Significance
Ion channels regulate ion flow by opening and closing their pore gates. K+ channels commonly possess two pore gates, one at the intracellular end for fast channel activation/deactivation and the other at the selectivity filter for slow C-type inactivation/recovery. The large-conductance calcium-activated potassium (BK) channel lacks a classic intracellular bundle-crossing activation gate and normally show no C-type inactivation. We found conditions to induce BK channels into a C-inactivated state and observed a unique closed state-coupled C-type inactivation, which is contrary to the commonly observed open state-coupled C-type inactivation in other K+ channels. We propose that the activation gate of BK channels may exist at or near the selectivity filter and that the channel’s normal closing may represent an early conformational stage of C-type inactivation.
Keywords: BK channel, maxi K channel, potassium channel, C-type inactivation, pore gate
Abstract
Ion channels regulate ion flow by opening and closing their pore gates. K+ channels commonly possess two pore gates, one at the intracellular end for fast channel activation/deactivation and the other at the selectivity filter for slow C-type inactivation/recovery. The large-conductance calcium-activated potassium (BK) channel lacks a classic intracellular bundle-crossing activation gate and normally show no C-type inactivation. We hypothesized that the BK channel’s activation gate may spatially overlap or coexist with the C-type inactivation gate at or near the selectivity filter. We induced C-type inactivation in BK channels and studied the relationship between activation/deactivation and C-type inactivation/recovery. We observed prominent slow C-type inactivation/recovery in BK channels by an extreme low concentration of extracellular K+ together with a Y294E/K/Q/S or Y279F mutation whose equivalent in Shaker channels (T449E/K/D/Q/S or W434F) caused a greatly accelerated rate of C-type inactivation or constitutive C-inactivation. C-type inactivation in most K+ channels occurs upon sustained membrane depolarization or channel opening and then recovers during hyperpolarized membrane potentials or channel closure. However, we found that the BK channel C-type inactivation occurred during hyperpolarized membrane potentials or with decreased intracellular calcium ([Ca2+]i) and recovered with depolarized membrane potentials or elevated [Ca2+]i. Constitutively open mutation prevented BK channels from C-type inactivation. We concluded that BK channel C-type inactivation is closed state-dependent and that its extents and rates inversely correlate with channel-open probability. Because C-type inactivation can involve multiple conformational changes at the selectivity filter, we propose that the BK channel’s normal closing may represent an early conformational stage of C-type inactivation.
Ion channels regulate ion flow across membranes through the voltage- or ligand-regulated opening and closing of a conformational constriction site (gate) at the central pore. For most K+ channels, the pore gate located near the pore’s intracellular end, called a “bundle-crossing” region, controls fast channel activation/deactivation (1–4) whereas the existence of another pore gate at the selectivity filter controls slow C-type inactivation and recovery (1, 5–8). C-type inactivation in the widely studied voltage-gated Drosophila melanogaster Shaker and mammalian Kv channels or the prokaryotic pH-gated KcsA channels is known to occur from the open state as a result of sustained membrane depolarization or acidic intracellular pH. Extensive structural studies have indicated that C-type inactivation results from changes in ion occupancy and structural rearrangements at or near the selectivity filter (1, 5–8). C-type inactivation is distinct from the fast N-type inactivation, which involves pore blockade by a positively charged N-terminal ball peptide (9, 10).
The large-conductance, calcium-activated potassium (BK) channel is activated by both membrane depolarization and ligand binding (intracellular calcium) (11–13). Similar to ligand-gated, cyclic nucleotide-gated (CNG) channels (14–17), the BK channel’s pore has been shown to be accessible to large intracellular blockers, even when the channel was in a closed state (18–20), indicating the absence of the classic intracellular bundle-crossing gate observed in most other ion channels. The observation that permeant ions, such as thallium, can affect BK channel activation further led to the notion that the BK channel’s activation gate may exist near or at the selectivity filter (21). A direct determination of the state dependence of ion accessibility at the selectivity filter using small cysteine modification reagents like cadmium and silver, as previously done in CNG channels (22), has been hampered by the complexity of BK channels, which have numerous endogenous cysteine residues whose modifications affect channel functions (23, 24). Thus, it is important to explore alternative approaches to localizing the activation gate in BK channels. An investigation of the relationship between activation/deactivation and C-type inactivation/recovery would be helpful in evaluating the relationship between their respective physical gates. However, WT BK channels don’t show an obvious sign of inactivation, even upon persistent membrane depolarization. The BK channel’s homotetrameric core subunit, BKα, is structurally overall vastly different from most other voltage-gated K+ channels. Nevertheless, the signature K+ selectivity filter motif TVGYGD is conserved in BK channels, and a certain low level of sequence similarity with other K+ channels exists in the extracellular mouth (turret), pore helix, and S6 transmembrane domain (Fig. 1A). It is reasonable to assume that the BK channel pore, particularly within and near the selectivity filter region, may share a similar structure with other K+ channels and thus is essentially capable of undergoing C-type inactivation. It was well established that C-type inactivation in Shaker channels can be hastened drastically by single-point mutations at position Thr449 or Trp434 or by a decrease in external K+ concentration (25–29). To explore the hypothesis that the BK channel’s activation gate may exist near or at the selectivity filter, we tested ways to induce C-type inactivation of BK channels by mutation and by a decrease in the external K+ concentration and studied the relationship between activation/deactivation and C-type inactivation/recovery.
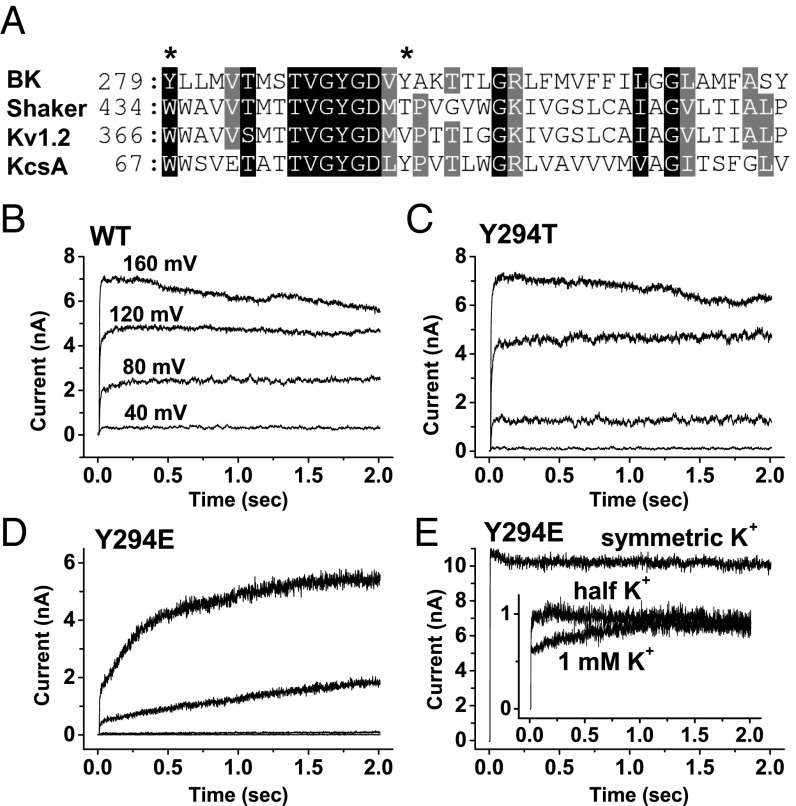
Fig. 1.
Effects of external K+ and mutations at the Y294 position on BK channel slow activation (recovery from inactivated state). (A) Sequence alignment of the pore regions of the BK channel and other channels. The Y294 and Y279 positions are indicated by asterisks. (B–E) Elicited BK channel K+ currents in the absence of external K+ for WT (B), Y294T (C), and Y294E (D) channels or in the presence of the indicated amounts of external K+ for Y294E (E) channels during a long (2-s) membrane depolarization period from a holding potential of 0 mV to 40, 80, 120, and 160 mV. The excised inside-out patches were recorded in bath solution without added Ca2+ and chelator. The external K+ was fully replaced by NMDG+, except in E for which either no NMDG+ was used or the residual amount of K+ (Inset) was indicated.
Results
Unusual Slow-Rising Currents Produced by Y294E Mutation and Depletion of External K+.
WT BK channels showed no indication of inactivation at different membrane voltages, even in the virtual absence of external K+ by substitution with the large N-methyl-d-glucamine (NMDG+) cation (Fig. 1B). To induce a detectable C-type inactivation, we mutated the Tyr residue at position 294 to Thr (Y294T), which mimics WT Shaker channels at this position. We found that Y294T and other mutations mentioned later at the Y294 position were normal in voltage and Ca2+ dependence of BK channel activation under symmetric K+ recording conditions. The Y294T mutant failed to produce a different channel from the WT channel, showing no inactivation in the virtual absence of external K+ upon membrane depolarization (Fig. 1C). However, the Y294E mutation, whose equivalents in Shaker channels greatly enhanced the inactivation rate (25), surprisingly slowed channel currents drastically instead of resulting in inactivation (Fig. 1D). Such slow-rising currents in the Y294E mutant were more than two orders of magnitude slower than those observed in the WT channels [e.g., ∼500 ms vs. ∼2 ms to reach half-maximum current at 160 mV (Fig. 1 B and D)] and were apparently voltage-dependent, being faster at a higher voltage of membrane depolarization (Fig. 1D).
It is noteworthy that a small amplitude (∼25% of the total current amplitude) of normal fast activation (τ = ∼2 ms), which was kinetically similar to WT activation (Fig. 1B), was also observed in the Y294E mutant (Fig. 1D) under the examined conditions (membrane depolarization from a holding potential of 0 mV at an estimated concentration of [Ca2+]i of <10 µM). This finding indicates the presence of two distinct closed states before membrane depolarization-induced channel opening. The slow-rising current was fully absent under the symmetric K+ or external half K+/half NMDG+ condition (Fig. 1E). The presence of as low as 1 mM external K+ significantly decreased the amplitude of the slow-rising current from ∼75% to ∼30% of the total current amplitude, suggesting a high sensitivity to external K+ concentration, which is a characteristic feature of C-type inactivation. We speculated that the observed slow-rising currents might be a consequence of the channel’s slow recovery from a preexisting C-type inactivated state whose voltage or state dependence is the opposite of what is known for other K+ channels that require membrane hyperpolarization or channel closure for recovery.
The Closed-State Dependence of Inactivation.
To probe the nonconductive inactivated state, we first depolarized the membranes at 180 mV for 2 s to allow the channels to fully recover from any preexisting inactivated state; then, we held the excised membrane patches at a certain voltage to allow the channel to inactivate. The time course of inactivation was determined by a brief (5-ms) membrane depolarization to 180 mV every 2 s to detect the available channels for activation (Fig. 2A). We observed that the amplitude of the activation current of the Y294E mutant was decreased by more than 80% in 10 s at a holding voltage of 0 mV in the virtual absence of [Ca2+]i, suggesting that most channels slowly (τ = ∼3 s) underwent inactivation (Fig. 2A). The decrease in amplitude of the available activation currents during inactivation can be roughly fitted by a single exponential function. At an elevated [Ca2+]i concentration of 85 μM, however, only a small decrease (∼10%) in the amplitude of the activation current was observed at the same holding voltage of 0 mV (Fig. 2B). Shifting the holding voltage to −40 mV and −80 mV accelerated the rate of decease in the activation current amplitude and also caused more channels to be unavailable for activation within 30 s (Fig. 2B). No significant change was observed upon a further negative shift in the holding voltage to −120 mV (Fig. 2B). These results suggest that channel inactivation is likely a closed state-dependent process that is sensitive to both membrane voltage and intracellular calcium concentration.
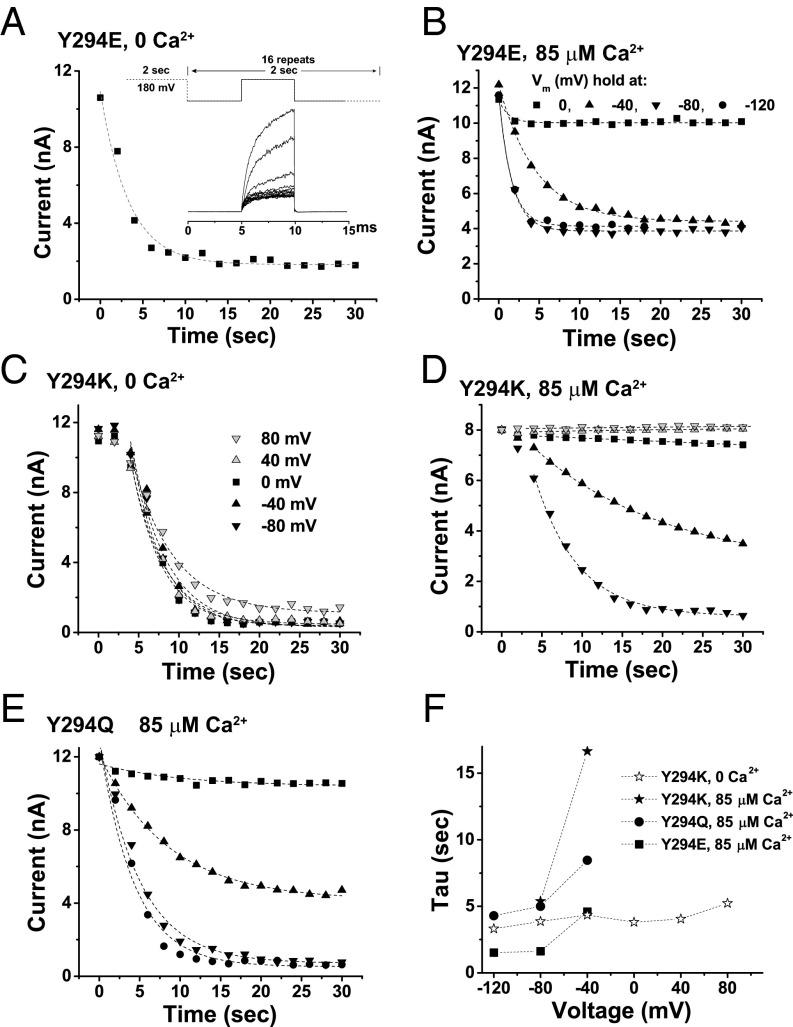
Fig. 2.
Effects of voltage and calcium on inactivation in mutant BK channels. (A) Time course of inactivation in Y294E mutant channels at 0 mV in the virtual absence of [Ca2+]i and external K+ (replaced by NMDG+). The channels were activated by a 2-second depolarization to 180 mV, and then the inactivation was induced by holding the membrane at a certain voltage. A brief (5-ms) membrane depolarization to 180 mV every 2 seconds was used to determine residual available channels for quick activation. (B–E) Time course of inactivation in the virtual absence of external K+ (replaced by NMDG+) at different holding membrane voltages either in the presence of 85 μM [Ca2+]i for the Y294E (B), Y294K (D), and Y294Q (E) mutant channels or in the virtual absence of [Ca2+]i for the Y294K (C) mutant channel. (F) Plot of the inactivation rates against holding membrane voltages for different Y294 mutant channels at the indicated [Ca2+]i concentrations.
Consistent with the above observation of fast and slow currents resulting from the channel’s fast activation and slow recovery (Fig. 1D), a significant proportion of the Y294E-mutant channels remained able to be activated, even at −120 mV in the presence of 85 µM [Ca2+]i (Fig. 2B). The proportion of Y294E channels that did not become inactivated was estimated to be ∼1/3 for the condition of −120 mV in the presence of 85 µM [Ca2+]i and ∼1/5 at 0 mV in the virtual absence of [Ca2+]i, as calculated from the relative amplitudes of the remaining currents. To investigate the state dependence of inactivation in more detail, we included two more Y294 mutants for analysis (the Y294K and Y294Q mutants), whose equivalents in Shaker channels greatly facilitated C-type inactivation as well (25, 27). Different from the Y294E mutant, these two C-type inactivation mutants showed nearly full inactivation upon enough membrane hyperpolarization for channel closure: e.g., −80 mV at 85 µM [Ca2+]i (Fig. 2 C–E). Similar to Y294E, both Y294K and Y294Q showed increases in inactivation rate and amplitude upon more membrane hyperpolarization from 0 to −80 mV at 85 µM [Ca2+]i (Fig. 2 D–F), but no significant difference was observed between −80 mV and −120 mV (Y294Q) (Fig. 2 E and F). In the virtual absence of [Ca2+]i, no difference in the rate and amplitude of inactivation was seen in the Y294K mutant from 40 to −120 mV (Fig. 2 C and F). There was an initial delay in inactivation for the Y294K mutant that was not observed in the other mutants.
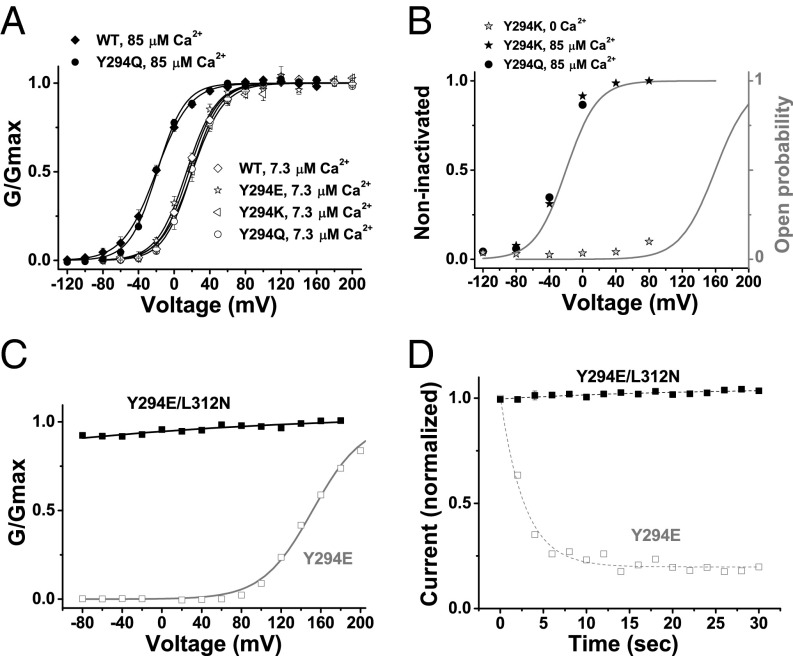
Under symmetric K+ conditions, the Y294 mutant channels did not differ significantly from the WT channels in the voltage dependence of channel activation, as measured at 85  M [Ca2+]i for mutants Y294E, Y294K, and Y294Q and at 7.3
M [Ca2+]i for mutants Y294E, Y294K, and Y294Q and at 7.3  M [Ca2+]i for Y294Q (Fig. 3A). A plot of the availability of channels for activation (noninactivated, steady state) at different holding voltages largely overlaps with the curve of the predicted open probability at different membrane voltages for Y294K at 0 µM [Ca2+]i and for Y294Q and Y294K at 85 µM [Ca2+]i (Fig. 3B). Therefore, we concluded that the inactivation was a closed state-dependent process. From this conclusion, it is inferred that there will be no inactivation and recovery if the channels are constitutively open. We reported recently that substitution of a deep pore Leu residue at position 312 with Asn (L312N) caused BK channels to be constitutively open, which rendered them highly conductive at even negative voltages and insensitive to voltage and calcium (30). Therefore, we tested the double mutant Y294E/L312N and found it to be similarly constitutively open (Fig. 3C). We observed that the Y294E/L312N double-mutant channels showed no inactivation at a holding voltage of −80 mV in the virtual absence of [Ca2+]i, a condition under which the Y294E single mutant can be maximally inactivated (Fig. 3D).
M [Ca2+]i for Y294Q (Fig. 3A). A plot of the availability of channels for activation (noninactivated, steady state) at different holding voltages largely overlaps with the curve of the predicted open probability at different membrane voltages for Y294K at 0 µM [Ca2+]i and for Y294Q and Y294K at 85 µM [Ca2+]i (Fig. 3B). Therefore, we concluded that the inactivation was a closed state-dependent process. From this conclusion, it is inferred that there will be no inactivation and recovery if the channels are constitutively open. We reported recently that substitution of a deep pore Leu residue at position 312 with Asn (L312N) caused BK channels to be constitutively open, which rendered them highly conductive at even negative voltages and insensitive to voltage and calcium (30). Therefore, we tested the double mutant Y294E/L312N and found it to be similarly constitutively open (Fig. 3C). We observed that the Y294E/L312N double-mutant channels showed no inactivation at a holding voltage of −80 mV in the virtual absence of [Ca2+]i, a condition under which the Y294E single mutant can be maximally inactivated (Fig. 3D).
Fig. 3.
Closed-state dependence of inactivation in mutant BK channels. (A) Voltage dependence of BK channel activation for WT and Y294 mutants at 7.3 or 85 μM [Ca2+]i under symmetric K+ recording conditions. The gating parameters V1/2 and z obtained from single Boltzmann function fit at 7.3 μM Ca2+ are 17 ± 2 mV and 1.64 ± 0.07 e (n = 4) for WT, 14 ± 2 mV and 1.56 ± 0.15 e (n = 4) for Y294E, 20 ± 3 mV and 1.54 ± 0.14 e (n = 6) for Y294K, and 20 ± 3 mV and 1.70 ± 0.09 e (n = 6) for Y294Q. The V1/2 and z at 85 μM are −20 ± 2 mV and 1.43 ± 0.19 e (n = 4) for WT and 19 ± 1 mV and 1.66 ± 0.09 e (n = 4) for Y294Q. Error bars represent ±SEM. (B) Relationship between holding membrane voltages and availability of channels for quick activation (noninactivated channels) in Y294Q- and Y294K-mutant channels in the absence and presence of [Ca2+]i. Voltage dependence of channel open probability at 0 and 85 μM [Ca2+]i is included to show state dependence of inactivation. The gray curves are estimated voltage dependence of the channel’s open probabilities at 0 and 85 μM Ca2+ based on those measured at the symmetric K+ recording condition. (C) Relationship between normalized conductance (G/Gmax) and voltages for Y294E (gray) single-mutant and Y294E/L312N double-mutant (black) channels in the virtual absence of [Ca2+]i. (D) Time courses of available currents upon brief membrane depolarization in Y294E-mutant (gray) and Y294E/L312N-mutant (black) channels. The excised patches were held at −80 mV in the virtual absence of [Ca2+]i.
The Voltage Dependence of Recovery from Inactivation.
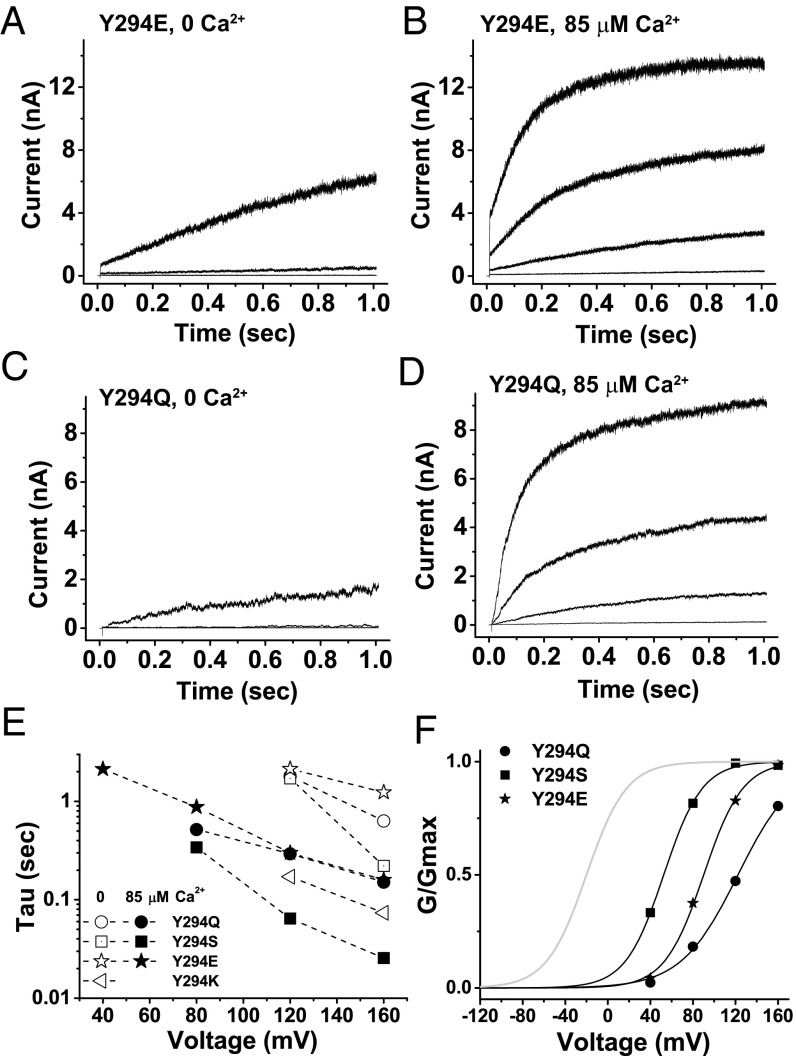
Recovery from the inactivated state in Y294-mutant channels was also dependent on membrane voltages and intracellular calcium: the higher the voltage or the level of calcium, the faster the recovery. As shown in Fig. 4 A and C, in the virtual absence of [Ca2+]i, only membrane depolarization to a high voltage, such as 160 mV, showed significant recovery in the Y294E and Y294Q mutants whereas 120 mV produced little current. The presence of 85 µM [Ca2+]i greatly accelerated the recovery rates of the Y294E- and Y294Q-mutant channels at the four tested membrane voltages (40, 80, 120, and 160 mV) (Fig. 4 B and D). In the virtual absence of external K+, similar slow activation (recovery from inactivation) was also observed in mutants Y294S and Y294K in a voltage- or calcium-dependent manner. The recovery (the slow increase in current) can be roughly fitted by a single exponential function, and the calculated τ values were plotted against membrane voltages at 0 and 85 µM [Ca2+]i (Fig. 4E). The rate of recovery from the inactivated state in these mutants gradually increased as the membrane voltage increased and was about 10 times higher at 85 μM [Ca2+]i than at 0 μM [Ca2+]i at the same voltages, which indicated a process coupled to channel opening that is sensitive to both voltage and [Ca2+]i. Under the same voltage and [Ca2+]i conditions, the rates of recovery in these mutant channels were in the order Y294K > Y294S > Y294Q > Y294E (Fig. 4E), which inversely correlated with the order of inactivation rates for the Y294E, Y294Q, and Y294K mutants (Fig. 2F). The recovery rates notably continued to increase as the tested voltages increased (40–160 mV) at 85 µM [Ca2+]i, suggesting a significant rightward (depolarizing direction) shift in the voltage dependence of channel recovery compared with normal channel activation/deactivation without inactivation. This distinct voltage dependence of channel recovery was also obvious from a plot of the voltage dependence of G/Gmax in the recovered channels at the end of a 1-s membrane depolarization at 85 µM [Ca2+]i, which was estimated to be shifted toward depolarization direction by 70–140 mV compared with the voltage dependence of channel inactivation or normal activation (Fig. 4F).
Fig. 4.
Effects of voltage and calcium concentration on the recovery from inactivation in Y294-mutant channels. Elicited K+ currents of Y294E (A and B) and Y294Q (C and D) channels at 0 and 85 μM [Ca2+]i in the absence of external K+ upon a 1-s membrane depolarization from a holding potential of −80 mV to 40, 80, 120, and 160 mV. (E) Calculated rates (Tau) of the observed slow recovery processes at different depolarization voltages for Y294Q, -S, -E, and -K mutants at 0 and 85 μM [Ca2+]i by single exponential fits. (F) Relationship between normalized conductance (G/Gmax) and voltages for Y294-mutant channel currents recovered from the inactivated state at the end of 1-s membrane depolarization in the presence of 85 μM [Ca2+]i.
Y279F Mutation-Induced Inactivation.
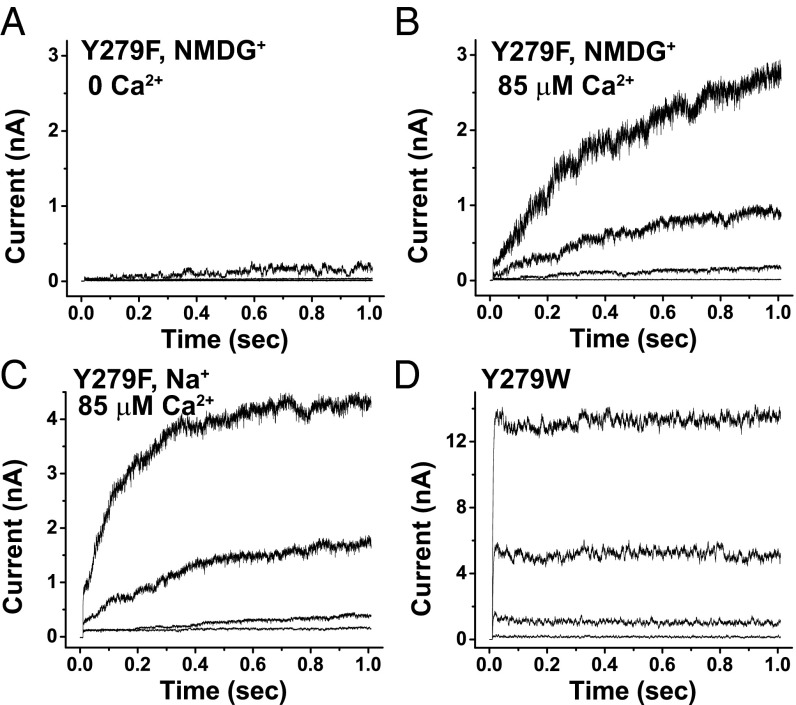
In Shaker channels, the W434F mutation can lead to constitutive C-type inactivation (28). In BK channels, the equivalent position is Y279. We observed slow recovery of Y279F mutant channels from the inactivated state; the channels were barely open at 160 mV over a 1-s period in the absence of both [Ca2+]i and external K+ (which was substituted with NMDG+) (Fig. 5A). Similar to other inactivation mutants, the Y279F mutant recovered from inactivation at a drastically faster rate in the presence of 85 μM [Ca2+]i (Fig. 5B). Replacement of external K+ with nonpermeable Na+ also produced slow-rising recovery currents in the Y279F channels although the rates seemed to be faster than with NMDG+ (Fig. 5C). As expected, Y279W channels, which mimic Shaker WT channels, showed no inactivation and produced no slow-rising recovery current (Fig. 5D).
Fig. 5.
Effects of external cations and mutations at the Y279 position on BK channel slow activation (recovery from inactivated state). (A and B) Elicited K+ currents of Y279F mutant channels at 0 and 85 μM [Ca2+]i in the absence of external K+ (replaced by NMDG+) upon 1-s membrane depolarization from a holding potential of −80 mV to 40, 80, 120, and 160 mV. (C) Similar to B, except Na+ was used to replace K+. (D) Elicited K+ currents of Y279W-mutant channels at the same conditions as in A.
Discussion
We found ways to induce C-type inactivation and identified an uncommon closed-state dependence of C-type inactivation in BK channels, which is different from what has been reported in most other K+ channels. Our findings establish a relationship between activation and C-type inactivation in BK channels and provide a strategy to indirectly probe the structural location of the activation gate in BK channels.
C-type, sometimes also called P/C-type, inactivation has been mostly studied in Shaker channels, Kv channels, and the relatively simple bacterial KcsA channels. BK channels are noninactivating K+ channels, except in the presence of a β2 or β3 auxiliary subunit whose N-terminal peptide sequence occludes the channel pore in a more N-type inactivation manner (31–33). We found that, even under nearly K+-free conditions in the external recording solution, WT BK channels did not show any significant inactivation up to 10 s. The presence of Tyr at the BK channel 294 position (equivalent to the 449 position in Shaker channels) might partly contribute to the noninactivation because the T449Y mutation slows down C-type inactivation in Shaker channels (25). We observed that the Y294T mutation was not sufficient to induce C-type inactivation, even in the virtual absence of external K+. Inclusion of K+ chelator in the external solution was reported to cause the BK channel to be in a nonreversible, nonconducting or defunct state (34), implying that the C-type inactivation machinery is essentially preserved but just hard to trigger in BK channels. The strong resistance of BK channels to C-type inactivation indicates a high energy barrier for transition from open or closed state to C-inactivated state, a situation that might arise from the BK channels’ high affinity for K+ binding in the external lock-in site (KdK+ of 2.7 μM using K+ chelator or 19 μM without chelator); the external lock-in site hinders Ba2+ exist to external side and its empty might be needed for C-type inactivation (34, 35). This finding is in contrast to the estimated 0.75 mM of K+ affinity for the lock-in site (36) and 0.5–2 mM of K+ affinity for inhibition of C-type inactivation (25, 29) in Shaker channels. Therefore, a small amount of contaminant K+ in our external recording solution could be sufficient to prevent channel inactivation.
By using both a nearly K+-free external solution and mutations at the residue 294 or 279 position, we were able to induce inactivation of BK channels. The observed inactivation had two characteristic features of C-type inactivation. First, it was sensitive to the external K+ concentration; replacement of the external K+ with the nonpermeable cation NMDG+ or Na+ was needed to induce inactivation, and an elevated external K+ dampened the inactivation. We observed slower recovery rates with NMDG+ than with Na+, which is in agreement with a previous report showing that NMDG+ caused a far greater increase in the Ba2+ off-rate than Na+ did in BK channels (35). Secondly, the inactivation was sensitive to mutations at the BK channel’s amino acid 294 and 279 positions, which are equivalent to the Shaker 449 and 434 positions. We observed a similar pattern of mutational effects, E > Q > K > T, on inactivation at the 294 position as at the 449 position of Shaker channels. Therefore, we consider it clearly C-type inactivation. However, it is clearly a closed state-dependent process whose amplitudes and rates correlated with the predicted closed probability, which is opposite to the known open-state dependence of C-type inactivation commonly observed in Shaker and Kv channels. The voltage dependence of recovery from inactivation in mutant BK channels was greatly shifted toward the membrane depolarization direction compared with the expected voltage dependence of a normal channel opening without inactivation. This feature is reminiscent of the effect of C-type inactivation on voltage sensor activation observed in other channels. In Shaker channels, the voltage dependence of voltage sensor activation is shifted in the hyperpolarizing direction in C-type–inactivated channels (37) because inactivation is coupled to the open state of the activation gate, which will allosterically stabilize the voltage sensor in the activated state. Similarly, in BK channels, a closed state-coupled C-type inactivation may correspondingly stabilize the channel in a closed state and the voltage sensors in a resting state, which results in a shift in voltage dependence toward the depolarization direction for channel recovery.
The closed state-coupled C-type inactivation that we observed in mutant BK channels is clearly distinct from other types of closed state-related inactivation. Closed-state preopen inactivation, which is commonly called U-type inactivation, is observed in many channels and occurs from preopen closed states at modestly depolarized membrane potentials (38, 39). However, U-type inactivation is clearly distinct from the BK channel-related closed-state C-type inactivation observed in this study and also the common open-state C-type inactivation observed in most other channels, in that (i) it is insensitive to mutations at the Shaker T449 and W434 positions, (ii) it is not facilitated by a decrease in external K+ concentration (38, 40), and (iii) it likely occurs through a selectivity filter-independent mechanism involving disengagement of the voltage sensor and activation gate when the voltage sensor is in a partially activated (preopen) state (38, 39).
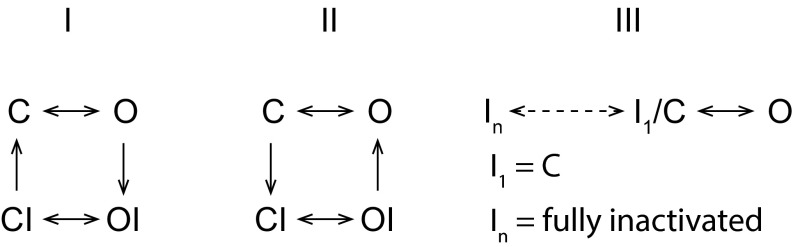
Channel activation and C-type inactivation are physically separate but mechanistically coupled gating processes in most K+ channels (Fig. 6, scheme I): One involves the intracellular bundle-crossing region (states C and O), and the other locates at the selectivity filter (state I). It remains not well understood how these two gating processes communicate with each other, particularly the mechanism(s) underlying the dependence of C-type inactivation on membrane depolarization or channel opening (O → OI) in these channels. Voltage-clamp fluorometry measurements have detected similar time courses of conformational changes in voltage sensors as with the onset and recovery of slow inactivation in Shaker channels (41, 42). However, it remains largely obscure how voltage-sensor movement is structurally coupled to C-type inactivation at or near the selectivity filter. The voltage sensor may indirectly interact with the selectivity filter region through its direct impact on the pore-lining S6 domain (8, 41, 42). Alternatively, it was proposed that the voltage sensor may directly interact with the selectivity filter region that confers C-type inactivation with voltage dependence (43). The bacterial KcsA channels, which lack voltage sensor domains, displayed a remarkable correlation between the degree of activation gate opening and the conformational changes and ion occupancy of the selectivity filter, suggesting an intrinsic open-state dependence of C-type inactivation (O → OI) in the absence of voltage sensors (7). It was further proposed that side-chain rearrangements of a residue on TM2 (KCsA) or S6 (Kv) located below the selectivity filter mechanically couple activation and C-type inactivation (44).
Fig. 6.
Schematic state diagrams depicting possible relationships among different channel states of closed (“C”), open (“O”), closed and inactivated (“CI”), and open and inactivated (“OI”). I1 means an initial step of inactivation.
It is intriguing how the state dependence of C-type inactivation in BK channels is the opposite of that in the widely studied Shaker, Kv, and KcsA channels. If the BK channels contain discrete C-type inactivation and activation gates (Fig. 6, scheme II), the C-type inactivation apparatus must be mechanically coupled to the closed state of the activation gate along the pore, which raises the question of how the BK channel is structurally different from other channels in the state dependence of communication between the C-type inactivation and activation gates. Because of the apparent lack of an intracellular bundle-crossing activation gate in BK channels and the absence of an open and inactivated state (OI) in our observation, we hypothesize that BK channel activation and C-type inactivation may have the same or spatially adjacent/overlapping mechanistic apparatus near or at the selectivity filter. Given this hypothesis, we would expect that the gating processes of C-type inactivation and normal channel closing are either equivalent (C = I) or closely coupled: e.g., the closed state is an initial step of the C-type inactivation (C = I1 in Fig. 6, scheme III). Because we observed both channel opening/closing and inactivation/recovery under the same inactivating condition, it is unlikely that they are exactly the same process. Alternatively, because of the likely involvement of multiple conformational changes at the selectivity filter in C-type inactivation (6, 7, 41), the BK channel’s closed state (C) may correspond to an early conformational stage (I1) of C-type inactivation (Fig. 6, scheme III). From voltage-clamp fluorometry data (41), it was suggested that the inactivation of Shaker channels comprises an initial closure of the inactivation gate, referred as “P-type” inactivation, which was related to an onset of macroscopic inactivation and exhibited no gating charge immobilization (41). This P-type inactivation is similar to what was observed in a constitutively inactivated W434F Shaker mutant, which still has a normal C ↔ O transition, as indicated by the largely intact voltage-dependent gating charge movements (28). It is plausible that the BK channel’s normal closure/deactivation may resemble P-type inactivation. P-type inactivation is followed by a much slower conformational change that stabilizes the voltage sensor and inactivated state of channels and results in gating charge immobilization in Shaker channels (41). We similarly observed a marked shift in the voltage dependence of recovery from inactivation compared with that of the normal BK channel opening, which was consistent with a stabilization of the voltage sensor by the inactivated state. This observation could be explained by a two-gate mechanism, as in Fig. 6, scheme II, or, alternatively, by the C-type inactivation process itself being voltage-dependent in BK channels (Fig. 6, scheme III).
Methods
Site-Directed Mutagenesis and Heterologous Expression of BK Channels in Culture Cells.
C-type inactivation mutants were constructed with the recombinant cDNA plasmid HF1-hSlo1, which encodes the c-Myc–tagged human BK channel α subunit (GenBank accession number AAB65837) (45). WT and mutant BK channels were heterologously expressed in HEK-293 cells. HEK-293 cells were obtained from ATCC, transfected with the designed plasmid(s) using Lipofectamine 2000 (Invitrogen), and used within 16–72 h for electrophysiological assays.
Electrophysiology.
The BK channel K+ currents were measured by patch-clamp recording in excised inside-out patches of HEK-293 cells with either symmetric K+ solutions of 136 mM KMeSO3, 6 mM KCl, and 20 mM Hepes (pH 7.20) or asymmetric solutions in which the external K+ was fully or partially replaced by NMDG+ or by Na+. To obtain the desired concentration of free Ca2+, the internal solution was supplemented with a certain amount of CaCl2 buffered by 5 mM HEDTA or nitrilotriacetic acid. The free Ca2+ concentration was measured with a Ca2+-sensitive electrode (Orion Research Inc.). For recordings with asymmetric K+ solutions, two to three observations were made usually for each type of measurement. The steady state of channel activation was expressed as G/Gmax, calculated from the relative amplitude of the tail currents (deactivation, held at −80 mV) or the ends of the activation currents. The voltage of half maximal activation (V1/2) and the equivalent gating charge (z) were obtained by fitting the relationship of G/Gmax with voltage with a single Boltzmann function: G/Gmax = 1/(1+e-zF(V-V1/2)/RT).
Acknowledgments
This work was supported in part by National Institutes of Health Grants NS078152 (to J.Y.) and NS07782 (to R.W.A.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Yellen G. The moving parts of voltage-gated ion channels. Q Rev Biophys. 1998;31(3):239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- 2.del Camino D, Yellen G. Tight steric closure at the intracellular activation gate of a voltage-gated K(+) channel. Neuron. 2001;32(4):649–656. doi: 10.1016/s0896-6273(01)00487-1. [DOI] [PubMed] [Google Scholar]
- 3.Lü Q, Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995;268(5208):304–307. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- 4.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 5.Kukuljan M, Labarca P, Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995;268(3 Pt 1):C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- 6.Kurata HT, Fedida D. A structural interpretation of voltage-gated potassium channel inactivation. Prog Biophys Mol Biol. 2006;92(2):185–208. doi: 10.1016/j.pbiomolbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Cuello LG, Jogini V, Cortes DM, Perozo E. Structural mechanism of C-type inactivation in K(+) channels. Nature. 2010;466(7303):203–208. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshi T, Armstrong CM. C-type inactivation of voltage-gated K+ channels: Pore constriction or dilation? J Gen Physiol. 2013;141(2):151–160. doi: 10.1085/jgp.201210888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: Effects of alterations in the carboxy-terminal region. Neuron. 1991;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- 10.Aldrich RW. Fifty years of inactivation. Nature. 2001;411(6838):643–644. doi: 10.1038/35079705. [DOI] [PubMed] [Google Scholar]
- 11.Pallotta BS, Magleby KL, Barrett JN. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- 12.Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- 13.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120(3):267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras JE, Holmgren M. Access of quaternary ammonium blockers to the internal pore of cyclic nucleotide-gated channels: Implications for the location of the gate. J Gen Physiol. 2006;127(5):481–494. doi: 10.1085/jgp.200509440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodor AA, Black KD, Zagotta WN. Tetracaine reports a conformational change in the pore of cyclic nucleotide-gated channels. J Gen Physiol. 1997;110(5):591–600. doi: 10.1085/jgp.110.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fodor AA, Gordon SE, Zagotta WN. Mechanism of tetracaine block of cyclic nucleotide-gated channels. J Gen Physiol. 1997;109(1):3–14. doi: 10.1085/jgp.109.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpen JW, Brown RL, Stryer L, Baylor DA. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J Gen Physiol. 1993;101(1):1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkens CM, Aldrich RW. State-independent block of BK channels by an intracellular quaternary ammonium. J Gen Physiol. 2006;128(3):347–364. doi: 10.1085/jgp.200609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang QY, Zeng XH, Lingle CJ. Closed-channel block of BK potassium channels by bbTBA requires partial activation. J Gen Physiol. 2009;134(5):409–436. doi: 10.1085/jgp.200910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson J, Begenisich T. Selectivity filter gating in large-conductance Ca(2+)-activated K+ channels. J Gen Physiol. 2012;139(3):235–244. doi: 10.1085/jgp.201110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piskorowski RA, Aldrich RW. Relationship between pore occupancy and gating in BK potassium channels. J Gen Physiol. 2006;127(5):557–576. doi: 10.1085/jgp.200509482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contreras JE, Srikumar D, Holmgren M. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc Natl Acad Sci USA. 2008;105(9):3310–3314. doi: 10.1073/pnas.0709809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Horrigan FT. Cysteine modification alters voltage- and Ca(2+)-dependent gating of large conductance (BK) potassium channels. J Gen Physiol. 2005;125(2):213–236. doi: 10.1085/jgp.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Xia X, Lingle CJ. Inhibition of large-conductance Ca2+-activated K+ channels by nanomolar concentrations of Ag+ Mol Pharmacol. 2010;78(5):952–960. doi: 10.1124/mol.110.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- 26.Ogielska EM, Aldrich RW. Functional consequences of a decreased potassium affinity in a potassium channel pore: Ion interactions and C-type inactivation. J Gen Physiol. 1999;113(2):347–358. doi: 10.1085/jgp.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlief T, Schönherr R, Heinemann SH. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflugers Arch. 1996;431(4):483–493. doi: 10.1007/BF02191894. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Yan Y, Sigworth FJ. How does the W434F mutation block current in Shaker potassium channels? J Gen Physiol. 1997;109(6):779–789. doi: 10.1085/jgp.109.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: A tale of two inactivation mechanisms. Neuron. 1995;15(4):951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Yan J, Aldrich RW. BK channel opening involves side-chain reorientation of multiple deep-pore residues. Proc Natl Acad Sci USA. 2014;111(1):E79–E88. doi: 10.1073/pnas.1321697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proc Natl Acad Sci USA. 1999;96(7):4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19(13):5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uebele VN, et al. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275(30):23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 34.Vergara C, Alvarez O, Latorre R. Localization of the K+ lock-In and the Ba2+ binding sites in a voltage-gated calcium-modulated channel: Implications for survival of K+ permeability. J Gen Physiol. 1999;114(3):365–376. doi: 10.1085/jgp.114.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neyton J, Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 1988;92(5):549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RE, Larsson HP, Isacoff EY. A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys J. 1998;74(4):1808–1820. doi: 10.1016/s0006-3495(98)77891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olcese R, Latorre R, Toro L, Bezanilla F, Stefani E. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J Gen Physiol. 1997;110(5):579–589. doi: 10.1085/jgp.110.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bähring R, Covarrubias M. Mechanisms of closed-state inactivation in voltage-gated ion channels. J Physiol. 2011;589(Pt 3):461–479. doi: 10.1113/jphysiol.2010.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bähring R, Barghaan J, Westermeier R, Wollberg J. Voltage sensor inactivation in potassium channels. Front Pharmacol. 2012;3:100. doi: 10.3389/fphar.2012.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamieson Q, Jones SW. Shaker IR T449 mutants separate C- from U-type inactivation. J Membr Biol. 2014;247(4):319–330. doi: 10.1007/s00232-014-9634-3. [DOI] [PubMed] [Google Scholar]
- 41.Loots E, Isacoff EY. Protein rearrangements underlying slow inactivation of the Shaker K+ channel. J Gen Physiol. 1998;112(4):377–389. doi: 10.1085/jgp.112.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi CS, Loots E, Isacoff EY. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron. 2000;27(3):585–595. doi: 10.1016/s0896-6273(00)00068-4. [DOI] [PubMed] [Google Scholar]
- 43.Panaghie G, Purtell K, Tai KK, Abbott GW. Voltage-dependent C-type inactivation in a constitutively open K+ channel. Biophys J. 2008;95(6):2759–2778. doi: 10.1529/biophysj.108.133678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuello LG, et al. Structural basis for the coupling between activation and inactivation gates in K(+) channels. Nature. 2010;466(7303):272–275. doi: 10.1038/nature09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc Natl Acad Sci USA. 1997;94(25):14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]