NdhN, NdhH, and NdhJ are essential for the stability and the activities of NAD(P)H dehydrogenase complexes.
Abstract
The cyanobacterial NAD(P)H dehydrogenase (NDH-1) complexes play crucial roles in variety of bioenergetic reactions such as respiration, CO2 uptake, and cyclic electron transport around PSI. Recently, substantial progress has been made in identifying the composition of subunits of NDH-1 complexes. However, the localization and the physiological roles of several subunits in cyanobacteria are not fully understood. Here, by constructing fully segregated ndhN, ndhO, ndhH, and ndhJ null mutants in Synechocystis sp. strain PCC 6803, we found that deletion of ndhN, ndhH, or ndhJ but not ndhO severely impaired the accumulation of the hydrophilic subunits of the NDH-1 in the thylakoid membrane, resulting in disassembly of NDH-1MS, NDH-1MS′, as well as NDH-1L, finally causing the severe growth suppression phenotype. In contrast, deletion of NdhO affected the growth at pH 6.5 in air. In the cytoplasm, either NdhH or NdhJ deleted mutant, but neither NdhN nor NdhO deleted mutant, failed to accumulate the NDH-1 assembly intermediate consisting of NdhH, NdhJ, NdhK, and NdhM. Based on these results, we suggest that NdhN, NdhH, and NdhJ are essential for the stability and the activities of NDH-1 complexes, while NdhO for NDH-1 functions under the condition of inorganic carbon limitation in Synechocystis sp. strain PCC 6803. We discuss the roles of these subunits and propose a new NDH-1 model.
Thylakoid membranes of cyanobacteria contain a NAD(P)H dehydrogenase (NDH-1) complex, homologous to complex I (NADH: ubiquinone oxidoreductase) from the mitochondria and eubacteria (Friedrich et al., 1995; Friedrich and Scheide, 2000). The NDH-1 complexes in cyanobacteria are involved in respiration and cyclic electron transport (CET) around PSI (Ogawa, 1991; Mi et al., 1992, 1995; Peltier and Cournac, 2002; Munekage et al., 2004). In addition, cyanobacterial NDH-1 complexes function in inorganic carbon concentrating mechanisms (Ogawa, 1991; Ohkawa et al., 2000).
In cyanobacteria, ndhA-ndhK encode proteins homologous to subunits of Escherichia coli complex I. However, homologous genes encoding the three subunits, NuoE-NuoG, constituting the catalytic domain of E. coli complex I, are not found in the genome of the cyanobacteria. There are six ndhD and three ndhF genes in Synechocystis sp. strain PCC 6803 (Synechocystis 6803). Different NDH-1 complexes consist of different types of NdhD and NdhF subunits, which are involved in diverse physiological functions. Four types of cyanobacterial NDH-1 complexes have been defined by reverse genetics (Klughammer et al., 1999; Shibata et al., 2001) and functional proteomics (Prommeenate et al., 2004; Battchikova et al., 2005). The large NDH-1 complex (NDH-1L) containing NdhD1/NdhF1 and the NDH-1L′ complex containing NdhD2/NdhF1 are involved in respiration and NDH-1 dependent CET around PSI (Ohkawa et al., 2000; Battchikova et al., 2011a). The expression of NDH-1L complex is stable under different growth conditions; however, the NDH-1L′ complex has not been detected on the protein level (Zhang et al., 2004).
All of these NDH-1 complexes contain a medium size NDH-1 complex (NDH-1M) as a skeleton. One type of NDH-1 complex, the NDH-1MS complex, is inducible at limiting inorganic carbon conditions and has a high uptake affinity for CO2, which is easily dissociated into NDH-1M and a small size NDH-1 complex (NDH-1S; Herranen et al., 2004). The NDH-1MS complex has been isolated from a Thermosynechococcus elongatus strain in which the C terminus of NdhL has been tagged with 6-His. This complex is easily dissociated into NDH-1M and NDH-1S complexes (Zhang et al., 2005). NDH-1MS has been characterized as a U-shape structure by analysis of single-particle electron microscopy after purification from the thylakoid membranes of T. elongatus (Arteni et al., 2006). CupA is responsible for the U-shape by binding at the tip of the membrane-bound arm of NDH-1MS in both T. elongatus and Synechocystis 6803 (Folea et al., 2008). As a homologous gene of cupA, cupB is involved in constitutive CO2 uptake system and forms a small complex NDH-1S′ (Shibata et al., 2001; Maeda et al., 2002). It has been found that CupB protein is localized in thylakoid membrane but is absent in that of NdhD4 deleted mutant (Xu et al., 2008). Based on the result that the purification of a 450-kD complex contained both NdhH and CupB protein, it has been suggested that the complex is NDH-1MS′ located in the thylakoid membranes.
Four additional subunits (NdhL-NdhO) specific for cyanobacteria and chloroplasts have been identified in Synechocystis 6803 and Arabidopsis (Arabidopsis thaliana; Prommeenate et al., 2004; Battchikova et al., 2005; Rumeau et al., 2005; Shimizu et al., 2008). Further electron microscopy investigations speculated that in Synechocystis 6803, the NdhL-NdhO subunits are located together, constituting the oxygenic photosynthesis specific (OPS) domain (Birungi et al., 2010). However, our previous study demonstrates that cyanobacterial NdhM is localized in the hydrophilic subcomplex of NDH-1 complexes and is essential for the function of NDH-1 complexes (He et al., 2016). Nowaczyk et al. (2011) reported two novel small subunits, NdhP and NdhQ, which were included in the purified NDH-1L complex by Ni2+ affinity chromatography and size-exclusion chromatography from T. elongatus. Recently, it has been demonstrated that NdhP and NdhQ are involved in respiration and CET and are required to stabilize the NDH-1L complex (Schwarz et al., 2013; Wulfhorst et al., 2014; Zhang et al., 2014; Zhao et al., 2015). In recent years, it has been reported that the newly identified OPS subunit, NdhS from Arabidopsis (also known as CRR31) or from Synechocystis 6803 contains Src homology 3 domain-like fold, which serves as the ferredoxin (Fd) docking site domain (Yamamoto et al., 2011; Battchikova et al., 2011b; Yamamoto and Shikanai, 2013), and the authors suggested that the chloroplast NDH complex could accept electrons from Fd rather than NAD(P)H. By studying the purified NDH-1L complex from the thermophilic cyanobacterium T. elongatus, we demonstrated that NDH-1L complex interacts with Fd via the subunit NdhS (He et al., 2015). Gao et al. (2016) identified the new NdhV subunit in Synechocystis 6803 and proposed that NdhV cooperates with NdhS to accept electrons from Fd. In addition, ndhH gene was shown to be vital to the survival of Synechocystis 6803 even under high-CO2 growth conditions (Pieulle et al., 2000). NdhJ was found in both plasma membrane and thylakoid membranes in Synechocystis 6803 (Berger et al., 1991; Pieulle et al., 2000) and Anacystis nidulans (Dworsky et al., 1995). The NdhN and NdhO subunits of Synechocystis 6803 were first identified by functional proteomics approach (Prommeenate et al., 2004; Battchikova et al., 2005). NdhH-NdhK, NdhN, and NdhO were copurified with PSI from Synechocystis 6803 cells by Ni2+ affinity chromatography (Kubota et al., 2010). Zhao et al. (2014) showed that NdhO destabilized the NDH-1M and repressed the NDH-1 activity. In higher plants, absence of NdhN or NdhO caused the complete impairment of chloroplast NDH activity (Rumeau et al., 2005; Peng et al., 2012). However, because null alleles of ndhN, ndhH, or NdhJ have never been fully segregated in Synechocystis 6803, the localization and the function of these cyanobacterial subunits are still unknown. Here, by using an indirect route, we successfully constructed the fully segregated ndhN, ndhH, and NdhJ null mutants. We demonstrate that absence of NdhN, NdhH, or NdhJ seriously impaired the hydrophilic subcomplexes of the NDH-1 complexes, resulting in the loss of the ability of CET and CO2 uptake. Neither the NdhN nor NdhO deleted mutants affect the stability of the NDH-1 subcomplex assembly intermediates consisting of NdhK, NdhM, NdhH, and NdhJ in the cytoplasm. Based on these results, we proposed a new model of the NDH-1 complexes.
RESULTS
Deletion of ndhN, ndhH, and ndhJ Impairs NDH-1 Activity
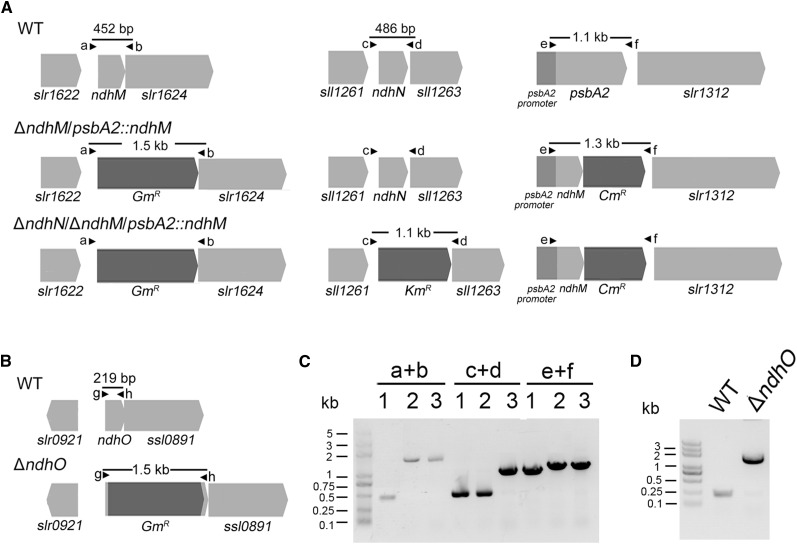
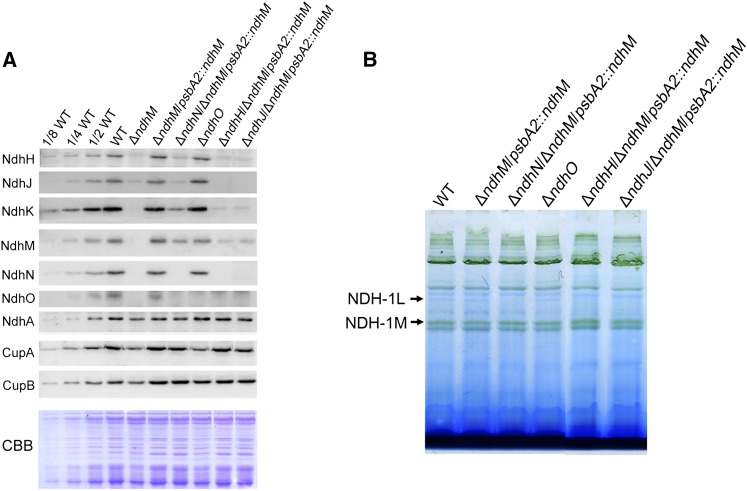
To investigate the function of ndhN, ndhO, ndhH, and ndhJ genes in NDH-1 complexes, we constructed the mutants defective in these ndh genes. Genetic manipulations to generate the ndhN, ndhH, ndhJ, and ndhO null mutants are described in “Materials and Methods.” The ndhO coding region was replaced with the gentamicin resistance (GmR) cassette. PCR analysis of the ndhO locus confirmed the complete segregation of the ndhO allele (Fig. 1, B and D). Immunoblotting analysis using the antibody specifically prepared against NdhO demonstrated the absence of the gene product in the mutant (Fig. 4A). For construction of the ndhN, ndhH, and ndhJ null mutants, we used the ΔndhM mutant strain in which the ndhM gene had been deleted and the hydrophilic subunits of NDH-1 complex could not be accumulated in the thylakoid membrane (He et al., 2016). The ndhN, ndhH, and ndhJ genes were inactivated in the ΔndhM background. After several attempts, the fully segregated double mutant strains were obtained. Subsequently, the double mutant strains were respectively rescued at psbA2 locus by transformation with a plasmid containing the fragment encoding for NdhM to produce the ΔndhN/ΔndhM/psbA2::ndhM, ΔndhH/ΔndhM/psbA2::ndhM, and ΔndhJ/ΔndhM/psbA2::ndhM mutant strains (Fig. 1A; Supplemental Fig. S1). To rule out the effect of ndhM, the control strain (ΔndhM/psbA2::ndhM) was generated. Immunoblotting analysis using the antibodies against NdhN, NdhH, and NdhJ demonstrated the absence of the gene products in these mutant strains (Fig. 4A).
Figure 1.
Construction and verification of the ndhN-deletion and ndhO-deletion mutants. A, Construction of the plasmids used to generate the ΔndhM/psbA2::ndhM and ΔndhN/ΔndhM/psbA2::ndhM. B, Construction of the plasmid to generate the ΔndhO construct. C and D, PCR segregation analysis of the ΔndhM/psbA2::ndhM, ΔndhN/ΔndhM/psbA2::ndhM, and ΔndhO mutants using the primers listed in Supplemental Table S1. Primer location is shown in A and B, and the expected DNA fragment size is based on the DNA template. 1, Wild type; 2, ΔndhM/psbA2::ndhM; 3, ΔndhN/ΔndhM/psbA2::ndhM.
Figure 4.
Analysis of the NDH-1 complex in different NDH-1 mutant backgrounds. A, Immunoblot analysis of Ndh subunits in thylakoid membranes of wild-type, ndhM, ndhN, ndhO, ndhH, and ndhJ deletion mutants. Each lane was loaded with thylakoid membrane proteins corresponding to 2 μg chlorophyll a, and the series of dilution is indicated. In the lower panel, a replicated gel stained with Coomassie Brilliant Blue (CBB) was used as a loading control. B, BN-PAGE analysis of thylakoid protein complexes isolated from wild-type, ndhN, ndhO, ndhH, and ndhJ deletion mutants. Each lane was loaded with thylakoid membrane proteins corresponding to 3 μg chlorophyll a.
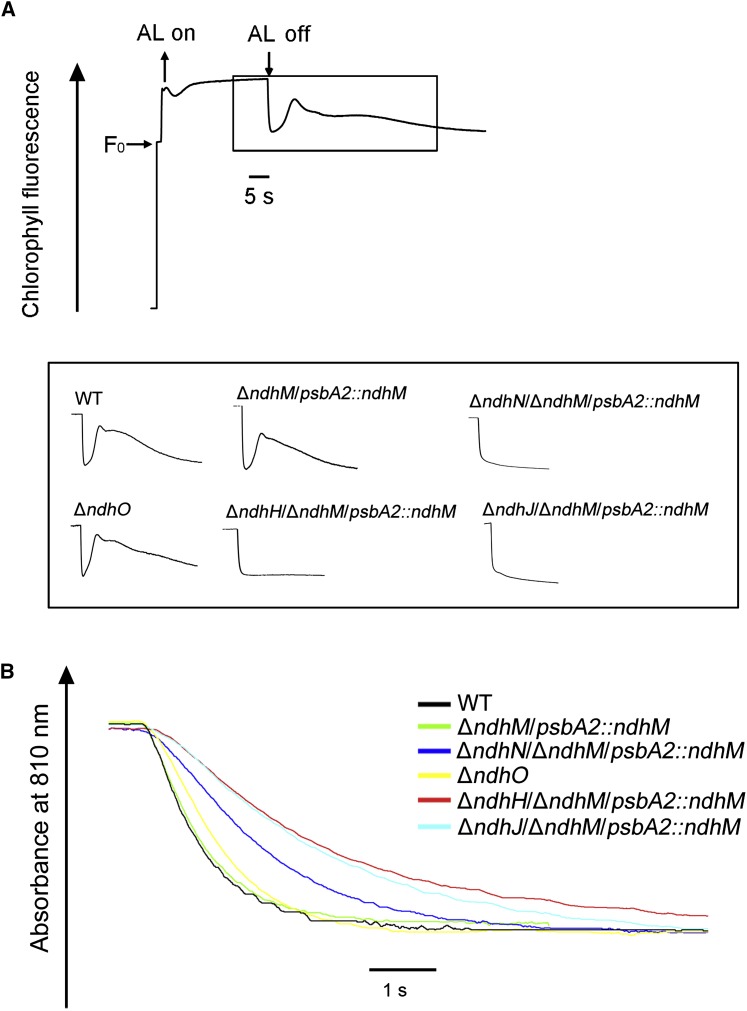
Next, we compared the NDH-1 CET activity among the ndhN, ndhO, ndhH, and NdhJ null mutants. We monitored the transient increase in chlorophyll a fluorescence after illumination with actinic light (AL), which is attributed to the NDH-dependent nonphotochemical reduction of the plastoquinone pool in the dark (Mi et al., 1995; Deng et al., 2003). In the absence of NdhN, NdhH, or NdhJ, the transient increase of chlorophyll fluorescence was completely arrested, whereas the NDH-1 CET activity was not affected in control strain (ΔndhM/psbA2::ndhM; Fig. 2A). A previous study showed that the NDH-1 CET activity was higher in the NdhO deleted mutant (Zhao et al., 2014). In contrast, our measurements showed that the transient increase of chlorophyll fluorescence was slightly smaller in the ΔndhO mutant compared with that in the wild type (Fig. 2A). A similar result was obtained by measuring the rereduction of P700+ in darkness. P700 was oxidized by far-red light (>720 nm) for 40 s and then the subsequent rereduction of P700+ in the dark was monitored. The operation of NDH-1 complexes transfers electrons from the reduced plastoquinone pool and accelerates the rereduction of P700+ (Mi et al., 1995). The rereduction rate of P700+ was decreased markedly in ndhH or ndhJ null mutants, partly in the ndhN null mutant strain, while only slightly in ΔndhO, compared with that in the wild-type strain. No difference was observed between the ΔndhM/psbA2::ndhM strain and the wild type (Fig. 2B). These results demonstrated that the three subunits (NdhN, NdhH, and NdhJ) are essential for the NDH-1 CET activity; however, the deletion of ndhO only slightly affected the NDH-1 CET activity.
Figure 2.
The effects on NDH-1 activity of the different NDH-1 mutants. A, Monitoring of the NDH-1 activity using chlorophyll fluorescence analysis. The upper curve shows a typical trace chlorophyll fluorescence in Synechocystis 6803. Cells were exposed to the AL (60 μmol photons m−2 s−1) for 60 s. After it was switched off, the transient increase in chlorophyll fluorescence level was ascribed to NDH-1 activity. The inset shows the magnified traces from the box area. B, Kinetics of P700+ rereduction in darkness after turning off far-red light in the presence of 10 μm DCMU. The chlorophyll a concentration was adjusted to 30 μg mL−1, and curves are normalized to the maximal signal.
Deletion of ndhN, ndhH, and ndhJ Results in the Growth Suppression Phenotype in Air or under Photoheterotrophic or Mixotrophic Conditions
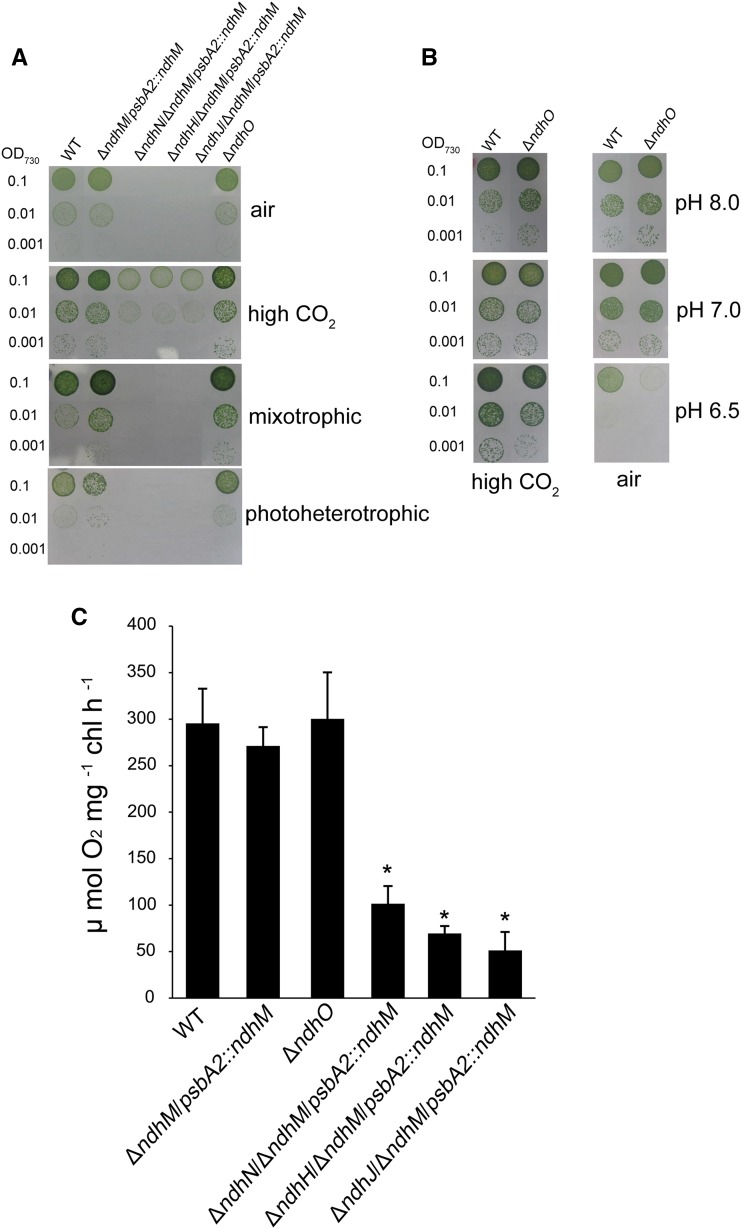
In addition to CET around PSI, NDH-1 complexes are also involved in CO2 uptake and respiration activities (Zhang et al., 2004). To examine the CO2 uptake and respiration activities, wild-type, ΔndhM/psbA2::ndhM, ΔndhN/ΔndhM/psbA2::ndhM, ΔndhH/ΔndhM/psbA2::ndhM, ΔndhJ/ΔndhM/psbA2::ndhM, and ΔndhO strains were grown on BG-11 plates in the presence of 2% CO2 in air or ambient air as well as under photoheterotrophic and mixotrophic conditions. The growth rates of ndhN, ndhH, and ndhJ deleted mutants were a bit slower than that of the wild type under autotrophic growth condition supplied with 2% CO2, while they grew very poorly under air condition or photoheterotrophic or mixotrophic conditions (Fig. 3A).
Figure 3.
Growth and the oxygen evolution rates of the wild type and different NDH-1 mutant strains. A and B, Growth of wild-type, ndhN, ndhH, ndhJ, and ndhO deletion mutants. The concentration of the cells was adjusted to OD730 = 0.1, 0.01, and 0.001. Three microliters of the cell suspensions was placed on the agar plate with different carbon sources, and 3 μL of wild-type and ΔndhO strains was spotted on the agar plates buffered at pH 8.0, 7.0, and 6.5. The cultures were grown for 7 d at 40 μmol photons m−2 s−1. High CO2, 2% CO2 (v/v) in air; mixotrophic, BG-11 medium + 5 mm Glc; photoheterotrophic, BG-11 medium + 5 mm Glc + 10 μm DCMU. C, The rates of O2 evolution of wild-type, ndhN, ndhH, ndhJ, and ndhO-deletion mutants using a light intensity of 400 μmol photons m−2 s−1 at 30°C. Values are means ± se of three replicates. Asterisks indicate a significant difference from the wild type under the same growth conditions (Student’s t test, P < 0.05).
The wild type and the control stain (ΔndhM/psbA2::ndhM) behaved similarly under all growth conditions (Fig. 3A). No significant differences were observed between the ΔndhO mutant and the wild-type cells under autotrophic (pH 8.0 or 7.0) or photoheterotrophic conditions (Fig. 3, A and B). The ΔndhO mutant grew as fast as the wild type under the presence of 2% CO2 at pH 6.5 (the concentration HCO3− becomes minor and CO2 is predominant); nevertheless, the growth was slower than the wild type under air condition at pH 6.5 (both the concentrations of HCO3− and CO2 are limited; Fig. 3B). Moreover, the ndhO mutant complemented with NdhO rescued a wild-type phenotype in air at pH 6.5 (Supplemental Fig. S3), confirming the slow growth phenotype caused by deletion of NdhO under the inorganic carbon limitation condition. Based on these results, we show that deletion of ndhO partially impaired the CO2 uptake activity. We further compared the capacity of photosynthesis among the wild type and the mutants as reflected by O2 evolution. Figure 3C shows that the rates of oxygen evolution were significantly reduced to about 30% in ndhN and about 20% in ndhH or ndhJ deleted mutants of that in wild-type strain, while the rate of ndhO deleted mutant was similar with that in the wild type. The above results suggest that in addition to NDH-1 CET, NdhN, NdhH, and NdhJ are essential for respiration and CO2 uptake and NdhO functions under the inorganic carbon limitation condition.
Disassembly of NDH-L, NDH-1MS, and NDH-1MS′ in ndhN, ndhH, and ndhJ Null Mutants
To investigate how NDH-1 activity is affected in the absence of NdhN, NdhH, NdhJ, or NdhO, we compared the accumulation of the NDH-1 complexes in the thylakoid membranes of the wild type and various NDH-1 mutant strains, including ndhM, ndhN, ndhH, ndhJ, and ndhO null mutants, by immunoblotting analysis using antibodies against NdhH, NdhJ, NdhK, NdhM, NdhN, NdhO, CupA, and CupB. As shown in Figure 4A, similar to the ΔndhM mutant, inactivation of ndhH or ndhJ almost completely abolished accumulation of the subunits of the hydrophilic domain of the NDH-1 complex, while the accumulation of the NdhA from the hydrophobic domain embedded in the membrane was not affected. The levels of NdhH, NdhJ, NdhK, NdhM, and NdhO were reduced to less than one-fourth of the wild type in the absence of NdhN, while the accumulation of the CupA and CupB was slightly affected in the mutant. In contrast, the accumulation of other Ndh subunits was barely affected in the absence of NdhO.
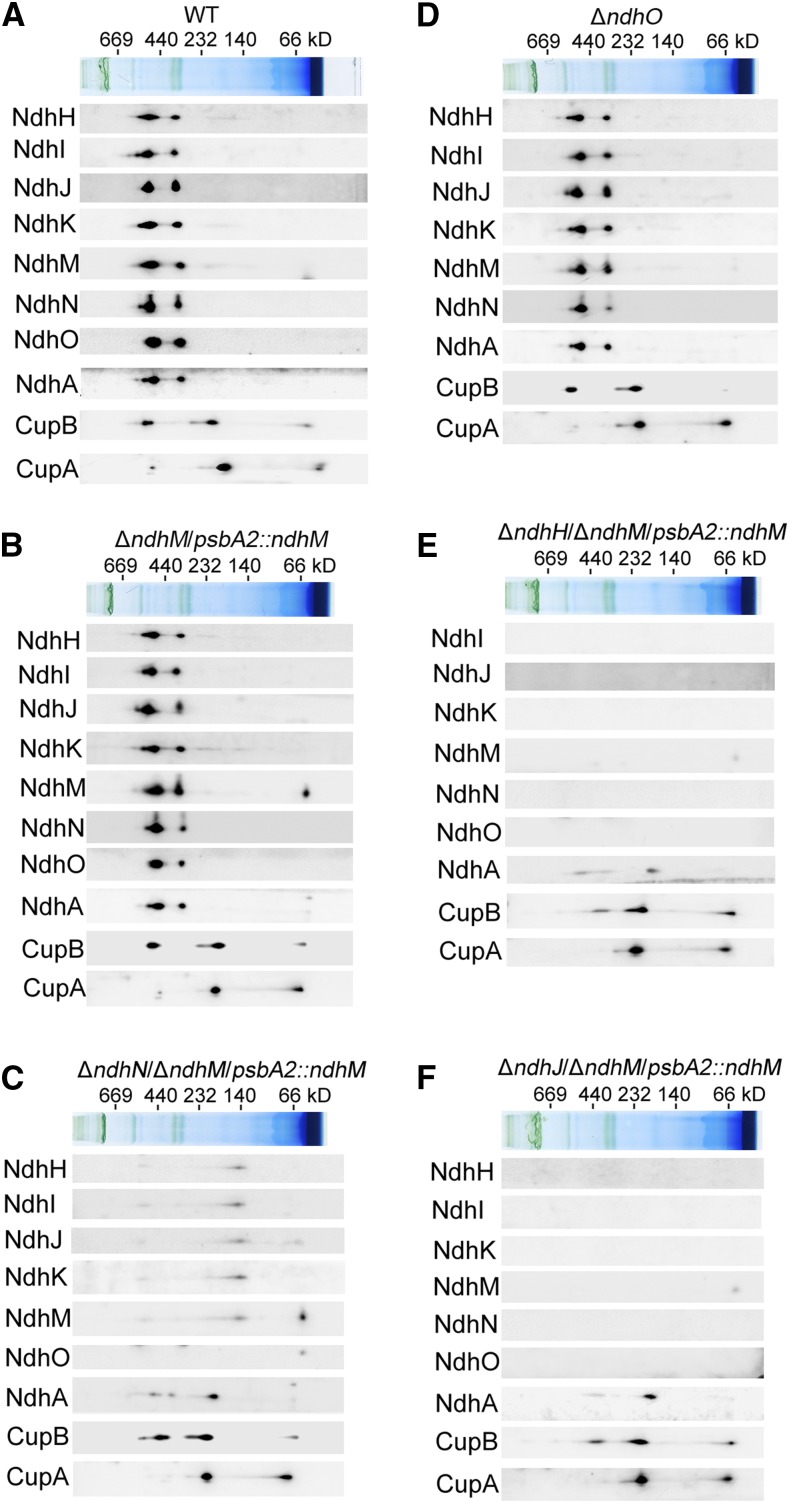
Furthermore, to reveal how the NDH-1 complexes are affected in different NDH-1 mutant strains, we separated the protein complexes from thylakoid membranes in the mutants by a 5 to 13% gradient Blue native-PAGE (BN-PAGE; Fig. 4B) and observed that the band corresponding to the NDH-1L complex disappeared in the ndhN, ndhH, and ndhJ null mutants, but not in the ndhO null mutant. Since the NDH-1L band was very weak in the BN gel, we further confirmed the results by two-dimensional SDS-PAGE and immunoblotting with antibodies against several Ndh subunits (Fig. 5). Indeed, both the NDH-1L and NDH-1M complexes were disassembled in the ndhN deleted mutant. The amount of the hydrophilic subunits, including NdhH, NdhI, NdhJ, NdhK, and NdhM, was evidently decreased and was present in a subcomplex with a molecular mass of ∼140 kD. The hydrophobic subunit, NdhA, was present in a subcomplex with a approximate molecular mass of 200 kD (Fig. 5C). In contrast, the accumulation of the NDH-1L and NDH-1M complexes in the ΔndhO and ΔndhM/psbA2::ndhM was not influenced (Fig. 5, B and D). Moreover, we also checked the low CO2-induced NDH-1MS and the constitutively expressed NDH-1MS′ complexes, which are essential for CO2 uptake activity. As shown in Figure 5A, NDH-1MS complex, NDH-1S complex, and free protein could be detected in the wild type using the antibody against CupA. However, in the absence of NdhN, NdhH, or NdhJ, only the NDH-1S complex and the free CupA could be detected, while the NDH-1MS complex was absent in the thylakoid membranes (Fig. 5, C, E, and F). Similarly, the NDH-1MS′ complex, NDH-1S′ complex, and free protein could also be detected in the wild type and control strains using the antibody against CupB. In the ndhN, ndhH, and ndhJ deleted mutants, the accumulation of the NDH-1S′ complex and the free CupB was not affected; however, the NDH-1MS′ complex with a molecular mass of ∼550 kD was degraded to a subcomplex with ∼400 kD, lacking the hydrophilic Ndh subunits (Fig. 5, C, E, and F). Nevertheless, the accumulation of the NDH-1MS and the NDH-1MS′ complex was not impaired in the ΔndhO mutant (Fig. 5D). Taken together, our results demonstrate that in the absence of NdhN, the amount of the hydrophilic subunits was significantly decreased and the remaining entire NDH-1 complexes were degraded to the hydrophilic subcomplex and hydrophobic subcomplex, while the absence of the NdhH or NdhJ caused the disassembly of the hydrophilic subcomplex of NDH-1 complexes; by contrast, the absence of NdhO appeared not to impair the stability of the NDH-1 complexes.
Figure 5.
Accumulation of Ndh subunits and their assembly into the NDH-1L and NDH-1M complexes in the thylakoid membranes of different NDH-1 mutant backgrounds. A-F, Thylakoid membrane proteins from the wild type and indicated mutant strains were separated by BN-PAGE and further subjected to 2D/SDS-PAGE. The proteins were immunodetected with the indicated antibodies against the Ndh subunits. The positions of molecular mass markers in the BN gel are indicated.
The Interaction of NdhN or NdhO with Other Ndh Subunits
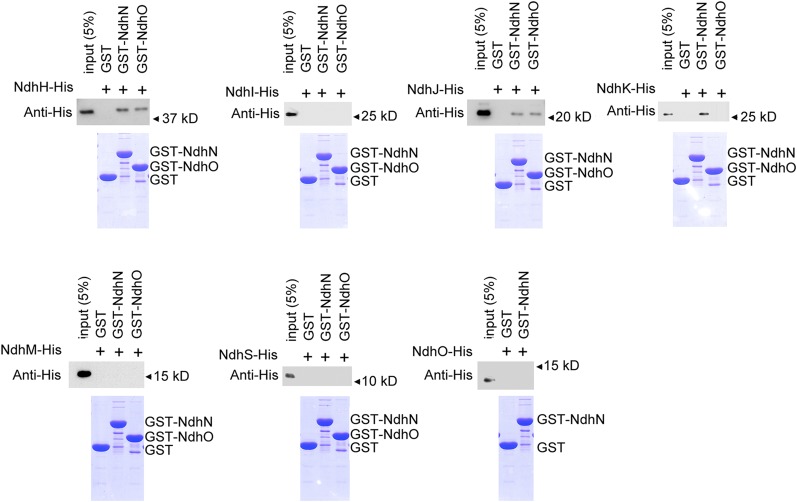
To determine the interaction of NdhN or NdhO with other hydrophilic Ndh subunits, we generated recombinant NdhN and NdhO fused with GST. The recombinant NdhH-(His)6, NdhI-(His)6, NdhJ-(His)6, NdhK-(His)6, NdhM-(His)6, NdhS-(His)6, and NdhO-(His)6 fusion proteins were then generated and tested for the interaction with GST-NdhN or GST-NdhO. GST-NdhN or GST-NdhO was bound to a GST affinity column and used as bait. Recombinant GST was used as negative control for unspecific binding. Proteins were eluted using Laemmli buffer and analyzed by immunoblotting (Fig. 6). These in vitro pull-down experiments show that NdhN interacts with NdhH, NdhJ, and NdhK, but not with NdhI, NdhM, NdhO, or NdhS subunits. As shown in Figure 6, NdhO only interacts with NdhH and NdhJ.
Figure 6.
Pull-down analysis of NdhN and NdhO with other Ndh subunits. Recombinant His-tagged NdhH, NdhI, NdhJ, NdhK, NdhM, and NdhS were incubated with recombinant GST, NdhN fused to GST, and NdhO fused to GST, respectively. The recombinant His-tagged NdhO was also incubated with recombinant GST and NdhN fused to GST. Proteins were bound to the GST affinity resin and then eluted with Laemmli buffer. Eluates were analyzed by SDS-PAGE and Coomassie blue staining, and the Ndh subunits were detected by immunoblot with anti-His antibody.
Analyses of NDH-1 Assembly Intermediates under Various Mutant Backgrounds
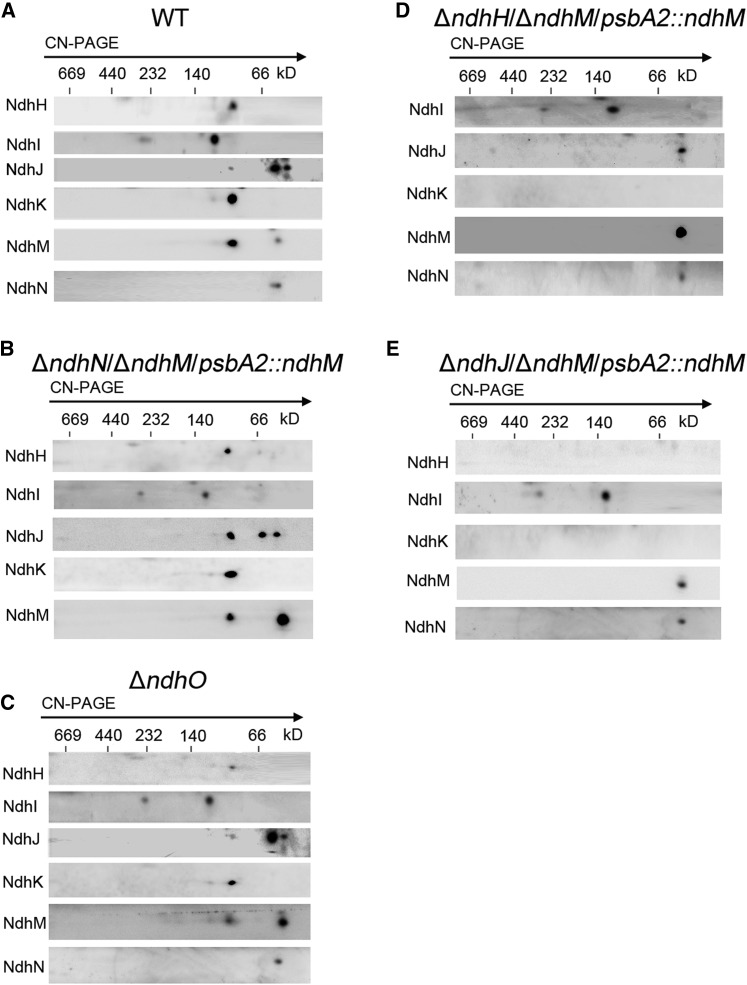
To know whether the defect of the four subunits (NdhN, NdhH, NdhJ, and NdhO) affects the assembly of NDH-1 complex, we investigated the components of the NDH-1 assembly intermediates by Clear native-PAGE (CN-PAGE) and subsequent immunoblot analyses for the cytoplasmic fraction under various mutant backgrounds. Our previous results demonstrated that the NDH-1 assembly intermediates, including NdhH, NdhK, and NdhM, were seriously affected in the ΔndhM or ΔndhK mutant (He et al., 2016). Here, we generated a new antibody against NdhJ of Synechocystis 6803 and showed that in the wild type, NdhH, NdhJ, NdhK, and NdhM subunits were present in a subcomplex with an apparent molecular mass of ∼100 kD, and NdhJ was also detected in a subcomplex of ∼60 kD as well as free protein, while NdhI was present in two subcomplexes with approximate molecular masses of 300 and 130 kD (Fig. 7A). However, the NdhN subunit was only detected as free protein, and in the absence of NdhN, the NDH-1 assembly intermediates, including NdhH, NdhJ, NdhK, and NdhM, or assembly intermediates containing NdhI were not affected (Fig. 7, A and B). Similarly, in the ΔndhO mutant, the NDH-1 assembly intermediates are formed efficiently in the cytoplasm (Fig. 7C). These results suggested that NdhN or NdhO appeared to be integrated to the NDH-1 complex in later steps of assembly (Fig. 8).
Figure 7.
Analysis of the cytoplasmic intermediate complexes isolated from the wild type and various NDH-1 mutant strains. A to E, Cytoplasmic protein complexes isolated from the wild type and the various mutants were separated by CN-PAGE and further subjected to 2D/SDS-PAGE. The proteins were immunodetected with antibodies against Ndh subunits (NdhH, NdhI, NdhJ, NdhK, NdhM, and NdhN). The positions of molecular mass markers are indicated.
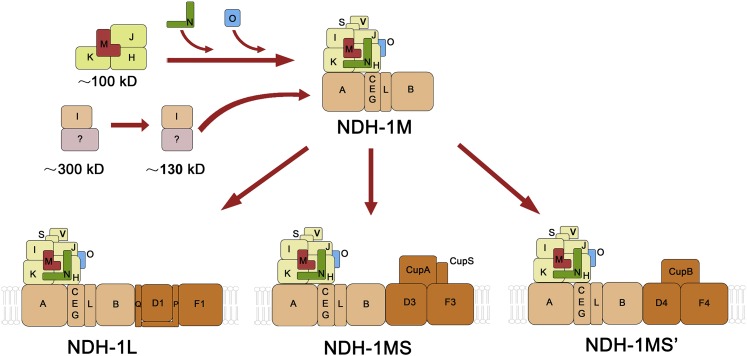
Figure 8.
A schematic model of cyanobacterial NDH-1 complex assembly. NdhH, NdhJ, NdhK, and NdhM are first assembled to form a 100-kD complex. Meanwhile, NdhI, as well as unknown factors, are assembled to form the 130- kD complex and the 300-kD complex. Subsequently, NdhN, NdhO, and NdhI are assembled to form the NDH-1M complex. The basic complex NDH-1M is combined with specific domains to assemble the functional complexes NDH-1L, NDH-1MS, and NDH-1MS′.
The mutant defective in NdhH failed to accumulate the 100-kD NDH-1 assembly intermediate consisting of NdhJ, NdhK, and NdhM. Only free NdhM, NdhJ, and NdhN could be detected in the mutant (Fig. 7D). Similar to the NdhH deleted mutant, the 100-kD assembly intermediate was also undetectable in the NdhJ deleted mutant (Fig. 7E). Nevertheless, the 300- and 130-kD assembly intermediates consisting of NdhI and other unknown proteins were not affected in all the mutant backgrounds (Fig. 7, B–E). These results indicated that NdhH and NdhJ are necessary for the stability of the NDH-1 assembly intermediate of 100 kD, and the assembly process of NdhI was independent of other Ndh subunits (Fig. 8).
DISCUSSION
NdhN, NdhH, and NdhJ Are Required for Stability of NDH-1 Complexes Involved in CET around PSI, Respiratory Electron Transport, and CO2 Uptake
Cyanobacterial NDH-1 complexes have multiple functions because of the diversity of the complexes based on their different subunits composition. Although great progress has been made in revealing the functions of novel subunits of NDH-1, the localization and the function of several subunits still need to be elucidated (Ogawa and Mi, 2007; Battchikova et al., 2011a; Ma and Ogawa, 2015). The NdhN and NdhO subunits exclusively exist in oxygenic photosynthetic organisms (Prommeenate et al., 2004; Rumeau et al., 2005). In higher plants, the chloroplast NDH complex interacts with PSI complex to form a supercomplex. The chloroplast NDH complex is divided into five subcomplexes, consisting of subcomplex A, membrane subcomplex, subcomplex B, lumenal subcomplex, and electron donor binding subcomplex (Ifuku et al., 2011). The hydrophilic subunits corresponding to NdhH-K and NdhM-O, including NdhL, are grouped into subcomplex A. The membrane-spanning subunits corresponding to NdhA-G form the membrane subcomplex. Both the subcomplex A and membrane subcomplex are conserved in cyanobacteria (Peng et al., 2009). Knockout of ndhN or ndhO results in complete loss of NDH activity and entire collapse of subcomplex A of chloroplast NDH complex (Rumeau et al., 2005; Peng et al., 2009, 2012).
Because the fully segregated ndhN null mutant could not be directly obtained in Synechocystis 6803, the function of cyanobacterial NdhN remains elusive. In this work, we deleted the ndhN gene in the ΔndhM strain and then we rescued the ndhM gene at the psbA2 locus. By investigation of the mutant, we found that deletion of NdhN caused the significant decrease of hydrophilic subunits levels (Fig. 4A), resulting in disassembly of NDH-1MS, NDH-1MS′, as well as NDH-1L (Fig. 5C), thus causing the inactivation of NDH-1 (Fig. 1). The severe growth suppression phenotype of NdhN, NdhH, or NdhJ deleted mutants under air condition or in the presence of Glc (Fig. 3A) suggests that NdhN, NdhH, and NdhJ are essential for NDH-1 CET, CO2 uptake, and respiration.
In contrast, the NDH-1 activity was only slightly impaired in the ΔndhO mutant, although it was conflict with the observation in the previous study (Zhao et al., 2014). The deletion of NdhO indeed didn’t affect the growth at pH 8.0 at high CO2 condition (2% CO2 in air), but the growth was suppressed under atmospheric levels of CO2, especially at pH 6.5 (Fig. 3B), where HCO3− was minor (Ohkawa et al., 2000). The result suggests that NdhO functions in CO2 uptake under the condition of inorganic carbon limitation. On the other hand, as shown in Figure 6, NdhO interacts with NdhH and NdhJ. However, Zhao et al. (2014) showed that NdhO interacts with NdhI and NdhK, but not with other Ndh subunits, using the yeast two-hybrid system. Further experiments including 3D structure analysis are necessary to resolve the controversy.
Localization of NdhN, NdhH, and NdhJ in NDH-1 Complexes
A previous study demonstrated that NdhL-NdhO subunits are grouped together in the central part of membrane domain of cyanobacterial NDH-1 complexes (Birungi et al., 2010), while it was suggested that NdhL, NdhM, NdhN, and NdhO are subunits of subcomplex A of chloroplast NDH in Arabidopsis (Peng et al., 2009). In this work, we proposed that cyanobacterial NdhN or NdhO, together with NdhM, are localized in the hydrophilic arm, corresponding to the subcomplex A in Arabidopsis.
In Arabidopsis, various NDH assembly intermediates exist in chloroplast stroma. Three assembly intermediates with approximate apparent molecular masses of 800, 500, and 300 kD contain NdhH in the stroma. The folding of NdhH requires the Cpn60 complex containing Cpn60β4 (Peng et al., 2011). The native NdhH, NdhO, CRR41, and other unknown proteins form the 500-kD assembly intermediate, which provides a scaffold for the assembly of NdhI, NdhJ, NdhK, and NdhM to form the 400-kD assembly intermediate (Peng et al., 2012). However, in the cytoplasm of Synechocystis 6803, NdhH, NdhJ, NdhK, and NdhM form the 100-kD assembly intermediate (Fig. 7). The molecular mass for the sum of four subunits is 107.6 kD, indicating that the 100-kD assembly intermediate does not contain other assembly factors. Interestingly, by constructing a Synechocystis 6803 strain with a His6 tag in the C terminus of NdhJ, Prommeenate et al. (2004) isolated three NdhJ-containing complexes with approximate apparent molecular masses of 460, 330, and 110 kD, implying the fragility of the NDH-1L. Further mass spectrometry analysis demonstrated that the 110-kD subcomplex was composed of NdhH, NdhJ, NdhK, and NdhM (Prommeenate et al., 2004). Our result showed the direct evidence that deletion any of the four subunits causes the disassembly of the 100 kD assembly intermediate (Fig. 7). Our recent studies demonstrated that the formation of 100 kD assembly intermediate was not impaired in the ΔndhI mutant (He et al., 2016). For some unknown reason, Peng et al. (2012) could not detect NdhI in the 2D CN/SDS-PAGE in chloroplast stroma. We showed that NdhI and other unknown proteins form the 300- and 130-kD assembly intermediates in the cytoplasm of Synechocystis 6803, which were not affected in all the mutant backgrounds (Fig. 7), indicating that the formation of these assembly intermediates appeared to be dependent on other assembly factors (Fig. 8), probably Slr1097, homolog of CRR6 in Arabidopsis, involved in the maturation of NdhI (Dai et al., 2013).
In addition, we showed that the cyanobacterial NDH-1 assembly intermediates were not affected in ndhO mutant, while in Arabidopsis, it was shown that NdhO together with NdhH are essential for the formation of the 500- and 400-kD assembly intermediates (Peng et al., 2012). The NdhN was present as free protein, and we could not detect NdhO in the cytoplasm of the wild type, suggesting that NdhN and NdhO are incorporated to the NDH-1 complexes after the formation of the NDH-1 assembly intermediates (Fig. 8). Consistent with chloroplast NDH complex, the cyanobacterial NDH-1 complex assembly intermediates were not affected in the ndhN null mutant.
Recent years have seen steady progress toward the atomic resolution structures of intact complex I from Thermus thermophilus (Baradaran et al., 2013), Bos taurus (Vinothkumar et al., 2014), and Yarrowia lipotytica (Zickermann et al., 2015). The cyanobacterial NDH-1 complex is homologous to the respiratory complex I except the OPS Ndh subunits. Cyanobacterial NdhH, NdhI, NdhJ, and NdhK are related to Nqo4, Nqo9, Nqo5, and Nqo6 in T. thermophilus, or mitochondrial 49-kD, TYKY, 30-kD, and PSST subunits, respectively, which are highly conserved from bacteria to humans. Nqo1 to Nqo3 form the dehydrogenase domain whose homologs do not exist in cyanobacteria, and the subunits Nqo4 to Nqo6 and Nqo9 connect it to the membrane arm. Nqo9 coordinates the Fe-S clusters N6a and N6b, while Nqo6 coordinates cluster N2 at the interface of Nqo4 (Sazanov and Hinchliffe, 2006). The 49-kD subunit, corresponding to NdhH, comprises a prominent four-helix bundle inclined toward the membrane surface, suggesting the binding of the peripheral arm of complex I to the membrane domain (Zickermann et al., 2015). Nqo5, homolog of NdhJ in Synechocystis 6803, wraps around Nqo4 on one side and interacts also with Nqo9. It was suggested that the role of Nqo5 is to stabilize the complex I (Sazanov and Hinchliffe, 2006). So far, the atomic resolution structure of cyanobacterial NDH-1 complex was not available. In our study, we found that NdhN interacts with NdhH, NdhJ, and NdhK, while NdhO interacts with NdhH and NdhJ (Fig. 6). Therefore, structurally, cyanobacterial NdhH-K are conserved with bacterial complex I and the position of NdhM-O is also conserved with chloroplast NDH. We suggest that NdhN is localized adjacent to the hydrophilic subunits and NdhO is localized between NdhH and NdhJ, as shown in the new NDH-1 complexes model (Fig. 8).
In conclusion, this study demonstrates that the cyanobacterial NdhN, NdhH, and NdhJ subunits, localized in the hydrophilic arm of the NDH-1 complexes, are required for stability of the NDH-1 complexes involved in CET around PSI, respiratory electron transport, and CO2 uptake. In addition, NdhO functions in the efficient NDH-1 activity under the condition of inorganic carbon limitation.
MATERIALS AND METHODS
Culture Conditions
The Glc-tolerant strain of the wild-type Synechocystis 6803 and mutants, ΔndhM (He et al., 2016), ΔndhM/psbA2::ndhM, ΔndhN/ΔndhM/psbA2::ndhM, ΔndhH/ΔndhM/psbA2::ndhM, ΔndhJ/ΔndhM/psbA2::ndhM, and ΔndhO were cultured at 30°C in BG-11 medium (Allen, 1968), buffered with Tris-HCl (5 mm, pH 8.0), and bubbled with 5% (v/v) CO2 in air. The solid medium used was BG-11 supplemented with 1.5% agar. Continuous illumination was provided by fluorescence lamps at 60 μmol photons m−2 s−1.
Construction of Mutant Strains
The upstream and downstream regions of ssl1690 (ndhO) were amplified by PCR creating appropriate restriction sites. A DNA fragment encoding a gentamicin resistance (GmR) cassette was also amplified by PCR, creating KpnI and BamHI sites using specific oligonucleotide primers (Supplemental Table S1). These three products were ligated into the MCS of pUC19 (Fig. 1B), which was used to transform the wild-type cells of Synechocystis 6803 as described by Williams and Szalay (1983). The transformants were spread on agar plates containing BG-11 medium and gentamicin (5 μg mL−1) buffered at pH 8.0, the plates were incubated in 2% (v/v) CO2 in air, and continuous illumination was provided by fluorescence lamps at 60 μmol photons m−2 s−1. The mutated ndhO in the transformants was segregated to homogeneity as determined by PCR amplification and immunoblotting. The same strategy was used for constructing the ΔndhN, ΔndhH, and ΔndhJ mutants; nevertheless, direct deletion of ndhN, ndhH, and ndhJ was never successful. Thus, a different strategy was used. First, we used the ΔndhM strain we recently constructed. The ndhN, ndhH, and ndhJ genes in these strains were deleted by replacing parts of the genes with a DNA fragment encoding kanamycin resistance (KmR) cassette, respectively. The generated ΔndhN/ΔndhM, ΔndhH/ΔndhM, and ΔndhJ/ΔndhM mutants were respectively rescued at psbA2 locus by transformation with the pPSBA2 vector inserted with a chloramphenicol resistance (CmR) gene (Lagarde et al., 2000) containing the fragment encoding for NdhM to produce the ΔndhN/ΔndhM/psbA2::ndhM, ΔndhH/ΔndhM/psbA2::ndhM, and ΔndhJ/ΔndhM/psbA2::ndhM mutant strains. The ΔndhM mutant was also transformed with this vector to generate the ΔndhM/psbA2::ndhM strain (Fig. 1A; Supplemental Fig. S1). To complement the ndhO mutant, the ndhO mutant was also rescued at psbA2 locus by transformation with the pPSBA2 vector containing the fragment encoding for NdhO to produce the ΔndhO/psbA2::ndhO strain (Supplemental Fig. S2). The PCR primers used to amplify the flanking sequence regions are listed in Supplemental Table S1. Full segregation of all mutants was confirmed by PCR.
Measurement of Chlorophyll Fluorescence and Redox Kinetics of P700
The transient increase in chlorophyll fluorescence after AL had been turned off was monitored by means of using a PAM Chl fluorometer (Walz), emitter-detector-cuvette assembly (ED-101US), and unit 101ED as previously described (Mi et al., 1995; Deng et al., 2003). After dark acclimation for 30 min, samples were exposed to actinic red light (∼630 nm, 60 μmol photons m−2 s−1) for 60 s, and the kinetics of PSII chlorophyll fluorescence after switching off actinic illumination was recorded as a measure of NDH activity.
The redox kinetics of P700 was measured as previously described (Mi et al., 1995; Deng et al., 2003). The rereduction of P700+ in darkness was measured using the PAM Chl fluorometer, ED-101US, and a unit ED-P700DW-II, by monitoring absorbance changes at 830 nm and using 875 nm as a reference. Cells were kept in the dark for 2 min, and 10 μm 3-(3,4-dichlorophenyl)-1,1dimethylurea (DCMU) was added to the cultures prior to measurement. P700 was oxidized by far-red light (>720 nm, 16 μmol photons m−2 s−1 from an LED lamp for 40 s), and the subsequent rereduction of P700+ in the dark was monitored.
Oxygen Evolution Measurements
The rate of O2 evolution was determined using a Clark type O2 electrode at 30°C as described by Wu et al. (2011). The cells were harvested by centrifugation and resuspended in a fresh growth medium at a chlorophyll concentration of 10 μg/mL. The measurements were performed with actinic white light (400 μmol photons m−2 s−1).
Isolation of Crude Thylakoid Membranes and Soluble Cell Fractions
Thylakoid membranes from Synechocystis 6803 were isolated as described by Gombos et al. (1994) with some modifications as follows. Cell cultures (1 liter) were harvested, resuspended in 5 mL disruption buffer (10 mm HEPES-NaOH, 5 mm sodium phosphate, pH 7.5, 10 mm MgCl2, 10 mm NaCl, and 20% [v/v] glycerol), broken by shaking with glass beads (150–212 μm), and then centrifuged at 5000g for 5 min at 4°C to remove glass beads and unbroken cells. The crude thylakoid membranes were obtained by centrifugation of the supernatant at 20,000g for 30 min at 4°C. The soluble fractions were separated by further centrifugation at 100,000g for 30 min. The thylakoid membranes were suspended in solubilization buffer (20 mm BisTris-HCl, pH 7.0, 10 mm MgCl2, and 20% [v/v] glycerol) at a final chlorophyll concentration of 1 mg mL−1.
Electrophoresis and Immunoblotting
BN-PAGE of the thylakoids membranes from Synechocystis 6803 was performed as described previously (Kügler et al., 1997) with slight modifications as follows. Membranes were washed with 330 mm sorbitol and 50 mm BisTris-HCl, pH 7.0, and solubilized in 25 mm BisTris-HCl, pH 7.0, 10 mm MgCl2, and 20% (v/v) glycerol at a chlorophyll a concentration of 0.5 mg mL−1. After incubation on ice for 40 min with 2% n-dodecyl β-d-maltoside and centrifugation at 20,000g for another 15 min, the supernatants were supplemented with one-tenth volume of BN sample buffer (5% Serva Blue G, 100 mm BisTris-HCl, pH 7.0, 30% [w/v] Suc, 500 mm ε-amino-n-caproic acid, and 10 mm EDTA). Solubilized membranes were then applied to a 0.75-mm-thick 5 to 13% acrylamide gradient gel. Electrophoresis was performed at 4°C by increasing the voltage gradually from 50 to 200 V during the 5-h run. The lanes of the BN gel were cut out and incubated in Laemmli SDS sample buffer containing 5% β-mercaptoethanol for 30 min. SDS-PAGE of the membrane protein was performed on a 12% polyacrylamide gel as described previously (Laemmli, 1970).
CN-PAGE of the soluble cell fractions from Synechocystis 6803 was performed as described previously (Peng et al., 2012) with slight modifications. A total of 50 μg cytoplasmic proteins was mixed with one-quarter volume of sample buffer (40 mm BisTris-HCl, pH 7.0, 0.008% Ponceau S, 200 mm ε-amino-n-caproic acid, and 60% [v/v] glycerol). Cytoplasmic proteins were separated by 5 to 13% acrylamide gradient CN-PAGE in 0.75-mm-thick gels. Electrophoresis was performed at 4°C by increasing the voltage gradually from 50 to 200 V during the 4-h run. The lanes of the CN gel were cut out and incubated in Laemmli SDS sample buffer containing 5% β-mercaptoethanol for 30 min. SDS-PAGE of the proteins was performed on a 12% polyacrylamide gel.
For immunoblotting, the proteins in gel were electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) and detected using protein-specific antibodies with the ECL assay kit (Thermo Scientific) according to the manufacturer’s protocol. Antibodies against the NdhN, NdhO, and NdhJ proteins of Synechocystis 6803 were raised in our laboratory. Primer sequences used to amplify the ndhN, ndhO, and ndhJ genes are listed in Supplemental Table S1. The PCR products were ligated into vector pET28a(+) (Novagen). The plasmids were used to transform Escherichia coli strain BL21 (DE3) pLysS for expression. Polyclonal antibodies were raised in rabbits from purified recombinant proteins. The antibodies against NdhH, NdhI, NdhK, NdhA, CupA, and CupB were previously raised in our laboratory.
Expression and Purification of Fusion Proteins
For testing the direct interactions of NdhN and NdhO with other Ndh subunits, the fragments containing ndhH, ndhI, ndhJ, ndhK, ndhM, ndhS, and ndhO genes were amplified by PCR and cloned into pET28a(+) to form His-tagged fusion protein constructs. The fragments containing ndhN and ndhO were cloned into pGEX-4T-1 to form the GST-tagged fusion constructs. Primer sequences used are listed in Supplemental Table S1. These constructs were transformed into E. coli strain BL21 (DE3) pLysS and induced by 1 mm isopropyl-β-d-thiogalactoside for 16 h at 16°C. These fusion proteins were purified using a nickel column (GE Healthcare) and glutathione Sepharose 4B (GE Healthcare) column according to the manufacturer’s instructions.
GST Pull-Down Assay
GST-NdhN, GST-NdhO, or GST and His-tagged fusion proteins were incubated with 20 μL glutathione Sepharose 4B rein for 2 h at 4°C in a buffer containing 20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 0.1 mm EDTA, 0.2% (v/v) Triton, and 10% (v/v) glycerol. The protein-bound resin was washed five times with a buffer containing 20 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.1 mm EDTA, and 0.5% (v/v) Nonidet P-40. The washed proteins were directly eluted with Laemmli buffer at 95°C for 5 min. The input and eluates were analyzed by immunoblotting and Coomassie staining.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Sll1262 (BAA18145.1), Slr0261 (BAA17939.1), Slr1281 (BAA18285), and Ssl1690 (BAA10471.1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Construction and verification of the ndhH-deletion and ndhJ-deletion mutants.
Supplemental Figure S2. Construction and verification of the complemented ndhO strain.
Supplemental Figure S3. Growth of the wild type, ndhO deletion mutant, and complemented ndhO strains in air.
Supplemental Table S1. Primer list and sequences.
Supplementary Material
Glossary
- CET
cyclic electron transport
- OPS
oxygenic photosynthesis specific
- AL
actinic light
- CN-PAGE
Clear native-PAGE
- DCMU
3-(3,4-dichlorophenyl)-1,1dimethylurea
- BN-PAGE
Blue native-PAGE
Footnotes
Articles can be viewed without a subscription.
References
- Allen MM. (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4: 1–4 [DOI] [PubMed] [Google Scholar]
- Arteni AA, Zhang P, Battchikova N, Ogawa T, Aro E-M, Boekema EJ (2006) Structural characterization of NDH-1 complexes of Thermosynechococcus elongatus by single particle electron microscopy. Biochim Biophys Acta 1757: 1469–1475 [DOI] [PubMed] [Google Scholar]
- Baradaran R, Berrisford JM, Minhas GS, Sazanov LA (2013) Crystal structure of the entire respiratory complex I. Nature 494: 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battchikova N, Eisenhut M, Aro E-M (2011a) Cyanobacterial NDH-1 complexes: novel insights and remaining puzzles. Biochim Biophys Acta 1807: 935–944 [DOI] [PubMed] [Google Scholar]
- Battchikova N, Wei L, Du L, Bersanini L, Aro E-M, Ma W (2011b) Identification of novel Ssl0352 protein (NdhS), essential for efficient operation of cyclic electron transport around photosystem I, in NADPH:plastoquinone oxidoreductase (NDH-1) complexes of Synechocystis sp. PCC 6803. J Biol Chem 286: 36992–37001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battchikova N, Zhang P, Rudd S, Ogawa T, Aro E-M (2005) Identification of NdhL and Ssl1690 (NdhO) in NDH-1L and NDH-1M complexes of Synechocystis sp. PCC 6803. J Biol Chem 280: 2587–2595 [DOI] [PubMed] [Google Scholar]
- Berger S, Ellersiek U, Steinmüller K (1991) Cyanobacteria contain a mitochondrial complex I-homologous NADH-dehydrogenase. FEBS Lett 286: 129–132 [DOI] [PubMed] [Google Scholar]
- Birungi M, Folea M, Battchikova N, Xu M, Mi H, Ogawa T, Aro E-M, Boekema EJ (2010) Possibilities of subunit localization with fluorescent protein tags and electron microscopy examplified by a cyanobacterial NDH-1 study. Biochim Biophys Acta 1797: 1681–1686 [DOI] [PubMed] [Google Scholar]
- Dai H, Zhang L, Zhang J, Mi H, Ogawa T, Ma W (2013) Identification of a cyanobacterial CRR6 protein, Slr1097, required for efficient assembly of NDH-1 complexes in Synechocystis sp. PCC 6803. Plant J 75: 858–866 [DOI] [PubMed] [Google Scholar]
- Deng Y, Ye J, Mi H (2003) Effects of low CO2 on NAD(P)H dehydrogenase, a mediator of cyclic electron transport around photosystem I in the cyanobacterium synechocystis PCC6803. Plant Cell Physiol 44: 534–540 [DOI] [PubMed] [Google Scholar]
- Dworsky A, Mayer B, Regelsberger G, Fromwald S, Peschek GA (1995) Functional and immunological characterization of both “mitochondria-like” and “chloroplast-like” electron/proton transport proteins in isolated and purified cyanobacterial membranes. Bioelectrochem Bioenerg 38: 35–43 [Google Scholar]
- Folea IM, Zhang P, Nowaczyk MM, Ogawa T, Aro E-M, Boekema EJ (2008) Single particle analysis of thylakoid proteins from Thermosynechococcus elongatus and Synechocystis 6803: localization of the CupA subunit of NDH-1. FEBS Lett 582: 249–254 [DOI] [PubMed] [Google Scholar]
- Friedrich T, Scheide D (2000) The respiratory complex I of bacteria, archaea and eukarya and its module common with membrane-bound multisubunit hydrogenases. FEBS Lett 479: 1–5 [DOI] [PubMed] [Google Scholar]
- Friedrich T, Steinmüller K, Weiss H (1995) The proton-pumping respiratory complex I of bacteria and mitochondria and its homologue in chloroplasts. FEBS Lett 367: 107–111 [DOI] [PubMed] [Google Scholar]
- Gao F, Zhao J, Wang X, Qin S, Wei L, Ma W (2016) NdhV is a subunit of NADPH dehydrogenase essential for cyclic electron transport in Synechocystis sp. strain PCC 6803. Plant Physiol 170: 752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos Z, Wada H, Murata N (1994) The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc Natl Acad Sci USA 91: 8787–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Xu M, Wu Y, Lv J, Fu P, Mi H (2016) NdhM subunit is required for the stability and the function of NAD(P)H dehydrogenase complexes involved in CO2 uptake in Synechocystis sp. strain PCC 6803. J Biol Chem 291: 5902–5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zheng F, Wu Y, Li Q, Lv J, Fu P, Mi H (2015) NDH-1L interacts with ferredoxin via the subunit NdhS in Thermosynechococcus elongatus. Photosynth Res 126: 341–349 [DOI] [PubMed] [Google Scholar]
- Herranen M, Battchikova N, Zhang P, Graf A, Sirpiö S, Paakkarinen V, Aro E-M (2004) Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803. Plant Physiol 134: 470–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Endo T, Shikanai T, Aro E-M (2011) Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant Cell Physiol 52: 1560–1568 [DOI] [PubMed] [Google Scholar]
- Klughammer B, Sültemeyer D, Badger MR, Price GD (1999) The involvement of NAD(P)H dehydrogenase subunits, NdhD3 and NdhF3, in high-affinity CO2 uptake in Synechococcus sp. PCC7002 gives evidence for multiple NDH-1 complexes with specific roles in cyanobacteria. Mol Microbiol 32: 1305–1315 [DOI] [PubMed] [Google Scholar]
- Kubota H, Sakurai I, Katayama K, Mizusawa N, Ohashi S, Kobayashi M, Zhang P, Aro E-M, Wada H (2010) Purification and characterization of photosystem I complex from Synechocystis sp. PCC 6803 by expressing histidine-tagged subunits. Biochim Biophys Acta 1797: 98–105 [DOI] [PubMed] [Google Scholar]
- Kügler M, Jänsch L, Kruft V, Schmitz U, Braun HP (1997) Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth Res 53: 35–44 [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lagarde D, Beuf L, Vermaas W (2000) Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl Environ Microbiol 66: 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Ogawa T (2015) Oxygenic photosynthesis-specific subunits of cyanobacterial NADPH dehydrogenases. IUBMB Life 67: 3–8 [DOI] [PubMed] [Google Scholar]
- Maeda S, Badger MR, Price GD (2002) Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol Microbiol 43: 425–435 [DOI] [PubMed] [Google Scholar]
- Mi H, Endo T, Ogawa T, Asada K (1995) Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 36: 661–668 [Google Scholar]
- Mi H, Endo T, Schreiber U, Ogawa T, Asada K (1992) Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol 33: 1233–1237 [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582 [DOI] [PubMed] [Google Scholar]
- Nowaczyk MM, Wulfhorst H, Ryan CM, Souda P, Zhang H, Cramer WA, Whitelegge JP (2011) NdhP and NdhQ: two novel small subunits of the cyanobacterial NDH-1 complex. Biochemistry 50: 1121–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. (1991) A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. Proc Natl Acad Sci USA 88: 4275–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Mi H (2007) Cyanobacterial NADPH dehydrogenase complexes. Photosynth Res 93: 69–77 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Pakrasi HB, Ogawa T (2000) Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J Biol Chem 275: 31630–31634 [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53: 523–550 [DOI] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Fujiwara M, Shikanai T (2012) Multistep assembly of chloroplast NADH dehydrogenase-like subcomplex A requires several nucleus-encoded proteins, including CRR41 and CRR42, in Arabidopsis. Plant Cell 24: 202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Fujiwara M, Takami T, Shikanai T (2009) Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell 21: 3623–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Myouga F, Motohashi R, Shinozaki K, Shikanai T (2011) A chaperonin subunit with unique structures is essential for folding of a specific substrate. PLoS Biol 9: e1001040. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pieulle L, Guedeney G, Cassier-Chauvat C, Jeanjean R, Chauvat F, Peltier G (2000) The gene encoding the NdhH subunit of type 1 NAD(P)H dehydrogenase is essential to survival of Synechocystis PCC6803. FEBS Lett 487: 272–276 [DOI] [PubMed] [Google Scholar]
- Prommeenate P, Lennon AM, Markert C, Hippler M, Nixon PJ (2004) Subunit composition of NDH-1 complexes of Synechocystis sp. PCC 6803: identification of two new ndh gene products with nuclear-encoded homologues in the chloroplast Ndh complex. J Biol Chem 279: 28165–28173 [DOI] [PubMed] [Google Scholar]
- Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G (2005) New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 17: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazanov LA, Hinchliffe P (2006) Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311: 1430–1436 [DOI] [PubMed] [Google Scholar]
- Schwarz D, Schubert H, Georg J, Hess WR, Hagemann M (2013) The gene sml0013 of Synechocystis species strain PCC 6803 encodes for a novel subunit of the NAD(P)H oxidoreductase or complex I that is ubiquitously distributed among cyanobacteria. Plant Physiol 163: 1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Kaneko T, Fukuzawa H, Tabata S, Kaplan A, Ogawa T (2001) Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci USA 98: 11789–11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Peng L, Myouga F, Motohashi R, Shinozaki K, Shikanai T (2008) CRR23/NdhL is a subunit of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol 49: 835–842 [DOI] [PubMed] [Google Scholar]
- Vinothkumar KR, Zhu J, Hirst J (2014) Architecture of mammalian respiratory complex I. Nature 515: 80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JGK, Szalay AA (1983) Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene 24: 37–51 [DOI] [PubMed] [Google Scholar]
- Wu Y, Zheng F, Ma W, Han Z, Gu Q, Shen Y, Mi H (2011) Regulation of NAD(P)H dehydrogenase-dependent cyclic electron transport around PSI by NaHSO₃ at low concentrations in tobacco chloroplasts. Plant Cell Physiol 52: 1734–1743 [DOI] [PubMed] [Google Scholar]
- Wulfhorst H, Franken LE, Wessinghage T, Boekema EJ, Nowaczyk MM (2014) The 5 kDa protein NdhP is essential for stable NDH-1L assembly in Thermosynechococcus elongatus. PLoS One 9: e103584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Ogawa T, Pakrasi HB, Mi H (2008) Identification and localization of the CupB protein involved in constitutive CO2 uptake in the cyanobacterium, Synechocystis sp. strain PCC 6803. Plant Cell Physiol 49: 994–997 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Peng L, Fukao Y, Shikanai T (2011) An Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 23: 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T (2013) In planta mutagenesis of Src homology 3 domain-like fold of NdhS, a ferredoxin-binding subunit of the chloroplast NADH dehydrogenase-like complex in Arabidopsis: a conserved Arg-193 plays a critical role in ferredoxin binding. J Biol Chem 288: 36328–36337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gao F, Zhao J, Ogawa T, Wang Q, Ma W (2014) NdhP is an exclusive subunit of large complex of NADPH dehydrogenase essential to stabilize the complex in Synechocystis sp. strain PCC 6803. J Biol Chem 289: 18770–18781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Battchikova N, Jansen T, Appel J, Ogawa T, Aro E-M (2004) Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell 16: 3326–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Battchikova N, Paakkarinen V, Katoh H, Iwai M, Ikeuchi M, Pakrasi HB, Ogawa T, Aro E-M (2005) Isolation, subunit composition and interaction of the NDH-1 complexes from Thermosynechococcus elongatus BP-1. Biochem J 390: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Gao F, Zhang J, Ogawa T, Ma W (2014) NdhO, a subunit of NADPH dehydrogenase, destabilizes medium size complex of the enzyme in Synechocystis sp. strain PCC 6803. J Biol Chem 289: 26669–26676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Rong W, Gao F, Ogawa T, Ma W (2015) Subunit Q is required to stabilize the large complex of NADPH dehydrogenase in Synechocystis sp. strain PCC 6803. Plant Physiol 168: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickermann V, Wirth C, Nasiri H, Siegmund K, Schwalbe H, Hunte C, Brandt U (2015) Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science 347: 44–49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.