Abstract
Butyrate is a histone deacetylase inhibitor implicated in many studies as a potential therapy for various forms of cancer. High concentrations of butyrate (>1.5 mM) have been shown to activate apoptosis in several cancer cell lines including prostate, breast, and leukemia. Butyrate is also known to influence multiple signaling pathways that are mediators of cytokine production. The purpose of this study was to evaluate the impact of high concentrations of butyrate on the cancer microenvironment vis-à-vis apoptosis, cellular migration, and capacity to modulate cytokine expression in cancer cells. The results indicate that high concentrations of butyrate induced a 2-fold activation of caspase-3 and reduced cell viability by 60% in U937 leukemia cells. Within 24 hours, butyrate significantly decreased the levels of chemokines CCL2 and CCL5 in HL-60 and U937 cells, and decreased CCL5 in THP-1 leukemia cells. Differential effects were observed in treatments with valproic acid for CCL2 and CCL5 indicating butyrate-specificity. Many of the biological effects examined in this study are linked to activation of the AKT and MAPK signaling pathways; therefore, we investigated whether butyrate alters the levels of phosphorylated forms of these signaling proteins and how it correlated with the expression of chemokines. The results show that butyrate may partially regulate CCL5 production via p38 MAPK. The decrease in p-ERK1/2 and p-AKT levels correlated with the decrease in CCL2 production. These data suggest that while promoting apoptosis, butyrate has the potential to influence the cancer microenvironment by inducing differential expression of cytokines.
Keywords: Butyrate, Acute Myeloid Leukemia, Inflammation, Cytokines, Migration
1. Introduction
Cytokines are regulatory proteins released from numerous immune and other cells of the body that mediate host defense and repair responses. Cytokines mainly regulate cells of the immune and hematopoietic systems, and modulate inflammatory responses [1, 2]. The study of cytokine function is complicated by characteristics which include: pleiotropy, redundancy, synergism, and receptor promiscuity [3]. Cytokines are divided into six subgroups: interferons, colony stimulating factors (CSF), growth factors, tumor necrosis factors (TNF), chemokines, and interleukins. It has become increasingly accepted by cancer researchers that in order to fully understand the tumorigenicity of cancer, the function of each cell type within the tumor and how these cells interact with the tumor microenvironment must be dissected. Exploring the crosstalk between these cells within the microenvironment via cytokines may explain their effects on tumor growth, angiogenesis, immune response, and metastasis [4-7].
Butyrate is a short-chain fatty acid produced in the human colon by bacterial fermentation activity [8, 9]. Butyrate is a histone deacetylase (HDAC) inhibitor [10, 11] implicated in many studies as a potential therapy for prostate [12], breast [13], and other forms of cancer [14] due to its ability at high concentrations (>1.5 mM) to cause cell death. Primarily highlighted for its use as a secondary chemotherapy, clinical use of butyrate holds substantial hope for reducing inflammation, reversing epigenetic aberrations, and suppressing the proliferation of cancer stem cells [15]. Butyrate is a feasible candidate to treat obesity, cardiovascular disease, neurological injury and even inherited diseases [15].
Previously, sodium butyrate (NaB) has been shown to induce apoptosis in leukemia cancer cell lines including U937 [16] and HL-60 cell lines [17]. Some reports imply that butyrate-induced apoptosis is associated with inhibition of telomerase activity [16], alterations in pro- and anti-apoptotic proteins, and regulation of signaling pathways involved in apoptosis [14]. A well-known target of butyrate is the p21Waf1/Cip1 gene. Activation of p21 results in cell cycle arrest at G1, a phenomenon that may lead to apoptosis or cell differentiation [18-20] independent of p53 [21]. Prior studies have shown that butyrate alters gene expression due to its HDAC inhibitory capabilities. Joseph et al. demonstrated that H460 human lung carcinoma cells treated with butyrate displayed differential expression of genes responsible for cytokine signaling and metastasis [22]. Also, butyrate increases the production of Interleukin-5 (IL-5) via epigenetic modifications in Jurkat cells [23] and TGF-β1 in colon carcinoma [24]. All of these studies utilized concentrations of butyrate at or below 2 mM. Often, higher concentrations may be needed to elicit the appropriate response. However, the biological implications of using these higher concentrations are largely unknown. Our goal was to understand whether high concentrations of butyrate that induce apoptosis are able to influence the tumor microenvironment vis-à-vis production and secretion of cytokines. We also elucidated the biological relevance of these alterations and attempted to pinpoint the associated signaling pathways required for butyrate-induced effects on cytokine production in leukemia cells. In summary, we concluded that butyrate alters the expression of cytokines and chemokines during apoptosis in leukemia cell lines.
2. Materials and Methods
2.1. Cell Culture
U937 leukemia cells were purchased from ATCC, HL-60 leukemia cells were from Dr. Ann Richmond at Vanderbilt University, and THP-1 leukemia cells were from Dr. Chandravanu Dash at Meharry Medical College. Cells were maintained in CO2 (5%) incubator in RPMI-1640 media (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Biowest, Kansas City, MO) containing 50 U/mL each of penicillin and streptomycin (Gibco, Grand Island, NY). Cell viability was determined to be better than 95% by trypan blue exclusion assay. Sodium butyrate (NaB) and valproic acid (VPA) (Sigma-Aldrich, St. Louis, MO) were reconstituted in sterile water at a stock concentration of 1 M.
2.2. Isolation of Monocytes
Human peripheral blood mononuclear cells (PBMC) were isolated from anonymous donors (New York Blood Center, Long Island, NY). PBMCs were harvested via Ficol-Percoll PLUS gradient (GE Health Care, Piscataway, NJ). Monocytes of greater than 85% purity were harvested and suspended in RPMI-1640 media supplemented with 10% FBS containing 50 U/mL each of penicillin and streptomycin as previously described [25].
2.3. Cell Viability Assay
Cells (5 × 106) were either untreated or treated with various concentrations of butyrate or valproic acid for 24 hours, harvested, and then diluted in 0.4% trypan blue at a ratio of 1:5. The cell number was counted with a hemocytometer.
2.4. Caspase-3 Activation
Cells (10 × 106) were untreated, treated with butyrate (5 or 10 mM) or valproic acid (2 or 4 mM) for 24 hours. Cells were lysed and equal amount of cell lysate proteins (120 μg) were assayed for evidence of activated caspase-3 using a caspase-3 colorimetric protease assay (Medical & Biological Laboratories Co. LTD, Woburn, MA) as previously described [25]. This assay utilized a substrate in which the amino acid sequence aspartic acid-glutamic acid-valine-aspartic acid is recognized by activated caspase-3 resulting in cleavage of substrate and exposure of a chromophore that can be detected with a spectrophotometer at 405 nm.
2.5. Apoptosis Detection Using Annexin V Staining
Cells (5 × 106) were either untreated or treated with butyrate (5 mM) or valproic acid (4 mM) over 24 hours. The cells were harvested, and suspended in 600 μL of Binding Buffer containing Annexin V-FITC conjugate as provided by the manufacturer (Raybiotech, Norcross, GA). Stained cells were run on Guava EasyCyte flow cytometer (EMD Millipore, Billerica, MA) and analyzed with FlowJo 7.6 Single Cell Analysis software as previously described [26].
2.6. Analysis of Cytokine Production
Culture supernatant from untreated or treated cells (5 × 106) was assayed for quantitative and qualitative levels of inflammatory cytokines and chemokines using a multi-analyte cytokine ELISA assay (SABiosciences, Frederick, MD). Briefly, tissue culture supernatants (50 μL/well) or standards (50 μL/well) were incubated in pre-coated 96-well microtiter plates at room temperature for 2 hours. After washing, 100 μL of the detection antibody mixture was added for 1 hour. Then, 100 μL of Avidin-HRP conjugate was added to each well for an additional 30 minutes at room temperature. Development and stop solutions were added and absorbance read at 450 nm. To quantify cytokine production, single-analyte ELISAs were used. In this assay, the protocol is previously described above. In addition, a range of 3.2 - 10,000 pg/mL recombinant cytokines was used to establish a standard curve for protein concentration.
2.7. Cellular Migration Assay
BD Biocoat Chambers (BD Biosciences, San Jose, CA) were used as a matrix. Media from butyrate-treated cells (5 × 106), FBS, or RPMI media only was added to the bottom wells to act as a chemoattractant (500 μL). Human monocytes (1 × 105) were suspended in 100 μL of RPMI media only and placed in the insert. The chambers incubated for 24 hours in the tissue incubator at 37°C. After the incubation period, the number of migrated cells in the lower chamber was determined by trypan blue exclusion assay.
2.8. Western Blot Analysis
U937 or HL-60 cells (10 × 106) were cultured overnight in serum-free media containing 0.5% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) and 100 U/mL each of penicillin and streptomycin to eliminate the influence of serum components on the activation of signaling proteins. The cells were then treated at various time points with either individual or varying combinations of the following compounds: butyrate, SB203580 (10 μM) (EMD Millipore, Billerica, MA), Anisomycin (10 μg/mL) (Sigma-Aldrich, St. Louis, MO), DMSO (0.5%) (Sigma-Aldrich, St. Louis, MO), LY294002 (20 μM) (Cayman Chemical, Ann Arbor, MI), and U0126 (20 μM) (Cayman Chemical, Ann Arbor, MI). Cell lysates were prepared and equal amounts of proteins were analyzed by Western blotting as previously described [27]. The following antibodies were used: p-ATF, Caspase-3, GAPDH, total and phosphorylated forms of AKT, p38 MAPK, JNK, and ERK1/2 (Cell Signaling, Danvers, MA). Blots were visualized by enhanced chemiluminescence (ECL) (Thermo Fisher Scientific, Waltham, MA).
2.9. Statistical Analysis
All data are presented as mean ± SEM. Results were compared using one-way ANOVA followed by Tukey's multiple comparison test. Differences were considered significant at two-tailed p < 0.05. All statistical analyses were performed with GraphPad Prism Version 6.0c (GraphPad software, La Jolla, CA). Data shown were obtained from at least five independent experiments unless otherwise indicated.
3. Results
3.1. Butyrate stimulates apoptosis in U937 leukemia cells
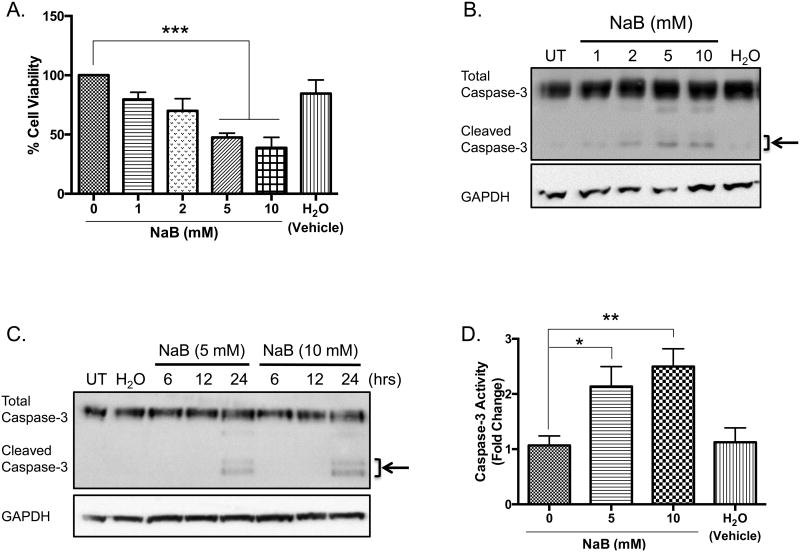
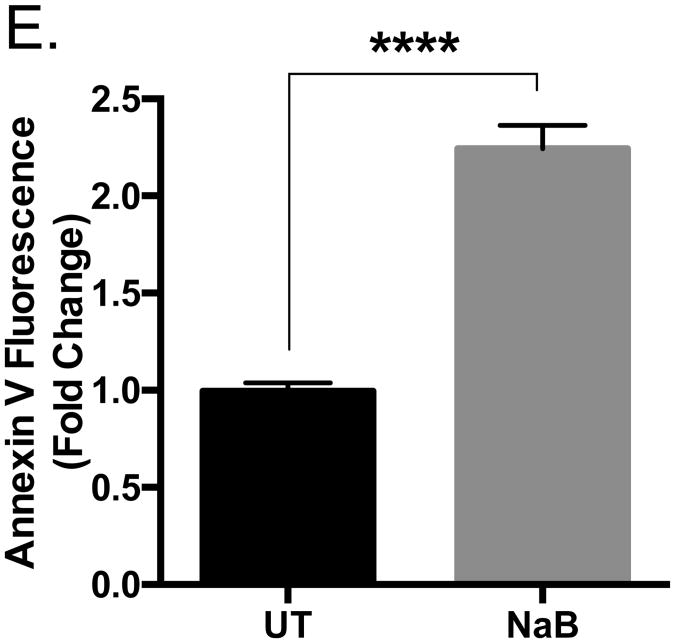
Previous studies indicate high concentrations (>1.5 mM) of butyrate induce apoptosis in various cancers [12-14, 16, 17, 28]. To confirm these reports, U937 cells were untreated or treated with butyrate or H2O, which serves as a vehicle, for 24 hours and analyzed for cellular viability using trypan blue exclusion assay. As figure 1A demonstrates, there is a significant decrease in the number of viable cells treated with 5 and 10 mM concentrations of butyrate when compared to the untreated cells by 52% and 61%, respectively. To investigate if the decline in cell viability was due to apoptosis, western blot analysis was utilized to examine cleavage of caspase-3. U937 cells were treated over 24 hours with varying concentrations of butyrate. The experimental results in figure 1B indicate that cleavage of caspase-3 occurs with as little as 2 mM butyrate with greatest cleavage at higher concentrations of butyrate, 5 and 10 mM. Additionally, figure 1C demonstrated that cleavage occurs 24 hours post-treatment compared to earlier time points of 6 and 12 hours. To confirm the catalytic activity of caspase-3, a colorimetric activity was employed. As illustrated in figure 1D, butyrate causes a 2.1 and 2.5 fold induction of activated caspase-3 activity at 5 and 10 mM, respectively, after a 24-hour treatment. Furthermore, Annexin V binding increased in butyrate-treated cells (Figure 1E and Supplementary S1). These data are clear evidence of butyrate-induced apoptosis in U937 leukemic cells.
Figure 1. Butyrate-induced apoptosis in U937 leukemic cells.
(A) U937 cells were treated with butyrate (NaB) or H2O for 24 hours. Cell viability was determined using trypan blue exclusion assay. n = 5. (B) Cells were untreated or treated with butyrate or H2O vehicle for 24 hours. After harvesting, total cell lysates were analyzed for caspase-3 by western blot analysis. (C) Cells were untreated or treated with 5 or 10 mM of butyrate or H2O vehicle for 6, 12, or 24 hours. Cell lysate proteins underwent western blot analysis for caspase-3. Arrows indicate the cleaved caspase-3 fragments at 17 and 19 kDa. (D) U937 cells were untreated or treated with 5 or 10 mM butyrate or H2O for 24 hours. A colorimetric caspase-3 assay was used to analyze caspase activity. n = 7. (E) Cells were treated with butyrate (5 mM) and Annexin V fluorescence was measured by flow cytometry. n = 7. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 when compared to the untreated (0, UT).
3.2. Butyrate induces apoptosis in HL-60 cells but minimally in monocytes and THP-1 cells
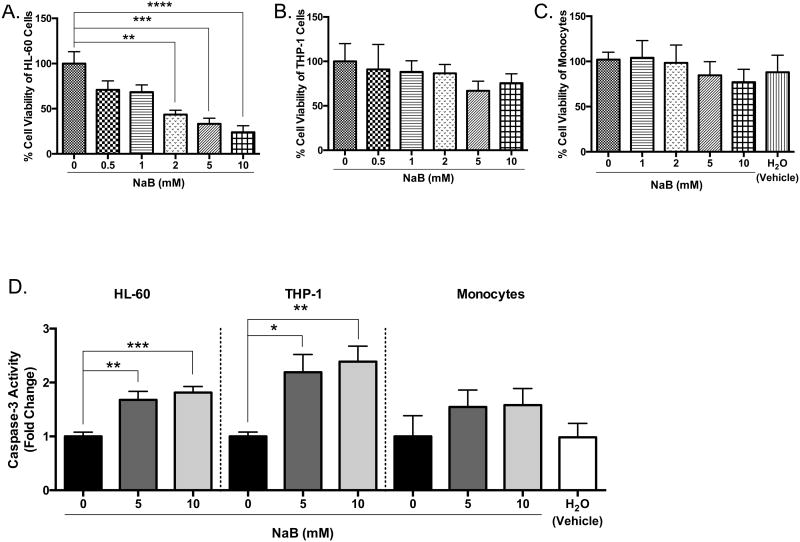
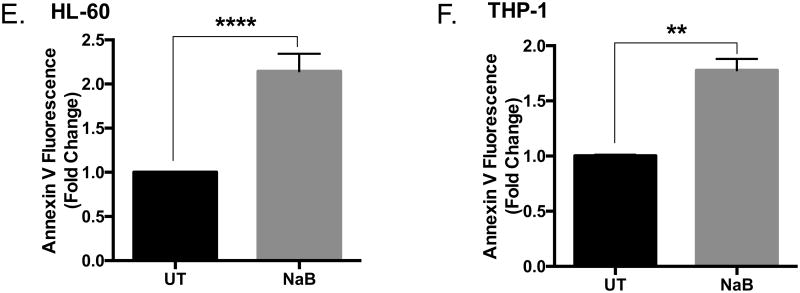
To determine if the biological changes induced by butyrate are similar in other types of acute myeloid leukemic cell lines, we employed HL-60 and THP-1 cell lines. HL-60 cells are less differentiated than U937 or THP-1 cells [29]. Based on the differentiating agent, HL-60 cells can be differentiated into monocytes or granulocytes; thus, they are considered myeloblastic/promyelocytic [30]. Here, HL-60 cells are susceptible to butyrate-induce apoptosis demonstrated by a significant decrease in cell viability starting at 2 mM concentrations (Figure 2A), induction of caspase-3 activity (Figure 2D), and increase in Annexin V binding (Figure 2E and Supplementary S1). Although THP-1 cells are described as a model cell line resembling primary monocytes/macrophages [31], research shows this cell line may display transformative properties due to differential expression of cytokines in response to LPS [32], and contrasting magnitude of response to various stimuli [33]. For the purpose of this work, we consider this cell line as transformed, but highly differentiated compared to U937 and HL-60 cells [31, 34]. Upon treatment of THP-1 cells with butyrate, we observed moderate decline in cell viability (Figure 2B) despite the significant increase in caspase-3 (Figure 2D) and Annexin V binding (Figure 2F and Supplementary S1). Although high concentrations of butyrate reduced the cell viability of monocytes, it was not statistically significant (Figure 2C). Butyrate induced caspase-3 activity in monocytes; however, this induction was not statistically significant (Figure 2D). These data demonstrate butyrate-induced apoptosis in U937 and HL-60 cells are more potent than apoptosis induced in THP-1 cells and monocytes. This indicates the magnitude of apoptosis provoked by butyrate will depend on the level of cellular differentiation.
Figure 2. Regulation of apoptosis by butyrate in HL-60, THP-1 leukemia cells, and monocytes.
(A) HL-60, (B) THP-1 leukemia cells, and (C) monocytes isolated from whole blood taken from anonymous donors were treated with butyrate for 24 hours. Cell viability was determined using trypan blue exclusion assay. n = 6, 5, and 9 respectively. (D) Cells were untreated or treated with 5 or 10 mM butyrate for 24 hours and subjected to colorimetric caspase-3 assay. n = 6, 5, and 5 respectively. (E) HL-60 and (F) THP-1 cells were treated with butyrate (5 mM) and Annexin V fluorescence was measured by flow cytometry. n = 7. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 when compared to the untreated (0).
3.3 Valproic acid induces apoptosis in U937 and HL-60 cells but not THP-1 cells
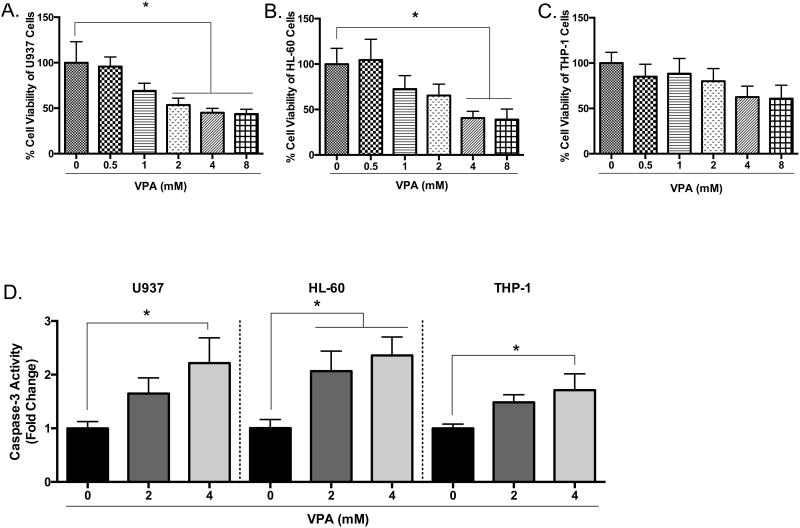
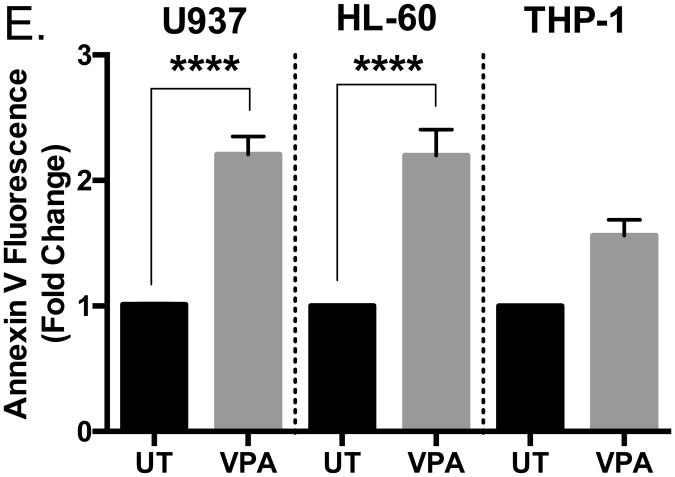
Next, we wanted to determine if another HDAC inhibitor might induce similar effects observed with butyrate treatment. We chose valproic acid (VPA) since it causes differentiation and apoptosis in hematopoietic malignancies and solid tumors [35-37]. As demonstrated via cell viability analysis, valproic acid induced a significant reduction in cell viability in U937 cells by 50% or greater beginning at concentrations as low as 2 mM following a 24-hour treatment (Figure 3A). In response to 4 mM of valproic acid, caspase-3 activation (Figure 3D) and Annexin V binding (Figure 3E and Supplementary S1) increased. HL-60 cells were also susceptible to valproic acid-induced apoptosis (Figures 3B, 3D, 3E, and Supplementary S1). Similar to the results of butyrate treatment, the number of viable THP-1 cells declined but not significantly (Figure 3C) despite increased caspase-3 activity (Figure 3D) and Annexin V binding (Figure 3E and Supplementary S1). These data provide valid evidence that butyrate and valproic acid activate apoptosis in U937 and HL-60 leukemic cells while producing minimal damage to the more differentiated THP-1 cells.
Figure 3. Valproic acid-induced apoptosis in leukemia cells.
Cells were treated with various concentrations of valproic acid (VPA) for 24 hours. (A) U937, (B) HL-60, and (C) THP-1 cell viability were determined using trypan blue exclusion assay. n = 5. (D) U937, HL-60, and THP-1 cells were untreated or treated with 2 or 4 mM of VPA for 24 hours and subjected to colorimetric caspase-3 assay. n = 7, 5, and 7 respectively. (E) Cells were treated with valproic acid (4 mM) and Annexin V fluorescence was measured by flow cytometry. n = 7. *p < 0.05, ****p < 0.0001 when compared to the untreated (0).
3.4. Butyrate induces differential expression of cytokines in leukemia cells
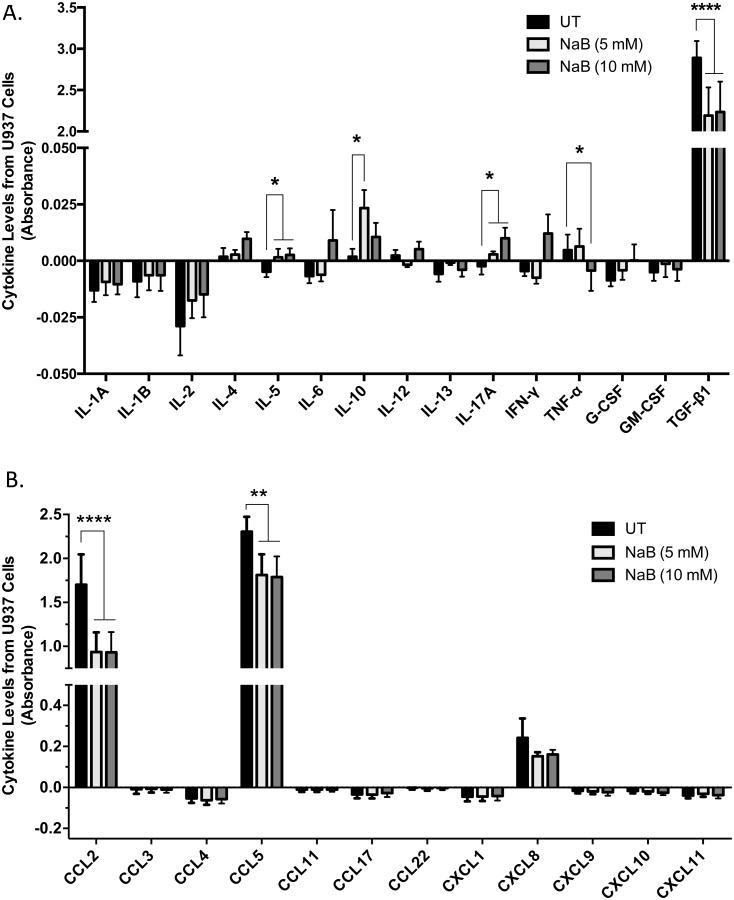
The myriad of cytokines located in the tumor microenvironment has a profound effect on the progression or suppression of a tumor [4-6]. To determine whether butyrate alters this array of cytokines thereby altering the tumor biology and/or patient response, we utilized ELISA to monitor the secretion of cytokines into the culture supernatant of cells untreated or treated with butyrate for 24 hours. Analysis of the levels of inflammatory cytokines in Figure 4A indicated IL-5 and IL-17A experienced a concentration-dependent increase in the culture media of U937 cells in response to butyrate. TNF-α level decreased at the highest concentration of butyrate, 10 mM. IL-10 level displayed an increase at the 5 mM concentration, but decreased at 10 mM. TGF-β1 while constitutively expressed in relatively high levels, displayed a significant decrease in response to both 5 and 10 mM concentrations of butyrate confirming previous studies indicating a decrease in gene expression of TGF-β1 [22].
Figure 4. Differential expression of cytokines in leukemia cells treated with butyrate.
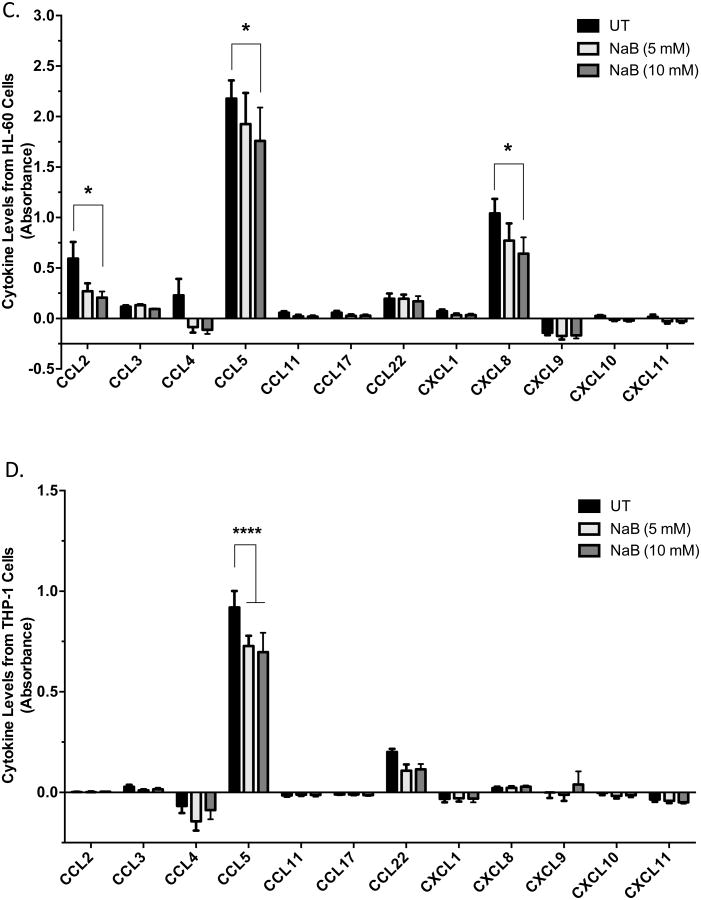
U937 cells were untreated or treated with butyrate for 24 hours and analyzed for the production of (A) inflammatory and (B) chemoattractant cytokines via multi-analyte ELISA. Data are presented as absorbance indicative of protein level. n = 8 and 5 respectively. Similarly, (C) HL-60 and (D) THP-1 cells were subjected to analysis of chemoattractant cytokines via multi-analyte ELISA. n = 5. *p < 0.05, **p < 0.01, ****p < 0.0001 when compared to the untreated (UT).
Chemokines may regulate tumorigenesis by attracting anti- and pro-tumor leukocytes to the tumor microenvironment [6]. Multi-analyte ELISA was utilized to investigate the expression of 12 chemotactic cytokines, many of which are implicated in regulating the tumor microenvironment [6]. As Figure 4B demonstrated, 5 and 10 mM concentrations of butyrate significantly decreased CCL2 and CCL5. It is also essential to note that both CCL2 and CCL5 are constitutively expressed in high levels compared to other chemokines analyzed. Chemokine profiles were also analyzed in HL-60 and THP-1 cells. HL-60 cells exhibited significant decreases in CCL2, CCL5 and CXCL8 when treated with 10 mM butyrate (Figure 4C). THP-1 cells constitutively express low levels of CCL2, but butyrate reduced the production of CCL5 (Figure 4D).
Treatment with butyrate (5 mM) was also shown to lower the mRNA levels of CCL2 and CCL5 by 3.8 and 1.4-folds respectively (Supplementary S2 and S3A) in U937 cells. Many other chemokines denoted changes in mRNA levels in response to butyrate. Surprisingly, CCL20 was highly upregulated by 19.9-fold, but this increase was not demonstrated at the protein level (Supplementary S3D). This indicates that regulating the expression of cytokines may involve both translational and post-translational mechanism yet to be identified.
3.5. Effects of butyrate are specific and dependent on the maturation of the cell
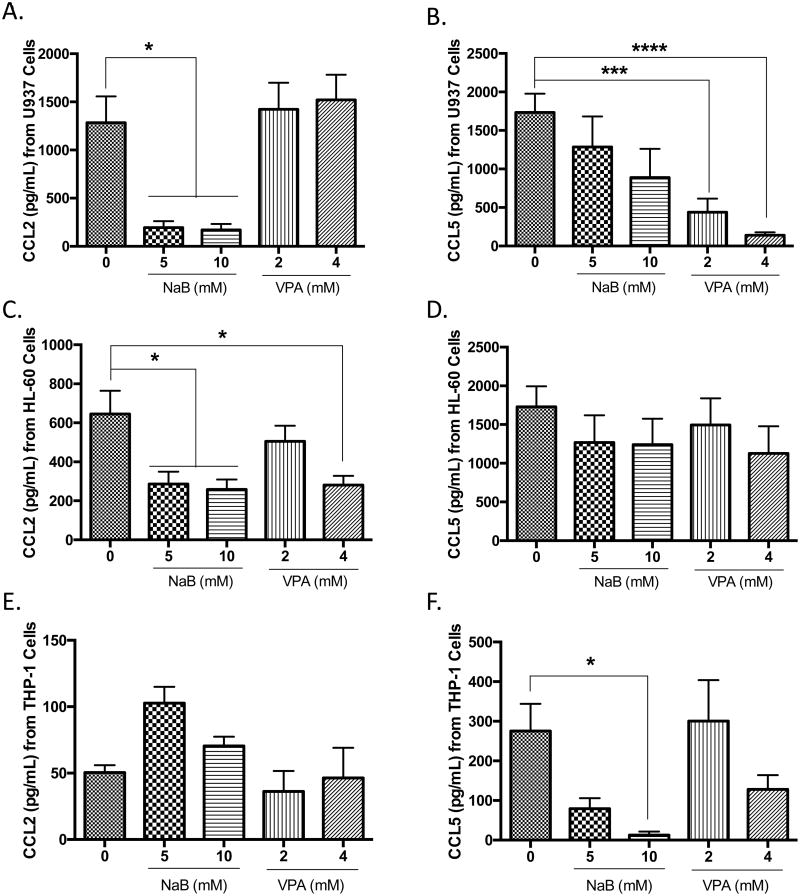
To determine if alterations in the expression of chemokines induced by butyrate were universal for all HDAC inhibitors, we investigated the protein concentrations of CCL2 and CCL5 in butyrate or valproic acid-treated U937, HL-60, and THP-1 cells. Butyrate significantly reduced CCL2 in U937 and HL-60 cells (Figures 5A and 5C). In contrast to the data generated from the cytokine profile, the significant decrease in the cytokine levels of CCL5 did not translate to significant decreases in protein level for butyrate-treated U937 and HL-60 cells (Figures 5B and 5D). Unlike butyrate, valproic acid did not suppress the production of CCL2 in U937 (Figure 5A), but significant suppression of CCL5 was observed in valproic acid-treated U937 cells (Figure 5B). Since the potency of apoptotic induction of the respective concentrations of valproic acid and butyrate are similar (Figures 1 and 3), this also demonstrates that the decrease in CCL2 in response to butyrate is not due to a decrease in cell viability; but in fact, butyrate utilizes a specific signaling mechanism to induce reduction in CCL2 secretion that is not induced in the presence of valproic acid.
Figure 5. Differential expression of CCL2 and CCL5 in leukemia cells treated with butyrate and valproic acid.
U937 cells were untreated or treated with butyrate or valproic acid for 24 hours and analyzed for the production of (A) CCL2 and (B) CCL5 via ELISA. n = 5. (B) HL-60 cells were analyzed for (C) CCL2 and (D) CCL5 in the presence and absence of butyrate or valproic acid. n = 5. Similarly, THP-1 cells were treated under the same conditions and analyzed for (E) CCL2 and (F) CCL5. n = 5. *p < 0.05, ***p < 0.001, ****p < 0.0001 when compared to the untreated (0).
Notably, the secretion of CCL2 in untreated THP-1 cells (Figure 5E) was constitutively lower when compared to untreated U937 and HL-60 cells. Results in THP-1 cells show a sharp contrast whereby treatment with 5 mM butyrate did not cause significant alterations in CCL2, but significantly reduce CCL5 (Figure 5F). Differences in response to butyrate and valproic acid may indicate that the regulation of CCL2 and CCL5 is dependent on the extent of cellular differentiation.
3.6. Culture media from butyrate-treated cells decreases the chemotactic response of monocytes
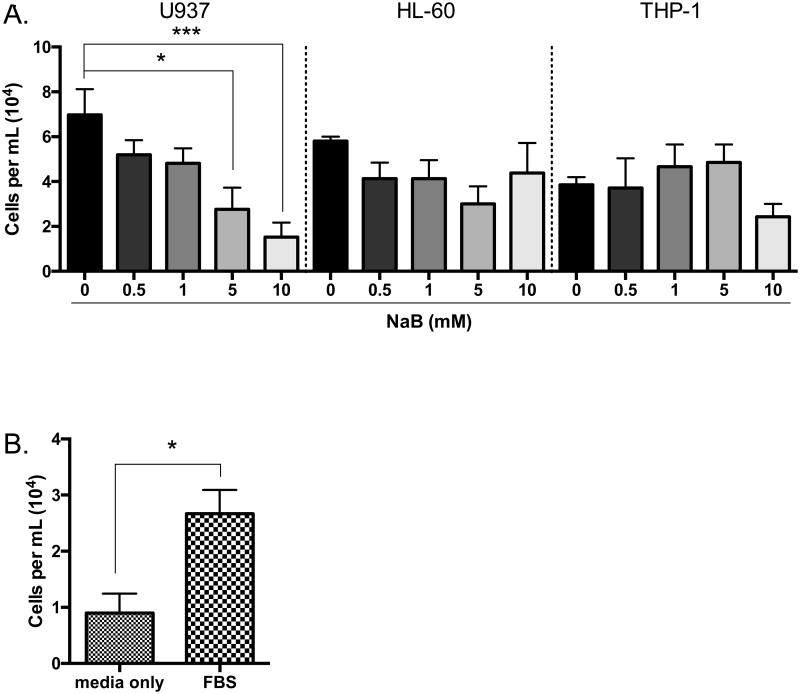
Monocytes may be recruited to tumor sites to mediate tumor-specific immunity [38, 39] or tumor-promoting mechanisms [40]. To determine the biological relevance of the decrease in chemokines induced by butyrate, the chemotactic responses of monocytes to culture media from untreated or butyrate-treated cells was analyzed via Borden chamber. Media from leukemia cells treated with butyrate for 24 hours were used as a chemoattractant in the bottom chamber. Monocytes placed in the upper chamber were allowed to migrate for 24 hours. Monocyte migration towards media from butyrate-treated U937 cells and HL-60 cells decreased in a concentration-dependent manner with significant reduction for U937 cells only (Figure 6A). The migration of monocytes towards media from THP-1 cells displayed little to no changes (Figure 6A). Figure 6B established the operability of our system using fetal bovine serum (FBS) as a positive control and RPMI media without FBS as a negative control. Based on these data, we infer butyrate decreases the release of chemoattractant cytokines resulting in reduced migration of monocytes. For all subsequent experiments, 5 mM concentration of butyrate was used unless otherwise stated.
Figure 6. Chemotaxis of monocytes decrease to media from butyrate-treated U937, HL-60, and THP-1 cells.
(A) U937, (B) HL-60, and (C) THP-1 cells were treated or untreated with butyrate for 24 hours. The migratory potential of monocytes was measured using the media of the treated cells as a chemoattractant in a Borden chamber. n = 5. (D) Fetal bovine serum (FBS), and media without FBS was used as a control. *p < 0.05, ***p < 0.001 when compared to the untreated (0, media only).
3.7. Butyrate regulates MAPK and AKT proteins
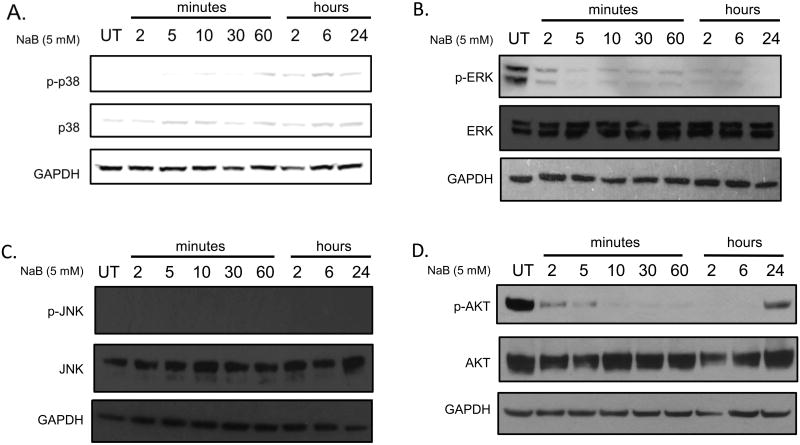
To elucidate the underlying mechanism involved in the regulation of CCL2 and CCL5 by butyrate, key signaling pathways involved in migration, apoptosis, and production of cytokines were analyzed in U937 cells. The mitogen-activated protein kinase (MAPK) proteins, p38, ERK, and JNK, possess diverse biological effects that include apoptosis, migration, differentiation, proliferation [41], and hematopoiesis [42]. Of particular interest, p38 and JNK are stress-induced proteins that may exhibit their roles as cytokine regulators [43]. AKT kinase has become a major focus of research due to its regulatory role in a wide variety of cellular processes that include protein synthesis, and cell survival [44]. Cells were treated with butyrate over a time course and analyses of phosphorylated and total forms of the proteins were conducted via Western blot. Butyrate significantly increases phosphorylation of p38 within 2 hours of treatment (Figure 7A, Supplementary S4A), but decreases p-ERK levels (Figure 7B, Supplementary S4B). Butyrate did not induce p-JNK (Figure 7C, Supplementary S4C). An initial decrease in p-AKT within 2 minutes show a decline in activation of AKT followed by re-activation 24 hours post-treatment (Figure 7D, Supplementary S4D).
Figure 7. Regulation of MAPK and AKT proteins in U937 cells by butyrate.
U937 cells were untreated or treated with butyrate (5 mM) over a time course and analyzed for phosphorylated and total forms of (A) p38 MAPK, (B) ERK1/2, (C) JNK, and (D) AKT via Western blot analysis.
3.8. p38 MAPK potentially mediates butyrate-induced regulation of CCL5 production, but not CCL2 production in U937 cells
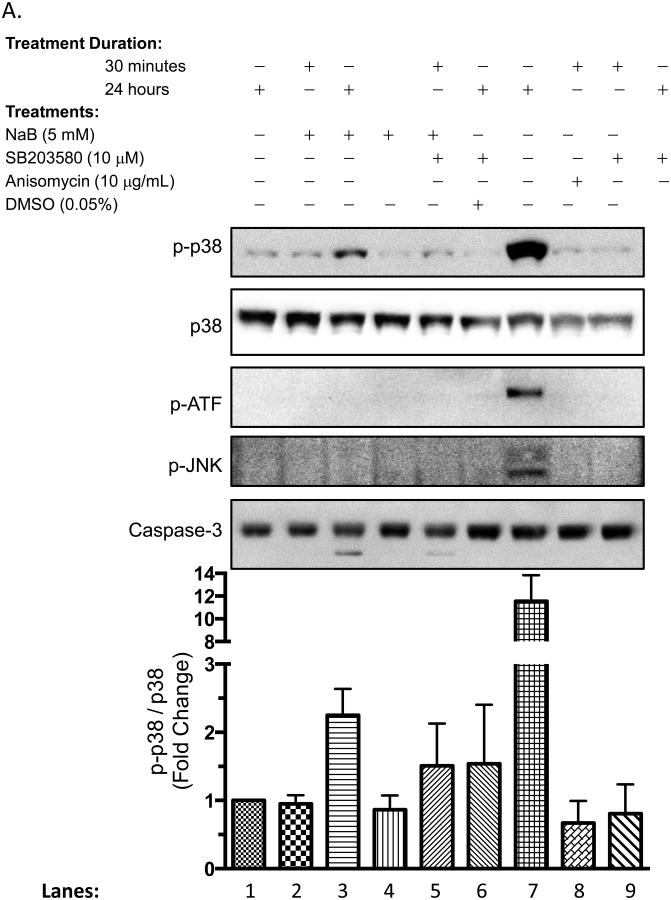
Butyrate induces p38 that regulate biological processes such as induction of β-globin [45], and regulation of apoptosis [41]. Yet, activation of this pathway by butyrate has not been linked to cytokine production. We exploited SB203580 (SB), a pharmacological inhibitor of p38, to directly correlate the role of p38 with the biological effects induced by butyrate. In Figure 8A, cells were subjected to varying conditions that included SB203580, butyrate, JNK and p38 inducer Anisomycin, and/or DMSO vehicle for 30 minutes or 24 hours. When cells were pretreated with SB203580 before butyrate treatment (Lane 5), phosphorylation of p38 decreases compared to butyrate treatment alone (Lane 3). Anisomycin successfully activated p38, JNK, and downstream protein activating transcription factor, ATF (Lane 7). Contrary, ATF was not activated by butyrate (Lanes 2 and 3). Lastly, butyrate-induced cleavage of caspase-3 decreased by 2-fold when p38 was blocked with SB203580 (Lanes 3 and 5). These data imply that butyrate-induced p38 utilizes another downstream protein other than ATF to render its biological effect. Additionally, the p38 MAPK pathway plays a role in the induction of apoptosis by butyrate, thus confirming previous reports [46-48].
Figure 8. p38 MAPK does not regulate the production of CCL2 and CCL5 in U937 cells.
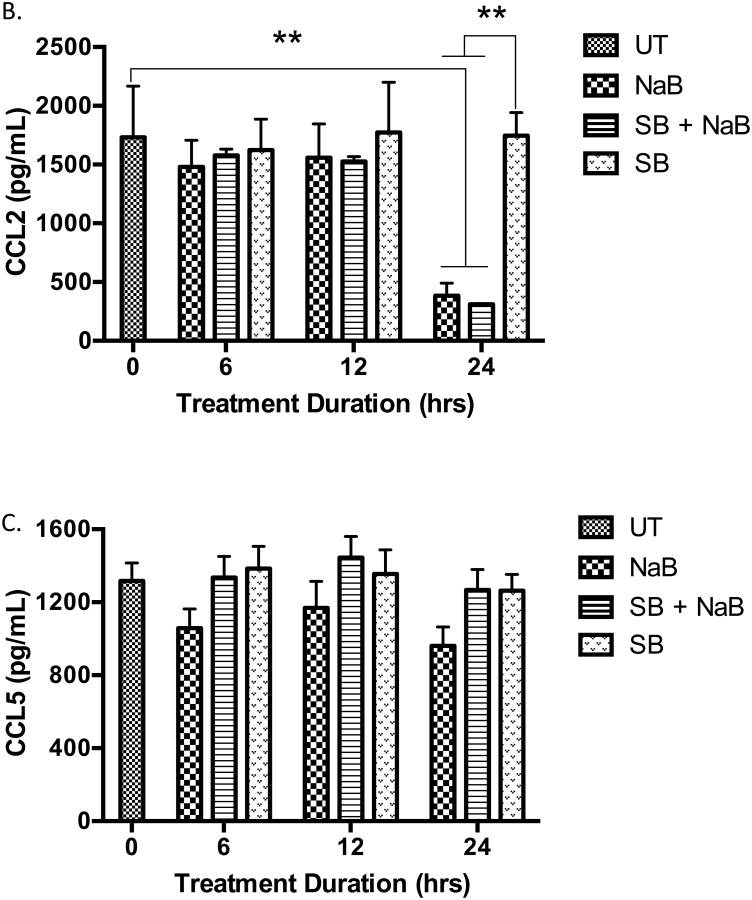
(A) Cells were untreated or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), p38 inducer Anisomycin, DMSO vehicle, and/or butyrate for 30 minutes or 24 hours. After harvesting and lysing the cells, Western blot analysis was carried out to analyze multiple proteins. Quantitative analyses of p-p38 and total p38 proteins were determined using densitometry. n = 5. (B) Cells were untreated or pre treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB) followed with treatment of 5 mM butyrate for 6, 12, or 24 hours and analyzed for CCL2 and (C) CCL5 via ELISA. n = 5. **p < 0.01 when compared to the untreated (UT, 0).
Next, the production of CCL2 and CCL5 were analyzed via ELISA using culture media from U937 cells treated with butyrate in the absence or presence of SB203580. The reduction of CCL2 induced by butyrate occurred 24 hours after administration of butyrate and not at earlier time points (Figure 8B). Blocking p38 MAPK did not abolish the decline in CCL2 production induced by butyrate. Although not statically significant, SB203580 overcomes butyrate-induced suppression of CCL5 to levels similar to that of the untreated cells (Figure 8C) indicating that butyrate may utilize p38 to regulate CCL5 in U937 cells. Importantly, levels of CCL5 in cells treated with SB203580 alone were near levels similar to the untreated cells and the viability of the cells did not change in the presence of SB203580 (Supplementary S5A). This indicates regulation of this chemokine is due to signaling mechanism induced by butyrate and not due to a decrease in cell population. Furthermore, blocking p38 did not influence the expression of the mRNA of the cytokines analyzed with PCR (Supplementary S2 and S3).
3.9. AKT and ERK may regulate the production of CCL2
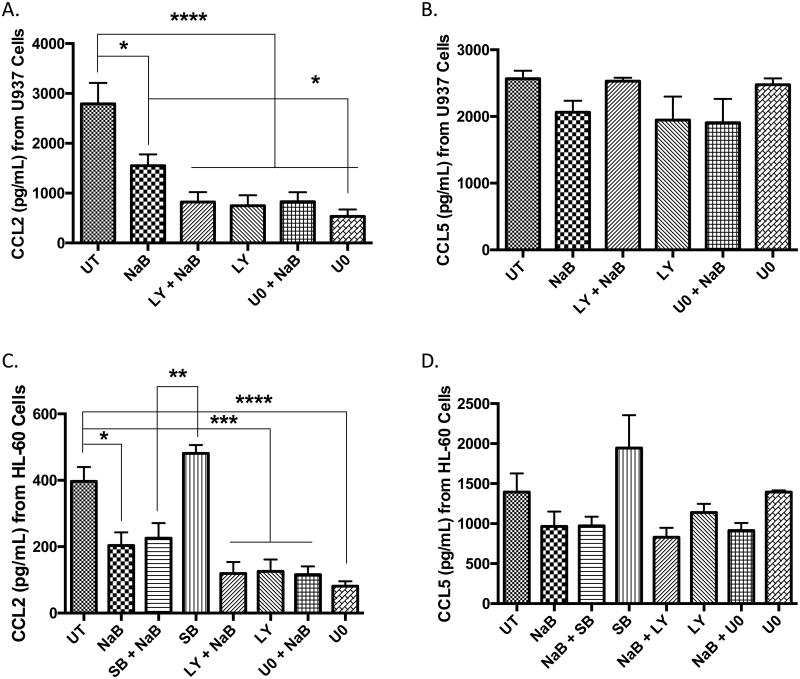
To determine whether the decline in phosphorylated ERK and AKT play a role in the production of CCL2 and CCL5, cells were pretreated with pharmacological inhibitors against PI3K (LY294002) and MEK (U0126) and then treated with butyrate for 24 hours. In Figure 9A, treatment with LY294002, U0126, and combinational treatments (inhibitor and butyrate) significantly reduced CCL2 production but not CCL5 (Figure 9B) in U937 cells. HL-60 cells displayed identical effects to butyrate as U937 cells including increased p-p38, and decreased activation of AKT and ERK (Supplementary S5C). HL-60 cells also established p38 does not contribute to CCL2 secretion (Figure 9C). The changes observed for CCL5 were not statistically significant (Figure 9D). Both inhibitors were successful in reducing activation of AKT and ERK (Supplementary S5D); but they were also toxic to the cells similar to butyrate in U937 cells only (Supplementary S5A). These data infer the AKT and ERK signaling pathways are major contributors to the regulation of CCL2 in that inactivation of these pathways leads to a decline in CCL2 expression.
Figure 9. Effect of p38, AKT, and ERK on the production of CCL2 and CCL5 by U937 and HL-60 cells.
Cells were untreated or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or 5 mM butyrate (NaB) for 24 hours. (A) U937 cells were analyzed for CCL2 and (B) CCL5 via ELISA. n = 5. Similarly, (C) HL-60 cells were analyzed for CCL2 and (D) CCL5 via ELISA. n = 5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
4. Discussion
In this study, we determined high concentrations of butyrate induce differential expression of inflammatory and chemoattractant cytokines into the tumor microenvironment. We demonstrated pro-inflammatory cytokines IL-5, IL-17, TNF-α and anti-inflammatory cytokine IL-10 are produced and significantly altered at low concentrations at the protein level in U937 cells in response to butyrate (Figure 4). Unlike the fore mentioned cytokines, TGF-β1 is constitutively expressed in high levels of protein to butyrate. Additionally, mRNA levels of TNFSF13B displayed a dramatic reduction (Supplementary S3B). The reduction of TGF-β1 and TNFSF13B in response to butyrate may pose some significant biological relevance. Based on the standard curves generated, this puts IL-5, -10, 17A, and TNF-α at or below 14, 5.9, 28.4, 21.4 pg/mL respectively. Based on the sensitivity of the assay, these measurements are close to the limit of detection. Overall, the biological influences due to the changes of these cytokines are still yet to be determined, but merit investigation since many cancers are initiated as a state of chronic inflammation [4].
Our work focuses heavily on the effects of butyrate on the acute leukemia cell linage. Yet, there remains a vital need for similar studies to be carried out in normal cells such as human lymphocytes and immature bone marrow cells. A review of the literature has indicated a potential effect. A study involving oral science suggests high concentrations of butyric acid (21-36 mM) produced by periodontopathic bacteria strongly induced apoptosis in lymphocytes and monocytes at similar magnitudes [49]. Another study demonstrated normal lymphocytes undergo a 30% decline in viable cells 72 hours after treatment with butyrate. The authors suggested variations in c-myc and p21 expression delayed butyrate-induced apoptosis for several hours [50]. The delay in the induction of apoptosis, evidence showing lymphocytes and monocytes behave similarly to butyrate, our data demonstrating the absence of butyrate-induced apoptosis on normal monocytes 24 hours post-treatment (Figure 2) and the high rate of degradation of butyrate in vivo [51] suggests lymphocytes and monocytes may be protected from the anti-cancer activities of butyrate. These studies may lend to a potential mechanism of resistance to butyrate in both lymphocytes and monocytes.
The hallmark function of chemokines is to regulate leukocyte trafficking to sites of inflammation and injury. Chemokines produced by tumor, stromal, and tumor-associated immune cells have been shown to promote tumorigenesis [6, 52, 53]. Studies have indicated macrophages as important regulators of tumor progression, metastasis, invasion, and angiogenesis [54]. The pro- or anti-tumorigenic effects of the influx of macrophages into the tumor microenvironment vary based on the composition of the tumor microenvironment. Therefore, the chemokine profile of the tumor microenvironment plays a vital role in recruitment and function of macrophages [54, 55].
Studies have demonstrated that tumor epithelial cells express high levels of CCL2, and that these levels correlate with the recruitment of monocytes into tumor tissues [56-58]. It has become clear that CCL2 is the main determinant of leukocyte recruitment into specific tumors and positively associated with tumor-associated macrophage accumulation [40]. Recent results in estrogen receptor positive breast cancer demonstrate the essential role of both CCL2 and CCL5 in cancer progression [59]. Similar to CCL2, CCL5 plays an immense role in tumor proliferation, metastasis, and angiogenesis [60]. Here, we have demonstrated U937 and HL-60 cells constitutively express high levels of CCL2 and CCL5 resulting in the chemotactic activity of monocytes. We have also shown butyrate has the capability to reduce these chemokines influencing the migration of monocytes (Figures 4 and 6). Consequently, butyrate is capable of altering the tumor microenvironment that could effect the migration of monocytes to the tumor potentially reducing tumor-associated macrophage populations.
Previous studies have shown butyrate alters gene expression by virtue of its ability to inhibit HDACs [22-24]. Even so, it is essential to elucidate other mechanisms that may be used by butyrate to influence cytokine production. Often constitutively activated in a number of cancers [44, 61], the AKT signaling pathway functions in survival and the synthesis of proteins [44] that is ideal to target for apoptosis and cytokine production. Previously, butyrate has been shown to suppress the PI3K pathway resulting in apoptosis that is linked to induction of PTEN in gastric cancer cell [62] and colon cancer cells [63]. Our data supports butyrate-induced inactivation of AKT, which may be relevant to the induction of apoptosis and CCL2 (Figures 7 and 9). However, U937 cells contain a frame shift mutation in the PTEN/MMAC1 gene resulting in premature termination [64] implying PTEN is likely not responsible for the reduction of phosphorylated AKT in U937 cells. The exact mechanism of action of butyrate on AKT signaling remains unclear as to whether it is an indirect inhibition due to suppression of specific upstream proteins or a direct interaction with AKT. Yet, it is plausible the AKT may regulate CCL2 via transcription factor Snail [65] that may be activated by GSK-3, a downstream protein of AKT [61]. The AKT signaling pathway continues to be an essential pathway of interest posing a prime target for treatment in leukemias [44].
The MAPK superfamily mediates biological processes that include proliferation, differentiation, apoptosis, and inflammation. For instance, the role of butyrate in K562 erytholeukemic cells has been widely studied for its ability to induce γ-globin that is regulated by p38 activation [45]. Blocking the signaling of AKT with LY294002 in the presence of butyrate reduced the phosphorylation of ERK in U937 cells [66]. These and other studies [45, 66-70] indicate MAPK signaling and crosstalk between the AKT and MAPK proteins are specific for the action of butyrate, which appears to be the case for CCL2 (Figure 9 and Supplementary S5).
Our observations indicate p38 MAPK is the only MAPK protein induced by butyrate and is positively linked to apoptosis (Figure 7A and 8A). It is also important to note results from contradictive studies indicating other members of the MAPK family (ERK and JNK) responsible for butyrate-induced apoptosis [71-73]. However, cell type and concentrations of butyrate used may explain these contradictive findings. The p38 pathway was also associated with the regulation of CCL5 identified through the use of the p38 inhibitor, SB203580; but p38 does not appear to mediate butyrate regulation of CCL2 (Figures 8 and 9). Although the results were not significant, the p38 pathway may be one of multiple mechanisms responsible for production of CCL5. The p38 signaling pathway is linked to the activation of transcription factors; thus, analysis of the mRNA levels of the cytokines indicates differential changes after butyrate treatment. However, it did not appear the changes in mRNA levels were due to the induction of p38 (Supplementary S2 and S3). Regardless, it remains possible that both post-transcriptional and post-translational regulatory mechanisms induced by butyrate would play a role in the regulation of cytokine expression observed.
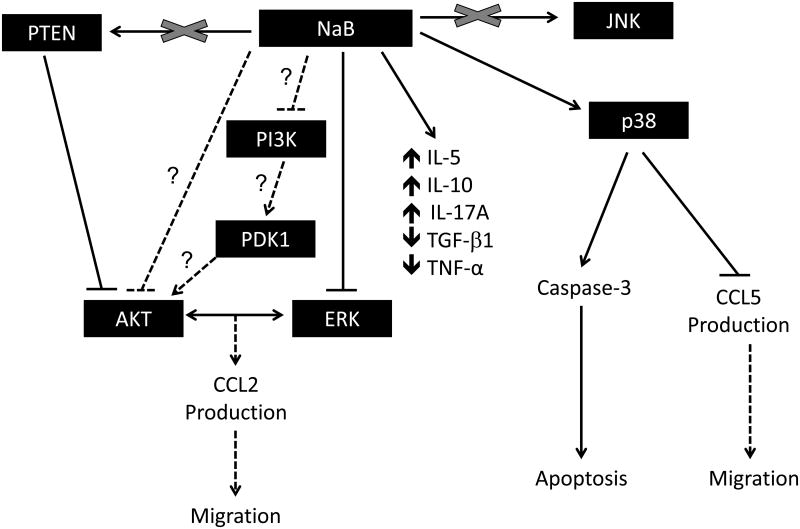
Based on our data and previous reports we propose the mechanistic model shown in Figure 10. As suggested, butyrate-induced p38 activation may be associated with enhanced apoptosis, decline in CCL5 expression and subsequent reduction in migration. Also, by employing direct or indirect mechanisms, butyrate induces negative regulation of PI3K/AKT and ERK pathways, which may be associated with downstream decline in cell viability, expression of CCL2, and migration. Furthermore, butyrate causes differential expression of IL-5, IL-10, IL-17A, TGF-β1 and TNF-α through yet to be identified mechanisms. Lastly, the biologic effects of butyrate reported here might be independent of both JNK and PTEN.
Figure 10. Potential mechanism of action of butyrate in U937 cells.
Based on our experimental data and previous publications regarding signaling pathways regulated by butyrate, we have proposed this potential mechanism of action for the induction of cytokines and how these pathways may also regulate migration and apoptosis in butyrate treated cells.
5. Conclusion
Collectively, these data depict butyrate as a viable adjunct therapy to induce apoptosis in leukemia cells, and reduce the migration of potential tumor-associated immune cells to the site of tumor by regulating cytokine production in the tumor microenvironment. These and other observations enhance the understanding of the action of butyrate providing valuable knowledge validating this drug as an effective adjuvant therapy for leukemia and other cancers.
Supplementary Material
S1: Butyrate and valproic acid increases Annexin V fluorescence in leukemia cells. Cells were treated with butyrate (5 mM) or valproic acid (4 mM) for 24 hours and Annexin V fluorescence was measured by flow cytometry. Plots are representative of 7 experiments.
S2: Modulation of mRNA levels of cytokines by butyrate. Heat map of the average cycle threshold of mRNA levels in U937 cells treated with butyrate and/or SB203580 (SB) for 24 hours generated from RT-PCR analysis.
S3: Quantitative analysis of the cytokine mRNA regulated by butyrate. Quantitative analysis of the cytokine mRNA found within detectable range in U937 cells. Data are separated into (A) chemokines, (B) TNF-related proteins, and (C) growth factors. Untreated cells are set to 1. n = 3. (D) U937 cells were untreated or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or 5 mM butyrate for 24 hours and assayed for the production of CCL20. n = 5. ****p <0 .0001 when compared to the untreated.
S4: Regulation of MAPK and AKT proteins in U937 cells by butyrate. U937 cells were untreated or treated with butyrate over a time course and analyzed for phosphorylated and total proteins via Western blot analyses. (A) Quantitative analyses of p38, and (B) ERK were determined using densitometry. n = 5 and 6 respectively. (C) ELISA was used to investigate phosphorylated and total JNK levels over a time course (10, 30, 60, 120 minutes). n = 3. (D) Quantitative analysis of AKT. n = 6 *p < 0.05 when compared to the untreated (UT).
S5: Effects of pharmacological inhibitors on cell viability and activation of protein. Cells were untreated (UT) or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or butyrate (NaB) (5 mM) for 24 hours. Cell viability of (A) U937 and (B) HL-60 cells were determined using trypan blue exclusion assay. (C) HL-60 cells were untreated or treated with butyrate over a time course and analyzed for phosphorylated and total proteins via Western blot analyses. (D) HL-60 cells were untreated or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or butyrate (N) for 24 hours. Western blot analysis was carried out to analyze multiple proteins.
Highlights.
During apoptosis, butyrate differentially regulates inflammatory cytokines.
Butyrate suppresses CCL2, CCL5, and subsequently monocyte migration.
Expression of CCL5 may be partially dependent on p38 MAPK.
Induction of CCL2 expression may be dependent on activation of AKT and ERK.
The regulation of cytokines is dependent on stage of maturation in leukemia.
Acknowledgments
I would like to acknowledge Dr. Chandravanu Dash for his donation of the THP-1 cell line; Dr. Ann Richmond for her donation of the HL-60 cell line; Dr. Deok-Soo Son for technical help; the New York Blood Center for supplying the necessary products for monocytes; and Drs. Ifeanyi Arinze and Amos Sakwe for scientific support.
Funding Sources: SRP is supported by T32 grant 2T32H007735-20 (NHLBI) and U54 CA091408-06 (NCI) and CTSA award No. UL1TR000445 (NCATS). STP is supported by T32 5T32HL007737 (NHLBI). AS is supported by U54 CA091408 (NCI), 5 U54 RR026140-03 (NCRR), 8 U54 MD007593-03 (NIMHD), 5P50 CA 090949 (NCI), and SCI CA182843 (NCI). SEA is supported by 5 U54 RR026140-07 (NCRR)/ 8 U54 MD007593-06 (NIMHD), and U54 CA091408-06 (NCI). There is no role of the sponsors in the manuscript design and the writing of the manuscript and in the decision to submit the manuscript for publication. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the sponsors.
Footnotes
Author Disclosure Statement: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002;277:29355–8. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 2.Liongue C, O'Sullivan LA, Trengove MC, Ward AC. Evolution of JAK-STAT Pathway Components: Mechanisms and Role in Immune System Development. PLoS One. 2012;7:e32777. doi: 10.1371/journal.pone.0032777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul WE. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989;57:521–4. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 4.Chai EZ, Siveen KS, Shanmugam MK, Arfuso F, Sethi G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015;468:1–15. doi: 10.1042/BJ20141337. [DOI] [PubMed] [Google Scholar]
- 5.Van der Jeught K, Bialkowski L, Daszkiewicz L, Broos K, Goyvaerts C, Renmans D, et al. Targeting the tumor microenvironment to enhance antitumor immune responses. Oncotarget. 2015;6:1359–81. doi: 10.18632/oncotarget.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow MT, Luster AD. Chemokines in cancer. Cancer immunology research. 2014;2:1125–31. doi: 10.1158/2326-6066.CIR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulliam SR, Uzhachenko RV, Adunyah SE, Shanker A. Common gamma chain cytokines in combinatorial immune strategies against cancer. Immunology letters. 2016;169:61–72. doi: 10.1016/j.imlet.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–79. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–8. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boffa LC, Vidali G, Mann RS, Allfrey VG. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978;253:3364–6. [PubMed] [Google Scholar]
- 11.Davie JR. Inhibition of histone deacetylase activity by butyrate. The Journal of nutrition. 2003;133:2485S–93S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Gao Z, Shima H. Growth of human prostate cancer cells is significantly suppressed in vitro with sodium butyrate through apoptosis. Oncol Rep. 2012;27:160–7. doi: 10.3892/or.2011.1470. [DOI] [PubMed] [Google Scholar]
- 13.Mandal M, Kumar R. Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1996;7:311–8. [PubMed] [Google Scholar]
- 14.Pajak B, Orzechowski A, Gajkowska B. Molecular basis of sodium butyrate-dependent proapoptotic activity in cancer cells. Advances in medical sciences. 2007;52:83–8. [PubMed] [Google Scholar]
- 15.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–28. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YH. Apoptosis of U937 human leukemic cells by sodium butyrate is associated with inhibition of telomerase activity. International journal of oncology. 2006;29:1207–13. [PubMed] [Google Scholar]
- 17.Calabresse C, Venturini L, Ronco G, Villa P, Chomienne C, Belpomme D. Butyric acid and its monosaccharide ester induce apoptosis in the HL-60 cell line. Biochemical and biophysical research communications. 1993;195:31–8. doi: 10.1006/bbrc.1993.2005. [DOI] [PubMed] [Google Scholar]
- 18.Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. The EMBO journal. 1999;18:1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosato RR, Almenara JA, Cartee L, Betts V, Chellappan SP, Grant S. The cyclin-dependent kinase inhibitor flavopiridol disrupts sodium butyrate-induced p21WAF1/CIP1 expression and maturation while reciprocally potentiating apoptosis in human leukemia cells. Mol Cancer Ther. 2002;1:253–66. [PubMed] [Google Scholar]
- 20.Dai Y, Rahmani M, Grant S. An intact NF-kappaB pathway is required for histone deacetylase inhibitor-induced G1 arrest and maturation in U937 human myeloid leukemia cells. Cell Cycle. 2003;2:467–72. [PubMed] [Google Scholar]
- 21.Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, et al. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem. 1997;272:22199–206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- 22.Joseph J, Mudduluru G, Antony S, Vashistha S, Ajitkumar P, Somasundaram K. Expression profiling of sodium butyrate (NaB)-treated cells: identification of regulation of genes related to cytokine signaling and cancer metastasis by NaB. Oncogene. 2004;23:6304–15. doi: 10.1038/sj.onc.1207852. [DOI] [PubMed] [Google Scholar]
- 23.Han S, Lu J, Zhang Y, Cheng C, Li L, Han L, et al. HDAC inhibitors TSA and sodium butyrate enhanced the human IL-5 expression by altering histone acetylation status at its promoter region. Immunology letters. 2007;108:143–50. doi: 10.1016/j.imlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen KA, Cao Y, Chen JR, Townsend CM, Jr, Ko TC. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Annals of surgery. 2006;243:619–25. doi: 10.1097/01.sla.0000216783.85214.c1. discussion 25-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampson JJ, 3rd, Donkor IO, Huang TL, Adunyah SE. Novel piperazine induces apoptosis in U937 cells. Int J Biochem Mol Biol. 2011;2:78–88. [PMC free article] [PubMed] [Google Scholar]
- 26.Apoptosis: Applied Reagents and Technologies. San Diego: PharMingen; 1998. [Google Scholar]
- 27.Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PloS one. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhard D, Ausserlechner MJ, Tonko M, Loffler M, Hartmann BL, Csordas A, et al. Apoptosis induced by the histone deacetylase inhibitor sodium butyrate in human leukemic lymphoblasts. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1991–2001. doi: 10.1096/fasebj.13.14.1991. [DOI] [PubMed] [Google Scholar]
- 29.Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. Journal of leukocyte biology. 1985;37:407–22. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 30.Birnie GD. The HL60 cell line: a model system for studying human myeloid cell differentiation. The British journal of cancer Supplement. 1988;9:41–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221:2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Schildberger A, Rossmanith E, Eichhorn T, Strassl K, Weber V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators of inflammation. 2013;2013:697972. doi: 10.1155/2013/697972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaddi K, Newton RC. Comparison of biological responses of human monocytes and THP-1 cells to chemokines of the intercrine-beta family. Journal of leukocyte biology. 1994;55:756–62. doi: 10.1002/jlb.55.6.756. [DOI] [PubMed] [Google Scholar]
- 34.Altieri DC, Edgington TS. The saturable high affinity association of factor X to ADP-stimulated monocytes defines a novel function of the Mac-1 receptor. J Biol Chem. 1988;263:7007–15. [PubMed] [Google Scholar]
- 35.Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase-dependent and -independent apoptotic signaling pathways. Leuk Res. 2002;26:495–502. doi: 10.1016/s0145-2126(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 36.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. The EMBO journal. 2001;20:6969–78. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer treatment reviews. 2008;34:206–22. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.van de Loosdrecht AA, Ossenkoppele GJ, Beelen RH, Broekhoven MG, Langenhuijsen MM. Cell cycle specific effects of tumor necrosis factor alpha in monocyte mediated leukemic cell death and the role of beta 2-integrins. Cancer Res. 1993;53:4399–407. [PubMed] [Google Scholar]
- 39.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis--tracing the accessory. Oncogene. 2014;33:3217–24. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 41.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 42.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. Journal of leukocyte biology. 2009;86:237–50. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 43.Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein & cell. 2010;1:218–26. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fransecky L, Mochmann LH, Baldus CD. Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Molecular and cellular therapies. 2015;3:2. doi: 10.1186/s40591-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramakrishnan V, Pace BS. Regulation of gamma-globin gene expression involves signaling through the p38 MAPK/CREB1 pathway. Blood cells, molecules & diseases. 2011;47:12–22. doi: 10.1016/j.bcmd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung JW, Cho SD, Ahn NS, Yang SR, Park JS, Jo EH, et al. Augmentation of sodium butyrate-induced apoptosis by p38 MAP kinase inhibition in rat liver epithelial cells. Antioxid Redox Signal. 2005;7:1767–72. doi: 10.1089/ars.2005.7.1767. [DOI] [PubMed] [Google Scholar]
- 47.Cho SD, Ahn NS, Jung JW, Yang SR, Park JS, Lee YS, et al. Critical role of the c-JunNH2-terminal kinase and p38 mitogen-activated protein kinase pathways on sodium butyrate-induced apoptosis in DU145 human prostate cancer cells. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2006;15:57–63. doi: 10.1097/01.cej.0000195704.05246.fc. [DOI] [PubMed] [Google Scholar]
- 48.Huang XZ, Li ZR, Zhu LB, Huang HY, Hou LL, Lin J. Inhibition of p38 mitogen-activated protein kinase attenuates butyrate-induced intestinal barrier impairment in a Caco-2 cell monolayer model. Journal of pediatric gastroenterology and nutrition. 2014;59:264–9. doi: 10.1097/MPG.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 49.Abe K. Butyric acid induces apoptosis in both human monocytes and lymphocytes equivalently. J Oral Sci. 2012;54:7–14. doi: 10.2334/josnusd.54.7. [DOI] [PubMed] [Google Scholar]
- 50.Kalousek I, Brodska B, Otevrelova P, Roselova P. BimEL-dependent apoptosis induced in peripheral blood lymphocytes with n-butyric acid is moderated by variation in expression of c-myc and p21(WAF1) Cell biochemistry and function. 2008;26:509–21. doi: 10.1002/cbf.1474. [DOI] [PubMed] [Google Scholar]
- 51.Conley BA, Egorin MJ, Tait N, Rosen DM, Sausville EA, Dover G, et al. Phase I study of the orally administered butyrate prodrug, tributyrin, in patients with solid tumors. Clin Cancer Res. 1998;4:629–34. [PubMed] [Google Scholar]
- 52.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. Journal of leukocyte biology. 2011;89:31–9. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 53.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HW, Choi HJ, Ha SJ, Lee KT, Kwon YG. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochimica et biophysica acta. 2013;1835:170–9. doi: 10.1016/j.bbcan.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. The Journal of clinical investigation. 1995;95:2391–6. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:3282–9. [PubMed] [Google Scholar]
- 58.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, et al. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer immunology, immunotherapy : CII. 2000;49:63–70. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svensson S, Abrahamsson A, Vazquez Rodriguez G, Olsson AK, Jensen L, Cao Y, et al. CCL2 and CCL5 are novel therapeutic targets for estrogen-dependent breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0204. [DOI] [PubMed] [Google Scholar]
- 60.Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators of inflammation. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue G, Zippelius A, Wicki A, Mandala M, Tang F, Massi D, et al. Integrated Akt/PKB Signaling in Immunomodulation and Its Potential Role in Cancer Immunotherapy. Journal of the National Cancer Institute. 2015;107 doi: 10.1093/jnci/djv171. [DOI] [PubMed] [Google Scholar]
- 62.Bai Z, Zhang Z, Ye Y, Wang S. Sodium butyrate induces differentiation of gastric cancer cells to intestinal cells via the PTEN/phosphoinositide 3-kinase pathway. Cell biology international. 2010;34:1141–5. doi: 10.1042/CBI20090481. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Zhou Y, Jackson LN, Johnson SM, Chow CW, Evers BM. Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation. Molecular biology of the cell. 2011;22:412–20. doi: 10.1091/mbc.E10-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu TC, Lin PM, Chang JG, Lee JP, Chen TP, Lin SF. Mutation analysis of PTEN/MMAC1 in acute myeloid leukemia. American journal of hematology. 2000;63:170–5. doi: 10.1002/(sici)1096-8652(200004)63:4<170::aid-ajh2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, et al. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene. 2003;22:6231–42. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- 67.Diakos C, Prieschl EE, Saemann MD, Bohmig GA, Csonga R, Sobanov Y, et al. n-Butyrate inhibits Jun NH(2)-terminal kinase activation and cytokine transcription in mast cells. Biochemical and biophysical research communications. 2006;349:863–8. doi: 10.1016/j.bbrc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- 68.Rivero JA, Adunyah SE. Sodium butyrate induces tyrosine phosphorylation and activation of MAP kinase (ERK-1) in human K562 cells. Biochemical and biophysical research communications. 1996;224:796–801. doi: 10.1006/bbrc.1996.1102. [DOI] [PubMed] [Google Scholar]
- 69.Witt O, Sand K, Pekrun A. Butyrate-induced erythroid differentiation of human K562 leukemia cells involves inhibition of ERK and activation of p38 MAP kinase pathways. Blood. 2000;95:2391–6. [PubMed] [Google Scholar]
- 70.Jung JW, Cho SD, Ahn NS, Yang SR, Park JS, Jo EH, et al. Ras/MAP kinase pathways are involved in Ras specific apoptosis induced by sodium butyrate. Cancer letters. 2005;225:199–206. doi: 10.1016/j.canlet.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 71.Scott DW, Longpre JM, Loo G. Upregulation of GADD153 by butyrate: involvement of MAPK. DNA and cell biology. 2008;27:607–14. doi: 10.1089/dna.2008.0773. [DOI] [PubMed] [Google Scholar]
- 72.Kurita-Ochiai T, Amano S, Fukushima K, Ochiai K. Cellular events involved in butyric acid-induced T cell apoptosis. J Immunol. 2003;171:3576–84. doi: 10.4049/jimmunol.171.7.3576. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Zhou L, Bao YL, Wu Y, Yu CL, Huang YX, et al. Butyrate induces cell apoptosis through activation of JNK MAP kinase pathway in human colon cancer RKO cells. Chemico-biological interactions. 2010;185:174–81. doi: 10.1016/j.cbi.2010.03.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: Butyrate and valproic acid increases Annexin V fluorescence in leukemia cells. Cells were treated with butyrate (5 mM) or valproic acid (4 mM) for 24 hours and Annexin V fluorescence was measured by flow cytometry. Plots are representative of 7 experiments.
S2: Modulation of mRNA levels of cytokines by butyrate. Heat map of the average cycle threshold of mRNA levels in U937 cells treated with butyrate and/or SB203580 (SB) for 24 hours generated from RT-PCR analysis.
S3: Quantitative analysis of the cytokine mRNA regulated by butyrate. Quantitative analysis of the cytokine mRNA found within detectable range in U937 cells. Data are separated into (A) chemokines, (B) TNF-related proteins, and (C) growth factors. Untreated cells are set to 1. n = 3. (D) U937 cells were untreated or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or 5 mM butyrate for 24 hours and assayed for the production of CCL20. n = 5. ****p <0 .0001 when compared to the untreated.
S4: Regulation of MAPK and AKT proteins in U937 cells by butyrate. U937 cells were untreated or treated with butyrate over a time course and analyzed for phosphorylated and total proteins via Western blot analyses. (A) Quantitative analyses of p38, and (B) ERK were determined using densitometry. n = 5 and 6 respectively. (C) ELISA was used to investigate phosphorylated and total JNK levels over a time course (10, 30, 60, 120 minutes). n = 3. (D) Quantitative analysis of AKT. n = 6 *p < 0.05 when compared to the untreated (UT).
S5: Effects of pharmacological inhibitors on cell viability and activation of protein. Cells were untreated (UT) or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or butyrate (NaB) (5 mM) for 24 hours. Cell viability of (A) U937 and (B) HL-60 cells were determined using trypan blue exclusion assay. (C) HL-60 cells were untreated or treated with butyrate over a time course and analyzed for phosphorylated and total proteins via Western blot analyses. (D) HL-60 cells were untreated or pre-treated for 30 minutes with p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), MEK inhibitor U0126 (U0) and/or butyrate (N) for 24 hours. Western blot analysis was carried out to analyze multiple proteins.