Highlight
Broad variations in the CO2 fixation kinetics of diatom Rubisco indicate novel mechanistic diversity and large differences in their carbon-concentrating mechanism.
Key words: Algae, carbon fixation, diatoms, kinetics, photosynthesis, Rubisco.
Abstract
While marine phytoplankton rival plants in their contribution to global primary productivity, our understanding of their photosynthesis remains rudimentary. In particular, the kinetic diversity of the CO2-fixing enzyme, Rubisco, in phytoplankton remains unknown. Here we quantify the maximum rates of carboxylation (k cat c), oxygenation (k cat o), Michaelis constants (K m) for CO2 (K C) and O2 (K O), and specificity for CO2 over O2 (SC/O) for Form I Rubisco from 11 diatom species. Diatom Rubisco shows greater variation in K C (23–68 µM), SC/O (57–116mol mol−1), and K O (413–2032 µM) relative to plant and algal Rubisco. The broad range of K C values mostly exceed those of C4 plant Rubisco, suggesting that the strength of the carbon-concentrating mechanism (CCM) in diatoms is more diverse, and more effective than previously predicted. The measured k cat c for each diatom Rubisco showed less variation (2.1–3.7s−1), thus averting the canonical trade-off typically observed between K C and k cat c for plant Form I Rubisco. Uniquely, a negative relationship between K C and cellular Rubisco content was found, suggesting variation among diatom species in how they allocate their limited cellular resources between Rubisco synthesis and their CCM. The activation status of Rubisco in each diatom was low, indicating a requirement for Rubisco activase. This work highlights the need to better understand the correlative natural diversity between the Rubisco kinetics and CCM of diatoms and the underpinning mechanistic differences in catalytic chemistry among the Form I Rubisco superfamily.
Introduction
Ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) plays a fundamental role in photosynthetic CO2 assimilation within the global carbon cycle. Rubisco activity within the terrestrial and ocean biospheres contributes approximately equally to the 10 Pmol of CO2 annually fixed into organic carbon (Raven, 2009). Often the rate of CO2 fixation is limited by Rubisco activity and, as such, has made the enzyme a primary target to enhance crop photosynthesis and yield through genetic manipulation (Mueller-Cajar and Whitney, 2008; Peterhansel et al., 2008; Carmo-Silva et al., 2015). However, improving Rubisco kinetics has proved difficult as a result of the complex assembly pathway of Rubisco in higher plants (Whitney et al., 2011; Hauser et al., 2015) and apparent trade-offs in its kinetic parameters (Tcherkez et al., 2006; Savir et al., 2010). Widening our understanding of the natural diversity in Rubisco is critical if solutions to improve its performance are to be found and understood (Parry et al., 2013). Of particular interest is Rubisco from organisms adapted to different environments. This includes marine phytoplankton whose efficient carbon-concentrating mechanisms (CCMs) enable them to endure, sometimes thrive in, nutrient- and CO2-depleted seawater (Nelson et al., 1995).
In nature, Rubisco is found in a variety of oligomeric forms and within a diverse array of organisms that include archaea, photosynthetic bacteria, cyanobacteria, algae, and plants (Whitney et al., 2011). Form I Rubisco consists of eight large and eight small subunits, and is subdivided into Forms IA–ID depending on its sequence and lineage (Tabita et al., 2008). Most research to date on Rubisco has focused on those sourced from the terrestrial biosphere, with comparatively little characterization of Rubisco from oceanic sources. The terrestrial biosphere is dominated by the ‘green’ chloroplast lineage pertaining to plants and green algae that contain Form IB Rubisco (Tabita et al., 2008). However, oceanic photosynthesis is primarily carried out by phytoplankton containing chloroplasts from a ‘red’ lineage that comprise Form ID Rubisco (Delwiche and Palmer, 1997; Yoon et al., 2002; Falkowski et al., 2004). A group of phytoplankton called diatoms are of particular interest due to their importance in ocean primary productivity (estimated to account for ~20% of global primary production; Nelson et al., 1995), thus influencing global biogeochemical cycles, and for producing silicified walls that preserve paleoclimate signals in the fossil record (Armstrong et al., 2001; Egan et al., 2013; Heureux and Rickaby, 2015).
Compared with other photosynthetic enzymes, Rubisco is considered inefficient due to its low CO2-saturated CO2 fixation rate (k cat c) and low affinity for CO2 (i.e. an elevated Michaelis constant, K m, for CO2; K C). The basis of this inefficiency arises from the complex catalytic mechanism of Rubisco that imposes biochemical trade-offs between k cat c, K C, and specificity for CO2 over its competitive inhibition by O2 (SC/O) (Tcherkez et al., 2006). Recent analyses show that the extent of these trade-offs is variable between the Form I Rubisco of plants, red algae, and cyanobacteria (Tcherkez, 2013, 2015). Notably Rubisco oxygenation produces 2-phosphoglycolate, which is toxic to the chloroplast (Zelitch et al., 2009), necessitating its removal via the photorespiratory pathway at a cost of energy and fixed carbon (Peterhansel et al., 2008). The loss of CO2 by photorespiration can be as high as 25% of the total carbon fixed in C3 flowering plants (Laing et al., 1974).
Despite the catalytic inefficiencies of Rubisco, it appears that it can adapt to the CO2:O2 ratio of its environment. This is particularly evident for Rubisco kinetics in C3 and C4 plants. While C3 plants rely on diffusion of CO2 from the air to chloroplast stroma, C4 plants utilize a CCM to elevate CO2 around Rubisco to avoid photorespiration and its associated cellular resource costs. In response to higher intracellular CO2, C4 Rubisco has evolved improvements in k cat c at the expense of reducing CO2 affinity (i.e. increasing K C) (Yeoh et al., 1980; Seemann et al., 1984; Ghannoum et al., 2005). The CCM and higher k cat c allow C4 plants to reduce their investment in Rubisco, lower the rate of photorespiration, and allow for carbon fixation rates similar to C3 plants under low stomatal apertures (Sage, 2004; Ghannoum et al., 2005; Way et al., 2014). These features improve the nitrogen, energy, and water use efficiencies of C4 plants.
In the oceans, the low levels of CO2, and its slow diffusion rate in water, have led many photosynthetic organisms to evolve CCMs that utilize the higher concentrations of bicarbonate. These mechanisms are different from the CCMs of C4 plants that arose during the low CO2 concentrations of the Oligocene period (Sage, 2001; Osborne and Sack, 2012) and the coupled warmer, arid environments that trigger stomatal closure and N limitation of the soil (Ehleringer et al., 1997; Long, 1999). The C4 plant CCM fixes HCO3 − by phosphoenolpyruvate (PEP) carboxylase, leading to production of C4 organic acids in the Rubisco-lacking mesophyll cells. These C4 organic acids then diffuse into the Rubisco-containing bundle sheath cells where they are decarboxylated (Sage, 2004). This process facilitates the concentration of CO2 to levels that effectively saturate Rubisco (Furbank and Hatch, 1987).
While some diatoms may also have a C4-like mechanism (Reinfelder et al., 2000, 2004) or a C3–C4 intermediate-like mechanism (Roberts et al., 2007), they contain a CCM suited to their single-celled physiology and high bicarbonate aquatic environment. In diatoms, the CCM consists of various bicarbonate transporters (Nakajima et al., 2013) and differing forms of carbonic anhydrase that serve to elevate CO2 levels within the pyrenoid, a low CO2-permeable subcellular compartment containing most of the cellular Rubisco (Reinfelder, 2010; Hopkinson et al., 2011). The efficiency of their CCMs allows diatoms to invest their scarce cellular resources conservatively in Rubisco. Accordingly the Rubisco content of diatoms is considerably lower [2–6% (w/w) of total cellular protein] than the 20–50% (w/w) Rubisco content of the soluble protein in plant leaves (Losh et al., 2013; Carmo-Silva et al., 2015).
Our understanding of phytoplankton CCM components and activity regulation remain rudimentary. This is despite the increasing interest in understanding how Form ID Rubisco and CCMs co-evolved and how carbon fixation rates by phytoplankton will respond to rising anthropogenic CO2 (Raven et al., 2012; Young et al., 2012, 2013). From the few Form ID Rubisco kinetics determined, there is a strong signal of positive selection within the evolution of the Rubisco large subunits in red algae, Haptophytes, and diatoms (Young et al., 2012). Rubiscos from red algae have the highest specificities for CO2 over O2 (SC/O; ~130–240mol mol−1) while the lower SC/O diatom Rubisco (~60–115mol mol−1) overlaps with the less diverse SC/O values of C3 plant and C4 plant Rubisco (~70–90mol mol−1) (Read and Tabita, 1994; Whitney et al., 2001, 2011; Haslam et al., 2005). K C measurements for diatom Rubisco (~28–40 μM, Badger et al., 1998; Whitney et al., 2001) exceed the concentration of CO2 in the surface ocean (~13 μM in air-equilibrated surface seawater at 20 °C), exemplifying the requirement for a CCM. Modeling of the CCM in phytoplankton is highly reliant on measurements of their Rubisco kinetics (Hopkinson, 2011, 2014). However, the paucity of catalysis measurements for diatom Rubisco—and other phytoplankton—continue to limit reliable assessments of CCM function in microalgae.
In this study, we evaluate the diversity of Rubisco kinetics in 11 different diatom species at the common assay temperature of 25 °C. From measurements of SC/O, k cat c, K C, and the K m for O2 (K O), we determine the catalytic turnover rate for O2 (k cat o) and unveil an unexpected large degree of kinetic variability across the species studied. Uncovered are novel relationships between kinetic parameters not previously observed for other plant and algal Form I Rubisco isoforms. Presented are a novel, robust data set of Rubisco kinetics in marine phytoplankton that provide new insight into potential constraints on microalgal photosynthesis that arise from variations in the effectiveness of their CCM to elevate CO2 around Rubisco.
Materials and methods
Species selection and sampling:
Eleven species of marine diatoms were selected from cultures maintained at Princeton University and the Australian National University (ANU). Strains from Princeton University were grown at 20 °C under continuous light (~150 μmol photons m−2 s−1) in 0.2 μm filtered seawater supplemented with Aquil medium (Sunda et al., 2005) and included the diatoms Thalassiosira weissflogii (CCMP 1336), Skeletonema marinoi (CCMP 1332), Chaetoceros calcitrans (CCMP 1315), Chaetoceros muelleri (CCMP 1316), and Phaeodactylum tricornutum (CCMP 642). Fragilariopsis cylindrus (CCMP 1102) was grown at 1 °C, 12:12 light:dark cycle at 75 μmol photons m−2 s−1. Strains from ANU were grown at 25 °C under a 16:8 light:dark cycle (~150μmol photons m−2 s−1) cultured in 0.2 μm filtered seawater supplemented with F/2 medium and included the diatoms Thalassiosisra oceanica (CS-427), Chaetoceros calcitrans (CS-178), Phaeodactylum tricornutum (CS-29), Bellerochea sp. (CS-874/01), and Cylindrotheca fusiformis (CS-13).
Materials
Unlabeled and 3H-labeled ribulose-1,5-bisphosphate (RuBP) was synthesized and purified as described (Kane et al., 1998), and used to prepare 14C-labeled carboxypenitol-P2 ([14C]CPBP) according to Pierce et al. (1980) and Zhu and Jensen (1991).
Rubisco extraction
Cells were harvested at or near exponential growth by gentle centrifugation (2000 g for 10min) and the 0.1–0.5ml cell pellets were snap-frozen in liquid nitrogen and stored at –80 °C. Pellets were re-suspended in 5ml of ice-cold extraction buffer containing 50mM EPPS-NaOH, pH 8.0, 1mM EDTA, 2mM DTT, and 1% (v/v) plant protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA), and cells were ruptured in a French press. Extract was centrifuged at 14 000 g, 4 °C for 2min and the supernatant was used to quantify Rubisco K C and k cat c or used to purify Rubisco and measure CO2/O2 specificity (SC/O)
CO2/O2 specificity
Rubisco was rapidly purified from ~1g (~4–5 pooled biological replicates) of the –80 °C stored cells extracted in ice-cold extraction buffer, and lysed using a French press. Polyvinylpolypyrrolidone [1% (w/v)] was added to the lysate to remove secondary metabolites prior to centrifugation (17 600 g, 4 °C, 5min). The soluble cellular protein was rapidly passed over a 1ml Bio-Scale Mini Macro-Prep High Q ion exchange column (Biorad, Hercules, CA, USA) equilibrated with column buffer (50mM EPPS-NaOH, pH 8.0, 1mM EDTA). Bound Rubisco was eluted in 1.5ml of column elution buffer (50mM EPPS-NaOH, pH 8.0, 1mM EDTA 0.8M NaCl) and concentrated to 0.5ml using an Amicon Ultra-15 centrifugal filter (30 000 NMWL, Millipore, Billerica, MA, USA). The protein was applied to the Superdex 200 (GE Life Sciences) column to purify and desalt the Rubisco further. Fractions containing Rubisco were pooled and concentrated again by centrifugal filtration to 0.25ml and glycerol was added to 20% (v/v) final concentration before freezing in liquid nitrogen and storing at –80 °C. Purified Rubisco preparations were used to measure CO2/O2 specificity using the method of Kane et al. (1994).
K c, k cat c, K o, and k cat o
Rubisco content was quantified by [14C]2-CABP (2-C-carboxyarabinitol 1,5-bisphosphate) binding as described by Sharwood et al. (2008) by incubating duplicate aliquots of soluble cellular protein extracts with 15mM NaHCO3, and 15mM MgCl2 and either 15 μM or 30 μM [14C]CPBP for 10–30min at 25 °C. The recovered amount of [14C]CABP-bound Rubisco in both reactions varied by <2% ensuring the Rubisco catalytic site content was accurately quantified. RuBP-dependent 14CO2 fixation assays were performed in 7ml septum-capped scintillation vials at 25 °C as described (Whitney and Sharwood, 2007) using soluble diatom protein extract following 10–15min activation with 15mM NaH14CO3 and 15mM MgCl2. After activation, kinetic measurements were made on soluble cellular protein in assay vials containing reaction buffer (0.5ml of 100mM EPPS-NaOH, pH 8, 15mM MgCl2, 0.6mM ribulose-P2, 0.1mg ml−1 carbonic anhydrase) equilibrated with 0, 21, 40, and 60% (v/v) O2 in N2 and five differing concentrations of 14CO2 (between 10 μM and 100 μM; specific activities of ~1800 cpm nmol–1 CO2 fixed). The assays were stopped after 1–3min with 0.5 vols of 25% (v/v) formic acid and processed for scintillation counting. Values for K C and maximal carboxylase activity (v c max) were extrapolated from the data using the Michaelis–Menten equation as described by Sharwood et al. (2008) and Whitney et al., (2011). Measures of k cat c were calculated by dividing v c max by the number of Rubisco active sites as determined by [14C]2-CABP binding. K O was determined from the slope of the linear increase in K C in response to O2. Values for k cat o were calculated using the equation k cat o=(k cat c×K O)/(K C×SC/O). All measurements were made in triplicate using material pooled from 2–3 biological replicates.
Statistics
One-way ANOVA was performed to determine whether significant differences (P-value ≤0.05) existed between the Rubisco kinetics parameters measured in the different groups in Fig. 2. Where significant differences were found, Tukey’s HSD tests were performed to determine which groups differed from each other. Linear regression was fit by least-square analysis, and the correlation coefficient (r 2) was based on standard error of estimate. Analysis of covariance (ANCOVA) was used to determine whether the linear fit was significant to 95% (P-value ≤0.05) in Table 2 and Figs 3 and 4.
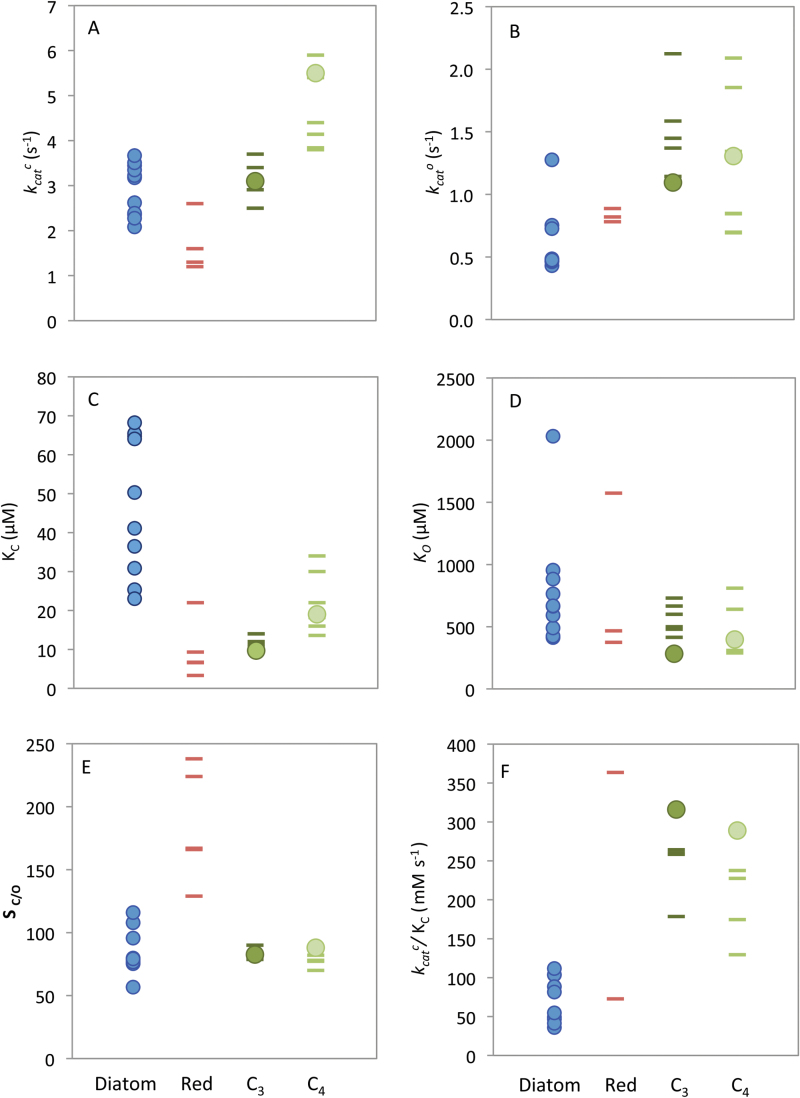
Fig. 2.
Rubisco kinetic parameters measured at 25 °C. Rubisco properties measured from diatoms (blue circles), tobacco and maize (green circles, see Table 1) compared with previously published values for red algae (red, maroon dashes), C3 plants (dark green dashes), and C4 plants (light green dashes) (Badger et al., 1998; Savir et al., 2010; Supplementary Table S1). Kinetic parameters include (A) the maximum rates of carboxylation (k cat c), (B) oxygenation rate (k cat o), the K m for (C) CO2 (K C) and (D) O2 (K O), (E) the specificity for CO2 over O2 (SC/O), and (F) the carboxylation efficiency (k cat c /K C, mM s−1).
Table 2.
Linear correlations between various Rubisco kinetic parameters from 11 diatom species
| k cat c | K C | K O a | SC/O | k cat o | k cat c/K C | k cat o/K O | K C /K O | K C 21%O2 | k cat c/K C 21%O2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| k cat c | 1 | 0.0212 (0.688) | 0.0133 (0.752) | 0.0182 (0.729) | 0.0503 (0.562) | 0.0388 (0.585) | 0.0016 (0.919) | 0.0299 (0.633) | 0.0293 (0.637) | 0.103 (0.365) |
| K C | 1 | 0.425 (0.0412) | 0.00965 (0.817) | 0.181 (0.254) | 0.854 (0.000131) | 0.223 (0.199) | 0.0715 (0.455) | 0.958 (<0.00001) | 0.760 (0.00102) | |
| K O a | 1 | 0.00818 (0.831) | 0.378 (0.0783) | 0.350 (0.0714) | 0.209 (0.216) | 0.275 (0.120) | 0.240 (0.150) | 0.217 (0.175) | ||
| SC/O | 1 | 0.178 (0.298) | 0.00189 (0.919) | 0.260 (0.196) | 0.00782 (0.835) | 0.0126 (0.791) | 0.00195 (0.742) | |||
| k cat o | 1 | 0.107 (0.390) | 0.125 (0.351) | 0.0914 (0.429) | 0.108 (0.389) | 0.0667 (0.502) | ||||
| k cat c/K C | 1 | 0.304 (0.124) | 0.0425 (0.568) | 0.803 (0.000448) | 0.963 (<0.00001) | |||||
| k cat o/K O | 1 | 0.00552 (0.849) | 0.176 (0.260) | 0.228 (0.194) | ||||||
| K C/K O | 1 | 0.210 (0.183) | 0.0888 (0.403) | |||||||
| K C air | 1 | 0.752 (0.0116) | ||||||||
| k cat c/K C air | 1 |
Correlation coefficient and probability of linear correlation, r 2 (P-value).
Relationships with statistically significant linear correlation (P<0.05) are shown in bold.
a Correlation when all data are included. This is different from the correlation shown in Fig. 2, which does not include the T. weissflogii outlier.
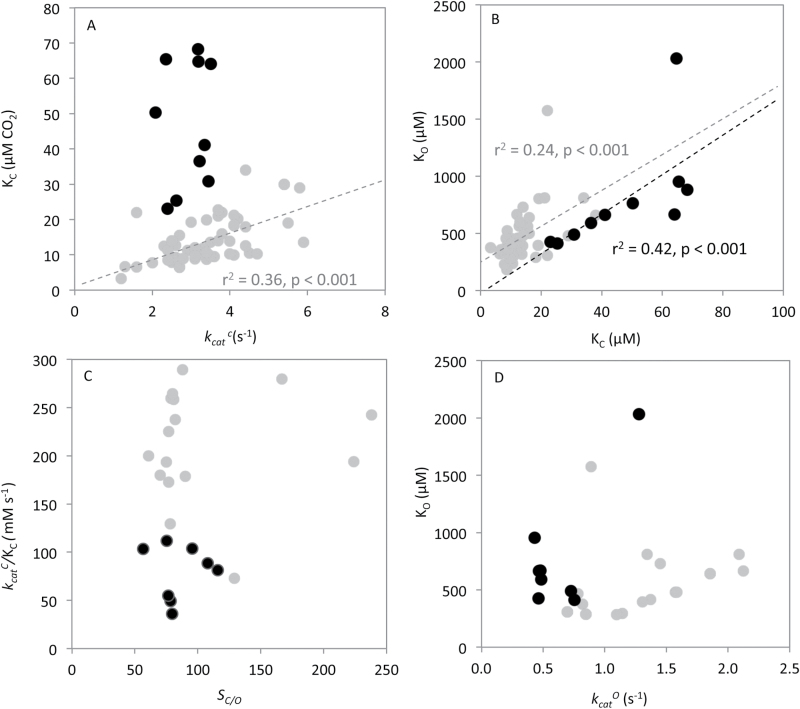
Fig. 3.
Comparing the catalytic relationships of diatom and other Form I Rubiscos. Comparison of (A) maximum carboxylation rate (k cat c) versus the K m for CO2 (K C), (B) K C versus the K m for O2 (K O), (C) the carboxylation efficiency (k cat c/K C) versus specificity for CO2 over O2 (SC/O), and (D) the maximum oxygenation rate (k cat o) and K O. Measured for diatoms (black circles) and compared with the compilation of plants and eukaryotic algae from Savir et al. (2010), Badger et al. (1998), Whitney et al. (2011), and Galmes et al. (2014) (gray circles).
Results
Our results for 11 species of diatoms represent the largest data set of Form ID Rubisco kinetics available to date. The diatom species selected in this study represent a wide range of habitats and evolutionary diversity (Table 1), and included multiple strains of the same species (P. tricornutum and C. calcitrans), and one polar pennate (F. cylindrus).
Table 1.
Diatom strains, location of isolation, and Rubisco kinetics measured at 25 °C
| k cat c | Kc | k cat o | K O | SC/O | K C 21%O2 | k cat c/K C | k cat c/K C 21%O2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Species | Location isolated | (s−1) | (μM) | (s−1) | (μM) | (mol mol−1) | (μM) | (mM s−1) | (mM s−1) |
| Diatoms (Bacillariophyta) | |||||||||
| Thalassiosira weissflogii (CCMP 1336)a | Gardiners Island, Long Island, NY, USA | 3.2±0.2 | 65±3 | 1.3 | 2032±458 | 79±1 | 73 | 49 | 44 |
| Thalassiosira oceania (CS-427)a | Little Swan port, Tasmania, Australia | 2.4±0.4 | 65±5 | 0.4 | 954±228 | 80±2 | 83 | 36 | 28 |
| Skeletonema marinoi (CCMP 1332)a | Milford, CT, USA | 3.2±1.1 | 68±8 | ND | 883±346 | ND | 88 | 47 | 36 |
| Chaetoceros calcitrans (CCMP 1315) | Collection site unknown | 2.6±0.2 | 25±1 | 0.8 | 413±53 | 57±11 | 41 | 103 | 64 |
| Chaetoceros muelleri (CCMP 1316) | Oceanic Institute, HI, USA | 2.4±0.3 | 23±1.5 | 0.5 | 425±67 | 96±2 | 37 | 104 | 65 |
| Chaetoceros calcitrans (CS-178) | Unknown (but should be synonymous with CCMP 1315) | 3.4±0.6 | 31±2 | 0.7 | 490±54 | 75±1 | 47 | 112 | 74 |
| Bellerochea cf. horologicalis (CS-874/01) | Ilbilbie, Queensland, Australia | 2.1±0.2 | 50±4 | ND | 764±190 | ND | 67 | 41 | 31 |
| Phaeodactylum tricornutum (UTEX 642) | Plymouth, UK | 3.2±0.9 | 36±1 | 0.5 | 592±44 | 108±2 | 52 | 88 | 62 |
| Phaeodactylum tricornutum (CS-29) | Unknown, UK | 3.3±0.6 | 41±1 | 0.5 | 664±54 | 116±2 | 57 | 81 | 59 |
| Fragilariopsis cylindrus (CCMP 1102) | Islas Orcadas Cruise, Station 12 | 3.5±0.3 | 64±8 | 0.5 | 667±255 | 77±1 | 88 | 55 | 40 |
| Cylindrotheca fusiformis (CS-13) | Halifax, Canada | 3.7±0.2 | ND | ND | ND | 79±1 | ND | ND | ND |
| Higher plants (controls) | |||||||||
| Nicotiana tabacum (tobacco, C3) | 3.1±0.3 | 9.7±0.1 | 1.1 | 283±15 | 82±1 | 18.3 | 316 | 167 | |
| Zea mays (maize, C4) | 5.5±0.2 | 19.0±0.6 | 1.4 | 397±59 | 88±2 | 31 | 289 | 177 |
a Belonging to the order Thalassiosirales;
Values shown are average of measurements from n ≥3 (± SD) biological repeat samples (see figure legends); ND, not determined
KC 21%O2 was calculated as K C(1+O2/K O) assuming air-saturated O2 levels in H2O of 252 µM
Rubisco activation and stability in diatom cellular protein extract
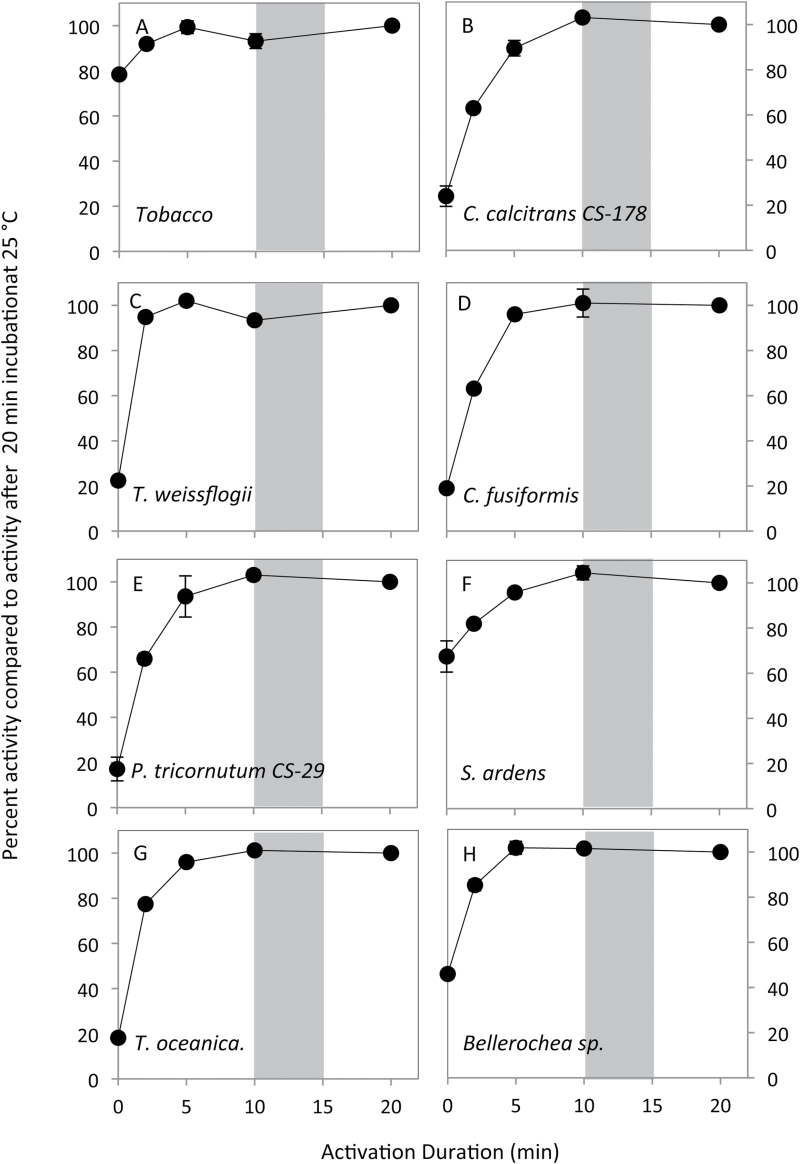
The maintained stability of Rubisco activity in isolated soluble leaf protein facilitates the accurate and reliable measure of maximum carboxylation rates (k cat c) and the Michaelis–Menten (half-saturation) constant (K m) for carboxylation (K C) without need of a purification step (Sharwood et al., 2008). These measurements require all eight catalytic sites in each L8S8 Rubisco molecule to be primed with CO2-Mg2+ (i.e. activated). In vivo, full activity is prevented by inhibitory binding of sugar phosphate molecules to the catalytic site (Andralojc et al., 2012). In illuminated leaves, the ~4:1 molar ratio of RuBP:catalytic sites leads to RuBP binding to non-activated catalytic sites being the almost exclusive cause of inactivation (Price et al., 1995). Upon cellular protein extraction, the levels of available RuBP deteriorate, facilitating RuBP dissociation (and fixation) and catalytic site activation. As shown in Fig. 1A (time zero), Rubisco from newly expanded upper canopy tobacco leaves is ~80% activated in vivo. At 25 °C, full activation (i.e. dissociation of all inhibitory RuBP) of tobacco Rubisco in all three leaf samples tested occurred within ~5min in vitro and full activity was maintained over the 20min test period.
Fig. 1.
Measurement of Rubisco activation status, maximal activity, and stability in vitro at 25 °C. Soluble cellular protein rapidly extracted from tobacco and each phytoplankton in CO2-free extraction buffer (containing 5mM MgCl2) was used to measure changes in the Rubisco 14CO2 fixation rate after activating the extract for 0–20min in buffer containing 15mM MgCl2 and 15mM NaHCO3. Gray shading indicates the time when protein extract was assayed to quantify k cat c, K C, and K O (Table 1). Data represent measures from duplicate biological samples (± SD).
In contrast to the control tobacco Rubisco in vitro activation assays, the activation status of Rubisco in each phytoplankton species was lower, varying between ~20% (Thalassiosira oceania, T. weissflogii, Cylindrotheca fusiformis, and Phaeodactylum tricornutum) to ~70% (Skeletonema ardens). Accordingly, longer incubation times at 25 °C were required to activate their Rubisco fully in vitro (Fig. 1B–H). Nevertheless, in all phytoplankton samples, Rubisco was fully activated within 10min of extraction at 25 °C and the activity was stable for at least a further 10min.
Rubisco kinetics are highly variable both between and within diatom species.
Measurements of k cat c, K C, maximum oxygenation rates (k cat o), K m for O2 (K O), and the specificity for carboxylase over oxygenase (SC/O) were measured at 25 °C to allow for direct comparison with other Rubisco kinetics in the literature. As shown in Table 1, there is significant variation in diatom Rubisco catalysis, with differences even found between Rubisco from the same genus (Chaetoceros). Also included in these analyses were control catalysis measurements for Rubisco from tobacco (C3 plant) and maize (C4 plant) whose values match those previously measured (Supplementary Table S1 at JXB online). In the following, we compare our results with kinetics measured at 25 °C for other Form ID Rubiscos from red algae and Form IB Rubisco from C3 and C4 plants (taken from Badger et al., 1998; Savir et al., 2010).
Among the diatom Rubiscos analyzed, there was a <2-fold variation in k cat c, which ranged from 2.1±0.2s−1 in Bellerochea cf. horologicalis to 3.7±0.2s−1 in C. fusiformis. As shown in Fig. 2A, k cat c varied significantly between the groups (one-way ANOVA, F=25.1, P<0.001). Further testing with Tukey HSD showed that diatom k cat c values were comparable with those of Rubisco from C3 plants but statistically lower than those from C4 plants (P<0.01) and higher than those from red algae (P<0.01). Diatom Rubisco also showed diversity in the oxygenation rates (Fig. 2B). The measured k cat o values of 0.4–1.6s−1 were significantly lower than those of C3 plants, but not lower than those of C4 plants or red algae (one-way ANOVA, F=5.25, P=0.008, Tukey HSD between diatoms and C3 plants P=0.007).
With regard to CO2 affinity, the K C values of diatom Rubisco varied >2.7 fold (25–68 μM; Fig. 2C) and were significantly higher than those of Rubisco from red algae, and C3 and C4 plants (one-way ANOVA, F=19.5, P<0.001, Tukey HSD P<0.01 for all three pairs). Only cyanobacteria have a lower CO2 affinitty than diatoms, with K C values in the range 200–260 μM, which accords with the effectiveness of their CCM (Price et al., 2008; Whitney et al., 2011; Hopkinson et al., 2014). In contrast, diatom Rubisco K O values were not statistically different from those of red algae, C3, or C4 plants (one-way ANOVA, F=1.44, P=0.26). Of particular interest was the low O2 affinity of T. weissflogii Rubisco whose K O exceeded 2mM O2.
Improving the SC/O of Rubisco, without unfavorably changing its other kinetic parameters, is a prized goal as it has a pervasive influence on Rubisco efficiency in organisms both with and without CCMs (Long et al., 2015). While the SC/O range in C3 and C4 plants shows limited diversity, a 2-fold variation was found in the SC/O of diatom Rubisco at 25 °C (i.e. 57–116mol mol−1; Table 1) and encompassed the range found previously in diatoms (Badger et al., 1998; Whitney et al., 2001; Haslam et al., 2005). Despite this large variation, the SC/O of diatom Rubisco was not significantly different from that of C3 and C4 plants, and failed to reach the high SC/O of Rubisco from red algae (Fig. 2E). In terms of carboxylation efficiency (k cat c /K C), we find that diatom Rubisco is low compared with red algae, and C3 and C4 plants (one-way ANOVA, F=11.5, P=0.0002, Fig. 2F, Tukey HSD P=0.04, P=0.001, and P=0.005, respectively), even in the presence of ambient O2 levels (i.e. k cat c/K C 21%O2; Table 1).
Novel kinetic relationships of diatom Rubisco
A number of trade-offs between Rubisco kinetic parameters have been observed across a range of primary producers (Tcherkez et al., 2006; Savir et al., 2010). From the relatively small data set examined, relationships between the varied Rubisco kinetic parameters (i.e. K C, k cat c, k cat o, SC/O, and K O) appear confined to a one-dimensional landscape, with simple power law correlations between parameters (Savir et al., 2010). We examined these relationships by comparing the kinetic parameters of diatom Rubisco with those measured in green algae, red algae, and plants using data sets of Tcherkez et al. (2006), Savir et al. (2010), Whitney et al. (2011), and Galmes et al. (2014) (see Supplementary Table S1).
As shown recently by Tcherkez (2015), the well-documented trade-off between K C and k cat c varies between organisms. For plant and algal Form I Rubisco, the correlation between K C and k cat c varies from that seen for Form I Rubisco from cyanobacteria and other prokaryotes (Tcherkez, 2013). With regard to eukaryotic Form I Rubisco, the diatom variants uniquely show no correlation between K C and k cat c (Fig. 3A). The linear correlation of r 2=0.36, P=1.1×10–6 between k cat c versus K C for plant and eukaryotic algal Form I Rubisco (Fig. 3A, gray circles), became insignificant when diatom data were included (Fig. 3A, black circles, r 2=0.013, P=0.36). This suggests that the catalytic mechanism of diatom Rubisco may differ relative to other eukaryotic variants—possibly through changes to one or more of the elemental steps in the Rubisco catalytic cycle.
A positive linear correlation between K C and K O was found among diatom Rubisco (r 2=0.43, P=0.041; Fig 3B) that was strengthened when the outlying high K O value (i.e. low O2 affinity) for T. weissflogii Rubisco was removed (r 2=0.83, P=5.8×10–4). Form 1 Rubisco from other plants and eukaryotic algae also displays a significant positive correlation (r 2=0.24, P=3.73×10–4), and the strength of this relationship increases (r=0.41, P=5.0×10–8) when the diatom measurements are included.
Diatom Rubiscos also showed no statistically significant relationship between carboxylase efficiencies and SC/O (r 2<0.002, P=0.5; Fig 3C). Although diatom carboxylation efficiencies are lower than those of higher plant Rubisco (Fig 2F), the diatom SC/O values are within a similar range, indicating that oxygenase efficiencies must also be correspondingly lower than those of plants, probably due to the lower k cat o rates of diatom Rubisco (Fig 2B).
While a weak positive relationship between k cat o and K O has been shown by Tcherkez (2015), we found that such a correlation did not extend to diatom Rubisco (r 2=0.38, P =0.078) (Fig. 3D, black circles) or when combined with plant and other eukaryotic algal Rubisco (Fig. 3D, gray circles). In addition, no relationship between k cat c and either K C/K O or SC/O was evident in the diatom Rubisco data set, although for these comparisons our values fall within the noise of the data analyzed by Savir et al. (2010). Linear regressions were tested between all kinetic parameters of diatom Rubiscos and are shown in Table 2.
Rubisco carboxylation efficiency versus Rubisco content
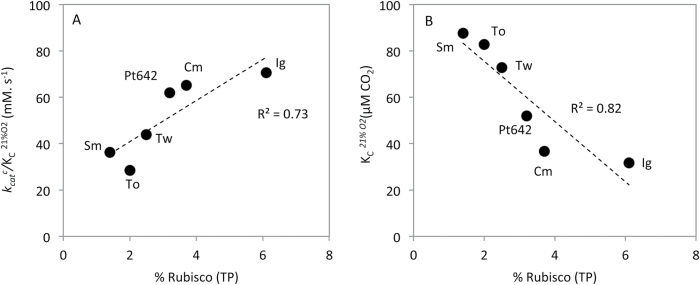
Compared with C3 plants, the faster carboxylation rates of Rubisco in C4 plants enable them to invest less of their N resources in Rubisco (Seemann et al., 1984; Ghannoum et al., 2005). We therefore compared the Rubisco content measured in a range of diatoms and the Haptophyte Isochrysis galbana in exponentially growing cells (same strains grown under the same conditions; see Losh et al., 2013) against our measured values of k cat c and K C (Table 1). We found no relationship between Rubisco content and k cat c, but observed a positive correlation between Rubisco content and carboxylation efficiency in the presence of 21% O2 (k cat c/K C 21%O2), which is driven by the negative correlation of Rubisco content with K C 21%O2 (Fig. 4). These relationships are also apparent when Rubisco content is plotted against carboxylation efficiency and K C in the absence of O2, but with a lower correlation coefficient.
Fig. 4.
Relationships of Rubisco content to catalysis. (A) Rubisco content as a percentage of total cellular protein (% TP) is positively correlated with carboxylation efficiency in 21% O2 (k cat c/K C 21%O2). (B) This is largely driven by the negative correlation of Rubisco content with K C 21%O2. Rubisco content was taken from Losh et al. (2013). I. galbana (Ig), C. muelleri (Cm), P. tricornutum 642 (Pt642), T. weissflogii (Tw), T. oceanica (To), and S. marinoi (Sm).
Discussion
In this study, we demonstrate the large catalytic diversity of Rubisco among 11 diatom species. A unique property of diatom Rubisco is that it lacks the relationship between CO2-fixing speed (k cat c) and CO2 affinity (K C) shared by many higher plant and algal Form I Rubisco isoforms. Our measured KC values for diatom Rubisco show larger diversity than observed in contemporary plant Form I Rubisco and, as a result, would require different CO2 concentrations to saturate Rubisco. Our data suggest that the current estimates of CO2 concentrations at the site of Rubisco in diatoms are significantly underestimated for some species. In addition, there is an unexpected negative relationship between K C 21%O2 and the Rubisco content in diatoms that suggests a trade-off in the allocation of resources (energy and nutrients) between investing in Rubisco production to sustain rates of photosynthesis for competitive growth or enhancing CCM capacity to sustain saturating CO2 levels for Rubisco function.
No significant relationship exists between k cat c and K C
A positive relationship between k cat c and K C has been found in all Rubiscos studied to date, although there are increasing indications from wider surveys of Rubisco kinetics (Galmes et al., 2014) that the relationship shared by plant and algal Rubisco differs from that observed for prokaryotic Form I Rubisco (Tcherkez, 2013, 2015). Remarkably, diatom Rubiscos in this study show no relationship between K C and k cat c, whereby their C3 plant-like k cat c values (Fig. 2A) contrast with atypically high K C values that generally exceed those of C4 plant Rubisco (Fig. 2C). The lack of relationship is surprising as the trade-off between K C and k cat c is thought to be due to a fundamental mechanistic constraint of their inter-related rate constants (Tcherkez et al., 2006, 2012). However, differences in the relationships of k cat c and K C between different photosynthetic groups may arise from differences in the intrinsic equilibrium of the RuBP enolization reaction (Tcherkez, 2013, 2015). More study is needed into the enolization, CO2/O2 addition, hydration, and cleavage reactions of Rubisco from diatoms (and other microalgae) to understand fully the extent and mechanistic foundation for the contrasting relationships between k cat c and K C found in nature.
High K C values require higher concentrations of CO2 to saturate
Diatom Rubisco K C values are significantly higher than the concentration of CO2 in seawater (~10 μM at 25 °C). Like C4 plants, cyanobacteria, and many other photosynthetic organisms, diatoms are rarely limited by CO2 for growth because they possess a CCM. The CCM varies between species, but all combine both morphological (such as the pyrenoid, carboxysome, and bundle sheath in diatoms, cyanobacteria, and C4 plants, respectively) and biochemical (e.g. carbonic anhydrases and bicarbonate transporters) specialization to provide high CO2 concentrations around the site of Rubisco. In C4 plants, CO2 concentrations within the bundle sheath chloroplasts are in excess of 160 μM (i.e. >5000 μbar; Furbank and Hatch, 1987) relative to the subsaturating CO2 concentrations in C3 chloroplasts (<10 μM). Similarly, the CCM of cyanobacteria provides highly saturating CO2 levels of >400 μM within the carboxysome for their Rubisco (assuming a pH of 7.35 and a 15mM inorganic carbon pool; Hopkinson et al., 2014; Whitehead et al., 2014).
Experimental determination of concentrations of CO2 and O2 in pyrenoids of diatoms is currently impossible. This has necessitated CO2 levels being modeled according to conceptual understanding of the diatom CCM and its Rubisco kinetics. Based on a K C 21%O2 of ~41 μM for P. tricornutum Rubisco (Whitney et al., 2001), a pyrenoid CO2 concentration of ~110 μM was modeled (Hopkinson, 2014). While only 9-fold higher than surface seawater CO2 concentrations, this CO2 level would give ~75% saturation of Rubisco carboxylase activity. As shown in Table 1, this assumed K C 21%O2 value is at the lower end of the values measured in our study, indicating that most diatom species would require higher concentrations of CO2 in the pyrenoid to attain similar saturation. For example, from our measures of K C 21%O2 (37–88 μM) the pyrenoid CO2 concentrations would need to range between ~148 μM and 352 μM for 80% saturation (or ~92–272 μM in the absence of O2). This adjustment would suggest that CO2 concentrations in the pyrenoid are similar to, and possibly exceed, those measured in C4 plant bundle sheath cells; see above and Furbank and Hatch (1987).
Energetic investment in the CCM and Rubisco
The single-cell physiology of phytoplankton and variable nutrient availability of marine ecosystems require their expedient use and probably restricts the energy and resources that can be expended on photosynthesis. The investment of cellular resources in Rubisco synthesis is probably limited to the minimum concentration required for growth (Losh et al., 2013) albeit in balance with the requirements of other cellular processes, such as the high energy costs of a CCM. Notably the resource investment by diatoms in Rubisco [e.g. 2–6% (w/w) of cellular protein; Fig. 3] is much smaller than that in the leaf soluble protein of C3 plants [25–50% (w/w)] and C4 plants [10–40% (w/w)] where it accounts for 5–25% of leaf N (Ghannoum et al., 2005; Carmo-Silva et al., 2015). The energy cost of a CCM depends on the capacity of phytoplankton to actively take up inorganic carbon at a rate that is proportional to the diffusive loss of CO2 from the cell, which is influenced by the internal:external CO2 gradient and the permeability of the pyrenoid to CO2 (Hopkinson et al., 2011; Raven et al., 2014; Raven and Beardall, 2016). Diatoms appear well adapted to maintaining near-saturating CO2 levels around Rubisco (Tortell et al., 2000; Rost et al., 2003; Chen and Gao, 2004; Kranz et al., 2015) by regulating CCM activity in response to varying extracellular CO2 (Chen and Gao, 2004; Hennon et al., 2015; Young et al., 2015). This ‘energy minimization’ strategy in CCM regulation and Rubisco production probably provides an N minimization strategy and a significant growth advantage to diatoms (Giordano et al., 2005; Raven et al., 2011).
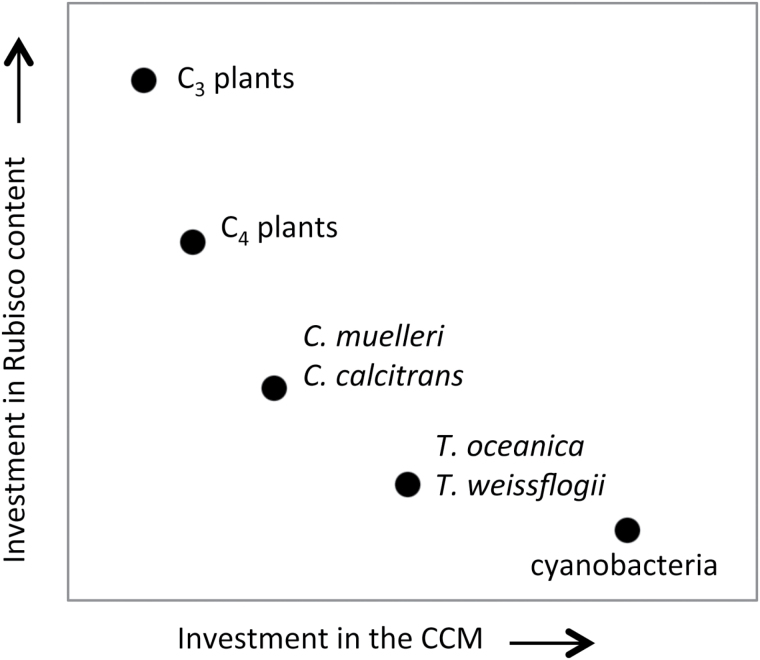
Our study provides evidence for linkages between Rubisco CO2 affinity and the efficiency of the CCMs in diatoms. Unlike in C4 plants where improvements in k cat c correlate with reduced N investment in Rubisco (Ghannoum et al., 2005), diatom Rubisco content was positively correlated with carboxylation efficiency (Fig. 4A) not k cat c. This correlation was governed primarily by the strong negative relationship between Rubisco content and K C (both with and without 21% O2; Fig. 4B). This leads us to hypothesize that diatoms balance resource allocation for photosynthesis between Rubisco content or the CCM. Diatoms such as Thalassiosira and Skeletonema species maintain low Rubisco content but require more resource allocation to their CCM to saturate their low-CO2 affinity (high K C) Rubiscos. Alternatively, diatoms such as Phaeodactylum and Chaetoceros species have higher Rubisco content and lower K C values, requiring lower CO2 concentrations for saturation of carboxylation (Fig. 4). As summarized in Fig. 5, this hypothesis infers an evolutionary trade-off between Rubisco kinetics and content in diatoms; that is, the level of energy invested by diatoms in their CCM to attain CO2 concentrations suited to their Rubisco CO2 affinity, not its k cat c, influences resource availability for cellular metabolism, that includes Rubisco synthesis.
Fig. 5.
Resource allocation hypothesis for the balance between Rubisco content and K C values. Organisms that invest their energy resources heavily in the CCM (e.g. cyanobacteria) to maintain high intracellular CO2 levels to saturate Rubisco and limit photorespiration are able to reduce their resource investment in Rubisco. At the other end of the spectrum, organisms without a CCM (e.g. C3 plants) have Rubisco that is undersaturated with CO2 and require large resource investments in Rubisco content to maintain adequate rates of carbon fixation for survival. Organisms with a CCM fall somewhere along the saturation curve, depending on the carboxylation speed of their Rubisco and their potential to balance the investment of resources in Rubisco biogenesis suitably or maintain elevated intercellular CO2 levels around Rubisco.
Co-evolution of Rubisco and CCMs
Variations in the mechanistic chemistry of diatom Rubisco and plasticity in CCM efficiency among diatom species may explain the non-canonical kinetic features identified for diatom Rubisco (Fig. 3). Although the common ancestor of diatoms was thought to have gained a chloroplast from secondary endosymbiosis of a red alga ~1.2 billion years ago (Yoon et al., 2002), diatoms only began to appear in the fossil record ~200 million years ago (Brown and Sorhannus, 2010). When, or how, the subsequent falling CO2:O2 levels triggered the evolution of marine CCMs remains uncertain (Raven et al., 2012). Notably, although sharing similar Form ID Rubiscos, the SC/O and carboxylation efficiencies of red algae are much higher than those of diatoms and, in some cases, those of higher plants (Whitney et al., 2001). Comparative analyses of the large subunit for a small number of Form ID Rubiscos showed a clear signal of positive selection both between and within the major algal groups (Young et al., 2012). In particular, positive selection was detected along the basal branch leading to diatoms, including between the centric diatom Thalassiosirales and Chaetocerales species, which accords with the very different K C values we observed in this study. Further work is needed to understand adaptation of Form ID Rubisco and to elucidate the amino acid changes in the large and/or small subunits responsible for the wide range of kinetic parameters observed in this study.
A requirement for Rubisco activase in diatoms
A unique outcome of this study was the finding of the low and variable activation status of Rubisco in diatoms (~20–70%, Fig. 1) that implies a requirement for accessory factors for functional maintenance. As in higher plants, deactivation of diatom Rubisco probably results from loss of Mg2+ and the carbamyl prosthetic group in the catalytic site, allowing for autoinhibition by substrate RuBP binding (to form ‘ER’ complexes). Likewise, binding of other inhibitory sugar phosphate molecules might occur to fully activated Rubisco (Andralojc et al., 2012). Removal of the sugar phosphate is facilitated by Rubisco activase (RCA) via conformational remodeling driven by ATP hydrolysis (Mueller-Cajar et al., 2014). In nature, differing types of RCA have independently evolved among plants, non-green algae (called CbbX; Mueller-Cajar et al., 2013), and photosynthetic prokaryotes (called CbbOQ; Tsai et al., 2016). While an RCA function in diatoms has not been demonstrated, they encode a chloroplast and nuclear cbbX gene (Kroth, 2015), consistent with our findings of a need for Rubisco activity regulation. The low activation status of diatom Rubisco somewhat parallels the low Rubisco activation status in C4 plants (~40–60%; von Caemmerer and Furbank, 2003) and in C3 plants grown at high CO2 (e.g. it is <50% in tobacco grown in air containing 0.3% v/v CO2; Whitney et al., 1999). Understanding how the regulatory properties of diatom RCA and the activation status of Rubisco differ in response to environmental conditions (e.g. temperature, illumination, CO2, and nutrients) and impact the resource use efficiency of diatoms have yet to be examined.
Photorespiration in diatoms
Analogous to other Form I Rubisco (Savir et al., 2010; Galmes et al., 2014), diatom Form ID Rubisco showed a positive relationship between K C and K O (Fig. 3B). This indicates that unwanted reductions in CO2 affinity (i.e. increasing K C) are complemented by favorable reductions in O2 affinity (i.e. increasing K O). These kinetics help to ensure that the rates of photorespiration are not exacerbated in diatoms. The corresponding effect of specificity for CO2 over O2 (SC/O) is quite variable, spanning values that match or exceed the SC/O of C3 and C4 plant Rubisco (Fig. 2E). As highlighted by Whitney et al. (2001), the lower carboxylation efficiencies under 21% O2 (k cat c/K C 21%O2) shared by all diatom Rubiscos relative to plant Rubisco (Table 1) precludes them from being more efficient enzymes within the context of a C3 plant chloroplast—irrespective of the higher SC/O values measured for some diatom Rubisco variants.
As in other CCM-containing organisms, the rates of photorespiration in diatoms will be suppressed under the higher CO2 concentrations maintained around Rubisco in the pyrenoid. To date, however, little is known about the photorespiration process and rate in diatoms (Schnitzler Parker et al., 2004; Rigobello-Masini et al., 2012). As indicated above, resolving our understanding of diatom CCMs, and the O2 levels in the pyrenoid, is vital to assessing the susceptibility of diatoms to photorespiration. One interpretation from the wide range in K O and SC/O values measured between the diatom species is that the CO2:O2 pressures within the pyrenoid may differ dramatically. This may arise through variation in the effectiveness of their CCM to concentrate CO2 or through reduced permeability to O2, or both. Particulaly curious is the elevated O2 insensitivity (i.e. high K O value) of Rubisco from the centric diatom T. weissflogii. This suggests that there may be different biochemical constraints on the Rubisco in this species worthy of further study.
Conclusions
Primary production in the oceans is dominated by phytoplankton, with diatoms accounting for ~20% of global primary production (Falkowski and Raven, 2007). Here we provide the largest data set of diatom Rubisco kinetics that identifies large catalytic diversity and novel relationships in their properties. The data suggest that the CCM of diatoms is highly diverse and capable of concentrating CO2 in the pyrenoid to higher levels than currently envisaged. Our findings also suggest that the CO2 affinity of diatom Rubisco is a key indicator for how these microalgae manage the allocation of their relatively scarce cellular resources between Rubisco biogenesis and components of their CCM. This work highlights the importance of further studying the phylogenetically diverse, non-terrestrial, Rubisco isoforms to decipher potential mechanistic differences in the catalytic chemistry of the Form I Rubisco superfamily.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Rubisco kinetics at 25 °C taken from other data sets and used in Fig. 2
Acknowledgements
We thank the reviewers for helpful comments that improved the article. Funding for JNY was through ANU visiting scholar (CE140100015) and NSF Grant 1040965. AH was funded through a Clarendon Scholarship, Oxford and ANU visiting scholar (CE140100015). RES was funded through ARC DECRA scheme (DE13010760). REMR was funded through ERC Starting Grant (SP2-GA-2008–200915), FMMM was funded through NSF Grant 104095, and SMW was funded through Australian Research Council Grant CE14010001
References
- Andralojc PJ, Madgwick PJ, Tao Y, et al. 2012. 2-Carboxy-d-arabinitol 1-phosphate (CA1P) phosphatase: evidence for a wider role in plant Rubisco regulation. Biochemical Journal 442, 733–742. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Lee C, Hedges JI, Honjo S, Wakeham SG. 2001. A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep Sea Research Part II: Topical Studies in Oceanography 49, 219–236. [Google Scholar]
- Badger MRT, Andrews J, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany 76, 1052–1071. [Google Scholar]
- Brown, JW. and Sorhannus, U. (2010) A molecular genetic timescale for the diversification of autotrophic Stramenophiles (Ochrophyta): Substantive underestimation of putative fossil ages. PLoS ONE 5(9):e12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva E, Scales JC, Madgwick PJ, Parry MAJ. 2015. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant, Cell and Environment 38, 1817–1832. [DOI] [PubMed] [Google Scholar]
- Chen X, Gao K. 2004. Photosynthetic utilisation of inorganic carbon and its regulation in the marine diatom Skeletonema costatum . Functional Plant Biology 31, 1027–1033. [DOI] [PubMed] [Google Scholar]
- Delwiche CF, Palmer JD. 1997. The origin of plastids and their spread via secondary symbiosis. In: Bhattacharya D, ed. The origins of algae and their plastids. Vienna: Springer-Verlag, 53–86. [Google Scholar]
- Egan KE, Rickaby REM, Hendry KR, Halliday AN. 2013. Opening the gateways for diatoms primes Earth for Antarctic glaciation. Earth and Planetary Science Letters 375, 34–43. [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299. [DOI] [PubMed] [Google Scholar]
- Falkowski P, Schofield O, Katz M, Van de Schootbrugge B, Knoll A. 2004. Why is the land green and the ocean red? In: Thierstein H, Young J, eds. Coccolithophores. Berlin: Springer, 429–453. [Google Scholar]
- Falkowski PG, Raven JA. 2007. Aquatic photosynthesis, Vol. 2 Princeton, NJ: Princeton University Press. [Google Scholar]
- Furbank RT, Hatch MD. 1987. Mechanism of C4 photosynthesis: the size and composition of the inorganic carbon pool in bundle sheath cells. Plant Physiology 85, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmes J, Kapralov MV, Andralojc PJ, Conesa MÀ, Keys AJ, Parry MAJ, Flexas J. 2014. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant, Cell and Environment 37, 1989–2001. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S. 2005. Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C(4) grasses. Plant Physiology 137, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Beardall J, Raven JA. 2005. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131. [DOI] [PubMed] [Google Scholar]
- Haslam RP, Keys AJ, Andralojc PJ, Madgwick PJ, Andersson I, Grimsrud A, Eilertsen HC, Parry MAJ. 2005. Specificity of diatom Rubisco. In: Omasa K, Nouchi I, De Kok LJ, eds. Plant responses to air pollution and global change. Springer: Japan, 157–164. [Google Scholar]
- Hauser T, Popilka L, Hartl FU, Hayer-Hartl M. 2015. Role of auxiliary proteins in Rubisco biogenesis and function. Nature Plants 1, 15065. [DOI] [PubMed] [Google Scholar]
- Hennon GMM, Ashowrth J, Groussman RD, Berthiaume C, Morales RL, Baliga NS, Orellana MW, Armbrust EV. 2015. Diatom acclimation to elevated CO2 via novel gene clusters and cAMP-signaling. Nature Climate Change 5, 761–765. [Google Scholar]
- Heureux AMC, Rickaby REM. 2015. Refining our estimate of atmospheric CO2 across the Eocene–Oligocene climatic transition. Earth and Planetary Science Letters 409, 329–338. [Google Scholar]
- Hopkinson B. 2014. A chloroplast pump model for the CO2 concentrating mechanism in the diatom Phaeodactylum tricornutum . Photosynthesis Research 121, 223–233. [DOI] [PubMed] [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM. 2011. Efficiency of the CO2-concentrating mechanism of diatoms. Proceedings of the National Academy of Sciences, USA 108, 3830–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson BM, Young JN, Tansik AL, Binder BJ. 2014. The minimal CO2-concentrating mechanism of Prochlorococcus spp. MED4 is effective and efficient. Plant Physiology 166, 2205–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane HJ, Viil J, Entsch B, Paul K, Morell MK, Andrews TJ. 1994. An improved method for measuring the CO2/O2 specificity of ribulose-bisphosphate carboxylase-oxygenase. Australian Journal of Plant Physiology 21, 449–461. [Google Scholar]
- Kane HJ, Wilkin J-M, Portis AR, John Andrews T. 1998. Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate. Plant Physiology 117, 1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz SA, Young JN, Hopkinson B, Goldman JAL, Tortell PD, Morel FMM. 2015. Low temperature reduces the energetic requirement for the CO2 concentrating mechanism in diatoms. New Phytologist 205, 192–202. [DOI] [PubMed] [Google Scholar]
- Kroth PG. 2015. The biodiversity of carbon assimilation. Plant Physiology 172, 76–81. [DOI] [PubMed] [Google Scholar]
- Laing WA, Ogren WL, Hageman RH. 1974. Regulation of soybean net photosynthetic CO2 fixation by the interaction of CO2, O2, and ribulose 1,5-diphosphate carboxylase. Plant Physiology 54, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long BM, Bahar NHA, Atkin OK. 2015. Contributions of photosynthetic and non-photosynthetic cell types to leaf respiration in Vicia faba L. and their responses to growth temperature. Plant, Cell and Environment 38, 2263–2276. [DOI] [PubMed] [Google Scholar]
- Long SP. 1999. Environmental responses. In: Sage RF, Monson RK, eds. C4 plant biology. Academic Press: San Diego, 215–249. [Google Scholar]
- Losh JL, Young JN, Morel FM. 2013. Rubisco is a small fraction of total protein in marine phytoplankton. New Phytologist 198, 52–58. [DOI] [PubMed] [Google Scholar]
- Mueller-Cajar O, Stotz M, Bracher A. 2014. Maintaining photosynthetic CO2 fixation via protein remodelling: the Rubisco activases. Photosynthesis Research 119, 191–201. [DOI] [PubMed] [Google Scholar]
- Mueller-Cajar O, Whitney S. 2008. Directing the evolution of Rubisco and Rubisco activase: first impressions of a new tool for photosynthesis research. Photosynthesis Research 98, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tanaka A, Matsuda Y. 2013. SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proceedings of the National Academy of Sciences, USA 110, 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DM, Tréguer P, Brzezinski MA, Leynaert A, Quéguiner B. 1995. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochemical Cycles 9, 359–372. [Google Scholar]
- Osborne CP, Sack L. 2012. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM. 2013. Rubisco activity and regulation as targets for crop improvement. Journal of Experimental Botany 64, 717–730. [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Niessen M, Kebeish RM. 2008. Metabolic engineering towards the enhancement of photosynthesis. Photochemistry and Photobiology 84, 1317–1323. [DOI] [PubMed] [Google Scholar]
- Pierce J, Tolbert NE, Barker R. 1980. Interaction of ribulose-bisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry 19, 934–942. [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. 2008. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany 59, 1441–1461. [DOI] [PubMed] [Google Scholar]
- Price GD, Evans JR, von Caemmerer S, Yu J-W, Badger MR. 1995. Specific reduction of chloroplast glyceraldehyde-3-phosphate dehydrogenase activity by antisense RNA reduces CO2 assimilation via a reduction in ribulose bisphosphate regeneration in transgenic tobacco plants. Planta 195, 369–378. [DOI] [PubMed] [Google Scholar]
- Raven J, Beardall J, Giordano M. 2014. Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynthesis Research 121, 111–124. [DOI] [PubMed] [Google Scholar]
- Raven J, Giordano M, Beardall J, Maberly SC. 2011. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynthesis Research 109, 1–16. [DOI] [PubMed] [Google Scholar]
- Raven JA. 2009. Contributions of anyoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquatic Microbial Ecology 56, 177–192. [Google Scholar]
- Raven JA, Beardall J. 2016. The ins and outs of CO2 . Journal of Experimental Botany 67, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Giordano M, Beardall J, Maberly SC. 2012. Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read BA, Tabita FR. 1994. High substrate specificity factor ribulose bisphosphate carboxylase/oxygenase from eukaryotic marine algae and properties of recombinant cyanobacterial rubisco containing ‘algal’ residue modifications. Archives of Biochemistry and Biophysics 312, 210–218. [DOI] [PubMed] [Google Scholar]
- Reinfelder JR. 2010. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annual Review of Marine Science 3, 291–315. [DOI] [PubMed] [Google Scholar]
- Reinfelder JR, Kraepiel AM, Morel FM. 2000. Unicellular C4 photosynthesis in a marine diatom. Nature 407, 996–999. [DOI] [PubMed] [Google Scholar]
- Reinfelder JR, Milligan AJ, Morel FMM. 2004. The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiology 135, 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigobello-Masini M, Penteado JCP, Tiba M, Masini JC. 2012. Study of photorespiration in marine microalgae through the determination of glycolic acid using hydrophilic interaction liquid chromatography. Journal of Separation Science 35, 20–28. [DOI] [PubMed] [Google Scholar]
- Roberts K, Granum E, Leegood RC, Raven JA. 2007. Carbon acquisition by diatoms. Photosynthesis Research 93, 79–88. [DOI] [PubMed] [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sültemeyer D. 2003. Carbon acquisition of bloom-forming marine phytoplankton. Limnology and Oceanography 48, 55–67. [Google Scholar]
- Sage RF. 2001. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biology 3, 202–213. [Google Scholar]
- Sage RF. 2004. Tansley review: the evolution of C4 photosynthesis. New Phytologist 161, 341–371. [DOI] [PubMed] [Google Scholar]
- Savir Y, Noor E, Milo R, Tlusty T. 2010. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proceedings of the National Academy of Sciences, USA 107, 3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler Parker M, Armbrust E, Piovia-Scott J, Keil RG. 2004. Induction of photorespiration by light in the centric diatom Thalassiosisra weissflogii (Bacillariophyceae): molecular characterization and physiological consequences. Journal of Phycology 40, 557–567. [Google Scholar]
- Seemann JR, Badger MR, Berry JA. 1984. Variations in the specific activity of ribulose-1,5-bisphosphate carboxylase between species utilizing differing photosynthetic pathways. Plant Physiology 74, 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharwood RE, von Caemmerer S, Maliga P, Whitney SM. 2008. The catalytic properties of hybrid rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source rubiscos and can support tobacco growth. Plant Physiology 146, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda WG, Price NM, Morel FMM. 2005. Trace metal ion buffers and their use in culture studies. In: Andersen RA, ed. Algal culturing techniques. Amsterdam: Elsevier, 35–63. [Google Scholar]
- Tabita FR, Satagopan S, Hanson TE, Kreel NE, Scott SS. 2008. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. Journal of Experimental Botany 59, 1515–1524. [DOI] [PubMed] [Google Scholar]
- Tcherkez G. 2013. Modelling the reaction mechanism of ribulose-1,5-bisphosphate carboxylase/oxygenase and consequences for kinetic parameters. Plant, Cell and Environment 36, 1586–1596. [DOI] [PubMed] [Google Scholar]
- Tcherkez G. 2015. The mechanism of Rubisco-catalyzed oxygenation. Plant, Cell and Environment 39 (5), 983–997. [DOI] [PubMed] [Google Scholar]
- Tcherkez GGB, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortell PD, Rau GH, Morel FM. 2000. Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnology and Oceanography 45, 1485–1500. [Google Scholar]
- Tsai YC, Lapina MC, Bhushan S, Mueller-Cajar O. 2016. Identification and characterization of multiple rubisco activases in chemoautotrophic bacteria. Nature Communications. 6, 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank R. 2003. The C4 pathway: an efficient CO2 pump. Photosynthesis Research 77, 191–207. [DOI] [PubMed] [Google Scholar]
- Way DA, Katul GG, Manzoni S, Vico G. 2014. Increasing water use efficiency along the C3 to C4 evolutionary pathway: a stomatal optimization perspective. Journal of Experimental Botany 65, 3683–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead L, Long BM, Price GD, Badger MR. 2014. Comparing the in vivo function of α-carboxysomes and β-carboxysomes in two model cyanobacteria. Plant Physiology 165, 398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SM, Baldet P, Hudson GS, Andrews TJ. 2001. Form I Rubiscos from non-green algae are expressed abundantly but not assembled in tobacco chloroplasts. The Plant Journal 26, 535–547. [DOI] [PubMed] [Google Scholar]
- Whitney SM, Houtz RL, Alonso H. 2011. Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiology 155, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SM, Sharwood RE. 2007. Linked Rubisco subunits can assemble into functional oligomers without impeding catalytic performance. Journal of Biological Chemistry 282, 3809–3818. [DOI] [PubMed] [Google Scholar]
- Whitney SM, von Caemmerer S, Hudson GS, Andrews TJ. 1999. Directed mutation of the Rubisco large subunit of tobacco influences photorespiration and growth. Plant Physiology 121, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh H-H, Badger MR, Watson L. 1980. Variations in Km(CO2) of ribulose-1,5-bisphosphate carboxylase among grasses. Plant Physiology 66, 1110–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Hackett JD, Pinto G, Bhattacharya D. 2002. The single, ancient origin of chromist plastids. Proceedings of the National Academy of Sciences, USA 99, 15507–15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JN, Bruggeman J, Rickaby REM, Erez J, Conte M. 2013. Evidence for changes in carbon isotopic fractionation by phytoplankton between 1960 and 2010. Global Biogeochemical Cycles 27, 505–515. [Google Scholar]
- Young JN, Kranz SA, Goldman JAL, Tortell PD, Morel FM. 2015. Antarctic phytoplankton down-regulate their carbon concentrating mechanisms under high CO2 with no change in growth rates. Marine Ecology Progress Series 532, 13–28. [Google Scholar]
- Young JN, Rickaby REM, Kapralov MV, Filatov DA. 2012. Adaptive signals in algal Rubisco reveal a hisotry of ancient atmospheric CO2 . Philosophical Transactions of the Royal Society B: Biological Sciences 367, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I, Schultes NP, Peterson RB, Brown P, Brutnell TP. 2009. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiology 149, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Jensen RG. 1991. Xyulose 1,5-bisphosphate synthesized by ribulose 1,5-bisphosphate carboxylase/oxygenase during catalysis binds to decarbamylated enzyme. Plant Physiology 97, 1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.