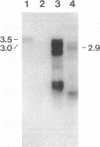
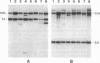
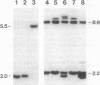
Abstract
Overexpression of the Activator (Ac) transposase gene in Arabidopsis thaliana resulted in a minimal germinal transposition frequency of 27% in which independent Dissociation (Ds) transposition events were observed. Molecular analysis of 45 F1 generation Ac/Ds plants indicated that high rates of somatic excision had occurred, and independent germinal insertions were identified in F2 generation progeny plants. A tandem cauliflower mosaic virus (CaMV) promoter fused to two different Ac coding sequences significantly increased the rate of Ds transposition. The CaMV-Ac fusions activated single and multiple copies of two different Ds elements, DsDHFR and Ds35S-1, and reciprocal crosses resulted in similar transposition frequencies. The improved rate of independent germinal transposition observed makes Arabidopsis an ideal system for insertional mutagenesis.
Full text
PDF
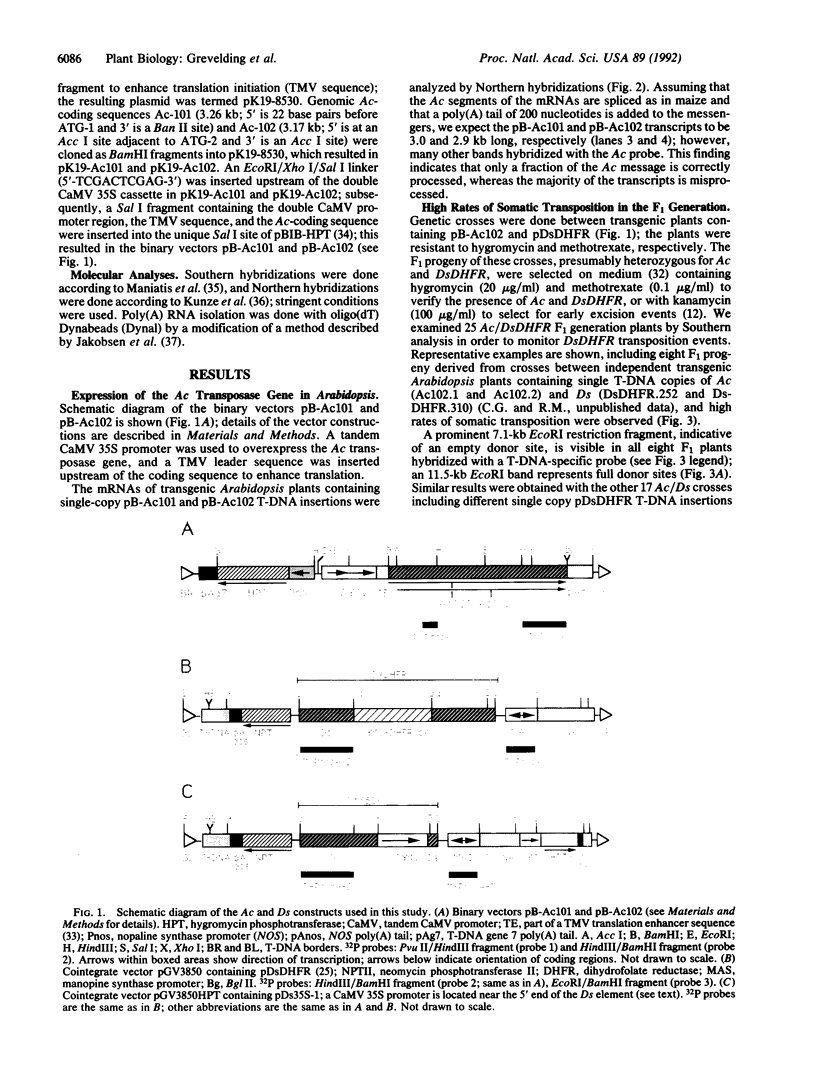



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B., Coupland G., Fedoroff N., Starlinger P., Schell J. Phenotypic assay for excision of the maize controlling element Ac in tobacco. EMBO J. 1987 Jun;6(6):1547–1554. doi: 10.1002/j.1460-2075.1987.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B., Schell J., Lörz H., Fedoroff N. Transposition of the maize controlling element "Activator" in tobacco. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4844–4848. doi: 10.1073/pnas.83.13.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D. Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res. 1990 Jan 11;18(1):203–203. doi: 10.1093/nar/18.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile F., Lassner M. W., Tong Y., Khush R., Yoder J. I. Sexual transmission of transposed activator elements in transgenic tomatoes. Genetics. 1989 Sep;123(1):181–189. doi: 10.1093/genetics/123.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Bowman J. L., DeJohn A. W., Lander E. S., Meyerowitz E. M. Restriction fragment length polymorphism linkage map for Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6856–6860. doi: 10.1073/pnas.85.18.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland G., Baker B., Schell J., Starlinger P. Characterization of the maize transposable element Ac by internal deletions. EMBO J. 1988 Dec 1;7(12):3653–3659. doi: 10.1002/j.1460-2075.1988.tb03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland G., Plum C., Chatterjee S., Post A., Starlinger P. Sequences near the termini are required for transposition of the maize transposon Ac in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9385–9388. doi: 10.1073/pnas.86.23.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann K. A., Marks M. D., Christianson M. L., Quatrano R. S. A Dwarf Mutant of Arabidopsis Generated by T-DNA Insertion Mutagenesis. Science. 1989 Mar 10;243(4896):1351–1354. doi: 10.1126/science.243.4896.1351. [DOI] [PubMed] [Google Scholar]
- Finnegan E. J., Taylor B. H., Craig S., Dennis E. S. Transposable elements can be used to study cell lineages in transgenic plants. Plant Cell. 1989 Aug;1(8):757–764. doi: 10.1105/tpc.1.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. A comparison of eukaryotic viral 5'-leader sequences as enhancers of mRNA expression in vivo. Nucleic Acids Res. 1987 Nov 11;15(21):8693–8711. doi: 10.1093/nar/15.21.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Somerville C. Construction and characterization of a yeast artificial chromosome library of Arabidopsis which is suitable for chromosome walking. Mol Gen Genet. 1991 May;226(3):484–490. doi: 10.1007/BF00260662. [DOI] [PubMed] [Google Scholar]
- Haring M. A., Rommens C. M., Nijkamp H. J., Hille J. The use of transgenic plants to understand transposition mechanisms and to develop transposon tagging strategies. Plant Mol Biol. 1991 Mar;16(3):449–461. doi: 10.1007/BF00023995. [DOI] [PubMed] [Google Scholar]
- Hehl R., Baker B. Induced transposition of Ds by a stable Ac in crosses of transgenic tobacco plants. Mol Gen Genet. 1989 May;217(1):53–59. doi: 10.1007/BF00330942. [DOI] [PubMed] [Google Scholar]
- Hehl R., Baker B. Properties of the maize transposable element Activator in transgenic tobacco plants: a versatile inter-species genetic tool. Plant Cell. 1990 Aug;2(8):709–721. doi: 10.1105/tpc.2.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houba-Hérin N., Becker D., Post A., Larondelle Y., Starlinger P. Excision of a Ds-like maize transposable element (Ac delta) in a transient assay in Petunia is enhanced by a truncated coding region of the transposable element Ac. Mol Gen Genet. 1990 Oct;224(1):17–23. doi: 10.1007/BF00259446. [DOI] [PubMed] [Google Scholar]
- Hwang I., Kohchi T., Hauge B. M., Goodman H. M., Schmidt R., Cnops G., Dean C., Gibson S., Iba K., Lemieux B. Identification and map position of YAC clones comprising one-third of the Arabidopsis genome. Plant J. 1991 Nov;1(3):367–374. doi: 10.1046/j.1365-313x.1991.t01-5-00999.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen K. S., Breivold E., Hornes E. Purification of mRNA directly from crude plant tissues in 15 minutes using magnetic oligo dT microspheres. Nucleic Acids Res. 1990 Jun 25;18(12):3669–3669. doi: 10.1093/nar/18.12.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Carland F. M., Maliga P., Dooner H. K. Visual detection of transposition of the maize element activator (ac) in tobacco seedlings. Science. 1989 Apr 14;244(4901):204–207. doi: 10.1126/science.244.4901.204. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Carland F., Lim E., Ralston E., Dooner H. K. Preferential transposition of the maize element Activator to linked chromosomal locations in tobacco. Plant Cell. 1990 Aug;2(8):701–707. doi: 10.1105/tpc.2.8.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R., Chan A., Daly M., McPherson J. Duplication of CaMV 35S Promoter Sequences Creates a Strong Enhancer for Plant Genes. Science. 1987 Jun 5;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Koncz C., Martini N., Mayerhofer R., Koncz-Kalman Z., Körber H., Redei G. P., Schell J. High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8467–8471. doi: 10.1073/pnas.86.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Stochaj U., Laufs J., Starlinger P. Transcription of transposable element Activator (Ac) of Zea mays L. EMBO J. 1987 Jun;6(6):1555–1563. doi: 10.1002/j.1460-2075.1987.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. G., Starlinger P. Mutational analysis of the N terminus of the protein of maize transposable element Ac. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6044–6048. doi: 10.1073/pnas.87.16.6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCLINTOCK B. Chromosome organization and genic expression. Cold Spring Harb Symp Quant Biol. 1951;16:13–47. doi: 10.1101/sqb.1951.016.01.004. [DOI] [PubMed] [Google Scholar]
- Nam H. G., Giraudat J., Den Boer B., Moonan F., Loos WDB., Hauge B. M., Goodman H. M. Restriction Fragment Length Polymorphism Linkage Map of Arabidopsis thaliana. Plant Cell. 1989 Jul;1(7):699–705. doi: 10.1105/tpc.1.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. H., Finnegan E. J., Dennis E. S., Peacock W. J. The maize transposable element Ac excises in progeny of transformed tobacco. Plant Mol Biol. 1989 Jul;13(1):109–118. doi: 10.1007/BF00027339. [DOI] [PubMed] [Google Scholar]
- Van Sluys M. A., Tempé J., Fedoroff N. Studies on the introduction and mobility of the maize Activator element in Arabidopsis thaliana and Daucus carota. EMBO J. 1987 Dec 20;6(13):3881–3889. doi: 10.1002/j.1460-2075.1987.tb02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. R., Jen G. C. Isolation of single-copy-sequence clones from a yeast artificial chromosome library of randomly-sheared Arabidopsis thaliana DNA. Plant Mol Biol. 1990 Apr;14(4):561–568. doi: 10.1007/BF00027501. [DOI] [PubMed] [Google Scholar]