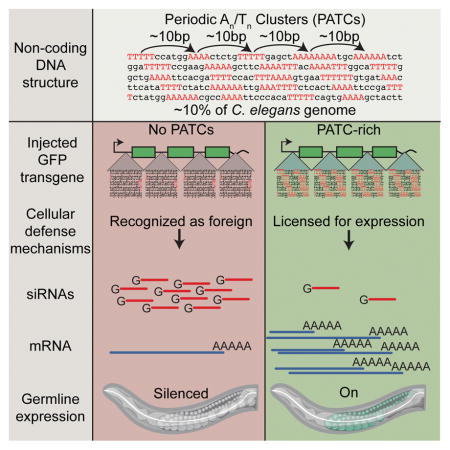
Summary
Cells benefit from silencing foreign genetic elements but must simultaneously avoid inactivating endogenous genes. Although chromatin modifications and RNAs contribute to maintenance of silenced states, the establishment of silenced regions will inevitably reflect underlying DNA sequence and/or structure. Here we demonstrate that a pervasive non-coding DNA feature in Caenorhabditis elegans, characterized by 10-basepair periodic An/Tn-clusters (PATCs), can license transgenes for germline expression within repressive chromatin domains. Transgenes containing natural or synthetic PATCs are resistant to position effect variegation and stochastic silencing in the germline. Among endogenous genes, intron length and PATC-character undergo dramatic changes as orthologs move from active to repressive chromatin over evolutionary time, indicating a dynamic character to the An/Tn periodicity. We propose that PATCs form the basis of a cellular immune system, identifying certain endogenous genes in heterochromatic contexts as privileged while foreign DNA can be suppressed with no requirement for a cellular memory of prior exposure.
Graphical Abstract
eTOC Paragraph
A non-coding DNA sequence pattern can prevent epigenetic silencing of transgenes in germ cells, revealing a functional role for an abundant class of non-coding DNA and a possible mechanism by which cells may recognize and silence foreign DNA
Introduction
Invasive DNA derived from viruses, retrotransposons, and DNA transposons constitute a substantial challenge to organisms. Uncontrolled replication of transposable elements will compromise the host’s genome (Malone and Hannon, 2009) and consequently, cellular defense mechanisms have evolved to detect and silence foreign DNA. These mechanisms are particularly well developed in germ cells, where deleterious changes will impact the fitness of subsequent generations. In eukaryotes, several classes of small RNAs (~20–30 nucleotides long) form complexes with Argonaute proteins to silence foreign nucleic acids by degrading target mRNAs (Zamore et al., 2000) and by transcriptional silencing via RNA-directed heterochromatin formation (Volpe et al., 2002). In germ cells, genome surveillance is mediated in part by a large class of small RNAs (piRNAs) that interact with Argonautes from the Piwi clade (e.g. Aravin et al., 2007; Batista et al., 2008; Das et al., 2008).
Small RNAs act as a recognition system for transcription from invasive DNA, but introduce the danger of silencing endogenous genes. In C. elegans, several potentially-related mechanisms have been proposed to protect endogenous genes against silencing. At a chromatin level, activating H3K36 histone marks deposited on germline-expressed genes provide a positive feed-forward mechanism promoting expression in subsequent generations (Rechtsteiner et al., 2010). Operating alongside and/or in parallel, RNA-based protection systems have been shown to use maternal RNAs to license forthcoming expression of genes (Johnson and Spence, 2011). A licensing mechanism based on small RNAs associated with the CSR-1 Argonaute protein has been proposed to protect endogenous genes from piRNA-mediated silencing (Shirayama et al., 2012). Notably, these mechanisms would only propagate germline expression decisions made in prior generations. What other features, such as genome position or DNA sequences, determine initial licensing for germline expression has remained an open question. Specifically, how can a gene be selected (or rejected) for expression if there is no chromatin mark or small RNA population to indicate whether the gene was expressed in prior generations? To probe this question, we focused on de novo expression and silencing of transgenes in the germline of C. elegans.
Transgenes have been a useful tool to determine the effects of large-scale genome organization in several organisms, most notably Drosophila (Elgin and Reuter, 2013). In C. elegans, transgenes are notoriously difficult to express in the germline, with rapid silencing of episomal DNA (Kelly et al., 1997) and progressive silencing of many single-copy genomic transgenes (e.g., Shirayama et al., 2012). Here we study a stochastic process in which some single-copy insertions variegate in somatic cells and are frequently silenced in germ cells. Variegation and silencing mirror a chromosomal pattern that corresponds to the organization of the genome into broad domains (e.g. Liu et al., 2010). We find that a non-coding DNA structure, called Periodic An/Tn Clusters (PATCs) (Fire et al., 2006) can license transgenes for expression in the germline. Thus, PATCs constitute an abundant class (comprising ~10% of the C. elegans genome) of functionally important non-coding DNA in nematodes that may safeguard endogenous genes from silencing in repressive chromatin environments. We propose that lack of PATCs in foreign DNA may be one characteristic used by nematodes to silence invasive genetic material.
Results
Germline expression is sensitive to large-scale genome organization
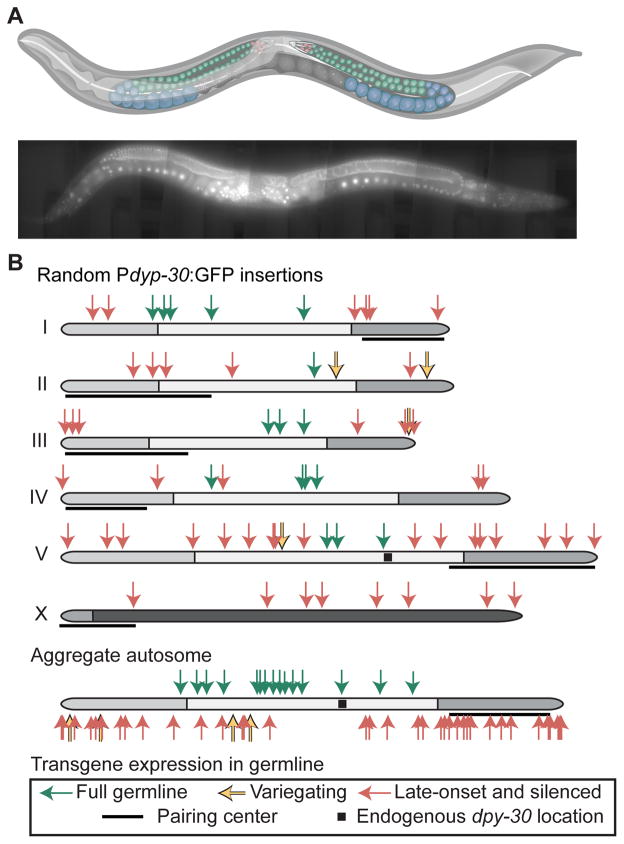
To explore transcriptionally permissive and repressive genomic regions for germline expression we inserted a ubiquitously expressed transgene (Pdpy-30:GFP:H2B) into random locations by transposition (Frøkjær-Jensen et al., 2014) (Figure 1A, Table S1). Of 67 insertion strains analyzed, all had visible somatic expression but most (51 strains) had limited germline expression. We categorized germline expression from animals maintained at 25°C into three classes: complete germline expression (mitosis, early, and late meiosis), early germline silencing (fluorescence only visible in late meiosis), and full germline silencing (no visible expression in any germ cells) (Figure 1A, Figure S1). Additionally, some strains showed transgene variegation and were categorized as “variable”.
Figure 1. Many Pdpy-30:GFP transgenes are specifically silenced in the germline.
A. Top. Schematic of C. elegans hermaphrodite with the female germline highlighted. Red = mitotic cells; green = early meiotic cells; blue = late meiotic cells. Bottom. Composite fluorescence image of animal expressing a Pdpy-30:GFP transgene (42x magnification, scale bar = 50 micron). Graphic of C. elegans modified from “Caenorhabditis elegans hermaphrodite adult-en.sv” by K.D. Schroeder from Wikimedia Commons under a CC-BY-SA 3.0 license.
B. Top. Germline expression at 25°C of Pdpy-30:GFP transgenes inserted randomly by Mos1 transposition. Genomic insertion sites, transgene copy number, and somatic expression of all insertions were previously verified (Frøkjær-Jensen et al., 2014). Germline fluorescence is indicated by colored arrows (key at bottom. Black squares indicate the endogenous location of dpy-30, gray shading indicates enrichment of repressive histone modifications (Liu et al., 2010), and pairing centers are indicated with black lines (MacQueen et al., 2005). Bottom. Aggregated normalized autosomes aligned with pairing centers to the right and Pdpy-30:GFP insertions.
See also Figure S1 and Table S2 for details of classification.
Strains with early or full germline silencing appeared to cluster in non-random chromosomal patterns (Figure 1B). First, all insertions into the X chromosome showed early germline silencing (Figure S1). This pattern is consistent with broad inactivation of the X chromosome in the early meiotic germline by homologs of the Polycomb Repressive Complex 2 (PRC2) (Fong et al., 2002) and de-repression at late meiotic stages (Kelly et al., 2002). Second, 15 0f 25 strains with transgenes inserted within the central 50% of autosomes were expressed, whereas only 1 of 30 transgenes inserted into the distal 25% of autosome arms were expressed in the germline (P < 0.001, Fisher’s exact test) (Figure 1B). This pattern of expression is in agreement with the observed central and distal autosomal domains based on recombination frequency (Brenner, 1974; Rockman and Kruglyak, 2009), histone modifications (Gu and Fire, 2010; Liu et al., 2010), and heterochromatin protein 1 (HP1) distribution (Garrigues et al., 2015). Targeted insertion of a Pdpy-30:GFP transgene (Frøkjær-Jensen et al., 2008, 2014) showed a similar pattern of germline expression and frequent position-dependent stochastic silencing on autosome arms (Figure S1).
Transgene expression in the soma is position-dependent and variegates
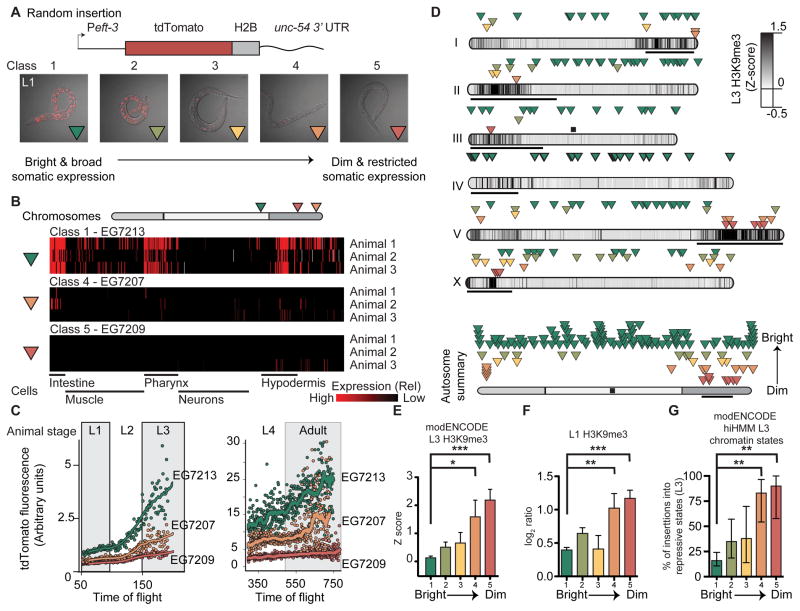
Although we were primarily interested in germline-specific silencing, we also tested whether somatic transgene expression was position-dependent. We generated random insertions with a strong ubiquitous promoter eft-3 (eef-1A.1) that expressed a bright red fluorophore (tdTomato) and screened transgenic animals for low expression at the L1 stage. Qualitatively, most strains showed reproducibly bright expression and we were unable to isolate any animals with fully silenced transgenes. However, a subset of strains (40 of ~800 insertions) showed “mottled” expression in a limited number of somatic cells (Figure 2A). We scored the fluorescence qualitatively at the L1 stage by assigning animals to five expression classes (Class 1 (brightest) to Class 5 (most silenced)) (Figure 2A, Table S2).
Figure 2. Somatic transgene silencing is frequent on chromosome arms.
A. Top. Schematic of the Peft-3:tdTomato transgene. Bottom. Overlay of tdTomato fluorescence and transmitted light images (identical exposure) of representative L1 animals from each expression class.
B. Automated analysis of single cell fluorescence in L1 animals (Liu et al., 2009) of one bright and two dimmer Peft-3:tdTomato:h2b insertions. Each horizontal line represents replicate imaging of different animals from the same strain. The brightness of each red vertical line indicates the level of tdTomato fluorescence in individual, identified cells. Gray indicates failed cell identification. The cells are clustered based on general classes of tissue and indicated below.
C. tdTomato fluorescence intensity at different larval stages (L1 to adult) based on flow cytometry (Dupuy et al., 2007). Average peak tdTomato fluorescence is indicated for each animal with a dot and the line indicates a smoothed average of peak fluorescence.
D. Top. Genomic location and average brightness of each strain (Green: bright & broad expression, red: dim and restricted expression). Darker chromosome shading indicates higher H3K9me3 density at the L3 stage (Liu et al., 2010), black bars indicate the approximate location of pairing centers (MacQueen et al., 2005), and the black box indicates the endogenous location of eft-3. Bottom. Aggregate normalized autosome with all insertions.
E. Average H3K9me3 Z score in L3 animals (Liu et al., 2010) in a 2 kb interval centered on insertions from each class.
F. Average H3K9me3 level (this work) in starved L1 animals in a 2 kb interval centered on insertions from each class. Log2 ratio between sample and input.
Panels E–F: Average ± SEM. Statistics: Mann-Whitney test (** P< 0.01, *** P < 0.001).
G. Percentage of inserts from each class (Class 1 – Class 5) that was inserted into repressive chromatin states (Polycomb or heterochromatin, states 10–13) identified at the L3 stage based on hierarchical non-parametric machine-learning (hiHMM) (Ho et al., 2014). Error bars indicate 95% confidence interval. Statistics: Fischer’s exact test of Class 1 (brightest) compared to Classes 2–5 (dimmer) (** P< 0.01).
See also Figure S2 and Table S2 for higher resolution images and expression quantification.
Insertions into chromosome centers generally belonged to the two brightest classes whereas most dim transgenes were inserted into distal regions (Figure 2D). Genes silenced in the L1 stage corresponded to local genomic environments (± 1 kb) previously found to be significantly enriched in repressive H3K9me3 histone marks at a later larval (L3) stage (Liu et al., 2010) (Figure 2E). We generated an H3K9 methylation dataset from L1 animals and verified this association between transgene expression and H3K9me3 histone marks (Figure 2F). Finally, silenced insertions were frequently inserted into chromatin identified as repressive based on global chromatin marks at the L3 stage (Polycomb repressed and heterochromatin states, hiHMM 10–13) (Ho et al., 2014) (Figure 2G, Figure S2C).
We also measured transgene variegation with quantitative imaging techniques on strains from Class 1, Class 4, and Class 5. One method automatically identified and assigned fluorescence levels to 363 of the 558 cells from fixed L1 animals (Liu et al., 2009). L1 imaging detected expression in significantly more cells and higher levels of expression in the Class 1 strain compared to Class 4 and Class 5 strains (Figure 2B, Figure S2A) and a higher degree of variation in dim strains compared to bright strains was evident (e.g. E lineage, Figure S2B). A second technique relied on imaging live worms across different developmental stages by flow cytometry (Dupuy et al., 2007), which confirmed higher transgene expression in Class 1 compared to Class 4 and Class 5 at the L1 stage (EG7213 = 19.2 ± 0.4, EG7207 = 11.3 ± 0.2, EG7209 = 7.9 ± 0.1, mean ± SEM) (Figure 2C). At later larval stages, the two Class 1 and Class 4 strains increased in absolute fluorescence, whereas the one Class 5 strain remained mostly dim throughout development (Figure 2C). Thus, transgene silencing in early larval stages is not necessarily a permanent state, possibly tracking developmental changes in local chromatin environments (Meister et al., 2010).
In sum, we observed evidence for transgene variegation and an influence of large-scale genome domains on somatic expression. At the same time, only a small subset of insertions variegated. These data extend the longstanding observation that it is easier to express transgenes in the soma compared to the germline of C. elegans (e.g., Kelly et al., 1997).
Transgene silencing in the germline depends on promoter elements
The silencing character of any given chromosomal region might conceivably be universal (so that any insertion would be shut down) or insertion-dependent (capable of silencing some transgenes but not others). We examined whether position-dependent germline silencing was transgene-dependent by using two germline-specific promoters: Pmex-5 and Ppie-1. Similar to Pdpy-30:GFP (Figure 1B), Pmex-5:GFP and Ppie-1:GFP transgenes were mostly silenced on the X-chromosome and frequently silenced on autosome arms compared to centers (GFP positive: Pmex-5, 11 of 17 arm, 25 of 25 center, P < 0.01; Ppie-1: 28 of 48 arm, 34 of 37 center, P < 0.01; Fischer’s test) (Figure 3A). Also, Ppie-1:GFP insertions were more frequently silenced in genomic regions with high levels of H3K9me3 (early embryo, EE) (Liu et al., 2010) (Figure 3B) and in regions that immunoprecipitate with a nuclear lamina protein (LEM-2) (Ikegami et al., 2010) (Figure 3C). However, the mex-5 and pie-1 promoters were significantly more active compared to Pdpy-30 from autosome arms (GFP-positive insertions: Pmex-5, 11 of 17 arm; Ppie-1: 28 of 48 arm; Pdpy-30: 2 of 31 arm, P < 0.01, Fischer’s, Figure 3C) and from repressive hiHMM chromatin states (Ho et al., 2014) (Figure S3F).
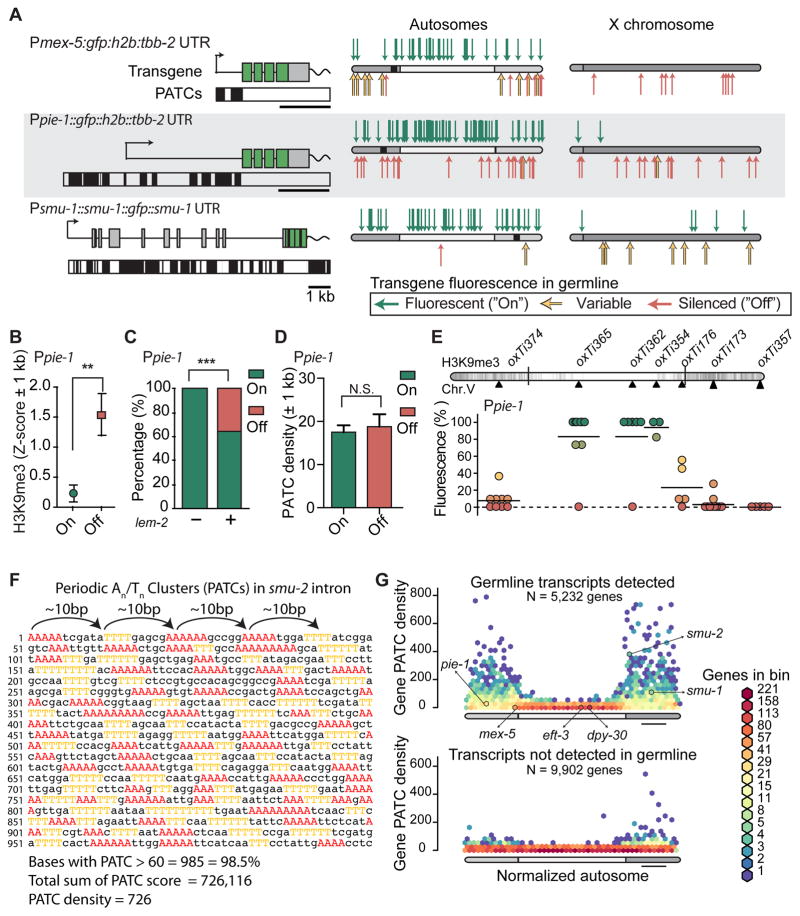
Figure 3. Transgenes containing PATCs are less frequently silenced.
A. Expression of Ppie-1:gfp, Pmex-5:gfp and smu-1:gfp transgenes. Left: transgene schematics with PATCs >60 indicated below as black boxes. Right. Location and germline expression of insertions on aggregated autosomes and the X-chromosome. Endogenous locations of pie-1, mex-5, and smu-1 are indicated with black squares.
B. Local chromatin environment (2kb interval centered on insertion sites) near Ppie-1 insertions. H3K9me3 signal from early embryos (Liu et al., 2010), which have been used as a proxy for germline tissue (Rechtsteiner et al., 2010). Mean ± SEM. Statistical test: Mann Whitney (** = P < 0.01).
C. Local chromatin interactions with nuclear lamin (2 kb interval) near Ppie-1 insertions. Nuclear lamin interactions based on ChIP-sequencing on mixed stage embryos with an antibody against the transmembrane nuclear protein lem-2 (Ikegami et al., 2010). Mean ± SEM. Fischer’s exact test (*** = P < 0.001).
D. Local PATC density (2 kb interval) near Ppie-1 insertions. Mean ± SEM. Statistical test: Mann Whitney (N.S. = not significant).
E. Germline expression of targeted, single-copy Ppie-1:GFP:H2B:tbb-2 UTR insertions into universal MosSCI sites on Chr. V (see also Figure S1). Circles indicate independent transgene insertions that are color coded for each insertion’s expression in the germline (11 animals scored, horizontal bar = mean of independent insertions). Darker chromosome shades correspond to higher H3K9me3 density in early embryos (Liu et al., 2010).
F. Example of a Periodic An/Tn Cluster (PATC) from intron 3 of smu-2. Clusters of three, four, or five adjacent As and Ts are colored. Clusters of As and Ts are separated by approximately 10 bps (~one helical DNA turn) and short An and Tn clusters therefore align along one face of the DNA helix over an extended region (here 1 kb). The PATC algorithm (Fire et al., 2006) assigns a PATC value to every nucleotide of a DNA sequence; higher values indicate that the nucleotide is part of an extended DNA stretch (“PATC-rich region”) with many clusters of An/Tn clusters in perfect 10-basepair register. Less than 0.1% of nucleotides in a random DNA sequence reach a PATC value of 60. The PATC density is defined as the average PATC value of nucleotides in a sequence (See (Fire et al., 2006) and Supplemental Information for details)).
G. Comparison of the PATC density of autosomal, protein-coding genes as a function of chromosome position. Top. Germline-expressed genes (based on > 2 FPKM expression in 1-cell oocytes, (Stoeckius et al., 2014)). Bottom. Genes with no detectable germline expression. Gene distributions were hexagonally binned on a logarithmic frequency scale. A subset of genes used in this study are indicated with arrows.
Targeted insertions of Ppie-1:GFP showed a similar pattern of germline expression and stochastic silencing (GFP-positive insertions: 13 of 18 center, 0 of 24 arm, P < 0.01, Fischer’s) (Figure 3E). One insertion site (oxTi176) near the transition between domains of low and high H3K9me3 showed highly variable Ppie-1:GFP (Figure 3E) and Pmex-5:GFP (Figures S3I) expression; it is possible that insertions into chromosomal locations bordering heterochromatin are particularly prone to stochastic silencing and position effect variegation, similar to what has been observed in Drosophila (Elgin and Reuter, 2013).
These data provide support for genome position as a strong determinant of germline expression and suggest that some germline promoters are more resistant to silencing imposed by heterochromatic domains.
A transgene rich in periodic A/T clusters is expressed from repressive chromatin domains
To resolve how endogenous genes are protected from stochastic silencing in the germline, we looked for a common DNA character that might safeguard genes from the surrounding repressive heterochromatin environment. Periodic An/Tn clusters (PATCs) are an abundant class of non-coding DNA that are enriched in germline expressed genes on autosome arms (Fire et al., 2006) and are anti-correlated with H3K9 methylation (Gu and Fire, 2010). PATCs are composed of short clusters of adenines and thymines spaced approximately 10 basepairs apart, thereby “coating” one face of the DNA helix with An/Tn clusters over extended runs (Figure 3F). It is notable that the relatively silencing-resistant pie-1 and mex-5 promoters contain PATCs (Figure 3A) whereas Pdpy-30 does not (not shown). We confirmed the previously established positive association between germline expression and PATCs (Fire et al., 2006) with more recent gene models (WS245) and gene expression profiles from isolated germlines and single-cell oocytes (Ortiz et al., 2014; Stoeckius et al., 2014; Wang et al., 2009) (Figure 3G, Figure S3).
As a starting point for investigating the role of PATCs, we generated random insertions of a gfp-tagged smu-1 gene (smu-1:gfp). smu-1 has moderately strong overall PATC content (Figure 3G) with PATCs distributed across the endogenous promoter, gene body, and 3′ UTR (Figure 3A). smu-1, and the related gene smu-2, have the highly unusual property that simple, extrachromosomal arrays (hereditary, highly repetitive episomal DNA structures) with these genes are readily expressed in both soma and germ cells (Spartz et al., 2004; Spike et al., 2001). When inserted randomly, all smu-1:gfp insertions were expressed in somatic cells and in the germline when cultured at 25°C (with one exception, which is likely a damaged transgene insertion). Notably, despite some initial silencing, X-linked smu-1:GFP transgene inserts were also expressed in the full germline after propagation for 3–4 generations (Figure 3A). Germline de-silencing over time is not a general feature of transgenes; X-linked Pmex-5 insertions remained silenced over the same number of generations (5 of 5 Pmex-5 strains, Table S2). These observations are unlikely to be be explained by relative promoter strength: smu-1 expression is generally low compared to mex-5, pie-1, and dpy-30 expression, as measured by endogenous gene expression (Stoeckius et al., 2014) or visual inspection of transgene fluorescence.
If PATCs contribute to prevent silencing, then insertion into a PATC-rich chromatin environment might also promote germline expression? We analyzed the local PATC content near Ppie-1:gfp insertions and found no positive association between high PATC-content and germline expression (Figure 3D). We also observed no association between somatic transgene expression (Peft-3:tdTomato) and local PATC environment (Figure S3J), suggesting that insertion into An/Tn clusters does not in itself protect from silencing.
These data show that at least one PATC-rich transgene (smu-1) is remarkably resistant to germline silencing and suggest that, if PATCs permit germline expression, only do so when they are part of the transgene itself.
PATCs in introns of gfp reduces stochastic germline silencing
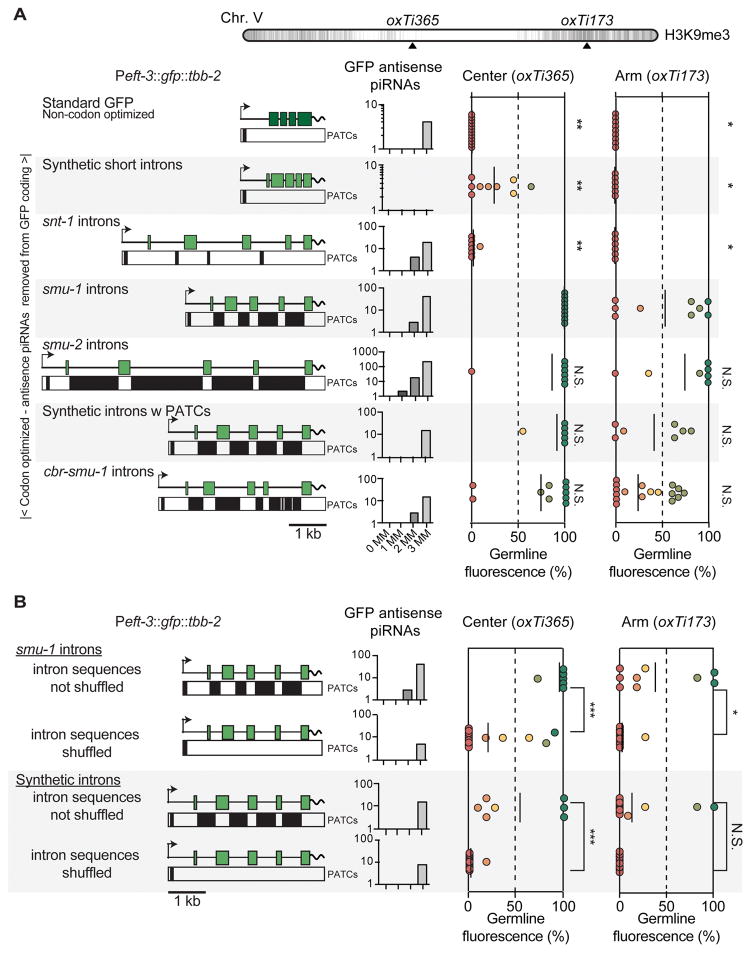
Are PATCs sufficient to safeguard transgenes from gene silencing? To test the effect of PATCs in a consistent context we used a ubiquitous promoter (Peft-3) and 3′ UTR (tbb-2) with few PATCs. We expressed a gfp with minimal piRNA homology that had been optimized for high expression (Figure 4A). Only the PATC content within introns was varied; in particular, the initial 68 basepairs of gfp were kept invariant to minimize possible effects on translation efficiency and all intronic splice junctions were identical between transgenes to minimize possible differences in silencing caused by spliceosome stalling (Dumesic et al., 2013). A standard gfp and optimized gfps with short synthetic introns or introns from a neuronal gene snt-1 were frequently silenced in the germline from the center of Chr. V (oxTi365, insertion at 25°C) (Figure 4A). In contrast, PATC-rich introns from smu-1, smu-2, or the C. briggsae ortholog of smu-1 (cbr-smu-1) significantly reduced stochastic transgene silencing. In the repressive chromatin environment on the arm of Chr. V (oxTi173), we observed a similar pattern of stochastic silencing, except that all transgenes were expressed at lower frequency (Figure 4A). Similarly, silencing of a gfp:cdk-1 transgene was reduced when fused to an optimized GFP containing PATCs (Figure S4C).
Figure 4. PATCs in GFP introns reduce germline silencing.
A. Germline expression of transgenes inserted into chromosome V at 25°C. Top. Location of MosSCI insertion sites on Chr. V. Left, Peft-3:gfp transgenes with PATCs >55 indicated as black boxes underneath. Center. piRNA homology of transgenes allowing 0, 1, 2, or 3 mismatches (MM). Right, germline expression from insertions into oxTi365 and oxTi173, respectively. The standard gfp (dark green) contains three synthetic introns and was not codon-optimized for C. elegans (Fire et al., 1998b). All other gfps (light green) were C. elegans codon-optimized and homologies with less than four mismatches to piRNAs were removed. All codon-optimized gfp transgenes were identical except for the four introns. “Synthetic introns” and “synthetic introns w PATCs” were generated by gene synthesis. Statistical test: Kruskal-Wallis ANOVA comparing all insertions at a given genomic location. Dunn’s multiple comparison t-test against gfp with smu-1 introns (* P < 0.05, ** P< 0.01).
B. Germline expression of transgenes with semi-randomly shuffled intron sequences (shuffling done with rules to prevent novel consensus splice site motifs while maintaining basepair composition of introns). Statistical test: Mann-Whitney rank test (* P < 0.05, ** P< 0.01, *** P< 0.005, N.S. = not significant).
Panels A–B. All transgenes were expressed in somatic cells. Peft-3:gfp transgenes with smu-1 introns and synthetic PATCs in panel A and panel B are the same transgene constructs; however, independent insertions were generated in parallel to the shuffled transgene insertions for comparison under identical conditions.
See also Figure S4.
Are the intronic PATCs responsible for reduced germline silencing, or do the introns harbor other signals that increase expression, for example, germline specific enhancers? First, no single smu-2 intron increased the frequency of germline expression (Figure S4A). Second, a gfp with smu-1 introns inserted with a minimal promoter (pes-10) did not result in visible germline or somatic expression (data not shown). These data argue against the presence of strong enhancers in the introns. Third, we generated synthetic introns with PATCs by gene synthesis. Peft-3:gfp transgenes with synthetic PATCs showed partial but significant resistance to germline silencing in both permissive and repressive chromatin domains (Figure 4A). Fourth, increasing the number of synthetic PATC introns reduced stochastic gene silencing in repressive environments, suggesting an additive effect of PATCs (Figure S4B), although one of our synthetic introns consistently decreased expression. Fifth, the ability of PATC-rich smu-1 introns or synthetic introns to prevent germline silencing was lost when we shuffled the intron sequences to eliminate the A-T clusters but maintained overall nucleotide composition (Figure 4B). This indicates that the basepair composition or specific length of these introns does not in itself improve germline expression.
By contrast, we were unable to confidently demonstrate that PATCs reduce somatic transgene variegation. We inserted Peft-3:tdTomato transgenes with no introns (cDNA), a codon-optimized tdTomato with short synthetic introns or introns from smu-1 with PATCs into central (oxTi365) and distal (oxTi173) locations. Somatic expression quantified by visual classification, with flow cytometry, and with automated identification in L1 animals showed at most a very modest increase in expression from transgenes with PATCs and only at later larval stages (Figure S4D–E).
In sum, native and synthetic PATC-rich introns placed in a foreign coding region can reduce position dependent silencing in the germline.
PATC-rich introns across a range of lengths and from many genes reduce germline silencing
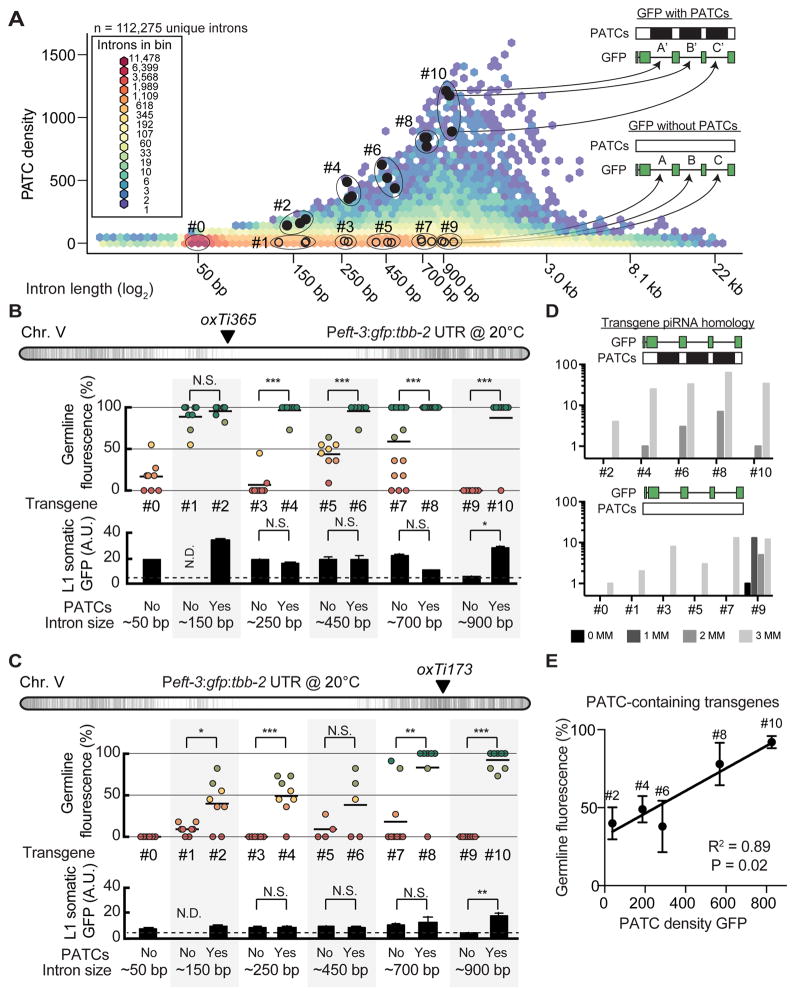
Introns from smu-1 and smu-2 could efficiently reduce transgene silencing but were derived from genes that are unusually resistant to germline silencing (Spartz et al., 2004; Spike et al., 2001). Furthermore, we maintained animals at 25°C, a temperature that empirically promotes germline expression of transgenes (Strome et al., 2001) but also reduces fecundity and is above the thermal tolerance of some C. elegans isolates (e.g. the Bergerac isolate) (Hirsh et al., 1976). To more fully characterize PATC introns, we investigated the role of intron length, intron diversity, and temperature on germline expression with five pairs of GFP with or without PATCs in introns. To select introns, we analyzed the PATC density of all protein-coding introns individually (112,275 introns, WS245): introns have a median length of 69 bps and a prominent peak of PATC-rich introns near ~900 bp (Figure 5A). We selected 30 introns spanning lengths from ~150 bp to ~900 bp that were derived from 29 different endogenous genes and inserted three introns into each Peft-3:gfp transgene. Each individual PATC-containing intron (solid black circles) was matched to an intron with no PATCs (open circles); the introns were matched to within 10% based on intron length and germline expression of the endogenous genes containing the two introns (Table S3).
Figure 5. PATC algorithm can identify introns that reduce germline silencing.
A. Hexagon plot showing the PATC density of all predicted, unique introns extracted from protein-coding genes (WS245). Introns tested in panel B are indicated by closed black circles (high PATC density) and open circles (low PATC density). Each individual intron with high PATC content (top) was matched to an intron with low PATC content (bottom) based on two parameters: (1) intron length and (2) germline expression of the parent gene (both within 10%).
B. Germline (top) and somatic (bottom) expression at 20°C from single-copy Peft-3:gfp transgene insertions at a central, permissive chromosome location (oxTi365).
C. Germline (top) and somatic (bottom) expression at 20°C from single-copy Peft-3:gfp transgene insertions at a distal, repressive chromosome location (oxTi173).
Panel B–C. Statistical test: Mann-Whitney (* P < 0.05, ** P< 0.01, *** P< 0.005, N.S. = not significant, N.D = no data).
D. piRNA homology with zero (0MM), one (1MM), two (2MM), or three (3MM) mismatches to the sequence of each numbered gfp.
E. Linear correlation between germline expression and PATC content for PATC-rich transgenes inserted at the repressive oxTi173 location. The P value indicates the statistical significance for a positive slope of the linear fit.
See also Table S3.
At the central insertion site (oxTi365) we observed variable germline expression at 20°C from transgenes with no PATCs (Figure 5B). In contrast, all five transgenes with PATCs were expressed at high frequencies (Figure 5B). The effects of PATCs on somatic transgene expression were mixed; transgenes with PATCs were generally well expressed but only one PATC-rich transgene had significantly higher expression than a poorly expressed matched control (Figure 5B). In repressive chromatin (oxTi173) we observed a strong and consistent effect of PATCs: transgenes containing PATCs were less frequently silenced in the germline (4 of 5 matched transgenes) and there was no pervasive enhancement of somatic expression (Figure 5C). These differences did not generally appear to be caused by differences in piRNA homology, with one possible exception (900 bp, non-PATC) (Figure 5D). We note that only transgenes with the longest introns (700–900 bps) were expressed at high frequency in repressive chromatin and we observed a good association (R2 = 0.89) between PATC content and resistance to germline silencing (Figure 5E). It is possible that longer introns or higher PATC densities are required to efficiently prevent germline silencing within highly repressive chromatin.
In sum, these data demonstrate a consistent ability for PATC-rich introns to reduce germline silencing and small effects, if any, on somatic transgene expression.
mRNA is depleted and small antisense RNAs are enriched in strains with silenced transgenes
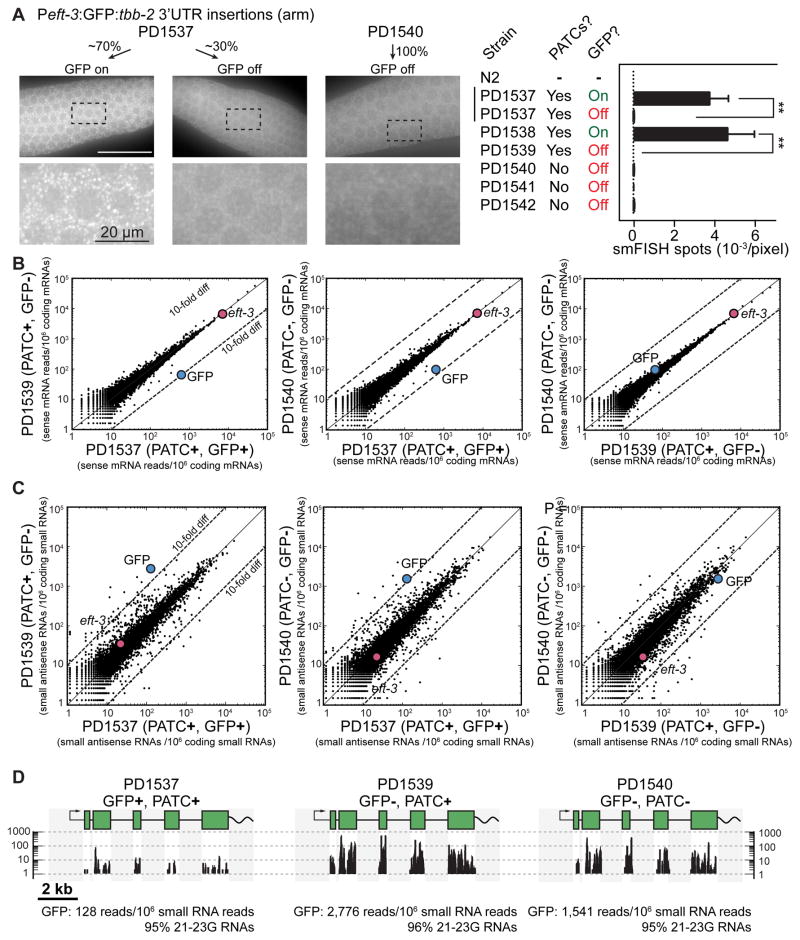
To determine at what stage transgenes were silenced we performed single molecule RNA fluorescence in situ hybridization (smFISH) (Raj et al., 2008) and RNA sequencing experiments on active and silenced Peft-3:gfp insertions. We observed many individual gfp transcripts (diffraction limited cytoplasmic spots; Figure 6A) and frequent transcriptional foci (brighter nuclear spots; Figure S5) by smFISH against gfp in germlines with GFP expression (PD1538). In contrast, we detected few transcripts and no transcriptional foci in GFP negative germlines from wild-type animals (negative control) or from fully silenced strains (e.g. PD1540). The same was true for a transgene with PATCs that was fully silenced (PD1539) or in GFP-negative animals from a transgene that was infrequentely silenced (PD1537) (Figure 6A and Figure S5).
Figure 6. Transgene mRNA expression and small RNA populations.
A. Left. Representative images from single molecule Fluorescence In Situ Hybridization (smFISH) on the germline from animals with frequent GFP expression (~70% fluorescent germlines, PD1537) and a fully silenced strain (PD1540) from Peft-3:gfp transgenes. Individual white spots indicate diffraction-limited single mRNA transcripts (Raj et al., 2008) and germline nuclei are visible as darker circles. Right. Quantification (blinded) of the number of smFISH spots (mean ± SEM) from transgenic animals and N2 animals. In many cases, germlines with silenced gfp showed easily distinguishable GFP expression in somatic cells and smFISH spots in those tissues. Statistical test: ANOVA, post-test Sidak’s multiple comparison test (** P< 0.01).
B. Sense mRNA expression of young adult animals with frequent germline expression (PD1537) or complete germline silencing (PD1539, PD1540) of Peft-3:gfp transgenes. Statistical tests: 2-proportion Z-test (two-tailed), number of gfp transcripts in PD1537 vs PD1539, P ≅ 0; PD1537 vs PD1540, P ≅ 0.
C. Top. Small antisense RNA expression in young adult animals with germline expressed (PD1537) or silenced (PD1539, PD1540) Peft-3:gfp transgenes. Statistical tests: 2-proportion Z-test (two-tailed), number of gfp transcripts in PD1537 vs PD1539, P ≅ 0; PD1537 vs PD1540, P ≅ 0.
Panel B + C. RNAs were aligned against all protein coding genes (WS245) and normalized to uniquely aligned RNAs. Colored dots indicate gfp and eft-3 expression. All strains had GFP expression in somatic cells.
D. Unique alignments of small RNAs (>95% 21–23G RNAs) detected against gfp. The native introns in gfp (250 bp), the eft-3 promoter, and tbb-2 3′UTR are also present in the C. elegans genome and therefore reads could not be aligned uniquely to these regions (shaded gray).
See also Figure S5.
To examine silencing on a bulk level, we sequenced total RNA isolated from synchronized young adult hermaphrodites and observed a depletion of gfp mRNA in animals with silenced Peft-3:gfp transgenes (Figure 6B). A strain with frequent GFP expression in the germline (PD1537) had approximately 10-fold more transcripts than animals with a fully silenced PATC-rich gfp (PD1539) or a fully silenced gfp with no PATCs (PD1540). We did not capture unspliced gfp pre-mRNAs sequences in RNA samples from strains with active or silenced transgenes. In combination with the lack of detectable transcripts in the nucleus by smFISH, we found no evidence for accumulation of unspliced transcripts in the germ cells of animals with silenced GFPs.
To further understand the mechanisms involved in transgene silencing, we isolated and sequenced populations of small RNAs from synchronized young adult hermaphrodites (Figure 6C). Animals carrying silenced GFPs with PATCs (PD1539) or lacking PATCs (PD1540) were 10–20 fold enriched for detectable small antisense RNAs against GFP (primarily 21–23G RNAs) compared to a strain with frequent GFP expression (PD1537). Fully silenced gfp transgenes (i.e. full stochastic silencing from the time of insertion) with or without PATCs were indistinguishable based on the level of gfp mRNA and small anti-sense RNAs (PD1539 versus PD1540) (Figure 6B, C).
Thus, PATCs within introns of a foreign gene confer significant but incomplete protection from stochastic silencing, in agreement with visible germline fluorescence (Figures 4,5,6). It is possible these observations reflect a lack of PATCs in Peft-3 and the tbb-2 3′ UTR; perhaps, full protection from stochastic silencing also requires PATCs in regions flanking the coding sequence, as observed for the smu-1 transgene (Figure 3A).
In sum, these data suggest that transgenes are most likely transcriptionally silenced with the abundance of small RNAs potentially indicative of an RNAi-like mechanism maintaining, or potentially initiating, the silenced state via secondary 22G siRNAs.
Evolutionary adjustment of PATC content for different genomic environments
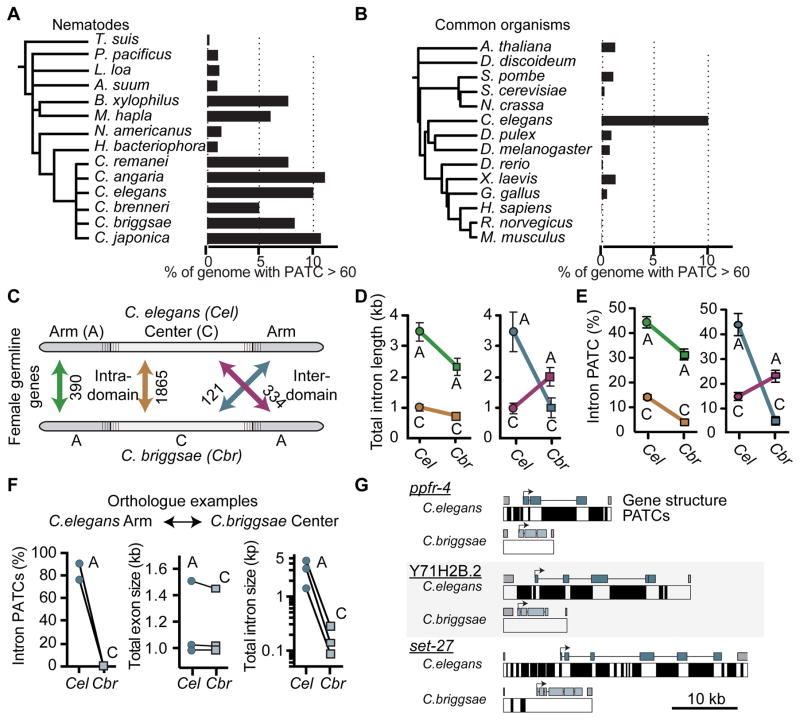
Genomes are under selective pressure and we expect functionally important sequence characteristics to be evolutionarily conserved in closely related species. PATCs are well conserved in the Caenorhabditis genus (5–11% of the total genome sequences are PATC-rich), whereas conservation is mixed in more distantly related nematodes (Figure 7A and Table S4). Outside of nematodes, most genomes do not contain comparable frequencies of PATCs, although some distantly related organisms have PATC-like structures in their genomes (e.g. the centipede S. maritima) (Figure 7B and Table S4).
Figure 7. C. elegans and C. briggsae intron size and PATC content contract and expand with chromosomal location.
A. PATC content of select nematode genomes.
B. PATC signal in commonly studied genetic model organisms and the human genome sequence. Panel A and B: frequency of DNA with a PATC value greater than 60.
C. Schematic of the unique orthologs analyzed. One class (“intra-domain”) contains orthologs that reside on the arm (green) or the center (brown) of both C. elegans and C. briggsae. A second class contains “inter-domain” orthologs (blue, red) that have moved between chromatin domains in the two species.
D. Comparison of the total intron length of genes for each ortholog class.
E. Comparison of the percentage of intronic bases with an average PATC > 60 for each ortholog class.
F. An example of the PATC content, exon length, and intron size for three genes residing in a distal repressive chromatin domain in C. elegans and a central permissive domain in C. briggsae on Chr. III.
G. Gene structure and PATCs > 60 of the three genes from Panel F. The DNA sequence from the last exon of the upstream gene (“5′ gene”) to the first intron of the downstream gene (“3′ gene”) are included.
See also Figures S6–7, Table S4, and Data S2.
If PATCs are functionally important, we might also predict the PATC content of genes to change in response to changes in chromatin environment, for example those caused by large-scale genome rearrangements. To test this prediction, we compared C. elegans and C. briggsae, whose most recent common ancestor existed approximately 20 million years ago (Ross et al., 2011). The overall genomic PATC-content is similar in the two species (Figure 7A) and PATCs from cbr-smu-1 were able to safeguard transgenes in C. elegans (Figure 4A) suggesting functional conservation. Based on recombination frequencies (Ross et al., 2011) a chromosome structure with distinct center and arm domains is conserved in C. briggsae, and PATCs are similarly enriched on autosomal arms (Figure S6A). We analyzed unique C. elegans and C. briggsae ortholog pairs and determined their PATC content as a function of genomic location and expression in the germline (Figure 7C). Orthologs pairs that remain on arms have longer introns (Figure 7D) and higher PATC frequency (Figure 7E) compared to ortholog pairs that remained at a central location over evolution. Ortholog pairs that change chromatin domain show reciprocal changes: the ortholog residing in repressive chromatin has ~3 fold longer introns (Figure 7D) and ~4-fold higher PATC content (Figure 7E) than the ortholog residing at a central domain. Examples of these large changes in intron size and PATCs as a function of genome position are illustrated for six ortholog pairs in Figure 7F–G and Figure S6B–C.
To investigate how PATCs may be generated and/or maintained, we analyzed a large set of single nucleotide variants (SNVs) identified in chemically mutagenized and natural variant strains of C. elegans (Thompson et al., 2013) (Figure S7). For PATC regions, the underlying periodicity can be used to associate each SNV with a phase (0–10 using An/Tn cluster starts as defining phase 0; Fire et al., 2006). Normalized mutation frequencies that result in a net loss of G/C content (G -> A & C -> T transitions and G->T and C->A transversions) within PATCs are most frequent where the G/C content is lowest. In contrast, mutations that increase G/C content are enriched in areas with already high G/C content. These two mutational profiles are offset by approximately 5 basepairs, yielding a tendency to strengthen the density of PATCs within regions already contained in An/Tn-rich clusters (Figure S7). These results are consistent with models (e.g. Holmquist, 1994) in which large-scale genomic domains in nematodes are relatively fixed and genes adapt under evolutionary pressure or due to net mutagenic bias to the chromatin domains they are embedded in.
In sum, changes in non-coding DNA reveal a surprisingly dynamic character (on an evolutionary time-scale) with PATCs generated or eliminated and concomitant intron expansion or contraction for genes expressed in the germline.
Discussion
In many cases, epigenetic control of gene expression is initiated by small RNAs and maintained by proteins bound to DNA. Here we show that distributed sequences of non-coding DNA can safeguard genes from epigenetic gene silencing. Specifically, these DNA structures are comprised of clusters of A/T sequences arranged so that they are on a single face of the DNA molecule. These periodic An/Tn clusters (PATCs) are found within introns or in intergenic regions and can promote germline expression of transgenes in repressive environments. We propose that PATCs similarly promote expression of endogenous genes from repressive chromatin environments.
Genome domains and position effect variegation in C. elegans
Using a synthetic transposon, we probed the C. elegans genome for chromatin environments affecting transgene expression. The chromatin domains for transgene expression described here are consistent with the large-scale structural stratification observed for other genome features:
C. elegans autosomes are partitioned into broad central and distal regions. Here we present functional evidence that these broad genomic domains influence gene expression. Transgenes inserted into distal autosomal regions are frequently silenced in the germline and subject to position effect variegation in somatic cells. Consistent with alternating regions of repressive and permissive chromatin marks (e.g. Gu and Fire, 2010; Liu et al., 2010) transgene silencing on arms is not uniform but rather a heterogeneous mix of active and silenced insertions.
The C. elegans X-chromosome is largely inactivated in early meiotic stages of the gonad (Fong et al., 2002; Kelly et al., 2002). We observed expression that closely mimicked this pattern: most X chromosome insertions were silenced in the early germline. There were two interesting exceptions. First, two Ppie-1 insertions near the left tip of the chromosome were expressed. The left tip of the X chromosome exhibits several features characteristic of distal regions of autosomes and is distinct from the remainder of the X chromosome (Fire et al., 2006; Fong et al., 2002; Kelly et al., 2002). Second, Psmu-1 insertions anywhere on the X chromosome were initially silenced but de-silenced over a few generations. Genes on the X-chromosome are largely devoid of PATCs, which may reflect the unique constraints on X-linked genes caused by X chromosome inactivation in the germline and somatic sex dosage compensation (Meyer, 2010). These data suggest that the smu-1:GFP transgene, possibly due to PATCs, can overcome chromosome-scale Polycomb-mediated epigenetic silencing.
A role for an abundant class of non-coding DNA in nematodes
An analysis of the large RNA polymerase II gene (ama-1) identified periodic sequence features (A-tracts), which were predicted to result in significant DNA bending and explain the unusual migration of C. elegans genomic DNA on electrophoretic gels (VanWye et al., 1991). Subsequent analysis on the completed C. elegans genome sequence identified a pervasive periodic 10 bp motif of An/Tn clusters that were associated with germline expressed genes (Fire et al., 2006). Here we present experimental support for how genes can acquire PATCs in repressive chromatin environments through biased mutations and evidence supporting a causal role for PATCs in permitting germline expression. Transgenes containing promoters with PATCs (Ppie-1:GFP and Pmex-5:GFP) were less prone to silencing than a transgene with few PATCs in the promoter (Pdpy-30:GFP). PATC distributed throughout the entire construct (smu-1:GFP) or only in the coding region (various GFPs) significantly reduced germline silencing compared to transgenes lacking PATCs. These effects were consistent across different PATCs: C. elegans and C. briggsae introns, synthetic introns, short and long introns, and from a multitude of different genes. Our data support a generally permissive role for PATCs in allowing germline expression in contrast to an instructive signal that directly drives germline expression; we imagine that distributed PATCs within a gene allow “proper” DNA access for transcriptional regulation (enhancers, promoters, transcriptional elongation, splicing, etc.). Importantly, transgenes with many internal PATCs are not immune from stochastic silencing; once silenced, we could not distinguish a PATC-rich transgene from a PATC-poor transgene based on fluorescence imaging, smFISH and RNA sequencing.
PATCs may protect endogenous genes from silencing
Why are PATCs necessary? We have studied the role of PATCs in the context of transgenes but we propose that their natural role is to protect endogenous genes from otherwise non-discriminating silencing mechanisms. There are several described mechanisms by which transgenes are silenced in C. elegans: histone methylation, RNAi, RNA epigenetic (RNAe), and unpaired chromosomes in meiosis (Kelly et al., 2002; Ketting et al., 1999; Leopold et al., 2015; Shirayama et al., 2012; Tabara et al., 1999). PATCs may function in parallel to protective pathways that are proposed to depend on a balance between silencing piRNAs bound to the PRG-1 Argonaute and activating small RNAs bound to the Argonaute protein CSR-1 (Shirayama et al., 2012). A balance between permissive and repressive pathways does not imply full activation or complete silencing; for example, expression of a Pmex-5:GFP transgene was increased five-fold in prg-1 mutants (Leopold et al., 2015). More generally, a class of endogenous genes with complementarity to 22G small RNAs were derepressed in prg-1 mutants, suggesting that piRNAs do not exclusively repress foreign DNA (Lee et al., 2012). Unless mechanisms to “reboot” expression exist, antagonistic repressive and permissive pathways that monitor expression in prior generations would be prone to enter a negative feedback loop, which would ultimately lead to a silenced state. Endogenous structures that counteract silencing, such as PATCs, may constitute a fail-safe mechanism to prevent negative feedback loops from forming. PATCs may be particularly important for endogenous genes located in repressive genomic environments, such as the autosomal arms, where unimpeded spreading of repressive histone marks from adjacent regions may bias the balance toward the repressive pathway. Since no homologs of canonical boundary elements or insulators have been identified in C. elegans (Heger et al., 2009), one possible role for distributed PATCs is to prevent heterochromatin spreading.
PATCs may defend the genome against viral or transposon DNA
What are the possible benefits to nematodes for evolving PATC structures? We propose that this unusual DNA structure may be an important component of a genomic immune system that protects the nematode from viral or transposon invasion. A cellular immune system must recognize self versus non-self. We suggest a model in which PATCs can protect endogenous genes from silencing by counteracting silencing pathways. For endogenous genes, the PATC signature can be incorporated into non-coding DNA with no effect on protein sequence. For such a defense system to be efficient, insertion into a PATC-rich chromatin domain should not confer anti-silencing properties; this requirement is consistent with the observed broad distribution of PATCs in endogenous genes and silencing of transgenes inserted into local PATC-rich domains. In the constant arms race between hosts and parasitic DNA elements, it would be costly for invasive DNA to evolve structures that mimic PATCs to avoid silencing, especially if PATCs are specific to nematodes. This protection of self may have allowed nematodes to evolve an aggressive defense system that includes large, repressive genome domains and a piRNA system that tolerates target mismatches. In this model, invasive foreign DNA lacking an elaborate structural feature of endogenous genes are silenced by default (even in central, less repressive regions of the genome) and uncontrolled propagation of invasive DNA is limited. Notably, such a genomic defense system would not require a cellular memory of prior exposure to any particular foreign DNA sequence or structure.
Experimental Procedures
Transgene insertions
Random miniMos insertions were generated in unc-119(ed3) animals and targeted mosSCI insertions in animals with Mos1 elements at defined locations (Frøkjær-Jensen et al., 2008, 2014). See Table S1 for strains and insertion sites.
Imaging
Germline fluorescence was classified by visual inspection on a fluorescence dissection microscope blind to strain identity (Table S2). Automated imaging of somatic fluorescence was performed on confocal microscopy images of fixed L1 animals (Liu et al., 2009) or mixed stage profiling of live animals on a COPAS-profiler2 (Dupuy et al., 2007) (Table S2). smFISH was performed according to the manufacturer’s protocol (Biosearch Technologies, CA) on dissected germlines, imaged at 100x magnification and deconvolved. mRNA and transcriptional foci were quantified blind to strain identity.
Molecular Biology
All plasmids were generated by standard techniques and annotated Genbank sequences are included in Data S1.
Total mRNA and small RNA sequencing
We isolated mRNA and small RNAs from animals grown at 25°C and sequenced libraries on a miSeq instrument (Illumina, CA). We aligned reads to C. elegans protein coding sequences (WS245). SRA accession: SRP072711.
PATC analysis
Individual genes and introns (Table S3) were analyzed with the original PATC algorithm (Fire et al., 2006) and whole genomes were analyzed with a modified algorithm that minimizes off-helical An/Tn signals (Table S4). See Data S2 for 25bp resolution PATC signals for C. elegans (WS245) and C. briggsae (WS245) genomes and Data S3 for intron sequences incorporated in gfp.
Supplementary Material
Article Highlights.
Periodic, non-coding DNA can prevent transgenes from stochastic silencing in germline
Non-coding content of genes is shaped by genomic context and heterochromatin domains
Conditioning of active DNA may allow cells to distinguish foreign from host genes
Acknowledgments
We thank J. Feldman, A. Villeneuve, S. Strome, A. Rechtsteiner, J. Lieb, K. Ikegami, C. Engert, S. Klemm, T. Machacek, V. Jantsch, C. Girard, G. Maro, D. Nix, Fire and Jorgensen lab members; and K. Hoe for expert technical assistance. This work was supported by the Carlsberg Foundation (C.F.J), Direktør Ib Henriksens Foundation (C.F.J.), a Stanford Graduate Fellowship (N.J.), National Institutes of Health grants R01GM095817 (E.M.J) and R01GM37706 (A.Z.F.), and the Howard Hughes Medical Institute (E.M.J.).
Footnotes
Author contributions
Conceptualization, C.F.J. and A.Z.F.; Software, L.H., M.W.D. and A.Z.F.; Formal Analysis, C.F.J. and A.Z.F.; Investigation, C.F.J., N.J., L.H., Y.L., D.Z., K.R., J.R.M.M., X.L., D.D.; Writing - Original Draft, C.F.J.; Writing - Review & Editing, C.F.J., E.M.J., A.Z.F.; Supervision, C.F.J., X.L., S.K.K., D.D., E.M.J., A.Z.F.; Funding Acquisition, C.F.J., E.M.J., A.Z.F.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA Pathway Provides an Adaptive Defense in the Transposon Arms Race. Science. 2007;318:761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs Interact to Form the piRNA Complex Required for Fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The Genetics of Caenorhabditis Elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, et al. Piwi and piRNAs Act Upstream of an Endogenous siRNA Pathway to Suppress Tc3 Transposon Mobility in the Caenorhabditis elegans Germline. Mol Cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic PA, Natarajan P, Chen C, Drinnenberg IA, Schiller BJ, Thompson J, Moresco JJ, Yates JR, Bartel DP, Madhani HD. Stalled spliceosomes are a signal for RNAi-mediated genome defense. Cell. 2013;152:957–968. doi: 10.1016/j.cell.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- Elgin SCR, Reuter G. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb Perspect Biol. 2013;5:a017780. doi: 10.1101/cshperspect.a017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Alcazar R, Tan F. Unusual DNA structures associated with germline genetic activity in Caenorhabditis elegans. Genetics. 2006;173:1259–1273. doi: 10.1534/genetics.106.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Bender L, Wang W, Strome S. Regulation of the different chromatin states of autosomes and X chromosomes in the germ line of C. elegans. Science. 2002;296:2235–2238. doi: 10.1126/science.1070790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Wayne Davis M, Hopkins CE, Newman BJ, Thummel JM, Olesen S-P, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, LaBella M, Pozniakovsky A, Moerman DG, Jorgensen EM. Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat Methods. 2014;11:529–534. doi: 10.1038/nmeth.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues JM, Sidoli S, Garcia BA, Strome S. Defining heterochromatin in C. elegans through genome-wide analysis of the heterochromatin protein 1 homolog HPL-2. Genome Res. 2015;25:76–88. doi: 10.1101/gr.180489.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SG, Fire A. Partitioning the C. elegans genome by nucleosome modification, occupancy, and positioning. Chromosoma. 2010;119:73–87. doi: 10.1007/s00412-009-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger P, Marin B, Schierenberg E. Loss of the insulator protein CTCF during nematode evolution. BMC Mol Biol. 2009;10:84. doi: 10.1186/1471-2199-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Ho JWK, Jung YL, Liu T, Alver BH, Lee S, Ikegami K, Sohn KA, Minoda A, Tolstorukov MY, Appert A, et al. Comparative analysis of metazoan chromatin organization. Nature. 2014;512:449–452. doi: 10.1038/nature13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Egelhofer TA, Strome S, Lieb JD. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 2010;11:R120. doi: 10.1186/gb-2010-11-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Spence AM. Epigenetic licensing of germline gene expression by maternal RNA in C. elegans. Science. 2011;333:1311–1314. doi: 10.1126/science.1208178. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Schaner CE, Dernburg AF, Lee M-H, Kim SK, Villeneuve AM, Reinke V. X-chromosome silencing in the germline of C. elegans. Dev Camb Engl. 2002;129:479–492. doi: 10.1242/dev.129.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF, Haverkamp TH, van Luenen HG, Plasterk RH. Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell. 1999;99:133–141. doi: 10.1016/s0092-8674(00)81645-1. [DOI] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D, Jr, Mello CC. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell. 2012;150:78–87. doi: 10.1016/j.cell.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold LE, Heestand BN, Seong S, Shtessel L, Ahmed S. Lack of pairing during meiosis triggers multigenerational transgene silencing in Caenorhabditis elegans. Proc Natl Acad Sci. 2015;112:E2667–E2676. doi: 10.1073/pnas.1501979112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Rechtsteiner A, Egelhofer TA, Vielle A, Latorre I, Cheung M-S, Ercan S, Ikegami K, Jensen M, Kolasinska-Zwierz P, et al. Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res. 2010 doi: 10.1101/gr.115519.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Long F, Peng H, Aerni SJ, Jiang M, Sánchez-Blanco A, Murray JI, Preston E, Mericle B, Batzoglou S, et al. Analysis of cell fate from single-cell gene expression profiles in C. elegans. Cell. 2009;139:623–633. doi: 10.1016/j.cell.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen AJ, Phillips CM, Bhalla N, Weiser P, Villeneuve AM, Dernburg AF. Chromosome Sites Play Dual Roles to Establish Homologous Synapsis during Meiosis in C. elegans. Cell. 2005;123:1037–1050. doi: 10.1016/j.cell.2005.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–668. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister P, Towbin BD, Pike BL, Ponti A, Gasser SM. The Spatial Dynamics of Tissue-Specific Promoters During C. Elegans Development. Genes Dev. 2010;24:766–782. doi: 10.1101/gad.559610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ. Targeting X chromosomes for repression. Curr Opin Genet Dev. 2010;20:179–189. doi: 10.1016/j.gde.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz MA, Noble D, Sorokin EP, Kimble J. A New Dataset of Spermatogenic vs. Oogenic Transcriptomes in the Nematode Caenorhabditis elegans. G3 GenesGenomesGenetics. 2014;4:1765–1772. doi: 10.1534/g3.114.012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtsteiner A, Ercan S, Takasaki T, Phippen TM, Egelhofer TA, Wang W, Kimura H, Lieb JD, Strome S. The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny. PLoS Genet. 2010;6:e1001091. doi: 10.1371/journal.pgen.1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman MV, Kruglyak L. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000419. doi: 10.1371/journal.pgen.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JA, Koboldt DC, Staisch JE, Chamberlin HM, Gupta BP, Miller RD, Baird SE, Haag ES. Caenorhabditis briggsae Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination. PLoS Genet. 2011;7:e1002174. doi: 10.1371/journal.pgen.1002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D, Jr, Mello CC. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell. 2012;150:65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Herman RK, Shaw JE. SMU-2 and SMU-1, Caenorhabditis elegans homologs of mammalian spliceosome-associated proteins RED and fSAP57, work together to affect splice site choice. Mol Cell Biol. 2004;24:6811–6823. doi: 10.1128/MCB.24.15.6811-6823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike CA, Shaw JE, Herman RK. Analysis of smu-1, a gene that regulates the alternative splicing of unc-52 pre-mRNA in Caenorhabditis elegans. Mol Cell Biol. 2001;21:4985–4995. doi: 10.1128/MCB.21.15.4985-4995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckius M, Grün D, Kirchner M, Ayoub S, Torti F, Piano F, Herzog M, Selbach M, Rajewsky N. Global characterization of the oocyte-to-embryo transition in Caenorhabditis elegans uncovers a novel mRNA clearance mechanism. EMBO J. 2014;33:1751–1766. doi: 10.15252/embj.201488769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, Saxton W. Spindle Dynamics and the Role of γ-Tubulin in Early Caenorhabditis elegans Embryos. Mol Biol Cell. 2001;12:1751–1764. doi: 10.1091/mbc.12.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 Gene, RNA Interference, and Transposon Silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Thompson O, Edgley M, Strasbourger P, Flibotte S, Ewing B, Adair R, Au V, Chaudhry I, Fernando L, Hutter H, et al. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 2013;23:1749–1762. doi: 10.1101/gr.157651.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWye JD, Bronson EC, Anderson JN. Species-specific patterns of DNA bending and sequence. Nucleic Acids Res. 1991;19:5253–5261. doi: 10.1093/nar/19.19.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, Martienssen RA. Regulation of Heterochromatic Silencing and Histone H3 Lysine-9 Methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao Y, Wong K, Ehlers P, Kohara Y, Jones SJ, Marra MA, Holt RA, Moerman DG, Hansen D. Identification of genes expressed in the hermaphrodite germ line of C. elegans using SAGE. BMC Genomics. 2009;10:213. doi: 10.1186/1471-2164-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: Double-Stranded RNA Directs the ATP-Dependent Cleavage of mRNA at 21 to 23 Nucleotide Intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.