Abstract
Spatial and temporal control of cell wall deposition plays a unique and critical role during growth and development in plants. To characterize membrane trafficking pathways involved in these processes, we have examined the function of a plant Rab GTPase, RabA4b, during polarized expansion in developing root hair cells. Whereas a small fraction of RabA4b cofractionated with Golgi membrane marker proteins, the majority of this protein labeled a unique membrane compartment that did not cofractionate with the previously characterized trans-Golgi network syntaxin proteins SYP41 and SYP51. An enhanced yellow fluorescent protein (EYFP)-RabA4b fusion protein specifically localizes to the tips of growing root hair cells in Arabidopsis thaliana. Tip-localized EYFP-RabA4b disappears in mature root hair cells that have stopped expanding, and polar localization of the EYFP-RabA4b is disrupted by latrunculin B treatment. Loss of tip localization of EYFP-RabA4b was correlated with inhibition of expansion; upon washout of the inhibitor, root hair expansion recovered only after tip localization of the EYFP-RabA4b compartments was reestablished. Furthermore, in mutants with defective root hair morphology, EYFP-RabA4b was improperly localized or was absent from the tips of root hair cells. We propose that RabA4b regulates membrane trafficking through a compartment involved in the polarized secretion of cell wall components in plant cells.
INTRODUCTION
In eukaryotic cells, proteins and membranes are sorted and delivered to specific cellular destinations through a series of interconnected membrane trafficking pathways. Much work has focused on understanding the various protein components required to organize and conduct these trafficking steps at the level of single cells. However, little is known about how these pathways are organized and regulated as cell morphology changes during differentiation in multicellular organisms. To begin to understand the molecular processes by which membrane trafficking pathways are regulated during polarization and cell morphogenesis in plants, we have focused on the development of the root hair.
After the site of root hair initiation has been determined, cell growth occurs in two major stages. During root hair initiation, a localized swelling, or bulge, forms at one end of the root epidermal cell. When the bulge is 20 to 40 μm long, the root hair transitions to growth by polarized expansion at the tip (Dolan et al., 1994). This type of growth, also known as tip growth, is accomplished through the targeted secretion of new cell wall materials to the expanding tip of the cell (Schnepf, 1986). Specific sorting and delivery of Golgi-synthesized polysaccharides, cell wall proteins, and cellulose synthase proteins to the tip-localized site of expansion requires an intact cytoskeleton, a Ca2+ gradient, and the proper regulation of membrane trafficking pathways (Miller et al., 1997; Ryan et al., 2001).
We were interested in characterizing membrane trafficking pathways that were involved in the polarized secretion observed during differentiation of plant root hair cells. To identify secretory membrane compartments involved this process, we took advantage of the characteristic that Rab GTPases are specifically recruited to different organelles in eukaryotic cells. Rab GTPases are small GTP binding proteins that cycle between an inactive GDP-bound state and an active GTP-bound state. In their membrane-associated, GTP-bound state, Rab GTPases undergo conformational changes allowing subsequent recruitment of additional cytosolic proteins to that subcellular compartment. In eukaryotic organisms, Rab GTPases regulate membrane trafficking events associated with distinct compartments (reviewed in Zerial and McBride, 2001). The type of subcellular compartment with which a Rab GTPase is associated can be predicted based on sequence similarity (Rutherford and Moore, 2002; Vernoud et al., 2003). Ara-4 and Pra3 are highly similar to the mammalian Rab11, and each has been shown to localize to or function within the broadly-defined trans-Golgi network (TGN; Ueda et al., 1996; Inaba et al., 2002). Ypt6 in yeast (Saccharomyces cerevisiae) has been implicated in protein recycling from endosomes to the Golgi and from late to early Golgi (Luo and Gallwitz, 2003). AtRab6 is closely related to yeast Ypt6 and can functionally complement ypt6 mutants when expressed in yeast (Bednarek et al., 1994). Mammalian Rab2 regulates membrane flow in Golgi intermediates, and its ortholog in tobacco (Nicotiana tabacum), NtRab2, also localizes to Golgi bodies in pollen tubes (Tisdale et al., 1992; Tisdale, 1999; Cheung et al., 2002). AtRab1 is required for transport between the endoplasmic reticulum (ER) and Golgi, which is similar to Rab1 function in mammals (Tisdale et al., 1992; Batoko et al., 2000). Rab5 regulates fusion of endosomal compartments in mammals, whereas its homolog in plants, Rha1, is responsible for trafficking of cargo molecules to the vacuole through late endosomal/prevacuolar compartments (Sohn et al., 2003). Therefore, localization of plant Rab GTPases generally coincides with that of their eukaryotic homologs.
Here, we show that the Arabidopsis thaliana Rab GTPase RabA4b, which is highly similar to Rab11, labels a novel compartment that accumulates at the tips of expanding root hair cells. Using time-lapse video fluorescence microscopy techniques, we observe that the polarized distribution of these membranes is dependent upon an intact F-actin cytoskeleton. Finally, we demonstrate that tip accumulation of the RabA4b-labeled compartment is altered or absent in root hair developmental mutants. Based on these findings, we propose that in A. thaliana, the RabA4b Rab GTPase regulates membrane trafficking steps involved in the polarized deposition of cell wall components in tip-growing root hair cells.
RESULTS
RabA4b Is Ubiquitously Expressed in A. thaliana
To identify A. thaliana Rab GTPases that regulate secretion of cell wall components in rapidly expanding root hair cells, we first identified AtRab GTPases that may be localized on TGN membranes based on similarity to TGN-localized Rab GTPases from other organisms. In yeast and mammals, Ypt31/32 and Rab11 homologs localize to TGN compartments. Therefore, plant Rab GTPases with significant similarity to these yeast and mammalian GTPases may also have a similar localization. Analysis of the A. thaliana genome revealed 26 Rab GTPases that shared significant similarity to Ypt31/32 and Rab11 (Rutherford and Moore, 2002; Vernoud et al., 2003). In Medicago truncatula, MtRab11G shares sequence similarity to mammalian Rab11 and was cloned from a root hair–specific cDNA library (Covitz et al., 1998). Another related member in pea (Pisum sativum), Pra3, was expressed at highest levels in the rapidly expanding hypocotyl cells of etiolated pea seedlings (Nagano et al., 1995). The A. thaliana Rab GTPase RabA4b is closely related to both MtRab11G and Pra3; therefore, we reasoned that RabA4b was a good candidate to begin investigation of polarized secretion in A. thaliana root hair cells.
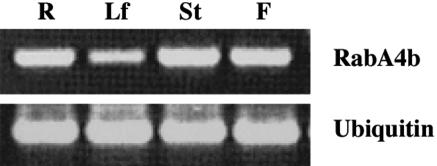
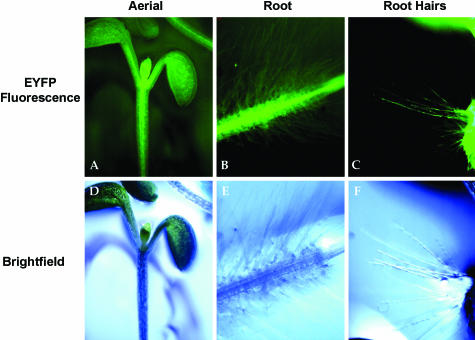
Although RabA4b shared a high degree of similarity to the Pra3 and MtRab11G GTPases, it was not clear whether RabA4b was actually expressed in A. thaliana root hair cells. To determine the expression pattern of RabA4b in A. thaliana, RNA was obtained from root, leaf, stem, and flower tissues of three-week-old plants. After cDNA levels were normalized by amplification of ubiquitin, RT-PCR was performed with RabA4b-specific primers. RabA4b transcripts were present in all examined tissues, although slightly lower levels were detected in leaves (Figure 1). To determine if RabA4b expression was restricted to specific tissues or cell types within the various plant organs, we constructed a promoter:enhanced yellow fluorescent protein (EYFP) reporter gene fusion to examine the expression pattern of RabA4b in planta. Roughly 2 kb of upstream promoter sequence of the RabA4b gene was PCR amplified, fused to EYFP in a plant expression vector, and stably transformed into A. thaliana. Expression of the promoter:EYFP fusion was examined using fluorescence microscopy in 7- to 10-d-old seedlings. EYFP fluorescence was observed in all cell types throughout seedling shoots and roots (Figures 2A, 2B, 2D, and 2E). Importantly, expression of the RabA4b promoter was also detected in root hair cells (Figures 2C and 2F). Taken together, the RT-PCR and promoter fusion results indicate both that RabA4b is widely expressed in different tissues and, more importantly for these studies, that the expression of this Rab GTPase is found in root hair cells of A. thaliana.
Figure 1.
RabA4b Is Expressed Ubiquitously in A. thaliana Plants.
Tissue from roots (R), leaves (Lf), stems (St), and flowers (F) of 3-week-old A. thaliana plants were used for RNA isolation. RT-PCR analysis of RabA4b expression was performed with primers specific for RabA4b and primers specific to ubiquitin as a loading control.
Figure 2.
Promoter:EYFP Analysis of RabA4b Expression in A. thaliana.
Seven- to ten-day-old seedlings expressing the RabA4b promoter:EYFP gene were imaged using a Zeiss M2-Bio fluorescence dissecting scope with a 1.0× lens either with epifluorescence illumination and appropriate EYFP filters ([A], [B], and [C]) or with transmitted light ([D], [E], and [F]). EYFP fluorescence was observed throughout the shoots ([A] and [D]) and roots ([B] and [E]) and also in root hair cells ([C] and [F]).
Subcellular Localization of the RabA4b GTPase in A. thaliana
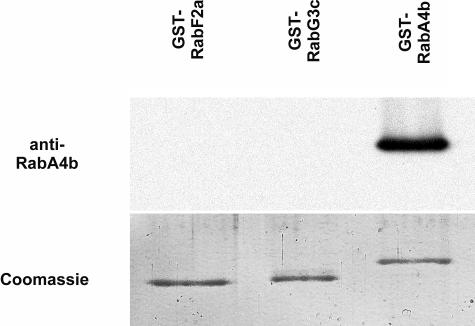
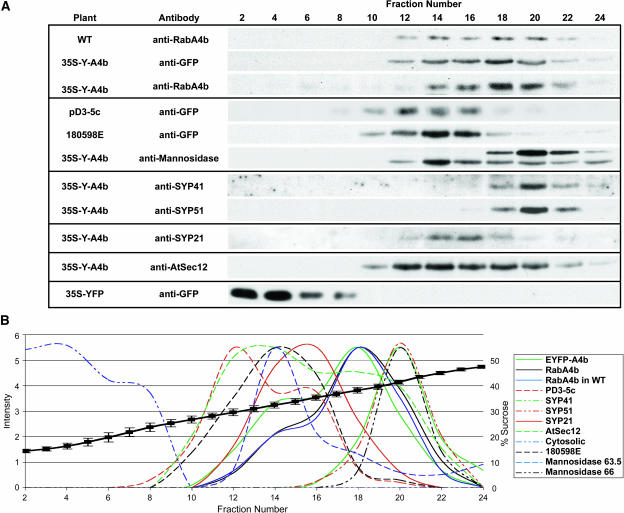
Rab GTPases specifically localize to subcellular membranes and regulate membrane trafficking through these compartments (reviewed in Zerial and McBride, 2001). We therefore wanted to determine the subcellular localization of the RabA4b Rab GTPase within plant cells. First, antibodies were raised against recombinant RabA4b protein that specifically recognized a single band in total protein extracts (see supplemental figure online). Furthermore, this antibody was shown to be specific for the RabA4b GTPase because it failed to recognize equivalent quantities of purified recombinant RabF2a or RabG3c (Figure 3). Membranes were isolated from root tissue from A. thaliana plants and then separated by sucrose density gradient fractionation. Fractionated membranes were probed with the RabA4b-specific antibody to determine the fractionation pattern of the compartment (Figure 4). Transformed A. thaliana plants were generated expressing EYFP-RabA4b as a fusion protein under control of the 35S promoter of Cauliflower mosaic virus. Membranes containing EYFP-RabA4b were detected by immunoblotting with antibodies specific for EYFP. The fusion protein was found to have the same fractionation pattern as the RabA4b protein in membranes from untransformed plants. Also, in plants expressing EYFP-RabA4b, the endogenous RabA4b protein colocalized with the EYFP fusion protein (Figure 4). These results confirmed that the EYFP-RabA4b fusion protein cofractionated with membranes containing endogenous RabA4b, and expression of the fusion protein did not noticeably alter the characteristics of these membranes. Because RabA4b showed significant similarity to Rab GTPases implicated in late-Golgi and/or TGN membrane trafficking steps (Rutherford and Moore, 2002; Vernoud et al., 2003), we asked if RabA4b-labeled membranes cofractionated with proteins previously determined to localize to Golgi or TGN membranes. First, we examined the fractionation patterns of two GFP fusion proteins, PD3-5c and 180598E, previously suggested to localize to Golgi compartments in A. thaliana (Cutler et al., 2000; Cutler, 2001). In addition, we used antibodies raised against a recombinantly expressed A. thaliana Golgi enzyme, α-1,2-mannosidase I. These antibodies label Golgi compartments, as determined by immunofluorescence techniques (data not shown). We observed that this antibody recognized two proteins of 63.5 and 66 kD on immunoblots (Figure 4A). The 63.5-kD α-1,2-mannosidase I protein band cofractionated with the Golgi markers PD3-5c and 180598E, with highest levels of antibody detection observed in fractions 12 and 14 (Figure 4B). Whereas minor peaks of RabA4b and EYFP-RabA4b were detected in fraction 14, the vast majority of RabA4b-labeled membranes were observed in later fractions peaking in fraction 18 (Figure 4B). Intriguingly, the 66-kD protein band recognized by the anti-α-1,2-mannosidase I antibody cofractionated with TGN-localized syntaxins (SYP41 and SYP51; Bassham et al., 2000; Sanderfoot et al., 2001), with all three of these marker proteins displaying clear peaks in fraction 20 (Figures 4A and 4B). RabA4b- and EYFP-RabA4b–labeled membranes also did not cofractionate with other markers tested, including SEC12, an ER-localized protein (Bar-Peled and Raikhel, 1997), and SYP21, a syntaxin localized to endosomes (Sanderfoot et al., 1998). In these fractionation studies, we did not observe two SYP21 peaks as has been previously described (Sanderfoot et al., 1998); however, this may be explained by differences in the fractionation methods. Taken together, these results indicate that although a small proportion of RabA4b can be detected on membranes that cofractionate with Golgi marker proteins, the majority of RabA4b resides on a novel compartment, and this compartment is distinct from TGN compartments defined by the presence of the syntaxin proteins SYP41 and SYP51.
Figure 3.
Specificity of the RabA4b Antibody.
Antisera raised to RabA4b was purified and tested on a protein blot with equal quantities (0.1 μg of protein) of recombinant RabA4b, RabAF2a, and RabG3c protein. The antibodies recognized only RabA4b and not other Rab proteins under these conditions.
Figure 4.
RabA4b Is Localized to a Novel Membrane Compartment.
(A) Transformed A. thaliana plants were generated expressing EYFP-RabA4b, GFP-PD3-5c (Cutler, 2001), or GFP-180598E (Cutler et al., 2000) under control of the 35S promoter of Cauliflower mosaic virus. Membranes were isolated from roots of 2- to 3-week-old untransformed seedlings grown in liquid culture under continuous shaking and then separated by sucrose density gradient fractionation (20 to 60%, w/v). Fractions (500 μL) were collected from the top (fraction 2) to the bottom (fraction 24), and proteins were analyzed by SDS-PAGE followed by immunoblotting with specific antibodies. Anti-RabA4b antibodies were used to detect membranes containing endogenously expressed RabA4b. In transformed plants, membranes containing EYFP-RabA4b and endogenous RabA4b were detected by immunoblotting with antibodies specific for EYFP and RabA4b, respectively. Cofractionation of these two proteins is consistent with localization of the EYFP-RabA4b fusion protein to membranes that also contain endogenously expressed RabA4b. RabA4b from wild-type plants also cofractionated with EYFP-RabA4b, showing that expression of the fusion protein did not alter the nature of this compartment. To detect Golgi membranes, antibodies specific to Golgi-localized α-1,2-mannosidase I were used. Interestingly, this antibody recognized two distinct proteins: a lower molecular mass protein (63.5 kD) that cofractionated with two other Golgi marker proteins, PD3-5c and 180598E (Cutler et al., 2000; Cutler, 2001), and a higher molecular mass protein (66 kD) that cofractionated with two TGN markers, SYP41 (Bassham et al., 2000) and SYP51 (Sanderfoot et al., 2001). EYFP-RabA4b–containing membranes displayed a fractionation pattern similar to, but distinct from, membranes containing these TGN markers, SYP41 and SYP51. RabA4b and EYFP-RabA4b also did not cofractionate with AtSec12, an ER-localized protein (Bar-Peled and Raikhel, 1997), or SYP21, a syntaxin localized to endosomes (Sanderfoot et al., 1998).
(B) To quantitatively measure the fractionation patterns of the various proteins in this analysis, band intensities were first collected for each immunoblot. These measurements were then normalized to compensate for overall variation in band intensities observed with the different antibodies. After normalization, these values were plotted for quantitative comparison of fractionation profiles.
EYFP-RabA4b Localizes to the Tips of Growing Root Hair Cells
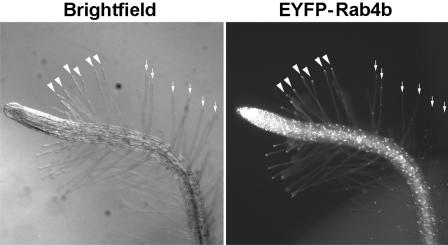
We next visualized the distribution of RabA4b within living plant cells. In most tissues, EYFP-RabA4b localized as punctate structures distributed randomly within the cell (Figures 5 and 6, main root axis). Interestingly, the EYFP-AtRab4b fusion protein labeled a compartment that accumulated at the tips of root hairs (Figures 5A, 5C, 5E, and 5G). This tip localization was observed in nine independent lines expressing the EYFP-RabA4b fusion protein (data not shown). Close examination of these cells indicated that enrichment of the EYFP-RabA4b–labeled compartment was confined to the tips of these root hairs and was not simply distributed evenly throughout the subapical cytosol region (Figure 5C, arrows).
Figure 5.
EYFP-RabA4b Localizes to the Tips of Root Hairs in A. thaliana.
Root hairs of A. thaliana seedlings expressing EYFP-RabA4b ([A], [C], [E], and [G]) or EYFP-RabF2a ([B], [D], [F], and [H]) were imaged at high magnification using a Zeiss M2-Bio fluorescence dissecting scope with a 1.0× lens either with transmitted light ([A] and [B]) or with epifluorescence illumination and appropriate EYFP filters ([C] and [D]). Arrows indicate the EYFP-RabA4b localized to the extreme tips of root hair cells ([A] and [C]). By contrast, EYFP-RabF2a was not preferentially localized to root hair tips ([B] and [D], arrowheads); rather, it was distributed in punctate structures along the length of the root hair ([D], arrows). Lower magnification images show the zone of growing root hairs where EYFP-RabA4b is tip localized ([E] and [G]) and EYFP-RabF2a is distributed along the length of each root hair ([F] and [H]).
Figure 6.
Only Growing Root Hairs Display Polarized EYFP-RabA4b.
Images of A. thaliana seedlings stably expressing EYFP-RabA4b were collected using a Zeiss M2-Bio fluorescence dissecting microscope equipped with a 1.0× lens. Successive images were collected either with transmitted light, (brightfield, left panel) or with epifluorescence illumination and appropriate EYFP filters (EYFP-RabA4b, right panel). Root hairs in the vicinity of the root meristem display tip-localized EYFP-RabA4b compartments (arrowheads), whereas the tip localization of EYFP-RabA4b was not seen in mature root hair cells (arrows).
It is possible that high levels of both secretory and endocytic membrane trafficking occur in this region and thus cause EYFP-RabA4b–labeled compartments to localize at the growing tips of root hair cells (Miller et al., 1997). If this were the case, plant Rab GTPases that label other membrane compartments would also be expected to localize to the tips of growing root hair cells. However, when we examined the distribution of another A. thaliana Rab GTPase, RabF2a, which labels early endocytic compartments in plants (Ueda et al., 2001; E. Nielsen, unpublished results), we found it was not similarly distributed (Figures 5B, 5D, 5F, and 5H). In expanding root hair cells, EYFP-RabF2a did not accumulate at root hair tips (Figure 5D, arrowheads) but instead was localized in small punctate structures spread along the length of the root hair (Figure 5D, arrows). Therefore, the tip localization observed for EYFP-RabA4b did not simply reflect high levels of endomembrane trafficking in this region of growing root hair cells. Rather, this compartment was specifically focused at the tips of these root hair cells.
Specific accumulation of the EYFP-RabA4b fusion to compartments at the tips of root hairs would support the idea of a role for this compartment in secretion of cell wall materials in these expanding cells. If this were the case, accumulation of the EYFP-RabA4b compartment should correlate with root hair growth in a developmentally regulated manner. We therefore examined the accumulation of EYFP-RabA4b in developing root hair cells along the length of the root. During root hair development, root hairs closer to the root apex, in zones of active expansion, get progressively longer as they become located further away from the growing root apex (Figure 6, arrowheads); by contrast, mature root hairs that are no longer expanding are all of similar length (Figure 6, arrows). Tip localization of EYFP-RabA4b was observed only in younger root hairs (Figure 6, arrowheads). In mature root hair cells, EYFP-RabA4b no longer focused at the root hair tip but either spread thinly around the edge of the cell or accumulated in compartments in the base of the cell (Figure 6, arrows). Therefore, the tip localization of the EYFP-RabA4b–labeled compartment in root hair cells is developmentally regulated and is only associated with root hair tips during active expansion.
Tip Localization of EYFP-RabA4b Requires an Intact Actin Cytoskeleton
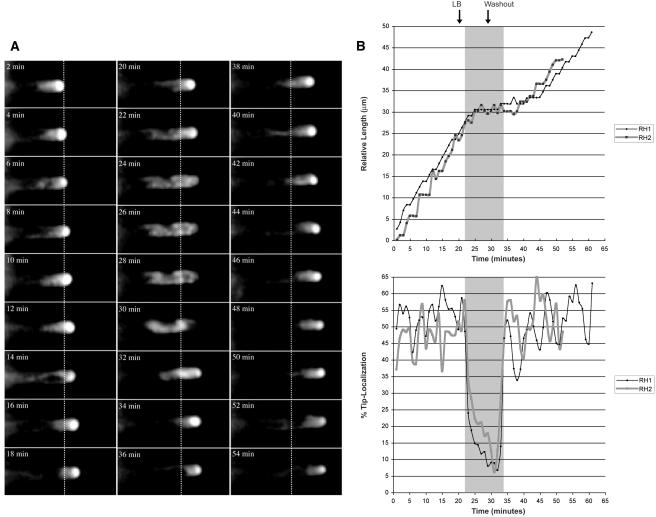
The actin cytoskeleton is known to be essential for maintaining tip growth in plant cells (Cai et al., 1997; Kropf et al., 1998; Bibikova et al., 1999); treating root hairs with latrunculin B (LB), an actin-depolymerizing agent, inhibits the growth of root hairs (Bibikova et al., 1999). To see if depolymerization of F-actin affected tip localization of the EYFP-RabA4b compartment, we first established conditions that allowed us to visualize growing root hair cells in a microscope growth chamber. Under these conditions, expanding root hair cells were always observed to have tip-localized EYFP-RabA4b compartments (n > 20). EYFP-RabA4b tip localization was quantified by measuring the amount of fluorescence at the tip of the growing root hair cell and comparing it to the EYFP-RabA4b fluorescence detected along the entire length of the root hair cell. Under normal growing conditions, 40 to 60% of the EYFP-RabA4b fluorescence is at the tip (Figure 7B). Upon treatment with 200 nM LB, tip-focused EYFP-RabA4b localization rapidly became dispersed along the length of the root hair (Figures 7A and 7B; see supplemental video online). Analysis of successive images of growing root hair cells indicated that loss of tip localization was associated with inhibition of root hair growth, although a delay of ∼2 minutes was observed between dispersal of EYFP-RabA4b and growth inhibition (Figures 7A and 7B). These effects were reversible: washout of LB resulted in a reorganization of tip-localized EYFP-RabA4b compartments. Within 5 to 10 min of the removal of LB in washout experiments, significant levels of EYFP-RabA4b could again be observed in the tip of the root hair cell (Figures 7A and 7B). After ∼5 min, recovery of tip-localized EYFP-RabA4b compartment was followed by resumed root hair expansion at the same rate as before LB treatment (Figures 7A and 7B). Although the individual growth characteristics of different root hair cells varied to a degree, all treatments (n > 20) with LB resulted in a loss of tip-localized EYFP-RabA4b that coincided with cessation of expansion. These effects were specific for LB because root hairs treated with oryzalin to depolymerize microtubules, or control treatment with DMSO, did not display either loss of tip-localized EYFP-RabA4b or altered growth rates during the time frame examined in these experiments (Figure 8A). These experiments do not, however, address other potential roles for microtubules in root hair growth that might only be observed after longer-term incubation with microtubule inhibitors (Bibikova et al., 1999).
Figure 7.
Treatment with the Actin-Depolymerizing Drug LB Causes Loss of EYFP-RabA4b Tip Localization in Root Hairs.
(A) Seedlings expressing EYFP-RabA4b were grown in liquid media and transferred to a perfusion chamber for fluorescence microscopy. Normal root hair expansion was observed for 20 min. A dotted line denotes the relative position of the root hair tip at the beginning of analysis. Upon treatment with 200 nM LB (20 min time point), EYFP-RabA4b tip localization was rapidly lost, and fluorescence was observed along the entire length of the cell (24 to 30 min time points). This effect was reversible: washout of LB (28 min time point) resulted in reorganization of tip-localized EYFP-RabA4b after a short lag (34 min time point).
(B) Quantitative analysis of LB inhibition of root hair growth. Root hair length and EYFP-RabA4b tip fluorescence were quantified in two representative root hairs (RH1 and RH2) treated with LB. Root hair fluorescence was quantified using computational methods. Fluorescent signal located within the proximal 15% of the length of the root hair was defined as tip fluorescence, and this was presented as a percentage of the fluorescence detected in the entire root hair. Growth of each root hair was inhibited when tip fluorescence was lost. Upon recovery of tip fluorescence, root hair expansion resumed. Shaded area denotes the time period in which tip fluorescence was absent.
Figure 8.
Tip Localization of the EYFP-RabA4b Compartment Does Not Require Intact Microtubules.
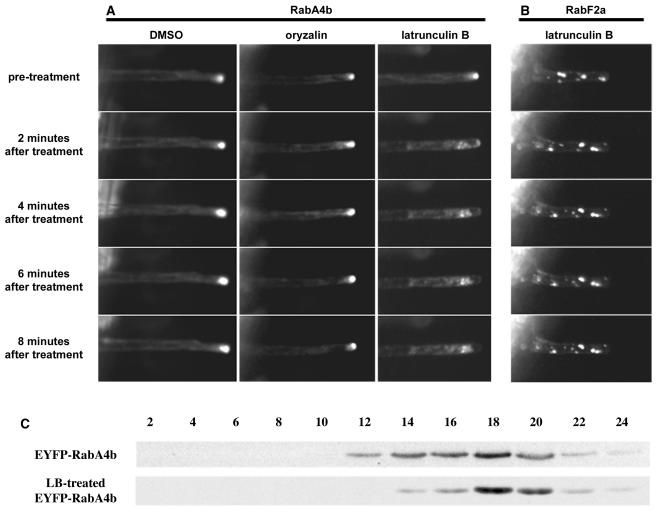
(A) Root hairs treated with LB show significant loss of tip localization within 2 min of treatment. In the same time frame, root hairs treated with 10 μM oryzalin to depolymerize microtubules and control treatment with DMSO display no obvious defects in tip localization.
(B) In contrast with root hairs treated with EYFP-RabA4b, the overall distribution of EYFP-RabF2a in root hairs was not significantly changed upon treatment with LB.
(C) LB treatment did not significantly alter the characteristics of EYFP-RabA4b–labeled compartments. Plants expressing EYFP-RabA4b were treated with LB for 15 min before subcellular fractionation of the membranes over a sucrose density gradient. The fractionation pattern of EYFP-RabA4b in membranes treated with LB was similar to that in nontreated plants (cf. with fractionation patterns in Figure 4). These results demonstrate that tip localization of RabA4b compartments is dependent on an intact actin cytoskeleton and that the nature of the compartment is not demonstrably changed by LB treatment.
To determine whether LB treatment affected localization of other membrane compartments, we examined the distribution of EYFP-RabF2a upon actin depolymerization. Whereas tip localization of EYFP-RabA4b was quickly lost upon LB treatment, the overall distribution of EYFP-RabF2a compartments did not change, implying that F-actin depolymerization does not alter the distribution of every subcellular compartment in root hair cells (Figure 8B). Furthermore, LB treatment did not appear to significantly alter the nature of these compartments because EYFP-RabA4b–labeled membranes isolated after LB treatment were still present, and their sedimentation pattern was not dramatically different than untreated membranes. (Figures 8C and 4). These results indicate that maintenance of tip-localized EYFP-RabA4b compartments requires an intact actin cytoskeleton and support the hypothesis that this compartment plays a role in the actin-dependent growth of root hairs.
Tip Localization of EYFP-RabA4b Is Altered in Root Hair Developmental Mutants
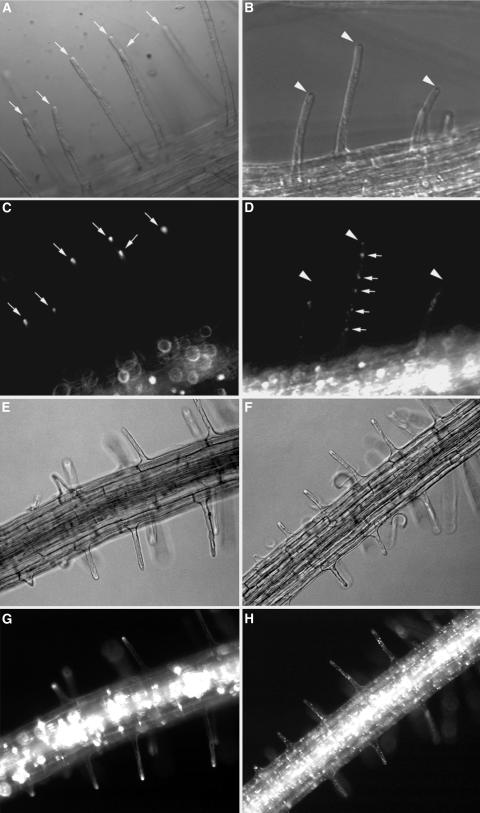
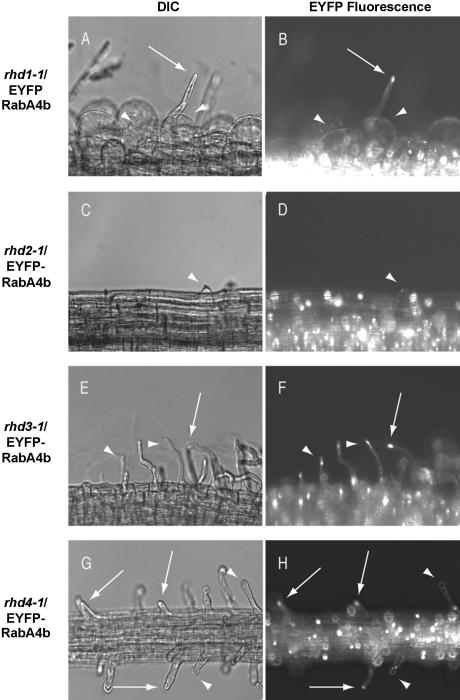
If tip-localized EYFP-RabA4b is required for normal growth and development of root hair cells in A. thaliana, loss or alteration of EYFP-RabA4b tip localization might be expected in mutants defective in root hair growth. To determine if EYFP-RabA4b compartments displayed altered distribution during defective root hair growth, we took advantage of previously described root hair developmental mutants. A. thaliana with root fair defective (rhd) mutations, rhd1-1, rhd2-1, rhd3-1, and rhd4-1, are impaired at various stages of root hair development (Figure 9; Schiefelbein and Somerville, 1990). To examine whether localization of EYFP-RabA4b was altered in these plants, we expressed the EYFP-RabA4b fusion protein in root hair mutant backgrounds and examined the localization of EYFP-RabA4b within the root hair cells of these plants. Mutant rhd1-1 plants displayed defects at early stages of root hair development (Schiefelbein and Somerville, 1990). Loss of RHD1 probably results in the weakening of the cell wall during root hair initiation, resulting in uncontrolled bulge formation. EYFP-RabA4b showed no specific localization in these bulging root hairs (Figures 9A and 9B, arrowheads). Occasionally, rhd1-1 root hairs overcame the defect and initiated root hairs from these enlarged bulges. In these cells, tip-localized EYFP-RabA4b could be observed (Figures 9A and 9B, arrows), suggesting that the rhd1-1 defect does not directly interfere with EYFP-RabA4b tip localization. In rhd2-1 mutants, root hair cells initiate bulges but are unable to switch to tip growth and terminate expansion at this stage. As would be consistent with a role in tip-focused expansion, we never observed accumulation of EYFP-RabA4b compartments in rhd2-1 root hair cells (Figures 9C and 9D, arrowheads). Both rhd3-1 and rhd4-1 root hair mutants improperly control root hair elongation once tip growth has initiated. In rhd3-1 plants, wavy root hairs are associated with abnormal distribution of vesicles in the subapical cytoplasm (Wang et al., 1997; Galway et al., 1999). Interestingly, although some tip localization of EYFP-RabA4b compartments was still seen in rhd3-1 mutant root hairs (Figures 9E and 9F, arrows), this localization often accumulated either slightly behind or to one side of the growing root hair tip (Figures 9E and 9F, arrowheads). In rhd4-1 plants, bulging or branching occurs along the length of the root hair, which suggests that proper restriction of cell expansion to the tips of these root hairs is lost during growth. In those root hairs where bulges were observed at the extreme growing tip, organized EYFP-RabA4b tip localization could not be detected (Figures 9G and 9H, arrowheads). However, in root hair cells in which bulges had formed earlier but where normal restriction of cell expansion associated with tip growth had resumed, tip-focused EYFP-RabA4b had also recovered (Figures 9G and 9H, arrows). These results indicate that when root hairs deviate from normal tip growth in different rhd mutants, normal tip localization of the EYFP-RabA4b compartment is also disturbed.
Figure 9.
Localization of the EYFP-RabA4b Fluorescence in the rhd Mutant Backgrounds.
The 35S-EYFP-RabA4b construct was transformed into the mutant root hair lines rhd1-1 ([A] and [B]), rhd2-1 ([C] and [D]), rhd3-1 ([E] and [F]), and rhd4-1 ([G] and [H]). Plants were grown in 0.25× MS + 0.3% phytagel and transferred to microscope slides. Root hairs were observed using a Nikon Eclipse E600 microscope with differential interference contrast ([A], [C], [E], and [G]) and epifluorescence ([B], [D], [F], and [H]) optics. Arrows indicate root hairs with normal tip localization of EYFP-RabA4b. Arrowheads point to abnormal distributions of the EYFP-RabA4b in root hairs.
DISCUSSION
In this article, we characterize the expression pattern and subcellular localization of the A. thaliana Rab GTPase RabA4b. Whereas a small proportion of this Rab GTPase cofractionated with Golgi marker proteins, the majority localized to a novel compartment and displayed a polarized distribution to the tips of growing root hair cells. In plants with normal root hairs, this polarized localization was tightly correlated to tip growth. However, tip localization of RabA4b-labeled compartments was altered or absent in root hair developmental mutants. These results provide evidence that this plant Rab GTPase is associated with novel membrane compartments that display polarized accumulation in tip-growing cells. This distribution is consistent with a role in polarized secretion of cell wall components to the plasma membrane in these tip-growing cells.
RabA4b Localizes to a Novel Plant Membrane Compartment
In many instances, a high degree of sequence conservation between plant Rab GTPases and Rab GTPases from yeast and animals correlates with localization to similar membrane compartments (Bednarek et al., 1994; Ueda et al., 1996; Batoko et al., 2000; Cheung et al., 2002; Inaba et al., 2002). RabA4b is most closely related to yeast Ypt31/32 and human Rab11a, Rab11b, and Rab25. In yeast, YPT31/32 have been implicated in vesicle transport steps associated with the trans-Golgi cisterna (Benli et al., 1996; Jedd et al., 1997). In Schizosaccharomyces pombe, YPT3 is localized to cell tips in an F-actin–dependent manner, and mutations in this gene result in defects in cell wall integrity (Cheng et al., 2002). Mammalian Rab11-like GTPases mediate membrane trafficking steps involved in the polarized recycling of plasma membrane proteins (Ullrich et al., 1996). However, Rab11a also plays an important role in the secretion of newly synthesized proteins (Chen et al., 1998; Chen and Wandinger-Ness, 2001). Previous immunolocalization experiments performed on the related P. sativum Rab GTPase, Pra3, also showed colocalization with a TGN marker protein, AtVTI11 (Zheng et al., 1999; Inaba et al., 2002). Intriguingly, we have not seen colocalization of RabA4b with some markers for the TGN (Figure 4), implying that the RabA4b compartment is distinct from previously described TGN compartments. What might the RabA4b compartment be? Whereas the majority of RabA4b appeared to localize to distinct compartments, a small proportion of this Rab GTPase was regularly observed cofractionating with Golgi marker proteins. This dual localization would be consistent with cargo sorting and trafficking between the Golgi and a novel RabA4b-labeled compartment. Although it is possible that these trafficking events are associated with cis-elements of the Golgi complex, it is more likely that this sorting and trafficking is from trans-Golgi elements. If this were the case, the role of RabA4b in membrane trafficking would be the same as functions attributed to the highly similar Rab GTPases Ypt31/32 and Rab11a in both yeast and mammals. Furthermore, post-Golgi secretory vesicles accumulate directly beneath the site of expansion in tip-growing root hair cells (Galway et al., 1997). These vesicles are thought to deliver newly synthesized cell wall components from Golgi complexes to the plasma membrane where they are deposited during cell expansion. Therefore, the tip-restricted localization of RabA4b-labeled membranes in growing root hair cells also supports a role for RabA4b in trafficking of cargo from trans-Golgi elements to the plasma membrane.
At present, we are not able to determine whether the RabA4b-labeled compartment is involved in delivery of secretory cargo from the Golgi to the plasma membrane or if its primary function is in endocytosis and recycling proteins and membranes in the growing root hair. However, EYFP-RabF2a, which labels plant endocytic compartments (Ueda et al., 2001; E. Nielsen, unpublished results), was not focused at the root hair tip like the EYFP-RabA4b compartment was (Figure 5). These results imply a role for RabA4b in secretion rather than endocytosis and indicate that if RabA4b labels an endocytic compartment, it is distinct from that defined by the presence of RabF2a.
Polarized Localization of RabA4b in Root Hair Cells
In A. thaliana, root epidermal cells emerge from the meristem in alternating columns of root hair forming cells (trichoblasts) and non-root-hair forming cells (atrichoblasts; Dolan et al., 1994; Galway et al., 1994). Formation of the root hair begins with a bulge in the cell wall at the end of the trichoblast closest to the root meristem. Bulge formation is not accompanied by accumulation of secretory vesicles in the underlying cytosol but does coincide with cell wall acidification and cell wall thinning (Bibikova et al., 1998; Ryan et al., 2001). Thus, deposition of new cell wall material is not thought to occur until after the transition to tip-focused growth (Schiefelbein and Somerville, 1990). This transition is accompanied by formation of a tip-focused Ca2+ gradient and accumulation of secretory vesicles directly beneath the site of expansion (Galway et al., 1997; Wymer et al., 1997). Because we did not detect accumulation of EYFP-RabA4b–labeled compartments in early root hair bulges of wild-type plants or in the arrested bulges of rhd2 mutants, our hypothesis is that EYFP-RabA4b labels compartments specifically involved in tip growth (Figure 9).
During tip growth, root hairs maintain a highly polarized subapical cytoplasmic region behind the tip of the cell (Galway et al., 1997). EYFP-RabA4b compartment distribution was restricted to an apical subdomain within this region (Figure 5). This distribution appears to be unique, although other proteins involved in membrane trafficking have been described to localize to root hair tips. When ectopically expressed, KNOLLE, normally acytokinesis-specific syntaxin, accumulates at the tips of root hair cells (Volker et al., 2001). This localization is intriguing because KNOLLE has a polarized distribution and localizes to phragmoplasts in dividing cells. But because this syntaxin is not normally expressed in root hair cells, the tip distribution observed in this cell type may not correspond to its true subcellular localization. In addition, it was not determined that the tip localization of the KNOLLE protein corresponded with expansion in these root hair cells. Several groups have described polarized accumulation of Rop GTPases in root hair cells (Molendijk et al., 2001; Jones et al., 2002). However, it appears that they localize to the plasma membrane and that localization is not dependent upon an intact actin cytoskeleton (Molendijk et al., 2001; see discussion below). Interestingly, the Rop GTPase tip localization is lost upon treatment with Brefeldin A, implying that some vesicle transport steps are required for the localization of these signaling GTPases. The plant dynamin-like protein ADL1c also displays tip localization in expanding root hair cells (Kang et al., 2003), and the ADL1c protein is associated primarily with the plasma membrane. These observations highlight the dynamic nature of membrane trafficking events in the tips of expanding root hair cells. Clearly, understanding how these various membrane trafficking and signaling components interact with one another will be central to elucidating the molecular mechanisms by which polarity is established and secretory pathways are organized during polarized expansion in plant cells.
Actin-Dependent Positioning of RabA4b in Root Hair Cells
We observed a correlation between polarized EYFP-RabA4b distribution and tip growth in root hair cells. This correlation implies that the membrane compartment labeled by the RabA4b GTPase plays a key role during polar growth in these cells. It is, however, important to understand how this polarized accumulation is accomplished. The apical vesicle-rich zone in growing root hairs is embedded in a dense meshwork of F-actin (Baluska et al., 2000). Presence of this network is thought to be responsible for delivering secretory vesicles to the growing tips of root hair cells (Ryan et al., 2001), and disrupting F-actin with LB inhibits root hair expansion (Bibikova et al., 1999; Ovecka et al., 2000). Additionally, mutations of the ACTIN2 gene of A. thaliana result in defective root hair growth (Ringli et al., 2002). When we treated root hair cells with LB, EYFP-RabA4b tip localization was abolished (Figure 7). Therefore, accumulation of the RabA4b compartment at the growing tips of expanding root hairs requires an intact F-actin cytoskeleton. Upon washout of LB, expansion resumed only after EYFP-RabA4b–labeled compartments reorganized at the tip (Figures 7A and 7B). Based on these observations, we propose that tip-localized RabA4b labels secretory vesicles in the vesicle-rich zone of root hairs and is involved inF-actin–dependent sorting and secretion of cell wall components from the Golgi to the site of cell expansion.
Microtubules (MT) are also required for normal root hair growth. Endoplasmic MTs were described as being required for fast growth in M. truncatula root hairs (Sieberer et al., 2002). However, most studies indicate that MTs are more important for maintaining directionality of root hair expansion rather than tip growth itself (Bibikova et al., 1999). Consistent with these findings, we did not observe changes in root hair growth, and tip localization EYFP-RabA4b compartments were not affected by treatment with the MT-depolymerizing drug oryzalin (Figure 8A). We do not exclude the possibility that long-term disruption of MTs may affect tip localization of EYFP-RabA4b–labeled membranes. However, based on the results we present here, maintenance of tip localization of the RabA4b compartment is primarily dependent upon an intact F-actin cytoskeleton. Therefore, in root hairs, MTs likely provide a stable framework that helps orient the actin cytoskeleton.
RabA4b Localization Is Altered in Root Hair Developmental Mutants
Much insight into root hair growth has come from studies of developmental mutants (Schiefelbein and Somerville, 1990; Parker et al., 2000). We reasoned that if tip localization of the EYFP-RabA4b–labeled compartment was important for root hair growth, then it might be mislocalized in mutants with defective root hair morphology. Mutation of the UDP-d-glucose4-epimerase encoded by RHD1 (Seifert et al., 2002) results in loss of control of bulge formation in trichoblasts. In rhd1-1 mutants, many trichoblast cells no longer organize tip-growing root hairs (Schiefelbein and Somerville, 1990). Failure to organize a tip-growing root hair in bulging rhd1-1 trichoblasts was accompanied by a lack of any focused accumulation of EYFP-RabA4b–labeled compartments within the trichoblast cell (Figure 9). Intriguingly, some rhd1-1 root hair cells were able to organize tip growth and subsequently form root hairs. Although these cells often had enlarged bulges at the base of the root hair, we observed tip-localized EYFP-RabA4b at the growing tips. This implies that defective substrate channeling of UDP-d-galactose leads to a loss of structural integrity in the cell wall but that this interferes with downstream organization of tip-growing root hairs only indirectly.
In rhd2-1 mutants, root hair cells initiate bulges but fail to transition to tip growth; no further elongation of root hairs occurs. In this mutant background, we were unable to observe any accumulation of EYFP-RabA4b within the bulges of root hair cells (Figure 9). Based on these results, we hypothesize that polarized accumulation of EYFP-RabA4b–labeled compartments only occurs after transition to tip growth. RHD2 has recently been cloned and has been found to encode an NADPH oxidase (Foreman et al., 2003). RHD2 activity is required for production of reactive oxygen species (ROS) in root hair cells. ROS production results in Ca2+ uptake at the root hair tip and the subsequent formation of a tip-focused Ca2+ gradient. Ca2+ gradient formation is, in turn, necessary for tip growth in the root hair cell (Wymer et al., 1997). Lack of accumulation of the EYFP-RabA4b compartment in the bulges of rhd2-1 root hair cells indicates that an oxidative burst and generation of a Ca2+ gradient are necessary for the proper positioning of these membranes.
The RHD3 gene encodes a novel GTP binding protein (Wang et al., 1997). In rhd3-1 mutants, root hairs are wavy and have disorganized subapical cytoplasmic domains (Galway et al., 1997; Wang et al., 1997). Other rhd3-1 phenotypes, such as smaller vacuoles and smaller cell sizes, are not restricted to root hair cells and are observed throughout the plant (Wang et al., 1997). More recently, RHD3 function has been implicated in trafficking steps between the ER and Golgi (Zheng et al., 2004). In the rhd3-1 mutant background, we often observed abnormal positioning of EYFP-RabA4b–labeled compartments in expanding root hair cells. RabA4b membranes were observed at sites distal to the root tip; in many cases, this localization was unevenly distributed to one side of the root hair (Figure 9). This altered distribution supports previous observations that the orientation of the site of expansion changes during root hair growth in rhd3-1 mutants (Galway et al., 1997). It is possible that the wavy growth characteristics seen in rhd3-1 root hairs are because of improper placement of EYFP-RabA4b compartments within these cells.
In rhd4-1 mutant plants, root hairs are shorter than in the wild type and vary in diameter along their length, forming bulges and constrictions (Schiefelbein and Somerville, 1990). In this mutant background, EYFP-RabA4b–labeled compartments were observed in the tips of some root hair cells but not others (Figure 9). Generally, tip localization of EYFP-RabA4b was lost in root hairs that appeared to be in the process of forming bulges. Interestingly, a periodic loss of tip organization during tip growth, perhaps correlated with the stochastic growth of root hairs (Wymer et al., 1997), could lead to the observed branching or bulges that are seen along the length of rhd4-1 root hairs (Galway et al., 1999). This would be consistent with the periodic loss of EYFP-RabA4b tip localization observed in these root hairs. Another study has shown that application of low concentrations of actin-depolymerizing drugs in root hairs led to unstable F-actin and local cell expansion or bulging (Ketelaar et al., 2003). An intriguing explanation is that the RHD4 gene product influences actin organization, and loss of function in this mutant results in the instability of the actin cytoskeleton in root hairs. In this situation, periodic disruptions of the organization of the apical vesicle-rich zone in root hair tips could result in episodes of diffuse expansion and bulge formation.
The Function of RabA4b within Root Hair Cells
Although we have isolated and characterized SALK T-DNA insertional mutants for all AtRabA4 family members (data not shown), we have not yet detected defects in root hair cell development in these plants. This is probably because of the presence of several closely related family members within the AtRabA4 subfamily, which may provide redundant functions. It is likely that construction of double or triple mutants will be necessary for proper examination of the effects that loss of these Rab GTPases has on the polarized growth of A. thaliana root hair cells.
One of the first observable events associated with root hair formation is the specific recruitment of Rho of plant (Rop) GTPases to the future bulge sites. Polar localization of AtRop2, AtRop4, and AtRop6 in root hair cells was found to specify sites of hair initiation, and GFP-Rop fusion proteins remained localized to tips of root hairs during polar growth (Molendijk et al., 2001; Jones et al., 2002). An NADPH oxidase was identified as being necessary for ROS production and subsequent Ca2+ gradient formation in root hairs. Indeed, in other systems, Rop GTPases are involved in signal transduction pathways that regulate a variety of cellular processes, such as cell death and secondary cell wall differentiation (Kawasaki et al., 1999; Potikha et al., 1999). In these two examples, Rop GTPase generated signals are transduced through activation of NADPH oxidase (Knaus et al., 1991). H2O2, generated by NADPH oxidases, in turn activates plasma membrane Ca2+ channels (Pei et al., 2000). This results in increased cytoplasmic Ca2+ and initiation of subsequent downstream responses. In tip-growing cells, it has been proposed that one of these downstream responses may be actin reorganization (Gu et al., 2003). We have shown that the actin cytoskeleton is necessary for the proper polarized accumulation of RabA4b-labeled membranes at the tips. Once this tip localization is achieved, localized deposition of cell wall materials by these compartments can lead to proper growth of the root hair. Understanding the molecular mechanisms by which RabA4b becomes polarly localized to the actin cytoskeleton at the tips of growing root hair cells will likely involve components that are regulated by Rop GTPases and/or respond to cytosolic Ca2+ concentrations.
METHODS
RT-PCR
Wild-type Columbia Arabidopsis thaliana plants were grown at 22°C with 16-h light for 3 weeks. Roots, stems, leaves, and flowers were separated and frozen in liquid nitrogen. Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA), and cDNA was made according to Omniscript RT kit (Qiagen) instructions. Amplification of ubiquitin10 (Callis, 1995) was used to equalize levels of cDNA from different tissues. PCR was performed with Taq polymerase using primers to RabA4b (At4g39990; A4b forward, 5′-GGGGTACCATGGCCGGAGGAGGCGGATACG-3′; A4b reverse, 5′-CGGGATCCTCAAGAAGAAGTACAACAAGTG-3′). The amplification program consisted of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C for 30 cycles, followed by a 7-min extension at 72°C.
Construction of Plasmids and Transformed Plants
The plant expression vector pCAMBIA (CAMBIA, Canberra, Australia) was used for plant transformations. The RabA4b cDNA was amplified using the A4b forward and reverse primers described above. The product was cloned into pCAMBIA with EYFP at the N terminus under the control of the 35S promoter of Cauliflower mosaic virus. RabF2a (At5g45130) cDNA was amplified using the primers F2a forward (5′-CGGGATCCATGGCTACGTCTGGAAACAAGA-3′) and F2a reverse (5′-GCTCTAGACTAAGCACAACACGATGAACTC-3′) and inserted into the same pCAMBIA expression vector. Using primers to the RabA4b upstream sequence (A4b promoter forward, 5′-GGAATTCTTGGGGTTCATGTCTGCATG-3′; A4b promoter reverse, 5′-CATGCCATGGTCACGCCAAACTATTGAAAC-3′), about 2 kb of promoter region was amplified. This was inserted into pCAMBIA to drive the expression of EYFP. Plants were transformed using Agrobacterium tumefaciens by the floral dip method (Clough and Bent, 1998).
Fusion Proteins and Antibody Production
Primers were used to amplify the full-length RabA4b, RabF2a, and RabG3c (At3g16100) cDNAs for expression as glutathione S-transferase (GST)-fusion proteins (GST-A4b forward, 5′-CGCGGATCCATGGCCGGAGGAGGCGGATAC-3′; GST-A4b reverse, 5′-TCCCCCGGGTCAAGAAGAAGTACAACAAGT-3′; F2a forward, described above; GST-F2a reverse, 5′-CCGGAATTCCTAAGCACAACACGATGAACT-3′; GST-RabG3c forward, 5′-CGCGGATCCATGGCTTCTCGGCGGCGAGTG-3′; GST-RabG3c reverse, 5′-CCGGAATTCTTAGCATTCGCACCCAGTTGA-3′). These fusion proteins were expressed in Escherichia coli BL21 cells purified with glutathione-sepharose beads. During purification, RabA4b was proteolytically cleaved from GST and eluted from the glutathione-sepharose beads. This cleaved RabA4b protein was then used for antiserum production in rabbits. Cloning of α-1,2-mannosidase I used primers specific for the lumenal domain of the 63.5-kD α-1,2-mannosidase I (At1g51590; mannosidase forward, 5′-ATAGGATCCATGCTTGTCTGGGATCGTA-3′; mannosidase reverse, 5′-ATAGAATTCTAAACGTTAATCTGATGACCAAAC-3′). This protein shares amino acid sequence identity to the 66-kD α-1,2-mannosidase encoded by At3g21160. GST-α-1,2-mannosidase I was expressed in DH5α cells, purified with glutathione-sepharose beads, and used for antiserum production. Before use, both the RabA4b and α-1,2-mannosidase antibodies were affinity purified with recombinant fusion proteins using standard antibody purification techniques.
Membrane Fractionation
Wild-type (Columbia) A. thaliana or A. thaliana expressing 35S-EYFP-RabA4b, GFP-180598E (Cutler et al., 2000), or GFP-PD3-5c (Cutler, 2001) were used for membrane fractionation. Seedlings were grown in liquid culture on a shaker under continuous light conditions for 2 to 3 weeks. Root membranes were isolated and prepared as described in Sanderfoot et al. (1998), except 20% (w/v) sucrose was used in the grinding buffer. Cleared, postnuclear supernatant was layered over 20 to 60% continuous sucrose gradients and spun for 16 h in a SW41 swinging bucket rotor at 35,000 rpm at 4°C. Twenty-four 0.5-mL fractions were collected from the top of the gradient, and sucrose concentrations were determined with a refractometer. For several proteins, we analyzed all fractions collected from these gradients, but we did not see changes in these fractionation profiles versus fractionation profiles when only every other fraction was included. Therefore, even numbered fractions were precipitated with methanol, solubilized in SDS sample buffer, separated on SDS-PAGE, and transferred to nitrocellulose blots for immunodetection with specific antibodies. To ensure that variability between fractionation profiles made from different plants was not an issue, we only compared the experiments that satisfied two criteria. First, we ensured that the sucrose densities in the fractions collected for each experiment were comparable. Then we determined that both RabA4b and SYP21 fractionation profiles were the same between experiments. Only after establishing these two points did we then examine the fractionation profiles of other proteins. Blots were stripped at 50°C with stripping buffer (10% SDS, 100 mM β-mercaptoethanol, and 20 mM Tris, pH 6.8) between probings. Antibodies used were anti-GFP (Clontech, Palo Alto, CA), anti-RabA4b, anti-TLG2a (SYP41; Bassham et al., 2000; Rose Biotechnology), anti-SYP51 (Sanderfoot et al., 2001), anti-α-1,2-mannosidase I, anti-AtSec12 (Rose Biotechnology, Palo Alto, CA), and anti-PEP12 (SYP21; Rose Biotechnology).
Visualization of EYFP-RabA4b Localization
Seedlings were grown in 0.25 MS + 0.3% phytagel, and roots were imaged using a Zeiss M2-Bio fluorescence dissecting microscope (Jena, Germany) equipped with a 1.0× lens. Successive images were collected either with transmitted light or with epifluorescence illumination and appropriate EYFP filters. Seedlings grown in 0.25× MS liquid culture were transferred to a slide and imaged using a Nikon Eclipse E600 microscope (Tokyo, Japan) with a 10× Plan Apo lens (numerical aperture 0.45) with either differential interference contrast or epifluorescence illumination and appropriate EYFP filters.
Treatment of Root Hairs with Cytoskeleton Inhibitors
For inhibitor studies, A. thaliana seeds were individually grown in 2 mL of 0.25× MS liquid media with shaking. Seven to nine days after germination, seedlings were placed into a slide chamber through which media could be exchanged via a peristaltic pump at a rate of 0.35 mL/min. After transfer, seedlings were allowed to recover for at least 1 h. EYFP fluorescence was observed using a Nikon Eclipse E600 microscope every 30 s. LB (Calbiochem, San Diego, CA), dissolved in DMSO, was added at a concentration of 200 nM for inhibition of root hair growth. Equivalent quantities of DMSO were added for the controls. Oryzalin (Chem Service, West Chester, PA) was added at a concentration of 10 μM. Root hair fluorescence was quantified using computational methods. Fluorescent signal located within the proximal 15% of the length of the root hair was defined as tip fluorescence, and this was presented as a percentage of the fluorescence detected in the entire root hair.
Analysis of Root Hair Defective Mutants
Root hair developmental mutants rhd1-1, rhd2-1, rhd3-1, and rhd4-1 were obtained from the ABRC. The 35S-EYFP-RabA4b construct was inserted into the different mutant backgrounds by Agrobacterium transformation, and EYFP fluorescence was observed in root hairs. Plants were grown in 0.25 MS + 0.3% phytagel and transferred to microscope slides. Root hairs were observed using a Nikon Eclipse E600 microscope with differential interference contrast and epifluorescence optics.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers At4g39990, At5g45130, and At3g16100.
Supplementary Material
Acknowledgments
The authors would like to thank Tony Sanderfoot and Natasha Raikhel for kindly sharing antibodies (SYP21, SYP41, and SYP51) and Sean Cutler for transformed A. thaliana lines (EGFP-PD3-5c and GFP-180598E) used in this study. This work was supported by the following grants: Department of Energy DE-FG02-03ER15412, National Aeronautics and Space Administration 01-UNSOL-LSD-003 (E.N.), and USDA Grant 2002-35304-12692 (S.Y.B.).
Online version contains Web-only data.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Erik Nielsen (enielsen@danforthcenter.org).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.021634.
References
- Baluska, F., Salaj, J., Mathur, J., Braun, M., Jasper, F., Samaj, J., Chua, N.H., Barlow, P.W., and Volkmann, D. (2000). Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 227, 618–632. [DOI] [PubMed] [Google Scholar]
- Bar-Peled, M., and Raikhel, N.V. (1997). Characterization of AtSEC12 and AtSAR1. Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol. 114, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham, D.C., Sanderfoot, A.A., Kovaleva, V., Zheng, H., and Raikhel, N.V. (2000). AtVPS45 complex formation at the trans-Golgi network. Mol. Biol. Cell 11, 2251–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.Q., Hawes, C., and Moore, I. (2000). A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12, 2201–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek, S.Y., Reynolds, T.L., Schroeder, M., Grabowski, R., Hengst, L., Gallwitz, D., and Raikhel, N.V. (1994). A small GTP-binding protein from Arabidopsis thaliana functionally complements the yeast YPT6 null mutant. Plant Physiol. 104, 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli, M., Doring, F., Robinson, D.G., Yang, X., and Gallwitz, D. (1996). Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 15, 6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bibikova, T.N., Blancaflor, E.B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657–665. [DOI] [PubMed] [Google Scholar]
- Bibikova, T.N., Jacob, T., Dahse, I., and Gilroy, S. (1998). Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development 125, 2925–2934. [DOI] [PubMed] [Google Scholar]
- Cai, G., Moscatelli, A., and Cresti, M. (1997). Cytoskeletal organization and pollen tube growth. Trends Plant Sci. 2, 86–91. [Google Scholar]
- Callis, J. (1995). Regulation of protein degradation. Plant Cell 7, 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Feng, Y., Chen, D., and Wandinger-Ness, A. (1998). Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell 9, 3241–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., and Wandinger-Ness, A. (2001). Expression and functional analyses of Rab8 and Rab11a in exocytic transport from trans-Golgi network. Methods Enzymol. 329, 165–175. [DOI] [PubMed] [Google Scholar]
- Cheng, H., Sugiura, R., Wu, W., Fujita, M., Lu, Y., Sio, S.O., Kawai, R., Takegawa, K., Shuntoh, H., and Kuno, T. (2002). Role of the Rab GTP-binding protein Ypt3 in the fission yeast exocytic pathway and its connection to calcineurin function. Mol. Biol. Cell 13, 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, A.Y., Chen, C.Y., Glaven, R.H., de Graaf, B.H., Vidali, L., Hepler, P.K., and Wu, H.M. (2002). Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14, 945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Covitz, P.A., Smith, L.S., and Long, S.R. (1998). Expressed sequence tags from a root-hair-enriched Medicago truncatula cDNA library. Plant Physiol. 117, 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S.R. (2001). Live Cell Analysis in Arabidopsis thaliana Using Randomly Generated Markers. (Stanford, CA: Stanford University Press).
- Cutler, S.R., Ehrhardt, D.W., Griffitts, J.S., and Somerville, C.R. (2000). Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97, 3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan, L., Duckett, C., Grierson, C., Linstead, P., Schneider, K., Lawson, E., Dean, C., Poethig, S., and Roberts, K. (1994). Clonal relations and patterning in the root epidermis of Arabidopsis. Development 120, 2465–2474. [Google Scholar]
- Foreman, J., Demidchik, V., Bothwell, J.H., Mylona, P., Miedema, H., Torres, M.A., Linstead, P., Costa, S., Brownlee, C., Jones, J.D., Davies, J.M., and Dolan, L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Galway, M.E., Heckman, J.W., Jr., and Schiefelbein, J.W. (1997). Growth and ultrastructure of Arabidopsis root hairs: The rhd3 mutation alters vacuole enlargement and tip growth. Planta 201, 209–218. [DOI] [PubMed] [Google Scholar]
- Galway, M.E., Lane, D.C., and Schiefelbein, J.W. (1999). Defective control of growth rate and cell diameter in tip-growing root hairs of the rhd4 mutant in Arabidopsis thaliana. Can. J. Bot. 77, 494–507. [Google Scholar]
- Galway, M.E., Masucci, J.D., Lloyd, A.M., Walbot, V., Davis, R.W., and Schiefelbein, J.W. (1994). The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev. Biol. 166, 740–754. [DOI] [PubMed] [Google Scholar]
- Gu, Y., Vernoud, V., Fu, Y., and Yang, Z. (2003). ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J. Exp. Bot. 54, 93–101. [DOI] [PubMed] [Google Scholar]
- Inaba, T., Nagano, Y., Nagasaki, T., and Sasaki, Y. (2002). Distinct localization of two closely related Ypt3/Rab11 proteins on the trafficking pathway in higher plants. J. Biol. Chem. 277, 9183–9188. [DOI] [PubMed] [Google Scholar]
- Jedd, G., Mulholland, J., and Segev, N. (1997). Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 137, 563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.A., Shen, J.J., Fu, Y., Li, H., Yang, Z., and Grierson, C.S. (2002). The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14, 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, B.H., Rancour, D.M., and Bednarek, S.Y. (2003). The dynamin-like protein ADL1C is essential for plasma membrane maintenance during pollen maturation. Plant J. 35, 1–15. [DOI] [PubMed] [Google Scholar]
- Kawasaki, T., Henmi, K., Ono, E., Hatakeyama, S., Iwano, M., Satoh, H., and Shimamoto, K. (1999). The small GTP-binding protein rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 96, 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar, T., de Ruijter, N.C., and Emons, A.M. (2003). Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus, U.G., Heyworth, P.G., Evans, T., Curnutte, J.T., and Bokoch, G.M. (1991). Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 254, 1512–1515. [DOI] [PubMed] [Google Scholar]
- Kropf, D.L., Bisgrove, S.R., and Hable, W.E. (1998). Cytoskeletal control of polar growth in plant cells. Curr. Opin. Cell Biol. 10, 117–122. [DOI] [PubMed] [Google Scholar]
- Luo, Z., and Gallwitz, D. (2003). Biochemical and genetic evidence for the involvement of yeast Ypt6-GTPase in protein retrieval to different Golgi compartments. J. Biol. Chem. 278, 791–799. [DOI] [PubMed] [Google Scholar]
- Miller, D.D., Ruijter, N.D., and Emons, A.M. (1997). From signal to form: Aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J. Exp. Bot. 48, 1881–1896. [Google Scholar]
- Molendijk, A.J., Bischoff, F., Rajendrakumar, C.S., Friml, J., Braun, M., Gilroy, S., and Palme, K. (2001). Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20, 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano, Y., Okada, Y., Narita, H., Asaka, Y., and Sasaki, Y. (1995). Location of light-repressible, small GTP-binding protein of the YPT/rab family in the growing zone of etiolated pea stems. Proc. Natl. Acad. Sci. USA 92, 6314–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovecka, M., Nadubinska, M., Volkmann, D., and Baluska, F. (2000). Actomyosin and exocytosis inhibitors alter root hair morphology in Poa annua. Biologia (Bratisl.) 55, 105–114. [Google Scholar]
- Parker, J.S., Cavell, A.C., Dolan, L., Roberts, K., and Grierson, C.S. (2000). Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell 12, 1961–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Potikha, T.S., Collins, C.C., Johnson, D.I., Delmer, D.P., and Levine, A. (1999). The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 119, 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli, C., Baumberger, N., Diet, A., Frey, B., and Keller, B. (2002). ACTIN2 is essential for bulge site selection and tip growth during root hair development of Arabidopsis. Plant Physiol. 129, 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, S., and Moore, I. (2002). The Arabidopsis Rab GTPase family: Another enigma variation. Curr. Opin. Plant Biol. 5, 518–528. [DOI] [PubMed] [Google Scholar]
- Ryan, E., Steer, M., and Dolan, L. (2001). Cell biology and genetics of root hair formation in Arabidopsis thaliana. Protoplasma 215, 140–149. [DOI] [PubMed] [Google Scholar]
- Sanderfoot, A.A., Ahmed, S.U., Marty-Mazars, D., Rapoport, I., Kirchhausen, T., Marty, F., and Raikhel, N.V. (1998). A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 95, 9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot, A.A., Kovaleva, V., Bassham, D.C., and Raikhel, N.V. (2001). Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol. Biol. Cell 12, 3733–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein, J.W., and Somerville, C. (1990). Genetic control of root hair development in Arabidopsis thaliana. Plant Cell 2, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf, E. (1986). Cellular polarity. Annu. Rev. Plant Physiol. 37, 23–47. [Google Scholar]
- Seifert, G.J., Barber, C., Wells, B., Dolan, L., and Roberts, K. (2002). Galactose biosynthesis in Arabidopsis: Genetic evidence for substrate channeling from UDP-D-galactose into cell wall polymers. Curr. Biol. 12, 1840–1845. [DOI] [PubMed] [Google Scholar]
- Sieberer, B.J., Timmers, A.C., Lhuissier, F.G., and Emons, A.M. (2002). Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs. Plant Physiol. 130, 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, E.J., Kim, E.S., Zhao, M., Kim, S.J., Kim, H., Kim, Y.W., Lee, Y.J., Hillmer, S., Sohn, U., Jiang, L., and Hwang, I. (2003). Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell 15, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale, E.J. (1999). A Rab2 mutant with impaired GTPase activity stimulates vesicle formation from pre-Golgi intermediates. Mol. Biol. Cell 10, 1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale, E.J., Bourne, J.R., Khosravi-Far, R., Der, C.J., and Balch, W.E. (1992). GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, T., Anai, T., Tsukaya, H., Hirata, A., and Uchimiya, H. (1996). Characterization and subcellular localization of a small GTP-binding protein (Ara-4) from Arabidopsis: Conditional expression under control of the promoter of the gene for heat-shock protein HSP81-1. Mol. Gen. Genet. 250, 533–539. [DOI] [PubMed] [Google Scholar]
- Ueda, T., Yamaguchi, M., Uchimiya, H., and Nakano, A. (2001). Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 20, 4730–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbe, S., Zerial, M., and Parton, R.G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud, V., Horton, A.C., Yang, Z., and Nielsen, E. (2003). Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 131, 1191–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker, A., Stierhof, Y.D., and Jurgens, G. (2001). Cell cycle-independent expression of the Arabidopsis cytokinesis-specific syntaxin KNOLLE results in mistargeting to the plasma membrane and is not sufficient for cytokinesis. J. Cell Sci. 114, 3001–3012. [DOI] [PubMed] [Google Scholar]
- Wang, H., Lockwood, S.K., Hoeltzel, M.F., and Schiefelbein, J.W. (1997). The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 11, 799–811. [DOI] [PubMed] [Google Scholar]
- Wymer, C.L., Bibikova, T.N., and Gilroy, S. (1997). Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 12, 427–439. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117. [DOI] [PubMed] [Google Scholar]
- Zheng, H., Kunst, L., Hawes, C., and Moore, I. (2004). A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J. 37, 398–414. [DOI] [PubMed] [Google Scholar]
- Zheng, H., von Mollard, G.F., Kovaleva, V., Stevens, T.H., and Raikhel, N.V. (1999). The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol. Biol. Cell 10, 2251–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.