Abstract
The blood-brain barrier (BBB) poses a unique challenge for drug delivery to the central nervous system (CNS). The BBB consists of a continuous layer of specialized endothelial cells linked together by tight junctions, pericytes, nonfenestrated basal lamina, and astrocytic foot processes. This complex barrier controls and limits the systemic delivery of therapeutics to the CNS. Several innovative strategies have been explored to enhance the transport of therapeutics across the BBB, each with individual advantages and disadvantages. Ongoing advances in delivery approaches that overcome the BBB are enabling more effective therapies for CNS diseases. In this review, we discuss: (1) the physiological properties of the BBB, (2) conventional strategies to enhance paracellular and transcellular transport through the BBB, (3) emerging concepts to overcome the BBB, and (4) alternative CNS drug delivery strategies that bypass the BBB entirely. Based on these exciting advances, we anticipate that in the near future, drug delivery research efforts will lead to more effective therapeutic interventions for diseases of the CNS.
Keywords: Central Nervous System (CNS), Blood-Brain Barrier (BBB), nanotechnology, ultrasound, immunotherapy
1. Introduction
1.1. Structure of the Blood-Brain Barrier
The human brain is comprised of a complex vascular network of over 100 billion capillaries [1]. The vasculature in the central nervous system (CNS) plays a vital role in protecting the brain from potentially neurotoxic substances [2]. The blood-brain barrier (BBB) is formed primarily by the tight junctions that join the endothelial cells of the CNS, creating a physical barrier that restricts the passage of solutes [3]. These specialized endothelial cells maintain a continuous, nonfenestrated basal lamina and interact with a number of perivascular elements, including astrocytes, pericytes, and perivascular macrophages which contribute to the barrier [4]. Astrocytes and pericytes help shape the early development of the BBB through the secretion of signaling proteins, such as sonic hedgehog and retinoic acid [3, 5]. As the BBB matures, astrocytes project end-foot processes along the perivascular space, whereas pericytes cover the basal lamina of the endothelium and contribute to the structural integrity of the BBB [6].
1.2. Transport Across the BBB
Substances may cross the BBB by two primary pathways: 1) paracellular transport, which involves passing in between the endothelial cells, and 2) transcellular transport, which involves passing across the luminal side of the endothelial cell, crossing the cytoplasm, and then passing across the abluminal side of the endothelial cell into the brain interstitium. The endothelial cell tight junctions typically prevent the paracellular transport of molecules in areas
with an intact BBB. Transcytosis, on the other hand, takes place through passive and active mechanisms. Passive transport across the endothelial cell is regulated by physicochemical properties such as molecular weight, electrical charge, and lipophilicity, and is typically limited to small, lipophilic molecules that are less than 500 Daltons in size [1, 7]. Other transport mechanisms are necessary for nutrients and proteins that are larger and less lipophilic. These include glucose, which undergoes carrier-mediated transport (CMT) via the glucose transporter 1 (GLUT-1) protein, insulin, which undergoes receptor-mediated transcytosis (RMT), and albumin, which undergoes adsorptive-mediated transcytosis (AMT) (for a review, see [8]).
1.3. Challenges for Drug Delivery Across the BBB
The BBB serves an important role by maintaining homeostasis and preventing macromolecules, infectious agents, and potential neurotoxins from entering the brain [1]. However, the BBB also significantly limits the ability of therapeutic agents to reach their targets in the CNS. More than 90% of all small-molecule drugs and nearly 100% of all larger therapeutics are prevented from crossing the BBB [1]. Furthermore, those few drugs that are capable of crossing the BBB may be actively transported back into the vasculature by efflux transporters, such as P-glycoprotein 1, a member of the ATP binding cassette family [3]. These active efflux transporters can recognize a wide range of compounds (>60% of all marketed drugs) and contribute to drug resistance [9]. In addition, metabolic degradation has also been shown to play a role in reducing the accumulation of drugs in the brain [3, 7].
A number of strategies have been developed to overcome the BBB and improve the delivery of therapeutic agents to the brain (see Fig. 1). These range from disrupting the barrier itself to modifying the transported agents and their carriers. An alternative strategy is to deliver drugs directly into the brain through a variety of routes that bypass the BBB entirely. In this review, we discuss both conventional and emerging strategies to overcome the BBB, including specific features, advantages, and limitations (see Table 1).
Fig. (1).
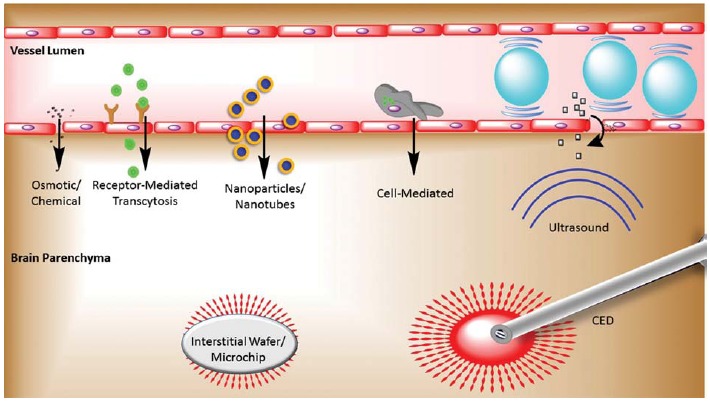
Strategies for delivering therapeutic agents across the BBB. Therapeutic agents are transported from the vessel lumen across the BBB via osmotic or chemical disruption of tight junctions, receptor-mediated transcytosis, nanoparticle-based carriers (including targeted nanoparticles), cell-mediated delivery, and FUS-mediated oscillation of microbubbles causing disruption of tight junctions and enhanced transcytosis. Interstitial wafers and microchips, in addition to catheter-based CED, bypass the BBB and deliver therapeutic agents directly to the brain parenchyma.
Table 1. Strategies for Drug Delivery Across the BBB.
| Method | Route | Pros | Cons |
|---|---|---|---|
| Osmotic Disruption of BBB | Paracellular | Transient | Invasive Transient cerebral edema Non-specific |
| Chemical Disruption of BBB | Paracellular | Transient | Conflicting results in clinical trials |
| Enhanced Transcellular Transport | Transcellular | Targeted | Low efficiency |
| Nanoparticle Delivery | Paracellular and Transcellular | Targeted Sustained and/or controlled release |
Cost Regulatory hurdles Potential toxicity |
| Cell-Based Delivery | Transcellular | Targeted | Toxicity to cell carrier Low therapeutic loading |
| Focused Ultrasound | Paracellular and Transcellular | Non-invasive Targeted |
Cost |
| Intrathecal and Intraventricular Delivery | Bypass BBB | Elevated concentrations in CSF | Invasive Limited parenchymal concentrations Rapid CSF turnover |
| Intranasal Delivery | Bypass BBB | Non-invasive Simple administration |
Irritation of nasal mucosa Low efficiency |
| Interstitial Wafers and Microchips | Bypass BBB | Sustained and/or controlled release | Invasive Limited distribution through ECS |
| Convection Enhanced Delivery | Bypass BBB | Enhanced distribution via bulk flow | Invasive Back flow of infusate Risk of catheter misplacement |
BBB = blood-brain barrier, CSF = cerebrospinal fluid, ECS = extracellular space
2. Conventional strategies for overcoming the BBB
2.1. Enhanced Paracellular Transport
2.1.1. Osmotic Disruption of the BBB
Hyperosmotic disruption of the BBB has been explored since the early 1970s, when Rapoport et al. [10] first demonstrated that hypertonic solutions applied to the pial surface of a rabbit brain allowed the extravasation of intravascular Evans Blue dye. Subsequent work in animal models and humans employed an intra-arterial osmotic agent to facilitate the delivery of various drugs of interest, which were typically also injected intra-arterially in order to maximize local concentrations of the drug by taking advantage of the “first pass” of the drug through the tumor circulation. Osmotic agents exert an effect on the BBB via several mechanisms (for a review, see [11]). First, water is drawn out of the endothelial cell and into the blood vessel lumen, causing shrinkage of the endothelial cells. Secondly, the net flow of water out of the brain leads to vasodilation, thereby stretching the endothelial cell membrane. Lastly, interactions between actin and cadherin cause the endothelial cell cytoskeleton to contract via a calcium-dependent mechanism. Each of these mechanisms places stress on the tight junctions that join the endothelial cells, ultimately causing widening of the junctions and allowing paracellular transport into the brain parenchyma [12].
A number of osmotic agents have been used for BBB disruption (BBBD), including arabinose [13], urea [14], and in particular mannitol [15-17]. Following the intra-carotid infusion of mannitol, BBB permeability persists for 15 minutes to 4 hours depending on the size of the molecule being transported, with the barrier reclosing sooner to larger molecules [18, 19]. Preclinical studies have demonstrated the benefits of this approach for neuro-oncology – following osmotic BBBD with mannitol, the intraparenchymal concentration of intraarterially delivered chemotherapeutic agents rises substantially [20]. However, while this is safe for some chemotherapies, such as methotrexate, other drugs such as cisplatin and doxorubicin can reach neurotoxic levels [21]. In addition to chemotherapeutic agents, other studies have utilized osmotic BBBD for the enhanced delivery of antibodies [22, 23], nanoparticles [24], recombinant proteins [25], stem cells [26], and viral vectors [27].
Since the first Phase I clinical trial in 1979 [16], numerous studies and clinical trials have reported the use of osmotic BBBD in patients. Adults with primary CNS lymphoma undergoing treatment with methotrexate and cyclophosphamide demonstrated improved survival and positive cognitive outcomes when the treatment was preceded by osmotic BBBD [28-30]. Four patients with multifocal CNS germinoma underwent consolidation therapy with carboplatin and etoposide following osmotic BBBD [31]. The patients all showed complete responses. Similarly, patients with malignant glioma were treated with a combination of methotrexate, cyclophosphamide, and procarbazine (in the pre-temozolomide era) following osmotic BBBD and demonstrated improved survival with minimal neurotoxicity [32, 33]. In 2000, a consortium of five centers reported their experience treating 221 adult patients with primary CNS lymphoma, primitive neuroectodermal tumors, germ cell tumors, brain metastases, low grade gliomas, and high grade gliomas [34]. The patients were treated with intra-arterial chemotherapy with or without osmotic BBBD, and the authors demonstrated high rates of stable disease and tumor response, with a correspondingly low complication rate.
Despite these positive reports, osmotic disruption of the BBB has yet to become widespread due to several critical limitations. One concern relates to the fact that osmotic BBBD results in transient cerebral edema, with a 1.5% increase in brain water [35]. Furthermore, osmotic BBBD is nonspecific, taking place throughout normal brain tissue as well. This allows protein components of the blood to enter the brain parenchyma, including albumin, which is toxic to neural tissue [36]. These features of osmotic BBBD can result in seizures and the exacerbation of neurological deficits.
2.1.2. Chemical Disruption of the BBB
Chemical BBBD involves intra-arterial vasoactive agents that generate a temporary inflammatory reaction in the endothelial cells. Alkylglycerols have been studied because they are characterized by non-ionic amphiphilicity, which allows them to rapidly integrate into and destabilize the endothelial cell membrane, resulting in BBB opening for 3-15 minutes [37, 38]. A number of other agents have been explored as well, including the cytokine interleukin-2 [39], leukotriene C4 [40], bradykinin [41, 42], and RMP-7 [43]. Bradykinin represents a popular agent for BBBD due to reports that at low concentrations, intra-arterial bradykinin selectively increases the BBB permeability in the region of a brain tumor [42, 44]. Bradykinin binds to the B2 receptors on endothelial cells, producing a transient increase in cytosolic Ca2+, which activates nitric oxide synthase – an enzyme that is more prevalent in tumor vasculature. The ultimate result is an increase in nitric oxide, a signaling molecule that produces vasodilation and increased vascular permeability [45, 46]. A synthetic bradykinin analog, RMP-7, demonstrates similar effects but with higher potency, greater specificity for the B2 receptor [47], and more resistance to degradation, resulting in a higher half-life and allowing for intravenous administration (for a review, see [48]). RMP-7 has therefore become the more commonly studied agent for chemical BBBD.
Early preclinical studies demonstrated that RMP-7 administration could enhance the delivery of variably sized molecular tracers to RG2 gliomas in rats [49]. When applied to the delivery of chemotherapeutic agents, RMP-7 improved the transport of carboplatin across the BBB by two-fold, resulting in improved survival of rats with RG2 gliomas [50-52]. Lipophilic chemotherapies such as paclitaxel, however, did not demonstrate improved transport [51]. As a result of these studies, carboplatin was studied in combination with RMP-7 in Phase I clinical trials involving adults [43] and children [53] with brain tumors. These trials found that RMP-7 is safe and tolerable, and that combining it with carboplatin does not increase the incidence of side effects. An early, single-arm Phase II trial also found a beneficial effect of BBBD via RMP-7 for patients with recurrent malignant glioma undergoing carboplatin treatment [54]. Unfortunately, when the RMP-7/carboplatin regimen advanced to a multicenter, placebo-controlled trial, the earlier studies could not be replicated and the combination was found to be ineffective [55].
2.2. Enhanced Transcellular Transport
A number of strategies are being developed for enhancing the transcellular transport of therapeutic agents across the endothelial cells of the CNS. These include modifying drugs to make them more lipophilic, as well as using prodrugs that are capable of crossing the BBB into the brain parenchyma where they become modified into a biologically active form, which can no longer cross the BBB. One of the more innovative methods for achieving transcellular transport, however, involves hijacking the endogenous RMT pathway. Therapeutic agents that would typically be unable to overcome the BBB are conjugated to ligands or antibodies that trigger RMT, thereby producing a “chimeric peptide” that is transported across the endothelial cell. This approach has been described as using a “molecular Trojan horse” to gain access to the brain parenchyma (for a review, see [56]).
A variety of receptors have been implicated in RMT across the BBB, including the insulin receptor [57], the transferrin receptor [58, 59], and the low density lipoprotein receptor [60]. Several studies have used an antibody for the transferrin receptor to achieve RMT-based delivery across the BBB [61-63]. Human transferrin receptor fused to a mouse-human chimeric IgG maintained IgG receptor binding activity and was able to cross the BBB intact [61]. A more recent study demonstrated that by using a monoclonal antibody to the transferrin receptor, a gene therapy construct (consisting of a glial fibrillary acidic protein gene promoter driving tyrosine hydroxylase expression) could be successfully delivered across the BBB [62]. However, in another study in which a lysosomal enzyme (iduronate 2-sulfatase) was fused to a human insulin receptor antibody, only a small amount of the fusion protein crossed the BBB of a nonhuman primate, with only 1% of the injected dose transported into the brain parenchyma [64].
A recently completed Phase I clinical trial studied GRN1005, a peptide-drug conjugate composed of paclitaxel and a low-density lipoprotein receptor-related protein 1 peptide, in patients with recurrent malignant glioma [65]. While reported toxicities included neutropenia, leucopenia, fatigue, and mucositis, these are similar to the toxicities of paclitaxel alone. There was no evidence of CNS toxicity, and the chimeric peptide appeared to deliver paclitaxel successfully across the BBB, thereby paving the way for future Phase II studies.
3. Emerging strategies for delivery across the BBB
Several new, promising approaches for drug targeting and delivery across the BBB have been described in recent years, including the use of carrier agents such as nanoparticles and immune cells, reversibly opening the BBB using MR-guided focused ultrasound (MRgFUS), and leveraging alternate administrative routes. These emerging strategies have great potential to enhance drug delivery to the CNS in spite of the BBB, and they will be discussed in the following sections.
3.1. Nanoparticle Carriers
Nanoparticles have become a major focus of drug delivery research, and in particular are promising carriers for drug delivery to the brain due to several unique characteristics, including small size, enhanced drug solubility, the ability for multi-functionality, a controlled drug release profile, and the potential for site-specific targeting [66]. The nanoparticle surface can be readily modified to effectively carry drugs across the BBB. Although the exact mechanisms by which these nanoparticles cross the BBB are not fully understood, various classes of nanoparticles, including metallic, polymeric and lipid nanoparticles, have been shown to cross the BBB and enter the brain through a variety of endocytotic mechanisms [67].
3.1.1. Metallic Nanoparticles
Metallic nanoparticles are commonly made of inorganic materials and include gold, silver, and iron oxide particles, in addition to metallic allotropes of non-metals, such as carbon fullerenes. Metallic nanoparticles are typically smaller than polymeric or lipid nanoparticles, which provides an advantage with regard to crossing the BBB. Metallic nanoparticles can be transported into the brain through several routes, including passive diffusion, CMT, or trans-synaptic transport [2]. Due to the solid and dense structure of metallic nanoparticles, drugs cannot be encapsulated within them. However, therapeutic agents such as anticancer drugs, antibodies, and siRNA can be conjugated to the surfaces of these nanoparticles.
Several in vivo studies have shown that metallic nanoparticles, including those comprised of gold [68], silver [69], and iron oxide [70], can cross the BBB. For instance, Cheng et al. [68] demonstrated that gold nanoparticles (5 nm in diameter) modified with the trans-activator of transcription peptide are capable of crossing the BBB and delivering both doxorubicin and gadolinium contrast agents to a murine intracranial glioma xenograft. Kong et al. [70] demonstrated that following the systemic administration of magnetic iron oxide nanoparticles and application of an external magnetic field, the nanoparticles can cross the mouse BBB via a transcellular mechanism and accumulate in a perivascular zone.
Nanoparticle shape plays an important role in crossing the BBB. Carbon nanotubes have generated significant interest due to their needle-like shape yet flexible structure, which facilitates the crossing of biological membranes such as the BBB [71, 72]. Kafa et al. [73] showed that functionalized multi-walled carbon nanotubes (MWNTs) were able to cross an in vitro model of the BBB. Successful crossing of the BBB and accumulation in the brain was confirmed in vivo, as well, following systemic administration of the MWNTs.
3.1.2. Polymeric Nanoparticles
In contrast to metallic nanoparticles, polymeric nanoparticles consist of “soft” nanomaterials, which are synthesized by organic chemistry. Polymeric nanoparticles are less rigid and less dense, enabling them to encapsulate a wide variety of therapeutics including chemotherapeutic drugs, proteins, nucleic acids, and contrast agents. Polymeric nanoparticles are excellent candidates for drug delivery vehicles due to the availability of a variety of polymer types as well as access to large surface areas containing functional groups to which biomolecules can be conjugated. There is also a high degree of flexibility with respect to tailoring the physicochemical properties of polymeric nanoparticles, including the size, surface charge, and aspect ratio (shape) of the particles, with the goal of crossing the BBB more effectively.
A number of therapeutic agents have been delivered to the brain using polymeric nanoparticles. For example, dalargin, kyotorphin, loperamide, tubocurarine, doxorubicin, clioquinol, D-penicillamine, paclitaxel, rivastigmine, dexamethasone, and 5-fluorouracil have been encapsulated in polymeric nanoparticles composed of dextran, chitosan, propylene glycol, polypropylene oxide, polyethylene glycol (PEG), poly (lactic-co-glycolic) acid (PLGA), a co-polymer of poly (oxyethylene)-poly (oxypropylene), poly (butylcyanoacrylate), poly (N-isopropylacrylamide), or dendrimers [66]. In some cases, these drug-loaded polymeric nanoparticles were coated with stabilizers, such as PEG and polysorbate-80, to prolong the circulation time and reduce particle clearance [74].
Transcytosis is the main mechanism by which polymeric nanoparticles accumulate in the brain [75]. This route has been examined using small polymeric nanoparticles composed of albumin or poly (butylcyanoacrylate) [76, 77]. Polymeric nanoparticles may therefore be used as carriers for therapeutic agents that would otherwise be unable to cross the BBB, by mechanisms such as CMT or RMT. For example, Ren et al. [78] reported that amphotericin B-loaded poly (lactic acid)-b-PEG nanoparticles coated with polysorbate 80 crossed the BBB by CMT, resulting in increased drug concentrations in the mouse brain. Vergoni et al. [79] used peptide-decorated PLGA nanoparticles to deliver loperamide to the brain by RMT, and confirmed the efficacy of this drug carrier when compared to intraventricular administration. More recently, Serramia et al. [80] delivered Nef siRNA to the CNS using dendrimers. The dendrimers were transcytosed across the BBB resulting in efficient Nef silencing, which in turn reduced human immunodeficiency virus-1 (HIV-1) infectivity in astrocytes. Gene silencing was achieved without significant cytotoxicity.
3.1.3. Liposomes
Liposomes are self-assembled vesicles made of amphiphilic phospholipids that mimic the lipid bilayer of the cell membrane. Liposomes are biocompatible carriers capable of carrying hydrophilic, hydrophobic, and amphoteric drug molecules [81, 82]. Liposomal formulations have been shown to be an effective system for crossing the BBB due to their resemblance to the lipid bilayer of the endothelial cell membrane. The basic mechanisms for liposomal transport across the BBB include AMT and RMT. Cationic liposomes, in particular, have been shown to cross the BBB via AMT [83]. Similarly to polymeric nanoparticles, the surface properties of liposomes can be modified to extend their circulation time. Modification of the liposomal surface with hydrophilic polymers sterically stabilizes the liposomes, allowing them to avoid opsonization and uptake by reticuloendothelial cells [84]. Qin et al. [85] reported that liposomes formulated with a glycosyl derivative of cholesterol showed enhanced delivery across the BBB by targeting the glucose transporters on the endothelial cell membranes of the brain.
3.1.4. Targeted Nanoparticles
The metallic, polymeric, and lipid nanoparticles described above typically cross the BBB via transcellular pathways. Conjugating the nanoparticles to a targeting moiety facilitates this passage across the BBB by offering several advantages: 1) a potential increase in nanoparticle lipophilicity, 2) an increase in nanoparticle stability, 3) site-specific targeting, and 4) uptake by the endothelium via RMT [86].
Liposomes can be conjugated to a variety of targeting moieties such as monoclonal antibodies, peptides, and plasma proteins for site-specific targeting [87]. Liposomes with mannose coatings, for example, have been extensively used for brain targeting [88, 89]. Du et al. [90] conjugated p-aminophenyl-α-d-mannopyranoside (MAN), a mannose analog, to the surface of liposomes to enhance their delivery to the brain. They found a significantly higher accumulation of the MAN-liposomes in the cerebellum and cerebral cortex of the mouse brain, compared to non-conjugated liposomes. Similarly, Qin et al. [91] evaluated the potential anti-depressant effect of edaravone-loaded liposomes conjugated to the cyclic RGD (cRGD) peptide in rats. The authors reported that the cRGD-liposomes showed excellent brain-targeting and enhanced drug delivery. Ying et al. [88] developed dual-targeting liposomes conjugated with MAN and transferrin in order to enhance their transport across the BBB and targeting to glioma cells. The authors found that this strategy significantly enhanced the transport of daunorubicin (encapsulated within the liposomes) across the BBB by up to 24.9% in both in vitro and in vivo models.
Kreuter et al. [77] showed that dalargin and loperamide-loaded poly(butyl cyanoacrylate) nanoparticles, coated with polysorbate 80 and with apolipoprotein B or E, achieved anti-nociceptive effects in ApoEtm1Unc and C57BL/6J mice. The authors concluded that the polysorbate 80-coated nanoparticles adsorb to apolipoproteins in the blood, thereby mimicking lipoprotein particles that are taken up by the brain capillary endothelial cells via RMT. In another study, the same group showed that apolipoprotein E-conjugated albumin nanoparticles entered into the brain parenchyma by an endocytic mechanism [76]. Similarly, Tosi et al. [92] used the near-infrared probe DY-675 to show that systemically administered PLGA nanoparticles conjugated with a simil-opioid glycopeptide (g7) achieved successful BBB-crossing and brain targeting via endocytosis.
As described above, various BBB-associated receptors, including transferrin, lactoferrin and folate, have been used to deliver nanoparticles that are specifically targeted to the CNS by hijacking the RMT pathway. In particular, transferrin receptors are of great interest because of their overexpression on the BBB endothelium [93]. Recently, Fornaguera et al. [94] showed that anti-transferrin monoclonal antibodies conjugated to loperamide-loaded PLGA nanoparticles were able to efficiently cross the BBB in vivo. Additionally, Clark et al. [95] prepared transferrin-conjugated gold nanoparticles with an acid-cleavable linkage between the transferrin and the nanoparticle core to facilitate the RMT of nanoparticles across the BBB. These nanoparticles bind to transferrin receptors with high affinity on the luminal side of the endothelial cell, and later separate upon acidification (which takes place during transcytosis), thus allowing release of the nanoparticles into the brain. Combining nanoparticle carriers with RMT thus represents a powerful method for enhancing drug targeting and efficiency. Nanoparticles may also be employed jointly with other emerging delivery strategies, including cell-mediated delivery and ultrasound-mediated BBBD (see below).
3.2. Cell-Based Drug Delivery
3.2.1. Immune Cells and Stem Cells as Therapeutic Carriers
Harnessing the innate mobility of cells is another exciting option for enabling therapeutic delivery across the BBB. To date, two broad cell types have been evaluated as therapeutic carriers, immune cells and stem cells, particularly neural stem cells (NSCs) and mesenchymal stem cells (MSCs). Both immune cells and stem cells have been demonstrated to cross the BBB and migrate into the CNS via an inflammation-mediated pathway. A number of advantages make these cells an attractive option for delivering therapeutics to the CNS (for a review, see [96]). Cells can deliver a variety of therapeutics, including genes, cytokines, enzymes, and nanoparticles. Moreover, both immune cells and stem cells are naturally recruited to sites of tissue damage and inflammation, a common feature of diseases of the CNS, including brain cancer [97-99]. Indeed, intravenous administration of these cell types has resulted in accumulation in brain tumors [100, 101]. Therefore, cells may serve as targeted carriers for delivering therapeutics to inflamed tissues across the BBB.
Immune cells migrate across endothelial barriers, including the BBB, by a process known as diapedesis, whereby the cell transiently tethers to and rolls along the endothelial cells and ultimately transmigrates through interactions between integrins (e.g. very late antigen-4), cell adhesion molecules (e.g. VCAM-1 and ICAM-1), and selectin molecules (e.g. P-selectin glycoprotein-1) [102-104]. The immune cells then generate actin-containing structures such as lamellipodia to extend into the endothelial cells, facilitating diapadesis. The method by which stem cells cross the BBB is controversial, but there are many reported similarities to immune cell diapedesis. MSCs express many, though not all, of the same types of receptors and adhesion molecules [104, 105]. Unlike immune cells, MSCs do not move laterally along the endothelial wall, and MSCs cross endothelial barriers much more slowly, taking 1-2 hours to transmigrate (while immune cells take minutes) [105]. Additionally, whereas immune cells use actin structures such as lamellipodia to initiate diapedesis, MSCs use membrane blebs [104].
3.2.2. Cell-Mediated Delivery for the Treatment of Brain Tumors
Immune and stem cell migration has been studied extensively as a means of delivering therapies for cancer patients with primary or metastatic brain lesions. In particular, cell-based therapies have been explored as a treatment for metastases, due to the associated inflammation that accompanies these tumors [106]. In a mouse model of metastatic brain cancer, stem cells have been shown to migrate to metastases and suppress growth [107]. Cell-based therapies are also relevant for the treatment of invasive glioblastoma (GBM) cells that have migrated away from the tumor core. NSCs in particular are recruited to migrating tumor cells, providing a mechanism for the targeted delivery of therapeutics to distant glioma cells following an intraparenchymal injection. NSCs are therefore an attractive option for the treatment of invasive cancer cells, although the signals that promote NSC migration to these cells remain unknown [100, 108]. NSCs have been used to deliver a soluble form of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which can trigger apoptosis of cancer cells by binding to death receptors [100]. MSCs may play a role as well – in a mouse glioma model, MSCs were used to deliver the pro-inflammatory cytokine interleukin (IL) 12, resulting in enhanced survival [109]. Recently, human adipose-derived MSCs were also engineered to express bone morphogenetic protein 4 (BMP4), a growth factor with the potential to inhibit the proliferation of GBM brain tumor-initiating cells [110]. The MSCs successfully targeted GBM cells in a mouse glioma model, resulting in reduced proliferation, limited migration, and improved survival. These results reinforce the possibility of treating brain cancer via cell-mediated delivery of therapeutics.
3.2.3. Limitations of Cell-Mediated Delivery
Although the ability of both immune cells and stem cells to cross the BBB make them a very attractive avenue for potentially delivering therapeutics to the CNS, there are a number of challenges to cell-mediated drug delivery. A chief problem is the potentially toxic effects of the cargo on the cell carrier itself [111]. As a result, the therapeutic agent must either be non-toxic to the carrier or the carrier must be shielded from its cargo until the cell has reached the target. Spatial and temporal control over the release of the therapeutic agent by the cell poses another challenge to cell-mediated delivery [112]. Additionally, the loading efficiency of therapeutics into cell carriers may be low, requiring large numbers of cells to deliver therapeutic doses.
3.2.4. Enhancing Cell-Mediated Delivery with Nanoparticle Carriers
Nanoparticles offer an option for overcoming many of the hurdles to successful cell-mediated therapy. By using the ability of macrophages and monocytes to phagocytose nanoparticles, nanoparticles can be effectively loaded with therapeutics and delivered to the interior of the cell [113, 114]. Nanoparticle formulations, at least temporarily, protect the cell carrier from its cargo. In one study, a nanoparticle formulation of doxorubin delayed the death of the T-cells in which they were loaded, although 60% of the T-cells were destroyed by 15 hours following delivery [111].
Loading cells with nanoparticles is affected by several features of the particles. Nanoparticles with positively charged surface residues are endocytosed more quickly than neutral or negatively charged particles [112, 115]. Size also influences cellular uptake of nanoparticles – larger particles are phagocytosed more efficiently than smaller particles. For example, 1 um diameter particles accumulated in macrophages 2.5x more than 0.5 um diameter particles [115]. Even the shape and angle at which the nanoparticle interacts with the immunocyte influences the kinetics of endocytosis. Particles with sharp edges are endocytosed by macrophages more quickly than those with dull edges [116].
Once endocytosed, the nanoparticles can be degraded within the lysosome, which may result in premature release of the therapeutic [115]. Such destruction may be mitigated by using positively charged nanoparticles, which offers some protection to lysosomal degradation [117]. Alternatively, intracellular degradation may be avoided by taking an entirely different approach – rather than relying on internalization of the nanoparticles, drug-loaded nanoparticles are used to coat the external surface of the cell. Nanoparticles can be attached to the surface of cells by binding to amine or thiol groups found on proteins within the cell membrane [118]. MSCs coated with a nanoparticle formulation of doxorubicin resulted in increased drug accumulation in tumor tissues, as well as increased tumor-cell apoptosis in a human glioma cell xenograft model [119].
A variety of mechanisms enhance the ability of drug-loaded cells to cross the BBB and enter the CNS. Magnetic nanoparticles have been used to enhance the accumulation of drug-loaded immune cells in the CNS. One study loaded monocytes with magnetic liposomes containing the anti-inflammatory drug, diclofenac [120]. Once endocytosed, a magnet placed near the head of the animal was used to manipulate the migration of the monocytes, thereby significantly increasing the dose of drug that reached the brain. Additionally, cell-mediated delivery has been combined with osmotic or chemical BBBD to enhance monocyte trafficking across the BBB [121]. The route of administration of dug-loaded cell carriers can also influence the accumulation within the CNS. In mice, delivery of monocytes via the carotid artery resulted in greater accumulation in the brain compared to tail vein injections [121].
Although the delivery of therapeutics across the BBB using cell-mediated transport is not without challenges, distinct advantages such as intrinsic targeting to sites of inflammation and the ability to cross the BBB after systemic administration make cell-mediated delivery of therapeutics an attractive avenue of research. Nevertheless, more work is necessary before this method of treating disease in the CNS will be ready for translation into a clinical tool.
3.3. Focused Ultrasound-Based Drug and Gene Delivery
A unique approach for drug delivery across the BBB involves reversibly opening the BBB using an emerging technology, focused ultrasound (FUS). Although widely used as a diagnostic tool, ultrasound was initially explored for its therapeutic applications. In fact, the effects of ultrasound on the BBB were realized as early as the 1950s, when Ballantine and colleagues [122] showed strong trypan blue staining in brain tissue that was exposed to a defocused ultrasound beam, without evidence of a discrete lesion. However, FUS was largely abandoned as a therapeutic tool due to the beam distortion and attenuation produced by the skull, necessitating a bone window through which the beam could be focused. This critical limitation was finally overcome in the 1990s, when a hemispheric phased array of transducers was developed in conjunction with software that uses a co-registered computed tomography (CT) scan to predict and ultimately compensate for the phase aberrations produced by the skull [123]. The resulting ability to noninvasively focus acoustic energy through the intact skull, along with the capability to monitor the temperature changes at the acoustic focus with magnetic resonance (MR) thermometry [124], renewed the scientific community’s interest in the potential neurological applications of FUS.
3.3.1. Bioeffects of FUS
To date, MRgFUS has primarily been studied as an ablative tool. A Phase I clinical trial was recently completed for the treatment of Essential Tremor (NCT01304758), and the use of MRgFUS for Parkinson’s disease is being studied as well (NCT01772693, NCT02246374, and NCT02263885). In these studies, ultrasound’s utility is based on its thermal effects. Focusing the ultrasound beam causes acoustic energy to become concentrated at the focal point, where the spatial intensity is elevated and the high rate of energy deposition produces temperature elevations of up to 60°C [125]. Coagulative necrosis and cellular death ensue in a tightly defined spatial region (on the order of several millimeters), making MRgFUS an effective tool for the noninvasive lesioning of deep brain targets.
However, FUS also produces mechanical effects, including radiation forces, acoustic streaming, and acoustic cavitation. Radiation forces and acoustic streaming occur when the ultrasound beam transfers its momentum to a reflecting or absorbing surface, producing displacements within the tissue or within a liquid medium, respectively. Cavitation results from the gas-filled bubbles that oscillate in the presence of the positive (compressive) and negative (rarefactive) components of the ultrasound wave. The bubbles may undergo stable oscillation (i.e. non-inertial cavitation), or as the pressure wave amplitude increases, may undergo inertial cavitation, where the bubbles collapse, producing shock waves and high-velocity jets [126].
3.3.2. Ultrasound-Mediated BBBD
Whereas the thermal effects of FUS predominate in the setting of continuous exposures, short pulses of focused ultrasound (pFUS) produce primarily mechanical effects, with temperature elevations of only 4-5°C. Therefore, pFUS has been used in a variety of non-ablative roles, including drug delivery – and in particular, transporting drugs across the BBB [127] (see Fig. 2). While early studies attempted to disrupt the BBB with pFUS alone [128], high intensities were required and the effects on tissue integrity were variable. The introduction of intravenous, commercially available ultrasound contrast agents (UCAs) – lipid- or protein-encased gas microbubbles that are 1-10 microns in diameter – was a critical step in enabling finer control over BBBD [129]. The microbubbles typically cluster near capillary walls, where in the presence of pulsed exposures at low frequencies and pressure amplitudes, they undergo stable cavitation. Microbubbles therefore localize the pFUS effects to the endothelial cells, and significantly reduce the energy needed for BBB disruption, enabling the use of low pressures that reduce the risk of heating of the skull.
Fig. (2).
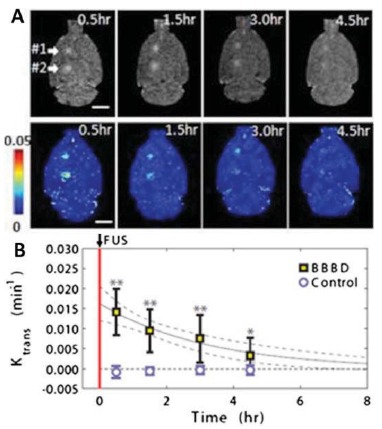
MRgFUS produces transient and localized BBBD. (A) Axial contrast-enhanced T1-weighted MRI sequences (top) and permeability maps generated via dynamic contrast-enhanced imaging (bottom) were obtained at four time points following sonication of a rat brain. Locations #1 and #2 were treated at 0.72 and 0.68 MPa, respectively. Ktrans values (min-1) are indicated by the color bar. (B) Mean Ktrans values as a function of time in sonicated and non-sonicated regions. Modified with permission from Park et al. [127].
The stable cavitation of intravenous microbubbles induces transient, reversible BBBD via several mechanisms. On the one hand, the resulting oscillations exert mechanical stresses on the tight junctions that join the specialized endothelial cells of the CNS, generating a paracellular transport route. Electron microscopy revealed the reduction of several tight junction proteins following sonication, including claudin 5, occludin, and ZO-1, with intercellular leakage of intravenously administered horseradish peroxidase [130]. On the other hand, transcellular pathways have been identified, as well. Horseradish peroxidase [131], endogenous immunoglobulin G (IgG) [132], and fluorescent dextrans [133] have been demonstrated in endothelial cell vesicles and vacuoles following sonication, suggesting a role for ultrasound-induced transcytosis. This process occurs, at least in part, through the upregulation of caveolin-1 and caveolin-2 [134, 135]. These studies also revealed the transient formation of channels and fenestrations within the endothelial cell membrane, a process called sonoporation, which may contribute to transcellular transport across the BBB [132, 133]. Recent work with two-photon microscopy demonstrated that BBBD occurs via distinct slow and fast mechanisms, which are felt to reflect the transcellular and paracellular pathways, respectively [136]. Additionally, multiphoton imaging has revealed that sonication produces temporary vasoconstriction prior to the leakage of a tracer, suggesting that transient ischemia may further contribute to BBBD [137].
Numerous preclinical studies throughout the 1990s and 2000s tested a range of pFUS frequencies, pressure amplitudes, burst lengths, and microbubble doses in rat and rabbit brains in order to determine the parameters that maximize BBB opening while minimizing structural tissue damage [129, 138-141]. Two recent studies confirmed the safety of repeated pFUS-induced BBB opening in primates: (1) repeated BBB opening in central visual field targets of rhesus macaques did not result in visual deficits or loss of visual acuity [142], and (2) repeated BBB opening in the basal ganglia of macaques over 4-20 months did not cause any long-term visual, cognitive, motivational, or motor deficits [143].
3.3.3. pFUS-Mediated Drug and Gene Delivery Across the BBB
Preclinical studies have demonstrated that pFUS can be employed to deliver a wide variety of therapeutic agents, including chemotherapeutic drugs, antibodies, immunotherapies, cellular therapies, and even gene therapies across the BBB. Much of this work has focused on enhancing the delivery of conventional chemotherapeutic agents. The feasibility of this approach was first shown using doxorubicin – the concentration of liposome-encapsulated doxorubicin (Doxil) reached therapeutic levels in a normal rat brain that was treated with pFUS, whereas it remained at sub-therapeutic levels in the control group [144]. Subsequent
studies demonstrated the therapeutic efficacy of pFUS-enhanced doxorubicin delivery in the rat 9L glioma model [145, 146], as well as in two syngeneic mouse models of GBM (GL261 and SMA-560) [147], where animals treated with both ultrasound and doxorubicin demonstrated slower tumor growth and improved survival. This work has resulted in an ongoing Phase I clinical trial examining the safety and efficacy of BBB disruption using MRgFUS with intravenous microbubbles in brain tumor patients being treated with doxorubicin (NCT02343991).
Other chemotherapeutic agents have been investigated as well. Intravenous methotrexate, for instance, reached higher intracranial concentrations in rabbit brains treated with pFUS than those of animals receiving intra-carotid infusions of the drug [148]. Similarly, the C6 glioma rat model was used to demonstrate increased 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) concentrations, slower tumor growth, and improved survival in animals treated with pFUS [149]. Also, temozolomide, a small molecule chemotherapeutic drug which is part of the standard of care for patients with GBM and typically crosses the BBB on its own with approximately 20% efficiency [150], demonstrates enhanced concentration, retention, and efficacy in the setting of pFUS-induced BBB opening in mice implanted with U87 human glioma cells [151].
In addition to small molecule drugs, antibodies may also benefit from ultrasound-based BBBD. Despite substantial potential, therapeutic antibodies have limited applications for diseases of the CNS due to their large size (on average, 150 kDa), which prevents transport across the BBB. Nevertheless, the potential use of MRgFUS to overcome this obstacle was highlighted in a feasibility study in which intravenously administered anti-dopamine D(4) receptor
antibodies were successfully delivered, functionally intact, across the BBB where they recognized their antigen [152]. This has important implications for the treatment of numerous cancer types, including metastatic breast cancer. Traztuzumab (Herceptin), a humanized anti-human epidermal growth factor receptor 2 (HER2) monoclonal antibody, has been used successfully to treat HER2+ breast cancer, but due to the BBB, it is an ineffective treatment for breast cancer metastases to the brain. Using MRgFUS, however, traztuzumab has been successfully delivered across the BBB in a mouse model [153] – and more recently in a rat model [154] – of breast cancer metastasis, producing lower tumor volumes and increased survival when compared to controls.
Antibody delivery to the CNS may prove useful not only in neuro-oncology, but in the treatment of neurodegenerative diseases as well. Anti-amyloid β antibodies, in particular, reduced the plaque burden and improved cognitive outcomes in the TgCRND8 murine model of Alzheimer’s disease [155]. Recent studies demonstrated the benefits of combining this treatment with pFUS-induced BBBD. Sonication resulted in glial activation, enhanced the delivery of endogenous IgG and IgM antibodies [156], and facilitated the delivery of exogenous anti-amyloid β antibodies [157, 158], resulting in reduced plaque number and size.
While the majority of studies have focused on the delivery of chemotherapeutic drugs and monoclonal antibodies, other forms of therapies are being examined as well. For instance, a recent study confirmed that pFUS can enhance the delivery of an immunotherapy agent, IL-12, resulting in an improved cytotoxic T-lymphocyte to regulatory T-lymphocyte ratio, reduced tumor growth, and improved survival in a C6 glioma model [159]. pFUS can also be used to facilitate cell-mediated delivery. The only study of stem cell entry into the brain after pFUS-mediated BBB disruption showed human neuro-progenitor cells within the brain following an intra-carotid artery injection [160], although relatively few injected cells were found within the sonicated region. Cellular entry following pFUS-mediated BBB disruption was validated by a later study showing a five-fold increase in targeted natural killer cells within a brain tumor following intravenous administration of the cells [161]. Other groups are working on gene therapy, in which exogenous genes are delivered into cells. Indeed, gene therapy has the potential to treat congenital disorders (e.g. lysosomal storage diseases), neurodegenerative diseases (e.g. Alzheimer’s disease and Parkinson’s disease), and brain tumors. While vector delivery and distribution has remained a persistent problem, recent studies have demonstrated enhanced transfection rates with pFUS-mediated delivery of plasmid DNA [162, 163] and adeno-associated viral vectors [164-166].
Recent advances have also resulted in microbubbles that can be engineered in a variety of ways. Targeting ligands can be conjugated to the microbubbles, allowing them to accumulate in a target region, thereby further minimizing the risk of off-target effects. Additionally, therapeutic agents may be packaged within microbubbles or bound to their surface, thereby only becoming released once the microbubbles are destroyed by pFUS. This concept was recently demonstrated using BCNU-loaded microbubbles – in healthy rats, encapsulation by microbubbles increased the circulation of BCNU and protected the drug from clearance by the reticuloendothelial system, while in a C6 glioma model, the BCNU-microbubble construct led to better tumor control and increased survival [167]. These effects were further enhanced by conjugating the vascular endothelial growth factor-A (VEGF-A) ligand to the microbubbles, thereby targeting the chemotherapeutic effects to regions of tumor angiogenesis [168]. As MRgFUS technology, drug carrier design, and targeting of therapeutics undergoes further refinement, ultrasound-based BBBD will occupy an increasingly significant position in the area of drug delivery.
4. Bypassing the BBB
The techniques described above serve to deliver therapeutic agents across the BBB by either disrupting the barrier itself, using cells to traffic through the barrier, or by modifying agents or carriers to enhance drug transport. However, an alternative strategy is to locally deliver drugs through a variety of routes that bypass the BBB entirely. These include drug delivery to the cerebrospinal fluid (CSF) via intrathecal or intraventricular routes, intranasal delivery, and interstitial delivery via either biodegradable wafers or convection enhanced delivery (CED) using stereotactically placed catheters.
4.1. Intrathecal and Intraventricular Delivery
One route that bypasses the BBB as well as the blood-CSF barrier (comprised of the epithelial cells of the choroid plexus), involves delivering therapeutic agents directly into the lumbar subarachnoid space (i.e. intrathecal delivery) or alternatively into the ventricular system (i.e. intraventricular delivery). Early studies attempted to deliver chemotherapeutic agents (e.g. methotrexate) directly into the ventricular system as a treatment for malignant gliomas and brain metastases [169]. However, despite bypassing the BBB and the blood-CSF barrier, the brain parenchyma is still separated from the CSF by a layer of ependymal cells as well as the glia limitans. This brain-CSF barrier has a much smaller surface area than the capillaries of the CNS, thus limiting the diffusion of drugs from the CSF into the brain parenchyma [170]. Rapid CSF turnover further limits the utility of intrathecal or intraventricular drug delivery for the treatment of parenchymal disease.
Intra-CSF drug delivery does, however, result in high leptomeningeal drug concentrations, allowing the effective treatment of various diseases of the meninges (for a review, see [171, 172]). These include carcinomatous meningitis [173, 174], lymphomatous meningitis [175], and meningeal gliomatosis [176, 177]. Although systemically administered chemotherapies often don’t reach the leptomeningeal space in sufficient concentrations, intrathecal or intraventricular administration delivers the necessary agent directly to the CSF.
Additionally, spasticity and chronic pain are commonly treated with intrathecal baclofen and opioids, respectively. Intrathecal baclofen has received FDA approval for the treatment for spasticity of either cerebral or spinal cord origin. It is commonly delivered via an implantable pump, which administers a continuous infusion of baclofen to the CSF via a tunneled catheter. Via this route, baclofen is delivered directly to the spinal cord where it binds to gamma aminobutyric acid B receptors; furthermore, serum levels remain low, thereby reducing the risk of sedation. Multiple studies have confirmed the efficacy of this approach for treating the spasticity associated with cerebral palsy [178, 179]. Similarly, clinical guidelines have been developed for the treatment of chronic cancer and non-cancer pain using an implantable pump for the intrathecal delivery of opioids [180, 181]. However, these implantable systems have been associated with a variety of complications, including CSF leak, catheter malfunction, and poor wound healing.
4.2. Intranasal Delivery
Over the past decade, the intranasal route has also been explored as a means of noninvasively delivering therapeutics to the brain [182]. Drugs delivered intranasally reach the brain by crossing the nasal olfactory epithelium or nasal mucosa [183, 184]. The olfactory region of the nasal submucosal space is connected to CSF flow tracts around the olfactory bulb, providing a direct pathway for drugs to reach the CSF [7]. Although the exact mechanism of intranasal drug delivery is not fully understood, the administrated drugs can take different paths, including paracellular and transcellular routes, to cross the nasal olfactory epithelium. Therapeutic agents may also travel along the olfactory and trigeminal nerves [185, 186]. Via these routes, drugs can be absorbed across the highly permeable nasal membrane and thereby avoid first-pass metabolism. Intranasal delivery offers several advantages, including the option for self-administration and lower drug dosages; additionally, drugs typically do not require further modification or carriers in order to reach their target [66, 187].
A number of therapeutic agents have been delivered to the brain via the intranasal route, including chemotherapeutic drugs, small molecules, proteins, and nanoparticles [182]. Ying et al. [188] reported that the intranasal administration of NAD+ in a rat model decreased infarct formation at 24 to 72 hours after ischemia, and significantly attenuated ischemia-induced neurological deficits. In addition, the same group also showed that the intranasal administration of gallotannin, a poly (ADP-ribose) glycohydrolase (PARG) inhibitor, significantly reduced the frequency of ischemic brain injury in rats [189]. In other studies, metallic nanoparticles, such as manganese oxide, gold, carbon-13, iron oxide, zinc oxide and titanium dioxide particles, were efficiently transported to the olfactory bulbs and into the deep brain structures following intranasal administration in rats (for a review, see [190]). Additionally, intranasal administration of estradiol-loaded chitosan nanoparticles resulted in lower plasma levels and higher CSF levels of estradiol when compared to intravenous administration [191]. Similarly, Kumar et al. [192, 193] delivered risperidone (RS) and olanzapine drugs through nanoemulsions to the brain via the intranasal route, reporting higher drug transport efficiency for nanoemulsions delivered intranasally as opposed to intravenously. In a phase I/II clinical trial conducted by da Fonseca et al. [194, 195], intranasal delivery of the signal transduction inhibitor perillyl alcohol (POH) was evaluated as an adjuvant therapeutic strategy for patients with malignant gliomas. The authors reported that the intranasal administration of POH was a safe, noninvasive, and low-cost method. In addition, tumor regression was reported in some patients, suggesting a potential antitumor role for POH. Furthermore, Yang et al. [196] studied headache patients who self-administered arginine vasopressin (AVP), a nonapeptide pituitary hormone, via the intranasal route. AVP concentrations were significantly increased in plasma as well as CSF, and patients experienced headache relief in a dose-dependent manner. Despite a number of promising advantages, intranasal delivery has some limitations. Frequent drug administration may irritate or damage the nasal mucosa. Additionally, the drugs can be cleared from the nasal cavity by the mucociliary clearance system. Moreover, drug delivery and absorption are limited in the setting of nasal congestion.
4.3. Interstitial Delivery
Although intrathecal, intraventricular, and intranasal drug delivery circumvent the BBB to some extent, the most direct way to deliver a drug to its target in the brain is to administer it directly into the interstitium. The two primary forms of interstitial delivery are interstitial biodegradable wafers and catheter-based CED.
4.3.1. Interstitial Wafers
Local drug delivery via interstitial wafers was made possible by the discovery that macromolecules could be incorporated into non-inflammatory polymers, with subsequent sustained release from the polymer over time [197]. During the four decades that have passed since this first report, a number of biodegradable polymers have been engineered, including the polyanhydride polymers poly [1,3-bis-(p-carboxyphenoxy propane)-co-(sebacic anhydride)] (CPP:SA) [198], poly (fatty acid dimer: sebacic acid) (FAD:SA) [199], and PLGA [200]. Macromolecules may be encapsulated within these polymers, which provide protection from clearance and degradation. The incorporated macromolecules are released by a combination of diffusion and polymer degradation, which can be tightly controlled by modifying the composition of the polymer (for a review, see [201]).
A number of chemotherapeutic agents have been explored in the context of biodegradable polymers, including methotrexate [202], carboplatin [203], and 5-fluorouracil [204]. However, the most commonly studied intracranial, implantable, biodegradable interstitial wafer is Gliadel® – p(CPP:SA) polymer loaded with 3.8% BCNU [205]. Gliadel® wafers are used to line the surgical cavity following the maximal resection of a malignant glioma. At the time Gliadel® was being developed in the 1980s and 1990s, BCNU had already been FDA approved and was commonly being used for the treatment of brain tumors. BCNU was therefore chosen as the optimal drug to be incorporated into the biodegradable polymer, with the goal of increasing its half-life, providing controlled release of the drug over several weeks, and directly delivering the drug to its target (i.e. the cancer cells just beyond the surgical resection cavity), thereby minimizing systemic toxicity.
The first human study involving Gliadel® was a Phase I-II trial of patients with recurrent glioma [205]. Three doses of BCNU (1.93%, 3.85%, and 6.35% by weight) were tested, with the intermediate dose demonstrating both safety and efficacy. This dose was therefore used in the subsequent Phase III trial, which was a prospective, randomized, placebo-controlled trial involving 27 centers with 222 patients with recurrent malignant glioma [206]. Median posttreatment survival was 31 weeks for the treatment group vs. 23 weeks for the placebo group (p = 0.006). Among patients with GBM, 56% of those in the treatment group were alive at 6 months, compared to only 36% in the placebo group (p = 0.02). As a result, Gliadel® received FDA approval in 1996 for the treatment of recurrent malignant glioma.
Subsequent trials examined the role of Gliadel® for newly diagnosed GBM. A Phase I trial involving three centers included patients who all received radiation postoperatively, without any negative impact on safety [207]. This was followed by a prospective, randomized, placebo-controlled trial in Europe [208]. Patients received either Gliadel® wafers or placebo, followed by radiation therapy. Median survival was 58 weeks for the treatment group vs. 40 weeks for the placebo group (p = 0.012). Two and three years later, significantly more patients were alive in the Gliadel® group. An even larger randomized, placebo-controlled Phase III trial involving 240 patients confirmed a survival benefit for Gliadel®, with a median survival of 14 months for treated patients compared to 11.6 months for patients in the placebo group [209]. Gliadel® subsequently received FDA approval in 2003 as an initial therapy for GBM.
The development and clinical translation of Gliadel® marks an important step, not only for the treatment of GBM, but for a variety of neurological disorders that might benefit from interstitial therapy. Researchers have continued to improve upon the initial wafer design, with a recent advance being the development of controlled-release microchips containing individual drug-containing reservoirs [210]. In contrast to interstitial wafers, microchips may deliver multiple drugs, each with a unique release profile, to the surrounding parenchyma. Microchips may be passively actuated, containing polymeric, biodegradable membranes that cover the reservoirs. Pulsatile drug release may be achieved by modifying the composition of the membranes, thereby allowing drugs housed within individual reservoirs to be released at different times [211]. Alternatively, microchips may incorporate microelectromechanical systems, whereby individual anode membranes undergo electrochemical dissolution when an electric potential is applied [212]. Preclinical studies have confirmed that microchips loaded with BCNU are effective in a rat gliosarcoma model [212, 213].
Nevertheless, some clinicians have been hesitant to implant interstitial wafers and microchips due to the risk of perioperative complications. One meta-analysis reported a 42.7% complication rate associated with Gliadel®, including CSF leak, infection, cerebral edema, and seizures [214]. On the other hand, a retrospective review of 1013 craniotomies (of which 288 involved Gliadel® wafers) demonstrated similar perioperative complication rates in the Gliadel® and non-Gliadel® groups. An additional limitation of Gliadel®, and interstitial wafers in general, is that while intraoperative implantation of the wafers bypasses the BBB, the drug must still overcome the “brain penetration barrier” produced by the brain interstitium. Preclinical studies demonstrated that the penetration distance (defined as the distance from the polymer at which the concentration of BCNU had fallen to 10% of its maximum value) was 5 mm on the first day following wafer implantation, and only 1 mm by day 3 [215]. Additional strategies will be needed in order to enhance drug distribution across the brain parenchyma once it has been delivered to the interstitium.
4.3.2. Convection-Enhanced Delivery
A second option for direct, interstitial delivery involves the stereotactic placement of one or multiple catheters through which therapeutic agents can be injected. Although this approach seems intuitive, most macromolecules (particularly those of high molecular weight) do not diffuse sufficiently from the point of injection, resulting in low volumes of distribution and limited efficacy [216, 217]. CED, however, uses micro-pumps to maintain a constant hydrostatic pressure gradient [218, 219], producing bulk flow by convection in order to homogenously distribute the infusate (for a review, see [220]). The ultimate penetration depth of the infusate depends on the balance of bulk flow, diffusion, and clearance. In theory, CED has the potential to homogenously distribute the infusate in a square-shaped pattern up to several centimeters away from the infusion site via bulk flow, in contrast to the 1-2 mm achieved by diffusion alone [170, 221].
A wide variety of agents may be delivered via CED. These range from small molecule chemotherapeutic agents [222-224] and imaging tracers [225-227] to larger compounds such as proteins [228], viruses [229, 230], and nanoparticles [231-236] (see Fig. 3). Unlike diffusion, bulk flow typically distributes compounds homogenously regardless of molecular weight; nevertheless, larger molecules are still restricted by the size limitations of the extracellular space (ECS) of the brain. While early reports suggested that the ECS consists of pores that are 38-64 nm in width [237], more recent evidence suggests that the average pore size is actually closer to 100 nm, but that surface characteristics also play an important role in distribution throughout the brain [229, 238].
Fig. (3).
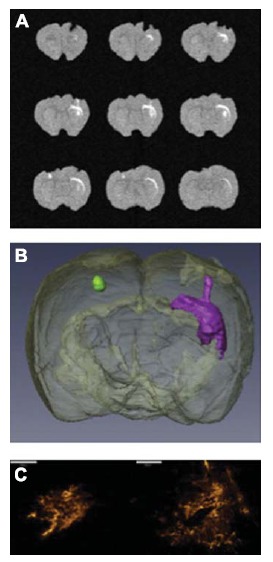
Distribution of gadolinium-labeled anionic liposomes following CED. (A) 3D axial T1-weighted gradient echo scans demonstrate the gadolinium in the nanocomplexes. (B) The data was reconstructed to provide a 3D model of the liposome distribution following CED into the striatum (green) and corpus callosum (purple). (C) Fluorescence microscopy was performed to visualize the anionic liposome distribution in the striatum (left) and corpus callosum (right) by using the incorporated rhodamine label. Scale bars = 500 µm. Modified with permission from Kenny et al. [236].
A number of Phase I and Phase II clinical trials have been conducted in order to explore the safety and efficacy of using CED for the direct interstitial delivery of various therapeutic agents. Several of these have focused on the delivery of conventional chemotherapies, such as the Phase I/II trial involving 15 patients with recurrent malignant glioma who underwent intratumoral CED of paclitaxel [239]. Although there was a 73% response rate to the drug, several complications were noted including transient aseptic meningitis, infections, and temporary neurological deficits. Those who did not demonstrate a tumor response were believed to have poor distribution of the drug due to leakage into the subarachnoid space, ventricles, and prior resection cavities. A Phase I trial that plans to study carboplatin delivered by CED in patients with recurrent high grade gliomas is currently in the recruitment phase (NCT01644955), and two pilot studies examining topotecan administered via CED are being planned as well (NCT02500459 and NCT00308165).
To date, the majority of completed clinical trials have examined CED for the delivery of miscellaneous, experimental agents. A Phase I/II trial involving 51 patients studied the safety and efficacy of CED of a chimeric monoclonal antibody that recognizes the DNA-histone H1 complex, and is conjugated to I131 [240]. The side effect profile was considered reasonable and the radioimmunotherapy treatment achieved between 90% and 110% of the prescribed administered activity in the majority of patients. A second set of Phase I [241] and Phase II [242] trials focused on the delivery of CpG-28, an oligodeoxynucleotide containing CpG motifs (CpG ODN), in patients with recurrent GBM. CpG ODNs have been found to have an immunostimulatory anti-cancer effect. However, while early results were promising, the Phase II trial found that of 31 patients, only 1 had a partial response and 3 had minor responses, with a progression-free survival of 19% at 6 months.
A large number of clinical trials have also examined the delivery of various toxins via CED. Phase I and II trials have found that Pseudomonas exotoxin fused to IL-4 [243], as well as a diphtheria toxin conjugated to the transferrin receptor [244, 245], demonstrate good safety profiles and some efficacy in patients with recurrent malignant glioma. Cintredekin besudotox (Pseudomonas exotoxin fused to IL-13), also demonstrated acceptable safety profiles in a series of Phase I trials involving 51 patients with recurrent malignant glioma [246], as well as in 22 patients with newly diagnosed malignant glioma (where CED was followed by radiation therapy with or without temozolomide) [247]. These positive results culminated in the only Phase III clinical trial involving CED to date – the PRECISE trial [248]. This trial compared CED administration of the Pseudomonas toxin/IL-13 conjugate to Gliadel wafers in 296 patients with recurrent GBM, and found no survival difference between the study groups. A subsequent analysis of the PRECISE trial, however, emphasized the fact that 68% of the catheters were misplaced, thereby limiting the conclusions that can be drawn about the efficacy of CED in general or cintredekin besudotox in particular [249].
In addition to catheter misplacement, drug delivery with CED may be suboptimal due to a variety of phenomena that take place at the catheter tip. For example, reflux may occur at high infusion rates as the infusate travels along the catheter itself, instead of into the brain parenchyma [250]. Air entrainment is a second obstacle to optimal CED infusion, as the drug being infused will likely enter the air pocket upon exiting the catheter, thereby limiting its homogenous distribution into the tissue. Additionally, drug entry into the CSF reduces the concentrations that persist in the parenchyma, thus limiting the drug’s efficacy. This was reflected in a study involving a Pseudomonas exotoxin fused to transforming growth factor-α (which targets the epidermal growth factor receptor) – only 3 of 16 catheters resulted in adequate distributions of infusate, as the majority resulted in leakage of infusate into the CSF [251]. A variety of catheters have been designed with the goal of optimizing the infusion during CED, including catheters with smaller diameters (thereby minimizing tissue expansion) [252, 253], a reflux-free step design cannula [254], a reflux-resistant indwelling catheter [255], and hollow fiber tubes [256], among others. These engineering innovations, along with new techniques to increase the accuracy of catheter placement, will enable clinicians and researchers to realize the full potential of interstitial delivery via CED.
Conclusion
The BBB serves an essential function in the brain, but its presence necessitates innovative therapeutic approaches for treating CNS diseases. In view of the aging population and increasing prevalence of neurological disorders, the demand for the improved delivery of CNS therapeutics is only going to increase with time. Current advances in drug delivery strategies are expanding our arsenal with which to treat diseases of the CNS. By integrating emerging therapeutic approaches with conventional concepts of interstitial delivery and the modulation of BBB permeability, advanced strategies to overcome the BBB are being developed. The drug delivery community is challenged to develop innovative strategies to overcome this barrier and improve therapeutic delivery to the CNS while minimizing associated toxicity.
Authors’ Contribution
D.S.H., A.S.W., N.B.R., J.G.P., N.P.C., V.F., J.A.W., G.F.W., and A.J.K. wrote the paper.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health (K12NS080223 (G.F.W), K25EB018370 (A.J.K.), K08NS09043 (G.F.W.)), DOD CDMRP Lung Cancer Research Program IDEA Award (W81XWH-14-1-0324) (J.A.W.), an Institutional Research Grant (IRG-97-153-10) from the American Cancer Society (A.J.K. and G.F.W.), a Passano Foundation Physician Scientist Award (G.F.W.), an Elsa U. Pardee Foundation Research Grant (A.J.K. and J.A.W.), a PhRMA Foundation Research Starter Grant in Pharmaceutics (A.J.K.), a AAPS Foundation New Investigator Grant Award (A.J.K.), a Dean’s Challenge Award to Accelerate Innovation and Discovery in Medicine (V.F., J.A.W. and G.F.W.), and an AMA Foundation Seed Grant (D.S.H.).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Pardridge W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z., Liu Z.W., Allaker R.P., et al. A review of nanoparticle functionality and toxicity on the central nervous system. Interface Focus. 2010;7:S411–S422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Tellingen O., Yetkin-Arik B., de Gooijer M.C., Wesseling P., Wurdinger T., de Vries H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez J.I., Dodelet-Devillers A., Kebir H., et al. The hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 6.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Demeule M., Regina A., Jodoin J., et al. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul. Pharmacol. 2002;38:339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport S.I., Hori M., Klatzo I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 1972;223:323–331. doi: 10.1152/ajplegacy.1972.223.2.323. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport S.I. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000;20:217–230. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport S.I., Robinson P.J. Tight-junctional modification as the basis of osmotic opening of the blood-brain barrier. Ann. N. Y. Acad. Sci. 1986;481:250–267. doi: 10.1111/j.1749-6632.1986.tb27155.x. [DOI] [PubMed] [Google Scholar]
- 13.Fredericks W.R., Rapoport S.I. Reversible osmotic opening of the blood-brain barrier in mice. Stroke. 1988;19:266–268. doi: 10.1161/01.str.19.2.266. [DOI] [PubMed] [Google Scholar]
- 14.Rapoport S.I., Thompson H.K. Osmotic opening of the blood-brain barrier in the monkey without associated neurological deficits. Science. 1973;180:971. doi: 10.1126/science.180.4089.971. [DOI] [PubMed] [Google Scholar]
- 15.Neuwelt E.A., Hill S.A., Frenkel E.P., et al. Osmotic blood-brain barrier disruption: pharmacodynamic studies in dogs and a clinical phase I trial in patients with malignant brain tumors. Cancer Treat. Rep. 1981;65(Suppl. 2):39–43. [PubMed] [Google Scholar]
- 16.Neuwelt E.A., Frenkel E.P., Diehl J.T., et al. Osmotic blood-brain barrier disruption: a new means of increasing chemotherapeutic agent delivery. Trans. Am. Neurol. Assoc. 1979;104:256–260. [PubMed] [Google Scholar]
- 17.Siegal T., Rubinstein R., Bokstein F., et al. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J. Neurosurg. 2000;92:599–605. doi: 10.3171/jns.2000.92.4.0599. [DOI] [PubMed] [Google Scholar]
- 18.Robinson P.J., Rapoport S.I. Size selectivity of blood-brain barrier permeability at various times after osmotic opening. Am. J. Physiol. 1987;253:R459–R466. doi: 10.1152/ajpregu.1987.253.3.R459. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda M., Nagashima T., Bhattacharjee A.K., Kondoh T., Kohmura E., Tamaki N. Quantitative analysis of hyperosmotic and hypothermic blood-brain barrier opening. Acta Neurochir. Suppl. (Wien) 2003;86:559–563. doi: 10.1007/978-3-7091-0651-8_114. [DOI] [PubMed] [Google Scholar]
- 20.Neuwelt E.A., Frenkel E.P., Rapoport S., Barnett P. Effect of osmotic blood-brain barrier disruption on methotrexate pharmacokinetics in the dog. Neurosurgery. 1980;7:36–43. doi: 10.1227/00006123-198007000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Neuwelt E.A., Glasberg M., Frenkel E., Barnett P. Neurotoxicity of chemotherapeutic agents after blood-brain barrier modification: neuropathological studies. Ann. Neurol. 1983;14:316–324. doi: 10.1002/ana.410140310. [DOI] [PubMed] [Google Scholar]
- 22.Neuwelt E.A., Barnett P.A., Hellstrom I., et al. Delivery of melanoma-associated immunoglobulin monoclonal antibody and Fab fragments to normal brain utilizing osmotic blood-brain barrier disruption. Cancer Res. 1988;48:4725–4729. [PubMed] [Google Scholar]
- 23.Hicks J.T., Albrecht P., Rapoport S.I. Entry of neutralizing antibody to measles into brain and cerebrospinal fluid of immunized monkeys after osmotic opening of the blood-brain barrier. Exp. Neurol. 1976;53:768–779. doi: 10.1016/0014-4886(76)90154-0. [DOI] [PubMed] [Google Scholar]
- 24.Muldoon L.L., Nilaver G., Kroll R.A., et al. Comparison of intracerebral inoculation and osmotic blood-brain barrier disruption for delivery of adenovirus, herpesvirus, and iron oxide particles to normal rat brain. Am. J. Pathol. 1995;147:1840–1851. [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao S., Miller P.J., Lapchak P.A. Enhanced delivery of [125I]glial cell line-derived neurotrophic factor to the rat CNS following osmotic blood-brain barrier modification. Neurosci. Lett. 1996;220:187–190. doi: 10.1016/s0304-3940(96)13265-1. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales-Portillo G.S., Sanberg P.R., Franzblau M., et al. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transplant. 2014;23:531–539. doi: 10.3727/096368914X678337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley C.P., Rubin D.G., Santillan A., et al. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J. Control. Release. 2014;196:71–78. doi: 10.1016/j.jconrel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahlborg S.A., Henner W.D., Crossen J.R., et al. Non-AIDS primary CNS lymphoma: first example of a durable response in a primary brain tumor using enhanced chemotherapy delivery without cognitive loss and without radiotherapy. Cancer J. Sci. Am. 1996;2:166–174. [PubMed] [Google Scholar]
- 29.McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. 2000. [PubMed]
- 30.Angelov L., Doolittle N.D., Kraemer D.F., et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J. Clin. Oncol. 2009;27:3503–3509. doi: 10.1200/JCO.2008.19.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuwelt E.A., Wiliams P.C., Mickey B.E., Frenkel E.P., Henner W.D. Therapeutic dilemma of disseminated CNS germinoma and the potential of increased platinum-based chemotherapy delivery with osmotic blood-brain barrier disruption. Pediatr. Neurosurg. 1994;21:16–22. doi: 10.1159/000120809. [DOI] [PubMed] [Google Scholar]
- 32.Gumerlock M.K., Belshe B.D., Madsen R., Watts C. Osmotic blood-brain barrier disruption and chemotherapy in the treatment of high grade malignant glioma: patient series and literature review. J. Neurooncol. 1992;12:33–46. doi: 10.1007/BF00172455. [DOI] [PubMed] [Google Scholar]
- 33.Neuwelt E.A., Howieson J., Frenkel E.P., et al. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood-brain barrier modification in glioblastoma. Neurosurgery. 1986;19:573–582. doi: 10.1227/00006123-198610000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Doolittle N.D., Miner M.E., Hall W.A., et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport S.I., Fredericks W.R., Ohno K., Pettigrew K.D. Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am. J. Physiol. 1980;238:R421–R431. doi: 10.1152/ajpregu.1980.238.5.R421. [DOI] [PubMed] [Google Scholar]
- 36.Nadal A., Fuentes E., Pastor J., McNaughton P.A. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc. Natl. Acad. Sci. USA. 1995;92:1426–1430. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erdlenbruch B., Schinkhof C., Kugler W., et al. Intracarotid administration of short-chain alkylglycerols for increased delivery of methotrexate to the rat brain. Br. J. Pharmacol. 2003;139:685–694. doi: 10.1038/sj.bjp.0705302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H.J., Zhang Y., Pardridge W.M. Blood-brain barrier disruption following the internal carotid arterial perfusion of alkyl glycerols. J. Drug Target. 2002;10:463–467. doi: 10.1080/1061186021000038337. [DOI] [PubMed] [Google Scholar]
- 39.Gutman M., Laufer R., Eisenthal A., et al. Increased microvascular permeability induced by prolonged interleukin-2 administration is attenuated by the oxygen-free-radical scavenger dimethylthiourea. Cancer Immunol. Immunother. 1996;43:240–244. doi: 10.1007/s002620050328. [DOI] [PubMed] [Google Scholar]
- 40.Black K.L., Chio C.C. Increased opening of blood-tumour barrier by leukotriene C4 is dependent on size of molecules. Neurol. Res. 1992;14:402–404. doi: 10.1080/01616412.1992.11740093. [DOI] [PubMed] [Google Scholar]
- 41.Cote J., Bovenzi V., Savard M., et al. Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS One. 2012;7:e37485. doi: 10.1371/journal.pone.0037485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inamura T., Black K.L. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J. Cereb. Blood Flow Metab. 1994;14:862–870. doi: 10.1038/jcbfm.1994.108. [DOI] [PubMed] [Google Scholar]
- 43.Ford J., Osborn C., Barton T., Bleehen N.M. A phase I study of intravenous RMP-7 with carboplatin in patients with progression of malignant glioma. Eur. J. Cancer. 1998;34:1807–1811. doi: 10.1016/s0959-8049(98)00155-5. [DOI] [PubMed] [Google Scholar]
- 44.Nomura T., Inamura T., Black K.L. Intracarotid infusion of bradykinin selectively increases blood-tumor permeability in 9L and C6 brain tumors. Brain Res. 1994;659:62–66. doi: 10.1016/0006-8993(94)90863-x. [DOI] [PubMed] [Google Scholar]
- 45.Nakano S., Matsukado K., Black K.L. Increased brain tumor microvessel permeability after intracarotid bradykinin infusion is mediated by nitric oxide. Cancer Res. 1996;56:4027–4031. [PubMed] [Google Scholar]
- 46.Matsukado K., Sugita M., Black K.L. Intracarotid low dose bradykinin infusion selectively increases tumor permeability through activation of bradykinin B2 receptors in malignant gliomas. Brain Res. 1998;792:10–15. doi: 10.1016/s0006-8993(97)01502-3. [DOI] [PubMed] [Google Scholar]
- 47.Bartus R.T., Elliott P., Hayward N., Dean R., McEwen E.L., Fisher S.K. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology. 1996;33:270–278. doi: 10.1016/0162-3109(96)00070-7. [DOI] [PubMed] [Google Scholar]
- 48.Emerich D.F., Dean R.L., Osborn C., Bartus R.T. The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier: from concept to clinical evaluation. Clin. Pharmacokinet. 2001;40:105–123. doi: 10.2165/00003088-200140020-00003. [DOI] [PubMed] [Google Scholar]
- 49.Inamura T., Nomura T., Bartus R.T., Black K.L. Intracarotid infusion of RMP-7, a bradykinin analog: a method for selective drug delivery to brain tumors. J. Neurosurg. 1994;81:752–758. doi: 10.3171/jns.1994.81.5.0752. [DOI] [PubMed] [Google Scholar]
- 50.Matsukado K., Inamura T., Nakano S., Fukui M., Bartus R.T., Black K.L. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery. 1996;39:125–133. doi: 10.1097/00006123-199607000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Bartus R.T., Snodgrass P., Marsh J., Agostino M., Perkins A., Emerich D.F. Intravenous cereport (RMP-7) modifies topographic uptake profile of carboplatin within rat glioma and brain surrounding tumor, elevates platinum levels, and enhances survival. J. Pharmacol. Exp. Ther. 2000;293:903–911. [PubMed] [Google Scholar]
- 52.Elliott P.J., Hayward N.J., Dean R.L., Blunt D.G., Bartus R.T. Intravenous RMP-7 selectively increases uptake of carboplatin into rat brain tumors. Cancer Res. 1996;56:3998–4005. [PubMed] [Google Scholar]
- 53.Warren K.E., Patel M.C., Aikin A.A., et al. Phase I trial of lobradimil (RMP-7) and carboplatin in children with brain tumors. Cancer Chemother. Pharmacol. 2001;48:275–282. doi: 10.1007/s002800100356. [DOI] [PubMed] [Google Scholar]
- 54.Gregor A., Lind M., Newman H., et al. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J. Neurooncol. 1999;44:137–145. doi: 10.1023/a:1006379332212. [DOI] [PubMed] [Google Scholar]
- 55.Prados M.D., Schold S.J., Fine H.A., et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro-oncol. 2003;5:96–103. doi: 10.1093/neuonc/5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardridge W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 2002;1:131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 57.Duffy K.R., Pardridge W.M. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420:32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- 58.Fishman J.B., Rubin J.B., Handrahan J.V., Connor J.R., Fine R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987;18:299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- 59.Pardridge W.M., Buciak J.L., Friden P.M. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J. Pharmacol. Exp. Ther. 1991;259:66–70. [PubMed] [Google Scholar]
- 60.Dehouck B., Fenart L., Dehouck M.P., Pierce A., Torpier G., Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin S.U., Friden P., Moran M., et al. Transferrin-antibody fusion proteins are effective in brain targeting. Proc. Natl. Acad. Sci. USA. 1995;92:2820–2824. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Schlachetzki F., Zhang Y.F., Boado R.J., Pardridge W.M. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum. Gene Ther. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y.J., Zhang Y., Kenrick M., et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 64.Boado R.J., Hui E.K., Lu J.Z., Sumbria R.K., Pardridge W.M. Blood-brain barrier molecular trojan horse enables imaging of brain uptake of radioiodinated recombinant protein in the rhesus monkey. Bioconjug. Chem. 2013;24:1741–1749. doi: 10.1021/bc400319d. [DOI] [PubMed] [Google Scholar]
- 65.Drappatz J., Brenner A., Wong E.T., et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432.CCR-12-2481. [DOI] [PubMed] [Google Scholar]
- 66.Alam M.I., Beg S., Samad A., et al. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010;40:385–403. doi: 10.1016/j.ejps.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 67.De Jong W.H., Borm P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng Y., Dai Q., Morshed R.A., et al. Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10:5137–5150. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rungby J., Danscher G. Localization of exogenous silver in brain and spinal cord of silver exposed rats. Acta Neuropathol. 1983;60:92–98. doi: 10.1007/BF00685352. [DOI] [PubMed] [Google Scholar]
- 70.Kong S.D., Lee J., Ramachandran S., et al. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release. 2012;164:49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J.T., Al-Jamal K.T. Functionalized carbon nanotubes: revolution in brain delivery. Nanomedicine (Lond.) 2015;10:2639–2642. doi: 10.2217/nnm.15.114. [DOI] [PubMed] [Google Scholar]
- 72.Al-Jamal K.T., Nerl H., Muller K.H., et al. Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale. 2011;3:2627–2635. doi: 10.1039/c1nr10080g. [DOI] [PubMed] [Google Scholar]
- 73.Kafa H., Wang J.T., Rubio N., et al. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials. 2015;53:437–452. doi: 10.1016/j.biomaterials.2015.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroeder U., Sommerfeld P., Ulrich S., Sabel B.A. Nanoparticle technology for delivery of drugs across the blood–brain barrier. J. Pharm. Sci. 1998;87:1305–1307. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 75.Barbu E., Molnar E., Tsibouklis J., Gorecki D.C. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrier. Expert Opin. Drug Deliv. 2009;6:553–565. doi: 10.1517/17425240902939143. [DOI] [PubMed] [Google Scholar]
- 76.Zensi A., Begley D., Pontikis C., et al. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release. 2009;137:78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Jr Kreuter. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 78.Ren T., Xu N., Cao C., et al. Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J. Biomater. Sci. Polym. Ed. 2009;20:1369–1380. doi: 10.1163/092050609X12457418779185. [DOI] [PubMed] [Google Scholar]
- 79.Vergoni A.V., Tosi G., Tacchi R., Vandelli M.A., Bertolini A., Costantino L. Nanoparticles as drug delivery agents specific for CNS: in vivo biodistribution. Nanomedicine (Lond.) 2009;5:369–377. doi: 10.1016/j.nano.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Serramia M.J., Alvarez S., Fuentes-Paniagua E., et al. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release. 2015;200:60–70. doi: 10.1016/j.jconrel.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 81.Samad A., Sultana Y., Aqil M. Liposomal drug delivery systems: an update review. Curr. Drug Deliv. 2007;4:297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 82.Micheli M.R., Bova R., Magini A., Polidoro M., Emiliani C. Lipid-based nanocarriers for CNS-targeted drug delivery. Recent Patents CNS Drug Discov. 2012;7:71–86. doi: 10.2174/157488912798842241. [DOI] [PubMed] [Google Scholar]
- 83.Schnyder A. Huwyler Jr. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2:99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabizon A., Goren D., Horowitz A.T., Tzemach D., Lossos A., Siegal T. Long-circulating liposomes for drug delivery in cancer therapy: a review of biodistribution studies in tumor-bearing animals. Adv. Drug Deliv. Rev. 1997;24:337–344. [Google Scholar]
- 85.Qin Y., Fan W., Chen H., et al. In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes. J. Drug Target. 2010;18:536–549. doi: 10.3109/10611861003587235. [DOI] [PubMed] [Google Scholar]
- 86.Jones A.R., Shusta E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai F., Fadda A.M., Sinico C. Liposomes for brain delivery. Expert Opin. Drug Deliv. 2013;10:1003–1022. doi: 10.1517/17425247.2013.766714. [DOI] [PubMed] [Google Scholar]
- 88.Ying X., Wen H., Lu W.L., et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 89.Rose J., Neal J., Kopacz D. Extended-duration analgesia: update on microspheres and liposomes. Reg. Anesth. Pain Med. 2005;30:275–285. doi: 10.1016/j.rapm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Du D., Chang N., Sun S., et al. The role of glucose transporters in the distribution of p-aminophenyl-a-d-mannopyranoside modified liposomes within mice brain. J. Control. Release. 2014;182:99–110. doi: 10.1016/j.jconrel.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Qin J., Zhang R.X., Li J.L., et al. cRGD mediated liposomes enhanced antidepressant-like effects of edaravone in rats. Eur. J. Pharm. Sci. 2014;58:63–71. doi: 10.1016/j.ejps.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 92.Tosi G., Bondioli L., Ruozi B., et al. NIR-labeled nanoparticles engineered for brain targeting: in vivo optical imaging application and fluorescent microscopy evidences. J. Neural Transm. 2011;118:145–153. doi: 10.1007/s00702-010-0497-1. [DOI] [PubMed] [Google Scholar]
- 93.Lajoie J.M., Shusta E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015;55:613–631. doi: 10.1146/annurev-pharmtox-010814-124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fornaguera C., Dols-Perez A., Calderó G., García-Celma M.J., Camarasa J., Solans C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier. J. Control. Release. 2015;211:134–143. doi: 10.1016/j.jconrel.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 95.Clark A.J., Davis M.E. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl. Acad. Sci. USA. 2015;112:12486–12491. doi: 10.1073/pnas.1517048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang F., Xu C-L., Liu C-M. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des. Devel. Ther. 2015;9:2089–2100. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sowers J., Johnson K., Conrad C., Patterson J., Sowers L. The role of inflammation in brain cancer. In: Aggarwal B.B., Sung B., Gupta S.C., editors. Inflammation and Cancer. Basel: Springer; 2014. pp. 75–105. [Google Scholar]
- 98.Behnan J., Isakson P., Joel M., et al. Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells. 2014;32:1110–1123. doi: 10.1002/stem.1614. [DOI] [PubMed] [Google Scholar]
- 99.Griffith J.W., Sokol C.L., Luster A.D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 100.Bovenberg M.S., Degeling M.H., Tannous B.A. Advances in stem cell therapy against gliomas. Trends Mol. Med. 2013;19:281–291. doi: 10.1016/j.molmed.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 101.Miao H., Choi B.D., Suryadevara C.M., et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One. 2014;9:e94281. doi: 10.1371/journal.pone.0094281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pawlowski N.A., Kaplan G., Abraham E., Cohn Z.A. The selective binding and transmigration of monocytes through the junctional complexes of human endothelium. J. Exp. Med. 1988;168:1865–1882. doi: 10.1084/jem.168.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyck R., Engelhardt B. Going against the tide – how encephalitogenic T cells breach the blood-brain barrier. J. Vasc. Res. 2012;49:497–509. doi: 10.1159/000341232. [DOI] [PubMed] [Google Scholar]
- 104.Liu L, Eckert MA, Riazifar H, Kang D-K, Agalliu D, Zhao W. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? 2013. [DOI] [PMC free article] [PubMed]
- 105.Rüster B., Göttig S., Ludwig R.J., et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 106.Schackert G., Simmons R.D., Buzbee T.M., Hume D.A., Fidler I.J. Macrophage infiltration into experimental brain metastases: occurrence through an intact blood-brain barrier. J. Natl. Cancer Inst. 1988;80:1027–1034. doi: 10.1093/jnci/80.13.1027. [DOI] [PubMed] [Google Scholar]
- 107.Bagci-Onder T., Du W., Figueiredo J-L., Martinez-Quintanilla J., Shah K. Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain. 2015;138:1710–1721. doi: 10.1093/brain/awv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aboody K.S., Brown A., Rainov N.G., et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryu C.H., Park S-H., Park S.A., et al. Gene therapy of intracranial glioma using interleukin 12–secreting human umbilical cord blood–derived mesenchymal stem cells. Hum. Gene Ther. 2011;22:733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 110.Li Q., Wijesekera O., Salas S.J., et al. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin. Cancer Res. 2014;20:2375–2387. doi: 10.1158/1078-0432.CCR-13-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinfeld U., Pauli C., Kaltz N., Bergemann C., Lee H-H. T lymphocytes as potential therapeutic drug carrier for cancer treatment. Int. J. Pharm. 2006;311:229–236. doi: 10.1016/j.ijpharm.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 112.Batrakova E.V., Gendelman H.E., Kabanov A.V. Cell-mediated drugs delivery. Expert Opin. Drug Deliv. 2011;8:415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rogers W.J., Basu P. Factors regulating macrophage endocytosis of nanoparticles: implications for targeted magnetic resonance plaque imaging. Atherosclerosis. 2005;178:67–73. doi: 10.1016/j.atherosclerosis.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 114.J. Panyam VL Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm. Res. 2003;20:212–220. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- 115.Nowacek A.S., Miller R.L., McMillan J., et al. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte—macrophage drug delivery. Nanomedicine (Lond.) 2009;4:903–917. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Champion J.A., Katare Y.K., Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thiele L., Diederichs J.E., Reszka R., Merkle H.P., Walter E. Competitive adsorption of serum proteins at microparticles affects phagocytosis by dendritic cells. Biomaterials. 2003;24:1409–1418. doi: 10.1016/s0142-9612(02)00525-2. [DOI] [PubMed] [Google Scholar]
- 118.Durymanov M.O., Rosenkranz A.A., Sobolev A.S. Current approaches for improving intratumoral accumulation and distribution of nanomedicines. Theranostics. 2015;5:1007–1020. doi: 10.7150/thno.11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L., Guan Y., Liu H., et al. Silica nanorattle–doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5:7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 120.Jain S., Mishra V., Singh P., Dubey P.K., Saraf D.K., Vyas S.P. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int. J. Pharm. 2003;261:43–55. doi: 10.1016/s0378-5173(03)00269-2. [DOI] [PubMed] [Google Scholar]
- 121.Wu J., Yang S., Luo H., Zeng L., Ye L., Lu Y. Quantitative evaluation of monocyte transmigration into the brain following chemical opening of the blood–brain barrier in mice. Brain Res. 2006;1098:79–85. doi: 10.1016/j.brainres.2006.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ballantine H.T., Jr, Bell E., Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J. Neurosurg. 1960;17:858–876. doi: 10.3171/jns.1960.17.5.0858. [DOI] [PubMed] [Google Scholar]
- 123.Hynynen K., Jolesz F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998;24:275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 124.Cline H.E., Hynynen K., Hardy C.J., Watkins R.D., Schenck J.F., Jolesz F.A. MR temperature mapping of focused ultrasound surgery. Magn. Reson. Med. 1994;31:628–636. doi: 10.1002/mrm.1910310608. [DOI] [PubMed] [Google Scholar]
- 125.Clement G.T. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42:1087–1093. doi: 10.1016/j.ultras.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 126.Krasovitski B., Frenkel V., Shoham S., Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park J., Zhang Y., Vykhodtseva N., Jolesz F.A., McDannold N.J. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release. 2012;162:134–142. doi: 10.1016/j.jconrel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vykhodtseva N.I., Hynynen K., Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med. Biol. 1995;21:969–979. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]
- 129.Hynynen K., McDannold N., Vykhodtseva N., Jolesz F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 130.Sheikov N., McDannold N., Sharma S., Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sheikov N., McDannold N., Jolesz F., Zhang Y.Z., Tam K., Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med. Biol. 2006;32:1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 132.Sheikov N., McDannold N., Vykhodtseva N., Jolesz F., Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 133.Meijering B.D., Juffermans L.J., van Wamel A., et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- 134.Deng J., Huang Q., Wang F., et al. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles. J. Mol. Neurosci. 2012;46:677–687. doi: 10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- 135.Xia C.Y., Zhang Z., Xue Y.X., Wang P., Liu Y.H. Mechanisms of the increase in the permeability of the blood-tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J. Neurooncol. 2009;94:41–50. doi: 10.1007/s11060-009-9812-9. [DOI] [PubMed] [Google Scholar]
- 136.Nhan T., Burgess A., Cho E.E., Stefanovic B., Lilge L., Hynynen K. Drug delivery to the brain by focused ultrasound induced blood-brain barrier disruption: quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. J. Control. Release. 2013;172:274–280. doi: 10.1016/j.jconrel.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raymond S.B., Skoch J., Hynynen K., Bacskai B.J. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J. Cereb. Blood Flow Metab. 2007;27:393–403. doi: 10.1038/sj.jcbfm.9600336. [DOI] [PubMed] [Google Scholar]
- 138.Hynynen K., McDannold N., Sheikov N.A., Jolesz F.A., Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 139.Hynynen K., McDannold N., Vykhodtseva N., et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J. Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 140.McDannold N., Vykhodtseva N., Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med. Biol. 2008;34:834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McDannold N., Vykhodtseva N., Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med. Biol. 2008;34:930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDannold N., Arvanitis C.D., Vykhodtseva N., Livingstone M.S. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Downs M.E., Buch A., Sierra C., et al. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One. 2015;10:e0125911. doi: 10.1371/journal.pone.0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Treat L.H., McDannold N., Vykhodtseva N., Zhang Y., Tam K., Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 145.Treat L.H., McDannold N., Zhang Y., Vykhodtseva N., Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aryal M., Vykhodtseva N., Zhang Y.Z., Park J., McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release. 2013;169:103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kovacs Z., Werner B., Rassi A., Sass J.O., Martin-Fiori E., Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J. Control. Release. 2014;187:74–82. doi: 10.1016/j.jconrel.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 148.Mei J., Cheng Y., Song Y., et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J. Ultrasound Med. 2009;28:871–880. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 149.Liu H.L., Hua M.Y., Yang H.W., et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl. Acad. Sci. USA. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ostermann S., Csajka C., Buclin T., et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 151.Liu H.L., Huang C.Y., Chen J.Y., Wang H.Y., Chen P.Y., Wei K.C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One. 2014;9:e114311. doi: 10.1371/journal.pone.0114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kinoshita M., McDannold N., Jolesz F.A., Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 153.Kinoshita M., McDannold N., Jolesz F.A., Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park E.J., Zhang Y.Z., Vykhodtseva N., McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control. Release. 2012;163:277–284. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Janus C., Pearson J., McLaurin J., et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 156.Jordao J.F., Thevenot E., Markham-Coultes K., et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jordao J.F., Ayala-Grosso C.A., Markham K., et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raymond S.B., Treat L.H., Dewey J.D., McDannold N.J., Hynynen K., Bacskai B.J. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PLoS One. 2008;3:e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen P.Y., Hsieh H.Y., Huang C.Y., Lin C.Y., Wei K.C., Liu H.L. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J. Transl. Med. 2015;13:93. doi: 10.1186/s12967-015-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Burgess A., Ayala-Grosso C.A., Ganguly M., Jordao J.F., Aubert I., Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alkins R., Burgess A., Ganguly M., et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Q., Deng J., Xie Z., et al. Effective gene transfer into central nervous system following ultrasound-microbubbles-induced opening of the blood-brain barrier. Ultrasound Med. Biol. 2012;38:1234–1243. doi: 10.1016/j.ultrasmedbio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 163.Huang Q., Deng J., Wang F., et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp. Neurol. 2012;233:350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 164.Thevenot E., Jordao J.F., O'Reilly M.A., et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 2012;23:1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang S., Olumolade O.O., Sun T., Samiotaki G., Konofagou E.E. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2015;22:104–110. doi: 10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hsu P.H., Wei K.C., Huang C.Y., et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8:e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ting C.Y., Fan C.H., Liu H.L., et al. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33:704–712. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- 168.Fan C.H., Ting C.Y., Liu H.L., et al. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34:2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 169.Dakhil S., Ensminger W., Kindt G., et al. Implanted system for intraventricular drug infusion in central nervous system tumors. Cancer Treat. Rep. 1981;65:401–411. [PubMed] [Google Scholar]
- 170.Blasberg R.G., Patlak C., Fenstermacher J.D. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J. Pharmacol. Exp. Ther. 1975;195:73–83. [PubMed] [Google Scholar]
- 171.Fleischhack G., Jaehde U., Bode U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin. Pharmacokinet. 2005;44:1–31. doi: 10.2165/00003088-200544010-00001. [DOI] [PubMed] [Google Scholar]
- 172.Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11:871–879. doi: 10.1016/S1470-2045(10)70034-6. [DOI] [PubMed] [Google Scholar]
- 173.Platini C., Long J., Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006;7:778–780. doi: 10.1016/S1470-2045(06)70864-6. [DOI] [PubMed] [Google Scholar]
- 174.Gururangan S., Petros W.P., Poussaint T.Y., et al. Phase I trial of intrathecal spartaject busulfan in children with neoplastic meningitis: a Pediatric Brain Tumor Consortium Study (PBTC-004). Clin. Cancer Res. 2006;12:1540–1546. doi: 10.1158/1078-0432.CCR-05-2094. [DOI] [PubMed] [Google Scholar]
- 175.Cheung C.W., Burton C., Smith P., Linch D.C., Hoskin P.J., Ardeshna K.M. Central nervous system chemoprophylaxis in non-Hodgkin lymphoma: current practice in the UK. Br. J. Haematol. 2005;131:193–200. doi: 10.1111/j.1365-2141.2005.05756.x. [DOI] [PubMed] [Google Scholar]
- 176.Ochiai H., Campbell S.A., Archer G.E., et al. Targeted therapy for glioblastoma multiforme neoplastic meningitis with intrathecal delivery of an oncolytic recombinant poliovirus. Clin. Cancer Res. 2006;12:1349–1354. doi: 10.1158/1078-0432.CCR-05-1595. [DOI] [PubMed] [Google Scholar]
- 177.Witham T.F., Fukui M.B., Meltzer C.C., Burns R., Kondziolka D., Bozik M.E. Survival of patients with high grade glioma treated with intrathecal thiotriethylenephosphoramide for ependymal or leptomeningeal gliomatosis. Cancer. 1999;86:1347–1353. doi: 10.1002/(sici)1097-0142(19991001)86:7<1347::aid-cncr34>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 178.Grabb P.A., Guin-Renfroe S., Meythaler J.M. Midthoracic catheter tip placement for intrathecal baclofen administration in children with quadriparetic spasticity. Neurosurgery. 1999;45:833–836. doi: 10.1097/00006123-199910000-00020. [DOI] [PubMed] [Google Scholar]
- 179.Murphy N.A., Irwin M.C., Hoff C. Intrathecal baclofen therapy in children with cerebral palsy: efficacy and complications. Arch. Phys. Med. Rehabil. 2002;83:1721–1725. doi: 10.1053/apmr.2002.36068. [DOI] [PubMed] [Google Scholar]
- 180.Bennett G., Burchiel K., Buchser E., et al. Clinical guidelines for intraspinal infusion: report of an expert panel. PolyAnalgesic Consensus Conference 2000. J. Pain Symptom Manage. 2000;20:S37–S43. doi: 10.1016/s0885-3924(00)00202-5. [DOI] [PubMed] [Google Scholar]
- 181.Deer T.R., Smith H.S., Cousins M., et al. Consensus guidelines for the selection and implantation of patients with noncancer pain for intrathecal drug delivery. Pain Physician. 2010;13:E175–E213. [PubMed] [Google Scholar]
- 182.Costantino H.R., Illum L., Brandt G., Johnson P.H., Quay S.C. Intranasal delivery: physicochemical and therapeutic aspects. Int. J. Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 183.Pardridge W.M. Blood-brain barrier delivery. Drug Discov. Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 184.Graff C.L., Pollack G.M. Nasal drug administration: potential for targeted central nervous system delivery. J. Pharm. Sci. 2005;94:1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 185.Allhenn D., Shetab Boushehri M.A., Lamprecht A. Drug delivery strategies for the treatment of malignant gliomas. Int. J. Pharm. 2012;436:299–310. doi: 10.1016/j.ijpharm.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 186.Lochhead J.J., Thorne R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 187.Wu H., Hu K., Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin. Drug Deliv. 2008;5:1159–1168. doi: 10.1517/17425247.5.10.1159. [DOI] [PubMed] [Google Scholar]
- 188.Ying W., Wei G., Wang D., et al. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front. Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- 189.Wei G., Wang D., Lu H., et al. Intranasal administration of a PARG inhibitor profoundly decreases ischemic brain injury. Front. Biosci. 2007;12:4986–4996. doi: 10.2741/2443. [DOI] [PubMed] [Google Scholar]
- 190.Mistry A., Stolnik S., Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009;379:146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 191.Wang X., Chi N., Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008;70:735–740. doi: 10.1016/j.ejpb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 192.Kumar M., Misra A., Babbar A.K., Mishra A.K., Mishra P., Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008;358:285–291. doi: 10.1016/j.ijpharm.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 193.Kumar M., Misra A., Mishra A.K., Mishra P., Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J. Drug Target. 2008;16:806–814. doi: 10.1080/10611860802476504. [DOI] [PubMed] [Google Scholar]
- 194.da Fonseca C.O., Schwartsmann G., Fischer J., et al. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg. Neurol. 2008;70:259–266. doi: 10.1016/j.surneu.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 195.Fonseca C., Linden R. Futuro Db, Gattass C, Quirico-Santos T. Ras pathway activation in gliomas: a strategic target for intranasal administration of perillyl alcohol. Arch. Immunol. Ther. Exp. (Warsz.) 2008;56:267–276. doi: 10.1007/s00005-008-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang J., Lu L., Wang H-C., et al. Effect of intranasal arginine vasopressin on human headache. Peptides. 2012;38:100–104. doi: 10.1016/j.peptides.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 197.Langer R., Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 198.Leach K.J., Mathiowitz E. Degradation of double-walled polymer microspheres of PLLA and P(CPP: SA)20: 80: in vitro degradation. Biomaterials. 1998;19:1973–1980. doi: 10.1016/s0142-9612(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 199.Shieh L., Tamada J., Chen I., Pang J., Domb A., Langer R. Erosion of a new family of biodegradable polyanhydrides. J. Biomed. Mater. Res. 1994;28:1465–1475. doi: 10.1002/jbm.820281212. [DOI] [PubMed] [Google Scholar]
- 200.Lavik E.B., Hrkach J.S., Lotan N., Nazarov R., Langer R. A simple synthetic route to the formation of a block copolymer of poly(lactic-co-glycolic acid) and polylysine for the fabrication of functionalized, degradable structures for biomedical applications. J. Biomed. Mater. Res. 2001;58:291–294. doi: 10.1002/1097-4636(2001)58:3<291::aid-jbm1019>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 201.Lesniak M.S., Langer R., Brem H. Drug delivery to tumors of the central nervous system. Curr. Neurol. Neurosci. Rep. 2001;1:210–216. doi: 10.1007/s11910-001-0020-z. [DOI] [PubMed] [Google Scholar]
- 202.Bouhadir K.H., Alsberg E., Mooney D.J. Hydrogels for combination delivery of antineoplastic agents. Biomaterials. 2001;22:2625–2633. doi: 10.1016/s0142-9612(01)00003-5. [DOI] [PubMed] [Google Scholar]
- 203.Olivi A., Ewend M.G., Utsuki T., et al. Interstitial delivery of carboplatin via biodegradable polymers is effective against experimental glioma in the rat. Cancer Chemother. Pharmacol. 1996;39:90–96. doi: 10.1007/s002800050542. [DOI] [PubMed] [Google Scholar]
- 204.Sun Z.J., Chen C., Sun M.Z., et al. The application of poly (glycerol-sebacate) as biodegradable drug carrier. Biomaterials. 2009;30:5209–5214. doi: 10.1016/j.biomaterials.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 205.Brem H., Mahaley M.S., Jr, Vick N.A., et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J. Neurosurg. 1991;74:441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 206.Brem H., Piantadosi S., Burger P.C., et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 207.Brem H., Ewend M.G., Piantadosi S., Greenhoot J., Burger P.C., Sisti M. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J. Neurooncol. 1995;26:111–123. doi: 10.1007/BF01060217. [DOI] [PubMed] [Google Scholar]
- 208.Valtonen S., Timonen U., Toivanen P., et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–48. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 209.Westphal M., Hilt D.C., Bortey E., et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Santini J.T., Jr, Cima M.J., Langer R. A controlled-release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 211.Richards Grayson A.C., Choi I.S., Tyler B.M., et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat. Mater. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 212.Li Y., Ho Duc H.L., Tyler B., et al. In vivo delivery of BCNU from a MEMS device to a tumor model. J. Control. Release. 2005;106:138–145. doi: 10.1016/j.jconrel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 213.Kim G.Y., Tyler B.M., Tupper M.M., et al. Resorbable polymer microchips releasing BCNU inhibit tumor growth in the rat 9L flank model. J. Control. Release. 2007;123:172–178. doi: 10.1016/j.jconrel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bregy A., Shah A.H., Diaz M.V., et al. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 2013;13:1453–1461. doi: 10.1586/14737140.2013.840090. [DOI] [PubMed] [Google Scholar]
- 215.Fung L.K., Shin M., Tyler B., Brem H., Saltzman W.M. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm. Res. 1996;13:671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- 216.Kroin J.S., Penn R.D. Intracerebral chemotherapy: chronic microinfusion of cisplatin. Neurosurgery. 1982;10:349–354. doi: 10.1227/00006123-198203000-00009. [DOI] [PubMed] [Google Scholar]
- 217.Sendelbeck S.L., Urquhart J. Spatial distribution of dopamine, methotrexate and antipyrine during continuous intracerebral microperfusion. Brain Res. 1985;328:251–258. doi: 10.1016/0006-8993(85)91036-4. [DOI] [PubMed] [Google Scholar]
- 218.Lieberman D.M., Laske D.W., Morrison P.F., Bankiewicz K.S., Oldfield E.H. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J. Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 219.Bobo R.H., Laske D.W., Akbasak A., Morrison P.F., Dedrick R.L., Oldfield E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lonser R.R., Sarntinoranont M., Morrison P.F., Oldfield E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015;122:697–706. doi: 10.3171/2014.10.JNS14229. [DOI] [PubMed] [Google Scholar]
- 221.Strasser J.F., Fung L.K., Eller S., Grossman S.A., Saltzman W.M. Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J. Pharmacol. Exp. Ther. 1995;275:1647–1655. [PubMed] [Google Scholar]
- 222.Shi M., Fortin D., Sanche L., Paquette B. Convection-enhancement delivery of platinum-based drugs and Lipoplatin(TM) to optimize the concomitant effect with radiotherapy in F98 glioma rat model. Invest. New Drugs. 2015;33:555–563. doi: 10.1007/s10637-015-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sewing A.C., Caretti V., Lagerweij T., et al. Convection enhanced delivery of carmustine to the murine brainstem: a feasibility study. J. Neurosci. Methods. 2014;238:88–94. doi: 10.1016/j.jneumeth.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 224.Anderson R.C., Kennedy B., Yanes C.L., et al. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J. Neurosurg. Pediatr. 2013;11:289–295. doi: 10.3171/2012.10.PEDS12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hardy P.A., Keeley D., Schorn G., et al. Convection enhanced delivery of different molecular weight tracers of gadolinium-tagged polylysine. J. Neurosci. Methods. 2013;219:169–175. doi: 10.1016/j.jneumeth.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 226.Asthagiri A.R., Walbridge S., Heiss J.D., Lonser R.R. Effect of concentration on the accuracy of convective imaging distribution of a gadolinium-based surrogate tracer. J. Neurosurg. 2011;115:467–473. doi: 10.3171/2011.3.JNS101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sampson J.H., Brady M., Raghavan R., et al. Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans. Neurosurgery. 2011;69:668–676. doi: 10.1227/NEU.0b013e3182181ba8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barua N.U., Bienemann A.S., Woolley M., et al. Convection-enhanced delivery of MANF - volume of distribution analysis in porcine putamen and substantia nigra. J. Neurol. Sci. 2015;357:264–269. doi: 10.1016/j.jns.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 229.Chen M.Y., Hoffer A., Morrison P.F., et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J. Neurosurg. 2005;103:311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- 230.Szerlip N.J., Walbridge S., Yang L., et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J. Neurosurg. 2007;107:560–567. doi: 10.3171/JNS-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 231.Dickinson P.J., LeCouteur R.A., Higgins R.J., et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J. Neurosurg. 2008;108:989–998. doi: 10.3171/JNS/2008/108/5/0989. [DOI] [PubMed] [Google Scholar]
- 232.Arshad A., Yang B., Bienemann A.S., et al. Convection-enhanced delivery of carboplatin PLGA nanoparticles for the treatment of glioblastoma. PLoS One. 2015;10:e0132266. doi: 10.1371/journal.pone.0132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mastorakos P., Zhang C., Berry S., et al. Highly PEGylated DNA nanoparticles provide uniform and widespread gene transfer in the brain. Adv. Healthc. Mater. 2015;4:1023–1033. doi: 10.1002/adhm.201400800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Foley C.P., Nishimura N., Neeves K.B., Schaffer C.B., Olbricht W.L. Real-time imaging of perivascular transport of nanoparticles during convection-enhanced delivery in the rat cortex. Ann. Biomed. Eng. 2012;40:292–303. doi: 10.1007/s10439-011-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ksendzovsky A., Walbridge S., Saunders R.C., Asthagiri A.R., Heiss J.D., Lonser R.R. Convection-enhanced delivery of M13 bacteriophage to the brain. J. Neurosurg. 2012;117:197–203. doi: 10.3171/2012.4.JNS111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kenny G.D., Bienemann A.S., Tagalakis A.D., et al. Multifunctional receptor-targeted nanocomplexes for the delivery of therapeutic nucleic acids to the brain. Biomaterials. 2013;34:9190–9200. doi: 10.1016/j.biomaterials.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 237.Thorne R.G., Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. USA. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nance E.A., Woodworth G.F., Sailor K.A., et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012;4:149ra19. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lidar Z., Mardor Y., Jonas T., et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J. Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 240.Patel S.J., Shapiro W.R., Laske D.W., et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56:1243–1252. doi: 10.1227/01.neu.0000159649.71890.30. [DOI] [PubMed] [Google Scholar]
- 241.Carpentier A., Laigle-Donadey F., Zohar S., et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro-oncol. 2006;8:60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Carpentier A., Metellus P., Ursu R., et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro-oncol. 2010;12:401–408. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Weber F., Asher A., Bucholz R., et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 2003;64:125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 244.Laske D.W., Youle R.J., Oldfield E.H. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat. Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 245.Weaver M., Laske D.W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 246.Kunwar S., Prados M.D., Chang S.M., et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 247.Vogelbaum M.A., Sampson J.H., Kunwar S., et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61:1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. [DOI] [PubMed] [Google Scholar]
- 248.Kunwar S., Chang S., Westphal M., et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncol. 2010;12:871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sampson J.H., Archer G., Pedain C., et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 2010;113:301–309. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 250.Chen M.Y., Lonser R.R., Morrison P.F., Governale L.S., Oldfield E.H. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J. Neurosurg. 1999;90:315–320. doi: 10.3171/jns.1999.90.2.0315. [DOI] [PubMed] [Google Scholar]
- 251.Sampson J.H., Akabani G., Archer G.E., et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro-oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Morrison P.F., Chen M.Y., Chadwick R.S., Lonser R.R., Oldfield E.H. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am. J. Physiol. 1999;277:R1218–R1229. doi: 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- 253.White E., Bienemann A., Malone J., et al. An evaluation of the relationships between catheter design and tissue mechanics in achieving high-flow convection-enhanced delivery. J. Neurosci. Methods. 2011;199:87–97. doi: 10.1016/j.jneumeth.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 254.Krauze M.T., Saito R., Noble C., et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J. Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rosenbluth K.H., Luz M., Mohr E., Mittermeyer S., Bringas J., Bankiewicz K.S. Design of an in-dwelling cannula for convection-enhanced delivery. J. Neurosci. Methods. 2011;196:118–123. doi: 10.1016/j.jneumeth.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 256.Oh S., Odland R., Wilson S.R., et al. Improved distribution of small molecules and viral vectors in the murine brain using a hollow fiber catheter. J. Neurosurg. 2007;107:568–577. doi: 10.3171/JNS-07/09/0568. [DOI] [PMC free article] [PubMed] [Google Scholar]


