Abstract
Recombinant antibodies spurred a revolution in medicine that saw the introduction of powerful therapeutics for treating a wide range of diseases, from cancers to autoimmune disorders and transplant rejection with more applications looming on the horizon. Many of these therapeutic monoclonal antibodies (mAbs) are based on human immunoglobulin G1 (IgG1), or at least contain a portion of the molecule. Most mAbs require interactions with cell surface receptors for efficacy, including the Fc γ receptors (FcγRs). High-resolution structural models of antibodies and antibody fragments have been available for nearly forty years, however, a thorough description of the structural features that determine the affinity with which antibodies interact with human receptors is not known. In this review we will cover the relevant history of IgG-related literature and how recent developments have changed our view of critical antibody-cell interactions at the atomic level with a nod to outstanding questions in the field and future prospects.
Keywords: CD64, CD32, CD16, N-glycosylation, carbohydrate, protein-protein interaction, TRIM21, FcRn, low affinity Fc gamma receptor
Graphical abstract
I. Introduction
Immunoglobulin G1 (IgG1) is a dual-function molecule. Interactions between the fragment antigen binding (Fab) and target antigens are of high affinity, developed through clonal selection and an affinity maturation process that optimizes the amino acid sequence of the variable regions of both the IgG heavy and light chains. In the case of an invading pathogen, multivalent pathogen-specific IgGs will coat the surface of the pathogen (opsonization) through Fab regions that recognize surface antigens. This process clusters and orients the fragment crystallizable (Fc) region of IgG to interact with cell surface receptors including the FcγRs. The IgG Fc receptor family is comprised of one high affinity receptor (nM affinity), FcγRI, and several low affinity receptors (μM affinity), FcγRIIa, FcγRIIb, and FcγRIIIa 1–4. Fc elicits antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Fc is also capable of eliciting intracellular antibody-mediated degradation in a wide variety of cell types 5. This process is triggered when antibody-coated virions enter the cytoplasm and are recognized by the cytosolic Fc-binding protein TRIM21.
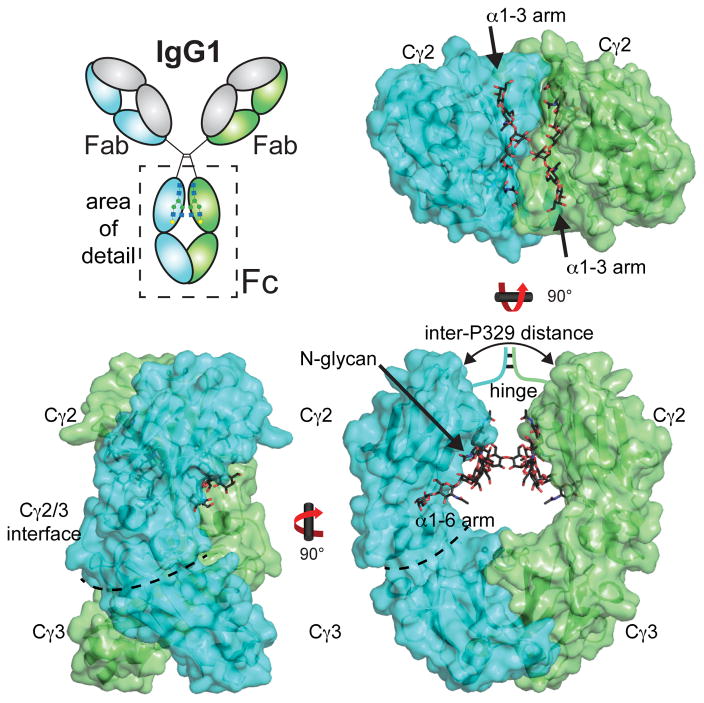
IgG1 is a heterotetramer made up of two “heavy” polypeptide chains and two “light” chains (Figure 1). The heterotetramer structure is covalently stabilized through disulfide bonds that link both heavy chains together in the hinge regions and link each “light” chain to a “heavy” chain. Fab and Fc both retain their individual functions after proteolytic separation, thus structure and acitivy-based investigation of the individual IgG components is informative of the entire molecule. Fc is released as a 52 kDa homodimer following papain digestion of the IgG1 molecule. Each monomer contains a Cγ2 domain and a Cγ3 domain (Figure 1). The Cγ3 domains of each monomer interact to form a strong non-covalent dimer interface. The Cγ2 domain is the site of many receptor interactions and contains a conserved asparagine 297-linked (N-linked) carbohydrate chain (glycan). This is a structural feature of note as the Fc N-glycan is required for interactions with receptors such as FcγRIIIa and FcγRIIa, but not FcγRI, TRIM21 and the neonatal Fc receptor (FcRn).
Figure 1.
Structure of IgG1 Fc (PDB 1L6X). Chain A of the homodimer is colored in cyan, and chain B in green.
IgG1 Fc is a popular target for studies of protein structure solved by x-ray crystallography and high-resolution models have been available for nearly forty years 6–26. Structural studies also revealed how Fc interacts with a wide variety of receptors through a diverse set of interfaces 9–11, 16, 17, 22, 27. A curious feature of all these models is the near complete resolution of the conserved Fc N-glycan 12–14, 21, 24. However, these models do not indicate why the N-glycan is necessary for proper IgG1 and mAb function. Preliminary studies indicate the behavior of the N-glycan is much more complex than these models reveal28, 29, and a hypothesis linking N-glycan structure with Fc receptor binding activity remains elusive.
It is clear that an approach integrating high-resolution structural methods and measurements of Fc affinity in solution will be required to thoroughly evaluate the Fc structure/activity relationship. Here we will present the results and interpretations of studies on human Fc using a wide range of solution and solid-state methods as well as in vitro measurements of Fc interactions with human receptors.
II. Structural aspects of IgG Fc
IIa. Cγ2 Domain Orientations
Domain orientations are a well characterized feature of many Fc models. The C-terminal half of the “heavy” polypeptide chain forms the IgG1 Fc homodimer (residues 225–447) 30. Though symmetric in solution with a two-fold rotational symmetry axis formed by dimer interface 31, Fc rarely crystallizes in a symmetric pose. Deviations from a symmetric structure are largely limited to the positions of the Cγ2 domains; the dimer interface formed by the Cγ3 domains appears structurally invariant. Differences in Cγ2 domain orientation suggest the Cγ2 domains are not rigid with respect to one another. This may be important for determining the role of Fc motions, particularly the Cγ2 domains, in receptor binding because Fc binds Fc gamma receptors (FcγRs) I, II and III via the lower hinge region between Cγ2 domains (Figure 2). Furthermore, FcγR I, II and III all form an interface with both Fc Cγ2 domains, thus, Cγ2 motion and relative domain orientation is thought to influence the Fc/FcγR interaction 15.
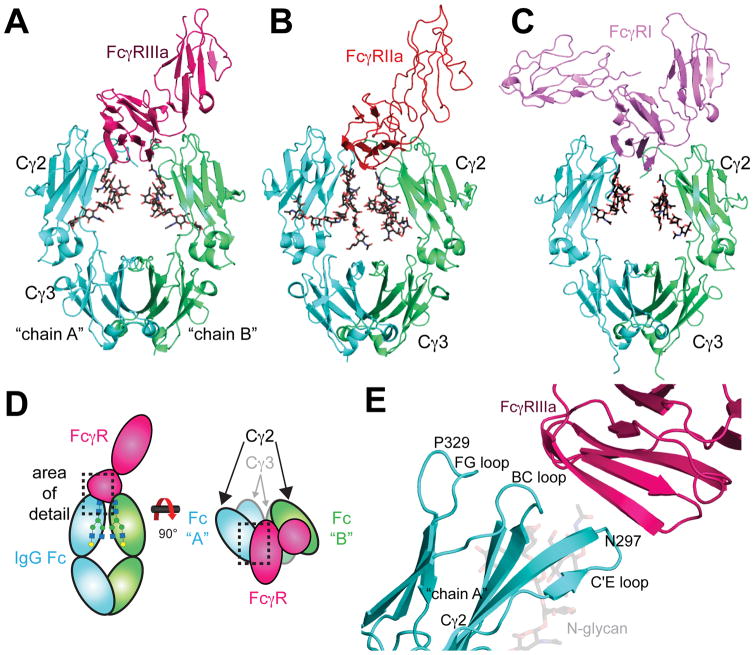
Figure 2.
A) Structural model of the Fc-FcγRIIIa interaction (PDB 3AY4) the (B) Fc-FcγRIIa interaction (PDB 3RY6) and (C) the Fc:FcγRI interaction (PDB 4X4M). Fc N-glycans are shown as black stick models. D) Schematic diagram of the Fc:FcγR interaction. E) Fc Cγ2 loops serve as the FcγRIIIa (pink) binding site. The C’E loop includes N297, the site of N-glycosylation. The Fc chain A monomer (cyan) shown to emphasize the locations of the loops structures (PDB 1E4K). The interaction between chain A and FcγRIIIa occurs primarily at the site of these Fc Cγ2 loops.
Several different parameters have been used to compare relative Fc domain orientations. These measurements provide a useful frame of reference for comparing Fc structures as determined by x-ray crystallography, but the biological relevance of these structural deviations is unclear. One commonly reported measurement is the distance between P329 residues 20, 21, 24. P329 is located in the FG loop of the Cγ2 domain (Figures 1,2). Inter-P329 distances describe the distance between Cγ2 domains in Fc. The smallest reported P329 distance is 18.9Å in an aglycosylated Fc structure 24. This observation lead to the hypothesis that aglycosylated Fc assumes a collapsed structure, and inferred that the N-glycan serves to prevent this collapsed conformation from forming and maintain the FcγR binding properties. However, an unpublished aglycosylated Fc structure, (PDB: 3DNK) has a P329 distance of 27.6 Å which is very similar to many glycosylated structures (27.4 Å for PDB 4KU1 8). It is currently unclear which observed Fc conformation more accurately reflects the likely ensemble of solution conformations. Extensive MD simulations indicate the Cγ2 domains access a significantly larger degree of motion than that described by x-ray crystallography (75–108° vs. 91–104°, respectively; 8)
Fc conformation is a complex property and it is unclear what range of conformations Fc samples and what effect this distribution has on receptor binding. Attempts to more fully describe Fc conformation include additional distance measurements (P238, F241, R301, and C1 atom of Man4 20, 21) or the definition of interdomain angles. Descriptions of simple three-point angles formed by the Cγ2 and Cγ3 domains 8, 9 or four-point dihedral angle between Cγ2-Cγ3 domains 8 allow description of the degree of Fc asymmetry. Regardless of the mode by which asymmetry is measured, it is clear from the multiple poses observed by x-ray crystallography and molecular dynamic simulations, the Cγ2 domains are mobile and the relative orientation in space is not required to be symmetric 8. The role of this conformational heterogeneity in Fc function remains undefined.
IIb. Cγ2-Cγ3 Interface
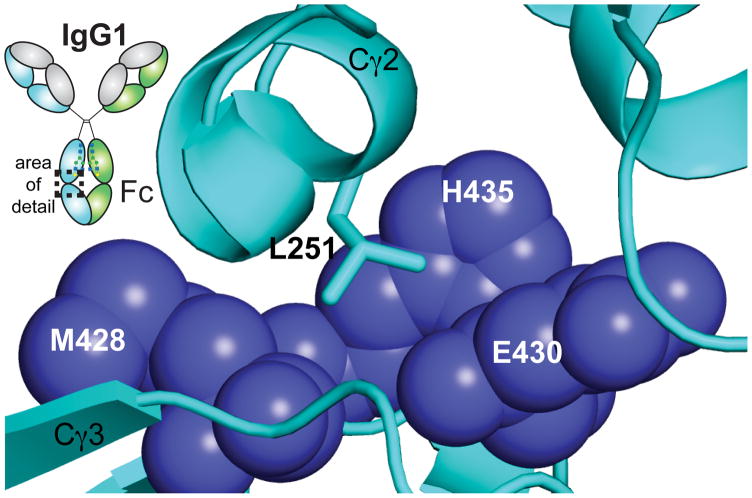
Two features likely contribute to restricting Cγ2 orientation. The disulfide-bonded hinge links the Cγ2 domains at the end of the Cγ2 distal to the pivot point formed by the Cγ2-Cγ3 domain interface. This interface likewise restricts the overall domain motions of the Cγ2 domains 8, 20. The Cγ2-Cγ3 interface is stabilized through two salt bridges, hydrogen bonds, and a hydrophobic “ball in socket” joint (Figure 3). Salt bridges formed by E380–K248 and E430–K338 are poised to restrict Cγ2 orientations. This hypothesis is supported by 200 ns MD simulations of the Fc E380A / E430A mutant that revealed increased flexibility of the Cγ2 domains when compared to wild-type Fc 8. Interestingly, The E380A mutant by itself appears to only affect FcRn binding while E430A reduces affinity for only FcγRIIIa 32. The side chain atoms of L251 forms the ball of the “ball-in-socket” joint and is found at the Cγ2-Cγ3 interface (Figure 3) 20.
Figure 3.
L251 (cyan stick model) forms the pivot point in a “ball-in-socket” joint that guides Cγ2 motions. E430, H435, and M428 of the Cγ3 domain form the socket (blue spheres).
IIc. The N297 Glycan
The structural role of conserved IgG1 Fc glycosylation at N297 is a topic of great interest. Therapeutic mAbs largely require appropriate N-glycosylation for activity, complicating drug manufacture as recombinant bacterial expression hosts do not express proteins with mammalian N-glycans. Furthermore, the Fc N-glycan is heterogeneous with respect to composition 33–35. The variability in Fc glycan composition, and glycans in general, originates because complex carbohydrate biosynthesis is not a template-driven process, unlike protein and nucleic acid biosynthesis. Compositional glycan diversity results from conserved, but variably complete, modifications by glycosyltransferases and glycosylhydrolases in the ER and Golgi complex 36, 37.
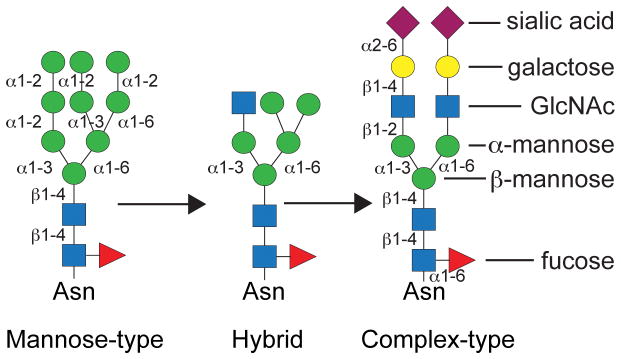
Two aspects of the Fc N-glycan composition indicate that it contributes to Fc interactions. The distribution of Fc N-glycan compositions changes in certain disease states and changes of the Fc N-glycan composition affect affinity for many FcγRs (both topics are covered in detail below). The Fc N-glycan is primarily of a biantennary, complex-type with a high level of core fucosylation (>95%; see Figure 4). A common motif found in all human Fc N-glycan structures is a heptasaccharide composed of chitobiose linked to N297 followed by a branching trimannose structure with terminating N-acetylglucosamine (GlcNAc) residues on the non-reducing branch termini (Figure 4). The N-glycan can be further decorated with terminal galactoses then sialic acids, and occasionally the addition of a bisecting GlcNAc residue. In general, the IgG Fc N-glycan is subject to less processing than most N-glycans, due to the interaction between N-glycan and polypeptide residues. In serum from healthy human subjects, the Fc N-glycan ranges from 30–35% GlcNAc terminated, ~50% Gal terminated, and 10–15% sialic acid terminated 38. The branched structure of the N-glycan is characterized by the linkage between the β-linked mannose residue at the branch point and the α-linked mannose residues that form the first residues of the branches at the non-reducing termini of the N-glycan. One of these branch mannose residues is linked by an α1–6 linkage and this forms the “6-arm” of the N-glycan. The other is linked through an α1–3 linkage and forms the “3-arm” of the N-glycan.
Figure 4.
Fc N-glycan maturation. The most common forms of the Fc N-glycan include 0, 1 or 2 galactose residues and 0 or 1 sialic acid residues.
Glycan changes have been noted in multiple diseases, but rheumatoid arthritis (RA) is of particular note. In advanced RA patients the GlcNAc terminated (G0F) form dominates 39, 40. Changes in the glycan distribution predate RA onset by as many as 3.5 years 41 and changes in the Fc N-glycan composition of RA patients temporarily return to normal during pregnancy-induced remission 42. One hypothesis to explain the correlation between RA and glycan composition is that the G0F glycoform is more pro-inflammatory than the galactosylated form 43. If galactose termini were available, a small percentage of Fc would be transformed to a sialylated form, which is believed to be potently anti-inflammatory (discussed further below). In either case it is clear that Fc glycosylation at N297 is vital to proper function of IgG1 Fc.
Aglycosylated or deglycosylated Fc does not bind the low affinity Fc gamma receptors (IIa, IIb, IIIa, IIIb) 44–46 but binding of the high affinity receptor, FcγRI, is preserved 14, 47. Glycan composition likewise impacts the affinity of Fc for FcγRs 24, 31, 38, 48–52. One well-described example is fucosylation of the (2)GlcNAcβ1–4(1)GlcNAcβ–N297 core chitobiose disaccharide that decreases the affinity of Fc for FcγRIIIa by 10–50 fold 53–56. Fc structures determined by X-ray crystallography revealed that Y296 becomes solvent exposed in fucosylated Fc 57, disrupting a contact between an FcγRIIIa N-glycan and the Fc N-glycan 16, 58.
Changes to the non-reducing termini of the glycan (distal to N297) also impact receptor binding 59. For example, the presence of GlcNAc and galactose residues at the Fc N-glycan termini improve the affinity of Fc for FcγRIIIa 31. Addition of these residues enhances interactions between the Fc N-glycan and polypeptide surface, likely stabilizing an Fc conformation that is predisposed to FcγR binding 60, 61.
An unexpected feature of N-glycan composition and its effect on FcγR affinity was revealed recently by Subedi et al. (2014). Though the N-glycan is required and changes to the termini affect affinity, it was noted that Fc, trimmed back to a glycan that consists of only a single GlcNAc residue, still binds FcγRIIIa with ~10-fold reduction in affinity when compared to Fc with a full-length G2F N-glycan 62. This result suggests that the (1)GlcNAc residue alone provides the predominant contribution of the N-glycan to FcγRIIIa binding, consistent with similar measurement on N-glycan contributions to intramolecular stability 63. The observation that (1)GlcNAc-Fc binds was surprising because aglycosylated Fc, as noted above, does not bind FcγRIIIa, nor does Fc enzymatically cleaved to contain an N-glycan of the (1)GlcNAc and (0)fucose residues 31, 64. The latter observation can be understood by considering the additional 10–50 fold negative impact of fucosylation on FcγR binding, as noted above, likely pushing the association of the Fc with a fucose-GlcNAc disaccharide beyond detection limits.
The effect of sialylation on Fc structure and Fc-mediated interactions is an open question and of great interest because sialyl-Fc was reported to be a keen mediator of an anti-inflammatory response with therapeutic potential 65. Sialylation of the Fc N-glycan, a less abundant modification in healthy human serum at ~5–10%, was reported to reduce the affinity of Fc for FcγRIIIa by 10-fold when enzymatically pushed to completion and the formation of high titers of disiaylyl Fc 33, 65, 66. However, these results have been challenged by other observations that found no change in binding affinity by sialylating wild-type Fc 14. It was proposed that sialylation shifted Fc specificity to favor an anti-inflammatory receptor, DC-SIGN67–69, however these results have also been recently challenged 70. Structures of sialyl Fc were also reported, and were found in two different forms: one much like other Fc structures showing no large-scale structural consequence of sialylation 13 and another showing some rearrangement of the Cγ2 domain orientation 21. This area of inquiry is nascent and without a clear definition of the effects of Fc sialylation, but it is clear that the behavior of the Fc N-glycan is complex 29.
III. Glycan Motions: The Fc N-glycan is Dynamic
IIIa. Motion of the Fc N-glycan
The Fc N-glycan was long thought to remain bound to the Fc polypeptide surface between the two Cγ2 domains of the homodimer based on structures from x-ray crystallography 6,7. This conformation would lead to steric occlusion of the N-glycan termini, and restriction from N-glycan modifying enzymes. However, the Fc N-glycan is sensitive to enzymatic modifications in the Golgi and in vitro, suggesting the Fc N-glycan must populate exposed conformations at some frequency.
A clear relationship between glycan composition and glycan function has long been of interest, with conflicting results over the years. Motion of the Fc N-glycan was first thought to be on the same timescale as Cγ2 domain motion suggesting the N-glycan was bound to the Cγ2 surface 71–73. Wormald and colleagues later measured relaxation rates of the IgG1 Fc N-glycan by solution nuclear magnetic resonance spectroscopy (NMR) and noted that they were lower than those of the bulk protein suggesting that the glycan was mobile 74. Shortly after this study, Kato and colleagues used a 13C-galactose labeling strategy to measure spectra of the galactose residue at the non-reducing termini of Fc and found a similar result for the galactose residue on 3-arm of the Fc N-glycan indicating a high degree of mobility relative to the Fc polypeptide 28. In contrast, the 13C label in the galactose residue on 6-arm of the glycan revealed a lineshape that was much broader, and similar in shape to that expected for a Cα atom, indicating restriction by the polypeptide as would be expected based on the location of the 6-arm galactose residue according to structures determined by x-ray crystallography.
The findings by Yamaguchi et al (1998) regarding immobility of the 6-arm of the N-glycan were challenged by Barb and Prestegard (2011) who used Fc remodeled with 13C2 galactose to thoroughly characterize the motion of the Fc N-glycan using solution NMR spectroscopy. These studies revealed that surprisingly both branches of the Fc N-glycan were mobile and experienced significant motion at physiological temperature 29. Slow, μs motions of 6-arm galactose resonances contributed to the broad 13C-galactose lineshapes, and thus explained the apparently conflicting results reported by Yamaguchi et al. The NMR data of Barb and Prestegard revealed that the 3-arm experienced one highly mobile state, while the 6-arm exchanges between two states on a μs timescale: one dominant, highly mobile, unrestricted state and a minor polypeptide-bound, restricted state.
IIIb. N-glycan motion is perturbed by Fc mutations
Together, data collected using NMR and x-ray crystallography provide a model of N-glycan motion. Interactions between polypeptide and N-glycan residues restrict the motion of the N-glycan termini, however the restriction of 6-arm residues is significantly greater than those of the 3-arm due to more extensive intramolecular contacts near the non-reducing terminus of the 6-arm. In addition, the intramolecular interactions between the Fc polypeptide and N-glycan restrict the degree of glycan motion 62. Residues F241, F243, D265, V264, K246, and R301 were identified as key residues in the glycan-polypeptide interaction 14, 62, 75. Mutations at these sites disrupt the interaction and increase the extent of glycan processing in the Golgi. X-ray crystallography indicates that disrupting these interactions has a small impact on Fc conformations sampled by Fc, potentially altering its ability to interact with receptors 14, 21.
Mutations to aromatic residues at the interface formed by N-glycan and polypeptide residues were designed to abrogate π-CH interactions, thought to be the predominant force behind many strong carbohydrate binding sites 76. The F241A mutation is designed to disrupt the interaction between F241 and (2)GlcNAc 75. Experimental structures of the F241A mutant have been determined by X-ray crystallography 14, 21. Though the structures are largely similar to those previously observed, it was noted that electron density of the 3-arm is reduced in the F241A mutant 14, suggesting increased N-glycan motion. F241A, F241I, F241S, F243I, F243S Fc mutants likewise show greater levels of sialic incorporation which is likewise consistent with increased motion and accessibility 14, 62.
IIIc. Association of Fc N-glycan motion and FcγRIIIa affinity
A quantitative analysis of N-glycan motions using NMR determined that the 6-arm of Fc F241S was significantly more mobile than that of wild-type Fc. 62. Increases in glycan motion were likewise observed with F241I, F243S, F241I/F243I and F241S/F243S mutants.
Glycan motion was found to be correlated with FcγRIIIa affinity 62. Residues F241, F243, and K246 were mutated to perturb the Fc glycan-polypeptide interaction. Fc F241I/F243I and Fc F241S/F243S double mutants had considerable decreases in glycan restriction with 20- and 60-fold decreases in FcγRIIIa affinity, respectively. Fc F241I, Fc F241S and Fc F243S showed less perturbation of glycan restriction, and FcγRIIIa binding was intermediate between the Fc wild-type and Fc double mutants (4, 3 and 4- fold reduced affinity, respectively). Fc K246F appeared to stabilize the Fc N-glycan, reducing mobility, while promoting FcγRIIIa interaction. A comparison of the glycan motion versus FcγRIIIa affinity revealed a strong linear correlation between the two parameters 62.
IIId. Motion of sialylated N-glycoforms
As noted above, sialyl Fc is potentially potently anti-inflammatory 38, 43, 65, 77–79, thus it was of interest to determine if sialylation modified the structure of the Fc N-glycan. Measurements by solution NMR spectroscopy found little change to the motion of the N-glycan upon sialylating the 3-arm, or both the 3- and 6-arms 80. This is consistent with more recent structures of sialyl Fc showing no contact between the 6-arm sialic acid and the Fc polypeptide 13, 21.
Sensitivity of Fc to modification by ST6Gal-I, the primary α2–6 sialyl transferase in humans, is informative of global motions of the N-glycan. The sialyl transferase St6Gal-I adds sialic acids to galactose terminated N-glycans 81–84. ST6Gal-I has a branch preference for the 3-arm of the Fc N-glycan, even when the glycan is released from the polypeptide surface 81, 85, 86. The conservation of relative branch modification by St6GalI was similar for Fc-conjugated and free N-glycans, indicating the innate branch specificity of St6GalI was not influenced by the Fc polypeptide 80. This result suggested the Fc N-glycan samples conformations that have both branch termini either simultaneously exposed or restricted from access by the enzyme.
IV. Fc-Fc Receptor Interactions
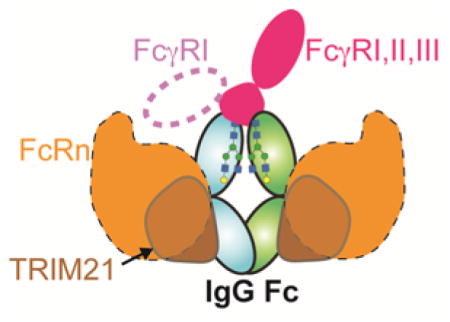
Interaction between the Fc region of immunoglobulins and Fc receptors links the humoral and cellular immune responses. The IgG Fc receptor family is comprised of one high affinity receptor (nM affinity), FcγRI, and several low affinity receptors (μM affinity), FcγRIIa, FcγRIIb, and FcγRIIIa 1–4. FcγRs are, in general, activating receptors except for the inhibitory FcγRIIb. Fc can also trigger responses through interactions with TRIM21 and C1q and Fc has been associated with DC-SIGN. Maintaining antibodies in the serum, preventing degradation, and transcytosis of IgG across the placenta is mediated by an additional interaction of Fc with the neonatal Fc receptor, FcRn 87, 88. The ability to target specific receptors is desirable to impart response specificity in future antibody-based biotherapeutics. In this section, we discuss the current evidence and models of these interactions.
IVa. FcγRIIIa (CD16)
High resolution models show how one FcγRIIIa protein binds to one Fc dimer, in an asymmetric interaction that occupies the lower hinge region, the BC-loop, the C’E loop (containing N297), and the FG loop of Fc (Figure 2) 16, 27, 89–91. The extracellular domains of FcγRIIIa and IIIb are 97% identical and IIIa has a 21 residue C-terminal extension. Thus, the binding of IIIa and IIIb is expected to be identical. The contact surface area between Fc and FcγRIIIa varies between 1200 Å2 to 1700 Å2 16, 91, including both protein-protein and protein-carbohydrate interactions. S239 and L235 on both Fc chains form contacts with FcγRIIIa. While Fc residues 327–330 on only one chain contact FcγRIIIa, as shown by high-resolution structures and functional analysis of mutant proteins 9, 10, 16, 92
While it is easy to disrupt receptor binding though mutation, several studies demonstrated increased binding including the Fc S239D/A330L/I332E variant that increased FcγRIIIa affinity 30 fold 10. Another Fc variant, L234F/L235E/P331S, has impaired affinity for FcγRIIIa and other FcγRs 9. Most likely this reduction in affinity is due to the L235E mutation, which replaces a hydrophobic contact with a highly charged group.
Recent approaches engineering Fc for maximal FcγR affinity include breaking Fc symmetry to capitalize on the asymmetry of the Fc:FcγR complex (Figure 2D). The majority of the FcγRIIIa interaction occurs between only one Fc heavy chain monomer (hereafter referred to as the “A” chain) 16, 27, 91. The asymmetric binding mode indicates that creating a synthetic heterodimer of different heavy chain polypeptides could more specifically influence the interaction between Fc and FcγRIIIa 93–96. For example, introducing a set of four alterations to the A chain of Fc (D270E, K326D, A330K, K334E), and seven into the B chain (L234Y, L235Y, G236W, S239M, H268D, S298A, A327D), improves binding to FcγRIIIa 1000-fold 95, 96. Interestingly, the structure of the A chain is not dramatically perturbed, having a backbone RMS of 0.67Å in the Cγ2 domain versus structures solved of only Fc 96. The B-chain, on the other hand, is more affected with an RMS of 1.13Å for the Cγ2 versus wild-type Fc.
In addition to the role of Fc N-glycosylation in Fc-FcγR interactions (see section IIc), FcγRIIIa N-glycosylation is also a measurable, but not required, factor. There are five glycosylation sites on FcγRIIIa. Of these sites, only N162 and N45 appear to be important for Fc-FcγRIIIa interactions. As mentioned previously, fucosylation of Fc blocks the FcγRIIIa N162 glycan from interacting with Y296 of Fc 16, 57. This interaction is specific for the N162-linked glycan on FcγRIIIa as removal of the glycan promotes interactions with fucosylated Fc 97. The N45 glycan is thought to have an inhibitory effect on binding; removal of the N45 glycan promotes Fc-FcγRIIIa interaction 97, 98. One theory for the inhibitory effect of the N45 glycan is that steric interference between the N45 glycan the chain B of Fc blocks Fc-FcγRIIIa interactions.
IVb. FcγRII (CD32)
FcγRIIa functions as an activating receptor and FcγRIIb inhibits immune responses 1, 2, 99. This functional difference is due to the presence of a cytosolic immune receptor tyrosine activating motif (ITAM) in FcγRIIa and an immune receptor tyrosine inhibitory motif (ITIM) in FcγRIIb. While FcγRIIa and FcγRIIb are functionally distinct, their extra cellular domains are structurally similar 100–102. At the amino acid level the extracellular domains of FcγRIIa and FcγRIIb have 89% sequence identity. This high degree of similarity is maintained in the folded proteins. Alignment of FcγRIIa and FcγRIIb structural models reveals an RMSD of only 1.1 Å (Figure 5). Despite the high degree of similarity, Fc variants are described that show isotype specificity 32, 94.
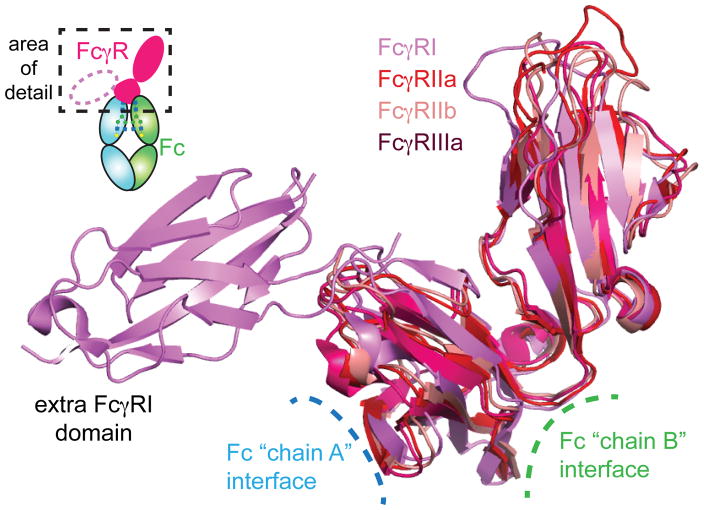
Figure 5.
The structural conservation among FcγRs is high. Ribbon diagrams highlight the interfaces with IgG Fc and the extra FcγRI domain. PDB: FcγRI (3RJD), FcγRIIa (1FCG), FcγRIIb (2FCB), FcγRIIIa (3AY4).
The mechanism of FcγRII engagement by Fc was initially unclear. Isolated FcγRIIa and IIb both crystallize as dimers 100, 102. This observation led to the speculation of a 1 Fc : 2 FcγRIIa complex that was reportedly supported by computational modeling of the Fc-FcγRIIa interaction 101. However, co-structures of Fc with FcγRIIa showed a single FcγRIIa receptor bound at the lower hinge region of IgG Fc, much like the previously mentioned FcγRIIIa 17. Sedimentation equilibrium, ITC, and NMR experiments confirmed the binding stoichiometry was 1:1 103–106. However, it should be noted that FcγRIIa is thought to exist as a dimer on the surface of cells and the in vivo characteristics of the complex have not been thoroughly characterized 17.
IVc. FcγRI (CD64)
The structure of the extracellular domains of FcγRIa has recently been solved 107. There are several differences between FcγRI and the rest of the FcγR family, including affinity (I ≫II~III) and FcγRI binds aglycosylated Fc with high affinity (high nM). Furthermore, FcγRI can bind monomeric Fc on cell surfaces, unlike II and III which only signal following Ig-dependent clustering of FcγR molecules on the cell surface 108.
Stronger affinity is not the only feature that distinguishes FcγRI. FcγRI has a prominent third extra cellular domain, which is not present in FcγRII or III 109. Early research suggested the third domain was responsible for improved affinity in mice 110. In this study, removal of the third domain in FcγRI removed the high affinity recognition of Fc. An experiment in mice revealed that including the third domain to a low-affinity receptor transformed the low affinity receptor into a high affinity receptor. Later, the second domain was also identified as playing a role in promoting high-affinity interactions between FcγRI and Fc 111. Recent studies support the role of the second domain in increasing binding, and contest the importance of the third domain 107, 112. Residues 171–176 of FcγRI form the FG-loop in FcγRI. The FG loop is located in the second extracellular domain of FcγRI and forms a perfect binding wedge to bridge the both Fc Cγ2 domains 113. Swapping the FcγRI FG loop for the same residues in FcγRIIIa increases the affinity of FcγRIIIa for Fc 15-fold 107.
The importance of the FG loops is highlighted in the recent report of a high resolution FcγRI:Fc structure 113. Two key features make the FcγRI FG loop suitable for Fc interactions: the shorter length of the FcγRI FG loop and the presence of a positively charged KHR motif. The FG loop in FcγRI is one residue shorter than the corresponding loop in FcγRII or III. In FcγRIII, the FG loop was found to bend away from the Fc glycans to accommodate the additional residue. However, in FcγRI the FcγRI FG loop is 5 Å closer to Fc. This may permit a tighter interaction between the FcγRI FG loop and Fc. This position alone likely does not completely explain the greater FcγRI affinity. The KHR motif in the FcγRI FG loop is positively charged. This allows for the formation of additional contacts between FcγRI and Fc. The positive charges on the FcγRI FG loop are important for forming salt bridges between K173 of FcγRI and D265 of Fc. Swapping any of the residues in the KHR motif for neutral or negatively charged amino acids results in 2- to 30-fold decreases in affinity 113.
IVd. DC-SIGN
DC-SIGN is an inhibitory receptor on dendritic cells and macrophages that interacts with high mannose type glycans to recognize pathogens. While not a traditional Fc receptor, it was proposed that interactions with DC-SIGN explain the anti-inflammatory effects of sialyl-Fc 65, 69. Removal of SIGN-R1, a DC-SIGN homolog in mouse, abrogates the restorative effects of intravenous treatment with donated immunoglobulins (IVIg), but adding human DC-SIGN restores its functionality 78, 114. It was suggested that Fc sialylation induces a structural change to unveil a new epitope recognized specifically by DC-SIGN 67.
Similar to the anti-inflammatory properties and structure of sialyl Fc, the interaction between sialyl Fc and DC-SIGN remains an unresolved topic in the literature. A small number of published studies refute the formation of a complex between sialyl-Fc and DC-SIGN. The strongest evidence supporting this view is that DC-SIGN, a C-type lectin, does not bind sialylated N-glycans in carbohydrate binding arrays 115. One study using carbohydrate arrays shows that sialylation of certain epitopes, like Lewis X, in fact prevents interaction with DC-SIGN 116. Furthermore, sialyl Fc does not compete with DC-SIGN ligands in carbohydrate binding experiments and binds no better than deglycosylated Fc 70. One theory is Fab cross-reactivity, and not Fc sialylation, allows IVIG to interact with DC-SIGN 70. No structures of the Fc:DC-SIGN complex are available as of the writing of this review.
IVe. FcRn
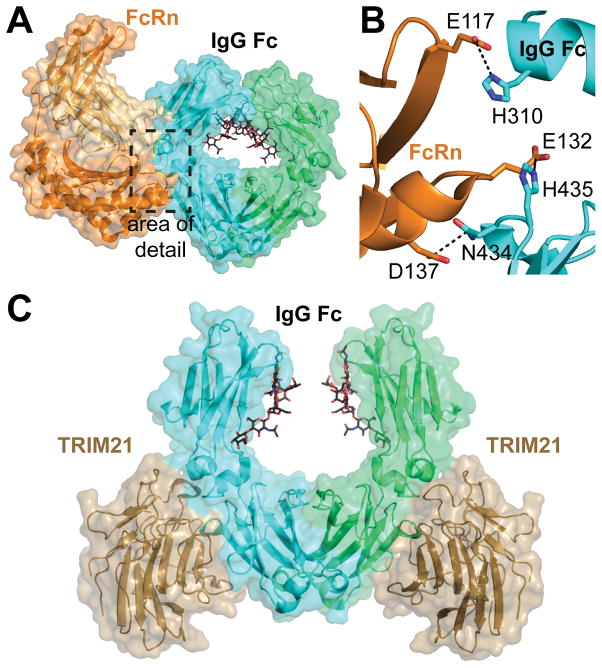
The neonatal Fc receptor (FcRn) is structurally and functionally unique among Fc receptors. Named initially after its role in transporting IgG across the placenta, FcRn is not known to serve a signaling function. Rather, FcRn is responsible for transcytosis of IgG and recycling endocytosed IgG back to the serum 1, 88, 91. FcRn is similar in structure to the major histocompatibility complex molecules 117. FcRn interacts with the Cγ2-Cγ3 interface of Fc, and not the lower hinge region like FcγRs 118 (Figure 6A). The interaction between Fc and FcRn is pH dependent, exhibiting tight binding to Fc at pH 6 and weak binding at pH 7.4 This pH dependence allows for tight binding between FcRn and IgG in lysosomes, then release of IgG in neutral environments. In mice, the pH dependence is thought to be due to salt bridges created by Fc H435/FcRn E132, Fc H436/FcRn D137, and Fc H310/FcRn E117 (Figure 6B). In humans the salt bridge pairs between H435-E132 and H310-E117 still exist, but residue 436 is a tyrosine.
Figure 6.
A) The neonatal Fc Receptor (FcRn, orange ribbon) binds to the Cg2/3 interface of IgG1 Fc (cyan ribbon) (PDB 1I1A). B) Key H-bond and ionic interactions formed between Fc and FcRn are responsible for the pH dependence of binding. Fc residues 245–260 were removed from this image for clarity. C) The PRYSPRY domain of TRIM21 (sand) recognizes the Cγ2/3 interface of IgG Fc (cyan, green) in a manner similar to FcRn (PDB 2IWG).
In principle, the serum half-life of Fc-based therapeutics can be improved by engineering Fc to bind more tightly to FcRN at pH 6 119. This has been achieved through an Fc YTE variant (M252Y/S254T/T256E) that binds FcRn with a 10-fold greater affinity, but at the cost of a 2-fold decrease in affinity for FcγRIIIa 119, 120. The structure of this Fc is largely conserved, with an RMSD of only 0.86 Å when compared to wild-type Fc 11, however, S254 mutations are known to impair FcγRIIIa interactions 32.
Additional Fc variants are reported to enhance the Fc:FcRn interaction 32. Fc T307A/E380A/N434A demonstrates a 16-fold increase in binding to isolated FcRn and a 3.3-fold increase in binding to cells expressing FcRn 121. T250Q/M428L has a 30-fold increase in serum half-life that is pH dependent 122. The Fc H433K/N434F variant also has a 16-fold increase in affinity for FcRn at pH 6.0, but unexpectedly revealed a 4-fold reduction of half-life in mice 123. Fc variants that increase FcRn affinity without affecting other FcR interactions likewise would be useful for increasing efficacy 88, 124. Those that do have impaired affinity for other FcRs are useful as Abdegs; antibodies that promote the degradation of pathogenic Igs by preventing their recycling by FcRn 125.
IVf. TRIM21
TRIM21 is a member of the tripartite motif family of pathogen defense proteins and binds Fc. TRIM21 contributes an important function in viral defense by binding to intracellular IgG-virus complexes. TRIM21 marks these complexes for degradation by the proteasome, destroying the virus and bound antibody 126. It should be noted that enveloped viruses can shed IgG before infecting a cell, and thus do not initiate a TRIM21-mediated response. Additionally, the anti-viral capabilities of TRIM21 can be overcome by superinfection 127. However, knockout studies in mice have shown that TRIM21 is necessary for antibody-dependent intracellular neutralization.
TRIM21 has low nanomolar affinity for IgG and is able to compete with protein A for Fc binding 127–130. TRIM21 binds to Fc at the Cγ2-Cγ3 interface in a manner similar to FcRn (Figure 6) 126. This binding location allows two TRIM21 proteins to interact with one Fc 129, 130. The TRIM21-Fc interaction is mediated by ionic interactions. Despite the similarity between the Fc motifs recognized by TRIM21 and FcRn, the TRIM21-Fc interaction does not appear to be pH dependent 130.
IVg. General Theories of The Fc N-glycan Requirement
Of the receptors discussed above, the low-affinity FcγRs and (potentially) DC-SIGN require Fc N-glycosylation, while TRIM21 and FcRn do not. Two hypothesis have been put forth to describe the structural consequence of Fc N-glycosylation in low-affinity FcγR binding 62. This is still very much an open question. One prevalent theory, based primarily on models solved by x-ray crystallography, suggests glycan composition affects the relative orientation of the Fc Cγ2 domains 15, 25, 67, 68. According to this hypothesis the Cγ2 domains of Fc sample a range of conformations, some predisposed to bind FcγRs with the receptor binding site easily accessible and others populating conformations that are unfavorable for Fc-FcγR interactions. In this model glycan composition tunes the Cγ2 orientation, with pro-inflammatory glycoforms assuming a small set of conformations predisposed to bind receptor and truncated or aglycosylated forms incapable of binding. Computational simulations of Fc motions are consistent with this hypothesis 8. One primary limitation of models built from x-ray crystallography data is that only the low-energy forms are observed, and the low energy forms may not be highly populated in solution at physiological temperature. Indeed, x-ray crystallography is blind to the predominant highly mobile state of the Fc N-glycan termini as discussed above (Section III)
An alternative hypothesis built upon solution measurements suggests local structural perturbations explain differential receptor binding affinities. This idea was first suggested by Jefferis and coworkers with data directly supporting this idea by the groups of Kato and Barb and developed further by Barb 57, 62, 75. In this model the role of the Fc N-glycan is to restrict local Fc conformation, including the C’E loop. This is an attractive hypothesis because N297, the site of N-glycan attachment, sits at the apex of the C’E loop; furthermore, significant contacts are made between FcgRII/IIIs and the Fc C’E loop loop.
The two models describing the role of the Fc N-glycan are not mutually exclusive. Solution NMR studies using molecules of this size (~55 kDa) are incapable of providing high resolution definitions of all atoms in the system, unlike x-ray crystallography, and may be blind to certain features and certain timescales of motion. Defining which of these models best accounts for the predominant forces behind the N-glycan contribution to FcγR binding will be informative for future targeted improvement of immunoglobulin G-based therapeutics.
Acknowledgments
We thank Prof. Eric S. Underbakke (Iowa State University) for a critical reading of the manuscript, and Dr. Peter Sun and Dr. Jinghua Lu (NIH/NIAID) for providing the Fc:FcγRI coordinates.
Funding Sources
This work was financially supported by the grant K22AI099165 from the National Institutes of Health, and by funds from the Roy J. Carver Department of Biochemistry, Biophysics & Molecular Biology at Iowa State University. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Author Contributions
The manuscript was written through contributions of both authors. All authors have given approval to the final version of the manuscript.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn F, Ravetch JV. FcγRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 4.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 5.McEwan WA, James LC. TRIM21-dependent intracellular antibody neutralization of virus infection. Progress in molecular biology and translational science. 2015;129:167–187. doi: 10.1016/bs.pmbts.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 7.Huber R, Deisenhofer J, Colman PM, Matsushima M, Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- 8.Frank M, Walker RC, Lanzilotta WN, Prestegard JH, Barb AW. Immunoglobulin G1 Fc domain motions: implications for Fc engineering. J Mol Biol. 2014;426:1799–1811. doi: 10.1016/j.jmb.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oganesyan V, Gao C, Shirinian L, Wu H, Dall’Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr. 2008;64:700–704. doi: 10.1107/S0907444908007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oganesyan V, Damschroder MM, Leach W, Wu H, Dall’Acqua WF. Structural characterization of a mutated, ADCC-enhanced human Fc fragment. Mol Immunol. 2008;45:1872–1882. doi: 10.1016/j.molimm.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Oganesyan V, Damschroder MM, Woods RM, Cook KE, Wu H, Dall’acqua WF. Structural characterization of a human Fc fragment engineered for extended serum half-life. Mol Immunol. 2009;46:1750–1755. doi: 10.1016/j.molimm.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Bowden TA, Baruah K, Coles CH, Harvey DJ, Yu X, Song BD, Stuart DI, Aricescu AR, Scanlan CN, Jones EY, Crispin M. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J Am Chem Soc. 2012;134:17554–17563. doi: 10.1021/ja306068g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispin M, Yu X, Bowden TA. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc Natl Acad Sci U S A. 2013;110:E3544–3546. doi: 10.1073/pnas.1310657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Baruah K, Harvey DJ, Vasiljevic S, Alonzi DS, Song BD, Higgins MK, Bowden TA, Scanlan CN, Crispin M. Engineering hydrophobic protein-carbohydrate interactions to fine-tune monoclonal antibodies. J Am Chem Soc. 2013;135:9723–9732. doi: 10.1021/ja4014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 2011;16:1071–1080. doi: 10.1111/j.1365-2443.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, Scott AM, Hogarth PM. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol. 2011;187:3208–3217. doi: 10.4049/jimmunol.1101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart R, Thom G, Levens M, Güler-Gane G, Holgate R, Rudd PM, Webster C, Jermutus L, Lund J. A variant human IgG1-Fc mediates improved ADCC. Protein Eng Des Sel. 2011;24:671–678. doi: 10.1093/protein/gzr015. [DOI] [PubMed] [Google Scholar]
- 19.Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, Sutton J, Chaparro-Riggers J, Chen W, Casas MG, Chin SM, Wong OK, Liu SH, Vergara G, Shelton D, Rajpal A, Pons J. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012;420:204–219. doi: 10.1016/j.jmb.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Teplyakov A, Zhao Y, Malia TJ, Obmolova G, Gilliland GL. IgG2 Fc structure and the dynamic features of the IgG CH2-CH3 interface. Mol Immunol. 2013;56:131–139. doi: 10.1016/j.molimm.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang LX, Bjorkman PJ. Structural characterization of anti-inflammatory Immunoglobulin G Fc proteins. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oganesyan V, Damschroder MM, Cook KE, Li Q, Gao C, Wu H, Dall’Acqua WF. Structural insights into neonatal Fc receptor-based recycling mechanisms. J Biol Chem. 2014;289:7812–7824. doi: 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baruah K, Bowden TA, Krishna BA, Dwek RA, Crispin M, Scanlan CN. Selective deactivation of serum IgG: a general strategy for the enhancement of monoclonal antibody receptor interactions. J Mol Biol. 2012;420:1–7. doi: 10.1016/j.jmb.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem Biol. 2012;7:1596–1602. doi: 10.1021/cb300130k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispin M, Bowden TA, Coles CH, Harlos K, Aricescu AR, Harvey DJ, Stuart DI, Jones EY. Carbohydrate and domain architecture of an immature antibody glycoform exhibiting enhanced effector functions. J Mol Biol. 2009;387:1061–1066. doi: 10.1016/j.jmb.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Davies AM, Jefferis R, Sutton BJ. Crystal structure of deglycosylated human IgG4-Fc. Mol Immunol. 2014;62:46–53. doi: 10.1016/j.molimm.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi Y, Kato K, Shindo M, Aoki S, Furusho K, Koga K, Takahashi N, Arata Y, Shimada I. Dynamics of the carbohydrate chains attached to the Fc portion of immunoglobulin G as studied by NMR spectroscopy assisted by selective 13C labeling of the glycans. J Biomol NMR. 1998;12:385–394. doi: 10.1023/a:1008392229694. [DOI] [PubMed] [Google Scholar]
- 29.Barb AW, Prestegard JH. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat Chem Biol. 2011;7:147–153. doi: 10.1038/nchembio.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang AC, Wang IY. Cleavage sites of human IgG1 immunoglobulin by papain. Immunochemistry. 1977;14:197–200. doi: 10.1016/0019-2791(77)90194-x. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 2006;1760:693–700. doi: 10.1016/j.bbagen.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 33.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Raju TS. Assessing Fc glycan heterogeneity of therapeutic recombinant monoclonal antibodies using NP-HPLC. Methods Mol Biol. 2013;988:169–180. doi: 10.1007/978-1-62703-327-5_10. [DOI] [PubMed] [Google Scholar]
- 35.Masuda K, Yamaguchi Y, Kato K, Takahashi N, Shimada I, Arata Y. Pairing of oligosaccharides in the Fc region of immunoglobulin G. FEBS Lett. 2000;473:349–357. doi: 10.1016/s0014-5793(00)01557-x. [DOI] [PubMed] [Google Scholar]
- 36.Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- 37.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 39.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 40.Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988;167:1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ercan A, Cui J, Chatterton DE, Deane KD, Hazen MM, Brintnell W, O’Donnell CI, Derber LA, Weinblatt ME, Shadick NA, Bell DA, Cairns E, Solomon DH, Holers VM, Rudd PM, Lee DM. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010;62:2239–2248. doi: 10.1002/art.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241–1248. doi: 10.1002/art.24003. [DOI] [PubMed] [Google Scholar]
- 43.Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A. 2007;104:8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund J, Tanaka T, Takahashi N, Sarmay G, Arata Y, Jefferis R. A protein structural change in aglycosylated IgG3 correlates with loss of huFc gamma R1 and huFc gamma R111 binding and/or activation. Mol Immunol. 1990;27:1145–1153. doi: 10.1016/0161-5890(90)90103-7. [DOI] [PubMed] [Google Scholar]
- 45.Walker MR, Lund J, Thompson KM, Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989;259:347–353. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jefferis R. The glycosylation of antibody molecules: functional significance. Glycoconj J. 1993;10:358–361. [PubMed] [Google Scholar]
- 47.Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol. 2013;190:4315–4323. doi: 10.4049/jimmunol.1200501. [DOI] [PubMed] [Google Scholar]
- 48.Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcγRs in vivo. Curr Opin Organ Transplant. 2011;16:7–14. doi: 10.1097/MOT.0b013e328342538f. [DOI] [PubMed] [Google Scholar]
- 49.Ghirlando R, Lund J, Goodall M, Jefferis R. Glycosylation of human IgG-Fc: influences on structure revealed by differential scanning micro-calorimetry. Immunol Lett. 1999;68:47–52. doi: 10.1016/s0165-2478(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 50.Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, Shitara K, Satoh M. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17:104–118. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- 51.Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Adv Exp Med Biol. 2011;780:113–124. doi: 10.1007/978-1-4419-5632-3_10. [DOI] [PubMed] [Google Scholar]
- 52.Radaev S, Sun PD. Recognition of IgG by Fcgamma receptor. The role of Fc glycosylation and the binding of peptide inhibitors. J Biol Chem. 2001;276:16478–16483. doi: 10.1074/jbc.M100351200. [DOI] [PubMed] [Google Scholar]
- 53.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 54.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 55.Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. MAbs. 2009;1:230–236. doi: 10.4161/mabs.1.3.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009;100:1566–1572. doi: 10.1111/j.1349-7006.2009.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumiya S, Yamaguchi Y, Saito J, Nagano M, Sasakawa H, Otaki S, Satoh M, Shitara K, Kato K. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol. 2007;368:767–779. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umaña P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand binding. J Immunol. 1997;159:3849–3857. [PubMed] [Google Scholar]
- 60.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 61.Barb AW. Intramolecular N-Glycan/Polypeptide Interactions Observed at Multiple N-Glycan Remodeling Steps through [(13)C,(15)N]-N-Acetylglucosamine Labeling of Immunoglobulin G1. Biochemistry. 2015;54:313–322. doi: 10.1021/bi501380t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subedi GP, Hanson QM, Barb AW. Restricted Motion of the Conserved Immunoglobulin G1 N-Glycan Is Essential for Efficient FcγRIIIa Binding. Structure. 2014 doi: 10.1016/j.str.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanson SR, Culyba EK, Hsu TL, Wong CH, Kelly JW, Powers ET. The core trisaccharide of an N-linked glycoprotein intrinsically accelerates folding and enhances stability. Proc Natl Acad Sci U S A. 2009;106:3131–3136. doi: 10.1073/pnas.0810318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allhorn M, Olin AI, Nimmerjahn F, Collin M. Human IgG/Fc gamma R interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS One. 2008;3:e1413. doi: 10.1371/journal.pone.0001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 66.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–1534. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A. 2013;110:9868–9872. doi: 10.1073/pnas.1307864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. Reply to Crispin et al. : Molecular model that accounts for the biological and physical properties of sialylated Fc. Proc Natl Acad Sci U S A. 2013;110:E3547. doi: 10.1073/pnas.1311721110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425:1253–1258. doi: 10.1016/j.jmb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Nezlin R. Internal movements in immunoglobulin molecules. Adv Immunol. 1990;48:1–40. doi: 10.1016/s0065-2776(08)60750-6. [DOI] [PubMed] [Google Scholar]
- 72.Sykulev Y, Nezlin R. The dynamics of glycan-protein interactions in immunoglobulins. Results of spin label studies. Glycoconjugate Journal. 1990;7:163–182. [Google Scholar]
- 73.Rosen P, Pecht I, Cohen JS. Monitoring the carbohydrate component of the Fc fragment of human IgG by 13C nuclear magnetic resonance spectroscopy. Mol Immunol. 1979;16:435–436. doi: 10.1016/0161-5890(79)90112-3. [DOI] [PubMed] [Google Scholar]
- 74.Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry. 1997;36:1370–1380. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- 75.Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R. Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol. 1996;157:4963–4969. [PubMed] [Google Scholar]
- 76.Chen W, Enck S, Price JL, Powers DL, Powers ET, Wong CH, Dyson HJ, Kelly JW. Structural and energetic basis of carbohydrate-aromatic packing interactions in proteins. J Am Chem Soc. 2013;135:9877–9884. doi: 10.1021/ja4040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 80.Barb AW, Meng L, Gao Z, Johnson RW, Moremen KW, Prestegard JH. NMR characterization of immunoglobulin G Fc glycan motion on enzymatic sialylation. Biochemistry. 2012;51:4618–4626. doi: 10.1021/bi300319q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- 82.Harduin-Lepers A, Recchi MA, Delannoy P. 1994, the year of sialyltransferases. Glycobiology. 1995;5:741–758. doi: 10.1093/glycob/5.8.741. [DOI] [PubMed] [Google Scholar]
- 83.Meng L, Forouhar F, Thieker D, Gao Z, Ramiah A, Moniz H, Xiang Y, Seetharaman J, Milaninia S, Su M, Bridger R, Veillon L, Azadi P, Kornhaber G, Wells L, Montelione GT, Woods RJ, Tong L, Moremen KW. Enzymatic basis for N-glycan sialylation: structure of rat α2,6-sialyltransferase (ST6GAL1) reveals conserved and unique features for glycan sialylation. J Biol Chem. 2013;288:34680–34698. doi: 10.1074/jbc.M113.519041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhn B, Benz J, Greif M, Engel AM, Sobek H, Rudolph MG. The structure of human α-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystallogr D Biol Crystallogr. 2013;69:1826–1838. doi: 10.1107/S0907444913015412. [DOI] [PubMed] [Google Scholar]
- 85.Barb AW, Brady EK, Prestegard JH. Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I. Biochemistry. 2009;48:9705–9707. doi: 10.1021/bi901430h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paulson JC, Hill RL, Tanabe T, Ashwell G. Reactivation of asialo-rabbit liver binding protein by resialylation with beta-D-galactoside alpha2 leads to 6 sialyltransferase. J Biol Chem. 1977;252:8624–8628. [PubMed] [Google Scholar]
- 87.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159–166. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Tian Z, Thirumalai D, Zhang X. Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering. J Drug Target. 2014;22:269–278. doi: 10.3109/1061186X.2013.875030. [DOI] [PubMed] [Google Scholar]
- 89.Ghirlando R, Keown MB, Mackay GA, Lewis MS, Unkeless JC, Gould HJ. Stoichiometry and thermodynamics of the interaction between the Fc fragment of human IgG1 and its low-affinity receptor Fc gamma RIII. Biochemistry. 1995;34:13320–13327. doi: 10.1021/bi00041a007. [DOI] [PubMed] [Google Scholar]
- 90.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469–16477. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 91.Radaev S, Sun P. Recognition of immunoglobulins by Fcgamma receptors. Mol Immunol. 2002;38:1073–1083. doi: 10.1016/s0161-5890(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 92.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z, Gunasekaran K, Wang W, Razinkov V, Sekirov L, Leng E, Sweet H, Foltz I, Howard M, Rousseau AM, Kozlosky C, Fanslow W, Yan W. Asymmetrical Fc engineering greatly enhances antibody-dependent cellular cytotoxicity (ADCC) effector function and stability of the modified antibodies. J Biol Chem. 2014;289:3571–3590. doi: 10.1074/jbc.M113.513366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mimoto F, Katada H, Kadono S, Igawa T, Kuramochi T, Muraoka M, Wada Y, Haraya K, Miyazaki T, Hattori K. Engineered antibody Fc variant with selectively enhanced FcγRIIb binding over both FcγRIIa(R131) and FcγRIIa(H131) Protein Eng Des Sel. 2013;26:589–598. doi: 10.1093/protein/gzt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mimoto F, Igawa T, Kuramochi T, Katada H, Kadono S, Kamikawa T, Shida-Kawazoe M, Hattori K. Novel asymmetrically engineered antibody Fc variant with superior FcγR binding affinity and specificity compared with afucosylated Fc variant. MAbs. 2013;5:229–236. doi: 10.4161/mabs.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mimoto F, Kadono S, Katada H, Igawa T, Kamikawa T, Hattori K. Crystal structure of a novel asymmetrically engineered Fc variant with improved affinity for FcγRs. Mol Immunol. 2014;58:132–138. doi: 10.1016/j.molimm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 97.Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 98.Shibata-Koyama M, Iida S, Okazaki A, Mori K, Kitajima-Miyama K, Saitou S, Kakita S, Kanda Y, Shitara K, Kato K, Satoh M. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2009;19:126–134. doi: 10.1093/glycob/cwn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 100.Maxwell KF, Powell MS, Hulett MD, Barton PA, McKenzie IF, Garrett TP, Hogarth PM. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat Struct Biol. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 101.Sondermann P, Jacob U, Kutscher C, Frey J. Characterization and crystallization of soluble human Fc gamma receptor II (CD32) isoforms produced in insect cells. Biochemistry. 1999;38:8469–8477. doi: 10.1021/bi982889q. [DOI] [PubMed] [Google Scholar]
- 102.Sondermann P, Huber R, Jacob U. Crystal structure of the soluble form of the human fcgamma-receptor IIb: a new member of the immunoglobulin superfamily at 1.7 A resolution. EMBO J. 1999;18:1095–1103. doi: 10.1093/emboj/18.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sondermann P, Oosthuizen V. The structure of Fc receptor/Ig complexes: considerations on stoichiometry and potential inhibitors. Immunol Lett. 2002;82:51–56. doi: 10.1016/s0165-2478(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 104.Sondermann P, Oosthuizen V. X-ray crystallographic studies of IgG-Fc gamma receptor interactions. Biochem Soc Trans. 2002;30:481–486. doi: 10.1042/bst0300481. [DOI] [PubMed] [Google Scholar]
- 105.Kato K, Sautès-Fridman C, Yamada W, Kobayashi K, Uchiyama S, Kim H, Enokizono J, Galinha A, Kobayashi Y, Fridman WH, Arata Y, Shimada I. Structural basis of the interaction between IgG and Fcgamma receptors. J Mol Biol. 2000;295:213–224. doi: 10.1006/jmbi.1999.3351. [DOI] [PubMed] [Google Scholar]
- 106.Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, Jefferis R. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539–45547. doi: 10.1074/jbc.M107478200. [DOI] [PubMed] [Google Scholar]
- 107.Lu J, Ellsworth JL, Hamacher N, Oak SW, Sun PD. Crystal structure of Fcγ receptor I and its implication in high affinity γ-immunoglobulin binding. J Biol Chem. 2011;286:40608–40613. doi: 10.1074/jbc.M111.257550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcγRI. J Immunol. 2011;186:2699–2704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- 109.Sears DW, Osman N, Tate B, McKenzie IF, Hogarth PM. Molecular cloning and expression of the mouse high affinity Fc receptor for IgG. J Immunol. 1990;144:371–378. [PubMed] [Google Scholar]
- 110.Hulett MD, Osman N, McKenzie IF, Hogarth PM. Chimeric Fc receptors identify functional domains of the murine high affinity receptor for IgG. J Immunol. 1991;147:1863–1868. [PubMed] [Google Scholar]
- 111.Hulett MD, Hogarth PM. The second and third extracellular domains of FcgammaRI (CD64) confer the unique high affinity binding of IgG2a. Mol Immunol. 1998;35:989–996. doi: 10.1016/s0161-5890(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 112.Asaoka Y, Hatayama K, Ide T, Tsumoto K, Tomita M. The binding of soluble recombinant human Fcγ receptor I for human immunoglobulin G is conferred by its first and second extracellular domains. Mol Immunol. 2013;54:403–407. doi: 10.1016/j.molimm.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Lu J, Chu J, Zou Z, Hamacher NB, Rixon MW, Sun PD. Structure of FcγRI in complex with Fc reveals the importance of glycan recognition for high-affinity IgG binding. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1418812112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwab I, Biburger M, Krönke G, Schett G, Nimmerjahn F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol. 2012;42:826–830. doi: 10.1002/eji.201142260. [DOI] [PubMed] [Google Scholar]
- 115.van Liempt E, Bank CM, Mehta P, Garciá-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Holla A, Skerra A. Comparative analysis reveals selective recognition of glycans by the dendritic cell receptors DC-SIGN and Langerin. Protein Eng Des Sel. 2011;24:659–669. doi: 10.1093/protein/gzr016. [DOI] [PubMed] [Google Scholar]
- 117.Burmeister WP, Huber AH, Bjorkman PJ. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372:379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- 118.Martin WL, West AP, Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell. 2001;7:867–877. doi: 10.1016/s1097-2765(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 119.Dall’Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 120.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 121.Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, Presta LG, Meng YG, Roopenian DC. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 122.Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–356. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 123.Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci U S A. 2006;103:18709–18714. doi: 10.1073/pnas.0606304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rath T, Baker K, Dumont JA, Peters RT, Jiang H, Qiao SW, Lencer WI, Pierce GF, Blumberg RS. Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol. 2013 doi: 10.3109/07388551.2013.834293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 126.McEwan WA, Mallery DL, Rhodes DA, Trowsdale J, James LC. Intracellular antibody-mediated immunity and the role of TRIM21. Bioessays. 2011;33:803–809. doi: 10.1002/bies.201100093. [DOI] [PubMed] [Google Scholar]
- 127.McEwan WA, Hauler F, Williams CR, Bidgood SR, Mallery DL, Crowther RA, James LC. Regulation of virus neutralization and the persistent fraction by TRIM21. J Virol. 2012;86:8482–8491. doi: 10.1128/JVI.00728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rhodes DA, Trowsdale J. TRIM21 is a trimeric protein that binds IgG Fc via the B30.2 domain. Mol Immunol. 2007;44:2406–2414. doi: 10.1016/j.molimm.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 129.James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci U S A. 2007;104:6200–6205. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A. 2008;105:6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]