from RV-fed dams.36 High intakes of a group of methyl vitamins (i.e., 10-fold the RDA of vitamins B12, B6, and folic acid) during pregnancy, similar to the HV diet, resulted in an obesogenic phenotype and alterations in the hypothalamic feeding pathways in the offspring, and folic acid alone accounted for many of these effects.114 Expression of an obesogenic phenotype in the offspring has been associated with changes in DNA methylation of hypothalamic regulatory genes and may be corrected by matching the pregnancy diet with the postweaning pup diet.115 In a mouse model, 5-fold increases in the folic acid content of the gestational diet (AIN-93G) exacerbated weight gain, adiposity, glucose intolerance, and insulin resistance in male offspring fed a high-fat diet post weaning and was associated with lower expression of adipokine genes and higher global DNA methylation in white adipose tissue.116 Together, this work shows the involvement of folic acid in modulating the long-term weight trajectory of the offspring and the epigenetic plasticity of central and peripheral circuitry due to high exposure in utero.
Several studies have also examined the relationship between maternal folic acid supplementation and its interaction with the macronutrient content of the diet on metabolic outcome. They show that methyl vitamin–induced changes in gene expression may be gene specific, and manipulations of the macronutrient composition of the gestational diet are important determinates. In mice, consumption of a protein-restricted diet during pregnancy results in altered promoter methylation patterns, but such changes are prevented when diets are supplemented with folic acid.117 Feeding a protein-restricted diet combined with 5-fold the RDA of folic acid was shown to prevent altered promoter methylation and expression of Ppar-α, acyl-CoA oxidase (an enzyme involved in β-oxidation and partly regulated by Ppar-α), and glucocorticoid receptor in hepatocytes but had no effect on expression of Ppar-γ. In contrast, a maternal high-fat diet supplemented with methyl donors (choline, betaine, folic acid, vitamin B12, L-methionine, and zinc) prevented high-fat-diet–induced weight gain, fat preference, and global hypomethylation in higher-order reward regions in mice.118
Only two studies have assessed the potential lasting effects of maternal vitamin excess during pregnancy on the mother. In a preliminary study,119 dams were fed an HV or high-folic-acid diet during pregnancy and then an RV diet during the postweaning period. Dams fed the HV diet during pregnancy had higher plasma concentrations of the appetite-stimulating hormones ghrelin (at weaning) and leptin (at 20 weeks post weaning), whereas the high-folic-acid maternal diet resulted in higher expression of the orexigenic gene neuropeptide Y in the hypothalamus at 20 weeks post weaning. Although body weight and food intake were not affected by either vitamin-supplemented diet, both diets altered the physiological phenotype of the dam to favor weight gain when exposed to an obesogenic environment. In a subsequent study,120 dams fed an HV diet, an HV diet with recommended levels of folic acid (1-fold folic acid), or an RV diet with high levels of methyl-group vitamins (10-fold the RDA of folic acid, vitamins B12, and B6) during pregnancy alone showed greater weight gain when exposed to a high-fat diet post weaning compared with dams fed an RV diet during pregnancy, and their weight gain was dependent on postweaning food intake. However, only dams fed the HV diet demonstrated hypofunctioning of the mesolimbic reward pathway and higher expression of several hypothalamic feeding-related neuropeptides and Ppar genes in peripheral tissue. Because the effects of the maternal diets on central homeostatic, hedonic, and peripheral systems involved in energy balance regulation were inconsistent, the primary mechanism(s) of action of the vitamin-induced weight gain remain unknown.
CONCLUSION
Data from animal and epidemiological studies strongly support the importance of adequate nutrition during pregnancy, as well as its interaction with the nutritional environment later in life, in modulating the predisposition to obesity and related disorders in the mother and child. In addition to concerns about the lasting effects of an overall energy abundance in developed countries, maternal vitamin consumption, particularly of methyl-group vitamins (i.e., folic acid and vitamins B12 and B6), has increased concomitantly with obesity trends in North America. Although periconceptional use of methyl vitamins, such as folic acid, is encouraged for prevention of congenital anomalies and developmental disabilities, there are potential adverse effects that should be considered if these supplements are widely consumed in a population not at risk for deficiency. Methyl-group vitamins are intimately involved in one-carbon metabolism and regulate several metabolic processes, such as DNA methylation, gene expression, and nucleotide and monoamine synthesis. Several animal studies have shown that, in excess but nontoxic quantities, these vitamins may promote weight gain and other metabolic disturbances by altering central homeostatic, hedonic, or peripheral metabolic pathways involved in energy balance regulation; however, the macronutrient composition of the maternal diet appears to be an important determinate of the effects of vitamins. Although evidence from human trials is insufficient, results from animal models combined with the emerging recognition of the plasticity of, and the role of vitamins in, epigenetic regulation of gene expression encourage a shift of research focus toward the lasting effects of high vitamin intakes during human pregnancy to help better guide recommendations for optimal pregnancy and long-term outcomes.
Acknowledgments
Funding. This research was supported by the Canadian Institute of Health Research, Institute of Nutrition, Metabolism and Diabetes (CIHR-INMD), Reference MOP-93624. C.E.C. was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship. D.S.H. was supported by the Consejo Nacional de Ciencia y Tecnología (Mexico).
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84:289–298. [DOI] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief. 2011;61:1–8. [PubMed] [Google Scholar]
- 4.Colapinto CK, O'Connor DL, Tremblay MS. Folate status of the population in the Canadian Health Measures Survey. CMAJ. 2011;183:E100–E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez MF, Field CJ, Olstad DL, et al. Use of micronutrient supplements among pregnant women in Alberta: results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Matern Child Nutr. 2015;11:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindzon G, O'Connor DL. Folate during reproduction: the Canadian experience with folic acid fortification. Nutr Res Pract. 2007;1:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health Canada. Prenatal nutrition guidelines for health professionals – folate contributes to a healthy pregnancy. Health Canada website. http://www.hc-sc.gc.ca/fn-an/pubs/nutrition/folate-eng.php. Updated April 12, 2013. Accessed November 28, 2014. [Google Scholar]
- 9.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjepkema M. Adult obesity. Health Rep. 2006;17:9–25. [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 12.Harris HE, Ellison GT, Clement S. Relative importance of heritable characteristics and lifestyle in the development of maternal obesity. J Epidemiol Community Health. 1999;53:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlin A, Rossner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14:159–173. [PubMed] [Google Scholar]
- 14.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26:149–159. [DOI] [PubMed] [Google Scholar]
- 16.Kew S, Ye C, Hanley AJ, et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care. 2014;37:1998–2006. [DOI] [PubMed] [Google Scholar]
- 17.Catalano PM. Increasing maternal obesity and weight gain during pregnancy: the obstetric problems of plentitude. Obstet Gynecol. 2007;110:743–744. [DOI] [PubMed] [Google Scholar]
- 18.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 1999;21:261–275. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Composition and components of gestational weight gain: physiology and metabolism. In: Rasmussen KM, Yaktine AL, eds. In: . Washington, DC: National Academies Press; 2009:71–110. [Google Scholar]
- 20.Baker JL, Gamborg M, Heitmann BL, et al. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88:1543–1551. [DOI] [PubMed] [Google Scholar]
- 21.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088 doi:10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertz F, Brekke HK, Ellegard L, et al. Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr. 2012;96:698–705. [DOI] [PubMed] [Google Scholar]
- 23.Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity – adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res. 2001;133:1–38. [DOI] [PubMed] [Google Scholar]
- 24.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. [DOI] [PubMed] [Google Scholar]
- 25.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71(5 suppl):1280S–1284S. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. [DOI] [PubMed] [Google Scholar]
- 27.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133(5 suppl 2):1592S–1596S. [DOI] [PubMed] [Google Scholar]
- 28.Saldana TM, Siega-Riz AM, Adair LS. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am J Clin Nutr. 2004;79:479–486. [DOI] [PubMed] [Google Scholar]
- 29.Clausen T, Slott M, Solvoll K, et al. High intake of energy, sucrose, and polyunsaturated fatty acids is associated with increased risk of preeclampsia. Am J Obstet Gynecol. 2001;185:451–458. [DOI] [PubMed] [Google Scholar]
- 30.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50; discussion 50–55. [PubMed] [Google Scholar]
- 31.Ferezou-Viala J, Roy AF, Serougne C, et al. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1056–R1062. [DOI] [PubMed] [Google Scholar]
- 32.Vucetic Z, Kimmel J, Totoki K, et al. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. [DOI] [PubMed] [Google Scholar]
- 34.Burgueno AL, Carabelli J, Sookoian S, et al. The impact of maternal high-fat feeding on liver and abdominal fat accumulation in adult offspring under a long-term high-fat diet. Hepatology. 2010;51:2234–2235. [DOI] [PubMed] [Google Scholar]
- 35.Szeto IM, Aziz A, Das PJ, et al. High multivitamin intake by Wistar rats during pregnancy results in increased food intake and components of the metabolic syndrome in male offspring. Am J Physiol Regul Integr Comp Physiol. 2008;295:R575–R582. [DOI] [PubMed] [Google Scholar]
- 36.Cho CE, Sanchez-Hernandez D, Reza-López SA, et al. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics. 2013;8:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho CE, Pannia E, Huot PS, et al. Methyl vitamins contribute to obesogenic effects of a high multivitamin gestational diet and epigenetic alterations in hypothalamic feeding pathways in Wistar rat offspring. Mol Nutr Food Res. 2015;59:476–489. [DOI] [PubMed] [Google Scholar]
- 38.Yajnik CS, Deshpande SS, Jackson AA, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff GL, Kodell RL, Moore SR, et al. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 40.Wills L. Treatment of “pernicious anaemia of pregnancy” and “tropical anaemia". Br Med J. 1931;1:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills JL, Signore C. Neural tube defect rates before and after food fortification with folic acid. Birth Defects Res A Clin Mol Teratol. 2004;70:844–845. [DOI] [PubMed] [Google Scholar]
- 42.Koren G, Goh YI, Klieger C. Folic acid: the right dose. Can Fam Physician. 2008;54:1545–1547. [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen P, Boskovic R, Yazdani P, et al. Comparing folic acid pharmacokinetics among women of childbearing age: single dose ingestion of 1.1 versus 5 mg folic acid. Can J Clin Pharmacol. 2008;15:e314–e322. [PubMed] [Google Scholar]
- 44.Wald NJ. Folic acid and the prevention of neural-tube defects. N Engl J Med. 2004;350:101–103. [DOI] [PubMed] [Google Scholar]
- 45.Varela-Moreiras G, Murphy MM, Scott JM. Cobalamin, folic acid, and homocysteine. Nutr Rev. 2009;67(suppl 1):S69–S72. [DOI] [PubMed] [Google Scholar]
- 46.Dror DK, Allen LH. Interventions with vitamins B6, B12 and C in pregnancy. Paediatr Perinat Epidemiol. 2012;26(suppl 1):55–74. [DOI] [PubMed] [Google Scholar]
- 47.Festin M. Nausea and vomiting in early pregnancy. BMJ Clin Evid. 2007;2007:pii 1405. [PubMed] [Google Scholar]
- 48.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 49.Molloy AM, Kirke PN, Brody LC, et al. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr Bull. 2008;29(2 suppl):S101–S111; discussion S112–S115. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Huffman SL. Review of fortified food and beverage products for pregnant and lactating women and their impact on nutritional status. Matern Child Nutr. 2011;7(suppl 3):19–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan KM, Ford ES, Azrak MF, et al. Multivitamin use in pregnant and nonpregnant women: results from the Behavioral Risk Factor Surveillance System. Public Health Rep. 2009;124:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo X, Willows N, Kuhle S, et al. Use of vitamin and mineral supplements among Canadian adults. Can J Public Health. 2009;100:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakur YA, Tarasuk V, Corey P, et al. A comparison of micronutrient inadequacy and risk of high micronutrient intakes among vitamin and mineral supplement users and nonusers in Canada. J Nutr. 2012;142:534–540. [DOI] [PubMed] [Google Scholar]
- 54.Yu SM, Keppel KG, Singh GK, et al. Preconceptional and prenatal multivitamin-mineral supplement use in the 1988 National Maternal and Infant Health Survey. Am J Public Health. 1996;86:240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams LM, Morrow B, Lansky A, et al. Surveillance for selected maternal behaviors and experiences before, during, and after pregnancy. Pregnancy Risk Assessment Monitoring System (PRAMS), 2000. MMWR Surveill Summ. 2003;52:1–14. [PubMed] [Google Scholar]
- 56.Lagiou P, Mucci L, Tamimi R, et al. Micronutrient intake during pregnancy in relation to birth size. Eur J Nutr. 2005;44:52–59. [DOI] [PubMed] [Google Scholar]
- 57.Sacco JE, Tarasuk V. Health Canada's proposed discretionary fortification policy is misaligned with the nutritional needs of Canadians. J Nutr. 2009;139:1980–1986. [DOI] [PubMed] [Google Scholar]
- 58.Fayyaz F, Wang F, Jacobs RL, et al. Folate, vitamin B12, and vitamin B6 status of a group of high socioeconomic status women in the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Appl Physiol Nutr Metab. 2014;39:1402–1408. [DOI] [PubMed] [Google Scholar]
- 59.MacFarlane AJ, Greene-Finestone LS, Shi Y. Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr. 2011;94:1079–1087. [DOI] [PubMed] [Google Scholar]
- 60.Pfeiffer CM, Caudill SP, Gunter EW, et al. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82:442–450. [DOI] [PubMed] [Google Scholar]
- 61.Health Canada. Do Canadian adults meet their nutrient requirements through food intake alone? Health Canada website. http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/surveill/nutrition/commun/art-nutr-adult-eng.pdf. Published 2012. Accessed July 29, 2014.
- 62.Morris MS, Picciano MF, Jacques PF, et al. Plasma pyridoxal 5'-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1446–1454. [DOI] [PubMed] [Google Scholar]
- 63.Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006;55:1387–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YI. Role of folate in colon cancer development and progression. J Nutr. 2003;133(11 suppl 1):3731S–3739S. [DOI] [PubMed] [Google Scholar]
- 65.Kim YI. Does a high folate intake increase the risk of breast cancer? Nutr Rev. 2006;64(10 pt 1):468–475. [DOI] [PubMed] [Google Scholar]
- 66.Zhou SS, Li D, Zhou YM, et al. B-vitamin consumption and the prevalence of diabetes and obesity among the US adults: population based ecological study. BMC Public Health. 2010;10:746 doi:10.1186/1471-2458-10-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li D, Sun WP, Zhou YM, et al. Chronic niacin overload may be involved in the increased prevalence of obesity in US children. World J Gastroenterol. 2010;16:2378–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69:1352–1353. [DOI] [PubMed] [Google Scholar]
- 69.Verhaar MC, Wever RM, Kastelein JJ, et al. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–241. [DOI] [PubMed] [Google Scholar]
- 70.Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv Nutr. 2010;1:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavebratt C, Almgren M, Ekstrom TJ. Epigenetic regulation in obesity. Int J Obes (Lond). 2012;36:757–765. [DOI] [PubMed] [Google Scholar]
- 72.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. [DOI] [PubMed] [Google Scholar]
- 73.van Vliet J, Oates NA, Whitelaw E. Epigenetic mechanisms in the context of complex diseases. Cell Mol Life Sci. 2007;64:1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther. 2012;92:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med. 2009;46:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dominguez-Salas P, Moore SE, Baker MS, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746 doi:10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevens A, Begum G, Cook A, et al. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151:3652–3664. [DOI] [PubMed] [Google Scholar]
- 79.Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14:17–22. [DOI] [PubMed] [Google Scholar]
- 80.Plagemann A, Harder T, Brunn M, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587(pt 20):4963–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vucetic Z, Kimmel J, Reyes TM. Chronic high-fat diet drives postnatal epigenetic regulation of µ-opioid receptor in the brain. Neuropsychopharmacology. 2011;36:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nikoshkov A, Sunkari V, Savu O, et al. Epigenetic DNA methylation in the promoters of the Igf1 receptor and insulin receptor genes in db/db mice. Epigenetics. 2011;6:405–409. [DOI] [PubMed] [Google Scholar]
- 83.Martinez JA, Milagro FI, Claycombe KJ, et al. Epigenetics in adipose tissue, obesity, weight loss, and diabetes. Adv Nutr. 2014;5:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun YN, Gao Y, Qiao SP, et al. Epigenetic DNA methylation in the promoters of peroxisome proliferator-activated receptor γ in chicken lines divergently selected for fatness. J Anim Sci. 2014;92:48–53. [DOI] [PubMed] [Google Scholar]
- 85.Sie KK, Li J, Ly A, et al. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol Nutr Food Res. 2013;57:677–685. [DOI] [PubMed] [Google Scholar]
- 86.Matthews RG, Haywood BJ. Inhibition of pig liver methylenetetrahydrofolate reductase by dihydrofolate: some mechanistic and regulatory implications. Biochemistry. 1979;18:4845–4851. [DOI] [PubMed] [Google Scholar]
- 87.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–533. [DOI] [PubMed] [Google Scholar]
- 88.Meguid MM, Fetissov SO, Varma M, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. [DOI] [PubMed] [Google Scholar]
- 89.Choi HJ, Jang YJ, Kim HJ, et al. Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol Pharmacol. 2000;58:633–640. [PubMed] [Google Scholar]
- 90.Siow YL, Dakshinamurti K. Effect of pyridoxine deficiency on aromatic L-amino acid decarboxylase in adult rat brain. Exp Brain Res. 1985;59:575–581. [DOI] [PubMed] [Google Scholar]
- 91.Hellmann H, Mooney S. Vitamin B6: a molecule for human health? Molecules. 2010;15:442–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bottiglieri T, Hyland K, Reynolds EH. The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs. 1994;48:137–152. [DOI] [PubMed] [Google Scholar]
- 93.Gavin G, McHenry EW. The B vitamins and fat metabolism: III. The effects of vitamin B6 upon liver and body fat. J Biol Chem. 1940;132:41–46. [Google Scholar]
- 94.McHenry EW, Gavin G. The B vitamins and fat metabolism: IV. The synthesis of fat from protein. J Biol Chem. 1941;138:471–475. [Google Scholar]
- 95.Sure B, Easterling L. The role of pyridoxine in economy of food utilization. J Nutr. 1949;39:393–396. [DOI] [PubMed] [Google Scholar]
- 96.Carter CW, Phizackerley PJ. The influence of pyridoxine on fat metabolism in the rat. Biochem J. 1951;49:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawada T, Aoki N, Kamei Y, et al. Comparative investigation of vitamins and their analogues on terminal differentiation, from preadipocytes to adipocytes, of 3T3-L1 cells. Comp Biochem Physiol A Comp Physiol. 1990;96:323–326. [DOI] [PubMed] [Google Scholar]
- 98.Huq MD, Tsai NP, Lin YP, et al. Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat Chem Biol. 2007;3:161–165. [DOI] [PubMed] [Google Scholar]
- 99.Yanaka N, Kanda M, Toya K, et al. Vitamin B6 regulates mRNA expression of peroxisome proliferator-activated receptor-gamma target genes. Exp Ther Med. 2011;2:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mardones-Santander F, Rosso P, Stekel A, et al. Effect of a milk-based food supplement on maternal nutritional status and fetal growth in underweight Chilean women. Am J Clin Nutr. 1988;47:413–419. [DOI] [PubMed] [Google Scholar]
- 101.Owen CG, Martin RM, Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. [DOI] [PubMed] [Google Scholar]
- 102.Lubetzky R, Vaisman N, Mimouni FB, et al. Energy expenditure in human milk- versus formula-fed preterm infants. J Pediatr. 2003;143:750–753. [DOI] [PubMed] [Google Scholar]
- 103.Butte NF, Wong WW, Ferlic L, et al. Energy expenditure and deposition of breast-fed and formula-fed infants during early infancy. Pediatr Res. 1990;28:631–640. [DOI] [PubMed] [Google Scholar]
- 104.Changamire FT, Mwiru RS, Peterson KE, et al. Effect of multivitamin supplements on weight gain during pregnancy among HIV-negative women in Tanzania. Matern Child Nutr. 2015;11:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Asemi Z, Samimi M, Tabassi Z, et al. Multivitamin versus multivitamin-mineral supplementation and pregnancy outcomes: a single-blind randomized clinical trial. Int J Prev Med. 2014;5:439–446. [PMC free article] [PubMed] [Google Scholar]
- 106.Vaidya A, Saville N, Shrestha BP, et al. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stewart CP, Christian P, Schulze KJ, et al. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011;141:1912–1917. [DOI] [PubMed] [Google Scholar]
- 108.Dougan MM, Willett WC, Michels KB. Prenatal vitamin intake during pregnancy and offspring obesity. Int J Obes. 2015;39:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davis JN, Koleilat M, Shearrer GE, et al. Association of infant feeding and dietary intake on obesity prevalence in low-income toddlers. Obesity (Silver Spring). 2014;22:1103–1111. [DOI] [PubMed] [Google Scholar]
- 110.Szeto IM, Das PJ, Aziz A, et al. Multivitamin supplementation of Wistar rats during pregnancy accelerates the development of obesity in offspring fed an obesogenic diet. Int J Obes (Lond). 2009;33:364–372. [DOI] [PubMed] [Google Scholar]
- 111.Szeto IM, Payne CM, Jahan-Mihan A, et al. Multivitamin supplementation during pregnancy alters body weight and macronutrient selection in Wistar rat offspring. J Dev Orig Health Dis. 2010;1:386–395. [DOI] [PubMed] [Google Scholar]
- 112.Reza-López SA, Anderson GH, Szeto IM, et al. High vitamin intake by Wistar rats during pregnancy alters tissue fatty acid concentration in the offspring fed an obesogenic diet. Metabolism. 2009;58:722–730. [DOI] [PubMed] [Google Scholar]
- 113.Reza-López SA, Poon AN, Szeto IM, et al. High multivitamin intakes during pregnancy and postweaning obesogenic diets interact to affect the relationship between expression of PPAR genes and glucose regulation in the offspring. J Nutr Biochem. 2013;24:877–881. [DOI] [PubMed] [Google Scholar]
- 114.Cho CE, Pannia E, Huot PS, et al. Methyl vitamins contribute to obesogenic effects of high multivitamin gestational diet and epigenetic alterations in hypothalamic feeding pathways in Wistar rat offspring. Mol Nutr Food Res. 2015;59:476–489. [DOI] [PubMed] [Google Scholar]
- 115.Cho CE, Sanchez-Hernandez D, Reza-López SA, et al. Obesogenic phenotype of offspring of dams fed a high multivitamin diet is prevented by a post-weaning high multivitamin or high folate diet. Int J Obes (Lond). 2013;37:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Y, He Y, Sun X, et al. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int J Mol Sci. 2014;15:6298–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lillycrop KA, Phillips ES, Jackson AA, et al. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. [DOI] [PubMed] [Google Scholar]
- 118.Carlin J, George R, Reyes TM. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One. 2013;8:e63549 doi:10.1371/journal.pone.0063549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.López SR. High Vitamin Intakes During Pregnancy and Characteristics of Metabolic Syndrome in Wistar Rat Dams and their Offspring [doctoral thesis]. Toronto: Nutritional Sciences, University of Toronto; 2011. [Google Scholar]
- 120.Pannia E, Cho CE, Kubant R, et al. A high multivitamin diet fed to Wistar rat dams during pregnancy increases maternal weight gain later in life and alters homeostatic, hedonic and peripheral regulatory systems of energy balance. Behav Brain Res. 2014;278:1–11. [DOI] [PubMed] [Google Scholar]


