Abstract
Healthy individuals maintain remarkably constant skeletal muscle mass across much of adult life, suggesting the existence of robust homeostatic mechanisms. Muscle exists in dynamic equilibrium whereby the influx of amino acids (AAs) and the resulting increases in muscle protein synthesis (MPS) associated with the intake of dietary proteins cancel out the efflux of AAs from muscle protein breakdown that occurs between meals. Dysregulated proteostasis is evident with aging, especially beyond the sixth decade of life. Women and men aged 75 y lose muscle mass at a rate of ∼0.7% and 1%/y, respectively (sarcopenia), and lose strength 2- to 5-fold faster (dynapenia) as muscle “quality” decreases. Factors contributing to the disruption of an otherwise robust proteostatic system represent targets for potential therapies that promote healthy aging. Understanding age-related impairments in anabolic responses to AAs and identifying strategies to mitigate these factors constitute major areas of interest. Numerous studies have aimed to identify 1) the influence of distinct protein sources on absorption kinetics and muscle anabolism, 2) the latency and time course of MPS responses to protein/AAs, 3) the impacts of protein/AA intake on muscle microvascular recruitment, and 4) the role of certain AAs (e.g., leucine) as signaling molecules, which are able to trigger anabolic pathways in tissues. This review aims to discuss these 4 issues listed, to provide historical and modern perspectives of AAs as modulators of human skeletal muscle protein metabolism, to describe how advances in stable isotope/mass spectrometric approaches and instrumentation have underpinned these advances, and to highlight relevant differences between young adults and older individuals. Whenever possible, observations are based on human studies, with additional consideration of relevant nonhuman studies.
Keywords: muscle, amino acids, metabolism, aging, leucine
Introduction—A Brief History of the Role of Amino Acids in Skeletal Muscle Metabolism
In the 1930s Nobel laureate George Whipple first proposed that tissue proteins, including skeletal muscle, existed in a dynamic equilibrium with circulating plasma proteins. This challenged the conventional belief of separate exogenous and endogenous metabolic pools. He showed that sugar-fed animals could produce plasma proteins at the cost of tissue and, conversely, could maintain muscle mass, good health, and sustained nitrogen equilibrium when transfused only with plasma proteins (1). Within a decade it was shown that the sole protein derivatives that are both essential and sufficient to maintain health and nitrogen equilibrium were the amino acids (AAs)8 threonine, valine, leucine, isoleucine, lysine, tryptophan, phenylalanine, methionine, and histidine—the “essential” AAs (EAAs) (2). By the 1970s, in vitro work in skeletal muscle preparations provided evidence that the role of AAs may not simply be as substrates for protein synthesis, with each having the potential to limit synthesis dependent on availability and the relative contribution of tissues (3). Clues as to the underlying AA metabolic pathways “sensing” AAs emerged in the 1980s when some of the signaling molecules responsible for the anabolic effects of AAs were defined (4). More recently, with the use of contemporary molecular biology techniques, researchers have more completely elucidated the molecular underpinnings of cellular AA sensing [via leucine (5)]. Developments in stable isotope methods and analytical MS allowed the measurement of rates of protein synthesis and breakdown during fasting and feeding (6) and permitted the determination of the latency, duration, and dose-response of muscle to AA feeding (7–9) directly in humans.
AAs as the Anabolic Constituents of Nutrition
After the identification of the anabolic effects of a mixed-macronutrient meal in promoting muscle protein synthesis (MPS) >30 y ago (6), it was soon shown that the AA constituents of protein were the bioactively anabolic constituents (10) that are both necessary and sufficient for the stimulation of MPS. For example, a number of studies indicated that boluses of leucine, phenylalanine, valine, and threonine (all EAAs) were all capable of stimulating MPS in humans. In contrast, arginine, glycine, and serine [nonessential AAs (NEAAs)] did not recapitulate this stimulation (11, 12). Importantly, EAAs cannot undergo de novo synthesis and must be acquired through the diet, whereas NEAAs can be synthesized via AA interconversions and scavenger pathways (13). It perhaps makes physiologic sense that the “signal” to build muscle (an energy-demanding process due to ATP demands of AA transport and peptide bonding) indicates the ingestion of foods containing those AAs the body cannot make itself, simultaneously predicting energy sufficiency. Moreover, the majority of NEAA constituents of protein (e.g., alanine, glutamate, and glutamine) are sequestered by splanchnic tissue, with glutamine being used as an energy source for enterocytes and alanine by the liver for gluconeogenesis (14, 15). In contrast, only a relatively small percentage of EAAs are taken up by splanchnic tissues (16), such that these are more available to maintain proteostasis in other systemically perfused organs (i.e., skeletal muscle).
Of all the EAAs, leucine has a particularly central role in regulating MPS. The provision of a small dose of leucine (3 g) to humans has been shown to provide a robust stimulation of MPS despite the absence of any other AA (17). Moreover, doses as low as 1.7 g may be sufficient to maximize the anabolic response of muscle in young adult humans. In a study that provided a suboptimal dose of EAAs (6.7 g) in which the proportion of leucine was adjusted from 26% (1.7 g) to 41% (2.8 g), there was no additional benefit of increasing the leucine content (18). Moreover, in a recent study that compared the response of MPS to 20 g whey protein or a low-dose leucine (1.2 g)–enriched EAA mix, increases in MPS were identical (19), highlighting the potent stimulatory effects of leucine. Although it has been established that leucine is a potent signaling molecule (mechanisms are discussed later) and thereby a stimulator of MPS, to our knowledge there has not yet been a dose-response study focused exclusively on leucine in humans.
Leucine (in addition to valine and isoleucine) is a BCAA, which, unlike the other EAAs, are primarily metabolized within skeletal muscle (20). Therefore, it has been proposed that some of the metabolites of BCAAs could have anabolic activity. The first stage of leucine metabolism is reversible transamination to its keto-acid, α-ketoisocaproate (KIC), regulated by the enzyme branched-chain amino transferase (3). Indeed, infusions of KIC alone can stimulate MPS similar to an infusion of leucine (21). This stimulation may, however, have been driven by the reversible transamination of KIC back to leucine, whereby leucine itself stimulated MPS rather than KIC, because plasma concentrations of leucine increased significantly with the infusion of KIC alone. After the reversible transamination of leucine, KIC can be irreversibly metabolized to isovaleryl-CoA, providing carbon skeletons for the formation of energy substrates (22). Although the vast majority of leucine catabolism follows this pathway, a small percentage (∼5%) can be converted to the metabolite β-hydroxyl-β-methylbutyrate (HMB) (23), a metabolite primarily involved in cholesterol synthesis through the production of β-hydroxy-β-methylglutaryl coenzyme A (24). This metabolite has sparked interest in recent years because it has potential anabolic, and anticatabolic, properties. The oral administration of 3 g HMB leads to the stimulation of MPS equivalent to that provided by leucine alone, with the concomitant inhibition of leg protein breakdown (17). Although it would take a large amount of leucine (∼60 g) to provide the equivalent amount of HMB, these data do provide the first evidence, to our knowledge, of anabolic activity for distal leucine metabolites. However, the benefit of consuming HMB over other AAs is somewhat contentious considering that MPS is almost maximally stimulated through equivalent small (∼3 g) doses of leucine alone (17).
Latency and Time Course of MPS Responses to AAs
The anabolic responses to protein/EAAs are dose-dependent and transient in nature. It is generally accepted that the maximal anabolic response to nutrition is achieved through the intake of between 20 and 40 g high-quality protein, such as meat (25), whey protein (8), or 10–20 g EAAs (26). Nonetheless, a bona fide dose-response to increasing protein quantities is somewhat overshadowed by studies that showed that the provision of just 3 g leucine alone (17) or low-dose leucine-enriched EAA feedings (19) provides a robust, even maximal, response in acute MPS. Thus, it seems most likely that the potent anabolic signaling properties of leucine (discussed in more detail later) are sufficient to maximize MPS in the short term through a process that uses intracellular EAAs, potentially leading to a decrease in intracellular EAA pools (12). When providing doses of EAAs beyond those being used as substrates for MPS (and for other tissues), excess circulating AAs are directed through oxidation and urea synthesis pathways (27), with the remaining carbon skeletons being made available for gluconeogenesis and ketogenesis in other tissues. This return to baseline of circulating AAs also appears to be dose-dependent, with higher doses requiring greater time for excess AAs to be utilized (28, 29).
Current studies have also determined the time course of responses to protein/EAA feeding. After protein/ EAA intake there is an initial lag of ∼45–90 min before the MPS response peaks at between ∼1.5 and 2 h; after this, MPS rapidly returns to postabsorptive rates despite the maintenance of substrate availability and anabolic signaling, the onset of the so-called muscle-full state (8, 9). This concept was proposed ∼20 y ago (7, 30), but recent improvements in analytic sensitivity, coupled with the development and refinement of hybrid GC–combustion–isotope ratio MS, and the availability of multiply labeled tracers have experimentally supported this theory (8). This latency and duration of responses to feeding may to some extent also depend on the amount or composition of protein/EAAs ingested (i.e., di/tripeptides from proteins are more rapidly absorbed than free AAs) (31). Nonetheless, it seems physiologically intuitive that skeletal muscles replenish protein stores lost during postabsorptive periods (via AA-induced stimulation of MPS), and that when this is complete the muscle senses this and “switches off” MPS. The concept of mechanisms that inhibit MPS and enforce a muscle-full state is further supported by the fact that increased protein alone is not sufficient to achieve muscle hypertrophy; it requires the additional stimulation of loading contractions (32). The actual mechanisms that underlie the transition from stimulated, postprandial MPS back to fasting rates despite nutrient availability have not been elucidated. Preclinical evidence suggests that this may be driven by a block in translation at the elongation phase, through increased energy stress, an increase in the AMP:ATP ratio, and the induction of adenosine 5′-monophosphate kinase (AMPK) signaling (33, 34). However, this mechanism is questionable in weight-stable adult humans, because there is a lack of evidence for increases in AMPK with feeding, whereas intracellular leucine concentrations remain high even when MPS returns to baseline levels (8). Other proposed mechanisms include endoplasmic reticulum stress, whereby the accumulation of un/misfolded, recently transcribed peptides affects ribosomal function.
“Anabolic Resistance” to Protein/AA Nutrition in Older Age
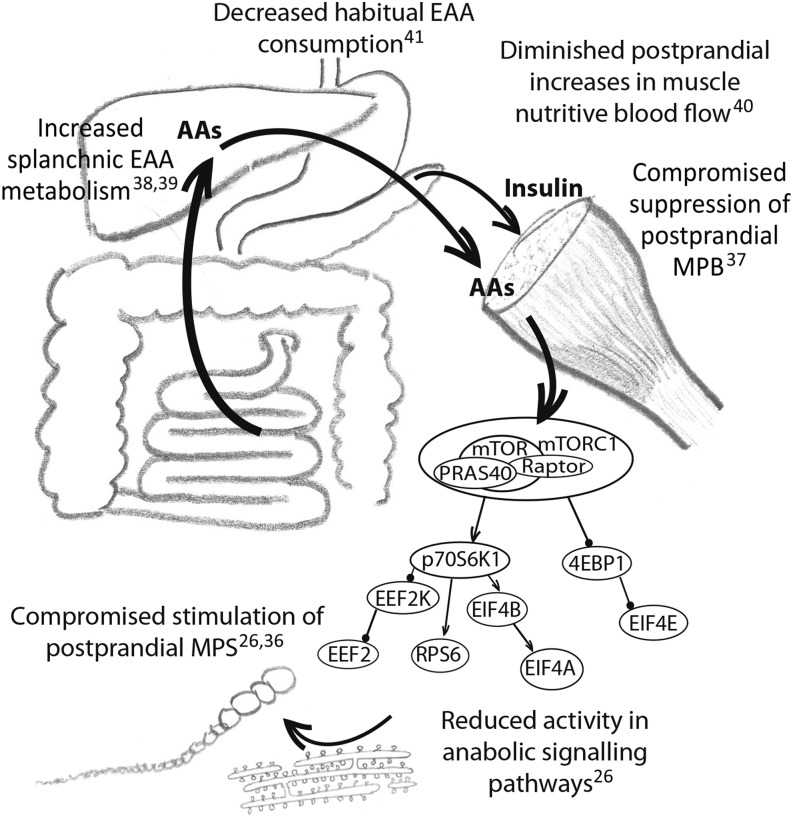
The role of AAs as key regulators of muscle protein metabolism has led to increasing interest in harnessing their inherent anabolic potential to mitigate the age-related loss of muscle mass and function. Increasing elderly populations across the globe have led to increased health care costs associated with frailty and ill health, prompting a search for potentially modifiable risk factors, such as nutritional behaviors (35). Diminishing muscle mass, such as that which occurs with aging, typically detectable from the age of ∼60–70 y onward, reflects a chronic deficit in net protein balance in which MPS fails to match muscle protein breakdown (MPB) across a prolonged period. Potential contributing factors are summarized in Figure 1.
FIGURE 1.
Factors that may affect skeletal muscle protein metabolism in older individuals, shifting the dynamic equilibrium toward a net loss of muscle mass. AA, amino acid; EAA, essential amino acid; EEF2, eukaryotic elongation factor 2; EEF2K, eukaryotic elongation factor 2 kinase; EIF, eukaryotic initiation factor; MPB, muscle protein breakdown; MPS, muscle protein synthesis; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; p70S6K1, ribosomal protein S6 kinase; PRAS40, proline-rich Akt substrate of 40 kDa; RPS6, ribosomal protein S6; 4EBP1, 4E-binding protein.
There are no detectable differences in the fasting rates of MPS between old and young men (26, 42–44), suggesting that this is not a major driver. Instead, there is a measurable blunting of the response to EAA feeding in older men (so-called anabolic resistance) (26), whereas anticatabolic effects of insulin secretion resulting from carbohydrate/protein/EAA ingestion are also blunted (45). This, in essence, means that it requires greater relative protein intakes in older than in younger individuals to stimulate MPS (46). Collectively, this desensitization to both of the key anabolic nutrient-driven stimuli (EAAs and insulin) regulating MPS and MPB likely promotes the chronic development of sarcopenia. However, although these changes are reproducible they are unlikely to represent the whole picture of age-related sarcopenia. The typical rate of progression of sarcopenia (−0.9% muscle mass/y) would represent an average negative balance of 10−4%/h. Assuming that basal fasting MPS and MPB in old and young individuals are identical and all net protein loss occurs during the postprandial period, the rate of loss would not exceed 10−3%/h. Thus, the measurable age-related differences in MPS and MPB described above must actually reflect only part of a complex age-related change in skeletal muscle proteostasis that avoids the rapid and catastrophic wasting otherwise predicted by detectably compromised postprandial responses. This is not to say that improving measurable anabolic responses to acute protein/AA feeding in older people is not a worthwhile research goal, because there is evidence that maintaining postprandial anabolism reduced muscle loss in preclinical studies (47).
Relatively greater amounts of ingested protein are needed to achieve acute postprandial MPS in old than in young individuals (46), and community-dwelling older adults with protein consumption in the lowest quintile (albeit above the RDA of 0.8 g ⋅ kg−1 ⋅ d−1) lost 40% more lean mass in 3 y than did those in the highest quintile (48). However, whether interventions aimed at enhancing anabolic responses to protein/EAAs yield positive effects on important physiologic variables such as lean mass or functional outcomes remains contentious, with some studies showing effects (49) and others not (50).
AA Absorption Profiles as a Putative Determinant of Muscle Anabolism
The appearance profile of protein/EAAs has long been considered important in the regulation of muscle protein anabolism. This is especially true in the context of distinct dietary protein sources, which greatly influence absorption kinetics and plasma AA availability. The kinetics of absorption has commonly been monitored by using milk or animal-based proteins made up of 2 distinct fractions: soluble whey and insoluble micellar casein (51). Upon consumption of milk proteins, absorption occurs in 2 phases: an initial “fast” absorption due to the whey fraction (which is quickly hydrolyzed and provides a rapid onset of aminoacidemia) and a delayed “slow” absorption due to casein [which provides a more prolonged and gradual increase in plasma AAs due to the formation of a gel or clot in the acidic environment of the stomach, delaying hydrolysis, gastric emptying, and absorption (52, 53)]. Rapid-onset aminoacidemia was believed to enhance muscle anabolism on the basis of early work highlighting close relations between blood AA concentrations and increases in MPS when a mixed amino acid infusion was provided to increase AA concentrations (54). After this, a number of studies proposed that rapid aminoacidemia contributes to the apparent anabolic favorability of whey over casein (20 g whey protein achieves postprandial MPS rates double that of 20 g casein) (55–57).
However, this notion remains contentious, with studies failing to provide consensus evidence of a benefit from any single delivery profile (see Table 1). In addition to having different rates of absorption (52), whey and casein also have different constituent AAs; whey provides greater amounts of EAAs, and in particular leucine, than does casein per gram of protein (57). This latter aspect may explain the apparent superior ability of whey protein to stimulate MPS when compared with casein (55–57); the composition or dose of EAAs provided (leucine in particular), rather than delivery profile or plasma appearance, may be the key determinant of MPS response to feeding. In support of this notion, no significant difference was found in MPS responses to casein compared with (rapidly absorbed) casein hydrolysate (61). Although the majority of this research has concentrated on so-called animal-based protein sources (whey and casein), there has also been interest in alternative sources of protein such as plant-based soy. Unfortunately, the lack of robust studies that used these protein sources and methodologic inconsistencies mean that the findings to date are somewhat conflicting; some suggest that equal quantities of whey and soy elicit similar rates of MPS that are significantly higher than the equivalent amount of casein (56), whereas others have shown identical rates of MPS when comparing soy and casein (68). Recent studies have found no difference in total MPS to equivalent 15-g doses of EAAs delivered either as a single bolus (providing rapid aminoacidemia within 45 min) or as small fractions delivered at 45-min intervals (providing low-amplitude aminoacidemia spread over >2 h) (9, 63). These findings suggest that when composition and dose are matched, delivery profile may not be a determining factor in anabolism. Interestingly, a prolongation of stimulated postprandial MPS has been shown with gradual or low-amplitude aminoacidemia, which may explain how adequate MPS can be achieved with such delivery profiles. Furthermore, the MPS response to 3 g of 40% leucine–enriched AAs, which provided minimal postprandial increases in total AA concentrations beyond that of leucine, was comparable to that of a 20-g whey bolus, which provided rapid aminoacidemia (19), whereas the provision of just 3 g leucine to young men after an overnight fast also resulted in an approximately maximal stimulation of MPS (17). The acute stimulation of MPS seems to be dependent on the dose of EAAs/leucine rather than the rates of plasma AA appearance.
TABLE 1.
Summary of studies that compare the anabolic responses across different protein/EAA absorption profiles1
| Study (ref) | Comparator | Study group | Study period | Study substrate | Technique | Conclusion |
| Boirie et al. (52) | Rapidly vs. slowly digested protein | Healthy adults, aged ∼24 y, at rest | 7 h | 30 g whey protein vs. 43 g casein (leucine matched) | Whole-body leucine kinetics (dual tracer) | Benefit; slow AA appearance |
| Dangin et al. (58) | Fast vs. slow AA appearance | Healthy men, aged ∼25 y, at rest | 7 h | 30 g whey/30 g free AAs representative of casein vs. 30 g casein/30 g whey in delayed fractions | Whole-body leucine kinetics (dual tracer) | Benefit; slow AA appearance |
| West et al. (59) | Rapidly vs. slowly digested protein | Healthy men, aged ∼21 y, after resistance exercise | 5 h | 25 g whey bolus vs. 2.5-g (×10) whey “pulses” at 20-min intervals | FSR by l-[ring-13C6]Phe incorporation | Benefit; rapid AA appearance |
| Burke et al. (60) | Fast vs. slow AA appearance | Healthy men after resistance exercise | 5 h | 25 g whey + 5 g leucine, single bolus or in 15 spread fractions at 15-min intervals | FSR by stable isotope incorporation | No difference between regimens |
| Dangin et al. (28) | Rapidly vs. slowly digested protein in young and old individuals | Healthy men, aged ∼20 y and ∼72 y, at rest | 7 h | ∼34 g casein vs. ∼34 g whey (nitrogen matched) vs. ∼22 g whey (leucine matched) | Whole-body leucine kinetics (dual tracer) | Benefit; rapid AA appearance |
| Tang et al. (56) | Rapidly vs. slowly digested protein | Healthy men, aged ∼23 y, after resistance exercise | 3 h | Whey, soy protein isolate, and casein, each to provide 10 g EAAs | FSR by l-[ring-13C6]Phe incorporation | Benefit; rapid AA appearance |
| Pennings et al. (57) | Rapidly vs. slowly digested protein | Healthy men, aged ∼74 y, at rest | 6 h | 20 g whey, 20 g casein, or 20 g casein hydrolysate | FSR by l-[1-13C]Phe incorporation | Benefit; rapid AA appearance |
| Koopman et al. (61) | Rapidly vs. slowly digested protein | Healthy men, aged ∼64 y, at rest | 6 h | 35 g casein vs. 35 g casein hydrolysate | FSR by l-[1-13C]Phe incorporation | No difference between regimens |
| Reitelseder et al. (62) | Rapidly vs. slowly digested protein | Healthy men, aged ∼28 y, after resistance exercise | 6 h | 0.3 g whey or casein/kg | FSR by l-[1-13C]Phe incorporation | No difference between regimens |
| Mitchell et al. (9) | Fast vs. slow EAA arrival | Healthy men, aged ∼20 y, at rest | 4 h | 15 g mixed EAAs given as single bolus or in 4 spread fractions at 45-min intervals | FSR by l-[ring-13C6]Phe incorporation | No difference between regimens |
| Mitchell et al. (63) | Fast vs. slow EAA arrival | Healthy men, aged ∼70 y, at rest | 4 h | 15 g mixed EAAs given as single bolus or in 4 pulsed fractions at 45-min intervals | FSR by l-[ring-13C6]Phe incorporation | No difference between regimens |
| el-Khoury et al. (64) | 3 meals vs. 12 “snacks” | Healthy men, aged ∼21 y, at rest | 24 h | Egg solids | Leucine oxidation (single tracer) | Benefit; fewer, larger meals |
| Areta et al. (65) | Protein ingestion in large, intermediate, and small fractions | Healthy men, aged ∼25 y, after resistance exercise | 12 h | 80 g whey; 2, 4, or 8 fractions | FSR by l-[ring-13C6]Phe incorporation | Benefit; intermediate-sized protein meals |
| Mamerow et al. (66) | Daily protein intake “focused” at 1 meal or spread across 3 meals | Healthy adults, aged ∼37 y | 7 d | Standardized mixed meals; ∼30 g dietary protein at each of 3 meals or 10 g at breakfast, 15 g at lunch, and 65 g at dinner | FSR by l-[ring-13C6]Phe incorporation across 24 h | Benefit; even spread of protein across 3 meals vs. focused at 1 meal |
| Arnal et al. (38) | Daily protein intake “focused” at 1 meal or spread across 4 meals | Healthy women, aged ∼26 y | 14 d | Mixed meals, ∼80% of daily intake of protein consumed (“pulsed”) at midday meal vs. spread evenly across 4 meals | Nitrogen balance and whole-body protein turnover | No difference between regimens |
| Arnal et al. (67) | Daily protein intake “focused” at 1 meal or spread across 4 meals | Healthy women, aged ∼64 y | 14 d | Mixed meals, ∼80% of daily intake of protein consumed (“pulsed”) at midday meal vs. spread evenly across 4 meals | Nitrogen balance and whole-body protein turnover | Benefit; protein focused at 1 meal vs. even spread feeding |
| Bouillanne et al. (39) | Daily protein intake “focused” at 1 meal or spread across 4 meals | Elderly malnourished or at-risk inpatients, aged ∼85 y | 6 wk | Mixed meals, ∼70% of daily intake of protein consumed (“pulsed”) at midday meal vs. spread evenly across 4 meals | Randomized controlled trial; body composition/clinical endpoints | Benefit; protein focused at 1 meal vs. even spread feeding |
Studies were identified by using the MEDLINE database with the following search keywords: “amino acid" [all fields] AND “Protein Synthesis" [all fields] AND (“BOLUS" [all fields] OR “PULSE" [all fields] OR “profile" [all fields]), and relevant articles were identified from this search. The conclusion of “benefit” was made upon demonstration of significant enhanced muscle protein synthesis, net protein balance, or body-composition/clinical endpoints depending on the technique used. AA, amino acid; EAA, essential amino acid; FSR, fractional synthetic rate; ref, reference.
An alteration in protein digestion kinetics has also been suggested as one of the mechanisms that contributes to the anabolic blunting observed with aging. Early findings suggested that in older adults the splanchnic metabolism may be altered, leading to an increased first-pass extraction of AAs by the splanchnic tissues, with the consequence that less enters the systemic circulation and so less is available for utilisation elsewhere in the body (69–71). However, subsequent studies have opposed these early findings. Small intakes (∼7 g EAAs) achieve similar plasma EAA concentrations in old and young individuals (18, 40), whereas the ingestion of larger doses (∼15 g EAAs, 30 g protein) results in higher plasma EAA concentrations in the elderly and this hyperaminoacidemia is prolonged, with a slower return to baseline values (9, 26, 63, 72). This likely reflects the inability of the elderly to “handle” or utilize large doses of AAs in a metabolically efficient manner, which may be due to several possible contributory factors: 1) MPS is blunted in older individuals (8, 9, 26, 63), 2) older individuals have less whole-body muscle mass (73), 3) the anticatabolic effect of insulin is reduced (45) thereby slowing the clearance of excess AAs from the plasma pool, and 4) the rates of phenylalanine hydroxylation are slower (72). Evidence thus exists to argue against suggestions that age-related changes in the digestion and absorption of ingested dietary protein substantially contribute to the development of sarcopenia.
Muscle Microvascular Blood Flow as a Potential Determinant of Muscle Anabolism
Another major area of emerging interest is the impact of nutrient intake on perfusion of the muscle microvasculature (so-called nutritive flow) in the context of delivery of AAs to the sarcolemmal AA transporters and resulting skeletal muscle anabolism. This field has been facilitated by developments in contrast-enhanced ultrasonography, which permits the assessment of microvascular blood volume (reflecting capillary recruitment) and microvascular flow velocity (reflecting proximal vasodilation), which together represent muscle perfusion. In young adults, increases in skeletal muscle microvascular blood flow are a reproducible feature of nutrient intake, either from mixed meals (74) or EAAs (37). Capillary recruitment (observed within 45 min) precedes increases in limb blood flow (shown at 2–3 h postintake) (37). It is likely that insulin plays a role in mediating postprandial microvascular responses to normal eating via NO-dependent vasodilation of precapillary arterioles (75, 76). Euglycemic, hyperinsulinemic clamps produce similar responses in muscle microvascular flow to consumption of a mixed meal. (77). However, AAs are able to achieve increases in limb blood flow independent of insulin (78), raising the prospect of multiple pathways effecting postprandial blood flow responses.
The physiologic relevance of postprandial blood flow responses is not well understood, and this remains an active area of research. In young men, the gradual onset of low-amplitude aminoacidemia failed to achieve a detectable increase in muscle microvascular blood volume; yet, MPS was identical when compared with a feeding regimen that achieved rapid aminoacidema and a significant increase in microvascular blood volume. Enhancing limb and microvascular blood flow under postprandial conditions via intra-arterial methacholine infusions does not further enhance muscle anabolic responses to feeding (79). Therefore, it would appear that surpassing the subtle vasoactive effects of nutrition does not further increase fed-state anabolism, at least in younger individuals, possibly because they have already reached a muscle-full state (8, 9, 79).
The loss of the postprandial increase in both femoral artery and muscle microvascular blood flow with advancing age may be hypothesized to compromise EAA delivery and subsequent MPS in older individuals (37). Experiments aimed at determining whether “rejuvenating” postprandial blood flow in older persons could positively modulate muscle anabolic responses to feeding have been equivocal (80, 81). The exposure of an older cohort to resistance exercise training led to marked improvements in macro- and microvascular responses to feeding (likely due to resistance exercise training–induced angiogenesis and enhanced endothelial function) but without positively modulating muscle anabolism (81). Although this remains an active area of research, age-related declines in vascular function do not appear to substantially contribute to anabolic resistance in skeletal muscle protein metabolism.
Molecular Regulation of Skeletal Muscle Protein Metabolism by AAs in Youth and Aging
In skeletal muscle cells (as in many other cells) certain EAAs do not just act as substrates or building blocks for MPS but also as signaling molecules to the mRNA translation machinery. The mechanisms by which EAAs stimulate intracellular signaling pathways in non–muscle (82) and muscle (83) cells have been a longstanding focus of research. The initiation of the anabolic signal is thought to occur after transmembrane transport of EAAs (84), particularly of the AA leucine (85). The mechanistic indication of the cellular pathways involved in EAA sensing was defined through experiments that used the immunosuppressant rapamycin; the provision of rapamycin, which inhibits the activation of mechanistic target of rapamycin complex 1 (mTORC1), was found to suppress anabolic responses to EAAs (86). Rapamycin exerts its effects by disrupting the activation of mTORC1, a multiprotein kinase complex [e.g., G-protein β subunit-like protein (GβL), proline-rich Akt substrate 40, and Raptor (87)], thereby impairing EAA-induced stimulation. Moreover, leucine stimulates mTORC1 pathways more potently than any other single EAA (85). Beyond this mechanistic work, it has been shown that when humans consume protein, the activity of mTORC1 pathway constituents increases in synergy with MPS, suggesting close links between EAA availability and the activation of MPS (8). Intriguingly, with sustained availability of EAAs, MPS returns to fasting values despite maintained mTORC1 signaling activity. The mechanisms that “uncouple” MPS and mTORC1 activity at this muscle-full set point remain undefined. The notion that the activation of mechanistic target of rapamycin (mTOR) represents the central node communicating EAA availability can be reconciled by the number of mTORC1 substrates involved in mRNA translation. For instance, the activation of mTORC1 by EAAs is associated with subsequent phosphorylation of mRNA translational initiation and elongation factor substrates including 4E-binding protein (4EBP1), ribosomal protein S6 kinase (p70S6K1), and eukaryotic initiation factors (eIF) 4G, 4A, and 4B with ensuing formation of the fully competent eIF3F scaffold to promote the assembly of a 48S preinitiation complex (88). From a practical standpoint, the phosphorylation of certain mTORC1 substrates are often used as a proxy for mTORC1 signaling (e.g., p70S6K1) because the kinase activity of mTORC1 has multiple inputs (beyond phosphorylation), including cellular localization and varying affinity to its binding partners (89).
Although mTORC1 is clearly a central node for EAA-sensing, the proximal mechanisms involved in its activation remain incompletely defined. It is known that MPS responses to EAAs are independent of the proximal insulin signaling pathway at the level(s) of phosphatidylinositol 3-kinase (PI3K)/protein kinase B, because pharmacologic inhibition of these constituents did not block AA-stimulated MPS (90). Similarly, other well-characterized signaling pathways upstream of mTORC1, such as tuberous sclerosis complex (TSC), have been investigated. TSC is a GTPase-activating protein (GAP) for the GTPase Ras-homolog enriched in brain (Rheb), which negatively regulates mTORC1 by promoting Rheb-GTP hydrolysis, converting Rheb into its inactive GDP-bound form (91). As a result, the inhibition of TSC gives rise to GTP-bound Rheb, which is a potent activator of mTORC1 kinase activity. Yet, a number of lines of evidence have shown that this pathway is not crucial for mTOR activity in response to AAs (85, 90). The first clues as to the regulation of AA-induced MPS came from another group of GTPases. The presence of EAAs promotes the formation of an active complex configuration of obligate heterodimer Ras-related GTPase (Rag) proteins in which RagA and RagB [there are 4 RAG proteins (A–D)] are GTP-bound and RagC and RagD are GDP-bound (note that RagA/B ⋅ GTP–RagC/D ⋅ GDP is the active complex and RagA/B ⋅ GDP–RagC/D ⋅ GTP the inactive complex). Under conditions of EAA availability, EAAs accumulate within lysosomes, activating vacuolar H+-ATPase, after which active Rag complexes (via the Ragulator) (92, 93) directly bind to the mTORC1 protein subunit Raptor, redistributing mTORC1 to lysosomal surfaces. This is thought to be a critical step in the regulation of cellular EAA sensing and ensuing increases in MPS. A second central point of control of MPS in response to EAAs appears to be at the level of charging of leucyl-transfer RNA (tRNA) synthetase (5). In response to leucine, leucyl-tRNA synthetase was found to translocate to the lysosomal membrane, where it bound to and acted as a GTPase activating protein (GAP) toward RagD. Whether this leucyl-tRNA pathway is a parallel recognition signal to the “inside-out” Ragulator lysosomal mechanisms or is acting somewhere within the same recognition pathway remains to be fully determined. This specific signaling trigger elicited via leucyl-tRNA synthetase charging may explain the more potent anabolic actions of leucine in comparison to other EAAs. However, other EAAs, independently of leucine, can equivalently stimulate those signaling pathways (85). Furthermore, the MPS response to oral EAAs in humans is not proportional to mTORC1 substrate activity (78), with maximal MPS being achieved without detectable mTORC1 phosphorylation (9, 63) (via immunoblotting). Thus, it seems likely that multiple parallel inputs signal EAA availability to regulate the stimulation of MPS in humans.
There is some evidence that age-related dysfunction in sensing and signaling EAA availability contributes to anabolic resistance. Although basal (fasting or rested) mTORC1 phosphorylation is higher in these aged ∼70 y than in those aged ∼30 y (94), the activation of the mTORC1 signaling pathway (e.g., as assessed by p70S6K1 phosphorylation) is impaired after protein/EAA intake in older compared with younger individuals (26, 95). To our knowledge, studies to date have provided insufficient clarity to identify in detail the apparent level of dysregulation in AA sensing and signaling; however, it is unlikely that this is due to differences in intracellular AA availability after intake of protein/EAAs in young compared with older individuals because intracellular EAA concentrations are higher in older individuals (26). As such, the etiology of this “signaling block” remains to be determined and could even be influenced by cellular aging or epigenetics.
Quantifying Muscle Anabolic Responses to Protein/EAAs: Different Approaches and Pitfalls
Much of the recent progress in defining the temporal responses of muscle protein metabolism to nutrition and exercise has been made possible by technological advances in the sensitivity, stability, and speed of mass spectrometers [e.g., coupling GC to combustion–isotope ratio MS and tandem MS (MS-MS)], added to the availability of multiply stable isotope–labeled AA tracers (e.g., 13C6 Phe/ring-D5 Phe); for this reason, we briefly present some technological advances in relation to these methods. The “gold standard” tracer incorporation approach to measuring MPS involves a primed constant infusion of a stable isotopically labeled AA. The rate of label incorporated into the protein (e.g., from a tissue biopsy, the “product”) is then calculated with reference to the pool chosen to represent the “precursor” pool for MPS; from this, a fractional rate of protein synthesis can be calculated—the so-called precursor-product approach. Ideally, labeling of the amino acyl-tRNA (the “true” precursor) would be measured; however, in practice, this is difficult to obtain due to the small size of the labile tRNA pool, which makes extraction difficult and inherently variable (96, 97). In view of this, it is generally accepted that intracellular free AA labeling represents an appropriate surrogate of the true precursor pool (6). Another approach is to “flood” all AA pools to the same extent (so-called flooding-dose), in which large doses of labeled AAs (50 mg/kg) are administered as a bolus, resulting in equilibration of the arterial, venous, and inter-/intracellular and amino-acyl tRNA pools to the same extent (98), which is maintained throughout the measurement period (i.e., 90 min; due to the high labeling, the measurement period can be reduced). However, this “flooding” does not hold for long in tissues that rapidly turn over (e.g., gut and liver), in which the intracellularly labeled pool is more rapidly diluted (99). Moreover, the choice of AA is important because flooding with EAAs can result in a stimulation of MPS, whereas flooding with NEAAs has no impact and yields values for MPS similar to those obtained by using primed constant infusion approaches (11, 12). It is noteworthy when using precursor approaches that relations between precursor pools alter in fasting and fed states. Moving from fasting to fed states causes the inward transport of AAs from the arterial side. This, coupled with an expansion of the intracellular AA pool and an inhibition of MPB, brings the ratio of labeling in those pools and the venous pool much closer, almost reaching unity. In the fasting state, AA labeling within arterial blood is greater than labeling within the intracellular pool which is itself greater than labeling of venous blood (100). Thus, the choice of precursor pool is critical to data interpretation, particularly if comparing fasting to fed states. An alternative to the precursor-product method for measuring protein metabolism is the arterial-venous (A-V) balance technique, in which both the A and V concentration and labeling are measured across the limb or organ alongside a measure of blood flow to the limb or organ (e.g., by Doppler ultrasound) (101, 102). The magnitude of dilution of the labeled tracer across the organ or limb, measured by comparing the enrichment and concentration in the arterial and venous pools, when combined with the blood flow provides a measure of the rate of phenylalanine released via MPB (rate of appearance). Measuring the concentration difference provides the net balance, which reflects the sum of MPS and MPB, and thereby one can derive synthesis (rate of disposal). A limitation of the A-V balance approach across a limb is that other tissues (e.g., bone, fat, and skin) may contribute to the A-V balance (although muscle comprises the majority of metabolically active tissue in the leg); however, some of these limitations can be overcome through the sampling of the intracellular pool of the muscle, the so-called 3-pool model (103). The use of the A-V balance approach provides qualitatively and directionally similar changes, although due to assumptions is not as robust as the incorporation method, especially when A-V sampling is not rigorously controlled or when blood flow is variable (80).
Limitations
The primary aim of this review was to describe the known metabolic and molecular mechanisms regulating responses to AA nutrition in both youth and aging, with particular reference to work undertaken within the author’s laboratory on this topic (see Table 2). From what has been described, it is clear that the control of MPS plays a key role in responses to AA nutrition and the associated anabolic resistance present in aging (26, 104); however, it should be acknowledged that to determine the true net anabolic effect after nutritional intake one would require knowledge of MPB as well. This review concentrates primarily on MPS, because it is considered the main driver of anabolism after AA nutrition (41), with the release of insulin via both carbohydrate and protein/AA intake considered key to the anticatabolic regulation of MPB. Moreover, there are few studies that combine both MPS and MPB measures under such conditions; therefore, MPB and nitrogen balance were not discussed. We chose, however, to focus on detailing the temporal response of MPS to EAAs in young and aged individuals, highlighting the known metabolic and molecular changes associated with this response. For the chronic effects of dietary protein intake and AA supplementation on skeletal muscle metabolism and mass and in relation to aging, the reader is directed toward relevant reviews (36, 105).
TABLE 2.
Key points: human skeletal muscle protein metabolism in response to AA nutrition1
| Key points |
| EAAs (especially leucine) are key modulators of skeletal muscle metabolism, and robust sensing mechanisms exist that ensure effective muscle proteostasis across most of life. |
| Rapid aminoacidemia is not a prerequisite for an adequate (maximal) postprandial anabolic response; more likely, compositional differences in protein sources are key. |
| A dose- rather than time-sensitive mechanism underlies the onset of the muscle-full state. |
| MPS can be maximized with relatively low-dose leucine or leucine-enriched supplements. |
| Anabolic resistance to protein/EAAs is a pervasive feature of aging. |
| Intake of EAAs modulates muscle blood flow to facilitate their delivery in youth but not in older age; interventions promoting the restoration of flow have not yet shown benefit. |
| EAA-sensing mechanisms involve mTORC1 and Rag-dependent signaling; there is poor proportionality in mTORC1 activity and MPS. EAA sensing may be impaired in aging. |
| Technical advances in analytical MS, tissue microvascular flow measures, and modern cell biology techniques continue to underpin progress in this field. |
AA, amino acid; EAA, essential amino acid; MPS, muscle protein synthesis; mTORC1, mechanistic target of rapamycin complex 1; Rag, Ras-related GTPase.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, amino acid; AMPK, adenosine 5′-monophosphate kinase; A-V, arterial-venous; EAA, essential amino acid; GAP, GTPase-activating protein; GβL, G-protein β subunit-like protein; HMB, β-hydroxyl-β-methylbutyrate; KIC, α-ketoisocaproate; MPB, muscle protein breakdown; MPS, muscle protein synthesis; mTOR, mechanistic target of rapamycin; mTORC1 mechanistic target of rapamycin complex 1; NEAA, nonessential amino acid; PI3K, phosphatidylinositol 3-kinase; p70S6K1, ribosomal protein S6 kinase; Rag, Ras-related GTPase; Rheb, Ras-homolog enriched in brain; tRNA, transfer RNA; TSC, tuberous sclerosis complex; 4EBP1, 4E-binding protein.
References
- 1.Holman RL, Mahoney EB, Whipple GH. Blood plasma protein given by vein utilized in body metabolism: II. A dynamic equilibrium between plasma and tissue proteins. J Exp Med 1934;59:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madden SC, Carter JR, Kattus AA, Miller LL, Whipple GH. Ten amino acids essential for plasma protein production effective orally or intravenously. J Exp Med 1943;77:277–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buse MG, Reid SS. Leucine: a possible regulator of protein turnover in muscle. J Clin Invest 1975;56:1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem 1982;257:1613–21. [PubMed] [Google Scholar]
- 5.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–24. [DOI] [PubMed] [Google Scholar]
- 6.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–23. [DOI] [PubMed] [Google Scholar]
- 7.Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 2001;532:575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 2010;92:1080–8. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell WK, Phillips BE, Williams JP, Rankin D, Lund JN, Smith K, Atherton PJ. A dose- rather than delivery profile-dependent mechanism regulates the “muscle-full” effect in response to oral essential amino acid intake in young men. J Nutr 2015;145:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1–13C]leucine. Clin Sci (Lond) 1989;76:447–54. [DOI] [PubMed] [Google Scholar]
- 11.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol 1992;262:E372–6. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol 1998;275:E73–8. [DOI] [PubMed] [Google Scholar]
- 13.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 2009;37:1–17. [DOI] [PubMed] [Google Scholar]
- 14.Battezzati A, Haisch M, Brillon DJ, Matthews DE. Splanchnic utilization of enteral alanine in humans. Metabolism 1999;48:915–21. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol 1993;264:E848–54. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol 1993;264:E109–18. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591:2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 19.Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, Kobayashi H, Greenhaff PL, Smith K, Atherton PJ. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab 2015;308:E1056–65. [DOI] [PubMed] [Google Scholar]
- 20.Wagenmakers AJ. Protein and amino acid metabolism in human muscle. Adv Exp Med Biol 1998;441:307–19. [DOI] [PubMed] [Google Scholar]
- 21.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 2010;140:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs HA, Lund P. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv Enzyme Regul 1976;15:375–94. [DOI] [PubMed] [Google Scholar]
- 23.Van Koevering M, Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol 1992;262:E27–31. [DOI] [PubMed] [Google Scholar]
- 24.Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab 2008;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 2009;109:1582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4. [DOI] [PubMed] [Google Scholar]
- 27.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89:161–8. [DOI] [PubMed] [Google Scholar]
- 28.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballevre O, Beaufrere B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol 2003;549:635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millward DJ, Pacy PJ. Postprandial protein utilization and protein quality assessment in man. Clin Sci (Lond) 1995;88:597–606. [DOI] [PubMed] [Google Scholar]
- 31.Adibi SA, Morse EL, Masilamani SS, Amin PM. Evidence for two different modes of tripeptide disappearance in human intestine: uptake by peptide carrier systems and hydrolysis by peptide hydrolases. J Clin Invest 1975;56:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 2012;590:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson GJ, Moulton CJ, Garlick PJ, Anthony TG, Layman DK. Post-meal responses of elongation factor 2 (eEF2) and adenosine monophosphate-activated protein kinase (AMPK) to leucine and carbohydrate supplements for regulating protein synthesis duration and energy homeostasis in rat skeletal muscle. Nutrients 2012;4:1723–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab 2011;301:E1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg I. Summary comments. Am J Clin Nutr 1989;50:1231–3. [Google Scholar]
- 36.Wall BT, Cermak NM, van Loon LJ. Dietary protein considerations to support active aging. Sports Med 2014;44(Suppl 2):S185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell WK, Phillips BE, Williams JP, Rankin D, Smith K, Lund JN, Atherton PJ. Development of a new Sonovue contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol Rep 2013;1:e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrere B, et al. Protein feeding pattern does not affect protein retention in young women. J Nutr 2000;130:1700–4. [DOI] [PubMed] [Google Scholar]
- 39.Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chretien P, Schauer N, Vincent JP, Cynober L, Aussel C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin Nutr 2013;32:186–92. [DOI] [PubMed] [Google Scholar]
- 40.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–73. [DOI] [PubMed] [Google Scholar]
- 41.Phillips BE, Hill DS, Atherton PJ. Regulation of muscle protein synthesis in humans. Curr Opin Clin Nutr Metab Care 2012;15:58–63. [DOI] [PubMed] [Google Scholar]
- 42.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 2004;286:E321–8. [DOI] [PubMed] [Google Scholar]
- 45.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 2009;90:1343–50. [DOI] [PubMed] [Google Scholar]
- 46.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 47.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 2009;587:5483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 49.Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 2012;60:16–23. [DOI] [PubMed] [Google Scholar]
- 50.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 2009;89:1468–75. [DOI] [PubMed] [Google Scholar]
- 51.Luiking YC, Abrahamse E, Ludwig T, Boirie Y, Verlaan S. Protein type and caloric density of protein supplements modulate postprandial amino acid profile through changes in gastrointestinal behaviour: a randomized trial. Clin Nutr 2016;35(1):48–58. [DOI] [PubMed] [Google Scholar]
- 52.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 1997;94:14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahé S, Roos N, Benamouzig R, Sick H, Baglieri A, Huneau JF, Tome D. True exogenous and endogenous nitrogen fractions in the human jejunum after ingestion of small amounts of 15N-labeled casein. J Nutr 1994;124:548–55. [DOI] [PubMed] [Google Scholar]
- 54.Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 2003;552:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 2012;108:958–62. [DOI] [PubMed] [Google Scholar]
- 56.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987–92. [DOI] [PubMed] [Google Scholar]
- 57.Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 2011;93:997–1005. [DOI] [PubMed] [Google Scholar]
- 58.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 2001;280:E340–8. [DOI] [PubMed] [Google Scholar]
- 59.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 2011;94:795–803. [DOI] [PubMed] [Google Scholar]
- 60.Burke LM, Hawley JA, Ross ML, Moore DR, Phillips SM, Slater GR, Stellingwerff T, Tipton KD, Garnham AP, Coffey VG. Preexercise aminoacidemia and muscle protein synthesis after resistance exercise. Med Sci Sports Exerc 2012;44:1968–77. [DOI] [PubMed] [Google Scholar]
- 61.Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr 2009;90:106–15. [DOI] [PubMed] [Google Scholar]
- 62.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, et al. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 2011;300:E231–42. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell WK, Phillips BE, Williams JP, Rankin D, Lund JN, Wilkinson DJ, Smith K, Atherton PJ. The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men: clinical efficacy of pulse vs. bolus supply. Am J Physiol Endocrinol Metab 2015;309:E450–7. [DOI] [PubMed] [Google Scholar]
- 64.el-Khoury AE, Sanchez M, Fukagawa NK, Gleason RE, Tsay RH, Young VR. The 24-h kinetics of leucine oxidation in healthy adults receiving a generous leucine intake via three discrete meals. Am J Clin Nutr 1995;62:579–90. [DOI] [PubMed] [Google Scholar]
- 65.Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 2013;591:2319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014;144:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrere B, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr 1999;69:1202–8. [DOI] [PubMed] [Google Scholar]
- 68.Luiking YC, Engelen MP, Soeters PB, Boirie Y, Deutz NE. Differential metabolic effects of casein and soy protein meals on skeletal muscle in healthy volunteers. Clin Nutr 2011;30:65–72. [DOI] [PubMed] [Google Scholar]
- 69.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 1999;277:E513–20. [DOI] [PubMed] [Google Scholar]
- 70.Moreau K, Walrand S, Boirie Y. Protein redistribution from skeletal muscle to splanchnic tissue on fasting and refeeding in young and older healthy individuals. J Am Med Dir Assoc 2013;14:696–704. [DOI] [PubMed] [Google Scholar]
- 71.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 1997;65:489–95. [DOI] [PubMed] [Google Scholar]
- 72.Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WH, van Loon LJ. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr 2009;139:1707–13. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength: a quantitative review. Front Physiol 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006;290:E1191–7. [DOI] [PubMed] [Google Scholar]
- 75.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–23. [DOI] [PubMed] [Google Scholar]
- 76.Rajapakse NW, Chong AL, Zhang WZ, Kaye DM. Insulin-mediated activation of the L-arginine nitric oxide pathway in man, and its impairment in diabetes. PLoS One 2013;8:e61840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sjøberg KA, Rattigan S, Hiscock N, Richter EA, Kiens B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. Am J Physiol Heart Circ Physiol 2011;301:H450–8. [DOI] [PubMed] [Google Scholar]
- 78.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 2008;295:E595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips BE, Atherton PJ, Varadhan K, Wilkinson DJ, Limb M, Selby AL, Rennie MJ, Smith K, Williams JP. Pharmacological enhancement of leg and muscle microvascular blood flow does not augment anabolic responses in skeletal muscle of young men under fed conditions. Am J Physiol Endocrinol Metab 2014;306:E168–76. [DOI] [PubMed] [Google Scholar]
- 80.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010;59:2764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips BE, Atherton PJ, Varadhan K, Limb MC, Wilkinson DJ, Sjoberg KA, Smith K, Williams JP. The effects of resistance exercise training on macro- and micro-circulatory responses to feeding and skeletal muscle protein anabolism in older men. J Physiol 2015;593:2721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol 2004;279:215–44. [DOI] [PubMed] [Google Scholar]
- 83.Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochem Soc Trans 2007;35:1298–301. [DOI] [PubMed] [Google Scholar]
- 84.Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem 2002;277:9952–7. [DOI] [PubMed] [Google Scholar]
- 85.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010;38:1533–9. [DOI] [PubMed] [Google Scholar]
- 86.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 2011;141:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 2009;587:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr 2014;99(Suppl):237S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 2012;18:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr 2004;134:1704–10. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 2003;5:578–81. [DOI] [PubMed] [Google Scholar]
- 92.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008;320:1496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 2008;10:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markofski MM, Dickinson JM, Drummond MJ, Fry CS, Fujita S, Gundermann DM, Glynn EL, Jennings K, Paddon-Jones D, Reidy PT, et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol 2015;65:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li M, Verdijk LB, Sakamoto K, Ely B, van Loon LJ, Musi N. Reduced AMPK-ACC and mTOR signaling in muscle from older men, and effect of resistance exercise. Mech Ageing Dev 2012;133:655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA 1991;88:5892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baumann PQ, Stirewalt WS, O’Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol 1994;267:E203–9. [DOI] [PubMed] [Google Scholar]
- 98.Garlick PJ, Wernerman J, McNurlan MA, Essen P, Lobley GE, Milne E, Calder GA, Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a ‘flooding dose’ of [1-13C]leucine. Clin Sci (Lond) 1989;77:329–36. [DOI] [PubMed] [Google Scholar]
- 99.Southorn BG, Kelly JM, McBride BW. Phenylalanine flooding dose procedure is effective in measuring intestinal and liver protein synthesis in sheep. J Nutr 1992;122:2398–407. [DOI] [PubMed] [Google Scholar]
- 100.Rennie MJ, Smith K, Watt PW. Measurement of human tissue protein synthesis: an optimal approach. Am J Physiol 1994;266:E298–307. [DOI] [PubMed] [Google Scholar]
- 101.Barrett EJ, Revkin JH, Young LH, Zaret BL, Jacob R, Gelfand RA. An isotopic method for measurement of muscle protein synthesis and degradation in vivo. Biochem J 1987;245:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biolo G, Chinkes D, Zhang XJ, Wolfe RR, Harry M. Vars Research Award: a new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enteral Nutr 1992;16:305–15. [DOI] [PubMed] [Google Scholar]
- 103.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol 1995;268:E75–84. [DOI] [PubMed] [Google Scholar]
- 104.Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 2015;10:e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nowson C, O’Connell S. Protein requirements and recommendations for older people: a review. Nutrients 2015;7:6874–99. [DOI] [PMC free article] [PubMed] [Google Scholar]