Abstract
Patients with metastatic melanoma who progress on ipilimumab can clearly derive benefit to subsequent anti-PD-1 (programmed death-1). However, patients experience heterogeneous outcomes with ipilimumab, including rapid or delayed progression, and it is unclear whether patterns of ipilimumab progression influence subsequent clinical responses to anti-PD-1. We retrospectively reviewed 116 patients with metastatic melanoma who progressed on ipilimumab and were subsequently treated with pembrolizumab. The study objectives were to determine whether progression-free survival (PFS) to ipilimumab associated with PFS, objective response rate (ORR), and clinical benefit rate (CBR; ORR + stable disease) to pembrolizumab. Patients with PFS ≥ 90 days to ipilimumab had generally superior outcomes with subsequent pembrolizumab compared to patients with PFS<90 days (ORR 49% vs. 35%, P = 0.12; CBR 66% vs. 46%, P = 0.03). Patients with prolonged ipilimumab benefit (PFS ≥ 180 days) had particularly excellent outcomes to pembrolizumab compared to rapid progressors (PFS < 45 days; ORR 55% vs. 25%, CBR 80% vs. 25%, median PFS 249 vs. 50 days). Using logistic regression models, PFS to ipilimumab was independently correlated with response to pembrolizumab (OR 1.22, 95% CI 1.02–1.51). This study shows that prolonged PFS to ipilimumab predicts excellent outcomes to subsequent pembrolizumab, offering valuable prognostic information for clinicians.
Keywords: Melanoma, immune therapy, ipilimumab, nivolumab, pembrolizumab, anti-PD-1, atezolizumab, sequencing
Introduction
The advent of more effective and less toxic immune therapies has revolutionized therapy for patients with metastatic melanoma. Once among the most recalcitrant and therapy-resistant of all cancers, melanoma has been at the leading edge in both immune and genetically targeted therapy advances. We are now faced with choosing between multiple effective therapies and identifying the most optimal treatment sequences.
Ipilimumab, a monoclonal antibody targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4), was the first agent to improve survival in metastatic melanoma (1, 2). Although the objective response rate is low, nearly 20% of patients survive for 5 years, greatly improved from historical controls (3, 4). Anti-PD-1–directed therapies have higher response rates than ipilimumab and also appear to produce durable responses (5, 6). Two of these agents, pembrolizumab and nivolumab, have received regulatory approval for use when patients progress after ipilimumab treatment (7, 8). More recently, both agents have demonstrated superiority to ipilimumab in patients naïve to both drugs and have now received regulatory approval in this setting (9, 10).
Although anti-PD-1 agents have now become the standard first-line immune therapy in most cases, a sizable number of patients have or will receive ipilimumab as initial therapy. Since most of these patients treated with ipilimumab will ultimately experience disease progression, additional therapy will be required. Whereas both pembrolizumab and nivolumab have demonstrated clinical activity in patients who progress on ipilimumab, it is unknown whether the pattern of progression on ipilimumab influences subsequent outcomes to anti-PD-1. We hypothesized that some patients possess an “immune-unresponsive phenotype”, and if they rapidly progress on ipilimumab they are less likely to derive benefit to anti-PD-1 therapies. Therefore, we speculated that the duration of benefit from ipilimumab would correlate with subsequent response to anti-PD-1 (e.g., patients with prolonged PFS with ipilimumab would tend to respond to subsequent anti-PD-1 and vice versa). Identifying this association could provide valuable prognostic information and may help stratify patients unlikely to benefit from immune therapy toward other treatment modalities (e.g., targeted therapy).
To investigate this question, we conducted a retrospective study of patients at Mayo Clinic and Vanderbilt University who had been treated sequentially with both ipilimumab and pembrolizumab from 2011 to January 2015. The primary objective of this study was to determine whether the duration of PFS to ipilimumab influenced patient outcomes to subsequent pembrolizumab therapy.
Methods
Patients
After approval by the institutional review board, the clinical data from 116 patients from May 2011 through January 2015 who received treatment with ipilimumab and pembrolizumab at Mayo Clinic (n = 76) and Vanderbilt University (n = 40) were collected. All patients who received at least one dose of both ipilimumab and pembrolizumab were included in the analysis. At the time of analysis, all surviving patients had been followed for a minimum of 80 days after treatment with pembrolizumab. For this study, we included only patients who received therapy sequentially; we did not include patients treated with combined ipilimumab and nivolumab.
Study Design
Demographic data including age, sex, site of metastatic disease, and lactate dehydrogenase were recorded. We collected treatment results, including objective response (by RECIST 1.1 criteria), progression-free survival, and overall survival for each therapy (11). Interval therapy between ipilimumab and pembrolizumab was also recorded. Tumor response was assessed by cross-sectional imaging after four cycles of ipilimumab, unless clinically deterioration necessitated imaging before all cycles were completed. Ipilimumab was administered at the FDA approved dose of 3 mg/kg. Pembrolizumab was administered at 2 mg/kg every 3 weeks as standard therapy or part of an expanded access program, or at various doses (2–10 mg/kg every 2–3 weeks) through clinical trials.
Statistics
Progression free survival (PFS) was calculated as the time from the first dose of therapy to the date of documented disease progression, and was assessed for ipilimumab and pembrolizumab, respectively. Overall survival (OS) was calculated as the time from therapy start to time of death for any reason. Patients were censored at their last follow-up. Per RECIST 1.1 criteria, complete response was defined as the resolution of all lesions and the absence of new lesions and partial response as a decrease in tumor burden by 30% from the baseline measurements. Objective response rate (ORR) was defined as the rate of complete or partial responses (CR or PR); clinical benefit rate (CBR) was defined as the aggregate of complete and partial responses, and stable disease (SD) lasting at least 3 months (CR + PR + SD).
The outcomes to pembrolizumab were assessed in relation to PFS on prior ipilimumab. We assessed PFS to ipilimumab as a continuous variable and correlated with response to pembrolizumab using ordinal logistic regression models, controlled for age, prior therapies, treatment center, metastatic stage, and lactate dehydrogenase (LDH). Ordinal regression models considered progressive disease, stable disease, and objective response (CR/PR) as ordinal outcomes. We also performed Cox proportional hazards analysis controlling for the same variables to determine whether PFS to ipilimumab predicted PFS to subsequent pembrolizumab. We stratified patients with ≥ 90 day PFS and < 90 day PFS and compared their response to subsequent anti-PD-1 using chi-square testing, and compared subsequent PFS and OS to anti-PD-1 between these two groups using the log rank test. We performed similar analyses stratifying by more extreme values of ipilimumab PFS: < 45 days (“rapid progression”) compared to ipilimumab PFS of > 180 days (“prolonged benefit”). For proof of concept, we also performed these analyses using cutoffs of 60/120 days and stratifying into tertiles. P-values in these analyses represented the likelihood of difference between any group.
Results
Patient Characteristics
A total of 116 patients from all Mayo Clinic sites and Vanderbilt University were included in the final analysis. Of these, 37% of patients were female (n = 42) and 63% of patients were male (n = 73) (Table 1). Ages ranged from 24 to 88 with a mean of 63 years. Some patients (59%, n = 69) received no treatment prior to ipilimumab.
Table 1.
Patient Demographics
| N | % | |
|---|---|---|
|
| ||
| Gender | ||
| Female | 43 | 37 |
| Male | 73 | 63 |
|
| ||
| Age | ||
| 24–88 | Mean 63 | |
|
| ||
| Site Of Metastatic Disease | ||
| Liver | 28 | 24 |
| Lung | 60 | 52 |
| Brain | 16 | 14 |
| Bone | 17 | 15 |
| Lymph | 49 | 42 |
| Other | 45 | 39 |
|
| ||
| Lines of treatment prior to Ipilimumab | ||
| 0 | 69 | 59 |
| 1 | 34 | 29 |
| 2+ | 13 | 11 |
|
| ||
| LDH at start of Ipilimumab | 195 (median) | |
|
| ||
| Lines of interval treatment between ipilimumab and PD-1 | ||
| 0 | 59 | 51 |
| >/=1 | 57 | 49 |
|
| ||
| Interval Radiation | 19 | 16 |
|
| ||
| Interval BRAF inhibitor | 22 | 19 |
|
| ||
| LDH at start of anti-PD-1 | 238 (median) | |
|
| ||
| Status at last follow-up | ||
| Alive | 77 | 66 |
| Dead | 39 | 34 |
Among all patients treated with ipilimumab, the median PFS was 94 days. Of these, 75% (n = 86) had progressive disease as their best response to ipilimumab, 6% (n = 7) had a partial response and 18% (n = 21) had stable disease. Following treatment with ipilimumab, 67 patients had an interim treatment, whereas the remaining patients were treated with pembrolizumab immediately after progression on ipilimumab. Of all patients then treated with pembrolizumab, 35% (n = 41) had a partial response, 7% (n = 8) had a complete response, 14% (n = 16) had stable disease, and 44% (n = 51) had primary disease progression on pembrolizumab, with a median PFS of 176 days. The median OS from the time of ipilimumab administration was not reached; at the time of analysis 67% of patients remained alive (n = 77). The median time between ipilimumab and pembrolizumab initiation was 257 days, and the median time of follow-up after starting pembrolizumab was 174 days.
Ipilimumab PFS correlated with subsequent pembrolizumab outcomes
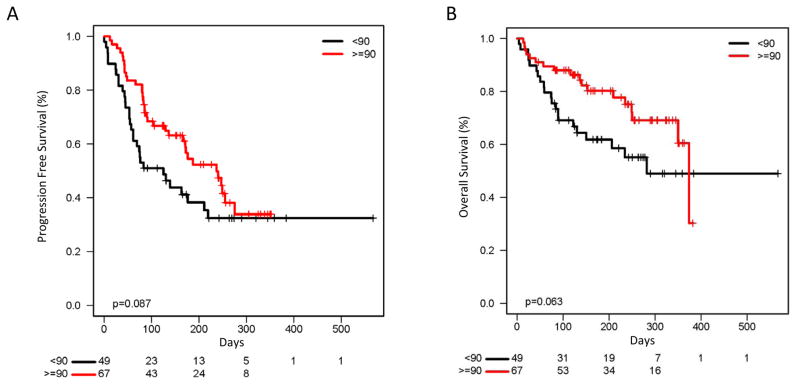
We evaluated whether outcomes to pembrolizumab varied in relation to prior PFS on ipilimumab. We performed a multivariate Cox proportional hazards analysis to control for known prognostic variables: age, metastatic stage, prior therapy, and LDH. Ipilimumab PFS, as measured in months, was significantly associated with decreased odds of pembrolizumab progression (odds ratio 0.85, P = 0.02). To identify whether particular PFS cutoffs were clinically useful, we stratified patients by PFS to ipilimumab of greater than or less than 90 days. Patients with ≥ 90 day PFS to ipilimumab had a similar ORR to pembrolizumab compared to those with <90 day PFS (49% vs. 35%, P = 0.12) but a greater clinical benefit rate (CBR; 66% vs. 46%, P = 0.03) (Table 2). The median PFS to pembrolizumab also appeared greater in the ≥ 90 day group (237 vs. 125 days, P = 0.09) although this was not statistically significant (Fig. 1A). Overall survival also appeared somewhat higher in the ≥ 90 day cohort (median OS 374 vs. 282 days, P = 0.06) (Fig. 1B).
Table 2.
Response to Pembrolizumab based on Progression Free Survival (PFS) to Ipilimumab
| Ipilimumab PFS | |||
|---|---|---|---|
| <90 days (n=57) | ≥90 days (n=59) | P value | |
| Objective Response Rate | 20 (35%) | 29 (49%) | 0.12 |
| Clinical Benefit Rate | 26 (46%) | 39 (66%) | 0.03 |
| <45 days (n=12) | ≥180 days (n=20) | ||
| Objective Response Rate | 3 (25%) | 11 (55%) | 0.09 |
| Clinical Benefit Rate | 3 (25%) | 16 (80%) | 0.002 |
Figure 1.
Figure 1A: Progression free survival to pembrolizumab in patients with ≥ 90 day PFS vs patients with < 90 day PFS to ipilimumab, (237 days vs 125 days, P = 0.09).
Figure 1B: Overall survival to pembrolizumab in patients with ≥ 90 day PFS vs patients with < 90 day PFS to ipilimumab, (374 days vs 282 days, P = 0.06)
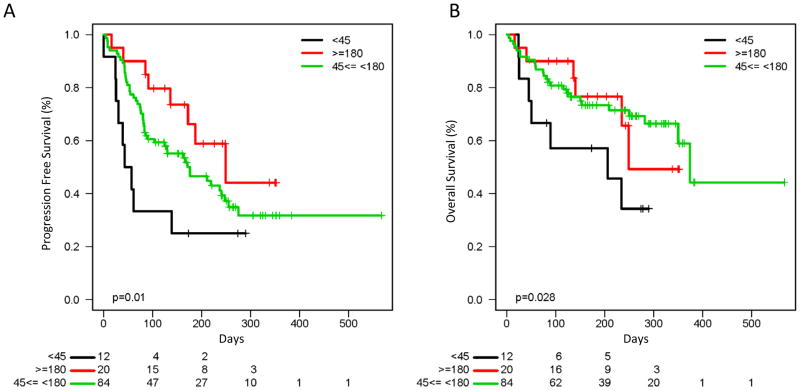
To assess patients with more extreme phenotypes, we then compared outcomes to pembrolizumab for rapid ipilimumab progressors (PFS < 45 days; n = 12) compared to those with prolonged prior ipilimumab benefit (PFS ≥ 180 days; n = 20). Patients with prolonged ipilimumab benefit had a seemingly higher ORR to pembrolizumab (55% vs. 25%, P = 0.09) and CBR (80% vs. 25%, p<0.01). Other outcomes were also superior in the prolonged benefit group compared to rapid progressors and to all other patients, including PFS (249 vs. 50 vs.176 days; P = 0.01), and OS (median 249 vs. 206 vs. 374 days; P = 0.03) (Fig. 2A and B). Similar stratification was observed when using other cutoffs to define rapid progression and prolonged benefit, including 60 and 120 days, and dividing patients into tertiles based on PFS (Supplementary Figs. S1 and S2). We also assessed whether prior response to ipilimumab correlated with PFS to pembrolizumab. Although only 7 patients experienced a RECIST-defined response to ipilimumab in this cohort, PFS and OS were (P = 0.027, P = 0.242 respectively)higher for these patients (Supplementary Fig. S3). Of these 7 patients, 4 experienced PR/CR to pembrolizumab, and the other 3 had stable disease (ongoing in 2).
Figure 2.
Figure 2A: Progression free survival to pembrolizumab based on prolonged benefit (≥ 180 days), rapid progression (< 45 days), and all others on ipilimumab (249 vs 50 vs 176 days, P = 0.01)
Figure 2B: Overall survival to pembrolizumab based on prolonged benefit (≥ 180 days), rapid progression (< 45 days), and all others on ipilimumab (249 vs 206 vs 374 days, P = 0.03)
A multivariable ordinal logistic regression model was used to investigate the correlation between ipilimumab PFS with response to pembrolizumab controlled for age, prior therapy, metastatic stage, and LDH. Ipilimumab PFS (measured in months) was independently correlated with subsequent pembrolizumab response (odds ratio 1.22, P = 0.04).
Conclusions
The advent of several effective immune checkpoint inhibitors has markedly improved melanoma outcomes. In this study, we assessed patients who were treated with pembrolizumab after progressing on ipilimumab, and found that after accounting for other known prognostic variables, ipilimumab PFS was independently associated with pembrolizumab outcomes. In particular, patients with prolonged benefit from ipilimumab had excellent response rates, PFS, and OS to pembrolizumab. In contrast, patients with rapid progression to ipilimumab tended to have a worse outcome to pembrolizumab. A subset of these patients, however, did experience a response and had prolonged benefit. This suggests that “immune-responsive” and “immune-resistant” phenotypes may be shared among distinct therapies.
In view of numerous clinically-active immune and targeted therapies, understanding the most effective sequences and combinations is a major priority. Either pembrolizumab or nivolumab as monotherapy, or the combination of ipilimumab and nivolumab, are all superior to single-agent ipilimumab (9, 10). A retrospective study by our group and others suggested that ipilimumab could benefit patients who previously progressed on high-dose interleukin-2 (IL-2), regardless of the degree of response or PFS to IL-2 (12). Two other retrospective studies have suggested that ipilimumab rarely benefits patients following progression on BRAF inhibitors, but that BRAF inhibitors may be effective after immune therapy failure (13, 14). This study, however, assessed the correlation between ipilimumab and pembrolizumab benefit and found a potentially useful association.
Whereas pembrolizumab, nivolumab, or even combined ipilimumab and nivolumab have already become the first-line immune therapy, these data have value for several reasons. First, they suggest that overlap exists between patients who benefit from different immune therapies, implying a shared immune phenotype. Second, our data suggest that prognostic information may be provided for the many patients who have or will be treated with ipilimumab in the first-line, either due to prolonged responses or to delays in practice pattern changes. In particular, this information may inform treatment for patients with more durable benefit from ipilimumab who ultimately progress, and provides a possible treatment alternative to ipilimumab re-induction. Third, these data suggest that assessing other treatment orders is critically important. For example, investigating the outcomes of patients treated with ipilimumab following anti-PD-1 failure will be particularly vital. A prior study has reported that 2 of 12 patients responded to ipilimumab following nivolumab failure (15), although a much larger experience will be needed for any firm conclusions.
This study has several limitations. Patients were treated largely with standard of care therapy (off clinical trials) and were therefore subject to nonstandardized timing for tumor assessments by cross-sectional imaging. Second, ipilimumab PFS may be difficult to accurately measure given the occasional atypical, immune-related responses. In this study we used RECIST 1.1 criteria to standardize PFS calculations. Finally, the follow-up time on pembrolizumab was relatively short, limiting our ability to evaluate prolonged survival data, although differences in outcomes were particularly striking in the first several months on therapy. Despite these limitations, we observed a correlation between ipilimumab PFS and subsequent responses to pembrolizumab.
In conclusion, we observed that the duration of PFS with ipilimumab correlated with subsequent pembrolizumab treatment responses. This study provides useful prognostic information for patients treated with immune therapies and suggests investigation into shared immune features that predict benefit (or lack thereof) from both ipilimumab and pembrolizumab.
Supplementary Material
Acknowledgments
Research Support: DBJ – K12 CA0906525
Footnotes
Conflicts of Interest: DBJ – Advisory board, Genoptix, BMS
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O’Day S, MDJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 8.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Joseph RW, Eckel-Passow JE, Sharma R, Liu P, Parker A, Jakob J, et al. Characterizing the clinical benefit of ipilimumab in patients who progressed on high-dose IL-2. J Immunother. 2012;35:711–5. doi: 10.1097/CJI.0b013e3182742c27. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman A, McDermott DF, Lawrence DP, et al. Outcomes of patients with malignant melanoma treated with immunotherapy prior to or after vemurafenib. J Clin Oncol. 2012;30:8569. [Google Scholar]
- 14.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med. 2012;10:107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–8. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.