Abstract
Each of the macronutrients, carbohydrate, protein and fat, has a unique set of properties that influence health, but all are a source of energy. The optimal balance of their contribution to the diet has been a long-standing matter of debate. Over the past half century, there has been a progression of thinking regarding the mechanisms by which each may contribute to energy balance. At the beginning of this time period, the emphasis was on metabolic signals that initiated eating events (i.e., determined eating frequency). This was followed by an orientation to gut endocrine signals that purportedly modulate the size of eating events (i.e., determined portion size). Most recently, research attention has been directed to the brain where the reward signals elicited by the macronutrients are viewed as potentially problematic (i.e., contribute to disordered eating). At this point the predictive power of the macronutrients for energy intake remains limited.
Keywords: Carbohydrate, protein, fat, diet, food, energy balance
Introduction
Consensus is difficult to achieve on most topics in the field of nutrition and the target seems to be retreating. With imperfect knowledge of the function of human somatic cells and growing recognition of the contribution of genetics, epigenetics, the gut microbiome and probabilistic behavioral inputs, establishing cause and effect, let alone best practices for individuals and populations, is problematic. One area of agreement is that body weight is a function of energy balance, and there is evolving acceptance that this is truly based on energy itself rather than its source. Body weight can be gained, lost or maintained on diets varying in macronutrient composition (142, 156). There are clearly different health implications of diets that emphasize one macronutrient over another (a topic where consensus is still elusive), but from a body weight perspective, energy is the common denominator. It is well known that the energy yield from each macronutrient differs, but a key question is whether the unique properties of proteins, fats and carbohydrates hold particular implications for energy balance. This review will consider the progression of thinking about the roles of proteins, fats and carbohydrates on appetitive sensations and feeding since the middle of the last century.
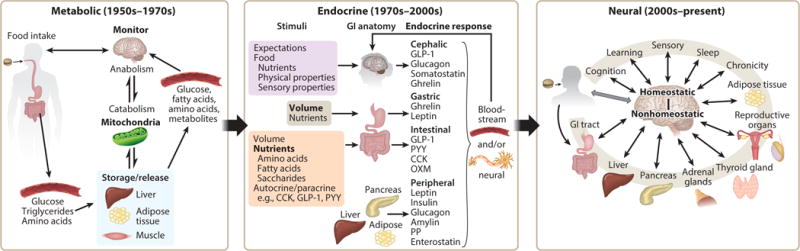
It is proposed that there were three eras over this interval (Figure 1). The boundaries between them are not distinct and elements of each are relevant in the present, but it is argued that there has been a transition of views about the role played by each macronutrient. In the period from the 1950’s –1970’s the emphasis was on metabolism, with the proposal of glucostatic, aminostatic and lipostatic theories predominating. Each of these views was based largely on a model where a decrease of one of the nutrients or a metabolite signaled the need for increased energy intake. Hence, regulation of energy intake was largely based on a signal that initiated an eating event. The sensation is termed “hunger.” The emphasis on hunger and meal initiation was logical at the time when overweight/obesity was not a health concern. Indeed, federal dietary recommendations emphasized obtaining enough energy (201). It follows that eating frequency was then viewed as the primary driver of energy intake. This set of theories lost favor, in part, because their predictive power for energy intake was limited. This is not surprising given that eating frequency is only half of the equation that defines total energy intake. The other half entails portion size, and this is where the field moved during the 1970’s through the 2000’s. During this era, there was a greater emphasis on gut signaling and its influence on appetite and feeding. Numerous gut peptides were identified and evidence emerged that as nutrients entered the GI tract, they stimulated secretion of hormones, leading to sensations of satiation or fullness that prompted the termination of an eating event (ghrelin is the exception). The orientation towards signals to terminate eating events again fit the times, as this was the era when the prevalence of overweight/obesity increased markedly and federal dietary recommendations shifted to warn against eating in excess of energy needs. However, the primary focus on portion size also resulted in limited predictive power for human energy intake. With recognition that decades of research had not yielded dietary recommendations that most of the population would follow, explanations of poor adherence were sought. The increased understanding of gut signaling systems, identification of “taste” receptors in the GI tract (and elsewhere), and advances in neural imaging capabilities led to a reorientation towards the brain. More specifically, attention turned to reward centers that reportedly drive excess intake. The brain was always implicated in the metabolic and endocrine theories of feeding, but it did not play the central maladaptive role ascribed to it now. In this latest trend, the role of the macronutrients shifted from their contribution to homeostasis to providing signals that provoked dysfunctional eating and addictive ingestive behaviors with consequent health complications. The strengths and weaknesses of evidence underlying this evolution of thinking from metabolic to endocrine to neural orientations for carbohydrate, protein and fat will each be reviewed.
Figure 1.
Metabolic responses to Carbohydrate
The glucostatic theory of food intake regulation stemmed from recognition that the body has limited capacity for carbohydrate storage but requires a constant glucose supply for the central nervous system (CNS). The lateral (LH) and ventromedial hypothalamus (VMH) were hypothesized to contain glucoreceptors sensitive to changes in circulating carbohydrate concentrations (180) measurable as differences in circulating arteriovenous (AV) glucose concentrations (179, 180, 261). The LH was proposed as a hunger center and the VMH a satiety center. Considerable evidence supported the theory. Neural activity was observed in the VMH upon glucose administration and in the lateral hypothalamus with insulin administration, while neither protein hydrolysate nor fat emulsion had such effects (6). Further, damage to the VMH (40, 122) or lowering of blood glucose concentrations (179) prompted food intake while damage to the LH produced aphagia and anorexia (76). Hunger was viewed as a mechanism for regulating glucose homeostasis (179, 180). Hunger following insulin administration was suggested as a homeostatic response against hypoglycemia (261) and introduced the possibility of hormonal influences on CNS glucoreceptors (247).
However, scientific favor for the theory began to wane as a larger picture emerged. The emphasis on hypothalamic glucoreceptors was diminished by evidence that brain glucose concentrations are unlikely to fluctuate widely in the short-term (56), and arguments that glucostasis is not the primary goal of feeding (99). Particularly problematic was the theory’s inability to explain why hyperglycemia did not lead to decreased food intake in animals (120) or lower hunger and food intake in humans (24, 236). Indeed, numerous rodent studies (76, 268) cast doubt on the presence of hypothalamic glucoreceptors and AV glucose differences as key mechanisms controlling food intake, both core concepts of the glucostatic theory. The theory also did not satisfactorily explain the observation that epinephrine administration quickly triggers hyperglycemia while concurrently reducing hunger without causing a difference in AV glucose. Additionally, it was noted in humans that hunger and hyperphagia occurred with hyperglycemia in diabetes mellitus. To account for this, Mayer modified the theory to propose hunger in this instance was due to a reduction in glucose utilization as a result of abnormal carbohydrate metabolism, rather than AV glucose fluctuations (181).
Another iteration of the theory focused on glycogen balance and considered glycogenolysis the primary determinant of food intake and body weight (93). This view derived from evidence that liver glycogen levels in mice tended to be lower prior to feeding (7), indicating a possible effect on food intake and timing of meal initiation. Because glycogen stores are generally not saturated, with a wide maintenance range (200–500 g), it was thought carbohydrate intake and its contribution to energy maintenance varied to ensure carbohydrate balance (2). However, the body’s ability to adjust substrates for energy utilization did not support a requirement of maintaining carbohydrate storage as an element of food intake regulation (244).
Throughout its history, the primary focus on carbohydrate (glucose) modulation of ingestive behavior and body weight centered on signals that initiated eating events. Continuous monitoring of blood glucose concentrations in rats and humans led to the concept that short-duration blood glucose dips act as a transient signal to initiate feeding (45). However, spontaneous meal initiation did not always occur following such a dip (146) and meal initiation frequently occurred without a change in blood glucose (15). Strong evidence against this hypothesis are euglycemic clamp studies that show independent manipulation of glucose or insulin does not alter appetitive ratings (47). Nevertheless this hypothesis continues to impact the direction of carbohydrate research in relation to human feeding (107, 132). More recently, low glycemic index (GI) foods have been purported to reduce appetite and increase satiety (126). Though reducing available carbohydrate lowers glycemic and insulinemic responses, low GI foods per se do not reliably reduce subjective hunger or increase satiety, nor is GI predictive of appetitive sensations (3, 166).
The glucostatic theory is still espoused by some (206). However, strong evidence challenging its importance under physiological conditions has relegated it to a lesser status than it once held.
Metabolic Responses to Protein
Evidence supporting a link between dietary protein consumption and appetite was formalized as the aminostatic theory of feeding. Mellinkoff noted an inverse association between serum amino acid (AA) concentrations and reports of hunger (184). In its original conception, it was a mechanism focused on eating frequency. Building on this theory, Booth et al. concluded that the relationship between dietary protein and appetite involved more than circulating AA concentrations because reported hunger ratings remain low after AA concentrations return to basal levels (37). Further, fasting AA concentrations are not predictive of hunger sensations.
Nevertheless, a central role for protein in maintenance of energy balance has considerable support. Some work indicates that rodents fed a protein deficient diet or animals experiencing protein stress (e.g., pregnancy) spontaneously select high protein diets under choice feeding conditions (66). Such a specific appetite does not exist for carbohydrate or fat (67). Among humans, both children and the elderly with compromised protein status express a preference for soup containing casein hydrolysate compared to soup alone despite its stronger bitter taste (265, 190). Other work with healthy humans indicates high protein intake results in lower hunger, while low protein intake promotes the desire to eat protein-containing savory foods (115). At the population level, protein intake is strikingly consistent cross-culturally, unlike carbohydrate and fat (193), and has remained so over the recent three decades of markedly increased overweight/obesity incidence of in the United States (196). The aforementioned observations suggest a biological basis for regulation based on protein, and this culminated in the proposition of the protein leveraging hypothesis (232). According to this hypothesis, when the proportion of protein needs are not met, food intake will increase until an appropriate amount of protein is ingested. Conversely, protein rich diets are purportedly ingested in low quantity as they provide the requisite amount of protein with relatively less total energy.
When protein in chow is low or essential AAs are imbalanced, mice consume more energy to compensate and reach a level of adequate protein intake (161). This phenomenon has been replicated in numerous species (85, 86); however, not in humans. Direct assessments of protein leveraging using diets containing 5–10%, 15% and 25–30% of energy from protein (112, 176), as well as protein from animal and plant sources (177), have yielded uniformly negative findings. One trial noted a small increase in energy intake on a diet containing 10% energy from protein, but no reduction of intake with the 25% version. The two other trials reported the opposite, i.e., a small reduction on a 30% protein-derived energy diet, but no increment on a 5% energy from protein diet.
One possible explanation for the failure to document protein leveraging is that protein’s role is overridden by other interrelated factors such as dietary protein source, food form, and protein quality. Consistent with Mellinkoff’s original findings, rapidly absorbed protein sources have been associated with stronger satiety than more slowly absorbed sources (e.g., whey versus casein) (118). The former leads to higher acute post-prandial circulating AA concentrations, thus implicating protein digestibility (34) (However, for some dietary protein sources, such as fish, slower digestion and delayed peak plasma AA concentrations have been proposed as explanations for protein’s satiety effects (259). Other work fails to reveal protein source differences on satiety (151, 152). These inconsistent findings indicate a single property such as digestibility or absolute blood AA concentration cannot fully account for protein’s effect on appetite.
A protein-satiety threshold has been proposed (200) but not confirmed. The concept of a satiety “ceiling effect” is also under consideration (157). In some instances, pure protein loads are studied, but this would rarely occur under customary dietary conditions. There are limited data on a minimum quantity or increment required to decrease hunger or induce satiety.
The importance of protein’s food delivery system also reveals that an independent effect of protein is not robust. When protein is ingested through a beverage, its reported stronger satiety effect, relative to carbohydrate or fat, is diminished or lost (8). One argument for the food form findings is the generally low concentration of protein in beverages relative to what may be present in solid foods. Though some work reveals a positive association between the protein content of a beverage and its ability to reduce hunger (150, 153), other work where a high concentration of protein has been tested does not support a strong protein satiety effect. Additionally, feeding predominately protein, carbohydrate or fat containing beverages does not differentially affect subsequent energy intake (64).
Given these variable findings, other aspects of protein metabolism such as diet-induced thermogenesis and protein’s effects on maintenance of lean body mass with implications for resting energy expenditure have been explored. Though outside the scope of this paper, it may be noted that a two-week, controlled feeding intervention trial revealed participants maintained weight on a 2-fold higher protein diet (15% versus 30% energy) compared to diet matched on energy despite greater diet-induced thermogenesis. When switched to a higher protein, ad libitum diet, participants lost a significant amount of weight as a result of reduced energy intake (273). These observations do not support a strong effect of protein’s high thermogenic effect of food (TEF) property on body weight. Others reviewing longer-term dietary interventions also concluded the higher TEF of high protein diets is unlikely to contribute substantially to weight loss (280). There is clear evidence that higher protein, energy-restricted diets lead to reduced loss of lean body mass. Maintenance of higher levels of metabolically active tissue should aid energy expenditure and moderate weight gain or aid weight loss. However, these effects are small (280). Both of these latter explanations are dependent on marked elevations of energy intake (e.g., tripling from 10% to 30% of dietary energy from protein), which have not proven feasible on a chronic basis in a large proportion of the population.
Metabolic Responses to Fat
In 1953, Kennedy proposed that the hypothalamic control of food intake was regulated by a lipostatic mechanism designed to prevent excess fat storage (140). Parabiotic studies in mice performed years later provided evidence of a circulating factor secreted in direct proportion to fat mass that regulates body mass on a long-term basis (121). The factor, now known as leptin, was cloned in 1994, followed by the cloning of its receptor in 1995 (250, 286). Soon after, circulating concentrations of leptin were shown to correlate with fat mass in both rodents and humans (173), providing further support of the lipostatic theory of regulation.
There is strong evidence both supporting and refuting leptin’s influence on appetite, energy intake, and body weight. The most compelling positive findings derive from studies in leptin-deficient rodents and humans. Administering leptin to ob/ob mice results in reduced food intake and weight loss (mainly due to loss of fat mass) (117). Administration of leptin to children with leptin deficiency results in decreased food intake and significant weight loss (mainly fat mass) (80, 81). These effects indicate leptin signaling can impact both food intake and fat storage. However, the majority individuals with obesity have elevated circulating leptin concentrations and develop leptin resistance, resulting in a poor and inconsistent response to leptin administration (277).
Evidence opposing the role of leptin as “the” long-term regulator of fat mass has also been provided by both rodent and human studies. Although serum leptin is elevated in overfed rats, concentrations return to normal within two days after the overfeeding ceases, while decreased food intake persists beyond this time (275). In addition, overfeeding obese rats that lack functional leptin receptors still induces a subsequent decrease in food intake (275). Additional work has raised questions about the link between leptin secretion and body fat stores. In both lean and obese individuals, plasma leptin concentrations decrease during energy restriction (12, 71, 144). However, decreased plasma leptin concentrations associated with energy restriction in normal weight men and women were only partially explained by weight loss and were not associated with changes in body fat (71). Further, in normal weight to obese women consuming an energy-restricted diet, leptin only decreased significantly within the first week and then remained low, while body fat decreased more linearly throughout the twelve week trial (12).
Leptin was originally thought to be a long-term regulator of energy status, but evidence of short-term leptin regulation has also been reported. Under or overfeeding individuals for a three day period influences leptin concentrations when a eucaloric diet is restored, suggesting that leptin responds to short-term alterations in energy balance (50). The magnitude of increase (with overfeeding) or decrease (with underfeeding) in leptin greatly exceeded that expected if leptin was altered solely based on changes in body fat. In addition, a seven day energy restricted diet induced a significantly greater decrease in circulating leptin than could be explained by weight lost during this period (71). Leptin also negatively correlated with hunger after short-term changes in food consumption in lean individuals (50). However, when leptin was administered to obese individuals after a 10% weight reduction, it resulted in increased fullness following meals and a lower perceived food consumption, but no actual difference in food intake (144). Although these studies suggest leptin serves as a short-term appetitive signal in normal weight individuals, plasma leptin concentrations exhibit a diurnal pattern (271) and thus do not directly reflect changes in appetitive sensations during a 24-hour period.
There is also evidence supporting short-term regulation of food intake by fatty acid oxidation. When mitochondrial β-oxidation is inhibited, rats on a medium (18%) fat diet exhibit a significant increase in food intake, while no effect is observed in rats on a low (3.3%) fat diet (226). In addition, rodents and humans decrease food intake when consuming a diet rich in medium chain triacylglycerols (MCTs) (198, 262). MCTs contain 8-C and 10-C saturated fatty acids, which can enter the portal vein directly, and are rapidly oxidized once they reach the liver. Inhibiting fatty acid oxidation in the liver increases food intake in rats administered MCTs, while no effect is observed in response to long chain triacylglycerols (LCTs) (198). In a human study, a preload containing MCTs significantly reduced food intake in non-dieting women during a meal provided 30 minutes later, but had no effect on the intake of dieters (219).
In contrast, several studies in both normal weight and obese individuals have shown that supplementing a meal with MCTs versus LCTs has no effect on food intake or appetite ratings at a subsequent meal (241, 263). However, when an additional MCT or LCT load of test oil was provided one hour before an ad libitum meal, individuals who received the MCTs consumed significantly less (241). In a longer term study of women with obesity administered a very low energy diet supplemented with either MCTs or LCTs for four weeks, hunger ratings were lower and satiety ratings were higher after consuming MCT supplemented meals, but only during the first two weeks at 5 and 40 minutes following the meal (148). These findings suggest that there may be very short-term effects of MCTs on food intake and satiety, but they do not extend to subsequent meals, and the body adapts to these changes if MCTs are consumed chronically.
Furthermore, the digestion of dietary fat influences appetite through its effects on gastric volume. In a study where intragastric distention was fixed to resemble postprandial fullness, an intraduodenal infusion of a lipid emulsion increased gastric volume and fullness ratings more than either a mixed nutrient or protein solution (82). In another study, both LCT and MCT infusions increased gastric volume and fullness sensations, but not when administered along with a lipase inhibitor (83). This suggests that the digestive products of TAG elicit these effects. Compared to a glucose supplemented meal, a high fat meal elicited higher fullness ratings at any given gastric volume, despite the faster gastric empting rate and lower energy content of the high carbohydrate meal (174). Collectively, these studies suggest that the digestive products of dietary fat induce greater fullness sensations than other macronutrients.
Observations that high fat diets lead to passive overconsumption and weight gain in humans (32) suggest that there are weak inhibitory mechanisms in place to prevent excess fat intake. In one exemplary study, individuals consumed more energy from an ad libitum meal containing an array of high fat foods versus foods high in carbohydrates, suggesting the satiating properties of fat are not as potent as those of other macronutrients (30). In addition, a breakfast supplemented with fat did not decrease hunger ratings or energy intake of a snack provided 90 minutes later when compared to the breakfast without the additional fat (30). A lack of inhibitory mechanisms to prevent excess fat consumption may have been beneficial when food was scarce and eating events did not occur often, but now that fat is abundant in the food supply, it seems to enable weight gain. The observation that fat preference positively correlates with percent body fat (183) suggests that the palatability of this macronutrient contributes to its overconsumption. Overall, the high palatability and availability of dietary fat seem sufficient to prompt food intake without eliciting a precise reduction of portion size through its satiety and the satiation properties.
Endocrine Responses to Carbohydrate
An endocrine function for the GI tract has been recognized since the seminal work on secretin by Bayliss and Starling in 1902 (19). The repertoire of gut peptides that control digestive processes has grown to over 20. They are secreted by15–20 types of enteroendocrine cells characterized by the peptides they release and their anatomic location. The GI tract is now viewed as the largest endocrine organ in the body. The effect of cholecystokinin (CCK) on gallbladder contraction was demonstrated in 1928 (129), but its influence on energy intake (meal size) in rodents was not established until 1973 (109). This finding prompted the search for other peptides that modulate appetite and ingestive behavior, with characterization of more than a dozen hormones (obestatin, nesfatin-1, CCK, PYY, GLP-1, GLP-2, OCM, Glicentin, GIP, NT, somatostatin, 5-HT, secretin) as holding anorexic properties and one (ghrelin) with orexigenic activity. These satiety hormones are secreted by enteroendocrine cells termed “open” because they have microvilli exposed to the lumen that contain receptors for nutrient signals derived from ingesta passing through the tract. These nutrient receptors are located on I-cells (source of CCK) in the proximal and L-cells (source of GLP-1, GLP-2, PYY) primarily in the distal tract. However, L-cells are also present in the duodenum. Other enteroendocrine cells, such as the P/D1-like cells in the stomach that secrete ghrelin, are “closed,” and don’t likely monitor the nutrient mixture in the gastric lumen. Ingestion of monosaccharides and complex carbohydrates (28, 29) are associated with ghrelin secretion, but this is probably not directly responsive to nutrient signaling as closed form enteroendocrine cells respond to neural and hormonal signals.
GI peptides may modulate appetitive sensations and glucose tolerance by neural, endocrine, paracrine and/or autocrine mechanisms. The initial work on endocrine signaling in the GI tract focused on the stomach and small intestine but more recently has expanded to range from the oral cavity to the colon. The promise that manipulation of these modulators of feeding and glucose tolerance could be harnessed to address the growing overweight/obesity and diabetes problems, identified in the late 1970’s in the US, prompted research that expanded understanding of gut biology, but has not yet yielded the hoped for clinical results.
The oral sweet taste receptor, first characterized in 2001, binds an array of sugars as well as low calorie sweeteners (LCS). In addition to providing a sensory signal that is inherently pleasant and guides food choice, rodent taste cells that bind sweeteners also express GLP-1 (69, 248). GLP-1 may be involved in sweet taste signaling and may also enter the circulation where it could exert a direct anorexic effect in the brain. The concentration of GLP-1 released from taste cells is limited, but because there is little or no dipeptidyl peptidase-IV present to degrade the molecule, as occurs following release from L-cells in the distal intestine, it may exert a larger effect than expected. The cephalic phase insulin response has traditionally been ascribed to vagal-mediated pancreatic secretion, but recent findings now suggest there may also be an endocrine contribution via taste cell release of GLP-1 (145). Alternatively, a signaling system activated by selected sweeteners independent of the sweet receptor may be contributing (110). Such a system has been described in mice, but its existence in humans is not known. The cephalic phase insulin response is significantly correlated with post-absorptive insulin concentrations (253) and has been associated with ingestive behaviors (213). However, the roles of glucose and insulin in mediating appetite and feeding under physiological conditions are questionable (47).
In 1958, it was reported that the presence of nutrients in the ileal lumen inhibit gastric motility (205) and augment satiation. In the early 1960’s, gastric infusions of isolcaloric test solutions of glucose and starch were reported to slow the rate of gastric emptying (128). Subsequent work revealed glucose ingested orally or administered gastrically had a greater effect on plasma concentrations of insulin than intravenous glucose (208). This effect, termed the ‘incretin effect,’ suggested signals from the gut are important in the hormonal regulation of postprandial glycemia (9). Since that time, additional evidence has documented that digestive products of dietary carbohydrates are effective stimuli for the secretion of multiple gut peptides in the small intestine, most notably the incretins, GIP and GLP-1. Recent evidence suggests glucose or fructose may trigger CCK release as well (149, 238), though treatment with the sweet taste inhibitor lactisol does not alter CCK release (108) and the TAS1R2 component of the sweet receptor is not co-expressed with CCK (60).
Ghrelin is an orixigenic hormone secreted in the stomach. Its release is not driven by luminal sensing of carbohydrate. Indeed, several reports note hunger ratings precede changes of ghrelin (98, 160). Additionally, post-prandial concentrations of ghrelin are not predictive of meal requests or the energy content of the meals (38).
Peptides are secreted in the intestine by several mechanisms. First, there is suggestive evidence for a learned anticipatory secretion that would presumably have a neural or hormonal basis (58, 260). Interestingly, suppression of the pre-meal GLP-1 rise leads to lower, rather than greater food intake. This is inconsistent with GLP-1’s reported anorexic effect, but may reflect the lack of suppression of orexigenic signals (65, 213). Second, glucose can pass through SGLT1 on L-cells depolarizing the cell and releasing peptides. Intravenous glucose administration does not suppress food intake acutely, whereas there is a direct relationship with intestinal glucose (216). This is consistent with the hypothesis that glucose transport is required for carbohydrate mediated reduction of food intake (72). Third, selected nutritive sweeteners and LCS, can bind to the T1R2/T1R3 sweet taste receptor on enteroendocrine cells and effect peptide release. Enteroendocrine cells that express the receptor also possess the elements required for taste transduction such as α-gustducin, TRPM5 and PLCβ2. However, the terminology of gut taste may be inappropriate, given that the signal generated is not conveyed by taste nerves, decoded in taste cortex, and does not lead to a sweet percept. Nutrient sensing more aptly characterizes the function of these cells distributed along the entirety of the GI tract. Once released, the peptides may act as autocrine or paracrine signals, bind to receptors on the afferent vagus nerve generating a neural signal or reach the brain via the circulation.
Identification of sweet taste molecule receptors on intestinal enteroendocrine cells raised the question of whether LCS would also be metabolically active in the GI tract. This is the case in cell culture (130), but support in animal models (101) and humans has been weak. Trials have reported no effect of sucralose on plasma GLP-1 or plasma glucose (170); no effect on GLP-1 and an increase in glucose (207) a rise of GLP-1 with no effect on glucose (41) and an elevation of GLP-1 with a lower glucose concentration (255). There is no resolution of the matter at this point. It is also not confirmed that nutrient signaling mechanisms identified through in vitro studies hold under physiological in vivo conditions.
Ingestion of high fructose corn syrup increased markedly over the past four decades leading some to hypothesize that this poses a particular risk for positive energy balance and weight gain. However, fructose has only a comparable effect to glucose on gastric emptying, incretin release, the rate of glucose absorption, post-absorptive glucose concentration (164), appetite, energy intake and energy compensation (236) in humans. Fructose is sweeter than glucose or sucrose, so it could have stronger rewarding properties. However, it is rarely experienced alone (245).
The density of enteroendocrine cells is highest in the colon, so signaling molecules that are not absorbed in more proximal regions or are generated in the colon may also prompt a robust hormone response. The concept that fermentable carbohydrates could be a source of signaling molecules that affect gut peptide concentrations associated with glucose and energy balance dates back to the late 1980s and early 1990s (111, 167). Presently, there is considerable interest in the role of the microbiota in fermenting dietary fibers to yield short chain fatty acids (SCFA) such as acetate, propionate and butyrate. SCFA influence the expression of G-protein coupled receptors and can bind to FFAR2 (GPR43) and FFAR3 (GPR41) on L-cells resulting in secretion of peptides such as GLP-1 and PYY, the two principle drivers of the ileal break, which slows gastric emptying and GI transit and augments satiety. However, animals lacking these two receptors have normal responses to SCFA and improved glucose tolerance (249) revealing redundancy in the system and uncertainty about the mechanism. Evidence linking fermentable fiber, the microbiota and gut peptides in humans reveals inconsistent associations between gut peptide concentrations, appetite, and energy intake (78). This may reflect the fact that the microbiota generates many bioactive compounds that exert effects in multiple organs (53). Additionally, much of the work on this topic is short term and the effects of fermentable fiber may be on proliferation of enteroendocrine cells, which would reflect chronic feeding patterns (149). Fiber’s impact on satiation may be overestimated (52, 137) and its contribution to energy intake underestimated. Whether increasing fiber intake to moderate energy balance will have the desired effect is uncertain.
Taken together, strong evidence indicates simple and complex dietary carbohydrates prompt the secretion of gut “satiety” peptides. The expectation would be that carbohydrate should be highly satiating and consumed in limited portion sizes. However, this has not been the common experience, especially when present in beverages. This appears to belie the importance of endocrine signaling for carbohydrates on ingestive behavior. The reasons are many but include: 1) the possibility that dietary patterns influence enteroendocrine cell density, and thus peptide concentrations, in the GI tract (224, 276), 2) executive function can, and frequently does, override physiological signals, and 3) no single peptide acts independently and many factors influence the hormonal response pattern to eating. Whether continued exploration of carbohydrate driven endocrine mechanisms that are primarily related to only one facet of intake (portion size) to modulate appetite and intake for preventive or therapeutic purposes will be productive is uncertain.
Endocrine Responses to Protein
In 1970 Booth et al. demonstrated intake of a midday and subsequent meal were lower when the earlier meal was higher in protein (35). Subsequent studies in humans supported these findings (36, 220) In addition to decreased energy intake, self-reports of hunger and desire to eat were significantly lower with high protein compared to a high carbohydrate meals (123). Hypotheses for the increased satiety due to protein included circulating AAs (related to Mellinkoff’s aminostatic theory discussed previously) (184), increased thermogenesis with protein intake (75), sensory specific satiety (264) and stimulation of gut hormone release (192). However, the associations between protein exposure and gut peptide release are weak and inconsistent. This stems, in part, from influences of the quantity, quality, and type of protein ingested on gut peptide secretion.
Research into the effect of the quantity of protein consumed on appetitive peptide concentrations began in the mid 2000’s. Findings were suggestive of positive effects, but not compelling. Meals containing 14%, 25% and 50% protein (21) increased GLP-1, PYY, and glucagon dose dependently but had no effect on GIP, CCK, or ghrelin. Feelings of fullness also increased dose dependently; however, this had no effect on ad libitum energy intake. A review of acute feeding studies in humans noted that higher protein interventions led to lower hunger, ghrelin, and increased fullness in only 35%, 37%, and 55% of trials, respectively. GLP-1 or PYY concentrations of the high protein groups were elevated in only 47% of trials, and food intake at a challenge meal was lower in only 18% of trials with a high-protein intervention (157). These inconsistent results may be due to limitations of pre-load design protocols and inter-study differences in protein intake levels. Weaknesses of the pre-load paradigm include assessment of laboratory-based, atypical eating behavior, variability of test meals (both composition and timing), inconsistent challenge loads (75), short time window between eating events (243), and fixed meal times instead of meal request. These studies also manipulate protein load relative to meal composition (e.g., percent of energy, percent by weight) and consumer characteristics (e.g., energy need, percent body weight) hampering isolation of protein effects.
Protein source and quality have also been explored as predictors of satiety, reportedly mediated by gut endocrine signals. Results from these studies are numerous and inconclusive (1, 48) Different protein types and combinations of proteins result in variable peptide release (68). Ghrelin secretion notably varies with protein source. When the amount of protein is controlled, dairy, plant, and animal protein lead to a reduction, reduction to a lesser extent, and increase in plasma ghrelin (16, 77), respectively, though appetite ratings remain similar. Effects of whey and casein are frequently contrasted (118, 202), because whey is digested and absorbed more rapidly than casein (34). Whether these digestive peptides directly stimulate appetitive peptide release or whether the digestion rate of dietary protein modulates release is uncertain. Liquid whey preload reportedly leads to significantly lower energy intake, increased fullness, decreased desire to eat, and elevated GIP, GLP-1, and CCK compared to an isocaloric casein preload (118). The increase in CCK with the whey preload has been attributed to the 64 AA peptide product of whey digestion, caseinomacropeptide (CMP) (197). Early research with rat models indicated CMP stimulates release of CCK (26); however, subsequent human studies found CMP alone or with an energy containing preload had no effect on subjective satiety, CCK release, or food intake (116). A review on the satiating effect of dairy proteins (22) concluded whey protein stimulates secretion of GLP-1 more than other proteins, but there is no clear evidence CCK, PYY, and ghrelin concentrations are affected by protein source.
Discovery of endogenous satiety peptides lead to experimentation with exogenous administration of synthetic forms of these peptides. In these experiments, administration of peptides reduced energy intake and/or increased feelings of satiety (18, 95, 227). However, a meta-analysis examining the difference between exogenous concentrations and endogenous production levels of PYY, GLP-1, and CCK found concentrations of exogenous PYY and GLP-1 that elicited a satiation response were significantly above physiologic concentrations, leaving open questions about their nutritional relevance in humans (175). Macronutrient composition was not evaluated for the above meta-analysis, limiting the ability to determine the effect of protein on satiety peptide levels. Whether gut peptide hormones are released primarily to signal satiety or to regulate digestive processes primarily with appetitive sensations only associated is receiving greater consideration (131). The effect of one macronutrient like protein and release of any one or combination of appetite peptides is unlikely to be the primary driver controlling energy intake.
Endocrine Responses to Fat
A link between lipid consumption, gut peptide secretion and satiety was reported as early as 1937, when an extract from intestinal secretions collected after an oral olive oil dose suppressed feeding in rabbits (172). Because later research documented that dietary lipids are potent stimuli for release of peptides such as CCK and PYY, some researchers hypothesized that lipids are the primary regulator of energy balance (20).
The endocrine mechanisms by which dietary lipids reportedly influence ingestive behavior and digestive processes are mediated by signals that begin in the oral cavity. In rodents, oral fat exposure reduces the variability of gastric emptying under conditions of different load rates (136) and augments pancreatic exocrine (124, 154) and endocrine (57) secretions. In humans, oral fat exposure elicits gastrin, gastric acid, and ghrelin secretion (119, 279, 281) and enhances gastric emptying (1119). Additionally, oral exposure stimulates release of gut peptides (e.g., CCK (278) and GLP-1 (281)) (49) (119). These products are also released by the detection of medium and long chain fatty acids in the small intestine in a dose dependent manner (165, 171, 86). In one study, an intestinal infusion of C12:0 but not C10:0 or control significantly reduced “desire to eat” and “hunger” ratings (though it increased nausea, typical of MCFA administration) (86). This corresponded to male participants consuming 2857 kJ less energy at a single buffet meal compared to control. Some studies show that LCFA are even more potent stimulators of gut peptide release as PYY secretion responds in proportion to increasing chain length of fatty acids and CCK concentrations increase more in the first 30 min after ingestion of LCFA compared to MCFA (85). Even so, there appears to be no differential effect of fatty acid chain length on hunger ratings or energy consumption in lean subjects (212).
While not a result of dietary fat intake, short-chain fatty acids (SCFA) are generated by the microbiota through fiber fermentation (257) and also stimulate secretion of gut peptides, such as PYY and GLP-1 (61). However, administration of acetate (C2:0) and propionate (C3:0) does not reduce energy intake (133, 223). Indeed, SCFA are a source of energy (8 kJ/g) and can increase one’s energy balance by an additional ≈150 kcal per day through energy-harvesting by the microbiota (135).
The effect of fatty acid saturation on secretion of gut peptides and appetitive sensations is also not established. Some work suggests consumption of meals high in SFA (C12:0+16:0+18:0) leads to lower hunger and greater fullness scores compared to loads containing either MUFA (C18:1) or PUFA (C:18:2+C18:3) (147), while other work indicates that there is no significant association between fatty acid saturation and either gut peptide secretion, appetitive sensations, or food intake (4, 242). Even when differences are noted, energy compensation in the period following test meal consumption offsets the reduction in energy intake, which indicates the effect of fatty acids and gut peptides on energy intake may only be acute (155).
Interpretation of the literature relating lipid intake to appetitive sensations and diet is difficult to assess due to differential inter-individual responsiveness. For example, sex and BMI may affect the gut peptide response to unsaturated lipids. While not well studied, almond oil containing mainly oleic acid (18:1) increases CCK concentrations and decreases hunger ratings in females but not males (44), and a duodenal infusion of a lipid composed primarily of long chain unsaturated fatty acids (intralipid) did not increase CCK and GLP-1 in males, nor did it result in any differences in energy consumption (39). An experiment with a similar experimental paradigm and a more aggressive dosing scheme observed increased gut peptide concentrations but still no difference in energy intake in treated males (211). Additionally, adiposity may be an important determinant of responsiveness as an intestinal infusion of oleic acid increases CCK concentrations to a greater degree in lean as compared to obese individuals (239), and ghrelin concentrations in obese males do not decline as much after a meal as in lean males following administration of long-chain fatty acids (231).
Taken together, the evidence suggests dietary lipids stimulate secretion of gut peptides reported to hold satiation/satiety effects, but they have limited impact on long-term energy intake. There are several reasons for this. First, they act primarily on meal size but generally not on eating frequency (63), Indeed, high-fat diets are often associated with positive energy balance and weight gain. This, has been attributed to tolerance or adaptation of the GI response with chronic consumption of a high fat diet (73). Second, there may be certain subgroups of responders (possibly related to sex or BMI) and gradations of responsiveness within categories without a dominant pattern (33, 43), Third, the range of lifestyles and conditions within a population may make certain gut peptides the primary determinant in particular situations but not others (282) and fourth, other physiological systems may “override” satiety signals from gut peptides (191). Thus, under customary dietary conditions, the consequence of intestinal lipid signaling and gut peptide secretion on appetite and energy intake is limited.
Neural Responses to Carbohydrate
The role of carbohydrates in energy balance has transitioned from being the macronutrient to be emphasized for weight management (99) to a more neutral role (127) to the present where they are often regarded as especially problematic (13, 168). This is particularly true for refined carbohydrates which some contend stimulate appetite and activate brain reward systems leading to addictive ingestive behaviors that promote positive energy balance. However, the veracity of this view is not established.
Reports on food addiction rarely specify the active component, but refer to high sugar, high fat, energy-dense, highly-processed foods. Given the lack of a widely agreed upon definition of “processed” and disagreement over how to calculate energy density (70, 272) this definition is left wanting. Interestingly, the degree of processing is often highlighted as it reportedly reflects the rate of nutrient absorption. However, it is unclear how sugar and fat are concurrently implicated as they have markedly different rates of gastric emptying and absorption into the circulation.
High palatability is another attribute linked to addiction with opposing claims that foods have been formulated to be “hyperpalatable” (11, 105). and that highly processed foods have lost the sensory appeal they held when “natural”. Neither position has scientific standing because liking and preference are learned attributes (188) as reflected by the many distinct global cuisines and evidence that familiarity is a strong predictor of acceptability (seen with sugar (163), fat (178), salt (25)).
Sweetness is another often cited dimension of addictive foods. Studies with mice indicate tasting sugar without digestion stimulates dopamine release (14). If sweetness is the effective signal, then addictions would be expected for LCS. In humans, dopamine release is greater for sucrose compared to LCS solutions (97, 234). However, some studies on diet soda consumers show similar activation of brain reward systems when consuming sucrose and saccharin sweetened beverages and a significantly greater dopinamergic response compared to non-diet soda drinkers (97, 113). One explanation holds that regular use of LCS decreases the response of reward centers to sucrose and thereby leads to increased ingestion (222, 246). Whether reduced responsivity (42) (if it occurs) leads to greater intake as a means to achieve a desired level of stimulation or lower intake due to lower reward value is an open question. The two largest meta-analyses of randomized controlled trials indicate LCS were associated with lower BMI (185, 218), and this has been supported by a more recent randomized control trial (209).
Another challenge to the view that sweetness is the driver of addictive feeding stems from work with trpm5−/− mice lacking the ability to taste sweetness. These mice significantly prefer a sucrose over a sucralose solution (62) and have significantly increased activation of the dopaminergic system, indicating that the post-ingestive energy contribution of sugar may be the primary driver of reward center activity (62).
Other work indicates LCS activate the opioid system responsible for liking, but that nutritive sweeteners activate dopamine pathways responsible for working to attain substances (92, 234). This finding raises questions about the hypothesis that sweetness as provided by LCS is addictive because the motivation to attain the substance, not just liking it, is a necessary component of addiction.
Measurement issues hamper study of sweetness and addiction. The Yale Food Addiction Scale, based on DSM-IV drug abuse diagnostic tools, is the most widely used criteria to classify individuals as food addicted (10). However, the reliability of the scale has not been established (96, 104). Further, much of the literature on this issue draws on measurements of dopamine. In rodents, some evidence indicates that the taste of sugar elicits the release of dopamine dose-dependently (12). However, dopamine may be released in the absence of a rewarding stimulus and may also remain unchanged in the presence of a rewarding stimulus (230). Additionally, the amount of dopamine released depends on the novelty of food and decreases after repeated exposure to the same food, in contrast to drugs abuse which elicit continued dopamine release (12). The absolute magnitude of reward system response to sugar is also markedly smaller compared to drugs of abuse (10, 12). Thus, the dopaminergic system is an imperfect index of reward system activation and addiction. In light of the limited support for addiction to any one food (120, 287), some have suggested framing the issue as “eating addiction” (120). This would be in line with recent recognition of other behavioral addictions such as computer gaming (217). However, the question remains as to whether “addiction” or some other descriptor such as disorder is more apt.
Finally, the putative implications of sweet food addiction are not clear. A primary basis for concern about sugar addiction is a posited link to increased energy intake and weight gain. Surveys reveal only about 5% of the population (3% of males and 6.7% of females) would be classified as food addicted (203). A meta-analysis determined the prevalence of food addiction as 11.1% of the normal weight population from six included studies compared with 24.9% of the overweight/obese population from fourteen included studies (214). Given the prevalence of overweight/obesity is approximately 68% of the US population (94), this trait could not be ascribed a major role in the etiology of this pervasive problem. Others have narrowed the argument to focus on individuals with binge eating disorder. Several rat models have implicated sugar addiction in the context of binge eating disorder (BED) (54). However, in humans, it is possible to have BED and not be food addicted according to current classification methods and those classified as addicted do not necessarily have BED (106). Thus, BED lacks sensitivity and specificity as a marker. Addictive behavior does not seem to fit the two popular models of weight gain in the population. A small systematic positive energy balance does not follow from the expected more gluttonous intake pattern by addicted individuals in an environment where energy is readily available. Alternatively, if weight gain stems from periodic (holiday) eating (229, 285) this suggests the addictive trait manifests only episodically rather than being a stable trait of an individual.
Taken together, the recent demonization of sugar as a driver of addictive eating behaviors that promote obesity requires resolution of many outstanding issues. Among these are what the offending agent actually is (e.g., sweetener, sweetness, palatability), how to reliably and accurately measure and quantify responses to a stimulus, intra- and inter-individual variability in susceptibility, and health implications.
Neural Responses to Protein
AA are precursors for transmitters in serotonergic, dopaminergic, and opioid brain reward pathways (210). The serotonergic system contributes to the hedonic impressions of food and satiation more than to hunger. The rate of serotonin synthesis is limited by tryptophan availability. Though proteins may be rich in tryptophan, its availability in the brain is determined by the plasma ratio of tryptophan to other large neutral AAs as all compete for uptake across the blood-brain barrier (88). Dietary carbohydrate augments tryptophan uptake in the brain by stimulating insulin secretion which enhances absorption of the AAs competing for access to the transporter into skeletal muscle (284). In addition, insulin promotes the uptake of free fatty acids into adipocytes, allowing unbound albumin to bind with tryptophan, hence preventing its uptake by peripheral tissues. Intake of a tryptophan-rich food reportedly reduces the preference for sweet foods in individuals with high anxiety (266). However other studies have not demonstrated such effects (27). Although diet can influence tryptophan availability, the importance of this pathway is uncertain since the brain maintains a pool of serotonin to cover needs during inter-meal intervals.
The dopaminergic pathway is involved in food-based reward as well and is most closely linked to hunger. The synthesis of dopamine is dependent on its AA precursors tyrosine and phenylalanine (89). Similar to tryptophan, the availability of tyrosine in the brain is modifiable by diet and depends on the plasma concentrations of its AA competitors. However, the influence of dietary protein on dopamine signaling is not as well understood. In a recent study involving overweight adolescent breakfast skippers, a high protein breakfast elicited greater and sustained plasma homovanillic acid concentrations, an index of central dopamine production, than a normal protein breakfast (125). While this may suggest that increased protein intake is rewarding, other work has not documented such effects (51).
The opioid pathway is associated with the motivational aspects of feeding (17). Opioid peptides can be formed from hydrolysis of dietary proteins in the GI tract (103). When absorbed, opioid peptides can traverse the blood-brain barrier where they produce opiate-like effects (210). However, the functionality of this pathway with respect to human feeding is, again, not established.
The early focus on dietary protein as a modulator of appetite by providing precursor AAs for neurotransmitter synthesis was later augmented by documentation that dietary protein elicits complex signals in the form of gut neuropeptides and hormones that converge on the brain via the vagus nerve and through the circulation (134). These signals integrate with input from peripheral sensory systems to influence the neural contribution to energy balance. Input from neural circuits responsible for motivation, cognition and hedonic impressions contribute further and guide food choice. The effects of dietary protein on food cravings are not well characterized. It is thought to reduce reward driven feeding behaviors despite being a precursor of the transmitters subserving reward pathways.
Centrally, the satiating effect of dietary protein is largely mediated by activation of neurons in the nucleus of the solitary tract (NTS) and arcuate nucleus (ARC) of the hypothalamus (134). Activation of noradrenergic and adrenergic neurons following high protein intake can enhance satiety and decrease energy intake by increasing sensitivity to gut hormones such as CCK (79, 134). In addition, high protein intake can decrease mRNA expression of the vagal orexin-1 receptor, hence reversing the inhibitory effects of orexin on CCK-mediated satiety (194). High protein intake also upregulates the catabolic POMC pathway and downregulates the anabolic NPY/agRP pathway in the ARC (143, 189, 221). Leucine may be the principal AA modulating the satiety effects of high protein diets. It is associated with activation of mammalian target of rapamycin (mTOR) and suppression of AMP-activated protein kinase (AMP-APK), intracellular pathways involved in satiety signaling in the ARC (221). In addition to a role of the ARC and NTS in feeding regulation, other neural circuits such as those mediating food-based reward are garnering significant attention.
The hedonic properties of foods that guide selection and consumption are determined by interconnections between several regions of the brain, primarily the orbitofrontal cortex, amygdala, insula, nucleus accumbens and dorsal striatum (141). Historically, sweet and fatty foods have been considered rewarding (87) with limited consideration of protein. This may stem from the neutral (D-Ala, -G~u, L-His, and D- and L-Arg, -As~, -Ile, -Lys, -Pro, -Ser, -Thr, -Val) or unpleasant (L-Leu, -Phe, -Trp, -Tyr, and D- and L-Cys, -Met) taste of most AAs and proteins. Some AAs (e.g., Gly, D-His, -Phe, -Trp, -Tyr, and L-Ala, -Leu) (237), peptides (aspartame) and proteins (monellin and thaumatin) (228) are sweet, but these are not strongly preferred. In addition, mice (91) and humans (204) avoid or negatively rate hydrolyzed casein in a dose-dependent manner. Even umami-tasting L-glutamate is not preferred over sucrose when in the form of monosodium glutamate (258). Further, though they contribute approximately the same energy as carbohydrate, proteins hold less appeal based on their metabolism. TRPM knockout mice that lack sweet taste transduction mechanisms prefer glucose over the sweet-tasting amino-acid L-serine (215).
Magnetic resonance imaging studies in animals and humans provide evidence of suppression of regions associated with food motivation and reward after protein intake. In rats, protein consumption is associated with a decrease in the blood oxygen level dependent (BOLD) signal in the amygdala (186). Moreover, in overweight female adolescent breakfast skippers, high protein breakfasts lead to greater reductions in 3 hour post-breakfast activation of insula and middle prefrontal cortex (158), and reductions in pre-dinner activation of hippocampal and parahippocampal regions (159) compared to a normal protein breakfast. In addition, the reductions in activation of the aforementioned brain regions coincide with increases in perceived afternoon fullness (159) and correlate with reductions in appetite (158). The protein status of an individual may influence brain reward responses to food cues as well. Healthy women with a low protein status had higher BOLD responses in the orbitofrontal cortex and striatum in response to food cues compared to those with a high protein status (114). In the same study, women in a low protein state had a higher ad libitum intake of protein following the intervention than those in a higher protein state. This indicates that protein status may have implications for modulating reward-driven feeding behaviors. Interpretation of this type of data requires caution as associations are not necessarily straight-forward. For example, reduced activation of the hippocampal regions after a high protein meal (159) may reflect changes in hippocampal-dependent learning and memory rather than decreased reward. The hippocampus is also involved in the cognitive regulation of feeding behavior such as remembering when one last ate, conditioned associations with food, remembering the interoceptive sensory cues and how to act on them etc. (23).
In summary, there is a proposed multifold role for protein in the neural regulation of appetite and feeding, but the importance of that role has yet to be delineated. Effects may be the manifestations of the orosensory properties of protein, protein-induced metabolic processes converging on neural circuits and central neural activity which ultimately regulate feeding. High protein foods have generally not been implicated in disordered eating.
Neural Responses to Fat
Work conducted fifty years ago demonstrated that rodents prefer diets containing lard or petrolatum over standard chow (3). Subsequent studies confirmed that both high energy yield and sensory qualities (such as taste, texture and smell) are characteristics that render fat highly palatable and contribute to the preference for and intake of high-fat foods (3). An important role for taste properties, in particular, has been documented in rodents by trials showing A) sham-fed rats prefer corn oil over mineral oil (187); B) rats prefer non-esterified fatty acids to triacylglycerol (138); and C) rats prefer triacylglycerol to triacylglycerol plus a lipase inhibitor (blocking hydrolysis of fatty acids, the presumptive sensory signal) (138). This hedonic taste response in rodents stands in sharp contrast to human responses which are typically aversive (256). However, as is the case with most bitter compounds, sampling non-esterified fatty acids in isolation and at high concentrations generally elicits an aversive response while, in low concentrations and in certain contexts, they may augment palatability in humans just as bitter notes contribute positively to the flavor profile of foods such as chocolate, coffee and wine. No evidence for flavor enhancement currently exists, whereas avoidance of foods with high concentrations of non-esterified fatty acids, such as rancid foods, is well recognized in humans.
Other work in rodents indicates that the energy yield from fat, independent of oral sensory stimulation, is sufficient to motivate intake (254). Indeed, intragastric self-administration of lipid emulsions mimics addictive-type ingestive responses. These include increased licking rates during extinction and progressive ratio schedules of reinforcement as well as attenuated striatal dopamine concentrations during extinction trials that increase again when fat exposure is returned to maintenance concentrations (90). Thus, sensory and metabolic cues enhance the appeal of high fat foods. Whether these properties also uniquely contribute to intake of fat and energy, in excess of need, is less clear. Common experience indicates fat is the least satiating of the three macronutrients (256). This raises questions about the homeostatic control of feeding generally, (162) or, if a system exists (283), when and how it has been disrupted. One view is that chronic consumption of high fat diets results in decreased responsiveness to satiety signals (33, 55). Recent work hypothesizes this adaptation to an obesogenic environment is a hippocampal-dependent phenomenon. A number of early animal studies reveal lesions of the hippocampus, an area within the mesolimbic pathway involved in learning and memory as well as the regulation of eating behaviors (267), result in dysregulation of food intake and obesity (5, 40, 122, 139). Another mechanism holds that dietary fat may modify food intake through activation of the mesolimbic dopamine reward pathway (195). Oleoylthanolamide (OEA), promotes satiety (100, 102, 199) and has been posited as a lipid messenger linking excess energy intake and dopamine deficiency. Consumption of a high fat diet leads to a significant reduction of OEA synthesis, resulting in dopamine dysregulation. This is supported by evidence that intraperitoneal administration of OEA is sufficient to restore dopamine release in high fat fed mice and the dopamine release is dependent on the PPARα-gastrointestinal-brain axis (254). Importantly, the fat specificity of these mechanisms is not established. The dopamine reward pathway is suppressed by excessive food intake, not simply foods high in fat.
In contrast to an adaptation or injury explanation for a neural basis for high fat feeding, it has also been posited that selected individuals may have heightened responsiveness to the palatability of high fat foods, promoting intake in excess of energy need (31). Indeed, addictive-type eating responses to high fat foods have been described, just as for high sugar foods. However, there is no consensus as to whether there is a sufficient evidence base for high fat food addiction (74, 169, 269). One of the defining symptoms in addiction is withdrawal from the rewarding substance. Symptoms of withdrawal are associated with increased stress that results in relapse - increased seeking behavior for the reward (233), and this has been reported in mice with fat withdrawal. Animals fed a high fat diet for 4 weeks exhibit a significant elevation in ΔFosB, and abrupt withdrawal from the high fat diet to a less palatable chow diet results in decreased concentrations of active cAMP response element binding protein (CREB). This result is associated with a significant increase in arousal and anxiety-like behaviors (169). Both CREB and ΔFosB alter neuronal responsiveness to dopamine signaling, leading to a reduction in the rewarding effects of a stimulus (169, 182).
Through a dietary reinstatement model, it was further observed that the mice experiencing withdrawal were willing to endure an aversive environment to gain access to the highly preferred high fat diet. The authors speculated that acute withdrawal from a highly preferred high fat diet may elevate the stress state and reduce reward, which contributes to the drive for dietary relapse (251). Despite the similarities between food and drugs of abuse on reward centers, one clear distinction is that the magnitude of responses to foods is a fraction of that observed to drugs of abuse (182).
One study demonstrated that individuals develop a preference for fat during infancy; and this preference is thought to predispose an individual to consume a predominately high fat diet in adulthood (252). Mice acutely exposed to a high fat diet during early life exhibit significant alterations in biomarkers of dopamine responses (252). Additionally, in a ten day macronutrient choice preference test, mature adult mice showed a significant preference for a high fat diet. But, despite the increased proportional intake of the high fat diet, there were no differences in total daily energy intake or weight gain (252). Interestingly, breastfed human infants are exposed to a high fat diet (~50% of energy from fat) of human breast milk or formula, yet this is associated with no correlation or a lower incidence of overweight and obesity (46). Thus, translation of the rodent literature to humans must be made cautiously.
Functional magnetic resonance neuroimaging (fMRI) studies yield mixed findings on responses to fat. Some work suggests there are individuals highly responsive to the reward value of foods linked to their metabolic status (74, 141), while other work reveals hypo-responsiveness to food reward. Obese individuals subjectively rated high-fat foods as being more pleasant than their lean counterparts, yet fMRI data suggest obese individuals show less activation in the dorsal striatum in response to consumption of palatable foods and reduced striatal D2 dopamine receptor density (240, 270). Consistent with both the hyper- and hypo-sensitivity theories, it has been hypothesized that individuals who are predisposed to obesity may experience increased hedonic drive during the onset of adiposity gain. The increased exposure and consumption of high fat foods down-regulates the dopamine reward pathway, leading to a reduction in the hedonic value of those foods (74). Among obese individuals, this reduction in reward value purportedly leads to the increased motivational drive to obtain and consume high fat foods (74). Support for this hypothesis is presently lacking in humans.
In summary, the rewarding properties of fats, both sensory and metabolic, have been associated with low satiety and weight gain. Proposed mechanisms implicate low and high responsiveness of individuals at both the neural and behavioral levels. None has yet to be substantiated.
Conclusion
Perspectives on the roles of the macronutrients in appetite and energy intake have changed over time. Their digestion products and/or circulating metabolites have been viewed as A) signals to initiate eating events, thus determining eating frequency; B) signals to terminate ingestive events thereby controlling portion size; and C) signals that activate brain reward systems that may dysregulate healthful eating. Drawing on these views, a wide variety of diets have been proposed accentuating or minimizing each macronutrient to achieve a desired effect on appetite and/or energy intake. Common experience over the past six decades reveals none has been widely successful. This is likely due to their failure to adequately address effects on eating frequency and portion size concurrently, as well as the fact that ingestive behavior is guided by many cognitive and environmental factors in addition to sensory appeal, appetite and metabolic, endocrine and neural signals stemming from macronutrient intake, digestion and metabolism. Furthermore, overweight and obesity result from many inputs in addition to energy intake, thus weakening the predictive power of macronutrient distribution on this outcome. There may be health reasons to emphasize one macronutrient over another in a diet, but from the perspective of energy balance, total energy intake, rather than its source (142, 156), is the critical factor to address.
Contributor Information
Alicia L Carreiro, Email: acarreir@purdue.edu.
Jaapna Dhillon, Email: jdhillon@purdue.edu.
Susannah Gordon, Email: susannahlgordon@gmail.com.
Ashley G Jacobs, Email: jacobs38@purdue.edu.
Kelly A Higgins, Email: higgin20@purdue.edu.
Breanna M McArthur, Email: bmcarth@purdue.edu.
Benjamin W Redan, Email: ben.redan@gmail.com.
Rebecca L Rivera, Email: rcusack@purdue.edu.
Leigh R Schmidt, Email: lcrschmidt@purdue.edu.
Richard D Mattes, Email: mattes@purdue.edu.
References
- 1.Abou-Samra R, Keersmaekers L, Brienza D, Mukherjee R, Mace K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr J. 2011;10(1):139. doi: 10.1186/1475-2891-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jéquier E. Glycogen storage capacity and de novo lipogenesis during masive carbohydrate overfeding in man. Am J Clin Nutr. 1988;48:240–47. doi: 10.1093/ajcn/48.2.240. [DOI] [PubMed] [Google Scholar]
- 3.Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: The response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15:171–88. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- 4.Alfenas RCG, Mattes RD. Effect of fat sources on satiety. Obes Res. 2003;11:183–87. doi: 10.1038/oby.2003.29. [DOI] [PubMed] [Google Scholar]
- 5.Anand BK, Brobeck JR. Hypothalamic Control of Food Intake in Rats and Cats. Yale J Biol Med. 1951;24:123–40. [PMC free article] [PubMed] [Google Scholar]
- 6.Anand BK, Dua S, Singh B. Electrical activity of the hypothalamic ‘feeding centres’ under the effect of changes in blood chemistry. Electroencephalogr & Clin Neurophysiol. 1961;13:54–59. doi: 10.1016/0013-4694(61)90074-8. [DOI] [PubMed] [Google Scholar]
- 7.Anliker J, Mayer JM. The Regulation of Food Intake: Some experiments relating behavioral, metabolic, and morphologic aspects. Am J Clin Nutr. 1957;5:148–53. doi: 10.1093/ajcn/5.2.148. [DOI] [PubMed] [Google Scholar]
- 8.Apolzan JW, Leidy HJ, Mattes RD, Campbell WW. Effects of food form on food intake and postprandial appetite sensations, glucose and endocrine responses, and energy expenditure in resistance trained v. sedentary older adults. Br J Nutr. 2011;106(7):1107–16. doi: 10.1017/S0007114511001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronoff SL, Berkowitz K, Shreiner B, Want L. Gluceose metabolism and regulation: beyone insulin and glucagon. Diab Spec. 2004;17(3):183. [Google Scholar]
- 10.Avena NM, Gold JA, Kroll C, Gold MS. Further developments in the neurobiology of food and addiction: Update on the state of the science. Nutrition. 2012;28(4):341–3. doi: 10.1016/j.nut.2011.11.002. doi: http://dx.doi.org/10.1016/j.nut.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avena NM, Gold MS. Variety and hyperpalatability: are they promoting addictive overeating? The Am J Clin Nutr. 2011;94(2):367–8. doi: 10.3945/ajcn.111.020164. [DOI] [PubMed] [Google Scholar]
- 12.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience & Biobehavioral Reviews. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. doi: http://dx.doi.org/10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139(3):623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139(3):813–20. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36(5):950–62. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- 16.Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey Protein but Not Soy Protein Supplementation Alters Body Weight and Composition in Free-Living Overweight and Obese Adults. J Nutr. 2011 doi: 10.3945/jn.111.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 18.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 19.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–53. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beglinger C. Effect of Cholecystokinin on Gastric Motility in Humansa. Ann N Y Acad Sci. 1994;713:219–25. doi: 10.1111/j.1749-6632.1994.tb44068.x. [DOI] [PubMed] [Google Scholar]
- 21.Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013;97(5):980–89. doi: 10.3945/ajcn.112.047563. [DOI] [PubMed] [Google Scholar]
- 22.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of Dairy Proteins on Appetite, Energy Expenditure, Body Weight, and Composition: a Review of the Evidence from Controlled Clinical Trials. Ad Nutr: Int Rev J. 2013;4(4):418–38. doi: 10.3945/an.113.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benoit SC, Davis JF, Davidson TL. Learned and cognitive controls of food intake. Brain Res. 2010;1350:71–76. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein LM, Grossman MI. An experimental test of the glucostatic theory of regulation of food intake. J Clin Invest. 1956;35:627–33. doi: 10.1172/JCI103318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertino M, Beauchamp GK, Engelman K. Increasing dietary salt alters salt taste preference. Physiol & Behav. 1986;38(2):203–13. doi: 10.1016/0031-9384(86)90155-1. doi: http://dx.doi.org/10.1016/0031-9384(86)90155-1. [DOI] [PubMed] [Google Scholar]
- 26.Beucher S, Levenez F, Yvon M, Corring T. Effects of gastric digestive products from casein on CCK release by intestinal cells in rat. J Nutr Biochem. 1994;5(12):578–84. doi: http://dx.doi.org/10.1016/0955-2863(94)90012-4. [Google Scholar]
- 27.Beulens JWJ, Bindels JG, de Graaf C, Alles MS, Wouters-Wesseling W. Alpha-lactalbumin combined with a regular diet increases plasma Trp-LNAA ratio. Physiol Behav. 2004;81:585–93. doi: 10.1016/j.physbeh.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Blom WA, Lluch A, Vinoy S, Stafleu A, van den Berg R, et al. Effects of gastric emptying on the postprandial gherlin response. Am J Physiol Endocrinol Metab. 2006;290(2):E389–95. doi: 10.1152/ajpendo.00238.2005. [DOI] [PubMed] [Google Scholar]
- 29.Blom WAM, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HFJ. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am Soc Clin Nutr. 2005;81:367–75. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 30.Blundell JE, Burley VJ, Cotton JR, Lawton CL. Dietary fat and the control of energy intake: evaluating the effects of fat on meal size and postmeal satiety. Am J Clin Nutr. 1993;57(5 Suppl):772S–77S. doi: 10.1093/ajcn/57.5.772S. discussion 777S-78S. [DOI] [PubMed] [Google Scholar]
- 31.Blundell JE, Finlayson G. Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiol Behav. 2004;82:21–5. doi: 10.1016/j.physbeh.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc. 1997;97(7 Suppl):S63–S69. doi: 10.1016/s0002-8223(97)00733-5. [DOI] [PubMed] [Google Scholar]
- 33.Blundell JE, Stubbs RJ, Golding C, Croden F, Alam R, et al. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol Behav. 2005;86:614–22. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 34.Boirie Y, Dangin M, Gachon P, Vasson M, Maubois J, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–35. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth DA, Chase A, Campbell AT. Relative effectiveness of protein in the late stages of appetite suppression in man. Physiol & Behav. 1970;5(11):1299–302. doi: 10.1016/0031-9384(70)90044-2. doi: http://dx.doi.org/10.1016/0031-9384(70)90044-2. [DOI] [PubMed] [Google Scholar]
- 36.Booth DA. Food intake compensation for increase or decrease in the protein content of the diet. Behav Bio. 1974;12(1):31–40. doi: 10.1016/s0091-6773(74)90996-1. [DOI] [PubMed] [Google Scholar]
- 37.Booth DA, Campbell AT, Chase A. Temporal bounds of post-ingestive glucose induced satiety in man. Nature. 1970;228:1104–5. doi: 10.1038/2281104a0. [DOI] [PubMed] [Google Scholar]
- 38.Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes. 2007;31(11):1696–703. doi: 10.1038/sj.ijo.0803665. [DOI] [PubMed] [Google Scholar]
- 39.Boyd KA, O’Donovan DG, Doran S, Wishart J, Chapman IM, et al. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am J Physiol Gastrointest Liver Physiol. 2003;284:G188–96. doi: 10.1152/ajpgi.00375.2002. [DOI] [PubMed] [Google Scholar]
- 40.Brooks CM, Lambert EF. A study of the effect of limitation of food intake and the method of feeding on the rate of weight gain during hupothalamic obesity in the albino rat. Am J Physiol – Leg Content. 1946;147:695–707. doi: 10.1152/ajplegacy.1946.147.4.695. [DOI] [PubMed] [Google Scholar]
- 41.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diab Care. 2009;32:2184–86. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. Curr Drug Abuse Rev. 2011;4:182–89. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burley VJ. Commentary on Cotton J R, Burley V J, Westrate J A, Blundell J E. 1994. Dietary fat and appetite: similarities and differences in the satiating effect of meals supplemented with either fat or carbohydrate. Journal of Human Nutrition and Dietetics; 7, 11–24. J Hum Nutr Diet Off J Br Diet Assoc. 2007;20:200–01. doi: 10.1111/j.1365-277X.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 44.Burton-Freeman B, Davis PA, Schneeman BO. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. Am J Clin Nutr. 2004;80:1207–14. doi: 10.1093/ajcn/80.5.1207. [DOI] [PubMed] [Google Scholar]
- 45.Campfield LA, Brandon P, Smith FJ. On-line continuous measurement of blood glucose and meal pattern in free-feeding rats: The role of glucose in meal initiation. Brain Res Bull. 1985;14:605–16. doi: 10.1016/0361-9230(85)90110-8. [DOI] [PubMed] [Google Scholar]
- 46.Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, et al. Myths, Presumptions, and Facts about Obesity. N Engl J Med. 2013;368:446–54. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman IM, Goble EA, Wittert GA, Morley JE, Horowitz M. Effect of intravenous glucose and euglycemic insulin infusions on short-term appetite and food intake. Am Physiol Soc. 1998;274:R596–603. doi: 10.1152/ajpregu.1998.274.3.R596. [DOI] [PubMed] [Google Scholar]
- 48.Charlton KE, Tapsell LC, Batterham MJ, Thorne R, O’Shea J, et al. Pork, beef and chicken have similar effects on acute satiety and hormonal markers of appetite. Appetite. 2011;56(1):1–8. doi: 10.1016/j.appet.2010.10.013. doi: http://dx.doi.org/10.1016/j.appet.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–48. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85(8):2685–91. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 51.Choi S, Disilvio B, Fernstrom MH, Fernstrom JD. Meal ingestion, AAs and brain neurotransmitters: effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol Behav. 2009;98:156–62. doi: 10.1016/j.physbeh.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Clark MJ, Slavin JL. The effect of fiber on satiety and food intake: a systematic review. J Am Coll Nutr. 2013;32:200–11. doi: 10.1080/07315724.2013.791194. [DOI] [PubMed] [Google Scholar]
- 53.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28(8):1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104(1):87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit redueced satiety in response to CCK and bombesin. Peptides. 1998;19(8):1407–15. doi: 10.1016/s0196-9781(98)00096-5. Sec. 10 (Neural Response to Fat)10. [DOI] [PubMed] [Google Scholar]
- 56.Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965;181:103–13. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crystal SR, Teff KL. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav. 2006;89:213–20. doi: 10.1016/j.physbeh.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Dailey MJ, Stingl KC, Moran TH. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology. 2012;153(1):132–42. doi: 10.1210/en.2011-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalen JE, Devries S. Diets to prevent coronary heart disease 1957–2013 what have we learned? Am J Med. 2014;127(5):364–9. doi: 10.1016/j.amjmed.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Daly K, Al-Rammahi M, Moran A, et al. Sensing of amino acids by the gut-expressed taste receptorT1R1-T1R3 stimulates CCK secretion. 2013. Am J Physiol Gastro Liver Physiol. 2013;304:G271–82. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darzi J, Frost GS, Robertson MD. Do SCFA have a role in appetite regulation? Proc Nutr Soc. 2011;70:119–28. doi: 10.1017/S0029665110004039. [DOI] [PubMed] [Google Scholar]
- 62.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930–41. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 63.de Graaf C, Blom WAM, Smeets PAM, Stafleu A, Hendriks HFJ. Biomarkers of satiation and satiety. Am J Clin Nutr. 2004;79:946–61. doi: 10.1093/ajcn/79.6.946. [DOI] [PubMed] [Google Scholar]
- 64.de Graaf C, Hulshof T, Weststrate JA, Jas P. Short-term effects of different amounts of protein, fats, and carbohydrates on satiety. Am J Clin Nutr. 1992;55(1):33–38. doi: 10.1093/ajcn/55.1.33. [DOI] [PubMed] [Google Scholar]
- 65.de Ruyter JC, Katan MB, Kuijper LD, Liem DG, Olthof MR. The effect of sugar-free versus sugar-sweetened beverages on satiety, liking and wanting: an 18 month randomized double-blind trial in children. PLoS One. 2013;8(10):e78039. doi: 10.1371/journal.pone.0078039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deutsch JA, Moore BO, Heinrichs SC. Unlearned specific appetite for protein. Physiol Behav. 1989;46(4):619–24. doi: 10.1016/0031-9384(89)90341-7. [DOI] [PubMed] [Google Scholar]
- 67.DiBattista D. Effects of time-restricted access to protein and to carbohydrate in adult mice and rats. Physiol Behav. 1991;49(2):263–69. doi: 10.1016/0031-9384(91)90042-m. [DOI] [PubMed] [Google Scholar]
- 68.Diepvens K, Haberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond) 2008;32:510–18. doi: 10.1038/sj.ijo.0803758. [DOI] [PubMed] [Google Scholar]
- 69.Dotson CD, Geraedts MCP, Munger SD. Peptide regulators of peripheral taste function. Semin Cell Dev Biol. 2013;24(3):323–39. doi: 10.1016/j.semcdb.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drewnowski A, Almiron-Roig E, Marmonier C, Lluch A. Dietary energy density and body weight: is there a relationship? Nutr Rev. 2004;62(11):403–13. doi: 10.1111/j.1753-4887.2004.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 71.Dubuc GR, Phinney SD, Stern JS, Havel PJ. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism. 1998;47(4):429–34. doi: 10.1016/s0026-0495(98)90055-5. [DOI] [PubMed] [Google Scholar]
- 72.Duca FA, Lam TKT. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab. 2014;16(Suppl 1):68–76. doi: 10.1111/dom.12340. [DOI] [PubMed] [Google Scholar]
- 73.Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem. 2013;24:1663–77. doi: 10.1016/j.jnutbio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, et al. Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord. 2011;12:141–51. doi: 10.1007/s11154-011-9166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisenstein J, Roberts S, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002;60:189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- 76.Epstein AN, Teitelbaum P. Specific loss of the hypoglycemic control of feeding in recovered lateral rats. Am J Phys. 1967;213:1159–67. doi: 10.1152/ajplegacy.1967.213.5.1159. [DOI] [PubMed] [Google Scholar]
- 77.Erdmann J, Leibl M, Wagenpfeil S, Lippl F, Schusdziarra V. Ghrelin response to protein and carbohydrate meals in relation to food intake and glycerol levels in obese subjects. Reg Pep. 2006;135:1–2. 23–9. doi: 10.1016/j.regpep.2006.03.003. doi: http://dx.doi.org/10.1016/j.regpep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15(3):189–96. doi: 10.1007/s11154-014-9288-6. [DOI] [PubMed] [Google Scholar]
- 79.Faipoux R, Tomé D, Gougis S, Darcel N, Fromentin G. Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats. J Nutr. 2008;138:1172–8. doi: 10.1093/jn/138.6.1172. [DOI] [PubMed] [Google Scholar]
- 80.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 81.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feinle C, et al. Effects of duodenal fat, protein or mixed-nutrient infusions on epigastric sensations during sustained gastric distension in healthy humans. Neurogastro Motil. 2002;14(2):205–13. doi: 10.1046/j.1365-2982.2002.00318.x. [DOI] [PubMed] [Google Scholar]
- 83.Feinle C, et al. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastro. 2001;120(5):1100–07. doi: 10.1053/gast.2001.23232. [DOI] [PubMed] [Google Scholar]
- 84.Felton AM, Felton A, Lindenmayer DB, Foley WJ. Nutritional goals of wild primates. Functional Ecology. 2009;23(1):70–8. doi: 10.1111/j.1365-2435.2008.01526.x. [DOI] [Google Scholar]
- 85.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Rades T, et al. Comparative effects of intraduodenal infusions of lauric and oleic acids on antropyloroduodenal motility, plasma cholecystokinin and peptide YY, appetite, and energy intake in healthy men. Am J Clin Nutr. 2008;87:1181–87. doi: 10.1093/ajcn/87.5.1181. [DOI] [PubMed] [Google Scholar]
- 86.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, et al. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287:R524–33. doi: 10.1152/ajpregu.00039.2004. [DOI] [PubMed] [Google Scholar]
- 87.Fernandes MF, Sharma S, Hryhorczuk C, Auguste S, Fulton S. Nutritional Controls of Food Reward. Can J Diab. 2013;37:260–68. doi: 10.1016/j.jcjd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Fernstrom JD. Effects on the diet on brain neurotransmitters. Metabolism. 1977;26:207–23. doi: 10.1016/0026-0495(77)90057-9. [DOI] [PubMed] [Google Scholar]
- 89.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–47S. doi: 10.1093/jn/137.6.1539S. discussion 1548S. [DOI] [PubMed] [Google Scholar]
- 90.Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J Physiol. 2012;590:953–72. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Field KL, Kimball BA, Mennella JA, Beauchamp GK, Bachmanov AA. Avoidance of hydrolyzed casein by mice. Physiol Behav. 2008;93:189–99. doi: 10.1016/j.physbeh.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Finlayson G, Dalton M. Current progress in the assessment of ‘liking’ vs. ‘wanting’ food in human appetite. Comment on ‘“You say it’s liking, i say it’s wanting…”. On the difficulty of disentangling food reward in man’. Appetite. 2012;58(1):373–8. doi: 10.1016/j.appet.2011.10.011. discussion 252. [DOI] [PubMed] [Google Scholar]
- 93.Flatt JP. Carbohydrate balance and body-weight regulation. P Nutr Soc. 1996;55:449–65. doi: 10.1079/pns19960041. [DOI] [PubMed] [Google Scholar]
- 94.Flegal K, Carroll MD, Ogden C, Curtin L. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA-J Am Med Assoc. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 95.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. Journal of Clinical Investigation. 1998;101(3):515. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Food Forum Food and Nutrition Board Institute of Medicine. Relationships Among the Brain, the Digestive System, and Eating Behavior: Workshop Summary. Washington (DC): National Academies Press (US); 2015. Edtion ed. Copyright 2015 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- 97.Frank GKW, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, et al. Sucrose activates human taste pathways differently from artificial sweetener. NeuroImage. 2008;39(4):1559–69. doi: 10.1016/j.neuroimage.2007.10.061. doi: http://dx.doi.org/10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 98.Frecka JM, Mattes RD. Possible entrainment of ghrelin to habitual meal patterns in humans. Am Physiol Soc. 2008;294(3):G699–707. doi: 10.1152/ajpgi.00448.2007. [DOI] [PubMed] [Google Scholar]
- 99.Friedman MI, Stricker EM. The physiological psychology of hunger: A physiological perspective. Psych Rev. 1976;83:409–31. [PubMed] [Google Scholar]
- 100.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 101.Fujita Y, Wideman RD, Speck M, Asadi A, King DS, et al. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am Physiol Soc. 2009;94(3):717–25. doi: 10.1152/ajpendo.90636.2008. [DOI] [PubMed] [Google Scholar]
- 102.Gaetani S, Oveisi F, Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2003;28:1311–16. doi: 10.1038/sj.npp.1300166. [DOI] [PubMed] [Google Scholar]
- 103.Ganapathy V, Miyauchi S. Transport systems for opioid peptides in mammalian tissues. AAPS J. 2005;7:E852–56. doi: 10.1208/aapsj070482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food. Addiction Scale. 2009;52(2):430–6. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 105.Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106(7):1208–12. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gearhardt AN, White MA, Masheb RM, Grilo CM. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Comprehensive Psychiatry. 2013;54(5):500–5. doi: 10.1016/j.comppsych.2012.12.009. doi: http://dx.doi.org/10.1016/j.comppsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geiselman PJ, Novin D. Role of carbohydrate in appetite, hunger and obesity. Appetite. 1982;3:203–23. doi: 10.1016/s0195-6663(82)80017-2. [DOI] [PubMed] [Google Scholar]
- 108.Gerspach AC, Steinert RE, Schonenberger L, et al. The role of the gut sweet taste receptor in regulating GLP-1, PPY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301:E17–25. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- 109.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488–95. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 110.Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, et al. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am J Physiol Regul Integr Comp Physiol. 2015;309:R552–60. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goodlad RA, Lenton W, Ghatei MA, Adrian TE, Bloom SR, Wright NA. Effects of an elemental diet, inert bulk and different types of dietary fibre on the response of the intestinal epithelium to refeeding in the rat and relationship to plasma gastrin, enteroglucagon, and PYY concentrations. Gut. 1987;28(2):171–80. doi: 10.1136/gut.28.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, et al. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS One. 2011;6(10):e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Green E, Murphy C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol Behav. 2012;107(4):560–67. doi: 10.1016/j.physbeh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Griffioen-Roose S, Smeets PA, van den Heuvel E, Boesveldt S, Finlayson G, de Graaf C. Human protein status modulates brain reward responses to food cues. Am J Clin Nutr. 2014;100:113–22. doi: 10.3945/ajcn.113.079392. [DOI] [PubMed] [Google Scholar]
- 115.Griffioen-Roose S, Mars M, Siebelink E, Finlayson G, Tomé D, de Graaf C. Protein status elicits compensatory changes in food intake and food preferences. Am J Clin Nutr. 2012;95(1):32–8. doi: 10.3945/ajcn.111.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gustafson DR, McMahon DJ, Morrey J, Nan R. Appetite is not influenced by a unique milk peptide: caseinomacropeptide (CMP) Appetite. 2001;36(2):157–63. doi: 10.1006/appe.2000.0392. doi: http://dx.doi.org/10.1006/appe.2000.0392. [DOI] [PubMed] [Google Scholar]
- 117.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94(16):8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hall W, Millward D, Long S, Morgan L. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 119.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol. 2004;180(2):273–81. doi: 10.1677/joe.0.1800273. [DOI] [PubMed] [Google Scholar]
- 120.Hebebrand J, Albayrak Ö, Adan R, Antel J, Diequez C, et al. Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci & Biobehav Rev. 2014;47(0):295–306. doi: 10.1016/j.neubiorev.2014.08.016. doi: http://dx.doi.org/10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 121.Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959;145(2):336–52. doi: 10.1113/jphysiol.1959.sp006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec. 1940;78:149–72. [Google Scholar]
- 123.Hill AJ, Blundell JE. Macronutrients and satiety: the effects of a high-protein or high-carbohydrate meal on subjective motivation to eat and food preferences. Nutr and behav (USA) 1986 [Google Scholar]
- 124.Hiraoka T, Fukuwatari T, Imaizumi M, Fushiki T. Effects of oral stimulation with fats on the cephalic phase of pancreatic enzyme secretion in esophagostomized rats. Physiol Behav. 2003;79:4–5. 713–17. doi: 10.1016/s0031-9384(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 125.Hoertel HA, Will MJ, Leidy HJ. A randomized crossover, pilot study examining the effects of a normal protein vs. high protein breakfast on food cravings and reward signals in overweight/obese “breakfast skipping”, late-adolescent girls. Nutr J. 2014;13:80. doi: 10.1186/1475-2891-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holt S, Brand J, Soveny C, Hansky J. Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin responses. Appetite. 1992;18:129–141. doi: 10.1016/0195-6663(92)90190-h. [DOI] [PubMed] [Google Scholar]
- 127.Hu T, Bazzano LA. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. Nutr Metab Cardiovasc Dis. 2014;24(4):337–43. doi: 10.1016/j.numecd.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunt J. The site of receptors slowing gastric emptying in response to starch in test meals. The Journal of physiology. 1960;154(2):270–6. doi: 10.1113/jphysiol.1960.sp006578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ivy AC, Oldberg E. A Hormone Mechanism for Gallbladder Contraction and Evacuation. Amer J Physiol. 1928;86:599. [Google Scholar]
- 130.Jang H-J, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim B-J, et al. Gut-expressed gustducin and taste receptros regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104(38):15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends in Endocrin & Meta. 2013;24(2):92–100. doi: 10.1016/j.tem.2012.11.006. doi: http://dx.doi.org/10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 132.Jenkins DJA, Jenkins AL, Wolever TMS, Vuksan V, Rao AV, et al. Low glycemic index: Lente carbohydrates and physiological effects of altered food frequency. Am J Clin Nutr. 1994;59(suppl):706S–709S. doi: 10.1093/ajcn/59.3.706S. [DOI] [PubMed] [Google Scholar]
- 133.Johnston CS, Kim CM, Buller AJ. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care. 2004;27:281–82. doi: 10.2337/diacare.27.1.281. [DOI] [PubMed] [Google Scholar]
- 134.Journel M, Chaumontet C, Darcel N, Fromentin G, Tomé D. Brain Responses to High-Protein Diets. Adv Nutr Int Rev J. 2012;3:322–29. doi: 10.3945/an.112.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaplan JM, Siemers W, Grill HJ. Effect of oral versus gastric delivery on gastric emptying of corn oil emulsions. Am J Physiol. 1997;273:R1263–70. doi: 10.1152/ajpregu.1997.273.4.R1263. [DOI] [PubMed] [Google Scholar]
- 137.Karalus M, Clark M, Greaves KA, Thomas W, Vickers Z, et al. Fermentable fibers do not affect satiety or food intake by women who do not practice restrained eating. J Acad Nutr Diet. 2012;112:1356–62. doi: 10.1016/j.jand.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 138.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R447–54. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 139.Kennedy GC. The Hypothalamic Control of Food Intake in Rats. Proc R Soc Lond B Biol Sci. 1950;137:535–49. doi: 10.1098/rspb.1950.0065. [DOI] [PubMed] [Google Scholar]
- 140.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140(901):578–96. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 141.Kenny PJ. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron. 2011;69:664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kinsell LW, Gunning B, Michaels GD, Richardson J, Cox SE, Lemon C. Calories do count. Metabolism. 1964;13:195–204. doi: 10.1016/0026-0495(64)90098-8. [DOI] [PubMed] [Google Scholar]
- 143.Kinzig KP, Hargrave SL, Hyun J, Moran TH. Energy balance and hypothalamic effects of a high-protein/low-carbohydrate diet. Physiol Behav. 2007;92:454–60. doi: 10.1016/j.physbeh.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kissileff HR, Thornton JC, Torres MI, Pavlovich K, Mayer LS, et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012;95(2):309–17. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kokrashvili Z, Yee KK, Ilegems E, Iwatsuki K, Li Y, et al. Endocrine Taste Cells Br J Nutr. 2014;111:S23–29. doi: 10.1017/S0007114513002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kovacs EMR, Westerterp-Plantenga MS, Saris WHM, Melanson KJ, Goossens I, Brouns F. Associations between spontaneous meal initiations and blood glucose dynamics in overweight men in negative energy balance. Brit J Nutr. 2002;87:39–45. doi: 10.1079/BJN2001473. [DOI] [PubMed] [Google Scholar]
- 147.Kozimor A, Chang H, Cooper JA. Effects of dietary fatty acid composition from a high fat meal on satiety. Appetite. 2013;69:39–45. doi: 10.1016/j.appet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 148.Krotkiewski M. Value of VLCD supplementation with medium chain triglycerides. Int J Obes Relat Metab Disord. 2001;25(9):1393–400. doi: 10.1038/sj.ijo.0801682. [DOI] [PubMed] [Google Scholar]
- 149.Kuwahara A. Contributions of colonic short-chain fatty acid receptors in energy homeostasis. Front Endocrinol. 2014;5:144. doi: 10.3389/fendo.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lambert TC, Hill AJ, Blundell JE. Investigating the satiating effect of protein with disguised liquid preloads. Appetite. 1989;12:220. [Google Scholar]
- 151.Lang V, Bellisle F, Oppert J, Craplet C, Bornet F, Slama G. Satiating effect of proteins in healthy subjects: a comparison of egg albumin, casein, gelatin, soy protein, pea protein, and wheat gluten. Am J Clin Nutr. 1998;67:1197–204. doi: 10.1093/ajcn/67.6.1197. [DOI] [PubMed] [Google Scholar]
- 152.Lang V, Bellisle F, Alamowitch C, Craplet C, Bornet FR, et al. Varying the protein source in mixed meal modifies glucose, insulin and glucagon kinetics in healthy men, has weak effects on subjective satiety and fails to affect food intake. Eur J Clin Nutr. 1999;53(12):959–65. doi: 10.1038/sj.ejcn.1600881. [DOI] [PubMed] [Google Scholar]
- 153.Latner JD, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite. 1999;33(1):119–28. doi: 10.1006/appe.1999.0237. [DOI] [PubMed] [Google Scholar]
- 154.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115(11):3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lawton CL, Delargy HJ, Brockman J, Smith FC, Blundell JE. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr. 2000;83:473–82. [PubMed] [Google Scholar]
- 156.Leibel RL, Hirsch J, Appel BE, Checani GC. Energy intake required to maintain body weight is not affected by wide variation in diet composition. Am J Clin Nutr. 1992;55:350–55. doi: 10.1093/ajcn/55.2.350. [DOI] [PubMed] [Google Scholar]
- 157.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 158.Leidy HJ, Lepping RJ, Savage CR, Harris CT. Neural Responses to Visual Food Stimuli After a Normal vs. Higher Protein Breakfast in Breakfast-Skipping Teens: A Pilot fMRI Study. Obesity. 2011;19:2019–25. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leidy HJ, Ortinau LC, Douglas SM, Hoertel HA. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677–88. doi: 10.3945/ajcn.112.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lemmens SG, Martens EA, Kester AD, Westerterp-Plantenga MS. Changes in gut hormone and glucose concentrations in relation to hunger and fullness. Am J Clin Nutr. 2011;94:717–25. doi: 10.3945/ajcn.110.008631. [DOI] [PubMed] [Google Scholar]
- 161.Leung PMB, Rogers QR. Effect of amino acid imbalance and deficiency on dietary choice patterns of rats. Physiol & Behav. 1986;37(5):747–58. doi: 10.1016/0031-9384(86)90180-0. doi: http://dx.doi.org/10.1016/0031-9384(86)90180-0. [DOI] [PubMed] [Google Scholar]
- 162.Levitsky DA. The non-regulation of food intake in humans: Hope for reversing the epidemic of obesity. Physiol Behav. 2005;86:623–32. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 163.Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol & Behav. 2004;83(3):421–9. doi: 10.1016/j.physbeh.2004.08.028. doi: http://dx.doi.org/10.1016/j.physbeh.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 164.Little TJ, Gupta N, Maynard Case R, Thompson DG, McLaughlin JT. Sweetness and bitterness taste of meals per se does not mediate gastric emptying in humans. Am J Physiol Regul Integr Comp Physiol. 2009;297:R632–39. doi: 10.1152/ajpregu.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Little TJ, Russo A, Meyer JH, Horowitz M, Smyth DR, et al. Free fatty acids have more potent effects on gastric emptying, gut hormones, and appetite than triacylglycerides. Gastroenterology. 2007;133:1124–31. doi: 10.1053/j.gastro.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 166.Liu AG, Most MM, Brashear MM, Johnson WD, Cefalu WT, Greenway FL. Reducing the glycemic index or carbohydrate content of mixed meals reduces postprandial glycemia and insulinemia over the entire day but does not affect satiety. Diab Care. 2012;35:1633–1637. doi: 10.2337/dc12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Longo WE, Ballantyne GH, Savoca PE, Adrian TE, Bilchik AJ, Modlin IM. Short-chain fatty acid release of peptide YY in the isolated rabbit distal colon. Scan J Gastro. 1991;26(4):442–48. doi: 10.3109/00365529108996507. [DOI] [PubMed] [Google Scholar]
- 168.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110(9):1307–21. doi: 10.1016/j.jada.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 169.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–32. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma J, Chang J, Checklin HL, Young RL, Jones KL, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr. 2010;104:803–06. doi: 10.1017/S0007114510001327. [DOI] [PubMed] [Google Scholar]
- 171.Ma J, Checklin HL, Wishart JM, Stevens JE, Jones KL, et al. A randomised trial of enteric-coated nutrient pellets to stimulate gastrointestinal peptide release and lower glycaemia in type 2 diabetes. Diabetologia. 2013;56:1236–42. doi: 10.1007/s00125-013-2876-2. [DOI] [PubMed] [Google Scholar]
- 172.Maclagan NF. The role of appetite in the control of body weight. J Physiol. 1937;90:385–94. doi: 10.1113/jphysiol.1937.sp003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 174.Marciani L, et al. Additive effects of gastric volumes and macronutrient composition on the sensation of postprandial fullness in humans. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mars M, Stafleu A, de Graaf C. Use of satiety peptides in assessing the satiating capacity of foods. Physiology & Behavior. 2012;105(2):483–88. doi: 10.1016/j.physbeh.2011.08.033. doi: http://dx.doi.org/10.1016/j.physbeh.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 176.Martens EA, Lemmens SG, Westerterp-Plantenga MS. Protein leverage affects energy intake of high-protein diets in humans. Am J Clin Nutr. 2013;97(1):86–93. doi: 10.3945/ajcn.112.046540. [DOI] [PubMed] [Google Scholar]
- 177.Martens EA, Tan SY, Dunlop MV, Mattes RD, Westerterp-Plantenga MS. Protein leverage effects of beef protein on energy intake in humans. Am J Clin Nutr. 2014;99(6):1397–406. doi: 10.3945/ajcn.113.078774. [DOI] [PubMed] [Google Scholar]
- 178.Mattes RD. Fat preference and adherence to a reduced-fat diet. The American Journal of Clinical Nutrition. 1993;57(3):373–81. doi: 10.1093/ajcn/57.3.373. [DOI] [PubMed] [Google Scholar]
- 179.Mayer J, Bates WM. Blood glucose and food intake in normal and hypo-physectomized alloxan-treated rats. Am J Physiol. 1952;168:812–19. doi: 10.1152/ajplegacy.1952.168.3.812. [DOI] [PubMed] [Google Scholar]
- 180.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 181.Mayer J. Regulation of energy intake and the body weight. Annals of the New York Academy of Sciences. 1955;63:15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- 182.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat Neurosci. 2003;6:1208–15. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 183.Mela DJ, Sacchetti DA. Sensory preferences for fats: relationships with diet and body composition. Am J Clin Nutr. 1991;53(4):908–15. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 184.Mellinkoff Sm, Frankland M, Boyle D, Greipel M. Relationship between serum amino acid concentration and fluctuations in appetite. J Appl Physiol. 1956;8(5):535–38. doi: 10.1152/jappl.1956.8.5.535. [DOI] [PubMed] [Google Scholar]
- 185.Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100(3):765–77. doi: 10.3945/ajcn.113.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Min DK, Tuor UI, Koopmans HS, Chelikani PK. Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology. 2011;141:1832–41. doi: 10.1053/j.gastro.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 187.Mindell S, Smith GP, Greenberg D. Corn oil and mineral oil stimulate sham feeding in rats. Physiol Behav. 1990;48:283–87. doi: 10.1016/0031-9384(90)90314-t. [DOI] [PubMed] [Google Scholar]
- 188.Morris MJ, Beilharz J, Maniam J, Reichelt A, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 189.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–71. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Murphy C, Withee J. Age and biochemical status predict preference for casein hydrolysate. J Gerontol. 1987;42:73–77. doi: 10.1093/geronj/42.1.73. [DOI] [PubMed] [Google Scholar]
- 191.Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1:14. doi: 10.3978/j.issn.2305-5839.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nakamura E, Hasumura M, Uneyama H, Torii K. Luminal Amino Acid-Sensing Cells in Gastric Mucosa. Digestion. 2011;83(suppl 1):13–8. doi: 10.1159/000323399. [DOI] [PubMed] [Google Scholar]
- 193.Nations FaAootU. FAOSTAT database. 2002 [WWW document]. URL http://faostat.fao.org (accessed December 2014)
- 194.Nefti W. Les modifications de la sensibilité du nerf vague aux neuropeptides gastro-intestinaux induites par des situations nutritionnelles chez la souris: bases cellulaires et conséquences sur le comportement alimentaire. Thesis Paris: Agroparistech. 2009:138. [Google Scholar]
- 195.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005:1445–49. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 196.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States. JAMA. 2011–2012;311(8):806–14. doi: 10.1001/jama.2014.732. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Onwulata C, Huth P. Whey processing, functionality and health benefits. 1st. Ames, Iowa: Wiley-Blackwell; 2008. p. xiv, 400. [Google Scholar]
- 198.Ooyama K, Kojima K, Aoyama T, Takeuchi H. Decrease of food intake in rats after ingestion of medium-chain triacylglycerol. J Nutr Sci Vitaminol(Tokyo) 2009;55(5):423–27. doi: 10.3177/jnsv.55.423. [DOI] [PubMed] [Google Scholar]
- 199.Oveisi F, Gaetani S, Eng KT-P, Piomelli D. Oleoylethanolamide inhibits food intake in free-feeding rats after oral administration. Pharmacol Res Off J Ital Pharmacol Soc. 2004;49:461–66. doi: 10.1016/j.phrs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 200.Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care. 2014;17(1):5–11. doi: 10.1097/MCO.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Page L, Phipard EF. Essentials of an Adequate Diet:Facts for Nutrition Programs. US Dept Ag ARS-62-4 1956 [Google Scholar]
- 202.Pal S, Radavelli-Bagatini S, Hagger M, Ellis V. Comparative effects of whey and casein proteins on satiety in overweight and obese individuals: a randomized controlled trial. Eur J Clin Nutr. 2014;68(9):980–6. doi: 10.1038/ejcn.2014.84. [DOI] [PubMed] [Google Scholar]
- 203.Pedram P, Wadden D, Amini P, et al. Food Addiction: Its Prevalence and Significant Association with Obesity in the General Population. PloS one. 2013;8(9):e74832. doi: 10.1371/journal.pone.0074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pedrosa M, Pascual CY, Larco JI, Esteban MM. Palatability of hydrolysates and Other substitution formulas for cow’s milk-allergic children: a comparative study of taste, smell, and texture evaluated by healthy volunteers. J Investig Allergol Clin Immunol. 2006;16:351–56. [PubMed] [Google Scholar]
- 205.Penagini R, Spiller RC, Misiewicz JJ, Frost PG. Effect of ileal infusion of glycochenodeoxycholic acid on segmental transit, motility, and flow in the human jejunum and ileum. Gut. 1989;30(5):609–17. doi: 10.1136/gut.30.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pénicaud L, Leloup C, Lorsignol A, Alquier T, Guillod E. Brain glucose sensing mechanism and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2002;5:539–543. doi: 10.1097/00075197-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 207.Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36:2530–35. doi: 10.2337/dc12-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. Journal of Clinical Investigation. 1967;46(12):1954. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Peters JC, Beck J, Cardel M, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: A randomized clinical trial. Obesity. 2015;24:297–304. doi: 10.1002/oby.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peuhkuri K, Sihvola N, Korpela R. Dietary proteins and food-related reward signals. Food Nutr Res. 2011;55 doi: 10.3402/fnr.v55i0.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, et al. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R668–77. doi: 10.1152/ajpregu.00606.2005. [DOI] [PubMed] [Google Scholar]
- 212.Poppitt SD, Strik CM, MacGibbon AK, McArdle BH, Budgett SC, McGill AT. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol Behav. 2010;101(1):161–67. doi: 10.1016/j.physbeh.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 213.Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 2008;50:194–206. doi: 10.1016/j.appet.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pursey K, Stanwell P, Gearhardt A, Collins CE, Burrows T. The Prevalence of Food Addiction as Assessed by the Yale Food Addiction Scale: A Systematic Review. Nutrients. 2014:4552–90. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient Selection in the Absence of Taste Receptor Signaling. J Neurosci. 2010;30:8012–23. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ritter RC, Brenner L, Yox DP. Neuroanatomy & Physiol Of Abdominal Vagal Afferents. Ann Arbor: CRC Press; 1992. Participation of vagal sensory neurons in putative satiety signals from the upper gastrointestinal tract; pp. 221–48. [Google Scholar]
- 217.Robbins TW, Clark L. Behavioral addictions. Current Opinion in Neurobiology. 2015;30:66–72. doi: 10.1016/j.conb.2014.09.005. doi: http://dx.doi.org/10.1016/j.conb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 218.Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Intl J Obes. 2015:1–14. doi: 10.1038/ijo.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rolls BJ, Gnizak N, Summerfelt A, Laster LJ. Food intake in dieters and nondieters after a liquid meal containing medium-chain triglycerides. Am J Clin Nutr. 1988;48(1):66–71. doi: 10.1093/ajcn/48.1.66. [DOI] [PubMed] [Google Scholar]
- 220.Rolls BJ, Hetherington M, Burley VJ. The specificity of satiety: The influence of foods of different macronutrient content on the development of satiety. Physiology and Behavior. 1988;43(2)(88):145–53. 90230–2. doi: 10.1016/0031-9384. [DOI] [PubMed] [Google Scholar]
- 221.Ropelle ER, Pauli JR, Fernandes MFA, Rocco SA, Marin RM, et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes. 2008;57:594–605. doi: 10.2337/db07-0573. [DOI] [PubMed] [Google Scholar]
- 222.Rudenga KJ, Small DM. Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite. 2012;58(2):504–07. doi: 10.1016/j.appet.2011.12.001. doi: http://dx.doi.org/10.1016/j.appet.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ruijschop RMAJ, Boelrijk AEM, te Giffel MC. Satiety effects of a dairy beverage fermented with propionic acid bacteria. Int Dairy J. 2008;18:945–50. [Google Scholar]
- 224.Sakar Y, Duca FA, Langelier B, Devime F, Blottiere H, et al. Impact of high-fat feeding on basic helix-loop-helix transcription factors controlling enteroendocrine cell differentiation. Int J Obes. 2014;38:1440–48. doi: 10.1038/ijo.2014.20. [DOI] [PubMed] [Google Scholar]
- 225.Santesso N, Akl EA, Bianchi M, Mente A, Mustafa R, et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr. 2012;66(7):780–8. doi: 10.1038/ejcn.2012.37. Epub 2012/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Scharrer E, Langhans W. Control of food intake by fatty acid oxidation. Am J Physiol. 1986;250(6 Pt 2):R1003–06. doi: 10.1152/ajpregu.1986.250.6.R1003. [DOI] [PubMed] [Google Scholar]
- 227.Schick RR, Schusdziarra V, Mössner J, Neuberger J, Schröder B, et al. Effect of CCK on food intake in man: physiological or pharmacological effect? Zeitschrift für Gastroenterologie. 1991;29(2):53. [PubMed] [Google Scholar]
- 228.Schiffman SS, Reilly DA, Clark TB. Qualitative differences among sweeteners. Physiol Behav. 1979;23:1–9. doi: 10.1016/0031-9384(79)90113-6. [DOI] [PubMed] [Google Scholar]
- 229.Schoeller DA. The effect of holiday weight gain on body weight. Physiol & Behav. 2014 doi: 10.1016/j.physbeh.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 230.Schultz W. Getting Formal with Dopamine and Reward. Neuron. 2002;36(2):241–63. doi: 10.1016/s0896-6273(02)00967-4. doi: http://dx.doi.org/10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 231.Seimon RV, Taylor P, Little TJ, Noakes M, Standfield S, et al. Effects of acute and longer-term dietary restriction on upper gut motility, hormone, appetite, and energy-intake responses to duodenal lipid in lean and obese men. Am J Clin Nutr. 2014;99:24–34. doi: 10.3945/ajcn.113.067090. [DOI] [PubMed] [Google Scholar]
- 232.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6(2):133–42. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 233.Sinha R, Garcia M, Paliwal P, Kreek M, Rounsaville BJ. STress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 234.Smeets PA, Weijzen P, de Graaf C, Viergever MA. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage. 2011;54(2):1367–74. doi: 10.1016/j.neuroimage.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 235.Smyth CJ, Lasichak AG, Levey S. The effect of orally and intravenously administered amino acid mixtures on voluntary food consumption in normal men. J Clin Invest. 1947;26:439–45. doi: 10.1172/JCI101827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr. 2007;86:1586–94. doi: 10.1093/ajcn/86.5.1586. [DOI] [PubMed] [Google Scholar]
- 237.Solms J. Taste of amino acids, peptides, and proteins. J Agric Food Chem. 1969;17:686–88. [Google Scholar]
- 238.Steinert RE, Feinle-Bisset C, Geary N, Beglinger C. Digestive physiology of the pig symposium: secretion of gastrointestinal hormones and eating control. J Anim Sci. 2013;91:1963–73. doi: 10.2527/jas.2012-6022. [DOI] [PubMed] [Google Scholar]
- 239.Stewart JE, Seimon RV, Otto B, Keast RSJ, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr. 2011;93:703–11. doi: 10.3945/ajcn.110.007583. [DOI] [PubMed] [Google Scholar]
- 240.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–60. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.St-Onge MP, Mayrsohn B, O’Keeffe M, Kissileff HR, Choudhury AR, Laferrère B. Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur J Clin Nutr. 2014;68(10):1134–40. doi: 10.1038/ejcn.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Strik CM, Lithander FE, McGill AT, MacGibbon AK, McArdle BH, Poppitt SD. No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: a randomised trial of fatty acid saturation. Nutr J. 2010;9:24. doi: 10.1186/1475-2891-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Stubbs RJ, Johnstone AM, O’Reilly LM, Poppitt SD. Methodological issues relating to the measurement of food, energy and nutrient intake in human laboratory-based studies. Proceed Nutr Soc. 1998;57(03):357–72. doi: 10.1079/pns19980053. [DOI] [PubMed] [Google Scholar]
- 244.Stubbs RJ, Murgatroyd PR, Goldberg GR, Prentice AM. Carbohydrate balance and the regulation of day-to-day food intake in humans. Am J Clin Nutr. 1993;57:897–903. doi: 10.1093/ajcn/57.6.897. [DOI] [PubMed] [Google Scholar]
- 245.Sun SZ, Anderson GH, Flickinger BD, et al. Fructose and non-fructose sugar intakes in the US population and their associations with indicators of metabolic syndrome. Food Chem Toxicol. 2011;49:2875–82. doi: 10.1016/j.fct.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 246.Swithers SE, Martin AA, Clark KM, Laboy AF, Davidson TL. Body weight gain in rats consuming sweetened liquids. Effects of caffeine and diet composition. Appetite. 2010;55(3):528–33. doi: 10.1016/j.appet.2010.08.021. doi: http://dx.doi.org/10.1016/j.appet.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Szabo O, Szabo AJ. Evidence for an insulin-sensitive receptor in the central nervous system. Am J Physiol. 1972;223:1349–53. doi: 10.1152/ajplegacy.1972.223.6.1349. [DOI] [PubMed] [Google Scholar]
- 248.Takai S, Yasumatsu K, Inoue M, Iwata S, Yoshida R, et al. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29(6):2268–80. doi: 10.1096/fj.14-265355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tang C, Ahmed K, Gille A, Lu S, Gröne HJ, et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21:173–77. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 250.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 251.Teegarden SL, Bale TL. Decreases in Dietary Preference Produce Increased Emotionality and Risk for Dietary Relapse. Biol Psychiatry. 2007;61:1021–29. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 252.Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162:924–32. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Teff KL, Mattes RD, Engleman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism. 1993;42(12):1600–08. doi: 10.1016/0026-0495(93)90157-j. [DOI] [PubMed] [Google Scholar]
- 254.Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limón P, et al. A Gut Lipid Messenger Links Excess Dietary Fat to Dopamine Deficiency. Science. 2013;341:800–02. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- 255.Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, et al. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur J Clin Nutr. 2014;69(2):162–66. doi: 10.1038/ejcn.2014.208. [DOI] [PubMed] [Google Scholar]
- 256.Tucker RM, Mattes RD, Running CA. Mechanisms and effects of “fat taste” in humans. BioFactors. 2014;40:313–26. doi: 10.1002/biof.1162. [DOI] [PubMed] [Google Scholar]
- 257.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 258.Uematsu A, Tsurugizawa T, Kitamura A, Ichikawa R, Iwatsuki K, et al. Evaluation of the “liking” and “wanting” properties of umami compound in rats. Physiol Behav. 2011;102:553–58. doi: 10.1016/j.physbeh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 259.Uhe AM, Collier GR, O’Dea K. A comparison of the effects of beef, chicken and fish protein on satiety and amino acid profiles in lean male subjects. J Nutr. 1992;122(3):467–72. doi: 10.1093/jn/122.3.467. [DOI] [PubMed] [Google Scholar]
- 260.Vahl TP, Drazen DL, Seeley RJ, D’Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-a secretion in rats. Endocrinology. 2010;151(2):569–75. doi: 10.1210/en.2009-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Van Itallie TB, Beaudoin R, Mayer J. Arteriovenous glucose differences, metabolic hypoglycemia, and food intake in man. J Clin Nutr. 1953;1:208–17. doi: 10.1093/ajcn/1.3.208. [DOI] [PubMed] [Google Scholar]
- 262.Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr. 1998;68(2):226–34. doi: 10.1093/ajcn/68.2.226. [DOI] [PubMed] [Google Scholar]
- 263.Van Wymelbeke V, Louis-Sylvestre J, Fantino M. Substrate oxidation and control of food intake in men after a fat-substitute meal compared with meals supplemented with an isoenergetic load of carbohydrate, long-chain triacylglycerols, or medium-chain triacylglycerols. Am J Clin Nutr. 2001;74(5):620–30. doi: 10.1093/ajcn/74.5.620. [DOI] [PubMed] [Google Scholar]
- 264.Vandewater K, Vickers Z. Higher-protein foods produce greater sensory-specific satiety. Physiol & Behavior. 1996;59(3):579–83. doi: 10.1016/0031-9384(95)02113-2. doi: http://dx.doi.org/10.1016/0031–9384(95)02113-2. [DOI] [PubMed] [Google Scholar]
- 265.Vazquez M, Pearson PB, Beauchamp GK. Flavor preferences in malnourished Mexican infants. Physiol Behav. 1982;28:513–19. doi: 10.1016/0031-9384(82)90148-2. [DOI] [PubMed] [Google Scholar]
- 266.Verschoor E, Finlayson G, Blundell J, Markus CR, King NA. Effects of an acute alpha-lactalbumin manipulation on mood and food hedonics in high- and low-trait anxiety individuals. Br J Nutr. 2010;104:595–602. doi: 10.1017/S0007114510000838. [DOI] [PubMed] [Google Scholar]
- 267.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci. 2011;108:15037–42. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wagner JW, De Groot J. Changes in feeding behaviour after intracerebral injections in the rat. Am J Physiol. 1963;204:483–87. doi: 10.1152/ajplegacy.1963.204.3.483. [DOI] [PubMed] [Google Scholar]
- 269.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, et al. The Influence of ΔFosB in the Nucleus Accumbens on Natural Reward-Related Behavior. J Neurosci. 2008;28:10272–77. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, et al. Brain dopamine and obesity. Lancet Lond Engl. 2001;357:354–57. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 271.Wardlaw SL, Burant CF, Klein S, Meece K, White A, et al. Continuous 24-hour leptin, proopiomelanocortin, and amino acid measurements in human cerebrospinal fluid: correlations with plasma leptin, soluble leptin receptor, and amino acid levels. J Clin Endocrinol Metab. 2014;99(7):2540–48. doi: 10.1210/jc.2013-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Weaver CM, Dwyer J, Fulgoni VL, et al. Processed foods: contributions to nutrition. The American Journal of Clinical Nutrition. 2014;99(6):1525–42. doi: 10.3945/ajcn.114.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82(1):41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 274.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53(6):495–502. doi: 10.1038/sj.ejcn.1600782. [DOI] [PubMed] [Google Scholar]
- 275.White CL, Purpera MN, Ballard K, Morrison CD. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol Behav. 2010;100(4):408–16. doi: 10.1016/j.physbeh.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wichman A, Allahyar A, Greiner TU, Plovier H, Lundén G, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–90. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 277.Wilding JP. Leptin and the control of obesity. Curr Opin Pharmacol. 2001;1(6):656–61. doi: 10.1016/s1471-4892(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 278.Wisén O, Björvell H, Cantor P, Johansson C, Theodorsson E. Plasma concentrations of regulatory peptides in obesity following modified sham feeding (MSF) and a liquid test meal. Regul Pept. 1992;39:43–54. doi: 10.1016/0167-0115(92)90007-h. [DOI] [PubMed] [Google Scholar]
- 279.Wøjdemann M, Traberg P, Stadil F, Sternby B, Larsen S, et al. Effect of sham feeding and acute suppression of acid secretion on human gastric lipase secretion. Am J Gastroenterol. 1998;93(2):244–48. doi: 10.1111/j.1572-0241.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 280.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 281.Woods SC, D’Alessio DA, Tso P, Rushing PA, Clegg DJ, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy. Balance Physiol Behav. 2004;83(4):573–78. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 282.Woods SC, Langhans W. Inconsistencies in the assessment of food intake. Am J Physiol Endocrinol Metab. 2012;303:E1408–18. doi: 10.1152/ajpendo.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food Intake and the Regulation of Body Weight. Annu Rev Psychol. 2000;51:255–77. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 284.Wurtman RJ, Fernstrom JD. Control of brain monoamine synthesis by diet and plasma amino acids. Am J Clin Nutr. 1975;28:638–47. doi: 10.1093/ajcn/28.6.638. [DOI] [PubMed] [Google Scholar]
- 285.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, Neil PM, Sebring NG. A Prospective Study of Holiday Weight Gain. N Eng J Med. 2000;342(12):861–7. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 287.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13(4):279–86. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]


