Summary
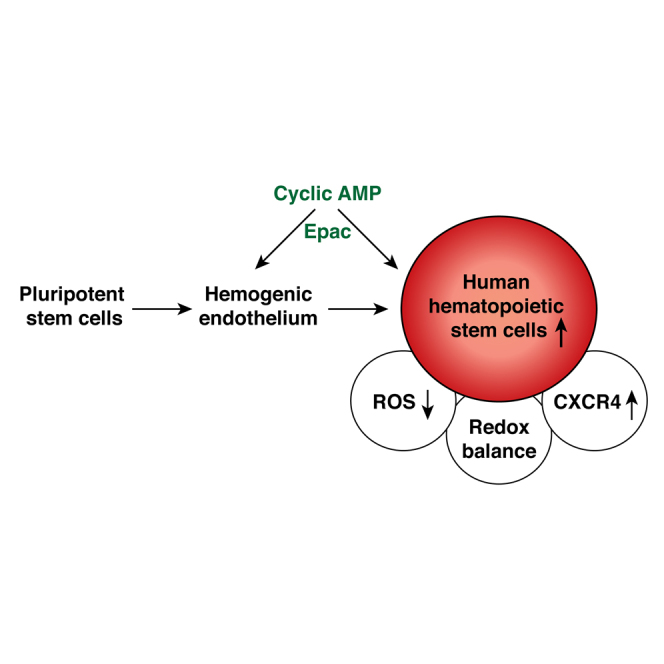
Hematopoietic cells emerge from hemogenic endothelium in the developing embryo. Mechanisms behind human hematopoietic stem and progenitor cell development remain unclear. Using a human pluripotent stem cell differentiation model, we report that cyclic AMP (cAMP) induction dramatically increases HSC-like cell frequencies. We show that hematopoietic cell generation requires cAMP signaling through the Exchange proteins activated by cAMP (cAMP-Epac) axis; Epac signaling inhibition decreased both hemogenic and non-hemogenic endothelium, and abrogated hematopoietic cell generation. Furthermore, in hematopoietic progenitor and stem-like cells, cAMP induction mitigated oxidative stress, created a redox-state balance, and enhanced C-X-C chemokine receptor type 4 (CXCR4) expression, benefiting the maintenance of these primitive cells. Collectively, our study provides insights and mechanistic details on the previously unrecognized role of cAMP signaling in regulating human hematopoietic development. These findings advance the mechanistic understanding of hematopoietic development toward the development of transplantable human hematopoietic cells for therapeutic needs.
Graphical Abstract
Highlights
-
•
cAMP induction increases HSC-like cell generation from human pluripotent stem cells
-
•
cAMP signaling through Epac axis modulates hemogenic endothelium
-
•
cAMP upregulates anti-oxidative mechanisms and creates redox-state balance
-
•
cAMP induction enhances CXCR4 expression in hematopoietic progenitors
Woods and colleagues show that cyclic AMP induction leads to efficient upregulation of human pluripotent stem cell-derived hematopoietic progenitors. Cyclic AMP, through the Epac axis, modulates hemogenic endothelium and hPSC-derived hematopoietic cells. Cyclic AMP induction creates redox-state balance, protecting the developing hematopoietic progenitors from oxidative stress. This study reveals the previously unrecognized role of cyclic AMP in regulating human hematopoietic development.
Introduction
Hematopoietic stem cells (HSCs) replenish the hematopoietic system throughout the lifetime of an individual, and can be transplanted into patients to treat malignant and non-malignant blood disorders. The need to develop an alternative source of HSCs to matched adult donors, such as HSCs generated in vitro from pluripotent stem cells, requires increased understanding of the mechanisms of HSC development. During development, the first hematopoietic cells emerge from hemogenic endothelium in the embryonic aorta-gonad-mesonephros (AGM) region through endothelial-to-hematopoietic transition (EHT) (Zovein et al., 2008). The concurrence of neural crest stem cells in the AGM region coincides with the time of HSC emergence, suggesting a link between neural crest/catecholamines and hematopoietic development (Nagoshi et al., 2008). Recently, catecholamine signaling was reported to regulate HSC emergence in the AGM region, as the deletion of GATA binding protein 3 (GATA3), a crucial regulator of catecholamine production, compromised HSC development, which could be rescued with administration of catecholamine derivatives (Fitch et al., 2012). However, the mechanism of catecholamine signaling, through its second messenger, cyclic AMP (3′-5′-cyclic AMP; cAMP) and its downstream signaling pathways have not been critically evaluated in the context of hematopoietic development.
In the adult hematopoietic system, a situation parallel to the hematopoietic developmental context exists. Catecholamines and sympathoadrenergic innervation (Afan et al., 1997, Mendez-Ferrer et al., 2010) of the bone marrow (BM) niche regulates HSC mobilization and migration (Katayama et al., 2006, Lucas et al., 2013, Mendez-Ferrer et al., 2008) of catecholamine receptor-expressing hematopoietic stem and progenitor cells (Heidt et al., 2014, Spiegel et al., 2007). Together, these studies during developmental hematopoiesis and adult hematopoiesis provide evidence for neural regulation of hematopoietic cells and establish catecholamine-mediated signaling as a key component of the hematopoietic program.
Activation of specific G-protein-coupled receptors by catecholamines, as well as neurotransmitters, growth factors, and hormones, activate the cAMP-signaling pathway (Beavo and Brunton, 2002, Sutherland and Rall, 1958), followed by cell-type dependent responses mediated by cAMP effectors protein kinase A (PKA) (Walsh et al., 1968) and Exchange proteins activated by cAMP (Epac) (de Rooij et al., 1998). Epac have been shown to modulate endothelial cell remodeling, enhance endothelial cell adhesion, and regulate the integrity of endothelial cell junctions (Cullere et al., 2005, Fukuhara et al., 2005, Kooistra et al., 2005). However, the role of Epac signaling in hemogenic endothelium is unknown.
cAMP-mediated regulation of adult hematopoiesis is emphasized in studies showing that cAMP increases C-X-C chemokine receptor type 4 (CXCR4) expression and motility of hematopoietic progenitors (Goichberg et al., 2006), HSCs from Gsα-deficient mice do not engraft (Adams et al., 2009), and Gsα-deficient osteocytes alter the BM niche, leading to defective hematopoiesis (Fulzele et al., 2013). In human hematopoietic cells, prostaglandin E2 (PGE2)-mediated cAMP activation enhances human cord blood engraftment (Cutler et al., 2013, Goessling et al., 2011). Recently, cAMP was shown to regulate hematopoietic emergence and homing in studies where cAMP was upregulated by adenosine in zebrafish and mouse (Jing et al., 2015), PGE2 in zebrafish and mouse (Diaz et al., 2015, Goessling et al., 2009, Hoggatt et al., 2009, North et al., 2007), and shear stress in murine AGM (Kim et al., 2015). However, the role and mechanism of cAMP signaling, as mediated through PKA and Epac, in regulating human developmental hematopoiesis has not been adequately studied, and no study has been performed on the role of cAMP in the human hematopoietic developmental context.
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (Thomson et al., 1998) and induced pluripotent stem cells (iPSCs) (Takahashi et al., 2007), provide an ideal in vitro model to recapitulate human hematopoietic development. We have shown that hPSC-derived HSC-like cells possess lymphoid and myeloid differentiation ability, a key feature of HSCs (Ronn et al., 2015). Recent studies have functionally demonstrated an endothelial precursor to blood (hemogenic endothelium) from hPSC differentiation cultures (Ditadi et al., 2015, Slukvin, 2013), further establishing hPSCs as a suitable model to study human hematopoietic cell development. However, the signals regulating hemogenic endothelium and newly emergent HSCs in the human developmental context remain undefined. In addition, for functional transplantable HSCs it is vital to reduce reactive oxygen species (ROS) and oxidative stress, as reduced ROS is crucial for HSC functionality (Ito et al., 2006, Jang and Sharkis, 2007, Yahata et al., 2011).
As cAMP-mediated regulation of human hematopoietic cell emergence remains elusive, we set out to investigate the role of cAMP signaling in the development of hematopoietic progenitors from hPSCs. Here, we demonstrate that cAMP induction during hPSC-to-hematopoietic differentiation increases the frequency of cells with HSC-like surface phenotype and increases the colony-forming unit (CFU) potential. We demonstrate that cAMP regulation of hemogenic endothelium is dependent on the cAMP-Epac signaling axis. Furthermore, we propose that the cAMP-mediated increase in HSC-like cells is in part coupled to cAMP-mediated mitigation of oxidative burden and increasing hematopoietic cell function.
Results
cAMP Induction Increases the Frequency of HSC-like Cells Derived from hPSCs
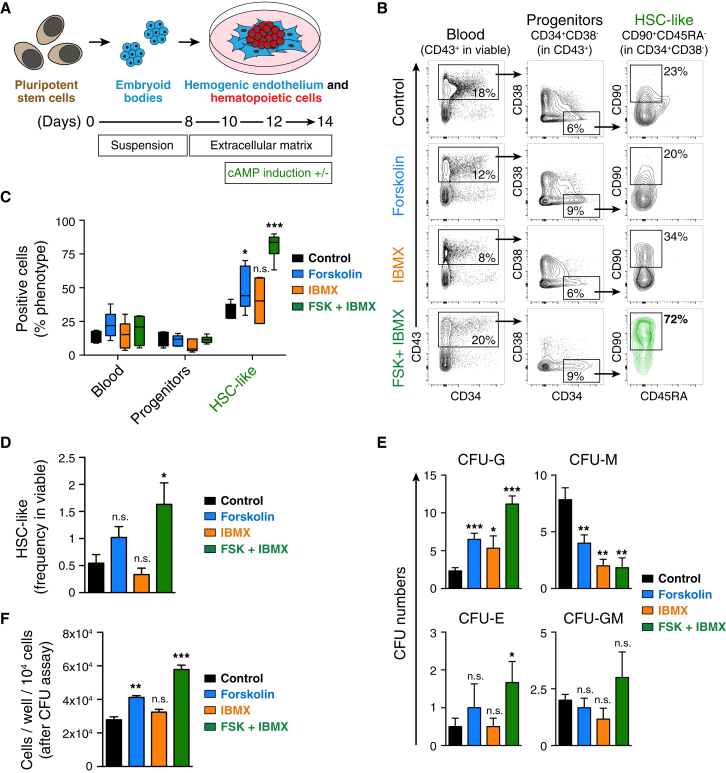
To assess the role of cAMP signaling in human hematopoietic development, we differentiated hPSCs using our previously described protocol (Ronn et al., 2015) whereby mesoderm-biased embryoid bodies (EBs) were plated onto the extracellular matrix allowing for adherence and expansion of hemogenic endothelium, to generate hematopoietic cells (Figure 1A). To elevate intracellular cAMP, we applied a combination of forskolin and 3-isobutyl-1-methylxanthine (IBMX) from day 10. Forskolin specifically increases intracellular cAMP levels by activating the catalytic subunit of adenylyl cyclase (Seamon et al., 1981). IBMX is a phosphodiesterase (PDE) inhibitor that specifically prevents PDE-mediated dephosphorylation of cAMP to AMP (Beavo et al., 1970). Thus, combining forskolin with IBMX elevates intracellular cAMP by increasing cAMP production and preventing its dephosphorylation. Fluorescence-activated cell sorting (FACS) analysis of the hPSC-derived hematopoietic cells revealed that cAMP induction with forskolin + IBMX significantly increased the numbers and frequency of our previously described HSC-like cells (CD43+CD34+CD38−CD90+CD45RA−) (Figures 1B–1D).
Figure 1.
cAMP Induction Increases HSC-like Frequency during hPSC-to-Hematopoietic Differentiation
(A) Conditions and timeline applied to differentiate hPSCs toward mesoderm commitment and hematopoietic differentiation.
(B) Flow cytometric analysis of hematopoietic cells at day 14 of differentiation. Representative flow cytometry plots (biexponential axis) of cells cultured in control medium (MesoTotal), and cells treated with forskolin, IBMX, or forskolin + IBMX are shown.
(C) Percentage of the hematopoietic surface phenotypes indicated in (B). Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05, ∗∗∗p < 0.001; n.s., not significant.
(D) Frequency of the putative HSC-like cells (in viable fraction). Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05; n.s., not significant.
(E) Total CFU numbers after 12-day CFU assay of differentiated hematopoietic cells treated with forskolin, IBMX, and forskolin + IBMX. The CFU distribution of three independent experiments is shown as mean ± SEM CFU-G (granulocyte), CFU-M (macrophage), CFU-E (erythroid), CFU-GM (granulocyte/macrophage). Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(F) Cell numbers obtained per well (per 1 × 104 seeded cells) after CFU assay. Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗∗p < 0.01, ∗∗∗p < 0.001; n.s., not significant.
See also Figure S1.
To confirm that the upregulation of HSC-like cells was specifically due to increased cAMP levels, we used two different synthetic cAMP analogs, dibutyryl-cAMP and 8-Br-cAMP, to elevate intracellular cAMP during hPSC-to-hematopoietic differentiation. Both cAMP analogs upregulated the HSC-like phenotype (Figure S1A). Also, to rule out any unspecific effects of forskolin we used 1,9-dideoxyforskolin, an inactive analog of forskolin that does not activate adenylyl cyclase. We observed that HSC-like phenotype was induced only with forskolin + IBMX, not with 1,9-dideoxyforskolin + IBMX (Figure S1B), thus verifying the specificity of forskolin-mediated effects through cAMP.
Assessment of the differentiation capacity of hematopoietic cells in a CFU assay showed an increase in granulocyte CFU (CFU-G) and erythroid colonies, while the numbers of macrophage CFU (CFU-M) were decreased (Figure 1E). cAMP induction, although decreasing the numbers of CFU-M colonies, significantly increased the total cell number resulting from the colony assay (Figure 1F) compared with the non-induced control.
Together, these data show that elevation of intracellular cAMP upregulates hPSC-derived HSC-like surface phenotype, and also alters the distribution of colony types, favoring mixed, granulocyte, and erythroid colonies at the expense of macrophage colonies.
cAMP Signaling through the Epac Axis Is Required for HSC-like Cell Generation from an Endothelial Cell Intermediate
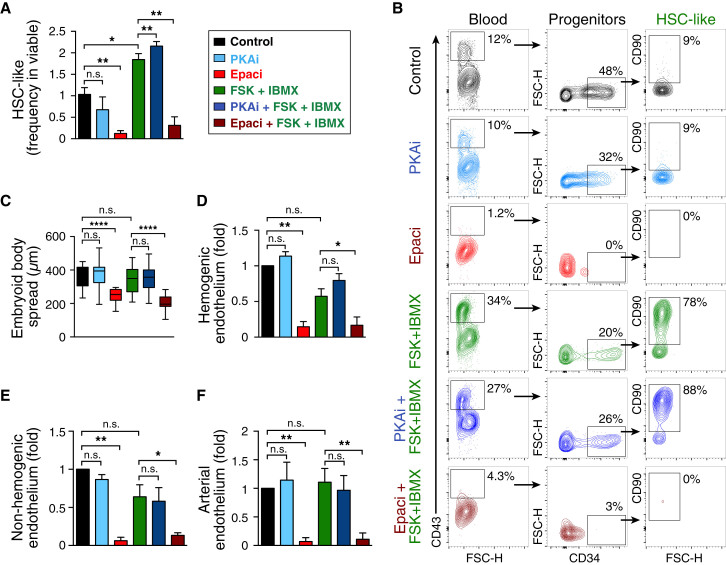
Intracellular cAMP downstream effectors, PKA and Epac, influence cellular functions by regulating the activation of various transcription factors and signaling molecules (Cheng et al., 2008). We set out to elucidate the mechanism of cAMP-mediated upregulation of phenotypic HSC-like cells during hPSC differentiation toward the hematopoietic lineage. Epac inhibition abrogated the hematopoietic cell generation efficiency as assessed by FACS measuring the frequency of pan-hematopoietic marker CD43+ cells and HSC-like cells (Figures 2A, 2B, and S2A). Inhibition of PKA, on the other hand, had no negative effect on hematopoietic cells (Figures 2A, 2B, and S2A). The cell viability after PKA and Epac inhibition was similar to that of controls, at 90%–95% (Figure S2B). We observed that inhibition of Epac in the absence or presence of forskolin + IBMX reduced the radial spreading of adherent endothelial-like cells (emerging from the plated EBs) while PKA inhibition did not have any effect (Figure 2C), the radial spread being measured as indicated in Figure S2C. The reduced EB radial spread after Epac inhibition was in accordance with a trend in reduced total cell number at day 14 after Epac inhibition (Figure S2D), suggesting that Epac inhibition affected cell proliferation in a manner not associated with cell survival. As bona fide hematopoietic cells are reported to emerge from such endothelial spread (Eilken et al., 2009), we evaluated the effect of Epac inhibition on the frequency of the previously described hemogenic endothelium phenotype cells (CD43−CD34+CXCR4−CD73−VEcad+) (Ditadi et al., 2015), whereby we applied the cAMP modulators at days 6 and 8 and analyzed the cells at day 10 (schematic regimen described in Figure S2E and Experimental Procedures). Epac inhibition in the absence or presence of forskolin + IBMX decreased the hemogenic endothelial fraction (Figure 2D) without compromising the viability of hemogenic endothelium (Figure S2F). Epac inhibition also reduced the non-hemogenic endothelium (CD43−CD34+VEcad+) (Figure 2E) and arterial endothelium (CD43−CD34+CD90+CD73+CXCR4+) (Figure 2F). In contrast, the various endothelial cell types were insensitive to PKA inhibition (Figures 2D–2F and S2A).
Figure 2.
cAMP-Mediated HSC-like Upregulation Occurs through the cAMP-Epac Axis
(A) Quantification of HSC-like frequency in viable (at day 14) after PKA or Epac inhibition. Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant.
(B) Representative flow cytometry plots (biexponential axis) showing the CD43+ blood cells and HSC-like cells (day 14) generated after forskolin + IBMX-mediated cAMP induction and PKA or Epac inhibition (PKAi, Epaci), with or without forskolin + IBMX.
(C) Quantification of radial spread of EBs (distance between center of EB and outer edge of the cellular spread, day 13). Data represent mean ± SEM of 100 EBs from three independent experiments. Statistical analysis was performed using the t test. ∗∗∗∗p < 0.0001; n.s., not significant.
(D) Analysis of hemogenic endothelial (CD43−CD34+CXCR4−CD73−VEcad+) phenotype (day 10), after cAMP induction and PKA or Epac inhibition (PKAi, Epaci) with or without cAMP induction. Data represent mean ± SEM of three independent experiments; mean fold change respective to control is shown. Statistical analysis was performed using the t test. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant.
(E) Analysis of general (non-hemogenic) endothelium (CD43−CD34+VEcad+) at day 10, after cAMP induction and PKA or Epac inhibition (PKAi, Epaci) with or without cAMP induction. Data represent mean ± SEM of three independent experiments; mean fold change respective to control is shown. Statistical analysis was performed using the t test. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant.
(F) Analysis of arterial endothelium (CD43−CD34+CD90+CD73+CXCR4+) at day 10, after cAMP induction and PKA or Epac inhibition (PKAi, Epaci) with or without cAMP induction. Data represent mean ± SEM of three independent experiments; mean fold change respective to control is shown. Statistical analysis was performed using the t test. ∗∗p < 0.01; n.s., not significant. For (D)–(F) the analysis was done at day 10, as the hemogenic endothelium is less abundant after this time point.
See also Figure S2.
These results indicate that the cAMP-Epac axis plays a pivotal role in the development of hematopoietic progenitors and stem-like cells in hPSC differentiation cultures by regulating hemogenic endothelial cell expansion.
cAMP Induction Reduces Oxidative Stress and Induces CXCR4 Upregulation in hPSC-Derived Hematopoietic Cells
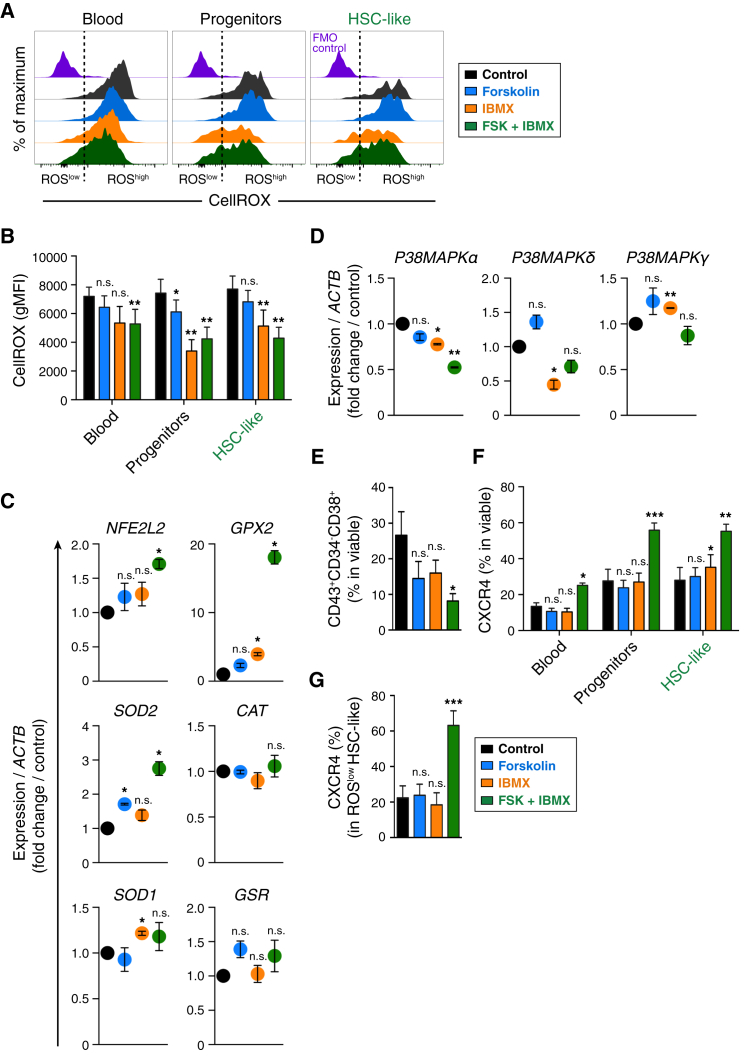
The deleterious effects of elevated ROS and its ensuing oxidative damage on the function of mammalian HSCs are well documented (Ito et al., 2006, Jang and Sharkis, 2007, Suda et al., 2011, Yahata et al., 2011). As cAMP induction decreased the prevalence of macrophages in our system (Figure 1E), and as cAMP elevation coupled with PDE inhibition has been reported to promote immune homeostasis (Katakami et al., 1988, Sinha et al., 1995), we rationalized that cAMP induction with forskolin + IBMX might mitigate the ROS burden in our hPSC-to-hematopoietic differentiation system, thus protecting the HSC-like cells from ROS-mediated effects mentioned above. Analysis of ROS levels showed that cAMP induction decreased the ROS levels in hPSC-derived hematopoietic cells compared with the control setting (Figure 3A). cAMP induction along with PDE inhibition (forskolin + IBMX), and protecting endogenously available cAMP with PDE inhibition alone (IBMX), significantly lowered the ROS levels in various hematopoietic phenotypes, including the HSC-like cells (Figure 3B), thus indicating reduced oxidative stress of these hematopoietic cellular fractions.
Figure 3.
cAMP Induction Reduces Oxidative Stress and Induces CXCR4 in hPSC-Derived Hematopoietic Cells
(A) Flow cytometry analysis for detection of reactive oxygen species (ROS) in differentiated hPSC-to-hematopoietic cells at day 14 of differentiation. Representative flow cytometry plots (biexponential x axis) show ROS levels in the hematopoietic surface phenotypes. FMO control, fluorescence-minus-one (staining control).
(B) Quantification of geometric mean fluorescence intensity (gMFI) of CellROX dye as indicated in (A). Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05, ∗∗p < 0.01; n.s., not significant.
(C and D) qRT-PCR expression analysis of the indicated redox-state-regulating genes (C) and p38MAPK-related genes (D) in PSC-derived hematopoietic cells. Relative expression of each gene to housekeeping gene ACTB (β-ACTIN) was calculated, and mean fold change respective to control condition (set at 1) is shown. Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05, ∗∗p < 0.01; n.s., not significant.
(E) Analysis of mature hematopoietic progenitors (CD43+CD34−CD38+) after cAMP induction (day 14). Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05; n.s., not significant.
(F) Expression of CXCR4 across indicated hematopoietic surface phenotypes. Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; n.s., not significant.
(G) Expression of CXCR4 in HSC-like surface phenotype (ROSlow fraction). Data represent mean ± SEM of three independent experiments. Statistical analysis was performed using the t test. Significance compared with the control setting: ∗∗∗p < 0.001, n.s., not significant.
See also Figure S3.
The decreased ROS level after cAMP induction prompted us to determine the status of genes that regulate the redox state of cells and thus help to reduce the oxidative stress. Transcriptional analysis of the redox-state-regulating genes in hPSC-derived hematopoietic cells showed that nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) was upregulated after cAMP induction with forskolin + IBMX (Figure 3C). NFE2L2 is a global regulator of the oxidative stress response, as it binds to anti-oxidant response element in the upstream promoter region of several anti-oxidative genes and initiates their transcription (Itoh et al., 1997, Tsai et al., 2013), thus initiating the mitigation of ROS-induced oxidative stress in the cells. In our system, NFE2L2 upregulation after cAMP induction was in concert with the upregulation of anti-oxidant genes, such as superoxide dismutase (SOD1, SOD2), glutathione peroxidase (GPX2) (18-fold increase), catalase (CAT), and glutathione S-reductase (GSR) (Figure 3C).
Stress conditions activate p38 mitogen-activated protein kinases (p38MAPK) (Raingeaud et al., 1995). ROS/stress-mediated p38MAPK activation leads to HSC self-renewal defects and reduced HSC long-term repopulation potential (Ito et al., 2006, Jang and Sharkis, 2007). Thus we analyzed the levels of stress-activated p38 pathway components P38-MAPKα, δ, and γ and found that cAMP induction generally reduced the levels of these p38MAPK isoforms (Figure 3D), indicative of reduced stress. As elevated ROS levels have been shown to induce differentiation of hematopoietic progenitors toward mature lineages (Jang and Sharkis, 2007, Suda et al., 2011), we analyzed the abundance of mature hematopoietic cells (CD43+CD34−CD38+) in our differentiation assay. cAMP induction reduced the prevalence of mature hematopoietic progenitors (Figure 3E), indicating the role of cAMP in preventing cell maturation and agreeing to the reduced ROS levels after cAMP induction (Figures 3A and 3B). Together, these data indicate that cAMP induction with forskolin + IBMX upregulated anti-oxidant defense mechanisms and downregulated stress-activated genes, thus creating a redox balance in our system that is favorable for hematopoietic progenitor and stem-like cell maintenance.
The chemokine receptor CXCR4 is pivotal for retaining quiescent HSCs in the BM niche, as well as for HSC homing to BM (Lai et al., 2014, Nie et al., 2008, Peled et al., 1999). Given that cAMP and PGE2 treatment has been shown to increase CXCR4 expression in adult HSCs/human cord blood CD34+ cells (Goessling et al., 2011, Goichberg et al., 2006, Hoggatt et al., 2009), we analyzed CXCR4 levels in our hPSC-derived hematopoietic cells following cAMP induction. Analysis of CXCR4 expression revealed that cAMP induction with forskolin + IBMX enhanced CXCR4 expression across various hematopoietic surface phenotypes, including the HSC-like fraction (Figure 3F). Importantly, CXCR4 expression was enhanced in the ROSlow HSC-like surface phenotype upon treatment with forskolin + IBMX (Figure 3G). We investigated whether cAMP-mediated CXCR4 modulation was regulated through PKA or Epac. cAMP-induced upregulation of CXCR4 was insensitive to inhibition of PKA, indicating that CXCR4 regulation does not depend on PKA. On the contrary, Epac inhibition reduced CXCR4 expression, indicating that CXCR4 regulation was mediated by the cAMP-Epac axis (Figure S3). Our data showing that PKA does not regulate CXCR4 expression agree with a previous finding where cAMP-mediated CXCR4 elevation (in human mobilized peripheral blood CD34+ cells) was insensitive to PKA inhibition (Goichberg et al., 2006).
Together, these findings show that cAMP induction reduced the oxidative burden by creating a redox-state balance in hPSC-derived hematopoietic cells and upregulated CXCR4 in the HSC-like cells, both essential for HSC functionality.
Discussion
Using an hPSC differentiation system to model human hematopoietic cell emergence and development, our findings suggest that cAMP and signaling through its Epac axis is an important factor for the generation of hemogenic endothelium, from which the first hematopoietic cells arise. Thus cAMP is a crucial second messenger molecule regulating the in vitro hPSC-derived HSC-like cells. Interestingly, cAMP induction upregulated the frequency of HSC-like cells in the differentiation cultures and also mitigated oxidative stress, created redox-state balance, and enhanced CXCR4 expression in hPSC-derived hematopoietic cells, thus suggesting multiple independent functions of cAMP signaling in endothelial and hematopoietic cells. Because of the importance of low ROS levels in maintaining repopulating human HSCs (Jang and Sharkis, 2007, Yahata et al., 2011), the identification of cAMP-mediated ROS reduction in hPSC differentiation systems provides a mechanism for how ROS can be regulated in in vitro systems to better mimic in vivo HSC development. We speculate that the presence of mature immune cells elevates ROS activity in our differentiation system. The reduction in mature cells and decreased macrophage numbers following cAMP induction agrees with cAMP-mediated redox-state balance, leading to decreased ROS levels. ROS reduction was in concert with the increase of anti-oxidant gene response, decrease in the p38 stress pathway components, and reduced prevalence of mature hematopoietic cells. In terms of cAMP-mediated ROS reduction and reduction of mature progenitors, the reduced abundance of differentiated cells could in part result in decreased ROS, or, alternatively, low ROS levels could be playing a role to reduce differentiation, either way both benefiting the system for increasing the prevalence of HSC-like cells.
Thus, benefits of cAMP, in terms of decreased ROS/p38 and maintenance of redox balance together, created a better environment for the HSC-like cells, presumably helping their survival, preventing premature senescence, and maintaining their functionality, as has been shown for adult HSCs. cAMP induction reduced p38 signaling, which is beneficial for the maintenance of human hematopoietic stem and progenitor cells (Baudet et al., 2012, Zou et al., 2012). The ability of cAMP to regulate inflammation and ROS has been shown previously, either by reducing pro-inflammation cytokines and enhancing the T helper 2 responses in inflamed human blood cells (Harris et al., 2002, Snijdewint et al., 1993, Yoshimura et al., 1997) or via suppressing inflammation/ROS through the cAMP-Epac axis (Remans et al., 2004, Xu et al., 2008). In our assay, cAMP-mediated ROS/p38 reduction, increased anti-oxidant response, and reduced differentiation could in part be attributed to these inflammation-regulating actions of cAMP.
We speculate that ROS reduction has an important, but not exclusive, regulatory role in the cAMP-mediated maintenance of HSC-like cells. Moreover, cAMP-mediated ROS/p38 reduction and increased CXCR4 receptor expression provide a correlational benefit to HSC-like cells, as low ROS levels specifically increased the CXCR4high HSC-like cells. These findings suggest that cAMP induction imparts important functional properties to the derived hematopoietic cells, as low oxidative stress (Ito et al., 2006, Jang and Sharkis, 2007, Yahata et al., 2011) and high CXCR4 expression (Nie et al., 2008, Peled et al., 1999) are properties of HSCs with long-term transplantation potential.
Multiple factors, through binding to GPCRs, orchestrate the regulation of cAMP levels in a cell- and context-dependent manner, an effect that is most likely to be preserved during embryonic hematopoietic emergence and development (Diaz et al., 2015, Goessling et al., 2011, Hoggatt et al., 2009, Jing et al., 2015, Kim et al., 2015, Li et al., 2014, North et al., 2007). Thus, the regulatory networks involved in cAMP-mediated regulation of hematopoietic development are likely to be multifactorial and interconnected. Detailed dissection of such signals is therefore required to elucidate the signaling networks that trigger cAMP-mediated benefits to hematopoietic cells.
Our findings on the pro-hematopoietic effects of cAMP on hPSC-derived hematopoietic cell specification are in agreement with reports describing the pivotal role of cAMP signaling in promoting and enhancing mouse and zebrafish hematopoietic development (Diaz et al., 2015, Jing et al., 2015, Kim et al., 2015), as well as human CD34+ cord blood/mobilized peripheral blood survival and engraftment (Goessling et al., 2011, Li et al., 2014). These studies demonstrated that cAMP activation via fluid shear stress in the murine system (Diaz et al., 2015, Kim et al., 2015), adenosine signaling in the zebrafish hematopoietic system (Jing et al., 2015), and PGE2 in human CD34+ cells (Goessling et al., 2011, Li et al., 2014), instructs hematopoietic specification, mediated through the cAMP-PKA axis. In our in vitro model of human hematopoietic development, using an hPSC differentiation system, we show that by inhibiting the downstream effectors of cAMP signaling, PKA and Epac, only Epac signaling is required for hematopoietic development, notwithstanding that apart from affecting the underlying endothelium, Epac inhibition appears to affect the newly formed HSC-like cells. As experimental modulation of cAMP signaling to instruct HSC fate from hPSCs has not yet been achieved (Traver, 2015), our finding describing the pro-hematopoietic benefits of cAMP on hPSC-derived human hematopoietic cells is a key step toward refining modalities of human HSC generation using cAMP. Toward understanding the in vivo emergence and development of human HSCs, our findings on human PSC-derived hematopoietic cell generation necessitate further evaluation of cAMP and its signaling components during definitive human hematopoiesis, i.e., either directly in human fetuses or by developing culture systems to propagate human AGM region in vitro, notwithstanding the ethical and technical challenges related to this pursuit.
cAMP-mediated Epac upregulation has been reported to reorganize cortical actin, enhance vascular endothelial cadherin-mediated cell adhesion, and induce integrin-mediated cell adhesion, leading to decreased endothelial cell permeability and enhanced endothelial barrier function (Cullere et al., 2005, Fukuhara et al., 2005, Rangarajan et al., 2003). Developmentally, in the early embryo hematopoietic cells (including cells with the potential to form HSCs) emerge from hemogenic endothelium in the AGM region through EHT (Chen et al., 2009, Zovein et al., 2008). From our Epac inhibition experiments, we demonstrate the critical role of Epac signaling in modulating hPSC-derived hemogenic endothelium and HSC-like cells. Epac being one of the downstream effectors of cAMP that regulates endothelial cell-cell adhesion, permeability, and barrier functions may suggest a link between endothelial cell mechanobiology and hemogenic endothelial cell function. However, due to the inability to currently evaluate pure populations of human endothelial cells with hematopoietic potential, confirmation of Epac's role, whether specifying hematopoietic function at the hemogenic endothelium (HE) cell stage, or acting indirectly on HE via an upstream cell intermediate, is still necessary.
Collectively, our findings suggest that cAMP regulates the generation and function of human HSCs via multiple separate mechanisms in both the endothelial and hematopoietic cell fractions. By demonstrating the role of cAMP-Epac axis in hematopoietic cells and HSC-like cells using an in vitro human development model, our study provides insights into understanding the previously unknown role of the cAMP-signaling component in human hematopoietic development. Taken together, these findings advance our current understanding of human hematopoietic developmental mechanisms toward the development of transplantable hematopoietic cells for therapeutic purposes.
Experimental Procedures
Human Pluripotent Stem Cell Culture
Human iPSC line RB9-CB1 derived from cord blood endothelial cells (Ronn et al., 2015, Woods et al., 2011) was cultured on irradiated mouse embryonic fibroblasts in DMEM/Nutrient Mixture F-12 (DMEM/F12) supplemented with 20% KnockOut-serum replacement (KO-SR), 2 mM L-glutamine, 0.1 mM non-essential amino acids, 0.1 mM 2-mercaptoethanol, and 10 ng/ml basic fibroblast growth factor, all from Thermo Fisher Scientific. The cells were incubated in a humidified incubator at 37°C and 5% CO2.
Embryoid Body Formation and Hematopoietic Differentiation
EBs were prepared after incubating the pluripotent stem cell colonies with 2 mg/ml Dispase (Thermo Fischer Scientific), followed by gentle pipetting. The detached colonies were washed twice with 20% KO-SR containing DMEM/F12, before plating in ultra-low-adherence suspension culture dishes (Corning Life Sciences) to form EBs for 8 days. During suspension culture, the MesoTotal HPC/HSC Differentiation System (Primorigen Biosciences) was used to specify the EBs toward mesoderm commitment. At day 8, EBs were plated on 0.08 μg/mm2 Matrigel (BD Biosciences), and further differentiation toward hematopoietic cells was carried out until day 14 in MesoTotal medium. Application of fresh medium and cAMP induction with 10 μM forskolin (Stemgent) and 500 μM IBMX (3-isobutyl-1-methylxanthine; Santa Cruz Biotechnology) was carried out at days 10 and 12 of differentiation. For inhibition of PKA and Epac, 50 μM PKA inhibitor (Rp-8-CPT-cAMPS) (Staples et al., 2001, Yu et al., 2014) and 20 μM Epac inhibitor (ESI-09) (Almahariq et al., 2013, Zhu et al., 2015) (both from BioLog Life Science Institute) were used (20 min pre-treatment followed by cAMP induction) on days 10 and 12 of differentiation, and the cells were analyzed on day 14. These inhibitors occupy cAMP-binding domains of their respective receptor (PKA or Epac), thus blocking further cAMP binding and inhibiting the functions of PKA or Epac. For analysis of hemogenic endothelium, PKA and Epac were inhibited on day 6 (EB stage) and day 8 (adherent stage) and the cells were analyzed on day 10 (schematic regimen, Figure S2E). We selected day 10 to analyze hemogenic endothelium, as this population is less abundant after day 12 (data not shown). For measurement of EB radial spread, the distance between center of an EB and outer edge of its cellular spread was analyzed on day 13 of differentiation (Figure S2C) using ImageJ (developed at the NIH).
Colony Formation Assay
After 14-day hPSC-to-hematopoietic differentiation, the differentiated hematopoietic cells were plated (1 × 104 cells/9.5 cm2) in Methocult H4230 (STEMCELL Technologies) supplemented with 2.5 μg/ml human Stem Cell Factor, 2.5 μg/ml human interleukin-3, 5 μg/ml human granulocyte-macrophage colony-stimulating factor, and 500 U/ml erythropoietin, all recombinant human cytokines from PeproTech. For cells treated with forskolin or IBMX, or a combination of both, similar treatment was continued in the CFU assay (chemicals added to the CFU medium on the first day of CFU assay), and after 12 days hematopoietic colonies were scored microscopically to evaluate various CFU phenotypes.
Flow Cytometry
For analysis of surface markers, cells harvested using TrypLE Select (Thermo Fisher Scientific) were labeled with primary antibodies for 30 min at 4°C. The following fluorophore-conjugated antibodies were used: CD43-FITC (catalog #555475, clone 1G10), CD45RA-V450 (#560363, clone HI100), CD73-PE (#550257, clone AD2), anti-VE-cadherin-Percp-Cy5.5 (#561566, clone 55-7H1) (all from BD Biosciences), and CD34-PE.Cy7 (#343515, clone 581), CD38-APC (#303509, clone HIT2), CD90-PE (#328110, clone 5 × 1010), and CXCR4-BV421 (#306517, clone 12G5) (all from BioLegend). After incubation with the antibodies for 30 min, cells were washed, resuspended in 2% fetal bovine serum (Thermo Fischer Scientific) containing PBS, and acquired with BD FACSCanto (BD Biosciences). To detect oxidative stress, we used CellROX Deep Red (#C10422, Life Technologies) according to the manufacturer's instructions. For live/dead cell discrimination, 7-amino-actinomycin D (BD Biosciences) was applied to the cells before acquisition. Dot plots were derived from gated events based on size and scatter characteristics and doublet-exclusion, fluorescence-minus-one controls were used to identify gating boundaries. Acquired events were analyzed using FlowJo software.
RNA Isolation and qRT-PCR
Total RNA from cells was extracted using an RNeasy Micro Kit (Qiagen) and 500 ng of total RNA was reverse transcribed to cDNA using SuperScript III reverse transcriptase (Life Technologies) according to the manufacturer's instructions. qPCR was performed with gene-specific primers (Table S1) (Dannenmann et al., 2015) using SYBR GreenER qPCR SuperMix (Life Technologies) in a 7900HT Fast Real-Time PCR system (Life Technologies), and the relative expression to housekeeping gene β-ACTIN was analyzed by comparative CT method (Livak and Schmittgen, 2001).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software). Quantitative data represent mean ± SEM, unless otherwise stated, and n represents the number of biological replicates. For statistical evaluation, Student's t test (two-tailed) was used, statistical significance in the figures being indicated by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Author Contributions
Conceptualization, S.S. and N.B.W.; Investigation and data presentation, S.S.; Data analysis, S.S. and N.B.W.; Writing – Original Draft, S.S. and N.B.W.; Writing – Review & Editing, S.S., R.R., C.G., N.B.W.; Methodology, S.S., R.R., C.G., R.M., N.B.W.; Funding Acquisition, N.B.W.; Supervision, S.S. and N.B.W.
Acknowledgments
We are grateful for the support from ES/iPSC core facility, Lund University. This work was funded by grants from: The Swedish Research Council, Swedish Cancer Society, Swedish Children's Cancer Society, AFA Insurance (Sweden), Lund University Medical Faculty, The HematoLinné Program Grant, and Stem Therapy Program Grant.
Published: April 21, 2016
Footnotes
Supplemental Information includes three figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.03.006.
Supplemental Information
References
- Adams G.B., Alley I.R., Chung U., Chabner K.T., Jeanson N.T., Lo Celso C., Marsters E.S., Chen M., Weinstein L.S., Lin C.P. Hematopoietic stem cells depend upon Gsα-mediated signalling to engraft bone marrow. Nature. 2009;459:103–107. doi: 10.1038/nature07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afan A.M., Broome C.S., Nicholls S.E., Whetton A.D., Miyan J.A. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br. J. Haematol. 1997;98:569–577. doi: 10.1046/j.1365-2141.1997.2733092.x. [DOI] [PubMed] [Google Scholar]
- Almahariq M., Tsalkova T., Mei F.C., Chen H., Zhou J., Sastry S.K., Schwede F., Cheng X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 2013;83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet A., Karlsson C., Safaee Talkhoncheh M., Galeev R., Magnusson M., Larsson J. RNAi screen identifies MAPK14 as a druggable suppressor of human hematopoietic stem cell expansion. Blood. 2012;119:6255–6258. doi: 10.1182/blood-2012-01-403949. [DOI] [PubMed] [Google Scholar]
- Beavo J.A., Brunton L.L. Cyclic nucleotide research—still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Beavo J.A., Rogers N.L., Crofford O.B., Hardman J.G., Sutherland E.W., Newman E.V. Effects of xanthine derivatives on lipolysis and on adenosine 3′,5′-monophosphate phosphodiesterase activity. Mol. Pharmacol. 1970;6:597–603. [PubMed] [Google Scholar]
- Chen M.J., Yokomizo T., Zeigler B.M., Dzierzak E., Speck N.A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Ji Z., Tsalkova T., Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim. Biophys. Sin. (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X., Shaw S.K., Andersson L., Hirahashi J., Luscinskas F.W., Mayadas T.N. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Cutler C., Multani P., Robbins D., Kim H.T., Le T., Hoggatt J., Pelus L.M., Desponts C., Chen Y.B., Rezner B. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenmann B., Lehle S., Hildebrand D.G., Kubler A., Grondona P., Schmid V., Holzer K., Froschl M., Essmann F., Rothfuss O. High glutathione and glutathione peroxidase-2 levels mediate cell-type-specific DNA damage protection in human induced pluripotent stem cells. Stem Cell Rep. 2015;4:886–898. doi: 10.1016/j.stemcr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J., Zwartkruis F.J., Verheijen M.H., Cool R.H., Nijman S.M., Wittinghofer A., Bos J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Diaz M.F., Li N., Lee H.J., Adamo L., Evans S.M., Willey H.E., Arora N., Torisawa Y.S., Vickers D.A., Morris S.A. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J. Exp. Med. 2015;212:665–680. doi: 10.1084/jem.20142235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditadi A., Sturgeon C.M., Tober J., Awong G., Kennedy M., Yzaguirre A.D., Azzola L., Ng E.S., Stanley E.G., French D.L. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat. Cell Biol. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken H.M., Nishikawa S., Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Fitch S.R., Kimber G.M., Wilson N.K., Parker A., Mirshekar-Syahkal B., Gottgens B., Medvinsky A., Dzierzak E., Ottersbach K. Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell. 2012;11:554–566. doi: 10.1016/j.stem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele K., Krause D.S., Panaroni C., Saini V., Barry K.J., Liu X., Lotinun S., Baron R., Bonewald L., Feng J.Q. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121:930–939. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W., North T.E., Loewer S., Lord A.M., Lee S., Stoick-Cooper C.L., Weidinger G., Puder M., Daley G.Q., Moon R.T. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W., Allen R.S., Guan X., Jin P., Uchida N., Dovey M., Harris J.M., Metzger M.E., Bonifacino A.C., Stroncek D. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goichberg P., Kalinkovich A., Borodovsky N., Tesio M., Petit I., Nagler A., Hardan I., Lapidot T. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood. 2006;107:870–879. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. Prostaglandins as modulators of immunity. Trends. Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Heidt T., Sager H.B., Courties G., Dutta P., Iwamoto Y., Zaltsman A., von Zur Muhlen C., Bode C., Fricchione G.L., Denninger J. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J., Singh P., Sampath J., Pelus L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jang Y.Y., Sharkis S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L., Tamplin O.J., Chen M.J., Deng Q., Patterson S., Kim P.G., Durand E.M., McNeil A., Green J.M., Matsuura S. Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J. Exp. Med. 2015;212:649–663. doi: 10.1084/jem.20141528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakami Y., Nakao Y., Koizumi T., Katakami N., Ogawa R., Fujita T. Regulation of tumour necrosis factor production by mouse peritoneal macrophages: the role of cellular cyclic AMP. Immunology. 1988;64:719–724. [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Battista M., Kao W.M., Hidalgo A., Peired A.J., Thomas S.A., Frenette P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kim P.G., Nakano H., Das P.P., Chen M.J., Rowe R.G., Chou S.S., Ross S.J., Sakamoto K.M., Zon L.I., Schlaeger T.M. Flow-induced protein kinase A-CREB pathway acts via BMP signaling to promote HSC emergence. J. Exp. Med. 2015;212:633–648. doi: 10.1084/jem.20141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra M.R., Corada M., Dejana E., Bos J.L. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Lai C.Y., Yamazaki S., Okabe M., Suzuki S., Maeyama Y., Iimura Y., Onodera M., Kakuta S., Iwakura Y., Nojima M. Stage-specific roles for CXCR4 signaling in murine hematopoietic stem/progenitor cells in the process of bone marrow repopulation. Stem Cells. 2014;32:1929–1942. doi: 10.1002/stem.1670. [DOI] [PubMed] [Google Scholar]
- Li L., Kim H.T., Nellore A., Patsoukis N., Petkova V., McDonough S., Politikos I., Nikiforow S., Soiffer R., Antin J.H. Prostaglandin E2 promotes survival of naive UCB T cells via the Wnt/beta-catenin pathway and alters immune reconstitution after UCBT. Blood Cancer J. 2014;4:e178. doi: 10.1038/bcj.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucas D., Scheiermann C., Chow A., Kunisaki Y., Bruns I., Barrick C., Tessarollo L., Frenette P.S. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S., Lucas D., Battista M., Frenette P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma'ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi N., Shibata S., Kubota Y., Nakamura M., Nagai Y., Satoh E., Morikawa S., Okada Y., Mabuchi Y., Katoh H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Nie Y., Han Y.C., Zou Y.R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T.E., Goessling W., Walkley C.R., Lengerke C., Kopani K.R., Lord A.M., Weber G.J., Bowman T.V., Jang I.H., Grosser T. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A., Petit I., Kollet O., Magid M., Ponomaryov T., Byk T., Nagler A., Ben-Hur H., Many A., Shultz L. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Raingeaud J., Gupta S., Rogers J.S., Dickens M., Han J., Ulevitch R.J., Davis R.J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Rangarajan S., Enserink J.M., Kuiperij H.B., de Rooij J., Price L.S., Schwede F., Bos J.L. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J. Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans P.H., Gringhuis S.I., van Laar J.M., Sanders M.E., Papendrecht-van der Voort E.A., Zwartkruis F.J., Levarht E.W., Rosas M., Coffer P.J., Breedveld F.C. Rap1 signaling is required for suppression of Ras-generated reactive oxygen species and protection against oxidative stress in T lymphocytes. J. Immunol. 2004;173:920–931. doi: 10.4049/jimmunol.173.2.920. [DOI] [PubMed] [Google Scholar]
- Ronn R.E., Guibentif C., Moraghebi R., Chaves P., Saxena S., Garcia B., Woods N.B. Retinoic acid regulates hematopoietic development from human pluripotent stem cells. Stem Cell Rep. 2015;4:269–281. doi: 10.1016/j.stemcr.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K.B., Padgett W., Daly J.W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B., Semmler J., Eisenhut T., Eigler A., Endres S. Enhanced tumor necrosis factor suppression and cyclic adenosine monophosphate accumulation by combination of phosphodiesterase inhibitors and prostanoids. Eur. J. Immunol. 1995;25:147–153. doi: 10.1002/eji.1830250125. [DOI] [PubMed] [Google Scholar]
- Slukvin I.I. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle. 2013;12:720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint F.G., Kalinski P., Wierenga E.A., Bos J.D., Kapsenberg M.L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- Spiegel A., Shivtiel S., Kalinkovich A., Ludin A., Netzer N., Goichberg P., Azaria Y., Resnick I., Hardan I., Ben-Hur H. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Staples K.J., Bergmann M., Tomita K., Houslay M.D., McPhee I., Barnes P.J., Giembycz M.A., Newton R. Adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent inhibition of IL-5 from human T lymphocytes is not mediated by the cAMP-dependent protein kinase A. J. Immunol. 2001;167:2074–2080. doi: 10.4049/jimmunol.167.4.2074. [DOI] [PubMed] [Google Scholar]
- Suda T., Takubo K., Semenza G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sutherland E.W., Rall T.W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Traver D. Going with the flow: how shear stress signals the emergence of adult hematopoiesis. J. Exp. Med. 2015;212:600. doi: 10.1084/jem.2125insight4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.J., Dudakov J.A., Takahashi K., Shieh J.H., Velardi E., Holland A.M., Singer N.V., West M.L., Smith O.M., Young L.F. Nrf2 regulates haematopoietic stem cell function. Nat. Cell Biol. 2013;15:309–316. doi: 10.1038/ncb2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D.A., Perkins J.P., Krebs E.G. An adenosine 3′,5′-monophosphate-dependent protein kinase from rabbit skeletal muscle. J. Biol. Chem. 1968;243:3763–3765. [PubMed] [Google Scholar]
- Woods N.B., Parker A.S., Moraghebi R., Lutz M.K., Firth A.L., Brennand K.J., Berggren W.T., Raya A., Izpisua Belmonte J.C., Gage F.H. Brief report: efficient generation of hematopoietic precursors and progenitors from human pluripotent stem cell lines. Stem Cells. 2011;29:1158–1164. doi: 10.1002/stem.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.J., Reichner J.S., Mastrofrancesco B., Henry W.L., Jr., Albina J.E. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J. Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T., Takanashi T., Muguruma Y., Ibrahim A.A., Matsuzawa H., Uno T., Sheng Y., Onizuka M., Ito M., Kato S. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Kurita C., Nagao T., Usami E., Nakao T., Watanabe S., Kobayashi J., Yamazaki F., Tanaka H., Nagai H. Effects of cAMP-phosphodiesterase isozyme inhibitor on cytokine production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Gen. Pharmacol. 1997;29:633–638. doi: 10.1016/s0306-3623(96)00580-0. [DOI] [PubMed] [Google Scholar]
- Yu X., Li F., Klussmann E., Stallone J.N., Han G. G protein-coupled estrogen receptor 1 mediates relaxation of coronary arteries via cAMP/PKA-dependent activation of MLCP. Am. J. Physiol. Endocrinol. Metab. 2014;307:E398–E407. doi: 10.1152/ajpendo.00534.2013. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Chen H., Boulton S., Mei F., Ye N., Melacini G., Zhou J., Cheng X. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window”. Sci. Rep. 2015;5:9344. doi: 10.1038/srep09344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Zou P., Wang J., Li L., Wang Y., Zhou D., Liu L. Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells. Ann. Hematol. 2012;91:813–823. doi: 10.1007/s00277-011-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A.C., Hofmann J.J., Lynch M., French W.J., Turlo K.A., Yang Y., Becker M.S., Zanetta L., Dejana E., Gasson J.C. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.